Abstract
Structurally reconfigurable RNA structures enable dynamic transitions of the functional states in response to diverse molecular stimuli, which are fundamental in genetic and epigenetic regulations. Inspired by nature, rationally designed RNA structures with responsively reconfigurable motifs have been developed to serve as switchable components for building engineered nanomachines, which hold promise in synthetic biological applications. In this review, we summarize recent progress in the design, synthesis, and integration of engineered reconfigurable RNA structures for nanomachines. We highlight recent examples of their targeted applications such as biocomputing and smart theranostics. We also discuss their advantages, challenges as well as possible solutions. We further provide an outlook of their potential in future synthetic biology.
Keywords: RNA nanomachine, Structure, Reconfiguration, Biocomputing, Theranostics
INTRODUCTION
Nucleic acid base-pairing and protein–nucleic acid interactions in living systems are precisely encoded by nucleic acid sequences, which enable complex biomolecular networks and lay the foundation of synthetic biology (Ma et al. 2012; Purnick and Weiss 2009). In recent decades, great efforts have been made to engineer biological structures and nanomachines for diverse applications, such as biocomputing, nanofabrication and smart theranostics (Ge et al. 2018; Hu et al. 2019; Liu et al. 2018; Wang et al. 2020). Particularly, RNA molecules can form predictable secondary and tertiary structures via self-base pairing (Shapiro et al. 2008; Shi et al. 2014), allowing finite structural reconfigurations in response to trans-acting molecular stimuli (e.g., proteins, metabolites, and other nucleic acids) and environmental cues, which can trigger dynamic switches of their functional states (Bayer and Smolke 2005). This mechanism is fundamental in the genetic and epigenetic regulations of living systems.
Recent advances in nucleic acid structural nanotechnology (Fig. 1) have enabled a variety of nanostructures relying on diverse naturally existing or engineered RNA units at the level of secondary and tertiary motifs, including hairpins, kissing loops, kinks (Ohno et al. 2011) and three-way junctions. These RNA units can be further assembled into 2D and 3D architectures such as polygons, arrays, and filaments (Grabow and Jaeger 2014). There have been several comprehensive reviews about structural RNA nanotechnology, involving the fundamental aspects of designing, synthesizing, folding and self-assembly of engineered RNA nanostructures (Grabow and Jaeger 2014; Guo 2010; Jasinski et al. 2017; Krishnan and Bathe 2012; Qiu et al. 2013). Particularly, the development of RNA nanotechnology allows researchers to develop nanomachines based on structurally reconfigurable RNA structures, which provide a powerful toolbox for diverse synthetic biological applications (Ishikawa et al. 2013; Li et al. 2017; Osada et al. 2014).
Figure 1.
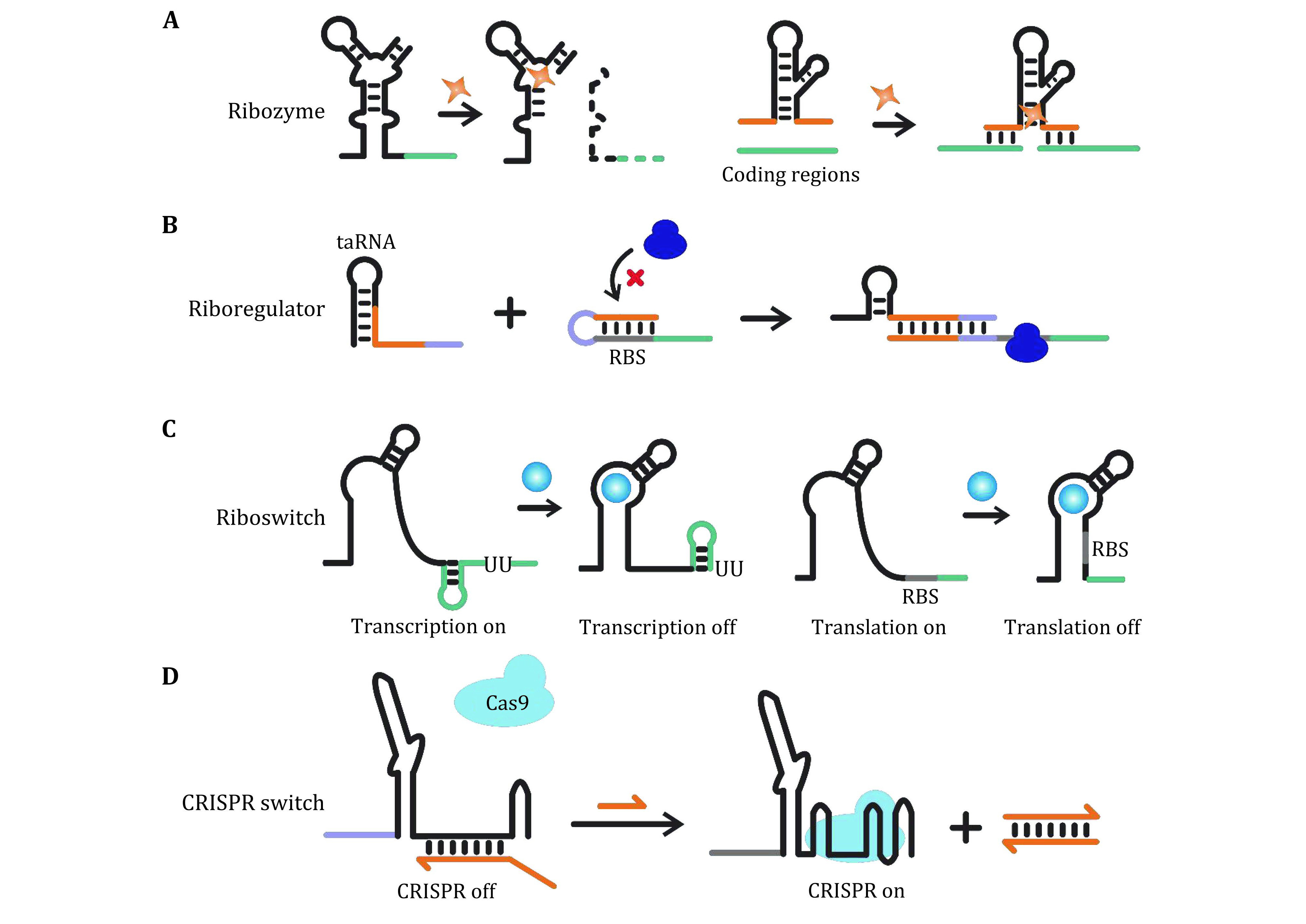
RNA structures with functions that can be switched via structural reconfigurations. A Ribozymes and aptamers which are structurally responsive to specific molecules. B Riboregulators for trans-acting regulation of gene expression. C Riboswitches for cis-acting regulation of gene expression. D General schematic of CRISPR switches allowing controllable activities of the CRISPR-Cas system
Engineered RNA structures possess several important advantages in building artificial nanomachines. First, they have excellent programmability, that is, their structures and functionality can be precisely prescribed by their nucleotide sequences. Secondly, RNA motifs are highly modularizable, which can serve as independent and standardized parts allowing nearly arbitrary combinations into complicated systems. Moreover, like their natural counterparts, engineered RNA structures can be synthesized in biological systems. For example, in vitro transcription and self-folding of a single-stranded (ss-) RNA into 2D shapes or networks have been demonstrated (Geary et al. 2014; Han et al. 2017; Yu et al. 2015). Recently, the synthesis of RNA nanostructures inside living cells has also been realized via a similar approach (Li et al. 2018).
In this review, we focus on recent studies on structurally reconfigurable designer RNA structures. We briefly introduce the basic principles in the design and fabrication of nanomachines driven by RNA structure reconfiguration. We demonstrate some recent applications in gene circuit rewiring, biocomputing, nanofabrication, and smart theranostics based on these RNA nanomachines. We also discuss several limitations and future directions of this field.
FUNCTIONAL UNITS BASED ON STRUCTURALLY RECONFIGURABLE RNA MOTIFS
In this section, we introduce several basic RNA motifs (Fig. 1), whose functionality can be switched via structural reconfigurations in response to external stimuli. They have been widely exploited for programmable gene regulation in synthetic biology, and can serve as functional modules for the fabrication of nanomachines.
Ribozymes and RNA aptamers
Ribozymes (or RNA enzymes) are a class of RNA structures with enzymatic activities. They often cooperate with metal cofactors and perform specific phosphodiester bond cleavage and formation at the translational level (Birikh et al. 1997; Sigel and Pyle 2007). For example, hammerhead ribozymes are a class of small catalytic RNAs existing in tobacco ringspot viruses, which allow self-cleavage in satellite RNAs in response to magnesium ions signals (Beilstein et al. 2015) (Fig. 1A).
Due to recent advances in systematic evolution of ligands by exponential enrichment (SELEX) technology, a variety of artificially selected RNA aptamers and ribozymes have emerged, which can bind the molecules of interest, or show certain catalytic activities when incorporated with given molecules (Darmostuk et al. 2015; Ellington and Szostak 1990; Kawazoe et al. 2001; Ozer et al. 2014). For example, there have been a variety of ribozymes showing activities such as nucleic acid cleavage and ligation. These activities usually depend on their structural configurations, thus are sensitive to external conditions that can alter their structures. On this basis, many ribozyme-based systems have been constructed, which are responsive to environmental factors like metal ions and pH (Lau and Ferre-D'Amare 2016; Li et al. 2009).
Recently, a series of RNA aptamers that can mimic fluorescent proteins (fluorescence light-up aptamers) have attracted much interest. These RNA aptamers light up when binding to corresponding fluorophores (Neubacher and Hennig 2019; Song et al. 2014), and are named after vegetables according to their fluorescence colors (e.g., spinach, broccoli, corn, and mango). Like their protein counterparts, these RNA aptamers can be fused to other RNA molecules of interest, thus are suitable for RNA imaging and detection in live cells. These fluorescence aptamers can be further integrated with other structurally reconfigurable aptamers, enabling switchable fluorescence in response to the molecules of interest (Jepsen et al. 2018; Paige et al. 2012; Pothoulakis et al. 2014). Recently, an aptamer-initiated fluorescence complementation (AiFC) method has been developed for RNA imaging (Wang et al. 2018b). In this method, a broccoli motif is split into two fragments unable to emit fluorescence, which can recover the intact form and light up the fluorescence upon binding to the target RNA, enabling high-contrast and real-time imaging of endogenous RNAs. On the other hand, these fluorescent RNA aptamers can be utilized in other synthetic nucleic acid nanodevices as output signals (Chakraborty et al. 2016; Jiao et al. 2020).
