Abstract
While many studies have investigated the use of recombinant adeno-associated vectors (rAAV) in the posterior chamber for treatment of inherited retinal diseases, fewer studies have looked at rAAV’s ability to transduce cells within the anterior chamber. This study focuses on evaluating the tropism and tolerability of three rAAV serotypes - rAAV2/6, rAAV2/9, and rAAV2/2[MAX] – expressing a green fluorescent protein (GFP) reporter following intracameral injection in the non-human primate (NHP) African green monkey (Chlorocebus sabaeus) model. Injection of high dose (1×1012 vg/eye) rAAV vector resulted in transient inflammation characterized by aqueous flare and cellular infiltrate that resolved without intervention in all serotypes. Post-mortem histology revealed widespread expression of GFP in cells of the trabecular meshwork and iris in high dose rAAV2/6, rAAV2/9, and particularly rAAV2/2[MAX] eyes, indicating that rAAV vectors of these serotypes have broad tropism for cells of the anterior chamber and may facilitate the treatment of blinding disorders, such as glaucoma.
INTRODUCTION
The anterior chamber of the eye functions to focus light upon the retina to facilitate vision, consisting of the cornea, trabecular meshwork, iris, ciliary body, and lens. Anterior chamber dysfunctions, such as corneal opacities, endothelial dystrophies, and glaucoma together account for ~12% of global blindness and have limited treatment options (1). While corneal dystrophies oftentimes have a defined genetic etiology, many diseases such as keratoconus, diabetic keratopathy, or glaucoma are complex multifactorial disorders characterized by abnormal alterations in downstream signaling pathways, such as changes in extracellular matrix depositions and the TGFβ and Wnt signaling pathways (2–5). Whether an anterior chamber disorder is caused by a single gene defect (e.g., Fuch’s endothelial dystrophy) or has multiple genetic and environmental risk factors, diseases of the anterior chamber may be amenable to treatment through gene therapy approaches by gene augmentation or manipulation of pathway biology, respectively.
Traditionally, ocular gene therapy has primarily been focused on two major injection routes – intravitreal and subretinal – in order to target differing layers of the retina or retinal pigment epithelium (RPE) for the treatment of inherited retinal diseases, with relatively little research focused on the use of rAAV vector to transduce cells of the anterior chamber (6,7). While it is possible for rAAV injected intravitreally to transduce the anterior chamber owing to the posterior-to-anterior directional flow of the aqueous humor, intracameral injections offer a direct administration route to anterior chamber structures, such as the corneal endothelium, iris, and trabecular drainage pathways without unwanted dilution of the vector in the vitreous (8). While the majority of ophthalmic gene therapy studies in the past two decades have focused on targeting transgene delivery to cells of the retina for the treatment of neurodegenerative disease, there have been several attempts to define the tropism of rAAV for cells of the anterior chamber that together informed serotype selection for the present study (9,10). Specifically, rAAV serotypes rAAV2/2, 2/6 and 2/9 have been shown to transduce human trabecular meshwork cells in vitro with varying degrees of efficiency, and also to mediate efficient gene transfer to cells of the cornea in rabbits following epithelial debridement and topical application (11) (12). A small number of studies have also evaluated the tropism of self-complementary rAAV2/2 vectors in rat and non-human primate (e.g. macaca fascicularis) models, showing sustained transduction of corneal endothelium, iris, ciliary body, and trabecular meshwork (13,14). Lastly, efforts have also been made in mouse and rat models to improve the tropism of rAAV vectors delivered intracamerally through the use of capsid mutant serotypes, such as rAAV2/2(TrpYF), which contains three Y-F point mutations (Y444F, Y500F, Y730F) that decrease proteasomal degradation of the virion, leading to improved transduction of the corneal endothelium, chamber angle, and iris (15). Here we aim to build upon these studies by directly comparing the tropism of multiple rationally selected rAAV serotypes in the NHP anterior chamber, including rAAV2/6, rAAV2/9 and rAAV2/2[MAX], a capsid mutant vector that contains several point mutations (Y272F, Y444F, T491V, Y500F, Y730F) and the 7m8 peptide insertion (N587-R588insLALGETTRPA) that further decrease proteasomal degradation and tissue penetrance.
The African green monkey (AGM), or Caribbean vervet, is an Old-World non-human primate species, a subpopulation of which was introduced to the West Indies in the 1600s, most notably St. Kitts, where a colony of AGMs reside at the St. Kitts Biomedical Research Foundation. Given their biologic isolation, St. Kitts AGMs are not carriers of pathogens common to continental NHPs such as simian immunodeficiency virus and Marberg virus, and as a species are not carriers of macacine herpesvirus I (16,17). Moreover, they exhibit a comparatively low prevalence of AAV exposure to multiple serotypes making them a favorable test system for AAV vector evaluation. Additionally, having undergone a major population bottleneck during their introduction to the island, a substantial founder effect has occurred, leading to levels of homozygosity that enable genotype/phenotype correlations with respect to disease relevant genes, furthering their value for biomedical research. Herein, we investigate three rAAV serotypes injected intracamerally - rAAV2/2[MAX], rAAV2/6, and rAAV2/9 - into the anterior chamber to determine both the safety and efficacy of transduction of the anterior chamber tissues in the AGM.
METHODS
Vector Production:
Vector production was carried out following a previously described protocol (18). Briefly, rAAV vectors were manufactured in adherent HEK293T (ATCC no. CRL-11268; Manassass, VA, USA) cells seeded in hyperflasks (Corning, Corning, NY) using a triple plasmid transfection approach, wherein each cell receives: 1) a plasmid containing inverted terminal repeats flanking a self-complementary green fluorescent protein (GFP) transgene cassette, 2) a helper plasmid coding for adenovirus helper genes necessary for cis-packaging, and 3) a Rep/Cap plasmid expressing viral replicatory and capsid genes necessary for packaging of each vector serotype, namely rAAV2/2[MAX], rAAV2/6, or rAAV2/9. Cell lines used were not tested for mycoplasma contamination. Plasmids were transfected using polyethylenimine (PEI, Polysciences, #N23966–100, PA, USA) in a 1:1:1 equimolar ratio based on plasmid size to a total of 500 μgs DNA per hyperflask (Corning, Corning, NY). Following a 72-hour incubation to allow for viral production, cells were recovered from each flask by manual displacement and concentrated via centrifugation (370,000×g, 1.5 hours). rAAV virions were recovered from the resulting HEK293T cell pellets using repetitive (4x) freeze-thaw lysis before being purified using an iodixanol density gradient separation, as described previously (18,19). The purified vector was then concentrated through buffer exchange as described previously and titered via a PicoGreen assay (Thermo Fisher Scientific, Waltham, MA) (18,20). Following purification and titration, all vector preparations were screened to confirm the absence (<5EU/ml) of endotoxin (Endosafe Nexgen-PTS, Charles River, Wilmington, MA) and then assigned an anonymous identifier (e.g. ‘A’, ‘B’ or ‘C’) so as to mask the injecting surgeon and in vivo investigators with respect to vector serotype and dose (‘low’ or ‘high’).
