ABSTRACT
Objective.
To develop an evidence map on visceral leishmaniasis prevention, control, diagnosis, treatment, and prognosis.
Methods.
Systematic reviews on visceral leishmaniasis were searched using MEDLINE/PubMed and Virtual Health Library. After selection, each included systematic review was assessed, characterized, and categorized by intervention type and by outcomes, according to the methodology offered by the PAHO/WHO Latin American and Caribbean Center on Health Sciences Information (BIREME). The methodological quality was assessed using the AMSTAR2 tool to determine the confidence level of the evidence obtained.
Results.
Among the prevention and control interventions, insecticide spraying, bednets, dog collars, and dog culling were the most assessed, emphasizing that insecticidal dog collars can reduce visceral leishmaniasis incidence in dogs. Regarding diagnosis, polymerase chain reaction (PCR), rK39 immunochromatographic test (rK39 ICT), and direct agglutination test (DAT) presented high sensitivity and specificity. As for treatment, pentavalent antimonials and amphotericin B were the most analyzed drugs and showed therapeutic success; however, serious adverse events can occur due to their use. The prognostic factors identified were anemia, edema, bleeding, jaundice, age, and HIV coinfection.
Conclusions.
The evidence map developed shows rK39 ICT and DAT as promising diagnostic alternatives and reinforces the efficacy of liposomal amphotericin B and pentavalent antimonials. Insecticide-impregnated dog collars appear as a promising measure for the control of visceral leishmaniasis, but there is also a need for future studies and reviews with higher methodological quality, especially on prevention and control interventions.
Keywords: Leishmaniasis, visceral; neglected diseases; evidence-based medicine
RESUMEN
Objetivo.
Elaborar un mapa de evidencia sobre la prevención, el control, el diagnóstico, el tratamiento y el pronóstico de la leishmaniasis visceral.
Métodos.
Se realizaron búsquedas de revisiones sistemáticas sobre la leishmaniasis visceral en MEDLINE/PubMed y la Biblioteca Virtual en Salud. Tras la selección, cada revisión sistemática incluida fue sometida a evaluación, caracterización y categorización según tipo de intervención y resultados, de acuerdo con la metodología ofrecida por el Centro Latinoamericano y del Caribe de Información en Ciencias de la Salud de la OPS/OMS (BIREME). La calidad metodológica se evaluó con la herramienta AMSTAR2 para determinar el nivel de confianza de la información obtenida.
Resultados.
Entre las intervenciones de prevención y control, las más evaluadas fueron la fumigación con insecticidas, los mosquiteros, los collares para perros y el sacrificio de perros y se hizo hincapié en que los collares insecticidas para perros pueden reducir la incidencia de leishmaniasis visceral en perros. En cuanto al diagnóstico, la reacción en cadena de la polimerasa (PCR, por su sigla en inglés), la prueba inmunocromatográfica rK39 (rK39 ICT) y la prueba de aglutinación directa (DAT, por su sigla en inglés) mostraron alta sensibilidad y especificidad. Con respecto al tratamiento, los fármacos más analizados que arrojaron éxito terapéutico fueron los antimoniales pentavalentes y la anfotericina B; sin embargo, su uso puede provocar efectos adversos graves. Los factores pronósticos que se identificaron fueron anemia, edema, sangrado, ictericia, edad y coinfección por el VIH.
Conclusiones.
El mapa de evidencia elaborado presenta la prueba inmunocromatográfica rK39 y la prueba de aglutinación directa como alternativas diagnósticas prometedoras, y consolida la eficacia de la anfotericina B liposomal y los antimoniales pentavalentes. Los collares de perro impregnados de insecticida parecen ser una medida prometedora para el control de la leishmaniasis visceral, si bien también son necesarios estudios y revisiones adicionales de mayor calidad metodológica, especialmente sobre intervenciones de prevención y control.
Palabras clave: Leishmaniasis visceral, enfermedades desatendidas, medicina basada en la evidencia
RESUMO
Objetivo.
Desenvolver um mapa de evidências de prevenção, controle, diagnóstico, tratamento e prognóstico da leishmaniose visceral.
Métodos.
Foram realizadas buscas por revisões sistemáticas sobre leishmaniose visceral no MEDLINE/PubMed e na Biblioteca Virtual em Saúde. Após a seleção, cada revisão sistemática incluída foi avaliada, caracterizada e classificada por tipo de intervenção e por desfechos, de acordo com a metodologia oferecida pelo Centro Latino-Americano e do Caribe de Informação em Ciências da Saúde (BIREME) da OPAS/OMS. A qualidade metodológica foi avaliada utilizando a ferramenta AMSTAR2 para determinar o nível de confiança das evidências obtidas.
Resultados.
Entre as intervenções de prevenção e controle, pulverização com inseticida, mosquiteiros, coleiras para cães e abate de cães foram as mais frequentemente avaliadas, com destaque para as coleiras inseticidas na redução da incidência de leishmaniose visceral em cães. Quanto ao diagnóstico, a reação em cadeia da polimerase (PCR), o teste imunocromatográfico rK39 (rK39 ICT) e o teste de aglutinação direta (DAT, sigla em inglês para direct agglutination test) apresentaram alta sensibilidade e especificidade. Em relação ao tratamento, os antimoniais pentavalentes e a anfotericina B foram os medicamentos mais analisados e demonstraram sucesso terapêutico, embora seu uso possa resultar em eventos adversos graves. Os fatores prognósticos identificados foram anemia, edema, sangramento, icterícia, idade e coinfecção com HIV.
Conclusões.
O mapa de evidências desenvolvido mostra o rK39 ICT e o DAT como alternativas promissoras para o diagnóstico e reforça a eficácia da anfotericina B lipossomal e dos antimoniais pentavalentes. As coleiras impregnadas com inseticida aparecem como medida promissora para o controle da leishmaniose visceral, mas estudos e revisões futuras com mais qualidade metodológica, especialmente sobre intervenções de prevenção e controle, são necessários.
Palavras-chave: Leishmaniose visceral, doenças negligenciadas, medicina baseada em evidências
Visceral leishmaniasis (VL), also known as kala-azar, is the most severe of the clinical presentations of the leishmaniases. The disease is caused by protozoan parasites of the Leishmania donovani complex, including L. infantum, which is most frequent in the Americas, Mediterranean region, and West and Central Asia. L. donovani is restricted to the Indian Subcontinent and East Africa (1). VL has a broad clinical spectrum, but the most typical signs and symptoms are fever, splenomegaly, hepatomegaly, pallor, and weight loss (2). Transmission occurs by the bite of infected sandflies of several species. Domestic dogs can be one crucial reservoir in areas with L. infantum transmission, although several species of mammals also act as sylvatic reservoirs (3), whereas VL caused by L. donovani is restricted to humans. VL has complex transmission dynamics, which are influenced by socioeconomic and environmental factors, further complicating the effectiveness of control measures (4).
An evidence map is a systematic way of organizing the knowledge available on a subject, making it accessible (5). Identifying evidence gaps, helping researchers to find relevant topics for future studies, and facilitating health policy planning are the advantages of using this tool. Evidence maps have in common the visual representation of knowledge through products with user-friendly formats that benefit health professionals, researchers, and policymakers (6). The aim of this study is to develop an evidence map about VL.
MATERIALS AND METHODS
This evidence map was developed according to the methodology from the Pan American Health Organization/World Health Organization (PAHO/WHO) Latin American and Caribbean Center on Health Sciences Information (BIREME), which has previously been applied to traditional, complementary, and integrative medicine (7). The working group was formed of six researchers on VL and one librarian with experience on evidence maps, which delimited the theme and scope, criteria for inclusion and exclusion of studies, and data to be extracted.
Two searches were performed, in MEDLINE/PubMed and the Virtual Health Library (VHL), applying the same search strategy: “Visceral Leishmaniasis” OR “Black Fever” OR “Fever, Black” OR “Kala-Azar” OR “Kala Azar” OR “Kalazar” OR “Calazar.” The searches were conducted in August 2020 and April 2021 and filtered for systematic reviews (SRs). There were no restrictions to language or country. The publication period analyzed covers 1990 to 2021 in VHL and 2005 to 2021 in MEDLINE/PubMed.
