Abstract
KRAS is one of the most commonly mutated oncogenes in cancers and therapeutics directly targeting the KRas have been challenging. Among the different known mutants, KRasG12C has been proved to be successfully targeted recently. Several covalent inhibitors selectively targeting KRasG12C have shown promising efficacy against cancers harboring KRASG12C mutation in clinical trials and AMG510 (sotorasib) has been approved for the treatment of KRASG12C-mutated locally advanced or metastatic non-small cell lung cancer. However, the overall responsive rate of KRasG12C inhibitors was around 50% in patients with non-small cell lung cancer and the efficacy in patients with colorectal cancer or appendiceal cancer appears to be less desirable. It is of great importance to discover biomarkers to distinguish patients who are likely benefitted. Moreover, adaptive resistance would occur inevitably with the persistent administration like other molecularly targeted therapies. Several combinatorial regimens have been studied in an effort to potentiate the efficacy of KRasG12C inhibitors in preclinical settings. This review summarized the recent progress of covalent KRasG12C inhibitors with a focus on identifying biomarkers to predict or monitor the efficacy and proposing rational drug combinations based on elucidation of the mechanisms of drug resistance.
Keywords: Biomarkers, Covalent KRasG12Cinhibitors, Drug resistance, Human cancers, KRASG12Cmutation
Abbreviations
- AKT
protein kinase B
- ALK
anaplastic lymphoma kinase
- APC
adenomatous polyposis coli
- AURKA
Aurora kinase A
- CDK1
cyclin-dependent kinase 1
- CDK4
cyclin-dependent kinase 4
- CRC
colorectal cancer
- CXCL8/10/11
C-X-C motif chemokine ligand 8/10/11
- DCR
disease control rate
- DUSP
dual-specificity phosphatases
- EGFR
epidermal growth factor receptor
- EMT
epithelial–mesenchymal transition
- ERK
extracellular regulated protein kinases
- FDA
U.S. Food and Drug Administration
- FGFR
fibroblast growth factor receptor
- GAPs
GTPase activating/accelerating proteins
- GDP
guanosine diphosphate
- GEFs
guanine nucleotide exchange factors
- GI
gastrointestinal/gastro-intestinal
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- HER2
human epidermal growth factor receptor-2
- IC50
half maximal inhibitory concentration
- IFN-γ
inactivation of interferon-γ
- IL-6/10
interleukin-6/10
- KEAP1
Kelch-like ECH-associated protein 1
- KRAS
v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog
- LKB1
liver kinase B1
- MAPK
mitogen-activated protein kinase
- MET
hepatocyte growth factor receptor
- MHC
major histocompatibility complex
- MMP2
matrix metallopeptidase 2
- NSCLC
non-small cell lung cancer
- ORR
overall response rate
- OS
overall survival
- PD-1/PDL-1
programmed cell death protein-1/programmed death ligand-1
- PDX
patient-derived xenograft
- PFS
progression-free survival
- PI3K
phosphoinositide-3-kinase
- Raf
Ras-associated factor
- RAL-GEFs
Ral-specific guanine nucleotide exchange factors
- RAS
rat sarcoma protein
- RB
retinoblastoma
- RET
proto-oncogene tyrosine-protein kinase receptor Ret
- RNAi
RNA interference
- RTK
receptor tyrosine kinase
- SAR
structure activity relationships
- SHP2
Src homology containing protein tyrosine phosphatase 2
- SOS1
SOS Ras/Rac guanine nucleotide exchange factor 1
- STK11
Serine–Threonine Kinase 11
- TGFβ
transforming growth factor beta
- TGI
tumor growth inhibition
- TP53
tumor protein p53
Introduction
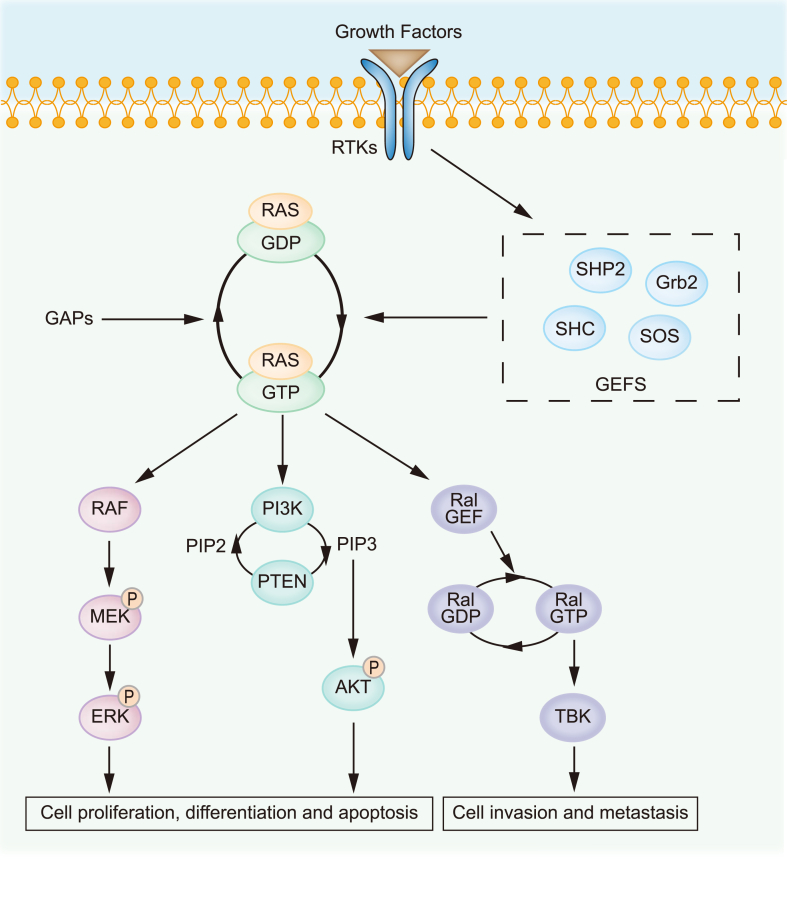
RAS gene was first discovered in 19641 as a viral gene transferred from the rodent genome and is part of an RNA sarcoma virus with potent oncogenic functions.2 There are three RAS family members in human: HRAS, NRAS and KRAS. KRAS appears in forms of KRAS4A and KRAS4B as a result of alternative splicing,3 while the latter one is the predominant form in human.4 KRAS encodes a 21 kDa GTP-binding protein and known as Ras-like GTPase.4 It switches between active and inactive state by binding to GTP or GDP respectively.5 This process is regulated by guanine nucleotide exchange factors (GEFs) which facilitate the release of GDP, and GTPase activating/accelerating proteins (GAPs) that strengthen the relatively poor intrinsic GTPase activity of KRas.3 In response to extracellular stimuli, KRas binds to GTP and becomes active after conformational switching,6 which connects activated membrane receptors to mitogen-activated protein kinase (MAPK) pathway,7 phosphoinositide-3-kinase (PI3K) pathway8 and Ras-like small GTPases (Ral) pathway4 (Fig. 1), thus playing important roles in regulating cell growth, survival, differentiation, proliferation and migration.2 In consistency to its pivotal physiological roles, KRas is indispensable for the development of mice embryo and plays a key role in cardiovascular homeostasis.9 However, knockout of NRAS or HRAS showed no effect on the viability of mice10 and replacement of the KRAS with HRAS did not lead to embryonic lethality,11 indicating the redundant while distinctive roles of RAS family members.
Figure 1.
The Ras signaling pathway. Activated Ras by upstream RTKs or by gain-of-function mutation possesses high affinity with GTP and further activates multiple downstream signaling pathways, including Raf/MEK, PI3K/Akt and Ral/GEF cascades, which regulate important processes such as cell growth, differentiation, apoptosis, invasion, and metastasis.
