Abstract
The combination of lithographic methods with 2D DNA origami self-assembly has led, among others, to the development of photonic crystal cavity arrays and the exploration of sensing nanoarrays where molecular devices are patterned on the sub-micron scale. Here we extend this concept to the third dimension by mounting 3D DNA origami onto nano-patterned substrates, followed by silicification to provide hybrid DNA-silica structures exhibiting mechanical and chemical stability while achieving feature sizes in the sub-10-nm regime. Our versatile and scalable method relying on self-assembly at ambient temperatures offers the potential to 3D-position any inorganic and organic components compatible with DNA origami nanoarchitecture, demonstrated with gold nanoparticles. This way of nanotexturing could provide a route for the low-cost production of complex and 3D-patterned surfaces and integrated devices designed on the molecular level while reaching macroscopic dimensions.
Substrates and surfaces structured on the micron and nanometer scale are ubiquitous in modern life. They are used in information technology, bio-sensors, water-repellent surfaces for cloths, and solar cells. To achieve three-dimensional (3D) architectures in chip technology, for example, multiple lithography steps are executed on top of each other. Replacing top-down lithography in parts or entirely through self-assembly processes could reduce production times and energy costs. Structural DNA nanotechnology, particularly DNA origami self-assembly1, 2, has proven useful in the bottom-up fabrication of well-defined complex designer two-dimensional and three-dimensional nanostructures with single-nanometer feature resolution3, 4, 5, 6, 7, 8, 9. DNA origami-assisted lithographic methods can successfully transfer spatial information of discrete DNA origami shapes10, 11, 12, 13, 14, 15 or extended 3D periodic DNA lattices16 into inorganic substrates. Recently, micrometer-scale periodic 3D DNA patterns assembled from DNA bricks17 were transferred to Silicon via reactive ion etching, successfully reaching line pitches as small as 16.2 nm, which is already smaller than what is achievable with state-of-the-art quadruple patterning or extreme-ultraviolet lithography16.
DNA origami’s strength lies in its ability to serve as a molecular breadboard for positioning molecules and nanoparticles in space with sub-nm precision18, 19, 20, 21, 22, 23. By combining DNA origami self-assembly with lithographic nanopatterning, Kershner et al. established so-called DNA origami placement (DOP), a technique based on site- and shape-selective deposition of DNA origami object onto lithographically patterned substrates, creating large-scale arrays of precisely placed DNA structures24. DOP overcame some of the drawbacks mentioned above and demonstrated the ultimate power of dictating the nanoscale arrangement of nanocomponents such as metallic nanoparticles25, organic dyes26, 27, proteins28 and peptides29 over 2D arrays and patterns. Planar triangular or disc-shaped DNA origami were positioned on substrates patterned by e-beam lithography with high accuracy and orientation control27, 30. To further circumvent the complex e-beam steps in this patterning procedure, highly parallel and low-cost methods such as self-assembling nanosphere lithography31, 32, 33 or nanoimprint lithography30, 34 of centimeter-sized substrates were recently applied. However, all DOP methods developed so far are limited to planar DNA origami and can only fabricate 2D arrays and patterns.
Herein, we demonstrate site-directed placement of various 3D DNA origami shapes in nanometer-precise patterns over micro-to millimeter scales. We employed two different approaches to achieve the upright positioning of various DNA origami shapes via connector-mediated binding (hollow tubes) or direct binding (barrels, tetrapods) via self-aligning. Both approaches are compatible with the nanopatterning techniques we tested, e-beam and nanosphere lithography. To mechanically and chemically stabilize the arrays, the DNA structures were silicified on their respective substrates resulting in hybrid DNA–silica structures with controllable heights up to 50 nm and a feature size down to ~ 6 nm. As a proof of concept, we site-specifically decorated the structures with gold nanoparticles and, finally, connected the individually placed DNA origami in the xy-plane with further DNA struts to create continuous periodic networks.
Fabrication design
The various steps of the fabrication process are illustrated in Fig. 1. We deposited 3D DNA origami shapes, which were designed in-silico35, 36 and folded in buffer containing MgCl2 (Fig. 1a), on DNA origami binding sites (Fig. 1b). We adapted protocols for placing planar DNA origami on such patterned surfaces, which can be produced with e-beam lithography24 or nanosphere lithography32. Effectively, both methods result in a hydrophobic trimethylsilyl surface with hydrophilic spots carrying hydroxyl groups. Notably, the DNA origami structures are placed on the surfaces in the presence of Mg2+, allowing salt bridging between the negatively charged surface hydroxyl groups and the negatively charged DNA backbone30, 37, 38. We employed two approaches to achieve upright placement of 3D DNA shapes: i) hollow nanotubes, for example, have a small footprint in the desired upright position. In contrast, such a DNA tube exhibits a large contact area with the hydrophilic spots in the undesired flat-lying orientation. Indeed, we mainly observed flat-lying tubes if they were administered directly onto the pre-patterned surfaces. We thus used a two-step process where planar DNA origami sheets were deposited first as connectors and the 3D DNA structures were annealed to these connector sheets in a subsequent step (Fig. 1c). For this, specific anchor strands extend from the ends of the tubes and bind to strands protruding from the planar connector origami sheets. ii) the barrels are designed such that the bottom-to-be faces of the structure are larger than its side and are, therefore, more likely to attach to the hydrophilic regions. Or, as in the case of the tetrapod, the four main faces are identical. These two objects were deposited directly on the patterned substrates (Fig. 1d). After successful deposition, samples from both approaches can optionally be incubated with pre-hydrolyzed N-trimethoxysilylpropyl-N, N, N-trimethylammonium chloride (TMAPS) and tetraethylorthosilicat (TEOS) enabling the growth of a rigid silica shell to allow for drying of the products39 (Fig. 1e).
