Highlights
-
•
As of April 2021, a low proportion of the Canada had been infected by severe acute respiratory syndrome coronavirus-2.
-
•
Early on, the number of reported cases represented only one in eight infected persons.
-
•
By April 2021, the number of persons infected was similar to the number of reported cases.
Keywords: COVID-19, SARS-CoV-2, Seroprevalence, Canada, Antibodies, Serosurvey
Abstarct
Objectives
To estimate the proportion of the population infected by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in Canada through April 2021, 16 months into the coronavirus disease 2019 (COVID-19) pandemic and 4 months after COVID-19 vaccines became available.
Methods
Publication databases, preprint servers, public health databases and the grey literature were searched for seroprevalence surveys conducted in Canada from 1 November 2019 to 10 July 2021. Studies were assessed for bias using the Joanna Briggs Checklist. Numbers of infections derived from seroprevalence estimates were compared with reported cases to estimate under-ascertainment ratios.
Results
In total, 12 serosurveys with 210,321 participants were identified. Three (25%) serosurveys were conducted at national level, one (8.3%) was conducted at provincial level, and eight (66.7%) were conducted at local level. All 12 serosurveys had moderate or high risk of bias. The proportion of the population infected by April 2021 was low (2.6%). The proportion of the population infected was higher in surveys of residents of long-term care facilities (43.0–86%), workers at long-term care facilities (22.4–32.4%), and workers in healthcare institutions (1.4–14%).
Conclusions
As of April 2021, the proportion of the population infected by SARS-CoV-2 was low in the overall population of Canada, but was high in healthcare facilities, particularly long-term care facilities, supporting the need for vaccines.
Introduction
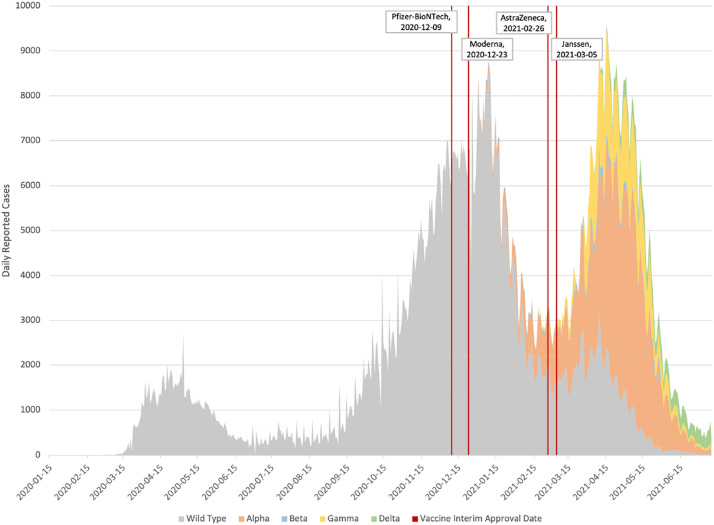
The first reported case of coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), in Canada was in Toronto on 25 January 2020, in a traveller from Wuhan, China (Canadian Institute for Health Information, 2021). As of April 2021, 1,219,418 cases of COVID-19 and 24,219 deaths (Government of Canada, 2021) had been reported in Canada (population 38,048,738) (Statistics Canada, 2021b). COVID-19 vaccines became available in Canada in December 2020, administered first to healthcare workers and residents of long-term care facilities. Vaccine availability remained limited in early 2021, with only 29% of the population aged ≥16 years receiving a first dose of vaccine by April 2021.Through mid-2021, Canada had experienced three waves of COVID-19 (Figure 1). The latter waves were of increasing magnitude, and associated with the dissemination of SARS-CoV-2 variants B.1.1.7 (Alpha) and B.1.617.2 (Delta), which were more transmissible than the wild-type SARS-CoV-2 strain (Government of Canada, 2021). To reduce community transmission, restrictive pandemic measures were enacted by each level of government, based upon the scope of their responsibilities. The federal government focused on international travel restrictions and border closures, with all other measures enacted by provincial/territorial/municipal governments. This led to substantial variability, ranging from the probusiness policies of Alberta to the most restrictive policies of the Atlantic provinces (Hale et al., 2021).
Figure 1.
Trends in cases of coronavirus disease 2010 in Canada (31 January 2020–10 July 2021).
The number of cases of COVID-19 reported in Canada is derived from provincial public health surveillance of laboratory-confirmed SARS-CoV-2. Laboratory confirmation is based upon molecular testing (real-time, reverse transcription polymerase chain reaction) to detect SARS-CoV-2 genetic material from nasopharyngeal specimens (Ontario Ministry of Health, 2021). The number of reported cases does not include all individuals infected with SARS-CoV-2 for several reasons. Asymptomatic and mild infections are unlikely to be laboratory tested for SARS-CoV-2 due to reduced access or lack of awareness for the need of testing, yet represent an important source of community transmission, estimated to be the cause of up to 44% of cases (He et al., 2020). Furthermore, limited testing capacity requires screening protocols which vary by jurisdiction, but generally prioritize access to symptomatic individuals (Hale et al., 2021). Laboratory-based surveillance also relies on reporting of laboratory-confirmed cases to surveillance. For these and other reasons, public health case counts under-ascertain the number of individuals infected with SARS-CoV-2.
Most patients infected with SARS-CoV-2 will generate a detectable immune response within a few weeks of infection (Charlton et al., 2021). Serological assays have been developed that can measure SARS-CoV-2 antibodies either by total antibody level or by individual isotypes – immunoglobulin G (IgG), immunoglobulin M (IgM) and immunoglobulin A (IgA) – generated in response to SARS-CoV-2 proteins [nucleocapsid (N) or spike (S) protein] (Charlton et al., 2021). These assays are primarily used to estimate population-level exposure to SARS-CoV-2, but also have limited clinical uses. The long-term persistence of detectable antibodies is not known, although assays have been observed to lose sensitivity after approximately 4 months due to antibody waning following natural infection, increasing the potential for false-negative results (Perez-Saez et al., 2021).
