Abstract
Purpose
This study aimed to examine the short-term effects of SARS-CoV-2 infection and return to sport (RTS) on neuromuscular performance, body composition, and mental health in well-trained young kayakers.
Methods
17 vaccinated kayakers (8 male, 9 female) underwent body composition assessment, peak power output bench press (BP), and 40-s maximum repetition BP tests 23.9 ± 1.6 days before and 22.5 ± 1.6 days after a SARS-CoV-2 infection. A linear transducer was used to examine the BP performance. The perception of training load and mental health were quantified with Borg's CR-10 scale and the Hooper questionnaire before and after infection. The difference and relationship of variables were used Wilcoxon test, Student t-test, Pearson's, and Spearman's r correlation coefficients.
Results
There was a significant increase in body mass, fat-free mass, and skeletal muscle mass, but no significant changes in body fat, fat mass, and all BP performance after infection (p < 0.05). There was a significant reduction in training hours per week, session rating of perceived exertion (sRPE), internal training load (sRPE-TL), fatigue, muscle soreness levels, and Hooper index, but no changes in sleep quality and stress levels after infection (p < 0.05). The training and mental health during the RTS period was significantly correlated (r = −0.85 to 0.70) with physical performance after infection.
Conclusion
A SARS-CoV-2 infection did not appear to impair the upper-body neuromuscular performance and mental health of vaccinated well-trained young kayakers after a short-term RTS period. These findings can assist coaches, and medical and club staff when guiding RTS strategies after other acute infections or similar restrictions.
Keywords: COVID-19, Athletes, Acute respiratory infection, Rehabilitation, Physical performance, Return to play
1. Introduction
Acute respiratory infections (ARinf), mainly caused by viruses, are the most typical form of acute illness among athletes and comprise about 50% of the disease attacks during major events.1 The waves of the pandemic caused by coronavirus disease 2019 (COVID-19) are still lingering and affecting populations in some regions, especially athletes.2,3 Athletes with ARinf including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) who experience symptoms such as fatigue, cough, and myalgia may face challenges when returning to sport and competition.1,4,5 Therefore, prompt and safe return to training after ARinf was an inescapable concern for affected athletes, coaches, and medical staff alike.6
The terminology “return to sport” (RTS) was widely used after injury and has recently been more explicitly defined as a continuum7 that begins with a return to participating (training) and ends with a full recovery to the former levels of athletic performance.6 Despite the numerous guidelines about RTS, there are limited evidence-based data on the effects of RTS among athletes after illness and the standardization is lacking.6,8 For a standardized RTS routine, the variable “days to return to training”, as the time point (day from onset of disease) when athletes proceed to the first training after an ARinf, was needly reported.1,6 Moreover, detraining refers to the partial or entire loss of morphological, physiological, and performance adaptations resulting from a decrease or cessation of training.9 Although numerous studies have investigated the effects of detraining due to the COVID-19 lockdown (without infection) on athletic performance,10 it is necessary to emphasize that athletes experiencing ARinf should take into account both the potential pathological effects of virus infection and the detraining effects due to lacking training stimulus.1,4,11,12
During the post-infection RTS period, it was paramount to consider the neuromuscular aspect of physical performance for athletes, including muscle function/strength, sprint/jump, and coordination, as this system plays a pivotal role in motion execution and was a significant risk factor for injury.10, 11, 12, 13 However, there was restricted data about RTS on neuromuscular performance among athletes after ARinf and most available studies also focus on the lower-body.14, 15, 16 Upper-body strength and power were of particular importance to some sports, like kayaking, cross-country skiing, rowing, etc.17,18 Furthermore, the effects of RTS during the pandemic on maintaining body composition in athletes remain mixed results.19, 20, 21 Given above, it is necessary to elucidate the influences of ARinf and RTS in athletes who demand high-level neuromuscular function and optimum body composition, such as kayakers.22,23
Professional athletes must possess a great deal of mental resilience to cope with training loads, performance demands, and external pressures.24 The COVID-19 waves and ensuing competitive postponement adversely affected the mental health of athletes,14,25 although some studies suggested that mental qualities remained stable in some cases.26,27 In fact, few studies described the effects of ARinf on athletic performance, and the limited data are lacking standardization.4,5 Furthermore, most present studies lack baseline (i.e., pre-infection) and training (e.g., period, load, and methods) data to delineate the relationships among RTS and exercise testing parameters after ARinf, and do not identify confounders (e.g., severity and duration of illness, vaccination status, etc.).4,5,8 Noteworthy is the fact that the athletes included in this study were affected by the third COVID-19 outbreak. At that time, the Omicron variation was the strain circulating in China.28 However, most previous studies appeared prior to Omicron's emergence, and vaccination data about the athletic population were limited.5 It is necessary to investigate how this new variant affects acute presentations in vaccinated athletes,5 and this might be the majority of scenarios in the future.2
In light of the above, there was an immediate need to provide evidence-based and standardized RTS information on how athletes in various sports disciplines and regions coped with the waves of new variants. This would contribute to creating a framework and strategy of RTS that could be used to deal with similar future restrictions caused by other ARinf.1,8 This study aimed to examine the short-term effects of SARS-CoV-2 infection and RTS on neuromuscular performance, body composition, and mental health in well-trained young kayakers with a history of vaccination during the third outbreak of the 2022–2023 season.
2. Methods
2.1. Participants
Seventeen (8 male, 9 female) well-trained sprint kayakers (age 17.5 ± 1.6 yrs, height 176.0 ± 7.0 cm, training experience 3.02 ± 1.60 yrs, typical training volume 21.23 ± 2.35 h wk−1; mean ± SD) volunteered to participate in the study. Participants were all from the same provincial training base of water sports in China and were competitors at the highest level of national competition in their age group. These athletes were regularly monitored by the training staff, and those who had contracted the virus were included as participants in the present study. The present study obtained explicit written consent from each participant and their respective guardians in the case of minors after a comprehensive explanation of the study. Approval for conducting the study was secured from the Ethics Committee of the Shanghai University of Sport (number of approval: 102772022RT102).
