Abstract
Background
Thymic stromal lymphopoietin (TSLP) has been shown to be able to amplify Tregs. Thus, TSLP induction has the potential to induce endogenous Tregs and control autoimmunity. In the previous research, we found that a new compound named 02F04 can induce TSLP production while simultaneously activating the liver X receptor (LXR). Because LXR activation leads to a decrease in Treg, we attempted to find a 02F04-derivative, druggable lead compound with a basic skeleton that induces TSLP production without activating LXR. As the results, we found HA-7 and HA-19 and, in this study, examined the molecular mechanisms in TSLP production.
Methods
A murine keratinocyte cell line PAM 212 was stimulated with HA-7 and HA-19, and then the expressions of cytokines were examined via ELISA and real-time fluorescence quantitative PCR.
Results
HA-7 and HA-19 induced TSLP production but almost not the expression of TNF-α, IL-13, IL-25, and IL-33 in PAM212 cells. These compounds inhibited LXR activities. The TSLP expression induced by HA-7 and HA-19 was inhibited by the Gq/11 inhibitor YM-254890, ROCK inhibitor Y-27632, and ERK inhibitor U0126. HA-7 and HA-19 also induced the formation of stress fiber and ERK phosphorylation, which were inhibited by YM-254890 and Y-27632.
Conclusions
Our findings indicated that HA-7 and HA-19 selectively induced TSLP production in PAM212 via Gq/11, Rho/ROCK and ERK pathways. Our findings also indicated that TSLP expression was differentially regulated from other cytokines, and the selective expression could be induced with low-molecular-weight compounds such as HA-7 and HA-19.
1. Introduction
Thymus stromal lymphopoietin (TSLP) is a pleiotropic cytokine first recognized as a master cytokine in allergies [1]. Recently, TSLP has been considered to play an important role in immune stability and the regulation of mucosal immune barriers [2,3]. TSLP is mainly produced by barrier epithelial cells [4] and is accompanied by the production of pro-allergic cytokines, such as IL-13 and IL-33 [5,6]. In addition, TSLP can induce the maturation of dendritic cells (DC) and increase the production of IL-23, leading to IL-23-driven autoimmune diseases [7]. Thus, the involvement of TSLP has been suggested in autoimmune diseases, such as psoriasis and rheumatoid arthritis [2,8,9]. In contrast, recent studies have shown that TSLP can induce the conversion of thymic Foxp3− CD4+ T cells to Foxp3+ regulatory T cells (Tregs) in a DC-independent manner [10]. Additionally, TSLP produced in the intestine induces the expansion of Tregs, allowing for the control of Th17 responses in colitis [11]. Furthermore, several studies have indicated that cancer cells produce TSLP, which correlates with the prevalence of Tregs [12]. Overall, TSLP is a multi-functional regulator of immune responses.
Autoimmune diseases are intractable and difficult to completely cure with drug therapy. To date, treatments for these diseases include anti-inflammatory steroids and immunosuppressive drugs. Recently, biopharmaceuticals, such as antibodies, have shown marked efficacy; however, their severe side effects are inevitable. In this regard, the use of Tregs or Treg inducers have been suggested as a new approach. TSLP is the first candidate Treg inducer. Recombinant TSLP attenuates autoimmune diseases in animal models [13]. In addition, vitamin D derivatives suppress autoimmune diseases in mice [14]. Therefore, TSLP induction can potentially induce endogenous Tregs and control autoimmune responses.
TSLP production is induced by environmental chemicals, apart from toll-like receptor stimulants. Previously, we reported that xylene and nonanoic acid induce TSLP production in mice, while valeric acid induces TSLP production in keratinocytes in vitro. Valeric acid-induced TSLP production is mediated by Gq/11, indicating that the chemical induces TSLP via a specific receptor. Therefore, using a chemical library, we attempted to identify druggable compounds that induce TSLP.
In our previous study, we have identified a novel TSLP inducer, 02F04, through phenotypic screening. We used a chemical library, which includes approximately 2200 compounds [15]. However, 02F04 is a natural product; thus, its structure is difficult to optimize for increased activity. In addition, 02F04 can induce the activation of the liver X receptor (LXR) [16], increasing the expression of ATP-binding transporter A1 (ABCA1), a target gene of LXR [17]. LXR agonists can reduce the transcriptional upregulation of several inflammatory genes, such as tumor necrosis factor (TNF), cyclooxygenase 2, inducible nitric oxide synthase, and matrix metalloproteinase 9 [18,19]. LXR activation reportedly leads to a decrease in Tregs [20]. Therefore, a more suitable lead compound for Treg inducers is a low-molecular-weight compound that selectively induces TSLP production without activating LXR. In this study, we attempted to find a 02F04-derivative, druggable lead compound with a basic skeleton that induces TSLP production without activating LXR.
2. Materials and methods
2.1. Chemicals
02F04 (Fig. 1A) was purchased from InterBioScreen Ltd. (Cat. No. STOCK1N-55172; Moscow, Russia). We synthesized the 02F04 derivatives, including HA-7 (Fig. 1B) and HA-19 (Fig. 1C). T0901317 was purchased from the Cayman Chemical Company (Ann Arbor, MI, USA). U0126 and Y-27632 were purchased from Promega Corporation (Madison, WI, USA), and Nacalai Tesque Inc. (Kyoto, Japan), respectively. YM-254890 was given by Astellas Pharma Inc. (Tokyo, Japan) and Taiho Pharmaceutical Co. Ltd. (Tokyo, Japan).
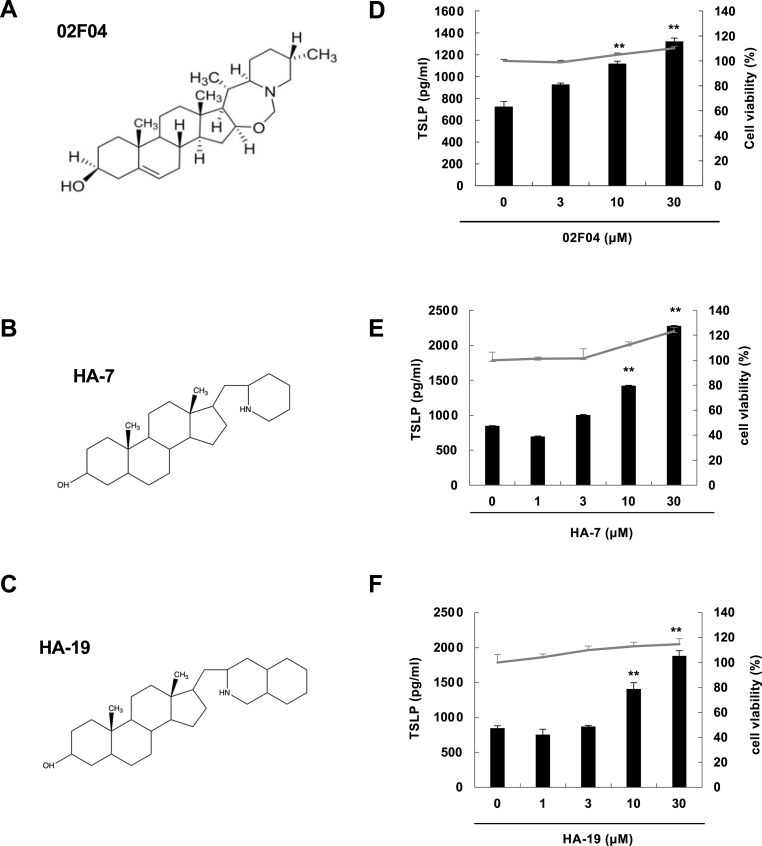
Fig. 1.
