Abstract
Background
Reference blood pressure (BP) values for Japanese children based on a large number of measurements by auscultation have not yet been established.
Methods
This was a cross-sectional analysis of data from a birth-cohort study. The data from the sub-cohort study conducted for children at the age of 2 years in the Japan Environment and Children’s Study from April 2015 to January 2017 were analyzed. BP was measured via auscultation using an aneroid sphygmomanometer. Each participant was measured in triplicate, and the average value of two consecutive measurements with a difference of less than 5 mmHg was recorded. The reference BP values were estimated using the lambda–mu–sigma (LMS) method and compared with those obtained via the polynomial regression model.
Results
Data from 3361 participants were analyzed. Although the difference between the estimated BP values by the LMS and the polynomial regression model was small, the LMS model was more valid based on the results of the fit curve of the observed values and regression models for each model. For 2-year-old children with heights in the 50th percentile, the 50th, 90th, 95th, and 99th percentile reference values of systolic BP (mmHg) for boys were 91, 102, 106, and 112, and that for girls were 90, 101, 103, and 109, respectively, and those of diastolic BP for boys were 52, 62, 65, and 71, and that for girls were 52, 62, 65, and 71, respectively.
Conclusion
The reference BP values for 2-year-old Japanese children were determined based on auscultation and were made available.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10157-023-02370-w.
Keywords: Blood pressure, Reference value, Auscultation, Aneroid sphygmomanometer, LMS method, Polynomial regression model
Introduction
Children with hypertension (HT) account for approximately 3% of the pediatric population [1, 2], but some studies have shown an increase in the number of these individuals [3], which is of concern [4]. Previous reports have shown that childhood HT can cause target organ damage, such as cardiovascular disease [5] in adulthood [6] and may increase the risk of atherosclerosis [7]. However, some studies have reported that blood pressure (BP) reduction can reduce the risk of target organ damage [8, 9]. Therefore, BP control during childhood is essential.
Appropriate reference values are required for proper BP management; the following factors should be considered when designating reference values for BP: first, an auscultatory method should be used to measure BP. BP obtained via aneroid manometers is comparable to that via mercury sphygmomanometers, while SBP measured with oscillometric devices are slightly higher than that measured with mercury sphygmomanometers [10]. Although oscillometric devices are widely used in BP screening in children, HT should be diagnosed by auscultation using the reference values [11]. The reference values for BP in children that are widely used in the United States and Europe were established via auscultation [11, 12]. Second, the reference value for BP should be appropriate for each race or ethnicity. Racial differences reportedly occur in BP, even in children [2]. Previous reports have introduced auscultatory BP reference values for children in Western [13–15] and other Asian countries [16]; however, no such reference values have been identified for Japanese children. Currently, the most widely used BP reference values in Japan are those from the US [13]. However, their applicability in Japanese children is insufficiently verified. Therefore, it is necessary to establish auscultatory BP reference values for Japanese children.
The Japan Environment and Children’s Study (JECS) is a nation-wide birth cohort study to discern the effects of fetal and childhood environmental factors on the health and development of children [17, 18]. This study began in 2010 and will survey approximately 100,000 pregnant women and their offspring at 15 Regional Centers and 300 Co-operating health care providers across Japan and will continue until the children reach the age of thirteen. In addition to surveying participants, the JECS plans detailed surveys of approximately 5000 children, named the Sub-Cohort Study, including auscultatory BP measurement at the 15 Regional Centers at the ages of 2, 4, 6, 8, 10, and 12 years. To date, only the detailed survey for 2-year-olds has been completed.
This study establishes reference BP values from the Sub-Cohort Study for 2-year-old Japanese children using BP measurements obtained via the auscultatory method.
Methods
Study design, study participants, and data collection
This was a cross-sectional analysis of data from the Sub-Cohort Study. The details of the JECS main study and the Sub-Cohort Study have been described elsewhere [18, 19] (Supplementary Method). Of the 100,303 children in the JECS main study, 5015 participated in the Sub-Cohort Study. The participants who met the eligibility criteria of the Sub-Cohort Study were randomly recruited. The present study was based on the dataset “jecs-ta-20190930”, released in October 2019.
We excluded children of the Sub-Cohort Study with: (1) chronic diseases; (2) two or more missing BP values or a diastolic BP (DBP) value of 0 mmHg or not applicable; (3) unstable posture or condition for measuring BP, such as children unable to maintain a sitting or supine position, those crying or sleeping during measurement, or those with a fever; (4) unstable BP values with a difference of ≥ 5 mmHg for two consecutive BP measurements; (5) either missing covariates or outliers for height measurements; (6) children under 24 months of age.
