Abstract
Prolonged stress beginning in adolescence can contribute to the dysregulation of the neuroendocrine system in adulthood. As the neuroendocrine and neuroimmune systems participate in bi-directional regulatory control, adolescent stress can prime the neuroimmune system to future inflammatory insults. Previous work from our group demonstrates that stress exaggerates the hippocampal response to inflammation, which can lead to deficits in learning and memory. In the current study, we sought to interrogate the interaction between an acute peripheral challenge of lipopolysaccharide (LPS) in male and female Wistar rats with a history of stress beginning in adolescence (CAS). Males from the CAS group were more vulnerable to the peripheral effects of LPS compared to non-stressed males including porphyrin staining and ruffled fur. In contrast, LPS generated similar peripheral effects in females regardless of adolescent stress history. Learning and memory were differentially impacted by LPS as a function of stress history and effects manifested differently when stratified by sex. Males with a history of adolescent stress exhibited deficits in initial learning. Females from the CAS group performed similar to controls during acquisition but exhibited a slight impairment during reversal learning. Males and females with a history of stress displayed memory impairment during the probe assessments as compared to their same-sex control group. We conclude that while stress beginning in adolescence enhanced the vulnerability of learning and memory to an inflammatory challenge, the phenotype of this effect manifested differently in males and females. These data demonstrate a sustained impact of adolescent stress on the neuroimmune system which is sufficient to influence cognitive performance in both sexes.
Keywords: stress, inflammation, cognition, sex
Chronic stress exposure and exposure to stress over the lifecourse can lead to sustained behavioral effects on neural function including deficits in learning and memory. Increased circulation of glucocorticoids, the primary messenger of the hypothalamic-pituitiary-adrenal (HPA) axis component of the stress response, leads to memory performance impairment in humans (Lupien et al., 2005). In addition, preclinical studies show sex differences in learning and memory tasks following stress. For example, unpredictable intermittent restraint stress triggers deficits in spatial memory in male rats, but not female rats (Peay et al., 2020). We have previously demonstrated that following a mixed modality chronic adolescent stress (CAS) paradigm, female rats exhibit an improved performance compared to non-stress controls during a learning task in adulthood, but females display slight impairment in cognitive flexibility during reversal learning (Hyer et al., 2021). This is consistent with other work demonstrating adolescent stress leads to cognitive rigidity in performance strategy alteration long into adulthood, well past the initial exposure to the stressor (Chaby et al., 2015). Adolescence is a developmentally sensitive period in which HPA axis disturbance can influence the response to a stressor in adulthood (McCormick et al., 2010). Male and female rodents exhibit divergent vulnerabilities to stress paradigms which may be contingent upon the stressor employed and timeline of the memory metrics tested. Identifying the extent to which males and females each experience susceptibility to stress-induced deficits in cognition will further our understanding of responses to stress stratified by sex, as well as individualize therapeautic strategies.
Along with stress-induced HPA axis dysregulation comes deficits in the system’s counterregulatory control of the immune response (Horowitz & Zunszain, 2015). Circulating glucocorticoids inhibit NF-κB transcription factor activity and the subsequent initiation of proinflammatory signaling cascades, but cumulative exposure to chronic stress and developmental stress disrupt this balance and lead to potentiated immune responses to exogenous inflammatory challenges (Bekhbat et al., 2020; De Bosscher et al., 2000). Considering the mutual control of the neuroendocrine and neuroimmune systems, it is reasonable to consider chronic stress as a risk factor for negative outcomes in individuals with elevated cytokine levels. To this end, depression can co-occur in inflammatory diseases in which cytokine levels are elevated including physical trauma such as traumatic brain injury (Villapol et al., 2017), lifestyle influences such as obesity (Kim et al., 2019), and severe infection-based inflammation from diseases such as COVID-19 (Lamontagne et al., 2021).
Inflammatory biomarkers assessed across mood disorders differ by sex (Rainville & Hodes, 2019). This is not surprising as males and females display divergent vulnerability to an inflammation challenge following adolescent stress, with stressed males given an acute challenge exhibiting elevated hippocampal inflammatory markers compared to non-stressed males, an effect not observed in females (Pyter et al., 2013). Similarly, stress does not increase cytokine mRNA expression in the hippocampus of female mice, but females express higher cytokine levels at baseline as compared to males (Hudson et al., 2014). As such, the sex differences in inflammatory response may contribute to sex differences in learning and memory performance following stress to an extent that precludes assessment of both sexes in a single analysis. Within the current study, we sought to interrogate sensitization by stress to a potent peripheral immune challenge on learning and memory in male and female rats in parallel, using a rodent model of chronic adolescent stress (CAS) that induces long-lasting effects into adulthood (Fig. 1A). This line of work expands on previous work from our group demonstrating sex differences in stress sensitization to inflammation and extends understanding of the influence of this interaction to examine the long-term impact of stress that begins in adolescence on the impact of an acute immune challenge on learning and memory.
