Abstract
Background Wound healing is a dynamic and complex process. Therefore, no single agent can efficiently mediate all aspects of the wound healing process. Split-thickness graft has become a workhorse of plastic surgery for wound or raw area cover. In this study, we evaluate the effectiveness of autologous platelet-rich plasma (PRP) on the donor site and its effect in pain, purities, and epithelization.
Materials and Methods This is a prospective study. A total of 15 patients were included who underwent split skin grafting for burns, trauma, or post-tumor excision raw area. PRP was prepared using standard described procedure. The donor site raw area after harvesting split-thickness graft was measured and the surface area was divided into two equal halves. One half was dressed using PRP and the other half was dressed using paraffin gauze piece only. The dressings were changed weekly for 3 weeks.
Observation We found significant reduction in severity of pain and pruritis in the PRP group as compared with control group. Epithelization was faster in the PRP group on day 7 and 14, but the overall healing time was nearly the same by day 21. The side-by-side dressing thus show a definite improvement in the post-split-thickness skin graft wound care and PRP as a good dressing alternative.
Conclusion Autologous PRP is very effective adjuvant in management of skin graft donor site. Its role in relieving pain and pruritis over donor site significantly improves patient's discomfort postoperatively. It helps in early and painless wound healing. However, we recommend for larger clinical study for better understanding of the efficacy of this blood product.
Keywords: PRP, split-thickness skin graft, skin graft donor site
Introduction
Wound healing is a dynamic and complex process controlled by interacting signals that regulate a myriad of cellular and molecular events. Therefore, no single agent can efficiently mediate all aspects of the wound healing process. 1 Split-thickness graft has become a workhorse of plastic surgery for wound or raw area cover caused by burns, trauma, and defects after scar or tumor resection. The problems associated with complications, such as prolonged healing time, severe scarring, and wound pain, at the donor site after split-thickness skin graft (STSG) have garnered significant attention over the last decade. Many treatments aimed at shortening the healing time, alleviating pain, and reducing scarring have been applied to the donor site after STSG. 2 3 Autologous platelet-rich plasma (PRP) is widely used in plastic and reconstructive surgery for various skin conditions, such as acute wounds, chronic wounds, maxillofacial bone defects, and aesthetic issues, as PRP stimulates the regeneration of soft and hard tissues. 4 The extraction of PRP begins with any peripheral venous access. The extraction of platelet concentrate from patient blood occurs via plasmapheresis, whereby PRP is concentrated to 300% of normal blood levels. 5 PRP contains many platelets, the α-granules of which contain many molecules, such as transforming growth factor-β (TGF-β), epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), coagulation factors, calcium, serotonin, histamine, and hydrolytic enzymes. These growth factors—particularly TGF-β—are essential for wound healing. 6 7 8 The management of the donor site is also of equal importance to reduce morbidity in patients. Various dressing modalities such as paraffin gauze dressing, wet or dry collagen sheet, and hydrocolloid dressings have been described. In this study, we evaluate the effectiveness of autologous PRP on the donor site and its effect in pain, purities, and epithelization.
Patients and Methods
This is a prospective study, done in JNMCH, AMU, between July 2020 and July 2021. A total of 15 patients were included in this study who underwent split skin grafting for burns, trauma, or post-tumor excision raw area. The split-thickness grafts harvested were of intermediate thickness (0.3–0.45mm). All patients were between the age of 18 and 60 years. There were 10 males and 5 females.
Inclusion Criteria
Any patient with healthy raw area/wound bed requiring split-thickness skin grafting.
Raw areas between 100 and 200 cm 2 requiring single sheet graft for easy comparison.
Exclusion Criteria
β hemolytic streptococcus culture-positive patients.
Immunocompromised patients.
Preparation of Platelet-Rich Plasma
There have been many methods described for PRP preparation. In this study, 45 mL of patients' blood was taken in a 50 mL disposable syringe containing 5 mL anticoagulant sodium dextrose solution. This was then divided into two tubes for centrifugation ( Fig. 1 ). The blood was then centrifuged for 10 minutes at 2000 rpm at 15 cm radius ( Fig. 2 ). This produced three layers—the bottom most being red blood cell, the middle layer (buffy coat) layer being white blood cell and platelet, and the top layer being acellular plasma ( Fig. 3 ). The top layer and buffy layer were separated and further centrifuged for 10 minutes at 2000 rpm. This further separated into PPP (platelet poor plasma) at the top and PRP in the bottom ( Fig. 4 ). The PPP was discarded and the PRP was used for dressing of donor area ( Fig. 5 ).
Fig. 1.