BP measurement procedure and BP value selection
The standardized BP measurement in the Sub-Cohort Study is shown in Supplementary Table S1. This method complied with the American Academy of Pediatrics (AAP) guidelines [11], and the doctors or nurses took all BP measurements. Each participant was measured thrice to ensure stable measurement value according to the BP definition in previous major BP management guidelines [11, 12, 20, 21]. BP measuring postures and conditions are collected simultaneously. The Sub-Cohort Study used aneroid sphygmomanometers (DS66 DuraShockTM hand aneroid [Welch Allyn Inc, Syracuse, NY, USA]). Each sphygmomanometer was visually inspected every test day before the investigation, and the investigator repaired or replaced it as required. Although the accuracy guarantee by the manufacturer was ten years, JECS strictly operated them within the accuracy guarantee period and replaced them every 4 years.
We adopted the BP value when comparing the average of two consecutive measurements with a difference of < 5 mmHg. The difference among the three measurements was < 5 mmHg, and the average of the second and third BP values was adopted. Moreover, when the second BP measurement was missed, we adopted the average of the other two when the difference between the first and the third one was < 5 mmHg.
Covariates
Data on the sex, gestational weeks, birth weight, and diagnosis of chronic disease in the children were collected from the JECS main study. Diagnosis of chronic disease was defined based on medical record transcripts at birth and one month of age and parent-reported doctor-diagnosed disease until the questionnaires at 2 years of age. Chronic diseases included congenital heart disease, congenital renal and urinary disease, neurofibromatosis, tuberous sclerosis, and hypothyroidism. The height, weight, measurement season, and age of the children and BP were measured simultaneously. When any covariates were missed, we excluded the child from our analysis. Furthermore, we excluded children if the height deviated more or less than five standard deviations (SDs) from the Japanese standard growth chart [22].
Analytic plan
We excluded children with either missing or unstable BP measurements and covariates to estimate the standardized BP reference table. We then described the participants’ background based on gestational weeks, birth weight, height, and weight at BP measurement, measurement seasons, systolic BP (SBP), and DBP. The mean and SD were calculated for continuous variables, and the number of cases and proportion in the entire target population were calculated for categorical variables.
Next, we separated the SBP and DBP values and analyzed each BP using two methods for making BP reference values: the polynomial regression model and the lambda-mu-sigma (LMS) model. In the polynomial regression model, based on the previous study in the USA [13], the analysis was estimated by sex, age (years), and height Z score (Zht). However, in the present study, the age of the children was approximately 2 years. We estimated the analysis using only sex and Zht and excluded children < 24 months of age. Then, according to the previous study, we estimated the expected BP values using sex, Zht, squared Zht, cubed Zht, and quartet Zht as each SBP and DBP. The Zht was calculated based on standard Japanese data [22]. The observed fit curve, residual density, and normal Q–Q were plotted to confirm the fit of the estimated models.
Third, we estimated the reference BP values by the LMS method. The LMS model was developed for each sex with the height Z score as the explanatory variable in a generalized additive model using the LMS method [23]. The LMS method should indicate a more appropriate estimation when each BP distribution was not equal to each Zht. The parameters L, M, and S were set to Box–Cox power, median, and coefficient of variance, and the combination with the minimum Bayesian information criteria was selected for each equivalent degree of freedom. The observed fit curve, residual density, and normal Q–Q were plotted to confirm the fit of the estimated models.
Fourth, for making the reference tables, on applying the Zht, corresponding to the 5th, 10th, 25th, 50th, 75th, 90th, and 95th percentile points to SBP and DBP percentile curves, respectively, the expected BP values corresponding to each percentile point for the height and BP were obtained by each model. Both the polynomial regression and LMS models retrospectively estimated similar values for SBP and DBP and found similar trends in the observed fit curve, residual density, and normal Q–Q plot; however, the LMS model had a better fit of approximately two SD ranges of height graphically (Supplementary Figures S1 and S2). The coefficients of each model are listed in Supplementary Tables S2 and S3.
We used R statistical software, version 3.6.3 (The R Foundation for Statistical Computing, Vienna, Austria) for statistical analyses.
