Abstract
Vaccination strategies to control COVID-19 have been ongoing worldwide since the end of 2020. Understanding their possible effect is key to prevent future disease spread. Using a modelling approach, this study intends to measure the impact of the COVID-19 Portuguese vaccination strategy on the effective reproduction number and explore three scenarios for vaccine effectiveness waning. Namely, the no-immunity-loss, 1-year and 3-years of immunity duration scenarios. We adapted an age-structured SEIR deterministic model and used Portuguese hospitalisation data for the model calibration. Results show that, although the Portuguese vaccination plan had a substantial impact in reducing overall transmission, it might not be sufficient to control disease spread. A significant vaccination coverage of those above 5 years old, a vaccine effectiveness against disease of at least 80% and softer non-pharmaceutical interventions (NPIs), such as mask usage and social distancing, would be necessary to control disease spread in the worst scenario considered. The immunity duration scenario of 1-year displays a resurgence of COVID-19 hospitalisations by the end of 2021, the same is observed in 3-year scenario although with a lower magnitude. The no-immunity-loss scenario presents a low increase in hospitalisations. In both the 1-year and 3-year scenarios, a vaccination boost of those above 65 years old would result in a 53% and 38% peak reduction of non-ICU hospitalisations, respectively. These results suggest that NPIs should not be fully phased-out but instead be combined with a fast booster vaccination strategy to reduce healthcare burden.
Keywords: Epidemiology, Compartmental models, Vaccination, Vaccine effectiveness waning, COVID-19, SEIR model
1. Introduction
During the pre-COVID-19 vaccine period, the pandemic has tested the capacity of health systems to deal with a high influx of COVID-19 patients. In order to reduce disease transmission, most countries opted to implement non-pharmaceutical interventions (NPIs), such as general lockdowns, the closure of schools and the mandatory use of masks, among others [1]. Portugal had two major lockdowns during the COVID-19 pandemic during 2020 and 2021. The first occurred mid March 2020 during the first SARS-CoV-2 epidemic wave, and the second on January 2021 during the third epidemic wave [2]. During these periods, the public health system was severely stressed with high numbers of hospitalisations. This surge of cases coincided with the appearance of the first cases of the Alpha variant of concern (VOC). This variant was the most commonly identified in the Portuguese population, until the identification of the Delta VOC. This new VOC became dominant, reaching 89.1% of confirmed COVID-19 cases in Portugal by the end of June 2021 [3]. The Delta VOC has been tied to higher disease transmission [4] and severe disease [5], [6].
With the development of COVID-19 vaccines, the focus has changed from the sole use of NPIs to a mixture of both strategies. The COVID-19 vaccines have shown promising effects and mass vaccination programmes were implemented worldwide. Although such programmes are promising, several modelling studies have shown that as vaccination is rolled out, proper vaccine allocation is necessary [7], [8], [9], [10], [11] and the maintenance of NPIs is still required to prevent the increase of hospitalisations and deaths, particularly before completing full vaccination [12], [13], [14], [15], [16]. In Portugal, vaccination took place in two main phases: the first focused in healthcare professionals and vulnerable individuals (residents in nursing homes, individuals at higher risk of severe disease due to preexisting medical conditions and those 80 years old and over, starting December 2020), and the second one for the general population, organised by age groups, from 79 years old down to 12 years old, starting March 2020. On September 26, 2021, 84% of the population had received the primary complete vaccination scheme [17]. Four vaccines were administered: Cominarty (Pfizer) and Spikevax (Moderna), both in a 2-dose scheme with a 28 day interval, Vaxzevria (Astrazeneca), using a 2-dose schedule with an interval from 8 to 12 weeks, and COVID-19 Janssen, with a single dose. Early estimates, with original virus or during Alpha variant circulation, have shown that these vaccines were highly effective in the real world. Vaccine effectiveness was estimated to be 89.1% against infection and 99% against death in fully vaccinated individuals [18]. Continued monitoring of vaccine effectiveness has however revealed that the vaccines might not grant long-lasting protection. Vaccine induced protection has been shown to last at least 5–6 months [19], [20], [21] and older individuals and at risk groups had higher rates of immunity loss [19]. The potential loss of immunity led the Portuguese government to create new boosting vaccine strategies, prioritising individuals at higher risk, including healthcare professionals and older adults aged 65 years old and over.