As available COVID-19 vaccines are based on the S protein, vaccine-induced immunity only generates antibodies to the S protein. This allows serological assays that measure antibodies to the N protein the ability to distinguish immunity derived from natural infection from that derived from vaccination. Many jurisdictions in Canada have conducted population-based SARS-CoV-2 serosurveys in accordance with the World Health Organization UNITY protocol, which guides the conduct of seroprevalence surveys to assess the prevalence of SARS-CoV-2 infection and monitor population immunity (World Health Organization, 2020).
This review was conducted to understand the extent of SARS-CoV-2 infection in Canada through April 2021, and thereby estimate the extent of under-ascertainment by public health surveillance in the first 16 months of the pandemic and after 4 months of vaccine availability. The aims were to: (1) identify SARS-CoV-2 seroprevalence surveys conducted in Canada; and (2) provide a comparison of seroprevalence estimates over time, and by region and study method.
Methods
Information sources and search strategy
This systematic literature review was conducted in accordance with PRISMA guidelines (Table S1, see online supplementary material), and the protocol and search strategy were registered with PROSPERO (CRD42021246958) and the National Collaborating Centre for Methods and Tools (ID 401) (Major et al., 2021; Page et al., 2021).
PubMed and Scopus were searched using a search strategy developed in consultation with a health sciences librarian. Pre-prints were searched on the MedRXIV and BioRXIV servers. A grey literature search was conducted by hand searching specific websites and online COVID-19 data repositories. Data disseminated by press release was searched using Google News advanced search. The search dates used were November 2019 to 10 July 2021 in order to factor in a time lag for publication of reports that would enable estimation of the extent of SARS-CoV-2 infection through April 2021. References in both of Canada's official languages (English and French) were included. Detailed search strategies can be found in Tables S2–S6 (see online supplementary material).
This review included studies that reported SARS-CoV-2 serological surveys conducted in Canada in humans, that estimated seroprevalence for a defined population in a distinct geographical area within a specified time period. Studies were included if they reported seroprevalence, sample size, sampling interval dates and sample frame. Studies included in this review used a validated serological assay to measure antibodies to SARS-CoV-2, either total antibodies or by individual isotype (IgG, IgM, IgA), generated in response to exposure to one or more of the SARS-CoV-2 proteins [N, S, receptor-binding domain (RBD)]. Cohort and cross-sectional studies were included, while all other designs (e.g. case–control studies, case reports, review articles, assay validation studies) were excluded. The inclusion and exclusion criteria are shown in Table S7 (see online supplementary material).
Data extraction and quality assessment
A single reviewer (LJJ) executed the searches and entered references into Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia: www.covidence.org). A single reviewer (MM) screened articles, and a second reviewer (LJJ) verified and provided translation support for French language articles. Discrepancies regarding study inclusion were settled by consensus and expert input from co-authors (MM, LJJ, MO, FA). If two or more studies reported seroprevalence estimates from the same serological survey and period, the most current and complete study was included. Data items from the included studies were extracted and entered into an Excel (Microsoft Corp., Redmond, WA, USA) template by one author (LJJ), then reviewed by a second author (MM). Missing data items were marked as not reported. Data items collected for each included study were grouped into four categories: publication level information, serological assay characteristics, study characteristics and outcome level information (Table S8, see online supplementary material).
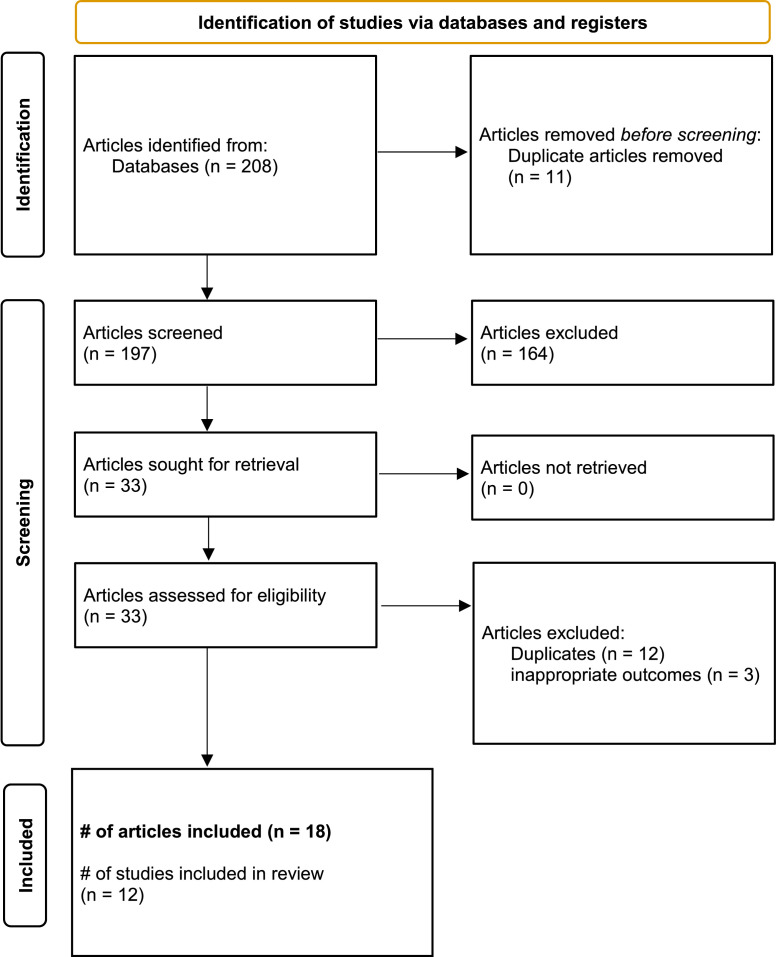
An individual article was defined as a published or non-published study or report that provided one or more seroprevalence estimates. Individual articles that were part of a repeated cross-sectional assessment using identical methodology were grouped as companion studies but were analysed individually. The number of studies, articles and distinct seroprevalence estimates that were included in this study are shown in the PRISMA flowchart (Figure 1), and the full data extraction sheet is given in Tables S12–S14 (see online supplementary material). Studies were defined as national, provincial or regional, based upon the jurisdiction of sampling, with studies sampling most provinces/territories being classified as national and studies sampling most health regions within a province classed as provincial.