The selection of SRs was done by independent analysis performed using Rayyan, a free web and mobile app useful for the initial screening of studies, using a semi-automated process (8). The reviewers considered eligible SRs on VL to be those that presented explicit methods of identification, selection, evaluation, and synthesis of individual studies. Publications that did not refer to diagnosis, treatment, prognosis, and prevention and control in human or canine VL were excluded. Conflicts were resolved through discussion among reviewers to reach a consensus.
The information extracted from each SR was: title, intervention(s), outcome(s), effect(s), population, year of publication, type of review, review design, confidence level, design and country of individual studies, electronic address, database, ID, and citation. The AMSTAR2 tool (A MeaSurement Tool to Assess Systematic Reviews) was used to determine the methodological quality of SRs, classified as high, moderate, low, and critically low. High quality means an accurate and comprehensive summary of the results, while moderate quality suggests only an accurate summary. Low-quality may indicate that there was not an accurate and comprehensive summary, and critically low quality reviews should not be relied on (9). Interventions and outcomes were organized in groups. All these data were registered in a characterization table.
Data processing was performed using Tableau Software® (10). This evidence map is an interactive matrix of interventions and outcomes in bubble plot format. The size and the color of the bubble indicate the number and the confidence level of SRs, respectively. More information is obtained by clicking the mouse button on each bubble. Graphs for intervention groups and related outcomes, considering only effects and the number of SRs, were also created.
RESULTS
The searches resulted in 218 articles, of which 152 did not refer to VL or were not SRs. Of the remaining 66 articles, 33 were excluded because they did not refer to diagnosis, treatment, prognosis, or prevention and control of VL. Then, 33 SRs were included and characterized, as summarized in Table 1. Concerning the level of confidence, 1 review was classified as moderate, 4 reviews were classified as low, and 28 reviews as critically low. The critical and non-critical items that represent weaknesses for SRs about VL are listed in Table 2. The evidence map is available from: https://public.tableau.com/app/profile/bireme/viz/leishmaniose-visceral-en/evidence-map.
TABLE 1. Characterization of the included systematic reviews.
|
Systematic review (reference) |
Level of confidence |
|---|---|
|
Moderate |
|
|
Low |
|
|
Critically low |
Source: Prepared by the authors based on the results of the evidence map.
TABLE 2. Strengths and weaknesses of the included systematic reviews, according to the criteria of the AMSTAR2 tool.
|
Criteria |
Answers (%)a |
|||
|---|---|---|---|---|
|
Yes |
Partial yes |
No |
No meta-analysis |
|
|
Presence of components of PICO |
84.8 |
0 |
15.2 |
NA |
|
Written protocol* |
3.0 |
57.6 |
39.4 |
NA |
|
Justification of the inclusion of the study designs |
15.2 |
0 |
84.8 |
NA |
|
Comprehensive literature search strategy* |
6.1 |
45.5 |
48.8 |
NA |
|
Study selection in duplicate |
75.8 |
0 |
24.2 |
NA |
|
Data extraction in duplicate |
57.6 |
0 |
42.4 |
NA |
|
List of excluded studies* |
15.2 |
6.1 |
78.8 |
NA |
|
Adequate description of the included studies |
27.3 |
21.2 |
51.5 |
NA |
|
Satisfactory technique for assessing the risk of bias* |
30.3 |
6.1 |
63.6 |
NA |
|
Report on the sources of funding for the studies included |
3.0 |
0 |
97.0 |
NA |
|
Use of appropriate methods for statistical combination of results* |
27.3 |
0 |
33.3 |
39.4 |
|
Assess the impact of risk of bias |
18.2 |
0 |
42.4 |
39.4 |
|
Account for risk of bias when interpreting/discussing* |
36.4 |
0 |
63.6 |
NA |
|
Explanation for any heterogeneity |
45.5 |
0 |
54.5 |
NA |
|
Investigation of publication bias and discussion* |
24.2 |
0 |
36.4 |
39.4 |
|
Report sources of conflict of interest |
90.9 |
0 |
9.1 |
NA |
Notes:
“Answers (%)” represents the percentage of SRs with a specific answer.
Asterisk indicates critical items.
PICO, Population, Intervention, Control, and Outcome; NA, not applicable.
Source: Prepared by the authors based on the results of the evidence map.
Prevention and control
The SRs that evaluated dog culling had conflicting results. The included studies show a significant reduction in the seroconversion or incidence of VL in areas where dog culling was carried out. Meanwhile, other investigations did not find significant difference between the intervention and control groups analyzed (11, 12). Similarly, two SRs diverge on the effect of dog culling on human seroconversion. One of them had the results of a study in which reservoir control reduced seroconversion in humans; another review concluded that this intervention does not provide protection (11, 13).
Three SRs analyzed insecticide-impregnated dog collars. One of the reviews indicated the absence of significant difference between the incidence rates obtained in the intervention studies, whereas the other two reviews, including a meta-analysis, highlight the statistically significant reduction in the incidence of VL in dogs through use of collars impregnated with deltamethrin (12, 14, 15). On the other hand, this intervention presented conflicting results regarding seroconversion in humans (11).
It was observed that insecticide thermal fogging and insecticides on the walls of houses can reduce the density of sandflies and cause the death of sandflies, respectively. Still, the resulting impact depends on the characteristics of the houses where the insecticide was applied. A protected home must have low density of sandflies, without damp, dark places or cracks and holes (16). Insecticidal spraying on houses using alpha-cypermethrin or lambda-cyhalothrin promoted a reduction in the density of sandflies; however, it had no significant effect on seroconversion in humans (12, 13). The lack of effect of insecticide spraying on the control of zoonotic VL in community trials contrasts with anecdotal reports of the effectiveness of long-lasting insecticides such as DDT (11).
The use of bednets impregnated with deltamethrin reduces the density and landing of sandflies on humans, besides causing the death of the vectors. However, their use does not significantly reduce seroconversion and the incidence of human VL (12, 13, 17). It was impossible to determine conclusions about the use of bednets due to divergent results on the existence of a protective effect of this intervention (18). The poor performance of aerial spray or short-lasting insecticides is likely due to the assumption that the life cycle of sandflies and transmission occurs indoors. Indeed, after biting, they land and may die if the surface has been recently sprayed. However, most species are exophilic and may enter dwellings every night or may bite outdoors and therefore escape the action of the insecticides currently in use.
Permethrin spot-on formulation and the fucose-mannose ligand antigen (FML) vaccine proved helpful in decreasing seroconversion and the incidence of VL in dogs (12, 14). Health education activities using educational material improved the knowledge of health professionals and school-age children about VL (19).
Dog culling associated with insecticide spraying of houses did not provide a statistically significant reduction in human seroconversion, or in the incidence of human VL, in comparison with control groups (12, 13). The inconclusive result was due to methodological weaknesses, such as absence of a control group and loss to follow-up in the included individual studies (11). Additionally, the combination of dog culling, treatment of human cases, and insecticide spraying of houses did not significantly reduce VL incidence (12). The effects of the prevention, control, and combined interventions on the respective outcomes are shown in Figure 1.
FIGURE 1. Effects of the prevention, control, and combined interventions on the respective visceral leishmaniasis outcomes.
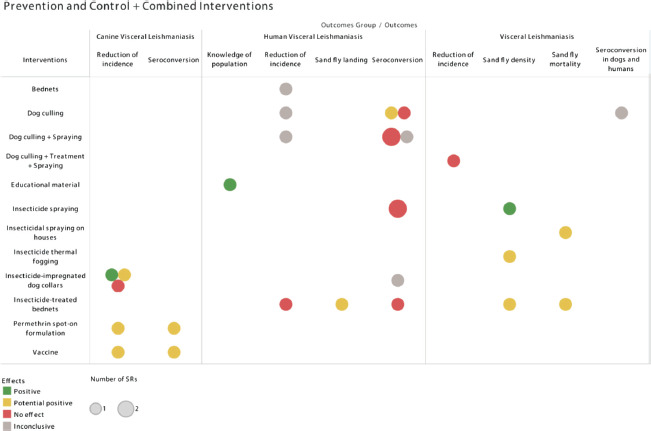
Source: Prepared by the authors based on the results of the evidence map
Diagnosis
For VL diagnosis in humans, one SR analyzed several antigens regarding their applicability in the development of diagnostic tests focusing on the East African region (20). Tests under development based on rK28 antigen obtained sensitivity and specificity above 94% and 97%, respectively. The use of urine samples in molecular tests had positivity lower than 60% (21). Urine samples applied to rK39 immunochromatographic test (rK39 ICT) and enzyme-linked immunosorbent assay (ELISA) presented, respectively, sensitivity of 97% and 91% and specificity of 98% and 99% (22). The performance of other biological samples in diagnostic tests could not be determined due to the lack of sufficient data or heterogeneity of the studies (21).