2.2. Study design
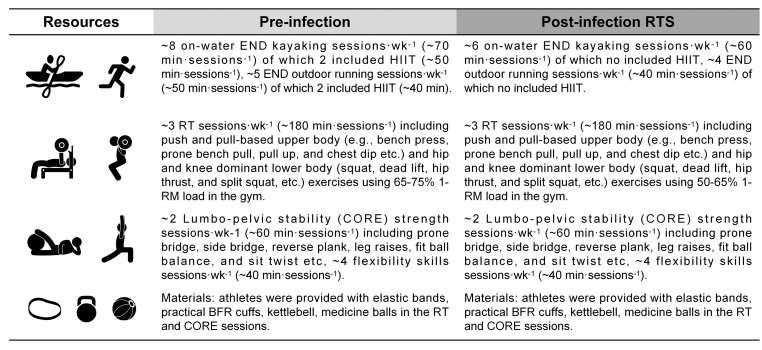
Participants were tested two times before and after the SARS-CoV-2 infection (Table 1). The pre-test was a regular physical test conducted before the start of the general preparation period. All athletes were at the same training stage and fitness level, and they maintained their regular training routine before the infection. Athletes detrained in succession by onset of symptoms (December 17 to 23, 2022). The training base was closed, and the normal training programs were all cancelled (December 23, 2022 to January 1, 2023), all participants were resting in dormitories with no physical activity (Fig. 1). A progressive ∼2 weeks RTS routine after infection was started (January 2, 2023). The post-test proceeded after infection and the RTS period. All tests were conducted under standardized resting conditions at the same time of day, 24 h apart with body composition assessment being assessed first. The perception of training load and mental health were measured daily and averaged weekly before and during the RTS period after infection. The training routine followed by the kayakers during the different periods was available (Fig. 2).
Table 1.
Participants characteristics.
| N = 17 | ||
|---|---|---|
| Age (years) | 17.5 ± 1.6 | |
| Female (n,%) | 9 (52.9%) | |
| Height (cm) | 176.0 ± 7.0 | |
| Vaccination status (dose) | 3 | 2 (11.78%) |
| 2 | 12 (70.6%) | |
| 1 | 3 (17.7%) | |
| SBP/DBP (mmHg) | 125.2 ± 8.1/70.5 ± 7.3 | |
| SpO2 (%) | 98.3 ± 0.8 | |
| Symptoms duration (days) | 2.9 ± 1.1 | |
| RTT (days) | 10.4 ± 1.8 | |
| RTS (days) | 12.1 ± 1.1 | |
| Interval between pre-test and detected infection (days) | 23.9 ± 1.6 | |
| Interval between detected infection and post-test (days) | 22.5 ± 1.6 | |
Abbreviation: SBP, systolic blood pressure; DBP, diastolic blood pressure; SpO2, resting peripheral oxygen saturation, RTT, return to training; RTS, return to sport.
Fig. 1.
Timeline and data collection. The number in circles denotes sequences of the testing procedure. Number 1, body composition analysis; Number 2, neuromuscular performance test; Number 3 and 6, daily collected training load and mental health; Number 4, COVID-19 specific questionnaire; Number 5, blood pressure and SpO2. Each colored block denotes different periods. Abbreviation: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; RTT, return to training; RTS, return to sport.
Fig. 2.
Training routine followed by the kayakers during the pre- and post-infection RTS period. Abbreviation: END, endurance training; HIIT, high-intensity interval training; RT, resistance training; BFR, blood flow restriction, 1-RM, one-repetition maximum, RTS, return to sport. Note: no difference in compliance with the training sessions during two periods (97.2 ± 5.8 vs 96.8 ± 9.0 [%]).
All participants were first-time infected with SARS-CoV-2. Athletes were subjected to a nasal swab antigen test through the training base's medical staff after the onset of any COVID-19-related symptoms, for confirming the infection positively. According to the International Olympic Committee consensus statement, our cases were defined as confirmed general (upper/lower) mild to moderate ARinf.1,4 To determine the severity of the illness, a specific questionnaire was administered to gather information about the duration of symptoms, the symptoms, and vaccination status. Participants were excluded if they had a medical history (e.g., heart disease, blood vessel disease, and asthma, etc.), smoking history, any significant musculoskeletal injury, blood pressure over 150/90 mmHg (YE655A, YuWell, China), or resting peripheral oxygen saturation (SpO2%) below 95 (YX306, Yuwell, China). Blood pressure and SpO2% were measured three times 2 min apart by medical staff and the mean was taken. All participants had a Polymerase Chain Reaction test to confirm that they were negative before the post-test and had no residual symptoms at the moment of testing.
2.3. Test procedure
Body mass (BM), body fat (BF), fat mass (FATM), fat-free mass (FFM), and skeletal muscle mass (SKM) were assessed with an IOI353 device based on 8-electrode bioelectrical impedance analysis (IOI353, Jawon Medical, Korea), which provides a comparable measurement for assessing body fat mass compared with Dual Energy X-ray Absorptiometry.29 Participants were to attain a state of fasting and refrain from caffeine and alcohol intake for a minimum of 8 h before the analysis.
Peak power output (explosive strength) and 40-s maximum repetition (muscular endurance) were tested in the bench press (BP), as described before.30 The subjects lay supine on a flat bench with their feet resting flat on the floor. After lowering the barbell to the chest, they pushed up at maximum velocity to full elbow extension. Subjects were not allowed to bounce the bar off their chest or lift their shoulders or trunk off the bench. Before the formal tests, a warm-up consisting of 10 repetitions with a barbell (20 kg) was performed, and one familiarization set was performed by 3 single repetitions at 40% of the one-repetition maximum (1RM), which was established by a 1RM test separate 24 h before. For the formal tests, each subject performed 3 repetitions, separated by a 2-min rest, with the instruction to lift 50% of their 1RM as quickly as possible. If the subjects reached the highest peak power (PP) in formal trial number 3, more trials were conducted until no further increase in PP was recorded. The pre- and post-test used the same load. The highest value was used for the analysis. PP was recorded by a linear encoder (GymAware, Kinetic Performance Technologies, Canberra, Australia). The 40-s maximal repetition test of BP identified the number of repetitions athletes could perform in 40 s (40-s) with 40% of their 1RM. The same linear encoder was used to measure mean power output (MP), mean propulsion velocity (MV), and maximal repetitions (R) during the movement of the barbell.