Riboswitches
In nature, riboswitches refer to cis-acting RNA motifs located in the regulatory regions (e.g. 5' or 3' untranslated regions, UTRs) of mRNAs (Fig. 1C). They can be structurally reconfigured upon binding with specific small molecules (Bailor et al. 2010), and then sequester or expose key regulatory sites (e.g., ribosome binding site, RBS), allowing switches of translation initiation or transcription termination (Batey et al. 2004; Nudler and Mironov 2004). Inspired by naturally existing riboswitches, artificially designed RNA motifs allowing responsive structural reconfigurations have been designed, which can serve as binary switches enabling transduction of input signals into various outputs, thus have been widely used in synthetic biology (Dwidar et al. 2019; Ogawa and Maeda 2008).
Riboswitches at the transcriptional level
Many riboswitches regulate gene expression at the transcriptional level by producing transcription-terminating helices. For example, a series of synthetic small transcription activating RNAs (STARs) have been constructed to disrupt the formation of an intrinsic transcription terminator placed upstream of a gene in Escherichia coli (E. coli) (Chappell et al. 2015; Meyer et al. 2016) (Fig. 2A). Moreover, multiple orthogonal STARs can be built and assembled in tandem in one RNA molecule, enabling RNA-only transcriptional logic gates.
Figure 2.
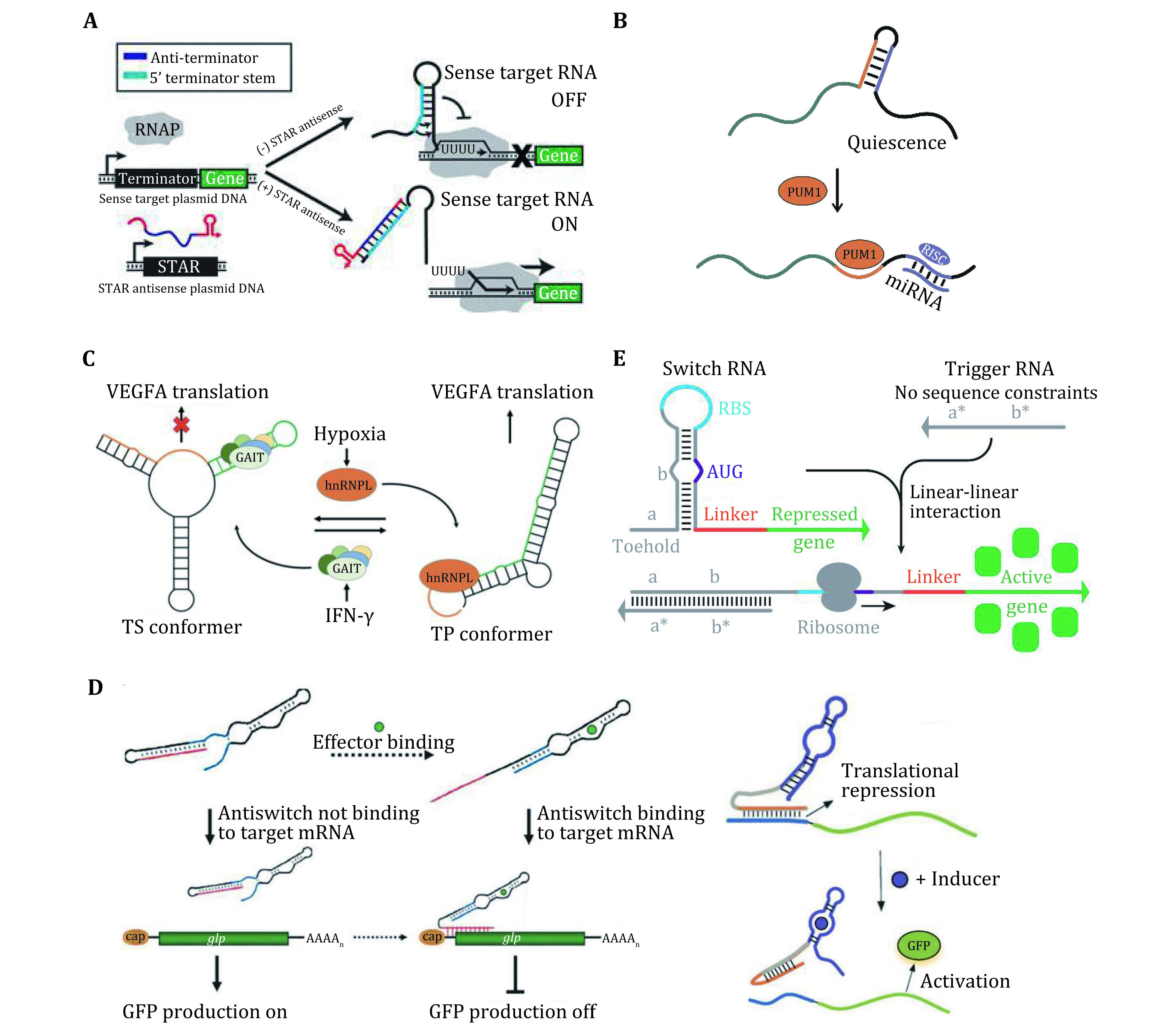
A Schematic of an anti-terminator STAR. This system inverts the structural configuration of the attenuator through the addition of an anti-terminator sequence. Adapted from Meyer et al. (2016). B Pumilio protein-mediated mRNA secondary structural switch controls the accessibility of microRNA-binding sites and regulates the protein expression. RISC, RNA-induced silencing complex. C Translation control of gene expression through a dual protein-dependent RNA secondary structural switch that responds to interferon-γ (IFN-γ). Adapted from Liu et al. (2016a). D Engineered riboregulators responsive to small molecules, which control the gene translation in a trans-acting manner. Adapted from Agapakis and Silver (2009) and Munzar et al. (2019). E Toehold switch system comprising a switch RNA for repressing translation, and a trigger RNA with arbitrary sequence which can reconfigure the switch RNA via a toehold-mediated linear-linear interaction. Adapted from Green et al. (2014)
Riboswitches at the post-transcriptional level
A variety of riboswitches have been found involved in regulating post-transcriptional processing (Abbink et al. 2005; Winkler and Breaker 2005). For example, splicing, gene silencing by microRNA (miRNA), and RNA editing. Although the detailed mechanisms behind these systems are poorly understood, these riboswitches enable controllable exposure, occlusion, or modulation of the processing sites to regulate post-transcriptional processes. For example, a riboswitch (Kedde et al. 2010) has recently been identified in the 3’-UTR of p27 mRNA. This riboswitch is a secondary structure that simultaneously sequesters two regions: one is a microRNA (miRNA) binding site, the other is a pumilio-recognition element (PRE) which can bind a pumilio RNA-binding protein (PUM1). The binding of PUM1 and PRE can trigger a structural reconfiguration of the riboswitch and expose the miRNA binding site, leading to miRNA-induced gene silencing (Fig. 2B).
Riboswitches at the translational level
Riboswitches functioning at the translational level have also been intensively exploited category of switches in synthetic biology. They usually rely on structurally reconfigurable motifs at the 5' or 3' UTR, which can sequester or expose the RBS in response to specific stimuli, thus switch the translational activity of the downstream coding regions (Isaacs et al. 2006). For example, a riboswitch is responsive to thiamine or its pyrophosphate derivative without the need for protein cofactors (Winkler et al. 2002). The binding of the effector can trigger a reconfiguration of the riboswitch, which sequesters the RBS and leads to a suppression of gene expression in E. coli.
Recently, a protein-dependent riboswitch has been identified in the 3ʹ UTR of VEGFA mRNA in myeloid cells that regulates translation of VEGFA in response to proteins associated with two disparate stress stimuli (Fig. 2C). The interferon-γ (IFN-γ)-activated inhibitor of translation (GAIT)-complex binds a structural GAIT element within a family of inflammatory mRNAs and silences their translation by promoting the formation of a translational-silencing (TS) conformer (Ray et al. 2009). During oxidative stress, the heterogeneous nuclear ribonucleoprotein L (hnRNP L) overrides GAIT silencing by triggering a secondary structural RNA switch to a translation-permissive (TP) conformer, in which the GAIT element is occluded. The RNA alternates between two mutually exclusive conformers in response to the binding of the GAIT complex or hnRNP L, thereby functioning as an AND NOT Boolean logic-gate switch in which the presence of one protein, but not the other, yields an output of gene repression.
Riboregulators
In contrast to riboswitches that are cis-acting motifs, riboregulators usually refer to trans-acting RNAs (or antisense RNAs) that can bind and reconfigure the regulatory sites (e.g., riboswitches) of other mRNAs, leading to post-transcriptional/translational regulations of the latter (Fig. 1B). This class of RNA structures has also been widely employed in synthetic biology (Ueno et al. 2017, 2018; Wang et al. 2018a).
For example, Bayer et al. designed a set of riboregulators called "antiswitches" (Fig. 2D) (Bayer and Smolke 2005), which can function in Saccharomyces cerevisiae. The riboregulators contain an aptamer domain, whose structure can be reconfigured in response to specific ligands, leading to switchable sequester/exposure of the antisense domain, and thus switchable binding/unbinding of the target mRNAs. By the rational design, the translation of the target mRNAs can be activated (by the on-antiswitch) or repressed (by the off-antiswitch) in the presence of the ligands, which demonstrates the capability of these riboregulators for bi-directional modulation.
In these earlier strategies, the riboregulators usually rely on U-turn loop structures to drive loop-linear interactions between RNAs (Callura et al. 2012; Daniel et al. 2013). The riboregulators need to contain sequences antisense to the riboswitches (including the RBS), which largely limits their design freedom. Lately, Yin and coworkers (Green et al. 2014) established a new set of riboswitches (referred to as switch RNA) and riboregulators (referred to as trigger RNA), dubbed "Toehold Switch" (Fig. 2E). In this system, a "toehold" sequence is introduced on the switch RNA at the 5' UTR, thus the trigger RNA can reconfigure the switch RNA via a toehold-mediated linear–linear interaction. This strategy allows us to design riboregulators with arbitrary sequences. Moreover, the toehold switches provide a high dynamic range (>400) as well as a high level of orthogonality. They can be applied in the bacterial genome to regulate endogenous genes.