Results
Participants’ background
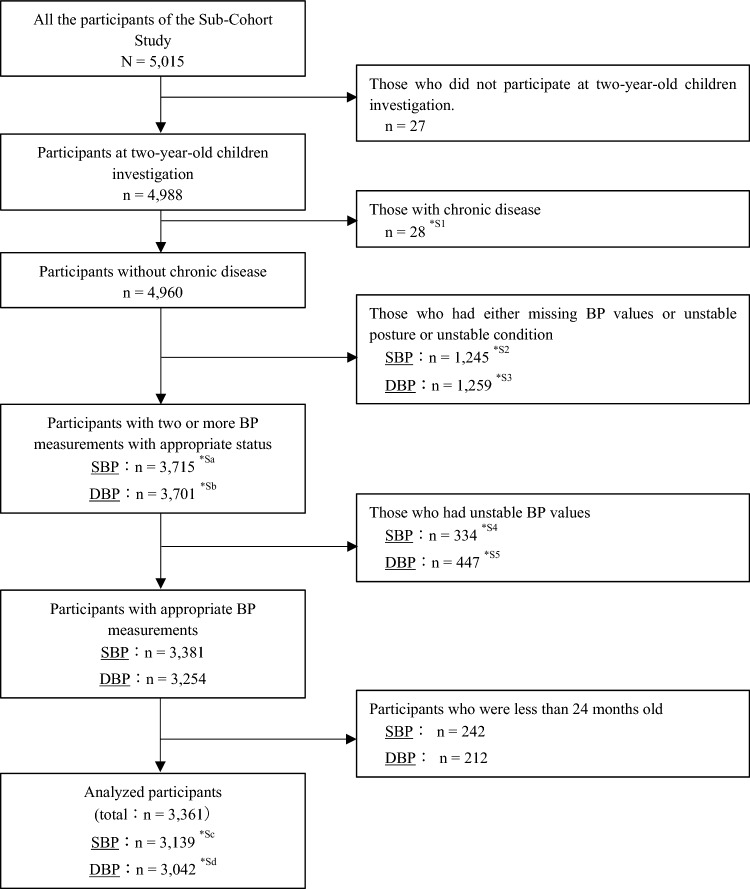
Of the 5,015 participants in the Sub-Cohort Study, 4988 were included in this study. The SBP and DBP of 3139 and 3042 participants (3361 in total) were adapted for our analysis. Among the excluded children, 28 had a chronic disease. In the SBP and DBP groups, 1256 and 1259 had either missing BP values or unstable measurement or unstable condition, 334 and 447 were measured with unstable BP values, and 242 and 212 were under 2 years of age, respectively (Fig. 1.) Detailed explanations for each factor are shown in Supplementary Table S4.
Fig. 1.
Flowcharts for selecting participants for SBP and DBP analysis. SBP systolic blood pressure, DBP diastolic blood pressure, BP blood pressure, *S1–*S5 the detailed contents and number of each item are shown in Supplementary Table S4; *Sa-*Sd the detailed breakdowns of each number are shown in Supplementary Table S4
Of those 3361 children, 1672 (49.7%) were boys and 144 (4.3%) had preterm birth. The winter measurements were less than those in the other seasons (Table 1 and Supplementary Table S5). Supplementary Table S6 lists the age in months at the time of the BP measurement.
Table 1.
Participants’ demographic background
| Name of regional center | n | Sex | Height | Weight | SBP | DBP | |||
|---|---|---|---|---|---|---|---|---|---|
| Male | (cm) | (kg) | Available | (mmHg) | Available | (mmHg) | |||
| Hokkaido | 207 | 90 (43.5%) | 83.9 (2.9) | 11.5 (1.2) | 195 (94.2%) | 88.5 (8.7) | 181 (87.4%) | 51.6 (7.8) | |
| Miyagi | 295 | 149 (50.5%) | 84.6 (2.9) | 11.8 (1.2) | 277 (93.9%) | 92.0 (6.4) | 265 (89.8%) | 52.8 (7.8) | |
| Fukushima | 404 | 198 (49.0%) | 84.1 (2.8) | 11.6 (1.1) | 375 (92.8%) | 95.4 (7.8) | 359 (88.9%) | 53.6 (8.7) | |
| Chiba | 158 | 65 (41.1%) | 83.7 (2.7) | 11.5 (1.2) | 141 (89.2%) | 93.1 (9.5) | 140 (88.6%) | 51.7 (8.6) | |
| Kanagawa | 184 | 88 (47.8%) | 84.1 (3.4) | 11.6 (1.4) | 172 (93.5%) | 93.0 (7.5) | 169 (91.8%) | 54.0 (7.5) | |
| Koshin | 264 | 135 (51.1%) | 83.7 (2.7) | 11.4 (1.1) | 249 (94.3%) | 88.4 (7.7) | 241 (91.3%) | 53.4 (6.2) | |
| Toyama | 203 | 112 (55.2%) | 84.8 (3.0) | 11.6 (1.2) | 172 (84.7%) | 88.2 (8.7) | 177 (87.2%) | 52.2 (8.0) | |
| Aichi | 177 | 83 (46.9%) | 83.9 (2.9) | 11.3 (1.1) | 161 (91.0%) | 86.7 (8.8) | 156 (88.1%) | 45.4 (8.0) | |
| Kyoto | 155 | 80 (51.6%) | 84.5 (3.0) | 11.8 (1.2) | 151 (97.4%) | 87.4 (7.1) | 142 (91.6%) | 48.4 (7.1) | |
| Osaka | 326 | 156 (47.9%) | 83.9 (3.1) | 11.4 (1.2) | 321 (98.5%) | 88.9 (5.8) | 320 (98.2%) | 47.5 (5.8) | |
| Hyogo | 201 | 122 (60.7%) | 84.1 (3.1) | 11.3 (1.2) | 197 (98.0%) | 91.3 (6.8) | 194 (96.5%) | 51.8 (6.6) | |
