Abstract
Introduction
Elderly people living in care facilities suffer from difficulties in accessing preventive and curative dental care. This leads to poor oral health, which is an increased risk of systemic diseases, in a fragile and dependent population. All this contributes to a progressive loss of autonomy and a decreased quality of life. The use of information and communication technologies through oral telemedicine could help to overcome these barriers. We described the protocol for evaluating the diagnostic performance of two intraoral cameras against a gold standard clinical examination.
Methods and analysis
We conduct a pilot multicentric and prospective diagnostic study (a minimal-risk, minimal-burden interventional research called ONE-1 (for Oral graNd Est step 1)) on two intraoral diagnostic tools (Soprocare camera and consumer camera) compared with a reference intraoral examination. Patients in four elderly care facilities will be included, with randomisation of participant selection and randomisation of the order of the three intraoral examinations performed by a dental surgeon. We will evaluate the diagnostic performance of each device with the asynchronous analysis of videos by two independent dental surgeons against the clinical gold standard examination performed by a single, third dental examiner. The primary outcome is the presence of at least one tooth decay in the dentition of each study participant. Second, we will evaluate the presence of other dental or oral diseases, and the time required to perform each examination. Finally, we will evaluate the organisation of patient follow-up.
Ethics and dissemination
The protocol has been approved by the French ethics committee (Protection to Persons Committee, Nord-Ouest IV on 9 June 2021 and on 28 November 2022). Results will be disseminated through conferences’ presentations and publications in peer-reviewed journals.
Trial registration number
Keywords: Telemedicine, Public health, ORAL MEDICINE
STRENGTHS AND LIMITATIONS OF THIS STUDY.
First evaluation to compare two intraoral cameras with a gold standard intraoral examination.
Evaluation of pragmatic tools for the detection of oral diseases in the elderly.
Evaluation of appropriate referral tools accepted from the elderly.
Pilot study: the video recording will be performed by one trained dental surgeon and not by non-oral health professionals.
Introduction
Background and rationale
Oral health is part of general health, which is considered as an essential component of quality of life.1 One of the main levers to achieve this health status is access to dental care.2 Over the past 20 years, the French government has taken measures to improve oral healthcare access. Following the implementation of these measures, the Direction de la Recherche, des Études, de l'Évaluation et des Statistiques (Direction of Research, Study, Evaluation, and Statistics) estimates that 98% of the French population has access to a dentist within 15 min.3 The lack of reliable routine epidemiological surveillance data on oral health is an unfortunate reality, and one of the fundamental causes that has led to the neglect of global oral health.4 As a result, inequalities in access to oral health and the severity of oral diseases persist at both national and regional levels in France.5
These are exacerbated by socioeconomic status (income, occupation and educational level).2 6 This is particularly true for patients with vulnerabilities such as those with disability,7 those in detention,8 those with social needs9 or the elderly living in care facilities.10 Several studies conducted in France revealed that elderly people living in care facilities suffer from poor oral health, with a greater need for care than the general population. A study conducted in a department of France showed that two-thirds of elderly people living in care facilities needed dental care.11 Numerous other regional and national studies have confirmed the poor oral health status of these elderly people, with 50%–96% of residents requiring dental care or adapted prostheses. Similarly, oral hygiene is poorly integrated and support from medical teams is inadequate.12–14 The likelihood of using a dental surgeon (when care is needed or the need for care is expressed) is reduced by a quarter in people living in care facilities (OR=0.7; p<0.001) compared with people living at home.15 Another recent study among 114 French elderly care facility residents showed that only 18% of them were judged to be in good health according to the Oral Health Assessment Tool, and only 9% rating their oral health to be ‘good’ according to the General Oral Health Assessment Index.16 This suggests a high level of preventive and curative needs in the ageing population.