Venous blood with anticoagulant sodium dextrose.
Fig. 2.

Blood after first and second centrifugation.
Fig. 3.

Application of platelet-rich plasma.
Fig. 4.

Intraoperative photo (P – proximal, PRP, D – distal, paraffin gauze only).
Fig. 5.

Postoperative photo day 7 (P – proximal, PRP, D – distal, paraffin gauze only).
Donor Site Dressing
The donor site raw area after harvesting STSG was measured and the surface area was divided into two equal halves. Proximal half raw area was dressed after spraying PRP followed by paraffin gauze and the distal half raw area was dressed using paraffin gauze piece only. Approximately 0.1 mL of PRP was sprayed per cm 2 . The dressings were changed on day 7, 14, and 21 and parameters noted. All dressings were changed after thoroughly wetting the paraffin gauze dressings with normal saline soaked pads and great care was taken to cause minimal pain and trauma to underlying tissue. No repeat application of PRP was done.
Outcome
The wound was assessed for pain, pruritis, and epithelization every 7th day for 3 weeks. The coverage of the epithelium and fibrous tissues without secretions, rupture, or granulation formation on the wound was considered as successful wound healing. Patients were given injectable nonsteroidal anti-inflammatory drugs analgesics for 5 days postoperatively and from day 6 oral analgesics were given on SOS basis. The wound healing time was simultaneously recorded by two specialists, and the wound healing rate was calculated by comparing the healed area to the pretreatment area after 7, 14, and 21 days of applying wound dressing to STSG donor sites. We placed a transparent film on the wound, marked around its margins on the film for both raw area and healed area; then, we cut the transparent film along the line, traced on graph paper and calculated the surface area according to the number of squares, which then helped calculate the wound healing rate comparing percentage raw area to day 0.
For the assessment of pain and pruritis, patients were asked to rate using visual analog scale (VAS) and pruritis numerical rating scale (PNRC) comparing proximal half (PRP) to distal half (only paraffin gauze).
The VAS score ranged from 0 to 10, where 0 denoted no pain, 1 to 3 denoted mild pain within tolerable limits, 4 to 6 denoted pain that affected sleep, and 7 to 10 denoted activity and maximum pain that seriously affected appetite and sleep.
Another scale, PNRC, was used to measure the severity of pruritis. Numbers “0” represents no itch to number “10” that represents worst imaginable itch.
All patients were discharged on day 7, after first donor site dressing and further dressings were done in outpatient department.
Result
A total of 15 patients were included in the study (age: 24–54 years) with an average age of 37.47 years. Six patients had raw area over left leg followed by three patients each in right leg and abdomen, two patients with right upper limb, and one patient with left upper limb raw area. Raw area in seven patients was secondary to second-degree or third-degree burns, six were following road traffic accident, and two were secondary to cutaneous tumor excision.
In all patients, thigh was used as donor for STSG with right thigh in 10 patients and left thigh in 5 patients. The donor site postharvest was divided in two equal halves. Proximal half was dressed with local application of PRP and distal half with paraffin gauze. Dressing was changed on day 7, 14, and 21 ( Table 1 , Figs. 5 6 7 ).
Table 1. Demography of patient.
| Characteristic | Number of patients | |
|---|---|---|
| Etiology | Burn | 7 |
| Road traffic accident | 6 | |
| Tumor | 2 | |
| Recipient site | Right leg | 3 |
| Left leg | 6 | |
| Abdomen | 3 | |
| Right upper limb | 2 | |
| Left upper limb | 1 | |
| Donor site | Right thigh | 10 |
| Left thigh | 5 | |
Fig. 6.

