Abstract
Introduction
Emicizumab is a bispecific monoclonal antibody developed to address the unmet needs of clotting factor replacement therapy and has become the benchmark for optimal prophylaxis in managing patients with haemophilia A with inhibitors. We describe the emicizumab rollout and pharmacokinetic strategies and their use in paediatric patients.
Methods
The evolving real‐world experience in using emicizumab has confirmed its safety, efficacy and pharmacokinetic profile in paediatric, adolescent and adult patients receiving emicizumab at various prophylactic dosing regimens. The emicizumab current global rollout includes over 100 countries with 29 low to middle‐income countries accessing emicizumab through the World Federation of Haemophilia (WFH) Humanitarian Aid Program. The diversity of emicizumab dosing and pharmacokinetic tools such as the Calibra® and the WAPPS‐Hemo platforms make it possible to achieve prophylaxis goals in line with the WFH Haemophilia treatment guidelines recommendations, with minimal drug wastage. The emerging experience from long term clinical trials and long‐term real‐world follow‐up confirm the safety, efficacy, and pharmacokinetic profile of emicizumab in paediatric haemophilia A patients. A few questions, including inhibitor recurrence, concurrent use of emicizumab with various replacement therapies and inhibitor eradication, are being addressed through multiple ongoing clinical studies.
Conclusion
The current global rollout of emicizumab is remarkable, and versatile dosing regimens and evolving pharmacokinetic tools such as the Calibra® and WAPPS‐Hemo platforms make it a treatment choice available also for pharmacokinetic guided personalised treatment. Data from paediatric studies are consistent with those seen in adolescent and adult Haemophilia A.
Keywords: emicizumab, haemophilia A, rollout, pharmacokinetic, paediatric, real‐world evidence

1. INTRODUCTION
For several decades, replacement therapy with plasma‐derived or recombinant clotting factors was the standard of care in managing patients with haemophilia A. 1 However, patients on replacement therapy have several unmet needs, including the requirement for intravenous access to infuse clotting factor, the immunogenicity of replacement FVIII and clotting factor pharmacokinetics (PK) characterised by peaks and troughs, with the latter associated with breakthrough bleeds. 2 Non‐replacement therapies are a new treatment paradigm developed to address these unmet needs, and the first non‐factor therapeutic licenced is emicizumab.
Emicizumab (Hemlibra®, Roche, USA) is a bispecific monoclonal antibody that brings together activated Factor IX and Factor X to restore the haemostatic function of the absent Factor FVIII in haemophilia A patients. 3 It is indicated to prevent bleeds in haemophilia A patients with and without inhibitors. Data from four Phase 3 studies (HAVEN 1, HAVEN 2, HAVEN 3 and HAVEN 4) demonstrated that emicizumab is safe and efficacious with an improved stable PK profile when given subcutaneously weekly, fortnightly, or every four weeks in haemophilia A with and without inhibitors. 3 , 4 , 5 , 6 In addition, several ongoing clinical trials evaluate the safety and efficacy of emicizumab in haemophilia patients with mild or moderate levels of FVIII (HAVEN 6, clinicalTrials.gov Identifier NCT04158648) as well as in young paediatric patients (HAVEN 7, clinicalTrials.gov Identifier NCT04431726).
Since its first approval by the European Medicine Agency (EMA) in January 2018 7 and the United States Food and Drug Administration (FDA) in October 2018, 8 experience in using emicizumab has increased exponentially around the world. While the early switchers to emicizumab were predominantly inhibitor patients, there is now a rapid increase in its use for prophylaxis in non‐inhibitor haemophilia A patients, those challenged by venous access and high bleed rates despite receiving adequate FVIII prophylaxis. In the first part of the review, we update the global rollout of emicizumab, review the safety and efficacy profile of emicizumab in clinical trials and its use in the real world, and assess its impact on the care of patients with haemophilia beyond bleed prevention.
The 2020 World Federation of Haemophilia (WFH) Haemophilia Treatment Guideline has declared prophylaxis the new standard of care for haemophilia patients globally. 9 The guideline further recommends individualised prophylaxis for optimal patient outcomes. Strategies to implement personalised patient care include PK consideration, such as raising the trough level to match the individual lifestyle and physical activities. In the second part of this review, we update what is new in our understanding of the pharmacokinetic optimisation when using emicizumab and how this may improve patient outcomes.
HAVEN 2 was a paediatric Phase 3 study that evaluated the safety and efficacy of emicizumab in haemophilia A paediatric patients with inhibitors. 6 The PK, safety, and efficacy profiles of emicizumab paediatric patients in HAVEN 2 were similar to that of adolescents and adults in HAVEN 1 and HAVEN 3. However, the experience of emicizumab in young patients is limited, necessitating the current ongoing HAVEN 7 Phase 3 study and several real‐world studies evaluating emicizumab safety and efficacy in very young patients. Therefore, in the third part of this review, we update the infant paediatric trials and summarise the real‐world experience of using emicizumab in this patient population. We also give guidance on the role of immune tolerance induction in the era of emicizumab and suggest an approach to the first fifty exposures days in previously untreated patients on emicizumab (Figure 1).
FIGURE 1.