Behind this situation ensue some major concerns. Functional: oral diseases such as periodontal diseases, tooth decay, xerostomia or tooth loss can lead to deficiencies and malnutrition.17–19 Medically: poor oral health can induce bacteraemia and general diseases such as lung disease, cancer, rheumatoid arthritis or cardiovascular diseases.20 21 Periodontal pathogens can also promote progressive neurodegenerative diseases such as Alzheimer’s disease.21–23 From a social perspective, poor oral health status is correlated with poor mental status, low self-esteem, depression18 and poor psychosocial functions.24 All these factors in turn lead to a gradual slide into dependency and loss of autonomy, which further increases difficulties in maintaining good oral health.25 From a public health perspective, maintaining a high level of oral health in elderly people living in care facilities is an important health and economic challenge.6
Today, access to information, communication and social connection is made possible by information and communication technologies (ICTs). In the field of health, ICTs also play an important role, grouped under the term eHealth, defined, in 2005, by the WHO.26 These devices are also used for teledentistry. Previous research in France, such as the e-DENT Project or the Tel-e-Dent Project,27 28 has shown that teledentistry would allow remote screening and consultation sessions to be carried out in populations with specific needs or particular risks, or who are isolated. We therefore considered that teledentistry could be well adapted to elderly people living in care facilities. However, to our knowledge, each of these projects has worked with a single preferred tool (intraoral camera or modified endoscope), but no study has been conducted to compare more than one teledentistry tool, that is, multiple intraoral cameras with very different costs compared with the reference clinical examination (traditional face-to-face oral examination). While teledentistry has already been shown to be a good solution for improving access to oral health at lower cost,29–31 the tools have yet to demonstrate their diagnostic value. If this is the case, then teledentistry could help the elderly care facilities to follow the guidelines issued in 2011 by the Haute Autorité de Santé (French National Authority of Health) in instituting and increasing oral health monitoring of residents,32 and also the recent recommendations on the fundamental rights of elderly people living in care facilities.33 It therefore seems relevant to compare two types of intraoral cameras with very different costs. Our hypothesis is that both cameras have a sufficient diagnostic performance to decide on the oral care pathway for elderly people living in care facilities.
Objectives
Main objective: to evaluate the diagnostic performances of two types of intraoral cameras compared with an intraoral clinical examination (gold standard or GS) for the detection of at least one tooth decay among elderly people living in care facilities.
Secondary objectives
At a patient level: (1) to assess the performances of two types of intraoral cameras compared with the intraoral clinical examination (GS) for the detection of each of the following conditions: (a) at least one filled tooth, (b) at least one missing tooth, (c) gingivitis, (d) abscess, (e) dental plaque, (f) dental calculus; (2) to assess the concordance between each intraoral camera and the intraoral clinical examination (GS) in the evaluation of the number of (a) decayed, (b) filled, (c) missing teeth; (3) from the video acquisitions obtained from each of the intraoral cameras, to assess the interobserver concordance for the diagnosis of each dental disease (at least one decayed, filled or missing tooth) and periodontal disease (Löe & Silness Gingival Index (0–3), periodontal abscess (presence or absence), Silness & Löe Plaque Index (0–3), or calculus (absent; present in small amounts; present in large amounts)); (4) from the video acquisitions obtained from each of the intraoral cameras, to assess the interobserver concordance for the evaluation of the number of (a) decayed, (b) filled, (c) missing teeth.
At the tooth level: (5) to evaluate the performances of two types of intraoral cameras compared with the intraoral clinical examination (GS) for the detection of each of the following conditions: (a) a decayed, (b) filled, (c) missing tooth.
For all the patients: (6) to compare the video recording time of each intraoral camera.
For patients in whom dental and/or periodontal disease will be identified by the intraoral clinical examination: (7) to assess 2 months after the clinical examination the percentage of patients (a) who received dental and/or periodontal care, and (b) for whom a dental appointment has been scheduled.
Methods and analysis
Study design
This study is a multicentric and prospective diagnostic study (a minimal-risk, minimal-burden interventional research) on two teledentistry diagnostic tools. Inclusions began in June 2022 and will last 2 years, in four care facilities for dependent elderly people in a department of Eastern France particularly affected by the ageing of the population and medical desertification. The expected end of the study is December 2024.
Information meetings led by the coordinating investigator (CC) will take place before inclusion in each elderly care facility concerned. The aim of these meetings will be to explain the research and its progress to the staff of the elderly care facility. Recruitment material (leaflets and posters) will be available at the reception of each elderly care facility recruited on the basis of voluntary participation by their management and the acceptance of the coordinators.
In each elderly care facility
First, in consultation with the facility’s coordinating physician, the investigator (PB) will identify all eligible residents for the study. If it is necessary to solicit the patient’s representative, an explanatory letter will be sent to him/her. Second, a random draw will be made among all eligible and volunteer patients. Then, a study information document will be given to the randomly selected patients and an appointment for inclusion will be made. The study started in a single facility care on 16 June 2022 and will cover an inclusion period of 2 years, with a maximum patient follow-up of 174 days. The course of the study is summarised in figure 1.
