Abstract
Background
Glanzmann thrombasthenia (GT) is a rare bleeding disorder caused by inherited defects of the platelet αIIbβ3 integrin. Platelet transfusions can be followed by an immune response that can block integrin function by interfering with fibrinogen binding.
Objectives
In this study, we aimed to determine the prevalence of such isoantibodies and better characterize their pathogenic properties.
Methods
Twelve patients with GT were evaluated for anti-αIIbβ3 isoantibodies. Sera from patients with GT with or without anti-αIIbβ3 isoantibodies were then used to study their in vitro effect on platelets from healthy donors. We used several approaches (IgG purification, immunofluorescence staining, and inhibition of signaling pathways) to characterize the pathogenic properties of the anti-αIIbβ3 isoantibodies.
Results
Only 2 samples were able to severely block integrin function. We observed that these 2 sera caused a reduction in platelet size similar to that observed when platelets become procoagulant. Mixing healthy donor platelets with patients’ sera or purified IgGs led to microvesiculation, phosphatidylserine exposure, and induction of calcium influx. This was associated with an increase in procoagulant platelets. Pore formation and calcium entry were associated with complement activation, leading to the constitution of a membrane attack complex (MAC) with enhanced complement protein C5b-9 formation. This process was inhibited by the complement 5 inhibitor eculizumab and reduced by polyvalent human immunoglobulins.
Conclusion
Our data suggest that complement activation induced by rare blocking anti-αIIbβ3 isoantibodies may lead to the formation of a MAC with subsequent pore formation, resulting in calcium influx and procoagulant platelet phenotype.
Keywords: anti-αIIbβ3 isoantibodies, coagulation, complement activation, Glanzmann thrombasthenia, platelet transfusion, procoagulant platelet
Essentials
-
•
Patients with GT may produce anti-αIIbβ3 isoantibodies after platelet transfusions.
-
•
Rare anti-αIIbβ3 can block fibrinogen binding and induce procoagulant platelet formation.
-
•
Procoagulant platelets induced by complement activation might cause transfusion refractoriness.
-
•
Complement directed therapeutic interventions might rescue platelet transfusion efficiency.
1. Introduction
Taking into account the number of copies and function, αIIbβ3 integrin is a major transmembrane receptor for fibrinogen (Fg) expressed at the platelet surface [1,2]. Glanzmann thrombasthenia (GT) is a rare autosomal recessive bleeding disorder caused by inherited defects of the ITGA2B or ITGB3 gene encoding the platelet αIIbβ3 integrin [3,4]. This disease is characterized by a lack of platelet aggregation in response to all physiologic stimuli, except to ristocetin [5,6]. Generally, bleeding is largely mucocutaneous in nature, and if minor, local measures, sometimes in conjunction with antifibrinolytics, are sufficient; in contrast, platelet transfusions are used to control or to prevent life-threatening blood loss [7,8]. Despite recent advances, platelet transfusions remain the first option to stop or prevent major bleeding in GT. Unfortunately, platelet transfusion therapy can be followed by an immune response that is usually directed against the deficient αIIbβ3 complex [9,10]. These isoantibodies are of clinical concern as they can render platelet transfusion ineffective [11]. Among 382 Iranian patients, 80% had received at least once platelet transfusions to control bleedings, emphasizing the urgency of the problem [12]. Development of anti-αIIbβ3 isoantibodies occurs in approximately 20% to 30% of patients and is most frequently observed in GT type I due to reduced expression of the integrin at the platelet surface (<5%) [9,13].
Unfortunately, the physiopathological mechanism leading to platelet transfusion refractoriness is not known for this disease. Nevertheless, it generally refers to excessive platelet clearance by the reticuloendothelial system of the spleen. Previous detailed characterizations of these isoantibodies also show that some can block platelet function by interfering with Fg binding [[14], [15], [16]]. Past studies in France showed that an IgG antibody isolated from the plasma of a patient with polytransfused GT inhibited agonist-induced aggregation of normal human platelets, except with ristocetin [15]. This IgG antibody also strongly inhibited thrombin-induced clot retraction, thereby inducing a thrombasthenia-like state in normal human platelets. These isoantibodies can block integrin function of transfused platelets from healthy donors [16]. However, initial studies were largely performed on isolated cases and there is no consensus pertaining to the frequency of such isoantibodies. Current transfusion approaches for patients with GT are mostly based on positivity of anti-αIIbβ3 isoantibodies but do not account for their functional properties. To which extent blocking isoantibodies contributes to platelet transfusion refractoriness in these patients remains unclear.
In this work, we aimed to determine the prevalence of anti-αIIbβ3 isoantibodies that can block integrin function in a French population of patients with GT. Sera from patients with GT with or without anti-αIIbβ3 isoantibodies were used to study their in vitro effect on platelets from healthy donors. Our results show that only 2 sera from patients with GT with anti-αIIbβ3 isoantibodies were able to severely block Fg binding to the integrin. Surprisingly, their sera also reduced platelet size similar to that observed when platelets are activated with calcium ionophore. Furthermore, we describe the in vitro characterization of the pathogenic properties of these 2 positive sera, showing that they were able to generate procoagulant platelets in healthy donor platelets via complement activation. Finally, our results suggest that complement directed therapeutic interventions may partially reverse this procoagulant phenotype induced by anti-αIIbβ3 isoantibodies.
2. Methods
2.1. Patients and sera studied
Blood samples from patients and healthy subjects were collected and obtained in accordance with the Declaration of Helsinki. The study was approved by our local committee. In our regional center, we have the opportunity to follow up 15 patients, of which 9 have developed anti-αIIbβ3 isoantibodies. Samples from patients and controls were handled in parallel when possible. All included patients with GT satisfied diagnostic criteria for the disease—association with mucocutaneous bleeding, defects in αIIbβ3 expression, and an absence of platelet aggregation to all agonists, except ristocetin. Data for patients included the following information: (i) age of the patient, (ii) subtype of GT or residual αIIbβ3 expression, and (iii) mutation screening of the ITGA2B or ITGB3 genes. Sera from patients and control donors were prepared and decomplemented as previously described [17]. Seventeen sera were selected from a cohort of 12 patients with GT regularly followed up in our French reference center. Except one (GT9), all patients with GT had previously received platelet concentrates.
2.2. Indirect MoAb-specific immobilization of platelet antigens
The following murine monoclonal antibodies (MoAbs) were used: P2 (Immunotech) directed against the αIIbβ3 complex and Y2/51 (Dako) specific for β3. Platelet-rich plasma (PRP) was obtained from healthy donors and platelets were washed once before being resuspended at 2 × 109 platelets/mL. Volumes (20 μL) of the platelet suspension were sensitized with serum from patients or control donors. After resuspension, each of the selected MoAbs were added separately. The mixture was incubated for 1 hour at room temperature. The platelets were then washed once and solubilized in a lysis solution composed of 0.5% Nonidet P-40. In parallel, microtiter plates were coated with a goat anti-mouse IgG (Jackson Immunoresearch). After 4 washes, blocking buffer was added to each well and incubated for 1 hour, which was then washed 4 times. The supernatant from lysed platelets was diluted in 200 μL of milk PBS-Tween buffer. One hundred five microliters of the respective diluted supernatants was added in duplicate to each coated well. The plates were incubated for 90 minutes at 4 °C and washed 4 times. A volume of 105 μL of alkaline phosphatase-labeled goat antihuman IgG/M F(ab’2) (Jackson Immunoresearch) was added to each well. After incubation, the plate was washed 4 times and the substrate (para-nitrophenylphosphate in diethanolamine buffer) was added. After 60 minutes in the dark, absorbance was measured at 405 nm in a TECAN microplate reader (Tecan Group Ltd).
All results for the patients were calculated as a ratio of their optical density compared to the mean value of a series of 39 control sera. For results to be considered positive, this ratio must exceed by 3 SDs of the mean of these controls. Positive results were classified as follows: weakly positive when the patient ratio was up to 2-fold increased compared to threshold and highly positive when the ratio was greater than a 2-fold increase. As an additional internal control, a day-to-day serum from a normal donor was tested in parallel with those from the patients. Finally, for comparison, sera were grouped into high positive anti-αIIbβ3 Abs, low positive ratios, and negative results.
2.3. Preparation of citrated platelet-rich plasma and washed human platelets
Platelets were isolated from citrated blood and tested within 4 hours. In brief, after 1 centrifugation step (1000-1100 rpm, 10 minutes at room temperature, and without brake), citrated platelet-rich plasma (cPRP) was gently separated and used for further analysis.