The Joanna Briggs Institute Critical Appraisal Checklist for Studies Reporting Prevalence Data (JB Checklist) was adapted to assess the potential risk of bias for each included article (Munn et al., 2015). Risk assessment appraisals were conducted by one author (MM), in consultation with other members of the study team (LJJ, FA, MO). Overall risk of bias for included articles was classified as low, moderate, high or unclear. The checklist and criteria for appraisals are shown in Table S9 (see online supplementary material).
Synthesis methods
Data from the included articles were assessed using the SWiM (Synthesis Without Meta-analysis) reporting guidelines for systematic reviews (Campbell et al., 2020). Seroprevalence estimates were grouped by jurisdiction; classified as national, provincial or local; then ranked by sampling interval to assess trends over time. Articles were grouped by target sample frame, classified as population-wide or population-specific. Sample sizes were also pooled to assess representation by age and jurisdiction, and reported in structured tables.
Descriptive methods were used to investigate sources of heterogeneity. Structured tables were created to visually inspect study characteristics, sampling method and assay characteristics, and to test algorithms used to determine their impact on seropositivity. Articles were also grouped by risk of bias.
For serosurveys that used population-wide sample frames, seroprevalence estimates were used to estimate the cumulative number of cases of SARS-CoV-2 in Canada. To assess the extent of under-ascertainment by public health surveillance, national and provincial seroprevalence estimates were converted to an ‘estimated’ case count based upon the last day of the sampling interval, then compared with the corresponding cumulative reported case counts on that date, to assess concordance. Estimated case counts were derived by multiplying seroprevalence estimates by the population estimate for the jurisdiction, obtained from Statistics Canada, on the final day of the sampling interval (Statistics Canada, 2021b). Confidence intervals for the seroprevalence estimates were used to produce confidence intervals for the estimated case counts using the same method. Reported case counts were extracted from the national COVID-19 case surveillance reporting databases (Government of Canada, 2021). Ratios of estimated case counts to reported cases were calculated for each sampling interval to visually assess differences between estimated case counts and reported cases. To assess trends over time, regional reported seroprevalence assessments were grouped, ranked by sampling timeframe, and displayed in bar graphs.
Serosurveys that assessed population-specific sample frames, such as healthcare workers and long-term care residents, were grouped into structured tables and ranked by sampling date. Population-wide and population-specific serosurveys that reported estimates for children aged <19 years were combined and ordered by sampling date.
Results
Study characteristics
This systematic literature review yielded 208 published or unpublished studies, of which 33 underwent full-text screening (Figure 2). Fifteen (45%) of these 33 articles met the exclusion criteria. The primary reasons for exclusion were duplicates (n=12) or inappropriate outcome measures (n=3). Of the 18 included articles, six (33%) had been published in peer-reviewed journals, two (11%) had been published as pre-prints, and 10 (55%) had been published as reports posted on websites (Table S13, see online supplementary material). The seroprevalence results of the 18 articles were provided by 12 different serosurveys: four articles reported results (each at one different time point) from one serosurvey, four articles reported results (each at one or two different time points) from one serosurvey, two articles reported results (each at two time points) from two different serosurveys, and eight articles reported results (each at one time point) from eight different serosurveys (Table S13, see online supplementary material). The dates for seroprevalence estimates in the 18 articles ranged from 5 March 2020 to April 2021, and seroprevalence estimates ranged from 0% to 56% (Table S13, see online supplementary material).
Figure 2.
PRISMA flowchart.
From: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71.
The 12 serosurveys in the 18 articles had a total sample size of 210,321 participants (Table S14, see online supplementary material). Of the 12 serosurveys, three (25%) were of national scope (with seroprevalence stratified by sex, age group, province and selected urban centres), one (8.3%) was Ontario-based (seroprevalence stratified by sex and age group), and eight (66.6%) were conducted in large urban centres (Table 1). Of the 12 serosurveys, six (50%) targeted a general population sample frame and six (50%) targeted specific populations, primarily children, healthcare workers, and residents of long-term care facilities (Table 1). The study participants in the 12 serosurveys included 57.4% from Ontario, 28.5% from western Canada (British Columbia, Alberta, Saskatchewan and Manitoba, including Northern Territories), 8.5% from eastern Canada (New Brunswick, Nova Scotia, Newfoundland and Prince Edward County) and 5.6% from Quebec; these regional groupings represent 40%, 32%, 6% and 22% of Canada's population, respectively (Table S13, see online supplementary material) (Statistics Canada, 2021a). In the 12 serosurveys, children aged <19 years comprised 2.9% of the participants compared with 21% of the population (Statistics Canada, 2021a). Adults aged 20–64 years represented 75.4% of serosurvey participants compared with 60% of the population (Table 2) (Statistics Canada, 2021a).
Table 1.
Characteristics of 12 serosurveys conducted in Canada (March 2020–April 2021).
| Characteristic | Serosurveys n (%) (total n=12) |
|---|---|
| Geographical scope | |
| National | 3 (25%) |
| Provincial | 1 (8.3%) |
| Local | 8 (66.7%) |
| Target population | |
| Population-wide | 6 (50%) |
| Population-specific | 6 (50%) |
| Sampling method | |
| Probability sampling | 2 (16.7%) |
| Non-probability sampling | 10 (83.3%) |
| Testing algorithm to determine seropositivity | |
| Positive to one test alone | 8 (66.6%) |
| Positive to two or more tests | 4 (33.3%) |
| Risk of bias | |
| High | 8 (66.6%) |
| Moderate | 4 (33.3%) |
| Low | 0 |
Table 2.
Age of participants in the 12 severe acute respiratory syndrome coronavirus-2 serosuryeys conducted in Canada (March 2020–April 2021).
| Age groups | Sample size (n=199,621)a |
|---|---|
| Children (0–18 years) | 5792 (2.9%) |
| Adults (19–64 years) | 150,497 (75.4%) |
| Elderly (≥65 years) | 43,332 (21.7%) |
Excluding Statistics Canada (2021c) due to lack of reporting of sample stratification.