Figure 1.
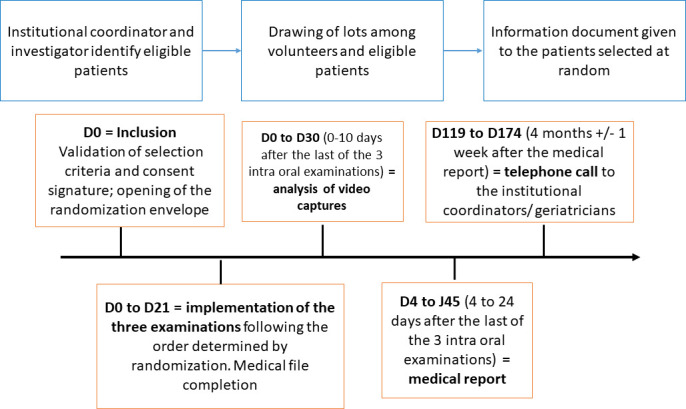
Sequences of the whole study from inclusion day (D0).
For every patient
Inclusion visit: D0
During this visit, the investigator (PB) will ensure that the resident meets the selection criteria of the study. The investigator will introduce the study to the patient (and/or the patient’s representative if applicable). After a period of reflection, the investigator will obtain free, informed and written consent from the patient (or representative, if applicable).
Intraoral examinations: D0–D21
The patients included will be comfortably installed in a room dedicated to telemedicine, or else in a room made available in the facility and isolated from the lounges or dining rooms, or in the patient’s room with his or her agreement or that of his or her family.
All three examinations will be administered by only one and same dental surgeon (YS) and performed in the order established after opening the envelope. If the patient will not wish to have all the examinations done on the same day, the other examinations will be done at another appointment within 21 days. The dental surgeon will have been previously trained in communication with patients in vulnerable age-related situations, possibly with cognitive and behavioural disorders and to the use of each camera. He will be equipped with a disposable mask, gown and gloves.
Analysis of video recordings: D0–D30
All videos recorded by the two cameras will be reviewed asynchronously by two independent dental surgeons (other than the dental surgeon who will perform the clinical examination). This part will be done within 10 days of the last intraoral examination (face-to-face or after the last intraoral camera pass depending on the draw). They will complete a teledentistry examination grid.
Drafting of the medical report: D4–D45
Four to 24 days after the last intraoral clinical examination (face-to-face or intraoral camera pass), a joint report will be made by the dental surgeons. This report will compile all dental, periodontal and prosthetic treatment needs, as well as oral prevention needs. The possibilities and organisation of the treatment will be discussed between the geriatrician and the patient (or legal guardian).
Follow-up: D119–D174
A telephone call to the geriatrician will be made 4 months (±1 week) after the analysis report has been submitted in order to:
Find out whether the patient was able to access the care recommended in the report, if necessary.
If the care could not be carried out, whether it was possible to schedule them with the practitioner of the patient’s choice (or their representative) (only for people in need).
Study setting
This study is conducted according to the principles of the Declaration of Helsinki. It is an interventional research with minimal risks and minimal burden. So, the experimental protocol (ONE-1) has been approved by the French ethics committee (Protection to Persons Committee (CPP) Nord-Ouest IV in June 2021 and in November 2022).
Patient and public involvement
Patients were and will be not involved in the design, or conduct, or reporting, nor dissemination plans of this research.
How will the results be disseminated to study participants?
The overall results of the study will be presented to health professionals, as well as to elderly volunteers and their families, at each institution in meetings with the investigators.
Each participant (or their representative) will receive an individual report of the examinations performed.
Publications or presentations of the results will be sent to each institution (geriatrician and nurse coordinator).
Study population and sample size calculation
This study will be conducted in four elderly care facilities of the French department of Meuse.
The population included in this study consists of elderly people living in four care facilities.
A sample size of 50 residents was considered to be adequate to meet the overall objectives of this study, although this calculation is not strictly necessary for a pilot study.34 35 The minimum inclusion of the study will be up to 12 participants in three of the four elderly care facilities and 24 in the fourth and largest structure. In order to take into account potential exclusions during the study, a maximum of 30 participants for the three smallest elderly care facilities and 60 for the largest one will be included (ie, a maximum inclusion of 180 people for the whole study).