The chemical structures of 02F04, HA-7 and HA-19 and their inducing effects on TSLP protein in PAM212 cells.
The chemical structures of 02F04 (A), HA-7 (B), and HA-19 (C). PAM212 cells were stimulated with 02F04 (D), HA-7 (E), or HA-19 (F) at the indicated concentrations for 24 h. TSLP levels in the culture supernatants were determined by ELISA, and the cytotoxicity test was performed through an MTT assay. Data are presented as means ± SEMs (n = 4). **p < 0.01 and ***p < 0.001 vs. the corresponding control.
2.2. Cell culture
The murine keratinocyte cell line PAM212 was provided by Dr. Yuspa (National Institutes of Health, Bethesda, MD, USA). The cells were cultured in alpha minimal essential medium supplemented with 10% heat-inactivated fetal bovine serum, 15 μg/mL penicillin G potassium, and 50 μg/mL streptomycin (Meiji Seika Pharma Co., Ltd., Tokyo, Japan) in a humidified atmosphere of 5% CO2 and 95% air at 37 °C.
2.3. Cell stimulation
PAM212 cells were seeded at a density of 1 × 105 cells/ml in 48-well plates for the analysis of TSLP protein levels in the culture supernatant, at 0.8 × 105 cells/ml in 24-well plates for RNA extraction and Western blot sample preparation, and at 0.25 × 105 cells/ml in a chamber slide (Thermo Fisher Scientific Inc., Waltham, MA, USA) for immunostaining analysis. After a 24-h cell culture, HA-7 or HA-19 was added at the indicated concentration for stimulation, along with inhibitors. These were dissolved in dimethyl sulfoxide (DMSO); the final concentration of DMSO was adjusted to 0.1%.
2.4. Cell viability
Cell viability was evaluated through a thiazolyl blue tetrazolium bromide (MTT) assay, as previously described [21]. The supernatant was removed from the well plate to be tested, and a medium containing 0.5 mg/ml MTT was added to each well. After a 2-h incubation, the medium containing MTT was removed, and the intracellular formazan crystals were dissolved in DMSO. The absorbance at 570 nm was measured using the iMark™ Microplate Absorbance Reader (Bio Rad Laboratories, Hercules, CA, USA). The treatment was considered non-cytotoxic at a cell viability of >80%.
2.5. Measurement of cytokine production
The PAM212 cells were stimulated with HA-7 or HA-19, with or without inhibitors. After 24 h, the culture supernatants were recovered. TSLP levels in the supernatants were determined using ELISA kits purchased from DuoSet® ELISA Development Systems (R&D Systems Inc., Minneapolis, MN, USA).
2.6. RNA extraction and real-time PCR
The cells were washed with PBS, and total RNA was extracted using RNAiso Plus (Takara Bio Inc., Shiga, Japan). The total RNA was reverse transcribed using PrimeScript™ RT Master Mix (Takara Bio Inc., Shiga, Japan) to synthesize cDNA samples. The obtained cDNA samples were amplified using the CFX Manager System (Bio Rad Laboratories, Hercules, CA, USA) with SYBR FAST qPCR Master Mix (2X). The level of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA was used to normalize the level of the extracted mRNA, and the fold change was calculated using the ΔΔCt method. The following primers were used: mouse GAPDH, 5′-TGTGTCCGTCGTGGATCTGA-3’ (forward) and 5′-TTGCTGTTGAAGTCGCAGGAG-3’ (reverse); mouse TSLP, 5′-GAGATTTGAAAGGGGCTAAGTT-3’ (forward) and 5′-GCCATTTCCTGAGTACCGTC-3’ (reverse); mouse TNF-α, 5′-CCTCCCTCTCAGTTCTA -3′(forward) and 5′-ACTTGGTGGTTTGCTACGAC-3′(reverse); mouse IL-13, 5′-GCTTATTGAGGAGCTGAGCAACA-3’ (forward) and 5′-GGCAGGTCCACTCCATA-3’ (reverse); mouse IL-25, 5′-ATGTACCAGGCTGTTGCATTCTTG-3’ (forward) and 5′-CTAAGCCATGACCCGGGGCC-3’ (reverse); mouse IL-33, 5′-GATGGGAAGAAGGTGATGGGTG-3’ (forward) and 5′-TTGTGAAGGACGAAGAAGGC-3’ (reverse); and mouse ABCA1, 5′-GCAGATCAAGCATCCCAACT-3’ (forward) and 5′-CCAGAGAATGTTTCATTGTCCA-3’ (reverse).
2.7. Western blotting
The cells were washed with PBS, which was later combined with the prepared protein lysate (20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid [pH: 7.4], 1% (v/v) Triton X-100, 10% (v/v) glycerol, 50 mM sodium fluoride, 2.5 mM p-nitrophenyl phosphate, 10 μg/mL phenylmethylsulfonyl fluoride, 1 mM Na3VO4, 10 μg/mL leupeptin, and 1 mM ethylenediaminetetraacetic acid). The denatured cell lysate was subjected to 10% (w/v) sodium dodecyl sulfate–polyacrylamide gel electrophoresis and then transferred to a nitrocellulose membrane. After being blocked with 4% (w/v) Block Ace (DS Pharma Biomedical Co., Ltd., Osaka, Japan), the membrane was incubated with the following primary antibodies: phospho-p44/42 mitogen-activated protein kinase (MAPK) (extracellular signal-regulated kinase [ERK]1/2) (Thr202/Tyr204) rabbit monoclonal antibodies (mAb) (1:1000, #9102; Cell Signaling Technology Inc., Danvers, MA, USA) or phospho-p44/43 MAPK (ERK1/2) rabbit mAb (1:1000, #9102; Cell Signaling Technology Inc., Danvers, MA, USA) and anti-rabbit IgG (1:2000,#ZA0324; Vector Laboratories, Inc., USA.), an HPR-linked antibody, as the secondary antibody (GE Healthcare Life Sciences, Buckinghamshire, England). The targeted protein was detected using ECL™ Western Blotting Detection Reagents (PerkinElmer, Waltham, MA, USA) and ChemiDoc™ Imaging system to analysis (Bio-Rad Laboratories, Inc., Japan).
2.8. F-actin staining
The cells were stimulated with HA-7 or HA-19, along with YM-254890 or Y-27632, for 24 h. Subsequently, these were fixed with 4% paraformaldehyde for 10 min at room temperature and permeabilized with 0.1% Triton X-100 at room temperature for 5 min. F-actin and nuclei were stained with rhodamine phalloidin (Cytoskeleton. Inc., Denver, CO, USA) for 1 h and 4′,6-diamidino-2-phenylindole (DAPI; Dojindo Laboratories, Kumamoto, Japan) for 15 min. Immunofluorescence images were obtained using a confocal laser scanning microscope (TCS SP8; Leica Microsystems, Wetzlar, Germany).
