Abstract
Introduction:
Randomized clinical trials have shown that anti-osteoporotic treatments can increase bone mineral density (BMD) and reduce the incidence of fragility fractures. However, data on the real-life effectiveness of anti-osteoporotic medications are still scarce.
Methods:
We conducted a cohort study on women at high risk of fracture. We retrieved clinical and densitometric data from the DeFRA database, which derives from the DeFRA tool, a web-based fracture risk assessment tool. Multivariable Cox regression survival models were employed to analyze the effectiveness of different anti-osteoporotic drugs on fracture. In sensitivity analyses, we conducted 1:1 propensity score matching analyses.
Results:
Data on 50,862 women were available. Among these, 3574 individuals had at least two consecutive visits. The crude fracture rate was 91.9/1000 person-year for non-treated patients. The crude fracture rate in bisphosphonate users was 72.1/1000 person-year, in denosumab users was 58.2/1000 person-year, and in teriparatide users was 19.3/1000 person-year. Overall, we found that bisphosphonate use was associated with a 30% lower risk of fracture compared to no treatment [adjusted hazard ratio (aHR): 0.70, 95% confidence interval (CI): 0.50–0.98]. Treatment with denosumab and teriparatide were associated with 60% and 90% lower risk of fracture, respectively (aHR: 0.43, 95% CI: 0.24–0.75 and aHR: 0.09, 95% CI: 0.01–0.70). Bisphosphonate use was associated with a lower risk of fracture only after 1 year of treatment.
Conclusion:
In conclusion, we found that all anti-osteoporotic medications considered in the study effectively reduced the risk of fracture in the real-life. The effect of bisphosphonate on fracture risk was apparent only after the first year of treatment. Our findings do not support the use of bisphosphonates in patients at imminent risk of fracture.
Keywords: anabolics, bisphosphonates, denosumab, osteoporosis, teriparatide
Introduction
Osteoporosis is a systemic disorder characterized by decreased bone mass, which leads to an increased risk of fragility fractures. 1 The primary objective of treatment should be preventing fragility fractures. Clinicians have access to various anti-osteoporotic pharmacological agents (e.g. bisphosphonates, denosumab, teriparatide, and in some countries, romosozumab) that can effectively prevent osteoporotic fractures. Efficacy of all these drugs compared to calcium plus vitamin D alone or, for some of these, against active comparators, has been broadly demonstrated in clinical trials. 2 However, robust data on effectiveness of one treatment over another are still lacking. 3 Indeed, real-life studies are commonly flawed by confounding by indication bias and protopathic bias. As a matter of fact, anabolic agents are commonly prescribed to patients with more severe osteoporosis, 4 which are, by definition, at higher risk of experiencing fractures; hence, controlling for pre-treatment risk of fracture is mandatory in such analyses.
Low bone mineral density (BMD) is among the major determinants of fracture risk. It has been demonstrated that BMD contributes substantially to fracture risk. 5 Halting the BMD loss over time was associated with lower risk of fracture. 6 Nevertheless, it is still unclear whether the increase in BMD can result in incremental fracture risk reduction. Aggregated data from clinical trials suggest that positive changes in BMD are associated with fracture risk reduction in a quasi-linear way.5,7 However, real-life data seem to not support this finding. As an example, denosumab, which is widely known to be associated with a positive effect on BMD without a plateau effect, 8 did not show superiority over alendronate in terms of fracture prevention. 9
Non-skeletal factors, such as falls risk, comorbidities, and treatment with glucocorticoids, can contribute to fracture risk.10,11 Several algorithms have been developed to estimate the absolute risk of fracture and, possibly, to help detect an intervention threshold. Among these algorithms, the most widely utilized is the Fracture Risk Assessment Tool (FRAX). 12 In Italy, the DeFRA was developed by The Italian Society for Osteoporosis, Mineral Metabolism and Bone Diseases (SIOMMMS) and the Italian Society of Rheumatology (SIR).11,13 The DeFRA has been validated against the FRAX and demonstrated a similar receiver operating characteristic (ROC) curve for identifying patients at risk.13,14
The primary objective of this study was to assess the real-life effectiveness of anti-osteoporotic treatment in a representative cohort of Italian women at high risk of fracture using a data set derived from the web-based DeFRA algorithm.
Material and methods
Cohort characteristics and data collection
We conducted a longitudinal cohort study on women at high risk of fracture. The reporting of this study conforms to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement (the checklist is available as supplementary material). Data were extracted from the DeFRA database, which gathers data on women, starting from June 2017 to January 2020. The DeFRA data set derives from the DeFRA tool, which is a fracture risk estimation algorithm derived from the FRAX developed in 2010 and further adapted to the Italian population in 2015. 15 All Italian clinicians can calculate the 10-year fracture risk with the DeFRA tool by entering densitometric data and clinical features of their patients on the website ‘https://defra-osteoporosi.it/’; similar to the operation of FRAX. However, registration is required to access the web-based tool. In addition, the DeFRA tool allows the recall of prior visits upon fracture risk calculation and to record follow-up visits. We extracted the patients’ data entered by the physicians assessing fracture risk. The DeFRA allows the calculation of fracture risk in all women aged >50 years (no other exclusion/inclusion criteria). As of January 2020, a total of 2366 physicians registered and entered patients’ data in the DeFRA data set (see supplementary materials for additional data source information). The DeFRA considers the following variables for fracture risk estimation: age, weight, height, number, and site of prior fragility fractures (categorized as non-vertebral and clinical vertebral fractures), parental history of hip and clinical vertebral fracture, glucocorticoid intake (recorded as a semiquantitative variable), treatment with adjuvant hormone therapy for breast cancer, the presence of rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), psoriatic arthritis (PsA) and other connective tissue diseases, number of falls, smoking status (as a semiquantitative variable), alcohol intake, dairy products intake, sun exposure, calcium and vitamin D supplements intake, and femoral neck BMD T-scores.