This sample size will necessarily have to be precisely recalculated for the large-scale study we will conduct as soon as the barriers and limitations highlighted in this study are in accordance with the data of the study conducted in France by Queyroux et al.28
Materials and equipment
Materials
For GS intraoral examination
It is a classic oral examination performed with an intraoral mirror, a dental probe and an examination lamp.
For intraoral camera examinations
The first camera is a Soprocare camera (Acteon Group, La Ciotat, France). It is certified as a class I medical device with a CE (Conformité Européenne) mark (0459) for dental use. It has been developed and made in accordance with the quality control certification EN ISO 13485. This camera offers good resolution (752×582 PAL: 768×494 NTSC) and definition (470 lines) with a 1/4’ high sensibility sensor and 7-LED (light-emitting diode) lighting system. A magnification up to 100 can be obtained. It has four preset focus positions and three operating modes (perio, cario and daylight).
The second camera is a ‘consumer’ camera (six-LED Xiaocui Digital Micro-Check Camera). It is CE marked, Restriction of Hazardous Substances Directive compliant without medical device certification. It is used to provide images for patients’ health education. It offers a high definition of 2 megapixels with a six-LED lighting system. However, it has only a wide-angle lens going from 2 to 4 cm.
Equipment
For GS intraoral examination
The patient is comfortably seated in a chair or armchair to perform the examination. The dental surgeon will be equipped with a disposable mask, gown and gloves.
For intraoral camera examinations
The two cameras will be connected to the Covotem software (Maincare Society, Canejan, France) via the Pulsy platform (public interest grouping validated by the Grand Est Regional Agency for telemedicine).
Inclusion and exclusion criteria for participants
Inclusion will be based on four eligibility criteria: (1) being of legal age; (2) being an associate or beneficiary of the health system; (3) being hosted in one of the four elderly care facilities; (4) having received and signed (or legal guardian) the informed consent. The inclusion of specially protected people is justified by the fact that they have more difficulty in expressing pain, discomfort possibly of dental, periodontal or injury-related origin due to an unsuitable prosthesis. This will have an immediate impact on their quality of life, let alone their ability to eat and their general health.
The criteria for non-inclusion of the participants were: (1) to be deprived of liberty by judicial or administrative decision or to be the subject under psychiatric care according to articles L. 3212-1 and L. 3213-1 of the French public health code36; (2) to have an insufficient command of the French language; (3) to be wearing a complete bimaxillary prosthesis; (4) to be totally toothless.
The participant will be excluded from the study if the patient shows insufficient cooperation or other impossibilities to perform the clinical or teledental examination.
Selection process and examination pathways
Selection of study participants
Within each elderly care facility and after consultation with each coordinating physician, the investigator will identify the patients eligible for the study and those who volunteer to participate. Once an ID number has been assigned to those patients, a random draw of 30 ID numbers will be made in three of the facilities and 60 in the largest, from the list of ID numbers. The inclusion will begin with the lowest number and will stop after the adequate inclusion of 12 or 24 participants depending on the elderly care facility.
Exclusion of a patient
In the event that a patient has to be excluded from the study, he/she will be replaced by the next number on the list. The envelope already opened will be used for the next patient.
Sequence of examinations
Thirty to 60 sealed envelopes (depending on the size of the elderly care facility) will be prepared for each elderly care facility. Each envelope will indicate the sequence of examinations to be conducted. These envelopes will be numbered from 1 to 30 (or 1 to 60).
The order of the examinations will be determined randomly from the following six possible combinations: (1) GS examination, Soprocare camera, Xiaocui camera; (2) GS examination, Xiaocui camera, Soprocare camera; (3) Soprocare camera, Xiaocui camera, GS examination; (4) Soprocare camera, GS examination, Xiaocui camera; (5) Xiaocui camera, GS examination, Soprocare camera; (6) Xiaocui camera, Soprocare camera, GS examination.
Outcome measures
The primary study outcome will be the presence of at least one decayed tooth in the dentition of each elderly study participant evaluated due to the three diagnostic techniques (during the GS examination, from the video recording acquired with the Soprocare camera, from the video recording acquired with the Xiaocui camera).
The secondary study outcomes will be:
The presence of at least one filled tooth, the absence of at least one tooth, the presence of gingivitis, of an abscess, of dental plaque and calculus in the elderly of the study evaluated by the intraoral clinical examination and the video records of each of the two cameras.
