Abstract
Lignocellulosic biomass is the generally explored substrate to produce bioethanol for environmental sustainability due to its availability in abundance. However, the complex network of cellulose-hemicellulose-lignin present in it makes its hydrolysis as a challenging task. To boost the effectiveness of conversion, biomass is pre-treated before enzymatic hydrolysis to alter or destroy its original composition. Enzymes like Cellulases are widely used for breaking down cellulose into fermentable sugars. Enzymatic hydrolysis is a complex process involving many influencing factors such as pH, temperature, substrate concentration. This review presents major four pre-treatment methods used for hydrolysing different substrates under varied reaction conditions along with their mechanism and limitations. A relative comparison of data analysis for most widely studied 10 kinetic models is briefly explained in terms of substrates used to get the brief insight about hydrolysis rates. The summary of pre-treatment methods and hydrolysis rates including cellulase enzyme kinetics will be the value addition for upcoming researchers for optimising the hydrolysis process.
Keywords: Lignocellulosic biomass, Pre-treatment, Cellulase enzyme, Kinetic models, Environmental sustainability
1. Introduction
Increased usage of fossil fuels has resulted from population growth and the rapid advancement of technology, which leads to increased greenhouse gas (GHG) emissions and threatens the stability of the climate [1]. To address these issues, nations around the world have started to produce sustainable alternative fuels in the form of biofuels using biomass [2]. The bioconversion of lignocellulosic materials into biofuels presents hitherto unheard-of prospects for substituting fossil fuel products and can significantly lower carbon emissions, hence reducing the effects of the current trends in climate change [3,4]. A plentiful resource that is recyclable and renewable is lignocellulosic biomass. Up to 50–80% of cellulose and hemicellulose can be converted into fermentable sugars using enzymes and it is an eco-friendly process [6]. Biomass such as agricultural waste, forest residues, domestic waste are non-petroleum, plenty and are easily available for harvesting in a sustainable and cost effective way [7]. Utilizing biomass can reduce the disposal issue of renewable resources and thus follows environment friendly and sustainable agricultural growth concepts [8].
Bioethanol is the most popular biofuel choice. The Indian government has laid a plan for 20% ethanol blending along with gasoline by 2025. The socio-economic and environmental benefits of producing bioethanol from non edible feedstock like lignocellulosic biomass as represented in Fig. 1.
Fig. 1.
Socio-economic and environmental benefits of bioethanol production.
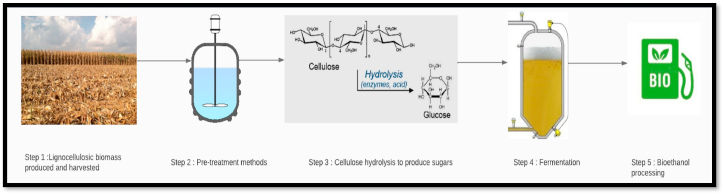
Cellulosic bioethanol production, entails several steps, including the delivery and collection of biomasses, pre-treatment methods to degrade hemicellulose and lignin, cellulose hydrolysis, fermentation, and bioethanol processing [9]. Fig. 2 shows the diagrammatic representation of steps involved in bioethanol production. Based on the type of biomass, different pre-treatment methods are used to destroy the physicochemical structure and make cellulose more available for enzymatic hydrolysis. There are typically two ways to carry out the processes of saccharification and fermentation: “simultaneous saccharification and fermentation (SSF) and separate hydrolysis and fermentation (SHF)”. For both enzymatic hydrolysis and fermentation, the SHF technique can attain the ideal conditions. SSF is the preferred option when the product inhibits the enzyme activity, while SHF is the preferred option to achieve higher conversion rates [10].
Fig. 2.
Shows the diagrammatic representation of the biofuel production process.
Techno-economic difficulties with cellulosic ethanol's conversion process are still one of the main barriers preventing its commercialization [[11], [12], [13]]. Although scientific advancements have been made, new technologies are still required to remove barriers to the development of effective and affordable conversion techniques that will create ethanol that is cost-competitive [14,15]. The mechanism involves breakdown of only cellulose and hemi-cellulose fractions of lignocellulose biomass as their long polysaccharide chains are broken down into hexose and pentose sugars which further can be converted into ethanol molecules [16]. A recent study on agro-industrial waste like de-oiled castor bean cake showed that roughly 32 R/L would be the cost of bioethanol production [17]. Pre-treatment methods and enzymatic hydrolysis are crucial procedures that considerably affect the efficiency and cost of the five steps required to produce cellulosic ethanol.
A complex heterogeneous catalytic process, enzymatic hydrolysis involves reaction kinetics and molecular mass transfer kinetics. It is a preferred choice to convert biomass to sugars as enzyme-substrate reactions are very specific and doesn't require high temperatures for carrying out the processes [18]. Numerous experimental studies have been conducted in recent years by researchers to improve hydrolysis at a lower cost. A high-throughput screening procedure based on microarray technology was created for the experimental approach from conventional studies. For instance, some researchers apply high-throughput screening optimization technology to improve various pre-treatment factors [[19], [20], [21], [22]]. Other researchers have conducted quantitative analyses of the impact of individual or mixed enzymes on enzyme breakdown. Currently, the fungus Trichoderma reesei was employed to produce the most widely used cellulase. The working conditions are better understood with a large number of experimental trials. These repeated tests are required because the influencing factors of enzymatic hydrolysis are numerous and all interconnected (for example, pre-treatment, enzymatic hydrolysis, and substrate characteristics) [23,24]. Raguskas et al. have explained the structural changes in cellulosic substrates during enzyme hydrolysis and various substrate and enzyme related factors that influence the conversion process [25].
The pre-treatment and hydrolysis process can be better understood using experimental tools. However, using them is time-consuming, labour-intensive, and harmful to the environment. Theoretical models were thus developed and are frequently used to reduce the workload and further give insights into the reaction process [26,27]. These models help in understanding the reaction mechanisms more thoroughly thus making the reactor design process easier. Kinetic models can predict profiles of pre-treatment and hydrolysis under various conditions, making them a useful tool for figuring out the best pre-treatment and hydrolysis at a reasonable cost.
Empirical models and mechanistic models are the two main categories of enzymatic hydrolysis kinetic models. To understand the process quantitatively, empirical models are developed using statistical tools [28]. In contrast, using the mechanistic model and mass transfer and reaction theory, the reaction kinetics is built [29]. The empirical model can partially explain the characteristics of simple hydrolysis. The mechanistic model offers benefits in terms of general adaptability, can fully expose the hydrolysis mechanism, and can achieve optimal parameters. By altering and refining the parameters, the current model can be utilized directly for verification in previous experimental studies with comparable mechanisms, which will simplify experimental runs and increases the validity of mechanistic models. A semi-mechanistic model can typically accurately forecast the reaction outputs. However, a kinetic mechanistic model taking into account more parameters should be developed to identify the primary influencing component to completely expose the reaction's process. The review of the different mechanistic models using different feedstocks is emphasised in this work.
The present study gives insight about the composition of cellulose, hemicellulose and lignin in various feedstocks, recalcitrant structure of lignocellulose biomass, several methods used for pre-treatment to break the chains along with their mechanism and types of kinetic models studied so far in understanding hydrolysis mechanism for different feedstocks. The novelty of this review paper is that it provides a holistic information about composition, hydrolysis and model mechanisms for various feedstocks. It helps scholars’ helpful pointers for future study to choose pre-treatment methods and kinetic models for their particular substrate to establish sustainable bioethanol production technology.
2. Structural and compositional characteristics of lignocellulosic biomass
Cellulose, hemicellulose and lignin are the primary components that constitute lignocellulosic biomass polymer. Table 1 summarises the composition and nature of each component.
Table 1.
Nature and composition of components of lignocellulosic biomass.
| Components | Composition | Nature of the component | References |
|---|---|---|---|
| Cellulose (40–50%) | A linear polymer made up of (1, 4)-linked d-glucose monomer units | Large cellulose chains are in crystalline nature (resistance to hydrolysis) | [5,[30], [31], [32], [33]] |
| Degree of polymerisation- 5000 to 15,000 glucose molecules | Smaller chains are in amorphous form (rapidly hydrolysed) | ||
| Hemicellulose (25–35%) | Polymer made up of five and six carbon sugars | It is amorphous and hydrophilic in nature (less resistant to chemicals and dissolved by water) | [33] |
| Degree of polymerisation – 50 to 300 | |||
| Lignin (15–20%) | Non-carbohydrate polymer made up of aromatic polymers | It is hydrophobic and cannot dissolve in water | [34,35] |
The structural cell wall organization of these elements is in the recalcitrant metastable phase and these elements are intricately related to one another as seen in Fig. 3. It is significantly more difficult to hydrolyse cellulose when the complex lignin-hemicellulose shield is present because it forms a protective covering around the cellulose microfibrils and shields them from enzyme attack [36]. Pectin, a small component of the cell wall that plays a role in cell wall recalcitrance, is one of the most structurally complicated plant cell wall glycans. It is possible to increase biomass yields and sugar release by decreasing pectin production during the processing of biomass [37]. Understanding how different cellulosic biomass compositions and structures respond to hydrolysis will help to clarify how ethanol can be produced from biomass and identify the primary causes of the resistance of biomass degradation. Table 2 summarises the various percentages of cellulose, hemicellulose, and lignin present in different feedstocks. The substrate containing more amount of cellulose and less amount of lignin can be chosen for bioethanol production as it is easy for processing.
Fig. 3.
The cell wall of lignocellulosic biomass, including its makeup and structure [38].
Table 2.