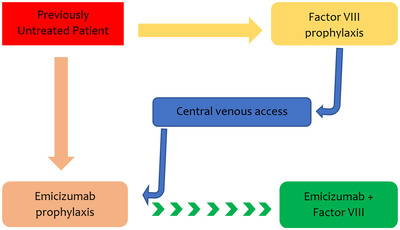
Suggested algorithm for prophylaxis of previously untreated (PUP) paediatric haemophilia A patients in the first 50 exposure days to replacement therapy. Parents have two choices of initial prevention, factor VIII or emicizumab. If venous access becomes an issue, we recommend transitioning to emicizumab for ease of administration and better adherence. This may or may not be accompanied by other factor VIII if the treater or caregiver believes it is essential for tolerance induction
2. EMICIZUMAB ROLLOUT
Since its first marketing authorisation in the United States of America (USA) and the European Union in 2018, the emicizumab rollout has progressed rapidly, reaching 107 countries for the inhibitor indication and 95 countries for the non‐inhibitor indication in the third quarter of 2021 (see Figure 2). The most recently reported number of patients using emicizumab in this short period now exceeds 12,500 patients. 10 In the USA, emicizumab is the number one agent prescribed for prophylaxis in inhibitor patients, who have had limited options for bleed prevention to date. Globally, the introduction of emicizumab has facilitated the acceptance of prophylaxis as the new global standard of care in inhibitor and non‐inhibitor haemophilia A patient management.
FIGURE 2.
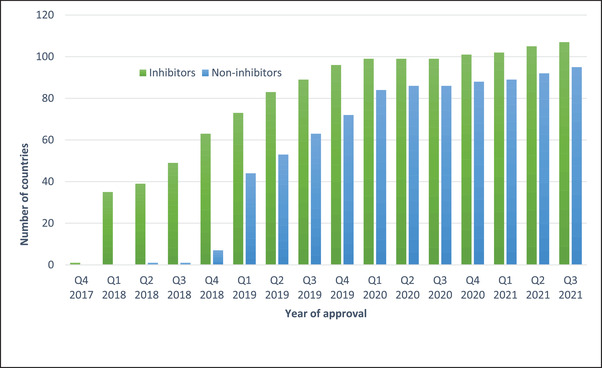
Regulatory approval of emicizumab for inhibitor and non‐inhibitor indications by year of approval. Data supplied courtesy of Roche Global, Switzerland
The global uptake of emicizumab prophylaxis beyond developed countries has been remarkable, with the impact of the World Federation of Haemophilia Humanitarian Aid Program (WFH HAP) particularly noteworthy. By the third quarter of 2021, the WFH HAP had distributed over 2528730 milligrams of emicizumab to 806 patients in 29 countries in Africa, Latin America, Asia and Eastern Europe. 11 Most emicizumab recipients in the WFH HAP are paediatric patients less than 12 years of age (55%), which is particularly significant as this may prevent or delay the onset of bleed‐induced haemophilic arthropathy needs to be demonstrated in future studies. In addition, most recipients of WFH HAP emicizumab (64%) are non‐inhibitor patients, which may partly reflect the difficulty of diagnosing patients with inhibitors in developing countries and lack of exposure to replacement therapy due to limited access to treatment.
The safety of emicizumab continues to be evaluated in both the clinical trial participants and real‐world experience. The recently published summary of long‐term follow‐up (median follow‐up of 130 weeks) of patients in HAVEN 1‐4 clinical trials indicates that overall emicizumab has an acceptable long term safety profile. 12 The noteworthy safety events from the 399 patients exposed to emicizumab in the four clinical trials were (1) one death in HAVEN 1 (judged by an investigator not to be related to emicizumab), (2) three microangiopathies in HAVEN 1 (due to co‐administration of emicizumab and activated prothrombin complex concentrate) and (3) four thrombotic events (two in HAVEN 1 and one each in HAVEN 2 and 3). Mitigating measures were implemented following these events in HAVEN 1, and these events did not recur in the subsequent HAVEN 2, 3 and 4 clinical trials and the STASEY study. Across the HAVEN studies, the most common adverse event was the injection site reaction which occurred in 27% of participants in which most (93%) were mild in severity. Data from the STASEY trial (ClinicalTrials.gov Identifier: NCT03191799) and HAVEN 5 (ClinicalTrials.gov Identifier: NCT03315455) programme concurs with the safety experience in HAVEN 1‐4 clinical trials.
Beyond the clinical trials, emicizumab safety has been documented in multiple real‐world experiences in patients with and without inhibitors. 13 , 14 , 15 , 16 , 17 , 18 , 19 In these studies, the emicizumab safety profile was similar to that seen in clinical trials. A few cases of anti‐emicizumab antibodies have been reported to date 20 , 21 ; however, the overall outlook is that immunogenicity is very low when using emicizumab compared to that seen with factor replacement therapies. 22 , 23 Breakthrough bleeds do occur when using emicizumab; however, current data do not show an increased risk of adverse events when these bleeds are treated with diverse replacement haemostatic agents. Following several reports of thromboses, deaths and microangiopathies associated with emicizumab in the compassionate use programs and off label use, a group of global haemophilia experts reviewed the available data and put these events into context when using other haemostatic agents. 24 , 25 , 26 Except for microangiopathy, which is unique to an emicizumab‐aPCC interaction, the deaths and thromboses were comparable to those seen when using other haemostatic agents in haemophilia A patients.
The efficacy of emicizumab in bleed prevention was very high in the HAVEN clinical trials, with most patients experiencing a mean of two bleeds per annum or less when the drug was given weekly, two‐weekly, or four‐weekly subcutaneously. 12 In addition, the proportion of patients with 1‐3 bleeds during the study was high, ranging from 70% to 100% in HAVEN 1‐4 trials and the HAVEN 5 and Stasey studies. Target joint resolution was also high at 99.2%, with patients able to successfully undertake both major and minor surgeries during emicizumab treatment.
