Abstract

Macromolecular crowding affects the activity of proteins and functional macromolecular complexes in all cells, including bacteria. Crowding, together with physicochemical parameters such as pH, ionic strength, and the energy status, influences the structure of the cytoplasm and thereby indirectly macromolecular function. Notably, crowding also promotes the formation of biomolecular condensates by phase separation, initially identified in eukaryotic cells but more recently discovered to play key functions in bacteria. Bacterial cells require a variety of mechanisms to maintain physicochemical homeostasis, in particular in environments with fluctuating conditions, and the formation of biomolecular condensates is emerging as one such mechanism. In this work, we connect physicochemical homeostasis and macromolecular crowding with the formation and function of biomolecular condensates in the bacterial cell and compare the supramolecular structures found in bacteria with those of eukaryotic cells. We focus on the effects of crowding and phase separation on the control of bacterial chromosome replication, segregation, and cell division, and we discuss the contribution of biomolecular condensates to bacterial cell fitness and adaptation to environmental stress.
1. Introduction
In bacteria and archaea, as in eukaryotes, macromolecular and supramolecular assemblies are at the core of all biochemical processes enabling cells to carry out their activities. Many of these assemblies are dynamic structures whose functions depend upon the ability of their constituent macromolecules to reversibly dissociate and reassociate. These dynamics regulate the biochemical activity of the interacting networks and/or facilitate structural modifications linked to function. The bacterial cell cycle machinery is an excellent example of an organized structure in which molecular assemblies involved in the initiation of replication, chromosome segregation, and cell division coordinate with one another for bacterial survival and genomic integrity.1
Although the intact cell represents an attractive system for studying the structural and functional organization of subcellular machines, interpreting the results obtained from such studies must consider that these interacting systems function inside the cell in a heterogeneous and highly volume-occupied or crowded environment.2−6 These microenvironments may influence the reactivity and location of proteins and other biological macromolecules involved in essential processes, thus acting as nonspecific modulating factors of bacterial cellular functions. The purpose of this review is to emphasize that the mode of operation of critical bacterial cell cycle events depends not only on the specific molecular interactions between their components but also on nonspecific interactions with elements of their intracellular microenvironments.
The cell interior of a simple organism such as Escherichia coli is highly crowded, as approximately 20–30% of its volume is occupied by macromolecules,7,8 although no single macromolecule needs to be highly concentrated for it to function. Therefore, a given protein X in the cytoplasm will be primarily subjected to the influence of excluded volume effects due to crowding by soluble macromolecules, leading to preferential (size- and shape-dependent) exclusion from highly volume-occupied elements. This exclusion may significantly alter the extent and rate of macromolecular reactions mediated by X. High macromolecular crowding can also drive phase transitions, resulting in the formation of membrane-free biomolecular condensates. During its life cycle, X will be subjected to additional background interactions with elements of its immediate surroundings, including ribosomes [ribosomal RNAs (rRNAs) contain most of the nucleic acid in a bacterial cell], the nucleoid (within which X will encounter a high local concentration of DNA and nucleoid-associated proteins), and the cytoplasmic membrane (within which X will encounter a high local concentration of lipids and membrane proteins).
These background interactions (nonspecific interactions between macromolecular reactants and other constituents of the local environment) can lead to excluded-volume effects (and beyond) due to natural crowding. These interactions also can result in partitioning between immiscible phases and surface adsorption that collectively contribute to the total free energy of the system. These effects thereby substantially influence the energetics, dynamics, and spatiotemporal organization of macromolecular interactions and reactions. The relative contribution of these effects on macromolecular reactivity likely differs between each of the intracellular environments.5,9
1.1. Macromolecular Crowding
The primary element of intracellular complexity is the presence of locally high concentrations of multiple macromolecular species. The importance of these background interactions arising from steric repulsion in volume-occupied native-like media lies in their generality; they are universally present, independently of the presence or absence of other types of interactions.9−11 Crowding refers to the amount of free energy required to transfer a macromolecule from a dilute solution to a crowded environment. This is equivalent to the amount of energy expended to create a cavity large enough to accommodate the introduced macromolecule (the entropic cost of changing the available volume around a macromolecule).12,13 Macromolecular aggregates exclude less volume to other macromolecules than isolated molecules, so it is less costly (in free energy) to add an n-mer to a crowded fluid than n monomers (Figure 1). Therefore, a fundamental chemical consequence of crowding is the nonspecific enhancement of reactions and processes leading to a reduction of total excluded volume. These reactions include the formation of macromolecular complexes in solution, binding of macromolecules to surface sites, formation of insoluble aggregates, and compaction or folding of proteins5,9,11,14 (Figure 1C). These predictions have been experimentally confirmed at physiologically significant regimes of volume occupancy (on the order of 10% or more), using a variety of macromolecules with different properties as crowders (for a detailed description of crowders and their use, we refer the readers to these comprehensive reviews5,10,15,16). Interestingly, the impact of the configurational entropic effects on the conformation of proteins has been used to design fluorescence-based crowding sensors.17−19 When the crowding (excluded volume) increases, the sensor takes on a more compact shape, which leads to increased Förster resonance energy transfer (FRET) from cerulean (CFP) to citrine (YFP).
Figure 1.

Molecular effects of crowding. (A and B) Crowding increases the chemical potential (activity) of a test protein (T) in solution in a size- and shape-dependent manner. The squares represent a volume element containing spherical macromolecules (in black) that occupy about 30% of the total volume, as is typical of bacterial cytoplasm. The available volume to the center of T is indicated by the blue-colored regions, and its complement (in red) is referred to as the excluded volume. If T is very small relative to the background macromolecules (A), the available volume is almost equal to the total unoccupied volume. But if the size of T is comparable to that of the other solutes (B), the available volume is considerably smaller and the contribution of steric repulsion to reduced entropy and increased free energy is correspondingly greater. Clearly, one of the ways in which the system can reduce its free energy is to maximize the available volume (or, alternatively, to minimize the excluded volume). Reproduced from ref (20). Copyright 2001 Elsevier Inc. under Creative Commons CC-BY license [https://creativecommons.org/licenses/by/4.0/]. (C) Thermodynamic cycles illustrating how dilute or crowded solutions determine free energy differences for (i) a binary heteroassociation between molecules A and B, (ii) a ligand L interacting with its binding site, and (iii) a two-state folding of a protein (red). Reproduced from ref (15). Copyright 2008 Annual Reviews.
Significantly, the expected magnitude of crowding effects increases rapidly as the size of the tracer (protein) species increases relative to the size of the crowding species.9,12 Therefore, the concerted formation of a large oligomer would be much more sensitive to excluded volume effects than the formation of a homo- or hetero-dimer, as observed experimentally (refs (5 and 9) and references therein). Along these lines, the most significant effects of crowding include decreasing the equilibrium solubility of macromolecules, with an increasing tendency to condense and enhance the formation of higher-order protein assemblies.21−24 This can also induce the spontaneous alignment and bundling of self-assembling fibers, particularly relevant for cytoskeletal organization.23,25,26
As the cell interior is far more complex than systems studied theoretically or experimentally in vitro, the potential implications of additional specific and nonspecific interactions, other than volume exclusion, on macromolecular reactivity in crowded environments has been contemplated since the early investigations on crowding.27,28 More recent studies have shown that additional attractive interactions between background molecules and the reactants studied could compensate (to a varying degree) for the repulsive steric interaction due to volume exclusion (refs (29−32) and references therein). While excluded-volume effects are ubiquitous, the impact of compensating attractive interactions is highly variable and system-dependent, as they vary with the chemical nature of the interacting species and the type of reactions studied. In this regard, analyses of the effect of crowding composition on protein solubility and fiber formation have revealed that when the aggregating protein is small relative to crowders, attractive protein–crowder interactions can eventually inhibit protein polymer formation (and, likewise, inhibit association of relatively small proteins). However, when the tracer protein is larger than the dominant crowding species, nonspecific attractive interactions between tracer and crowder are likely insufficient to overcome the magnitude of the excluded volume effect, thus promoting polymer formation and aggregation.31
Finally, crowding can affect macromolecular reaction rates by two distinct mechanisms (ref (15) and references therein). In the case of slow, transition-state limited reactions, crowding generally increases the association rate constant and has little effect on the dissociation rate constant. In the case of fast reactions, the limiting factor of the association rate is generally the rate of encounter of the reactants, usually dominated by translational diffusion, which decreases monotonically with increased crowding. The combination of these effects may result in a biphasic dependence regime in which the association rate initially increases with crowder concentration, toward reaching a maximum, and then subsequently decreases upon increasing crowding.15,33
1.2. Macromolecular Partitioning and Liquid–Liquid Phase Separation (LLPS)
A second element of intracellular complexity relates to the presence of multiple microenvironments, resulting in the partitioning of macromolecular species between immiscible phases with different concentrations of each macromolecule in each phase. A variety of membrane-less organelles found during the past decade within the cell interior that cluster specific biomolecules away from their surroundings represent examples of these local microenvironments.34 They have been tentatively identified as immiscible liquid phases, which most likely arise through LLPS, a physicochemical process well studied in polymer chemistry. The latter are also linked to the formation of biomolecular condensates, dynamic structures containing a wide range of proteins and nucleic acids. Such condensates are thought to provide special microenvironments in which the rates and equilibria of critical biochemical reactions may be modulated.35,36
These condensates have primarily been studied in eukaryotic cells.34,35 However, recent progress indicates that they are also assembled in prokaryotic cells where they play key roles.37 As bacteria typically lack membrane-bound organelles, phase separation provides a compelling novel mechanism for spatial and functional organization in this domain of life. Chromosome replication and segregation, and their tight coupling to cytokinesis, provide examples of LLPS with implications for bacterial fitness (vide infra).
A protein can undergo phase separation and form dynamic droplet-like structures above a critical concentration threshold, which is a function of temperature, pH, ionic strength, and physiologically relevant ligands (e.g., nucleotides) and protein modifications (Figure 2A).36 These droplets form a microcompartment that allows diffusion of molecules within the container and promotes the dynamic exchange of molecules with the dilute surrounding phase. The protein-containing droplets are stable above the critical concentration, but the protein system reverts to a one-phase regime when the protein concentration decreases below the critical concentration. Proteins that contain multivalent domains, which are mostly involved in protein–protein and protein–nucleic acid complexes, and those having intrinsically disordered regions are prone to form these droplet-like dynamic structures.38 RNA can further promote this process by interacting through RNA-binding domains.39,40 Although intrinsically disordered regions in proteins have been traditionally considered the main drivers in the formation of condensates, there is growing evidence that in many instances they have a secondary role, acting as modulators of condensation events promoted by folded domains.41
Figure 2.