INTEGRATION OF RNA UNITS INTO NANOMACHINES
Till now, a variety of reconfigurable RNA units possessing diverse functions have been established, which encourage researchers to integrate them into smart nanomachines or nanorobots. This pursuit requires that the functionality of the RNA units should be well maintained in the integrated systems, with undesired crosstalk minimized. Moreover, non-RNA molecules, especially proteins, can be integrated and cooperate with the RNA units, enabling functions unable to be provided by pure RNAs. Here we discuss two representative approaches for the integration.
Assembly of RNA units with RNA scaffolds
Owing to the excellent modularity of RNA motifs and the advances of RNA nanotechnology, diverse functional RNA units can be nearly arbitrarily assembled into complex systems with the assistance of RNA scaffolds, which enable multifunctional nanodevices and smart nanomachines (Khaled et al. 2005; Shu et al. 2011). Moreover, the structural stability of the functional RNA units can be largely enhanced.
For example, Guo's group (Li et al. 2016; Shu et al. 2015) utilized three-way junctions (derived from the packaging RNA, or pRNA, in a bacteriophage) and four-way junctions as building blocks to build multimeric RNA nanostructures (Fig. 3A). These structures as scaffolds can be used to integrate multiple ribozymes and aptamers into multifunctional nanodevices (Afonin et al. 2014; Delebecque et al. 2011; Hao et al. 2014). Rolling circle transcription has also been used to synthesize RNA nanoparticles carrying periodically arranged functional motifs (Geary et al. 2014; Jang et al. 2015; Lee et al. 2012) (Fig. 3B). More recently, RNA origami structures, folded by a long ssRNA (up to ~6000 nt) via transcription have shown promise in serving as scaffolds to assemble nanomachines (Geary et al. 2014; Grabow and Jaeger 2014; Han et al. 2017; Li et al. 2018) (Fig. 3C). Theoretically, they allow spatial arrangement of the functional RNA motifs in nanometer precision, and more importantly, can be synthesized in living systems.
Figure 3.
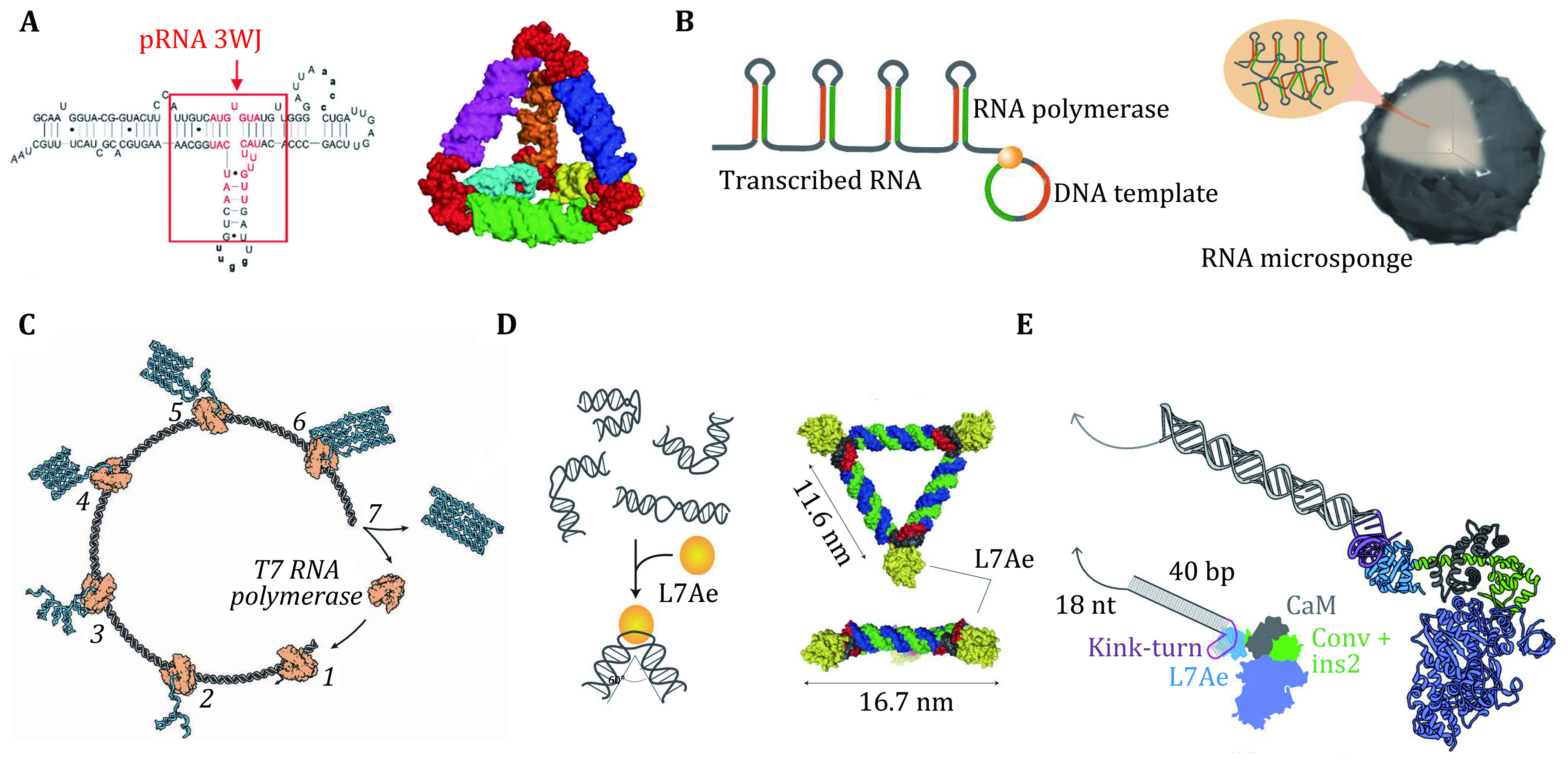
A An RNA tetrahedron assembled by the three-way junction (3WJ) motifs from pRNA. Adapted from Li et al. (2016). B Rolling circle transcription (RCT) for the self-assembly of RNA-microsponges. Adapted from Yuan et al. (2019). C RNA origami structure generated via a cotranscriptional folding pathway. The T7 RNA polymerase binds to the template DNA (step 1) and the RNA folds as it is being synthesized (steps 2 to 7). Adapted from Geary et al. (2014). D Molecular design of a triangular RNP assembled based on protein-RNA interactions. Adapted from Durbin et al. (2019). E Design and characterization of an engineered myosin with an RNA lever arm. Adapted from Saper and Hess (2020)
Integration with proteins
Many natural nanomachines in living organisms are in form of ribonucleoproteins (RNPs, or RNA-protein complexes, such as ribosomes, telomerase, and small nuclear ribonucleoproteins) (Greider and Blackburn 1987; Maniatis and Reed 1987). Inspired by them, natural RNA motifs or artificially selected RNA aptamers that can bind specified proteins have been harnessed to assemble hybrid nanomachines akin to natural RNPs.
For example, the kink-turn (K-turn) RNA motifs can interact with K-turn binding protein L7Ae and conduct structural reconfigurations, which can be utilized to build RNP nanomachines that can transform in response to L7Ae (Ohno et al. 2011; Osada et al. 2014) (Fig. 3D). Recently, a new type of myosin motors incorporating RNA lever arms has been constructed based on the binding of K-turn and L7Ae (Omabegho et al. 2017) (Fig. 3E). The structural changes in the protein motor domain are amplified and redirected by the RNA structures. The speed and direction of motor motion can be dynamically controlled by the programmable transitions of RNA lever arm structure in response to strand-displacement reactions. In multimeric assemblies, the motors can walk processively along actin filaments. Moreover, this system can implement orthogonal responses of RNA variants to specific oligonucleotides.
Integration with CRISPR-Cas system
Clustered regularly interspaced short palindromic repeats (CRISPR)-CRISPR associated protein (Cas) system can be regarded as a natural ribonucleoprotein machine for adaptive immunity in microorganisms (Gasiunas et al. 2012), which has been intensively exploited as a powerful gene-editing tool. For example, a typical CRISPR-Cas9 system relies on guide RNAs (gRNAs) which bind the Cas9 endonuclease and guide the cleavage of target double-stranded (ds-) DNA in a sequence-specific manner. Herein, by engineering the structure of guide RNAs, their ability of Cas binding and/or targeting sequence recognition can be altered (Fig. 1D), enabling nanomachines with switchable targeting and cleavage activity (Jin et al. 2019; Liu et al. 2016b; Wang et al. 2019; Zalatan et al. 2015).
For example, our group has developed a switchable CRISPR-Cas9 system (Hao et al. 2020), which can respond to multiple nucleic acid inputs and reconfigure the structure of the gRNA through toehold-switch-mediated strand displacement (Fig. 4A). This system can implement orthogonal suppression and activation of the Cas9 targeting ability in response to specific DNA inputs. The combination of toehold switches enables diverse intracellular Cas9 activation programs with simultaneous and orthogonal responses.
Figure 4.