The real‐world experience with emicizumab efficacy is still limited to date, with all published efficacy data showing similar results to those reported in the clinical trials. 15 , 16 , 17 , 27 , 28 , 29 However, treatment failure in the absence of anti‐emicizumab antibodies has now been reported in a child with recurrent intracranial bleeds. 30 This was not seen during the clinical trial conduct. In addition, recent real‐world experience from the Israeli group indicates that long‐term, the efficacy of emicizumab tends to reduce in adults with more breakthrough bleeds and need to go back to replacement in some cases. At the same time, this is not the case in children. 17 In the global rollout of emicizumab, there is now growing experience and confidence that switching patients from their replacement therapies to emicizumab is not associated with clinically relevant anti‐emicizumab antibodies or risk of thrombosis or bleeding. This observation is consistent with data seen when switching patients between various replacement therapies. 31
Beyond the acceptable safety and efficacy profiles, there is evolving evidence that emicizumab positively impacts the management and lives of patients with haemophilia. For example, emicizumab prophylaxis in PwHA without FVIII inhibitors resulted in persistent and meaningful improvements in Haem‐A‐QoL score and less work disruption than previous treatment. 27 , 32 Furthermore, apart from resolving almost all target joints, 12 recent data suggests it improves joint health as measured by joint scores. 33 Thus, not only does subcutaneous injection reduce the treatment burden, but it may also improve prophylaxis adherence and outcomes. The data to support the latter benefits are currently collected in many real‐world experiences.
The cost of emicizumab remains the single most significant barrier for its access in both developing and developed countries. Multiple studies have shown that bleed prevention with emicizumab is more cost‐effective than bypassing agents. 34 , 35 The introduction of Emicizumab to healthcare systems has the potential to generate additional savings by (1) delaying FVIII inhibitor development in previously untreated patients (PUPs) with the severe disease by up to 13 years, 36 (2) offering cost saving of $2 million per patient over 20 years by reducing immune tolerance induction and breakthrough bleeds, 36 (3) eliminating the need for surgical implantation of central venous access devices, especially in paediatrics and reducing severe bleeds requiring hospitalisation. The most considerable cost saving could be associated with maintaining joint health, but still, we do not know if this will be the case.
The recent Institute for Clinical and Economic Review (ICER) Haemophilia A assessment concluded that emicizumab provides comparable or better clinical benefits and cost savings than factor VIII prophylaxis in people with severe haemophilia A without factor VIII inhibitors, depending on the cost per country. 37 Therefore, one of the policy recommendations included within the final ICER Report states that considering the evidence of equivalent to improved comparative effectiveness, patient preference and lower overall cost, payers should work with clinicians and patients to encourage the use of Hemlibra over factor VIII for prophylaxis, unless it is contraindicated.
Notwithstanding its high therapeutic efficacy, acceptable safety profile, impressive pharmacokinetics and dosing versatility, there are a few unmet needs associated with the use of emicizumab. These include (1) its indication for prevention but not treatment of bleeds, (2) emicizumab interference with clot‐based assays, (3) haemostatic monitoring in the peri‐surgical setting and (4) absence of reversal agents for its effect. Several studies are currently ongoing to address some of these challenges, and these include (1) the MOTIVATE study, exploring concurrent use of emicizumab and clotting factor for ITI (www.clinicalTrials.gov identifier: NCT040230192), the INHIBIT clinical trial (www. clinicaltrials.gov identifier NCT040303572) and (2) the PRIORITY study exploring the possible effect of emicizumab in preventing inhibitor recurrence (www. clinicaltrials.gov NCT04030052)
3. PHARMACOKINETIC‐BASED EMICIZUMAB DOSING
For prophylactic factor replacement to be adequate, it must be tailored based on the patient's bleeding risk factors, bleeding history, current joint status, patient's preferences as to physical activity and life goals, and individual variability in the pharmacokinetics (PK) of factor concentrates. 9 Even back when targeting the historical trough level of 0.01 IU/mL (1%), just about 50% of patients achieved the target by adopting the one‐size‐fits‐all regimen of 20‐40 IU/kg given two to three times a week for factor VIII or 20‐50 IU/kg given twice a week for factor IX. 38 Nowadays, population PK‐based individualised treatment is recommended by the WFH haemophilia treatment guidelines for all patients, jointly with individualised target factor levels matching the patient's own individual goals and needs. 9
On the opposite end of the spectrum, emicizumab has, for the first time represented the opportunity to dramatically simplify the bleed prevention approach in haemophilia A patients. 12 From the PK point of view, emicizumab is an antibody, and, as such, it has a very favourable and rather predictable PK profile, 5 , 39 , 40 , 41 In addition, emicizumab is slowly and consistently absorbed when administered subcutaneously, 42 reaching and maintaining very stable levels for many weeks, to the point that the effect may last for many months after stopping treatment.