The DeFRA has been validated against FRAX in post-menopausal women and in patients with type II diabetes.13,14 Area under the ROC curve (AUC) of DeFRA for fracture risk prediction was similar to the AUC of FRAX.
Patients with follow-up visits were included in the longitudinal analysis. Medication use was recorded in the data set by the physician who entered the data. Therapies were recorded as ‘prescribed’ in the first visit and as ‘ongoing’ in the follow-up visits. Inconsistencies between prescribed on first visit and ongoing in follow-up visits were considered as medication changes or discontinuations. We did not perform adjudication through pharmacy fill records. The follow-up time was defined as the time between the first evaluation (osteoporosis screening or referral) and the fracture event or the last evaluation available in the records.
Statistical analysis
Group comparisons were performed with Student’s T and Mann–Whitney U tests (for normally and non-normally distributed continuous variables, respectively). Associations between continuous variables were tested using Pearson’s correlation coefficients and multivariate linear regression. All differences were considered significant when the p value was less than 0.05. Multivariable Cox regression survival models were employed to analyze the effectiveness of different anti-osteoporotic drugs on fracture (either vertebral or non-vertebral fractures). In sensitivity analyses, we generated 1:1 matched cohorts of patients with prescription of bisphosphonates, denosumab, teriparatide, or without any pharmacological prescription at baseline and 1:1 matched cohort based on the T-score variation over time (increase in T-score vs decrease or stability in T-score values). Propensity score estimates were assessed using a logistic regression model derived from the clinical variables: age, lumbar spine T-score, femoral neck T-score, and 10-year fracture risk percentage estimated with DeFRA. We performed a 1:1 matching (using SPSS Version 26 and Python-based PSM/FUZZY extensions) with caliper 0.2 and nearest-neighbor matching. Kaplan–Meier curves were made for treated patients and non-treated patients, respectively. Log-rank test was employed at different time points: 180, 365, and 730 days. All statistical analyses were performed using SPSS Version 26 (SPSS, Inc., Chicago, IL, USA).
The study was conducted according to the protocol DEFRA 1876CESC approved by the Ethics Committee of the University of Verona Hospital, in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Data were anonymized in full compliance with the Italian code of protection of personal data (Legislative Decree 196/03, http://www.camera.it/parlam/leggi/deleghe/03196dl.htm). No identifiers related to patients were provided to the researchers. Results derived from all analyses were produced as aggregated summaries, which are not possible to assign, either directly or indirectly, to the individual patients. Informed consent was not required using encrypted retrospective information.
Results
Cohort characteristics
Data on 50,862 women were available. Among these, 3574 individuals had at least two consecutive visits, 249 had three follow-up visits, 27 had four follow-up visits, and 4 had five follow-up visits. All the individuals included in the longitudinal cohort had not previously been treated with any anti-osteoporotic therapy. 3065 individuals did not start any treatment other than calcium and/or vitamin D, 329 patients started a bisphosphonate (249 alendronate, 52 risedronate, 23 ibandronate, and 5 zoledronic acid), 154 denosumab and 26 teriparatide. Median follow-up time was 558 days [interquartile range (IQR): 243–727 days]. The baseline descriptive characteristics of the longitudinal cohort stratified by treatment started are presented in Table 1. The characteristics of the overall cohort are presented in the Supplementary Material. The main contributor to the higher fracture risk in the treated cohort (bisphosphonates, denosumab, and teriparatide) compared to non-treated cohort was the number of prevalent vertebral or hip fractures. Almost 88.5% of teriparatide users had ⩾2 vertebral or hip fractures at baseline compared to only 1.1% of patients that did not initiate any pharmacological treatment at baseline.
Table 1.
Baseline descriptive characteristics of the cohort stratified by anti-osteoporotic treatment started.