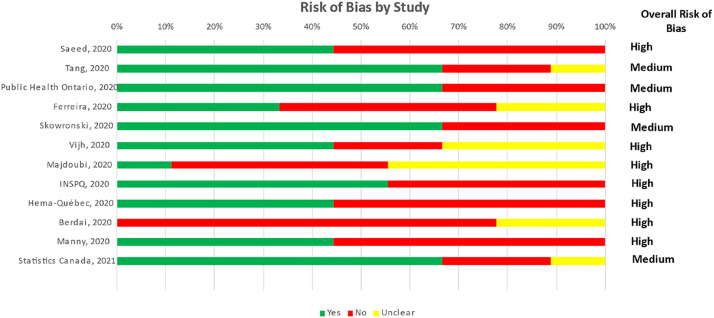
Using the JB Checklist, four studies were assessed as moderate risk of bias and eight were assessed as high risk of bias (Figure 3). The main sources of bias were attributed to the representativeness of the sampling frame, sensitivity and specificity of the assay, lack of orthogonal testing algorithm to determine seropositivity, and non-probability sampling (Table S10, see online supplementary material).
Figure 3.
Risk-of-bias assessment of the 12 severe acute respiratory syndrome coronavirus-2 serosurveys in Canada from March 2020 to April 2021.
Population-based seroprevalence estimates
Of the six serosurveys that targeted a general population sampling frame, two used residual specimens collected from the healthcare system, two used sera from blood donors, and two procured specimens prospectively (Table 3). Four of the six serosurveys determined seropositivity by testing positive using a single assay, while the remaining two serosurveys used an orthogonal approach (i.e. seropositivity to two or more assays). Estimates obtained using dried blood spots (DBS) were higher than those from residual sera or blood donors at national level (1.7% vs 0.7%) and in Ontario (2.35% vs 1.1% and 0.88%), but not in Quebec (1.56% vs 2.2%) in July 2020 (Table 3). The highest national seroprevalence estimates were reported by Tang et al. (2021)1 using DBS (2.5% in September 2020) and Statistics Canada (2021c) (2.6% in April 2021) (Table 3). The highest regional seroprevalence estimates were reported in western Canada in November 2020, with Manitoba at 8.6% and Saskatchewan at 4.2% (Table 3). The lowest seroprevalence estimates were reported in the eastern provinces, ranging from 0% to 1.3% (Table S13, see online supplementary material).
Table 3.
Population-wide under-ascertainment of cases of coronavirus disease 2019 by jurisdiction and sampling interval.
| Jurisdiction | Study | Dates samples were collected | Specimens | Seroprevalence (95% CI) | Estimated cases (n) (95% CI) | Reported cases (n) | Under-ascertainment ratio (95% CI) |
|---|---|---|---|---|---|---|---|
| Canada | Saeed et al., 2021a | 9 May–21 Jul 2020 | Blood donor | 0.7 (0.63–0.76) | 265,859 (239,273–288,647) | 111,684 | 2.4x (2.1–2.6) |
| Tang et al., 2021 | May–Jul 2020 | Dried blood spot | 1.7 (NR) | 645,658 | 116,298 | 5.6x | |
| Tang et al., 2021 | Aug–Sept 2020 | Dried blood spot | 2.54 (NR) | 965,333 | 158,758 | 6.1x | |
| Canadian Blood Services, 2020a | 12–31 Oct 2020 | Blood donor | 0.88 (0.73–1.04) | 334,470 (277,458–395,283) | 235,444 | 1.4x (1.2–1.7) | |
| Canadian Blood Services, 2021aa | 7–25 Nov 2020 | Blood donor | 1.51 (1.31–1.71) | 573,921 (497,905–649,937) | 347,466 | 1.7x (1.4–1.9) | |
| Canadian Blood Services, 2021ba | 1–27 Jan 2021 | Blood donor | 1.99 (1.84–2.15) | 757,170 (700,097–818,048) | 761,226 | 1.0x (0.9–1.1) | |
| Statistics Canada, 2021c | Nov 2020–Apr 2021 | Dried blood spot | 2.6 (1.6–3.2) | 989,267 (608,780–1,217,560) | 1,219,418 | 0.8x (0.5–1.0) | |
| Ontario | Public Health Ontario, 2020c | 27 Mar–30 Apr 2020 | Residual laboratory sera | 0.5 (0.1–1.5) | 73,445 (14,689–220,336) | 16,187 | 4.5x (0.9–13.6) |
| Public Health Ontario, 2020c | 26–31 May 2020 | Residual laboratory sera | 1.5 (0.7–2.2) | 220,852 (103,064–323,917 | 27,859 | 7.9x (3.7–11.6) | |
| Public Health Ontario, 2020c | 5–30 Jun 2020 | Residual laboratory sera | 1.1 (0.8–1.3) | 161,958 (117,788–191,405) | 35,068 | 4.6x (3.4–5.5) | |
| Saeed et al., 2021 | 9 May–21 Jul 2020 | Blood donor | 0.88 (0.78–0.98) | 129,659 (114,925–144,393) | 37,942 | 3.4x (3.0–3.8) | |
| Public Health Ontario, 2020b | 4–31 Jul 2020 | Residual laboratory sera | 1.1 (0.8–1.3) | 162,074 (117,872;191,542) | 39,209 | 4.1x (3.0–4.9) | |
| Tang et al., 2021 | May–Jul 2020 | Dried blood spot | 2.35 (NR) | 346,249 | 39,209 | 8.8x (NR) | |
| Public Health Ontario, 2020a | 1–31 Aug 2020 | Residual laboratory sera | 1.1 (0.8–1.3) | 162,074 (117,872–191,542) | 42,309 | 3.8x (2.8–4.5) | |
| Public Health Ontario, 2020d | 3–30 Sept 2020 | Residual laboratory sera | 0.7 (0.4–0.9) | 103,138 (58,936–132,606) | 51,710 | 2.0x (1.1–2.6) | |
| Public Health Ontario, 2020d | 1–30 Oct 2020 | Residual laboratory sera | 1.2 (0.9–1.4) | 176,802 (132,602–206,269) | 74,715 | 2.4x (1.8–2.8) | |
| Canadian Blood Services, 2020 | 12–31 Oct 2020 | Blood donor | 0.87 (0.65–1.08) | 128,182 (95,768–159,122) | 75,730 | 1.7x (1.3–2.1) | |