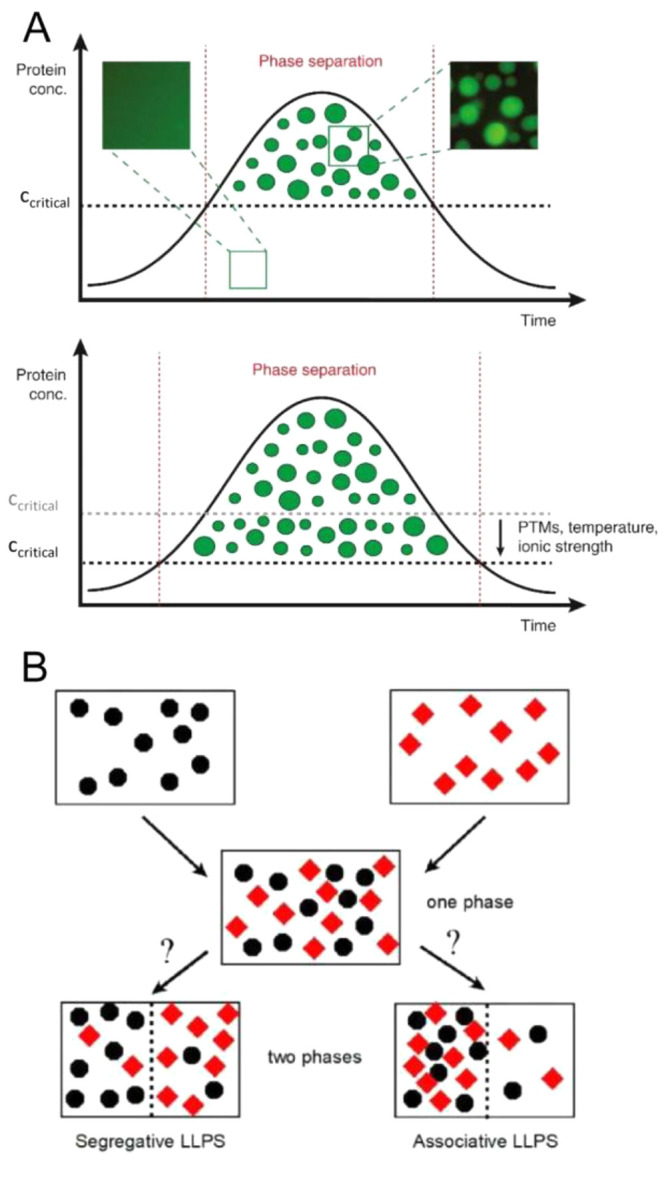
Phase separation. (A) Top: A scheme showing the time-dependent formation of liquid droplets of a protein above the critical concentration for phase separation. These protein microcompartments are dynamic and can exchange molecules with the surrounding phase. Below the critical concentration, they dislodge to form a one-phase state. The insets above show original data from a phase separation experiment with purified GFP-tagged FUS (a prion-like RNA-binding protein). Bottom: Post-translational modifications (PTMs) or changes in temperature or ionic strength can lower the critical threshold for phase separation and allow droplet formation at a much lower protein concentration. Reproduced with permission from ref (42). Copyright 2017 Elsevier Ltd. (B) Liquid–liquid phase separation in a solution containing two macromolecular solute species. Black circles denote species 1, and red diamonds denote species 2. Segregative phase transitions occur when the heterointeraction between molecules of species 1 and 2 is more repulsive than self-interactions between molecules of either species 1 or species 2. Associative phase transitions occur when heterointeractions between molecules of species 1 and species 2 are more attractive than self-interactions between molecules of either species 1 or species 2. Reproduced from ref (43). Copyright 2020 American Chemical Society under an ACS AuthorChoice license [https://pubs.acs.org/page/policy/authorchoice_termsofuse.html].
Crowding can promote these phase transition processes (recently reviewed in ref (44)). These studies have revealed two major features.5,38,43 If the proteins prone to phase separate establish attractive and nonspecific interactions with each other and with molecular additives such as nucleic acids or crowders, these interactions will lead to the formation of an associative LLPS. This phenomenon is also termed complex coacervation,45 in which one phase is enriched in both proteins and molecular additives and the second phase is depleted of both macromolecular species. On the other hand, if the crowders enhance protein associations via volume exclusion, then this nonspecific interaction will lead to a segregative LLPS. In this case, one phase is enriched (relative to the total composition) in the protein and depleted (relative to the total composition) of the crowder, while the second phase is enriched in the crowder and depleted of the protein (Figure 2B).
Significantly, in some instances, such droplet-like structures evolve with time (“age”) to form more solid-like or hydrogel structures, and/or the concentrated molecules within them can form fibrils, etc.40 These transitions are mostly related but not restricted to disease states.46 These observations have focused on studying the final state of matter resulting from the phase separation process. However, it is compelling to consider LLPS as an active process that may be modulated nonspecifically by crowding and specifically by proteins (i.e., those regulating essential cellular processes), which eventually dynamically act on the membrane (see below). Disentangling these interactions is a challenging task, especially in cellular systems, as phase separation and solubility may cooperate in poorly controlled ways, partly due to the difficulties of measuring precisely the composition dependence of phase diagrams in complex cell-like reconstituted systems and cellular environments.36,47,48 These experimental complications lead to ambiguous interpretations of in vivo observations related to phase separation and condensate formation.47
1.3. Interfacial (Surface) Effects
Surface interactions represent a special case of macromolecular partitioning.4 A protein near a membrane is in an environment significantly different from one that is distant from the surface.49,50 The same applies to the surface of large supramolecular structures such as cytoskeletal fibers. Proteins are localized at the surfaces of these structures by attractive electrostatic and/or hydrophobic interactions in addition to repulsive volume-exclusion interactions.5,49 Theory and experiments have shown that adsorbed macromolecules have a stronger tendency to self- or heteroassociate than those in bulk solution and that the tendency to associate increases substantially with the strength of attraction between the soluble macromolecule and the surface.4,5 Therefore, surfaces can act as scaffolds for protein organization in which nonspecific attraction between soluble proteins and the surfaces of membranes and fibers leads to enhanced surface adsorption of protein and self- and heteroassociation of adsorbed protein. Interestingly, these interfacial interactions can facilitate the formation of surface-associated assemblies and clusters, some of which could be compatible with phase-separated condensates.51−53
Quantitative characterization and correct interpretation of the combined effects of crowding, phase separation, surface interactions, and physicochemical homeostasis on reconstituted systems of increasing complexity will narrow the gap between in vitro and in vivo studies and provide further insights on the control of cellular functions and the emergent properties of the living cell. Moreover, this approach will aid the building and integration of functional modules from the bottom up in the context of synthetic cell research.54,55
2. Structure of Bacterial Cytoplasm and Physicochemical Homeostasis
2.1. Physicochemical Homeostasis
Physicochemical homeostasis is the ability of a system to maintain steady internal physical and chemical conditions such as (macro)molecular crowding, pH, ionic strength, and turgor pressure. Control of these generic factors is important for the catalytic performance, architecture, and vitality of any cell, regardless of its specific function or ecological habitat. We present the physicochemical homeostasis in connection to the volume regulation of the cell, because osmotic perturbations offer a means to alter and study the physical and chemical state of the cytoplasm. Moreover, osmotic up- or downshifts affect the macromolecular crowding and apparent viscosity, internal pH, ionic strength, and turgor pressure, and it is almost impossible to separate these properties from each other (see extended abstract published in Poolman 2023).56 Finally, we connect the physicochemical homeostasis to the energy status of the cell and focus on various interdependencies of these cellular parameters rather than on the mechanisms of the (membrane) proteins involved [for comprehensive reviews on these topics, we refer to refs (57−62)).
2.1.1. Quantitative Aspects of Macromolecular Crowding
In bacteria, proteins make up the majority of the cell’s macromolecules (∼55% w/w) and, together with rRNA (∼15% w/w), are the most space-consuming molecules.63 They occupy macromolecular volume fractions (Φ) in the range of 0.13–0.24, depending on the growth conditions.7,17,64−66 The excluded volume fraction of the cytoplasm can be even higher when bacteria are exposed to severe hypertonicity, and barriers for diffusion can form due to aggregation of biomolecules.67 Intriguingly, in plasmolyzing E. coli, the cytoplasm appears as a meshwork allowing the free passage of small molecules while restricting the diffusion of bigger ones. As described in the Introduction, the background interactions (mostly nonspecific) between proteins and other macromolecules and their surroundings within the highly volume occupied bacterial interior can significantly influence the equilibria and rate of macromolecular reactions when compared to the same reactions in uncrowded media.
The high crowding of the cytoplasm speeds up slow (transition-state limited) reactions, allowing processes to occur rapidly and enabling bacteria to grow with doubling times well below 1 h. But there is an optimum to the crowding, because too high an excluded volume (Φ) slows down diffusion-limited reactions.68 Computational modeling of a model cell shows that protein synthesis, involving the interaction of large macromolecules (e.g., tRNA and mRNA with ribosomes), is more hindered by high crowding than metabolic pathways involving diffusion of small molecules to the active site of enzymes.69 For example, maximal biochemical fluxes for ribosomal systems peak at Φ = ∼0.12, whereas metabolic systems plateau at Φ values from 0.1 to 0.6. The (micro)organisms studied to date have macromolecular volume fractions in the range of 0.15–0.20, which seemingly is the optimum to maximize the overall reactions rates without translational diffusion becoming a limiting factor.
2.1.2. pH Homeostasis
Protons, which participate in biochemical reactions as reactants and/or regulators of enzyme activity, can influence liquid–liquid phase separation and serve as a source of electrochemical energy, known as proton motive force (PMF). The PMF is composed of the membrane potential (ΔΨ, typically negative inside the cell relative to the outside) and the pH gradient (ΔpH, typically inside alkaline relative to the outside). In the equation
| 1 |
2.3RT/F equals 58 mV (at T = 298 K) and is abbreviated as Z, F is the Faraday constant, R the gas constant, and T is the absolute temperature. The generation of PMF is inseparable from the regulation of the internal pH. Bacteria and archaea generate PMF by electron transfer or respiration, light-driven proton translocation, ATP-driven proton pumps, or coupling of electrogenic transport to a metabolic reaction,70 and each of these mechanisms increases the internal pH (the ΔpH component of the PMF). Neutralophiles maintain a roughly neutral cytoplasmic pH (7.0–7.5) when growing in environments at pH 5.5–9.0,71 which implies control of proton fluxes. In fact, at alkaline pH the net translocation of protons will be from outside to inside rather than inside to outside because the cytoplasm needs to be acidified. Consequently, the ΔpH is reversed when cells grow at alkaline pH, and the ΔpH makes a larger contribution to the PMF at acidic than at neutral pH; the opposite relationship is observed for the ΔΨ such that the PMF and internal pH of neutralophilic bacteria can be kept relatively constant (see Figure 1b of ref (71)).
Protons are pumped out by respiration or other mechanisms and pumped back into the cell by PMF-consuming processes such as ATP synthesis or nutrient uptake. These processes are not necessarily in balance and prokaryotic cells have additional mechanisms to fine-tune the internal pH, but first we should estimate what is needed for bacteria to maintain a neutral internal pH. A cell like E. coli with a radius of 0.4 μm and length of 2.2 μm has a volume of ∼1 fL. At pH 7.2 the number of free protons is only about 10. A few protons entering or leaving such a cell would have a large impact on the internal pH in the absence of intracellular buffering capacity. In reality, a bacterial cell typically has inorganic and organic phosphates in the tens of millimolar range, and in several cases the total phosphate pool is well above 100 mM;72 the latter would buffer ∼10 million protons, but additional buffer components can be involved.
Does the internal buffering capacity play an important role in pH homeostasis? The internal buffering capacity has been determined experimentally for a number of Gram-negative and Gram-positive bacteria,73 and for E. coli it is ∼100 nmol H•+·(pH unit·mg of cell protein)−1 around neutral pH.74 The rate of proton extrusion by respiring Escherichia coli cells is 200–1000 nmol H•+·(min·mg of cell protein)−1,75 which corresponds to 1 to 5 million H+·(s·cell)−1. These numbers imply that the internal pH would change by 1 pH unit within seconds if the cell lacked additional mechanisms to compensate for the proton extrusion by the respiratory chain. In addition to passive influx of protons (leakage), the cell translocates protons back into the cell via membrane transport (uptake of nutrients, product excretion, and others), but most of these systems have not evolved to maintain a constant internal pH. For pH homeostasis the cell needs regulatory mechanisms that act fast (high turnover number) and have a specific pH dependence; that is, they are gated by the internal pH.
Cells use different transport mechanisms to simultaneously maintain a relatively constant PMF and internal pH by interconverting ΔΨ and ΔpH. Key regulators of bacterial pH homeostasis are cation/H+ antiporters, anion/H+ antiporters and metabolite decarboxylation pathways. pH-sensing cation/H+ antiporters, acidify the cytoplasm by exporting K+ or Na+ in exchange for protons when the internal pH gets too high.71 One well-studied K+/H+ antiporter is Kef from E. coli.76,77 Another well characterized bacterial system is the Na+/H+ antiporter NhaA from E. coli, which has a turnover number of >1000 s–1, which exchanges 2H+ for 1Na+ ions, and whose activity displays a steep pH dependence.78,79 The transport by NhaA is electrogenic, implying that it is driven by ΔΨ and chemical gradients of protons and Na+ ions. Assuming that a typical E. coli cell contains ∼1000 molecules of NhaA, this antiporter alone would allow a respiring cell [translocating 1 to 5 million H+·(s·cell)−1] to maintain its internal pH within limits. We note that NhaA is driven by ΔΨ, whereas respiration is inhibited by a high ΔΨ. Hence, there is an additional level of regulation (“respiratory control”) of the internal pH beyond pH sensing and gating by the antiporter. Furthermore, a cell typically has multiple ion/H+ antiporters, and a large fraction of the protons enters the cell via solute-H+ importers for the uptake of nutrients and synthesis of ATP.70
pH-sensing ion/H+ antiporters acidify the cytoplasm, whereas chloride/H+ antiporters (pumping H+ out and Cl– in) and metabolite decarboxylation operate during acid stress and alkalinize the bacterial cytoplasm.70,71,80 Decarboxylation pathways are found in both respiratory and fermentative bacteria, and they serve to decarboxylate carboxylic acids and amino acids. How do these pathways contribute to pH homeostasis and lead to the generation of a PMF? The chemistry of a decarboxylation reaction requires a proton, and thus, the internal pH is increased (and a ΔpH is formed) when the reaction takes place inside the cell. The substrate and product of the reaction differ in charge because a carboxylate group is removed, but the molecules are otherwise structurally similar. Hence, they can be transported by the same protein, as has been shown for numerous substrate/product antiporters.70 The substrate and decarboxylated product carry a different net charge, and thus, a ΔΨ is generated when an antiporter exchanges these molecules.81−84Figure 3 shows the case for malate decarboxylation, and here ΔΨ is generated by malate/lactic acid exchange or malate uniport, in addition to passive diffusion of lactic acid across the membrane. In both scenarios, the equivalent of 1 proton is pumped per molecule decarboxylated. Bacterial amino acid decarboxylases have remarkably low pH optima,85,86 and their activity increases when the internal pH drops due to enhanced proton influx. Hence, the enzymes have a built-in self-regulatory mechanism to deal with lower pH values and thus contribute to pH homeostasis by pH-dependent decarboxylation.