Composition of different lignocellulosic feedstocks.
| Sl. No | Feedstocks | Cellulose (%) | Hemicellulose (%) | Lignin (%) | References |
|---|---|---|---|---|---|
| 1 | Rice straw | 38 | 32 | 12 | [39] |
| 2 | Rice Husk | 37.1 | 29.4 | 24.1 | [40] |
| 3 | Waste papers | 65 | 13 | 1 | [41] |
| 4 | Bamboo | 45 | 24 | 20 | [42] |
| 5 | Sugarcane bagasse | 40–45 | 30–35 | 20–30 | [43] |
| 6 | Sweet sorghum bagasse | 45 | 27 | 21 | [44] |
| 7 | Barley straw | 38 | 35 | 16 | [45] |
| 8 | Wheat straw | 33–40 | 20–25 | 15–20 | [46] |
| 9 | Corn stover | 38 | 23 | 20 | [47] |
| 10 | Corn cob | 41 | 31 | 12 | [48] |
| 11 | Pine | 42 | 21 | 30 | [49] |
| 12 | Poplar | 44 | 20 | 29 | [50] |
| 13 | Elephant grass | 36 | 24 | 28 | [51] |
| 14 | Coastal Bermuda Grass | 30 | 29 | 23 | [52] |
| 15 | Napier grass | 47 | 31 | 22 | [53] |
| 16 | Salvadora oleoides saw dust | 24 | – | 21.8 | [54] |
| 17 | Gmelina arborea saw dust | 23 | – | 23.3 | [54] |
| 18 | Water hyacinth | 24.5 | 34.1 | 8.6 | [55] |
| 19 | Hazelnut shell | 25.2 | 28.2 | 42.1 | [56] |
| 20 | Spruce wood | 43 | 29.4 | 27.6 | [56] |
| 21 | Beech wood | 44.2 | 33.5 | 21.8 | [56] |
3. Study of different pre-treatment methods
It is generally agreed upon that various pre-treatment methods intend to damage the intricate polymer structure of lignocellulose. Hemicellulose or lignin degradation is the main impact of pre-treatment on substrates. The structural qualities, such as the cellulose area and porosity, alter as a result of the composition change. Now cellulose is more accessible for efficient enzymatic hydrolysis. “Chemical pre-treatment”, “physical pre-treatment”, “physicochemical pre-treatment”, “biological pre-treatment”, or a combination of the aforementioned methods are the primary pre-treatment techniques used as shown in Fig. 4. Different methods used, their mode of action, advantages, and disadvantages are summarized in Table 3. This summary i. e Table 3 serves as a recommendation for choosing a suitable and efficient pre-treatment strategy.
Fig. 4.
Different pre-treatment methods used to treat lignocellulosic biomass.
Table 3.
Types of pre-treatment methods.
| Sr. No | Pre-treatment method | Key Highlights | References | |
|---|---|---|---|---|
| 1 | Chemical pre-treatment | Acid Pre-treatment | Hemicellulose can be broken down by acid pre-treatment into pentoses, which can then open the chain of lignocellulose's fibre bundles. Diluted sulphuric acid pre-treatment is the most well-liked. The suitable temperature is typically 100–180 °C, whereas acid concentration is between 0.5 and 10%. Since acid is corrosive, it exerts a load on the reaction equipment at high temperatures and can further degrade sugars into by-products making the fermentation process difficult. | [[57], [58], [59]] |
| Alkali pre-treatment | This method eliminates acetyl groups, lignin or damages the lignin structure to promote polysaccharide reactivity. Sodium hydroxide, sodium carbonate, calcium hydroxide, hydrogen peroxide, ammonia, and other common alkaline reagents are a few examples. However, the alkali pre-treatment reaction takes a long time, and neutralisation is required after the reaction. | [[60], [61], [62], [63], [64]] | ||
| Organic Solvent Pre-treatment | To achieve delignification ad cellulose accessibility, some organic solvents like acetone, glycerine, ionic liquids (ILs), ethanol, aqueous tetrahydrofuran, green solvent of imidazole and IL etc are frequently used. This method is environmentally safe with a low level of toxicity. However, it's expensive and problems with organic solvent recovery exist. | [[65], [66], [67]] | ||
| 2 | Physical pre-treatment | Traditional coarse crushing | In the process of bio-converting biomass, substrates are often mechanically crushed beforehand to lower the particle size. To obtain a particle size at mm and μm scale, conventional mechanical crushing often requires cutting, milling, and grinding. It is an environmentally friendly method. No chemical liquid waste. Studies show that a smaller biomass particle size can result in a greater enzymatic hydrolysis efficiency and glucose output. | [68,69] |
| Ultrafine grinding | In this method, biomass is milled to obtain a particle size in the micron range. This method increases the cellulose surface area and enlarges the pore size, thus more enzyme is adsorbed onto the substrate making enzyme hydrolysis effective. However, this method involves high energy consumption. Ball milling is another method used that facilitates enzyme hydrolysis by removing lignin, reducing cellulose crystallinity, and increasing the specific surface area. | [[70], [71], [72], [73], [74], [75]] | ||
| Alternative physical pre-treatment | Microwave, ultrasound, and light irradiation are examples of physical treatments that have been used to improve hydrolysis. These pre-treatments primarily employ the physical forces to destroy biomass structure and release cellulose. | [[76], [77], [78], [79], [80]] | ||
| 3 | Physicochemical pre-treatment | Hydrothermal pre-treatment | It is a method to decompose biomass with water under high temperature and high-pressure conditions. It is a green, pollution -free method, but high temperature causes by-products. | [[81], [82], [83]] |
| Steam explosion pre-treatment | In this method, the fibrous nature of the lignocellulose is attacked through saturated steam at low pressure and high temperature. Steam explosion treatment is generally advantageous in that there are no adverse environmental effects and low waste stream recycling costs. | [84,85] | ||
| AFEX treatment | In order to depolymerize the fibre structure, AFEX (Ammonia fibre expansion) uses the expansion effect generated from the rapid decompression of steam nitrogen when the liquid nitrogen reaches steam conditions at high heat and pressure. However some disadvantages including high operating cost, liquid nitrogen cost, release of toxic fumes into the environment, and the problems with ammonia recycling and recovery limit the usage of this treatment method. | [[86], [87], [88]] | ||
| 4 | Biological pre-treatment | Using bacteria or fungi | It involves the degradation of lignin components by aerobic bacteria or fungi. It requires less energy, no release of hazardous compounds, and less inhibitors formation. However, it involves issues like low sugar yields, lengthy reaction times (30–60 days), and microbial instability. | [[89], [90], [91], [92]] |
From Table 3 it can be understood that each method has its own advantages and disadvantages. Based on the various factors mentioned in Fig. 5, one has to choose a suitable pre-treatment method to make the further process of hydrolysis and fermentation easier. Studies have shown that to overcome these drawbacks, a combined pre-treatment is expected to have a beneficial synergistic effect. Combination pre-treatment comes in a variety of forms, such as numerous chemical pre-treatments, physical and chemical pre-treatment, and biological and chemical pre-treatment [73,93,94]. Even while combined pre-treatment has several benefits from a production and financial standpoint, the complexity of the reaction process rises in direct proportion. The viability of various pre-treatment can be significantly increased by simplifying their operation and exposing their mechanism.
Fig. 5.
Different factors to be considered for selecting a pre-treatment method.
Table 4 compares various pre-treatment in detail. The lignocellulosic biomass undergoes simple crushing as the initial step in each of these pre-treatment techniques to reduce the size. However, depending on the type of substrate and the pre-treatment technique used for the manufacture of biofuel, the size reduction method must be carefully chosen [112]. Even though several conventional pre-treatment, like acid and alkaline pre-treatment, are frequently employed to satisfactorily solubilize hemicellulose content and remove lignin, to improve the prognosis for the field of bioconversion, new pre-treatment techniques and novel microorganisms should be explored [15,113]. Additionally, comparing the variations in composition and structural characteristics under various pre-treatment techniques can assist in the analysis of the mechanism and the identification of the primary influencing factors of enzymatic hydrolysis.
Table 4.
Different pre-treatment methods for different substrates with reaction conditions required.