Mathematical modelling and potential future scenarios development are tools that allow the simulation of the possible healthcare service impact by considering the introduction of new changes to COVID-19 dynamics, namely the appearance of a new VOC, vaccination strategies and immunity loss. Hence, our objectives with this modelling study are twofold. First, to ascertain the change in transmission upon instantaneous complete vaccination, according to the Portuguese vaccination programme, on the effective reproduction number () and secondly to develop epidemic scenarios of trajectories of COVID-19 intensive care unit cases (ICU) and non-ICU hospitalisations regarding potential waning of both vaccine induced protection and infection induced immunity. The results and conclusions of this study will help to support public health policy making in order to avoid future COVID-19 related healthcare burden.
2. Methods
We have considered a simplified version of the age-structured SEIR model developed in our previous work [22]. Details of the model are described in Appendix A. Individuals start off as susceptible (S) and, upon contact with an infectious individual, they have a probability to become infected. Upon infection, an individual is considered infectious after a period of time given by the average of the latency period for SARS-CoV-2 [23]. After this period an individual can either become symptomatic or asymptomatic with a given probability. Asymptomatic individuals infect at a reduced rate [24]. All asymptomatic individuals are assumed to recover from the infection while symptomatic individuals can either recover or require hospital admission. Hospitalised individuals can either recover, require intensive care or die. Individuals in intensive care units can either die or recover. To assess the effect of vaccination and immunity loss we added a further branch to the model: susceptible and recovered individuals are vaccinated at rate and move to an analogous compartment in the vaccine protected branch of the model. Here we considered that the vaccine is leaky [25], i.e., susceptible vaccinated individuals have reduced probability of being infected upon an infectious contact and if infected, have a reduced chance of developing symptoms . The leaky model has been used in other modelling studies [12] and has been shown to be similar to other approaches when vaccine coverage is high [26].Vaccinated individuals are similar in all manners to non-vaccinated individuals with the exception of the parameters mentioned above. Vaccinated and recovered individuals lose their vaccine/infection confered protection at given rates. These individuals are then assumed susceptible. See the supplementary material for more information on model description and parameters.
In the simulations presented, individuals were vaccinated per age-group attempting to capture the progression of the national COVID-19 vaccination programme. Individuals in each age-group were assumed to be vaccinated according to the information available of vaccine coverage, planned vaccination schedule and vaccines recommended for the respective age-group. In the model, we assumed that an individual is protected by the vaccine during a period after completing the vaccination scheme, assuming the targeted coverage described in Table 1 [17]. For the age-groups not corresponding to the ones used in the model a vaccination coverage was assigned which is proportional to their size. For younger age groups a mixture of planned vaccination dates and potential vaccines to be administered were used. Beginning of vaccination in each age-group started two weeks after the beginning of administration of the vaccine in the previous group and was terminated 28 days after, assuming that each age-group received both COVID-19 Janssen vaccine or Spikevax /Cominarty vaccines. Non-calibrated parameters were obtained from COVID-19 literature, while other were obtained by fitting the results of the model to data. The parameters of the model and their description may be found in the supplementary material.
Table 1.
Portuguese vaccination roll-out plan. Beginning and end dates of the vaccination scheme and targeted final vaccination coverage by age-group [17].
| Age-group | Begin date | End date | Targeted final vaccination coverage |
|---|---|---|---|
| 70+ | 2021–02-08 | 2021–08-29 | 99% |
| 60–69 | 2021–02-08 | 2021–08-29 | 99% |
| 50–59 | 2021–07-01 | 2021–09-15 | 95% |
| 40–49 | 2021–07-15 | 2021–09-29 | 95% |
| 30–39 | 2021–08-01 | 2021–10-15 | 95% |
| 20–29 | 2021–08-15 | 2021–10-15 | 85% |
| 12–19 | 2021–09-01 | 2021–10-29 | 80% |
The duration of infection induced immunity and vaccine induced protection are considered in the model and are given by the and parameters, respectively. An individual who loses its vaccine induced protection has the same characteristics as a non-vaccinated individual. In what follows, we will assume that both the infection induced immunity and vaccine induced protection have the same duration.
We fitted the model to the data on hospitalisations both in non-ICU and ICU, by age-group, from January 24, 2021 until September 29, 2021. Changes in contacts due to the introduction and lifting of NPIs and overall changes in population behaviour were estimated using a similar method described in [22], [27]. Details of the fitting procedure can be found in the supplementary material.