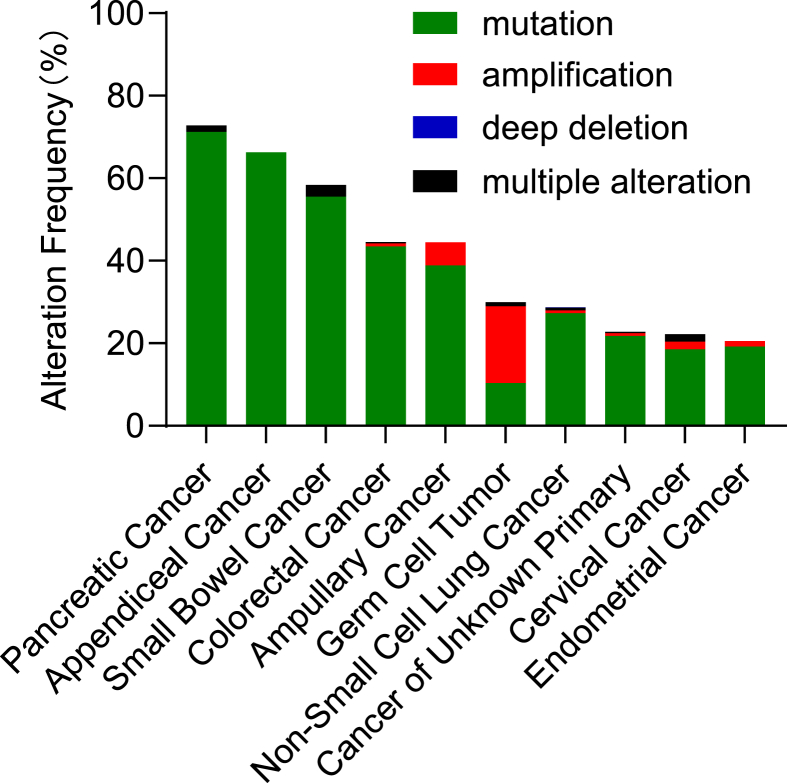
KRas is frequently hyper-activated in cancers as a result of gain-of-function mutation or abnormal activation of upstream receptors.12 Although mutations occur in all three RAS members,3 mutations in KRAS are the most frequent, which accounts for about 85% of RAS mutations in human cancers, especially in pancreatic cancer,13 colorectal cancer14 and lung cancer4 (Fig. 2). KRAS mutations mainly occur at codon 12, 13 or 61.4 Mutations at codon 12 are the most common, including G12C, G12V and G12D.15 G12C mutation occurs in 15% of lung cancer and 8% of colorectal cancer. Mutation at G12 interferes the binding of Ras with GAP and inhibits GTP hydrolysis stimulated by GAP,4 thus keeping Ras in the active GTP-bound form.8 Mutated KRAS leads to the continued phosphorylation and activation of the extracellular signal-regulated kinase (ERK), which in turn phosphorylates multiple cytoplasmic proteins, cytoskeletal proteins, and transcription factors that initiate genetic programs associated with cell growth and survival.16, 17, 18 Activated Ras has long been considered a prominent tumor driver.19 Ras activation had been identified in various carcinogen-induced animal tumor models nearly four decades ago.20, 21, 22 The roles of RAS in the multistep processes of carcinogenesis were further revealed in multiple transgenic and gene-targeted mouse models.2 There is also strong evidence that continued expression of mutated RAS is necessary for tumor maintenance.23 Suppression of Ras by RNA interference (RNAi) impaired the growth of RAS-mutated human cancer cells.24 Moreover, loss of tumor protein p53 (p53), liver kinase B1 (LKB1, also called Serine–Threonine Kinase 11, STK11) or adenomatous polyposis coli (APC), which occur frequently in human cancer, enhanced tumor initiation and progression triggered by RAS.25, 26, 27 Recent studies also revealed that complete knockout of either KRAS isoform would prevent the development of the tumor.28 Mutated KRAS also regulates tumor microenvironment and in turn promotes tumor development.29 For example, KRas reduced the production of T-cell-attracting chemokine CXCL10 and CXCL11, which was accompanied by inactivation of interferon-γ (IFN-γ) pathway and reduced antigen presentation in the tumor tissues.30
Figure 2.
Frequency of KRAS alterations in human cancers. The 10 cancer types with most frequent KRAS alterations are presented. Data are derived from 13,602 samples in “PanCancer Studies” analyzed by cBioPortal (www.cbioportal.org/, accessed in July 2020).
Despite its extreme importance in the development of tumors, direct inhibition of the mutated small GTPase has been challenging in the last few decades due to its high affinity with GTP and lack of a pocket for small molecules to bind with.31 Therefore, mutated KRas has long been recognized as ‘undruggable’. Meanwhile, strategies to target the KRas indirectly have been proposed and pursued, including inhibiting farnesyltransferase,32 blocking downstream signaling33, 34, 35 and exploiting the venerability of KRAS-mutated cancer via synthetic lethality.36 Direct targeting KRas has been proposed by preventing the formation of Ras-GTP complex with GAP analogues.37,38 The important binding sites of the KRas, including GEF binding site39 and the binding domains with the downstream effectors,40 are also potentially to be targeted by small molecule compounds. However, none of the strategies have been proved successful in clinical settings. ARS853 was first reported in 2016,41 which emerged as a game changer by covalently binding to the mutated cysteine residue and selectively inhibiting the KRasG12C. Several covalent inhibitors of KRasG12C, such as AMG510 and MRTX849, have been rapidly developed and advanced in clinical trials. These inhibitors irreversibly bind to the switch II pocket and lock the protein in the inactive KRas-GDP state.41, 42, 43, 44 They offer a promising opportunity to modulate one of the major oncogenic RAS mutants with improved efficacy and selectivity45 as well as reduced toxicity.46 Remarkably, AMG510 has shown promising efficacy against patients carrying the mutation47,48 and has been approved by U.S. Food and Drug Administration (FDA) for the treatment of locally advanced or metastatic non-small cell lung cancer (NSCLC) carrying KRASG12C. Moreover, combination of covalent KRasG12C inhibitors with other targeted therapies, immunotherapies or chemotherapies49 are in rigorous investigation. Despite the encouraging progress, the responsive rate was not satisfying and acquired resistance is likely to develop rapidly. Nearly half of the NSCLC patients carrying KRASG12C had poor response to AMG510,47 suggesting other factors independent of KRas may affect the efficacy. Disease progression was found in patients with NSCLC, colorectal cancer and appendiceal cancer, who initially had stable disease for at least 12 weeks or an objective response to MRTX849.50 Therefore, it is necessary to identify predictive biomarkers to stratify patients who are more likely benefitted from the therapy. Moreover, combinatorial therapy based on the elucidated mechanisms of intrinsic or acquired resistance may improve the efficacy and expand responsive population.
Advances in the covalent inhibitors of KRasG12C
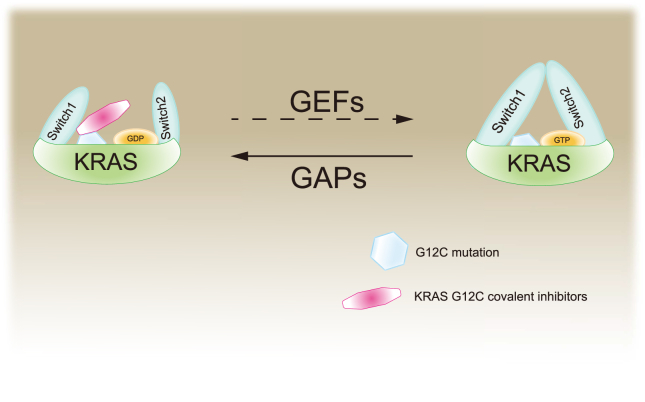
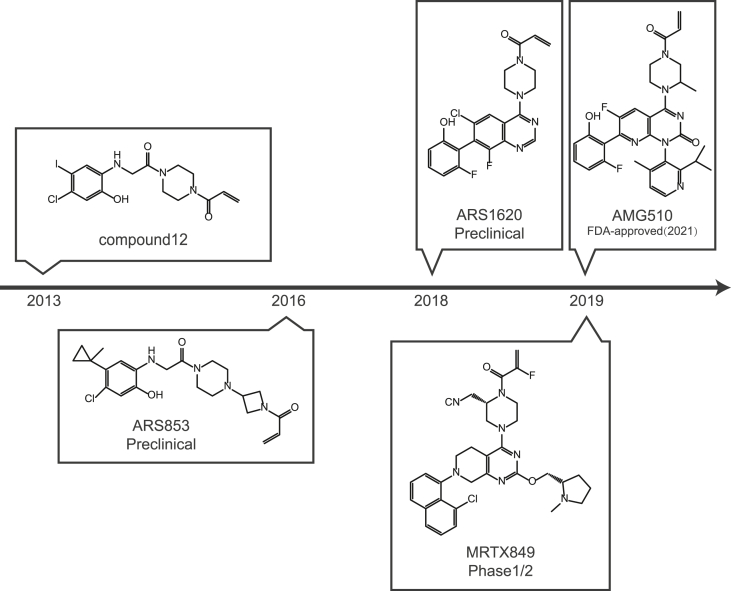
Though KRas has long been recognized as a promising target for cancer therapy, direct targeting KRas has not been feasible because of the high affinity between KRas and GTP. Alternative strategies include inhibition of the expression or post-translational modification,3 targeting downstream effectors29 and synthetic lethality.51 However, none of the strategies have been successful up to date and the KRas had been considered “undruggable”. Recently, small molecules that irreversibly bind to and inactivate a common oncogenic mutated KRASG12C shed new light on targeting KRas.52 These compounds selectively bind to the mutated cysteine residue of KRasG12C via a covalent bond and disrupt both switch-I and switch-II pocket, subverting the native nucleotide preference to GTP over GDP and impairing binding of the KRas to RAF proto-oncogene serine/threonine-protein kinase (Raf, Fig. 3).52 Although the initial lead compounds were limited by their potency and pharmaceutical properties, the discovery and development of the covalent inhibitors have been greatly progressed and a handful of candidates including AMG510, ARS3248 and MRTX849 are currently in clinical trials (Fig. 4 and Table 1). Promising efficacy has been observed in patients with lung cancer or colorectal cancer harboring KRASG12C.53 FDA has approved AMG510 (sotorasib, LUMAKRAS™) as the first and only targeted treatment for patients with KRASG12C-mutated locally advanced or metastatic NSCLC in May 2021.