| Pre-treatment method | Substrate | Catalyst | Pre-treatment condition | Effect of biomass | Advantage | Yield (sugar or ethanol) | Reference |
|---|---|---|---|---|---|---|---|
| Chemical pre-treatment | Switchgrass | Tetra butyl ammonium hydroxide Ionic liquid | 323 K for 180 min | Separation of cellulose from hemicellulose and lignin | Energy required for pre-treatment is less | ∼95% glucose yield | [95] |
| Corn stover | Extractive Ammonia | 393 K, 6:1 catalyst: biomass ratio, 30 min | Cellulose is more available for hydrolysis, lignin chains are broken | Less enzyme is required along with high concentration of solids | 18.2% ethanol yield | [15] | |
| Maize | Dilute sulphuric acid | 10% solid loadings, 453 K, pH: acidic, 200 min | Degradation of hemicellulose and lignin | Acid treatment parameters were confirmed | >90% glucose yield | [96] | |
| Wheat straw | Alkaline oxidative | Sodium hydroxide (2%) and Hydrogen peroxide (2%), 333 K, 300 min | 55–60% lignin removal | Less time is required for reaction in semi-batch mode | 66–72% reducing sugar yield | [97] | |
| Physical pre-treatment | Rice straw | Ball milling | Ball milling for 480 min at 1:2 dilution ratio | Reduced particle size and better access of surface area | Favourable for enzyme conditions | 81.7% glucose yield | [98] |
| P. hysterophorus. | Ultrasound | 1.48 W/cm2 of acoustic intensity, 150 kPa of the acoustic pressure amplitude, 303 K | Improved enzyme substrate binding | Increase in rate of hydrolysis | 71% reducing sugar yield | [99] | |
| Physicochemical pre-treatment | Agave leaf and bagasse residues | Ammonia fibre expansion | 6% glucan (w/w) loading, 383 K | Breakage of bonds in polymer chain | Less complex hydrolysis conditions | >85% sugar conversion, ∼40 g/L ethanol yield | [100] |
| Sugarcane bagasse | Supercritical CO2 | 45-65 wt% moisture content, 100–250 bar, 313–353 K, 30–120 min | Breakage of polymer chains | 280% increase in fermentable sugar amount, nontoxic | 74.2% hydrolysis efficiency | [101] | |
| Napiergrass and Energycane | Hot water | Hydrothermal at 433, 453, 473, and 493 K for 15 min | Hemicellulose degradation | Better sugar yields at low enzyme concentrations with favourable operating conditions | Upto 70% glucose yields | [102] | |
| Sugarcane trash | Alkalinized steam explosion | 477 K, 10 min, 1:20 (w/w) dilution ratio | Depolymerisation of lignin | Co-production of biopolymers and biofuels | 92% cellulose hydrolysis | [103] | |
| Biological pre-treatment | Corncob | Fungal consortium (white and brown rot fungi) | White rot fungi for 25 days and brown rot fungi for 7 days | Recalcitrant nature of biomass is disturbed | Fast hydrolysis with improved glucose yield | 83% glucose yield | [104] |
| Wheat bran | P. chrysogenum F.00814 strain | Liquid to solid ratio of 5:1, pH 5.0, 303 K, 3–5 days | – | Mixed culture is advantageous compared to pure culture | 87% bioconversion rate of carbohydrates,7.6% ethanol yield | [90] | |
| Sugarcane bagasse | Ceriporiopsis submervispora | 301 K for 60 days | Better glucose recovery | – | 47% sugar yield | [105] | |
| Sawdust | Pleurotus pulmonarius | 301 K for 30 days | Sugar concentration increased 20 times | – | – | [106] | |
| Corn stover | Fungal consortium | Pre-treatment for 42 days with fungi | More than 40% lignin removal and increase in sugar formation | – | – | [107] | |
| Corn stover | Ceriporiopsis subvermispora | Solid state fermentation at 301 K for 42 days | sugar yield increased | 67% glucose yield | [47] | ||
| Aspen biomass | Armillaria gemina SKU2114 | 48 h of hydrolysis | Improved hydrolysis rate | 62% glucose yield | [108] | ||
| Rice straw | Pholiota adiposa | Saccharification for 48 h | 75% sugar released | – | – | [109] | |
| Paddy straw | Pleurotus florida | 300 K for 28 days. | Hydrolysis efficiency up to 75.3% | – | – | [110] | |
| Straw | Fungal consortium | Incubated at 303 and 328 K for 6 days | increase in sugar production | – | – | [111] | |
| Combined pre-treatment | Vegetable wastes | Ultrasound + microwave + Ternary deep eutectic solvents | ultrasound (room temperature, 30 min) + microwave (353 K, 20 min) | Lignin degradation, cellulose readily available for hydrolysis | efficient lignin removal at low cost | 2.63% and 3.11% reducing sugar yields | [78] |
| Corn stover | H2SO4+ NaOH | 1% H2SO4+1% NaOH, 393 K, 60 min | Separation of cellulose | Better sugar yields and maximum lignin removal | 88.4% glucose yield | [94] |
4. Kinetic model studies
Kinetic models of hydrolysis are important to understand as they help in designing and optimising the processes [114]. This review summarises the various models used to study hydrolysis of lignocellulosic substrates. The models reported here are the different studies performed by several authors for a variety of substrates making certain valid assumptions. Depending upon the type of system, either homogeneous or heterogeneous, hydrolysis method (chemical or enzymatic) and the substrate chosen for study, one can refer to these models to develop their own models which can fit well with the experimental results.
4.1. Pseudo first-order rate kinetics
Ajani et al. (2011) have used this model to study the kinetics of cellulose hydrolysis from different agricultural derived biomass by varying two parameters i. e temperature and acid concentration in a homogeneous system. Their studies suggest that glucose formation improves with an increase in acid concentration and temperature. Experimental data was used to fit the models and activation energies were calculated for “Banana Skin”, “Cowpea shells”, “Maize stalks” and “rice husk” [115].
The rate of cellulose degradation follows first-order kinetics and is expressed by equation (1) [115].
| (1) |
Where “A0 -G = waste cellulose concentration at time t, A0 = total initial waste cellulose concentration, G-glucose content, k = specific rate constant, t = time”.
Temperature dependence Arrhenius model is expressed by equation (2) [115].
| (2) |
Where “kR = pre-exponential constant, Ea = activation energy, R = ideal gas constant, T = actual temperature and t = time”.
4.2. Semi-mechanistic kinetic model
Semi-mechanistic models use less experimental information necessary to describe the process in terms of mathematical expressions. Martha Suzana et al. (2016) have used this model to study pre-treatment processes for cellulose and hemicellulose fractions degradation in a batch reactor for pretreated sugarcane straw. Authors assumed that there are no mass transfer limits and the classification between crystalline and amorphous form is neglected. The models were able to fit well with the experimental data. Authors have reported series of differential equations for hemicellulose degradation including intermediate steps showing degradation profiles of xylo-oligomers, monomers, furfural and final degradation products. Similarly, the degradation equations of cellulosic fraction during hydrothermal pretreatment were derived.
First-order rate equations were derived to determine the rate constants and using the values obtained, optimum temperature and optimum pre-treatment conditions for lignocellulosic degradation of sugarcane straw were studied [116].
4.3. Model development for delignification process
N. Prathyusha et al. (2016) have reported model development for delignification process for individual and multi feedstocks (Sorghum, bamboo, wheat straw) and studied the effect of alkali loading, pretreatment temperature and enzyme loading on the extent of delignification. But the models built are used to find the degree of conversion possible only and not the rate of reaction. So they have developed a kinetic model for sorghum saccharification to study conversion rate which can be used for design purposes and is expressed as a first-order differential equation as in equation (3) [117].
| (3) |
where “S(t) represents the Sorghum biomass saccharification conversion at a given time t, and.
Smax represents the maximum conversion and is a time constant for the process”. This model has an analytic solution that can be written [117] as
| (4) |
Authors have followed the approach of developing a specific model for a range of operating parameters for single and multiple substrates and the value of maximum conversion obtained is used for studying the rate of reaction for individual substrates [117]. In all the models developed (all the equations not shown), author has assumed that feedstock consists of cellulose, hemi-cellulose, lignin only. Further regression analysis was performed to find the correlation between the model parameters.
4.4. Michaelis and Menten (MM) model for cellulases
This is the most commonly used model to study enzyme-catalyzed reactions. Many authors have reported the use of the classical MM model to study enzyme hydrolysis for lignocellulose biomass. The basic assumptions used in their studies are “(1) the substrate is a soluble reactant; (2) the system is homogeneous; (3) the concentration of the enzyme is constant; (4) the formation of the enzyme-substrate complex is rapid and reversible; (5) the breakdown of the enzyme-substrate complex into products is the limiting step of the overall reaction; and (6) the formation of the products is irreversible”. The equation is represented [118] as
| (5) |
Where “ = Velocity of reaction, VMax = the maximum rate of reaction at fixed enzyme concentration, S is the substrate concentration, and KM is the Michaelis and Menten constant”.
The Michaelis and Menten model has also been applied in the form reported in Equation (6) by considering the competitive enzyme inhibition by product.
| (6) |
Where “P is the product concentration, and Ki is the product inhibition constant “.
Ekaterina I. Makarova et al. (2017) have used the MM model to study Miscanthus and oat hulls hydrolysis treated with solutions of acid and base in direct and reverse sequences. Initial solid loading from 30 to 120 g/L was used to study kinetics. The effects of the type of substrate and pretreatment method were studied to evaluate the reducing sugar yield. The fitting results of the developed models showed good agreement with the experimental data [118].
Efri Mardawati et al. (2017) have also reported that enzymatic hydrolysis of oil palm petiole followed the MM model with kinetic parameters “Km = 6.433 g/L″ and “Vm = 0.042 g/L/min” respectively [119].
The mechanism of cellulose hydrolysis using enzymes is explained briefly with the following basic steps.
-
•
Diffusion of enzyme from bulk aqueous phase to the surface of substrate
-
•
Formation of enzyme-substrate complex due to adsorption of enzyme
-
•
Cellulose breakdown
-
•
Diffusion of breakdown products into bulk aqueous phase
-
•
End product glucose formation in the bulk aqueous phase [25].
4.5. Chrastil's model
This model was developed to describe kinetics of a heterogeneous system. The structure of the lignocellulose substrate causes diffusion-related resistance. According to this model, glucose concentration can be described by Equation (7):
| (7) |
Where “G(t) and G∞ are the glucose concentration at time t and at the equilibrium (maximum conversion degree), respectively, k is the rate constant, E0 the initial enzyme concentration, and m a diffusion resistance constant. For zero resistance, m = 1 and for controlled resistances m < 1 [120]”. This model can be used for the design purposes of reactors involving enzyme hydrolysis.