| Canadian Blood Services, 2021a | 7–25 Nov 2020 | Blood donor | 0.77 (0.56–0.97) | 113,448 (82,508–142,915) | 107,883 | 1.1x (0.8–1.3) | |
| Canadian Blood Services, 2021b | 1–27 Jan 2021 | Blood donor | 1.82 (1.61–2.04) | 268,545 (237,559–301,006) | 260,370 | 1.0x (0.9–1.2) | |
| Statistics Canada, 2021c | Nov 2020–Apr 2021 | Dried blood spot | 2.5 (1.1–4.4) | 368,880 (162,307–649,229) | 463,364 | 0.8x (0.4–1.4) | |
| Quebec | Héma-Quebec, 2020b | 25 May–9 Jul 2020 | Blood donor | 2.2 (1.9–2.56) | 188,585 (162,869–219,445) | 56,216 | 3.4x (2.9–3.9) |
| Tang et al., 2021 | May–July 2020 | Dried blood spot | 1.56 (NR) | 133,763 | 59,131 | 2.3x | |
| Statistics Canada, 2021c | Nov 2020–Apr 2021 | Dried blood spot | 3.2 (2.1–4.1) | 274,386 (180,066–351,557) | 349,773 | 0.8x (0.5–1.0) | |
| British Columbia | Saeed et al., 2021 | 9 May–21 Jul 2020 | Blood donor | 0.56 (0.42–0.69) | 28,797 (21,598–35,483) | 3328 | 8.7x (6.5–10.7) |
| Canadian Blood Services, 2020a | 12–31 Oct 2020 | Blood donor | 0.86 (0.5–1.23) | 44,254 (25,729–63,293) | 14,733 | 3.0x (1.7–4.3) | |
| Canadian Blood Services, 2021aa | 7–25 Nov 2020 | Blood donor | 1.51 (1.04–1.97) | 77,701 (53,516–101,372) | 29,086 | 2.7x (1.8;3.5) | |
| Canadian Blood Services, 2021ba | 1–27 Jan 2021 | Blood donor | 1.48 (1.16–1.81) | 76,265 (59,775–93,270) | 65,719 | 1.2x (0.9–1.4) | |
| Statistics Canada, 2021c | Nov 2020–Apr 2021 | Dried blood spot | 1.6 (0.5–2.9) | 82,449 (25,765–149,438) | 129,482 | 0.6x (0.2–1.2) | |
| Alberta | Saeed et al., 2021a | 9 May–21 Jul 2020 | Blood donor | 0.48 (0.31–0.62) | 21,255 (13,727–27,454) | 9728 | 0.8x (0.5–1.0) |
| Canadian Blood Services, 2020a | 12–31 Oct 2020 | Blood donor | 0.76 (0.38–1.14) | 33,653 (16,827–50,480) | 28,245 | 0.7x (0.3–1.0) | |
| Canadian Blood Services, 2021aa | 7–25 Nov 2020 | Blood donor | 1.79 (1.24–2.34) | 79,263 (54,908–103,617) | 50,801 | 1.6x (1.1–2.0) | |
| Canadian Blood Services, 2021ba | 1–27 Jan 2021 | Blood donor | 3.41 (2.89–3.94) | 151,276 (128,208–174,789) | 122,360 | 1.2x (1.0–1.4) | |
| Statistics Canada, 2021c | Nov 2020–Apr 2021 | Dried blood spot | 4.0 (2.6–5.7) | 177,450 (115,343–252,867) | 190,734 | 0.9x (0.6–1.3) | |
| Saskatchewan | Canadian Blood Services, 2020a | 12–31 Oct 2020 | Blood donor | 0.17 (0–0.59) | 2,002 (0–6,949) | 3144 | 0.6x (0–2.2) |
| Canadian Blood Services, 2021aa | 7–25 Nov 2020 | Blood donor | 4.17 (2.57–5.77) | 49,114 (30,269–67,958) | 7047 | 7.0x (4.3–9.6) | |
| Canadian Blood Services, 2021ba | 1–27 Jan 2021 | Blood donor | 2.46 (1.59–3.33) | 28,999 (18,743–39,255) | 22,794 | 1.3x (0.8–1.7) | |
| Statistics Canada, 2021c | Nov 2020–Apr 2021 | Dried blood spot | 2.9 (1.6–4.3) | 34,186 (18,861–50,690) | 41,098 | 0.8x (0.5–1.2) | |
| Manitoba | Canadian Blood Services, 2020a | 12–31 Oct 2020 | Blood donor | 2.96 (1.7–2.43) | 40,832 (23,451–58,352) | 5723 | 7.1x (4.1–10.2) |
| Canadian Blood Services, 2021aa | 7–25 Nov 2020 | Blood donor | 8.56 (6.51–10.62) | 118,083 (89,803–146,500) | 14,907 | 7.9x (6.0–9.8) | |
| Canadian Blood Services, 2021ba | 1–27 Jan 2021 | Blood donor | 3.92 (2.92–4.93) | 54,133 (40,323–68,080) | 28,996 | 1.9x (1.4–2.3) | |
| Statistics Canada, 2021c | Nov 2020–Apr 2021 | Dried blood spot | 2.4 (1.2–3.6) | 33,142 (16,571–49,714) | 36,629 | 0.9x (0.5–1.4) |
References did not include data from Quebec (Saeed et al., 2021; Canadian Blood Services, 2020; Canadian Blood Services, 2021a; Canadian Blood Services, 2021b; Canadian Blood Services, 2021c)
bReference only sampled 12/18 public health regions in the province (Héma-Quebec, 2020).
When seroprevalence estimates were compared with the cumulative SARS-CoV-2 case counts reported to public health surveillance, a varying degree of under-ascertainment was exhibited over the first 18 months of the pandemic. Under-ascertainment was particularly high early in the pandemic, between March and November 2020, independent of jurisdiction, assay or study method (Table 3). Using national data, the under-ascertainment ratio was highest at 6.1x in September 2020. Regionally, the ratio was highest at 8.8x in Ontario in July 2020 (Table 3). National and regional under-ascertainment ratios declined over time, with limited evidence of under-ascertainment of cases infected with SARS-CoV-2 by April 2021. The decline in under-ascertainment by public health surveillance nationally was also evident in Ontario, Quebec, British Columbia, Alberta, Saskatchewan and Manitoba (Table 3).