Figure 3.
The mechanism of the activation of KRas and KRasG12C covalent inhibitors. KRas switches between active and inactive state by binding to GTP and GDP, respectively. GEFs facilitate the release of GDP, while GAPs strengthen the relatively poor intrinsic GTP enzyme activity of KRas. KRasG12C covalent inhibitors are mainly considered to lock KRasG12C in the inactive KRas-GDP state.
Figure 4.
A chronicle of discovery and development of KRasG12C covalent inhibitors.
Table 1.
Registered clinical trials of KRasG12C inhibitors (www.clinicaltrials.gov.).
| NCT Number | Phases | Status | Interventions | Conditions |
|---|---|---|---|---|
| NCT03600883 | I/II | Recruiting | AMG 510 | KRAS p.G12C Mutant Advanced Solid Tumors |
| NCT04006301 | I | Completed | JNJ-74699157 | Neoplasms|Advanced Solid Tumors|Non-small Cell Lung Cancer|Colorectal Cancer |
| NCT04303780 | III | Recruiting | AMG 510, Docetaxel | KRAS p.G12C Mutated/Advanced Metastatic NSCLC |
| NCT04380753 | I | Recruiting | AMG 510 | Advanced/Metastatic Solid Tumors With KRAS p.G12C Mutation |
| NCT04625647 | II | Not yet recruiting | Sotorasib | Lung Adenocarcinoma|Lung Non-Small Cell Carcinoma|Recurrent Lung Non-Squamous Non-Small Cell Carcinoma|Stage IV Lung Cancer AJCC v8|Stage IVA Lung Cancer AJCC v8|Stage IVB Lung Cancer AJCC v8 |
| NCT04667234 | Available | AMG 510 | Non Small-cell Lung Cancer|Locally Advanced Unresectable NSCLC|Locally Advanced Metastatic NSCLC | |
| NCT04685135 | III | Recruiting | MRTX849, Docetaxel | Metastatic Non Small Cell Lung Cancer|Advanced Non Small Cell Lung Cancer |
| NCT03785249 | I/II | Recruiting | MRTX849, Pembrolizumab, Cetuximab, Afatinib | Advanced Cancer|Metastatic Cancer|Malignant Neoplastic Disease |
| NCT04165031 | I/II | Terminated | LY3499446, Abemaciclib, Cetuximab, Erlotinib, Docetaxel | Advanced Solid Tumor|Non-Small Cell Lung Cancer|Colorectal Cancer |
| NCT04185883 | I | Recruiting | Sotorasib, PD1 inhibitor, MEK inhibitor, SHP2 allosteric inhibitor, Pan-ErbB tyrosine kinase inhibitor, PD-L1 inhibitor, EGFR inhibitor, Chemotherapeutic regimen, PD-1 inhibitor, mTOR inhibitor, CDK inhibitor, VEGF inhibitor | Advanced Solid Tumors|Kirsten Rat Sarcoma (KRAS) p.G12C Mutation |
| NCT04330664 | I/II | Recruiting | MRTX849, TNO155 | Advanced Cancer|Metastatic Cancer|Malignant Neoplastic Disease |
| NCT04449874 | I | Recruiting | GDC-6036, Atezolizumab, Cetuximab, Bevacizumab, Erlotinib | Non-Small Cell Lung Cancer|Colorectal Cancer|Advanced Solid Tumors |
| NCT04585035 | I/II | Recruiting | D-1553, Other | Solid Tumor, Adult|NSCLC|CRC |
| NCT04613596 | II | Recruiting | MRTX849, Pembrolizumab | Advanced Non-Small Cell Lung Cancer|Metastatic Cancer |
| NCT04699188 | I/II | Recruiting | JDQ443, TNO155, spartalizumab | KRAS G12C Mutant Solid Tumors|Carcinoma, Non-Small-Cell Lung|Carcinoma, Colorectal|Cancer of Lung|Cancer of the Lung|Lung Cancer|Neoplasms, Lung|Neoplasms, Pulmonary|Pulmonary Cancer|Pulmonary Neoplasms |
| NCT04793958 | III | Recruiting | MRTX849, Cetuximab | Advanced Colorectal Cancer|Metastatic Colorectal Cancer |
ARS853
ARS853 was first reported in 2016, which was obtained from the modification of compound 12 and ARS107.41 ARS853 covalently binds to the Cys12 of mutated KRas, and extends into the Switch II pocket region located between the central β-sheet of KRas and the α2/α3 helices.41 ARS853 may also impede the activity of KRas by interfering with GTP binding and stabilizing the bound GDP.41 Treatment of KRASG12C-mutated lung cancer cells with ARS853 at 10 μM decreased KRas-GTP by over 95%, which also caused significant decrease in phosphorylation of AKT, c-Raf and ERK.54 ARS853 inhibited the proliferation of lung cancer H358 cells harboring KRASG12C with a half maximal inhibitory concentration (IC50) of 2.5 μM.54 Though ARS853 is not active in vivo4 which limits its further development, ARS853 is widely used as a tool compound and lays the foundation for the further development of KRasG12C inhibitors.55
ARS1620
ARS1620 is a second-generation KRasG12C inhibitor with similar characteristics to ARS853.44 ARS1620 is a quinazoline-based compound, which is a versatile lead scaffold that is able to overcome the structure activity relationship (SAR) restrictions of ARS853 and possesses better drug-like properties.56 ARS1620 covalently binds to Cys12 and inhibits the KRasG12C activity with high potency and atropisomeric selectivity.56 It reduced the level of KRas-GTP as well as the phosphorylation of ERK, AKT and S6 in a concentration-dependent manner and exhibited over 10 times improvement in potency compared to ARS853 to inhibit the proliferation of KRASG12C-mutated cancer cells.56 ARS1620 displayed greatly improved oral bioavailability with an F value over 60% in mice compared to its parent compound of lower than 2%.56 Matthew et al. found that ARS1620 enabled over 75% target occupancy for an extended period of time, which is necessary and sufficient to achieve therapeutic efficacy in vivo.56 In fact, administration of ARS1620 at 200 mg/kg achieved tumor growth inhibition (TGI) by over 70% in mice bearing xenografts derived from 5 types of NSCLC cells respectively.56 ARS1620 is currently in preclinical study.