4.6. HCH-1 three-parameter model
Mechanistic models are built to understand the reaction mechanism between substrate and enzyme. Russell F Brown et al. (2010) have reported various two-parameter and three-parameter mechanistic models and compared them to describe the enzymatic hydrolysis of pretreated biomass. Two parameter models use only substrate and enzyme concentration. Three parameter models use additional factors like inhibition, number of active sites, mass transfer coefficients, etc. AFEX-treated wheat straw was the substrate used. According to the author MM model provides the best fit for two-parameter model and among three-parameter models, HCH-1model provides the best fit because the model describes the number of reactive sites covered by the enzymes represented by the fractional coverage parameter () [121] as expressed in equation (8). The equation representing the model is given by
| (8) |
Where “[E]: cellulase concentration, k: rate constant; Km: Michaelis-Menten constant; [S]: substrate concentration; V: rate of reaction; ε: coverage parameter, : ratio of free substrate to total substrate, dimensionless”.
4.7. Langmuir adsorption model
Langmuir isotherm is the widely used model for understanding enzyme adsorption on a substrate.
Langmuir adsorption model is given by the following equation (9) [122].
| (9) |
Where “Eb is the absorbed enzyme, Ebm is the maximum absorb capacity, Ef is the free enzyme concentration in a liquid phase and the Ka is the association constant”.
Ye Yuan et al. (2018) have used this model to study enzymatic hydrolysis kinetics and enzyme adsorption kinetics on pre-treated corn stover. Dilute acid and base were used for pretreatment [122]. Mahdi Khodaverdi et al. (2012) have reported that the Langmuir model was applied to model the adsorption of cellulase onto the defatted and bleached cotton linter [123]. Their studies suggested that pre-treatment increased the adsorption of enzyme onto the substrate.
4.8. Intermediate and end product inhibition
Kadam et al. (2004) have reported a reaction scheme for modelling cellulose hydrolysis as shown in Fig. 6. A Kinetic model was developed and validated for understanding enzyme hydrolysis in dilute acid pretreated corn stover. The model explains that each enzymatic reaction is inhibited by the sugar it produces (glucose, xylose, cellobiose) in a homogeneous reaction.
Fig. 6.
Reaction scheme for modelling cellulose hydrolysis [124].
Where “r1 = reaction rate of cellulose to cellobiose; r2 = reaction rate of cellulose to glucose; r3 = reaction rate for cellobiose to glucose”.
Authors have derived equations to calculate reaction rates using the values of reaction rate constant, enzyme concentrations (bound and free), substrate reactivity, substrate concentration, product concentration and inhibition constant for all the three reactions as shown in Fig. 6.
4.9. Valjamae and Kopelman model for heterogeneous systems
While the Michaelis-Menten model is appropriate to explain enzyme kinetics in a homogeneous system, Valjamae and Kopelman have developed models to explain cellulose hydrolysis in a heterogeneous system. The enzyme deactivation phase is considered in these two models considering the fractal exponent whose value is between 0 and 1.
Megawati et al. (2020) have used these models to express the passion fruit hydrolysis kinetics with cellulase as enzymes.
A time course of enzymatic hydrolysis of cellulose using cellulase is described by Valjamae as Eq. (10),
| (10) |
Where “Sp = product glucose concentration, S0 = initial glucose concentration, t = time, k = reaction rate constant, and h = fractal exponent”.
The enzyme deactivation phase and its equilibrium are represented by the fractal parameter, which is the fractal exponent [114].
The other empirical equation for the heterogeneous kinetics model was explored by Kopelman, as in Eq. (11)
| (11) |
Where residual sugar concentration”.
Valjamae model considers the concentration of the glucose produced while the Kopelman model considers the concentration of the residual glucose in deducing the kinetic equations [114].
4.10. Deactivation and reactivation reaction rate mechanism
During the course of enzyme hydrolysis, after a certain period of time we can see decline in the enzyme activity. Enzymes become inactive as they adsorb on the substrates and also due to reactivity with the product formed. Augustine Omoniyi Ayeni et al. (2021) have studied this mechanism for pretreated corn cob to sugar.
Defining the real hydrolysis rate (12) [125] by considering the cellulase activity loss:
| (12) |
Where = real hydrolysis rate, maximum reaction rate, S = substrate concentration,
, Ro = residual enzyme activity, I0 = inactivation extent, t1/2 = half-life”.
Comprehensively, modelling cellulose hydrolysis requires consideration of different parameters taken into account to optimize hydrolysis process. Cellulose surface area, pore volume, end product inhibition, rate of delignification, rate of hemicellulose degradation, reaction conditions, type of system in enzymatic hydrolysis, diffusion aspects, reactive sites of enzyme and volume of the reaction mixture are some of the crucial parameters studied so far. Optimized parameters can be obtained from experiments and by identifying the important influencing factors for a particular substrate and processes one can perform kinetic model analysis. Table 5 gives a quick summary of the kinetic models used for different substrates along with pre-treatment methods and model operating parameters.
Table 5.
Summary of kinetic models along with substrates used, parameters used and key highlights.
| Sr. No | Model name | Substrate used | Pre-treatment method | Operating parameters used to fit kinetic data | Order of reaction | Key points | References |
|---|---|---|---|---|---|---|---|
| 1 | Psuedo-first order rate kinetics | Banana skin, cowpea shells, maize stalks, rice husk | Acid hydrolysis (Sulphuric acid) | Temperature, acid concentration | First | Increase in temperature and acid concentration improves sugar yields | [115] |
| 2 | Semi-mechanistic kinetic model | Sugarcane straw | Hydrothermal | Temperature, concentration profiles of monomers formed | First | Reaction rate increased with increase in temperature | [116] |
| 3 | Model for delignification | Sorghum, Wheat straw, Bamboo | Alkali peroxide | Alkali loading, pre-treatment temperature and enzyme loading | First | Extent of delignification increases with increase in alkaline loading. Increase in temperature and enzyme concentration increases the conversion. | [117] |
| 4 | MM model for cellulase | Miscanthus and oat hulls, oil palm petiole | Chemical (combined method using Nitric acid and Sodium hydroxide) | Substrate loading | First | Rate of enzyme hydrolysis increases with increase in substrate concentration | [118,119] |
| 5 | Chrastil's model | Apple Pomace | Combined (Alkaline + acid + enzymatic) | Mixing speed, substrate concentration | – | Better sugar yields are obtained at lower mixing speeds, maximum constant substrate concentration | [120] |
| 6 | HCH-1 three parameter model | AFEX treated wheat straw | AFEX | Enzyme loading, Substrate concentration |
First | Sugar yield increases with increased substrate concentration | [121] |
| 7 | Langmuir adsorption model | Corn stover, defatted and bleached cotton linter | Dilute alkali and acid, cellulosic solvent | Substrate concentration, enzyme loading | – | Sugar yields were better at lower substrate loadings and a wide range of enzyme concentrations | [122,123] |
| 8 | Intermediate and end product inhibition | Corn stover | Dilute acid | Substrate concentration, temperature, product concentration | First | Model predicts well the hydrolysis performance | [124] |
| 9 | Valjamae and Kopelman model | Passion fruit peel | Solvent extraction | Glucose concentration, enzyme ratio | – | Glucose concentration increases with increase in enzyme volume ratio | [114] |
| 10 | Deactivation and reactivation reaction rate mechanism | Corn cob | Alkaline peroxide oxidation | Substrate concentration, hydrolysis time | Second | Product concentration reaches maximum at optimum substrate loading and hydrolysis time | [125] |
From Table 5 and it can be inferred that pre-treatment of lignocellulose substrate further improves the hydrolysis rate of cellulose to glucose, which is crucial step for achieving higher bioethanol yields. The studies involving enzyme hydrolysis show that product formation is maximum at optimum substrate loading, enzyme loading and hydrolysis time. After this optimum point, the product concentration decreases due to end product inhibition, formation of by-products, diffusion related resistances. All the models summarized in Table 5 predict well the hydrolysis rates and thus are helpful in further optimization and reactor design studies. This Table 5 is a quick reference summary to researchers working on pre-treatment and kinetic studies for hydrolysing various types of lignocellulose substrates. Based on the specific pre-treatment method and substrate chosen for study, one can use these models or develop their own models by making specific changes as per the objectives of the study.
5. Conclusion and outlook
The potential for biofuel production is huge with the necessary infrastructure in place. From the understanding of cellulose hydrolysis so far, it is evident that it is a complex process. In this study, composition of around 21 different feeedstocks is reported for their cellulose and lignin percentage. Raw material with high cellulose and low lignin content is ideal choice for sustainable bioethanol production. Different pre-treatment methods are required for different substrates to achieve high sugar yields. A thorough analysis of the structure of lignocellulosic pattern in different substrates can further guide choice of the appropriate pre-treatment method to make cellulose more available for hydrolysis. The choice of pre-treatment method should reflect the right balance of its suitability to destroy the lignocellulosic structure in terms of availability of micro-organisms and chemicals, process hazards, environment-friendly aspects, process cost, reaction time with optimized sugar and ethanol yields. This may require choosing a combined pre-treatment method or individual type depending upon the substrates and the objectives of researchers. In this study 4 major pre-treatment methods are discussed along with their mechanism of action and limitations for use. Around 20 different substrates used for pre-treatment as reported in literature are tabulated along with process parameters and sugar yields to get a quick overview about the methods and results. This review also summarises 10 various kinetic models reported so far in understanding cellulose hydrolysis including cellulase enzyme kinetics. Further, the kinetic models elucidated in this work reflect upon the diversity of work done to understand the reaction rates and parameters to optimize the hydrolysis process. Experimental values have to be in line with the predicted values for the kinetic model chosen for study. The key step is to identify the main influencing factor and the rate-limiting step and develop a relevant model accordingly as there is no one model to explain the complex heterogeneous hydrolysis process. This review paper reflects important aspects about pre-treatment and kinetic model studies to help researchers get ready information about the composition, type of pre-treatment methods, their pros and cons and most widely studied kinetic models for different substrates. This data can help researchers to further develop processes and models for sustainable bioethanol production from lignocellulosic biomass.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Data availability statement
No data was used for the research described in the article.