Animals and Anesthesia
12 African green monkeys (Chlorocebus sabaeus) exhibiting normal health were screened by slit lamp exams and tonometry to identify animals with normal ocular anatomy and IOPs greater than 18 mmHg. Exams were performed under sedation with ketamine (8 mg/kg) and xylazine (1.6 mg/kg) and sensitivity to anesthesia associated IOP fluctuations was assessed by measuring IOP 5 and 15 minutes after administration and any animals exhibiting greater than a 4 mmHg decrease over this time period were excluded. Neutralizing antibody (NAb) screening was additionally conducted to select animals with an absence of AAV2, AAV6 and AAV9 NAbs at a serum dilution of 1:20 by luciferase inhibition assay. Animals with an absence of Nabs were chosen to complete the study (N=6). All experiments adhere to the Association for Research in Vision and Ophthalmology statement for the use of animals in ophthalmic and vision research.
Intracameral Injection
Under anesthesia, 0.5% proparacaine hydrocholoride was applied topically followed by lid speculum placement and aseptic preparation of the eye with 5% Betadine solution rinsed with a sterile saline. Each eye was injected with 100 μL vector suspended in HBSS + 0.014% Tween-20 using a 31-gauge 5/16” needle placed into a 0.3 mL syringe. The needle was inserted temporally 2 mm anterior to the limbus in the coronal plane and when removed, a topical triple antibiotic (neomycin, polymyxin, bacitracin) ophthalmic ointment was applied.
Tonometry
Tonometry was competed at baseline, and days 0 (dosing), 3, 7, 14, 21, 28, 25, and 42 (Table 3) with three measurements at each time point obtained at a consistent circadian interval (between 8–10 am). IOP measurements were recorded within 5 minutes of sedation with the animal placed in the supine position using a TonoVet tonometer (Icare, Oy, Finland) applying the dog calibration setting.
Table 3. Timeline of ocular assessments.
All AGMs underwent baseline, tonometry, pachymetry, slit- lamp biomicroscopy, fundus photography, and optical coherence tomography. This study concluded at day 42, wherein all assessments were repeated. Various assessments were completed at differing timepoints listed throughout the study.
| Evaluation | Timeline | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Day 0 | Day 3 | Day 7 | Day 14 | Day 21 | Day 28 | Day 35 | Day 42 | |
| Vector Administration | - | X | - | - | - | - | - | - | - |
| Tonometry | X | X | X | X | X | X | X | X | X |
| Pachymetry | X | - | - | X | - | X | - | - | X |
| Slit Lamp | X | X | X | X | X | X | X | X | X |
| Fundus Photography | X | - | X | - | X | - | - | - | X |
| Optical Coherence Tomography | X | - | - | X | - | X | - | - | X |
Slit Lamp Biomicroscopy
Slit lamp exams were completed at baseline, and days 0, 3, 7, 14, 21, 28, 35, and 42 post-injections. The nonhuman primate ophthalmic exam scoring system (21) was applied to quantitively define conjunctival congestion, swelling, and discharge, pannus, corneal clouding, inflammatory keratic precipitates, aqueous cell, hypopyon, fibrin strands, aqueous flare, iris hyperemia, iris exfoliation, iris synechia, anterior and posterior lens deposits and opacity, vitreous cell and haze, retinal vasculitis, and papillitis. Each of these parameters were scored according to incident severity with scores summed to derive a total clinical score (Supplemental table 1).
Pachymetry
An Accutome AccuPach 5 ultrasound pachymeter (Keeler, Malvern, PA) was used to measure corneal thickness at baseline, days 7, 21, and 42 post-injections, with three measurements of corneal thickness obtained in succession to derive an average measure per eye per time point.
Optical Coherence Tomography (OCT)
OCT was completed at baseline and days 7, 21, and 42 using a Heidelberg Spectralis OCT Plus (Heidelberg, Germany) with eye tracking and HEYEX image capture and analysis software. The macula, peri-macula, temporal arcades, and optic nerve were imaged by obtaining a scan encompassing the central posterior pole at a dense scan interval and retinal thickness and volume derived from the HEYEX software, following automated segmentation.
General Ophthalmic Imaging
Color fundus imaging and anterior segment photography was performed with a Topcon TRC-50EX (Topcon, Livermore, CA) retinal camera equipped with Canon 6D digital imaging hardware and New Vision Fundus Image Analysis software. Color fundus photos were captured with a shutter speed of 1/25 sec, ISO of 400, and flash of 18.
Euthanasia and Tissue Collection
Animals were euthanized with ketamine/xylazine followed by sodium pentobarbital delivered intravenously (100 mg/kg), after which eyes were enucleated and a suture placed in the limbal conjunctiva at the 12:00 o’clock position to maintain orientation during histology. Globes were then intravitreally injected with 500 μL of 4% paraformaldehyde (PFA) to facilitate reinflation, and the globe submerged in 4% PFA. After 6 hours, eyes were transferred to PBS with 0.05% sodium azide and sent to Medical College of Wisconsin for tissue processing.
Tissue Processing
Eyes were quartered and cryoprotected in 30% sucrose overnight. Tissue was then submerged in optimal cutting temperature media (Sakura, Torrence, CA) and frozen on dry ice before being sectioned on a Leica CM1860 cryostat (Leica, Buffalo Grove, IL) in 12 μm sections onto Fisherbrand Superfrost Plus slides and left to dry overnight. Sections were subsequently stored at −20°C until use, at which point they were thawed for 2 hours at room temperature, and the optimal cutting temperature media washed off using PBS. Sections were then incubated 1:200 with anti-GFP antibody (Millipore, MAB2580, St. Louis, MO) and counterstained with Hoechst 3342 for nuclei visualization. Sections were imaged with a Nikon Eclipse 80i confocal microscope (Nikon, Tokyo, Japan) at 20x, and processed as maximum intensity projections and merged in FIJI.