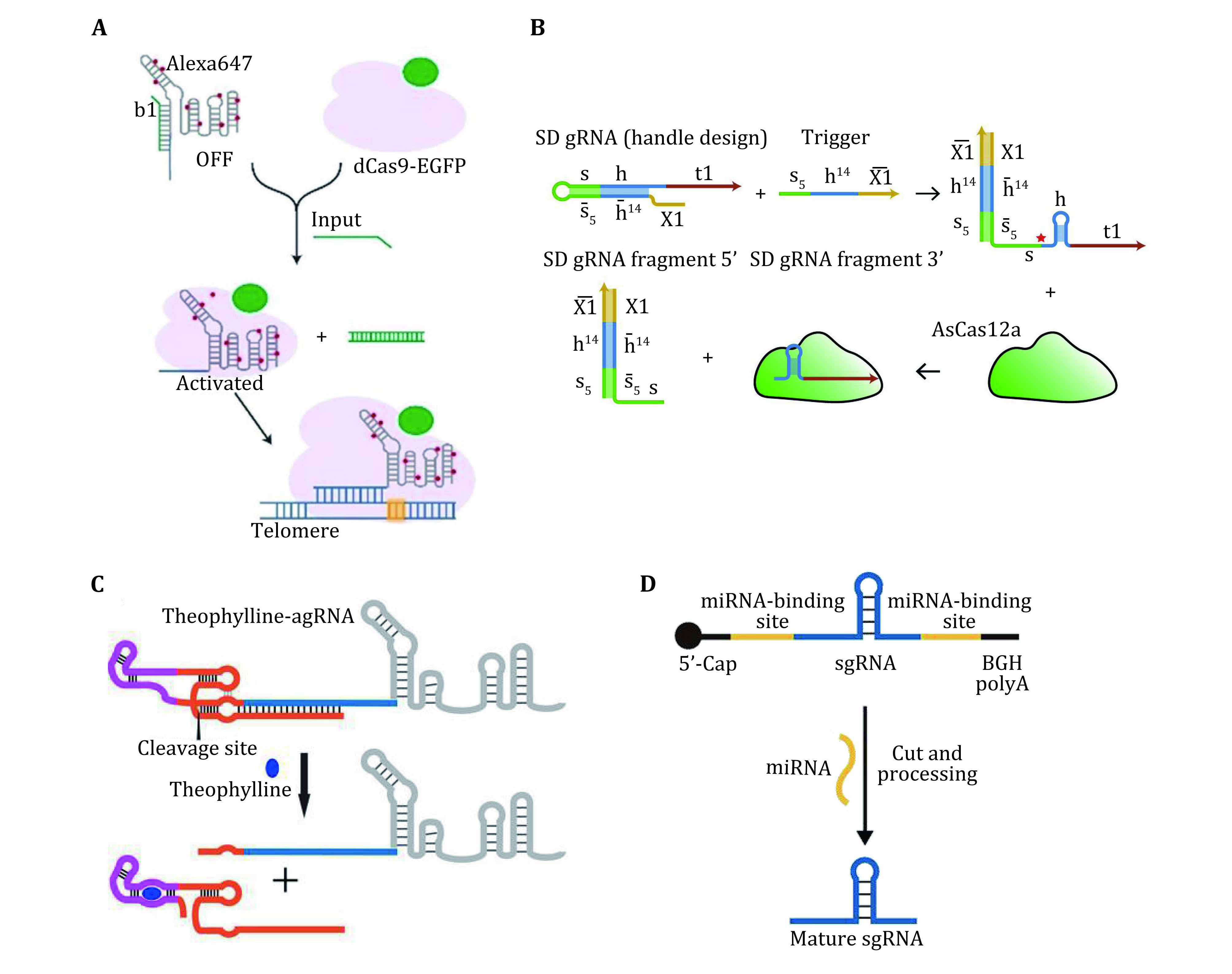
A Design of a trigger YES gate for the activation of telomere imaging. Adapted from Hao et al. (2020). B Principle of strand displacement switchable gRNAs. RNA trigger binds the SD gRNA, thereby restoring the gRNA handle. Binding of Cas12a leads to cleavage of the gRNA, and creates an active Cas12a-gRNA complex. Adapted from Oesinghaus and Simme (2019). C Schematic of the activation of theophylline aptazymes modified sgRNA in the presence of theophylline. Adapted from Tang et al. (2017). D Schematic representation of the activation of sgRNA by miRNA triggered cleavage. Adapted from Zhu et al. (2020)
Similarly, Oesinghaus et al. has reported a programmable activation strategy for the Cas12a system (Oesinghaus and Simme 2019). This system relies on designed strand displacement gRNAs (SD gRNAs) that can be specifically activated by trigger RNAs. This system enables logical transcriptional control of gene expression in E. coli (Fig. 4B).
By augmenting guide RNAs with self-cleaving ribozyme motifs (aptazymes) responsive to small molecules, Tang et al. (Tang et al. 2017) developed a CRISPR-Cas9 system allowing small molecule-controlled genome editing and small molecule-dependent transcriptional activation in mammalian cells (Fig. 4C).
In another example, Wang et al. designed an inactive sgRNA precursor (pre-sgRNA) (Wang et al. 2019), which could be cleaved by Argonaute (AGO) proteins upon binding with specific microRNAs (miRNAs), releasing functional sgRNAs generating that could guide the Cas9 to regulate the expression of reporter genes (Fig. 4D). By designing the targeting and miRNA binding sequence of pre-sgRNA, this system can be applied to control the expression of specified endogenous genes or mutate specific DNA bases in response to cell type-specific miRNAs.
TARGETED APPLICATIONS
Given the advances of nanomachines empowered by structurally reconfigurable RNA structures, their potential for biological and biomedical applications has also attracted broad interest. Here we summarize recent progress in their applications in cellular logic computation, diagnosis, and therapy.
Cellular logic computation
A cell is in principle an elaborate natural biocomputer that collects physical/chemical information from the environment, performs calculations via signal pathways or molecular circuitry, and uses this data to perform actions (Regev and Shapiro 2002). So far, most designed biological circuits are highly case specific, making them difficult to adapt and transplant from one organism to another. Hence, it is highly desirable to develop a more general synthetic circuitry scheme. Toehold-based RNA switches provide a powerful tool to realize such organism independent circuitry given their ubiquity in all forms of life and their predictable base pairing interactions. Yin and coworkers (Green et al. 2014) exploited orthogonal toehold switches to regulate 12 genes independently and to construct a circuit that can conduct four-input AND logic calculation (Fig. 5A). However, it remains challenging to scale up these circuits owing to the limited number of designable, orthogonal components. Later, they reported a "ribocomputing" system composed of de-novo-designed RNA parts, which can perform complex logic calculations in living cells with high dynamic range (Green et al. 2017). This ribocomputing system can be scaled up to calculate four-input AND, six-input OR, and a complex 12-input expression (Fig. 5B). On this basis, they introduced a new type of de-novo synthetic riboregulators, termed three-way junction (3WJ) repressors (Kim et al. 2019), which can help detect transcripts with nearly arbitrary sequences, repress gene expression by up to 300-fold and yield orthogonal sets of up to 15 devices. The modular repressors can be integrated into biological circuits that execute universal NAND and NOR logic and evaluate the four-input expression in E. coli (Fig. 5C).
Figure 5.
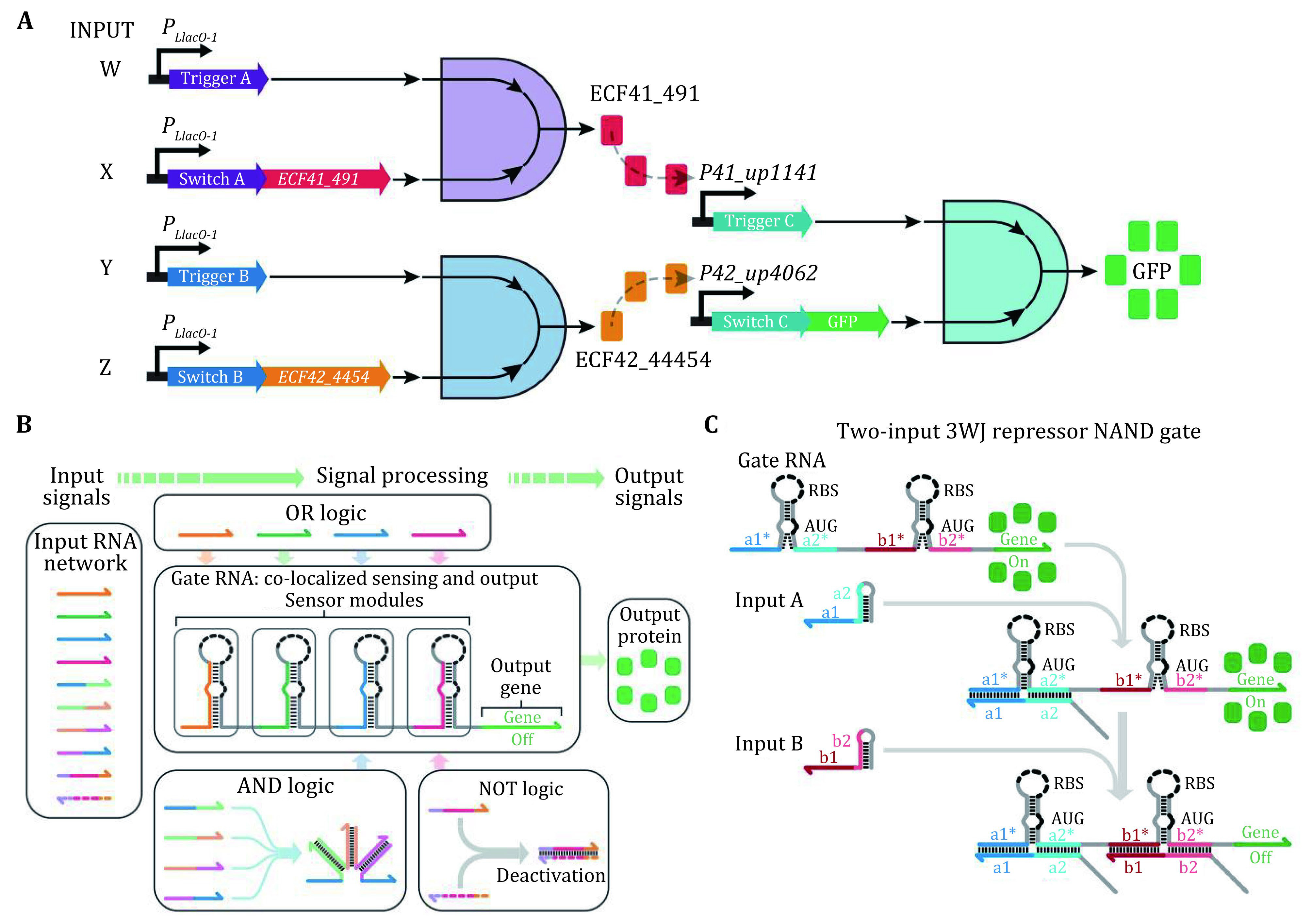
A Design schematic for the four-input AND circuit by three toehold switches, two orthogonal transcription factors, and a GFP reporter. Adapted from Green et al. (2014). B Ribocomputing system using RNA molecules as input signals and fluorescent protein as the output signal. Signal processing is carried out by a gate RNA that co-localizes sensing and output modules. Adapted from Simmel et al. (2019). C Design of a 3WJ repressor NAND gate. In the gate RNA, two switch modules are inserted in-frame and upstream of the reporter gene and both input RNAs must bind to the gate to prevent gene expression. Adapted from Kim et al. (2019)
Diagnosis
The increasing ability of synthetic biologists to repurpose and engineer reconfigurable RNA components for nanomachines has led to new opportunities for molecular diagnostics, especially for nucleic acid detection (Chan and Ng 2015; Khan 2006; Slomovic et al. 2015). The most prominent advantage might be that they can work under ambient conditions, e.g., detect nucleic acids under isothermal conditions without the need for thermal cycling equipment. Thus, they as easy-to-use platforms have the potential for point-of-care diagnosis (Thavarajah et al. 2020).