| Tottori | 98 | 44 (44.9%) | 83.6 (2.8) | 11.2 (1.0) | 93 (94.9%) | 82.1 (6.2) | 93 (94.9%) | 51.3 (5.5) | |
| Kochi | 223 | 114 (51.1%) | 83.5 (2.7) | 11.5 (1.1) | 200 (89.7%) | 92.9 (7.2) | 187 (83.9%) | 55.8 (7.7) | |
| Fukuoka | 277 | 140 (50.5%) | 83.8 (2.6) | 11.4 (1.2) | 262 (94.6%) | 88.9 (6.3) | 258 (93.1%) | 51.2 (6.8) | |
| Minamikyushu/Okinawa | 189 | 96 (50.8%) | 83.5 (2.7) | 11.5 (1.1) | 173 (91.5%) | 95.0 (8.8) | 160 (84.7%) | 52.9 (8.5) | |
| Total | 3361 | 1672 (49.7%) | 84.0 (2.9) | 11.5 (1.2) | 3139 (93.4%) | 90.6 (8.1) | 3042 (90.5%) | 51.7 (7.9) | |
Data are presented as mean (SD) or n (%)
SBP systolic blood pressure, DBP diastolic blood pressure
The estimated reference BP values
In Table 2, we described the estimated BP values by the LMS method. For 2-year-old children with heights in the 50th percentile, the 50th, 90th, 95th, and 99th percentile reference values of systolic BP (mmHg) for boys were 91, 102, 106, and 112, and that for girls were 90, 101, 103, and 109 for girls, respectively, and those of DBP for boys were 52, 62, 65, and 71, respectively, and 52, 62, 65, and 71 for girls, respectively. The estimated BP values by the polynomial regression model are shown in Supplementary Table S7.
Table 2.
Reference BP values by LMS method
| Age (year) | Sex | BP percentile | Systolic BP (mmHg) | Diastolic BP (mmHg) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Percentile of heighta | Percentile of heighta | |||||||||||||||
| 5th | 10th | 25th | 50th | 75th | 90th | 95th | 5th | 10th | 25th | 50th | 75th | 90th | 95th | |||
| 2 | Boys | 50th | 90 | 90 | 91 | 91 | 92 | 92 | 92 | 51 | 51 | 51 | 52 | 52 | 53 | 53 |
| 90th | 101 | 101 | 102 | 102 | 103 | 103 | 104 | 61 | 61 | 62 | 62 | 63 | 63 | 63 | ||
| 95th | 105 | 105 | 105 | 106 | 106 | 107 | 107 | 64 | 64 | 65 | 65 | 66 | 66 | 66 | ||
| 99th | 111 | 111 | 112 | 112 | 113 | 113 | 114 | 70 | 70 | 70 | 71 | 71 | 72 | 72 | ||
| Girls | 50th | 89 | 89 | 90 | 90 | 91 | 91 | 92 | 51 | 51 | 51 | 52 | 52 | 53 | 53 | |
| 90th | 99 | 99 | 100 | 101 | 101 | 102 | 102 | 61 | 61 | 62 | 62 | 63 | 63 | 64 | ||
| 95th | 102 | 102 | 103 | 103 | 104 | 105 | 105 | 64 | 64 | 65 | 65 | 66 | 66 | 67 | ||
| 99th | 107 | 108 | 108 | 109 | 110 | 110 | 111 | 69 | 70 | 70 | 71 | 72 | 72 | 73 | ||
BP blood pressure, LMS method lambda-mu-sigma method
aThe Z score height values converted to their corresponding percentile values
Discussion
In the present study, we created reference BP values for 2-year-old Japanese children based on a detailed survey of the JECS. These are the first measurements by auscultatory methods from a sufficient number of Japanese children nationwide.