| Characteristics | No treatment (n, 3065) | Teriparatide (n, 26) | Denosumab (n, 154) | Bisphosphonates (n, 329) | |
|---|---|---|---|---|---|
| Age, years (SD) | 65 (8) | 75 (10) | 74 (8) | 68 (9) | |
| Weight, kg (SD) | 60.0 (10.5) | 60.7 (13.6) | 59.9 (11.4) | 58.7 (9.8) | |
| Height, cm (SD) | 159 (7) | 160 (7) | 158 (7) | 159 (7) | |
| Femoral neck T-score (IQR) | –2.5 (–3.0, –1.9) | –2.4 (–3.2, –2.0) | –2.4 (–3.0, –1.8) | –2.5 (–2.9, –1.9) | |
| 10-year % hip fracture risk, % (IQR) | 3.5 (1.8, 6.4) | 27.0 (19.0, 49.0) | 14.0 (6.8, 28.0) | 5.5 (2.6, 10.0) | |
| 10-year % MOF risk, % (IQR) | 9.6 (6.9, 14.0) | 64.0 (47.0, 97.0) | 32.0 (18.0, 57.0) | 13.0 (8.3, 22.0) | |
| Follow-up, days (IQR) | 553 (234, 728) | 726 (560, 748) | 692 (365, 734) | 569 (226, 713) | |
| Smoking, n (%) | No | 2702 (88.2) | 23 (88.5) | 136 (88.3) | 296 (90.0) |
| <10/day | 211 (6.9) | 1 (3.8) | 11 (7.1) | 23 (7.0) | |
| ⩾10/day | 152 (5.0) | 2 (7.7) | 7 (4.5) | 10 (3.0) | |
| Alcohol intake, n (%) | No | 2687 (87.7) | 20 (76.9) | 113 (73.4) | 286 (86.9) |
| <3 IU/day | 367 (12.0) | 6 (23.1) | 41 (26.6) | 42 (12.8) | |
| ⩾3 IU/day | 11 (0.4) | 0 (0) | 0 (0) | 1 (0.3) | |
| Family history of fracture, n (%) | No | 2246 (73.3) | 20 (76.9) | 118 (76.6) | 242 (73.6) |
| Yes | 819 (26.7) | 6 (23.1) | 36 (23.4) | 87 (26.4) | |
| Prior vertebral o hip fractures, n (%) | None | 2714 (88.5) | 0 (0) | 47 (30.5) | 245 (74.5) |
| 1 | 255 (8.3) | 3 (11.5) | 55 (35.7) | 63 (19.1) | |
| 2 | 63 (2.1) | 4 (15.4) | 26 (16.9) | 11 (3.3) | |
| >2 | 33 (1.1) | 19 (73.1) | 26 (16.9) | 10 (3.0) | |
| Prior non-vertebral, non-hip fractures, n (%) | None | 2701 (88.1) | 23 (88.5) | 136 (88.3) | 278 (84.5) |
| 1 | 273 (8.9) | 2 (7.7) | 11 (7.1) | 39 (11.9) | |
| 2 | 68 (2.2) | 0 (0) | 2 (1.3) | 11 (3.3) | |
| >2 | 23 (0) | 1 (3.8) | 5 (3.2) | 1 (0.3) | |
| Comorbidities, n (%) | No | 2896 (94.5) | 24 (92.3) | 134 (87.0) | 275 (83.6) |
| Yes | 169 (5.5) | 2 (7.7) | 20 (13.0) | 54 (16.4) | |
| Glucocorticoids (prednisone equivalent), n (%) | No | 2959 (96.5) | 24 (92.3) | 137 (89.0) | 287 (87.2) |
| >2.5 but <5 mg/day | 78 (2.5) | 1 (3.8) | 11 (7.1) | 32 (9.7) | |
| ⩾5 mg/day | 28 (0.9) | 1 (3.8) | 6 (3.9) | 10 (3.0) | |
| Vitamin D, n (%) | 0 IU/day | 2324 (75.8) | 3 (11.5) | 11 (7.1) | 76 (23.1) |
| <250 IU/day | 80 (2.6) | 2 (7.7) | 6 (3.9) | 28 (8.5) | |
| 250–400 IU/day | 37 (1.2) | 1 (3.8) | 3 (1.9) | 7 (2.1) | |
| 400–800 IU/day | 182 (5.9) | 9 (34.6) | 52 (33.8) | 42 (12.8) | |
| 800–1200 IU/day | 284 (9.3) | 9 (34.6) | 57 (37.0) | 117 (35.6) | |
| >1200 IU/day | 158 (5.2) | 2 (7.7) | 25 (16.2) | 59 (17.9) | |
| Calcium, n (%) | 0 mg/day | 2872 (93.7) | 15 (57.7) | 72 (46.8) | 213 (64.7) |
| <300 mg/day | 19 (0.6) | 2 (7.7) | 3 (1.9) | 8 (2.4) | |
| 300–600 mg/day | 130 (4.2) | 6 (23.1) | 61 (39.6) | 74 (22.5) | |
| >600 mg/day | 44 (1.4) | 3 (11.5) | 18 (11.7) | 34 (10.3) | |
| Falls, n (%) | None | 2952 (96.3) | 16 (61.5) | 113 (73.4) | 289 (87.8) |
| 1 | 79 (2.6) | 6 (23.1) | 22 (14.3) | 29 (8.8) | |
| 2 | 22 (0.7) | 4 (15.4) | 7 (4.5) | 5 (1.5) | |
| ⩾3 | 12 (0.4) | 0 (0) | 12 (7.7) | 6 (1.8) | |
SD, standard deviation.
Anti-osteoporotic medications effectiveness
A total of 427 fractures of any kind (389 vertebral plus 38 non-vertebral fracture) were documented for 3065 patients who did not receive any treatment over 4643 patient years (prevalence of new fractures 13.9%). The crude fracture rate was 91.9/1000 person-year. Around 37, 17, and 1 fracture were reported for patients treated with bisphosphonates (n = 329), denosumab (n = 154), and teriparatide (n = 26). The crude fracture rate in such patients was 72.1/1000 person-year, 58.2/1000 person-year and 19.3/1000 person-year for bisphosphonates, denosumab, and teriparatide, respectively.