The number of decayed, filled, missing teeth in the elderly of the study recorded assessed by the intraoral clinical examination and the video recording from both of the cameras.
The presence of each dental condition (at least one tooth decayed, filled, missing) and each periodontal condition (Löe & Silness Gingival Index (0–3), periodontal abscess (presence or absence), Silness & Löe Plaque Index (0–3), or calculus (absent; present in small amounts; present in large amounts)) assessed by two independent reviewers from video records of each camera.
The number of teeth showing each dental condition (at least one tooth decayed, filled, missing) assessed by two independent reviewers from video records of each camera.
The presence of a decayed, filled or missing tooth, assessed from each tooth of the elderly of the study during the clinical examination and the records of each camera.
The video recording time evaluated for each patient of the study.
If a dental or periodontal condition has been found during the intraoral clinical examination, the coordinating investigator (CC) will assess by telephone whether treatment has been carried out within 2 months of this examination. If treatment was not performed during this 2-month follow-up period, the coordinating investigator will record whether an appointment has been scheduled for this necessary care.
Data acquisition
For the Soprocare camera, and in this study, the image acquisition will be in ‘intraoral’ setting allowing to see one to five teeth and in daylight mode. It will be connected to a computer by USB (Universal Serial Bus) 2.0 with the proprietary software.
For the second camera called ‘consumer’ camera (six-LED Xiaocui Digital Micro-Check Camera), it is a USB plug and play and does not require any proprietary software.
The two cameras will be connected to the Covotem software, which will store the data on a secure server and manage the data of each patient. It will also provide the information system necessary for data transfer, the secure identification of the various participants and the production of a report for each patient.
Examinations
GS intraoral examination
During this step, the qualified dental surgeon (YS) will have a chart to annotate the dental and periodontal status, the duration of this examination and the time between the other examinations if not performed on the same day. All data from the intraoral examinations will be manually transcribed into the case report form, that is, clinical values and the duration of the three examinations.
Intraoral camera examinations
During this step, the qualified dental surgeon (YS) will only have a chart to note the duration of each of the two examinations performed and the time between each examination if not performed on the same day.
Data recording and variables
First, a single trained and qualified dental surgeon (YS) will fill in a file about the patient’s health condition and the dedicated questionnaire containing basic demographic data and type of diet, with the patient’s help. The patient will be seated on a chair. Then, three examinations will be successively performed, on the same day if possible.
GS intraoral examination
The qualified dental surgeon (YS) will look for dental diseases (decayed, filled or missing tooth) and periodontal diseases (Löe & Silness Gingival Index (0–3), periodontal abscess (presence or absence), Silness & Löe Plaque Index (0–3), or calculus (absent; present in small amounts; present in large amounts)).
Intraoral camera examinations
The same trained dental surgeon (YS) will collect the required data. He will record four intraoral videos in this order: upper right, upper left, lower left and lower right, for each of the two cameras (one video per section of the mouth, including the palatal/lingual, buccal and occlusal surfaces of each tooth). The patient will be already seated on a chair facing a laptop computer. The dental surgeon will stand behind the patient and use his right hand to hold the camera, and his left hand to spread the cheeks and move the camera head. The images will appear directly on the laptop screen. He will measure the time required for each camera pass per patient.
Asynchronous teledentistry for intraoral cameras
The analysis: two dental surgeons (CC and PB) will provide an asynchronous, remote and independent diagnosis (dental and periodontal status described above) using only the information recorded at the site where the intraoral examinations were performed.
Statistical analysis plan
All statistical analyses will be performed by Statistical Analysis System V.9.4 software (SAS Institute).
Descriptive analysis
A description of the sample will be made by calculating absolute and relative frequencies of categorical variables; and means and SDs or medians, and statistical ranges of discrete and continuous variables.
Percentages of patients who received a dental or periodontal treatment, or for whom an appointment is scheduled, will be treated by the same methods.
Diagnostic performances for the detection for each of studied events/quantification for each of studied events using the clinical examination as the GS
Categorical outcomes: for each camera, the diagnostic performances (sensitivity, specificity, positive predictive value and negative predictive value) of each camera will be calculated using the clinical examination as a reference.
Numerical outcomes: for each patient, the difference in number of events counted with the clinical examination and each camera will be recorded.
The diagnostic performances of each camera will be evaluated (1) for each observer in charge of the video analysis and (2) after comparing the results produced by the two observers and resolving any discrepancies.