Selecting an appropriate survey group is essential to create a reliable reference BP value. The appropriate reference BP value for each race or ethnic group must be created based on their respective data. Previous studies have shown that BP values differ between urban and rural areas [24] and vary based on temperature and season [25]. As shown in Table 1 and Supplementary Table S5, the detailed survey data of JECS were collected year-round at 15 Regional Centers across Japan. The participants of the detailed survey of the JECS were considered appropriate for creating reference BP values for Japanese children.
A proper BP measurement device is indispensable for BP measurements. We used aneroid sphygmomanometer to measure the BP. Applying an aneroid sphygmomanometer to measure BP was considered acceptable for the following reasons. First, the gold standard method for measuring BP is auscultation using a mercury sphygmomanometer, and the reference BP values for US children [11, 13], which are the most widely used values worldwide, are based on BP values measured by this method. However, since mercury sphygmomanometers can no longer be used due to their negative environmental impact, aneroid sphygmomanometers are widely used in clinical practice. Second, the difference between BP readings by an aneroid and mercury sphygmomanometer has been reported to be very small [26], and the use of either device is recommended by the AAP guidelines [11].
An appropriate measurement technique is critical for the BP measurements used to create reference BP values. The technique used in our study was based on the AAP guidelines [11] and was considered reliable. Additionally, to obtain appropriate BP values, it is important not only to calm the participants during measurements but also to select which measurement to use from multiple available measurements. Each participant was measured thrice, and the average value of two consecutive measurements with a difference of < 5 mmHg was adopted as the BP value. Comparing this selection method with those of previous major guidelines [11, 12, 20, 21] (Supplementary Table S8) our method was considered capable of selecting stable BP values similar to the other methods.
Differences in reference DBP values were observed between the present study and US guidelines [11]. Taking the 50th percentile reference BP values for 2-year-old children with heights in the 50th percentile as an example, the SBP (mmHg) for Japanese boys and girls were 91 and 90, and those for the US boys and girls were 89 and 89, respectively, which were almost the same in Japan and the USA. The DBP (mmHg) was 52 for both boys and girls in Japan. However, DBP for US boys and girls were 44 and 48, which were 8 and 4 mmHg lower, respectively, compared with Japanese children. Although the reference BP values created in the present study differed from those of the US guidelines, our reference BP values were sufficiently reliable because our reference BP values were measured and aggregated in a standardized and appropriate manner based on a sufficient number of Japanese children.
Differences were observed between the reference BP values obtained using aneroid manometers in this study and those using oscillometric devices in the previous study. In the report on the reference BP values of Japanese children using an automated BP recorder (Dinamap Model 8104), the 95th percentile BP values for 2-year-olds were 115/69 mmHg for boys and 121/70 mmHg for girls [27]. In the present study, the 95th percentile BP values for 2-year-olds at the 50th percentile height were 106/65 mmHg for boys and 103/65 mmHg for girls, with a large difference in SBP. These differences are possibly attributed to variations in BP measurement methods, devices, BP value selections, and study participants.
A reliable analysis model is required to create the reference BP values. We used LMS and polynomial regression models for the analysis, both of which were considered valid in this study. The reference BP values for US children [13] were created using a polynomial regression model. The LMS method has been used in the previously reported BP reference for children [14–16]. In the drawing of the residual density plot and the normal Q-Q plot, which were performed to confirm the fit of each estimated regression model, both models generally followed a normal distribution (Supplementary Fig. S1 and S2). The difference in the expected BP values estimated by each model was small.
The LMS model could more accurately estimate the reference BP value in this study as it was considered more valid for a wider range of Zht than the polynomial regression model.
This study has some limitations. First, we analyzed BP measurements in 2-year-olds. Further study is needed to create the reference BP values for older children, as the data after 2 years will be aggregated from now on. Second, not all participants were exactly 24 months old when BP was measured (Supplementary Table S6). Although most participants were slightly more than 2 years at the time of BP measurement, the measurements from children with a small age range were summarized as the data for 2 years. Third, the differences in mean measured BP values at each of the 15 Regional Centers were observed up to 13.3 (range 82.1–95.4) mmHg for SBP and 10.4 (range 45.4–55.8) mmHg for DBP. In JECS, the BP measurement methods were standardized. Biases due to examiners' skills and noise, such as anxiety from measurement environment conditions for each child, are unavoidable in clinical settings; it is meaningful to create reference values while considering these biases. Further investigation is required to determine whether there are regional differences in blood pressure.