Overall, 389 vertebral fractures were recorded for 3065 patients who did not receive any treatment. The crude vertebral fracture rate was 82.3/1,000 person-year. As regards bisphosphonates, denosumab, and teriparatide the crude vertebral fracture rate was 69.2/1,000 person-year, 45.3/1,000 person-year, and 19.3/1,000 person-year, respectively.
Cox regression survival analysis (vertebral plus non-vertebral) is presented in Table 2 (Cox regression analyses stratified by site are presented in Supplementary Materials). We found that after adjusting for traditional fracture risk factors, treatment with bisphosphonates was associated with a 30% lower risk of any kind of fracture (vertebral plus non-vertebral) compared to no treatment [adjusted hazard ratio (aHR): 0.70, 95% confidence interval [CI]: 0.50–0.98]. Patients who started denosumab had 60% lower risk of any fracture compared to no treatment (aHR: 0.43, 95% CI: 0.24–0.75) and patients who started teriparatide had 90% lower risk of any fracture compared to no treatment (aHR: 0.09, 95% CI: 0.01–0.70). In Cox regression analysis, teriparatide prescription was associated with lower, yet not significant, risk of fracture compared to bisphosphonates (aHR: 0.13, 95% CI: 0.01–1.00, p = 0.052).
Table 2.
Cox multivariable regression analysis (vertebral and non-vertebral fractures).
| Beta | SE | Sign. | Exp(B) | 95% CI for Exp(B) | ||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| A: No treatment as reference | ||||||
| Bisphosphonates | −0.352 | 0.174 | 0.043 | 0.703 | 0.500 | 0.989 |
| Denosumab | −0.845 | 0.287 | 0.003 | 0.429 | 0.245 | 0.754 |
| Teriparatide | −2.353 | 1.021 | 0.021 | 0.095 | 0.013 | 0.703 |
| B: Bisphosphonates as references | ||||||
| Denosumab | −0.493 | 0.316 | 0.119 | 0.611 | 0.329 | 1.136 |
| Teriparatide | −2.001 | 1.029 | 0.052 | 0.135 | 0.018 | 1.015 |
| No treatment | 0.352 | 0.174 | 0.043 | 1.422 | 1.012 | 2.000 |
CI, confidence interval; SE, standard error.
Adjusted for age, weight, height, number and site of prior fragility fractures, parental history of hip and clinical vertebral fracture, glucocorticoid intake, the presence of comorbidities, number of falls, smoking status, alcohol intake, dairy products intake, sun exposure, calcium and vitamin D supplements intake, and femoral neck T-scores.
In sensitivity analyses, using propensity score matching analysis, we matched patients who were prescribed with anti-osteoporotic therapies (i.e. bisphosphonates n = 292, denosumab n = 123) with patients who did not start any pharmacological treatment. Histograms of propensity scores, standardized differences, and distributions of propensity scores are presented in Supplementary Materials. We then generated Kaplan–Meier survival curves from the newly 1:1 matched cohort. The Kaplan–Meier estimate curve for bisphosphonates versus no treatment (Figure 1) reached statistical significance for vertebral fractures and for fractures of any kind (vertebral fracture plus non-vertebral fractures; log-rank p < 0.0001) but did not reach statistical significance for non-vertebral fractures alone (log-rank p = 0.136). Regarding denosumab, the Kaplan–Meier estimate curve (Figure 2) reached statistical significance for vertebral fracture and fractures of any kind (log-rank p = 0.037) but did not reach statistical significance for non-vertebral fractures (log-rank p = 0.094). Kaplan–Meier estimate for teriparatide are presented in Supplementary Materials.
Figure 1.
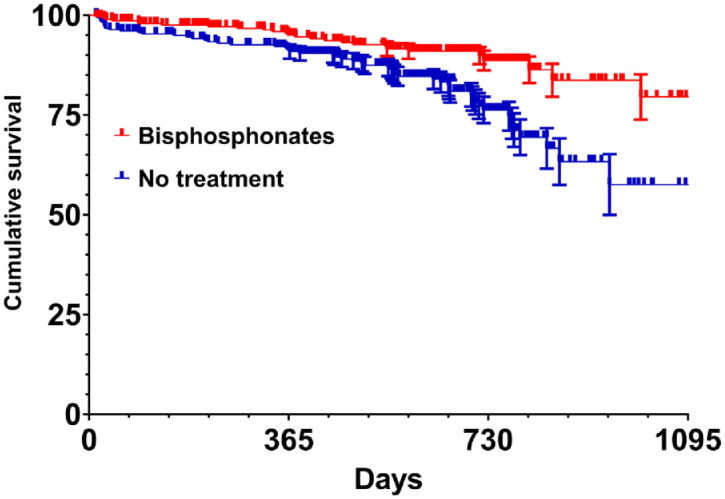
Kaplan–Meier curves displaying the fracture (vertebral and non-vertebral fractures) probability for 1:1 matched groups of bisphosphonate users and individuals without treatment (log-rank p < 0.0001).
Figure 2.