The perception of training load and mental health of the athletes were evaluated using the Borg's CR-10 scale and the Hooper questionnaire, respectively. The Borg's CR-10 scale, modified by Foster et al.,31 was used to monitor the athletes' session rating of perceived exertion (sRPE) 30 min after each training session. The good validity and reliability of sRPE method to monitor training load was confirmed in the previous studies.32 sRPE stands for the perception of training intensity was divided into 3 zones as low (≤4), moderate (5–6), and high intensity (≥7) in accordance with the zones, as described before.33 The internal training load (sRPE-TL) was obtained by multiplying each athlete's sRPE value by the duration of each session in minutes (sRPE-TL = sRPE [AU] x session duration [mins], as suggested before.31,33
The Hooper questionnaire required athletes to rate their perceived levels of sleep quality, stress, fatigue, and delayed onset muscle soreness (DOMS) each morning, approximately 30 min before training sessions.34 The scale ranged from 1 (very, very low) to 7 (very, very high) for stress, fatigue, and DOMS categories. For sleep quality, 1 represented “very, very good” and 7 represented “very, very bad”. The Hooper Index (HI), calculated as the sum of the four rates (one for each item) for each day, was used to assess the overall daily scores. Each athlete selected their rating by touching the appropriate score on the portable computer tablet (iPad©, Apple Inc., California, USA). By adopting this approach, potential factors that may impact an athlete's appraisal were effectively minimized.35
2.4. Statistical analysis
Data are presented as mean ± standard deviation (SD) for continuous variables and numbers (n) with percentage (%) for categorical variables. The Shapiro-Wilk test was performed to assess the normality of the distribution. To assess the differences between pre- and post-test, paired t-tests were used for normal data and Wilcoxon signed-rank tests for non-normal data. To understand possible relationships between training, mental health during the RTS period, and physical performance parameters (post-test), Pearson's and Spearman's r correlation coefficients were used. Statistical significance was accepted at p < 0.05. The Cohen's d effect size (ES) was computed to display the magnitude of differences between variables and interpreted as trivial (0–0.19), small (0.20–0.49), medium (0.50–0.79), and large (≥0.80).36 The statistical analysis was performed using IBM SPSS version 27 (IBM company, Armonk, New York, USA).
3. Results
According to the specific questionnaire, athletes self-reported the duration of symptoms, the symptoms, and vaccination status, which were presented in Table 1 and Fig. 3.
Fig. 3.
The prevalence of self-reported symptoms in athletes infected with SARS-CoV-2.
There were significant increases in BM, BMI, FFM, and SKM as well as decreases in training hours, RPE, sPRE, fatigue, DOMS levels, and HI post-infection (p < 0.05). There were no significant changes in BF, FATM, all BP performance, sleep quality, and stress levels pre and post-infection (p > 0.05) (Table 2, Table 3).
Table 2.
Body composition and neuromuscular performance variables of well-trained young kayakers pre- and post-infection.
| Pre | Post | MD (95%CI) | p | ES | |
|---|---|---|---|---|---|
| BM (kg) | 68.82 ± 9.99 | 69.91 ± 9.28 | −1.09 ± 1.28 (−1.75 to −0.43) | 0.003a | −0.85 |
| BMI (kg·m−2) | 22.12 ± 2.07 | 22.47 ± 1.91 | −0.35 ± 0.43 (−0.57 to −0.13) | 0.004a | −0.81 |
| BF (%) | 18.95 ± 4.81 | 19.09 ± 5.11 | −0.14 ± 1.40 (−0.85 to 0.58) | 0.695 | −0.10 |
| FATM (kg) | 13.01 ± 3.85 | 13.34 ± 3.99 | −0.32 ± 1.17 (−0.93 to 0.28) | 0.273 | −0.28 |
| FFM (kg) | 55.81 ± 8.83 | 56.56 ± 8.44 | −0.75 ± 1.25 (−1.39 to −0.11) | 0.024a | −0.60 |
| SKM (kg) | 31.20 ± 5.08 | 31.66 ± 4.84 | −0.46 ± 0.71 (−0.83 to −0.09) | 0.018a | −0.64 |
| PPa, BP (w) | 559.65 ± 149.21 | 547.18 ± 158.76 | 12.47 ± 131.58 (−55.18 to 80.12) | 0.701 | 0.09 |
| PP, BP (w·kg−1) | 8.17 ± 2.02 | 7.74 ± 1.59 | 0.43 ± 2.03 (−0.61 to 1.47) | 0.394 | 0.21 |
| MPa, 40-s BP (w) | 254.30 ± 101.45 | 238.98 ± 52.93 | 15.31 ± 68.18 (−19.74 to 50.36) | 0.459 | 0.22 |
| MP, 40-s BP (w·kg−1) | 3.63 ± 0.90 | 3.41 ± 0.55 | 0.22 ± 0.76 (−0.17 to 0.61) | 0.159 | 0.40 |
| MV, 40-s BP (m·s−1) | 0.76 ± 0.06 | 0.74 ± 0.06 | 0.01 ± 0.05 (−0.01 to 0.04) | 0.231 | 0.30 |
| R, 40-s BP (n) | 43.71 ± 7.10 | 42.29 ± 4.25 | 1.41 ± 6.49 (−1.93 to 4.75) | 0.141 | 0.41 |
Abbreviation: MD, mean differences; 95% CI, confidence interval at 95%; ES, effect size; BM, body mass; BMI, body mass index; BF, body fat; FATM, fat mass; FFM, fat-free mass; SKM, skeletal muscle mass; BP, bench press; 40-s, 40-s; PPa, absolute peak power output; MPa, absolute mean power output; MV, mean velocity, R, maximal repetitions.
Indicated significant changes comparing pre- and post-infection.
Table 3.
Training load and mental health variables of well-trained young kayakers pre- and post-infection.
| Pre | Post | MD (95%CI) | p | ES | |
|---|---|---|---|---|---|
| Thours (h·wk−1) | 21.23 ± 2.35 | 19.18 ± 1.62 | 2.05 ± 2.61 (0.71–3.39) | 0.001a | 0.88 |
| sRPE (AU) | 4.59 ± 0.95 | 3.85 ± 0.68 | 0.73 ± 0.76 (0.34–1.12) | 0.001a | 0.97 |
| sRPE-TL (AU) | 376.89 ± 112.28 | 304.05 ± 57.69 | 72.84 ± 89.15 (27.00–118.67) | 0.004a | 0.82 |
| Sleep quality (AU) | 3.20 ± 0.92 | 3.09 ± 0.88 | 0.11 ± 0.29 (−0.04 to 0.26) | 0.379 | 0.26 |
| Stress (AU) | 2.87 ± 0.89 | 2.85 ± 0.83 | 0.03 ± 0.47 (−0.21 to 0.27) | 0.821 | 0.06 |
| Fatigue (AU) | 3.61 ± 0.67 | 3.22 ± 0.76 | 0.39 ± 0.46 (0.16–0.63) | 0.003a | 0.86 |
| DOMS (AU) | 3.96 ± 0.42 | 3.59 ± 0.72 | 0.37 ± 0.60 (0.06–0.68) | 0.014a | 0.71 |
| HI (AU) | 13.65 ± 2.63 | 12.75 ± 2.97 | 0.90 ± 1.27 (0.25–1.55) | 0.010a | 0.71 |
Abbreviation: MD, mean differences; 95% CI, confidence interval at 95%; ES, effect size; AU, arbitrary unit; Thours, training hours per week; sRPE, session rating of perceived exertion; sRPE-TL, internal training load obtained by sRPE; DOMS, delayed onset muscle soreness; HI, Hooper index.