2.9. Statistical analysis
All data are presented as means ± standard errors of the means (SEMs). The two sets of data used a two-tailed distribution, and a two-sample heteroscedasticity hypothesis test was conducted using Student's t-test. The significance of multiple samples was analyzed using Dunnett's post-hoc test.
3. Results
3.1. HA-7 and HA-19 induced the production of TSLP
We synthesized approximately 60 steroid alkaloids from 02F04 and determined their TSLP-producing activities. We identified HA-7 (Fig. 1B and HA-19 (Fig. 1C) as the active compounds. Incubating PAM212 cells with HA-7 or HA-19 for 24 h (Fig. 1 E and F), as well as 02F04 (Fig. 1D), increased TSLP protein levels in the medium in a concentration-dependent manner. Non-cytotoxic TSLP production was observed at concentrations >10 μM after stimulation with HA-7 and HA-19 (Fig. 1 E and F). Viability which determined by MTT method was rather increased.
3.2. HA-7 and HA-19 induced a slow and sustained production of TSLP
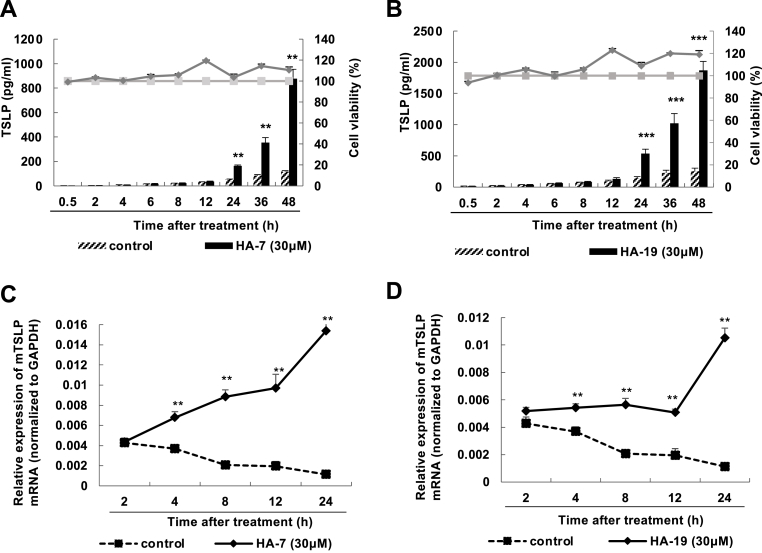
Production of TSLP induced by HA-7 and HA-19 was observed at 24 h and increased until at least 48 h after stimulation (Fig. 2 A and B). Increase in mRNA was observed at 4 h and increased until 24 h after stimulation (Fig. 2C and D). These indicated that the compounds, as well as 02F04 [16], induced a slow and sustained production of TSLP.
Fig. 2.
Time changes in HA-7 and HA-19-induced TSLP production.
PAM212 cells were stimulated with HA-7 (A and C) and HA-19 (B and D) at a concentration of 30 μM and at specified times. TSLP levels in the culture supernatants were determined by ELISA, and the cytotoxicity test was performed through an MTT assay (A and B). The mRNA levels of TSLP was determined by qRT-PCR (C and D). Data are presented as means ± SEMs (n = 4). **p < 0.01 and ***p < 0.001 vs. the corresponding control.
3.3. Selective expression of TSLP
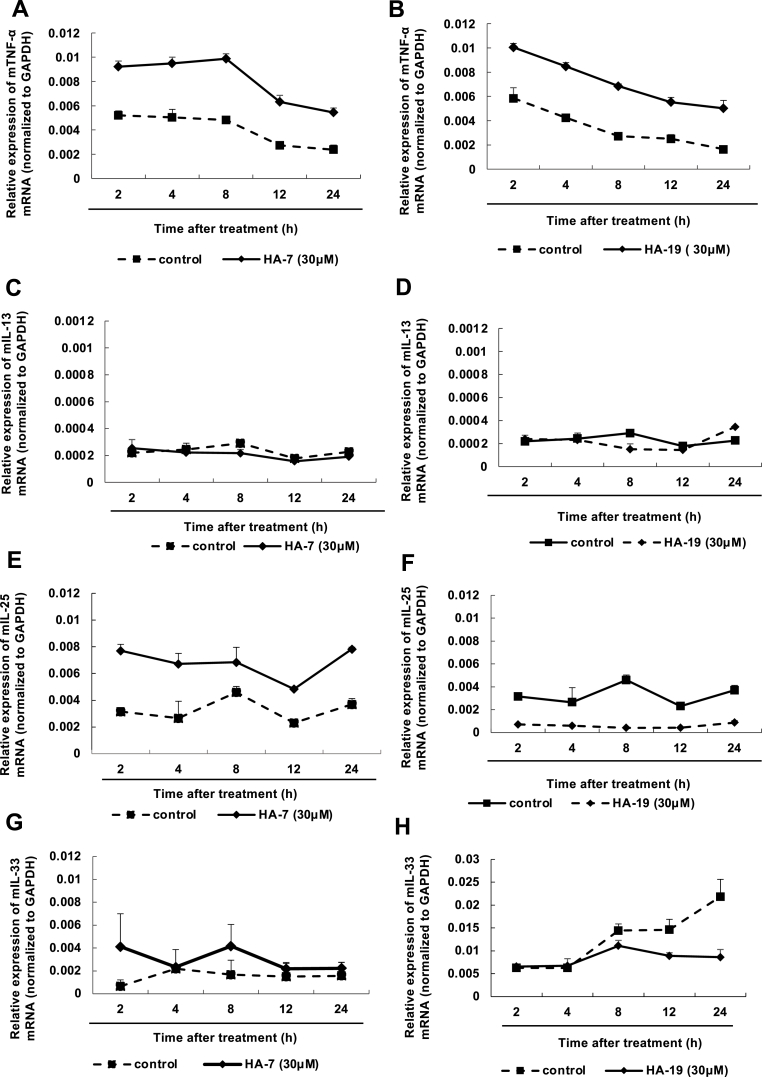
We checked whether PAM212 cells stimulated with HA-7 or HA-19 induced the expression of other cytokines and proteins. The cells were incubated with HA-7 or HA-19 for 2–24 h, and the mRNA expressions of TNF-α, IL-13, IL-25, and IL-33 were determined (Fig. 3). The expression of TNF- α slightly increased from 2 to 24 h without any peak, indicating that HA-7 and HA-19 affected basal expression (Fig. 3 A and B). In addition, neither compound significantly induced the expression of IL-13, IL-25, and IL-33 mRNA (Fig. 3C–H) and only slightly increased in the protein levels of TNF-α, IL-25 and IL-33 in the medium at 48 h (Supplementary Table 1). These finding suggested that HA-7 and HA-19 selectively induced TSLP in PAM212 cells.
Fig. 3.