RESULTS
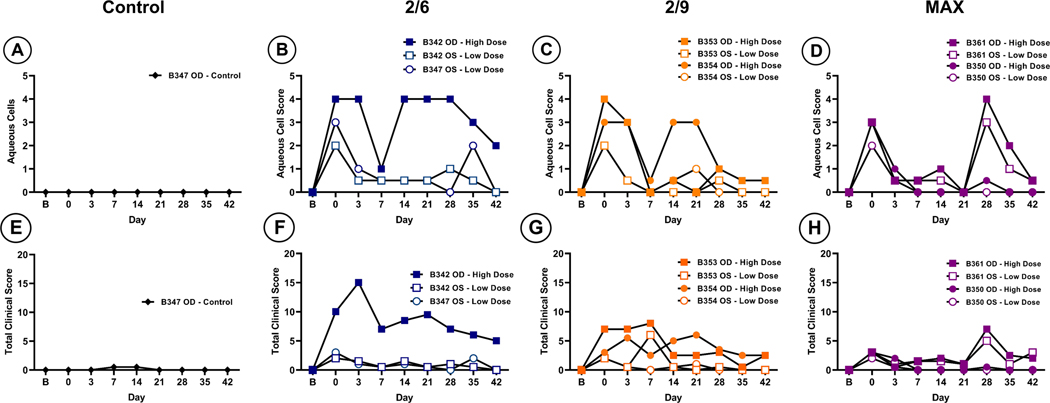
Intracameral administration of rAAV causes transient inflammation that resolves without pharmacological intervention in naïve AGMs
To limit the potential for an adaptive immune response following rAAV vector injection, sera from twelve NHP subjects were screened prior to enrollment to confirm the absence of NAbs raised against wild-type AAV2, AAV6, or AAV9 infections (Table 1). Six subjects (B342, B347, B353, B354, B350 and B361) with no detected NAbs for respective serotypes at serum dilutions of 1:20 were subsequently selected and assigned to receive intracameral injections of either buffer (control, N=1 eye) or rAAV2/2[MAX], rAAV2/6 or rAAV2/9 at either a low (1×1011vg/eye) or high (1×1012 vg/eye) dose (N=2 eyes per group; see Table 2). Slit lamp examinations were completed on all subjects at baseline (one day prior to injection), immediately following injection (day 0) and at day 3, 7, 14, 21, 28, 35, and 42 post-injection (Table 3); images were graded based on the applied nonhuman primate ophthalmic exam scoring system (21) by observers masked to treatment. Despite the seronegative status of the subjects, aqueous cell scores increased in all rAAV treated eyes regardless of dose or serotype immediately following injection (day 0) and was maintained until day 3, indicative of an acute innate immune response to the vector capsid (Figure 1, B–D). Importantly, this inflammation resolved by day 7 in all animals without pharmacological intervention. Between days 14 and 28 in high dose rAAV treated eyes, a secondary, potentially adaptive phase of inflammation was observed, but this also resolved without pharmacological intervention by the end of the study at day 42 (Figure 1, B–D). Total clinical scores, including aqueous cell and various metrics of conjunctival, corneal, iris, lens, and posterior chamber health (Figure 1, E–H) remained generally low (total clinical score <10) in all treated eyes, with the exception of B342 OD, which received an injection of high dose rAAV2/6 (Figure 1, F). This eye exhibited a total clinical score of 15 at day 3 due to cell and fibrin strand presence, keratic precipitates, as well as iris hyperemia and anterior lens deposits, which resolved progressively between days 5 and 42 (Figure 1, F). Eyes treated with rAAV2/9 (animals B353 and B354) exhibited total clinical score fluctuations between 0–7 with varying levels of cell and fibrin strand presence in addition to iris hyperemia and anterior lens deposits (Figure 1, G). rAAV2/2[MAX] treated eyes presented clinical scores of ≤ 7 wherein most activity was seen at day 0 immediately following vector administration. These findings mostly resolved by day 14, however, a secondary phase was also found at day 28 in B361 OD, which was treated with high dose rAAV2/2[MAX] and was found to have increased scores, reflecting presence of keratic precipitates and iris hyperemia (Figure 1, H). In depth findings, indicating individual values for each parameter investigated, can be found in Supplemental Table 1.
Table 1. Serum testing of AGMs for rAAV neutralizing antibodies.
12 AGMs were tested for pre-existing neutralizing antibodies specific to the capsids of rAAVs 6,9, and 2. Two AGMs per serotype were chosen to continue with the study, noted in blue.
| AAV6 Nab | Assigned Group | AAV9 Nab | Assigned Group | AAV2 Nab | Assigned Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serum Dilutions | Serum Dilutions | Serum Dilutions | ||||||||||||
| ID | 1:5 | 1:20 | 1:80 | ID | 1:5 | 1:20 | 1:80 | ID | 1:5 | 1:20 | 1:80 | |||
| B342 | - | - | - | 2/6 | B342 | - | - | - | x | B342 | + | - | - | x |
| B343 | - | - | - | x | B343 | - | - | - | x | B343 | + | + | - | x |
| B344 | - | - | - | x | B344 | - | - | - | x | B344 | + | + | - | x |
| B346 | - | - | - | x | B346 | - | - | - | x | B346 | + | + | - | x |
| B347 | - | - | - | 2/6 | B347 | - | - | - | x | B347 | + | - | - | x |
| B350 | - | - | - | x | B350 | + | - | - | x | B350 | - | - | - | MAX |
| B353 | - | - | - | x | B353 | - | - | - | 2/9 | B353 | + | - | - | x |
| B354 | - | - | - | x | B354 | - | - | - | 2/9 | B354 | + | - | - | x |
| B361 | - | - | - | x | B361 | - | - | - | x | B361 | + | - | - | MAX |
| B366 | - | - | - | x | B366 | - | - | - | x | B366 | + | + | - | x |
| B367 | - | - | - | x | B367 | - | - | - | x | B367 | + | + | - | x |
| B368 | - | - | - | x | B368 | - | - | - | x | B368 | + | - | - | x |
Table 2. AGM treatment assignments.
Animals underwent 100 μL intracameral injections with one of three rAAV serotypes: rAAV2/6, rAAV2/9, or rAAV2/2[MAX]. Each right eye (OD) was injected with a high dose of vector, while left eyes (OS) were injected with a low dose of vector. One eye, B347 OD, was left as an uninjected control eye.
| Animal ID | Treatment | Eye | Dose (vg/eye) | Volume |
|---|---|---|---|---|
| B347 | rAAV2/6 | OD | Uninjected Control | 100 μL |
| OS | 1×1011 | |||
| B342 | rAAV2/6 | OD | 1×1012 | |
| OS | 1×1011 | |||
| B353 | rAAV2/9 | OD | 1×1012 | |
| OS | 1×1011 | |||
| B354 | rAAV2/9 | OD | 1×1012 | |
| OS | 1×1011 | |||
| B361 | rAAV2/2[MAX] | OD | 1×1012 | |
| OS | 1×1011 | |||
| B350 | rAAV2/2[MAX] | OD | 1×1012 | |
| OS | 1×1011 |
Figure 1. Slit-lamp biomicroscopy of the anterior chamber for indications of inflammation.