AMG510
AMG510 (Sotorasib), is developed by Amgen,15,57 which is the first KRasG12C inhibitor approved by the FDA for treatment of KRASG12C-mutated locally advanced or metastatic NSCLC. AMG510 possesses a novel quinazolinone scaffold occupying the KRas switch II pocket and the acrylamide moiety forming a covalent bond with Cys12.15 The aromatic ring of AMG510 is able to bind to His95, thus strengthening the interaction with the KRasG12C.42 AMG510 is highly selective for covalent modification of the KRasG12C among 6451 cysteine-containing peptides profiled. Although the structure of AMG510 is similar to ARS1620, it is about 10-time more potent than the latter in inhibiting the KRasG12C.42 AMG510 inhibited KRas signaling represented by reduced p-ERK in all KRASG12C-mutated cell lines tested but not in cell lines harboring other types of mutations in KRAS.58 AMG510 selectively inhibited the signaling and proliferation with IC50 values ranging from 0.010 μM to 0.123 μM in a panel of 22 cell lines tested harboring heterozygous or homozygous KRASG12C other than non-KRASG12C mutations or wild-type KRAS.42 In mice bearing xenografts derived from human tumor cells, AMG510 significantly inhibited the tumor growth. AMG510 achieved regression of MIAPACA2 xenografts at the dose of 30 mg/kg, which was at least 3.3-fold lower than that of ARS1620.42 AMG510 displayed reasonable oral bioavailability observed in all species.15 AMG510 entered clinical trials in August 2018 due to the encouraging preclinical results and phase 3 clinical trials were initiated in March 2020 for locally advanced, unresectable or metastatic NSCLC harboring KRASG12C mutation (NCT04303780). Considering that the cancers in these patients had been refractory to previous treatments, it is valuable that a confirmed response was observed in 32.2% of the patients with NSCLC, and disease control for a few months or more was obtained in 88.1% of the patients, leading to a median progression-free survival (PFS) of 6.3 months.59 The common side effects of AMG510 are diarrhea, nausea, vomiting, fatigue, and elevations of aminotransferase levels.59 Up to now, no dose-limiting toxic effects have been observed even with extended treatment of AMG510. The FDA approved AMG510 (LUMAKRAS™) for the treatment of adult patients with KRASG12C-mutated locally advanced or metastatic NSCLC, as determined by the clinical trial in patients who have received at least one prior systemic therapy.60 Clinical trials evaluating AMG510 as monotherapy or in combination with various agents in patients with NSCLC or other solid tumors are under way (NCT04303780 and NCT04185883).59
MRTX849
MRTX849 (Adagrasib) is a member of the latest generation of KRasG12C inhibitors, which was developed by Mirati.43 It irreversibly binds to mutated Cys12 in the KRasG12C switch II pocket and locks it in the inactive KRas-GDP state.43 MRTX849 showed over 1000-fold selectivity against KRASG12C among tested kinases with an IC50 of 4.7 nM to inhibit the proliferation of KRASG12C-mutated MIAPACA2 cells.43 MRTX849 treatment resulted in reduction in tumor volume by more than 30% in 17 out of 26 tumor xenografts originated from lung cancer, colon cancer, pancreatic cancer, cervical cancer and esophagus cancer cells harboring KRASG12C mutation.43 MRTX849 has a predicted oral bioavailability in humans of around 50% and a half-life of about 20 h in rodent and non-rodent repeat-administration toxicology studies.61 The clinical results from the Phase 1/2 trial was first presented in 2019 AACR–NCI–EORTC meeting.48 Among the 12 patients (6 cases of NSCLC, 4 cases of CRC, and 2 cases of appendiceal cancer) treated with MRTX849, response has been observed in 3 NSCLC patients and 1 CRC patient. The most common adverse events associated with MRTX849 were gastrointestinal side effects such as diarrhea and nausea.6 The latest preliminary results, including updated clinical data of the drug, were announced by Mirati at the 2020 AACR–NCI–EORTC meeting.62,63 In patients with advanced NSCLC, the overall response rate (ORR) was up to 45% (23/51) and the disease control rate (DCR) was up to 96% (49/51) across Phase 1/1b and Phase 2 clinical trials. In patients with CRC, the ORR was 17% (3/18) and the DCR was 94% (17/18) across Phase 1/1b and Phase 2 clinical trials. Four patients with pancreatic, ovarian, endometrial or cholangiocarcinoma tumors had a confirmed partial response to the therapy.64 MRTX849 has been well tolerated as a monotherapy and in combination with BI1701963 (NCT04111458), pembrolizumab (a PD-1 inhibitor), cetuximab (an anti-EGFR antibody) and TNO-155 (a SHP-2 inhibitor).62,63
Challenges in the development of covalent inhibitors of KRasG12C
Direct targeting the KRasG12C by small-molecule covalent inhibitors represented by FDA approved AMG510 provided encouraging evidence for successful targeting the ‘undruggable’ KRas.65 However, tumors harboring KRASG12C mutation display differential sensitivity to the inhibitors, which was reflected by ORR of 48%–50% in NSCLS patients and 6%–14% in other cancer patients.66 Therefore, it is important to identify patients that are more likely benefitted from the treatment. As KRas sits in a complex network to regulate multiple processes of cancer, adaptive resistance has been observed in pre-clinical settings and in patients who initially responded well to the KRasG12C inhibitor MRTX849.50 Efforts have been devoted to elucidate the mechanisms leading to resistance and develop strategies to overcome the resistance.
Identification of predictive biomarkers for the efficacy of the KRasG12C inhibitors
Patients with cancers harboring KRASG12C mutation are the most likely to respond to KRasG12C covalent inhibitors. AMG510 has been shown to be active in tumor cells harboring KRASG12C mutation, while non-KRASG12C mutated cell lines were insensitive to AMG510.42 However, the potency of AMG510 was variable among the KRASG12C mutated cell lines.42 Similarly, the response is highly variable especially in colorectal cancer patients carrying KRASG12C mutation, which reflected the heterogeneity of cancers originated from different tissues types and even from the same tissue type.67,68
Recent study has reported that higher KRas-GTP level was found in the KRasG12C inhibitors-sensitive cells compared to resistant cells, which was accompanied with higher score of KRas-dependency signature.69 Considering that trapping KRasG12C in a GDP-bound conformation by ARS853 is lowering its affinity for nucleotide exchange factors, there is possibility that nucleotide exchange activity modulates the effect of ARS853.54 Indeed, KRAS mutations including Y40A, N116H and A146V that increase nucleotide exchange attenuated the effect of ARS853 on KRasG12C-GTP, while KRASY32S caused a slight augmentation in its effect. Therefore, co-occurrence of mutations in KRas that potentiates nucleotide exchange would reduce the potency of KRasG12C inhibitors.54 A recent phase II clinical trial found that improved efficacy with AMG510 was seen in patients concurrently carrying mutated STK11 and wild-type KEAP1 (n = 22) with median PFS of 11.0 months and median overall survival (OS) of 15.3 months.70 However, Hallin J et al. demonstrated the heterogeneity and complexity of KRAS-mutated cancers and suggested that individual binary biomarkers might not be able to predict therapeutic response.43 Further studies are warranted in both preclinical and clinical settings.
Identification of biomarkers to monitor the efficacy of KRasG12C inhibitors
Important downstream effectors of active KRas, including p-ERK, p-AKT and p-S6, have been proposed to be potential biomarkers to monitor the efficacy of KRasG12C inhibitors. The decrease of p-ERK could be observed in most KRASG12C cell lines after being treated with KRasG12C inhibitors. A recent study revealed the decrease in p-ERK correlated well with occupancy of the KRasG12C by AMG510 in H358 cells.42 The level of target genes of ERK, such as dual-specificity phosphatases (DUSP) decreased after KRasG12C inhibitor treatment,43,44,68 which could also be considered as a potential biomarker candidate. AKT is an intermediate effector downstream of KRas via PI3K signaling pathway. However, suppression of phosphorylated AKT was only observed in a few lines of NSCLC cells harboring KRASG12C mutation.42,44 It seems that most of the KRASG12C mutated cells have distinctive mechanisms to activate PI3K pathway and p-AKT may not be able to serve as a biomarker for the efficacy.44,68 Meanwhile, S6 is the integrated signaling node of both MAPK and PI3K pathways, indicating a potential indicator of KRas inhibition. Sustained shutdown of p-S6 may be a good predictor of the effective suppression of cell viability.71 Patricelli et al. observed reduced level of p-S6 after treatment with ARS853 for both short time and long time41 and similar phenomenon was observed after MRTX849 treatment,43 which indicated that the level of p-S6 might potentially monitor the efficacy. However, the outcomes were controversial in the experiments with ARS1620.44 Similar to p-AKT, the level of p-S6 was not affected in most cells after treated with ARS1620.44 It appears that alternative biomarkers instead of afore mentioned in the signaling pathway need to be discovered to better monitor the efficacy of KRasG12C inhibitors.