Polymerase chain reaction (PCR) showed excellent performance in diagnosing VL in humans, with sensitivity of 93.1% and 95.3% and specificity of 95.6% and 92.6% in blood and bone marrow samples, respectively (23). Among individuals with HIV, the sensitivity and specificity of PCR on blood samples were 92% and 96% (24). Among asymptomatic individuals, sensitivity varies from 92.1% to 96%, and specificity from 99.6% to 100% (25). Other molecular tests, such as loop-mediated isothermal amplification (LAMP) and nucleic acid sequence-based amplification (NASBA), have also shown potential for use to diagnose VL. LAMP had a sensitivity of 80% to 100% and a specificity of 95.8% to 100%, while NASBA on blood samples had 80% to 93% sensitivity and 57% to 100% specificity (23, 26).
Immunoblotting was evaluated by only one SR, which indicates it as an option for detecting VL among individuals with HIV due to its sensitivity and specificity of 84% and 82%, respectively. For this same population group, ELISA had low sensitivity and high specificity, 66% and 90% (24). Considering the overall population, ELISA with rK39 antigen showed better performance, with sensitivity of 92% and specificity of 81%. ELISA with antigens prepared from promastigotes had a sensitivity of 87% and a specificity of 77% (27). Similarly, indirect fluorescent antibody technique (IFAT) performed relatively better in the overall population than in people with HIV, with respective sensitivity of 88% and 51% and specificity of 90% and 93% (24, 27).
The term “agglutination tests” included direct agglutination test (DAT) and latex agglutination test (KAtex). Among the serological tests, DAT was recommended as an important diagnostic tool for humans. The sensitivity observed in the overall population ranged between 94% and 100%, while specificity ranged from 85.9% to 100% (12, 27–29). Among individuals with HIV, the sensitivity is 81%, and the specificity is 90%. Hence, DAT proved efficient in detecting VL, with superior performance to IFAT and ELISA, for example (24). KAtex had a lower performance than DAT, with a verified sensitivity of 63.6% and 77%, and specificity of 90% and 97% (30, 31).
rK39 ICT showed excellent performance, due to high sensitivity (85.7% to 93.9%) and specificity (80.9% to 100%) (12, 27, 28, 30). The performance of other rapid tests, such as rK26 and rKE16, remains inconclusive due to the low number of studies, which makes any recommendation unfeasible (30). The main challenges for serological tests for VL are HIV-coinfection and diseases with similar presentation. Although not evaluated in this study, parasitological tests are required for the diagnosis of VL in HIV-positive patients with symptoms resembling VL. The situation is also complex when severe diseases that lead to similar symptoms, such as leukemia and lymphoma, are a differential diagnosis. In these cases, a bone marrow examination can confirm or rule out these diagnoses.
For VL diagnosis in dogs, the application of molecular and serological tests on urine samples resulted in 51% and 62% positivity, respectively. Other biological specimens did not present conclusive data, similar to what was observed for humans (21). ELISA is characterized by moderate accuracy, with global sensitivity and specificity of 89% and 87%, superior to that verified for IFAT, with respective values of 88% and 63%. On the other hand, the performance of the immunochromatographic dual-path platform (DPP) is close to that presented for ELISA due to the sensitivity of 83% and the specificity of 73% (32).
rK39 ICT was evaluated as a valuable tool in confirming the clinical diagnosis due to the different sensitivity levels verified for detecting dogs with clinical signs and asymptomatic dogs, of 86.7% and 59.3%, respectively. The specificity calculated through meta-analysis was 98.7% (33). Agglutination tests perform well in detecting VL in dogs, with sensitivity and specificity parameters that vary between 85% and 100% and 89% and 100% (12). The effects of the diagnostic interventions concerning the respective applicability and performance outcomes in detecting VL are shown in Figure 2.
FIGURE 2. Effects of the diagnostic interventions on applicability and performance outcomes in detecting visceral leishmaniasis.
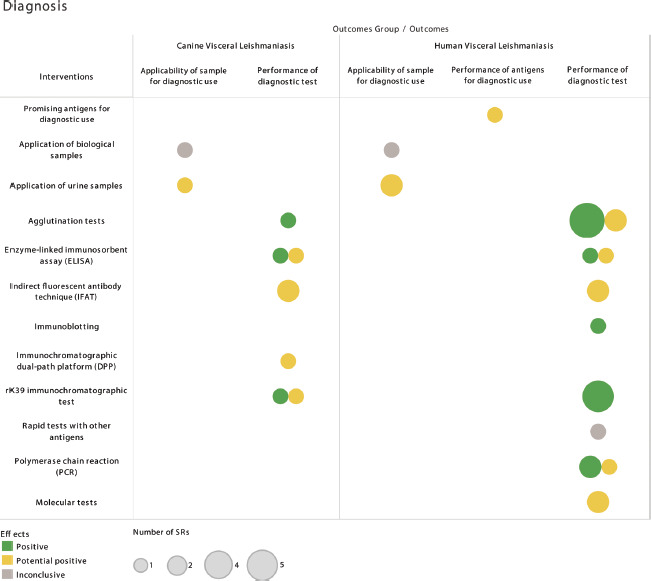
Notes: Biological samples evaluated for humans: feces; saliva, oral swab and sputum; semen and vulval secretion; milk and nasal secretion. Biological samples evaluated for dogs: feces; saliva, oral swab and sputum; conjunctival swab and lacrimal fluid/ocular; semen and vulval secretion; milk; nasal secretion; hair/bristles; and ear swab/cerumen.
Source: Prepared by the authors based on the results of the evidence map.
Treatment
Amphotericin B in the deoxycholate formulation showed high rates of cure. The dose of 1 mg/kg for 15 to 20 days was superior to other dose regimens due to a cure rate that reached 99% (34). The drug is effective for individuals with VL and HIV coinfection and is effective for treatment in different geographic regions (35, 36). Amphotericin B colloidal dispersion obtained high percentages of cure in Latin America (12). Liposomal amphotericin B showed high percentage of cure, which varies according to the dose, emphasizing the clinical response rate of 91% obtained for coinfection. This drug is useful in different areas of VL transmission, covering the Indian Subcontinent and Latin America (12, 34–38).
Pentavalent antimonials enable therapeutic success in approximately 80% of cases in the overall population (37). However, clinically relevant adverse events and resistance in some regions in India have led to recommendations to discontinue or limit their use (36, 38). The toxicity and mortality caused by these drugs justify the recommendation to administer liposomal amphotericin B over pentavalent antimonials in individuals with VL and HIV (35). The combination of pentavalent antimonials with recombinant interferon-gamma showed cure rates ranging from 69% to 100%, but further studies are needed to confirm this combination is superior to antimonials alone (39). On the other hand, the combination of pentavalent antimonials with paromomycin, evaluated in Ethiopia, produced a therapeutic success of 90.1% (37).
Miltefosine exhibits high cure rates ranging from 83% to 94% in children and adults in India (36). In Ethiopia, it was also found that this drug has resulted in 94.1% cure at the end of treatment, and the percentage of cure after six months of follow-up was 60% (37). Miltefosine is promising in areas with L. donovani transmission, but there is still no evidence of the same effectiveness in areas with L. infantum (38). Similarly, paromomycin, pentamidine, and sitamaquine are therapeutic options in India and Ethiopia (36, 37). One SR evaluated sitamaquine in Latin America, and no effect was observed (12). The intake of nutritional supplements is reported as not informed because the article about this theme was an SR that identified no eligible studies for review (40).