Figure 1. Assembly of 3D hybrid DNA-silica nanostructured substrates.
a) Design of 3D DNA origami shapes and connection interfaces for on-surface assembly. b) Substrates are patterned by e-beam30 or nanosphere lithography32 to produce hydrophilic hydroxyl patterns on HMDS-primed hydrophobic background (Si/SiO2 or glass). c, d) Alignment and upright positioning of 3D DNA origami structures on patterned surfaces. DNA is represented in a cylinder model. Shapes that cannot self-align in an upright position are placed in a two-step process with planar DNA origami as connectors (Fig. 1c). Other shapes are directly deposited to the patterned substrate (Fig. 1d right). e) Growing silica shells on the 3D DNA origami enables subsequent drying of the now rigidified objects.
Connector-mediated binding
The two-step placement method, based on sequence-specific DNA binding on a surface, enables us to position any 3D DNA origami shape in a defined directionality. As an example shape, we designed a DNA origami nanotube with a length of 50 nm and a diameter of 40 nm (Fig. 2a). Here, a rolled-up single layer of 48 DNA duplexes forms the tube (Supplementary Note 1, Supplementary Fig. 1 and Supplementary Table 1). Its native wall thickness is defined by the width of a DNA double helix, i.e. 2.1 nm. Figure 2a displays a in silico rendering of the tube and its built-in binding strands as well as the connector sheet. We used a variant of the “Rothemund triangle”. For one, this is a commonly used origami structure present in many laboratories and second, it has been successfully positioned on lithographically patterned substrates before30. Single-stranded DNA linkers extend from the center of the triangle, roughly matching the circular footprint of the DNA tube8. Hybridization between these linkers and complementary anchor strands extending from the tube’s ends – we labeled both ends of the tubes with anchor strands to increase the probability of binding – brings the tubes and the triangles together in a way that the tubes “stand” on top of the triangles (Supplementary Note 2, Supplementary Fig. 2). Figures 2b through 2d display transmission electron microscopy (TEM) images of the DNA objects where panel b shows a side view of a tube lying flat on the TEM grid, panel c the triangle and panel d the tube assembled on top of a triangle (additional TEM characterization in Supplementary Fig. 3).
Figure 2. Assembly of 3D hybrid nanostructured substrates by on-surface annealing of DNA origami nanotubes to a flat connector origami.
a) Design of the DNA origami tubes and triangles. Single-stranded DNA linkers extend from the center of the triangle roughly matching the circular footprint of the tube. Complementary anchor strands extend from the ends of the tubes. b-d) Uranyl-formate negative-stain TEM images of b) DNA tubes carrying 48 T11 ssDNA linkers, c) a triangle, carrying 27 A12 ssDNA anchors and d) a tube annealed with a triangle. e) AFM characterization of dried Si/SiO2 chip with an array of DNA origami triangles carrying 27 A12 ssDNA anchors. f) AFM and g-i) SEM characterization of dried Si/ SiO2 chip with an array of silica-coated DNA tubes standing on top of triangles. Scale bars in g) and h): 1 μm. Scale bars in b – d, i) and in the inserts in h, i): 50 nm.
We fabricated Si/SiO2 chips with square arrays of triangular binding sites situated 250 nm apart from each other via e-beam lithography. We deposited DNA triangles, carrying 27 ssDNA linkers each 12 nt in length, on the patterned chips. Generally, the mechanisms of origami-to-site binding are complex and small details in variations during the substrate fabrication can affect placement yields significantly30. The experimental conditions for high-quality positioning of planar origami on Si/SiO2 substrates (Tris buffer, pH of 8.35, Mg2+ concentration of 35 mM, incubation time of 1h at RT, see Supplementary Table 2 for all of the buffers used in the study) have been already reported in ref.30. To reproduce the successful placement of triangles, we used these parameters and only adjusted the size of the binding sites and the DNA origami concentration (Supplementary Note 3). We achieved up to ~ 94% of sites occupied with a single triangle and ~ 5% of sites with multiple/aggregated triangles (Fig. 2e, Supplementary Fig. 4).
Next, we incubated these pre-patterned surfaces with DNA tubes bearing 48 ssDNA anchors, each 11 nt in length and complementary to the linker DNA on the triangles at 37°C for 1h in a Tris buffer with 12.5 mM MgCl2 (see Supplementary Note 4.1, Supplementary Fig. 5, S6 for details on in-solution assembly optimization and Supplementary Note 4.2, Supplementary Fig. 7–10 for details on on-surface optimization). After annealing, the chips with the full assemblies were exposed to a silica-coating procedure described in ref.39. Subsequent air-drying and AFM and SEM imaging revealed rigid 3D DNA-silica nanotubes arranged in 22 μm × 22 μm square arrays on the Si/SiO2 surface (Fig. 2f–i). We observed up to 75 % occupancy of binding sites with individual standing tubes. In contrast, the remaining 25 % of sites were either doubly occupied (4 %), empty (2 %) or occupied with higher-order aggregates (Supplementary Fig. 10–13). Processes that potentially lead to multiple binding events and practical tips to minimize these are discussed in Supplementary Note 4. The height of the silicified tube determined by AFM is ~ 42 nm, which is in good agreement with the designed tube length of ~ 50 nm. Such a shrinking of silicified 3D DNA origami structures was already observed before7. The wall thickness of the upright silica-DNA tubes is 6 ± 1 nm, as determined by SEM (insert in Fig. 2i, Supplementary Fig. 14). This is below the state-of-the-art 10 nm resolution in 3D silica nanofabrication achievable by focused ion beam or thermal scanning probe lithography40, 41 and comparable with the resolution of nanopatterning techniques based on site-specific vertical epitaxy42 or self-assembly of block copolymers43, 44. Despite their fine structure, surface-bound DNA-silica nanostructures show excellent thermal and chemical stability in DI water, in organic solvents, at low and high pH values and at high temperatures (we tested up to 500° C; Supplementary Note 5 and Supplementary Fig. 15 and 16).