Funding statement
Dr. Minal Deshmukh was supported by the Department of Science & Technology [SP/YO/2019/1589 (G)].
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
References
- 1.Gustavsson L., Nguyen T., Sathre R., Tettey U.Y.A. Climate effects of forestry and substitution of concrete buildings and fossil energy. Renew. Sustain. Energy Rev. 2021;136(August 2020) doi: 10.1016/j.rser.2020.110435. [DOI] [Google Scholar]
- 2.Leibensperger C., Yang P., Zhao Q., Wei S., Cai X. The synergy between stakeholders for cellulosic biofuel development: perspectives, opportunities, and barriers. Renew. Sustain. Energy Rev. 2021;137(November 2020) doi: 10.1016/j.rser.2020.110613. [DOI] [Google Scholar]
- 3.Zhao Y., Damgaard A., Xu Y., Liu S., Christensen T.H. Bioethanol from corn stover – global warming footprint of alternative biotechnologies. Appl. Energy. 2019;247(December 2018):237–253. doi: 10.1016/j.apenergy.2019.04.037. [DOI] [Google Scholar]
- 4.Liu B., Rajagopal D. Life-cycle energy and climate benefits of energy recovery from wastes and biomass residues in the United States. Nat. Energy. 2019;4(8):700–708. doi: 10.1038/s41560-019-0430-2. [DOI] [Google Scholar]
- 5.Kumar R. Encyclopedia of sustainability science and technology. Encycl. Sustain. Sci. Technol. 2020 doi: 10.1007/978-1-4939-2493-6. [DOI] [Google Scholar]
- 6.Somerville C., Youngs H., Taylor C., Davis S.C., Long S.P. Feedstocks for lignocellulosic biofuels. Science. 2010;329(5993):790–792. doi: 10.1126/science.1189268. [DOI] [PubMed] [Google Scholar]
- 7.Gurunath R.B., Gobinath R., Giridhar P.V. Bioethanol production from lignocellulosic biomass: past, present and future trends. Res. J. Biotechnol. 2022;17(10):124–132. doi: 10.25303/1710rjbt1240132. [DOI] [Google Scholar]
- 8.El-Maghrabi N., Fawzy M., Mahmoud A.E.D. Efficient removal of phosphate from wastewater by a novel phyto-graphene composite derived from palm byproducts. ACS Omega. 2022;7(49):45386–45402. doi: 10.1021/acsomega.2c05985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Panahi H.K.S., Dehhaghi M., Kinder J.E., Ezeji T.C. A review on green liquid fuels for the transportation sector: a prospect of microbial solutions to climate change. Biofuel Res. J. 2019;6(3):995–1024. doi: 10.18331/brj2019.6.3.2. [DOI] [Google Scholar]
- 10.Rastogi M., Shrivastava S. Recent advances in second generation bioethanol production: an insight to pretreatment, saccharification and fermentation processes. Renew. Sustain. Energy Rev. 2017;80(May):330–340. doi: 10.1016/j.rser.2017.05.225. [DOI] [Google Scholar]
- 11.Solarte-Toro J.C., Romero-Garcia J.M., Martinez-Patino J.C., Ruiz-Ramos E., Castro-Galiano E., Cardona-Alzate C.A. Acid pretreatment of lignocellulosic biomass for energy vectors production: a review focused on operational conditions and techno-economic assessment for bioethanol production. Renew. Sustain. Energy Rev. 2019;107(February):587–601. doi: 10.1016/j.rser.2019.02.024. [DOI] [Google Scholar]
- 12.Kesharwani R., Sun Z., Dagli C., Xiong H. Moving second generation biofuel manufacturing forward: investigating economic viability and environmental sustainability considering two strategies for supply chain restructuring. Appl. Energy. 2019;242(February):1467–1496. doi: 10.1016/j.apenergy.2019.03.098. [DOI] [Google Scholar]
- 13.Olofsson J., Barta Z., Borjesson P., Wallberg O. Integrating enzyme fermentation in lignocellulosic ethanol production: life-cycle assessment and techno-economic analysis. Biotechnol. Biofuels. 2017;10(1):1–14. doi: 10.1186/s13068-017-0733-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baral N.R., et al. Approaches for more efficient biological conversion of lignocellulosic feedstocks to biofuels and bioproducts. ACS Sustain. Chem. Eng. 2019;7(10):9062–9079. doi: 10.1021/acssuschemeng.9b01229. [DOI] [Google Scholar]
- 15.Da Costa Sousa L., et al. Next-generation ammonia pretreatment enhances cellulosic biofuel production. Energy Environ. Sci. 2016;9(4):1215–1223. doi: 10.1039/c5ee03051j. [DOI] [Google Scholar]
- 16.Broda M., Yelle D.J., Serwanska K. Bioethanol production from lignocellulosic biomass—challenges and solutions. Molecules. 2022;27(24) doi: 10.3390/molecules27248717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deshmukh M., Pande A., Marathe A. Heliyon Different particle size study of castor deoiled cake for biofuel production with an environmental sustainability perspective. Heliyon. 2022;8(January) doi: 10.1016/j.heliyon.2022.e09710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deshmukh M., Pande A. Comparative study for production of biofuel from potato peel waste as feedstock by different enzymes. Enzyme Eng. 2022;11(1):1–6. doi: 10.35841/2329-6674.22.11.1000175. [DOI] [Google Scholar]
- 19.Tyufekchiev M., Kolodziejczak A., Duan P., Foston M., Schmidt-Rohr K., Timko M.T. Reaction engineering implications of cellulose crystallinity and water-promoted recrystallization. Green Chem. 2019;21(20):5541–5555. doi: 10.1039/c9gc02466b. [DOI] [Google Scholar]
- 20.Cheng M.H., Kadhum H.J., Murthy G.S., Dien B.S., Singh V. High solids loading biorefinery for the production of cellulosic sugars from bioenergy sorghum. Bioresour. Technol. 2020;318(August) doi: 10.1016/j.biortech.2020.124051. [DOI] [PubMed] [Google Scholar]
- 21.Gao X., Kumar R., Demartini J.D., Li H., Wyman C.E. Application of high throughput pretreatment and co-hydrolysis system to thermochemical pretreatment. Part 1: dilute acid. Biotechnol. Bioeng. 2013;110(3):754–762. doi: 10.1002/bit.24751. [DOI] [PubMed] [Google Scholar]
- 22.Lindedam J., et al. Evaluation of high throughput screening methods in picking up differences between cultivars of lignocellulosic biomass for ethanol production. Biomass Bioenergy. 2014;66:261–267. doi: 10.1016/j.biombioe.2014.03.006. [DOI] [Google Scholar]
- 23.Pihlajaniemi V., Sipponen M.H., Liimatainen H., Sirvio J.A., Nyyssola A., Laakso S. Weighing the factors behind enzymatic hydrolyzability of pretreated lignocellulose. Green Chem. 2016;18(5):1295–1305. doi: 10.1039/c5gc01861g. [DOI] [Google Scholar]
- 24.Zhang H., Li J., Huang G., Yang Z., Han L. Understanding the synergistic effect and the main factors influencing the enzymatic hydrolyzability of corn stover at low enzyme loading by hydrothermal and/or ultrafine grinding pretreatment. Bioresour. Technol. 2018;264:327–334. doi: 10.1016/j.biortech.2018.05.090. [DOI] [PubMed] [Google Scholar]
- 25.Ragauskas A.J. Physicochemical structural changes of cellulosic substrates during enzymatic saccharification. J. Appl. Biotechnol. Bioeng. 2016;1(3) doi: 10.15406/jabb.2016.01.00015. [DOI] [Google Scholar]
- 26.Lischeske J.J., Stickel J.J. A two-phase substrate model for enzymatic hydrolysis of lignocellulose: application to batch and continuous reactors. Biotechnol. Biofuels. 2019;12(1):1–15. doi: 10.1186/s13068-019-1633-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng Y., Pan Z., Zhang R., Jenkins B.M. Kinetic modeling for enzymatic hydrolysis of pretreated creeping wild ryegrass. Biotechnol. Bioeng. 2009;102(6):1558–1569. doi: 10.1002/bit.22197. [DOI] [PubMed] [Google Scholar]
- 28.Li Z., et al. Diagnostic accuracy of a fast computational approach to derive fractional flow reserve from coronary CT angiography. JACC Cardiovasc. Imaging. 2020;13(1):172–175. doi: 10.1016/j.jcmg.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Wojtusik M., Vergara P., Villar J.C., Ladero M., Garcia-Ochoa F. Enzymatic hydrolysis of several pretreated lignocellulosic biomasses: fractal kinetic modelling. Bioresour. Technol. 2020;318(August) doi: 10.1016/j.biortech.2020.124050. [DOI] [PubMed] [Google Scholar]
- 30.Alonso D.M., et al. Increasing the revenue from lignocellulosic biomass: maximizing feedstock utilization. Sci. Adv. 2017;3(5) doi: 10.1126/sciadv.1603301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chundawat S.P.S., Beckham G.T., Himmel M.E., Dale B.E. Deconstruction of lignocellulosic biomass to fuels and chemicals. Annu. Rev. Chem. Biomol. Eng. 2011;2(January):121–145. doi: 10.1146/annurev-chembioeng-061010-114205. [DOI] [PubMed] [Google Scholar]