Treatment interventions reviewed for canine VL include amphotericin B, with remission rates higher than 80%. However, there are not enough data for indication due to serious adverse events in dogs. Pentavalent antimonials promote clinical remission between 65% and 100% but present moderate adverse events. The combination of pentavalent antimonials with allopurinol has been advantageous for reducing relapses. The other therapeutic options did not obtain conclusive effects, mainly due to the heterogeneity of the individual studies (14). The effect of the treatment interventions on the respective outcomes are shown in Figure 3.
FIGURE 3. Effects of the treatment interventions on the respective visceral leishmaniasis outcomes.
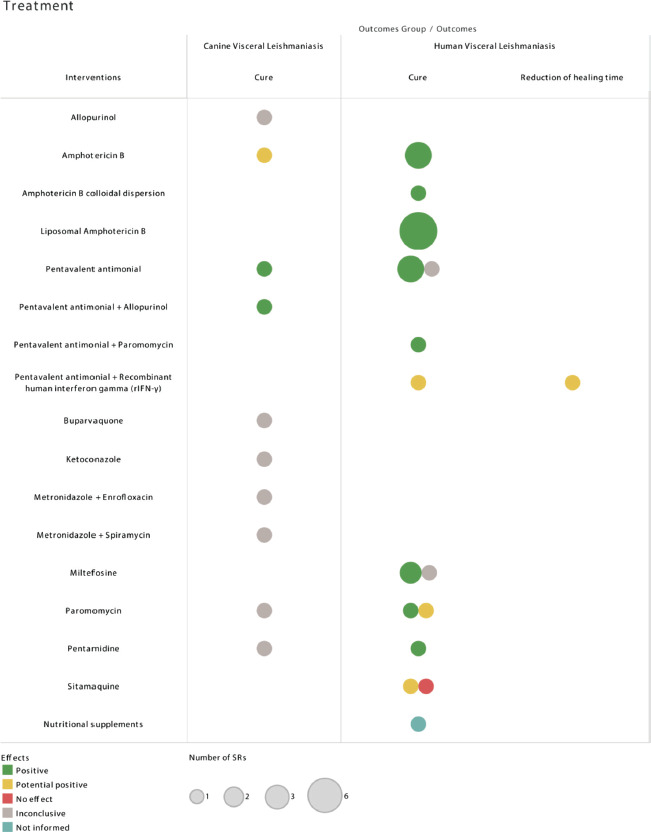
Notes: The interventions with “potential positive” effect need further studies to confirm the positive results.
Amphotericin B and liposomal amphotericin B were considered the best treatment options for people with visceral leishmaniasis–HIV coinfection.
Source: Prepared by the authors based on the results of the evidence map.
Regarding adverse events reported in humans, pentavalent antimonials can cause nephrotoxicity, bleeding, diarrhea, vomiting, pneumonia, pancreatitis, hepatotoxicity, cardiotoxicity, myalgia, headache, fever, colic, paresthesia, and death. Amphotericin B deoxycholate can lead to nephrotoxicity, diarrhea, cardiotoxicity, fever, and death, whereas liposomal amphotericin B had the same adverse outcomes, except for cardiotoxicity and vomiting. Miltefosine, sitamaquine, paromomycin, and pentamidine can cause hepatotoxicity and death. Additionally, miltefosine can lead to nephrotoxicity, diarrhea, vomiting, and pancreatitis. Pentamidine also presents insulin-dependent diabetes as an adverse event. Anemia, nephrotoxicity, and death are adverse outcomes of the combination of pentavalent antimonials with paromomycin (41).
Prognosis
Prognostic factors associated with poor clinical outcome and death were assessed by two SRs. Both reviews presented anemia, edema, hemorrhages, jaundice, age, and HIV coinfection as prognostic factors. Splenomegaly, sex, disease duration, malnutrition, and tuberculosis–VL coinfection were important prognostic factors in East Africa; thrombocytopenia, neutropenia, vomiting, diarrhea, dyspnea, and coinfections were relevant in the Americas (42, 43).
DISCUSSION
The VL evidence map includes essential topics and provides a foundation for the development of more effective policies. It not only mentions the available interventions but also the quality of the evidence. The effort to develop simple, accurate, and low-cost diagnostic tests and introduce these into clinical practice is highlighted. Likewise, this evidence map emphasizes that there are drugs which provide high cure rates even though the toxicity of the treatment is considerable. Despite this, the high cost and invasive nature of the techniques are still challenges. Furthermore, guidelines must be developed considering the best options for immunocompromised individuals.
It is worth noting that the differences inherent to the transmission areas can generate confusion in the interpretation of this evidence map. Drugs may perform well in areas where L. donovani is dominant but have no effect in L. infantum areas. This is more evident in terms of prevention and control interventions, mainly due to socio-environmental characteristics and the existence of zoonotic VL.
Prevention and control strategies showed weak evidence for zoonotic VL, but these interventions have been widely applied around the world. Bangladesh, India, and Nepal have implemented a policy that combines control interventions with diagnosis and treatment, which has promoted a dramatic reduction in the incidence of VL in the region, near to eradication. Surveillance and community involvement were part of the policy and contributed to this achievement (44), although it is not clear which of the applied strategies contributed the most to the programs’ success (45). This experience makes it clear that the maintenance of vector control measures enables interruption of disease transmission, especially when combined with other strategies. However, the epidemiology of anthroponotic VL is much less complex than that of zoonotic VL, which still needs much more knowledge for efficient control measures.
There is not enough evidence to support dog culling for the control of human disease, likely due to the quick replacement of young dogs whose susceptibility is even greater (46) and to the weaknesses of screening tests, which would result in the culling of healthy animals or not identifying those that pose the highest risk of transmission to humans. The treatment of canine VL is also questionable as a strategy for VL control, as dogs might relapse, the reduction of infectiveness is limited, and repeated use of drugs might cause resistance. Consequently, using insecticide-impregnated dog collars and spot-on formulation may be considered as the most promising prevention and control strategies (47). However, it has to be kept in mind that its implementation involves challenges in accessing homes, loss of collars, and the need for periodic replacement (48). Therefore, there is need for well-designed, large studies on the effectiveness of this strategy for the prevention of human VL, followed by cost-effectiveness analysis. Such a study is currently underway (personal communication with Guilherme L. Werneck, State University of Rio de Janeiro, 2022).
Several SRs had inconclusive results or were of critically low quality due to methodological weaknesses in the compiled evidence; hence, serious gaps remain in the knowledge about VL. Gaps in knowledge may be a consequence of limited resources for research, as VL is a neglected infectious disease, or of poor study design or implementation. Likewise, gaps in knowledge can be due to the complexity of the subject: VL is caused by a complex organism causing a serious disease with diverse clinical presentations and complications, with a poorly understood pathogenesis, is hard to treat and prevent, and is transmitted by vectors with elusive ecology. Besides more rigorous study protocols for conducting research, achievable by more scientific training, more generous investments in research are necessary in order to avoid distortions and ensure accurate results. This would result in the availability of evidence for public policies and policymakers’ trust in scientific knowledge. Indeed, a recent study shows how weak science on the subject is by describing a trend of increased VL mortality in Brazil (49).
Besides the factor of the quality of the evidence, this study has its own limitations: the inclusion of only SRs, and not capturing information contained in publications not indexed in the searched databases. Moreover, some of the identified gaps may have been already elucidated by individual studies or may not yet have been integrated in SRs and therefore were falsely identified as knowledge gaps.
We recommend early diagnosis and treatment of individuals with VL. DAT and rK39 ICT can be considered suitable options for diagnosis of VL in humans. The development of medicines with higher efficacy, lower toxicity, lower cost, and more convenient administration must be a priority for funders and researchers. Among the prevention and control measures, use of insecticide-impregnated dog collars with close surveillance should be further investigated, with improved study design in the Americas.
Conclusion
The evidence map on VL developed reflects the complexity of the disease and shows the ways to confront it. Diagnostic tools such as rK39 ICT and DAT should be applied, and liposomal amphotericin B and pentavalent antimonials should be administered according to patient characteristics to avoid complications. The use of insecticide-impregnated collars appears to be a promising measure for the control of VL in countries where the disease is a zoonosis.