Figure 1.
(A) Schematic of the experimental timeline. Adolescent male and female Wistar rats underwent a chronic mixed modality stress paradigm. As adults, rats were given an acute LPS challenge. Rats were assessed for anxiety-like behavior in the Open Field and trained on the Barnes Maze task. (B) During the defeat paradigm, female rats experienced a longer latency to pin (B) and fewer pins (C) compared to males. (D) For both males and females, regardless of stress history, LPS treatment caused a reduction in weight. In males, weight did not return to baseline until twelve days post injection. For females, weight returned to baseline after seven days post injection. Overall, males weighed more than females. Males with a history of chronic stress were more susceptible to the physical effects of LPS treatment compared to non-stress males. They showed higher levels of (E) ruffled fur and (F) porphyrin staining. Females with a history of stress showed similar, but attenuated, deficits compared to non-stress controls. There were no effects of stress history or sex on (G) diarrhea. Symbols represent mean ± SEM. *, main effect of sex (p<0.05); #, main of effect of stress (p<0.05); †, main effect of day (p<0.05). Panel A was created using the Biorender illustration tool available at Biorender.com.
We determined the extent to which chronic adolescent stress (CAS) altered memory performance of male and female Wistar rats, as compared to same-sex controls, which had recovered from a potent peripheral immune challenge in adulthood (Fig. 1A). All procedures were approved by the Institutional Animal Care and Use Committee at Virginia Commonwealth University. Male (n=13) and female (n=15) Wistar rats were born in-house to timed pregnant dams (Charles River, North Carolina) and weaned on postnatal day (PND) 21. On PND 35, seven males and seven females were individually housed and subsequently exposed to the CAS paradigm (see below). Following chronic stress, animals remained individually housed for the duration of the study. Beginning on PND 60, the remaining males (n=6) and females (n=8) in the non-stress group were individually housed to isolate the effect of individual housing in adulthood from the combined influence of stress that begins in adolescence. We have previously demonstrated that CAS exerts effects beyond those of individual housing (Bourke & Neigh, 2011). All animals were housed in static cages on a 14:10 light-dark cycle in a temperature (20–23°C) and humidity (60%) controlled colony room. Animals had access to food and water ad libitum.
The mixed modality psychosocial stress paradigm has been previously reported in detail (Bekhbat et al., 2020; Hyer et al., 2021). Briefly, males and females were isolated on PND 35 and on PND 38 began randomized, alternating days of restraint and social defeat until PND 49 (six sessions each; Fig. 1A). For the physical restraint, rats were placed into a plexiglass tube small enough that they could not turn 180° for 60 minutes. For the social defeat, rats were placed on one side of a barrier in the home cage of a same-sex, adult, Long Evans rat. Both male and female Long Evans rats demonstrate territorial aggression, allowing for the interrogation to include female rodents which are often excluded from social defeat paradigms. After two minutes, the barrier was lifted, and the rats were allowed to freely interact for either five minutes or until the Long Evans pinned the Wistar rat three times – whichever came first. Rats then remained on opposite sides of a mesh barrier for 25 additional minutes such that they were exposed to visual and olfactory cues but could not physically interact. The latency to first pin and total number of pins was recorded for each session to verify both males and females experienced adverse social experience. We have previously demonstrated that this stress paradigm is sufficient to induce anxiety- and depressive-like behaviors in female rats (Bourke & Neigh, 2011).
To determine the extent to which a robust acute inflammatory challenge interacted with stress that began in adolescence to precipitate a sustained alteration in cognitive function, a single intraperitoneal injection of lipopolysaccharide (LPS; 7.5×105 EU) was given on PND 110 (Fig. 1A). This dose has previously been shown to alter inflammatory outcomes in male and female Wistar rats with a history of chronic stress and that the physiology and timecourse differs by sex (Bekhbat et al., 2019). Following the injection, all animals were tracked daily for weight and health metrics for six days. Health metrics included ruffled fur, porphyrin staining, impaired breathing, immobility, diarrhea, and non-responsivity. These health metrics were chosen as they are signs of LPS-induced sickness behavior observed within rodents and are peripheral indications of circulating inflammatory mediators and overall rat health (Shrum et al., 2014; Zhang et al., 2019). Ruffled fur, porphyrin staining, and diarrhea were scored between 0–2 with higher scores indicating increased frequency. Breathing, immobility, and non-responsivity were scored between 1 and 3 with higher scores indicating negative outcomes. After the injection, one CAS male died shortly following injection of LPS. A second CAS male was euthanized three days later after losing >7% body mass in <24 hours and showing poor health metrics consistent with the euthanasia criteria defined in the IACUC protocol.