In order to assess the impact of the Portuguese vaccination strategy on disease spread, we determined the by the next generation approach [28], [29]. We have considered different levels of transmission associated with the presence of the Delta variant: = 2.5, 3.2, 4.0 and 5.0 [4]. Vaccine effectiveness was considered to vary from 50% to 100% by varying and fixing the parameter as 0.5, in order to obtain the desired range. We also explored the calculation with and without NPIs in place, such as the use of mask and social distancing. To this end, we assumed a 47% reduction in effective contacts according to mask effectiveness studies [30]. In these calculations we assumed that 11% of the population had already been infected and thus removed from the susceptible group [31]. The remaining susceptible population was then divided into vaccinated and non-vaccinated according to the vaccine coverage plan presented in Table 1. We have also considered the vaccination of those between the ages of 5–11, which is not currently part of the vaccination plan. We assumed a final coverage of 80% in this group. Other parameters used are described in the supplementary material.
Secondly, we developed scenarios of trajectories of COVID-19 hospitalisations for three levels of vaccine induced protection waning. We explored a 1-year and a 3-years vaccine protection duration scenario and a no-loss-of-immunity scenario. Although little information is available about the duration of vaccine induced protection we chose these values according to recent studies that report at least 5–6 months of vaccine protection [19], [20], [21]. In each scenario we also explored the effect of giving a booster dose to those above 65 years of age. We assumed that this new vaccination scheme starts on October 11, 2021 and reaches 99% coverage within a month. We also included a 14-day delay to account for immune response.
The increased level of transmission due to the Delta variant was also included in the simulations. This was achieved by changing the value of parameter , in order to be in line with the estimated in COVID-19 literature [4] and fixing all other known parameters. For these simulations we assumed an and that no NPIs were in place after September 30, 2021.
3. Results
In this section we present the results for the impact values and the waning immunity scenarios.
3.1. Rt Impact
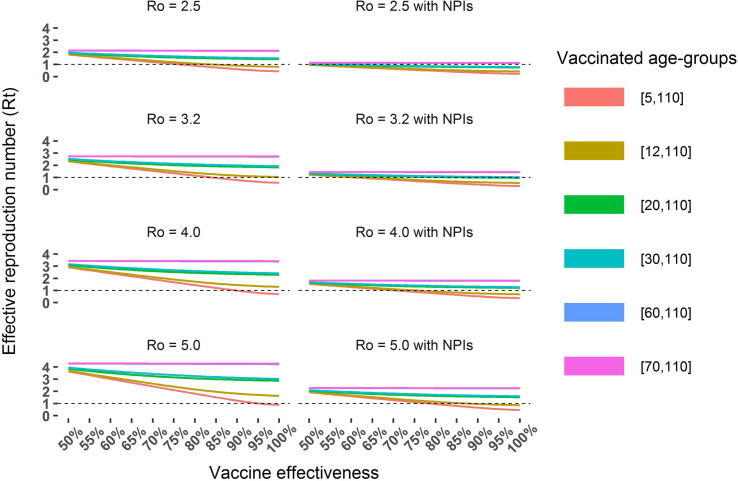
Fig. 1 depicts the computations for two scenarios with different levels of vaccine effectiveness, vaccination coverage and an increase of the reproduction number due to the circulation of the Delta variant, . We can observe in both scenarios that administering the vaccine to more age-groups has a considerable impact in reducing the effective reproduction number. Moreover, this reduction is more noticeable when all population with 5 or more years is vaccinated.
Fig. 1.
Effective reproduction number as a function of vaccine coverage (by age-group), vaccine effectiveness and basic reproduction number. Two scenarios were considered: no NPIs in place (left) and overall effective contacts reduced by 47% (right) to take into account social distancing and mask usage [30]. The vaccination coverage was assigned to each age-group according to the final targeted coverage in the Portuguese vaccination plan, presented in Table 1. Coverage of the age group 5–11 was assumed 80%.
Fig. 1 also describes the in both scenarios considered, i.e. with and without NPIs in place. In the scenario with no NPIs, we can observe that values of below 1 can only be achieved with the vaccination of at least the age-groups above 12 years old, a vaccine effectiveness above 85% and considering that the Delta variant has a similar transmission dynamics as the original strain () [32], [33]. The results for the scenario with NPIs in place show that an below one can be achieved for all considered levels of transmission. For example, assuming the reproduction number of the Delta variant to be = 5, the effective reproduction number below 1 is achievable for vaccine effectiveness values above 80%, vaccination of those with 5 or more years of age and with NPIs that induce a 47% reduction in the effective number of contacts, as depicted in the right bottom panel of Fig. 1.