Disclaimer.
Authors hold sole responsibility for the views expressed in the manuscript, which may not necessarily reflect the opinion or policy of the Revista Panamericana de Salud Pública/Pan American Journal of Public Health and/or those of the Pan American Health Organization.
Acknowledgments.
The authors would like to acknowledge BIREME/PAHO/WHO, the Foundation for Research Support of the State of Piauí (FAPEPI), and Lis Cardoso Marinho Medeiros for her insightful suggestions.
Footnotes
Author contributions.
All authors conceived the original idea, collected the data, and analyzed the data. ABI, BGAA, DLC, CHNC, and CVMA contributed data or analysis tools, interpreted the results, wrote the paper, and reviewed the paper. All authors reviewed and approved the final version.
Conflict of interest.
None declared.
REFERENCES
- 1.World Health Organization . Geneva: WHO; 2010. [cited 2021 Nov 1]. Control of the leishmaniasis: report of a meeting of the WHO Expert Committee on the Control of Leishmaniases. Technical Report Series; Report No. 949. Available from: https://apps.who.int/iris/bitstream/handle/10665/44412/WHO_TRS_949_eng.pdf. [Google Scholar]; World Health Organization. Control of the leishmaniasis: report of a meeting of the WHO Expert Committee on the Control of Leishmaniases. Geneva: WHO; 2010. Technical Report Series; Report No. 949. Available from: https://apps.who.int/iris/bitstream/handle/10665/44412/WHO_TRS_949_eng.pdf [cited 2021 Nov 1].
- 2.Pearson RD, Sousa ADQ. Clinical spectrum of leishmaniasis. Clin Infect Dis. 1996;22(1):1–13. doi: 10.1093/clinids/22.1.1. [DOI] [PubMed] [Google Scholar]; Pearson RD, Sousa ADQ. Clinical spectrum of leishmaniasis. Clin Infect Dis. 1996;22(1):1–13. 10.1093/clinids/22.1.1 [DOI] [PubMed]
- 3.Baneth G, Koutinas AF, Solano-Gallego L, Bourdeau P, Ferrer L. Canine leishmaniosis - new concepts and insights on an expanding zoonosis: part one. Trends Parasitol. 2008;24(7):324–330. doi: 10.1016/j.pt.2008.04.001. [DOI] [PubMed] [Google Scholar]; Baneth G, Koutinas AF, Solano-Gallego L, Bourdeau P, Ferrer L. Canine leishmaniosis - new concepts and insights on an expanding zoonosis: part one. Trends Parasitol. 2008;24(7):324–30. 10.1016/j.pt.2008.04.001 [DOI] [PubMed]
- 4.Valero NNH, Uriarte M. Environmental and socioeconomic risk factors associated with visceral and cutaneous leishmaniasis: a systematic review. Parasitol Res. 2020;119(2):365–384. doi: 10.1007/s00436-019-06575-5. [DOI] [PubMed] [Google Scholar]; Valero NNH, Uriarte M. Environmental and socioeconomic risk factors associated with visceral and cutaneous leishmaniasis: a systematic review. Parasitol Res. 2020;119(2):365–84. 10.1007/s00436-019-06575-5 [DOI] [PubMed]
- 5.Katz DL, Williams AL, Girard C, Goodman J, Comerford B, Behrman A, et al. The evidence base for complementary and alternative medicine: Methods of evidence mapping with application to CAM. Altern Ther Health Med. 2003;9(4):22–30. https://pubmed.ncbi.nlm.nih.gov/12868249/ [PubMed] [Google Scholar]; Katz DL, Williams AL, Girard C, Goodman J, Comerford B, Behrman A, et al. The evidence base for complementary and alternative medicine: Methods of evidence mapping with application to CAM. Altern Ther Health Med. 2003;9(4):22–30. https://pubmed.ncbi.nlm.nih.gov/12868249/ [PubMed]
- 6.Miake-Lye IM, Hempel S, Shanman R, Shekelle PG. What is an evidence map? A systematic review of published evidence maps and their definitions, methods, and products. Syst Rev. 2016;5:28. doi: 10.1186/s13643-016-0204-x. [DOI] [PMC free article] [PubMed] [Google Scholar]; Miake-Lye IM, Hempel S, Shanman R, Shekelle PG. What is an evidence map? A systematic review of published evidence maps and their definitions, methods, and products. Syst Rev. 2016;5:28. 10.1186/s13643-016-0204-x [DOI] [PMC free article] [PubMed]
- 7.Schveitzer MC, Abdala CVM, Portella CFS, Ghelman R. Traditional, complementary, and integrative medicine evidence map: A methodology to an overflowing field of data and noise. Rev Panam Salud Publica. 2021;45:e48. doi: 10.26633/rpsp.2021.48. [DOI] [PMC free article] [PubMed] [Google Scholar]; Schveitzer MC, Abdala CVM, Portella CFS, Ghelman R. Traditional, complementary, and integrative medicine evidence map: A methodology to an overflowing field of data and noise. Rev Panam Salud Publica. 2021;45:e48. 10.26633/rpsp.2021.48 [DOI] [PMC free article] [PubMed]
- 8.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed]
- 9.Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:1–9. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]; Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:1–9. 10.1136/bmj.j4008 [DOI] [PMC free article] [PubMed]
- 10.Deardorff A. Tableau (version. 9.1) J Med Libr Assoc. 2016;104(2):182–183. doi: 10.5195/jmla.2016.75. [DOI] [Google Scholar]; Deardorff A. Tableau (version. 9.1). J Med Libr Assoc. 2016;104(2):182–3. 10.5195/jmla.2016.75 [DOI]
- 11.Costa CHN. How effective is dog culling in controlling zoonotic visceral leishmaniasis? A critical evaluation of the science, politics and ethics behind this public health policy. Rev Soc Bras Med Trop. 2011;44(2):232–242. doi: 10.1590/s0037-86822011005000014. [DOI] [PubMed] [Google Scholar]; Costa CHN. How effective is dog culling in controlling zoonotic visceral leishmaniasis? A critical evaluation of the science, politics and ethics behind this public health policy. Rev Soc Bras Med Trop. 2011;44(2):232–42. 10.1590/s0037-86822011005000014 [DOI] [PubMed]
- 12.Romero GAS, Boelaert M. Control of visceral leishmaniasis in Latin America - A systematic review. PLoS Negl Trop Dis. 2010;4(1):e584. doi: 10.1371/journal.pntd.0000584. [DOI] [PMC free article] [PubMed] [Google Scholar]; Romero GAS, Boelaert M. Control of visceral leishmaniasis in Latin America - A systematic review. PLoS Negl Trop Dis. 2010;4(1):e584. 10.1371/journal.pntd.0000584 [DOI] [PMC free article] [PubMed]
- 13.González U, Pinart M, Sinclair D, Firooz A, Enk C, Vélez ID, et al. Vector and reservoir control for preventing leishmaniasis. Cochrane Database Syst Rev. 2015;2015(8):CD008736. doi: 10.1002/14651858.cd008736.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]; González U, Pinart M, Sinclair D, Firooz A, Enk C, Vélez ID, et al. Vector and reservoir control for preventing leishmaniasis. Cochrane Database Syst Rev. 2015;2015(8):CD008736. 10.1002/14651858.cd008736.pub2 [DOI] [PMC free article] [PubMed]
- 14.Noli C, Auxilia ST. Treatment of canine Old World visceral leishmaniasis: A systematic review. Vet Dermatol. 2005;16(4):213–232. doi: 10.1111/j.1365-3164.2005.00460.x. [DOI] [PubMed] [Google Scholar]; Noli C, Auxilia ST. Treatment of canine Old World visceral leishmaniasis: A systematic review. Vet Dermatol. 2005;16(4):213–32. 10.1111/j.1365-3164.2005.00460.x [DOI] [PubMed]