To determine if estrous cycle was altered by stress history, or if it influenced experimental outcomes, vaginal lavage was used over a period of eight days to establish two full estrous cycle patterns for each female (Cora et al., 2015). Females were gently restrained and saline (200μl) was flushed into the vaginal canal. The sample was placed on a slide and the presence of round cells, needle cells, and/or neutrophils was used to determine which stage was active.
Eight days following the LPS injection, rats were assessed on the open field task. The open field task was conducted as described previously (Bekhbat et al., 2020). All rats were allowed to freely explore a square open field arena (90 × 90 cm) for 10 minutes. Metrics recorded included percent of time spent in the center of the maze, percent of time spent in the corners of the maze while in the periphery, velocity, and distance travelled. All behavioral trials were recorded with an overhead camera and metrics tracked using EthoVisionXT 13.0 (Noldus, Leesburg, VA).
Ten days following the LPS injection, rats were tested for learning and memory performance using the Barnes Maze task. The Barnes Maze task was conducted as reported previously (Hyer et al., 2021). Briefly, acquisition learning took place over five days with two trials per day. Rats were allowed to explore a circular maze (121 cm in diameter) with 20 equidistant holes around the perimeter for three minutes or until they entered the hole that contained the goal box. Following an intertrial interval of two minutes, rats repeated the procedure. The goal box was removed during the probe task to assess memory and was completed 48 hours following the final acquisition trial and consisted of five minutes on the maze. Reversal learning began 48 hours later and lasted for three days with two trials per day. Procedures were identical to acquisition learning except the goal box was moved to the opposite side of the maze. The second memory probe was conducted 48 hours after the final reversal trial. Metrics recorded using EthoVisionXT 13.0 (Noldus, Leesburg, VA) included the summed latency to locate the goal box across both trials, total number of errors, and average velocity.
For physiological endpoints occurring over multiple days, including weight and health metrics, repeated measures three-way analysis of variance (ANOVA) was used to assess the influence of sex, stress, and day (repeated factor). Due to the known physiological differences in response to LPS by sex, data for behavioral endpoints were stratified by sex to assess outcomes with two-way ANOVAs in order to avoid an indirect confound of the difference in response to LPS by sex being conflated with a sex difference in learning and memory. All behavioral comparisons are within sex and discussed relative to the same-sex control. Any sex difference comparisons for behavioral outcomes are qualitiative only. Open field and probe data were analyzed using unpaired t-tests to assess the affect of stress. Defeat data was analyzed using a repeated measures two-way ANOVA. The number of days spent in each estrous cycle stage were calculated per female rat and compared using a two-way ANOVA.
Male Wistar rats were pinned sooner than the female rats during social defeat (F(1,12)=16.61; p=0.0015; Fig. 1B) and female defeaters initiated fewer pins than male defeaters (F(1,12)=16.00; p=0.0018; Fig. 1C). Weight differed by sex (F(1,22)=321.6; p<0.0001; Fig. 1D) and by day post-LPS exposure (F(18,396)=46.82; p<0.0001; Fig. 1D). In addition, weight was altered by interactions between day post-LPS exposure and sex (F(18,396)=13.85; p<0.0001; Fig. 1D) and stress history (F(18,396)=1.88; p=0.02; Fig. 1D). Post hoc analyses indicated that, in males, LPS injection reduced weight (F(1.232,11.09)=21.73; p=0.0004; Fig. 1D) regardless of stress history until 13 days post-injection. In females, weight was reduced following the injection (F(1.136,14.77)=39.41; p<0.0001; Fig. 1D), regardless of stress history, until eight days post-injection. Stress history did not affect the average total number of days spent in proestrus, estrus, metestrus, and diestrus across an eight-day period (p>0.05).