Figure 3.

Decarboxylation of malate by malolactic enzyme MleA, and electrogenic transport of malate via antiport or uniport by MleP. Passive diffusion of lactic acid across the membrane is shown by the dashed arrow. The energetics of malate–/lactic acid antiport and malate– uniport plus lactic acid diffusion are equivalent. Reproduced with permission from ref (70). Copyright 2019 Wiley-VCHVerlag GmbH&Co. KGaA,Weinheim.
In summary, the above analysis shows that a relatively high buffering capacity of the cytoplasm is important for absorbing fluctuations in the internal pH, but pH sensing cation/H+ antiporters are essential for pH homeostasis under alkaline stress, whereas anion/H+ antiporters and metabolite decarboxylation are required under acid stress. Additional levels of regulation can come from the pH dependence of respiration, ATP synthesis/hydrolysis by F0F1-ATPase, and other processes.70,71,87 For longer time scales, pH-dependent regulation of the expression of genes for proton translocating systems can also play a role.
2.1.3. Ionic Strength Homeostasis
The ionic strength of a cell is the effective (and not total) ion concentration of the cytoplasm, expressed in molar units (M). In the equation
| 2 |
i is the ion identification number, z is the charge of the ion, and c is the concentration (mol/L) of free ion. The ionic strength screens electrostatic interactions of (macro)molecules and is used to tune enzyme activity and gate membrane functions. The actual ionic strength of the cell is typically not known because a large fraction of the ions is bound to macromolecules. The vast majority of prokaryotes have an overall anionic proteome,88 and together with nucleic acids they bind a large fraction of the cations of the cell. The fraction of bound versus free ions is most often not known but can be obtained by comparing the total ion concentration by atomic emission spectrometry with the free ion concentration by specific optical probes. Fluorescence-based sensors have been developed to determine the actual ionic strength inside single cells.18 These probes allow observation of spatiotemporal changes in ionic strength in the hundreds of millimolar range and have been used to determine how the internal ionic strength of cells adjusts in response to osmotic challenges.
The ionic strength influences the structure of intrinsically disordered proteins,89 the activity of enzymes,90 ion channels91 and transporters,92 protein aggregation,93 phase separations,94 protein binding to (poly)nucleic acids,95 and many other processes. Hence, a given cell maintains its ionic strength within limits, but the actual amounts of ions vary considerably among different species. The most abundant cations in (micro)organisms are K+ (∼0.2 M in E. coli; ∼20 million K+ per cell) and Mg2+ (20–40 mM total; 1–2 mM free ion),63 but halophiles can also have a high concentration of Na+. The reported concentrations of K+ in E. coli, Lactococcus lactis, and the halophilic archaeon Haloferax volcanii are ∼0.2, 0.8, and 2.1 M, respectively,88 which suggests that across prokaryotes the ionic strength varies more than the internal pH does, but within a species the ionic strength is constrained.
When cells are exposed to an osmotic upshift, the cell volume decreases because water diffuses out. This results in an increase in internal ionic strength and a decrease in internal pH (the proton concentration increases, and a change in ionic strength affects the apparent pKa of buffer components). The primary driver of cell volume regulation in E. coli and other bacteria upon osmotic upshift is the controlled accumulation of potassium and its counterion glutamate,73,96,97 which increases the cell volume but does not reduce the increased ionic strength. Excessively high ionic strength can impair enzyme function and be detrimental for the cell. Therefore, in a secondary response to the osmotic upshift, bacteria like E. coli and Bacillus subtilis replace the K+ ions by zwitterionic or neutral compatible solutes such as betaine (N-trimethylglycine), proline, and trehalose, thereby maintaining the osmotic pressure and ability to regulate the cytoplasmic volume but reducing the internal ionic strength.97,98 The osmoregulatory transporters BetP (Corynebacterium glutamicum), ProP (E. coli), OpuA (L. lactis), and homologues in archaea and bacteria can accumulate high levels of zwitterionic compatible solutes, which increases cell volume and reduces ionic strength.62,70,92,99,100 Importantly, these transporters sense ionic strength (or K+ ions) and are activated instantaneously when the internal ionic strength reaches a threshold value. Thus, like pH-gated cation/H+ antiporters that regulate the internal pH, ionic strength-gated compatible solute transporters regulate cell volume and indirectly influence internal ionic strength and pH.
In general, an ionic strength dependency suggests a role of electrostatic interactions according to the classical electrolyte and double layer theories.3,101,102 These theories predict that electrostatic interactions between charged surfaces are screened by a thermal distribution of small ions (ionic cloud), which reduce the range of Coulombic forces as measured by the Debye’s length, usually designated by 1/k. As activation of osmoregulatory transporters takes place at relatively high ionic strengths (e.g., from 0.2 to 0.5 M), the contribution of the electrostatic force is small. Yet, osmoregulatory transporters such as OpuA are switched from off to on (maximally active state) over this range of ionic strengths, most likely by disrupting multivalent electrostatic interactions between protein residues and an anionic membrane surface92,103 (vide infra).
2.1.4. Turgor Pressure
Cell turgor (ΔΠ) is the hydrostatic pressure difference that balances the difference in internal and external osmolyte concentration. In the equation
| 3 |
Vw is the partial molal volume of water, a is the water activity, c is the total osmolyte concentration, and the subscripts in and out refer to inside and outside of the cell, respectively. A cell plasmolyzes when ΔΠ is zero. Although cell turgor is required for expansion of the cell wall, there is little information on what the lower limit of turgor pressure is before cell growth ceases. Depending upon the species, a bacterial cell may develop up to a few tens of atmospheres of pressure across the cell envelope. Wall-less bacteria such as Mycoplasma sp. are not protected against turgor pressure by a peptidoglycan layer, and thus, ΔΠ is low.104 The turgor pressure in thin-walled Gram-negative bacteria is in the range of 1–3 atm, which amounts to a difference in osmolyte concentration (cin – cout) of 40–120 mM (∼40 mM/atm). The turgor pressure of thicker-walled Gram-positive bacteria such as B. subtilis, L. lactis, and Listeria monocytogenes can be as high as 20 atm,67,68,105,106 corresponding to cin – cout of ∼800 mM. Variations in turgor pressure during nutrient shifts in E. coli and Caulobacter crescentus give rise to elastic changes in surface area, which are thought to be caused by changes in cell width rather than length.107 Thus, mechanical forces originating from turgor pressure can regulate the width of bacterial cells and influence macromolecular crowding in the cytoplasm.
Turgor pressure variations are typically much larger when cells are confronted with hypertonic stress (osmotic upshift conditions). In E. coli turgor pressure decreases from ∼3 to 1.5 and <0.5 atm when the osmolality of the growth medium is increased from 0.03 to 0.1 and >0.5 Osm.108 Although a turgor pressure of <0.5 atm may be sufficient to sustain the growth of E. coli, it is possible that Gram-positive bacteria have a higher turgor pressure minimum, because of the potential requirement for higher mechanical (expansion) force acting on the thicker cell wall.109,110
Upon a sudden osmotic upshift, turgor pressure and cytoplasmic volume decrease. In addition, the ionic strength, crowding, and (macromolecular) viscosity increase while the internal pH and water activity decrease (Figure 4). Cells counter the detrimental effects of hypertonicity by activating (gating) specific transport proteins that accumulate large amounts of compatible solutes or by synthesis of these molecules,111−113 which hydrates the cytoplasm and reverses the physicochemical changes. Various osmoregulatory mechanisms have been described to protect cells against hypertonic stress. Here, we focus on the ATP-binding cassette transporter OpuA of L. lactis, to illustrate how a single protein integrates various signals and elicits a response to the stress that encompasses several physicochemical properties.
Figure 4.
Osmotic challenges and changes in the physicochemistry of the cell. Hypertonicity leads to cell shrinkage and a lowering of the turgor pressure (Δπ); cells plasmolyze when Δπ is zero. During plasmolysis, the cell membrane shrinks away from the cell wall, leading to the collapse of the cytoplasm. The effect of hypertonicity on the overall physicochemistry of the cytoplasm is indicated in the bottom right of the figure. Hypotonicity leads to water uptake and swelling of cells, which increases Δπ and ultimately leads to cell lysis. Figure modified from ref (56). Copyright the Author(s) 2023. Published by Oxford University Press under the terms of the Creative Commons Attribution-NonCommercial License [http://creativecommons.org/licenses/by-nc/4.0/]. Top right: Illustration by David S. Goodsell, RCSB Protein Data Bank114 depicting the high crowding environment of the bacterial cell, the exclusion of large macromolecular complexes [e.g., (poly)ribosomes in purple] from the nucleoid, and the two-membrane system plus peptidoglycan layer of a Gram-negative bacterium.
When the volume of the bacterium decreases and the ionic strength reaches threshold values, OpuA is activated and large amounts of betaine are taken up.92 Passive influx of water follows the accumulation of betaine, and consequently, the volume of the cell increases and the ionic strength decreases. The electrostatic gating force acts between a specific osmosensing domain on the protein and the negative membrane plane.61,70 Hence, the threshold ionic strength for activation of the transporter can be tuned by varying the fraction of anionic lipids in the membrane.115 Macromolecular crowding does not activate OpuA but acts synergistically with ionic strength,116 presumably by adversely affecting the electrostatic interactions of differently charged protein–membrane surfaces via excluded volume effects. It was long thought that ionic strength gating was the only mechanism that controlled OpuA activity and the transporter would be switched off after restoration of normal cell volume. The second messenger cyclic-di-AMP has recently been shown to act as a backstop for the protein to prevent rampant accumulation of betaine,103 that is, when the volume has been restored but the ionic strength of the stress-adapted cells is still above the gating threshold. Importantly, cyclic-di-AMP also plays a key role in the control of potassium transport, the other key component of cell volume regulation in bacteria.117−120
Figure 4 shows that hypotonicity leads to swelling of the cell and an increase in ΔΠ. A lipid membrane can stretch up to ∼5% area before lysis tension is reached.121 To excrete osmolytes when turgor pressure becomes too high, microorganisms activate mechanosensitive (MS) channels.59 Bacteria have different types of MS channels; for example, E. coli has seven, but other microbes have a smaller number.122 The best-studied MS channels are MscL and MscS, which jettison solutes with little discrimination, except for size, and thereby lower the ΔΠ and the risk of cell lysis. The sensing mechanism of these MS channels is completely different from that of the osmoregulatory transporters (vide supra). The increase in tension in the membrane following water influx is sensed as a decrease in lateral pressure on the protein, which facilitates the transition from the closed to the open state. The closed-to-open transition of MscL involves an iris-like expansion, which leads to a final open pore diameter of ∼2.8 nm and a conductance of ∼3 nS and requires a gating tension of ∼10 mN/m.123 The closed-to-open transition of MscS involves the rotation and tilt of pore-lining helices,124 which leads to a final open pore diameter of ∼1 nm and a conductance of ∼1.25 nS and requires a lower gating tension than that for MscL.59,125 The MS channels act (gate) on short time scales (∼20 ms),126 which is required to counter the rapid swelling upon hypoosmotic shifts. Both MscL and MscS are gated by membrane tension (γ) and the pressure across the membrane (Δp) does not play a role as stimulus,127 but the two parameters are connected as shown in the Young–Laplace equation:
| 4 |
Here, r1 and r2 are the principal radii of the membrane, which change when the cell volume changes.