3.2. Wane simulations
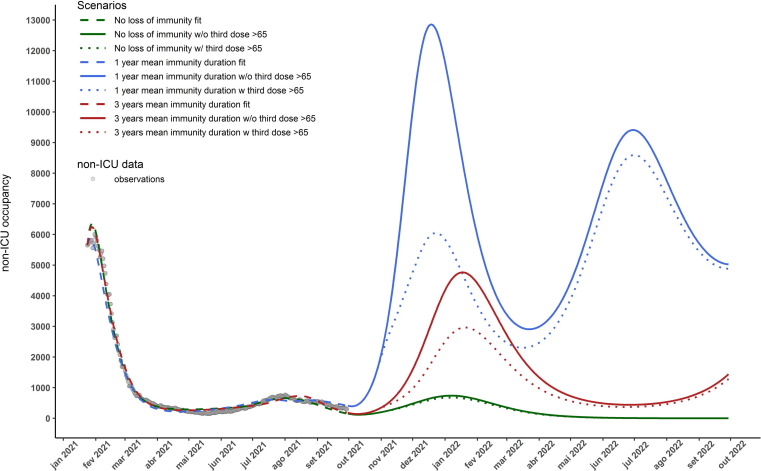
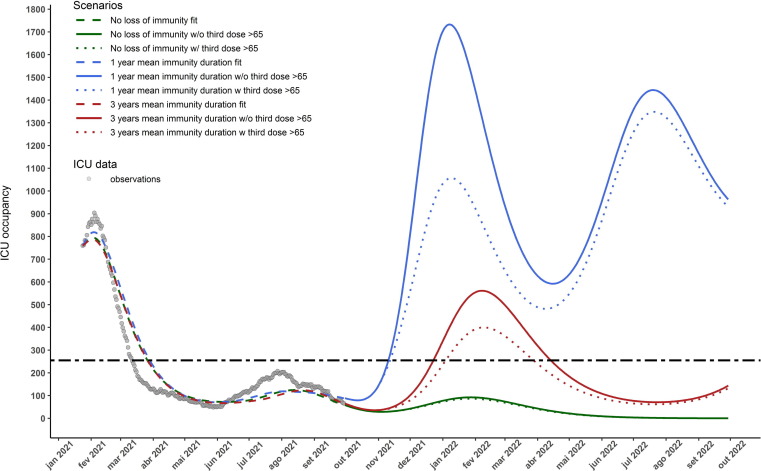
Fig. 2 presents the results for the vaccine protection duration scenarios and booster shots. The 1-year immunity duration scenario displays a new wave of non-ICU hospitalisations at the end of 2021, that can be mitigated by 53% at its peak if a booster shot is administered to those above 65 years of age. The 3-year immunity scenario displays a new wave of hospitalisations with a much smaller magnitude then the previous mentioned scenario but similar to the January 2021 wave. In this scenario the administration of the booster shot to those above 65 years old can reduce the peak non-ICU hospitalisation cases by . The no-immunity-loss scenario also displays a new wave of hospitalisations by the end of 2021, although with a much smaller magnitude than the previous scenarios. It does not exceed 1000 non-ICU occupied beds at its peak. Similar results are obtained for ICU occupations as depicted in Fig. 3 . Only in the no-immunity-loss scenario is expected not to exceed the maximum acceptable occupancy of ICU beds (255) by the end of 2021 [34]. More detailed results by age-group of the fitting procedure and simulation scenarios are presented in Appendix B.
Fig. 2.
Number of COVID-19 non-ICU hospitalised cases for the 3 considered scenarios: no loss of vaccine induced protection (green), vaccine effectiveness duration of 1 and 3 years (blue and red respectively). Dashed lines depict the model calibration for each immunity loss scenario, the solid lines represent the trajectory of the total number of non-ICU hospitalisation cases in each scenario. The dotted lines represent the trajectories where a third dose of the vaccine was administered to those above 65 years old and assuming that this group achieves 99% coverage with this new dose within one month of October 11, 2021. It is also assumed that no NPIs are in place after September 30, 2021 and that vaccine/infection granted immunity lasts an equal amount of time and is the same for each age-groups. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 3.