Postoperative photo day 14 (P – proximal, PRP, D – distal, paraffin gauze only).
Fig. 7.
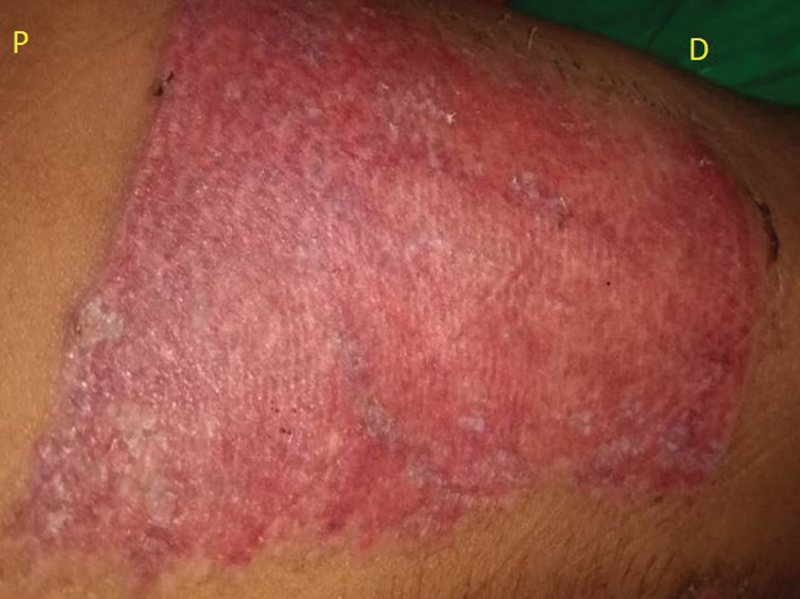
Postoperative photo day 21 (P – proximal, PRP, D – distal, paraffin gauze only).
On day 7, the PRP group had a pain score (VAS) of 1.73 as compared with control group with a score of 4.67. On day 14 and 21, the PRP group had an average pain score of 1 each as compared with 2.73 and 1.4 in control group ( p -value ≤0.0006).
According to the PNRC, day 7 showed an average score 1.53 and 1.2 in PRP and control group, respectively. On day 14, patients had maximum pruritis with an average score of 1.8 in PRP group and 4.8 in control group. Day 21 had a PNRC score of 1.47 (PRP) as compared with 2.27 (control) ( p -value ≤0.02).
Wound healing was calculated in percentage raw area remaining during each dressing. On day 7 and 14, the average percentage raw area was 44.53 and 16% for PRP group as compared with 68.47 and 30.4% for control group ( p -value ≤0.03 for day 7 and 14). However, on day 21, the average was 0.2% for PRP group and 0.67% for control group ( Figs. 8 , 9 , 10 ).
Fig. 8.

Effect of platelet-rich plasma (PRP) on pain. VAS, visual analog scale.
Fig. 9.

Effect of platelet-rich plasma (PRP) on pruritis.
Fig. 10.