2.2. Structure and Dynamics of Cytoplasm
2.2.1. Macromolecular Composition of Cytoplasm
The bacterial cytoplasm is a complex and dynamic milieu that consists of water, ions, metabolites, macromolecules, and membraneless structures such as the nucleoid (DNA, DNA associated proteins, and RNA), inclusion bodies (irreversible assemblies of macromolecules), biomolecular condensates (reversible assemblies of macromolecules), and membrane-associated cytoskeletal elements. These complex assemblies are universally present in prokaryotes, although well-defined cytoskeletal structures are not found in the simplest bacteria and biomolecular condensates have so far only been studied in a few bacterial species. In addition, various metabolic enzymes across diverse microorganisms form intracellular bodies in the form of fibers and other types of functional mega-assemblies,128,129 which can be organism specific. The complex assemblies of macromolecules are mostly segregated from each other (vide infra), but they are not compartmentalized via a membrane. A variety of mechanisms underlie the physical separation of the cytoplasmic components, including macromolecular crowding, protein-based scaffolds, liquid–liquid phase separation, and spatial organization via biochemical gradients, but physicochemical factors such as the internal pH and ionic strength also play a role. Subcellular compartmentalization by lipid-based membranes is rare in prokaryotes, but anammoxosomes, magnetosomes, and acidocalcisomes are notable exceptions.130 Protein-based nano- and microcompartments are found in bacteria and archaea,131,132 and these protein-bounded structures encapsulate dedicated cargo proteins to create a specific environment for enzyme functioning.
In E. coli the chromosome and nucleoid-associated proteins localize around the cell center,133,134 where they form heterogeneous phase-like structure(s)135 that exclude translating ribosomes. These polysomes or polyribosomes (Terminology) localize at the cell poles and cytoplasmic periphery.133,136,137 Aggregated or misfolded proteins also localize at the cell poles but typically not evenly between the old and new pole.138−140 Single-molecule diffusion measurements with nanoscale resolution have shown that each cell has a so-called slow and fast pole.141,142 The slow diffusion at one pole coincides with the old pole of a dividing cell, where aggregated and misfolded proteins are more abundant and most likely hinder the diffusion more than at the newly formed pole.143 In terms of the structure of the bacterial cytoplasm, there is increasing evidence for the formation of phase-separated liquid droplets or biomolecular condensates,144−149 which are metastable structures where certain proteins partition and others are excluded (see also section 1). The function of biomolecular condensates in bacteria is mostly unexplored territory, but by analogy to mammalian cells they are likely involved in selective recruitment of client proteins, improving the efficiency of enzymatic reactions, and sequestering and processing of RNA and protein molecules, which can help E. coli cells resist environmental stresses.149 There are only a few studies where condensates in bacteria have been shown to increase the catalytic efficiency by concentrating enzymes and/or its substrate(s). One example is the sequestration and activity of a client kinase upon phase separation by ATP depletion in C. crescentus,150 showing that ATP depletion can promote LLPS, enforce protein compartmentalization, and sustain enzyme activity. Another example is the activity of a bacterial polynucleotide phosphorylase, which is enhanced when the enzyme colocalizes with RNase E within biomolecular condensates (in this case ribonucleoprotein bodies151).
The total of protein and RNA molecules in the cytoplasm of bacteria can reach volume fractions of 15–20% in growing cells and even higher in osmotically stressed cells.7,14,17,64 An excluded volume of 20% is equivalent to 3 million globular particles with a radius of 2.5 nm in a volume of 1 fL, which reflects the number and average size of proteins in an Escherichia coli cell. If the molecules were evenly distributed, their surface-to-surface distance would be ∼1.9 nm, which is smaller than the radius of the proteins and thus should significantly affect their diffusion.
The macromolecules, ions, and other small molecules of the cytoplasm form a gel-like medium with colloidal properties (Terminology). We postulated two decades ago that macromolecules are not evenly distributed in the cytoplasm and that regions of higher and lower crowding are present; transient networks of electrolyte pathways would wire the cytoplasm, guide the flow of biochemical ions, and increase local diffusivity.61,101 The high excluded volume, together with hyperstructures,152 metabolons,153 intracellular bodies,128 and liquid–liquid phase separation,35,154 would shape the cytoplasmic structure outside the regions of lower crowding. There is increasing evidence for this view of a dynamic and heterogeneously structured cytoplasm, as we show below.
One way to characterize the dynamic structure of the cytoplasm is to determine the mobility or translational diffusion of a molecule. In fact, the translational diffusion coefficient of a molecule inside the cell is frequently used as a proxy of macromolecular crowding under different metabolic or stress conditions. However, the intracellular environment is not a homogeneous medium with a single diffusion coefficient for a given molecule; many factors may retard the diffusion of a protein in a crowded cell, as illustrated in Figure 5. Moreover, the thermodynamic nonideality of the cytoplasm makes the diffusion coefficient not simply a sum of its contributors. Recently developed microscopy and computational methods allow the diffusion coefficient of molecules inside cells to be determined with high spatial and temporal resolution.141,142 Below we discuss how these technologies have enabled the characterization of the dynamic structure of the cytoplasm.
Figure 5.
Factors that affect protein diffusion inside cells. (A) Hard sphere collisions of the probe (blue) with other freely diffusing molecules (crowders) lowers its diffusion coefficient. (B) Movement through the hydrodynamic wake of another molecule slows down the probe. (C) Complex formation with another particle leads to a lower diffusion coefficient due to the increased effective size of the complex. (D) Immobile barriers such as membranes confine particles in a given part of the cell. The dimensionality of diffusion is reduced at small distances from the barriers. (E) Sieving effects occur when the mesh size of immobile barriers is smaller than the size of the probe, leading to a size-dependent alteration of diffusion. (F) Weak intermolecular forces and steric repulsion between the different biopolymers induce spatial heterogeneity, leading to location-dependent diffusion coefficients of the probe. Reproduced from ref (68). Copyright 2018 Schavemaker, Boersma and Poolman under Creative Commons Attribution License (CC BY) [CC BY 4.0 Deed | Attribution 4.0 International | Creative Commons].
2.2.2. Dynamics and Translational Diffusion
Single-particle tracking in bacteria (E. coli and C. crescentus) and lower eukaryotes (such as Saccharomyces cerevisiae) indicates that the cytoplasm is an adaptable fluid that can change from a fluid-like to a more solid-like (“colloidal glassy”) state when cells are deprived of metabolic energy. Pioneering studies by the Jacobs-Wagner lab showed that the E. coli cytoplasm acts as a glass-forming fluid in which the diffusion of molecules is disproportionally limited by the size of the tracked component,155 which is an example of sieving effects (Figure 5E). Cellular metabolism fluidizes the cytoplasm, which allows larger components to diffuse over larger regions of the cell. When E. coli cells are exposed to osmotic (upshift) stress, the cytoplasmic volume decreases and consequently the excluded volume of the macromolecules increases beyond 20%.67,156 The decrease in the translational diffusion coefficient of green fluorescent protein (GFP) is proportional to the magnitude of the osmotic up-regulation, and under extreme conditions (≥250 mM NaCl or >500 mM sorbitol in the case of E. coli), the excluded volume taken by the macromolecules is so high that diffusion barriers (Figure 5D, mobility barriers) are formed and part of the GFP becomes trapped in discrete pools.157
Analogous diffusion studies have been performed in the cytosol of the budding yeast S. cerevisiae. Macromolecules are less able to move around in the yeast cytosol when cells are starved of sugar,158 which has been attributed to a decrease in cell volume and the accompanying increase in macromolecular crowding. In addition to steric effects, altered physical interactions between macromolecules (Figure 5C), e.g. due to an increase in ionic strength or lower pH at the smaller cytosolic volumes, can also play a role in the translational diffusion of proteins.159 In another study,160 the more solid-like state of the cytosol of energy-starved cells is attributed to acidification of the cytoplasm, which leads to widespread assembly of macromolecules and thereby a reduced diffusion of large particles. Munder and colleagues conclude that acidification and osmotic stress result in different states of the cytoplasm, and thus, the underlying mechanism of reduced diffusion may differ.160 Altogether, these and other studies161−164 in prokaryotes and eukaryotes show that metabolic activity directly or indirectly affects the apparent viscosity and structural organization of the cytoplasm. Indirect metabolic effects may include stress conditions that affect the stability of the proteome.165 If a fraction of proteins or protein domains unfold as a result of, e.g., heat stress, these denatured polypeptides may exhibit properties akin to those of intrinsically disordered proteins and increase the (local) viscosity. In a recent study,166 Di Bari et al. show that the unfolding of just a small fraction of proteins can cause a slowdown of protein diffusivity by forming an entangling interprotein network across the cytoplasm, which is dominated by hydrophobic interactions.
The recently developed technique of single-molecule displacement mapping has been used to resolve the dynamics of a wide range of selected target proteins differing in mass, oligomeric state, abundance, and number of interaction partners (expressed as loneliness factor) with nanoscale resolution,141,142 which has provided new insight into the dynamic structure of the bacterial cytoplasm. It was shown that the translational diffusion coefficient (D) of proteins in E. coli scales with the complex molecular mass, that is, the mass of the tagged polypeptide chain multiplied by the oligomeric state, and not with their abundance in the cell or their loneliness factor.141 Furthermore, the diffusion in the E. coli cytoplasm does not follow the Einstein–Stokes equation:167
| 5 |
The dependence of the diffusion coefficient on the complex mass of proteins follows a power law relationship D = αMβ, where M is the complex mass and α and β are fitting parameters. The exponent β would be −0.33 in the Einstein–Stokes equation, assuming the proteins are globular and not interacting with each other. A value of β = −0.6 has been found for the diffusion of proteins in the cytoplasm of E. coli.141,143 The stronger than predicted dependence on molecular mass reflects the high macromolecular crowding of the cytoplasm and the collisions with other macromolecules, for which the term “macromolecular viscosity” has been introduced. The deviation of the diffusion coefficients from the Einstein–Stokes equation is explained by the proposal that the cytoplasm is a dilatant, non-Newtonian fluid. A characteristic of dilatant fluids is that viscosity increases with stress applied to the fluid. Larger components inside the cell impose a higher pressure on the environment, which in response becomes more viscous. In this view, the viscosity of the cytoplasm is considered as a function of the analyzed macromolecule, which will be subjected to a perceived viscosity depending on its size (Figure 6). This has led to a modified version of the Einstein–Stokes equation (eq 6):
| 6 |
where ηMW represents the perceived viscosity as a function of the molecular weight. The perceived macromolecular viscosity varies from 9.9 cP to 18.1 cP for protein ranging in mass from 26 kDa to 318.9 kDa.
Figure 6.
Structure ofEscherichia colicytoplasm and impact of confinement, protein aggregation, and perceived viscosity on the translational diffusion of proteins (red particles). The image in the middle shows a diffusion map obtained by single-molecule displacement mapping (right), a method to determine the mobility of (macro)molecules,141,142 which is overlaid with a schematic of the cytoplasm. The figure emphasizes three factors that affect the translational diffusion of molecules: (i) confinement; (ii) aggregation of macromolecules at the cell poles; and (iii) perceived viscosity. Since diffusion of proteins scales with their complex mass, bigger particles will be affected more by the crowding of the cytoplasm than smaller molecules (hence they perceive a different viscosity) and move relatively more slowly, leading to the deviation from the Einstein–Stokes equation. Dapp = apparent diffusion coefficient of molecules; the pixel size indicates the spatial resolution at which the diffusion of molecules in the cell can be determined. Reproduced from ref (143). Copyright 2023 Mantovanelli et al. under the terms of the Creative Commons Attribution License [https://creativecommons.org/licenses/by/4.0/].