Effect of HA-7 and HA-19-induced cytokine expression in PAM212 cells.
PAM212 cells were stimulated with HA-7 (A, C, E, and G) or HA-19 (B, D, F, and H) at a concentration of 30 μM and at specified times. The mRNA levels of each cytokine were determined by qRT-PCR. Data are presented as means ± SEMs (n = 4).
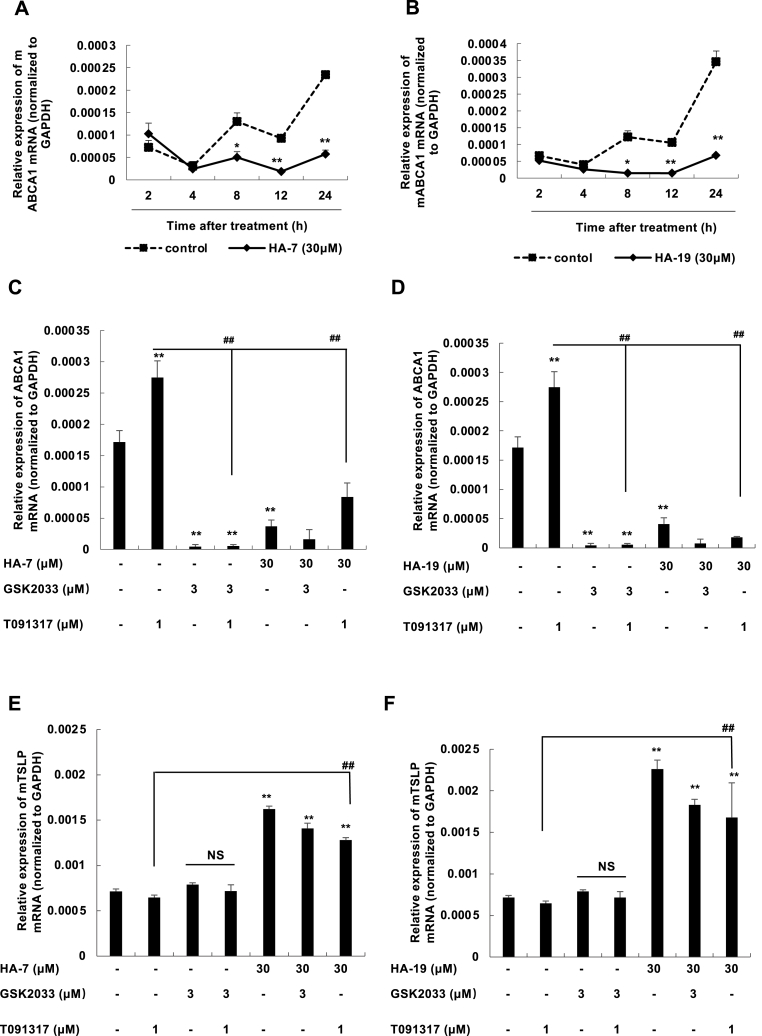
3.4. HA-7 and HA-19 did not induce but inhibited ABCA1 expression in relation to LXR
In a previous study, we have reported that 02F04 has an agonistic effect on LXR [16]. Therefore, we examined ABCA1 expression to determine whether HA-7 and HA-19 influenced LXR activity. HA-7 and HA-19 did not increase but decreased the basal expression of ABCA1 mRNA (Fig. 4 A and B). The compounds completely inhibited ABCA1 expression induced by the LXR agonists T091317 and GSK2033 (Fig. 4C and D). These results indicated that HA-7 and HA-19 had inhibitory effects on LXR. More importantly, since the LXR inhibitor GSK2033 failed to induce TSLP expression at the mRNA level (Fig. 4 E and F), its activity to inhibit LXR was not related to activities that induce TSLP production.
Fig. 4.
HA-7 and HA-19 inhibit the expression of ABCA1 in LXR.
PAM212 cells were stimulated with HA-7 (A) or HA-19 (B) at a concentration of 30 μM and at specified times. The mRNA levels of ABCA1 were determined by qRT-PCR (A and B). The cells were treated with GSK2033 at 3 μM and T091317 at 1 μM with or without HA-7 (C and E) or HA-19 (D and F) at 30 μM for 24 h. The mRNA expressions of ABCA1 (C and D) and TSLP (E and F) were determined by qRT-PCR. Data are presented as means ± SEMs (n = 4). **p < 0.01 vs. the non-stimulated group; ##p < 0.01 vs. T0901317 alone.
3.5. Activation and involvement of ERK in HA-7 and HA-19-induced TSLP expression
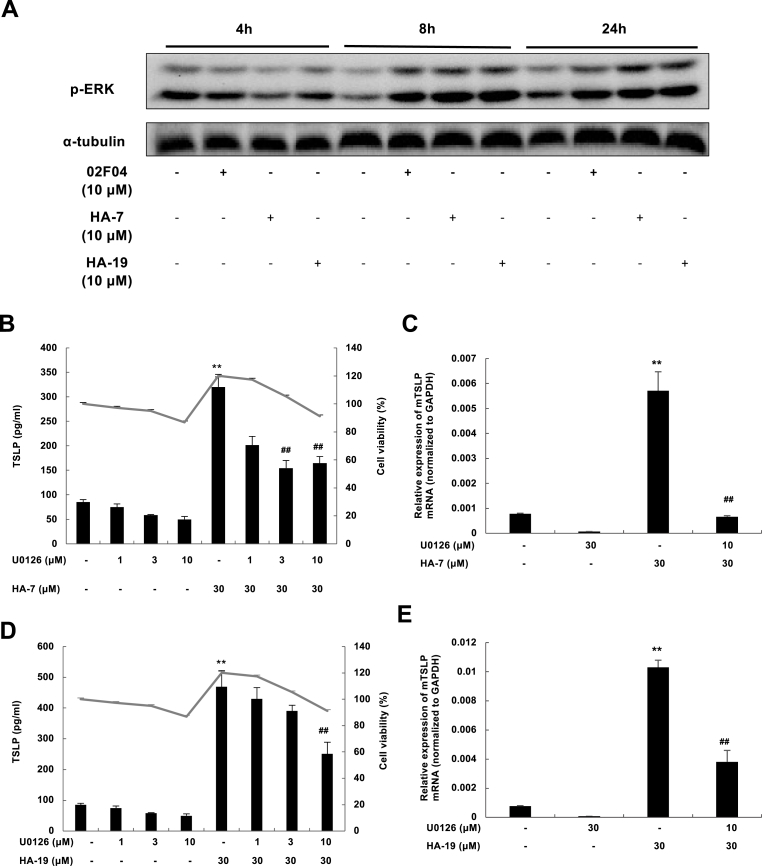
Stimulation with HA-7 and HA-19, as well as 02F04, induced an increase in phospho-ERK after 8–24 h (Fig. 5A). The ERK inhibitor U0126 inhibited HA-7- and HA-19-induced TSLP production (Fig. 5B and D) and increase in mRNA levels (Fig. 5C and E).
Fig. 5.
HA-7 and HA-19 induced ERK phosphorylation and the effect of U0126 on TSLP expression.