For example, Collins and coworkers developed toehold-based RNA switches as programmable RNA sensors (Green et al. 2014), which can be rationally designed to bind and sense virtually any RNA sequence. A freeze-dried, paper-based, cell-free protein expression platform, allows for the deployment of these toehold switch sensors outside of a research laboratory by providing a sterile and abiotic method for the storage and distribution of genetic circuits at room temperature (Pardee et al. 2014). They combined these technologies to create a platform for rapidly and inexpensively developing and deploying diagnostic sensors.
A later report examined the diagnostic capability of a toehold switch in which the trigger was Zika virus RNA, and the mRNA under control encoded an enzymatic "reporter" protein (Pardee et al. 2016). These paper based switches could reliably detect the Zika virus RNA with great sensitivity (Fig. 6A). Furthermore, the switches worked even after long term storage at ambient temperature. Although designed for use in detecting bacteria, paper based toehold switches also can quantify mRNA and validate the platform on clinical stool samples by comparison to RT-qPCR. They further highlight the potential clinical utility of the platform by showing that it can be used to rapidly and inexpensively detect toxin mRNA in the diagnosis of Clostridium difficile infections (Fig. 6B) (Takahashi et al. 2018). Alexander A. Green developed single-nucleotide-specific programmable riboregulators (SNIPRs) which provide over 100-fold differences in gene expression in response to target RNAs differing by a single nucleotide in E. coli and resolve single epitranscriptomic marks in vitro (Hong et al. 2020). By exploiting the programmable SNIPR design, integrating SNIPRs with portable paper-based cell-free reactions enables convenient isothermal detection of cancer-associated mutations from clinical samples (Fig. 6C).
Figure 6.
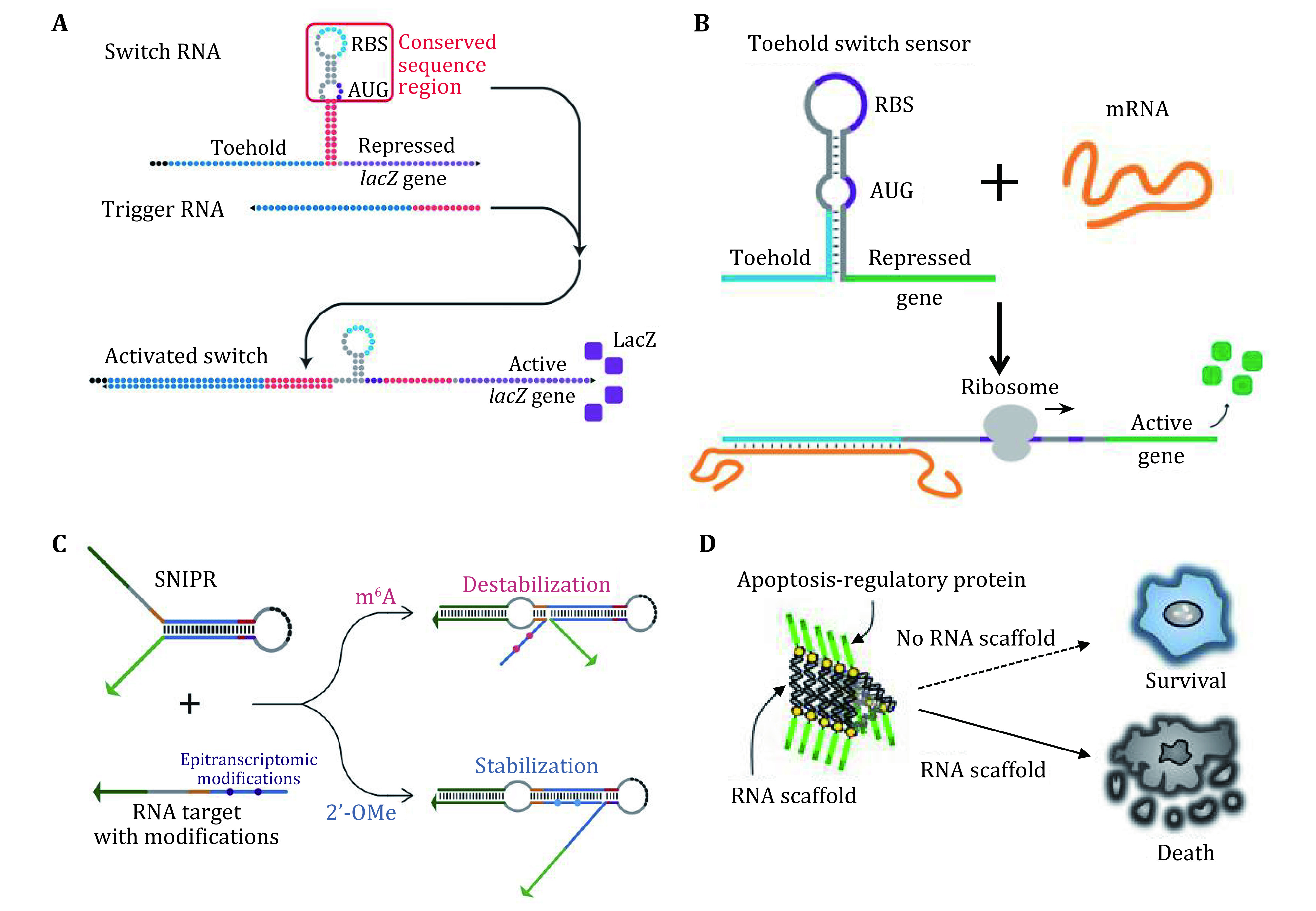
A Zika virus toehold switch sensor. The target RNA from the Zika virus can trigger the reconfiguration of the switch RNA and activate the expression of the reporter gene lacZ. Adapted from Pardee et al. (2016). B Schematic of toxin mRNA toehold switch sensor function. Adapted from Takahashi et al. (2018). C Principle of SNIPR for detection of epitranscriptomic marks, which can identify epigenetically modified nucleobases in target RNAs. Adapted from Hong et al. (2020). D An RNP nanomachine for inducing tumor cell apoptosis by oligomerization of apoptosis regulatory proteins. Adapted from Shibata et al. (2017)
Nanomachines based on CRISPR-Cas systems have also been harnessed in molecular diagnosis. For example, the miRNA-induced Cas9-sgRNA activating system developed by Wang et al. (Wang et al. 2019) can be used for high-fidelity sensing of miRNA activity at cellular levels and monitoring of differentiation status of stem cells. CRISPR-Cas12 system presents indiscriminate ssDNA cleavage activity upon RNA-guided DNA binding (Chen et al. 2018). Thus, an ssDNA labeled with both a fluorescence dye and a quencher can be used as a reporter, which is cleaved by Cas12 and generates a fluorescent signal in the presence of the target DNA. This principle recently has been utilized for target-specific isothermal signal amplification in nucleic acid assays (e.g., detection of SARS-CoV-2 viral nucleic acids) (Broughton et al. 2020; Chen et al. 2018). Similarly, CRISPR-Cas13 mediates collateral cleavage of RNAs upon RNA-guided RNA recognition, which has also been harnessed for detection of pathogenic nucleic acids (e.g., nucleic acids from Zika virus, dengue virus, or other pathogenic bacteria) (Gootenberg et al. 2017; Myhrvold et al. 2018).
Therapeutics
Nanomachines based on reconfigurable RNA structures have also shown potential in biomolecular therapeutics. For instance, RNA nanostructures can be used to incorporate RNA aptamers targeting tumor markers (e.g., human epidermal growth factor receptor, or EGFR) and therapeutic RNAs such as small interfering RNAs (siRNAs) and anti-miRNAs, enabling cancer-targeted therapeutics (Jasinski et al. 2017).
Controllable CRISPR-Cas9 nanomachines have also shown potential in therapeutics. Recently, a CRISPR-Cas9 system incorporating riboswitches has been used to simultaneously regulate multiple genes in an oncogenic pathway, allowing reprogramming of the fate of cancer cells (Liu et al. 2016b), which has implications for cancer therapeutics.
More recently, a type of protein-driven RNA nanostructured devices has been constructed, which can be activated in vitro by RNA-binding-protein-inducible structural change, which can control the assembly and oligomerization of apoptosis-regulatory proteins via specific RNA–protein interactions, leading to selective killing of target cells (Shibata et al. 2017) (Fig. 6D).
CONCLUSIONS AND PERSPECTIVES
In summary, we have reviewed the development of structurally reconfigurable RNA structures and nanomachines empowered by them. These studies show that by rationally programming RNA sequences, RNA structures with tailored thermodynamic and structural properties can be obtained, along with prescribed functionality (Chakraborty et al. 2014). Especially, reconfigurable RNA modules, which possess fast response kinetics to biological and environmental stimuli, can be employed in nanomachines to sense signals including nucleic acids, proteins metabolites, or other small molecules, and carry out specific tasks correspondingly.
Despite such progress, there remain challenges for the transition of the engineered RNA nanomachines into practical theranostic applications. First, compared to many inorganic or polymeric materials, RNA molecules are highly degradable due to ubiquitous RNases in practical environments, leading to very short lifetimes of the nanomachines and constrained application scenarios. Thus, in-situ fabrication of RNA nanomachines in living cells is highly desired, which allows them to be continuously produced utilizing the transcriptional machinery of the hosts. This approach also ensures that they are recycled rapidly after use, thus minimizes the biosafety risks resulted from long-term accumulation. On the other hand, the stability of RNA structures can be largely improved by introducing chemical modifications (Jasinski et al. 2017) or non-natural nucleotides, structural optimization (Guo et al. 2020; Khisamutdinov et al. 2014), and incorporation with protective materials.