Following the LPS injection, health metrics were influenced by both sex and stress history such that females tended to recover faster than males while stress history exacerbated the peripheral LPS response. Ruffled fur was altered by sex (F(1,22)=39.63; p<0.0001; Fig. 1E), stress history (F(1,22)=21.44; p=0.0001; Fig. 1E), and day post-injection (F(4,88)=4.58; p=0.002; Fig. 1E). Males with a history of stress maintained poor fur quality for the duration of the experiment compared to non-stress males (F(1,9)=11.38; p=0.008). Females with a history of stress gradually decreased quality of fur post-injection compared to non-stress controls (F(4,52)=5.46; p=0.001; Fig. 1E), until returning to baseline by day six. Porphyrin staining was similarly influenced by sex (F(1,22)=17.63; p=0.0004; Fig. 1F), stress history (F(1,22)=7.24; p=0.01; Fig. 1F), and day post-injection (F(4,88)=10.74; p<0.0001; Fig. 1F). Males with a history of stress took longer for porphyrin to return to baseline compared to non-stress controls (F(4,36)=5.27; p=0.001; Fig. 1F). Females with a history of stress showed increased porphyrin immediately following the LPS injection while non-stress controls showed no staining (F(4,52)=4.15; p=0.005; Fig. 1F). Difficulty breathing following LPS exposure was altered by sex (F(1,22)=23.40; p<0.0001), stress history (F(1,22)=23.40; p<0.0001), and day post-injection (F(4,88)=22.26; p<0.0001; Supp. Fig. 1A). In males, stress history impaired breathing (F(4,36)=15.76; p<0.0001) two days post-injection (p<0.0001) before returning to baseline. Breathing was not impaired in females. Immobility was impacted by sex (F(1,22)=36.75; p<0.0001; Supp. Fig. 1C) and day post-LPS exposure (F(4,88)=51.83; p<0.0001) such that males had more immobility two days post-injection regardless of stress history (p=0.01) while females remained fully mobile. Diarrhea frequency (Fig. 1G) and responsivity (Supp. Fig. 1B) were not altered in either males or females following the LPS injection.
A history of stress beginning in adolescence did not increase anxiety-like behaviors in the open field assessement. There was no effect of stress history (p>0.05) on percent time spent in the center of the open field for non-stressed vs CAS males (3.59 ± 1.29 vs 4.15 ± 1.30), percent time spent in the corners of the periphery (71.82 ± 3.96 vs 73.39 ± 2.47), or velocity (9.59 ± 1.64 cm/s vs 10.89 ± 0.67 cm/s). There was no effect of stress beginning in adolescence (p>0.05) on percent time spent in the center of the open field (4.33 ± 1.01 vs 4.17 ± 0.93), percent time spent in the corners of the periphery (64.31 ± 2.00 vs 65.68 ± 2.07), or velocity (13.37 ± 0.71 cm/s vs 12.24 ± 0.58 cm/s) for CAS females compared to same-sex controls.
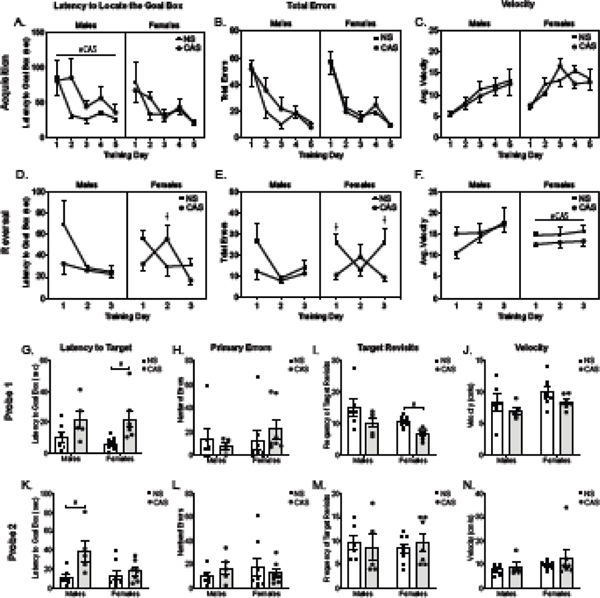
In males, stress history increased the latency to locate the goal box during acquisition learning compared to males without a history of stress (F(1,45)=4.12; 0=0.048; Fig. 2A) following recovery from the potent acute LPS challenge. There was no effect of stress history (p>0.05) on total errors (Fig. 2B) or velocity (Fig. 2C) during acquisition learning in males or females. There was no effect of stress history (p>0.05) on reversal learning in males.
Figure 2.
During acquisition learning, males with a history of stress had an increased latency to locate the goal box compared to non-stress males (A). There were no effects of stress history on (B) errors or (C) velocity. Acquisition learning in females was not altered by stress history. During reversal learning, females with a history of stress (D) showed a slight impairment in the latency to locate the goal box compared to non-stress females. (E) Non-stress females showed more errors on days one and three compared to CAS females. (F) CAS females appeared to move slightly slower than non-stress females. Performance in males was not altered by stress history during reversal learning (D). Females with a history of stress had longer latencies to locate the goal box position during probe 1 (G), while stressed males took longer during probe 2(K). I) During probe 1, females with a history of stress were less likely to revisit the goal box compared to non-stress animals. There were no differences in (H, L) error rates or (J, N) velocity in either probe. Symbols represent mean ± SEM. #, main effect of stress (p<0.05); †, interaction between stress and training day (p<0.05).