Number of COVID-19 ICU hospitalised cases for the 3 considered scenarios: no loss of vaccine granted protection (green), vaccine effectiveness duration of 1 and 3 years (blue and red respectively). Dashed lines depict the model calibration for each immunity loss scenario, the solid lines represent the trajectory of the total number of hospitalisation cases in each scenario. The dotted lines represent the trajectories where a third dose of the vaccine was administered to those above 65 years old and assuming that this group achieves 99% coverage with this new dose within one month of October 11, 2021. It is also assumed that no NPIs are in place after September 30th 2021 and that vaccine/infection granted immunity lasts an equal amount of time and is the same for age-group. The horizontal dashed line depicts the ICU capacity of COVID-19 hospitalisations in Portugal. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
In this study we proposed a model that could capture the dynamics of COVID-19 spread during the period from January 24 to September 30, 2021, i.e., during the implementation of the COVID-19 vaccination programme up to the goal of 84% of vaccine coverage. The model takes into account the heterogeneous nature of the Portuguese vaccination plan, the lifting and implementation of NPIs and the characteristics of disease complications in each age-group.
The calculations enabled the analysis of possible outcomes of disease spread according to the vaccine effectiveness, vaccine allocation and increased transmission associated with the new Delta variant. By considering a different array of possible values for each of these parameters we were able to explore the uncertainty and investigate possible outcomes of disease transmission. The results suggest that the herd-immunity threshold [35] might be higher than expected according to early assumptions ( = 2.5) due to the presence of new and more transmissible variants. On the other hand, herd-immunity might also not be attainable since vaccines do not grant immunity to infection. COVID-19 reports in Portugal highlight that the was estimated as 1.11 for the period between November 29, 2021 and December 3, 2021, vaccination coverage is above 85% in all eligible age-groups and early vaccine effectiveness studies estimate an effectiveness greater than 80% against COVID-19 hospitalisation and mortality and are also effective against SARS-CoV-2 infection with effectiveness close to 53%. Hence, considering the present setting, our results suggest that solely vaccinating the targeted age-groups might not be sufficient to bring the bellow one and prevent epidemic disease spread, even considering high vaccination coverage rates.
These results are in agreement with other Portuguese modelling studies [12], [36] and international studies [13], [14], [15], [16] which also present epidemic spread in the presence of vaccination. Consequently the Portuguese government decided to vaccinate those between 5 and 11 years old (during late December 2021), which according to our results would further reduce disease transmission and could even control disease spread in the worst hypothetical scenario considered ( and no NPIs) if vaccine effectiveness is high (>95%). Additionally under the hypothesis of vaccine immunity waning with time, one must consider that vaccine effectiveness changes throughout the pandemic, independently of vaccine coverage. This means that the impact of vaccine effectiveness and coverage on transmission can be transient in time. Being higher when effectiveness is high (after a booster dose campaign) and lower after more than 5–6 months of complete vaccination scheme. If epidemic control cannot be attained under these conditions, other measures should be adopted. The impact results considering the presence of NPIs show that epidemic control might be feasible under the present setting if soft NPIs are maintained, such as the use of mask and social distancing. We can observe that an can be achieved in the worst scenario () with those above 5 years old vaccinated and a vaccine effectiveness above 80%. Furthermore, we plan to update parameter values in these calculations as more detailed information becomes available regarding VOC transmission parameters. In future work we also plan to study the evolution of Rt taking into account the dynamical changes in susceptibility by considering the loss of immunity conferred by both infection and vaccination.
The immunity-loss simulations proposed enabled us to study outcomes of COVID-19 hospitalisations and ICU cases with regard to the uncertain nature of vaccine induced immunity waning and a new planned booster shot. These results show that a new wave of COVID-19 hospitalisations is expected by the end of 2021, and its magnitude is related to the duration of vaccine protection. We also showed that this wave can be heavily mitigated by administering a booster shot to those above 65 years of age (53% reduction at peak in non-ICU hospitalisations). The 1-year immunity loss scenario displays a possible wave of higher magnitude than past others in both non-ICU and ICU occupied beds, reaching 12,857 non-ICU occupied beds at peak, assuming constant severity. This is a consequence of immunity loss of those that were vaccinated first, which included older individuals and those with higher risk of severe disease. Note also that after this wave a new resurgence is expected by mid 2022 and in this wave the effect of the booster shot is dampened due to the potential immunity loss of those who received the booster dose. It is also important to state that we considered that loss of immunity after the booster dose is also 1 or 3 years, according to each scenario, this assumption will be updated as new data becomes available.