PAM212 cells were stimulated with 02F04, HA-7 and HA-19 at 10 μM for 4, 8, and 24 h (A). ERK1/2 phosphorylation was measured by western blotting. (B-E) PAM212 cells were stimulated with HA-7 (B and C) or HA-19 (D and E) at 30 μM for 24 h with or without U0126 at the indicated concentrations. TSLP proteins in the culture supernatant and the cytotoxicity (B and D) were determined by ELISA and an MTT assay, respectively. The mRNA levels of TSLP (C and E) were determined by qRT-PCR. Data are presented as means ± SEMs (n = 4). *p < 0.05 and **p < 0.01 vs. the non-stimulated group; ##p < 0.01 vs. HA-7 or HA-19 alone.
3.6. The Gq/11 and Rho-associated kinase (ROCK) signaling pathways participate in TSLP induction by HA-7 and HA-19
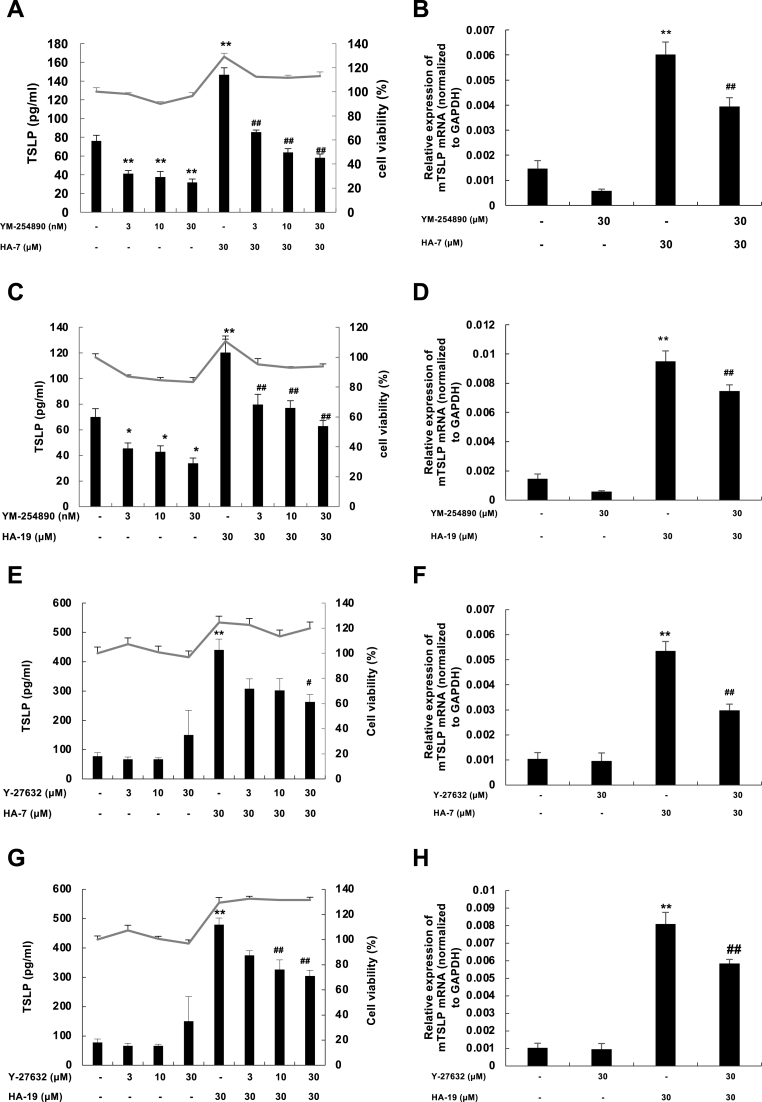
We used the Gq/11 inhibitor YM-254890 and the ROCK inhibitor Y-27632 to further confirm whether the induction mechanisms of HA-7 and HA-29 were the same as that of 02F04. The inhibitors suppressed 02F04-induced TSLP production [15,16]. Specifically, YM-254890 significantly inhibited HA-7 (Fig. 6A) and HA-19-induced (Fig. 6C) TSLP production in a concentration-dependent and non-cytotoxic manner. This inhibitor also suppressed the expression of TSLP mRNA (Fig. 6 B and D). Meanwhile, Y-27632 inhibited HA-7- and HA-19-induced production of TSLP protein (Fig. 6 E and G) and mRNA (Fig. 6 F and H). These findings indicated the involvement of the Gq/11 and ROCK signaling pathways in the TSLP induction by HA-7 and HA-19.
Fig. 6.
Effect of YM-254890 and Y-27632 on HA-7- and HA-19-induced TSLP expression.
PAM212 cells were stimulated with HA-7 (A, B, E, and F) or HA-19 (C, D, G, and H) at 30 μM for 24 h with or without YM-254890 (A–D) or Y-27632 (E–H) at the indicated concentrations. TSLP proteins in the culture supernatant (A, C, E, and G) were determined by ELISA, and the cytotoxicity test was performed using an MTT assay. The mRNA levels of TSLP (B, D, F, and H) were determined by qRT-PCR. Data are present as means ± SEMs (n = 4). *p < 0.05 and **p < 0.01 vs. the non-stimulated group; ##p < 0.01 vs. HA-7 or HA-19 alone.
3.7. HA-7 and HA-19 activate the ROCK signaling pathway via Gq/11
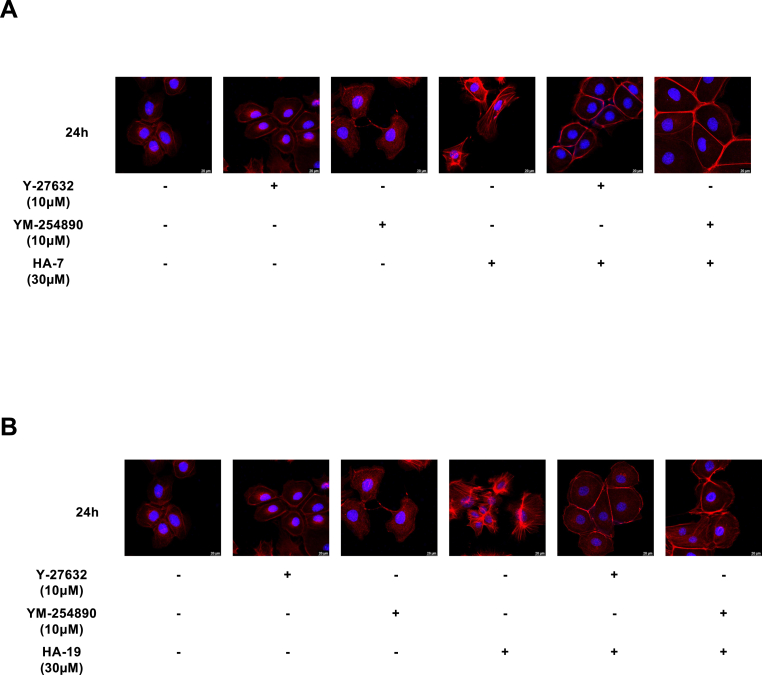
Stress fiber formation is an index of Rho/ROCK activation. Therefore, we immunostained stress fibers to confirm whether HA-7 and HA-19 activated the ROCK signaling pathway and whether Gq/11 was involved in this. HA-7 and HA-19 inhibited stress fiber formation 24 h after stimulation; in turn, Y-27632 significantly inhibited the compounds, indicating that HA-7 and HA-19 activated the Rho-ROCK pathway (Fig. 7). Likewise, YM-254890 inhibited the stress fiber formation; as such, Gq/11 acted upstream of Rho-ROCK signaling. These results suggested that HA-7 and HA-19 induce TSLP production through the Gq/11-ROCK-ERK signaling pathway in PAM212 cells.