Indicated significant changes comparing pre- and post-infection.
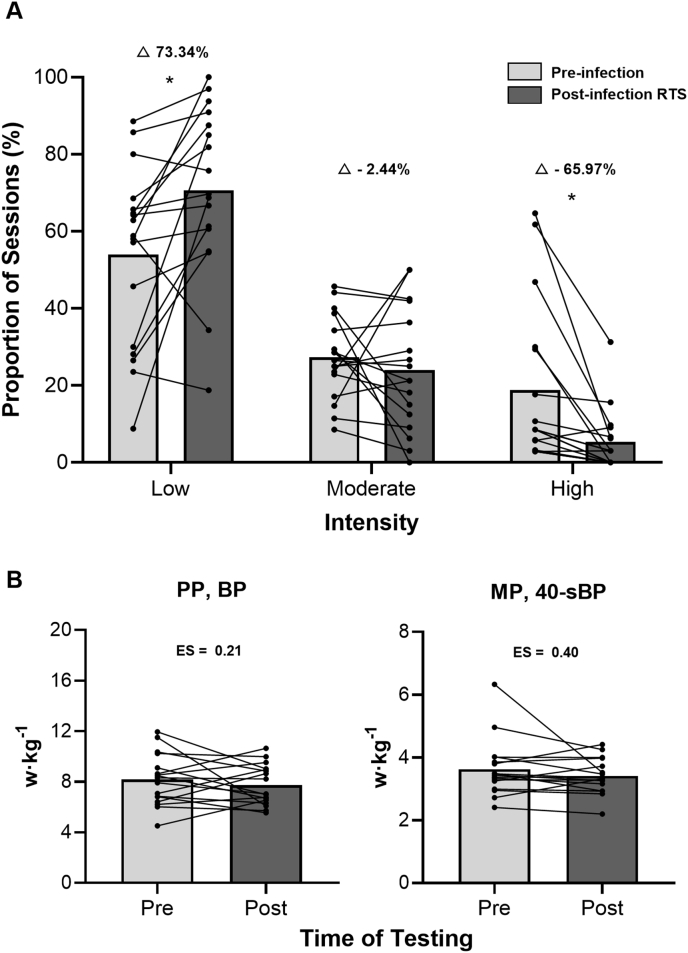
The number of sessions significantly decreased (33.59 ± 2.09 vs 31.47 ± 3.08 [n]; p < 0.001; ES = 0.87) as well as no significant changes in the duration of sessions (73.43 ± 10.13 vs 70.78 ± 6.17 [mins]; p = 0.251; ES = 0.32) pre- and post-infection. Regarding training intensity (Fig. 4, A), there were significant increases in low-intensity sessions (53.95 ± 23.21 vs 70.66 ± 22.17 [%]; p = 0.007; ES = −0.75) and significant reductions in high-intensity sessions (18.75 ± 20.63 vs 5.35 ± 8.03 [%]; p < 0.001; ES = 0.91). There were no significant changes in moderate-intensity sessions (27.30 ± 10.84 vs 23.99 ± 15.84 [%]; p = 0.445; ES = 0.19).
Fig. 4.
The perception of training intensity (session rating of perceived exertion [sRPE]) performed during the pre- and post-infection RTS period (A); Pre- and post-test BP performance data (B). Bars denote mean values, and dots and lines denote individual changes. sRPE (AU) were divided into 3 zones as low (≤4), moderate (5–6), and high (≥7).33 Abbreviation: RTS, return to sport; Δ%, relative changes, ES, effect size; BP, bench press; 40-s, 40-s; PP, peak power output; MP, mean power output. ∗p < 0.05.
There were significant correlations found between training (symptom duration [Sdays], days to return to training [RTTdays], RTS duration [RTSdays], and training hours per week [Thours]) and mental health during the RTS period and the physical performance after infection which ranged from r = −0.85 (p < 0.001) to r = 0.70 (p < 0.01) (Table 4).
Table 4.
Correlations between training and mental health during the post-infection RTS period, body composition, and neuromuscular performance (post-test).
| Variables | Post-test parameters | Correlation coefficients | Variables | Post-test parameters | Correlation coefficients |
|---|---|---|---|---|---|
| Sdays | PP, BP | −0.56 ∗ | Sleep | MP, 40-s BP | −0.83 ∗∗∗ |
| RTTdays | BF | 0.57 ∗ | Stress | MP, 40-s BP | −0.77 ∗∗∗ |
| RTSdays & Thours | BM | 0.62 & 0.64 ∗∗ | Fatigue | MP, 40-s BP | −0.75 ∗∗∗ |
| FFM | 0.67 & 0.69 ∗∗ | DOMS | BF | 0.60 ∗ | |
| SKM | 0.68 & 0.70 ∗∗ | FATM | 0.49 ∗ | ||
| PP, BP | 0.51 & 0.54 ∗ | MP, 40-s BP | −0.74 ∗∗∗ | ||
| MV, 40-s BP | 0.55 & 0.57 ∗∗ | HI | MP, 40-s BP | −0.85 ∗∗∗ |
Abbreviation: Sdays, symptoms duration (days); RTTdays, days to return to training (days); RTSdays, return to sport duration (days); Thours, training hours per week in RTS (h·wk−1); DOMS, delayed onset muscle soreness (AU); HI, hooper index (AU); BM, body mass (kg); BF, body fat (%); FATM, fat mass (kg); FFM, fat-free mass (kg); SKM, skeletal muscle mass (kg); 40-s, 40-s; BP, bench press; PP, peak power output (w·kg−1); MP, mean power output (w·kg−1); MV, mean velocity (m·s−1). Significant correlations: ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
4. Discussion
Our main findings showed a SARS-CoV-2 infection did not significantly change BP performance, body fat, and fat mass after a short-term RTS period. There were significant increases in body mass, BMI, fat-free mass, and skeletal muscle mass as well as decreases in training load, fatigue, DOMS levels, and HI after infection. Furthermore, training and mental health during the RTS period were found to be significant correlates of physical performance after infection.