All eyes except for the uninjected control (A) exhibited a transient phase of inflammation immediately after vector administration (B-D). In most animals this phase resolved by day 7. One high dose treated eye in each treated group (B342, B354, and B361) exhibited a second phase of inflammation that occurred at days 14 and 28 possibly coinciding with gene expression. In MAX treated eyes, both high and low dose treated eyes in subject B361 showed a second phase of inflammation (D). Total clinical scores compiled from multiple parameters showed increase incidence of clinical findings high dose treated eyes (F-H). Most instances resolved by day 42 without intervention.
Intracameral administration of rAAV temporarily alters intraocular pressure
To assess whether intracameral administration of rAAV2/2[MAX], rAAV2/6, or rAAV2/9 in the NHP anterior chamber affected intraocular pressure, rebound tonometry was completed on all subjects at baseline (one day prior to injection), immediately following injection (day 0) and at 3, 7, 14, 21, 28, 35, and 42 days post-injection (Table 3). Intraocular pressure readings were found to be steady in all baseline and injected eyes across the time course and exhibited only minor fluctuations that remained within the normal IOP range (12–22 mmHG) of the NHP (Figure 2, A–D) (22,23). A minor increase in IOP was observed immediately following vector administration on day 0–3 in all treated animals, as expected following the introduction of 100 μl buffer (HBSS + 0.014% Tween) containing either a low (1×1011vg/eye) or high (1×1012vg/eye) dose of rAAV vector into the anterior chamber. Importantly, this increase returned to baseline by day 3 and no relationship was observed between subsequent IOP fluctuations and vector dose or serotype (Figure 2, A–D).
Figure 2. Intraocular pressures as measured by rebound tonometry.
A transient increase in IOP was seen immediately following vector injection at day 0 (B-D). One MAX treated animal (B361) exhibited a decrease in IOP at day 28 that resolved by day 35 (D).
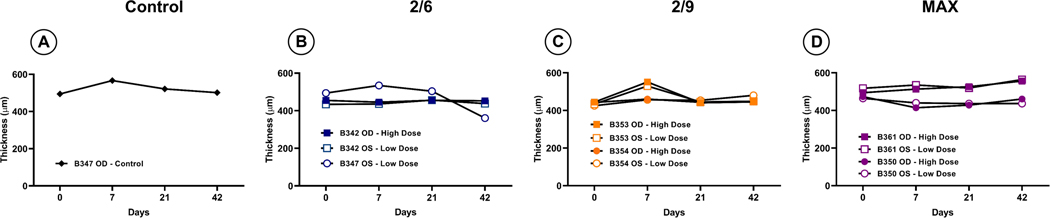
Intracameral rAAV administration does not impact retinal thickness or fundus appearance
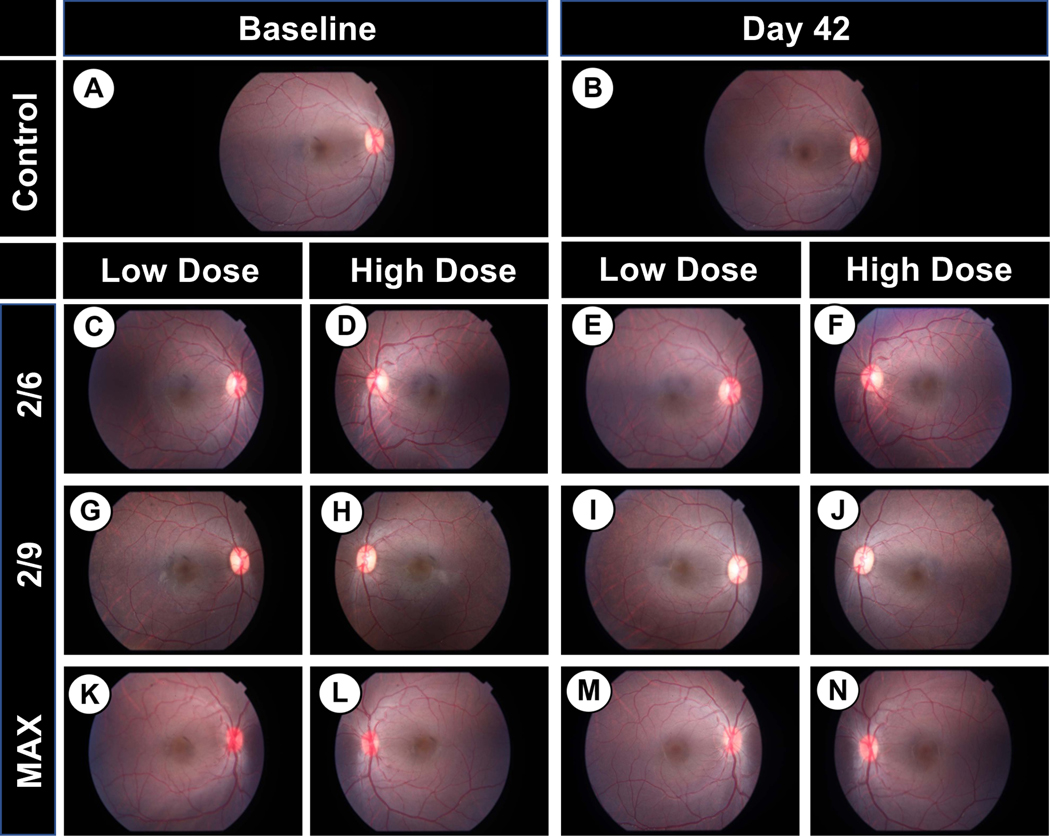
Optical coherence tomography was carried out throughout the study (Table 3) to assess the effects of intracameral rAAV injection on retinal morphology. Retinal thicknesses in the macula and surrounding retina were observed to be maintained throughout the course of this study, with only minor percent changes in thickness (Figure 3, B–H). Importantly, there was no observable trend in retinal thickness measurements at any sector as either a function of vector dose or serotype compared to the uninjected control eye (B347 OD), indicating that intracameral injection of the evaluated doses of rAAV does not alter central retinal thickness. Similarly, fundus photography of the posterior pole including the macula and optic disk revealed no evidence of pathologies (e.g., detachments, disc pallor, pigmentary alterations) when comparing baseline (pre-injection) and day 42 images in any groups, regardless of treatment (control vs. rAAV), dose (low vs. high) or serotype (rAAV2/2[MAX], rAAV2/6 or rAAV2/9) (Figure 4, A–N).