Fig. 7.
Effect of YM-254890 and Y-27632 on HA-7- or HA-19-induced polymerization of actin fibers.
PAM212 cells were stimulated with HA-7 (A) or HA-19 (B) at 30 μM for 24 h with or without YM-254890 at 10 μM or Y27632 at 10 μM. F-actin and nuclei were stained with rhodamine phalloidin and DAPI, respectively. Immunofluorescence images were obtained using a confocal laser scanning microscope.
3.8. HA-7 and HA-19 activate ERK via Gq/11 and the ROCK signaling pathway
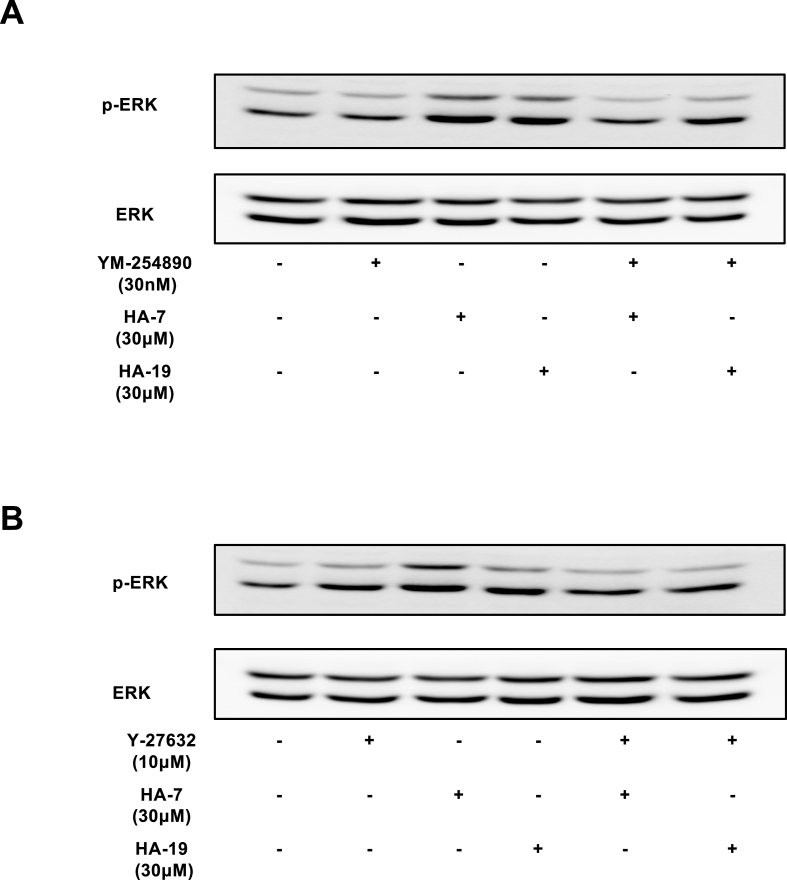
We examined the effects of YM-254890 and Y-27632 on HA-7- and HA-19-induced phosphorylation of ERK to confirm the interaction between Gq/11, ROCK, and ERK. YM-254890 and Y-27632 significantly inhibited HA-7 and HA-19-induced phosphorylation of ERK (Fig. 8 A and B). These findings indicated that both intracellular signals, Gq/11, and ROCK, acted upstream of ERK activation.
Fig. 8.
Effect of YM-254890 and Y-27632 on HA-7- or HA-19-induced ERK phosphorylation.
PAM212 cells were stimulated with HA-7 or HA-19 at 30 μM for 24 h with or without YM-254890 at 10 μM (A) or Y27632 at 10 μM (B). ERK1/2 phosphorylation was measured by western blotting.
4. Discussion
Tregs are key regulators of inflammation [22] and are critical for peripheral immune tolerance and the prevention of autoimmunity and tissue damage, making Treg-centered immunotherapy a current research hotspot [23,24]. Recent studies have found that TSLP is a potent inducer of Tregs; therefore, TSLP can be applied to treat autoimmune diseases [10]. With this idea, using low-molecular-weight compounds that selectively induce TSLP production in vivo may be a novel approach to induce Tregs and attenuate autoimmune diseases. PAM212 cells, a mouse stratum corneum cell, are widely used to investigate the mechanism of inflammation [25,26] including TSLP production [16]. Since PAM212 cells were sensitive to TSLP production, we used the cell line for this study.
In a previous study, we have reported that the steroid alkaloid 02F04 induces TSLP production in PAM212 cells; however, 02F04 has a complex structure comprising six condensations rings structures and had LXR activities [16]. Therefore, we searched for the lead compound of TSLP inducers, which had a simpler structure. This way, various derivatives can be easily synthesized without LXR agonist activity. HA-7 and HA-19 were identified by following this standard. In this study, we showed that HA-7 and HA-19, as well as 02F04 [15]and valeric acid [27], can selectively induce TSLP production in PAM212 cells via the Gq/11-ROCK-ERK pathway.
TSLP was induced in PAM212 cells by the stimulation with TNF-α for hours [28]. HA-7 and HA-19 slowly but continuously induced TSLP expression for 48 h. In contrast, the compounds only a little induced epithelial cell-derived cytokines, specifically IL-25 and IL-33, and TNF-α, probably via the activation of ERK. Time changes in the expressions of these cytokines evidently differ from those of TSLP, indicating that HA-7 and HA-19 have unique activities that selectively but continuously induce TSLP expression. Viability of PAM 212 cells which was determined by MTT method was slightly increased by the treatment with HA-7 and HA-19 for 24 and 48 h. It might be resulted from the continuous activation of ERK. Further examination was needed to clarify HA-7 and HA-19 have a proliferative activity.
We also observed time changes in the expression of TSLP in PAM212 cells following stimulation with 02F04 and valeric acid. Several environmental chemicals, such as volatile organic solvents (e.g., xylene and trimethyl xylene), also induce TSLP in vivo [[29], [30], [31]], indicating that keratinocytes may have a system to detect chemicals. Previously, we analyzed the molecular mechanisms of valeric acid and 02F04 and found that these chemicals induced TSLP via Gq/11-dependent pathways, indicating that specific binding proteins, such as receptors, may mediate the corresponding responses.
While 02F04 stimulated LXR activity, HA-7, and HA-19 inhibited this activity. LXR activation suppresses the expression of inflammatory genes [32], while LXR inhibition hinders the development of triple-negative breast cancer [32]. However, LXR activation leads to a decrease in Tregs [12]. Therefore, HA-7 and HA-19 lacked LXR are better candidates for Treg induction via TSLP production.