We then studied how the array period of the patterns affects the placement yields. It was shown before that the patterning yields of origami triangles depend on the array period – the occupation rate decreases as the period decreases – and on the relative location of the binding site – the occupation rate decreases towards the center of a given array30. We examined triangles and triangle-tube structures binding to sites in square arrays with periods ranging between 170 nm and 400 nm on the same Si/SiO2 chip (Supplementary Fig. 17–S18, respectively). In addition, we evaluated the placement at different periods in various areas of the patterns: in the center, close to a corner and close to an edge (Supplementary Fig. 19). We did not observe the expected drop of triangles binding rates with a decrease of period from 400 nm to 170 nm (Fig. 3a,e, Supplementary Fig. 17, Supplementary Fig. 19a–d), presumably a consequence of the higher concentration of origami triangles used here compared to the previous work (potential effects are discussed in Supplementary note 6). The highest percentage of 95% of sites binding a single triangle for the 250 nm period is slightly decreased to 93% for the 400 nm period and to 91% for the 170 nm period.
Figure 3.
Pattern diversity. AFM and SEM characterization of dried Si/SiO2 surfaces with square arrays of DNA origami prepared with e-beam lithography with periods of 400 nm (a-d) and 170 nm period (e-h). a, e) AFM characterization arrays of DNA origami triangles carrying ssDNA linkers. b, f) AFM and c, d, g, h) SEM characterization of arrays of silica-coated DNA tubes standing upright on the triangles. Scale bars in the inserts in c and g): 200 nm. All other scale bars: 400 nm.
Consequently, the site occupancy and alignment of standing tubes in arrays of corresponding periods did not vary significantly. We achieved up to 71 % and 74 % of sites with individual upright nanotubes for the 170 nm and 400 nm arrays, respectively (Fig. 3b–d and f–h, Supplementary note 6, Supplementary Fig. 18, Supplementary Fig. 19e–h). This opens a route to create diverse patterns and arrays of integrated 3D DNA-silica nanodevices on one chip (Supplementary Fig. 20).
Direct binding
Another approach to binding the 3D origami shapes to substrates relies on direct binding and self-aligning. To achieve this, it is necessary to design the objects so that certain faces are more likely to attach to the hydrophilic binding sites or that all main faces are identical.
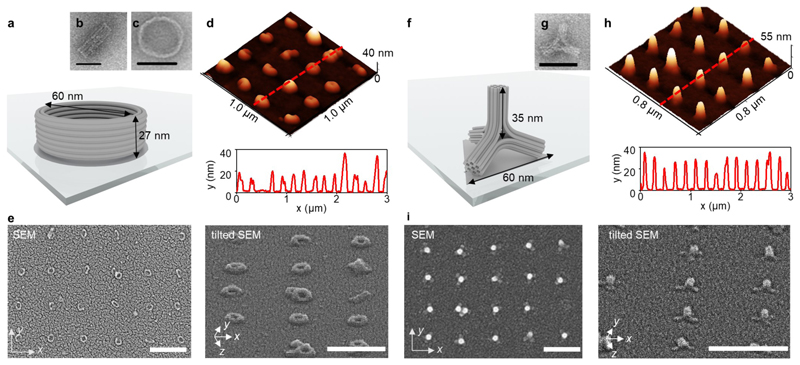
As an example of the first case, we demonstrate upright positioning of DNA origami barrels. The DNA origami barrel is a doughnut-shaped structure designed previously45 and constructed from horizontally aligned, circular DNA duplexes. The barrel has a diameter of 60 nm and a height of 27 nm (Fig. 4a-c, Supplementary Fig. 21). The relatively thick walls and a low aspect ratio of 1:2.2 (height to width) promote the horizontal binding of the structures. Moreover, the binding sites were tuned to match the diameter of the barrels. We fabricated a series of Si/SiO2 chips via e-beam lithography with circular binding sites, varying the diameter between 75 % and 200 % of the actual barrel diameter of 60 nm. We obtained the best yield of single-bound upright barrels (70 %) with spot sizes of 50 nm while the percentage of the barrels lying on their side is reduced to 16 % (Fig. 4d,e and Supplementary Note 7, Supplementary Fig. 22–25).
Figure 4. Assembly of 3D hybrid nanostructured substrates by direct deposition.
a) Design of the DNA origami barrels, illustrated as a cylinder model. b, c) Uranyl formate negative-stain TEM images of b) a DNA origami barrel lying on its side, c) an upright DNA origami barrel. d, e) AFM and SEM characterization of a dried Si/SiO2 substrate with a square array of silicified DNA origami barrels. f) Design of the DNA origami tetrapods. g) Uranyl formate negative-stain TEM images of the DNA origami tetrapod. d, e) AFM and SEM characterization of a dried Si/SiO2 substrate with a square array of silicified DNA origami tetrapods. Scale bars in e) and i): 400 nm.
As an example of the second design case, we employed a DNA origami tetrapod that consists of four equivalent arms, resulting in a four-fold symmetric object (Fig. 4f, g and Supplementary Fig. 26 and 27). Each arm comprises interconnected 24-helix bundles (24HBs) as described in Supplementary Note 8. As expected, after incubating the origami tetrapods on patterned substrates, we observed individual objects standing on three legs on the binding sites (Fig. 4f). After optimization of binding site shape and size, we achieved occupancy yields of 79 % while single therapods occupied 43 % of the sites and 36 % of the sites carried multiple tetrapods (Supplementary note 9, Supplementary Fig. 28–30). Square arrays with a 200 nm period of silica-coated and dried tetrapods imaged by SEM and AFM are presented in Fig. 4h and i, respectively. The height of an individual silica-coated tetrapod obtained from AFM measurements of dried samples is ~ 30 nm, which is below the designed 50 nm. Most likely, the tetrapod “sits” flat on the surface, reducing the effective height of the structures. We assume that a similar approach can be used for the individual placement of all 3D shapes with equilateral faces, such as tetrahedrons, cubes, octahedrons, etc. Overall, while the arrangement of deposited 3D structures in this one-step method is inferior compared to the two-step process, it can be helpful for positioning a large variety of 3D shapes with low-aspect-ratio as cuboids, cylinders or cones.
Sphere lithography and nanoparticle positioning
For many applications, using a cleanroom-free, large-scale DNA origami placement method such as the benchtop technique of nanosphere – DNA origami lithography can be advantageous32. Here, a layer of close-packed nanospheres creates a crystalline pattern of contact points for the selective passivation of the supporting substrate32. After chemically rendering the “free” Si/SiO2 or glass surface hydrophobic, the nanospheres are lifted off and DNA origami structures can subsequently bind to the untreated, close-packed, hexagonal pattern defined by the previous contact points.