Strategies to overcome resistance to KRasG12C inhibitors
Though the clinical response is encouraging for the KRasG12C inhibitors, the efficacy could be circumvented by both intrinsic and adaptive resistance. Indeed, disease progression was found in patients with NSCLC, CRC and appendiceal cancer, who initially had stable disease for at least 12 weeks or an objective response to MRTX849.50 Alternative KRas mutation or high-level amplification of the KRasG12C allele, activation of upstream RTKs, downstream effectors and other isoforms in Ras family could lead to resistance to KRasG12C inhibitors, and co-targeting these pathways and KRasG12C might be potential strategies to overcome the resistance and improve the efficacy of KRasG12C inhibitors.
Co-targeting upstream signaling
As KRas sits in the center of RTK-mediated signaling, bypass the Ras activity via activation of upstream may result in resistance to KRasG12C inhibitors. The clinical studies showed that the ORR of MRTX849 or AMG510 was 48%–50% in patients with KRASG12C mutated NSCLC, and only 6%–14% in patients with other types of cancer,66 indicating that these patients are usually intrinsically resistant to KRasG12C inhibitors. To identify mechanism underlying intrinsic resistance to KRasG12C inhibitors in CRC patients, Amodio et al. measured the levels of active RTKs in colorectal cancer and NSCLC cell lines using p-RTK arrays and found enhanced activation of EGFR in CRC cell lines.67 Consistently, combination of an EGFR inhibitor with a KRasG12C inhibitor achieved tumor regression in CRC patient-derived xenograft (PDX).67
In addition to intrinsic hyper-activation of EGFR in CRC cells, adaptive activation of multiple RTKs, including EGFR, human epidermal growth factor receptor-2 (HER2), and fibroblast growth factor receptor (FGFR) was detected in multiple KRASG12C-mutated cells treated with ARS1620 for 48 h in a highly heterogeneous pattern.68 For instance, primary dependency on EGFR family signaling was found in SW1463 cells, while MIAPACA2 cells appeared to be more dependent on FGFR signaling than EGFR/HER family members. Moreover, rapid but non-uniform adaptation to ARS1620 was detected using scRNA-seq at a single-cell resolution. Similarly, MET amplification, activating mutations in RET and oncogenic fusions involving ALK, RET and FGFR3 were found in patient relapsed during the treatment of MRTX849.50 Although targeting a dominant RTK-driven feedback reactivation is an attractive strategy to overcome adaptive resistance, it is difficult to propose a uniform drug combination as RTKs were activated heterogeneously across cancers carrying KRASG12C mutation. SHP2 is a primary effector of receptor tyrosine kinase signaling, which dephosphorylates Ras and results in increased Ras–Raf association and activation of downstream MAPK signaling.72 In this scenario, the RTK-associated phosphatase SHP2 may represent a common target to inhibit feedback reactivation of multiple RTKs and improve the efficacy across heterogeneous KRASG12C-mutated cancers. Indeed, co-targeting KRasG12C with a SHP2 inhibitor showed synergistic effect both in vitro and in vivo, which blocked downstream signals and finally led to tumor regression.68,69,73 These studies indicated that RTK activation could lead to the resistance to KRasG12C inhibitors, and co-targeting RTK or SHP2 and KRasG12C could circumvent the resistance. In fact, the combination of SHP2 inhibitors or EGFR inhibitors with KRasG12C inhibitors are currently under clinical investigation in KRASG12C mutated solid tumors including lung cancer and colorectal cancer (Table 1).
Co-targeting downstream signaling
MAPK pathway, which sits downstream of KRas, is a key regulator of cell proliferation, survival and differentiation.74,75 The effectors of MAPK pathway, MEK1/2 and ERK1/2, were initially robustly inactivated in all cell lines after ARS1620 treatment, while phosphorylation of MEK and ERK restored upon prolonged treatment.44 Moreover, activating mutation in MAP2K1 and oncogenic fusions in RAF1 were found in clinical samples with adaptive resistance to MRTX849.50 It has been shown that combination of a KRASG12C inhibitor with a MEK inhibitor could drive tumor regression,46 which indicated this strategy may overcome the resistance led by the rebound of MEK.
Sustained activation of another important parallel signaling, PI3K-AKT pathway, may also mediate the resistance to KRasG12C inhibitors. PI3K pathway is often aberrantly activated in KRASG12C cell lines. Inhibition of KRASG12C led to decreased p-AKT in only 3 of the 12 cell lines, suggesting that PI3K pathway may not be regulated by KRas alone in most KRAS mutated cells.44 Moreover, loss-of-function mutations in PTEN were found in clinical samples with acquired resistance to MRTX849,50 which may also lead to activation of PI3K signaling. Accordingly, concurrent inhibition of KRasG12C and PI3K improved the efficacy and achieved tumor regression.44 mTOR is a crucial downstream of both PI3K and MAPK pathways. However, transiently reduced p-S6 was observed in few cell lines after treated with KRasG12C inhibitors.44 The sustained mTOR activation indicates that mTOR activity might be independent of KRas. Sandra et al. performed a high-throughput combinatorial drug screening to evaluate the synergy of ARS1620 in combination with a panel of 112 small molecules of high clinical relevance and founded that inhibitors targeting PI3K and mTORC1 pathway were the most synergistic with ARS1620.44 Similarly, the combination of an mTOR inhibitor with a KRasG12C inhibitor could significantly reduce the level of both p-S6 and p-ERK, which may overcome the resistance to KRasG12C inhibitors.46 AMG510 in combination with MEK inhibitor or mTOR inhibitor is being tested in clinical trials (NCT04185883, Table 1).
The Ras-MAPK pathway plays an important role in cell cycle transition. Previous studies have identified CDK1 and CDK4 as synthetic lethal targets in KRAS-mutated CRC and NSCLC respectively,76,77 indicating that cell cycle regulators might be potential targets for tumors harboring KRAS mutation. Cyclin D1-CDK4/6 complex phosphorylates the tumor suppressor RB, resulting in the release of E2F and transcription of genes involved in G1-S transition. To identify potential combination therapies to overcome the primary resistance to KRas inhibitors, high-throughput screenings have been performed and found that signaling adaptation could limit the efficacy of KRasG12C inhibitors and combination with CDK4/6 inhibitors overcame this resistance.78 A recent study revealed that the expression of aurora kinase A (AURKA), a serine/threonine kinase regulating mitosis by binding to the centrosome, was initially downregulated when KRasG12C mutated gastrointestinal (GI) cancer cells were treated with KRasG12C inhibitors but rebounded after long-term treatment.79 Simultaneous inhibition of AURKA and KRasG12C displayed synergistic anti-proliferative activity. Thus targeting AURKA may potentially be a novel therapeutic strategy for treating KRAS-mutated GI cancer.80 CDK inhibitors in combination of AMG510 are currently tested in clinical trials (Table 1).
Co-targeting alternative pathways
There are three members in the RAS family and they perform distinctive activity in cells. Rapid adaptive feedback reactivation in Ras pathway has been observed in the majority of KRASG12C mutated cancer cells upon treatment of ARS1620 or AMG510.68 The activity of NRas and HRas was revealed to increase in KRASG12C mutated cells treated with ARS1620, which could not be inhibited by KRasG12C-specific inhibitors and might attenuate the activity of KRasG12C inhibitors.68 Moreover, acquired KRAS alterations including G12D/R/V/W, G13D, Q61H, R68S, H95D/Q/R, Y96C, and high-level amplification of the KRasG12C allele were found in patients developed acquired resistance to MRTX849.50 Introduction of the clinically observed switch II pocket mutations (R68S, H95D, H95Q, H95R, and Y96C) conferred marked resistance to MRTX849,50 which may contribute to clinical resistance to KRasG12C inhibitor. A novel clinical KRASY96D mutation has also been reported to affect the binding of MRTX849 to the switch-Ⅱ pocket, and thus confers resistance to KRasG12C inhibitors.81 Though pan-Ras inhibitors might overcome the resistance,82 the related toxicity should be concerned. The next-generation KRas covalent inhibitors are warranted to be discovered.
The epithelial-mesenchymal transition (EMT) is a process that epithelial cells lose cell polarity as well as cell-cell adhesion to become mesenchymal cells with gained migratory and invasive properties.83 The EMT is considered a key driver of invasion and metastasis in tumor for a long time, while recent studies revealed its role in mediating resistance to chemotherapy.84 Similarly, EMT was observed in cells with acquired resistance to AMG510. Moreover, induction of EMT by TGF-β rendered sensitive cells resistant to KRasG12C inhibitors.69 Therefore, EMT might mediate resistance to KRasG12C inhibitors and targeting EMT might enhance the efficacy.