Fourth, although BP values may vary depending on whether an individual adopts the supine and sitting positions, the differences could not be assessed, as the number of participants in the supine position was only eight among all participants. Therefore, the BP values for children who cannot adopt sitting positions need to be evaluated individually. Finally, an aneroid sphygmomanometer requires regular semiannual maintenance [26]. JECS does not stipulate that the maintenance interval of sphygmomanometers is to be performed semiannually. However, they are inspected visually before each BP measurement. JECS only uses newly acquired sphygmomanometers within the 2-year period.
Conclusion
The reference BP values for 2-year-old Japanese children were created based on auscultatory BP measurements from the JECS’s detailed survey using the LMS method and are now available for routine practice.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We are grateful to all participants of the JECS and to all individuals involved in data collection. The JECS was funded by Ministry of the Environment, Japan. There is no grant number for this funded research. The findings and conclusions of this article are solely the responsibility of the authors and do not represent the official views of Ministry of the Environment, Japan. The sponsor had no role in the study design, in the collection, analysis, or interpretation of data, in the writing of the report, or in the decision to submit the article for publication. Please refer to the information on clinical trial registration for this study (https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000035091). We would like to thank Editage (www.editage.com) for English language editing. Members of the JECS Group as of 2022: Michihiro Kamijima (Principal Investigator, Nagoya City University, Nagoya, Japan), Shin Yamazaki (National Institute for Environmental Studies, Tsukuba, Japan), Yukihiro Ohya (National Center for Child Health and Development, Tokyo, Japan), Reiko Kishi (Hokkaido University, Sapporo, Japan), Nobuo Yaegashi (Tohoku University, Sendai, Japan), Koichi Hashimoto (Fukushima Medical University, Fukushima, Japan), Chisato Mori (Chiba University, Chiba, Japan), Shuichi Ito (Yokohama City University, Yokohama, Japan), Zentaro Yamagata (University of Yamanashi, Chuo, Japan), Hidekuni Inadera (University of Toyama, Toyama, Japan), Takeo Nakayama (Kyoto University, Kyoto, Japan), Tomotaka Sobue (Osaka University, Suita, Japan), Masayuki Shima (Hyogo Medical University, Nishinomiya, Japan), Hiroshige Nakamura (Tottori University, Yonago, Japan), Narufumi Suganuma (Kochi University, Nankoku, Japan), Koichi Kusuhara (University of Occupational and Environmental Health, Kitakyushu, Japan), and Takahiko Katoh (Kumamoto University, Kumamoto, Japan).
Author contributions
NF, HM, OU, KI, YH, TS, KY, and YG contributed to the study conception and design. Data analysis was performed by NF, HM, KP, OU, KY-H, MS, MSA, YM, LY, NM, and YO. The first draft was written by NF and OU. KI, YH, TS, KY, SI, HM, and YG verified and contributed to the revision of all further drafts. All authors read and approved the final manuscript.
Funding
This study was funded by the Ministry of the Environment, Japan. The findings and conclusions of this article are solely the responsibility of the authors and do not represent the official views of the above government. We received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors for this research.
Data availability
Data are unsuitable for public deposition due to ethical restrictions and the legal framework of Japan. It is prohibited by the Act on the Protection of Personal Information (Act No. 57 of May 30, 2003, Amendment September 9, 2015) to publicly deposit data containing personal information. Ethical Guidelines for Epidemiological Research enforced by Japan’s Ministry of Education, Culture, Sports, Science and Technology and Ministry of Health, Labour and Welfare also restrict the open sharing of epidemiologic data. All inquiries about access to data should be sent to: jecs-en@nies.go.jp. The person responsible for handling enquiries sent to this e-mail address is Dr. Shoji F. Nakayama, JECS Programme Office, National Institute for Environmental Studies.
Declarations
Conflict of interest
The authors have no conflicts to declare.
Ethical approval
The present study was conducted in accordance with the guidelines specified in the Declaration of Helsinki. The JECS protocol was reviewed and approved by the Japan Ministry of the Environment’s Institutional Review Board on Epidemiological Studies (No. 100406001) and the Ethical Committees of all participating institutions.
Informed consent
Written informed consent was obtained from all participants.