During reversal learning, there was an interaction between stress history and training day (F(2,39)=4.82; p=0.01) on latency to locate the new goal box location in females (Fig. 2D). Females with a history of stress took longer to find the new goal box on day two compared to non-stress controls (p=0.04; Fig. 2D). There was an interaction between stress history and training day on total errors during reversal learning in females (F(2,39)=3.70; p=0.03; Fig. 2E). On day one (p=0.03) and three (p=0.02), non-stress females committed more errors than females with a history of stress. Stressed females did exhibit a lower average velocity during the reversal learning days (F(1,39)=4.36; p=0.04; Fig. 2F).
Chronic stress history increased the latency to locate the goal box during the first probe for females (t(13)=3.00; p=0.01; Fig. 2G) and decreased the number of times females revisited the location of the goal box during the first probe (t(13)=4.35; p=0.00; Fig. 2I). During probe one, primary errors (Fig. 2H) and velocity (Fig. 2J) were not increased by a history of stress in females (p>0.05). There was no effect of stress (p>0.05) on females during the second probe. Stress history impaired memory in males during the second probe trial as evidenced by an increase in the latency to locate the goal box location (t(9)=2.57; p=0.03; Fig. 2K). Primary errors were not increased by a history of stress (p>0.05) during either probe one (Fig. 2H) or probe two (Fig. 2L). Goal box location revisits was not altered by stress history (p>0.05) in males for probe one (Fig. 2I) or probe two (Fig 2M). Velocity was not altered by stress history (p>0.05) in either probe one (Fig. 2J) or two (Fig. 2N) for eiter sex.
In this study, rats exposed to stress beginning in adolescence and then challenged with a potent peripheral inflammatory stimulus in adulthood demonstrated an impairment in reference memory whether male or female. In contrast, the impact on learning qualitatively differed by sex. Females with a history of stress beginning in adolescence were able to learn as efficiently as the non-stressed females during the acquisition training. Stress did increase vulnerability to LPS in males during acquisition learning, but the group acquired the task. Conversely, only females with a history of stress beginning in adolescence exhibited increased latency to target and decreased target revisits during probe 1, as compared to their same-sex control group. Collectively, we report sex differences in metrics of stress severity and health outcomes following LPS and residual effects of acute LPS on reference memory within each sex. Additionally, females with a history of adolescent stress demonstrated a slight impairment during reversal learning compared to non-stress females, while there were no significant differences between stressed and non-stressed males. However, stressed males did exhibit an an impairment in reference memory during probe 2 compared to non-stressed males.
The current study was not designed with the intention of directly testing for a sex difference; therefore, it was not properly powered to detect sex differences in memory outcomes through direct statistical comparison. Our analysis and interpretation reflect this limitation. However, we were able to use the data we generated to determine the sample sizes necessary for the design of future studies aimed at detecting a sex difference following a similar complex physiological exposure with behavioral outcomes. We made these calculations for two of the behavioral outcomes where we observed a qualitative difference between the sexes. If a future study aimed to directly assess a sex difference in probe 2 latency to target, a power analysis of our data indicates that >60 animals would be needed to detect a significant sex difference and reach the criterion α = 0.05 and power = 0.8 using a two-way ANOVA (stress and sex as factors). Conducting this same analysis for probe 1 target revisits, indicated that the current sample size would be powered to detect a sex difference within a two-way ANOVA, indicating that future studies interested in this specific domain of a potential sex difference need to target a total sample size of at least 25 animals. Given the observed sex differences in physiological responses to both chronic stress and LPS which we have previously reported and appear here in Figures 1B–G, we designed our statistics plan to assess learning and memory outcomes within each sex. Sex difference comparisons of learning and memory based on the work reported here can be qualitative only, but we have provided foundational information for design of appropriately powered future studies that aim to directly compare sex after complex physiological exposures.
In previous work from our group, in which we characterized stress-induced sex differences in Barnes Maze performance in the absence of LPS, there were no observed effects of stress on latency to locate the goal box for either sex during the probe trials (Hyer et al., 2021) suggesting that LPS resulted in vulnerability to performance deficits within the current study. Similar to the current results, stressed females in (Hyer et al., 2021) exhibited slight impairment of cognitive flexibility, while stressed males did not. Interestingly, adult male rats that experienced mid-adolescenct social stress and were later assessed for cognitive performance as adults, displayed deficits in strategy shifting and cognition (Snyder et al., 2015). However, the same deficits were not observed in males that experienced early-adolescent social stress. The CAS paradigm employed in the current study overlaps the early-adolescent to mid-adolescent ages from (Snyder et al., 2015), suggesting timing and type of stressor likely influences susceptibility to cognitive performance deficits in adulthood. Adolescent males and females were subjected to the same CAS paradigm with equal sessions of restraint stress and social defeat. Female rats experienced fewer total pins during the defeat sessions compared to males. While previous work from our group demonstrates long-lasting effects of the CAS paradigm on female rats (Bourke & Neigh, 2011), it is possible that males and females experienced stress along a continuum. There were no post-stress or terminal assessments of stress markers within the current study (i.e., plasma corticosterone), but it has been previously shown that CAS females have an exaggeraged corticosterone response to an acute stressor in adolescence (Bourke & Neigh, 2011) and a blunted response in adulthood (Rowson et al., 2019), suggesting long-term consequences of CAS on the female HPA axis. Given the potential stress experience differences between the sexes, assessments of behavioral outcomes were stratified by sex to limit the influence of this confounding variable.