Similar observations of size-dependence of diffusion were made in a recent study, employing fluorescence correlation spectroscopy and computer simulations. Here, it was concluded that the size-dependence of diffusion is consistent with eq 5 when the specific dumbbell shape of the protein fusions is taken into account.168 Furthermore, pioneering studies on protein diffusion in E. coli have been made by ensemble measurements, using fluorescence recovery after photobleaching (FRAP),67,156,157,169−171 reviewed by Mika and Poolman.172 Although the ensemble measurements provide less detail and spatial resolution than single-molecule analyses such as single-molecule displacement mapping, the data are in agreement with the notion that the bacterial cytoplasm behaves as a non-Newtonian dilatant fluid and has macromolecular viscosity that is a function of the probe size. Finally, the diffusion of proteins in the mass range of 26–319 kDa is in agreement with the apparent average mesh size of ∼50 nm of the E. coli chromosome.173 Thus, the tested proteins with a Stokes radius up to 5 nm may not be affected by the meshwork of the chromosomal DNA.
Importantly, the translational diffusion of the selected proteins is location-dependent in E. coli, with the cell poles displaying slower diffusion throughout the whole set of investigated proteins and one pole showing faster diffusion than the other.141,143 The extent of the slowdown in the pole regions exceeds the confining effects of the cell membrane boundary, as inferred from computer simulations, and instead is most likely a consequence of hindrance by large macromolecular complexes due to accumulation of damaged proteins primarily at the old cell pole (Figure 6).143 Preliminary experiments on protein diffusion in the Gram-positive pathogen L. monocytogenes point toward a similar location-dependent mobility.105,174 It still is an outstanding question whether symmetrically dividing unicellular microorganisms age.175 The differences in diffusion coefficients and protein probe concentrations between old and new cell poles suggest that exclusion of aggregates and other supramolecular complexes from the nucleoid leads to bacterial aging.139 The selective segregation of aggregates to the old cell pole may maintain the viability of the whole population.
In general the diffusion coefficients for proteins like GFP are similar across bacterial species,68 which points toward similar levels of macromolecular crowding. Furthermore, both the Gram-negative bacterium E. coli and the Gram-positive bacterium L. lactis respond to osmotic stress by a drop in protein diffusion, which is mitigated when the medium contains osmoprotectants (Terminology). For both organisms a drop in cell size and diffusion coefficient happens even after a small osmotic upshift (0.1–0.2 Osm).176 This suggests that the cell wall, which is initially stretched, causes the cytoplasm to shrink when the turgor pressure is decreased (see also ref (109)). There are also important differences between the two organisms. L. lactis is less susceptible to osmotic challenge than E. coli, as it requires higher medium osmolalities to decrease the diffusion, which most likely relates to the order of magnitude higher turgor pressure of L. lactis relative to that of E. coli.176 An even more striking difference is that in L. lactis the GFP diffusion coefficient drops much more rapidly with volume than in E. coli. This suggests a different adaptability of the cytoplasmic fluid, but the underlying cause is unknown.
2.2.3. Diffusion-Limited Reactions and Surface Properties of (Macro)molecules
How common are diffusion-limited reactions in the cytoplasm of prokaryotic cells? Schavemaker et al.68 reviewed cases where protein diffusion plays a determining role in the physiology and biochemical organization of the cell. Reactions are diffusion limited when the association rate constant (kon) depends only on the translational diffusion coefficient. The kon,diffusion of a protein diffusing in the cytoplasm with D = 10 μm2/s and needing to interact with another molecule is ∼108 M–1 s–1. As most proteins are not reactive over their entire surface, a more realistic diffusion-limited kon is in the range of 105–106 M–1 s–1.68 Here the assumption is that only a fraction of the surface (the interaction interface) of a molecule is reactive and the interaction between two molecules is not steered through specific (oppositely charged) surfaces. Protein pairs such as Barnase–Barstar from Bacillus amyloliquefaciens manage to have a kon of 108–1010 M–1 s–1 and apparently behave beyond the diffusion limit. The interaction of Barnase (cationic, pI ∼9.2) with Barstar (anionic, pI ∼4.9) is driven by electrostatic attraction,177−179 which allows the kon for the binding of the ribonuclease to the inhibitor protein to be orders of magnitude higher than the nonelectrostatic diffusion limit. For such interactions, the magnitude of the diffusion coefficient is crucial, with the initial interaction of the proteins likely to be the slowest step. Other diffusion-limited reactions in prokaryotes can include enzymes with very high kon values,68 the ternary complex of amino acyl-tRNA, EF-TU plus GTP finding the ribosome,180,181 proteins present in the cell at low copy numbers (longer distances to cover), and proteins transiently binding to membranes or other large structures (e.g., the Min oscillation system182).
Most of the processes in the cell are most likely reaction rather than diffusion limited, despite the high crowding in the bacterial cytoplasm. This changes when cells are exposed to osmotic upshift and the crowding increases further. Consequently, the diffusion coefficient of macromolecules decreases by orders of magnitude (Figure 7) and many reactions will become diffusion limited. In extreme cases, diffusion barriers (Figure 5D) are formed and molecules are trapped in supramolecular aggregates.157 Remarkably, under conditions where proteins are trapped, small molecules like fluorescent sugars (NBD-glucose in Figure 7) are little affected by osmotic upshifts and can readily diffuse throughout the entire cytoplasmic volume even at 1 M or higher concentrations of NaCl stress (Figure 7). These data indicate that the cytoplasm acts as a molecular sieve (Figure 5E) during both high and low osmotic stress but with a different mesh size. The remarkable diffusion of NBD-glucose in plasmolyzed cells is also consistent with the notion of electrolyte pathways wiring the cytoplasm.101 The rapid diffusion of small molecules (ions, metabolites, signaling molecules) may keep the cell biochemically active, even when the majority of enzymes are trapped. This may allow the cell to recover from extreme osmotic stress, provided it can take up or synthesize compatible solutes.
Figure 7.

Effect of osmotic upshift (NaCl stress) on the diffusion coefficient of the red fluorescent protein mPlum and NBD-glucose (FSugar). The D values are normalized relative to the diffusion coefficients in the absence of NaCl (D0); data taken from ref (67). Copyright 2010 Blackwell Publishing Ltd. The images on the right show a photobleaching experiment of E. coli cells untreated (left) or upshifted with 500 mM NaCl (right).
The cytoplasm consists of various types of nucleic acids and >1000 types of protein, but only 50 protein types make up 85% of the cytoplasmic proteome of E. coli.183 These abundant proteins have a large impact on the structure of the cytoplasm through, e.g., weak and nonspecific interactions with other molecules (https://www.ebi.ac.uk/intact/home). However, analysis of protein diffusion as a function of loneliness factor in the E. coli cell does not reveal a correlation between a protein’s diffusion coefficient and the number of interaction partners.141 The boundary conditions for the importance of generic nonspecific interactions (Figure 5C) between macromolecules have been probed in a study of diffusion of surface-modified fluorescent proteins. The diffusivity of a set of GFP variants with a net charge ranging from −30 to +25 has been analyzed in E. coli (Gram-negative bacterium), L. lactis (Gram-positive bacterium), and H. volcanii (archaeon).88 These three organisms differ in their cytoplasmic ionic strength, as shown by measurements on the K+ ion concentrations, which, as mentioned above, are ∼0.2, 0.8, and 2.1 M, respectively. In E. coli the diffusion coefficient of GFP variants depends on the net charge and its distribution over the surface of the protein, with cationic proteins diffusing up to 100-fold slower than anionic ones. The decrease in GFP mobility is due to the binding of cationic GFP to ribosomes. This effect is weaker in L. lactis and H. volcanii due to electrostatic screening. Interestingly, the number of cationic proteins in E. coli with a net charge >+10 (surface charge comparable to that of the slowed cationic GFPs) is only 35, of which 18 are ribosomal proteins, 14 are DNA/RNA associated, and 3 have unknown functions. The same holds true for the vast majority of (micro)organisms, with endosymbionts of plants and insects being notable exceptions.88 Protein–protein interaction pairs such as cationic Barnase and anionic Barstar are rare in bacteria. Thus, the proteome of bacteria is generally anionic and appears to have evolved by avoiding highly cationic surfaces; the cationic proteins would lower the overall diffusivity and might affect the functioning of the ribosomes. The highly cationic proteomes of some endosymbionts indicate that these organisms have special mechanisms to avoid slow diffusion and perturbation of ribosomal function.
2.2.4. Nucleoid Structure
The bacterial nucleoid excludes ribosomes and some other proteins (see section 2.2.1), suggesting that it acts like a molecular sieve. As mentioned above, translating ribosomes (polysomes) are excluded from the nucleoid and localize mostly at the cell poles and cytoplasmic periphery.173 Nevertheless, ribosomal subunits with Stokes radii in the range of 15–20 nm can penetrate the DNA meshwork of the nucleoid as shown in E. coli and other bacteria.137,184,185 This also holds for metabolic enzymes with Stokes radii in the range of 5 nm.141 When the molecule size is close to the average mesh size of the nucleoid, the diffusivity of the particle becomes limited but smaller molecules diffuse freely through the meshwork (Figure 5E).
What are the molecular sieving properties of the nucleoid and what biophysical properties of the cytoplasm are important for its structure? The apparent mesh size of the E. coli chromosome is around 50 nm.173 Obviously, the volume of the cell, confinement by the cell membrane, ionic strength (polyvalent cations in particular), and macromolecular crowding play key roles in the structure and phase properties of the nucleoid, in addition to specific proteins associating with the DNA. The high excluded volume of the cytoplasm causes repulsion between macromolecules, which results in a compacting force through steric effects.33 This can lead to condensation of DNA, nucleoid size reduction, and DNA segregation,186 which can be antagonized by DNA-associated proteins.187 Furthermore, the overall quality of the cytoplasm as solvent will play a role. In polymer chemistry the quality of a solvent is classified as good when it exhibits a high degree of solubility and compatibility with a polymer, that is, if it allows the polymer to be well dissolved and dispersed. A poor solvent has limited solubility or affinity for a polymer and can induce phase separation in polymer solutions. Using the mesh size of the nucleoid and DNA concentration in the cell, Xiang and colleagues173 concluded that the cytoplasm behaves as a poor solvent for the chromosome. Computer simulations show that the poor solvent leads to chromosome compaction and domain formation. RNAs may contribute to the poor solvent effects, which would connect chromosome compaction and domain formation to transcription.
The volume of growing E. coli cells is ∼1 μm3, and the average volume of the nucleoid with one chromosome (∼4.6 × 106 base pairs) is estimated to be ∼0.7 μm3.173 From these numbers one can calculate the average DNA concentration in the nucleoid region of around 7 mg/mL,173 but 10-fold higher concentrations have also been reported (see footnote 6 in Murphy and Zimmerman188). In the older studies, cells appear larger and the nucleoid occupies a smaller fraction of the cytoplasmic volume. Interestingly, when the genome size of bacteria is plotted against cell volume189 there is an enormous variation in the amount of DNA per unit of cell volume. For instance, the tiny Bdellovibrio bacteriovorus(190) accommodates a chromosome of ∼3.8 × 106 base pairs in a volume that is more than 10 times smaller than the E. coli cytoplasm. Thus, irrespective of the volume of the nucleoid region, the DNA must be compacted even more than in E. coli. Similarly, other small bacteria such as Haemophilus influenzae, Mycoplasma sp., and Pelagibacter sp. have much more DNA per unit of volume than E. coli (see supplement of Bailoni and colleagues189). The more compacted DNA will result in a smaller mesh size of the corresponding chromosome, which may affect the exclusion of proteins from the nucleoid and the distribution of macromolecules inside these cells, and possibly their aging. Indeed, the chromosome of B. bacteriovorus is highly compacted in a polarized nucleoid that excludes freely diffusing proteins during the nonproliferative stage of the cell cycle.191
2.2.5. Fluidization of the Cytoplasm
What causes the fluidization of the cytoplasm by metabolism? Both in E. coli and the lower eukaryote S. cerevisae, depletion of metabolic energy reduces mobility of proteins, which has been attributed to a lowering of the ATP pool,155 a lowering of the internal pH,192 and an increase in macromolecular crowding.158 The mechanistic basis for the fluidization of the cytoplasm is complex, as ATP levels, internal pH, and crowding are connected and each of these physicochemical parameters can affect molecular interactions (e.g., protein aggregation) but also chromosome compaction. Multiple antibiotics studies have shown that changes in nucleoid compactness influence the diffusivity of molecules.193−196 Furthermore, when an enzyme undergoes large conformational changes in its catalytic cycle, it induces hydrodynamic flows in the surrounding fluid or membrane.197 Such pulsating flows can act on any passive particles in the solution or lipid bilayer. The collective hydrodynamic effects of active macromolecules can increase diffusion of all particles in the medium and in special cases result in directed flows. The collective conformational changes of enzymes and other macromolecules will be higher when cells are in a metabolically active state than when metabolic activity is low.