The 3-year immunity duration scenario displays a resurgence of hospitalisations similar to the one observed during the beginning of 2021. In this case, giving a booster to those above 65 years of age also substantially reduces the number of hospitalised individuals (38%). The no-immunity-loss scenario depicts a small size wave of hospitalisations, and since vaccine grants long lasting immunity, the booster shot does not substantially impact the number of hospitalisations. Similar results have been reported by modelling studies that consider the presence of immunity waning, i.e., the presence of immunity loss might result in larger outbreaks, especially in the presence of a VOC with higher transmission [37], [38]. Another study suggests that if the rate of administration of booster shots is not high enough, it might lead to a resurgence of cases, that can be worse than previous waves [39]. It is important to note that our simulations assume a maximum potential of disease spread (), before vaccination and susceptibility and also that vaccine/infection induced immunity waning is age-group independent and no NPIs are introduced, even the use of face mask. This value of was chosen according to estimated values of the reproduction numbers for the Delta variant [4]. A limitation of the analysis is that vaccinated individuals which lose their vaccine induced protection are assumed to have the same characteristics as non-vaccinated individuals within the same age-group. In a similar way, we assume that individuals who receive a booster shot are equally protected to those who have been vaccinated with a full scheme. Recent studies suggest that a third-dose booster shot increases vaccine protection to levels similar to receiving a complete scheme [40], [41] which is in line with the present assumptions.
Our results show that vaccine effectiveness, coverage, vaccination speed and vaccine induced protection waning play key roles in understanding disease spread and healthcare impact. Currently, vaccine allocation, effectiveness and coverage in Portugal have not been enough to control disease spread. Hence, NPIs should be maintained and combined with vaccination programmes that include more age-groups and booster doses. Vaccine effectiveness should be monitored in order to assess scenarios for the periodicity of booster shots. As more data becomes available we will extend the present model to study optimal booster dose schedules according to different scenarios of vaccine induced protection waning and infection induced immunity waning and the impact of new VOC.
This analysis was invoked by questions from Portuguese decision-makers, hence they are time sensitive. Yet, it is useful to reflect on how the results compare with what happened in reality. In the case of Portugal, a mixture of vaccination strategies and NPIs was indeed required to maintain a controlled COVID-19 epidemic activity throughout the autumn of 2021 [42]. Our analysis identified two subsequent waves of hospitalisations. These waves were concurrent in time but with different magnitudes than the ones observed (see supplementary material). This is probably due to various mechanisms: vaccination conferred protection against severe disease decayed slower with time than we anticipated [43], omicron and subsequent variants presented a lower risk of severe disease and immune escape [44]. We would also note that the scenarios presented were designed to provide insights regarding the evolution of the COVID-19 dynamics impact given the assumptions of the model and chosen immunity duration values. Nevertheless, we believe the results provide valuable highlights and show the importance of modeling in supporting decision making with the need to readjust models alongside pandemic evolution. Bosse et al suggested that the combination of mathematical modeling and human judgment can complement each other [45]. To address such challenges, a pre-existing modeling toolkit with easily extendable options is currently being developed.
Funding
The authors acknowledge financial support from the Fundação para a Ciência e Tecnologia - FCT through project “Projection of the Impact of Non-pharmacological real-time Control and mitigation measures for the COVID-19 epidemic” (COVID-19 in-CTRL) - project n° 692 from the 2nd edition of RESEARCH 4 COVID-19. The first author also acknowledges FCT within the PhD grants program “DOCTORATES 4 COVID-19”, Grant No 2020.10172.BD. The second author also acknowledges FCT within projects UIDB/04621/2020 and UIDP/04621/2020. The third author also acknowledges FCT within the Strategic Project UIDB/00297 /2020 (Centro de Matemática e Aplicações, Universidade Nova de Lisboa).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2022.10.007.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Greer S.L., King E.J., da Fonseca E.M., et al. The comparative politics of COVID-19: The need to understand government responses. Global Public Health. 2020;15:1413–1416. doi: 10.1080/17441692.2020.1783340. [DOI] [PubMed] [Google Scholar]
- 2.COVID-19: Relatório de situação (2021).