We utilized this method of bottom-up nanopatterning for the placement of DNA tetrapods and DNA tubes. For directly bound tetrapods we achieved occupancy yields of 69 % while the yield of single placement is 44 %, very close to the results obtained with e-beam lithography-based direct patterning (Fig. 5a–c, Supplementary Fig. 31). For the two-step placement of the tubes we obtained a different picture: Despite almost full occupancy of the circular binding sites (~ 97 %) with the triangular-shaped connector origami we only achieved ~ 36 % of sites with single tubes accompanied by a dramatic increase in multiple binding events (Supplementary Note 10, Supplementary Fig. 32–33). This could be a result of the geometrical mismatch between the circular binding sites and the triangular DNA origami connectors, which leaves free binding space where the DNA tubes can bind directly. It is noteworthy to hightlight that the hexagonal arrays of sphere-lithography assembled DNA tetrapods or tubes cover a total area of more than 4 mm2 (Supplementary Fig. 31 and Supplementary Fig. 34, respectively), demonstrating that it is possible to arrange complex 3D DNA-silicified molecular breadboards over macroscopic areas in a conventional wet lab environment.
Figure 5. Assembly of hybrid silica-AuNPs-DNA nanostructures prepared via nanosphere lithography.
a) Hexagonal arrays of DNA origami tetrapods on Si/SiO2 substrates. (b, c) SEM characterization of a dried Si/SiO2 surface with a hexagonal pattern of tetrapods. (d - i) AuNPs-DNA nanostructures were prepared by on-surface annealing of 20 nm AuNPs to DNA origami tetrapods pre-adsorbed to their binding sites. (e, g) Scheme of DNA origami tetrapods hosting 20 nm AuNPs in two geometrical configurations. In the nanoclover configuration (e), the DNA-coated AuNPs attach to DNA handles extending from the concave, central parts of the tetrapod. In the nanoquadruped configuration (g), the AuNPs attach to handles extending from the ends of the tetrapod’s legs. d, f, h, i) SEM characterization of a dried Si/SiO2 surface with therapods carrying AuNPs arranged as nanoclovers (d, f) and nanoquadrupeds (h, i). Scale bars in c, f, i): 1 μm. Scale bars in b, d, h) and in the insets in c, f, i): 100 nm.
Moreover, and important for potential applications of DNA-assembled 3D substrates, nanometer-precise modification with nanoscale components can be directly implemented on the DNA origami templates. As a proof-of-concept, we attached 20 nm gold nanoparticles (AuNPs) to the DNA origami tetrapods patterned with nanosphere lithography (Fig 5a-c and Supplementary Note 11). We designed two different configurations for the AuNPs on the tetrapods that we termed “nanoclover” (Fig. 5d–f and Supplementary Fig. 35) and “nanoquadruped” (Fig. 5g–i and Supplementary Fig. 36). SEM imaging of the silica-coated assemblies proved both site-selective deposition of DNA tetrapods onto the patterned substrates and the precise binding of DNA-coated AuNPs46 to each DNA tetrapod (Fig. 5d–i and Supplementary Fig. 37–40). Note that in the nanoclover configuration, only three AuNP capturing sites are freely accessible after the tetrapods have landed on the patterned spots. One face of the tetrapod is thus blocked once the AuNPs are added. At close inspection, only three particles are visible in the top-view micrographs and the tilted SEM views. The nanoquadruped in contrast, displays all four binding sites after surface contact; indeed,we observe four AuNPs bound accordingly.
Individually placed 3D DNA shapes can also be used as seeds for further assembly in subsequent annealing steps with other DNA origami structures. In Supplementary Note 12 and Supplementary Fig. 41–44 we present micron-sized honeycomb networks and arrangements of individual hexagons forming a Sierpiński triangle by combining arrays of tetrapod structures with 24-helix bundles.
Conclusions
In conclusion, we advanced the placement of individual DNA origami objects on patterned surfaces from 2D to 3D. Using colloidal gold nanoparticles and with the help of wet-chemistry coating, we fabricated hybrid DNA – gold – silica structures with controllable dimensions up to 50 nm and feature sizes of a few nanometer covering large-scale arrays. Such near nanometer-precise spatial positioning of nanoscale building blocks could be crucial for future applications of this method, such as scalable self-assembled meta-surfaces, tunable shadow masks for pattern transfer, or, possibly in combination with conventional lithography, for (opto-)electronic architectures containing highly diverse materials. In the context of emulating nature’s complex designs, we could further envision surfaces and thin materials relying on nano- and microstructuring that, for example, mimic the low reflectivity of moth eyes and the brilliant colors of butterfly wings.
Methods
DNA Origami design, preparation and purification
DNA origami nanotubes and tetrapods were designed using cadnano35. Design details of the DNA nanotube can be found in Supplementary Note 1. Design details of tetrapods and 24HBs can be found in Supplementary Note 7 and Supplementary Note 9, respectively.
Staple strands were purchased from IDT Technologies (HPLC purified, 100 μM each in water). Cadnano files and list of sequences of oligonucleotides can be found in the Supplementary Data. The scaffold strands (p7249, p8634) were produced from M13 phage replication in Escherichia coli. All chemicals were obtained from Sigma Aldrich unless otherwise stated. DNA origami structures used in this work (nanotubes, triangles, barrels, tetrapods and 24HBs) were folded by mixing scaffold strands with an excess of staple strands (and miniscaf short scaffold-parity strands in case of DNA origami barrels) in folding buffer (buffers used in this work can be found in Supplementary Table 2). Samples were annealed in a PCR machine (Tetrad 2 Peltier thermal cycler, Bio-Rad) and purified from excess staples by Amicon filtration47 (triangles, nanotubes), PEG precipitation48 (tetrapods, 24HBs) or ultracentrifugation49 (barrels). A full description of the folding and purification of each type of DNA origami can be found in Supplementary Note 13.