How could a change in ATP concentration by itself affect protein mobility? ATP has been postulated to act as a biological hydrotrope198 (Terminology). A hydrotrope is capable of solubilizing (hydrophobic) substances in an aqueous solution without the need for micelle formation. ATP and GTP at physiological millimolar concentrations have been shown, at least in vitro, to have hydrotropic properties and keep proteins soluble and minimize their aggregation,199,200 which may keep the cytoplasm more fluid. NMR spectroscopy has shown that ATP interacts weakly with various proteins, which may provide protection to protein surfaces.201 It has also been postulated that the dynamics of enzymes catalyzing metabolic reactions can have a “stirring” role in the cytoplasm.163 Many enzymes are ATP or GTP dependent, and depletion of these nucleotides will reduce the conformational dynamics of these proteins, which indirectly may affect other enzymes. There is debate whether or not enzymes at work (irrespective of ATP) are able to self-propel or to break free from supramolecular structures,202−204 which would also have a fluidizing effect. Recent studies on the diffusion of single molecules do not show catalysis-induced diffusion of alkaline phosphatase and urge a revisit of previous findings and models.205 However, there is increasing evidence that enzymatic activity generates a microflow in the surrounding medium,206 which will impact the diffusivity and dynamic structure of the cytoplasm.
3. The Bacterial Nucleoid
The bacterial nucleoid, with its several megabases of chromosomal DNA, is remarkably confined and compact despite the lack of a dedicated membrane to enclose it.207,208 Initially described as a collection of loops emanating from a dense core organized by proteins and RNA,209,210 the nucleoid has since been revealed to be a condensed phase (Figure 8) formed by LLPS through the interaction of multivalent cations and proteins in the presence of crowding agents.211−213 Mobility within this dynamic structure allows organization of the chromosomal loci as required during the cell cycle.214 Interestingly, its size and positioning within the cell are regulated by crowding and cell geometry.134,215 Atomic force microscopy and simulations with varying DNA concentrations show that self-crowding modifies nucleoid shape and properties depending on supercoiling density, which is essential for DNA replication.216 Nucleoid size also changes in response to antibiotics.217,218 For example, inhibition of translation with chloramphenicol results in ultracompaction of the nucleoid, presumably because of the loss of coupled translation with membrane insertion of proteins (transertion).193,219 Although most bacteria have a single, circular chromosome, in a few cases the genetic material distributes in two or more chromosomes.220
Figure 8.

The bacterial genome is organized as a phase-separated nucleoid. HU is a histone-like protein that packages DNA into a dense core surrounded by a less dense phase of DNA and associated proteins. Transcriptional foci are dynamic condensates comprised of RNA polymerase and other transcription factors. The single-stranded DNA binding protein (SSB) also forms compartments. Abbreviations: dsDNA, double-stranded DNA; ssDNA, single-stranded DNA. Figure adapted and modified with permission from ref (221). Copyright 2021 Elsevier Ltd.
The role of phase separation in the organization of DNA-based structures and regulation of protein-nucleic acid complexes in different organisms, including bacteria, has been comprehensively reviewed recently.221 Quantitative simulations propose that nucleoids are assembled and organized by segregative phase separation, probably as a first level of compaction, as a result of demixing of the chromosome and the macromolecules within the cytoplasm. These simulations show that different geometries of molecular crowders result in different repulsive interactions important for nucleoid organization.222 By analogy to the mitochondrial genome in eukaryotic cells, the bacterial chromosome is further organized by nucleoid associated proteins (NAPs), which bind to DNA with little sequence specificity,207 in contrast to mammalian nuclear genomes that assemble into orderly spaced nucleosomes. It is worth noting that the highly crowded conditions within the nucleoid result from the high density of NAPs that coat the chromosome (ca. 30% of the chromosome in E. coli), limiting its available protein-free regions.223 In fact, a phenomenological model of cytoplasm length-scale-dependent viscosity that considers crowding, including NAPs on DNA, shows that it alters the nonspecific binding of transcription factors and their 1D diffusion along DNA in E. coli.224 Some of the NAPs exhibit phase separation behavior, including the histone-like heat-unstable nucleoid protein (HU, see also section 7), a DNA-binding protein from starved cells (Dps, see also section 7), single-stranded DNA binding protein (SSB, see sections 6.1 and 7), and RNA polymerase (RNAP).
HU is one of the most abundant NAPs, and this protein is conserved across all bacteria.225 Upon interaction with MukB, HU ensures proper positioning of the chromosomal replication origin oriC in E. coli.(220) Two isoforms, HU-A and HU-B, contain intrinsically disordered regions and domains for homo- and heterodimerization. In vitro, these proteins form coacervates with DNA, causing phase separation, favored by PEG as crowding agent.226 Using fluorescently labeled HU, multiple dynamic submicron-sized condensates have been observed in E. coli cells that rearrange, probably through separation and fusion, over a time scale of a few tens of seconds. DNA and protein concentration, increasing temperature, and lower pH and salt concentrations are among the factors that enhance the condensation of HU-B. HU-A also assembles into homotypic and heterotypic condensates with HU-B, although it is less prone to coacervation with DNA than HU-B. This is consistent with the prevalence of HU-A mainly as dimers and discrete complexes with DNA, whereas HU-B self-associates into dimers, tetramers, and octamers and forms multiple higher order complexes with DNA, emphasizing the importance of weak multivalent interactions for condensation. HU condensates recruit a variety of nucleic acids, and phase separated HU-DNA droplets colocalize with DNA polymerase in vitro.
HU proteins also form heterotypic condensates with Dps,226 a NAP that contains disordered regions and assembles into dodecamers in vitro. In the presence of DNA, Dps demixes into condensates of smaller size compared to those of HU. Despite being dynamic and hence liquid-like, Dps condensates display a mixture of round and irregular shapes, compatible with a lower tendency to fuse. The distinct properties of HU and Dps condensates may be due to differences in interfacial surface tensions or shear relaxation characteristics. When assembled in the presence of DNA, condensates involving the two proteins consist of multiple droplets of Dps encircled by a larger droplet of HU-A or HU-B, a remarkable behavior probably arising from the different properties of HU and Dps condensates. Moreover, this arrangement seems to be dependent on DNA binding by Dps, as crowding-driven homogeneous condensates, in which both proteins fully colocalize, are obtained in the absence of nucleic acids.
Dissimilarities in the condensation features of the two HU isoforms and Dps suggest an interesting mechanism to spatiotemporally tune the level of phase separation through the HU-A:HU-B:Dps ratio. For example, during the early logarithmic growth phase, accumulation of HU-A would decrease phase separation by DNA in nucleoids to allow constant replication and gene expression. In contrast, higher levels of HU-B in the late logarithmic phase and of Dps in the stationary phase or during starvation227 would favor phase separation, promoting DNA compaction and providing resistance to stress (see section 7).
RNAP forms clusters in E. coli that behave as biomolecular condensates arising from LLPS.145 This ability to form condensates is notable, as bacterial RNAP lacks the disordered C-terminal domain present in eukaryotic Pol II.228 RNAP condensates are prevalent in cells during the logarithmic phase and gradually disband once cells reach the stationary phase. The RNAP clusters seem to be independent of the folding of the chromosome into a compact structure, emerging instead from weak protein–protein interactions that involve the transcriptional antiterminator protein NusA. NusA exhibits phase separation in vitro and in vivo (Figure 9), enhanced by its modular architecture with multiple folded domains connected by flexible linkers and the presence of protein and RNA binding domains.145 Biomolecular condensation of NusA is driven by crowding, and phase diagrams in solutions containing 100 g/L dextran show that it is regulated by protein concentration and salt (ionic strength). LLPS of NusA in vivo is observed through cellular foci nucleated by protein–protein or protein–RNA interactions, whose size depends on protein concentration. Experiments in vivo suggest that another protein in the antitermination complex, NusB, may also be involved in the phase separation of RNAP.
Figure 9.

Formation of biomolecular condensates by NusA and dynamics of components of RNAP clusters. (A) Phase diagram for purified NusA in the presence of dextran. Open circles correspond to conditions in which the protein is dissolved, as in the image on the right (top), while closed circles indicate conditions in which the protein is condensed, as in the image on the right (bottom). (B, top) A cartoon depicting how single molecules of NusA are tracked over time in living E. coli cells. Cells expressing NusA fused to the photoconvertible fluorescent protein mMaple are continuously activated with 405 nm light, which photoconverts mMaple from a green-emitting form to a red-emitting form, allowing single NusA-mMaple molecules to be tracked over time. (B, bottom) Distribution of Dapp (apparent diffusion coefficients) for fluorescent fusions of RpoC, NusA, or LacI that were tracked over time, showing faster movement of the former two compared with DNA-bound LacI. Figure adapted from ref (145). Copyright 2020 the Authors. Published by PNAS under Creative Commons Attribution-NonCommercial-NoDerivatives License 4.0 (CC BY-NC-ND) [CC BY-NC-ND 4.0 Deed | Attribution-NonCommercial-NoDerivs 4.0 International | Creative Commons].
Single-molecule tracking demonstrated that proteins within the RNAP condensates are highly dynamic,145 indicating the general usefulness of this technique to study biomolecular condensates in living bacterial cells. Broad distributions of diffusion coefficients were observed for the RNAP β′ subunit (RpoC) and NusA. In the case of RpoC, this distribution is likely the result of different activity states, including molecules engaged in transcription or nonspecifically bound to DNA, in agreement with other reports.229,230 These proteins had higher diffusion coefficients with a wider distribution compared with that of a DNA locus, indicative of slower diffusion compared to the proteins (Figure 9). Some overlap between the distributions of the proteins and the DNA was observed, likely corresponding to protein molecules of RpoC engaged in active mRNA transcription and NusA molecules engaged in transcriptional antitermination. The ability of RNAP to undergo LLPS in bacterial cells has important implications for transcriptional regulation and subsequent rRNA processing in bacteria, in response to internal and external cues.
4. Crowding and Phase Separation in Membrane Environments
Numerous cellular processes involve biological membranes, which facilitate the local concentration of highly ordered functional complexes at defined positions. In bacteria, the cytoplasmic membrane is an asymmetric bilayer of variable phospholipid and glycolipid compositions, depending on the species, and occupied by a high amount of integral and membrane-associated proteins231 (Figure 10). Gram-negative bacteria such as E. coli have an additional outer membrane that protects cells against harsh environments, contributes to their mechanical stability, and excludes many types of antibiotics, enhancing antibacterial resistance.232 The outer membrane consists of an asymmetric bilayer, with an inner leaflet of phospholipids and an outer one of lipopolysaccharides (LPSs) and membrane proteins.233 Bacterial membranes, whether from Gram-positive or Gram-negative bacteria, can contain hopanoids (sterols, equivalent of cholesterol in mammalian cells), pentacyclic triterpenoid lipids that are thought to enhance membrane integrity and impermeability by condensing the membrane.231
Figure 10.

Structure of the bacterial cell envelope and fluid state of the membrane. (A and B) Models of Gram-positive (A) and Gram-negative (B) cell envelopes. Adapted in part from ref (237). Copyright 2019 the Authors. Published by Springer under the terms of the Creative Commons Attribution 4.0 International License [http://creativecommons.org/licenses/by/4.0/]. (C) Reversible phase separation induced by reduction of membrane fluidity. Bilayers are typically in the liquid-disordered phase (Ld, blue), but they can phase separate into liquid-disordered and liquid-ordered phases (Lo, green) when, e.g., hopanoids are present. Both are fluid phases. Extreme fluidity reduction triggers massive phase separation into highly ordered Lo phases within large parts of the membrane, forcing membrane proteins into the fluid phases. Under these conditions the membrane maintains its integrity and semipermeability. Adapted and modified with permission from ref (234). Copyright 2022 the Author.