Second, the complexity of de-novo designed RNA nanostructures is so far quite limited compared to DNA nanostructures (e.g., DNA origami structures) (Geary et al. 2014; Grabow and Jaeger 2014; Han et al. 2017; Li et al. 2018). For nucleic acid self-assembly, the target structures are designed to be thermodynamically stable, but often not kinetically favored. The folding and assembly of complex RNA structures are more likely to be kinetically trapped, resulting in incorrect products. This could be attributed to that the base-pairing of RNA is stronger than that of DNA. Therefore, it is highly desired to develop easy-to-use designing principles and tools for RNA nanostructures, which can well avoid kinetic traps and allow more complex RNA nanostructures (Geary et al. 2014; Grabow and Jaeger 2014; Han et al. 2017; Li et al. 2018).
The combination with artificial evolution strategies (e.g., SELEX) may help construct more complicated RNA systems with optimized performances. However, the efficiency of traditional evolution techniques is poor. It often takes years to find a new RNA structure with the desired functionality. Therefore, there is a need to develop high-throughput yet high-speed selection strategies for evolving input-output responses and dynamic functions.
Taken together, we envision that in near future, the development of cutting-edge technologies in diverse fields would largely enrich the catalog of designer RNA structures, which enable novel nanomachines with functions that are robust, scalable, and applicable to a wide range of living organisms.
Conflict of interest
Kai Jiao, Yaya Hao, Fei Wang, Lihua Wang, Chunhai Fan and Jiang Li declare that they have no conflict of interest.
Acknowledgements
This work was supported by the National Key Research and Development Program (2020YFA0908900), the National Natural Science Foundation of China (21775157, 21775104, 11705270, 21834007), the Shanghai Municipal Science and Technology Commission (19JC1410300), the LU JIAXI International team program supported by the K.C. Wong Education Foundation, the National Major Special Project for the Development of Transgenic Organisms (2018ZX08011-04B), the Open Large Infrastructure Research and the Youth Innovation Promotion Association of CAS (2012205, 2016236).
Compliance with Ethical Standards
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Chunhai Fan, Email: fanchunhai@sjtu.edu.cn.
Jiang Li, Email: lijiang@zjlab.org.cn.
References
- Abbink TEM, Ooms M, Haasnoot PCJ, Berkhout B The HIV-1 leader RNA conformational switch regulates RNA dimerization but does not regulate mRNA translation. Biochemistry. 2005;44(25):9058–9066. doi: 10.1021/bi0502588. [DOI] [PubMed] [Google Scholar]
- Afonin KA, Viard M, Koyfman AY, Martins AN, Kasprzak WK, Panigaj M, Desai R, Santhanam A, Grabow WW, Jaeger L, Heldman E, Reiser J, Chiu W, Freed EO, Shapiro BA Multifunctional RNA nanoparticles. Nano Lett. 2014;14(10):5662–5671. doi: 10.1021/nl502385k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agapakis CM, Silver PA Synthetic biology: exploring and exploiting genetic modularity through the design of novel biological networks. Mol Biosyst. 2009;5(7):704–713. doi: 10.1039/b901484e. [DOI] [PubMed] [Google Scholar]
- Bailor MH, Sun XY, Al-Hashimi HM Topology links RNA secondary structure with global conformation, dynamics, and adaptation. Science. 2010;327(5962):202–206. doi: 10.1126/science.1181085. [DOI] [PubMed] [Google Scholar]
- Batey RT, Gilbert SD, Montange RK Structure of a natural guanine-responsive riboswitch complexed with the metabolite hypoxanthine. Nature. 2004;432(7015):411–415. doi: 10.1038/nature03037. [DOI] [PubMed] [Google Scholar]
- Bayer TS, Smolke CD Programmable ligand-controlled riboregulators of eukaryotic gene expression. Nat Biotechnol. 2005;23(3):337–343. doi: 10.1038/nbt1069. [DOI] [PubMed] [Google Scholar]
- Beilstein K, Wittmann A, Grez M, Suess B Conditional control of mammalian gene expression by tetracycline-dependent hammerhead ribozymes. ACS Synth Biol. 2015;4(5):526–534. doi: 10.1021/sb500270h. [DOI] [PubMed] [Google Scholar]
- Birikh KR, Heaton PA, Eckstein F The structure, function and application of the hammerhead ribozyme. Eur J Biochem. 1997;245(1):1–16. doi: 10.1111/j.1432-1033.1997.t01-3-00001.x. [DOI] [PubMed] [Google Scholar]
- Broughton JP, Deng XD, Yu GX, Fasching CL, Servellita V, Singh J, Miao X, Streithorst JA, Granados A, Sotomayor-Gonzalez A, Zorn K, Gopez A, Hsu E, Gu W, Miller S, Pan CY, Guevara H, Wadford DA, Chen JS, Chiu CY CRISPR-Cas12-based detection of SARS-CoV-2. Nat Biotechnol. 2020;38(7):870–U854. doi: 10.1038/s41587-020-0513-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callura JM, Cantor CR, Collins JJ Genetic switchboard for synthetic biology applications. Proc Natl Acad Sci USA. 2012;109(15):5850–5855. doi: 10.1073/pnas.1203808109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty K, Veetil AT, Jaffrey SR, Krishnan Y Nucleic acid-based nanodevices in biological imaging. Ann Rev Biochem. 2016;85(1):349–373. doi: 10.1146/annurev-biochem-060815-014244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty S, Mehtab S, Krishnan Y The predictive power of synthetic nucleic acid technologies in RNA biology. Acc Chem Res. 2014;47(6):1710–1719. doi: 10.1021/ar400323d. [DOI] [PubMed] [Google Scholar]
- Chan K, Ng TB In-vitro nanodiagnostic platform through nanoparticles and DNA–RNA nanotechnology. Appl Microbiol Biotechnol. 2015;99(8):3359–3374. doi: 10.1007/s00253-015-6506-4. [DOI] [PubMed] [Google Scholar]
- Chappell J, Takahashi MK, Lucks JB Creating small transcription activating RNAs. Nat Chem Biol. 2015;11(3):214–U165. doi: 10.1038/nchembio.1737. [DOI] [PubMed] [Google Scholar]
- Chen JS, Ma EB, Harrington LB, Da Costa M, Tian XR, Palefsky JM, Doudna JA CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science. 2018;360(6387):436–439. doi: 10.1126/science.aar6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel R, Rubens JR, Sarpeshkar R, Lu TK Synthetic analog computation in living cells. Nature. 2013;497(7451):619–623. doi: 10.1038/nature12148. [DOI] [PubMed] [Google Scholar]
- Darmostuk M, Rimpelova S, Gbelcova H, Ruml T Current approaches in SELEX: an update to aptamer selection technology. Biotechnol Adv. 2015;33(6):1141–1161. doi: 10.1016/j.biotechadv.2015.02.008. [DOI] [PubMed] [Google Scholar]
- Delebecque CJ, Lindner AB, Silver PA, Aldaye FA Organization of intracellular reactions with rationally designed RNA assemblies. Science. 2011;333(6041):470–474. doi: 10.1126/science.1206938. [DOI] [PubMed] [Google Scholar]
- Durbin JK, Miller DK, Niekamp J, Khisamutdinov EF Modulating immune response with nucleic acid nanoparticles. Molecules. 2019;24(20) doi: 10.3390/molecules24203740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwidar M, Seike Y, Kobori S, Whitaker C, Matsuura T, Yokobayashi Y Programmable artificial cells using histamine-responsive synthetic riboswitch. J Am Chem Soc. 2019;141(28):11103–11114. doi: 10.1021/jacs.9b03300. [DOI] [PubMed] [Google Scholar]
- Ellington AD, Szostak JW In vitro selection of RNA molecules that bind specific ligands. Nature. 1990;346(6287):818–822. doi: 10.1038/346818a0. [DOI] [PubMed] [Google Scholar]
- Gasiunas G, Barrangou R, Horvath P, Siksnys V Cas9-crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proc Natl Acad Sci USA. 2012;109(39):E2579–E2586. doi: 10.1073/pnas.1208507109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge ZL, Gu HZ, Li Q, Fan CH Concept and development of framework nucleic acids. J Am Chem Soc. 2018;140(51):17808–17819. doi: 10.1021/jacs.8b10529. [DOI] [PubMed] [Google Scholar]
- Geary C, Rothemund PWK, Andersen ES A single-stranded architecture for cotranscriptional folding of RNA nanostructures. Science. 2014;345(6198):799–804. doi: 10.1126/science.1253920. [DOI] [PubMed] [Google Scholar]
- Gootenberg JS, Abudayyeh OO, Lee JW, Essletzbichler P, Dy AJ, Joung J, Verdine V, Donghia N, Daringer NM, Freije CA, Myhrvold C, Bhattacharyya RP, Livny J, Regev A, Koonin EV, Hung DT, Sabeti PC, Collins JJ, Zhang F Nucleic acid detection with CRISPR-Cas13a/C2c2. Science. 2017;356(6336):438–442. doi: 10.1126/science.aam9321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabow WW, Jaeger L RNA self-assembly and RNA nanotechnology. Acc Chem Res. 2014;47(6):1871–1880. doi: 10.1021/ar500076k. [DOI] [PubMed] [Google Scholar]
- Green AA, Kim JM, Ma D, Ilver PAS, Collins JJ, Yin P Complex cellular logic computation using ribocomputing devices. Nature. 2017;548(7665):117–121. doi: 10.1038/nature23271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AA, Silver PA, Collins JJ, Yin P Toehold switches: de-novo-designed regulators of gene expression. Cell. 2014;159(4):925–939. doi: 10.1016/j.cell.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greider CW, Blackburn EH The telomere terminal transferase of tetrahymena is a ribonucleoprotein enzyme with 2 kinds of primer specificity. Cell. 1987;51(6):887–898. doi: 10.1016/0092-8674(87)90576-9. [DOI] [PubMed] [Google Scholar]