Population-specific seroprevalence estimates
Assessments of population-specific seroprevalence were grouped into three categories: long-term care residents, healthcare workers, and children aged <19 years. The highest seroprevalence estimates were observed in residents of two long-term care facilities (LTCFs) in Vancouver at 86.1% and 43.0% (Table S12A, see online supplementary material) following facility-wide outbreaks in May 2020 before the licensure of vaccines (Vijh et al., 2021). Staff working in these two LTCFs on the same date had the highest reported seroprevalence amongst workers within healthcare institutions at 32.4% and 22.4% (Table S12B, see online supplementary material) (Vijh et al., 2021). Another study that compared the seroprevalence of intensive care workers in Montreal in July–September 2020 between high- and low-prevalence settings reported seropositivity of 14.0 and 3.1, respectively (Table S12B, see online supplementary material) (Institut National de Santé Publique du Quebec, 2020).
Seroprevalence estimates for children were obtained from different settings and recruitment methods, ranging from age-stratified population-based serosurveys to studies that only recruited children within hospital settings; as such, meaningful comparison was difficult. Seroprevalence estimates from a single study by Statistics Canada from November 2020 to April 2021 which used random national sampling reported higher prevalence in children aged <19 years (3.3%) compared with adults aged 20–59 years (2.9%) or >60 years (1.4%) (Table S13, see online supplementary material), and demonstrated an increase over time from 0% in March 2020 to 3.3% in April 2021 (Table S12C, see online supplementary material) Statistics Canada (2021c).
Discussion
Through April 2021, SARS-CoV-2 seroprevalence in Canada, and thereby the proportion of the population that had been infected by SARS-CoV-2, was low. The low seroprevalence mirrors the relatively low incidence of reported infections in Canada. While the Canadian serosurveys varied considerably in terms of their methods of recruitment, assays used and testing algorithms, limiting the ability to make direct comparisons between studies, some similarities could be observed. Across all studies, seroprevalence estimates increased over time, with a peak in autumn 2020 followed by a plateau or decline by spring 2021 (Table 3 and Table S13, see online supplementary material). Correspondingly, there was a reduction in under-ascertainment ratios over time for most of the population-based serosurveys, with reported cases at parity with seroprevalence by April 2021.
The downward trend observed for under-reporting multipliers over the course of the pandemic was consistent with a similar analysis conducted in the USA (Angulo et al., 2021). This is likely to be due to the initial SARS-CoV-2 testing capacity and protocols which restricted testing to symptomatic travellers, and likely led to substantial under-identification of infected individuals during the early part of the pandemic (Ontario Ministry of Health, 2021). As capacity improved and guidelines evolved to include testing of asymptomatic high-risk groups, an increased proportion of infected people were identified. Another explanation could involve the characteristics of the assays used in the studies, and the durability of immunity following natural infection. Antinucleocapsid antibody levels have been observed to wane as early as 4 months following natural infection, which has been known to lead to underestimation of cases by increasing the probability of false-negative results as time between natural infection and serology testing increases (Perez-Saez et al., 2021). This was demonstrated within the Ontario serosurvey when Public Health Ontario retested sera samples from their August 2020 samples using a reduced assay threshold to assess assay sensitivity. A 16% increase in seropositivity was detected, which they attributed to waning of antinucleocapsid antibodies as time from infection increased (Bolotin et al., 2021).
The high seroprevalence in residents of LTCFs and healthcare workers reflects their higher frequency of exposure to the virus, particularly for LTCF residents who are vulnerable to poor health outcomes from SARS-CoV-2 infection. The widespread occurrence of LTCF outbreaks during the first wave of the pandemic, and the high seroprevalence observed in LTCF staff, support the implementation of strict infection control policies to limit transmission in this healthcare setting. As the pandemic progressed, the increasing seroprevalence observed in children and young adults raises concern about their contribution to community transmission of the virus within the community.
Seroprevalence rates observed in Canada were lower than rates observed in other countries. Population-wide seroprevalence studies in the USA report approximately double the seroprevalence compared with that found in Canada during comparable time periods, with estimates up 10x higher in New York, Connecticut, Louisiana and Florida (Angulo et al., 2021). National age-stratified seroprevalence estimates conducted by the US Centers for Disease Control and Prevention in April 2021 reported positivity of 27% in children aged <17 years, 24% in adults aged 18–49 years, 20% in adults aged 50–64 years, and 13% in adults aged >65 years, approximately 10x higher than Canadian national estimates by age group at that time (Centers for Disease Control and Prevention, 2021), when comparing assay results capturing natural, not vaccine-induced, immunity in both countries. A global seroprevalence review revealed population-wide seroprevalence estimates from areas in Eastern Europe, Russia and the Middle East to be substantially higher compared with Canada (13–16%), with an overall median seroprevalence rate of 4.5% (Bobrovitz et al., 2021).
The average under-ascertainment ratio calculated within the global review was 18.1x higher than the reported incidence of COVID-19, which was higher than that observed in Canada, but demonstrates a similar issue with relying upon reported infections to monitor population exposure to SARS-CoV-2 (Bobrovitz et al., 2021). The difference observed between Canada and other jurisdictions may be due to Canada's stringent public health response to the pandemic. Canada implemented extensive and prolonged lockdowns, which included cessation of in-person education, primary healthcare service disruptions, closure of non-essential business, travel restrictions, and closure of US border crossing; these measures likely reduced community transmission from November 2020 to April 2021 (Canadian Institute for Health Information, 2021; Hale et al., 2021).
Differences in recruitment, testing and study methods used in the Canadian seroprevalence surveys had advantages and disadvantages in evaluating the population-level exposure to SARS-CoV-2. The use of residual sera from healthcare services laboratories has the advantage of easy access to a large, geographically representative sample frame, as well as being a relatively fast and convenient specimen source, particularly within the conditions of a pandemic lockdown. However, this may disproportionately represent individuals with medical co-morbidities and under-represent children based upon medical-seeking behaviours and clinical standards for ordering specimen collection, particularly in children. Similarly, testing of residual sera from blood donor banks is a convenient and large specimen source. However, screening protocols for donating blood may lead to over-representation of healthier, urban populations (Atsma and de Vegt, 2011), and understandably would not provide any representation of children. Due to their advantages, study designs using residual sera and blood donors have been endorsed by the World Health Organization for SARS-CoV-2 seroprevalence surveys (World Health Organization, 2020).