Washed platelets were isolated from ACD-A blood by successive centrifugation steps with inclusion of apyrase from Sigma-Aldrich (0.06 U/mL) to avoid platelet activation. Briefly, the first centrifugation allowed the preparation of PRP. Then, platelets were washed in a buffer (pH 6.5) containing 103 mM NaCl, 5 mM KCl, 1 mM MgCl2, 36 mM citric acid, 50 mg glucose, and 0.35% bovine serum albumin (w/v). The same buffer containing 2 mM CaCl2 was added to the final suspension to reach 500 × 109 platelets/L.
2.4. Isolation of patients’ antibodies
Samples of serum from patients or control donors were applied to minicolumns of protein A Sepharose (Nab Protein A Plus Spin Kit, Thermofisher) according to the manufacturer’s instructions. Eluates were then concentrated using the Protein Concentrators kit (ThermoFisher). Purified IgGs were then used at a physiological concentration that is expected in human serum (12-14 g/L). Equal volumes of the patients’ purified IgGs or the nonretained fractions were added to control cPRP.
2.5. Platelet fibrinogen and PAC-1 binding
The capacity of patients’ sera to inhibit Fg or PAC-1 binding in normal platelets was assessed. Control cPRP at a final concentration of 250 × 109 platelets/L was incubated for 20 minutes with the same volume of patients or control’s serum tested in parallel. Dilutions were performed using a reaction buffer containing 137 mM NaCl, 2.7 mM KCl, 1 mM MgCl2, 0.3 mM NaH2PO4, 5 mM Hepes, 12 mM NaHCO3, 30% bovine serum albumin, and glucose.
Fg binding was measured by incubating 10 μL of cPRP with serum at 37 °C for 5 minutes with 10 μL of Alexa Fluor 488–labeled human fibrinogen (417 μg/mL; Molecular probes, Eugene), with or without activation with 10 μL of ADP (10 μM; Calbiochem).
Binding of PAC-1 (Franklin Lakes), an activation-dependent antibody that recognizes the ligand binding site in αIIbβ3, was also detected by flow cytometry upon stimulation with TRAP-14 (50 μM), a protease-activated receptor-1 (PAR-1) activating peptide containing 14 amino acids (SFLLRNPNDKYEPF). Briefly, 100 μL of PAC-1 at 1 μg/mL was added to 10 μL of the cPRP and serum mixture with or without 10 μL of TRAP-14 (50 µM) for 15 minutes at room temperature. Then, the mixture was stained with 10 μL of fluorescein (FITC)-AffiniPure F(ab’)2 Fragment Donkey Anti-Mouse IgM (0.01 μg/μL) from Jackson ImmunoResearch Laboratories for 15 minutes at room temperature.
For platelet fibrinogen and PAC-1 binding studies, reaction was stopped by adding 400 to 500 μL of not fixed reaction buffer. Platelets were analyzed on a Cytomics FC500 flow cytometer (Beckman Coulter) and results were expressed as mean fluorescence intensity (MFI). Data were analyzed using the CXP analysis software from Beckman Coulter after gating on the platelet population. MFI of activated platelets was calculated after taking into account the MFI at resting state.
2.6. Flow cytometric analysis of platelet microvesicle release and phosphatidylserine exposure
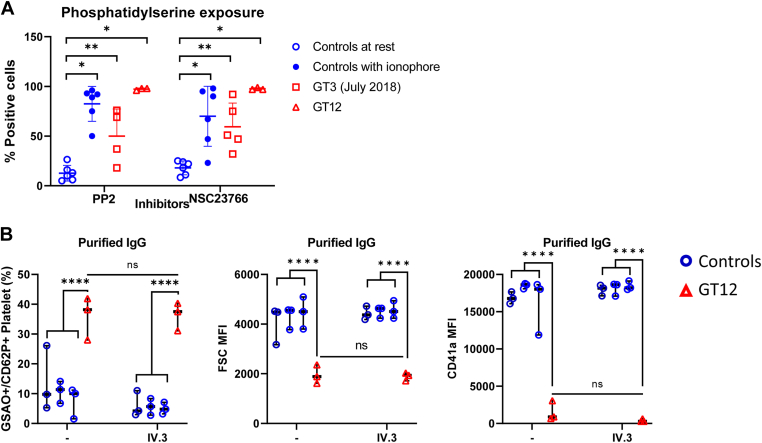
Platelet microvesicle (MV) release and phosphatidylserine (PS) exposure were analyzed by flow cytometry. cPRPs were stimulated or not with ionophore-A23187 Free Acid (100 μM; Calbiochem) for 5 minutes in buffer containing 1 UI/mL heparin. The samples were then incubated with FITC–conjugated annexin V (BD Pharmingen Inc) for PS detection and analyzed using a flow cytometer (FC500; Beckmann Coulter). As MVs are smaller than platelets, they were easily distinguished according to their light scattering pattern. The cut-off is placed at the lowest forward scatter (FSC) value of the platelet population at resting state. After platelet activation, all the events localized below this cut-off were classified as MVs or positive events. We also investigated the effect of inhibitors of Src PP2 and Rac1 NSC23766 from Sigma-Aldrich in integrin-dependent PS exposure in control platelets mixed with anti-αIIbβ3 isoantibodies.
2.7. Calcium signaling
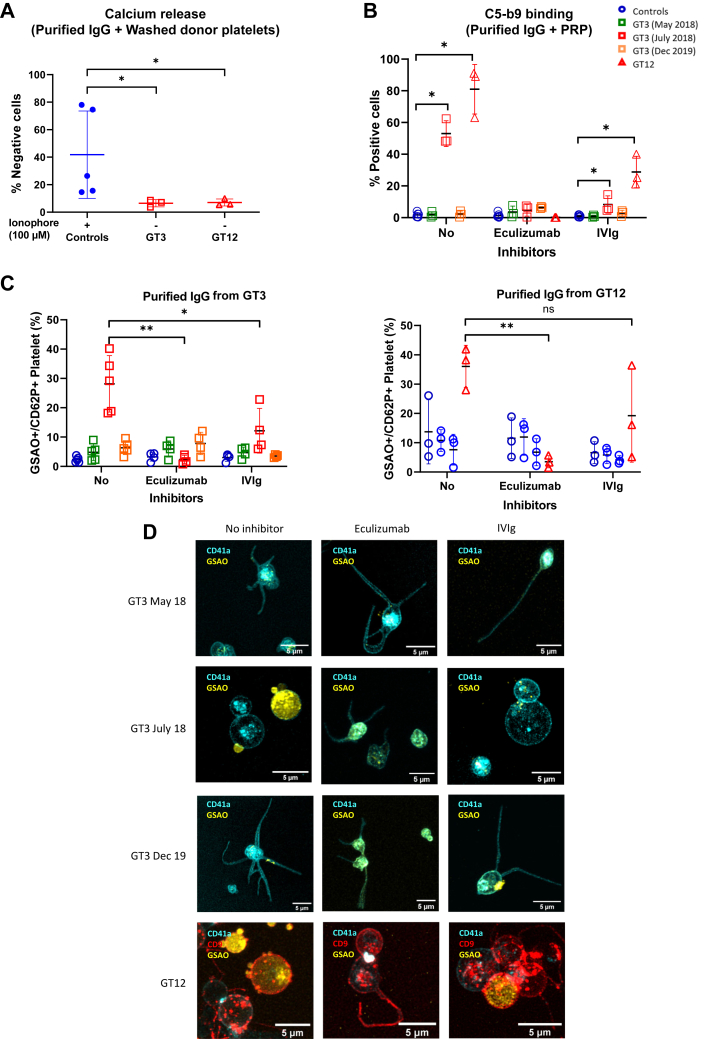
Intracellular calcium levels were determined by flow cytometry. Briefly, cPRPs were incubated with a calcium-sensitive dye Fura Red/AM (10 μM, AAT Bioquest) for 30 minutes at room temperature. The concentration of the calcium-sensitive dye solvent, dimethylsulfoxide, did not exceed 0.1%; additional controls confirmed that the vehicle did not affect the results. cPRP treated with ionophore (100 μM, 5 minutes) served as a positive control. Test results were expressed as percentage of negative cells.