Figure 3.
Average percent change in retinal thickness from baseline to day 42 for each treatment group. A representative cSLO and OCT scan indicates the examined areas (A). Retinal thicknesses stayed largely consistent throughout the study with most deviations staying within 9% of baseline measurements. Exceptions to this trend are seen in the control eye (B), rAAV2/6 low treated group (D), and both rAAV2/2[MAX] treated groups (G, H).
Figure 4. Representative fundus photography at baseline and 42 days.
No changes were found between any eyes at baseline vs. 42 days in either the control (A,B), Low dose treated (C, E, G, I, K, M) or high dose treated eyes (D, F, H, J, L, N).
Intracameral rAAV administration does not consistently alter corneal thickness
Pachymetry was completed at various time points (Table 3) to determine if intracameral administration of rAAV affects corneal thickness in the NHP (Figure 5). Only one animal (B347 OS) that received a low dose of rAAV2/6 showed a decrease in thickness (28%) which occurred between day 21 and day 42 (Figure 5, B). rAAV2/9 treated eyes (B353 OD & OS) showed an 18 and 19% increase, respectively, in corneal thickness at day 7 that resolved by day 42 without intervention (Fig 5, C). MAX treated eyes exhibited consistent corneal thickness throughout all timepoints. (Figure 5, D).
Figure 5. Average corneal thicknesses as determined by pachymetry.
Corneal thickness remained fairly constant with minor fluctuations throughout the study. One low dose treated rAAV2/6 eye (B347 OS) (B) showed the largest decrease in thickness at day 42. An increase in thickness was noted at day 7 in both control and rAAV2/9 treated eyes in subject B353 (A, C). All other measurements remained similar to baseline (A-D).
rAAV transgene expression persists within the iridocorneal angle and iris after 42 days
42 days post-injection all subjects were euthanized and the globes recovered for histological examination in order to determine the cellular tropism of each rAAV serotype. Each of the high dose serotypes injected demonstrated GFP expression evident in the cells surrounding the iridocorneal angle and iris (Figure 6, E–H). High dose injected MAX AGMs exhibited the highest number of transduced cells within the angle (Figure 6H). While no transduction was evident within the cornea or retina (Figure S2, B–D & N–P) of high dose treated AGMs, transduction was evident in the iris (Figure S2, F–H), ciliary body and ciliary muscles of the high dose treated animals (Figure S2, J–K). No evidence of GFP expression was found in any low dose treated iridocorneal angles for any serotype (Supplemental Figure 1, D–F). Additionally, no GFP expression was evident in the cornea, ciliary body, or retina of low dose treated AGMs (Figure S3, B–C, J–L, & N–P respectively). Trace amounts of GFP expression was evident within the iris of low dose treated groups (Figure S3, F–H).
Figure 6. Representative brightfield and confocal microscopy of the control and high dose treated eyes.
Transduction of the iris, and iridocorneal angle was found in rAAV2/6 (B,F) and rAAV2/9 (C,G) treated eyes, while the most transduction was found in rAAV2/2[MAX] treated eyes (D,H). A small amount of non-specific staining is seen in the control eye (A). Scale bar = 100μm
Discussion
Numerous congenital and acquired diseases involve dysfunction or death of anterior chamber cells, including various endothelial dystrophies (e.g., Fuch’s) and open-angle glaucoma, one of the leading causes of blindness worldwide. As such there is a need to develop methodologies to target gene delivery to cells of the anterior chamber in order to facilitate long-term treatment of chronic diseases; unfortunately, despite extensive work in recent decades focusing on improving subretinal and intravitreal delivery of genetic material using a variety of viral vectors (e.g., rAAV, lentivirus and adenovirus) and non-viral vectors (e.g., naked DNA or nanoparticles), limited work has focused on exploring gene delivery in the anterior chamber. Herein, we evaluated three rAAV serotypes following intracameral injection in a clinically relevant large animal model, the AGM, focusing firstly on evaluation of both anterior and posterior chamber health using comprehensive ocular examinations consisting of retinal imaging, slit lamp biomicroscopy, pachymetry, tonometry, and secondarily on cellular transduction using post-mortem histology. Over a period of 42 days post-dosing, no major complications were observed following intracameral rAAV administration, with all noted events (e.g., transient IOP increase, aqueous cell, etc.) resolving naturally without pharmacological or surgical intervention. Expression of the GFP reporter transgene was observed in all high dose rAAV injected eyes, predominantly in cells of the trabecular meshwork and iris, indicating that rAAV vectors may be promising tools for the treatment of open-angle glaucoma and other anterior segment pathologies.
Slit lamp biomicroscopy defined a self-limited inflammatory response immediately after vector administration that was more evident in the high dose treated eyes across all serotype groups, as revealed by aqueous cell scoring and total clinical scores. It is possible that the immune system responded to vector injection itself, as similar immediate trends of inflammation have been seen previously in studies where rAAV has been intravitreally injected in humans and other species, including NHPs, dogs, sheep, and rodents, despite being sero-negative (24,25). Alternatively, it is possible that even the low endotoxin levels present within our vector preparations may be sufficient to elicit an immediate inflammatory response, despite detected levels being below thresholds considered acceptable for human use (< 5 EU/mL). Specifically, previous studies have shown a similar degree and time course of inflammation in AGMs, with resolution within 18 days, when purified endotoxin was administered intravitreally, suspended in a vehicle (PBS), at a level of 0.08 EU/eye (21) Indeed, this level of endotoxin is similar to that contained within each of our vector preparations used herein (0.0723–0.0851 EU/eye).
While the injection of foreign material – rAAV capsids containing extremely low-levels of endotoxin – into the anterior chamber likely explains the acute inflammation observed, many of the NHPs in this study exhibited a secondary phase of inflammation occurring between days 14–21. The likeliest explanation of this secondary inflammatory peak is the triggering of an innate immune response to expression of the fluorescent reporter transgene itself, where GFP has been shown to be immunogenic (26). The timing of the secondary inflammatory peak fits well with our understanding of expression kinetics from a self-complementary vector genome, as employed in this study, where it is expected that transgene expression occurs at a faster rate than when a single-stranded expression cassette is used (27). This observation would also be in line with other previous studies, which have shown that when compared to an injection of empty vector capsid, injection of rAAV vectors expressing a transgene triggers a larger immune response. Another contributing factor to the observed second peak of immune response may be our choice of fluorescent reporter transgene, where GFP is known to be at least mildly toxic in the central nervous system and rAAV-derived GFP expression (e.g. after intrathecal delivery) has previously been show to causes a progressive inflammatory response in NHPs due to sustained expression of non-self-protein (28)(29,30). These findings have been corroborated in several additional studies, where T-cell mediated inflammation following GFP expression in the eyes or liver of dogs and NHP models has been reported, indicating that we may potentially have observed a lesser secondary immune peak if we had either selected a less toxic reporter gene or used a humanized version of GFP to reduce recognition of non-self (31,32)(33). Importantly; however, despite one or two phases of inflammation being observed in several injected eyes, total clinical scores returned to baseline by study conclusion at day 42 without pharmacological intervention (e.g. topical/systemic antibiotics or immune suppressants) indicating that intracameral injection of rAAV is well tolerated in NHPs, at least in AAV naïve subjects.