KRas may have profound influence on tumor immune microenvironment such as allowing tumor cells to escape the anti-tumor immune response and thus promoting the growth and metastasis of tumor cells.85,86 Recent study has reported that the combination of AMG510 and immunotherapies achieved improved efficacy compared to monotherapy. For example, combination of AMG510 and anti-PD-1 antibody led to complete regression in nine out of ten mice tested and generated T cell memory.42 AMG510 induced an inflammatory microenvironment by increasing tumor infiltration of macrophages and dendritic cells as well as the expression of MHC class I antigens on tumor cells.42 The combination of KRasG12C inhibitors and PD-1/PDL1 antibodies is being tested in clinical trials (Table 1).
Perspectives
KRAS is one of the most frequently mutated oncogenes in human cancer and is identified as an attractive target for cancer therapy. However, KRas protein was considered undruggable until the approval of AMG510 for treating patients with KRASG12C-mutated locally advanced or metastatic NSCLC. These covalent KRasG12C inhibitors trap the protein in the inactive GDP-bound state, which represents a major step towards targeting the undruggable KRas and provided valuable insights into targeting other common KRAS mutations such as G12D, G13C, or G13D.87 Encouragingly, Mirati reported the promising preclinical activity of a potential first-in-class G12D selective inhibitor, MRTX1133, with similar features of covalent KRASG12C inhibitors.62,63
Although early-phase clinical trial results of AMG510 and MRTX849 among NSCLC patients are promising, the objective response rate is around 50%, which indicates around half NSCLC patients harboring KRASG12C mutation don't respond to the treatment, and the activity of AMG510 and MRTX849 in patients with colorectal cancer and appendiceal cancer appears to be less favorable.66 In addition to KRASG12C mutation, additional biomarkers were needed to stratify patients. Reliable biomarkers also need to be investigated in the future clinical trials to monitor the efficacy. Moreover, the mechanisms of response or resistance should be comprehensively elucidated in both preclinical and clinical settings. It has been reported that activation of upstream RTKs and SHP2, downstream MEK, AKT and mTOR, as well as alternative mutations in KRas would mediate resistance to KRasG12C inhibitors. Indeed, combination of KRasG12C inhibitors with RTK inhibitors, SHP2 inhibitors, MEK inhibitors or mTOR inhibitors is currently tested in clinical trials (Table 1). In particular, activated Ras may possess profound influences on tumor immune microenvironment such as allowing cancer cells to evade the antitumor immune response.85 Recent studies found that the combination of a KRasG12C inhibitor and an anti-PD-L1 antibody displayed enhanced efficacy in vivo.42 Better understanding the mechanisms of action of KRasG12C inhibitors on tumor cells and the tumor micro-environment would be helpful to optimize the therapy based on KRasG12C inhibitors.
In summary, the emerging KRasG12C inhibitors have provided great opportunities for the treatment of KRAS-mutated cancers. It is still of great importance to improve the clinical efficacy through identifying predicting biomarkers and proposing rational drug combinations to circumvent drug resistance.
Author contributions
Ling-hua Meng and Yu-xiang Wang conceived the project and revised the manuscript. Hui-yu Li and Wei-liang Qi summarized the literature and composed the manuscript. All authors proofread the manuscript and approved to submit the final manuscript.
Conflict of interests
The authors declare no competing interests.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81773760, 81973345, and 82104199).
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Yu-xiang Wang, Email: yxwang@simm.ac.cn.
Ling-hua Meng, Email: lhmeng@simm.ac.cn.
References
- 1.Colicelli J. Human RAS superfamily proteins and related GTPases. Sci STKE. 2004;2004(250) doi: 10.1126/stke.2502004re13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malumbres M., Barbacid M. RAS oncogenes:the first 30 years. Nat Rev Cancer. 2003;3(6):459–465. doi: 10.1038/nrc1097. [DOI] [PubMed] [Google Scholar]
- 3.Khan I., Rhett J.M., O'Bryan J.P. Therapeutic targeting of RAS: new hope for drugging the "undruggable". Biochim Biophys Acta Mol Cell Res. 2020;1867(2) doi: 10.1016/j.bbamcr.2019.118570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu P., Wang Y., Li X. Targeting the untargetable KRAS in cancer therapy. Acta Pharm Sin B. 2019;9(5):871–879. doi: 10.1016/j.apsb.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bos J.L., Rehmann H., Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129(5):865–877. doi: 10.1016/j.cell.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 6.Nagasaka M., Li Y., Sukari A., et al. KRAS G12C Game of Thrones, which direct KRAS inhibitor will claim the iron throne? Cancer Treat Rev. 2020;84 doi: 10.1016/j.ctrv.2020.101974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banno K., Yanokura M., Iida M., et al. Carcinogenic mechanisms of endometrial cancer: involvement of genetics and epigenetics. J Obstet Gynaecol Res. 2014;40(8):1957–1967. doi: 10.1111/jog.12442. [DOI] [PubMed] [Google Scholar]
- 8.Lu S., Jang H., Gu S., et al. Drugging Ras GTPase: a comprehensive mechanistic and signaling structural view. Chem Soc Rev. 2016;45(18):4929–4952. doi: 10.1039/c5cs00911a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koera K., Nakamura K., Nakao K., et al. K-Ras is essential for the development of the mouse embryo. Oncogene. 1997;15(10):1151–1159. doi: 10.1038/sj.onc.1201284. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura K., Ichise H., Nakao K., et al. Partial functional overlap of the three ras genes in mouse embryonic development. Oncogene. 2008;27(21):2961–2968. doi: 10.1038/sj.onc.1210956. [DOI] [PubMed] [Google Scholar]
- 11.Potenza N., Vecchione C., Notte A., et al. Replacement of K-Ras with H-Ras supports normal embryonic development despite inducing cardiovascular pathology in adult mice. EMBO Rep. 2005;6(5):432–437. doi: 10.1038/sj.embor.7400397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson C.Y., Tolias P. Recent advances in cancer drug discovery targeting RAS. Drug Discov Today. 2016;21(12):1915–1919. doi: 10.1016/j.drudis.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Biankin A.V., Waddell N., Kassahn K.S., et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491(7424):399–405. doi: 10.1038/nature11547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neumann J., Zeindl-Eberhart E., Kirchner T., et al. Frequency and type of KRAS mutations in routine diagnostic analysis of metastatic colorectal cancer. Pathol Res Pract. 2009;205(12):858–862. doi: 10.1016/j.prp.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 15.Lanman B.A., Allen J.R., Allen J.G., et al. Discovery of a covalent inhibitor of KRAS G12C (AMG 510) for the treatment of solid tumors. J Med Chem. 2019;63(1):52–65. doi: 10.1021/acs.jmedchem.9b01180. [DOI] [PubMed] [Google Scholar]
- 16.Rodenhuis S. Ras and human tumors. Semin Cancer Biol. 1992;3(4):241–247. [PubMed] [Google Scholar]
- 17.Croce C.M. Oncogenes and cancer. N Engl J Med. 2008;358(5):502–511. doi: 10.1056/NEJMra072367. [DOI] [PubMed] [Google Scholar]
- 18.Khosravi-Far R., Der C.J. The Ras signal transduction pathway. Cancer Metastasis Rev. 1994;13(1):67–89. doi: 10.1007/BF00690419. [DOI] [PubMed] [Google Scholar]
- 19.Hansen R., Peters U., Babbar A., et al. The reactivity-driven biochemical mechanism of covalent KRAS G12C inhibitors. Nat Struct Mol Biol. 2018;25(6):454–462. doi: 10.1038/s41594-018-0061-5. [DOI] [PubMed] [Google Scholar]
- 20.Balmain A., Pragnell I.B. Mouse skin carcinomas induced in vivo by chemical carcinogens have a transforming Harvey-ras oncogene. Nature. 1983;303(5912):72–74. doi: 10.1038/303072a0. [DOI] [PubMed] [Google Scholar]