The clinical correlates of emicizumab's PK and pharmacodynamic(PD) profile are demonstrated in the HAVEN study program results 12 and several subsequent real‐world evidence data sets. 15 , 16 , 17 , 18 , 28 , 43 , 44 , 45 Given at a fixed dose of 1.5 mg/kg weekly (or equivalent dosing for longer time intervals), emicizumab provides robust and consistent protection from bleeding. Additionally, standard clotting assays available to most clinical laboratories are unsuitable for measuring emicizumab or its effect and cannot be used for dose adjustments. For all these reasons, emicizumab held the promise of genuinely representing the one‐size‐fits‐all solution for prophylaxis in haemophilia. Same dose for all, no need for laboratory monitoring, no PK profiling, and no treatment tailoring. Of course, this was (and is) a precious and welcome promise. But, one may argue, does it hold true after a few years, thousands of patients, and mounting real‐world clinical experience in the use of Emicizumab? Let's review the emerging evidence together.
First, whilst the PK of emicizumab is very predictable (i.e. its level in the blood spans over a narrow range), its PD shows more variability. 5 , 40 Even in the presence of emicizumab, clotting continues to be a very complex cascade of tightly regulated biochemical reactions, where the activation of factor X (which Emicizumab contributes to) is a critical step, yet one of many. Thus, the clotting effect of the same dose of emicizumab varies in different patients.
Second (and directly stemming from the observation of the PD variability), a sizeable minority of haemophilia treatment centres managed to adopt some form of measurement of the clotting activity of Emicizumab in their patients. 18 , 45 Likely, this will pave the way to some form of tailoring.
Third, the ‘factor VIII equivalent’ effect of emicizumab when given at 1.5 mg/Kg weekly (or equivalent dosing at longer intervals) is a stable 0.10‐0.20 IU/mL (10‐20%). 5 , 40 This confers a level of protection many times higher than we could dream of before the Emicizumab era and yet represents a paradigm shift for not providing post‐infusion peaks. The true significance of peaks remains mainly unknown, but they are considered helpful for safe high‐intensity physical activity, impact sports, or surgery, among other benefits. However, there may be a benefit for factor level in the normal range, which is never achieved with emicizumab, which generate clotting factor activity equivalent to 10‐20%.
Fourth, there is growing concern that the one‐size‐fits‐all regimen may not be leading to higher compliance. 46 Compliance with treatment has always been a severe concern in haemophilia management. The most common explanation for poor compliance with prophylaxis is the burden of treatment of an intravenous regimen requiring multiple venipunctures per week. The reduced burden associated with the advent of EHL Factor IX and Emicizumab seems to indicate that patients with haemophilia are no exception to the general rule that in general compliance to prescribed regimen tends to be lower than optimal and decreases with increasing time on treatment.
Finally, the practical implementation of the 1.5 mg/kg (or equivalent) regimens is not achievable in many patients using full vials. Injecting only part of a vial content introduces the risk for errors in the administered amount. In addition, it implies wasting medication, two concepts challenging to accept for patients and treaters in the haemophilia community. Furthermore, the linear relationship among the three available regimens (1.5 mg/week = 3 mg/2weeks = 6 mg/4weeks) suggests many other combinations of dose and interval (e.g. 2 mg/9 days or 4 mg/18 days) will yield the same emicizumab plasma level. Indeed, back of the napkin adjustments is reported to happen among current emicizumab users 47 widely.
The WAPPS‐Hemo research group has developed, responding to suggestions from the user base, a tool intended to support different levels of Emicizumab treatment, potentially addressing some of the observations proposed above. The tool called Calibra® is freely available at http://Calibra.app and includes a patient module, myCalibra®, available on the Apple and Google applications stores. The scientific base for Calibra®’s set of functionalities has been previously published. 41 , 47 In essence, as shown in Figure 3, Calibra® allows the treating physician to input the patient weight and select the intended treatment regimen. Calibra® then calculates the appropriate theoretical dose and suggests an alternative combination of dose and injection frequencies resulting in full vial(s) usage. Options available to the physician and patient indicate the available vial size(s) to use, if one or more measures, and one or multiple vials. Calibra® also allows ‘manual’ input of the desired dose, vial size and quantity. Once the amount is selected, Calibra® calculates the appropriate treatment interval, how many injections per year will be avoided, and how much drug waste will be avoided. The chosen regimen is available for offloading to myCalibra®. myCalibra® prompts the patient when it is time to inject, allows the patient to record the injection of emicizumab, and log any bleed they may experience. myCalibra® can generate an injection report, and all the data are available to the doctor in the myWAPPS dashboard. In the future, measurements of clotting activity after emicizumab injection could be modelled if becoming part of routine clinical practice. Whether Calibra® or similar apps will positively impact patient compliance and satisfaction is yet to be explored.
FIGURE 3.
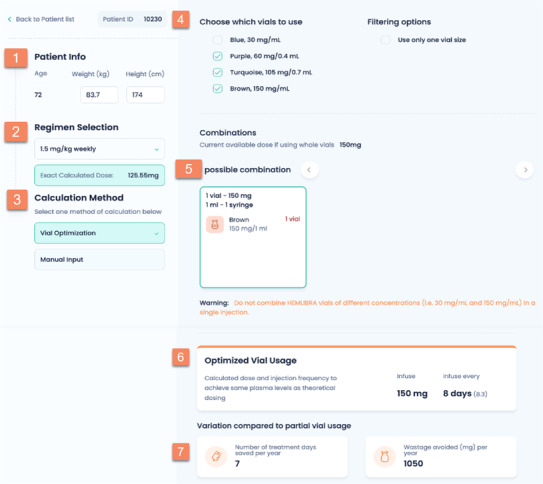
The picture reproduces the main page of the Calibra® application, and shows the logical flow to optimize the use of emicizumab vials for a given patients based on his weight and selected dose
4. UPDATE ON PAEDIATRIC CLINICAL STUDIES AND THE REAL‐WORLD EXPERIENCE
Prevention is the recommended standard of care for patients with haemophilia A, aiming to prevent life‐threatening bleeds, mitigate the risk of chronic joint damage and improve quality of life 48 Emicizumab is administered subcutaneously, which makes it especially beneficial for treating paediatric populations.