- 32.Benselfelt T., Engstrom J., Wagberg L. Supramolecular double networks of cellulose nanofibrils and algal polysaccharides with excellent wet mechanical properties. Green Chem. 2018;20(11):2558–2570. doi: 10.1039/c8gc00590g. [DOI] [Google Scholar]
- 33.Kanchanalai P., Temani G., Kawajiri Y., Realff M.J. Reaction kinetics of concentrated-acid hydrolysis for cellulose and hemicellulose and effect of crystallinity. Bioresources. 2016;11(1):1672–1689. doi: 10.15376/BIORES.11.1.1672-1689. [DOI] [Google Scholar]
- 34.Bhagia S., Li H., Gao X., Kumar R., Wyman C.E. Flowthrough pretreatment with very dilute acid provides insights into high lignin contribution to biomass recalcitrance. Biotechnol. Biofuels. 2016;9(1):1–15. doi: 10.1186/s13068-016-0660-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shinde S.D., Meng X., Kumar R., Ragauskas A.J. Recent advances in understanding the pseudo-lignin formation in a lignocellulosic biorefinery. Green Chem. 2018;20(10):2192–2205. doi: 10.1039/c8gc00353j. [DOI] [Google Scholar]
- 36.Kumar R., et al. Cellulose-hemicellulose interactions at elevated temperatures increase cellulose recalcitrance to biological conversion. Green Chem. 2018;20(4):921–934. doi: 10.1039/c7gc03518g. [DOI] [Google Scholar]
- 37.Kothari N., et al. Cellulose hydrolysis by: Clostridium thermocellum is agnostic to substrate structural properties in contrast to fungal cellulases. Green Chem. 2019;21(10):2810–2822. doi: 10.1039/c9gc00262f. [DOI] [Google Scholar]
- 38.Saldarriaga-Hernandez S., et al. Biotransformation of lignocellulosic biomass into industrially relevant products with the aid of fungi-derived lignocellulolytic enzymes. Int. J. Biol. Macromol. 2020;161:1099–1116. doi: 10.1016/j.ijbiomac.2020.06.047. [DOI] [PubMed] [Google Scholar]
- 39.Lu P., Lo Hsieh Y. Preparation and characterization of cellulose nanocrystals from rice straw. Carbohydr. Polym. 2012;87(1):564–573. doi: 10.1016/j.carbpol.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 40.Kalita E., Nath B.K., Deb P., Agan F., Islam M.R., Saikia K. High quality fluorescent cellulose nanofibers from endemic rice husk: isolation and characterization. Carbohydr. Polym. 2015;122:308–313. doi: 10.1016/j.carbpol.2014.12.075. [DOI] [PubMed] [Google Scholar]
- 41.Chen Y., Knappe D.R.U., Barlaz M.A. Effect of cellulose/hemicellulose and lignin on the bioavailability of toluene sorbed to waste paper. Environ. Sci. Technol. 2004;38(13):3731–3736. doi: 10.1021/es035286x. [DOI] [PubMed] [Google Scholar]
- 42.Li H., Qu Y., Yang Y., Chang S., Xu J. Microwave irradiation - a green and efficient way to pretreat biomass. Bioresour. Technol. 2016;199:34–41. doi: 10.1016/j.biortech.2015.08.099. [DOI] [PubMed] [Google Scholar]
- 43.Cardona C.A., Quintero J.A., Paz I.C. Production of bioethanol from sugarcane bagasse: status and perspectives. Bioresour. Technol. 2010;101(13):4754–4766. doi: 10.1016/j.biortech.2009.10.097. [DOI] [PubMed] [Google Scholar]
- 44.Kim M., Day D.F. Composition of sugar cane, energy cane, and sweet sorghum suitable for ethanol production at Louisiana sugar mills. J. Ind. Microbiol. Biotechnol. 2011;38(7):803–807. doi: 10.1007/s10295-010-0812-8. [DOI] [PubMed] [Google Scholar]
- 45.Sun J.X., Xu F., Sun X.F., Xiao B., Sun R.C. Physico-chemical and thermal characterization of cellulose from barley straw. Polym. Degrad. Stabil. 2005;88(3):521–531. doi: 10.1016/j.polymdegradstab.2004.12.013. [DOI] [Google Scholar]
- 46.Talebnia F., Karakashev D., Angelidaki I. Production of bioethanol from wheat straw: an overview on pretreatment, hydrolysis and fermentation. Bioresour. Technol. 2010;101(13):4744–4753. doi: 10.1016/j.biortech.2009.11.080. [DOI] [PubMed] [Google Scholar]
- 47.Wan C., Li Y. Microbial pretreatment of corn stover with Ceriporiopsis subvermispora for enzymatic hydrolysis and ethanol production. Bioresour. Technol. 2010;101(16):6398–6403. doi: 10.1016/j.biortech.2010.03.070. [DOI] [PubMed] [Google Scholar]
- 48.Chen S., Zhang X., Singh D., Yu H., Yang X. Biological pretreatment of lignocellulosics: potential, progress and challenges. Biofuels. 2010;1(1):177–199. doi: 10.4155/bfs.09.13. [DOI] [Google Scholar]
- 49.Sannigrahi P., Miller S.J., Ragauskas A.J. Effects of organosolv pretreatment and enzymatic hydrolysis on cellulose structure and crystallinity in Loblolly pine. Carbohydr. Res. 2010;345(7):965–970. doi: 10.1016/j.carres.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 50.Kim Y., Mosier N.S., Ladisch M.R. Enzymatic digestion of liquid hot water pretreated hybrid poplar. Biotechnol. Prog. 2009;25(2):340–348. doi: 10.1002/btpr.137. [DOI] [PubMed] [Google Scholar]
- 51.Scholl A.L., et al. Elephant grass pretreated by steam explosion for inducing secretion of cellulases and xylanases by Penicillium echinulatum S1M29 solid-state cultivation. Ind. Crop. Prod. 2015;77:97–107. doi: 10.1016/j.indcrop.2015.08.051. [DOI] [Google Scholar]
- 52.Lee J.M., Shi J., Venditti R.A., Jameel H. Autohydrolysis pretreatment of Coastal Bermuda grass for increased enzyme hydrolysis. Bioresour. Technol. 2009;100(24):6434–6441. doi: 10.1016/j.biortech.2008.12.068. [DOI] [PubMed] [Google Scholar]
- 53.Reddy K.O., et al. Extraction and characterization of cellulose single fibers from native african napier grass. Carbohydr. Polym. 2018;188:85–91. doi: 10.1016/j.carbpol.2018.01.110. [DOI] [PubMed] [Google Scholar]
- 54.Kalita E., Nath B.K., Agan F., More V., Deb P. Isolation and characterization of crystalline, autofluorescent, cellulose nanocrystals from saw dust wastes. Ind. Crop. Prod. 2015;65:550–555. doi: 10.1016/j.indcrop.2014.10.004. [DOI] [Google Scholar]
- 55.Ruan T., Zeng R., Yin X.Y., Zhang S.X., Yang Z.H. Water hyacinth (Eichhornia crassipes) biomass as a biofuel feedstock by enzymatic hydrolysis. Bioresources. 2016;11(1):2372–2380. doi: 10.15376/BIORES.11.1.2372-2380. [DOI] [Google Scholar]
- 56.Demirbaş A. Thermochemical conversion of biomass to liquid products in the aqueous medium. Energy Sources. 2005;27(13):1235–1243. doi: 10.1080/009083190519357. [DOI] [Google Scholar]
- 57.Kang S., Fu J., Zhang G. From lignocellulosic biomass to levulinic acid: a review on acid-catalyzed hydrolysis. Renew. Sustain. Energy Rev. 2018;94(June):340–362. doi: 10.1016/j.rser.2018.06.016. [DOI] [Google Scholar]
- 58.Lorenci Woiciechowski A., et al. Lignocellulosic biomass: acid and alkaline pretreatments and their effects on biomass recalcitrance – conventional processing and recent advances. Bioresour. Technol. 2020;304(October 2019) doi: 10.1016/j.biortech.2020.122848. [DOI] [PubMed] [Google Scholar]
- 59.Sen B., Chou Y.P., Wu S.Y., Liu C.M. Pretreatment conditions of rice straw for simultaneous hydrogen and ethanol fermentation by mixed culture. Int. J. Hydrogen Energy. 2016;41(7):4421–4428. doi: 10.1016/j.ijhydene.2015.10.147. [DOI] [Google Scholar]
- 60.Cabrera E., Munoz M.J., Martin R., Caro I., Curbelo C., Diaz A.B. Alkaline and alkaline peroxide pretreatments at mild temperature to enhance enzymatic hydrolysis of rice hulls and straw. Bioresour. Technol. 2014;167:1–7. doi: 10.1016/j.biortech.2014.05.103. [DOI] [PubMed] [Google Scholar]
- 61.Li J., Lu M., Guo X., Zhang H., Li Y., Han L. Insights into the improvement of alkaline hydrogen peroxide (AHP) pretreatment on the enzymatic hydrolysis of corn stover: chemical and microstructural analyses. Bioresour. Technol. 2018;265:1–7. doi: 10.1016/j.biortech.2018.05.082. [DOI] [PubMed] [Google Scholar]
- 62.Kim J.S., Lee Y.Y., Kim T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2016;199:42–48. doi: 10.1016/j.biortech.2015.08.085. [DOI] [PubMed] [Google Scholar]
- 63.Mathew A.K., Chaney K., Crook M., Humphries A.C. Alkaline pre-treatment of oilseed rape straw for bioethanol production: evaluation of glucose yield and pre-treatment energy consumption. Bioresour. Technol. 2011;102(11):6547–6553. doi: 10.1016/j.biortech.2011.03.067. [DOI] [PubMed] [Google Scholar]
- 64.Ho M.C., Ong V.Z., Wu T.Y. Potential use of alkaline hydrogen peroxide in lignocellulosic biomass pretreatment and valorization – a review. Renew. Sustain. Energy Rev. 2019;112(December 2018):75–86. doi: 10.1016/j.rser.2019.04.082. [DOI] [Google Scholar]