- Guo PX The emerging field of RNA nanotechnology. Nat Nanotech. 2010;5(12):833–842. doi: 10.1038/nnano.2010.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Vieweger M, Zhang K, Yin H, Wang H, Li X, Li S, Hu S, Sparreboom A, Evers BM, Dong Y, Chiu W, Guo P Ultra-thermostable RNA nanoparticles for solubilizing and high-yield loading of paclitaxel for breast cancer therapy. Nat Commun. 2020;11(1):972. doi: 10.1038/s41467-020-14780-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D, Qi X, Myhrvold C, Wang B, Dai M, Jiang S, Bates M, Liu Y, An B, Zhang F, Yan H, Yin P Single-stranded DNA and RNA origami. Science. 2017;358(6369):eaao2648. doi: 10.1126/science.aao2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao CH, Li X, Tian C, Jiang W, Wang GS, Mao CD Construction of RNA nanocages by re-engineering the packaging RNA of Phi29 bacteriophage. Nat Commun. 2014;5:3890. doi: 10.1038/ncomms4890. [DOI] [PubMed] [Google Scholar]
- Hao Y, Li J, Li Q, Zhang L, Shi JY, Zhang X, Aldalbahi A, Wang L, Fan C, Wang F Programmable live-cell CRISPR imaging with toehold-switch-mediated strand displacement. Angew Chem Int Ed Engl. 2020;59(46):20612–20618. doi: 10.1002/anie.202009062. [DOI] [PubMed] [Google Scholar]
- Hong F, Ma D, Wu K, Mina LA, Luiten RC, Liu Y, Yan H, Green AA Precise and programmable detection of mutations using ultraspecific riboregulators. Cell. 2020;180(5):1018–1032 e1016. doi: 10.1016/j.cell.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu QQ, Li H, Wang LH, Gu HZ, Fan CH DNA nanotechnology-enabled drug delivery systems. Chem Rev. 2019;119(10):6459–6506. doi: 10.1021/acs.chemrev.7b00663. [DOI] [PubMed] [Google Scholar]
- Isaacs FJ, Dwyer DJ, Collins JJ RNA synthetic biology. Nat Biotechnol. 2006;24(5):545–554. doi: 10.1038/nbt1208. [DOI] [PubMed] [Google Scholar]
- Ishikawa J, Furuta H, Ikawa Y RNA tectonics (tectoRNA) for RNA nanostructure design and its application in synthetic biology. Wiley Interdiscip Rev-RNA. 2013;4(6):651–664. doi: 10.1002/wrna.1185. [DOI] [PubMed] [Google Scholar]
- Jang M, Kim JH, Nam HY, Kwon IC, Ahn HJ Design of a platform technology for systemic delivery of siRNA to tumours using rolling circle transcription. Nat Commun. 2015;6:7930. doi: 10.1038/ncomms8930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinski D, Haque F, Binzel DW, Guo PX Advancement of the emerging field of RNA nanotechnology. Acs Nano. 2017;11(2):1142–1164. doi: 10.1021/acsnano.6b05737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepsen MDE, Sparvath SM, Nielsen TB, Langvad AH, Grossi G, Gothelf KV, Andersen ES Development of a genetically encodable FRET system using fluorescent RNA aptamers. Nat Commun. 2018;9(1):18. doi: 10.1038/s41467-017-02435-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao K, Zhu B, Guo L, Zhou H, Wang F, Zhang X, Shi J, Li Q, Wang L, Li J, Fan C Programming switchable transcription of topologically constrained DNA. J Am Chem Soc. 2020;142(24):10739–10746. doi: 10.1021/jacs.0c01962. [DOI] [PubMed] [Google Scholar]
- Jin MK, de Loubresse NG, Kim Y, Kim J, Yin P Programmable CRISPR-Cas repression, activation, and computation with sequence-independent targets and triggers. ACS Synth Biol. 2019;8(7):1583–1589. doi: 10.1021/acssynbio.9b00141. [DOI] [PubMed] [Google Scholar]
- Kawazoe N, Teramoto N, Ichinari H, Imanishi Y, Ito Y In vitro selection of nonnatural ribozyme-catalyzing porphyrin metalation. Biomacromolecules. 2001;2(3):681–686. doi: 10.1021/bm000148a. [DOI] [PubMed] [Google Scholar]
- Kedde M, van Kouwenhove M, Zwart W, Vrielink JAFO, Elkon R, Agami R A Pumilio-induced RNA structure switch in p27-3' UTR controls miR-221 and miR-222 accessibility. Nat Cell Biol. 2010;12(10):1014–1020. doi: 10.1038/ncb2105. [DOI] [PubMed] [Google Scholar]
- Khaled A, Guo SC, Li F, Guo PX Controllable self-assembly of nanoparticles for specific delivery of multiple therapeutic molecules to cancer cells using RNA nanotechnology. Nano Lett. 2005;5(9):1797–1808. doi: 10.1021/nl051264s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AU Ribozyme: a clinical tool. Clin Chim Acta. 2006;367(1-2):20–27. doi: 10.1016/j.cca.2005.11.023. [DOI] [PubMed] [Google Scholar]
- Khisamutdinov EF, Jasinski DL, Guo P RNA as a boiling-resistant anionic polymer material to build robust structures with defined shape and stoichiometry. ACS Nano. 2014;8(5):4771–4781. doi: 10.1021/nn5006254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Zhou Y, Carlson PD, Teichmann M, Chaudhary S, Simmel FC, Silver PA, Collins JJ, Lucks JB, Yin P, Green AA De novo-designed translation-repressing riboregulators for multi-input cellular logic. Nat Chem Biol. 2019;15(12):1173–1182. doi: 10.1038/s41589-019-0388-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan Y, Bathe M Designer nucleic acids to probe and program the cell. Trends Cell Biol. 2012;22(12):624–633. doi: 10.1016/j.tcb.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Lau MWL, Ferre-D'Amare AR In vitro evolution of coenzyme-independent variants from the glmS ribozyme structural scaffold. Methods. 2016;106:76–81. doi: 10.1016/j.ymeth.2016.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JB, Hong J, Bonner DK, Poon Z, Hammond PT Self-assembled RNA interference microsponges for efficient siRNA delivery. Nat Mater. 2012;11(4):316–322. doi: 10.1038/nmat3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Zhang KM, Pi FM, Guo SJ, Shlyakhtenko L, Chiu W, Shu D, Guo PX Controllable self-assembly of RNA tetrahedrons with precise shape and size for cancer targeting. Adv Mater. 2016;28(34):7501–7507. doi: 10.1002/adma.201601976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Green AA, Yan H, Fan C Engineering nucleic acid structures for programmable molecular circuitry and intracellular biocomputation. Nat Chem. 2017;9(11):1056–1067. doi: 10.1038/nchem.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Zheng M, Wu S, Tian C, Liu D, Weizmann Y, Jiang W, Wang G, Mao C In vivo production of RNA nanostructures via programmed folding of single-stranded RNAs. Nat Commun. 2018;9(1):2196. doi: 10.1038/s41467-018-04652-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YL, Vergne J, Torchet C, Maurel MC In vitro selection of adenine-dependent ribozyme against Tpl2/Cot oncogene. Febs J. 2009;276(1):303–314. doi: 10.1111/j.1742-4658.2008.06780.x. [DOI] [PubMed] [Google Scholar]
- Liu SR, Hu CG, Zhang JZ Regulatory effects of cotranscriptional RNA structure formation and transitions. Wiley Interdiscip Rev-RNA. 2016a;7(5):562–574. doi: 10.1002/wrna.1350. [DOI] [PubMed] [Google Scholar]
- Liu XG, Zhang F, Jing XX, Pan MC, Liu P, Li W, Zhu BW, Li J, Chen H, Wang LH, Lin JP, Liu Y, Zhao DY, Yan H, Fan CH Complex silica composite nanomaterials templated with DNA origami. Nature. 2018;559(7715):593–598. doi: 10.1038/s41586-018-0332-7. [DOI] [PubMed] [Google Scholar]
- Liu YC, Zhan YH, Chen ZC, He AB, Li JF, Wu HW, Liu L, Zhuang CL, Lin JH, Guo XQ, Zhang QX, Huang WR, Cai ZM Directing cellular information flow via CRISPR signal conductors. Nat Meth. 2016b;13(11):938–944. doi: 10.1038/nmeth.3994. [DOI] [PubMed] [Google Scholar]
- Ma SY, Tang N, Tian JD DNA synthesis, assembly and applications in synthetic biology. Curr Opin Chem Biol. 2012;16(3-4):260–267. doi: 10.1016/j.cbpa.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T, Reed R The role of small nuclear ribonucleoprotein-particles in pre-messenger-RNA splicing. Nature. 1987;325(6106):673–678. doi: 10.1038/325673a0. [DOI] [PubMed] [Google Scholar]
- Meyer S, Chappell J, Sankar S, Chew R, Lucks JB Improving fold activation of small transcription activating RNAs (STARs) with rational RNA engineering strategies. Biotechnol Bioeng. 2016;113(1):216–225. doi: 10.1002/bit.25693. [DOI] [PubMed] [Google Scholar]
- Munzar JD, Ng A, Juncker D Duplexed aptamers: history, design, theory, and application to biosensing. Chem Soc Rev. 2019;48(5):1390–1419. doi: 10.1039/C8CS00880A. [DOI] [PubMed] [Google Scholar]
- Myhrvold C, Freije CA, Gootenberg JS, Abudayyeh OO, Metsky HC, Durbin AF, Kellner MJ, Tan AL, Paul LM, Parham LA, Garcia KF, Barnes KG, Chak B, Mondini A, Nogueira ML, Isern S, Michael SF, Lorenzana I, Yozwiak NL, MacInnis BL, Bosch I, Gehrke L, Zhang F, Sabeti PC Field-deployable viral diagnostics using CRISPR-Cas13. Science. 2018;360(6387):444–448. doi: 10.1126/science.aas8836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubacher S, Hennig S RNA structure and cellular applications of fluorescent light-up aptamers. Angew Chem Int Ed Engl. 2019;58(5):1266–1279. doi: 10.1002/anie.201806482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudler E, Mironov AS The riboswitch control of bacterial metabolism. Trends Biochem Sci. 2004;29(1):11–17. doi: 10.1016/j.tibs.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Oesinghaus L, Simme FC Switching the activity of Cas12a using guide RNA strand displacement circuits. Nat Commun. 2019;10(1):2092. doi: 10.1038/s41467-019-09953-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa A, Maeda M An artificial aptazyme-based riboswitch and its cascading system in E. coli. Chembiochem. 2008;9(2):206–209. doi: 10.1002/cbic.200700478. [DOI] [PubMed] [Google Scholar]