- 15.Yimam Y, Mohebali M. Effectiveness of insecticide-impregnated dog collars in reducing incidence rate of canine visceral leishmaniasis: A systematic review and meta-analysis. PloS One. 2020;15(9):e0238601. doi: 10.1371/journal.pone.0238601. [DOI] [PMC free article] [PubMed] [Google Scholar]; Yimam Y, Mohebali M. Effectiveness of insecticide-impregnated dog collars in reducing incidence rate of canine visceral leishmaniasis: A systematic review and meta-analysis. PloS One. 2020;15(9):e0238601. 10.1371/journal.pone.0238601 [DOI] [PMC free article] [PubMed]
- 16.Calderon-Anyosa R, Galvez-Petzoldt C, Garcia PJ, Carcamo CP. Housing Characteristics and Leishmaniasis: A Systematic Review. Am J Trop Med Hygiene. 2018;99(6):1547–1554. doi: 10.4269/ajtmh.18-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]; Calderon-Anyosa R, Galvez-Petzoldt C, Garcia PJ, Carcamo CP. Housing Characteristics and Leishmaniasis: A Systematic Review. Am J Trop Med Hygiene. 2018;99(6):1547–54. 10.4269/ajtmh.18-0037 [DOI] [PMC free article] [PubMed]
- 17.Wilson AL, Dhiman RC, Kitron U, Scott TW, van den Berg H, Lindsay SW. Benefit of insecticide-treated nets, curtains, and screening on vector-borne diseases, excluding malaria: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2014;8(10):e3228. doi: 10.1371/journal.pntd.0003228. [DOI] [PMC free article] [PubMed] [Google Scholar]; Wilson AL, Dhiman RC, Kitron U, Scott TW, van den Berg H, Lindsay SW. Benefit of insecticide-treated nets, curtains, and screening on vector-borne diseases, excluding malaria: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2014;8(10):e3228. 10.1371/journal.pntd.0003228 [DOI] [PMC free article] [PubMed]
- 18.Bern C, Courtenay O, Alvar J. Of cattle, sand flies and men: a systematic review of risk factor analyses for South Asian visceral leishmaniasis and implications for elimination. PLoS Negl Trop Dis. 2010;4(2):e599. doi: 10.1371/journal.pntd.0000599. [DOI] [PMC free article] [PubMed] [Google Scholar]; Bern C, Courtenay O, Alvar J. Of cattle, sand flies and men: a systematic review of risk factor analyses for South Asian visceral leishmaniasis and implications for elimination. PLoS Negl Trop Dis. 2010;4(2):e599. 10.1371/journal.pntd.0000599 [DOI] [PMC free article] [PubMed]
- 19.de Sousa CTV, de Andrade CAF, da Hora DL, Hora EL da, Momesso Neto IA, de Matos MC, et al. Health education in South America regarding leishmaniasis: a systematic review. Rev Patol Trop. 2015;44(2):111–123. https://www.arca.fiocruz.br/bitstream/icict/35058/2/ve_Souza_Claudia_etal_INI_2015.pdf [Google Scholar]; Sousa CTV de, Andrade CAF de, Hora DL da, Hora EL da, Momesso Neto IA, Matos MC de, et al. Health education in South America regarding leishmaniasis: a systematic review. Rev Patol Trop. 2015;44(2):111–23. https://www.arca.fiocruz.br/bitstream/icict/35058/2/ve_Souza_Claudia_etal_INI_2015.pdf
- 20.Kühne V, Rezaei Z, Pitzinger P, Büscher P. Systematic review on antigens for serodiagnosis of visceral leishmaniasis, with a focus on East Africa. PLoS Negl Trop Dis. 2019;13(8):e0007658. doi: 10.1371/journal.pntd.0007658. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kühne V, Rezaei Z, Pitzinger P, Büscher P. Systematic review on antigens for serodiagnosis of visceral leishmaniasis, with a focus on East Africa. PLoS Negl Trop Dis. 2019;13(8):e0007658. 10.1371/journal.pntd.0007658 [DOI] [PMC free article] [PubMed]
- 21.Sereno D, Akhoundi M, Sayehmri K, Mirzaei A, Holzmuller P, Lejon V, et al. Noninvasive Biological Samples to Detect and Diagnose Infections due to Trypanosomatidae Parasites: A Systematic Review and Meta-Analysis. Int J Mol Sci. 2020;21(5):1684. doi: 10.3390/ijms21051684. [DOI] [PMC free article] [PubMed] [Google Scholar]; Sereno D, Akhoundi M, Sayehmri K, Mirzaei A, Holzmuller P, Lejon V, et al. Noninvasive Biological Samples to Detect and Diagnose Infections due to Trypanosomatidae Parasites: A Systematic Review and Meta-Analysis. Int J Mol Sci. 2020;21(5):1684. 10.3390/ijms21051684 [DOI] [PMC free article] [PubMed]
- 22.Asfaram S, Hosseini Teshnizi S, Fakhar M, Banimostafavi ES, Soosaraei M. Is urine a reliable clinical sample for the diagnosis of human visceral leishmaniasis? A systematic review and meta-analysis. Parasitol Int. 2018;67(5):575–583. doi: 10.1016/j.parint.2018.05.008. [DOI] [PubMed] [Google Scholar]; Asfaram S, Hosseini Teshnizi S, Fakhar M, Banimostafavi ES, Soosaraei M. Is urine a reliable clinical sample for the diagnosis of human visceral leishmaniasis? A systematic review and meta-analysis. Parasitol Int. 2018;67(5):575–83. 10.1016/j.parint.2018.05.008 [DOI] [PubMed]
- 23.de Ruiter CM, van der Veer C, Leeflang MMG, Deborggraeve S, Lucas C, Adams ER. Molecular tools for diagnosis of visceral leishmaniasis: Systematic review and meta-analysis of diagnostic test accuracy. J Clin Microbiol. 2014;52(9):3147–3155. doi: 10.1128/jcm.00372-14. [DOI] [PMC free article] [PubMed] [Google Scholar]; de Ruiter CM, van der Veer C, Leeflang MMG, Deborggraeve S, Lucas C, Adams ER. Molecular tools for diagnosis of visceral leishmaniasis: Systematic review and meta-analysis of diagnostic test accuracy. J Clin Microbiol. 2014;52(9):3147–55. 10.1128/jcm.00372-14 [DOI] [PMC free article] [PubMed]
- 24.Cota GF, de Sousa MR, Demarqui FN, Rabello A. The diagnostic accuracy of serologic and molecular methods for detecting visceral leishmaniasis in HIV infected patients: meta-analysis. PLoS Negl Trop Dis. 2012;6(5):e1665. doi: 10.1371/journal.pntd.0001665. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cota GF, de Sousa MR, Demarqui FN, Rabello A. The diagnostic accuracy of serologic and molecular methods for detecting visceral leishmaniasis in HIV infected patients: meta-analysis. PLoS Negl Trop Dis. 2012;6(5):e1665. 10.1371/journal.pntd.0001665 [DOI] [PMC free article] [PubMed]
- 25.Vieira AVB, Farias PCS, Bezerra GSN, Xavier AT, Lima Júnior MSC, Silva ED da, et al. Evaluation of molecular techniques to visceral leishmaniasis detection in asymptomatic patients: a systematic review. Expert Rev Mol Diagn. 2021;21(5):493–504. doi: 10.1080/14737159.2021.1900736. [DOI] [PubMed] [Google Scholar]; Vieira AVB, Farias PCS, Bezerra GSN, Xavier AT, Lima Júnior MSC, Silva ED da, et al. Evaluation of molecular techniques to visceral leishmaniasis detection in asymptomatic patients: a systematic review. Expert Rev Mol Diagn. 2021;21(5):493–504. 10.1080/14737159.2021.1900736 [DOI] [PubMed]
- 26.Bezerra GSN, Barbosa Júnior WL, Vieira AVB, Xavier AT, Lima Júnior MSDC, Xavier EM, et al. Loop-mediated isothermal amplification methods for diagnosis of visceral leishmaniasis (kala-azar)–a systematic review. Expert Rev Mol Diagn. 2020;20(5):455–465. doi: 10.1080/14737159.2020.1736564. [DOI] [PubMed] [Google Scholar]; Bezerra GSN, Barbosa Júnior WL, Vieira AVB, Xavier AT, Lima Júnior MSDC, Xavier EM, et al. Loop-mediated isothermal amplification methods for diagnosis of visceral leishmaniasis (kala-azar)–a systematic review. Expert Rev Mol Diagn. 2020;20(5):455–65. 10.1080/14737159.2020.1736564 [DOI] [PubMed]