- 65.Zhang K., Pei Z., Wang D. Organic solvent pretreatment of lignocellulosic biomass for biofuels and biochemicals: a review. Bioresour. Technol. 2016;199:21–33. doi: 10.1016/j.biortech.2015.08.102. [DOI] [PubMed] [Google Scholar]
- 66.Smith M.D., et al. Cosolvent pretreatment in cellulosic biofuel production: effect of tetrahydrofuran-water on lignin structure and dynamics. Green Chem. 2016;18(5):1268–1277. doi: 10.1039/c5gc01952d. [DOI] [Google Scholar]
- 67.Fockink D.H., Andreaus J., Ramos L.P., Łukasik R.M. Pretreatment of cotton spinning residues for optimal enzymatic hydrolysis: a case study using green solvents. Renew. Energy. 2020;145:490–499. doi: 10.1016/j.renene.2019.06.042. [DOI] [Google Scholar]
- 68.Fougere J.D., Lynch M., Zhao J., Zheng Y., Li K. Impact of mechanical downsizing on the physical structure and enzymatic digestibility of pretreated hardwood. Energy Fuel. 2014;28(4):2645–2653. doi: 10.1021/ef5001387. [DOI] [Google Scholar]
- 69.Rezania S., et al. Review on fermentative biohydrogen production from water hyacinth, wheat straw and rice straw with focus on recent perspectives. Int. J. Hydrogen Energy. 2017;42(33):20955–20969. doi: 10.1016/j.ijhydene.2017.07.007. [DOI] [Google Scholar]
- 70.Ji G., Gao C., Xiao W., Han L. Mechanical fragmentation of corncob at different plant scales: impact and mechanism on microstructure features and enzymatic hydrolysis. Bioresour. Technol. 2016;205:159–165. doi: 10.1016/j.biortech.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 71.Zhang H., Gao X., Wu P., Xu X. A cross-media distance metric learning framework based on multi-view correlation mining and matching. World Wide Web. 2016;19(2):181–197. doi: 10.1007/s11280-015-0342-4. [DOI] [Google Scholar]
- 72.Zhong Y., Frost H., Bustamante M., Li S., Liu Y.S., Liao W. A mechano-biocatalytic one-pot approach to release sugars from lignocellulosic materials. Renew. Sustain. Energy Rev. 2020;121(April 2019) doi: 10.1016/j.rser.2019.109675. [DOI] [Google Scholar]
- 73.Balch M.L., et al. Lignocellulose fermentation and residual solids characterization for senescent switchgrass fermentation by: Clostridium thermocellum in the presence and absence of continuous in situ ball-milling. Energy Environ. Sci. 2017;10(5):1252–1261. doi: 10.1039/c6ee03748h. [DOI] [Google Scholar]
- 74.Squinca P., Bilatto S., Badino A.C., Farinas C.S. Nanocellulose production in future biorefineries: an integrated approach using tailor-made enzymes. ACS Sustain. Chem. Eng. 2020;8(5):2277–2286. doi: 10.1021/acssuschemeng.9b06790. [DOI] [Google Scholar]
- 75.Wu Y., et al. Application of intermittent ball milling to enzymatic hydrolysis for efficient conversion of lignocellulosic biomass into glucose. Renew. Sustain. Energy Rev. 2021;136(September 2020) doi: 10.1016/j.rser.2020.110442. [DOI] [Google Scholar]
- 76.Kohli K., Katuwal S., Biswas A., Sharma B.K. Effective delignification of lignocellulosic biomass by microwave assisted deep eutectic solvents. Bioresour. Technol. 2020;303(January) doi: 10.1016/j.biortech.2020.122897. [DOI] [PubMed] [Google Scholar]
- 77.Gan Y.Y., et al. Microwave-assisted wet torrefaction of microalgae under various acids for coproduction of biochar and sugar. J. Clean. Prod. 2020;253 doi: 10.1016/j.jclepro.2019.119944. [DOI] [Google Scholar]
- 78.Ji Q., Yu X., Yagoub A.E.G.A., Chen L., Zhou C. Efficient removal of lignin from vegetable wastes by ultrasonic and microwave-assisted treatment with ternary deep eutectic solvent. Ind. Crop. Prod. 2020;149(December 2019) doi: 10.1016/j.indcrop.2020.112357. [DOI] [Google Scholar]
- 79.Ong V.Z., Wu T.Y., Lee C.B.T.L., Cheong N.W.R., Shak K.P.Y. Sequential ultrasonication and deep eutectic solvent pretreatment to remove lignin and recover xylose from oil palm fronds. Ultrason. Sonochem. 2019;58(January) doi: 10.1016/j.ultsonch.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 80.Kumar B., Bhardwaj N., Verma P. Microwave assisted transition metal salt and orthophosphoric acid pretreatment systems: generation of bioethanol and xylo-oligosaccharides. Renew. Energy. 2020;158:574–584. doi: 10.1016/j.renene.2020.05.006. [DOI] [Google Scholar]
- 81.Hamraoui K., Gil A., El Bari H., Siles J.A., Chica A.F., Martin M.A. Evaluation of hydrothermal pretreatment for biological treatment of lignocellulosic feedstock (pepper plant and eggplant) Waste Manag. 2020;102:76–84. doi: 10.1016/j.wasman.2019.10.020. [DOI] [PubMed] [Google Scholar]
- 82.Diaz M.J., Cara C., Ruiz E., Perez-Bonilla M., Castro E. Hydrothermal pre-treatment and enzymatic hydrolysis of sunflower stalks. Fuel. 2011;90(11):3225–3229. doi: 10.1016/j.fuel.2011.06.040. [DOI] [Google Scholar]
- 83.Tan L., Liu Z., Zhang T., Wang Z., Liu T. Enhanced enzymatic digestibility of poplar wood by quick hydrothermal treatment. Bioresour. Technol. 2020;302(January) doi: 10.1016/j.biortech.2020.122795. [DOI] [PubMed] [Google Scholar]
- 84.Singh J., Suhag M., Dhaka A. Augmented digestion of lignocellulose by steam explosion, acid and alkaline pretreatment methods: a review. Carbohydr. Polym. 2015;117:624–631. doi: 10.1016/j.carbpol.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 85.Yuan Z., Li G., Wei W., Wang J., Fang Z. A comparison of different pre-extraction methods followed by steam pretreatment of bamboo to improve the enzymatic digestibility and ethanol production. Energy. 2020;196 doi: 10.1016/j.energy.2020.117156. [DOI] [Google Scholar]
- 86.Mittal A., et al. Ammonia pretreatment of corn stover enables facile lignin extraction. ACS Sustain. Chem. Eng. 2017;5(3):2544–2561. doi: 10.1021/acssuschemeng.6b02892. [DOI] [Google Scholar]
- 87.Zhao C., Shao Q., Chundawat S.P.S. Recent advances on ammonia-based pretreatments of lignocellulosic biomass. Bioresour. Technol. 2020;298 doi: 10.1016/j.biortech.2019.122446. [DOI] [PubMed] [Google Scholar]
- 88.Haghighi Mood S., et al. Lignocellulosic biomass to bioethanol, a comprehensive review with a focus on pretreatment. Renew. Sustain. Energy Rev. 2013;27:77–93. doi: 10.1016/j.rser.2013.06.033. [DOI] [Google Scholar]
- 89.Sindhu R., Binod P., Pandey A. Biological pretreatment of lignocellulosic biomass - an overview. Bioresour. Technol. 2016;199:76–82. doi: 10.1016/j.biortech.2015.08.030. [DOI] [PubMed] [Google Scholar]
- 90.Farkas C., et al. Microbial saccharification of wheat bran for bioethanol fermentation. J. Clean. Prod. 2019;240 doi: 10.1016/j.jclepro.2019.118269. [DOI] [Google Scholar]
- 91.Zabed H.M., et al. Recent advances in biological pretreatment of microalgae and lignocellulosic biomass for biofuel production. Renew. Sustain. Energy Rev. 2019;105(June 2018):105–128. doi: 10.1016/j.rser.2019.01.048. [DOI] [Google Scholar]
- 92.Liao J.C., Mi L., Pontrelli S., Luo S. Fuelling the future: microbial engineering for the production of sustainable biofuels. Nat. Rev. Microbiol. 2016;14(5):288–304. doi: 10.1038/nrmicro.2016.32. [DOI] [PubMed] [Google Scholar]
- 93.Yao F., Shen F., Wan X., Hu C. High yield and high concentration glucose production from corncob residues after tetrahydrofuran + H2O co-solvent pretreatment and followed by enzymatic hydrolysis. Renew. Sustain. Energy Rev. 2020;132(February) doi: 10.1016/j.rser.2020.110107. [DOI] [Google Scholar]
- 94.Liu Z.H., et al. Synergistic maximization of the carbohydrate output and lignin processability by combinatorial pretreatmentf. Innov. Green Process Eng. Sustain. Energy Environ. 2017 - Top. Conf. 2017 AIChE Annu. Meet. 2017;2017(Octob):248–264. doi: 10.1039/c7gc02057k. [DOI] [Google Scholar]
- 95.Parthasarathi R., et al. Activation of lignocellulosic biomass for higher sugar yields using aqueous ionic liquid at low severity process conditions. Biotechnol. Biofuels. 2016;9(1):1–13. doi: 10.1186/s13068-016-0561-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Saulnier B.K., Phongpreecha T., Singh S.K., Hodge D.B. Impact of dilute acid pretreatment conditions on p-coumarate removal in diverse maize lines. Bioresour. Technol. 2020;314 doi: 10.1016/j.biortech.2020.123750. [DOI] [PubMed] [Google Scholar]