- 3.Departamento de Doenças Infeciosas. Instituto Nacional de Saúde Doutor Ricardo Jorge; 2021. Diversidade genética do novo coronavírus SARS-CoV-2 (COVID-19) [Google Scholar]
- 4.Liu Y., Rocklöv J. The reproductive number of the delta variant of SARS-CoV-2 is far higher compared to the ancestral SARS-CoV-2 virus. J Travel Med. 2021;28 doi: 10.1093/jtm/taab124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y., Chen R., Hu F., et al. Transmission, viral kinetics and clinical characteristics of the emergent SARS-CoV-2 Delta VOC in Guangzhou, China. EClinicalMedicine. 2021;40 doi: 10.1016/j.eclinm.2021.101129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodrigues E.F., Moreno J., Leite P.P., et al. B.1.617.2 SARS-CoV-2 (Delta) variant is associated with increased risk of hospitalization and death compared with B.1.1.7 SARS-CoV-2 (Alpha) variant. medRxiv. 2022 [Google Scholar]
- 7.Ferranna M., Cadarette D., Bloom D.E. COVID-19 vaccine allocation: Modeling health outcomes and equity implications of alternative strategies. Engineering. 2021;7:924–935. doi: 10.1016/j.eng.2021.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mukandavire Z., Nyabadza F., Malunguza N.J., et al. Quantifying early COVID-19 outbreak transmission in south africa and exploring vaccine efficacy scenarios. PLOS ONE. 2020;15:1–11. doi: 10.1371/journal.pone.0236003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bubar K.M., Reinholt K., Kissler S.M., et al. Model-informed COVID-19 vaccine prioritization strategies by age and serostatus. Science. 2021;371:916–921. doi: 10.1126/science.abe6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi Y., Kim J.S., Kim J.E., et al. Vaccination prioritization strategies for COVID-19 in Korea: A mathematical modeling approach. Int J Environ Res Public Health. 2021;18:4240. doi: 10.3390/ijerph18084240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foy B.H., Wahl B., Mehta K., et al. Comparing COVID-19 vaccine allocation strategies in India: A mathematical modelling study. Int J Infect Dis. 2021;103:431–438. doi: 10.1016/j.ijid.2020.12.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Viana J., van Dorp C., Gomes M., et al. Controlling the pandemic during the SARS-CoV-2 vaccination rollout. Nat Commun. 2021;12(1) doi: 10.1038/s41467-021-23938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhe X., Wu B., Topcu U. Control strategies for COVID-19 epidemic with vaccination, shield immunity and quarantine: A metric temporal logic approach. PLOS ONE. 2021;16(3):1–20. doi: 10.1371/journal.pone.0247660. e0247660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang B., Wang J., Cai J., et al. Integrated vaccination and physical distancing interventions to prevent future COVID-19 waves in Chinese cities. Nat Hum Behav. 2021;5(6):695–705. doi: 10.1038/s41562-021-01063-2. [DOI] [PubMed] [Google Scholar]
- 15.Li Y., Ge L., Zhou Y., et al. Toward the impact of non-pharmaceutical interventions and vaccination on the COVID-19 pandemic with time-dependent SEIR model. Front Artif Intell. 2021;4 doi: 10.3389/frai.2021.648579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borchering R.K., Viboud C., Howerton E., et al. Modeling of Future COVID-19 Cases, Hospitalizations, and Deaths, by Vaccination Rates and Nonpharmaceutical Intervention Scenarios - United States, April-September 2021. MMWR Morb Mortal Wkly Rep. 2021;70(19):719–724. doi: 10.15585/mmwr.mm7019e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DGS (2021) Relatório de vacinação.
- 18.Zheng C., Shao W., Chen X., et al. Real-world effectiveness of COVID-19 vaccines: A literature review and meta-analysis. Int J Infect Dis. 2022;114:252–260. doi: 10.1016/j.ijid.2021.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andrews N., Tessier E., Stowe J., et al. Vaccine effectiveness and duration of protection of Comirnaty, Vaxzevria and Spikevax against mild and severe COVID-19 in the UK. medRxiv. 2021 [Google Scholar]
- 20.Tartof S.Y., Slezak J.M., Fischer H., et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: A retrospective cohort study. The Lancet. 2021;398:1407–1416. doi: 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chemaitelly H., Tang P., Hasan M.R., et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N Engl J Med. 2021;385(24) doi: 10.1056/NEJMoa2114114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caetano C., Morgado M.L., Patrício P., et al. Mathematical modelling of the impact of non-pharmacological strategies to control the COVID-19 epidemic in Portugal. Mathematics. 2021;9:1084. doi: 10.3390/math9101084. [DOI] [Google Scholar]