Neither sex exhibited anxiety-like behavior during the open field assessment. It has been shown that CAS increases percent time spent in corners when measured during adolescence, but this effect may be lost during adulthood (Bekhbat et al., 2020). Despite a lack of anxiety-like behavior in the open field, both stressed males and females display an exaggerated neuroimmune transcriptome in response to an acute immune challenge (Bekhbat et al., 2020). While stress exaggerated the peripheral effects of LPS in both sexes in the current study, males overall exhibited greater signs of physiological distress following LPS in the metrics assessed. These peripheral results are consistent with previous work showing males with a history of CAS given an acute inflammatory challenge exhibited exaggerated plasma IL-1β levels, while stressed females did not (Bekhbat et al., 2019). Differences between the sexes may begin within the innate immune system as female rodents have a more efficient response to a peripheral infection compared to males (Scotland et al., 2011). Both stressed and non-stressed rats exhibited a decrease of weight compared to baseline, however this effect on weight was extended in the males. Despite an initial decrease in weight, the open field data shows that both males and females retained motor ability and that both sexes were physically able to complete the Barnes Maze task.
Other studies have shown that both acute and chronic LPS dosing have negative effects on memory performance (Hauss-Wegrzyniak et al., 2000; Zhao et al., 2019). These observed deficits may be dependent on the time post-inflammatory challenge at which memory performance was assessed, as males and females exhibit sex-specific deficits in early versus delayed memory performance following an immune challenge (Tchessalova & Tronson, 2019). Sex differences in hippocampal differential gene expression observed in these animals 12 weeks after the immune challenge concluded suggests dysregulation within the hippocampus contributes to vulnerability to the observed memory impariments (Tchessalova & Tronson, 2020). The reported effects from the current study do not recapitulate previous work, but demonstrates the combined effects of a history of adolescent stress and a history of acute LPS exposure on memory performance. The hippocampus is a stress-sensitive brain region that undergoes sex specific remodeling following stress (Shors et al., 2001) and our group has demonstrated hippocampal changes in gene expression are altered by CAS and an immune challenge (Bekhbat et al., 2020). While the hippocampus is recognized as a key regulatory region of memory processes, the prefrontal cortex (PFC) (Hinwood et al., 2013) and amygdala (Espejo et al., 2016) are also susceptible to stress and involved in memory circuitry. Direct injection of LPS into the PFC of stressed male rats induces higher elevations of TNF-α compared to non-stressed immune challenged rats, demonstrating a sensitization of the PFC during stress, as well (de Pablos, 2006). Teasing apart the contribution of these key brain regions is out of the scope of this study, but demonstrates the importance of extending our understanding of the long-term consequences of the CAS paradigm on vulnerability to inflammation within other key regulatory regions.
The work reported here highlights an important and understudied area of translationally relevant inquiry: developmental stress history and sex may collectively shape cognitive function following recovery from a severe acute inflammatory event. Physiological levels of cytokines are needed for proper learning and memory mechanisms to occur however, they can be detrimental to hippocampal function outside of this physiological range (Goshen et al., 2007). As such, future work is needed to further interrogate the mechanisms by which memory is impacted by immune responses in each sex. Understanding the impact of developmental stress history on vulnerability to sustained effects of immune and inflammatory challenges within each sex will provide foundational understanding necessary to identify mechanistic intervention points.
Supplementary Material
Supplementary Figure 1. (A) Difficulty breathing was impacted by sex, stress history and day post-injection, with stressed males exhibiting difficulty at two days post-injection. There were no observed breathing impairments in females. (B) Responsivity was not altered in males or females. (C) Immobility was altered by sex and day post-injection. Males overall demonstrated increased immobility on day two. Symbols represent mean ± SEM. *, effect of sex (p<0.05); #, effect of stress (p<0.05); †, interaction between stress and training day (p<0.05); †Males, effect seen within males overall.