2.8. Procoagulant platelet formation
cPRP from healthy donors was incubated with an equal volume of purified IgG from patients or control donors for 20 minutes at 37 °C prior to the addition of 2.5 mM Gly-Pro-Arg-Pro peptide (Sigma-Aldrich) and 2.5 mM calcium chloride in Hanks’ balanced salt solution (HBSS, pH: 7.35). In some experiments, cPRP was pretreated with monoclonal anti-C5 antibody eculizumab (50 μg/mL), polyvalent human immunoglobulins (25 mg/mL), or FcγRIIa function-blocking monoclonal antibody IV.3 (10 μg/mL) for 15 minutes before incubation with purified IgG. After 10 minutes at room temperature, the reaction was stopped by 3-fold dilution with HBSS, followed by staining with antibodies to CD45 (HI30; StemCell Technologies), CD41a (HIP8; BD Biosciences), CD62P (Psel.KO2.3; eBioscience)—or isotype control (eBioscience), and GSAO or GSCA. For experiments with purified IgG from GT12, CD9 (MEM-61; Invitrogen) was included as an additional platelet marker. After staining, samples were fixed with PAMFix (Platelet Solutions Ltd), centrifuged, and resuspended. Samples were rested for 1 hour in the dark at room temperature prior to analysis on a BD LSRFortessa X-20 with acquisition of 10000 platelet events. The remainder of the fixed samples were centrifuged at 200 g for 10 minutes, resuspended in 200 μL of HBSS, and placed into a chamber slide. The intensity of staining of CD41a (BV510), GSAO (Alexa-647), and CD9 (Alexa-488 in experiments with purified IgG from GT12) and the platelet morphology was visualized on a Leica SP8 confocal microscope. The images collected were processed and analyzed using Huygens Professional deconvolution software and Fiji/ImageJ.
2.9. Thrombus formation using a microchip flow-chamber assay
A total of 450 μL of recalcified citrated normal whole blood preincubated with tirofiban (100 ng/mL) was perfused using the T-TAS system (Fujimory Kogyo) at a constant flow rate (600 s−1) through a 80-μm depth flow chamber precoated with tissue thromboplastin and collagen (AR-chip; Fujimory Kogyo). Occlusion time was defined as the lag time for the flow pressure to reach 60 kPa from baseline pressure.
2.10. Complement deposition
For measuring the formation of a membrane complex attack, platelets were stained with rabbit antihuman C5b-9 antibody (Santa-Cruz Biotechnology Inc). Mean fluorescent intensities were measured using a Cytomics FC500 flow cytometer. Monoclonal anti-C5 antibody eculizumab (50 μg/mL) and polyvalent human immunoglobulins (25 mg/mL; inhibitor of complement activation) were purchased from Alexion Pharmaceuticals and LFB, respectively.
2.11. Statistical analysis
Statistical analyses were performed using GraphPad Prism 9.2 (GraphPad Software) as detailed in the figure legends. P values of < .05 were considered significant.
3. Results
3.1. Population study and development of anti-αIIbβ3 isoantibodies
A total of 12 patients with GT (10 females, 2 males) from different families were screened for anti-αIIbβ3 isoantibodies (n = 17 sera; Table and Supplementary Figure S1). Seven of them were homozygous for the founder c.1544+1G>A mutation in ITGA2B and failed to express αIIbβ3 on the surface of their platelets [18,19]. Of these patients, 6 had documented evidence of platelet transfusions associated with or without red blood cell concentrates. All the 7 patients had positive anti-αIIbβ3 serum isoantibodies (n = 9—two of them have been tested twice). Of the 9 samples, 4 were positive with a ratio of above a 2-fold increase (high responders) and the other 5 had a ratio of up to 2-fold increase compared to threshold (low responders).
Table.
Characteristics of the 12 patients with GT screened for platelet antibody formation against αIIbβ3.
| Clinical and biological characteristics |
Anti-αIIbβ3 antibodies |
Platelet transfusion |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Age (years) | Gender Race/ethnicity |
Type | Levels of αIIbβ3 | Gene | Molecular defect | Date | P2 value | Cut-off | Y2/51 value | Cut-off | Results | |
| GT1 | 23 | F-Arabian | Variant | 18-23% | ITGB3 | p.R63C/p.R169∗ | Oct. 17 | 1.1 | 1.2 | 1.1 | 1.3 | Neg | Yes |
| GT2 | 40 | F-Caucasian | I | <5% | ITGA2B | c.2095-19T>A/p.Q924∗ | Oct. 18 | 1.2 | 1.2 | 1.2 | 1.3 | Neg | Yes |
| Feb. 19 | 1.8 | 1.2 | 1.7 | 1.3 | Low | ||||||||
| GT3 | 19 | F-Caucasian | I | <5% | ITGA2B | p.Q228∗/p.R584∗ | May 18 | 1.0 | 1.2 | 1.0 | 1.3 | Neg | Yes |
| Jul. 18 | 23.8 | 1.2 | 22.0 | 1.3 | High | ||||||||
| Dec. 19 | 1.8 | 1.3 | 2.7 | 1.3 | Low | ||||||||
| GT4 | 64 | F-Arabian | I | <5% | ITGA2B | p.E355L | Nov. 17 | 0.9 | 1.2 | 0.9 | 1.3 | Neg | Yes |
| GT5 | 66 | F-Caucasian | I | <5% | ITGB3 | p.P189S | Jun. 17 | 1.2 | 1.2 | 1.0 | 1.3 | Neg | Yes |
| GT6 | 8 | F-Gypsy | I | <5% | ITGA2B | c.1544+1G>A | May 18 | 1.4 | 1.2 | 1.6 | 1.3 | Low | Yes |
| Dec. 18 | 2.1 | 1.2 | 2.0 | 1.3 | Low | ||||||||
| GT7 | 21 | F-Gypsy | I | <5% | ITGA2B | c.1544+1G>A | May 12 | 2.5 | 1.2 | 2.4 | 1.3 | Low | Yes |
| Nov. 18 | 1.2 | 1.2 | 1.3 | 1.3 | Low | ||||||||
| GT8 | 10 | F-Gypsy | I | <5% | ITGA2B | c.1544+1G>A | Jun. 18 | 1.6 | 1.2 | 2.0 | 1.3 | Low | Yes |
| GT9 | 18 | F-Gypsy | I | <5% | ITGA2B | c.1544+1G>A | Sept. 18 | 7.5 | 1.2 | 7.1 | 1.3 | High | No |
| GT10 | 33 | M-Gypsy | I | <5% | ITGA2B | c.1544+1G>A | Feb. 17 | 15.9 | 1.2 | 3.8 | 1.3 | High | Yes |
| GT11 | 48 | F-Gypsy | I | <5% | ITGA2B | c.1544+1G>A | Apr. 17 | 4.5 | 1.2 | 5.7 | 1.3 | High | Yes |
| GT12 | 73 | M-Gypsy | I | <5% | ITGA2B | c.1544+1G>A | Jan. 21 | 6.9 | 1.5 | 7.6 | 1.5 | High | Yes |
Of the other 5 patients, 4 had type I disease with little or no surface expression of αIIbβ3 and 1 had type II disease. Genotyping revealed that 3 of them had causative mutations in ITGA2B, whereas 2 others possessed mutations in ITGB3. All these patients had received treatment with platelet concentrates. In contrast to the patients with the founder c.1544+1G>A mutation, only 2 patients had serum samples with detectable platelet isoantibodies (GT2 and GT3). These isoantibodies occurred in patients with type I disease and only 1 was a high responder.
3.2. Sera containing anti-αIIbβ3 isoantibodies produced by 2 patients with GT may severely block fibrinogen binding to the integrin
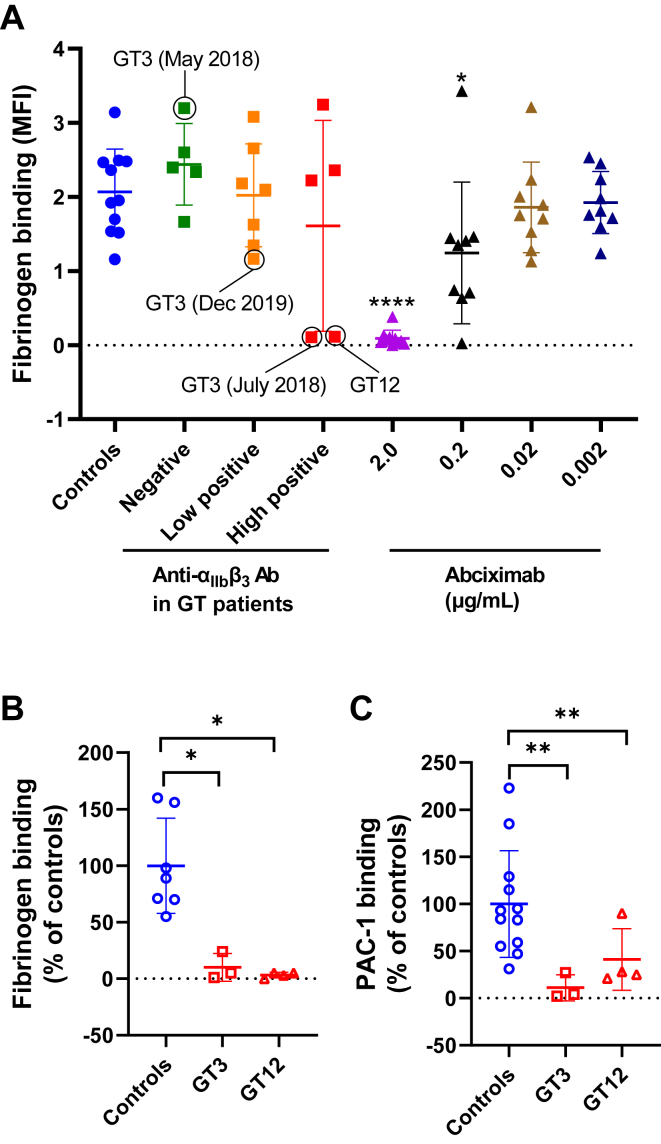
In order to evaluate whether all of these sera were able to inhibit fluorescent Fg binding, samples were incubated with platelets from healthy donors and analyzed by flow cytometry after activation with ADP. There was no significant difference in fibrinogen binding between controls and patients’ positive or negative sera (Figure 1A). However, 2 highly positive samples (from GT3 and GT12) were able to severely block integrin function similar to that observed with 2.0-μg/mL abciximab (a monoclonal antibody that prevents platelet aggregation by blocking αIIbβ3; Figure 1A, B). Additionally, when stimulated by TRAP-14 (50 μM), control platelets mixed with these sera did not bind fluorescent PAC-1 confirming their effect on integrin function (Figure 1C). There was also a concentration-dependent effect as integrin function was completely restored when GT3’s serum was diluted 1:10 (v/v; data not shown).