Annealing of DNA origami in buffer solution
For the triangular origami, 9, 18, or 27 staples close to the central hole were modified by extending 8, 12 or 20 nt polyA sequences from the 5′ end (Supplementary Fig. 2). These A8, A12, A20-modified DNA staples were introduced into the DNA scaffolds in place of the original DNA staples. After folding, excess staples were removed by filtering the DNA origami solution through 0.5 mL Amicon 100 kDa filter units.
For the nanotubes, 24 staples on each edge of a tube were modified by extending 11 nt polyT sequences from the 3′ end (Supplementary Note 2 and Supplementary Fig. 2). These T11-modified DNA staples were introduced into the DNA scaffolds in place of the original DNA staples and folded and purified as described in Supplementary Note 10.
The binding of T-modified DNA nanotubes to A-modified DNA triangles in suspension was studied at a final concentration of tubes and triangles of ~5 nM and optimized by varying the length and number of linkers. The solutions were mixed in the placement buffer (Supplementary Table 2) in 50 µl aliquots, annealed 1 h either at 37 °C in the thermal cycler or at room temperature, and imaged by gel electrophoresis and TEM.
Preparation of DNA-coated AuNPs
Gold nanoparticles (AuNPs, BBI International) with 20 nm diameter were functionalized with a mixture of 5’-thiolated 19 nt and 8 nt poly-T single-stranded DNA (Biomers). The particles were centrifuged at 7000 g for 10 min and supernatant was removed and the particles resuspended in 0.1% SDS to 10 nM concentration. The particles were then mixed with a 1:9 mixture of T19 and T8 thiolated single-stranded DNA (strand concentration 100 µM) in a 3:2 ratio and frozen at -80° C for 30 min. After freezing, the functionalized particles were purified from excess DNA with gel electrophoresis in a 1% agarose gel ran for 90 min at 80 V. Particles were collected by cutting out the migrating band and squeezing out the liquid with particles with a microscopy slide wrapped in parafilm. To form the nanoclover structure with attachment of AuNPs to the tetrapod 6 or 7 staples per attachment site at each intersection of 3 legs of the tetrapod were modified with 10 nt poly-A extensions on the 3’ ends (30 staples in total, Supplementary Fig. 35) and for the nanoquadruped structure the same modification was done to 6 staples per site on the end surface of each leg (24 staples in total, Supplementary Fig. 36).
Preparation of the substrates and DNA origami placement
Si/SiO2 substrates patterned with e-beam lithography were prepared by adaptation of procedure from ref.30 with slight modification. The 4-inch Si/SiO2 wafer with 100 nm thermal oxide (Microchemicals) was diced into 1 cm × 1 cm chips. Clean chips were primed with 10 mL of hexamethyldisilazane (HMDS) in a 4 L desiccator. The time of priming was optimised to maintain a Si/SiO2 surface contact angle of 70°-75° after HMDS deposition. Binding sites were patterned into poly(methyl methacrylate) resist by electron-beam lithography. Examples of binding sites design are shown in Supplementary Fig. 44. Then the chips were developed with a 1:3 solution of methyl isobutyl ketone (MIBK) and isopropanol (IPA). The HMDS in developed areas was removed with O2 plasma for 6 s in a plasma cleaner (PICO). The resist was stripped by ultrasonication in N-methyl pyrrolidone (NMP) at 50°C for 30 min. The substrates were briefly rinsed with 2-propanol, then dried in a nitrogen stream and used immediately.
Si/SiO2 or glass chips patterned via nanosphere lithography were prepared following the protocol reported in ref.32 1 cm2 glass chips were purchased from Epredia. The polystyrene (PS) nanospheres with a diameter of 350 nm or 400 nm (Thermo Scientific™ Nanosphere™ Size standards 3350A or 3400A) were purified by centrifugation and resuspension in 25% ethanol/water. The PS nanospheres were drop-casted onto an O2 plasma activated chip surface and dried at a ~ 45° angle at RT, forming a close-packed monolayer/multilayer of nanospheres. The chips were then primed with 0.5 mL HMDS in a 1 L desiccator under a vacuum. The PS nanospheres were lifted off the surface by ultrasonication in water at RT for 5 min. Finally, the surface was blown dry with a nitrogen gun and baked at 120 °C for 5 min to stabilize the HMDS on the surface and used immediately.
Binding of DNA origami to the patterned substrates was achieved as previously reported30 by depositing of a 20-60 μL drop of freshly folded and purified DNA origami in placement buffer (Supplementary Table 2) to the surface of the chips. After incubation for 1 h at 25 °C in an incubator with 100% humidity, excessive DNA origami was removed from the surface by 8 buffer replacement steps and purification with 0.1% Tween 20. After this step, chips with absorbed DNA origami could be air dried, or coated with silica and air-dried or further steps of DNA origami or nanoparticles placement could be performed. Air drying of the chips was performed by treating the samples with an ethanol dilution series as previously reported30. For silica coating, the chips were placed in glass jars filled with silica coating buffer (Supplementary Table 2) with 4% pre-hydrolyzed silica precursors (2% TMAPS and 2% TEOS), prepared according to the protocol published in Ref.30. The chips were left in this buffer for 3 days at RT, then purified in distilled water and air dried from Ethanol.
Two-step surface annealing with DNA origami/DNA-coated AuNPs was performed after the initial placement of first DNA origami as described above. Then second origami/DNA-coated AuNPs were introduced to the surface buffer and annealed for 1 h at 37° C. After surface annealing, chips were incubated in silica-coating buffer, rinsed and air-dried from Ethanol as described above.
Step by step protocols of preparation of the patterned substrates, placement of DNA origami and surface annealing with DNA origami/AuNPs can be found in Supplementary Note 14 and Supplementary Note 15, respectively.
Characterization techniques
UV-vis absorption measurements were performed with a NanoDrop ND-1000 Spectrophotometer (Thermo Scientific).
Tapping-mode AFM of dried Si/SiO2 or glass substrates with triangular DNA origami was carried out on a Dimension ICON AFM (Bruker). OTESPA silicon tips (300 kHz, Vecco Probes) were used for imaging in air. Images are analysed with the Software Gwyddeon.