Degree of agreement between the two cameras for the detection/quantification of the events studied
Categorical outcomes: from the results of the video analysis by each observer, the level of agreement—of each camera and the GS clinical examination—on each dental or periodontal disease will be evaluated through a kappa coefficient.
Numerical variables: from the results of the video analysis by each observer, the level of concordance in counting the number of teeth with each of the dental diseases studied will be evaluated by an intraclass correlation coefficient.
Comparison
The comparison of the recording time of each intraoral camera will be performed with the Student’s t-test and the Wilcoxon signed-rank test.
Measures to reduce bias
To reduce and avoid analysis bias, two draws will be made in this study:
A randomisation for the selection of participants among volunteers within each elderly care facility.
A randomisation to determine the order of the three intraoral examinations performed for each participant: two with intraoral cameras and the intraoral clinical examination done face-to-face (GS).
Then, this study will investigate the use of asynchronous teledentistry (also known as store and forward dental telemedicine), which consists of a delayed image examination.
Two diagnostic interpretations of the videos recorded with the two intraoral cameras will be performed asynchronously and independently by two dental surgeons who have different practices and do not work in the same clinic.
Ethics and dissemination
The data will be collected and analysed anonymously. Patient consent is required for such a study, and will be collected, according to the French legislation. This study is conducted according to the principles of the Declaration of Helsinki. It is an interventional research with minimal risks and minimal burden. So, the experimental protocol (ONE-1) has been approved by the French ethics committee (CPP Nord-Ouest IV in June 2021 and in November 2022). It was registered on the ClinicalTrials.gov platform (NCT05089214). All methods were applied in accordance with the relevant guidelines and regulations. In this French regional study, consent to participate is required, as approved by the CPP. The consent of all study subjects will be collected after all clear, appropriate, and fair information on the objectives of the study and their right to refuse to participate has been provided (and to the legal representative if necessary). It will also be emphasised that the choice to refuse will have no impact on their care within the institution.
The results of the study will be presented at scientific and medical conferences and will be published in peer-reviewed journals following the STARD (Standards for Reporting Diagnostic accuracy studies) guidelines. The publication of the study results will not depend on the nature of the results.
The coordinating investigator will make an oral and written presentation of this pilot study results to each patient who requests it to the geriatrician (and/or his/her representative).
Expected results
Significant barriers to accessing dental services exist. Elderly people living in care facilities are often physically and cognitively impaired, medically compromised and dependent on others to maintain their oral hygiene. Our work will support the need for a pragmatic approach with a willingness to include oral health as part of the overall primary healthcare. The implementation of teledentistry in elderly care facilities will contribute to a better management of the oral health of the elderly and their quality of life by allowing better triage of care and prevention needs, as well as rationalising the time and travel of all parties.
Supplementary Material
Acknowledgments
The authors thank the Agence Régionale de Santé Grand-Est (Direction de la Stratégie). They also thank Dr F Menager (Québec, Canada) for her assistance in English. The sponsor was CHRU de Nancy (Direction de la Recherche et de l’Innovation).
Footnotes
Twitter: @CLEMENTCELINE5
TV and YS contributed equally.
Contributors: AB, CC, PB and TR designed the study. CC, TV and YS drafted the manuscript. AB and TR revised the manuscript. CC and PB found the funding for the research. CC and BK supervised the study. CC, PB and YS will perform clinical examination and video acquisition or analysis. CC will participate in data analysis and interpretation. All authors have read and approved the final manuscript.
Funding: The Agence Régionale de Santé Grand-Est supports this study.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Spanemberg JC, Cardoso JA, Slob E, et al. Quality of life related to oral health and its impact in adults. J Stomatol Oral Maxillofac Surg 2019;120:234–9. 10.1016/j.jormas.2019.02.004 [DOI] [PubMed] [Google Scholar]
- 2.Northridge ME, Kumar A, Kaur R. Disparities in access to oral health care. Annu Rev Public Health 2020;41:513–35. 10.1146/annurev-publhealth-040119-094318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colet M, Sicart D. La démographie des chirurgiens-dentistes à l’horizon 2030. Etudes et Résultats, 595. DREES, 2007: 1–8. Available: https://drees.solidarites-sante.gouv.fr/publications/etudes-et-resultats/la-demographie-des-chirurgiens-dentistes-lhorizon-2030-un-exercice#:~:text=Si%20le%20numerus%20clausus%20%C3%A9tait,2006%20%C3%A0%2040%20en%202030 [Google Scholar]
- 4.Vergnes JN, Mazevet M. Oral diseases: a global public health challenge. Lancet 2020;395. 10.1016/S0140-6736(19)33015-6 [DOI] [PubMed] [Google Scholar]
- 5.Observatoire des territoires . Densité de chirurgien-dentistes libéraux (pour 100 000 personnes). 2020. Available: https://www.observatoire-des-territoires.gouv.fr/densite-de-chirurgiens-dentistes-liberaux [Accessed Feb 2022].