- 27.Maia Z, Lírio M, Mistro S, Mendes CMC, Mehta SR, Badaro R. Comparative study of rK39 Leishmania antigen for serodiagnosis of visceral leishmaniasis: Systematic review with meta-analysis. PLoS Negl Trop Dis. 2012;6(1):e1484. doi: 10.1371/journal.pntd.0001484. [DOI] [PMC free article] [PubMed] [Google Scholar]; Maia Z, Lírio M, Mistro S, Mendes CMC, Mehta SR, Badaro R. Comparative study of rK39 Leishmania antigen for serodiagnosis of visceral leishmaniasis: Systematic review with meta-analysis. PLoS Negl Trop Dis. 2012;6(1):e1484. 10.1371/journal.pntd.0001484 [DOI] [PMC free article] [PubMed]
- 28.Chappuis F, Rijal S, Soto A, Menten J, Boelaert M. A meta-analysis of the diagnostic performance of the direct agglutination test and rK39 dipstick for visceral leishmaniasis. BMJ. 2006;333(7571):723. doi: 10.1136/bmj.38917.503056.7c. [DOI] [PMC free article] [PubMed] [Google Scholar]; Chappuis F, Rijal S, Soto A, Menten J, Boelaert M. A meta-analysis of the diagnostic performance of the direct agglutination test and rK39 dipstick for visceral leishmaniasis. BMJ. 2006;333(7571):723. 10.1136/bmj.38917.503056.7c [DOI] [PMC free article] [PubMed]
- 29.Mohebali M, Keshavarz H, Shirmohammad S, Akhoundi B, Borjian A, Hassanpour G, et al. The diagnostic accuracy of direct agglutination test for serodiagnosis of human visceral leishmaniasis: a systematic review with meta-analysis. BMC Infect Dis. 2020;20(1):946. doi: 10.1186/s12879-020-05558-7. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mohebali M, Keshavarz H, Shirmohammad S, Akhoundi B, Borjian A, Hassanpour G, et al. The diagnostic accuracy of direct agglutination test for serodiagnosis of human visceral leishmaniasis: a systematic review with meta-analysis. BMC Infect Dis. 2020;20(1):946. 10.1186/s12879-020-05558-7 [DOI] [PMC free article] [PubMed]
- 30.Boelaert M, Verdonck K, Menten J, Sunyoto T, van Griensven J, Chappuis F, et al. Rapid tests for the diagnosis of visceral leishmaniasis in patients with suspected disease. Cochrane Database Syst Rev. 2014;2014(6):CD009135. doi: 10.1002/14651858.cd009135.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]; Boelaert M, Verdonck K, Menten J, Sunyoto T, van Griensven J, Chappuis F, et al. Rapid tests for the diagnosis of visceral leishmaniasis in patients with suspected disease. Cochrane Database Syst Rev. 2014;2014(6):CD009135. 10.1002/14651858.cd009135.pub2 [DOI] [PMC free article] [PubMed]
- 31.Fararouei M, Sarkari B, Khabisi SA, Rezaei Z. Diagnostic accuracy of urinary latex agglutination test (KAtex) for the diagnosis of visceral leishmaniasis: A meta-analysis. J Infect Dev Ctries. 2018;12(12):1045–1051. doi: 10.3855/jidc.10185. [DOI] [PubMed] [Google Scholar]; Fararouei M, Sarkari B, Khabisi SA, Rezaei Z. Diagnostic accuracy of urinary latex agglutination test (KAtex) for the diagnosis of visceral leishmaniasis: A meta-analysis. J Infect Dev Ctries. 2018;12(12):1045–51. 10.3855/jidc.10185 [DOI] [PubMed]
- 32.Peixoto HM, de Oliveira MRF, Romero GAS. Serological diagnosis of canine visceral leishmaniasis in Brazil: systematic review and meta-analysis. Trop Med Int Health. 2015;20(3):334–352. doi: 10.1111/tmi.12429. [DOI] [PubMed] [Google Scholar]; Peixoto HM, de Oliveira MRF, Romero GAS. Serological diagnosis of canine visceral leishmaniasis in Brazil: systematic review and meta-analysis. Trop Med Int Health. 2015;20(3):334–52. 10.1111/tmi.12429 [DOI] [PubMed]
- 33.Quinnell RJ, Carson C, Reithinger R, Garcez LM, Courtenay O. Evaluation of rK39 rapid diagnostic tests for canine visceral leishmaniasis: longitudinal study and meta-analysis. PLoS Negl Trop Dis. 2013;7(1):e1992. doi: 10.1371/journal.pntd.0001992. [DOI] [PMC free article] [PubMed] [Google Scholar]; Quinnell RJ, Carson C, Reithinger R, Garcez LM, Courtenay O. Evaluation of rK39 rapid diagnostic tests for canine visceral leishmaniasis: longitudinal study and meta-analysis. PLoS Negl Trop Dis. 2013;7(1):e1992. 10.1371/journal.pntd.0001992 [DOI] [PMC free article] [PubMed]
- 34.Rodrigo C, Weeratunga P, Fernando SD, Rajapakse S. Amphotericin B for treatment of visceral leishmaniasis: systematic review and meta-analysis of prospective comparative clinical studies including dose-ranging studies. Clin Microbiol Infect. 2018;24(6):591–598. doi: 10.1016/j.cmi.2017.11.008. [DOI] [PubMed] [Google Scholar]; Rodrigo C, Weeratunga P, Fernando SD, Rajapakse S. Amphotericin B for treatment of visceral leishmaniasis: systematic review and meta-analysis of prospective comparative clinical studies including dose-ranging studies. Clin Microbiol Infect. 2018;24(6):591–8. 10.1016/j.cmi.2017.11.008 [DOI] [PubMed]
- 35.Cota GF, de Sousa MR, Fereguetti TO, Rabello A. Efficacy of anti-leishmania therapy in visceral leishmaniasis among HIV infected patients: a systematic review with indirect comparison. PLoS Negl Trop Dis. 2013;7(5):e2195. doi: 10.1371/journal.pntd.0002195. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cota GF, de Sousa MR, Fereguetti TO, Rabello A. Efficacy of anti-leishmania therapy in visceral leishmaniasis among HIV infected patients: a systematic review with indirect comparison. PLoS Negl Trop Dis. 2013;7(5):e2195. 10.1371/journal.pntd.0002195 [DOI] [PMC free article] [PubMed]
- 36.Olliaro PL, Guerin PJ, Gerstl S, Haaskjold AA, Rottingen J-A, Sundar S. Treatment options for visceral leishmaniasis: a systematic review of clinical studies done in India, 1980-2004. Lancet Infect Dis. 2005;5(12):763–774. doi: 10.1016/s1473-3099(05)70296-6. [DOI] [PubMed] [Google Scholar]; Olliaro PL, Guerin PJ, Gerstl S, Haaskjold AA, Rottingen J-A, Sundar S. Treatment options for visceral leishmaniasis: a systematic review of clinical studies done in India, 1980-2004. Lancet Infect Dis. 2005;5(12):763–74. 10.1016/s1473-3099(05)70296-6 [DOI] [PubMed]
- 37.Gebreyohannes EA, Bhagvathula AS, Abegaz TM, Seid MA. Treatment outcomes of visceral leishmaniasis in Ethiopia from 2001 to 2017: a systematic review and meta-analysis. Infect Dis Poverty. 2018;7(1):1–9. doi: 10.1186/s40249-018-0491-7. [DOI] [PMC free article] [PubMed] [Google Scholar]; Gebreyohannes EA, Bhagvathula AS, Abegaz TM, Seid MA. Treatment outcomes of visceral leishmaniasis in Ethiopia from 2001 to 2017: a systematic review and meta-analysis. Infect Dis Poverty. 2018;7(1):1–9. 10.1186/s40249-018-0491-7 [DOI] [PMC free article] [PubMed]