TEM imaging of DNA origami was carried out using a JEM-1011 transmission electron microscope (JEOL) operating at 80 kV. For sample preparation 5 μL of DNA origami diluted to 5 nM concentration were deposited on glow-discharged TEM grids (formvar/carbon-coated, 300 mesh Cu; TED Pella, Inc; prod no. 01753 - f) for 30 sec. Grids were furthermore quickly washed once with 1 % uranyl formate solution (5 µL) and immediately afterwards stained with another 5 µL drop of uranyl formate for 10 s.
The Scanning Electron Microscope used in this work is the Raith e-LiNE Scanning Electron Microscope (Raith). The beam settings for imaging are 10 kV acceleration and 20 μm aperture. Samples were SEM imaged after 30 s sputtering using an Edwards Sputtercoater S150B 1990. The sputter target contained 60% gold and 40% palladium. Process parameters used for sputtering were 5 mbar Ar, 1.5 kV, 11 mA. 30 s sputtering results in the deposition of layer of gold/palladium with a thickness of a few nm. SEM imaging was performed on horizontal samples and samples tilted by 70°.
Stability test of silica-DNA nanostructures
Chemical stability of the hybrid silica-DNA tube nanostructures on Si/SiO2 chips was tested by incubating the chips for 1 h in DI water, in chloroform, in acetic acid/water mixture (pH = 1.95) and in NaOH/water mixture (pH = 10.50). In between these treatments the chips were rinsed with DI water and ethanol followed by air-drying. Thermal stability of the nanostructures was tested by heating the samples to 500 °C under vacuum in an annealing oven (AO 500, MBE-Komponenten GmbH). First, the chips were heated at 0.5° C/s from room temperature up to 500° C temperature. The chips were held at the maximum temperature for 1 h and then cooled down to room temperature at the rate of –0.5° C/s. After these procedures, the morphology of individual silica-DNA nanostructures was evaluated by tilted SEM imaging as described above. In total, we tested the stability of 3 chips with patterned silica-origami; two of these were sputter-coated with 60% gold and 40% palladium that allows to obtain tilted SEM images of the same pattern area before/after treatments. One chip was not sputter-coated prior to the treatments and was imaged only after the treatments.
Statistics & Reproducibility
For e-beam lithography experiments we performed at least two independent replications under optimized placement conditions. Experiments during the optimization process of placement parameters were performed in single replicates, in total >45 conditions were tested.
For nanosphere lithography experiments we performed >20 optimization experiments and for each type of assembly (origami on glass, origami and AuNP-origami on Si/SiO2 substrates one replication.
All AFM images and SEM images that were used to obtain the yield counts presented in Supplementary Fig. 4, 8, 9, 10, 17, 18, 19, 23, 24, 25, 29, 30, 31, 33, 38 and silica-DNA tube wall thickness in Supplementary Fig. 14 are available in ref.50. AFM raw data files in the original .spm format used by the Dimension ICON AFM used in the experiments are available in ref.50.
Supplementary Material
Acknowledgments
We thank Christian Obermayer for clean room assistance and Susanne Kempter for assistance with TEM. Besides all group members, we thank Nicolas Vogel (FAU Erlangen-Nürnberg) for the helpful discussions. I.M., E.E., G.P. and T.L. acknowledge funding from the ERC consolidator grant “DNA Funs” (Project ID: 818635) V.R., X.Y. and T.L. further acknowledge support from the cluster of excellence e-conversion EXC 2089/1-390776260. This work was funded by the Federal Ministry of Education and Research (BMBF) and the Free State of Bavaria under the Excellence Strategy of the Federal Government and the Länder through the ONE MUNICH Project Munich Multiscale Biofabrication.
Footnotes
Author Contributions Statement
T.L. and I.M. designed this study. I.M. and T.L. designed DNA origami samples, designed and optimized interfaces. I.M., E.E., V.R., assembled and purified DNA origami samples. G.P. and X.Y. designed, assembled, purified DNA origami tetrapods and 24HBs and designed the tetrapod-AuNPs and the tetrapod-24HB interfaces. I.M., E.E., V.R., performed placement experiments, surface annealing experiments, AFM and SEM measurements and data analysis with the assistance from M.D. and P.A.. I.M. and T.L. wrote the manuscript with input from all authors.
Competing Interests Statement
The authors declare no competing financial interest.
Data Availability
The data that support the findings of this study are openly available in ref.50. The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
References
- 1.Rothemund PWK. Folding DNA to create nanoscale shapes and patterns. Nature. 2006;440(7082):297–302. doi: 10.1038/nature04586. [DOI] [PubMed] [Google Scholar]
- 2.Douglas SM, Dietz H, Liedl T, Högberg B, Graf F, Shih WM. Self-assembly of DNA into nanoscale three-dimensional shapes. Nature. 2009;459(7245):414–418. doi: 10.1038/nature08016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seeman NC. DNA in a material world. Nature. 2003;421(6921):427–431. doi: 10.1038/nature01406. [DOI] [PubMed] [Google Scholar]
- 4.Yan H, Park SH, Finkelstein G, Reif JH, LaBean TH. DNA-templated self-assembly of protein arrays and highly conductive nanowires. Science. 2003;301(5641):1882–1884. doi: 10.1126/science.1089389. [DOI] [PubMed] [Google Scholar]