Prospective, randomized studies have the advantage of being more representative, but generally suffer from low response rates as subjects need to be motivated to comply with study activities and contribute specimen samples. The largest prospective seroprevalence survey in Canada recruited over 11,000 subjects, and collected specimens as well as conducting extensive surveys to assess the demographic and socioeconomic factors associated with SARS-CoV-2 exposure (Evans et al., 2022). The novel DBS assay used in this study provided some advantages in overcoming the need to travel to a laboratory for specimen collection, as subjects were able to self-administer the test at home and submit their samples by post. This may have facilitated participation of vulnerable populations, who may have been less likely to have access to primary care or to donate blood. This study described higher seropositivity in visible minorities, aboriginal populations and populations working in public-facing roles during the pandemic (Evans et al., 2022). The DBS assay also had the ability to test for three SARS-CoV-2 immune targets (N, S, RBD), which allows the distinction between naturally acquired immunity post infection and vaccine-mediated immunity; this has become an important factor as vaccines based upon the S protein became widely accessible in Spring 2021.
COVID-19 vaccines were not widely available in Canada at the time of this analysis (April 2021). At that time, they were only available to healthcare workers and residents of LTCFs, so this review period primarily covers a period when the general population was unvaccinated. The seroprevalence of SARS-CoV-2 antibodies in the most recent survey was 2.6% for antibodies by national infection and 1.0% for antibodies by vaccination, confirming low immunity from vaccine coverage (Statistics Canada, 2021c). Interestingly, this study also reported the highest population-wide seroprevalence in children aged 1–19 years at 3.3%, which was higher than other age groups at that time. It is likely that the random sampling method facilitated the recruitment of children and persons from geographically remote areas, who were under-represented in previous studies using residual sera from healthcare laboratories and blood donor specimens (Statistics Canada, 2021c), which led to lower seroprevalence estimates in Canada. The DBS assay may improve the response rate by providing the convenience of using an at-home, less-invasive specimen collection method compared with drawing blood, removing a substantial barrier for study participation and resulting in a more generalizable SARS-CoV-2 seroprevalence estimate for children. This may be a methodological recommendation for developing future seroprevalence surveys to improve study feasibility and reduce bias, particularly given the potential role of children in transmission dynamics.
In conclusion, through April 2021, 6 months into the pandemic and 4 months after the introduction of COVID-19 vaccines for selected high-risk groups in Canada, a low proportion of the general population had been infected by SARS-CoV-2. However, a high proportion of residents in some LTCFs had been infected by SARS-CoV-2 by April 2021, which emphasizes the need for continued public health measures such as vaccination, social distancing measures and the use of face masks to protect against new, more transmissible strains that continue to circulate and cause infections, particularly within healthcare institutions and LTCFs. The new DBS assay in use in Canada is a promising tool to evaluate the ongoing seroprevalence of the Canadian population in order to monitor the persistence of immunity from both natural infection and vaccination. Loss of assay sensitivity over time due to waning of immunity is an important barrier to conducting accurate longitudinal assessments of seroprevalence.
Acknowledgments
Acknowledgements
The authors wish to thank Jackie Stapleton, MLS, Liaison Librarian, University of Waterloo Library, for her assistance in development of the search strategies for this reviewand Arsh Maira Muhammad Muhyiddin, University of Waterloo, Health Studies (Honours, Co-op) student, for her assitance in development of Figure 1.
Conflict of interest statement
Maria Major is a full-time employee of Pfizer, Vaccines, Canada and a PhD student at the University of Waterloo, School of Public Health Sciences Waterloo, ON, Canada. Tuition and publication costs were paid by Pfizer Canada. Frederick J. Angulo is a full-time employee of Pfizer Vaccines, Medical Development, Scientific and Clinical Affairs, Collegeville, PA, USA. The other authors report no conflicts of interest.
Funding
This work was sponsored by Pfizer Inc.
Ethical approval
Not required.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijregi.2022.07.010.
Appendix. Supplementary materials
References
- Angulo FJ, Finelli L, Swerdlow DL. American Medical Association; Chicago, IL: 2021. Estimation of US SARS-CoV-2 infections, symptomatic infections, hospitalizations, and deaths using seroprevalence surveys. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atsma F, de Vegt F. The healthy donor effect: A matter of selection bias and confounding. Transfusion. 2011;51:1883–1885. doi: 10.1111/j.1537-2995.2011.03270.x. [DOI] [PubMed] [Google Scholar]
- Bobrovitz N, Arora RK, Cao C, Boucher E, Liu M, Donnici C, et al. Public Library of Science; San Francisco, CA: 2021. Global seroprevalence of SARS-CoV-2 antibodies: a systematic review and meta-analysis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolotin S, Tran V, Osman S, Brown KA, Buchan SA, Joh E, et al. SARS-CoV-2 seroprevalence survey estimates are affected by anti-nucleocapsid antibody decline. J Infect Dis. 2021;223:1334–1338. doi: 10.1093/infdis/jiaa796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M, McKenzie JE, Sowden A, Katikireddi SV, Brennan SE, Ellis S, et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ. 2020;368:l6890. doi: 10.1136/bmj.l6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canadian Blood Services. COVID-19 seroprevalence report December 18, 2020. Report #3: October 2020 survey. Ottawa, ON: Canadian Blood Services; 2020:23.
- Canadian Blood Services. COVID-19 seroprevalence report January 12, 2021. Report #4: November 2020 survey. Ottawa, ON: Canadian Blood Services; 2021a:28.
- Canadian Blood Services. COVID-19 seroprevalence report March 18, 2020. Report #6: January 2021 survey. Ottawa, ON: Canadian Blood Services; 2021b. Available at: https://www.covid19immunitytaskforce.ca/wp-content/uploads/2021/04/COVID-19-Report_January-2021_18March2021.pdf (accessed 1 August 2022).