- 6.Peres MA, Macpherson LMD, Weyant RJ, et al. Oral diseases: a global public health challenge. Lancet 2019;394:249–60. 10.1016/S0140-6736(19)31146-8 [DOI] [PubMed] [Google Scholar]
- 7.D’Addazio G, Santilli M, Sinjari B, et al. Access to dental care-A survey from dentists, people with disabilities and caregivers. Int J Environ Res Public Health 2021;18:1556. 10.3390/ijerph18041556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inquimbert C, Balacianu I, Huyghe N, et al. Applications of teledentistry in a french inmate population: a one-year observational study. PLoS One 2021;16:e0247778. 10.1371/journal.pone.0247778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vettore MV, Faerstein E, Baker SR. Social position, social ties and adult’s oral health: 13 year cohort study. J Dent 2016;44:50–6. 10.1016/j.jdent.2015.12.004 [DOI] [PubMed] [Google Scholar]
- 10.Gopalakrishnan A, Kahu E, Jones L, et al. Access and barriers to oral health care for dependent elderly people living in rest homes. Gerodontology 2019;36:149–55. 10.1111/ger.12392 [DOI] [PubMed] [Google Scholar]
- 11.Union Regionale des Caisses d’Assurance Maladie . État de santé bucco-dentaire des personnes âgées hébergées en établissement. 2003. Available: http://aspbd.fr/wp-content/uploads/2019/03/Epidemio_EHPAD_Pays_de_Loire.pdf [Accessed Feb 2022].
- 12.Bernard MF. Soins d’hygiène bucco-dentaire aux personnes âgées et dépendantes. Rueil Malmaison MA: Editions Lamarre; 2016. 164. [Google Scholar]
- 13.Bertrand MF, Macqueron N, Balard P, et al. Hiérachiser les facteurs de risque de dégradation de la santé orale des personnes âgées dépendantes en EHPAD. La Revue de Gériatrie 2015;40:261–70. Available: https://documentation.ehesp.fr/index.php?lvl=notice_display&id=311611 [Google Scholar]
- 14.Mangeney K, Barthélémy H, Vogel T, et al. La santé buccodentaire en EHPAD: état des lieux et suivi des recommandations de soins. NPG Neurologie - Psychiatrie - Gériatrie 2017;17:93–9. 10.1016/j.npg.2016.04.003 [DOI] [Google Scholar]
- 15.Thiebaut S, Lupi-pegurier L, Paraponaris A, et al. Comparaisondu recours à un chirurgien-dentiste entre les personnes âgéesinstitutionnnalisées et celles vivant à domicile, france, 2008-2009. Bulletin Épidémiologique Hebdomadaire 2013;7:60–4. Available: https://www.santepubliquefrance.fr/docs/comparaison-du-recours-a-un-chirurgien-dentiste-entre-les-personnes-agees-institutionnalisees-et-celles-vivant-a-domicile-france-2008-200 [Google Scholar]
- 16.Maille G, Saliba-Serre B, Ferrandez AM, et al. Objective and perceived oral health status of elderly nursing home residents: a local survey in Southern France. Clin Interv Aging 2019;14:1141–51. 10.2147/CIA.S204533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Algra Y, Haverkort E, Kok W, et al. The association between malnutrition and oral health in older people: a systematic review. Nutrients 2021;13:3584. 10.3390/nu13103584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindmark U, Ernsth Bravell M, Johansson L, et al. Oral health is essential for quality of life in older adults: a Swedish national quality register study. Gerodontology 2021;38:191–8. 10.1111/ger.12514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rapp L, Sourdet S, Lacoste-Ferré MH. Oral health and undernutrition in the frail elderly persons. J Nutr Health Aging 2021;25:484–91. 10.1007/s12603-020-1546-6 [DOI] [PubMed] [Google Scholar]