Effect of platelet-rich plasma (PRP) on wound healing.
Discussion
STSG is widely used in burn treatment and plastic surgery to cover and repair skin and soft tissue defects. 9 This procedure creates an acute wound with different levels of dermal appendage damage. The idea of treating STSG donor sites with simpler, more effective and reliable methods and materials has garnered a lot of attention. If the donor site heals rapidly, undesirable scarring, severe infection, and the pain during dressing changes can be minimized; this would thus reduce the hospital stay duration and treatment costs. 10 Many studies have reported on the methods of improving the healing of STSG donor sites. Sinha et al analyzed and summarized that hydrocolloid dressings were superior to other substrates in terms of their effects at the donor site. 11 Lairet et al reported that oxygen-diffusion dressings decreased the healing time at donor sites. 12 Sagray et al considered that providing the donor sites a moist environment enhances the re-epithelialization of wounds. 13 Each modern dressing has its own advantages and disadvantages compared with traditional way of dressing. For example, hydrocolloid dressings could provide a moist environment for the wound surface and reduce bacterial invasion. However, the adhesion of hydrocolloid dressing decreases with water and hence it needs to be changed more frequently. The use of foam dressings could alleviate the patient's pain, but these have little anti-infective effect that would increase the risk of wound infection. The oxygen-diffusion dressings could also improve the wetting degree of the wound surface, but the specific mechanisms are still unclear.
PRP is a fraction of blood plasma that releases many growth factors, such as TGF-β, PDGF, VEGF, fibroblast growth factor, EGF, and insulin-like growth factor. 14 Its use has been reported in some clinical treatments for acute, chronic, or refractory wounds of dermatology, dentistry, orthopaedics, neurosurgery, ophthalmology, and other surgical fields. In the treatment of chronic and nonhealing wounds, numerous studies reported different degrees of effectiveness of PRP gel for diabetic foot ulcers, venous ulcers, pressure ulcers, paraplegic ulcers, and other chronic wounds. 15 16
Sommeling et al 17 in their systematic review of 15 randomized controlled trials and 25 case–control studies found that there is no standard technique of PRP preparation. In our study, we adapted the double-spin technique. In the double-spin technique, a study by Ghoraba et al observed that the platelet count was increased approximately 3.5-folds and TGF-β1 was increased 2.4-folds. 18 In a similar study, Marukawa et al 19 used the double-spin technique for PRP preparation and noticed that the platelet count was increased approximately three times and the platelet released growth factors increased approximately two to three times. Conversely, Pietrzak and Eppley 20 suggested that a four- to fivefold increase in the baseline of platelet count is needed, but they show no clear evidence that lower or higher concentration may decrease or increase the positive effect of PRP.
Fang et al 21 in similar study evaluated the role of PRP gel versus paraffin dressing in wound healing and scaring in graft donor site. The time and frequency of dressing change were comparable between the two groups, and the mean wound healing times in the PRP group and petroleum gauze group were 13.89 ± 4.65 and 17.73 ± 5.06 days, respectively, and the difference was statistically significant ( p < 0.05). In addition, the total Vancouver scar scale scores of the PRP group at 4, 12, and 52 weeks were 6.41 ± 0.77, 4.42 ± 0.43, and 2.41 ± 0.39, respectively, which were statistically significantly lower ( p < 0.05) than those of the control group at 7.67 ± 0.64, 6.28 ± 0.62, and 4.29 ± 0.64, respectively.
In our series, the pain scores, while not differing at day 0 and 21 postoperative, significantly decreased by day 7 and 14 postoperatively in the PRP side as compared with the control side. In similar studies, Englert et al 22 found that postoperative pain was significantly reduced for the PRP treated wounds and Gardner et al 23 observed that intravenous narcotic use was statistically lower in the PRP-treated subjects. Kazakos et al 5 noticed that there was no pain difference between both groups at the end of the first week, while there were lower pain scores in the PRP treated group at the end of the second week.
In our study, we used PRP for dressing of the donor site wound post-STSG. The main advantage is the cost-effectiveness and minimal or no tissue reaction of the procedure.
We found significant reduction in severity of pain and pruritis in the PRP group as compared with control group. During dressing changes, it was also observed that the epithelization was faster in the PRP group on day 7 and 14, but the overall healing time was nearly the same by day 21. Complete wound healing would have occurred earlier in PRP group as seen on day 7 and 14, but since frequent dressings were not done for wound assessment to avoid disruption of epithelization, this is a pitfall of our study.
The results of the side-by-side dressing thus show a definite improvement in the post-STSG wound care and PRP as a good dressing alternative.
Conclusion
PRP functions as a vehicle of growth factors and thus this blood product is highly useful for application in several indications within plastic and reconstructive surgery. The complex interaction of multiple factors and physiological mechanisms contributing to tissue regeneration makes the use of autologous PRP very effective adjuvant in the management of skin graft donor site. Its role in relieving pain and pruritis over donor site significantly improves patient's discomfort postoperatively. It also helps in early and painless wound healing. Furthermore, its cost-effectiveness as compared with collagen or hydrocolloid dressings and ease of use make it a strong tool in a surgeon's armamentarium. However, we recommend for larger clinical study for better understanding of the efficacy of this blood product.