Footnotes
CRediT author Statement for: “Chronic stress beginning in adolescence decreases spatial memory following an acute inflammatory challenge in adulthood”
Molly M. Hyer: Conceptualization, Methodology, Formal Analysis, Writing-Original Draft, Writing-Reviewing & Editing
Amy J. Wegener: Writing-Original Draft, Writing-Reviewing & Editing, Visualization
Imogen Targett: Investigation, Writing-Reviewing & Editing
Samya K. Dyer: Investigation, Writing-Reviewing & Editing
Gretchen N. Neigh: Conceptualization, Funding acquisition, Writing-Review & Editing, Supervision
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bekhbat M, Howell PA, Rowson SA, Kelly SD, Tansey MG, & Neigh GN (2019). Chronic adolescent stress sex-specifically alters central and peripheral neuro-immune reactivity in rats. Brain, Behavior, and Immunity, 76, 248–257. 10.1016/j.bbi.2018.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekhbat M, Mukhara D, Dozmorov M, Stansfield J, Benusa S, Hyer MM, Rowson SA, Kelly S, Qin Z, Dupree J, Tharp G, Tansey MG, & Neigh GN (2020). Adolescent stress sensitizes the adult neuroimmune transcriptome and leads to sex-specific microglial and behavioral phenotypes. Neuropsychopharmacology, January. 10.1038/s41386-021-00970-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke CH, & Neigh GN (2011). Behavioral effects of chronic adolescent stress are sustained and sexually dimorphic. Hormones and Behavior, 60(1), 112–120. 10.1016/j.yhbeh.2011.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaby LE, Sheriff MJ, Hirrlinger AM, Lim J, Fetherston TB, & Braithwaite VA (2015). Does chronic unpredictable stress during adolescence affect spatial cognition in adulthood? PLoS ONE, 10(11), 1–12. 10.1371/journal.pone.0141908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cora MC, Kooistra L, & Travlos G. (2015). Vaginal Cytology of the Laboratory Rat and Mouse:Review and Criteria for the Staging of the Estrous Cycle Using Stained Vaginal Smears. Toxicologic Pathology, 43(6), 776–793. 10.1177/0192623315570339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bosscher K, Berghe WV, Vermeulen L, Plaisance S, Boone E, & Haegeman G. (2000). Glucocorticoids repress NF-κB-driven genes by disturbing the interaction of p65 with the basal transcription machinery, irrespective of coactivator levels in the cell. Proceedings of the National Academy of Sciences of the United States of America, 97(8), 3919–3924. 10.1073/pnas.97.8.3919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pablos RM (2006). Stress Increases Vulnerability to Inflammation in the Rat Prefrontal Cortex. Journal of Neuroscience, 26(21), 5709–5719. 10.1523/JNEUROSCI.0802-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espejo PJ, Ortiz V, Martijena ID, & Molina VA (2016). Stress-induced resistance to the fear memory labilization/reconsolidation process. Involvement of the basolateral amygdala complex. Neuropharmacology, 109, 349–356. 10.1016/j.neuropharm.2016.06.033 [DOI] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ounallah-Saad H, Renbaum P, Zalzstein Y, Ben-Hur T, Levy-Lahad E, & Yirmiya R. (2007). A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology, 32(8–10), 1106–1115. 10.1016/j.psyneuen.2007.09.004 [DOI] [PubMed] [Google Scholar]
- Hauss-Wegrzyniak B, Vraniak PD, & Wenk GL (2000). LPS-induced neuroinflammatory effects do not recover with time. NeuroReport, 11(8), 1759–1763. 10.1097/00001756-200006050-00032 [DOI] [PubMed] [Google Scholar]
- Hinwood M, Tynan RJ, Charnley JL, Beynon SB, Day TA, & Walker FR (2013). Chronic stress induced remodeling of the prefrontal cortex: Structural re-organization of microglia and the inhibitory effect of minocycline. Cerebral Cortex, 23(8), 1784–1797. 10.1093/cercor/bhs151 [DOI] [PubMed] [Google Scholar]
- Horowitz MA, & Zunszain PA (2015). Neuroimmune and neuroendocrine abnormalities in depression: Two sides of the same coin. Annals of the New York Academy of Sciences, 1351(1), 68–79. 10.1111/nyas.12781 [DOI] [PubMed] [Google Scholar]
- Hudson SP, Jacobson-Pick S, & Anisman H. (2014). Sex differences in behavior and pro-inflammatory cytokine mRNA expression following stressor exposure and re-exposure. Neuroscience, 277, 239–249. 10.1016/j.neuroscience.2014.07.007 [DOI] [PubMed] [Google Scholar]