4.1. Infection symptoms
Regarding most ARinf, caused by different pathogens (rhinoviruses, influenza, and SARS-CoV-2, etc), inflammatory mediators and cytokine storms can trigger a series of regional and generalized symptoms, such as rhinorrhoea, fever, headache, fatigue, and myalgia.1,11 Although ARinf symptoms are commonly non-specific, these traits (type, duration) could denote the severity of ARinf due to the relationship correlated with the degree of inflammatory response.37 Our athletes' symptom duration (i.e., ∼3 days) was shorter than the previous study (mean symptom duration of 7 days).8 Lemes et al.5 demonstrated that the vast majority of athletes (∼94%) who contract SARS-CoV-2 remained asymptomatic or presented only mild acute symptoms. Furthermore, the athlete's prevalence of severe COVID-19 cases was lower, at 1.3%, compared to young people in the general population (2.7%).38
It is worth mentioning that our athletes were all infected with SARS-CoV-2 for the first time and all had a history of vaccination. By the end of November 2022, less than 0.25% of China's 1.4 billion population had been infected.28 From December 1, 2022, to March 2, 2023, data from the Chinese Center for Disease Control and Prevention (chinacdc.cn) showed that a total of 20,551 valid genome sequences of SARS-CoV-2 cases were reported in China, all of which were Omicron variants. In COVID-19 infection with Omicron always causes milder symptoms, especially in vaccinated individuals.39 Our results suggest that professional young athletes who have been vaccinated do not seem to be highly susceptible to the most harmful impacts of new variants (i.e., Omicron). Furthermore, because the loss of gain ratio strongly advocates vaccination,40 we also support vaccination as an important tool for athletes preventing ARinf. However, more evidence about the influence of vaccinations on training in athletes is needed.5,40
4.2. Neuromuscular performance
No significant changes were observed in any BP performance indices before and after infection (Table 2). Previous studies indicated that short-term detraining (<4 weeks) was not enough to significantly decrease the neuromuscular performance in well- and highly-trained athletes.15,16,41 Moreover, there was only one earlier study of no significant changes on upper-body strength before and after SARS-CoV-2 infection (interval between infection diagnosis and tests median 45 days) in Swiss recruits.42 The ARinf could cause a decline in muscle protein content, muscular strength/endurance, enzyme activity, and aberrant mitochondrial function.4,11 However, muscle protein restoration may require two weeks,11 which could explain the absence of alteration in BP performance measured ∼22 days after infection, as athletes when testing may fully recover after an RTS period.15,16 In this sense, the interval between infection diagnosis and tests in previous studies ranged from 2 weeks to approximately 2 months.14, 15, 16,42 Although the acute effect of early neuromuscular performance following ARinf is unknown, we also do not advocate maximal neuromuscular testing directly after infection as this is irresponsible to these professional athletes due to the extremely high rate of injury following infection.12,13 Therefore, based on current evidence that the neuromuscular function of athletes could recover after a short-term RTS period. More importance should be given to how to carry out an effective RTS process after ARinf.1,8
Our athletes were advised to rest after infection, and then resume training using a progressive RTS procedure. The low-intensity proportion of training sessions increased by 73% during the RTS period compared to pre-infection (Fig. 4, A). Additionally, significant positive correlations (r = 0.51 to 0.57) were founded in RTSdays and Thours with BP performance as well as Sdays significant negative correlated (r = −0.56) with BP performance after infection (Table 4). These findings underscore the importance of maintaining adequate training volume in the RTS routine to mitigate detraining effects after ARinf among professional athletes.20,43 On the other hand, the high-intensity proportion of training sessions decreased by 66% during RTS period (Fig. 4, A), which could explain some signs of deconditioning (small ES = 0.21 to 0.40) observed in the BP performance results for some athletes especially in higher performance individuals (Fig. 4, B). Muriel et al.43 indicated that the decline in mean maximal power output (ES = 1.36 to 1.66) could be attributed to acute reductions (−41 to −52%) in high-intensity volumes in elite cyclists during the 7 weeks COVID-19 confinement period. Despite the RT being a range of loads between 50% and 65% 1RM in the RTS routine (Fig. 2), in certain higher performance athletes the higher loading variations (i.e., >65% 1RM) might be needed to alleviate the negative effects of detraining.44,45 It could be speculated that significant performance decreases (large ESs) may occur if these athletes continue to perform loads that can not provide adequate training stimulus for longer periods.9,45 Moreover, the plyometric and sprinting training was completely lacking during the RTS period, this might lead to possible changes in fiber-type morphology resulting in potential declines in neuromuscular performance.19,20 In applied terms, coaches and training staff should be aware of individual responses during the RTS period after an ARinf and are encouraged to provide individually planned RTS routines that are well-structured (i.e., containing a variety of training methods).3,20,41 Furthermore, the effects of ARinf on the upper and lower-body neuromuscular function may differ due to the relatively small muscle mass and differences in muscle fiber type, oxygen uptake, glucose and fat oxidation ability compared to the lower-body.17,18 Regarding the upper-body function was crucial for many other exercises (e.g., rowing, kayaking, water-polo, cross-country skiing, etc),18,22 it may be useful in future studies to refine the RTS strategy according to the importance of different body parts of the athletes for performance.
It must be noted that athletes were counseled against training during their ARinf period owing to the potential exacerbation of the infection.1,11 Despite athletes experiencing anxiety about regaining their previous levels of performance, engaging an over-aggressive RTS program (i.e., overtraining) after infection may result in weakened immunity, dysregulated systemic inflammatory response, and increase the risk of injuries.11,12,16,46 The above underlying deleterious effects may explain the transformation of some mildly or no symptom athletes into long-symptom after infection.5,16,47 Therefore, we advocate that training load should be cautiously managed to ensure the health of athletes during the RTS period after infection, and further research on RTS after ARinf in athletes is warranted.4,6
4.3. Body composition
There were significant increases in BM, BMI, FFM, and SKM, while BF and FATM remained unchanged before and after infection (Table 2). Patients with COVID-19 are often prone to weight loss due to various factors such as fatigue, fever, anorexia, and dysgeusia, which may be dependent on the progress of the illness.48 However, kayakers had a shorter duration of symptoms. Moreover, recent studies have shown that the body composition was not changed in athletes during the COVID-19 detraining period due to maintaining an adequate training stimulus and having well-available training facilities.20,21,44 Our results showed that RTSdays and Thours were positively correlated (r = 0.62 to 0.64) with BM, while RTTdays were positively correlated (r = 0.57) with BF after infection (Table 4). These results suggest that athletes performing a general training routine quickly could mitigate the detraining effect on body composition after infection. Interestingly, we found that the levels of DOMS during the RTS period were significantly positively correlated (r = 0.49 to 0.60) with BF and FATM after infection. It was presumed that a lack of adaptation to the RTS training load among certain athletes may lead to an elevation of DOMS levels, resulting in decreased physical activity and negative impacts on body composition.49 Furthermore, the RTS programs and nutrition plans for requirements of different sports disciplines (e.g., light-weight may facing greater challenges than heavy- or open-weight classes on managing body composition) in various periods (e.g., off-, pre-, and in-season) may need to be tailored and the coaches and training practitioners should take note of this.3,20
4.4. Mental health
There was a significant decrease in fatigue, DOMS, and HI, while sleep quality and stress remained unchanged (Table 3). The reduction in training load resulted in lower fatigue and DOMS levels leading to a lower HI.34 Besides, training schedules contribute to athletes maintaining athletes general daily routines, potentially resulting in fewer mental health problems.50 As a matter of fact, the outbreak of COVID-19 induced nearly no change in the management of professional training bases in China. Athletes generally train in the base from Monday to Saturday and go out of the base during the half to one day off on the weekend. The accommodation, daily diet, nutrition, and medical care of athletes are all undertaken by the training base, and they also receive regular psychological follow-ups from coaches and medical staff. It can be inferred that the COVID-19 pandemic did not significantly alter the training routine and lifestyle of professional athletes in this region, as they were already adapted to this way of daily routine and may have had less stress on their finances.