- 5.Aldaye FA, Palmer AL, Sleiman HF. Assembling materials with DNA as the guide. Science. 2008;321(5897):1795–1799. doi: 10.1126/science.1154533. [DOI] [PubMed] [Google Scholar]
- 6.Wang P, Huh J-H, Lee J, Kim K, Park KJ, Lee S, et al. Magnetic Plasmon Networks Programmed by Molecular Self-Assembly. Adv Mater. 2019;31(29):1901364. doi: 10.1002/adma.201901364. [DOI] [PubMed] [Google Scholar]
- 7.Liu X, Zhang F, Jing X, Pan M, Liu P, Li W, et al. Complex silica composite nanomaterials templated with DNA origami. Nature. 2018;559(7715):593–598. doi: 10.1038/s41586-018-0332-7. [DOI] [PubMed] [Google Scholar]
- 8.Sun W, Boulais E, Hakobyan Y, Wang WL, Guan A, Bathe M, et al. Casting inorganic structures with DNA molds. Science. 2014;346(6210):1258361. doi: 10.1126/science.1258361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kolbeck PJ, Dass M, Martynenko IV, van Dijk-Moes RJ, Brouwer KJ, van Blaaderen A, et al. A DNA origami fiducial for accurate 3D AFM imaging. bioRxiv. 2022 doi: 10.1021/acs.nanolett.2c04299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kabusure KM, Piskunen P, Yang J, Kataja M, Chacha M, Ojasalo S, et al. Optical characterization of DNA origami-shaped silver nanoparticles created through biotemplated lithography. Nanoscale. 2022;14(27):9648–9654. doi: 10.1039/d1nr06256e. [DOI] [PubMed] [Google Scholar]
- 11.Diagne CT, Brun C, Gasparutto D, Baillin X, Tiron R. DNA Origami Mask for Sub-Ten-Nanometer Lithography. ACS nano. 2016;10(7):6458–6463. doi: 10.1021/acsnano.6b00413. [DOI] [PubMed] [Google Scholar]
- 12.Surwade SP, Zhao S, Liu H. Molecular Lithography through DNA-Mediated Etching and Masking of SiO2. J Am Chem Soc. 2011;133(31):11868–11871. doi: 10.1021/ja2038886. [DOI] [PubMed] [Google Scholar]
- 13.Jin Z, Sun W, Ke Y, Shih C-J, Paulus GLC, Hua Wang Q, et al. Metallized DNA nanolithography for encoding and transferring spatial information for graphene patterning. Nature Communications. 2013;4(1):1663. doi: 10.1038/ncomms2690. [DOI] [PubMed] [Google Scholar]
- 14.Shen B, Linko V, Tapio K, Pikker S, Lemma T, Gopinath A, et al. Plasmonic nanostructures through DNA-assisted lithography. Science Advances. 2018;4(2):eaap8978. doi: 10.1126/sciadv.aap8978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Surwade SP, Zhou F, Wei B, Sun W, Powell A, O’Donnell C, et al. Nanoscale Growth and Patterning of Inorganic Oxides Using DNA Nanostructure Templates. J Am Chem Soc. 2013;135(18):6778–6781. doi: 10.1021/ja401785h. [DOI] [PubMed] [Google Scholar]
- 16.Shen J, Sun W, Liu D, Schaus T, Yin P. Three-dimensional nanolithography guided by DNA modular epitaxy. Nature Materials. 2021;20(5):683–690. doi: 10.1038/s41563-021-00930-7. [DOI] [PubMed] [Google Scholar]
- 17.Ke Y, Ong LL, Shih WM, Yin P. Three-Dimensional Structures Self-Assembled from DNA Bricks. Science. 2012;338(6111):1177–1183. doi: 10.1126/science.1227268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maune HT, Han S-p, Barish RD, Bockrath M, Iii WAG, Rothemund PWK, et al. Self-assembly of carbon nanotubes into two-dimensional geometries using DNA origami templates. Nature Nanotechnology. 2010;5(1):61–66. doi: 10.1038/nnano.2009.311. [DOI] [PubMed] [Google Scholar]
- 19.Kuzyk A, Schreiber R, Fan Z, Pardatscher G, Roller E-M, Högele A, et al. DNA-based self-assembly of chiral plasmonic nanostructures with tailored optical response. Nature. 2012;483(7389):311–314. doi: 10.1038/nature10889. [DOI] [PubMed] [Google Scholar]
- 20.Voigt NV, Tørring T, Rotaru A, Jacobsen MF, Ravnsbæk JB, Subramani R, et al. Single-molecule chemical reactions on DNA origami. Nature Nanotechnology. 2010;5(3):200–203. doi: 10.1038/nnano.2010.5. [DOI] [PubMed] [Google Scholar]
- 21.Knudsen JB, Liu L, Bank Kodal AL, Madsen M, Li Q, Song J, et al. Routing of individual polymers in designed patterns. Nature Nanotechnology. 2015;10(10):892–898. doi: 10.1038/nnano.2015.190. [DOI] [PubMed] [Google Scholar]
- 22.Hartl C, Frank K, Amenitsch H, Fischer S, Liedl T, Nickel B. Position Accuracy of Gold Nanoparticles on DNA Origami Structures Studied with Small-Angle X-ray Scattering. Nano Lett. 2018;18(4):2609–2615. doi: 10.1021/acs.nanolett.8b00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Funke JJ, Dietz H. Placing molecules with Bohr radius resolution using DNA origami. Nature Nanotechnology. 2016;11(1):47–52. doi: 10.1038/nnano.2015.240. [DOI] [PubMed] [Google Scholar]
- 24.Kershner RJ, Bozano LD, Micheel CM, Hung AM, Fornof AR, Cha JN, et al. Placement and orientation of individual DNA shapes on lithographically patterned surfaces. Nature Nanotechnology. 2009;4(9):557–561. doi: 10.1038/nnano.2009.220. [DOI] [PubMed] [Google Scholar]
- 25.Hung AM, Micheel CM, Bozano LD, Osterbur LW, Wallraff GM, Cha JN. Large-area spatially ordered arrays of gold nanoparticles directed by lithographically confined DNA origami. Nature Nanotechnology. 2010;5(2):121–126. doi: 10.1038/nnano.2009.450. [DOI] [PubMed] [Google Scholar]
- 26.Gopinath A, Miyazono E, Faraon A, Rothemund PWK. Engineering and mapping nanocavity emission via precision placement of DNA origami. Nature. 2016;535(7612):401–405. doi: 10.1038/nature18287. [DOI] [PubMed] [Google Scholar]
- 27.Gopinath A, Thachuk C, Mitskovets A, Atwater HA, Kirkpatrick D, Rothemund PWK. Absolute and arbitrary orientation of single-molecule shapes. Science. 2021;371(6531):eabd6179. doi: 10.1126/science.abd6179. [DOI] [PubMed] [Google Scholar]