One of the primary considerations we investigated was whether intracameral administration of vector would cause any changes in intraocular pressure, as this may limit future downstream applications of intracameral rAAV delivery in certain indications, such as development of gene therapy for open-angle glaucoma, where IOP is already elevated. IOP increased in all animals immediately following injections; however, this observation was expected owing to the acute increase in aqueous volume following injection of 100 μL fluid into the anterior chamber. While efforts were made to control for circadian and diurnal fluctuations in IOP by recording measurements at the same time of day, IOP fluctuations were observed throughout the course of the study; however, these measurements remained within the normal range of 12–22 mmHg observed in the AGM and other NHP species and are likely to represent normal pressure variations (21). We observed two deviations outside of the normative range during the study - B361 OS (low dose rAAV2/2[MAX] reached an IOP of 6–7 at Day 28 and B342 OD (high dose rAAV2/6) that exhibited an IOP of 27 at Day 3. These incidents corresponded to peaks of inflammation, as evidenced by high aqueous cell and total clinical scores – indicating that the large IOP fluctuations observed were due to inflammatory responses, which can lead to either decreased or increased pressure, reflecting decreased aqueous production and outflow obstruction, respectively (34–36)
As the vector in this study was injected intracamerally, we also completed pachymetry to determine if any changes in corneal thickness occurred. Previous studies indicated that the average normal corneal thickness for NHPs such as the rhesus macaque is approximately 456 μm for males and 449 μm for females (37) However, AGMs have been shown to have corneal thicknesses between 406–535 μm in males, and 393–574 μm in females (17) Corneal thicknesses remained consistent with baseline findings, with most measurements being within the acceptable range for AGMs. Two exceptions to normal corneal thickness were found in B347 OS (low dose rAAV2/6) wherein thickness decreased by 28% between days 21 and 42, and so falling out of normal range for male AGMs, and possibly reflecting technical variability.
While we were primarily focused on metrics of anterior chamber health, it is also important to investigate the health of the posterior chamber. Retinal thicknesses were generally stable throughout the study in all animals when comparing baseline measurements to day 42 measurements, and critically, measurements of foveal thickness ranged from 239–278 μm, which align with the previous reported normal range in the AGM of 274±8 μm (23) by histological measures and in the cynomolgus monkey by OCT of 244±21 μm and 286.27±53 μm(38,39). Of note, most animals maintained changes in retinal thickness that were similar to that of the control eye, and color fundus photography revealed no differences between baseline and day 42, with an absence of observed adverse retinal findings.
To determine tropism of each serotype in the AGM anterior chamber, histology of the anterior chamber was completed to assess distribution of transgene expression. GFP expression was evident within the angle, iris, and ciliary body of the high dose treated eyes with each serotype, with rAAV2/2[MAX] treated eyes showed the highest intensity of staining within the angle and iris compared to eyes administered rAAV2/6 or rAAV2/9. Interestingly, this transduction patterns is similar to that reported previously in brown Norway rats, wherein transgene expressed was primarily restricted to cells of the iris and iridocorneal angle (40). By contrast, in all low dose treated eyes no GFP expression was evident within the angle, with only sparse transduction evident within the iris. One possible explanation for the absence of GFP expression is that the low dose viral vector load falls below a threshold (or multiplicity of infection) necessary to efficiently transduce cells within the anterior chamber iridocorneal angle. Another possibility is that while the inflammatory response was overall less severe in low dose treated eyes, T cell mediated cytotoxicity may still possibly result in a decrease in the levels of GFP below the threshold for visible detection, leading to a situation where cells may have been successfully transduced, but are not obviously fluorescent (31–33). While it is not possible to distinguish in the present study between these options, the possible role of inflammation in diminishing transgene expression highlights the importance of considering prior immune exposure to rAAV when selecting prospective subjects, where it has been shown that approximately 72%, 46%, and 70% of individuals tested marked seropositive for antibodies for AAV2, 6, and 9 respectively (41).
In conclusion, treatment of NAb negative African green monkeys with intracameral injection of rAAV2/6, rAAV2/9, and rAAV2/2[MAX] packaging a self-complementary GFP transgene resulted in minimal adverse events, such as inflammation, that resolved within six weeks of dosing. Posterior and anterior chamber health was found to remain generally unaffected with minimal changes in retinal and corneal thicknesses observed. Future research investigating transduction with therapeutic transgenes will determine if transduction of the iridocorneal angle is sufficient to achieve a therapeutic effect.
Supplementary Material
Slit-lamp biomicroscopy was completed throughout the study examining the following parameters: CC (Conjunctival Congestion), CS (Conjunctival Swelling), CD (Conjunctival Discharge), PA (Pannus), CO (Corneal clouding), CA (Corneal Area), KP (Inflammatory Keratic Precipitates), AC (Aqueous Cell), H (Hypopyon), AF (Aqueous Flare), FS (Fibrin Strands), IH (Iris Hyperemia), IE (Iris Exfoliation), IS (Iris Synechia), ALD (Anterior Lens Deposits), PLD (Posterior Lens Deposits), LO (Lens Opacity), VC (Vitreous Cell), VH (Vitreous Haze), RV (Retinal Vasculitis), PP (Papillitis). Findings were graded according to a nonhuman primate ophthalmic examination scoring system. Total clinical score was then calculated as the summation of all graded findings.
Acknowledgements:
The authors would like to thank David Burke at Oregon Health & Science University for his assistance with the neutralizing antibody assays. We would also like to thank Gavin Marcoe for his assistance in vector manufacturing. Additionally, the authors would like to thank the supporting staff at Virscio and the St. Kitts Biomedical Research Foundation.
Funding:
This work was funded by intramural support from Medical College of Wisconsin’s Office of Research and NEI R01EY032478.