Footnotes
Conflict of Interest None declared.
References
- 1.Gharaee-Kermani M, Phan S H. Role of cytokines and cytokine therapy in wound healing and fibrotic diseases. Curr Pharm Des. 2001;7(11):1083–1103. doi: 10.2174/1381612013397573. [DOI] [PubMed] [Google Scholar]
- 2.Bian Y, Sun C, Zhang X. Wound-healing improvement by resurfacing split-thickness skin donor sites with thin split-thickness grafting. Burns. 2016;42(01):123–130. doi: 10.1016/j.burns.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 3.Kirsner R S, Eaglstein W H, Kerdel F A.Split-thickness skin grafting for lower extremity ulcerations Dermatol Surg 1997230285–91., quiz 92–93 [DOI] [PubMed] [Google Scholar]
- 4.Cervelli V, Gentile P, Scioli M G. Application of platelet-rich plasma in plastic surgery: clinical and in vitro evaluation. Tissue Eng Part C Methods. 2009;15(04):625–634. doi: 10.1089/ten.TEC.2008.0518. [DOI] [PubMed] [Google Scholar]
- 5.Kazakos K, Lyras D N, Verettas D, Tilkeridis K, Tryfonidis M. The use of autologous PRP gel as an aid in the management of acute trauma wounds. Injury. 2009;40(08):801–805. doi: 10.1016/j.injury.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Kakudo N, Kushida S, Kusumoto K. Platelet-rich plasma: the importance of platelet separation and concentration. Plast Reconstr Surg. 2009;123(03):1135–1136. doi: 10.1097/PRS.0b013e31819a3575. [DOI] [PubMed] [Google Scholar]
- 7.Rivers J K. Platelet-rich plasma: should we wait for the verdict? J Cutan Med Surg. 2014;18(03):147–150. doi: 10.2310/7750.2014.EDIT18.3. [DOI] [PubMed] [Google Scholar]
- 8.Andia I. Platelet rich plasma therapies: a great potential to be harnessed. Muscles Ligaments Tendons J. 2014;4(01):1–2. [PMC free article] [PubMed] [Google Scholar]
- 9.Tang Y W. Simultaneous very thick split-thickness and split-thickness skin grafting for treating burned limbs. J Burn Care Res. 2010;31(05):822–825. doi: 10.1097/BCR.0b013e3181eed464. [DOI] [PubMed] [Google Scholar]
- 10.Boonjindasup A, Pinsky M, Stewart C.Management of adult concealed penis using a meshed, split-thickness skin graft Can Urol Assoc J 201610(11-12):E407–E411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sinha S, Schreiner A J, Biernaskie J, Nickerson D, Gabriel V A. Treating pain on skin graft donor sites: Review and clinical recommendations. J Trauma Acute Care Surg. 2017;83(05):954–964. doi: 10.1097/TA.0000000000001615. [DOI] [PubMed] [Google Scholar]
- 12.Lairet K F, Baer D, Leas M L, Renz E M, Cancio L C. Evaluation of an oxygen-diffusion dressing for accelerated healing of donor-site wounds. J Burn Care Res. 2014;35(03):214–218. doi: 10.1097/BCR.0b013e31829b3338. [DOI] [PubMed] [Google Scholar]
- 13.Sagray B A, Lalani S, Mehan V. An alternative coverage for split thickness skin graft donor site wounds. J Foot Ankle Surg. 2011;50(03):369–371. doi: 10.1053/j.jfas.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Marx R E. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10(04):225–228. doi: 10.1097/00008505-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 15.de Leon J M, Driver V R, Fylling C P. The clinical relevance of treating chronic wounds with an enhanced near-physiological concentration of platelet-rich plasma gel. Adv Skin Wound Care. 2011;24(08):357–368. doi: 10.1097/01.ASW.0000403249.85131.6f. [DOI] [PubMed] [Google Scholar]
- 16.Rappl L M. Effect of platelet rich plasma gel in a physiologically relevant platelet concentration on wounds in persons with spinal cord injury. Int Wound J. 2011;8(02):187–195. doi: 10.1111/j.1742-481X.2011.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sommeling C E, Heyneman A, Hoeksema H, Verbelen J, Stillaert F B, Monstrey S. The use of platelet-rich plasma in plastic surgery: a systematic review. J Plast Reconstr Aesthet Surg. 2013;66(03):301–311. doi: 10.1016/j.bjps.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 18.Ghoraba S, Mahmoud W, Hammad S M, Ayad H. Clinical safety and efficacy of platelet-rich plasma in wound healing. Int J Clin Med. 2016;7:801–808. [Google Scholar]
- 19.Marukawa E, Oshina H, Iino G, Morita K, Omura K. Reduction of bone resorption by the application of platelet-rich plasma (PRP) in bone grafting of the alveolar cleft. J Craniomaxillofac Surg. 2011;39(04):278–283. doi: 10.1016/j.jcms.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 20.Pietrzak W S, Eppley B L. Platelet rich plasma: biology and new technology. J Craniofac Surg. 2005;16(06):1043–1054. doi: 10.1097/01.scs.0000186454.07097.bf. [DOI] [PubMed] [Google Scholar]
- 21.Fang Z, Yang X, Wu G. The use of autologous platelet-rich plasma gel increases wound healing and reduces scar development in split-thickness skin graft donor sites. J Plast Surg Hand Surg. 2019;53(06):356–360. doi: 10.1080/2000656X.2019.1635489. [DOI] [PubMed] [Google Scholar]
- 22.Englert S J, Estep T H, Ellis-Stoll C C. Postoperative surgical chest and leg incision sites using platelet gel: a retrospective study. J Extra Corpor Technol. 2008;40(04):225–228. [PMC free article] [PubMed] [Google Scholar]
- 23.Gardner M J, Demetrakopoulos D, Klepchick P R, Mooar P A. The efficacy of autologous platelet gel in pain control and blood loss in total knee arthroplasty. An analysis of the haemoglobin, narcotic requirement and range of motion. Int Orthop. 2007;31(03):309–313. doi: 10.1007/s00264-006-0174-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