Membranes are characterized by the dynamic localization of lipids and proteins. The diversity of lipid acyl chains [saturated, (poly)unsaturated, branched, and cyclopropane rings] results in different membrane packing densities and fluidities.234 Membranes are normally in the form of liquid phases with highly dynamic lipids, but liquid–liquid demixing is observed in membranes of living cells composed of saturated and unsaturated acyl chains, as well as with certain other types of lipids, such as the aforementioned hopanoids, organized in liquid-ordered and liquid-disordered domains234 (Figure 10). Lipid rafts, nanoscale domains associated with the formation of liquid-ordered regions, are well characterized in eukaryotic cells235 but have also been observed in B. subtilis cells.236
4.1. Remodeling of the Membrane by Crowding and Phase Separation
The effects of macromolecular crowding on the structure and dynamics of biological membranes, including those of bacteria, have been comprehensively reviewed recently.49 It is clear that the multifaceted effects of crowding pervade over multiple length scales. Crowding in the membrane reduces the diffusion of membrane proteins and increases their clustering, which can alter their function. Crowding in solution increases membrane adsorption of proteins and modulates the protein:lipid affinity accordingly. The asymmetric crowding of one side at a membrane, for example by binding a protein at the periphery of a membrane, can cause membrane remodeling by inducing curvature leading to vesicle formation, as well as induce lipid-phase separation. The high protein density in and at the membrane thus has the potential to affect a plethora of protein-associated processes and may play a role in tuning higher levels of membrane organization.
Physicochemical properties of biological membranes largely depend on changes in the environment such as temperature, osmolarity, etc. Consequently, proper cell function relies on homeostatic regulation to preserve vital membrane features such as fluidity. Living organisms regulate their lipid composition in response to changes in temperature through reversible phase separation, which can result in formation of specific membrane domains into which some proteins partition and others are excluded. In bacteria such as E. coli or B. subtilis, an overall low membrane fluidity induced by alteration of fatty acid composition triggers large-scale lipid phase separation and promotes segregation of normally dispersed integral membrane proteins into the fluid areas.238 Extreme changes in lipid fatty acid composition, more drastic than those in the normal adaptation mechanism to temperature shifts, can lead to very low membrane fluidity that reaches the limit for cell viability.238 This lipid phase separation results in partitioning of membrane-associated proteins into the liquid membrane regions, affecting protein function (Figure 10C). For example, membrane fluidity affects the localization of MreB and FtsZ, key proteins of the membrane-associated elongasome and divisome complexes, respectively, perturbing cell morphology. Whereas membrane fluidity changes do not affect cell division in B. subtilis, similar changes in E. coli cells result in a defect in divisome assembly. Conversely, membrane fluidity changes perturb the cell wall synthesis machinery in B. subtilis but not in E. coli. Low membrane fluidity has no detectable effects on chromosome replication and segregation in B. subtilis, although some effects on nucleoid compaction have been observed in E. coli, possibly related to perturbation of RNase E (see section 7).
Localization of the phospholipid cardiolipin at the cell poles of rod-shaped bacteria has been proposed to occur by microphase separation produced by osmotic pinning of the membrane to the cell wall.239 Unlike individual lipids, large lipid domains of finite size generated by such phase separation gain the ability to sense cell curvature, favoring their spontaneous localization to the most curved areas of the cell (the poles). The biophysical model of Mukhopadhyay et al. shows the dependence of lipid domain localization on size distribution, which with increasing lipid–lipid short-range interactions becomes larger and narrower.239 The relationship between localization and strength of the pinning is determined by the balance between the osmotic pressure difference along the membrane (resulting from gradients of osmolyte concentrations, environmental variables, and some growth processes) and the inward force exerted by the cell wall. Heterogeneity in membrane pinning facilitates localization of lipid domains in cellular regions with reduced osmotic pressure differences. In support of this idea, cardiolipin relocalizes from the cell poles, where osmotic pressure differential is high, to the midcell division septum, where osmotic pressure is predicted to be lower, during B. subtilis sporulation.240 This model also predicts a critical concentration for formation of cardiolipin domains. For example, E. coli with reduced cardiolipin content loses polar localization of both cardiolipin itself and the osmoregulatory integral membrane protein ProP.241
Imaging by atomic force microscopy (AFM) of the entire external membrane surface of live and metabolically active E. coli has identified large-scale networks of proteins. Key components of the outer membrane such as the porin OmpF are distributed throughout, interrupted by small gaps of phase-separated LPS that merge, grow, and split with time (according to a liquid phase behavior) while maintaining their location242 (Figure 11). The surface fraction occupied by the lipopolysaccharide phase is dependent on concentration and LPS-LPS interaction strength. Modulation of the levels of the most abundant proteins has a clear impact on the amounts of pores formed by the porins. Disruption of lipid asymmetry by mislocalized phospholipids at the surface induces formation of new phases that deform the membrane,242 likely altering its barrier function and rendering cells more susceptible to some antibiotics.243 Along the same lines, molecular dynamics simulations propose that polymyxin B, a lipopeptide with antimicrobial activity, loosens the packing of the LPS external membrane upon binding, which triggers the flipping of phospholipids from the inner to the outer leaflet.244 This results in phase separation of the outer leaflet, with defects at the boundaries between LPS and phospholipid domains because of the hydrophobic mismatch that facilitates internalization of polymyxin B toward the inner membrane.
Figure 11.

Outer membrane ofE. colicontains protein-free LPS patches. (A) AFM phase image with phase-separated LPS patches highlighted by dashed lines. The pores identify the protein network surrounding the patches, formed by porins as OmpF. (B) At time scales consistent with cell division, under these experimental conditions, patches merge, grow, and split apart. (C) Illustration of OmpF labeling by colicin N1–185mCherry, used to localize the porin within the membrane surface in the height image. The phase image of the same area is used to localize the patches. Quantification of the labels per area shows that OmpF colocalizes with the pore network. Reprinted in part with permission from ref (242). Copyright 2021 PNAS.
4.2. The Membrane as a Facilitator of Biomolecular Condensation
There is increasing evidence that many protein and protein–nucleic acid clusters assembled at the membrane display the hallmarks of biomolecular condensates. The role of membrane surfaces as key factors acting in the regulation of phase separation, along with post-translational modifications, has been analyzed in studies focused principally on eukaryotes (see Snead and Gladfelter51 and references therein). According to these studies, membranes generally lower the concentration threshold for biomolecular condensation,245 likely because they restrict diffusion to two-dimensions, although it is also possible that specific factors present in membrane boundaries may nucleate condensation and spatiotemporally regulate phase separation through changes in their distribution.51 In addition, it has been proposed that membranes can locally control the stoichiometry of elements within condensates and alter their dynamic properties and functions.51 Condensates in turn can drive membrane remodeling, suggesting that there may be an interdependence between lipid organization and the condensation of proteins and nucleic acids.51
Examples of biomolecular condensates at the bacterial membrane can also be found in vivo and in cytomimetic systems. One such example is the integral membrane ATP-binding cassette (ABC) transporter Rv1747 protein from Mycobacteria, a virulence factor whose cytoplasmic regulatory module forms biomolecular condensates.246 This module can assemble into higher-order oligomers, depending on the phosphorylation state of its intrinsically disordered domain that bridges two 2 phosphothreonine-binding Forkhead-associated domains. Interestingly, phosphorylation enhances the reversible phase separation of this protein and modifies the dynamic properties of the resulting condensates, probably because of its impact on the self-association of the transporter. This is in line with the idea that post-translational modifications are a key cellular mechanism enabling the control of biomolecular condensation,51 suggesting that this principle may be extended to the kingdom of bacteria. The cytosolic domain of the Rv1747 transporter also forms biomolecular condensates when attached, through a histidine tag, to supported lipid bilayers containing the lipid 1,2-dioleoyl-sn-glycero-3-[(N-(5-amino-1-carboxypentyl)iminodiacetic acid)succinyl] (DGS-NTA) (Figure 12). Foci of this transporter are also observed in the cellular membrane upon heterologous expression in bacteria and yeast. Notably, the full-length protein assembles into clusters in Mycobacterial membranes that are more dynamic than those of the cytoplasmic regulatory module, suggesting that the transmembrane and nucleotide-binding domains may regulate the material properties of the condensates. Condensation in this system appears to have a functional role, as serine/threonine protein kinases and phosphatases colocalize differently with biomolecular condensates of the transporter: the kinases are homogeneously distributed within the condensates, while the phosphatases form foci at condensate interfaces.
Figure 12.

Biomolecular condensates formed by integral or amphitropic proteins at the lipid membrane. (A) (top, left) Fluorescence images showing spontaneous clustering of Rv17471–310 on supported lipid bilayers. Nonphosphorylated His6-tagged OG-Rv17471–310 is anchored to the DGS-NTA(Ni2+) within the lipid bilayers. (bottom, left) Quantification of the phase separation by the fractional fluorescence intensity vs weight percentage of the NTA(Ni2+) lipid. (right) Clustering also occurs in yeast, as shown by the arrowheads in the fluorescence images of cells expressing msfGFP-Rv17471–310, in contrast to cells expressing msfGFP. Reprinted in part with permission from ref (246). Copyright 2019 PNAS. (B) Representative merged confocal images of the encapsulated FtsZ-SlmA-SBS nucleoprotein condensates into microfluidics-based microdroplets stabilized by the E. coli lipid mixture, showing preferential membrane location in a homogeneous crowding model generated with dextran (top) and in a compartmentalized cytoplasm model generated by a binary PEG/dextran LLPS system (bottom). The distribution of the condensates within the encapsulated systems is depicted on the right. Top, partly reproduced from ref (247). Copyright 2023 the Authors. Published by the Royal Society under the terms of the Creative Commons Attribution License [http://creativecommons.org/licenses/by/4.0/]. Bottom, partly reproduced with permission from ref (248). Copyright 2018 the Authors.
Other integral membrane proteins that assemble into condensates are SpmX and PodJ, both involved in the regulation of asymmetric division in C. crescentus (see section 6.3). Mediated by their respective intrinsically disordered regions, SpmX forms biomolecular condensates on its own and with the pole-organizing protein PopZ, resulting in the regulation of DivJ kinase activity in response to nutrient availability.150 Furthermore, SpmX antagonizes phase separation of the polar organelle development protein PodJ, which forms condensates whose fluidity is possibly regulated by the membrane.249
In addition to integral membrane proteins, amphitropic proteins able to interact peripherally with the membrane can also form biomolecular condensates in bacteria. As part of their functional interactions, some of these proteins also bind nucleic acids, concomitantly with the membrane or in a competitive manner, participating in the overall regulation of phase separation. This is the case for the single-stranded DNA-binding protein (SSB)250 involved in DNA replication (see section 6.1) and the nucleoid occlusion factors from B. subtilis and E. coli, Noc147,251 and SlmA,248,252 respectively, important for proper positioning of the cell division ring (see section 6.3). In the case of SSB, reversible foci lacking DNA at the membrane of E. coli cells disband upon DNA damage,250 compatible with biomolecular condensates negatively regulated by DNA binding.253 This suggests a model in which phase-separation at the membrane would serve as a mechanism to store SSB in an inactive state when the levels of its ssDNA substrate are low.253
The role of lipid membranes in the biomolecular condensation of bacterial proteins has been addressed through reconstitution of nucleoid occlusion factors SlmA and Noc in minimal membrane systems. When heterotypic nucleoprotein condensates of SlmA are encapsulated inside cell-like microfluidics microdroplets that display crowding and compartmentalization in the lumen and are stabilized by E. coli lipids, they preferentially localize at the membrane boundary248 (Figure 12). Condensation of these cell division proteins is enhanced by lipid surfaces, as determined by reconstitution in supported lipid bilayers.254 In fact, the enhancing effect of the lipid membrane is also observed with FtsZ alone, as incipient formation of FtsZ condensates in microdroplets occurs in conditions under which no condensates are formed in bulk.255 Similarly, condensates of Noc interact with the outer membrane of giant unilamellar vesicles (GUVs), with the membrane monolayer of water-in-oil droplets in which the protein is encapsulated, and with supported lipid bilayers.147 Membrane binding seems to stabilize Noc condensates that display a notable preference for the more flexible liquid disordered domains, and negatively charged lipids significantly increase phase separation. Noc condensates also change the physical properties of the membranes. Therefore, as in eukaryotic cells, some membrane-associated condensate forming proteins in bacteria have the ability to modulate, and be modulated by, their membrane partners.