- Hyer MM, Shaw GA, Goswamee P, Dyer SK, Burns CM, Soriano E, Sanchez CS, Rowson SA, McQuiston AR, & Neigh GN (2021). Chronic adolescent stress causes sustained impairment of cognitive flexibility and hippocampal synaptic strength in female rats. Neurobiology of Stress, 14. 10.1016/j.ynstr.2021.100303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JD, Yoon NA, Jin S, & Diano S. (2019). Microglial UCP2 Mediates Inflammation and Obesity Induced by High-Fat Feeding. Cell Metabolism, 30(5), 952–962.e5. 10.1016/j.cmet.2019.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamontagne SJ, Pizzagalli DA, & Olmstead MC (2021). Does inflammation link stress to poor COVID-19 outcome? Stress and Health, 37(3), 401–414. 10.1002/smi.3017 [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Fiocco A, Wan N, Maheu F, Lord C, Schramek T, & Tu MT (2005). Stress hormones and human memory function across the lifespan. Psychoneuroendocrinology, 30(3), 225–242. 10.1016/j.psyneuen.2004.08.003 [DOI] [PubMed] [Google Scholar]
- McCormick CM, Mathews IZ, Thomas C, & Waters P. (2010). Investigations of HPA function and the enduring consequences of stressors in adolescence in animal models. Brain and Cognition, 72(1), 73–85. 10.1016/j.bandc.2009.06.003 [DOI] [PubMed] [Google Scholar]
- Peay DN, Saribekyan HM, Parada PA, Hanson EM, Badaruddin BS, Judd JM, Donnay ME, Padilla-Garcia D, & Conrad CD (2020). Chronic unpredictable intermittent restraint stress disrupts spatial memory in male, but not female rats. Behavioural Brain Research, 383(October 2019). 10.1016/j.bbr.2020.112519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyter LM, Kelly SD, Harrell CS, & Neigh GN (2013). Sex differences in the effects of adolescent stress on adult brain inflammatory markers in rats. Brain, Behavior, and Immunity, 30, 88–94. 10.1016/j.bbi.2013.01.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainville JR, & Hodes GE (2019). Inflaming sex differences in mood disorders. Neuropsychopharmacology, 44(1), 184–199. 10.1038/s41386-018-0124-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowson SA, Bekhbat M, Kelly SD, Binder EB, Hyer MM, Shaw G, Bent MA, Hodes G, Tharp G, Weinshenker D, Qin Z, & Neigh GN (2019). Chronic adolescent stress sex-specifically alters the hippocampal transcriptome in adulthood. Neuropsychopharmacology, 44(7), 1207–1215. 10.1038/s41386-019-0321-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotland RS, Stables MJ, Madalli S, Watson P, & Gilroy DW (2011). Sex differences in resident immune cell phenotype underlie more efficient acute inflammatory responses in female mice. Blood, 118(22), 5918–5927. 10.1182/blood-2011-03-340281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Chua C, & Falduto J. (2001). Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. Journal of Neuroscience, 21(16), 6292–6297. 10.1523/jneurosci.21-16-06292.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrum B, Anantha RV, Xu SX, Donnelly M, Haeryfar SM, McCormick JK, & Mele T. (2014). A robust scoring system to evaluate sepsis severity in an animal model. BMC Research Notes, 7(1), 233. 10.1186/1756-0500-7-233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder KP, Barry M, & Valentino RJ (2015). Cognitive impact of social stress and coping strategy throughout development. Psychopharmacology, 232(1), 185–195. 10.1007/s00213-014-3654-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchessalova D, & Tronson NC (2019). Memory deficits in males and females long after subchronic immune challenge. Neurobiology of Learning and Memory, 158, 60–72. 10.1016/j.nlm.2019.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchessalova D, & Tronson NC (2020). Enduring and Sex-specific Changes in Hippocampal Gene Expression after a Subchronic Immune Challenge. Neuroscience, 428, 76–89. 10.1016/j.neuroscience.2019.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villapol S, Loane DJ, & Burns MP (2017). Sexual dimorphism in the inflammatory response to traumatic brain injury. Glia, 65(9), 1423–1438. 10.1002/glia.23171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Wei X, Zhang R, Si D, Petitte JN, Ahmad B, & Zhang M. (2019). A Novel Peptide Ameliorates LPS-Induced Intestinal Inflammation and Mucosal Barrier Damage via Its Antioxidant and Antiendotoxin Effects. International Journal of Molecular Sciences, 20(16), 3974. 10.3390/ijms20163974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Bi W, Xiao S, Lan X, Cheng X, Zhang J, Lu D, Wei W, Wang Y, Li H, Fu Y, & Zhu L. (2019). Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Scientific Reports, 9(1), 1–12. 10.1038/s41598-019-42286-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. (A) Difficulty breathing was impacted by sex, stress history and day post-injection, with stressed males exhibiting difficulty at two days post-injection. There were no observed breathing impairments in females. (B) Responsivity was not altered in males or females. (C) Immobility was altered by sex and day post-injection. Males overall demonstrated increased immobility on day two. Symbols represent mean ± SEM. *, effect of sex (p<0.05); #, effect of stress (p<0.05); †, interaction between stress and training day (p<0.05); †Males, effect seen within males overall.