Footnotes
Study group members are listed in the Acknowledgements.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Naoya Fujita, Email: fujita708@hkg.odn.ne.jp.
the Japan Environment, Children’s Study Group:
Michihiro Kamijima, Shin Yamazaki, Reiko Kishi, Nobuo Yaegashi, Koichi Hashimoto, Chisato Mori, Zentaro Yamagata, Hidekuni Inadera, Takeo Nakayama, Tomotaka Sobue, Masayuki Shima, Hiroshige Nakamura, Narufumi Suganuma, Koichi Kusuhara, and Takahiko Katoh
References
- 1.McNiece KL, Poffenbarger TS, Turner JL, Franco KD, Sorof JM, Portman RJ. Prevalence of hypertension and pre-hypertension among adolescents. J Pediatr. 2007;150:640–644. doi: 10.1016/j.jpeds.2007.01.052. [DOI] [PubMed] [Google Scholar]
- 2.Cheung EL, Bell CS, Samuel JP, Poffenbarger T, Redwine KM, Samuels JA. Race and obesity in adolescent hypertension. Pediatrics. 2017;139:e20161433. doi: 10.1542/peds.2016-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flynn J. The changing face of pediatric hypertension in the era of the childhood obesity epidemic. Pediatr Nephrol. 2013;28:1059–1066. doi: 10.1007/s00467-012-2344-0. [DOI] [PubMed] [Google Scholar]
- 4.Ingelfinger JR. Clinical practice. The child or adolescent with elevated blood pressure. N Engl J Med. 2014;370:2316–2325. doi: 10.1056/NEJMcp1001120. [DOI] [PubMed] [Google Scholar]
- 5.Kollias A, Dafni M, Poulidakis E, Ntineri A, Stergiou GS. Out-of-office blood pressure and target organ damage in children and adolescents: a systematic review and meta-analysis. J Hypertens. 2014;32:2315–2331. doi: 10.1097/HJH.0000000000000384. [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation. 2008;117:3171–3180. doi: 10.1161/CIRCULATIONAHA.107.730366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juhola J, Magnussen CG, Berenson GS, Venn A, Burns TL, Sabin MA, Srinivasan SR, Daniels SR, Davis PH, Chen W, Kähönen M. Combined effects of child and adult elevated blood pressure on subclinical atherosclerosis: the International Childhood Cardiovascular Cohort Consortium. Circulation. 2013;128:217–224. doi: 10.1161/CIRCULATIONAHA.113.001614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Litwin M, Niemirska A, Sladowska-Kozlowska J, Wierzbicka A, Janas R, Wawer ZT, Wisniewski A, Feber J. Regression of target organ damage in children and adolescents with primary hypertension. Pediatr Nephrol. 2010;25:2489–2499. doi: 10.1007/s00467-010-1626-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kupferman JC, Paterno K, Mahgerefteh J, Pagala M, Golden M, Lytrivi ID, Ramaswamy P. Improvement of left ventricular mass with antihypertensive therapy in children with hypertension. Pediatr Nephrol. 2010;25:1513–1518. doi: 10.1007/s00467-010-1511-4. [DOI] [PubMed] [Google Scholar]
- 10.Duncombe SL, Voss C, Harris KC. Oscillometric and auscultatory blood pressure measurement methods in children: a systematic review and meta-analysis. J Hypertens. 2017;35:213–224. doi: 10.1097/HJH.0000000000001178. [DOI] [PubMed] [Google Scholar]
- 11.Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, de Ferranti SD, Dionne JM, Falkner B, Flinn SK, Gidding SS. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140:e20171904. doi: 10.1542/peds.2017-1904. [DOI] [PubMed] [Google Scholar]
- 12.Lurbe E, Agabiti-Rosei E, Cruickshank JK, Dominiczak A, Erdine S, Hirth A, Invitti C, Litwin M, Mancia G, Pall D, Rascher W. 2016 European society of hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens. 2016;34:1887–1920. doi: 10.1097/HJH.0000000000001039. [DOI] [PubMed] [Google Scholar]
- 13.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114 Supplement 4th Report(2 Suppl 4th Report):555–76 [PubMed]
- 14.Krzyzaniak A, Krzywińska-Wiewiorowska M, Stawińska-Witoszyńska B, Kaczmarek M, Krzych L, Kowalska M, Szilágyi-Pągowska I, Palczewska I, Karch A, Jośko J, Ostrowska-Nawarycz L. Blood pressure references for Polish children and adolescents. Eur J Pediatr. 2009;168:1335–1342. doi: 10.1007/s00431-009-0931-2. [DOI] [PubMed] [Google Scholar]
- 15.Schwandt P, Scholze JE, Bertsch T, Liepold E, Haas GM. Blood pressure percentiles in 22,051 German children and adolescents: the PEP family heart study. Am J Hypertens. 2015;28:672–679. doi: 10.1093/ajh/hpu208. [DOI] [PubMed] [Google Scholar]