- 20.Bourgeois D, Inquimbert C, Ottolenghi L, et al. Periodontal pathogens as risk factors of cardiovascular diseases, diabetes, rheumatoid arthritis, cancer, and chronic obstructive pulmonary disease-is there cause for consideration? Microorganisms 2019;7:424. 10.3390/microorganisms7100424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bui FQ, Almeida-da-Silva CLC, Huynh B, et al. Association between periodontal pathogens and systemic disease. Biomed J 2019;42:27–35. 10.1016/j.bj.2018.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maitre Y, Mahalli R, Micheneau P, et al. Evidence and therapeutic perspectives in the relationship between the oral microbiome and Alzheimer’s disease: a systematic review. Int J Environ Res Public Health 2021;18:11157. 10.3390/ijerph182111157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borsa L, Dubois M, Sacco G, et al. Analysis the link between periodontal diseases and Alzheimer’s disease: a systematic review. Int J Environ Res Public Health 2021;18:9312. 10.3390/ijerph18179312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ortíz-Barrios LB, Granados-García V, Cruz-Hervert P, et al. The impact of poor oral health on the oral health-related quality of life (ohrqol) in older adults: the oral health status through a latent class analysis. BMC Oral Health 2019;19:141. 10.1186/s12903-019-0840-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rapp L, Sourdet S, Vellas B, et al. Oral health and the frail elderly. J Frailty Aging 2017;6:154–60. 10.14283/jfa.2017.9 [DOI] [PubMed] [Google Scholar]
- 26.World Health Organisation (WHO) . Fifty eight world health assembly. 2005. Available: https://apps.who.int/iris/bitstream/handle/10665/20378/WHA58_28-en.pdf?sequence=1 [Accessed Feb 2022].
- 27.Giraudeau N, Valcarcel J, Tassery H, et al. Projet e-DENT: téléconsultation bucco-dentaire en EHPAD. Eur Res Telemed 2014;3:51–6. 10.1016/j.eurtel.2014.04.005 [DOI] [Google Scholar]
- 28.Queyroux A, Saricassapian B, Herzog D, et al. Accuracy of teledentistry for diagnosing dental pathology using direct examination as a gold standard: results of the tel-e-dent study of older adults living in nursing homes. J Am Med Dir Assoc 2017;18:528–32. 10.1016/j.jamda.2016.12.082 [DOI] [PubMed] [Google Scholar]
- 29.Golder DT, Brennan KA. Practicing dentistry in the age of telemedicine. J Am Dent Assoc 2000;131:734–44. 10.14219/jada.archive.2000.0272 [DOI] [PubMed] [Google Scholar]
- 30.Mahdi SS, Allana R, Amenta F. Teledentistry-based program to improve oral hygiene indicators in rural pakistan-A protocol. J Contemp Dent Pract 2021;22:406–11. [PubMed] [Google Scholar]
- 31.Aquilanti L, Santarelli A, Mascitti M, et al. Dental care access and the elderly: what is the role of teledentistry? A systematic review. Int J Environ Res Public Health 2020;17:9053. 10.3390/ijerph17239053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haute autorite de sante. stratégies de prévention de la carie dentaire, synthèses et recommandations. 2011. Available: https://www.has-sante.fr/upload/docs/application/pdf/2010-10/corriges_synthese_carie_dentaire_version_postcollege-10sept2010.pdf [Accessed Feb 2022].
- 33.Défenseur des droits de la République française . Rapport sur les droits fondamentaux des personnes âgées accueillies en EHPAD. 2021. Available: https://www.defenseurdesdroits.fr/fr/rapports/2021/05/rapport-les-droits-fondamentaux-des-personnes-agees-accueillies-en-ehpad [Accessed Feb 2022].
- 34.Mariño R, Tonmukayakul U, Marwaha P, et al. Teleconsultation/telediagnosis using teledentistry technology: a pilot feasibility study. Journal Contribution 2014. Available: https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=0bb565c5015cdb5281446e8c18850c4040c9874a0bb565c5015cdb5281446e8c18850c4040c9874a [Google Scholar]
- 35.Thabane L, Ma J, Chu R, et al. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol 2010;10:1. 10.1186/1471-2288-10-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Code de la santé publique. Articles L. 3212-1 et L. 3213-1. Available: https://www.legifrance.gouv.fr/codes/section_lc/LEGITEXT000006072665/LEGISCTA000006140615/#LEGISCTA000006140615 [Accessed Feb 2022].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