Figure 1.
(A) Inhibition of fibrinogen binding on platelet surface by patients’ sera. Different negative or positive sera from 12 patients with GT were incubated with control platelets for 20 minutes at 37 °C. Then, fibrinogen binding was analyzed by flow cytometry after activation with 10-μM ADP. Circles correspond to samples from GT3 (collected at different dates) and GT12. These samples were able to severely block Fg binding. Results obtained with different concentrations of abciximab (anti-αIIbβ3 MoAb) are also represented (positive control). Results were compared with those of the control group. Fibrinogen (B) and PAC-1 binding (C) measured by flow cytometry in GT3, GT12, and different day controls (n = 7-12). Activation was obtained by incubation with ADP (10 μM) for fibrinogen binding and TRAP-14 (50 μM) for PAC-1. Results were expressed as percentage of the MFI of controls. Mann–Whitney–Wilcoxon test (P value): ∗< .05, ∗∗< .01, and ∗∗∗∗< .0001. ADP, adenosine diphosphate; GT, Glanzmann thrombasthenia; MFI, mean fluorescence intensity.
GT3 first tested positive for isoantibodies against αIIbβ3 in March 2010 within <12 days following her first and only transfusion (1 platelet and 2 red blood cell [RBC] concentrates). In May 2018, isoantibodies against αIIbβ3 had disappeared and GT3 tested negative for isoantibodies prior to receiving treatment for severe epistaxis (Table). Because GT3 was considered at high risk for anti-αIIbβ3 isoantibody development, she first received several doses of activated recombinant factor VII (rFVIIa; in association with RBC concentrates) rather than platelet concentrates to avoid stimulating these isoantibodies. rFVIIa provides an alternative treatment for patients with GT who developed antiplatelet antibodies [20]. Due to the ineffectiveness of rFVIIa after 24 hours of administration, she also received platelet concentrates every 12 hours for 48 hours and bleeding ceased 24 hours after the first platelet administration. She was then discharged home on day 6 of hospitalization. In July 2018, her MAIPA (monoclonal antibody–specific immobilization of platelet antigens—a laboratory test to detect antiplatelet antibodies in patient serum) revealed high positive anti-αIIbβ3 isoantibodies against αIIbβ3. Until now, she has not received further transfusion and her MAIPA showed persistence of low positive isoantibodies in December 2019.
Patient GT12 was first transfused at the age of 31 for severe repeated gastrointestinal bleedings. He received ∼5 to 10 RBC concentrates and <5 platelet concentrates during his life. His last transfusion was in January 2004 when he received 3 RBC concentrates. He was tested positive against αIIbβ3 in 2004 until when he has never received other platelet transfusions but was treated with rFVIIa. He was still highly immunized against αIIbβ3 in January 2021.
3.3. In vitro generation of procoagulant platelets by GT3 and GT12’s anti-αIIbβ3 isoantibodies
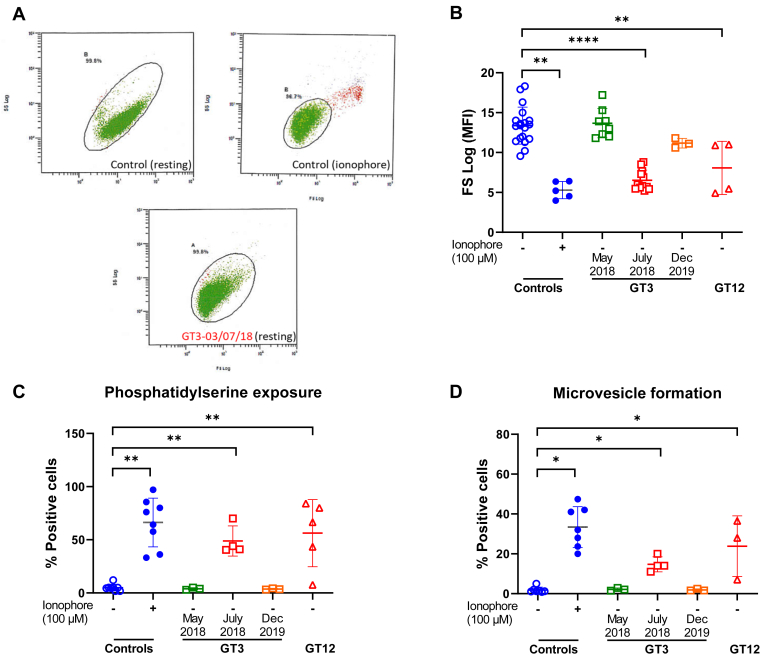
We also observed in the FSC/SSC plots measured by flow cytometry that high positive sera from patients with GT3 and GT12 caused a reduction of platelet size in healthy donor platelets similar to that observed when platelets are activated with calcium ionophore (Figure 2A, B), whereas a reduction of platelet size was not visualized in the unstimulated cPRP of these 2 patients (mean MFI: 14 ± 2; n = 6). It is well known that calcium ionophore promotes platelet PS exposure and microvesicle (MV) formation via high sustained intracellular calcium influx [21]. Thus, it was tempting to hypothesize that sera from patients with GT3 and GT12 containing high levels of anti-αIIbβ3 isoantibodies act as physiological calcium ionophore, also inducing PS exposure and MV release. To investigate this hypothesis, platelets from controls were incubated with these sera and analyzed by flow cytometry. As expected, mixing normal platelets with these sera generated MVs or exposed PS under unstimulated conditions (Figure 2C, D).
Figure 2.
(A) Dot plots of FSC (x axis) vs SSC fluorescence (y axis) show that GT3’s high positive serum (date: July 3, 2018) causes, at resting state, a change of platelet form similar to that observed when platelets are activated with calcium ionophore. (B) Histograms showing FSC values (MFI) obtained after mixing control platelets with GT3 (collected at different dates) and GT12’s sera. Also shown are results obtained with controls’ sera incubated with or without calcium ionophore (positive controls). (C, D) Analysis of PS exposure and MV formation by flow cytometry after incubation of controls’ citrated cPRP with controls, GT3, or GT12’s serum. Mann–Whitney–Wilcoxon test and Wilcoxon signed-rank test for controls incubated with ionophore or not. ∗< .05, ∗∗< .01, and ∗∗∗∗< .0001. cPRP, citrated platelet-rich plasma; FSC, forward scatter; MFI, mean fluorescence intensity; MV, microvesicle; PS, phosphatidylserine; SSC, side scatter.