- 23.Linton N., Kobayashi T., Yang Y., et al. Incubation period and other epidemiological characteristics of 2019 novel coronavirus infections with right truncation: A statistical analysis of publicly available case data. J Clin Med. 2020;9(2):538. doi: 10.3390/jcm9020538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li F., Li Y.Y., Liu M.J., et al. Household transmission of SARS-CoV-2 and risk factors for susceptibility and infectivity in Wuhan: A retrospective observational study. Lancet Infect Dis. 2021;21(5):617–628. doi: 10.1016/S1473-3099(20)30981-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halloran M.E., Haber M., Longini J., Ira M. Interpretation and Estimation of Vaccine Efficacy under Heterogeneity. Am J Epidemiol. 1992;136(3):328–343. doi: 10.1093/oxfordjournals.aje.a116498. [DOI] [PubMed] [Google Scholar]
- 26.Shim E., Galvani A.P. Distinguishing vaccine efficacy and effectiveness. Vaccine. 2012;30(47):6700–6705. doi: 10.1016/j.vaccine.2012.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glass D. European and US lockdowns and second waves during the COVID-19 pandemic. Math Biosci. 2020;330 doi: 10.1016/j.mbs.2020.108472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van den Driessche P., Watmough J. Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math Biosci. 2002;180:29–48. doi: 10.1016/s0025-5564(02)00108-6. [DOI] [PubMed] [Google Scholar]
- 29.Diekmann O., Heesterbeek J.A., Roberts M.G. The construction of next-generation matrices for compartmental epidemic models. J R Soc Interface. 2009;7(47):873–885. doi: 10.1098/rsif.2009.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang M., Gao L., Cheng C., et al. Efficacy of face mask in preventing respiratory virus transmission: A systematic review and meta-analysis. Travel Med Infect Dis. 2020;36 doi: 10.1016/j.tmaid.2020.101751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kislaya I., Gonçalves P., Barreto M., et al. Seroprevalence of SARS-CoV-2 infection in Portugal in May-July 2020: Results of the first national serological survey (ISNCOVID-19) Acta Médica Portuguesa. 2021;34:87. doi: 10.20344/amp.15122. [DOI] [PubMed] [Google Scholar]
- 32.Wu J.T., Leung K., Leung G.M. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: A modelling study. The Lancet. 2020;395(10225):689–697. doi: 10.1016/S0140-6736(20)30260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferguson N., Laydon D., Nedjati-Gilani G., et al. Report 9: Impact of non-pharmaceutical interventions (npis) to reduce covid-19 mortality and healthcare demand. 2020. [DOI] [PMC free article] [PubMed]
- 34.Monitorização das linhas vermelhas para a COVID-19. Instituto Nacional de Saúde Doutor Ricardo Jorge and DGS; 2021. [Google Scholar]
- 35.Randolph H.E., Barreiro L.B. Herd immunity: Understanding COVID-19. Immunity. 2020;52:737–741. doi: 10.1016/j.immuni.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Machado B., Antunes L., Caetano C., et al. The impact of vaccination on the evolution of COVID-19 in Portugal. Math Biosci Eng. 2021;19:936–952. doi: 10.3934/mbe.2022043. [DOI] [PubMed] [Google Scholar]
- 37.Zhou W., Tang B., Bai Y., et al. The resurgence risk of COVID-19 in the presence of immunity waning and ade effect: A mathematical modelling study. medRxiv. 2021 doi: 10.1016/j.vaccine.2022.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Childs L., Dick D.W., Feng Z., et al. Modeling waning and boosting of COVID-19 in Canada with vaccination. medRxiv. 2021 doi: 10.1016/j.epidem.2022.100583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Truszkowska A., Zino L., Butail S., et al. Predicting the effects of waning vaccine immunity against COVID-19 through high-resolution agent-based modeling. arXiv 2021. [DOI] [PMC free article] [PubMed]
- 40.Barda N., Dagan N., Cohen C., et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: An observational study. The Lancet. 2021;398(10316):2093–2100. doi: 10.1016/S0140-6736(21)02249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perez J.L. Centers for Disease Control and Prevention. 2021. ACIP november 19, 2021 presentation slides. [Google Scholar]
- 42.Portugal.gov.pt Comunicado do Conselho de Ministros de 25 de novembro de 2021. XXII Governo - República Portuguesa; 2021. [Google Scholar]
- 43.Kislaya I., Machado A., Magalhães S., et al. COVID-19 mRNA vaccine effectiveness (second and first booster dose) against hospitalisation and death during Omicron BA.5 circulation: Cohort study based on electronic health records, Portugal, May to July 2022. Eurosurveillance. 2022;27(37):2200697. doi: 10.2807/1560-7917.ES.2022.27.37.2200697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peralta-Santos A., Rodrigues E.F., Moreno J., et al. Omicron (BA.1) SARS-CoV-2 variant is associated with reduced risk of hospitalization and length of stay compared with Delta (B.1.617.2) medRxiv. 2022 [Google Scholar]
- 45.Bosse N.I., Abbott S., Bracher J., et al. Comparing human and model-based forecasts of COVID-19 in Germany and Poland. PLoS Comput Biol. 2022;18(9) doi: 10.1371/journal.pcbi.1010405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.