Footnotes
Ethical Approval:
All animal experiments were approved by the Medical College of Wisconsin’s Institutional Animal Care and Use Committee and adhere to the Association for Research in Vision and Ophthalmology statement for the use of animals in ophthalmic and vision research.
Competing Interests: The authors declare no competing interests.
Data Availability Statement:
The data necessary to evaluate the conclusions within this article are present in the paper and/or supplementary materials. Any other requests may be communicated to the corresponding author.
References
- 1.Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. British Journal of Ophthalmology. 2012. May;96(5):614–8. [DOI] [PubMed] [Google Scholar]
- 2.Liu J, Saghizadeh M, Tuli SS, Kramerov AA, Lewin AS, Bloom DC, et al. Different tropism of adenoviruses and adeno-associated viruses to corneal cells: implications for corneal gene therapy. 2008. [cited 2022 Dec 12]; Available from: http://www.molvis.org/molvis/v14/a245 [PMC free article] [PubMed] [Google Scholar]
- 3.Morgan JT, Murphy CJ, Russell P. What do mechanotransduction, Hippo, Wnt, and TGFβ have in common? YAP and TAZ as key orchestrating molecules in ocular health and disease. Vol. 115, Experimental Eye Research. 2013. p. 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vranka JA, Kelley MJ, Acott TS, Keller KE. Extracellular matrix in the trabecular meshwork: Intraocular pressure regulation and dysregulation in glaucoma. Vol. 133, Experimental Eye Research. Academic Press; 2015. p. 112–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hao XD, Gao H, Xu WH, Shan C, Liu Y, Zhou ZX, et al. Systematically Displaying the Pathogenesis of Keratoconus via Multi-Level Related Gene Enrichment-Based Review. Vol. 8, Frontiers in Medicine. Frontiers Media S.A.; 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kay CN, Ryals RC, Aslanidi G v., Min SH, Ruan Q, Sun J, et al. Targeting Photoreceptors via Intravitreal Delivery Using Novel, Capsid-Mutated AAV Vectors. PLoS One. 2013;8(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reid CA, Ertel KJ, Lipinski DM. Improvement of photoreceptor targeting via intravitreal delivery in mouse and human retina using combinatory rAAV2 capsid mutant vectors. Invest Ophthalmol Vis Sci. 2017;58(14):6429–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bogner B, Boye SL, Min SH, Peterson JJ, Ruan Q, Zhang Z, et al. Capsid mutated adeno-associated virus delivered to the anterior chamber results in efficient transduction of trabecular meshwork in mouse and rat. PLoS One. 2015;10(6):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohan RR, Schultz GS, Hong JW, Mohan RR, Wilson SE. Gene transfer into rabbit keratocytes using AAV and lipid-mediated plasmid DNA vectors with a lamellar flap for stromal access. Exp Eye Res. 2003. Mar 1;76(3):373–83. [DOI] [PubMed] [Google Scholar]
- 10.Hippert C, Ibanes S, Serratrice N, Court F, Malecaze F. Corneal Transduction by Intra-Stromal Injection of AAV Vectors In Vivo in the Mouse and Ex Vivo in Human Explants. PLoS One [Internet]. 2012. [cited 2022 Dec 13];7(4):35318. Available from: www.plosone.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma A, Tovey JCK, Ghosh A, Mohan R. AAV serotype influences gene transfer in corneal stroma in vivo. Exp Eye Res. 2010;91(3):440–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez-Estevez L, Asokan P, Borrás T. Transduction optimization of AAV vectors for human gene therapy of glaucoma and their reversed cell entry characteristics. Gene Ther. 2020. Apr 1;27(3–4):127–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buie LKK, Rasmussen CA, Porterfield EC, Ramgolam VS, Choi VW, Markovic-Plese S, et al. Self-complementary AAV virus (scAAV) safe and long-term gene transfer in the trabecular meshwork of living rats and monkeys. Invest Ophthalmol Vis Sci. 2010;51(1):236–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee SH, Sim KS, Kim CY, Park TK. Transduction Pattern of AAVs in the Trabecular Meshwork and Anterior-Segment Structures in a Rat Model of Ocular Hypertension. Mol Ther Methods Clin Dev. 2019. Sep 13;14:197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bogner B, Boye SL, Min SH, Peterson JJ, Ruan Q, Zhang Z, et al. Capsid mutated adeno-associated virus delivered to the anterior chamber results in efficient transduction of trabecular meshwork in mouse and rat. PLoS One. 2015. Jun 8;10(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dore KM. Vervets in the Caribbean. In: The International Encyclopedia of Primatology. John Wiley & Sons, Inc.; 2017. p. 1–3. [Google Scholar]
- 17.Goody R, Hu W, Whittaker S, Henry S, Brookes R, Struharik M, et al. Definition of Normal Ophthalmic Measures in the African Green Monkey. Investigative Ophthalmology & Visual Science: ARVO Meeting Abstract. 2013. Jun;54(15). [Google Scholar]
- 18.Reid CA, Lipinski DM. Small and Micro-Scale Recombinant Adeno-Associated Virus Production and Purification for Ocular Gene Therapy Applications. Methods in Molecular Biology. 1715:19–31. [DOI] [PubMed] [Google Scholar]
- 19.Zolotukhin S. Production of recombinant adeno-associated virus vectors. Hum Gene Ther. 2005;16(5):551–7. [DOI] [PubMed] [Google Scholar]
- 20.Piedra J, Ontiveros M, Miravet S, Penalva C, Monfar M, Chillon M. Development of a Rapid, Robust, and Universal PicoGreen-Based Method to Titer Adeno-Associated Vectors. Hum Gene Ther Methods. 2015;26(1):35–42. [DOI] [PubMed] [Google Scholar]
- 21.Corey TM, Woodley VV., O’connor M, Connolly E, Doyle S, Shrader S, et al. Evaluation of the Dose-Dependent Inflammatory Response and No-Observable Adverse Effect Level of Intravitreal Endotoxin in the African Green Monkey. Transl Vis Sci Technol. 2022. Aug 1;11(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hussein N, Ostler B, Gormus BJ, Wolf R, Walsh GP. Intraocular pressure changes and postural changes of intraocular pressure in experimentally induced Hansen’s disease of Rhesus, Mangabey, and African green monkeys. British Journal of Ophthalmology. 1990;74(11):647–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bouskila J, Palmour RM, Bouchard JF, Ptito M. Retinal structure and function in monkeys with fetal alcohol exposure. Exp Eye Res. 2018. Dec 1;177:55–64. [DOI] [PubMed] [Google Scholar]