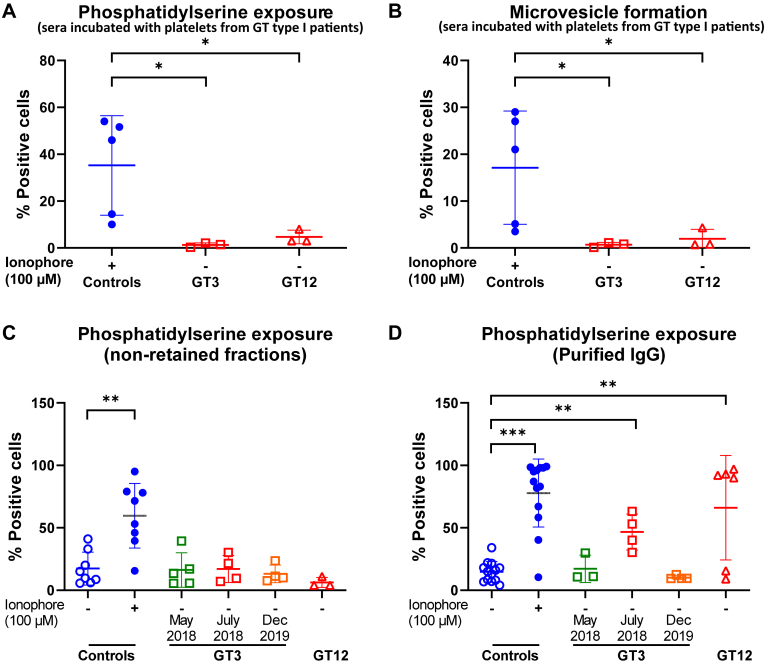
To prove that the αIIbβ3 integrin was required for this phenotype, sera were also incubated with platelets from patients with GT type I. Interestingly, MV formation and PS exposure were abolished in αIIbβ3-deficient platelets, indicating that the integrin is critical for MV release and PS exposure in this situation (Figure 3A, B). Furthermore, to see if this activity could also be produced by purified isoantibodies, sera from these patients were passed through a protein A-Sepharose column. The activity was only present in the purified IgGs samples obtained from high positive sera, as they were able to induce PS exposure of control platelets, similar to that observed with ionophore activation (Figure 3C, D). Together, these experiments indicated that αIIbβ3 interaction with isoantibodies was critical for this phenotype.
Figure 3.
Analysis of PS exposure (A) and MV formation (B) after incubation of cPRPs from 3 different patients with GT type I with sera of GT3, GT12, or controls incubated with ionophore (n = 5-6); nonretained flow-through (C) and IgG eluate fractions (D) were tested for their ability to induce PS exposure. Mann–Whitney–Wilcoxon test (P value): ∗< .05, ∗∗< .01, and ∗∗∗< .001. cPRP, citrated platelet-rich plasma; GT, Glanzmann thrombasthenia; IgG, immunoglobulin G; PS, phosphatidylserine.
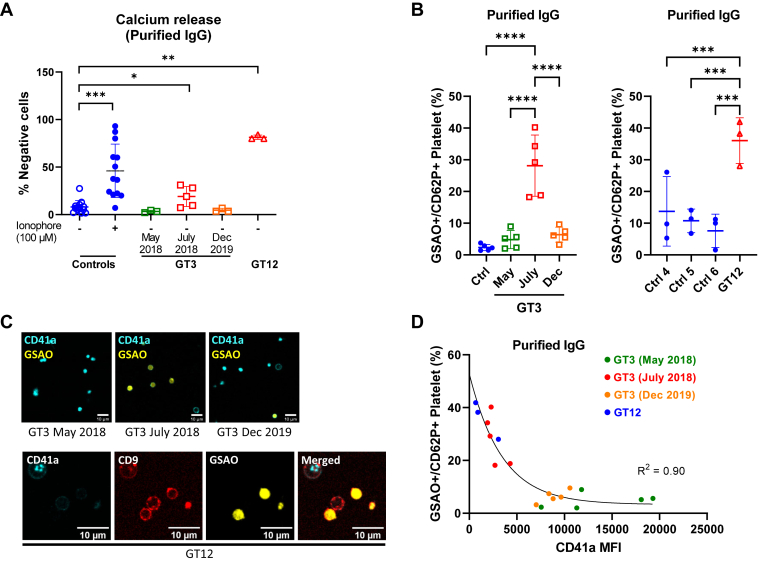
Previous studies also demonstrated the importance of intracellular calcium elevation in PS exposure and MV release. To demonstrate that patients’ purified IgGs also induce intraplatelet calcium mobilization, samples were incubated with normal platelets loaded with Fura Red/AM. Here, only incubation with the patients’ purified IgGs containing high positive anti-αIIbβ3 isoantibodies induced an increase in calcium influx (Figure 4A). These results confirm that platelet activation induced by the patients’ anti-αIIbβ3 isoantibodies also resulted in calcium influx.
Figure 4.
(A) Purified IgGs from the patients or control donors (n = 3-13) were tested for their ability to induce calcium release. Also shown are control platelets incubated with ionophore as positive control. (B) Procoagulant platelets (GSAO+/CD62P+) induced by purified IgG from controls, GT3, or GT12’s serum on healthy donor platelets (n = 3-5). ANOVA with Tukey’s multiple comparisons test. (C) Confocal microscope images showing the procoagulant morphology of donor platelets stained with CD41a (BV510) and GSAO (Alexa-647) and treated with purified IgGs from GT3 or GT12. Platelet marker CD9 (Alexa-488) was included in samples treated with GT12 purified IgGs. (D) Nonlinear relationship between anti-αIIbβ3 antibody interference with CD41a binding (clone HIP8) and induction of procoagulant response in donor platelets (n = 3-5) treated with GT3- or GT12-purified IgG. Mann–Whitney–Wilcoxon test and Wilcoxon signed-rank test for controls incubated with ionophore or not. ∗< .05, ∗∗< .01, ∗∗∗< .001, and ∗∗∗∗< .0001. GT, Glanzmann thrombasthenia; IgG, immunoglobulin G.
The cell death marker 4-[N-(S-glutathionylacetyl)amino]phenylarsonous acid (GSAO) rapidly enters a subpopulation of agonist-stimulated platelets via an organic anion-transporting polypeptide and is retained in the cytosol through covalent reaction with protein dithiols [22]. Labeling with GSAO, together with exposure of P-selectin, distinguishes necrotic from apoptotic platelets and correlates with procoagulant potential [23]. To determine whether PS+-MV+–releasing platelets represented procoagulant platelets, we used the binding of GSAO/P-selectin as a marker of procoagulant platelets [22,23]. This demonstrated induction of procoagulant phenotype in control donor platelets by purified patient IgGs (Figure 4B), while confocal imaging confirmed the characteristic balloon morphology of procoagulant platelets (Figure 4C) [24]. Assuming that proportional inhibition of HIP8 (CD41a) binding was directly linked to the amount of anti-αIIbβ3 isoantibodies that bind to the integrin, we saw a strong relationship between anti-αIIbβ3 isoantibody interference with nonconformation specific CD41a binding and induction of procoagulant platelets (Supplementary Figure S2 and Figure 4D), supporting the concept that allosteric interference by these anti-αIIbβ3 isoantibodies was related to the procoagulant phenotype.
To determine whether procoagulant platelets induced by these isoantibodies could also promote coagulation, we measured occlusion times of recalcified citrated normal whole blood, preincubated with tirofiban, using a microchip flow-chamber assay. “GT-like” platelets, which were incubated with patients’ purified IgGs showed significantly shorter clotting time than those of controls (Supplementary Figure S3).
Taken together, our data showed that anti-αIIbβ3 isoantibodies from GT3 and GT12 were able to induce a procoagulant phenotype in normal platelets.
3.4. Neither integrin outside-in signaling nor FcγRIIa pathway mediate calcium mobilization or generate procoagulant state in healthy platelets incubated with GT3 or GT12’s anti-αIIbβ3 isoantibodies
We next aimed to elucidate whether patients’ anti-αIIbβ3 isoantibodies induce intracellular calcium release leading to procoagulant platelets [25]. We hypothesized that binding of patients’ isoantibodies to the major platelet integrin may cause outside-in signaling leading to platelet activation [25]. Pang et al. have recently showed that integrin outside-in signaling facilitates platelet PS exposure and MV release via Src and Rac1-dependent signaling pathway [26]. We further investigated the effect of inhibitors of Src and Rac1 on control platelets mixed with the purified patient IgGs. Both the Src inhibitor PP2 and Rac1 inhibitor NSC23766 did not inhibit PS exposure, suggesting that patients’ anti-αIIbβ3 isoantibodies triggering the outside-in signaling pathway could not explain the observed platelet phenotype (Figure 5A).
Figure 5.
(A) PS exposure from controls’ platelets pretreated with Src inhibitor PP2 (40 μM) or Rac1 inhibitor NSC23766 (300 μM) and eluates. Data are presented as median and interquartile. Mann–Whitney–Wilcoxon test P values: ∗< .05, ∗∗< .01. (B) Procoagulant platelets (GSAO+/CD62P+) induced by IgGs in the presence of FcγRIIa-blocking monoclonal antibody IV.3 (10 μg/mL). ANOVA with Tukey’s multiple comparisons test: ∗∗∗∗< .0001. IgG, immunoglobulin G; PS, phosphatidylserine.
Immune complexes can bind and cross-link the low-affinity IgG receptor, FcγRIIa, on platelets causing platelet activation and aggregation through the immunoreceptor tyrosine-based activation motif (ITAM) [27]. FcγRIIa may also induce procoagulant platelet formation, which supports thrombin generation [28]. This was not the case here as the procoagulant phenotype induced by GT12 was not inhibited in the presence of anti-FcγRIIa blocking antibody IV.3 (Figure 5B).