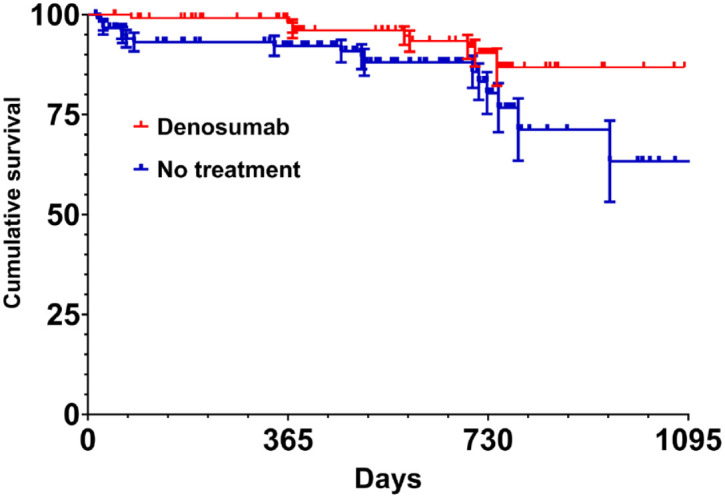
Kaplan–Meier curves displaying the fracture (vertebral and non-vertebral fractures) probability for 1:1 matched groups of denosumab users and individuals without treatment (log-rank p = 0.037).
In addition, we restricted the analyses to 180-, 365-, and 730-day periods. We found that bisphosphonates effectively reduced the risk for fractures of any kind at 730 days (log-rank p = 0.001) but not at 365 days (log-rank p = 0.091) and 180-days (log-rank p = 0.108). In contrast, denosumab reduced the risk of new fracture of any kind at both 730 days (log-rank p = 0.031) and 365 days (log-rank p = 0.039). In addition, we found a trend toward fracture risk reduction for denosumab at 180 days (log-rank p = 0.061).
We then stratified patients based on the variation of BMD over the time. Patients were divided in two groups: those with increasing BMD (n = 1140, 31.9%) and those with stable or decreasing BMD (n = 2434, 68.1%). A total of 106 (9.3% out of 1140) and 376 (15.4% out of 2434) fractures of any kind were recorded in patients with increasing and stable or decreasing BMD T-score levels, respectively. The crude fracture rate was 56.1/1000 person-year for women with increasing BMD and 96.7/1000 person-year for women with stable or decreasing BMD. We then 1:1 matched the two groups through a propensity score matching analysis. Figure 3 shows the Kaplan–Meier survival curve for women with increasing BMD and those with stable or decreasing BMD (log-rank p < 0.0001 for fracture of any kind and vertebral fractures and log-rank p = 0.298 for non-vertebral fractures).
Figure 3.
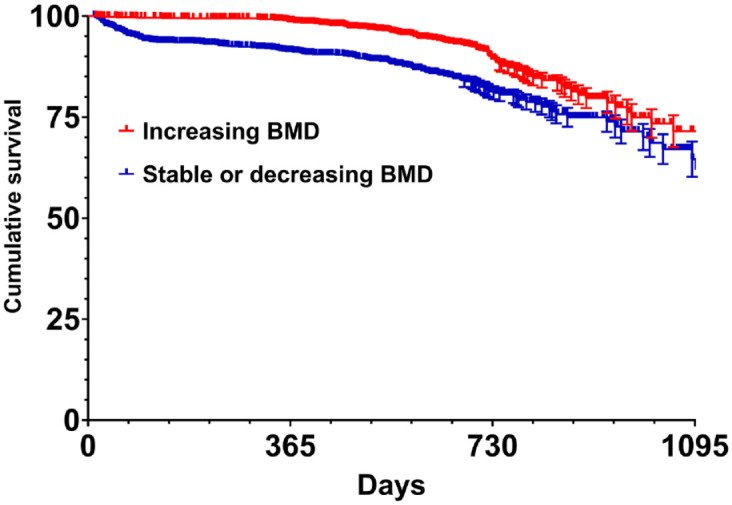
Kaplan–Meier curves displaying the fracture (vertebral and non-vertebral fractures) probability for 1:1 matched groups of patients with increasing BMD and stable or decreasing BMD (log-rank p < 0.0001).
We then restricted the analyses to the 180-, 365-, and 730-day periods. We found that the fracture risk was lower in patients with increasing BMD compared to those with stable or decreasing BMD within 180 days (log-rank p = 0.005), 365 days (log-rank p < 0.0001), and 730 days (log-rank p < 0.0001).
Discussion
Herein, we conducted a real-life study on the short-term effectiveness of anti-osteoporotic treatments in women at high risk of fracture. We conducted this analysis in a cohort of Italian women using a data set derived from a web-based FRAX. We have previously reported cross-sectional analyses on such database;4,16,17 in this study, we analyzed the data set longitudinally. We found that after adjusting for baseline fracture risk, bisphosphonates, denosumab, and teriparatide were associated with a 30%, 60%, and 90% lower risk of fracture compared to no treatment, respectively.