The results of a multicenter phase 3 study examining emicizumab prophylaxis in HA patients with inhibitors were encouraging for the HAVEN 1, which investigated adults and adolescents, and HAVEN 2, which analysed 2 to 12‐year‐olds. 3 , 49 The median age of the paediatric study patients was seven years, and only two patients were younger than 2 years of age. 49 In addition, a recent analysis of the Haemo‐QoL SF II questionnaire completed by children 8‐11 years old who took part in the HAVEN 2 study disclosed substantial and sustained improvements in HRQoL of paediatric HA patients with FVIII inhibitors and their caregivers. 50 Notably, the HAVEN 3‐4 trials on emicizumab prophylaxis in non‐inhibitor HA patients included mainly adults and adolescents. 4 , 5
Real‐world data on the safety and efficacy of emicizumab prophylaxis among the paediatric haemophilia population are still limited. A single publication by Barg et al. addressed emicizumab use in infants and toddlers, describing a prospectively followed cohort of 11 very young children with HA and inhibitors. 51 The HOHOEMI publication on non‐inhibitor children followed a total of 13 Japanese patients (aged four months to 10 years) who had been successfully treated by emicizumab prophylaxis every 2 or 4 weeks. 52 In this study, only three children out of 13 were aged < 2 years.
Several cohort studies from the US included paediatric patients. Ebbert and colleagues reported reducing annual bleeding rates (ABR) and good safety in a mixed cohort of 42 HA with and without inhibitors, of whom 40% were younger than 18 years of age. 53 A multicentre study reported 93 HA patients (19 of them with FVIII inhibitor), whose median age was 8.6 years. Patients <12 years without inhibitors (n = 49) accounted for much of this cohort. In this study, ABR dropped from 4.4 (inhibitors) and 1.6 (non‐inhibitors) to 0.4 (both groups) on emicizumab prophylaxis, 28 minor (21 port removals) and two significant procedures were successfully performed. No serious adverse events were noted. 45 Another recent mixed cohort by Warren et al. reported emicizumab prophylaxis in children whose initial age was 0.5 years. Yet, paediatric data and data regarding emicizumab's potential use in PUPs were not specified. 13 The most extensive prospective paediatric study reported to date is the Israeli cohort that sought to evaluate safety, efficacy, and laboratory monitoring of emicizumab prophylaxis in a cohort of 40 children with severe HA, including 22 non‐inhibitor patients and nine infants younger than one year. Bleeding, trauma, adverse events, and surgeries were documented during a median follow‐up of 45 weeks. Emicizumab levels activated partial thromboplastin time (aPTT) values, and thrombin generation was measured before and during therapy. Twenty patients experienced zero bleeds. Sixteen surgical interventions were performed in 12 patients, with no thrombotic complications or thrombotic microangiopathy. Elevation in the thrombin generation was observed following emicizumab prophylaxis, with lower values recorded in younger infants, yet as all bleedings were trauma‐related, laboratory monitoring could not predict bleeding risk. 13 Further longitudinal follow–up of the mixed Israeli cohort (107 patients with severe HA, including 58 children whose median (IQR) age was 6 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 years), followed for a median of 67 weeks (up to 144 weeks) disclosed zero bleeds in half of the patients. Most bleeding episodes (94%) among children were trauma‐related. One infant noted one major bleed with a fatal outcome, presenting with central venous line thrombosis. 18 A retrospective cohort study by Cohen et al. recently addressed prophylaxis switches from FVIII products into emicizumab therapy in 28 paediatric HA patients. The median age at the time of starting emicizumab was 6.7 years (range 0.2‐20.4), with the majority (n = 22, 78.6%) of patients starting under 12 years of age. The median follow‐up time was 2.2 years (range 0.7–3.6), with favourable outcomes. 54
Emicizumab prophylaxis is becoming the standard of care for paediatric patients with HA and inhibitors. Using emicizumab in place of ITI has been described in paediatric patients. 55 , 56 , 57 However, most inhibitors in tolerised patients would not recur. Ongoing inhibitor monitoring in tolerised patients with haemophilia A who transition to emicizumab is strongly recommended. 55 Regarding surgical interventions in paediatric patients during emicizumab prophylaxis, a recent study in children whose median (range) age was 6.6 years (0.7‐13.2) confirmed that minor procedures, especially central venous access device removals, can be safely done with antifibrinolytic agents without administration of additional haemostatic agents, regardless of FVIII inhibitor status. 58
Overall, the availability of emicizumab has significantly upended the traditional approach to managing children with haemophilia A. Its use may substantially delay initial exposure to FVIII, thereby altering the natural history of inhibitor development. Still, it remains unclear whether later exposure to FVIII or simultaneous exposure to low dose FVIII (at reasonable intervals) might modify the incidence of inhibitor development. Early administration of emicizumab prophylaxis is convenient (therefore, parental adherence to therapy may increase). It may avoid the need for central venous lines insertion to facilitate venous access, and it should reduce the potential risk of bleeding, including the hazard of intracerebral haemorrhage. The EMI‐PUPs study (NCT04030052) will aim to address these critical questions. Two other exciting studies currently on the way are the Haemophilia Prevention Trial NCT04303559‐ a phase 3 randomised multicentre trial that would compare inhibitor occurrence in children treated by Elocta versus emicizumab prophylaxis and the Emicizumab PUPs and NUWIQ ITI study (NCT04030052) addressing bleeding and inhibitor formation once both drugs are co‐administered. 59 Before recommending PUP therapy or transitioning previously treated children into emicizumab prophylaxis, a shared decision‐making process with parents and caregivers is advised (see Figure 1).