3.5. GT3 or GT12’s anti-αIIbβ3 isoantibodies induced complement activation, which was inhibited by eculizumab or human polyvalent immunoglobulins
We then hypothesized that another mechanism might explain the observed phenotype. As ionophore causes membrane pore formation and intracellular calcium entry, we hypothesized that patients’ anti-αIIbβ3 isoantibodies could follow a similar process. In particular, pore formation can be produced by complement activation leading to the formation of a membrane attack complex (MAC), also called the C5b-9 complex [29]. Patients’ purified IgGs were incubated with washed platelets from donors in the presence of decomplemented human plasma (heated at 56 °C for 20 minutes). As expected, intracellular calcium did not increase upon incubation with the purified patient IgGs, suggesting that the presence of a complement source was necessary to reproduce the phenotype (Figure 6A). To also confirm MAC formation, platelets incubated with purified patient IgGs were stained with an anti-C5b-9 antibody, confirming enhanced C5b-9 formation, which was inhibited in the presence of C5 inhibitor eculizumab and reduced by polyvalent human immunoglobulins intravenous immunoglobulin (IVIg) (Figure 6B). The procoagulant phenotype was also abrogated and the balloon morphology was inhibited in the presence of eculizumab or IVIg (Figure 6C, D, and Supplementary Figure S4). Together, these results suggest that complement activation induced by patients’ isoantibodies lead to the formation of a MAC, with subsequent pore formation in platelet membrane resulting in calcium influx.
Figure 6.
(A) Purified IgGs from patients or control donors incubated with ionophore (100 μM) were tested for their ability to induce calcium release from washed control platelets (WP) in absence of a complement source. (B) Anti-αIIbβ3 isoantibodies induce the formation of a MAC, which was inhibited by eculizumab (50 μg/mL) or human polyclonal immunoglobulins (IVIg; 25 mg/mL). Formation of a MAC was measured employing an anti-C5b-9 antibody. (C) Control cPRP (n = 4-5) was pretreated with or without eculizumab (50 μg/mL) or human polyclonal immunoglobulins (IVIg; 25 mg/mL) before incubation with purified IgGs from GT3, GT12, or control donors and assayed by flow cytometry for procoagulant platelet response. Mixed effects analysis with Tukey’s multiple comparisons test: ∗< .05 and ∗∗< .01. (D) Confocal microscope imaging of donor platelets stained with CD41a (BV510), CD9 (Alexa-488), and GSAO (Alexa-647) and treated with purified IgGs from patients in the presence of eculizumab (50 micrograms/mL) or IVIg (25 mg/mL). IgG, immunoglobulin G; IVIg, intravenous immunoglobulin; MAC, membrane attack complex.
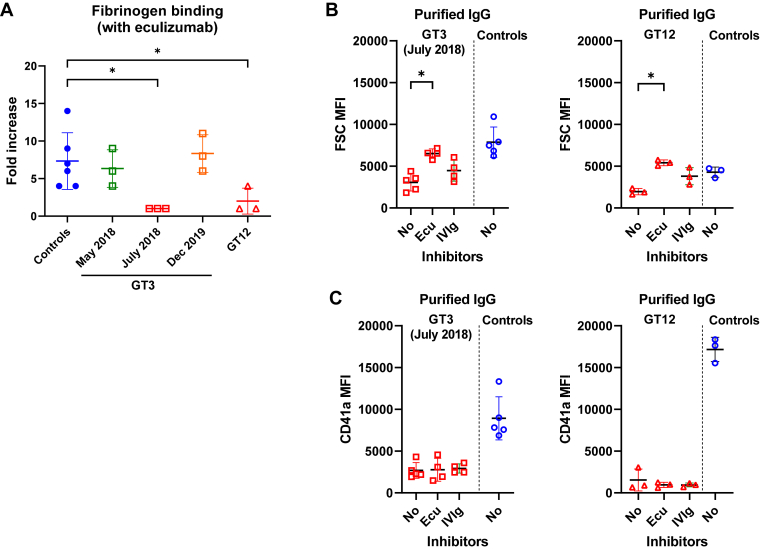
3.6. Eculizumab or human polyvalent immunoglobulins did not restore platelet fibrinogen binding blocked by anti-αIIbβ3 isoantibodies
It is known that procoagulant platelets disconnect from a thrombus by integrin inactivation, which relies on stimulating intracellular cleavage of the β3 chain by calpain activity in the presence of high sustained intracellular calcium [30]. To see if procoagulant platelet induction could explain the absence of Fg binding by inside-out integrin inactivation, we performed experiments in the presence of eculizumab. However, purified patient IgGs were still able to severely block Fg binding, suggesting that an allosteric interference of the anti-αIIbβ3 isoantibodies was implicated (Figure 7A). Consistent with this hypothesis, platelet CD41a binding was not restored by eculizumab, nor IVIGs (Figure 7B, C).
Figure 7.
(A) Inhibition of fibrinogen binding on the platelet surface by anti-αIIbβ3 isoantibodies in the presence of eculizumab (50 μg/mL). (B) FSC and (C) CD41a median fluorescence intensity values obtained after mixing control platelets with GT3 collected at different dates, or GT12’s purified IgGs in the presence of eculizumab (50 μg/mL) in the presence of eculizumab (50 ug/mL) or human polyclonal immunoglobulins (IVIg, 25 mg/mL). Mann–Whitney–Wilcoxon test and Wilcoxon signed-rank test for controls incubated with ionophore or not. ∗< .05. FSC, forward scatter; IgG, immunoglobulin G; IVIg, intravenous immunoglobulin.
3.7. Concentration or combinations of anti-αIIbβ3 isoantibodies from other patients with GT did not induce PS exposure
Among all GT-tested patients, GT3 and GT12 had high levels of anti-αIIbβ3 isoantibodies. Thus, we hypothesized that anti-αIIbβ3 isoantibodies from other patients with GT could also induce PS exposure by decreasing the number of antigenic targets. Samples with low levels of anti-αIIbβ3 isoantibodies were tested with cPRP containing a low platelet count (100 × 109 platelets/L). However, no PS exposure was observed (Supplementary Figure S5). Nonetheless, Rijkers et al. recently showed a synergistic complement activation employing a combination of monoclonal IgGs to different antigenic determinants on HLA (Platelet surface Human Antigen Leukocyte Class I is also implicated in anti-platelet allo-immunization) [29]. Combining anti-αIIbβ3 isoantibodies from 3 different patients with GT did not reproduce the PS exposure phenotype (Supplementary Figure S5). These results suggest that concentration and/or combination of anti-αIIbβ3 isoantibodies was not sufficient to induce the procoagulant phenotype observed with GT3 or GT12’s serum.
4. Discussion
Development of anti-αIIbβ3 isoantibodies after platelet transfusion remains a major concern in GT [10,16]. As yet, the pathogenic properties of these isoantibodies have been poorly characterized. Here, we have shown by in vitro studies that rare anti-αIIbβ3 isoantibodies produced by 2 patients with GT were able to severely block Fg binding to the integrin and generate procoagulant platelets in healthy donors.
Interestingly, Althaus et al. have recently shown that immunoglobulin G fractions from patients with COVID-19 induced procoagulant platelets by an Fcγ receptor IIA–dependent pathway [31]. Therefore, we tested whether the generation of procoagulant platelets induced by anti-αIIbβ3 also occurs via this signaling pathway. Since a blocking antibody directed to the Fcγ receptor IIA did not block the generation of procoagulant platelets, these isoantibodies do not appear to be dependent on this signaling pathway.
In contrast, blocking of complement activation resulted in complete inhibition of the induction of procoagulant platelets as measured by flow cytometry using a combination of GSAO and CD62P antibody. This suggests that isoantibodies can induce platelet procoagulant phenotype by promoting complement deposition. This is consistent with a report by Wiedmer et al., which showed that platelet membrane assembly of the C5b-9 proteins markedly accelerated the rate of plasma clotting, thereby suggesting a direct link between C5b-9–mediated procoagulant activity and immunologic damage to the platelet [32,33].
In our study, we also demonstrated that not all anti-αIIbβ3 isoantibodies block Fg binding. Indeed, combining positive sera from different patients with GT did not show enhanced PS exposure in healthy platelets. This suggests that a specific structural organization of platelet-bound IgG is crucial for initiation of the complement cascade. Moreover, additional properties of anti-αIIbβ3 isoantibodies such as specific subclass or composition of the glycan in the Fc part of IgGs may modulate complement activation [34,35]. Regarding the binding site, data suggest that the activating antibodies block access of binding site of the CD41a antibody clone. To investigate this further, we would need to test the patient isoantibodies against either a library of integrin mutants or perform displacement studies with multiple antibodies with known target epitopes. However, we were limited by the remaining amount of patients’ isoantibodies.