Interestingly, the survival curve for bisphosphonates against no treatment seemed to split beyond 1 year of treatment while teriparatide and denosumab appeared to reduce the risk soon after the start of medications. This result is in line with other previously published randomized clinical trials. For example, alendronate, in the pivotal clinical trial, effectively reduced the incidence of fracture only after 12 months of treatment.18,19 In contrast, denosumab was shown to successfully reduce the incidence of vertebral fractures within the first 6 months of therapy. 20 Notably, in the pivotal clinical trial, denosumab reduced the risk of fractures to a similar extent to our real-life analysis. 20
Pedersen et al. 9 have recently reported that treatment with denosumab was associated with a similar incidence of fragility fracture compared to alendronate, over a 3-year period, a result that is not in line with our finding. However, Pedersen et al. did not have access to densitometric data, possibly introducing a confounding by indication bias. Indeed, the authors controlled for age, sex, and relevant comorbidities, including history of fracture, alcohol-related disorders, obesity, and diseases from the Charlson Comorbidity Index but not for BMD levels at baseline. This lack of data might explain the results of the authors. In contrast, we had access to many bone-health-related covariates, including BMD T-score levels and glucocorticoid assumption, which is a well-known cause of bone fragility despite having normal or osteopenic BMD.21–23
Our findings are in line with other real-life epidemiological studies. Abrahamsen et al. 24 showed that bisphosphonate use was associated with a reduced risk of hip fracture independently from baseline characteristics. Again, Morin et al. 25 found that bisphosphonates were associated with a 30% lower risk of hip fracture, a proportion that is similar to our findings. Interestingly, Morin et al. approached the issue with a similar study design; namely, Cox regression in the main analysis and propensity score matching in sensitivity analyses. Choi et al. 26 used a 1:1 propensity score matching to control for confounders as well. The authors compared the effectiveness of zoledronic acid and denosumab on a large cohort of patients and found, likewise to us, no differences in terms of non-vertebral fracture risk reduction between the two drugs.
In a sensitivity analysis, we stratified patients by variations in BMD levels over time. We found that patients with increasing BMD, independently from the treatment applied, had a lower incidence of fracture compared to patients with stable or decreasing BMD. As a matter of fact, BMD is a valid surrogate outcome for treatment efficacy. Indeed, the Federal Drug Administration (FDA) considers BMD an acceptable primary endpoint for establishing efficacy for the treatment of male or glucocorticoid-induced osteoporosis for treatment with an established anti-fracturative efficacy in post-menopausal women. 27 Data from clinical trials suggested that an increasing BMD following an intervention is correlated with fracture risk reduction.5,7 Indeed, BMD can serve as good proxy of treatment validity and efficacy. In this analysis, we confirmed such results adding our real-life experience. Moreover, we demonstrated that even non-treated patients (with pharmacological agents), who, for any reason, had an increase in BMD levels over time were somehow protected from new fractures. Of note, this protective effect was seen as early as the first 6 months of follow-up.
Our study should be interpreted in the light of strengths and limitations. A key strength is that the sample is highly representative of the Italian female population at high risk of fracture. Indeed, the DeFRA is widely used for fracture risk assessment by both primary-care practitioners and bone specialists. We had access to densitometric data, which are usually not available in prescription claim analyses or insurance data analyses. However, the study has limitations common to all large data sets. First, we did not have access to adherence data which is an important determinant of treatment efficacy. Indeed, although we conducted a Cox regression analysis based on many clinical and densitometric data, and in sensitivity analyses, we matched treated patients with controls; some residual confounding might have impacted the analyses. Moreover, the relatively small sample size of patients treated with anti-osteoporotic medications limited our power to analyze treatment effectiveness of one treatment against another. In addition, we might have come across some kind of selection bias, which could have affected our results. Nonetheless, the overall fracture rate was in line with other studies conducted on the Italian population, underlining the representativeness of our cohort and the generalizability of the results.28,29 In addition, we did not perform radiological screening for asymptomatic vertebral fractures (e.g. VFA DXA or X-ray), limiting our sensitivity in detecting fractures. Finally, we did not perform medication fill adjudication through pharmacy records or independent verification of fractures.
In summary, we provided new real-life evidence for anti-osteoporotic treatment effectiveness. We found that bisphosphonates, denosumab, and teriparatide effectively reduced the risk of fracture. The delayed anti-fracturative effects of bisphosphonates (i.e. >6–12 months) makes these medications inappropriate for patients at imminent risk of fracture. In such patients, a rapid effect on fracture risk is desirable, and faster treatments are warranted. 30 In contrast, in patients with low risk of fracture, bisphosphonates are still the best option due to their low costs and wide availability.30,31
Supplemental Material
Supplemental material, sj-docx-1-tab-10.1177_1759720X221105009 for Real-life short-term effectiveness of anti-osteoporotic treatments: a longitudinal cohort study by Giovanni Adami, Irene Gavioli, Maurizio Rossini, Ombretta Viapiana, Giovanni Orsolini, Camilla Benini, Eugenia Bertoldo, Elena Fracassi, Davide Gatti and Angelo Fassio in Therapeutic Advances in Musculoskeletal Disease
Footnotes
Ethics approval and consent to participate: The study was conducted according to the protocol DEFRA 1876CESC approved by the Ethics Committee of the University of Verona Hospital, in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent for publication: This research was done without patient involvement. Patients were not invited to comment on the study design and were not consulted to develop patient relevant outcomes or interpret the results. Patients were not invited to contribute to the writing or editing of this document for readability or accuracy.