5. CONCLUSION
In conclusion, in a world of expanding choices, emicizumab became a very welcome addition to the armamentarium of paediatric, adolescents and adults’ haemophilia A treaters. The current global rollout of emicizumab is remarkable, with long term clinical trial data and real‐world experience confirming its acceptable safety and efficacy profile. The versatile dosing regimens and evolving pharmacokinetic tools such as the Calibra® and WAPPS‐Hemo platforms make it a treatment choice available also for pharmacokinetic guided treatment. Data from paediatric studies are consistent with those seen in adolescent and adult Haemophilia A.
ACKNOWLEDGEMENTS
The authors would like to thank Roche (Dr Fabian Sanabria) and the World Federation of Haemophilia (Dr Assad Affar) for sharing the most recent emicizumab rollout data included in this paper.
Mahlangu J, Iorio A, Kenet G. Emicizumab state‐of‐the‐art update. Haemophilia. 2022;28(Suppl. 4):103–110. 10.1111/hae.14524
[Correction added on July 6, 2022, after first online publication: The copyright line was changed.]
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Aledort L, Mannucci PM, Schramm W. Factor VIII replacement is still the standard of care in haemophilia A. Blood Transfus. 2019;17:479‐486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mahlangu J. An update of the current pharmacotherapeutic armamentarium for hemophilia A. Expert Opin Pharmacother. 2022;23:129‐138. [DOI] [PubMed] [Google Scholar]
- 3. Oldenburg J, Mahlangu JN, Kim B, et al. Emicizumab Prophylaxis in Hemophilia A with Inhibitors. N Engl J Med. 2017;377:809‐818. [DOI] [PubMed] [Google Scholar]
- 4. Mahlangu J, Oldenburg J, Paz‐Priel I, et al. Emicizumab Prophylaxis in Patients Who Have Hemophilia A without Inhibitors. N Engl J Med. 2018;379:811‐822. [DOI] [PubMed] [Google Scholar]
- 5. Pipe SW, Shima M, Lehle M, et al. Efficacy, safety, and pharmacokinetics of emicizumab prophylaxis given every 4 weeks in people with haemophilia A (HAVEN 4): a multicentre, open‐label, non‐randomised phase 3 study. Lancet Haematol. 2019;6:e295‐e305. [DOI] [PubMed] [Google Scholar]
- 6. Young G, Liesner R, Chang T, et al. A multicenter, open‐label phase 3 study of emicizumab prophylaxis in children with hemophilia A with inhibitors. Blood 2019;134:2127‐2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hemlibra European Medicines Agency (EMA) Marketing Authorisation 2018. https://www.ema.europa.eu/en/news/first‐class‐medicine‐prevent‐bleeding‐haemophilia‐patients‐inhibitors. Accessed November 20, 2021.
- 8. Hemlibra Food and Drug Administration (FDA) approval for patients with and without inhibitors 2018. https://www.fda.gov/drugs/drug‐approvals‐and‐databases/fda‐approves‐emicizumab‐kxwh‐hemophilia‐or‐without‐factor‐viii‐inhibitors. Accessed November 20, 2021.
- 9. Srivastava A, Santagostino E, Dougall A, et al. WFH Guidelines for the Management of Hemophilia, 3rd edition. Haemophilia 2020; 26 Suppl 6: 1‐158. [DOI] [PubMed] [Google Scholar]
- 10. Roche 2021 Annual Report. https://www.roche.com/dam/jcr:d7745cfb‐1133‐447e‐9a5b‐88b849495574/en/irp211020‐a.pdf. Accessed November 20, 2021.
- 11.Personal Communication, Humanitarian Aid Program Directorate, World Federation of Haemophilia, Montreal, Canada. Accessed November 20, 2021.