- 21.Sukumar S., Notario V., Martin-Zanca D., et al. Induction of mammary carcinomas in rats by nitroso-methylurea involves malignant activation of H-ras-1 locus by single point mutations. Nature. 1983;306(5944):658–661. doi: 10.1038/306658a0. [DOI] [PubMed] [Google Scholar]
- 22.Guerrero I., Calzada P., Mayer A., et al. A molecular approach to leukemogenesis: mouse lymphomas contain an activated c-ras oncogene. Proc Natl Acad Sci U S A. 1984;81(1):202–205. doi: 10.1073/pnas.81.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim K.H., Counter C.M. Reduction in the requirement of oncogenic Ras signaling to activation of PI3K/AKT pathway during tumor maintenance. Cancer Cell. 2005;8(5):381–392. doi: 10.1016/j.ccr.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 24.Brummelkamp T.R., Bernards R., Agami R. Stable suppression of tumorigenicity by virus-mediated RNA interference. Cancer Cell. 2002;2(3):243–247. doi: 10.1016/s1535-6108(02)00122-8. [DOI] [PubMed] [Google Scholar]
- 25.Ji H., Ramsey M.R., Hayes D.N., et al. LKB1 modulates lung cancer differentiation and metastasis. Nature. 2007;448(7155):807–810. doi: 10.1038/nature06030. [DOI] [PubMed] [Google Scholar]
- 26.Hingorani S.R., Wang L., Multani A.S., et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7(5):469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 27.Haigis K.M., Kendall K.R., Wang Y., et al. Differential effects of oncogenic K-Ras and N-Ras on proliferation, differentiation and tumor progression in the colon. Nat Genet. 2008;40(5):600–608. doi: 10.1038/ngXXXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen W.C., To M.D., Westcott P.M.K., et al. Regulation of KRAS4A/B splicing in cancer stem cells by the RBM39 splicing complex. bioRxiv. 2019 doi: 10.1101/646125. Preprint at . [DOI] [Google Scholar]
- 29.Ryan M.B., Corcoran R.B. Therapeutic strategies to target RAS-mutant cancers. Nat Rev Clin Oncol. 2018;15(11):709–720. doi: 10.1038/s41571-018-0105-0. [DOI] [PubMed] [Google Scholar]
- 30.van Maldegem F., Downward J. Mutant KRAS at the heart of tumor immune evasion. Immunity. 2020;52(1):14–16. doi: 10.1016/j.immuni.2019.12.013. [DOI] [PubMed] [Google Scholar]
- 31.Cox A.D., Fesik S.W., Kimmelman A.C., et al. Drugging the undruggable RAS: mission possible? Nat Rev Drug Discov. 2014;13(11):828–851. doi: 10.1038/nrd4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gibbs J.B., Graham S.L., Hartman G.D., et al. Farnesyltransferase inhibitors versus Ras inhibitors. Curr Opin Chem Biol. 1997;1(2):197–203. doi: 10.1016/s1367-5931(97)80010-5. [DOI] [PubMed] [Google Scholar]
- 33.Sanclemente M., Francoz S., Esteban-Burgos L., et al. c-RAF ablation induces regression of advanced Kras/Trp53 mutant lung adenocarcinomas by a mechanism independent of MAPK signaling. Cancer Cell. 2018;33(2):217–228. doi: 10.1016/j.ccell.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 34.Jones G.G., Del Río I.B., Sari S., et al. SHOC2 phosphatase-dependent RAF dimerization mediates resistance to MEK inhibition in RAS-mutant cancers. Nat Commun. 2019;10(1) doi: 10.1038/s41467-019-10367-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blasco M.T., Navas C., Martín-Serrano G., et al. Complete regression of advanced pancreatic ductal adenocarcinomas upon combined inhibition of EGFR and C-RAF. Cancer Cell. 2019;35(4):573–587. doi: 10.1016/j.ccell.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luo J., Emanuele M.J., Li D., et al. A genome-wide RNAi screen identifies multiple synthetic lethal interactions with the Ras oncogene. Cell. 2009;137(5):835–848. doi: 10.1016/j.cell.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.te Heesen H., Gerwert K., Schlitter J. Role of the arginine finger in Ras.RasGAP revealed by QM/MM calculations. FEBS Lett. 2007;581(29):5677–5684. doi: 10.1016/j.febslet.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 38.Hatakeyama K., Harada T., Kagamiyama H. IMP dehydrogenase inhibitors reduce intracellular tetrahydrobiopterin levels through reduction of intracellular GTP levels. Indications of the regulation of GTP cyclohydrolase I activity by restriction of GTP availability in the cells. J Biol Chem. 1992;267(29):20734–20739. [PubMed] [Google Scholar]
- 39.Nickerson S., Joy S.T., Arora P.S., et al. An orthosteric inhibitor of the RAS-SOS interaction. Enzymes. 2013;34(Pt. B):25–39. doi: 10.1016/B978-0-12-420146-0.00002-0. [DOI] [PubMed] [Google Scholar]
- 40.Shima F., Yoshikawa Y., Ye M., et al. In silico discovery of small-molecule Ras inhibitors that display antitumor activity by blocking the Ras-effector interaction. Proc Natl Acad Sci U S A. 2013;110(20):8182–8187. doi: 10.1073/pnas.1217730110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patricelli M.P., Janes M.R., Li L.S., et al. Selective inhibition of oncogenic KRAS output with small molecules targeting the inactive state. Cancer Discov. 2016;6(3):316–329. doi: 10.1158/2159-8290.CD-15-1105. [DOI] [PubMed] [Google Scholar]
- 42.Canon J., Rex K., Saiki A.Y., et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature. 2019;575(7781):217–223. doi: 10.1038/s41586-019-1694-1. [DOI] [PubMed] [Google Scholar]
- 43.Hallin J., Engstrom L.D., Hargis L., et al. The KRAS(G12C) inhibitor MRTX849 provides insight toward therapeutic susceptibility of KRAS-mutant cancers in mouse models and patients. Cancer Discov. 2020;10(1):54–71. doi: 10.1158/2159-8290.CD-19-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Misale S., Fatherree J.P., Cortez E., et al. KRAS G12C NSCLC models are sensitive to direct targeting of KRAS in combination with PI3K inhibition. Clin Cancer Res. 2019;25(2):796–807. doi: 10.1158/1078-0432.CCR-18-0368. [DOI] [PubMed] [Google Scholar]
- 45.Ni D., Li X., He X., et al. Drugging K-Ras(G12C) through covalent inhibitors: mission possible? Pharmacol Ther. 2019;202:1–17. doi: 10.1016/j.pharmthera.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 46.Molina-Arcas M., Moore C., Rana S., et al. Development of combination therapies to maximize the impact of KRAS-G12C inhibitors in lung cancer. Sci Transl Med. 2019;11(510) doi: 10.1126/scitranslmed.aaw7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Govindan R., Fakih M.G., Price T.J., et al. Phase 1 trial evaluating safety, efficacy, and PK of AMG 510, a novel KRASG12C inhibitor, in non-small cell lung cancer. J Thorac Oncol. 2019;14(11):S1191–S1192. [Google Scholar]
- 48.Janne P.A., Papadopoulous K., Ou S.I., et al. A Phase 1 clinical trial evaluating the pharmacokinetics (PK), safety, and clinical activity of MRTX849, a mutant-selective small molecule KRAS G12C inhibitor, in advanced solid tumors. International Conference on Molecular Targets and Cancer Therapeutics. 2019 October 26-30. [Google Scholar]
- 49.Hata A.N., Shaw A.T. Resistance looms for KRAS(G12C) inhibitors. Nat Med. 2020;26(2):169–170. doi: 10.1038/s41591-020-0765-z. [DOI] [PubMed] [Google Scholar]
- 50.Awad M.M., Liu S., Rybkin II, et al. Acquired resistance to KRAS(G12C) inhibition in cancer. N Engl J Med. 2021;384(25):2382–2393. doi: 10.1056/NEJMoa2105281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Downward J. RAS synthetic lethal screens revisited: still seeking the elusive prize? Clin Cancer Res. 2015;21(8):1802–1809. doi: 10.1158/1078-0432.CCR-14-2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ostrem J.M., Peters U., Sos M.L., et al. K-Ras (G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature. 2013;503(7477):548–551. doi: 10.1038/nature12796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Newswire P. 2019. Amgen Announces New Clinical Data Evaluating Novel Investigational KRAS(G12C) Inhibitor in Larger Patient Group at WCLC 2019.https://www.amgen.com/ [Google Scholar]