Significant negative correlations (r = −0.74 to −0.85) were found between mental health during the RTS period and BP performance after infection (Table 4). This could be explained by that athletes might be facing psychological challenges (e.g., stress from decreased performance and coaching expectations, overthinking postponed or canceled competitions, increased uncertainty about future budgetary and economic backing, etc.) upon return to training.24,25,51 From an application viewpoint, coaches and medical staff should be aware of and strengthen the individual caring (e.g., positive conversation or psychotherapy)3 because potential mental well-being issues experienced by some athletes during the RTS period after ARinf may affect subsequent training and performance.25 Furthermore, our athletes also maintained daily associations with teammates, coaches, and medical staff, and the availability of training facilities (e.g., on-water, outdoor, and gym sessions, training materials, etc.) was similar to that before the pandemic (Fig. 2). These findings reinforce the necessity for enhanced psychological support, along with other important aspects (e.g., social connections, facilities, finance, etc.), or additional interventions to help professional athletes deal with similar restrictions in the future.3,52
4.5. Limitations
Certain limitations of this study need to be recognized. These limitations are associated with the use of a single team and the absence of a control group. Moreover, due to the potential risk of injury from participating in high-intensity testing without complete recovery from the infection, no exercise performance assessments were conducted immediately following the infection. It is not possible to determine when the athletes will suffer infection and therefore earlier pre-infection testing (baseline) data could not be obtained. These limitations resulted from the study's observational nature and the unforeseeable and uncertain duration of the COVID-19 pandemic. In addition, controlling athletes' nutrition might help support the findings. To the best of our knowledge, there have been no published studies on the effect of SARS-CoV-2 infection and RTS on physical performance among professional athletes in China. However, despite these limitations, our study contributes to the field of research and initiates further studies.
5. Conclusions
Our study found that a SARS-CoV-2 infection did not appear to deteriorate the upper-body neuromuscular performance and mental health of well-trained young kayakers with a vaccination history after a short-term RTS period. These results should be interpreted by taking into consideration that post-testing was conducted ∼22 days after confirming the infection positively. Future studies should use standardized methods to examine the relationship between ARinf and other exercise/sports performance in different periods (e.g., off-season or competitive season).1,4 Taking into account the present results, athletes recovering from ARinf need to resume general training routine and habitual social connections as early as possible and the RTS strategy needs individually planned. Athletes, coaches, medical professionals as well as club/training staff ought to take into consideration the current information in dealing with similar acute respiratory infections in the future, such as flu or the waves due to new virus variants.
Data availability statement
If requested reasonably the authors of this study will consider providing raw data that supports their conclusions.
Ethics statement
The research protocols involving human subjects were subjected to rigorous review and received approval from the ethical committee at Shanghai University of Sport. All individuals who participated in the study provided explicit written consent after receiving adequate information about the nature and scope of the study.
CRediT authors statement
Conceptualization, S.J.D., Y.M.L.; methodology, S.J.D., Y.M.L.; investigation, S.J.D., J.F.D., M.Y·Y., Y.X.L.; resources, S.Q.Z., S.G.H., Y.M.L.; formal analysis, S.J.D.; visualization, S.J.D.; writing - original draft, S.J.D.; writing - review and editing, Y.M.L., G.P·N., Z.L.C., B·Y.Z.; supervision, Y.M.L., Z.L.C. All authors have read and approved the version of the manuscript.
Declaration of competing interest
A conflict of interest occurs when an individual's objectivity is potentially compromised by a desire for financial gain, prominence, professional advancement or a successful outcome. JESF Editors strive to ensure that what is published in the Journal is as balanced, objective and evidence-based as possible. Since it can be difficult to distinguish between an actual conflict of interest and a perceived conflict of interest, the Journal requires authors to disclose all and any potential conflicts of interest.
Funding
No funding was received.
Acknowledgments
The authors express their gratitude to the athletes and coaches who generously participated in the study, and provided support despite such a challenging case. Special appreciated the authors (e.g., Khoon Lay Gan, etc) from vecteezy.com for the exquisite vectors. The authors declare that they have no conflicts of interest to report.