- 12. Callaghan MU, Negrier C, Paz‐Priel I, et al. Long‐term outcomes with emicizumab prophylaxis for hemophilia A with or without FVIII inhibitors from the HAVEN 1‐4 studies. Blood 2021;137:2231‐2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Warren BB, Chan A, Manco‐Johnson M, et al. Emicizumab initiation and bleeding outcomes in people with hemophilia A with and without inhibitors: A single‐center report. Res Pract Thromb Haemost 2021;5:e12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Krumb E, Fijnvandraat K, Makris M, et al. Adoption of emicizumab (Hemlibra®) for hemophilia A in Europe: Data from the 2020 European Association for Haemophilia and Allied Disorders survey. Haemophilia 2021;27:736‐743. [DOI] [PubMed] [Google Scholar]
- 15. Hassan E, Jonathan L, Jayashree M. Real‐world experience on the tolerability and safety of emicizumab prophylaxis in paediatric patients with severe haemophilia A with and without FVIII inhibitors. Haemophilia. 2021;27:e698‐e703. [DOI] [PubMed] [Google Scholar]
- 16. Misgav M, Brutman‐Barazani T, Budnik I, et al. Emicizumab prophylaxis in haemophilia patients older than 50 years with cardiovascular risk factors: Real‐world data. Haemophilia. 2021;27:253‐260. [DOI] [PubMed] [Google Scholar]
- 17. Levy‐Mendelovich S, Brutman‐Barazani T, Budnik I, et al. Real‐World Data on Bleeding Patterns of Hemophilia A Patients Treated with Emicizumab. J Clin Med. 2021;10:4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barg AA, Budnik I, Avishai E, et al. Emicizumab prophylaxis: Prospective longitudinal real‐world follow‐up and monitoring. Haemophilia. 2021;27:383‐391. [DOI] [PubMed] [Google Scholar]
- 19. Barg AA, Livnat T, Budnik I, et al. Emicizumab treatment and monitoring in a paediatric cohort: real‐world data. Br J Haematol. 2020;191:282‐290. [DOI] [PubMed] [Google Scholar]
- 20. Valsecchi C, Gobbi M, Beeg M, et al. Characterization of the neutralising anti‐emicizumab antibody in a patient with hemophilia A and inhibitor. J Thromb Haemost. 2021;19:711‐718. [DOI] [PubMed] [Google Scholar]
- 21. Kaneda M, Kawasaki R, Matsumoto N, et al. Detailed analysis of anti‐emicizumab antibody decreasing drug efficacy, using plasma samples from a patient with hemophilia A. J Thromb Haemost 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schmitt C, Emrich T, Chebon S, et al. Low immunogenicity of emicizumab in persons with haemophilia A. Haemophilia 2021;27:984‐992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harroche A, Sefiane T, Desvages M, et al. Non‐inhibitory antibodies inducing increased emicizumab clearance in a severe haemophilia A inhibitor patient. Haematologica 2021;106:2287‐2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pipe SW, Kruse‐Jarres R, Mahlangu JN, et al. Establishment of a framework for assessing mortality in persons with congenital hemophilia A and its application to an adverse event reporting database. J Thromb Haemost 2021;19 Suppl 1:21‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Peyvandi F, Mahlangu JN, Pipe SW, et al. Application of a hemophilia mortality framework to the Emicizumab Global Safety Database. J Thromb Haemost 2021; 19 Suppl 1: 32‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hay CRM, Nissen F, Pipe SW. Mortality in congenital hemophilia A – a systematic literature review. J Thromb Haemost 2021;19 Suppl 1:6‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Skinner MW, Négrier C, Paz‐Priel I, et al. The effect of emicizumab prophylaxis on long‐term, self‐reported physical health in persons with haemophilia A without factor VIII inhibitors in the HAVEN 3 and HAVEN 4 studies. Haemophilia. 2021;27:854‐865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Samelson‐Jones BJ, Guelcher C, Kuhn J, et al. Real‐world cost estimates of initiating emicizumab in US patients with haemophilia A. Haemophilia. 2021;27:591‐598. [DOI] [PubMed] [Google Scholar]
- 29. Garcia J, Zia A. Real‐world case series and summary of current literature of infants and toddlers with severe hemophilia A with inhibitor on prophylaxis with emicizumab. Pediatr Blood Cancer 2021;68:e28942. [DOI] [PubMed] [Google Scholar]
- 30. Teo HKW, Wong WH, Lam JCM. Recurrent intracranial bleed in a child receiving prophylaxis with emicizumab. Haemophilia. 2021;27:e415‐e418. [DOI] [PubMed] [Google Scholar]
- 31. Nance D, Rodgers GM. Switching haemophilia products and inhibitor risk: a United States' perspective. Eur J Haematol. 2015;94:283. [DOI] [PubMed] [Google Scholar]
- 32. Abarca‐Villaseca V, Soto‐Arellano V. Breakthrough Bleeding Episodes at Minimum and Improvement in Quality of Life in a Child with Severe Hemophilia A with Inhibitors Treated with Emicizumab: A Case Report from Chile. Am J Case Rep. 2021;22:e929598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wu Y, Lu J, Zhou Y, et al. Long‐term joint outcomes of regular low‐dose prophylaxis in Chinese children with severe haemophilia A. Haemophilia. 2021;27:237‐244. [DOI] [PubMed] [Google Scholar]
- 34. Cortesi PA, Castaman G, Trifirò G, et al. Cost‐Effectiveness and Budget Impact of Emicizumab Prophylaxis in Haemophilia A Patients with Inhibitors. Thromb Haemost. 2020;120:216‐228. [DOI] [PubMed] [Google Scholar]
- 35. Lee H, Cho H, Han JW, et al. Cost‐utility analysis of emicizumab prophylaxis in haemophilia A patients with factor VIII inhibitors in Korea. Haemophilia. 2021;27:e12‐e21. [DOI] [PubMed] [Google Scholar]
- 36. Patel AM, Corman SL, Chaplin S, Raimundo K, Sidonio RF. Economic impact model of delayed inhibitor development in patients with hemophilia a receiving emicizumab for the prevention of bleeding events. J Med Econ. 2019;22:1328‐1337. [DOI] [PubMed] [Google Scholar]
- 37. Insititute for Clinical and Economic Review (ICER) . https://icer.org/news‐insights/press‐releases/icer‐publishes‐evidence‐report‐on‐therapies‐for‐hemophilia‐a/. Accessed November 30, 2021.