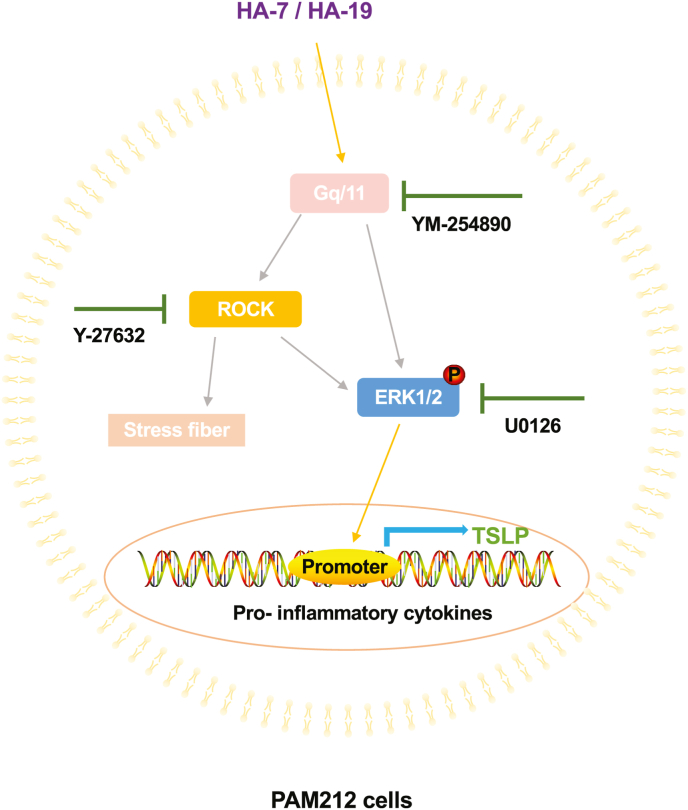
As previously stated, our results indicated that HA-7 and HA-19 may act on selective receptors. As such, we determined the signaling pathways induced by HA-7 and HA-19 to induce TSLP expression. Using the Gq/11 inhibitor YM-254890, the ROCK inhibitor Y-27632, and the ERK inhibitor U0126, we found that Gq/11, ROCK, and ERK mediated HA-7 and HA-19-induced TSLP expression. Most current studies have suggested that TSLP expression is inextricably linked to the activation of ERK signaling [33,34]. Since both YM-254890 and Y-27632 inhibited the phosphorylation of ERK induced by HA-7 and HA-19 and the formation of stress fibers, Gq/11 activation occurred upstream of ROCK signaling, and ERK occurred downstream of ROCK signaling [35, 36]. These findings indicated that HA-7 and HA-19 induced TSLP production via the Gq/11-ROCK-ERK pathway (Fig. 9). Binding proteins, such as the receptors of HA-7 and HA-19 had not yet been identified. We presumed that these receptors were Gq/11-coupled receptors. Currently, we are searching for the receptors by producing biotinylated HA-7.
Fig. 9.
TSLP production is induced by HA7 and HA-19 via the Gq/11-ROCK-ERK pathway.
There is limitation of this study. First, we analyzed the effects of HA-7 and HA-19 by using only mouse keratinocyte cell line PAM212 cells. It needs to examine the effects of human keratinocytes. Second, the activities of HA-7 and HA-19 to induce TSLP were still not enough to induced TSLP production in vivo. The identification of target protein will help to find more effective compounds.
To date, using cytokines is a promising strategy to regulate immune responses. TSLP is a candidate for inducing immunosuppression in autoimmune diseases by inducing Tregs. While many other cytokines are derived from macrophages and lymphocytes that circulate in the blood, TSLP is mainly derived from epithelial cells that are localized on the body surface. Our data suggested that TSLP expression was regulated uniquely compared with other cytokines, and selectively regulating cytokine production is possible with low-molecular-weight compounds. Several peptides and proteins, such as insulin, granulocyte colony-stimulating factor, and erythropoietin, have been used for therapy. Recently, hypoxia-inducible factor-prolyl hydroxylase inhibitors, such as enarodustat, have been developed as low-molecular-weight compounds expected to induce erythropoietin production. These are the first druggable, low-molecular-weight compounds to selectively induce immunological cytokines. More active compounds are expected to be developed using simpler structures.
Credit author statement
Yu Wang: Investigation, Writing - Original Draft, Ryosuke Segawa: Methodology, Validation, Investigation, Yan Weng: Methodology, Katsuya Nakai: Resources, Keiichiro Ohashi: Resources, Masahiro Hiratsuka: Writing - Review & Editing, Mieko Arisawa: Resources, Noriyasu Hirasawa: Conceptualization, Writing - Review & Editing.
Deckaration of interest statement
No conflict of interest exits in the submission of this manuscript.
Acknowledgements
This research was [partially] supported by Platform Project for Supporting Drug Discovery and Life Science Research (Basis for Supporting Innovative Drug Discovery and Life Science Research (BINDS)) from AMED, Japan, under Grant Number JP19am0101100.
Handling Editor: Y Renaudineau
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtauto.2022.100186.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
Data availability
Data will be made available on request.