An important question that remained unanswered is whether our observations in vitro are reflected in vivo. Assessing whether PS-expressing platelets are increased in vivo in peripheral blood from patients with GT3 and GT12 could add clinically relevant information. For that, we would need to test transfused platelets/thrombin generation in the patients in the presence of the activating antibody, which was not practical here. The potential prothrombotic effect of these isoantibodies if they come into contact with transfused platelets remains uncertain. Recently, Althaus et al. have shown that antibody-mediated procoagulant platelets in SARS-CoV-2 vaccination were associated with immune thrombotic thrombocytopenia [36]. It would be of interest to explore whether procoagulant platelets are induced in other conditions in which anti-αIIbβ3 antibodies are present, such as in ITP, where thrombotic complications have been reported [37,38]. Platelet PS exposure and loss of mitochondrial transmembrane potential have been previously demonstrated in this setting [39]. Reports of thrombosis in patients with GT are rare, but can occur [40]. However, to our knowledge, such complications have never been reported to be closely linked to platelet transfusions. Consistent with this, GT3 and GT12 have never presented with thrombotic complications.
In other conditions, antibody-mediated in vitro induction of a procoagulant phenotype correlates with improved hemostasis. In murine models of hemorrhage, treatment with platelet-mimicking procoagulant nanoparticles has been shown to reduce bleeding and improve survival [41]. However, it is not possible to confirm this as patients with GT3 and GT12 have not received further platelet transfusion after testing positive for isoantibodies on MAIPA.
Rijkers et al. have previously found that a subset of anti-HLA IgG induced complement activation and propose that this event can contribute to fast platelet clearance in vivo in patients refractory to platelet transfusions [29]. Ideally, the ability of these isoantibodies to induce complement deposition on donor platelets should be evaluated in relation with platelet transfusion refractoriness in future studies. Implementation of functional tests focusing on complement deposition may have the potential to help answer this question. Whether some high-titer isoantibodies may either interfere with platelet function or cause platelet elimination should also be evaluated.
IVIGs has been shown to be effective in the treatment of complement-dependent autoimmune diseases [42]. In a placebo-controlled, randomized blinded study, Fazekas et al. evaluated the efficacy of IVIGs in HLA alloimmunized patients and showed that IVIGs may improve platelet recovery [43]. Similarly, eculizumab reduced complement activation and MAC formation [44]. In a pilot trial, Vo et al. showed that eculizumab had the ability to overcome platelet transfusion refractoriness in patients with broad HLA alloimmunization [45]. Hence, this warrants future investigation into the potential benefit of complement inhibitors in patients with refractory GT receiving platelet transfusions.
In conclusion, we have shown that rare anti-αIIbβ3 isoantibodies can severely block Fg binding and generate procoagulant platelets via complement activation on healthy platelets in vitro (Supplementary Figure S6). Nonetheless, due to the rarity of the disease, we were limited by the number of patients included in the study, with only 2 with detectable platelet-activating isoantibodies.
Acknowledgments
Dr X. Pillois, with whom this study was initiated, suddenly died in the summer of 2021 and we dedicate this work to him.
Funding
The authors thank the LFB laboratory (Courtaboeuf, France) for providing polyvalent human immunoglobulins.
Author contributions
M.F. planned the study. V.M.Y.C. and C.S.M.L. contributed to experimental design. E.M. and M.M. performed most of the platelet function assays. Y.H. and M.F. followed patients clinically. C.S.M.L. and S.W. performed experiments on procoagulant platelets. M.F., C.S.M.L., V.M.Y.C., and X.P. all contributed to data interpretation and writing of the manuscript.
Relationship Disclosure
M.F. has been previously invited in meetings organized by the LFB laboratory. V.M.C. is supported by a NSW Health Cardiovascular Capacity Building Grant, Australia. All the other authors have no competing interests to disclose that might be perceived as posing a conflict or bias.
Footnotes
Handling Editor: Prof. Yotis Senis
Supporting Information
References
- 1.Bledzka K., Smyth S.S., Plow E.F. Integrin αIIbβ3: from discovery to efficacious therapeutic target. Circ Res. 2013;112:1189–1200. doi: 10.1161/CIRCRESAHA.112.300570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nurden A.T. Platelet membrane glycoproteins: a historical review. Semin Thromb Hemost. 2014;40:577–584. doi: 10.1055/s-0034-1383826. [DOI] [PubMed] [Google Scholar]
- 3.Botero J.P., Lee K., Branchford B.R., Bray P.F., Freson K., Lambert M.P., et al. Glanzmann thrombasthenia: genetic basis and clinical correlates. Haematologica. 2020;105:888–894. doi: 10.3324/haematol.2018.214239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nurden A.T., Fiore M., Nurden P., Pillois X. Glanzmann thrombasthenia: a review of ITGA2B and ITGB3 defects with emphasis on variants, phenotypic variability, and mouse models. Blood. 2011;118:5996–6005. doi: 10.1182/blood-2011-07-365635. [DOI] [PubMed] [Google Scholar]
- 5.Nurden A.T. Glanzmann thrombasthenia. Orphanet J Rare Dis. 2006;1:10. doi: 10.1186/1750-1172-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Minno G., Coppola A., Di Minno M.N., Poon M.C. Glanzmann’s thrombasthenia (defective platelet integrin alphaIIb-beta3): proposals for management between evidence and open issues. Thromb Haemost. 2009;102:1157–1164. doi: 10.1160/TH09-04-0225. [DOI] [PubMed] [Google Scholar]
- 7.Poon M.C., Di Minno G., d’Oiron R., Zotz R. New insights into the treatment of Glanzmann thrombasthenia. Transfus Med Rev. 2016;30:92–99. doi: 10.1016/j.tmrv.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Grainger J.D., Thachil J., Will A.M. How we treat the platelet glycoprotein defects; Glanzmann thrombasthenia and Bernard Soulier syndrome in children and adults. Br J Haematol. 2018;182:621–632. doi: 10.1111/bjh.15409. [DOI] [PubMed] [Google Scholar]
- 9.Fiore M., d’Oiron R., Pillois X., Alessi M.C. Anti-αIIb β3 immunization in Glanzmann thrombasthenia: review of literature and treatment recommendations. Br J Haematol. 2018;181:173–182. doi: 10.1111/bjh.15087. [DOI] [PubMed] [Google Scholar]
- 10.Santoro C., Rago A., Biondo F., Conti L., Pulcinelli F., Laurenti L., et al. Prevalence of allo-immunization anti-HLA and anti-integrin αIIbβa3 in Glanzmann thromboasthenia patients. Haemophilia. 2010;16:805–812. doi: 10.1111/j.1365-2516.2010.02230.x. [DOI] [PubMed] [Google Scholar]
- 11.Hod E., Schwartz J. Platelet transfusion refractoriness. Br J Haematol. 2008;142:348–360. doi: 10.1111/j.1365-2141.2008.07189.x. [DOI] [PubMed] [Google Scholar]
- 12.Toogeh G., Sharifian R., Lak M., Safaee R., Artoni A., Peyvandi F. Presentation and pattern of symptoms in 382 patients with Glanzmann thrombasthenia in Iran. Am J Hematol. 2004;77:198–199. doi: 10.1002/ajh.20159. [DOI] [PubMed] [Google Scholar]
- 13.Poon M.C., d’Oiron R. Alloimmunization in congenital deficiencies of platelet surface glycoproteins: focus on Glanzmann’s thrombasthenia and Bernard-Soulier’s syndrome. Semin Thromb Hemost. 2018;44:604–614. doi: 10.1055/s-0038-1648233. [DOI] [PubMed] [Google Scholar]
- 14.Coller B.S., Peerschke E.I., Seligsohn U., Scudder L.E., Nurden A.T., Rosa J.P. Studies on the binding of an alloimmune and two murine monoclonal antibodies to the platelet glycoprotein IIb-IIIa complex receptor. J Lab Clin Med. 1986;107:384–392. [PubMed] [Google Scholar]
- 15.Levy-Toledano S., Tobelem G., Legrand C., Bredoux R., Degos L., Nurden A., et al. Acquired IgG antibody occurring in a thrombasthenic patient: its effect on human platelet function. Blood. 1978;51:1065–1071. [PubMed] [Google Scholar]