- 38. McEneny‐King A, Iorio A, Foster G, Edginton AN. The use of pharmacokinetics in dose individualisation of factor VIII in the treatment of hemophilia A. Expert Opin Drug Metab Toxicol. 2016;12:1313‐1321. [DOI] [PubMed] [Google Scholar]
- 39. Schmitt C, Adamkewicz JI, Xu J, et al. Pharmacokinetics and Pharmacodynamics of Emicizumab in Persons with Hemophilia A with Factor VIII Inhibitors: HAVEN 1 Study. Thromb Haemost. 2021;121:351‐360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Donners A, Rademaker CMA, Bevers LAH, et al. Pharmacokinetics and Associated Efficacy of Emicizumab in Humans: A Systematic Review. Clin Pharmacokinet. 2021;60:1395‐1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Retout S, Schmitt C, Petry C, Mercier F, Frey N. Population Pharmacokinetic Analysis and Exploratory Exposure‐Bleeding Rate Relationship of Emicizumab in Adult and Pediatric Persons with Hemophilia A. Clin Pharmacokinet. 2020;59:1611‐1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kotani N, Yoneyama K, Kawakami N, Shimuta T, Fukase H, Kawanishi T. Relative and Absolute Bioavailability Study of Emicizumab to Bridge Drug Products and Subcutaneous Injection Sites in Healthy Volunteers. Clin Pharmacol Drug Dev. 2019; 8: 702‐712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mason JA, Young G. Emicizumab prophylaxis in infants with severe haemophilia A without inhibitors: Illustrative real‐world cases to support shared decision‐making. Haemophilia. 2021;27:724‐729. [DOI] [PubMed] [Google Scholar]
- 44. Barg AA, Avishai E, Budnik I, et al. The potential role of emicizumab prophylaxis in severe von Willebrand disease. Blood Cells Mol Dis. 2021;87:102530. [DOI] [PubMed] [Google Scholar]
- 45. McCary I, Guelcher C, Kuhn J, et al. Real‐world use of emicizumab in patients with haemophilia A: Bleeding outcomes and surgical procedures. Haemophilia. 2020;26:631‐636. [DOI] [PubMed] [Google Scholar]
- 46. Nieuwlaat R, Wilczynski N, Navarro T, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2014;2014:Cd000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yu JK, Iorio A, Chelle P, Edginton AN. Pharmacokinetic implications of dosing emicizumab based on vial size: A simulation study. Haemophilia. 2021;27:358‐365. [DOI] [PubMed] [Google Scholar]
- 48. Mancuso ME, Male C, Kenet G, et al. Prophylaxis in children with haemophilia in an evolving treatment landscape. Haemophilia. 2021;27:889‐896. [DOI] [PubMed] [Google Scholar]
- 49. Young G, Liesner R, Chang T, et al. A multicenter, open‐label phase 3 study of emicizumab prophylaxis in children with hemophilia A with inhibitors. Blood. 2019;34:2127‐2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mancuso ME, Mahlangu J, Sidonio R, Jr ., et al. Health‐related quality of life and caregiver burden of emicizumab in children with haemophilia A and factor VIII inhibitors‐Results from the HAVEN 2 study. Haemophilia. 2020;26:1009‐1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Barg AA, Avishai E, Budnik I, et al. Emicizumab prophylaxis among infants and toddlers with severe hemophilia A and inhibitors‐a single‐center cohort. Pediatr Blood Cancer. 2019;66:e27886. [DOI] [PubMed] [Google Scholar]
- 52. Shima M, Nogami K, Nagami S, et al. A multicentre, open‐label study of emicizumab given every 2 or 4 weeks in children with severe haemophilia A without inhibitors. Haemophilia. 2019;25:979‐987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ebbert PT, Xavier F, Seaman CD, Ragni MV. Emicizumab prophylaxis in patients with haemophilia A with and without inhibitors. Haemophilia. 2020;26:41‐46. [DOI] [PubMed] [Google Scholar]
- 54. Cohen CT, Diaz R. Emicizumab in pediatric hemophilia: Bleeding and surgical outcomes from a single‐center retrospective study. Pediatr Blood Cancer. 2021;68:e29325. [DOI] [PubMed] [Google Scholar]
- 55. Batsuli G, Greene A, Meeks SL, Sidonio RF, Jr . Emicizumab in tolerised patients with hemophilia A with inhibitors: A single‐institution pediatric cohort assessing inhibitor status. Res Pract Thromb Haemost 2021;5:342‐348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Carcao M, Escuriola‐Ettingshausen C, Santagostino E, et al. The changing face of immune tolerance induction in haemophilia A with the advent of emicizumab. Haemophilia. 2019;25:676‐684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Batsuli G, Zimowski KL, Tickle K, Meeks SL, Sidonio RF, Jr . Immune tolerance induction in paediatric patients with haemophilia A and inhibitors receiving emicizumab prophylaxis. Haemophilia. 2019;25:789‐796. [DOI] [PubMed] [Google Scholar]
- 58. Hassan E, Motwani J. Management and outcomes of paediatric patients on emicizumab prophylaxis undergoing surgical procedures: Experience from a large haemophilia centre in the UK. Haemophilia. 2021;27:e620‐e623. [DOI] [PubMed] [Google Scholar]
- 59. Young G. Management of children with haemophilia A: How emicizumab has changed the landscape. J Thromb Haemost. 2021;19:1629‐1637. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