References
- 1.Liu Y.J. Thymic stromal lymphopoietin: master switch for allergic inflammation. J. Exp. Med. Feb. 2006;203(2):269–273. doi: 10.1084/JEM.20051745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Varricchi G., et al. Thymic stromal lymphopoietin isoforms, inflammatory disorders, and cancer. Front. Immunol. 2018;9 doi: 10.3389/FIMMU.2018.01595. JUL, Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ziegler S.F., Roan F., Bell B.D., Stoklasek T.A., Kitajima M., Han H. Vol. 66. Academic Press Inc.; 2013. The biology of thymic stromal lymphopoietin (TSLP) pp. 129–155. (Adv. Pharmacol.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roan F., Obata-Ninomiya K., Ziegler S.F. Epithelial cell–derived cytokines: more than just signaling the alarm. J. Clin. Invest. 2019;1(4):1441–1451, Apr. doi: 10.1172/JCI124606. 129American Society for Clinical Investigation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang H.E., Reinhardt R.L., Bando J.K., Sullivan B.M., Ho I.C., Locksley R.M. Divergent expression patterns of IL-4 and IL-13 define unique functions in allergic immunity. Nat. Immunol. 2011;13(1):58–66. doi: 10.1038/ni.2182. Dec. 12011 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toki S., et al. TSLP and IL-33 reciprocally promote each other's lung protein expression and ILC2 receptor expression to enhance innate type-2 airway inflammation. Allergy. 2020;75(7):1606–1617, Jul. doi: 10.1111/ALL.14196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Volpe E., et al. Thymic stromal lymphopoietin links keratinocytes and dendritic cell-derived IL-23 in patients with psoriasis. J. Allergy Clin. Immunol. 2014;134(2) doi: 10.1016/J.JACI.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 8.Boissier M.C. Cell and cytokine imbalances in rheumatoid synovitis. Joint Bone Spine. May 2011;78(3):230–234. doi: 10.1016/J.JBSPIN.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 9.El-Ghareeb M.I., Helmy A., al Kazzaz S., Samir H. Serum TSLP is a potential biomarker of psoriasis vulgaris activity. Psoriasis Targets Ther. 2019;9:59–63. doi: 10.2147/PTT.S212774. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leichner T.M., et al. Skin-derived TSLP systemically expands regulatory T cells. J. Autoimmun. May 2017;79:39. doi: 10.1016/J.JAUT.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mosconi I., et al. Intestinal bacteria induce TSLP to promote mutualistic T-cell responses. Mucosal Immunol. 2013;6(6):1157–1167, Nov. doi: 10.1038/MI.2013.12. [DOI] [PubMed] [Google Scholar]
- 12.Poschke I., Mougiakakos D., Kiessling R. Camouflage and sabotage: tumor escape from the immune system. Cancer Immunol. Immunother. 2011;60(8):1161. doi: 10.1007/S00262-011-1012-8. 1171, Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Besin G., Gaudreau S., Menard M., Guindi C., Dupuis G., Amrani A. Thymic stromal lymphopoietin and thymic stromal lymphopoietin-conditioned dendritic cells induce regulatory T-cell differentiation and protection of NOD mice against diabetes. Diabetes. 2008;57(8):2107. doi: 10.2337/db08-0171. 2117, Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moosbrugger-Martinz V., Schmuth M., Dubrac S. A mouse model for atopic dermatitis using topical application of vitamin D3 or of its analog MC903. Methods Mol. Biol. 2017;1559:91–106. doi: 10.1007/978-1-4939-6786-5_8. [DOI] [PubMed] [Google Scholar]
- 15.Weng Y., et al. A steroid alkaloid derivative 02F04 upregulates thymic stromal lymphopoietin expression slowly and continuously through a novel Gq/11-ROCK-ERK1/2 signaling pathway in mouse keratinocytes. Cell. Signal. May 2019;57:58–64. doi: 10.1016/J.CELLSIG.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Weng Y., et al. Induction of thymic stromal lymphopoietin by a steroid alkaloid derivative in mouse keratinocytes. Int. Immunopharm. Feb. 2018;55:28–37. doi: 10.1016/J.INTIMP.2017.11.045. [DOI] [PubMed] [Google Scholar]
- 17.Sohrabi Y., et al. LXR activation induces a proinflammatory trained innate immunity-phenotype in human monocytes. Front. Immunol. 2020;11 doi: 10.3389/FIMMU.2020.00353/FULL. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghisletti S., et al. Cooperative NCoR/SMRT interactions establish a corepressor-based strategy for integration of inflammatory and anti-inflammatory signaling pathways. Genes Dev. 2009;23(6):681. doi: 10.1101/GAD.1773109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghisletti S., et al. Parallel SUMOylation-dependent pathways mediate gene- and signal-specific transrepression by LXRs and PPARγ. Mol. Cell. 2007;25(1):57. doi: 10.1016/J.MOLCEL.2006.11.022. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carbó J.M., et al. Pharmacologic activation of LXR alters the expression profile of tumor-associated macrophages and the abundance of regulatory T cells in the tumor microenvironment. Cancer Res. 2021;81(4):968–985. doi: 10.1158/0008-5472.CAN-19-3360. Feb. [DOI] [PubMed] [Google Scholar]
- 21.Segawa R., et al. A chalcone derivative suppresses the induction of TSLP in mice and human keratinocytes and attenuates OVA-induced antibody production in mice. Eur. J. Pharmacol. May 2019;851:52–62. doi: 10.1016/J.EJPHAR.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Toomer K.H., Malek T.R. Cytokine signaling in the development and homeostasis of regulatory T cells. Cold Spring Harbor Perspect. Biol. 2018;10(3) doi: 10.1101/CSHPERSPECT.A028597. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whiteside T.L. FOXP3+ Treg as a therapeutic target for promoting anti-tumor immunity. Expert Opin. Ther. Targets. 2018;22(4):353–363. doi: 10.1080/14728222.2018.1451514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mikami N., Kawakami R., Sakaguchi S. New Treg cell-based therapies of autoimmune diseases: towards antigen-specific immune suppression. Curr. Opin. Immunol. Dec. 2020;67:36–41. doi: 10.1016/J.COI.2020.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Shimizu K., Andoh T., Yoshihisa Y., Shimizu T. Histamine released from epidermal keratinocytes plays a role in α-melanocyte-stimulating hormone-induced itching in mice. Am. J. Pathol. 2015;185(11):3003–3010, Nov. doi: 10.1016/J.AJPATH.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 26.Gao Y., et al. Inhibition of phospholipases suppresses progression of psoriasis through modulation of inflammation. Exp. Biol. Med. 2021;246(11) doi: 10.1177/1535370221993424. 1253, Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mizuno N., et al. Pentanoic acid induces thymic stromal lymphopoietin production through Gq/11 and Rho-associated protein kinase signaling pathway in keratinocytes. Int. Immunopharm. Sep. 2017;50:216–223. doi: 10.1016/J.INTIMP.2017.06.024. [DOI] [PubMed] [Google Scholar]
- 28.Segawa R., et al. EGFR transactivation is involved in TNF-α-induced expression of thymic stromal lymphopoietin in human keratinocyte cell line. J. Dermatol. Sci. Mar. 2018;89(3):290–298. doi: 10.1016/J.JDERMSCI.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Satou N., et al. Induction of thymic stromal lymphopoietin production by xylene and exacerbation of picryl chloride-induced allergic inflammation in mice. Int. Arch. Allergy Immunol. Feb. 2012;157(2):194–201. doi: 10.1159/000327545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li F., Dong Y., Ni C., Kan H., Yan S. Fine particulate matter (PM2.5) is a risk factor for dermatitis by promoting the expression of thymic stromal lymphopoietin (TSLP) in keratinocytes. Indian J. Dermatol. Mar. 2020;65(2):92–96. doi: 10.4103/IJD.IJD_520_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamashita S., Segawa R., Satou N., Hiratsuka M., Leonard W.J., Hirasawa N. Induction of thymic stromal lymphopoietin production by nonanoic acid and exacerbation of allergic inflammation in mice. Allergol. Int. 2013;62(4):463–471. doi: 10.2332/ALLERGOLINT.13-OA-0552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carpenter K.J., et al. LXR-inverse agonism stimulates immune-mediated tumor destruction by enhancing CD8 T-cell activity in triple negative breast cancer. Sci. Rep. 2019;9(1) doi: 10.1038/S41598-019-56038-1. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jang Y., et al. UVB induces HIF-1α-dependent TSLP expression via the JNK and ERK pathways. J. Invest. Dermatol. 2013;133(11):2601–2608. doi: 10.1038/JID.2013.203. [DOI] [PubMed] [Google Scholar]
- 34.Feng S., Zhang L., Bian X.H., Luo Y., Qin G.H., Shi R.M. Role of the TSLP-DC-OX40L pathway in asthma pathogenesis and airway inflammation in mice. Biochem. Cell. Biol. 2018;96(3):306–316. doi: 10.1139/BCB-2017-0126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.