Author contributions: Giovanni Adami: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Validation; Visualization; Writing – original draft; Writing – review & editing.
Irene Gavioli: Investigation; Writing – original draft; Writing – review & editing.
Maurizio Rossini: Data curation; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
Ombretta Viapiana: Investigation; Writing – original draft; Writing – review & editing.
Giovanni Orsolini: Investigation; Writing – original draft; Writing – review & editing.
Camilla Benini: Investigation; Writing – original draft; Writing – review & editing.
Eugenia Bertoldo: Investigation; Writing – original draft; Writing – review & editing.
Elena Fracassi: Investigation; Writing – original draft; Writing – review & editing.
Davide Gatti: Investigation; Writing – original draft; Writing – review & editing
Angelo Fassio: Investigation; Validation; Visualization; Writing – original draft; Writing – review & editing.
ORCID iDs: Giovanni Adami  https://orcid.org/0000-0002-8915-0755
https://orcid.org/0000-0002-8915-0755
Maurizio Rossini  https://orcid.org/0000-0001-9692-2293
https://orcid.org/0000-0001-9692-2293
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no conflicts of interest. Giovanni Adami declares consultancy fee from Theramex and Galapagos outside the submitted work. Maurizio Rossini declares consultancy fees from Amgen, ABBvie, BMS, Eli Lilly, Galapagos, Novartis, Pfizer, Sandoz, Theramex and UCB outside the submitted work. All other authors declare no conflict of interest. The lead author (the manuscript’s guarantor) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Availability of data and material: The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Supplemental materials: Supplemental material for this article is available online.
Contributor Information
Giovanni Adami, Rheumatology Unit, University of Verona, Pz Scuro 10, 37134 Verona, Italy.
Irene Gavioli, Rheumatology Unit, University of Verona, Verona, Italy.
Maurizio Rossini, Rheumatology Unit, University of Verona, Verona, Italy.
Ombretta Viapiana, Rheumatology Unit, University of Verona, Verona, Italy.
Giovanni Orsolini, Rheumatology Unit, University of Verona, Verona, Italy.
Camilla Benini, Rheumatology Unit, University of Verona, Verona, Italy.
Eugenia Bertoldo, Rheumatology Unit, University of Verona, Verona, Italy.
Elena Fracassi, Rheumatology Unit, University of Verona, Verona, Italy.
Davide Gatti, Rheumatology Unit, University of Verona, Verona, Italy.
Angelo Fassio, Rheumatology Unit, University of Verona, Verona, Italy.
References
- 1. Black DM, Rosen CJ. Clinical practice: postmenopausal osteoporosis. N Engl J Med 2016; 374: 254–262. [DOI] [PubMed] [Google Scholar]
- 2. Lin S-Y, Hung M-C, Chang S-F, et al. Efficacy and safety of postmenopausal osteoporosis treatments: a systematic review and network meta-analysis of randomized controlled trials. J Clin Med 2021; 10: 3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Albert SG, Wood E. Meta-analysis of clinical fracture risk reduction of antiosteoporosis drugs: direct and indirect comparisons and meta-regressions. Endocr Pract: Off J Am Coll Endocrinol Am Assoc Clin Endocrinol 2021; 27: 1082–1092. [DOI] [PubMed] [Google Scholar]
- 4. Adami G, Giollo A, Rossini M, et al. Different fracture risk profile in patients treated with anti-osteoporotic drugs in real-life. Reumatismo 2020; 72: 71–74. [DOI] [PubMed] [Google Scholar]
- 5. Bouxsein ML, Eastell R, Lui L-Y, et al. Change in bone density and reduction in fracture risk: a meta-regression of published trials. J Bone Miner Res: Off J Am Soc Bone Miner Res 2019; 34: 632–642. [DOI] [PubMed] [Google Scholar]
- 6. Kanis JA, Johnell O, Oden A, et al. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos: Int J Establ Result Coop Eur Found Osteoporos 2008; 19: 385–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Black DM, Bauer DC, Vittinghoff E, et al. Treatment-related changes in bone mineral density as a surrogate biomarker for fracture risk reduction: meta-regression analyses of individual patient data from multiple randomised controlled trials. Lancet Diabetes Endocrinol 2020; 8: 672–682. [DOI] [PubMed] [Google Scholar]
- 8. Compston J. Safety of long-term denosumab therapy for osteoporosis. Lancet Diabetes Endocrinol 2017; 5: 485–487. [DOI] [PubMed] [Google Scholar]
- 9. Pedersen AB, Heide-Jørgensen U, Sørensen HT, et al. Comparison of risk of osteoporotic fracture in denosumab vs alendronate treatment within 3 years of initiation. JAMA Netw Open 2019; 2: e192416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kanis JA, Borgstrom F, De Laet C, et al. Assessment of fracture risk. Osteoporos Int 2005; 16: 581–589. [DOI] [PubMed] [Google Scholar]