- 16.Dong Y, Ma J, Song Y, Dong B, Wang Z, Yang Z, Wang X, Prochaska JJ. National blood pressure reference for Chinese Han children and adolescents aged 7 to 17 years. Hypertension. 2017;70:897–906. doi: 10.1161/HYPERTENSIONAHA.117.09983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawamoto T, Nitta H, Murata K, Toda E, Tsukamoto N, Hasegawa M, Yamagata Z, Kayama F, Kishi R, Ohya Y, Saito H. Rationale and study design of the Japan environment and children’s study (JECS) BMC Public Health. 2014;14:25. doi: 10.1186/1471-2458-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sekiyama M, Yamazaki S, Michikawa T, Nakayama SF, Nitta H, Taniguchi Y, Suda E, Isobe T, Kobayashi Y, Iwai-Shimada M, Ono M. Study design and participants’ profile in the sub-cohort study in the Japan Environment and Children’s Study (JECS) J Epidemiol. 2020 doi: 10.2188/jea.JE20200448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michikawa T, Nitta H, Nakayama SF, Yamazaki S, Isobe T, Tamura K, Suda E, Ono M, Yonemoto J, Iwai-Shimada M, Kobayashi Y, Suzuki G, Kawamoto T, the Japan Environment and Children’s Study Group Baseline profile of participants in the Japan Environment and Children’s Study (JECS) J Epidemiol. 2018 doi: 10.2188/jea.JE20170018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whelton PK, Carey RM, Aronow WS, Casey DE, Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC, Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA, Sr, Williamson JD, Wright JT., Jr 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Hypertension. 2018;71:1269–1324. doi: 10.1161/HYP.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 21.Umemura S, Arima H, Arima S, Asayama K, Dohi Y, Hirooka Y, Horio T, Hoshide S, Ikeda S, Ishimitsu T, Ito M, Ito S, Iwashima Y, Kai H, Kamide K, Kanno Y, Kashihara N, Kawano Y, Kikuchi T, Kitamura K, Kitazono T, Kohara K, Kudo M, Kumagai H, Matsumura K, Matsuura H, Miura K, Mukoyama M, Nakamura S, Ohkubo T, Ohya Y, Okura T, Rakugi H, Saitoh S, Shibata H, Shimosawa T, Suzuki H, Takahashi S, Tamura K, Tomiyama H, Tsuchihashi T, Ueda S, Uehara Y, Urata H, Hirawa N. The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2019) Hypertens Res. 2019;42:1235–1481. doi: 10.1038/s41440-019-0284-9. [DOI] [PubMed] [Google Scholar]
- 22.Isojima T, Kato N, Ito Y, Kanzaki S, Murata M. Growth standard charts for Japanese children with mean and standard deviation (SD) values based on the year 2000 national survey. Clin Pediatr Endocrinol. 2016;25:71–76. doi: 10.1297/cpe.25.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cole TJ, Green PJ. Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med. 1992;11:1305–1319. doi: 10.1002/sim.4780111005. [DOI] [PubMed] [Google Scholar]
- 24.Dong B, Wang Z, Ma J. Urban–rural disparity in blood pressure among Chinese children: 1985–2010. Eur J Public Health. 2016;26:569–575. doi: 10.1093/eurpub/ckv239. [DOI] [PubMed] [Google Scholar]
- 25.Modesti PA. Season, temperature and blood pressure: a complex interaction. Eur J Intern Med. 2013;24:604–607. doi: 10.1016/j.ejim.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Canzanello VJ, Jensen PL, Schwartz GL. Are aneroid sphygmomanometers accurate in hospital and clinic settings? Arch Intern Med. 2001;161:729–731. doi: 10.1001/archinte.161.5.729. [DOI] [PubMed] [Google Scholar]
- 27.Hashimoto N, Kawasaki T, Kikuchi T, Uchiyama M. Criteria of normal blood pressure and hypertension in Japanese preschool children. J Hum Hypertens. 1997;11:351–354. doi: 10.1038/sj.jhh.1000440. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are unsuitable for public deposition due to ethical restrictions and the legal framework of Japan. It is prohibited by the Act on the Protection of Personal Information (Act No. 57 of May 30, 2003, Amendment September 9, 2015) to publicly deposit data containing personal information. Ethical Guidelines for Epidemiological Research enforced by Japan’s Ministry of Education, Culture, Sports, Science and Technology and Ministry of Health, Labour and Welfare also restrict the open sharing of epidemiologic data. All inquiries about access to data should be sent to: jecs-en@nies.go.jp. The person responsible for handling enquiries sent to this e-mail address is Dr. Shoji F. Nakayama, JECS Programme Office, National Institute for Environmental Studies.