- 97.Hernandez-Guzman A., Navarro-Gutierrez I.M., Melendez-Hernandez P.A., Hernandez-Beltran J.U., Hernandez-Escoto H. Enhancement of alkaline-oxidative delignification of wheat straw by semi-batch operation in a stirred tank reactor. Bioresour. Technol. 2020;312 doi: 10.1016/j.biortech.2020.123589. [DOI] [PubMed] [Google Scholar]
- 98.Ji G., Xiao W., Gao C., Cao Y., Zhang Y., Han L. Mechanical fragmentation of wheat and rice straw at different scales: energy requirement in relation to microstructure properties and enzymatic hydrolysis. Energy Convers. Manag. 2018;171(May):38–47. doi: 10.1016/j.enconman.2018.05.087. [DOI] [Google Scholar]
- 99.Singh S., Agarwal M., Bhatt A., Goyal A., Moholkar V.S. Ultrasound enhanced enzymatic hydrolysis of Parthenium hysterophorus: a mechanistic investigation. Bioresour. Technol. 2015;192:636–645. doi: 10.1016/j.biortech.2015.06.031. [DOI] [PubMed] [Google Scholar]
- 100.Flores-Gomez C.A., Escamilla Silva E.M., Zhong C., Dale B.E., Da Costa Sousa L., Balan V. Conversion of lignocellulosic agave residues into liquid biofuels using an AFEXTM-based biorefinery. Biotechnol. Biofuels. 2018;11(1):1–18. doi: 10.1186/s13068-017-0995-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Benazzi T., Calgaroto S., Astolfi V., Dalla Rosa C., Oliveira J.V., Mazutti M.A. Pretreatment of sugarcane bagasse using supercritical carbon dioxide combined with ultrasound to improve the enzymatic hydrolysis. Enzym. Microb. Technol. 2013;52(4–5):247–250. doi: 10.1016/j.enzmictec.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 102.Wells J.M., Drielak E., Surendra K.C., Kumar Khanal S. Hot water pretreatment of lignocellulosic biomass: modeling the effects of temperature, enzyme and biomass loadings on sugar yield. Bioresour. Technol. 2020;300 doi: 10.1016/j.biortech.2019.122593. [DOI] [PubMed] [Google Scholar]
- 103.Mihiretu G.T., Chimphango A.F., Gorgens J.F. Steam explosion pre-treatment of alkali-impregnated lignocelluloses for hemicelluloses extraction and improved digestibility. Bioresour. Technol. 2019;294(September) doi: 10.1016/j.biortech.2019.122121. [DOI] [PubMed] [Google Scholar]
- 104.Wang R., You T., Yang G., Xu F. Efficient short time white rot-Brown rot fungal pretreatments for the enhancement of enzymatic saccharification of corn cobs. ACS Sustain. Chem. Eng. 2017;5(11):10849–10857. doi: 10.1021/acssuschemeng.7b02786. [DOI] [Google Scholar]
- 105.Machado A. da S., Ferraz A. Biological pretreatment of sugarcane bagasse with basidiomycetes producing varied patterns of biodegradation. Bioresour. Technol. 2017;225:17–22. doi: 10.1016/j.biortech.2016.11.053. [DOI] [PubMed] [Google Scholar]
- 106.Castoldi R., et al. Biological pretreatment of Eucalyptus grandis sawdust with white-rot fungi: study of degradation patterns and saccharification kinetics. Chem. Eng. J. 2014;258:240–246. doi: 10.1016/j.cej.2014.07.090. [DOI] [Google Scholar]
- 107.Song L., Yu H., Ma F., Zhang X. Biological pretreatment under non-sterile conditions for enzymatic hydrolysis of corn stover. Bioresources. 2013;8(2):3802–3816. doi: 10.15376/biores.8.3.3802-3816. [DOI] [Google Scholar]
- 108.Jagtap S.S., Dhiman S.S., Kim T.S., Li J., Lee J.K., Kang Y.C. Enzymatic hydrolysis of aspen biomass into fermentable sugars by using lignocellulases from Armillaria gemina. Bioresour. Technol. 2013;133:307–314. doi: 10.1016/j.biortech.2013.01.118. [DOI] [PubMed] [Google Scholar]
- 109.Jagtap S.S., Dhiman S.S., Kim T.S., Li J., Chan Kang Y., Lee J.K. Characterization of a β-1,4-glucosidase from a newly isolated strain of Pholiota adiposa and its application to the hydrolysis of biomass. Biomass Bioenergy. 2013;54:181–190. doi: 10.1016/j.biombioe.2013.03.032. [DOI] [Google Scholar]
- 110.Naresh Kumar M., Ravikumar R., Kirupa Sankar M., Thenmozhi S. New insight into the effect of fungal mycelia present in the bio-pretreated paddy straw on their enzymatic saccharification and optimization of process parameters. Bioresour. Technol. 2018;267:291–302. doi: 10.1016/j.biortech.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 111.Taha M., et al. Enhanced biological straw saccharification through coculturing of lignocellulose-degrading microorganisms. Appl. Biochem. Biotechnol. 2015;175(8):3709–3728. doi: 10.1007/s12010-015-1539-9. [DOI] [PubMed] [Google Scholar]
- 112.Kostas E.T., Beneroso D., Robinson J.P. The application of microwave heating in bioenergy: a review on the microwave pre-treatment and upgrading technologies for biomass. Renew. Sustain. Energy Rev. 2017;77(March):12–27. doi: 10.1016/j.rser.2017.03.135. [DOI] [Google Scholar]
- 113.Li X., et al. Preparation and investigation of highly selective solid acid catalysts with sodium lignosulfonate for hydrolysis of hemicellulose in corncob. RSC Adv. 2018;8(20):10922–10929. doi: 10.1039/c7ra13362f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Megawati M., Damayanti A., Putri R., Pratama A., Muftidar T. Kinetics of enzymatic hydrolysis of passion fruit peel using cellulase in bio-ethanol production. Reaktor. 2020;20(1):10–17. doi: 10.14710/reaktor.20.1.10-17. [DOI] [Google Scholar]
- 115.Ajani A., Agarry S., Agbede O. A comparative kinetic study of acidic hydrolysis of wastes cellulose from agricultural derived biomass. J. Appl. Sci. Environ. Manag. 2011;15(4):531–537. http://www.bioline.org.br/ja%5Cnhttp://www.ajol.info/index.php/jasem/article/view/88555 [Online]. Available: [Google Scholar]
- 116.dos Santos Rocha M.S.R., Pratto B., de Sousa R., Almeida R.M.R.G., da Cruz A.J.G. A kinetic model for hydrothermal pretreatment of sugarcane straw. Bioresour. Technol. 2017;228:176–185. doi: 10.1016/j.biortech.2016.12.087. [DOI] [PubMed] [Google Scholar]
- 117.Prathyusha N., et al. Modelling of pretreatment and saccharification with different feedstocks and kinetic modeling of sorghum saccharification. Bioresour. Technol. 2016;221:550–559. doi: 10.1016/j.biortech.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 118.Makarova E.I., Budaeva V.V., Kukhlenko A.A., Orlov S.E. Enzyme kinetics of cellulose hydrolysis of Miscanthus and oat hulls. 3 Biotech. 2017;7(5):1–9. doi: 10.1007/s13205-017-0964-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mardawatia E., Wira D.W., Djali M., Fetriyuna F., Suryadi E. Optimization and kinetic modelling of the enzymatic hydrolysis of oil palm petioles. KnE Life Sci. 2017;2(6):439. doi: 10.18502/kls.v2i6.1065. [DOI] [Google Scholar]
- 120.Procentese A., Russo M.E., Di Somma I., Marzocchella A. Kinetic characterization of enzymatic hydrolysis of apple pomace as feedstock for a sugar-based biorefinery. Energies. 2020;13(5) doi: 10.3390/en13051051. [DOI] [Google Scholar]
- 121.Brown R.F., Agbogbo F.K., Holtzapple M.T. Comparison of mechanistic models in the initial rate enzymatic hydrolysis of AFEX-treated wheat straw. Biotechnol. Biofuels. 2010;3:1–13. doi: 10.1186/1754-6834-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yuan Y., Zhai R., Li Y., Chen X., Jin M. Developing fast enzyme recycling strategy through elucidating enzyme adsorption kinetics on alkali and acid pretreated corn stover. Biotechnol. Biofuels. 2018;11(1):1–12. doi: 10.1186/s13068-018-1315-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Khodaverdi M., Karimi K., Jeihanipour A., Taherzadeh M.J. Kinetic modeling of rapid enzymatic hydrolysis of crystalline cellulose after pretreatment by NMMO. J. Ind. Microbiol. Biotechnol. 2012;39(3):429–438. doi: 10.1007/s10295-011-1048-y. [DOI] [PubMed] [Google Scholar]
- 124.Kadam K.L., Rydholm E.C., McMillan J.D. Development and validation of a kinetic model for enzymatic saccharification of lignocellulosic biomass. Biotechnol. Prog. 2004;20(3):698–705. doi: 10.1021/bp034316x. [DOI] [PubMed] [Google Scholar]
- 125.Ayeni A.O., et al. Kinetic study of activation and deactivation of adsorbed cellulase during enzymatic conversion of alkaline peroxide oxidation-pretreated corn cob to sugar. Kor. J. Chem. Eng. 2021;38(1):81–89. doi: 10.1007/s11814-020-0667-2. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.