- Canadian Institute for Health Information . CIHI; Otawa, ON: 2021. COVID-19 intervention timeline in Canada.https://www.cihi.ca/en/covid-19-intervention-timeline-in-canada Available at. (accessed 31 July 2022) [Google Scholar]
- Centers for Disease Control and Prevention . CDCAvailable at; Atlanta, GA: 2021. COVID data tracker (08/26/21). Nationwide commercial laboratory seroprevalence survey.https://covid.cdc.gov/covid-data-tracker/#national-lab (accessed 31 July 2022) [Google Scholar]
- Charlton C, Kanji J, Tran V, Kus J, Gubbay J, Osiowy C, et al. Health Canada; Ottawa, ON: 2021. Practical guidance for clinical laboratories for SARS-CoV-2 serology testing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans T, Cabot J, Earl S, Buckeridge D, Hankins C. Presenting final results from Canadas most representative seroprevalence study. Ottawa, ON. COVID-19 Immunity Task Force. 2022. Available at: https://www.covid19immunitytaskforce.ca/wp-content/uploads/2021/07/CITF_StatCan-presentation_Townhall_FINAL_ENG_for-web.pdf (accessed 1 August 2022).
- Government of Canada . Government of Canada; Ottawa, ON: 2021. Canadian COVID-19 case counts.https://health-infobase.canada.ca/covid-19/epidemiological-summary-covid-19-cases.html?stat=num&measure=total&map=pt#a2 Available at. (accessed 1 August 2022. [Google Scholar]
- Hale T, Angrist N, Goldszmidt R, Kira B, Petherick A, Phillips T, et al. A global panel database of pandemic policies (Oxford COVID-19 government response tracker) Nat Hum Behav. 2021;5:529–538. doi: 10.1038/s41562-021-01079-8. [DOI] [PubMed] [Google Scholar]
- He X, Lau EHY, Wu P, Deng X, Wang J, Hao X., et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26:672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- Héma-Quebec . Héma-Quebec; Montreal, QC: 2020. Étude de séroprévalence des anti-sars-cov-2 chez les donneurs de sang d'héma-Québec, vers la fin de la première vague de covid-19 –étude no. et-20-004, projet covid-20-02; p. 18. [Google Scholar]
- Institut National de Santé Publique du Quebec . Institut National de Santé Publique du Quebec; Quebec, QC: 2020. COVID-19: Étude de séroprévalence chez des travailleurs de la santé de centres hospitaliers au Québec.https://www.inspq.qc.ca/sites/default/files/publications/3084-seroprevalence-travailleurs-sante-covid19.pdf Available at. (accessed 1 August 2022) [Google Scholar]
- Major M, Majowicz S, Oremus M, Jimenez LJ, Angulo F, Horton S. Seroprevalence of SARS-CoV-2 in Canada. London, UK. NIHR. 2021. Available at: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021246958 (accessed 1 August 2022). [DOI] [PMC free article] [PubMed]
- Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc. 2015;13:147–153. doi: 10.1097/XEB.0000000000000054. [DOI] [PubMed] [Google Scholar]
- Ontario Ministry of Health . Ontario Ministry of Health; Toronto, ON: 2021. COVID-19 provincial testing guidance update.https://www.health.gov.on.ca/en/pro/programs/publichealth/coronavirus/docs/2019_testing_guidance.pdf Available at. (accessed 1 August 2022) [Google Scholar]
- Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. doi: 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Saez J, Zaballa M, Yerly S, Andrey DO, Meyer B, Eckerle I, et al. Persistence of anti-SARS-CoV-2 antibodies: immunoassay heterogeneity and implications for serosurveillance. Clin Microbiol Infect. 2021;27:1695. doi: 10.1016/j.cmi.2021.06.040. e7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Public Health Ontario . Public Health Ontario; Toronto, ON: 2020. COVID-19 seroprevalence in Ontario: March 27, 2020 to June 30, 2020; p. 10. [Google Scholar]
- Public Health Ontario . Public Health Ontario; Toronto, ON: 2020. COVID-19 seroprevalence in Ontario: July 4 to July 31, 2020; p. 9.https://www.publichealthontario.ca/-/media/documents/ncov/epi/2020/10/covid-19-epi-seroprevalence-in-ontario-july-31.pdf?la=en Available at. (accessed 1 August 2022) [Google Scholar]
- Public Health Ontario . Public Health Ontario; Toronto, ON: 2020. COVID-19 seroprevalence in Ontario: August 1 to August 31, 2020; p. 10. [Google Scholar]
- Public Health Ontario . Public Health Ontario; Toronto, ON: 2020. COVID-19 seroprevalence in Ontario: September 3 to October 30, 2020; p. 12. [Google Scholar]
- Saeed S, Drews SJ, Pambrun C, Yi QL, Osmond L, O'Brien SF. SARS-CoV-2 seroprevalence among blood donors after the first COVID-19 wave in Canada. Transfusion. 2021;61:862–872. doi: 10.1111/trf.16296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statistics Canada . Statistics Canada; Ottawa, ON: 2021. Population estimates on July 1st, by age and sex (Table 17-10-0005-01)https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1710000501 Available at. (accessed 1 August 2022) [Google Scholar]
- Statistics Canada . Statistics Canada; Ottawa, ON: 2021. Population estimates, quarterly.https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1710000901 Available at. (accessed 1 August 2022) [Google Scholar]
- Statistics Canada . Statistics Canada; Ottawa, ON: 2021. Study reveals children and youth had highest rates of SARS-CoV-2 infection in Canada before third wave.https://www.covid19immunitytaskforce.ca/study-reveals-children-and-youth-had-highest-rates-of-sars-cov-2-infection-in-canada-before-third-wave/ Available at. (accessed 1 August 2022) [Google Scholar]
- Tang X, Sharma A, Pasic M, Colwill K, Birnboim C, Nagelkerke N, et al. COVID symptoms, seroprevalence, and mortality during the first wave of SARS-CoV-2 in Canada. Soc Sci Res Netw. 2021 doi: 10.2139/ssrn.3752659. [DOI] [Google Scholar]
- Vijh R, Ghafari C, Hayden A, Schwandt M, Sekirov I, Morshed M, et al. Serological survey following SARS-COV-2 outbreaks at long-term care facilities in metro Vancouver, British Columbia: implications for outbreak management and infection control policies. Am J Infect Control. 2021;49:649–652. doi: 10.1016/j.ajic.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . WHO; Geneva: 2020. Population-based age-stratified seroepidemiological investigation protocol for coronavirus 2019 (COVID-19) infection.https://www.who.int/publications/i/item/WHO-2019-nCoV-Seroepidemiology-2020.2 Available at. (accessed 1 August 2022) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.