- 16.Kashiwagi H., Kiyomizu K., Kamae T., Nakazawa T., Tadokoro S., Takiguchi S., et al. Molecular analysis of a patient with type I Glanzmann thrombasthenia and clinical impact of the presence of anti-αIIbβ3 alloantibodies. Int J Hematol. 2011;93:106–111. doi: 10.1007/s12185-010-0731-5. [DOI] [PubMed] [Google Scholar]
- 17.Clofent-Sanchez G., Lucas S., Laroche-Traineau J., Rispal P., Pellegrin J.L., Nurden P., et al. Autoantibodies and anti-mouse antibodies in thrombocytopenic patients as assessed by different MAIPA assays. Br J Haematol. 1996;95:153–160. doi: 10.1046/j.1365-2141.1996.d01-1888.x. [DOI] [PubMed] [Google Scholar]
- 18.Jacquelin B., Tuleja E., Kunicki T.J., Nurden P., Nurden A.T. Analysis of platelet membrane glycoprotein polymorphisms in Glanzmann thrombasthenia showed the French gypsy mutation in the alphaIIb gene to be strongly linked to the HPA-1b polymorphism in beta3. J Thromb Haemost. 2003;1:573–575. doi: 10.1046/j.1538-7836.2003.00107.x. [DOI] [PubMed] [Google Scholar]
- 19.Fiore M., Pillois X., Nurden P., Nurden A.T., Austerlitz F. Founder effect and estimation of the age of the French Gypsy mutation associated with Glanzmann thrombasthenia in Manouche families. Eur J Hum Genet. 2011;19:981–987. doi: 10.1038/ejhg.2011.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poon M.C. The use of recombinant activated factor VII in patients with Glanzmann’s thrombasthenia. Thromb Haemost. 2021;121:332–340. doi: 10.1055/s-0040-1718373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morel O., Jesel L., Freyssinet J.M., Toti F. Cellular mechanisms underlying the formation of circulating microparticles. Arterioscler Thromb Vasc Biol. 2011;31:15–26. doi: 10.1161/ATVBAHA.109.200956. [DOI] [PubMed] [Google Scholar]
- 22.Pasalic L., Wing-Lun E., Lau J.K., Campbell H., Pennings G.J., Lau E., et al. Novel assay demonstrates that coronary artery disease patients have heightened procoagulant platelet response. J Thromb Haemost. 2018;16:1198–1210. doi: 10.1111/jth.14008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hua V.M., Abeynaike L., Glaros E., Campbell H., Pasalic L., Hogg P.J., et al. Necrotic platelets provide a procoagulant surface during thrombosis. Blood. 2015;126:2852–2862. doi: 10.1182/blood-2015-08-663005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agbani E.O., van den Bosch M.T.J., Brown E., Williams C.M., Mattheij N.J.A., Cosemans J.M.E.M., et al. Coordinated membrane ballooning and procoagulant spreading in human platelets. Circulation. 2015;132:1414–1424. doi: 10.1161/CIRCULATIONAHA.114.015036. [DOI] [PubMed] [Google Scholar]
- 25.Durrant T.N., van den Bosch M.T., Hers I. Integrin αIIbβ3 outside-in signaling. Blood. 2017;130:1607–1619. doi: 10.1182/blood-2017-03-773614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pang A., Cui Y., Chen Y., Cheng N., Delaney M.K., Gu M., et al. Shear-induced integrin signaling in platelet phosphatidylserine exposure, microvesicle release, and coagulation. Blood. 2018;132:533–543. doi: 10.1182/blood-2017-05-785253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qiao J., Al-Tamimi M., Baker R.I., Andrews R.K., Gardiner E.E. The platelet Fc receptor, FcγRIIa. Immunol Rev. 2015;268:241–252. doi: 10.1111/imr.12370. [DOI] [PubMed] [Google Scholar]
- 28.Lee C.S.M., Liang H.P.H., Connor D.E., Dey A., Tohidi-Esfahani I., Campbell H., et al. A novel flow cytometry procoagulant assay for diagnosis of vaccine-induced immune thrombotic thrombocytopenia. Blood Adv. 2022;6:3494–3506. doi: 10.1182/bloodadvances.2021006698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rijkers M., Schmidt D., Lu N., Kramer C.S.M., Heidt S., Mulder A., et al. Anti-HLA antibodies with complementary and synergistic interaction geometries promote classical complement activation on platelets. Haematologica. 2019;104:403–416. doi: 10.3324/haematol.2018.201665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mattheij N.J., Gilio K., van Kruchten R., Jobe S.M., Wieschhaus A.J., Chishti A.H., et al. Dual mechanism of integrin αIIbβ3 closure in procoagulant platelets. J Biol Chem. 2013;288:13325–13336. doi: 10.1074/jbc.M112.428359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Althaus K., Marini I., Zlamal J., Pelzl L., Singh A., Häberle H., et al. Antibody-induced procoagulant platelets in severe COVID-19 infection. Blood. 2021;137:1061–1071. doi: 10.1182/blood.2020008762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiedmer T., Esmon C.T., Sims P.J. On the mechanism by which complement proteins C5b-9 increase platelet prothrombinase activity. J Biol Chem. 1986;261:14587–14592. [PubMed] [Google Scholar]
- 33.Wiedmer T., Esmon C.T., Sims P.J. Complement proteins C5b-9 stimulate procoagulant activity through platelet prothrombinase. Blood. 1986;68:875–880. [PubMed] [Google Scholar]
- 34.Van Osch T.L.J., Oosterhoff J.J., Bentlage A.E.H., Nouta J., Koeleman C.A.M., Geerdes D.M., et al. Fc galactosylation of anti-platelet human IgG1 alloantibodies enhances complement activation on platelets. Haematologica. 2022;107:2432–2444. doi: 10.3324/haematol.2021.280493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Osch T.L.J., Pongracz T., Geerdes D.M., Mok J.Y., van Esch W.J.E., Voorberg J., et al. Altered Fc glycosylation of anti-HLA alloantibodies in hemato-oncological patients receiving platelet transfusions. J Thromb Haemost. 2022;20:3011–3025. doi: 10.1111/jth.15898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Althaus K., Möller P., Uzun G., Singh A., Beck A., Bettag M., et al. Antibody-mediated procoagulant platelets in SARS-CoV-2-vaccination associated immune thrombotic thrombocytopenia. Haematologica. 2021;106:2170–2179. doi: 10.3324/haematol.2021.279000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun S., Urbanus R.T., Ten Cate H., de Groot P.G., de Laat B., Heemskerk J.W.M., et al. Platelet activation mechanisms and consequences of immune thrombocytopenia. Cells. 2021;10:3386. doi: 10.3390/cells10123386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peerschke E.I., Yin W., Alpert D.R., Roubey R.A., Salmon J.E., Ghebrehiwet B. Serum complement activation on heterologous platelets is associated with arterial thrombosis in patients with systemic lupus erythematosus and antiphospholipid antibodies. Lupus. 2009;18:530–538. doi: 10.1177/0961203308099974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levy-Mendelovich S., Aviner S., Sharon N., Miskin H., Yacobovich J., Kenet G., et al. Pediatric immune thrombocytopenia: apoptotic markers may help in predicting the disease course. Pediatr Res. 2021;90:93–98. doi: 10.1038/s41390-020-01355-9. [DOI] [PubMed] [Google Scholar]
- 40.Nurden A.T. Should studies on Glanzmann thrombasthenia not be telling us more about cardiovascular disease and other major illnesses? Blood Rev. 2017;31:287–299. doi: 10.1016/j.blre.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 41.Sekhon U.D.S., Swingle K., Girish A., Luc N., de la Fuente M., Alvikas J., et al. Platelet-mimicking procoagulant nanoparticles augment hemostasis in animal models of bleeding. Sci Transl Med. 2022;14 doi: 10.1126/scitranslmed.abb8975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Appeltshauser L., Weishaupt A., Sommer C., Doppler K. Complement deposition induced by binding of anti-contactin-1 auto-antibodies is modified by immunoglobulins. Exp Neurol. 2017;287:84–90. doi: 10.1016/j.expneurol.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 43.Fazekas F., Deisenhammer F., Strasser-Fuchs S., Nahler G., Mamoli B. Randomised placebo-controlled trial of monthly intravenous immunoglobulin therapy in relapsing-remitting multiple sclerosis. Austrian immunoglobulin in multiple sclerosis study group. Lancet. 1997;349:589–593. doi: 10.1016/s0140-6736(96)09377-4. [DOI] [PubMed] [Google Scholar]
- 44.McKeage K. Eculizumab: a review of its use in paroxysmal nocturnal haemoglobinuria. Drugs. 2011;71:2327–2345. doi: 10.2165/11208300-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 45.Vo P., Purev E., West K.A., McDuffee E., Worthy T., Cook L., et al. A pilot trial of complement inhibition using eculizumab to overcome platelet transfusion refractoriness in human leukocyte antigen allo-immunized patients. Br J Haematol. 2020;189:551–558. doi: 10.1111/bjh.16385. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.