References
- 1.Schwellnus M., Adami P.E., Bougault V., et al. International Olympic Committee (IOC) consensus statement on acute respiratory illness in athletes part 1: acute respiratory infections. Br J Sports Med. 2022;56(19):1066–1088. doi: 10.1136/bjsports-2022-105759. [DOI] [PubMed] [Google Scholar]
- 2.Callaway E. COVID's future: mini-waves rather than seasonal surges. Nature. 2023 doi: 10.1038/d41586-023-01437-8. Published online May 1. [DOI] [PubMed] [Google Scholar]
- 3.Washif J.A., Kok L.Y., James C., et al. Athlete level, sport-type, and gender influences on training, mental health, and sleep during the early COVID-19 lockdown in Malaysia. Front Physiol. 2023:13. doi: 10.3389/fphys.2022.1093965. https://www.frontiersin.org/articles/10.3389/fphys.2022.1093965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaulback K., Pyne D.B., Hull J.H., Snyders C., Sewry N., Schwellnus M. The effects of acute respiratory illness on exercise and sports performance outcomes in athletes - a systematic review by a subgroup of the IOC consensus group on “Acute respiratory illness in the athlete.”. Eur J Sport Sci. 2022:1–19. doi: 10.1080/17461391.2022.2089914. Published online July 8. [DOI] [PubMed] [Google Scholar]
- 5.Lemes I.R., Smaira F.I., Ribeiro W.J.D., et al. Acute and post-acute COVID-19 presentations in athletes: a systematic review and meta-analysis. Br J Sports Med. 2022;56(16):941. doi: 10.1136/bjsports-2022-105583. [DOI] [PubMed] [Google Scholar]
- 6.Snyders C., Schwellnus M., Sewry N., et al. Symptom number and reduced preinfection training predict prolonged return to training after SARS-CoV-2 in athletes: aware IV. Med Sci Sports Exerc. 2023;55(1):1–8. doi: 10.1249/MSS.0000000000003027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doege J., Ayres J.M., Mackay M.J., et al. Defining return to sport: a systematic review. Orthop J Sports Med. 2021;9(7) doi: 10.1177/23259671211009589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Snyders C., Pyne D.B., Sewry N., Hull J.H., Kaulback K., Schwellnus M. Acute respiratory illness and return to sport: a systematic review and meta-analysis by a subgroup of the IOC consensus on ‘acute respiratory illness in the athlete.’. Br J Sports Med. 2022;56(4):223–232. doi: 10.1136/bjsports-2021-104719. [DOI] [PubMed] [Google Scholar]
- 9.Mujika I., Padilla S. Detraining: loss of training-induced physiological and performance adaptations. Part I: short term insufficient training stimulus. Sports Med. 2000;30(2):79–87. doi: 10.2165/00007256-200030020-00002. [DOI] [PubMed] [Google Scholar]
- 10.Córdova-Martínez A., Caballero-García A., Roche E., Pérez-Valdecantos D., Noriega D.C. Effects and causes of detraining in athletes due to COVID-19: a review. Int J Environ Res Publ Health. 2022;19(9):5400. doi: 10.3390/ijerph19095400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Börjesson M., Arvidsson D., Rensburg C.J.V., Schwellnus M. Return to play after infectious diseas. Return to Play in FootballAn Evidence-based Approach. 2017;1:755–769. Published online September. [Google Scholar]
- 12.Wezenbeek E., Denolf S., Willems T.M., et al. Association between SARS-COV-2 infection and muscle strain injury occurrence in elite male football players: a prospective study of 29 weeks including three teams from the Belgian professional football league. Br J Sports Med. 2022;56(14):818–823. doi: 10.1136/bjsports-2021-104595. [DOI] [PubMed] [Google Scholar]
- 13.Maestro A., Varillas-Delgado D., Morencos E., et al. Injury incidence increases after COVID-19 infection: a case study with a male professional football team. Int J Environ Res Publ Health. 2022;19(16) doi: 10.3390/ijerph191610267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wagemans J., Catteeuw P., Vandenhouten J., et al. The impact of COVID-19 on physical performance and mental health—a retrospective case series of Belgian male professional football players. Frontiers in Sports and Active Living. 2021;3 doi: 10.3389/fspor.2021.803130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dauty M., Grondin J., Daley P., Louguet B., Menu P., Fouasson-Chailloux A. Consequences of the SARS-CoV-2 infection on anaerobic performances in young elite soccer players. Int J Environ Res Publ Health. 2022;19(11):6418. doi: 10.3390/ijerph19116418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wezenbeek E., Denolf S., Bourgois J.G., et al. Impact of (long) COVID on athletes' performance: a prospective study in elite football players. Ann Med. 2023;55(1) doi: 10.1080/07853890.2023.2198776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calbet J.a.L., Holmberg H.C., Rosdahl H., van Hall G., Jensen-Urstad M., Saltin B. Why do arms extract less oxygen than legs during exercise? Am J Physiol Regul Integr Comp Physiol. 2005;289(5):R1448–R1458. doi: 10.1152/ajpregu.00824.2004. [DOI] [PubMed] [Google Scholar]
- 18.Ørtenblad N., Nielsen J., Boushel R., Söderlund K., Saltin B., Holmberg H.C. The muscle fiber profiles, mitochondrial content, and enzyme activities of the exceptionally well-trained arm and leg muscles of elite cross-country skiers. Front Physiol. 2018;9:1031. doi: 10.3389/fphys.2018.01031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grazioli R., Loturco I., Baroni B.M., et al. Coronavirus disease-19 quarantine is more detrimental than traditional off-season on physical conditioning of professional soccer players. J Strength Condit Res. 2020;34(12):3316–3320. doi: 10.1519/JSC.0000000000003890. [DOI] [PubMed] [Google Scholar]
- 20.Spyrou K., Alcaraz P.E., Marín-Cascales E., et al. Effects of the COVID-19 lockdown on neuromuscular performance and body composition in elite futsal players. J Strength Condit Res. 2021;35(8):2309. doi: 10.1519/JSC.0000000000004028. [DOI] [PubMed] [Google Scholar]
- 21.Czeck M.A., Roelofs E.J., Evanoff N.G., Dengel D.R. No changes in body composition in NCAA division I collegiate football players because of COVID-19 restrictions. J Strength Condit Res. 2022;36(6):1749. doi: 10.1519/JSC.0000000000004260. [DOI] [PubMed] [Google Scholar]
- 22.Pickett C.W., Nosaka Kazunori, James Zois, Hopkins Will G., Blazevich Anthony J. Maximal upper-body strength and oxygen uptake are associated with performance in high-level 200-M sprint kayakers. J Strength Condit Res. 2018;32(11):3186–3192. doi: 10.1519/JSC.0000000000002398. [DOI] [PubMed] [Google Scholar]