- 28.Cervantes-Salguero K, Freeley M, Gwyther REA, Jones DD, Chávez JL, Palma M. Single molecule DNA origami nanoarrays with controlled protein orientation. Biophysics Reviews. 2022;3(3):031401. doi: 10.1063/5.0099294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang D, Patel K, Perez-Garrido S, Marshall JF, Palma M. DNA Origami Nanoarrays for Multivalent Investigations of Cancer Cell Spreading with Nanoscale Spatial Resolution and Single-Molecule Control. ACS nano. 2019;13(1):728–736. doi: 10.1021/acsnano.8b08010. [DOI] [PubMed] [Google Scholar]
- 30.Gopinath A, Rothemund PWK. Optimized Assembly and Covalent Coupling of Single-Molecule DNA Origami Nanoarrays. ACS nano. 2014;8(12):12030–12040. doi: 10.1021/nn506014s. [DOI] [PubMed] [Google Scholar]
- 31.Deckman HW, Dunsmuir JH. Natural lithography. Appl Phys Lett. 1982;41(4):377–379. [Google Scholar]
- 32.Shetty RM, Brady SR, Rothemund PWK, Hariadi RF, Gopinath A. Bench-Top Fabrication of Single-Molecule Nanoarrays by DNA Origami Placement. ACS nano. 2021;15(7):11441–11450. doi: 10.1021/acsnano.1c01150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martynenko IV, Ruider V, Dass M, Liedl T, Nickels PC. DNA Origami Meets Bottom-Up Nanopatterning. ACS nano. 2021;15(7):10769–10774. doi: 10.1021/acsnano.1c04297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Penzo E, Wang R, Palma M, Wind SJ. Selective placement of DNA origami on substrates patterned by nanoimprint lithography. Journal of Vacuum Science & Technology B. 2011;29(6):06F205 [Google Scholar]
- 35.Douglas SM, Marblestone AH, Teerapittayanon S, Vazquez A, Church GM, Shih WM. Rapid prototyping of 3D DNA-origami shapes with caDNAno. Nucleic Acids Res. 2009;37(15):5001–5006. doi: 10.1093/nar/gkp436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim D-N, Kilchherr F, Dietz H, Bathe M. Quantitative prediction of 3D solution shape and flexibility of nucleic acid nanostructures. Nucleic Acids Res. 2011;40(7):2862–2868. doi: 10.1093/nar/gkr1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suzuki Y, Endo M, Sugiyama H. Lipid-bilayer-assisted two-dimensional self-assembly of DNA origami nanostructures. Nature Communications. 2015;6(1):8052. doi: 10.1038/ncomms9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cao HH, Abel GR, Jr, Gu Q, Gueorguieva G-AV, Zhang Y, Nanney WA, et al. Seeding the Self-Assembly of DNA Origamis at Surfaces. ACS nano. 2020;14(5):5203–5212. doi: 10.1021/acsnano.9b09348. [DOI] [PubMed] [Google Scholar]
- 39.Jing X, Zhang F, Pan M, Dai X, Li J, Wang L, et al. Solidifying framework nucleic acids with silica. Nat Protoc. 2019;14(8):2416–2436. doi: 10.1038/s41596-019-0184-0. [DOI] [PubMed] [Google Scholar]
- 40.Wen X, Zhang B, Wang W, Ye F, Yue S, Guo H, et al. 3D-printed silica with nanoscale resolution. Nature Materials. 2021;20(11):1506–1511. doi: 10.1038/s41563-021-01111-2. [DOI] [PubMed] [Google Scholar]
- 41.Seniutinas G, Balčytis A, Reklaitis I, Chen F, Davis J, David C, et al. Tipping solutions: emerging 3D nano-fabrication/-imaging technologies. Nanophotonics. 2017;6(5):923–941. [Google Scholar]
- 42.Tan C, Yu M, Tang J, Gao X, Yin Y, Zhang Y, et al. 2D fin field-effect transistors integrated with epitaxial high-k gate oxide. Nature. 2023;616(7955):66–72. doi: 10.1038/s41586-023-05797-z. [DOI] [PubMed] [Google Scholar]
- 43.Gottlieb S, Rösner B, Evangelio L, Fernández-Regúlez M, Nogales A, García-Gutiérrez MC, et al. Self-assembly morphology of block copolymers in sub-10 nm topographical guiding patterns. Molecular Systems Design & Engineering. 2019;4(1):175–185. [Google Scholar]
- 44.Ouk Kim S, Solak HH, Stoykovich MP, Ferrier NJ, de Pablo JJ, Nealey PF. Epitaxial self-assembly of block copolymers on lithographically defined nanopatterned substrates. Nature. 2003;424(6947):411–414. doi: 10.1038/nature01775. [DOI] [PubMed] [Google Scholar]
- 45.Wickham SFJ, Auer A, Min J, Ponnuswamy N, Woehrstein JB, Schueder F, et al. Complex multicomponent patterns rendered on a 3D DNA-barrel pegboard. Nature Communications. 2020;11(1):5768. doi: 10.1038/s41467-020-18910-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang T, Hartl C, Frank K, Heuer-Jungemann A, Fischer S, Nickels PC, et al. 3D DNA Origami Crystals. Adv Mater. 2018;30(28):1800273. doi: 10.1002/adma.201800273. [DOI] [PubMed] [Google Scholar]
- 47.Shaw A, Benson E, Högberg B. Purification of Functionalized DNA Origami Nanostructures. ACS nano. 2015;9(5):4968–4975. doi: 10.1021/nn507035g. [DOI] [PubMed] [Google Scholar]
- 48.Stahl E, Martin TG, Praetorius F, Dietz H. Facile and scalable preparation of pure and dense DNA origami solutions. Angew Chem Int Ed Engl. 2014;53(47):12735–12740. doi: 10.1002/anie.201405991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin C, Perrault SD, Kwak M, Graf F, Shih WM. Purification of DNA-origami nanostructures by rate-zonal centrifugation. Nucleic Acids Res. 2012;41(2):e40. doi: 10.1093/nar/gks1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martynenko I, Erber E, Ruider V, Dass M, Posnjak G, Yin X, et al. Dryad; 2023. Site-directed placement of three-dimensional DNA origami. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are openly available in ref.50. The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.