- 24.Bouquet C, Vignal Clermont C, Galy A, Fitoussi S, Blouin L, Munk MR, et al. Immune Response and Intraocular Inflammation in Patients with Leber Hereditary Optic Neuropathy Treated with Intravitreal Injection of Recombinant Adeno-Associated Virus 2 Carrying the ND4 Gene: A Secondary Analysis of a Phase 1/2 Clinical Trial. JAMA Ophthalmol. 2019. Apr 1;137(4):399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan YK, Dick AD, Hall SM, Langmann T, Scribner CL, Mansfield BC. Inflammation in Viral Vector-Mediated Ocular Gene Therapy: A Review and Report From a Workshop Hosted by the Foundation Fighting Blindness, 9/2020. 2021. [cited 2022 Nov 29]; Available from: 10.1167/tvst.10.4.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ansari AM, Ahmed AK, Matsangos AE, Lay F, Born LJ, Marti G, et al. Cellular GFP Toxicity and Immunogenicity: Potential Confounders in in Vivo Cell Tracking Experiments. Stem Cell Rev [Internet]. 2016. Oct 1 [cited 2022 Dec 13];12(5):553. Available from: /pmc/articles/PMC5050239/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCarty DM. Self-complementary AAV vectors; advances and applications. Vol. 16, Molecular Therapy. 2008. p. 1648–56. [DOI] [PubMed] [Google Scholar]
- 28.Timmers AM, Newmark JA, Turunen HT, Farivar T, Liu J, Song C, et al. Ocular Inflammatory Response to Intravitreal Injection of Adeno-Associated Virus Vector: Relative Contribution of Genome and Capsid. [cited 2022 Nov 29]; Available from: www.liebertpub.com [DOI] [PubMed] [Google Scholar]
- 29.Samaranch L, Sebastian WS, Kells AP, Salegio EA, Heller G, Bringas JR, et al. AAV9-mediated expression of a non-self protein in nonhuman primate central nervous system triggers widespread neuroinflammation driven by antigen-presenting cell transduction. Molecular Therapy. 2014;22(2):329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramsingh AI, Gray SJ, Reilly A, Koday M, Bratt D, Koday MT, et al. Sustained AAV9-mediated expression of a non-self protein in the CNS of non-human primates after immunomodulation. PLoS One [Internet]. 2018. Jun 1 [cited 2023 Mar 27];13(6). Available from: /pmc/articles/PMC5991358/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vandenberghe LH, Bell P, Maguire AM, Cearley CN, Xiao R, Calcedo R, et al. Dosage thresholds for AAV2 and AAV8 photoreceptor gene therapy in monkey. Sci Transl Med [Internet]. 2011. Jun 22 [cited 2023 Mar 27];3(88). Available from: https://www.science.org/doi/10.1126/scitranslmed.3002103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao G, Wang Q, Calcedo R, Mays L, Bells P, Wang L, et al. Adeno-Associated Virus-Mediated Gene Transferto Nonhuman Primate Liver Can Elicit DestructiveTransgene-Specific T Cell Responses. Hum Gene Ther. 2009;20:930–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boyd RF, Boye SL, Conlon TJ, Erger KE, Sledge DG, Langohr IM, et al. Reduced retinal transduction and enhanced transgene-directed immunogenicity with intravitreal delivery of rAAV following posterior vitrectomy in dogs. Gene Ther. 2016. Jun 1;23(6):548–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bodh SA, Kumar V, Raina UK, Ghosh B, Thakar M. Inflammatory glaucoma. Oman J Ophthalmol [Internet]. 2011. [cited 2022 Dec 14];4(1). Available from: http://www.ojoonline.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sen HN, Drye LT, Goldstein DA, Larson TA, Merrill PT, Pavan PR, et al. Hypotony in patients with uveitis: The Multicenter Uveitis Steroid Treatment (MUST) trial. Ocul Immunol Inflamm. 2012. Apr;20(2):104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tran VT, Mermoud A, Herbort CP. Appraisal and Management of Ocular Hypotony and Glaucoma Associated With Uveitis. Int Ophthalmol Clin. 2000;40(2):175–203. [DOI] [PubMed] [Google Scholar]
- 37.Casanova MI, Young L, Park S, Kim S, Roszak K, Leonard BC, et al. Normal Corneal Thickness and Endothelial Cell Density in Rhesus Macaques (Macaca mulatta). Transl Vis Sci Technol. 2022. Sep 1;11(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choi KE, Anh VTQ, Yun C, Kim YJ, Jung H, Eom H, et al. Normative data of ocular biometry, optical coherence tomography, and electrophysiology conducted for cynomolgus macaque monkeys. Transl Vis Sci Technol. 2021. Nov 1;10(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Denk Id N, Maloca Id P, Steiner G, Freichel C, Bassett S, Schnitzer TK, et al. Macular thickness measurements of healthy, naïve cynomolgus monkeys assessed with spectral-domain optical coherence tomography (SD-OCT). 2019. [cited 2022 Dec 4]; Available from: 10.1371/journal.pone.0222850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chern KJ, Nettesheim ER, Reid CA, Li NW, Marcoe GJ, Lipinski DM. Prostaglandin-based rAAV-mediated glaucoma gene therapy in Brown Norway rats. Commun Biol. 2022. Dec 1;5(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boutin S, Monteilhet V, Veron P, Leborgne C, Benveniste O, Franç Oise Montus M, et al. Prevalence of Serum IgG and Neutralizing Factors Against Adeno-Associated Virus (AAV) Types 1, 2, 5, 6, 8, and 9 in the Healthy Population: Implications for Gene Therapy Using AAV Vectors. Hum Gene Ther [Internet]. 2010;21(6):661–789. Available from: www.liebertpub.com. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Slit-lamp biomicroscopy was completed throughout the study examining the following parameters: CC (Conjunctival Congestion), CS (Conjunctival Swelling), CD (Conjunctival Discharge), PA (Pannus), CO (Corneal clouding), CA (Corneal Area), KP (Inflammatory Keratic Precipitates), AC (Aqueous Cell), H (Hypopyon), AF (Aqueous Flare), FS (Fibrin Strands), IH (Iris Hyperemia), IE (Iris Exfoliation), IS (Iris Synechia), ALD (Anterior Lens Deposits), PLD (Posterior Lens Deposits), LO (Lens Opacity), VC (Vitreous Cell), VH (Vitreous Haze), RV (Retinal Vasculitis), PP (Papillitis). Findings were graded according to a nonhuman primate ophthalmic examination scoring system. Total clinical score was then calculated as the summation of all graded findings.
Data Availability Statement
The data necessary to evaluate the conclusions within this article are present in the paper and/or supplementary materials. Any other requests may be communicated to the corresponding author.