- 54.Lito P., Solomon M., Li L.S., et al. Allele-specific inhibitors inactivate mutant KRAS G12C by a trapping mechanism. Science. 2016;351(6273):604–608. doi: 10.1126/science.aad6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hobbs G.A., Wittinghofer A., Der C.J. Selective targeting of the KRAS G12C mutant: kicking KRAS when it's down. Cancer Cell. 2016;29(3):251–253. doi: 10.1016/j.ccell.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 56.Janes M.R., Zhang J., Li L.S., et al. Targeting KRAS mutant cancers with a covalent G12C-specific inhibitor. Cell. 2018;172(3):578–589. doi: 10.1016/j.cell.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 57.Jiao D., Yang S. Overcoming resistance to drugs targeting KRAS(G12C) mutation. Innovation. 2020;1(2) doi: 10.1016/j.xinn.2020.100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rex K., Saiki A.Y., Sun J.R., et al. In vivo characterization of AMG 510-a potent and selective KRASG12C covalent small molecule inhibitor in preclinical KRASG12C cancer models. Cancer Res. 2019;79(13 Supplement) [Google Scholar]
- 59.Hong D.S., Fakih M.G., Strickler J.H., et al. KRAS(G12C) inhibition with sotorasib in advanced solid tumors. N Engl J Med. 2020;383(13):1207–1217. doi: 10.1056/NEJMoa1917239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.FDA Approves LUMAKRAS™ (Sotorasib), the First and Only Targeted Treatment for Patients with KRAS G12C-Mutated Locally Advanced or Metastatic Non-small Cell Lung Cancer. 2021. https://www.amgen.com/newsroom/press-releases/2021/05/fda-approves-lumakras-sotorasib-the-first-and-only-targeted-treatment-for-patients-with-kras-g12cmutated-locally-advanced-or-metastatic-nonsmall-cell-lung-cancer [Google Scholar]
- 61.Christensen J.G., Olson P., Briere T., et al. Targeting Kras(g12c) -mutant cancer with a mutation-specific inhibitor. J Intern Med. 2020;288(2):183–191. doi: 10.1111/joim.13057. [DOI] [PubMed] [Google Scholar]
- 62.Jänne P.A., Rybkin I.I., Spira A.I., et al. Activity and safety of adagrasib (MRTX849) in advanced/metastatic non-small-cell lung cancer (NSCLC) harboring KRAS G12C mutation. Eur J Canc. 2020;138(S2):S1–S2. [Google Scholar]
- 63.Johnson M.L., Ou S.H.I., Barve M., et al. Activity and safety of adagrasib (MRTX849) in patients with colorectal cancer (CRC) and other solid tumors harboring a KRAS G12C mutation. Eur J Canc. 2020;138(S2):S2. [Google Scholar]
- 64.Mirati Therapeutics Reports Investigational Adagrasib (MRTX849) Preliminary Data Demonstrating Tolerability and Durable Anti-tumor Activity as Well as Initial MRTX1133 Preclinical Data. 2020. https://ir.mirati.com/news-releases/news-details/2020/Mirati-Therapeutics-Reports-Investigational-Adagrasib-MRTX849-Preliminary-Data-Demonstrating-Tolerability-and-Durable-Anti-Tumor-Activity-as-well-as-Initial-MRTX1133-Preclinical-Data/default.aspx [Google Scholar]
- 65.McCormick F. Progress in targeting RAS with small molecule drugs. Biochem J. 2019;476(2):365–374. doi: 10.1042/BCJ20170441. [DOI] [PubMed] [Google Scholar]
- 66.Klempner S.J., Hata A.N. Can the help match the hype? KRAS(G12C)-specific inhibitors and beyond. Cancer Discov. 2020;10(1):20–22. doi: 10.1158/2159-8290.CD-19-1255. [DOI] [PubMed] [Google Scholar]
- 67.Amodio V., Yaeger R., Arcella P., et al. EGFR blockade reverts resistance to KRAS(G12C) inhibition in colorectal cancer. Cancer Discov. 2020;10(8):1129–1139. doi: 10.1158/2159-8290.CD-20-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ryan M.B., Fece de la Cruz F., Phat S., et al. Vertical pathway inhibition overcomes adaptive feedback resistance to KRAS(G12C) inhibition. Clin Canc Res. 2020;26(7):1633–1643. doi: 10.1158/1078-0432.CCR-19-3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Adachi Y., Ito K., Hayashi Y., et al. Epithelial-to-mesenchymal transition is a cause of both intrinsic and acquired resistance to KRAS G12C inhibitor in KRAS G12C mutant non-small cell lung cancer. Clin Cancer Res. 2020;26(22):5962–5973. doi: 10.1158/1078-0432.CCR-20-2077. [DOI] [PubMed] [Google Scholar]
- 70.Results from Phase 2 CodeBreaK 100 Show LUMAKRAS™ (Sotorasib) Is the First and Only KRAS G12C Inhibitor with Overall Survival Data. 2021. https://www.amgen.com/newsroom/press-releases/2021/06/results-from-phase-2-codebreak-100-show-lumakras-sotorasib-is-the-first-and-only-kras-g12c-inhibitor-with-overall-survival-data [Google Scholar]
- 71.Kelsey I., Manning B.D. mTORC1 status dictates tumor response to targeted therapeutics. Sci Signal. 2013;6(294) doi: 10.1126/scisignal.2004632. [DOI] [PubMed] [Google Scholar]
- 72.Bunda S., Burrell K., Heir P., et al. Inhibition of SHP2-mediated dephosphorylation of Ras suppresses oncogenesis. Nat Commun. 2015;6 doi: 10.1038/ncomms9859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fedele C., Li S., Teng K.W., et al. SHP2 inhibition diminishes KRASG12C cycling and promotes tumor microenvironment remodeling. J Exp Med. 2021;218(1) doi: 10.1084/jem.20201414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Santarpia L., Lippman S.M., El-Naggar A.K. Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy. Expert Opin Ther Targets. 2012;16(1):103–119. doi: 10.1517/14728222.2011.645805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seger R., Krebs E.G. The MAPK signaling cascade. Faseb J. 1995;9(9):726–735. [PubMed] [Google Scholar]
- 76.Costa-Cabral S., Brough R., Konde A., et al. CDK1 is a synthetic lethal target for KRAS mutant tumours. PloS One. 2016;11(2) doi: 10.1371/journal.pone.0149099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Puyol M., Martin A., Dubus P., et al. A synthetic lethal interaction between K-Ras oncogenes and Cdk4 unveils a therapeutic strategy for non-small cell lung carcinoma. Cancer Cell. 2010;18(1):63–73. doi: 10.1016/j.ccr.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 78.Santana-Codina N., Chandhoke A.S., Yu Q., et al. Defining and targeting adaptations to oncogenic KRAS(G12C) inhibition using quantitative temporal proteomics. Cell Rep. 2020;30(13):4584–4599. doi: 10.1016/j.celrep.2020.03.021. [DOI] [PubMed] [Google Scholar]
- 79.Xue J.Y., Zhao Y., Aronowitz J., et al. Rapid non-uniform adaptation to conformation-specific KRAS(G12C) inhibition. Nature. 2020;577(7790):421–425. doi: 10.1038/s41586-019-1884-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bishop L., Chen Z., Omar O., et al. Targeting Aurka effectively suppresses Kras-mutant tumorigenesis through regulating P70S6K phosphorylaton in gastrointestinal cancers. Gastroenterology. 2017;152(5):S41. [Google Scholar]
- 81.Tanaka N., Lin J.J., Li C., et al. Clinical acquired resistance to KRAS(G12C) inhibition through a novel KRAS switch-II pocket mutation and polyclonal alterations converging on RAS-MAPK reactivation. Cancer Discov. 2021;11(8):1913–1922. doi: 10.1158/2159-8290.CD-21-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Welsch M.E., Kaplan A., Chambers J.M., et al. Multivalent small-molecule pan-RAS inhibitors. Cell. 2017;168(5):878–889. doi: 10.1016/j.cell.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lamouille S., Xu J., Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fischer K.R., Durrans A., Lee S., et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature. 2015;527(7579):472–476. doi: 10.1038/nature15748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen N., Fang W., Lin Z., et al. KRAS mutation-induced upregulation of PD-L1 mediates immune escape in human lung adenocarcinoma. Cancer Immunol Immunother. 2017;66(9):1175–1187. doi: 10.1007/s00262-017-2005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weijzen S., Velders M.P., Kast W.M. Modulation of the immune response and tumor growth by activated Ras. Leukemia. 1999;13(4):502–513. doi: 10.1038/sj.leu.2401367. [DOI] [PubMed] [Google Scholar]
- 87.Goebel L., Müller M.P., Goody R.S., et al. KRasG12C inhibitors in clinical trials: a short historical perspective. RSC Med Chem. 2020;11(7):760–770. doi: 10.1039/d0md00096e. [DOI] [PMC free article] [PubMed] [Google Scholar]