- 38.Hodiamont CJ, Kager PA, Bart A, deVries HJC, van Thiel PPAM, Leenstra T, et al. Species-directed therapy for leishmaniasis in returning travellers: a comprehensive guide. PLoS Negl Trop Dis. 2014;8(5):e2832. doi: 10.1371/journal.pntd.0002832. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hodiamont CJ, Kager PA, Bart A, de Vries HJC, van Thiel PPAM, Leenstra T, et al. Species-directed therapy for leishmaniasis in returning travellers: a comprehensive guide. PLoS Negl Trop Dis. 2014;8(5):e2832. 10.1371/journal.pntd.0002832 [DOI] [PMC free article] [PubMed]
- 39.Mota CA, Oyama J, Souza Terron Monich M de, Brustolin AÁ, Perez de Souza JV, Murase LS, et al. Three decades of clinical trials on immunotherapy for human leishmaniases: a systematic review and meta-analysis. Immunotherapy. 2021;13(8):693–721. doi: 10.2217/imt-2020-0184. [DOI] [PubMed] [Google Scholar]; Mota CA, Oyama J, Souza Terron Monich M de, Brustolin AÁ, Perez de Souza JV, Murase LS, et al. Three decades of clinical trials on immunotherapy for human leishmaniases: a systematic review and meta-analysis. Immunotherapy. 2021;13(8):693–721. 10.2217/imt-2020-0184 [DOI] [PubMed]
- 40.Custodio E, López-Alcade J, Herrero M, Bouza C, Jimenez C, Storcksdieck genannt Bonsmann S, et al. Nutritional supplements for patients being treated for active visceral leishmaniasis. Cochrane Database Syst Rev. 2018;2018(3):CD012261. doi: 10.1002/14651858.cd012261.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]; Custodio E, López-Alcade J, Herrero M, Bouza C, Jimenez C, Storcksdieck genannt Bonsmann S, et al. Nutritional supplements for patients being treated for active visceral leishmaniasis. Cochrane Database Syst Rev. 2018;2018(3):CD012261. 10.1002/14651858.cd012261.pub2 [DOI] [PMC free article] [PubMed]
- 41.Singh-Phulgenda S, Dahal P, Ngu R, Maguire BJ, Hawryszkiewycz A, Rashan S, et al. Serious adverse events following treatment of visceral leishmaniasis: A systematic review and meta-analysis. PLoS Negl Trop Dis. 2021;15(3):e0009302. doi: 10.1371/journal.pntd.0009302. [DOI] [PMC free article] [PubMed] [Google Scholar]; Singh-Phulgenda S, Dahal P, Ngu R, Maguire BJ, Hawryszkiewycz A, Rashan S, et al. Serious adverse events following treatment of visceral leishmaniasis: A systematic review and meta-analysis. PLoS Negl Trop Dis. 2021;15(3):e0009302. 10.1371/journal.pntd.0009302 [DOI] [PMC free article] [PubMed]
- 42.Abongomera C, van Henten S, Vogt F, Buyze J, Verdonck K, van Griensven J. Prognostic factors for mortality among patients with visceral leishmaniasis in East Africa: Systematic review and meta-analysis. PLoS Negl Trop Dis. 2020;14(5):e0008319. doi: 10.1371/journal.pntd.0008319. [DOI] [PMC free article] [PubMed] [Google Scholar]; Abongomera C, van Henten S, Vogt F, Buyze J, Verdonck K, van Griensven J. Prognostic factors for mortality among patients with visceral leishmaniasis in East Africa: Systematic review and meta-analysis. PLoS Negl Trop Dis. 2020;14(5):e0008319. 10.1371/journal.pntd.0008319 [DOI] [PMC free article] [PubMed]
- 43.Belo VS, Struchiner CJ, Barbosa DS, Nascimento BWL, Horta MAP, da Silva ES, et al. Risk Factors for Adverse Prognosis and Death in American Visceral Leishmaniasis: A Meta-analysis. PLoS Negl Trop Dis. 2014;8(7):e2982. doi: 10.1371/journal.pntd.0002982. [DOI] [PMC free article] [PubMed] [Google Scholar]; Belo VS, Struchiner CJ, Barbosa DS, Nascimento BWL, Horta MAP, da Silva ES, et al. Risk Factors for Adverse Prognosis and Death in American Visceral Leishmaniasis: A Meta-analysis. PLoS Negl Trop Dis. 2014;8(7):e2982. 10.1371/journal.pntd.0002982 [DOI] [PMC free article] [PubMed]
- 44.Kumar V, Mandal R, Das S, Kesari S, Dinesh DS, Pandey K, et al. Kala-azar elimination in a highly-endemic district of Bihar, India: A success story. PLoS Negl Trop Dis. 2020;14(5):e0008254. doi: 10.1371/journal.pntd.0008254. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kumar V, Mandal R, Das S, Kesari S, Dinesh DS, Pandey K., et al. Kala-azar elimination in a highly-endemic district of Bihar, India: A success story. PLoS Negl Trop Dis. 2020:14(5):e0008254. 10.1371/journal.pntd.0008254 [DOI] [PMC free article] [PubMed]
- 45.World Health Organization Kala-azar in India–progress and challenges towards its elimination as a public health problem. Wkly Epidemiol Rec. 2021;96(26):267–279. https://apps.who.int/iris/bitstream/handle/10665/342263/WER9626-267-279-eng-fre.pdf [Google Scholar]; World Health Organization. Kala-azar in India–progress and challenges towards its elimination as a public health problem. Wkly Epidemiol Rec. 2021;96(26):267–79. https://apps.who.int/iris/bitstream/handle/10665/342263/WER9626-267-279-eng-fre.pdf
- 46.Nunes CM, deLima VMF, de Paula HB, Perri SHV, de Andrade AM, Dias FEF, et al. Dog culling and replacement in an area endemic for visceral leishmaniasis in Brazil. Vet Parasitol. 2008;153(1–2):19–23. doi: 10.1016/j.vetpar.2008.01.005. [DOI] [PubMed] [Google Scholar]; Nunes, CM, de Lima VMF, de Paula HB, Perri SHV, de Andrade AM, Dias FEF, et al. Dog culling and replacement in an area endemic for visceral leishmaniasis in Brazil. Vet Parasitol. 2008;153(1–2):19–23. 10.1016/j.vetpar.2008.01.005 [DOI] [PubMed]
- 47.Travi BL, Cordeiro-da-Silva A, Dantas-Torres F, Miró G. Canine visceral leishmaniasis: Diagnosis and management of the reservoir living among us. PLoS Negl Trop Dis. 2018;12(1):e0006082. doi: 10.1371/journal.pntd.0006082. [DOI] [PMC free article] [PubMed] [Google Scholar]; Travi BL, Cordeiro-da-Silva A, Dantas-Torres F, Miró G. Canine visceral leishmaniasis: Diagnosis and management of the reservoir living among us. PLoS Negl Trop Dis. 2018;12(1):e0006082. 10.1371/journal.pntd.0006082 [DOI] [PMC free article] [PubMed]
- 48.Alves EB, Figueiredo FB, Rocha MF, Werneck GL. Dificuldades operacionais no uso de coleiras caninas impregnadas com inseticida para o controle da leishmaniose visceral, Montes Claros, MG, 2012. Epidemiol Serv Saúde. 2018;27:e2017469. doi: 10.5123/s1679-49742018000400001. [DOI] [PubMed] [Google Scholar]; Alves EB, Figueiredo FB, Rocha MF, Werneck GL. Dificuldades operacionais no uso de coleiras caninas impregnadas com inseticida para o controle da leishmaniose visceral, Montes Claros, MG, 2012. Epidemiol Serv Saúde. 2018;27:e2017469. 10.5123/s1679-49742018000400001 [DOI] [PubMed]
- 49.Cota G, Erber AC, Schernhammer E, Simões TC. Inequalities of visceral leishmaniasis case-fatality in Brazil: A multilevel modeling considering space, time, individual and contextual factors. PLoS Negl Trop Dis. 2021;15(7):e0009567. doi: 10.1371/journal.pntd.0009567. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cota G, Erber AC, Schernhammer E, Simões TC. Inequalities of visceral leishmaniasis case-fatality in Brazil: A multilevel modeling considering space, time, individual and contextual factors. PLoS Negl Trop Dis. 2021;15(7):e0009567. 10.1371/journal.pntd.0009567 [DOI] [PMC free article] [PubMed]