- 11. Adami S, Bianchi G, Brandi ML, et al. Validation and further development of the WHO 10-year fracture risk assessment tool in Italian postmenopausal women: project rationale and description. Clin Exp Rheumatol 2010; 28: 561–570. [PubMed] [Google Scholar]
- 12. Kanis JA, Oden A, Johansson H, et al. FRAX and its applications to clinical practice. Bone 2009; 44: 734–743. [DOI] [PubMed] [Google Scholar]
- 13. Bonaccorsi G, Messina C, Cervellati C, et al. Fracture risk assessment in postmenopausal women with diabetes: comparison between DeFRA and FRAX tools. Gynecol Endocrinol 2018; 34: 404–408. [DOI] [PubMed] [Google Scholar]
- 14. Bonaccorsi G, Fila E, Cervellati C, et al. Assessment of fracture risk in a population of postmenopausal Italian women: a comparison of two different tools. Calcif Tissue Int 2015; 97: 50–57. [DOI] [PubMed] [Google Scholar]
- 15. Adami S, Bianchi G, Brandi ML, et al. Validation and further development of the WHO 10-year fracture risk assessment tool in Italian postmenopausal women: project rationale and description. Clin Exp Rheumatol 2010; 28: 561–570. [PubMed] [Google Scholar]
- 16. Orsolini G, Adami G, Adami S, et al. Short-term effects of TNF inhibitors on bone turnover markers and bone mineral density in rheumatoid arthritis. Calcif Tissue Int 2016; 98: 580–585. [DOI] [PubMed] [Google Scholar]
- 17. Adami G, Gatti D, Rossini M, et al. Factors associated with referral for osteoporosis care in men: a real-life study of a nationwide dataset. Arch Osteoporos 2021; 16: 56. [DOI] [PubMed] [Google Scholar]
- 18. Liberman UA, Weiss SR, Bröll J, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis: the Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med 1995; 333: 1437–1443. [DOI] [PubMed] [Google Scholar]
- 19. Black DM, Thompson DE, Bauer DC, et al. Fracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial. J Clin Endocrinol Metab 2000; 85: 4118–4124. [DOI] [PubMed] [Google Scholar]
- 20. Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 2009; 361: 756–765. [DOI] [PubMed] [Google Scholar]
- 21. Adami G, Saag KG. Glucocorticoid-induced osteoporosis: 2019 concise clinical review. Osteoporos: Int J Establ Result Coop Eur Found Osteoporos 2019; 30: 1145–1156. [DOI] [PubMed] [Google Scholar]
- 22. Adami G, Saag KG. Glucocorticoid-induced osteoporosis update. Curr Opin Rheumatol 2019; 31: 388–393. [DOI] [PubMed] [Google Scholar]
- 23. Adami G, Rahn EJ, Saag KG. Glucocorticoid-induced osteoporosis: from clinical trials to clinical practice. Ther Adv Musculoskelet Dis 2019; 11: 876468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Abrahamsen B, Eiken P, Prieto-Alhambra D, et al. Risk of hip, subtrochanteric, and femoral shaft fractures among mid and long term users of alendronate: nationwide cohort and nested case-control study. BMJ 2016; 353: i3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morin S, Rahme E, Behlouli H, et al. Effectiveness of antiresorptive agents in the prevention of recurrent hip fractures. Osteoporos: Int J Establ Result Coop Eur Found Osteoporos 2007; 18: 1625–1632. [DOI] [PubMed] [Google Scholar]
- 26. Choi N-K, Solomon DH, Tsacogianis TN, et al. Comparative safety and effectiveness of denosumab versus zoledronic acid in patients with osteoporosis: a cohort study. J Bone Miner Res: Off J Am Soc Bone Miner Res 2017; 32: 611–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Research C for DE. Table of surrogate endpoints that were the basis of drug approval or licensure. Silver Spring, MD: FDA, 2022. [Google Scholar]
- 28. Piscitelli P, Brandi ML, Chitano G, et al. Epidemiology of fragility fractures in Italy. Clin Cases Miner Bone Metab: Off J Ital Soc Osteoporos Miner Metab Skelet Dis 2011; 8: 29–34. [PMC free article] [PubMed] [Google Scholar]
- 29. Adami S, Isaia G, Luisetto G, et al. Fracture incidence and characterization in patients on osteoporosis treatment: the ICARO study. J Bone Miner Res: Off J Am Soc Bone Miner Res 2006; 21: 1565–1570. [DOI] [PubMed] [Google Scholar]
- 30. Adami G, Rossini M, Fassio A, et al. Comments on Kanis et al.: algorithm for the management of patients at low, high, and very high risk of osteoporotic fractures. Osteoporos: Int J Establ Result Coop Eur Found Osteoporos 2020; 31: 1015. [DOI] [PubMed] [Google Scholar]
- 31. Kanis JA, Harvey NC, McCloskey E, et al. Algorithm for the management of patients at low, high and very high risk of osteoporotic fractures. Osteoporos: Int J Establ Result Coop Eur Found Osteoporos 2019; 31: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tab-10.1177_1759720X221105009 for Real-life short-term effectiveness of anti-osteoporotic treatments: a longitudinal cohort study by Giovanni Adami, Irene Gavioli, Maurizio Rossini, Ombretta Viapiana, Giovanni Orsolini, Camilla Benini, Eugenia Bertoldo, Elena Fracassi, Davide Gatti and Angelo Fassio in Therapeutic Advances in Musculoskeletal Disease