- 23.Kukic F., Petrovic M., Greco G., Cataldi S., Fischetti F. Association of anthropometrics and body composition with maximal and relative force and power of kayak stroke in competitive kayak athletes. Int J Environ Res Publ Health. 2022;19(5):2977. doi: 10.3390/ijerph19052977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rice S.M., Purcell R., De Silva S., Mawren D., McGorry P.D., Parker A.G. The mental health of elite athletes: a narrative systematic review. Sports Med. 2016;46(9):1333–1353. doi: 10.1007/s40279-016-0492-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mon-López D., García-Aliaga A., Ginés Bartolomé A., Muriarte Solana D. How has COVID-19 modified training and mood in professional and non-professional football players? Physiol Behav. 2020;227 doi: 10.1016/j.physbeh.2020.113148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paravlic A.H., Simunic B., Pisot S., et al. Lower-limb muscle contractile properties, explosive power and the subjective response of elite soccer players to the COVID-19 lockdown. Int J Environ Res Publ Health. 2022;19(1):474. doi: 10.3390/ijerph19010474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keemss J., Sieland J., Pfab F., Banzer W. Effects of COVID-19 lockdown on physical performance, sleep quality, and health-related quality of life in professional youth soccer players. Frontiers in Sports and Active Living. 2022;4 doi: 10.3389/fspor.2022.875767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang L., Wang F.Z., Rodewald L.E., et al. Real-world effectiveness of primary series and booster doses of inactivated COVID-19 vaccine against Omicron BA.2 variant infection in China: a retrospective cohort study. J Infect Dis. 2023:jiad090. doi: 10.1093/infdis/jiad090. Published online April 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee L.C., Hsu P.S., Hsieh K.C., et al. Standing 8-electrode bioelectrical impedance analysis as an alternative method to estimate visceral fat area and body fat mass in athletes. Int J Graph Multimed. 2021;14:539–548. doi: 10.2147/IJGM.S281418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kristiansen M., Pedersen A.M.S.K., Sandvej G., et al. Enhanced maximal upper-body strength increases performance in sprint kayaking. J Strength Condit Res. 2022 doi: 10.1519/JSC.0000000000004347. Published online May 9. 10.1519/JSC.0000000000004347. [DOI] [PubMed] [Google Scholar]
- 31.Foster C., Florhaug J.A., Franklin J., et al. A new approach to monitoring exercise training. J Strength Condit Res. 2001;15(1):109–115. [PubMed] [Google Scholar]
- 32.Haddad M., Stylianides G., Djaoui L., Dellal A., Chamari K. Session-RPE method for training load monitoring: validity, ecological usefulness, and influencing factors. Front Neurosci. 2017;11:612. doi: 10.3389/fnins.2017.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lovell T.W.J., Sirotic A.C., Impellizzeri F.M., Coutts A.J. Factors affecting perception of effort (session rating of perceived exertion) during rugby league training. Int J Sports Physiol Perform. 2013;8(1):62–69. doi: 10.1123/ijspp.8.1.62. [DOI] [PubMed] [Google Scholar]
- 34.Hooper S.L., Mackinnon L.T. Monitoring overtraining in athletes. Recommendations. Sports Med. 1995;20(5):321–327. doi: 10.2165/00007256-199520050-00003. [DOI] [PubMed] [Google Scholar]
- 35.Burgess D., Drust B. Science and Soccer. third ed. Routledge; 2012. Developing a physiology-based sports science support strategy in the professional game. [Google Scholar]
- 36.Cohen J. second ed. Routledge; 1988. Statistical Power Analysis for the Behavioral Sciences. [DOI] [Google Scholar]
- 37.Markanday A. Acute phase reactants in infections: evidence-based review and a guide for clinicians. Open Forum Infect Dis. 2015;2(3):ofv098. doi: 10.1093/ofid/ofv098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leidman E., Duca L.M., Omura J.D., Proia K., Stephens J.W., Sauber-Schatz E.K. COVID-19 trends among persons aged 0–24 Years — United States, March 1–december 12, 2020. MMWR (Morb Mortal Wkly Rep) 2021;70(3):88. doi: 10.15585/mmwr.mm7003e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sigal A. Milder disease with Omicron: is it the virus or the pre-existing immunity? Nat Rev Immunol. 2022;22(2):69. doi: 10.1038/s41577-022-00678-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krzywański J., Mikulski T., Krysztofiak H., et al. Vaccine versus infection – COVID-19-related loss of training time in elite athletes. J Sci Med Sport. 2022;25(12):950–959. doi: 10.1016/j.jsams.2022.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Washif J.A., Ø Sandbakk, Seiler S., et al. COVID-19 lockdown: a global study investigating the effect of athletes' sport classification and sex on training practices. Int J Sports Physiol Perform. 2022;17(8):1242–1256. doi: 10.1123/ijspp.2021-0543. [DOI] [PubMed] [Google Scholar]
- 42.Crameri G.A.G., Bielecki M., Züst R., Buehrer T.W., Stanga Z., Deuel J.W. Reduced maximal aerobic capacity after COVID-19 in young adult recruits, Switzerland, May 2020. Euro Surveill. 2020;25(36) doi: 10.2807/1560-7917.ES.2020.25.36.2001542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muriel X., Courel-Ibáñez J., Cerezuela-Espejo V., Pallarés J.G. Training load and performance impairments in professional cyclists during COVID-19 lockdown. Int J Sports Physiol Perform. 2021;16(5):735–738. doi: 10.1123/ijspp.2020-0501. [DOI] [PubMed] [Google Scholar]
- 44.Villaseca-Vicuña R., Pérez-Contreras J., Merino-Muñoz P., Aedo-Muñoz E., Gonzalez-Jurado J., Zabaloy S. Effects of COVID-19 lockdown on body composition and physical performance of elite female football players. Women Sport Phys Activ J. 2022 doi: 10.1123/wspaj:2022-0002. Published online March 18. [DOI] [Google Scholar]
- 45.Schoenfeld B.J., Grgic J., Ogborn D., Krieger J.W. Strength and hypertrophy adaptations between low- vs. High-load resistance training: a systematic review and meta-analysis. J Strength Condit Res. 2017;31(12):3508–3523. doi: 10.1519/JSC.0000000000002200. [DOI] [PubMed] [Google Scholar]
- 46.Barker-Davies R.M., O'Sullivan O., Senaratne K.P.P., et al. The Stanford Hall consensus statement for post-COVID-19 rehabilitation. Br J Sports Med. 2020;54(16):949–959. doi: 10.1136/bjsports-2020-102596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nieman D.C. Coronavirus disease-2019: a tocsin to our aging, unfit, corpulent, and immunodeficient society. J Sport Health Sci. 2020;9(4):293–301. doi: 10.1016/j.jshs.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang A., Qiu Q., Kong X., et al. Clinical and epidemiological characteristics of COVID-19 patients in chongqing China. Front Public Health. 2020;8 doi: 10.3389/fpubh.2020.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iaia F.M., Hellsten Y., Nielsen J.J., Fernström M., Sahlin K., Bangsbo J. Four weeks of speed endurance training reduces energy expenditure during exercise and maintains muscle oxidative capacity despite a reduction in training volume. J Appl Physiol. 2009;106(1):73–80. doi: 10.1152/japplphysiol.90676.2008. 1985. [DOI] [PubMed] [Google Scholar]
- 50.Pensgaard A.M., Oevreboe T.H., Ivarsson A. Mental health among elite athletes in Norway during a selected period of the COVID-19 pandemic. BMJ Open Sport — Exercise Medicine. 2021;7(1) doi: 10.1136/bmjsem-2020-001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Han Q., Li X., Wang Z. How should athletes coping with COVID-19: focus on severity and psychological support. Front Psychol. 2021;12 doi: 10.3389/fpsyg.2021.559125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jia L., Carter M.V., Cusano A., et al. The effect of the COVID-19 pandemic on the mental and emotional health of athletes: a systematic review. Am J Sports Med. 2022 doi: 10.1177/03635465221087473. Published online April 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
If requested reasonably the authors of this study will consider providing raw data that supports their conclusions.