- Ohno H, Kobayashi T, Kabata R, Endo K, Iwasa T, Yoshimura SH, Takeyasu K, Inoue T, Saito H Synthetic RNA-protein complex shaped like an equilateral triangle. Nat Nanotech. 2011;6(2):115–119. doi: 10.1038/nnano.2010.268. [DOI] [PubMed] [Google Scholar]
- Omabegho T, Gurel PS, Cheng CY, Kim LY, Ruijgrok PV, Das R, Alushin GM, Bryant Z Controllable molecular motors engineered from myosin and RNA. Nat Nanotechnol. 2017;13(1):34–40. doi: 10.1038/s41565-017-0005-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osada E, Suzuki Y, Hidaka K, Ohno H, Sugiyama H, Endo M, Saito H Engineering RNA-protein complexes with different shapes for imaging and therapeutic applications. ACS Nano. 2014;8(8):8130–8140. doi: 10.1021/nn502253c. [DOI] [PubMed] [Google Scholar]
- Ozer A, Pagano JM, Lis JT New technologies provide quantum changes in the scale, speed, and success of SELEX methods and aptamer characterization. Mol TherNucleic Acids. 2014;3(8):e183. doi: 10.1038/mtna.2014.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paige JS, Nguyen-Duc T, Song W, Jaffrey SR Fluorescence imaging of cellular metabolites with RNA. Science. 2012;335(6073):1194. doi: 10.1126/science.1218298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee K, Green AA, Ferrante T, Cameron DE, DaleyKeyser A, Yin P, Collins JJ Paper-based synthetic gene networks. Cell. 2014;159(4):940–954. doi: 10.1016/j.cell.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee K, Green AA, Takahashi MK, Braff D, Lambert G, Lee JW, Ferrante T, Ma D, Donghia N, Fan M, Daringer NM, Bosch I, Dudley DM, O'Connor DH, Gehrke L, Collins JJ Rapid, low-cost detection of Zika virus using programmable biomolecular components. Cell. 2016;165(5):1255–1266. doi: 10.1016/j.cell.2016.04.059. [DOI] [PubMed] [Google Scholar]
- Pothoulakis G, Ceroni F, Reeve B, Ellis T The spinach RNA aptamer as a characterization tool for synthetic biology. ACS Synth Biol. 2014;3(3):182–187. doi: 10.1021/sb400089c. [DOI] [PubMed] [Google Scholar]
- Purnick PEM, Weiss R The second wave of synthetic biology: from modules to systems. Nat Rev Mol Cell Biol. 2009;10(6):410–422. doi: 10.1038/nrm2698. [DOI] [PubMed] [Google Scholar]
- Qiu M, Khisamutdinov E, Zhao Z, Pan C, Choi J-W, Leontis NB, Guo P RNA nanotechnology for computer design and in vivo computation. Philos Trans AMath Phys Eng Sci. 2013;371(2000):20120310. doi: 10.1098/rsta.2012.0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray PS, Jia J, Yao P, Majumder M, Hatzoglou M, Fox PL A stress-responsive RNA switch regulates VEGFA expression. Nature. 2009;457(7231):915–919. doi: 10.1038/nature07598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regev A, Shapiro E Cellular abstractions: cells as computation. Nature. 2002;419(6905):343–343. doi: 10.1038/419343a. [DOI] [PubMed] [Google Scholar]
- Saper G, Hess H Synthetic systems powered by biological molecular motors. Chem Rev. 2020;120(1):288–309. doi: 10.1021/acs.chemrev.9b00249. [DOI] [PubMed] [Google Scholar]
- Shapiro BA, Bindewald E, Kasprzak W, Yingling Y Protocols for the in silico design of RNA nanostructures. Methods Mol Biol. 2008;474:93–115. doi: 10.1007/978-1-59745-480-3_7. [DOI] [PubMed] [Google Scholar]
- Shi Y-Z, Wu Y-Y, Wang F-H, Tan Z-J RNA structure prediction: progress and perspective. ChinesePhys B. 2014;23(7):078701. doi: 10.1088/1674-1056/23/7/078701. [DOI] [Google Scholar]
- Shibata T, Fujita Y, Ohno H, Suzuki Y, Hayashi K, Komatsu KR, Kawasaki S, Hidaka K, Yonehara S, Sugiyama H, Endo M, Saito H Protein-driven RNA nanostructured devices that function in vitro and control mammalian cell fate. Nat Commun. 2017;8(1):540. doi: 10.1038/s41467-017-00459-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu D, Li H, Shu Y, Xiong GF, Carson WE, Haque F, Xu R, Guo PX Systemic delivery of anti-miRNA for suppression of triple negative breast cancer utilizing RNA nanotechnology. Acs Nano. 2015;9(10):9731–9740. doi: 10.1021/acsnano.5b02471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu D, Shu Y, Haque F, Abdelmawla S, Guo P Thermodynamically stable RNA three-way junction for constructing multifunctional nanoparticles for delivery of therapeutics. Nat Nano. 2011;6(10):658–667. doi: 10.1038/nnano.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigel RKO, Pyle AM Alternative roles for metal ions in enzyme catalysis and the implications for ribozyme chemistry. Chem Rev. 2007;107(1):97–113. doi: 10.1021/cr0502605. [DOI] [PubMed] [Google Scholar]
- Simmel FC, Yurke B, Singh HR Principles and applications of nucleic acid strand displacement reactions. Chem Rev. 2019;119(10):6326–6369. doi: 10.1021/acs.chemrev.8b00580. [DOI] [PubMed] [Google Scholar]
- Slomovic S, Pardee K, Collins JJ Synthetic biology devices for in vitro and in vivo diagnostics. Proc Natl Acad Sci US A. 2015;112(47):14429–14435. doi: 10.1073/pnas.1508521112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song WJ, Strack RL, Svensen N, Jaffrey SR Plug-and-play fluorophores extend the spectral properties of spinach. J Am Chem Soc. 2014;136(4):1198–1201. doi: 10.1021/ja410819x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi MK, Tan X, Dy AJ, Braff D, Akana RT, Furuta Y, Donghia N, Ananthakrishnan A, Collins JJ A low-cost paper-based synthetic biology platform for analyzing gut microbiota and host biomarkers. Nat Commun. 2018;9(1):3347. doi: 10.1038/s41467-018-05864-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang WX, Hu JH, Liu DR Aptazyme-embedded guide RNAs enable ligand-responsive genome editing and transcriptional activation. Nat Commun. 2017;8:15939. doi: 10.1038/ncomms15939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thavarajah W, Silverman AD, Verosloff MS, Kelley-Loughnane N, Jewett MC, Lucks JB Point-of-use detection of environmental fluoride via a cell-free riboswitch-based biosensor. Acs Synth Biol. 2020;9(1):10–18. doi: 10.1021/acssynbio.9b00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno K, Sakai Y, Shono C, Sakamoto I, Tsukakoshi K, Hihara Y, Sode K, Ikebukuro K Applying a riboregulator as a new chromosomal gene regulation tool for higher glycogen production in Synechocystis sp. PCC 6803. Appl Microbiol Biotechnol. 2017;101(23-24):8465–8474. doi: 10.1007/s00253-017-8570-4. [DOI] [PubMed] [Google Scholar]
- Ueno K, Tsukakoshi K, Ikebukuro K Riboregulator elements as tools to engineer gene expression in cyanobacteria. Appl Microbiol Biotechnol. 2018;102(18):7717–7723. doi: 10.1007/s00253-018-9221-0. [DOI] [PubMed] [Google Scholar]
- Wang F, Lv H, Li Q, Li J, Zhang XL, Shi JY, Wang LH, Fan CH Implementing digital computing with DNA-based switching circuits. Nat Commun. 2020;11(1):121. doi: 10.1038/s41467-019-13980-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wang H, Yang L, Lv L, Zhang Z, Ren B, Dong L, Li N A novel riboregulator switch system of gene expression for enhanced microbial production of succinic acid. J Ind Microbiol Biotechnol. 2018a;45(4):253–269. doi: 10.1007/s10295-018-2019-3. [DOI] [PubMed] [Google Scholar]
- Wang XW, Hu LF, Hao J, Liao LQ, Chiu YZ, Shi M, Wang YM A microRNA-inducible CRISPR-Cas9 platform serves as a microRNA sensor and cell-type-specific genome regulation tool. Nat Cell Biol. 2019;21(4):522–530. doi: 10.1038/s41556-019-0292-7. [DOI] [PubMed] [Google Scholar]
- Wang ZJ, Luo Y, Xie XD, Hu XJ, Song HY, Zhao Y, Shi JY, Wang LH, Glinsky G, Chen N, Lal R, Fan CH In situ spatial complementation of aptamer-mediated recognition enables live-cell imaging of native RNA transcripts in real time. Angew Chem Int Ed Engl. 2018b;57(4):972–976. doi: 10.1002/anie.201707795. [DOI] [PubMed] [Google Scholar]
- Winkler W, Nahvi A, Breaker RR Thiamine derivatives bind messenger RNAs directly to regulate bacterial gene expression. Nature. 2002;419(6910):952–956. doi: 10.1038/nature01145. [DOI] [PubMed] [Google Scholar]
- Winkler WC, Breaker RR Regulation of bacterial gene expression by riboswitches. Ann Rev Microbiol. 2005;59:487–517. doi: 10.1146/annurev.micro.59.030804.121336. [DOI] [PubMed] [Google Scholar]
- Yu JW, Liu ZY, Jiang W, Wang GS, Mao CD De novo design of an RNA tile that self-assembles into a homo-octameric nanoprism. Nat Commun. 2015;6:5724. doi: 10.1038/ncomms6724. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Gu Z, Yao C, Luo D, Yang DY Nucleic acid-based functional nanomaterials as advanced cancer therapeutics. Small. 2019;15(26):e1900172. doi: 10.1002/smll.201900172. [DOI] [PubMed] [Google Scholar]
- Zalatan JG, Lee ME, Almeida R, Gilbert LA, Whitehead EH, La Russa M, Tsai JC, Weissman JS, Dueber JE, Qi LS, Lim WA Engineering complex synthetic transcriptional programs with CRISPR RNA scaffolds. Cell. 2015;160(1-2):339–350. doi: 10.1016/j.cell.2014.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu CS, Liu CY, Qiu XY, Xie SS, Li WY, Zhu LY, Zhu LY Novel nucleic acid detection strategies based on CRISPR-Cas systems: from construction to application. Biotechnol Bioeng. 2020;117(7):2279–2294. doi: 10.1002/bit.27334. [DOI] [PubMed] [Google Scholar]