5. Comparison of Prokaryotic and Eukaryotic Cytoplasmic Structures
Comparison of prokaryotes with eukaryotes often provides insights into general principles of biochemical organization. Most studies on biomolecular condensates and biochemical organization of the cytoplasm have been conducted in eukaryotes due to their relatively large size, which permits formation of larger condensate structures and facilitates their imaging. The prokaryotic and eukaryotic domains of life share many biochemical similarities despite the hallmark macroscopic structural differences. Notable examples of the similarities are major metabolic pathways; the proteostasis machinery, with major chaperones having homologues in both domains; the machinery and mechanisms of macromolecular synthesis (DNA replication, transcription, and translation); as well as energy transducing machinery and signal transduction, which are highly conserved across different kingdoms of life.
Prokaryotes and eukaryotes are enormously diverse, and comparison based on model systems can become anecdotal. For example, the two most common model systems to represent prokaryotes and eukaryotes, E. coli and HeLa cells, differ significantly in size, but some plant cells have a cytosol that is only 100 nm in diameter, as the vacuole takes up most of the cytoplasm.256 This cytosol is almost 10 times smaller than the diameter of E. coli, and hence more similar to those of Pelagibacter species, which is one of the smallest (and most abundant) bacterial species on Earth. Eukaryotic organelles such as the endoplasmic reticulum (ER) and mitochondria have dimensions similar to bacteria, but there is a tremendous diversity among organelles depending on cell function. We will thus compare eukaryotes and prokaryotes with these limitations in mind.
5.1. Cell Volume
E. coli has a volume of 0.5–2.0 μm3, whereas a HeLa cell reaches 500–4000 μm3.63 This difference in cell size has a number of consequences for biochemical organization, most notably that a smaller volume limits the number of molecules needed to achieve high concentration: a HeLa cell needs ∼2,000 more molecules to reach the same concentration as E. coli. A small cell volume induces more confinement effects, and combined with the lower number of molecules, this reduces the size of biomolecular condensates and aggregates in bacteria. For example, polyQ-containing proteins grow into aggregates with dimensions corresponding to the cell diameter of E. coli (about 1 μm)257 and can be more than 5 μm in diameter in HEK293T cells, depending on the expression level.258 In general, the size of biomolecular condensates can be expected to scale with the cell volume.259 Although small bacterial cells generally have higher surface to volume ratios compared with most eukaryotic cells, the intracellular membrane systems of the latter compensate for this with a high membrane surface area that can promote more condensate adsorption or formation. For example, condensation of an RNA-binding protein is promoted at the ER membranes, and the properties of these condensates are modulated by the presence of RNA.260 Condensate-like clusters also occur at the plasma membrane during the formation of F-actin.261 In these cases, a condensate scaffold component is proposed to be tethered to the membrane. Such membrane tethering is analogous to the bacterial RNA degradosome that forms condensates on the bacterial cytoplasmic membrane,144 suggesting that condensate tethering is a more general strategy in all cells.
5.2. Diffusion and Active Transport
The small size of most bacteria allows them to rely solely on passive diffusion as the main mode of intracellular transport. As mammalian cells are larger, they evolved an additional active transport network where myosins carry cargo along actin filaments, and kinesins and dyneins along microtubules.262 The importance of the cytoskeleton is underscored by the notable abundance of actin and tubulin in such cells. The cytoskeletal network enables long(er) distances to be reached for large cargo (vesicles and organelles) rapidly, which is especially relevant in axons, flagella, and other cell extensions. It has recently been proposed that most vesicles, which are in the 25-nm-size range, similar to that of ribosomes and other supramolecular complexes, rely on passive diffusion in a normal mammalian cell.263 The mammalian cell is less crowded than bacterial cells such as E. coli and can therefore maintain >3 times higher diffusion coefficients (vide infra). The distance a particle travels by Brownian motion is determined by the diffusion coefficient:
| 7 |
where d is the distance traveled, n is the dimensionality of the confinement, and D is the translational diffusion coefficient.68 Although a mammalian cell is much larger than a bacterial cell, a molecule or complex rarely needs to travel from one end of the cell to the other but instead more locally between membranes or molecular complexes. Travel between compartments such as the ER, Golgi, mitochondria, and plasma membrane is shortened by large membrane surface areas, which increases the chance for membrane proximity and membrane contact sites. Hence, both mammalian and bacterial cells rely in large part on Brownian motion of their components.
In addition to being the highways of the cell, microtubules and other filamentous structures are major dynamic organizers of the cytoplasm of mammalian cells, as filaments are in bacteria. In both cell types, protein filaments are crucial in coordinating cell division (see section 6.3). In mammalian cells they provide mechanical strength and shape, which are largely provided by a rigid cell wall in most prokaryotes and eukaryotes such as fungi and plants. Cell walls are stronger and can withstand the higher pressures that these species have to endure. The cytoskeleton also provides additional organizational roles. For example, F-actin serves as a functional adhesion site for biomolecular condensates.264
5.3. Biomolecular Condensates
An emerging mode of dynamic organization is phase separation that results in biomolecular condensates. Here, proteins interact in a multivalent manner, driving phase separation. Proteins that undergo phase separation frequently have intrinsically disordered domains and heterotypic interactions with RNA. Intrinsically disordered regions (IDRs) modulate phase separation based on polymer-physics principles: comparatively unfavorable interaction with the solvent drives self-assembly of the polymers, reducing the energetic cost. Bioinformatics allows estimation of the percentage of proteins with extended disorder and found 28–42% in mammalian cells, 19–44% in yeast, and 4–29% in E. coli. While the numbers among studies vary widely, bacterial proteins have consistently less disorder than eukaryotes.63,265 IDRs in mammalian cells are often used in signaling, where their residues are phosphorylated or decorated with other post-translational modifications such as ubiquitination, glycosylation, lipidation, methylation, etc. Phosphorylation can determine whether a protein partitions into condensates:266 for example, NPM1 (nucleophosmin 1) phosphorylation drastically changes its interaction network, resulting in reduced partitioning in the nucleolus. In other cases, phosphorylation induces phase separation, such as condensation at a phosphorylated disordered domain of EGFR, a receptor tyrosine kinase.267 Kinases can also be recruited into condensates where they phosphorylate their target, which in turn modulates the condensate size.268 Also, in bacterial cells, many hydroxyl or nitrogen bearing amino side chains are phosphorylated and used for signal transduction. In C. crescentus, condensates are used as localized signaling hubs where phosphates are transferred between the participants for asymmetric patterning (described in more detail in section 6.3).269 The efficiency of this pathway depends on the material state of the condensate, with optimal performance and sufficient fluidity, which is governed by the IDRs and oligomerization domains of the scaffold protein PopZ.146
IDRs are, in principle, not needed for phase separation, as proteins with multiple interaction domains can form condensates similar to patchy colloids that undergo phase separation.270 Nonetheless, IDRs seem to be pervasive, for example, in the phase separation of the enzyme ribulose-1,5-biphosphate carboxylase (RuBisCO), which is mediated by the disordered protein Essential Pyrenoid Component 1 (EPYC1) of Chlamydomonas reinhardtii (green algae), which has multiple binding sites to connect multiple RuBisCOs.271 Similarly, disordered proteins assemble RuBisCOs in prokaryotic cells. For example, the intrinsically disordered protein CsoS2 assembles RuBisCOs through multivalent binding in the α-carboxysome from the γ-proteobacterium Halothiobacillus neapolitanus.272 In the case of the β-carboxysome from cyanobacterium Synechococcus elongatus, the RuBisCOs are linked by the protein CcmM.273 This protein has folded domains that bind RuBisCOs and has IDRs between the folded domains that function as linkers. Each RuBisCO specifically binds four CcmMs. The dynamic biomolecular condensate properties result from the disordered linker domain within CcmM, creating a network of RuBisCOs. McdB assists in positioning these carboxysomes in S. elongatus.274 This protein has been shown to phase separate also. This occurs through self-association with a coiled-coil dimerization and a trimerization domain while its IDR modulates its solubility. The functional relevance of self-assembly is not yet clear, but may involve tuning McdB binding to the carboxysome components, such as CcmM. NusA, an antitermination factor for RNA polymerase involved in rRNA synthesis, is one of the few proteins with high disorder in E. coli.145 It phase-separates in vitro and in vivo and may thereby nucleate RNAP foci (see also section 3).
RNA is a prevalent component in biomolecular condensates in eukaryotes, including stress granules and the nucleolus. mRNA half-lives are shorter in E. coli (4 min) than in mammalian cells (10 h) and comparable to S. cerevisiae (20 min).63 A short mRNA lifetime does not seem to prevent condensate formation, as RNA condensates containing stably incorporated mRNA are found in S. cerevisiae, such as P bodies,275 and in mammalian cells. RNA-containing droplets have also been found in various bacteria (see section 3), for example, in the form of the RNA degradosome.276 Moreover, RNAP condensates are formed through protein–protein interactions and are mostly involved in rRNA synthesis (in E. coli).145 This is particularly interesting because the nucleolus of mammalian cells is a separate compartment that produces rRNA.
In addition to these useful functions, biomolecular condensate formation can potentially be an intermediate step toward pathological protein aggregates.46 Notable examples of such behavior are the protein Huntingtin exon 1 associated with Huntington disease,277 tau associated with Alzheimer’s,278 and FUS associated with some forms of fALS.279 Preconcentrating such proteins enhances aggregation, although the probability of a transition to a fibrillar state will also depend on the chemical properties of the biomolecular condensate.280 Furthermore, it is unclear if these pathways are relevant beyond experiments with purified protein, high overexpression levels, or model cell lines. Nonetheless, biomolecular condensates have the potential to alter protein aggregation, and there is no reason to assume that this cannot occur in prokaryotes.
5.4. Molecular Density
Molecular density affects biochemical organization through macromolecular crowding effects, chemical interactions, and solvent quality. Molecular density is commonly measured by refractive index and, recently, by Raman imaging.281,282 The refractive index, which mostly reports on protein content, combined with volume measurements, indicates that E. coli maintains a macromolecular density of 300 mg/mL ± 15%.107 This compares to the 300–400 mg/mL biomacromolecule (protein + RNA) concentration obtained from cell dry weight.7,283 This is similar to fission yeast, which maintains a density of 280 mg/mL.284 In contrast, mammalian cells maintain a somewhat lower concentration of about 200 (90–260) mg/mL, as shown by a wide range of techniques and mammalian cell types.281 Normalized stimulated Raman imaging also reports on protein content.282,285 Using this method, the densities of the mammalian cytoplasm, nucleus, and nucleolus are 75, 85, and 115 mg/mL, respectively, and vary upon perturbations such as osmotic stress, ouabain treatment (inhibition of Na+/K+ ATPase), cytoskeleton disruption, cell senescence, and quiescence. Interestingly, the concentrations of protein in cell tissues vary: pancreatic islet maintains about 200 mg/mL, kidney glomerulus 100–200 mg/mL, skeletal muscle cells 200–300 mg/mL, and Zymogen granules in the pancreatic islet 300 mg/mL. Perhaps the matrix stiffness in different tissues reduces cell volume and thereby increases crowding.286 These findings suggest that measurements of immortal cell lines on glass slides or in suspension have less relevant densities. Determining protein concentration requires separate cell volume measurements, which can be challenging given the variety of cell shapes, and sample preparation (e.g., fixation) may generate artifacts. Nonetheless, if mammalian cells in tissues indeed have higher density, they may be more similar in density to cells of other domains of life.
The protein density is related to macromolecular crowding. Density is usually the weight per volume, whereas macromolecular crowding is the volume taken up by the bystander macromolecules providing steric hindrance. Macromolecular crowding is a function of the steric properties of the macromolecules, their number density, and how they are organized and can be measured by diffusion or dedicated probes.17 Diffusion of GFP suggests there is lower crowding in mammalian cells than bacterial cells (vide supra), which matches the density measurements. The lower crowding in mammalian cells has also been confirmed by a macromolecular crowding sensor (unpublished). The biochemical organization can strongly increase macromolecular crowding effects287 such as increased protein self-assembly.287
A common source of a change in density and crowding in most cells is osmotic stress. Fundamentally, the different domains of life have a similar response: release of water to the extracellular environment with a higher osmolality leads to a reduction of cell volume, which increases crowding, ionic strength, and internal osmolality.288,289 Cells recover volume through uptake of potassium ions and compatible solutes from the medium, as well as synthesis of other noncharged molecules such as sugars over the longer time frame. Full crowding recovery and adaptation takes half an hour to hours in E. coli and HEK293T, as shown with a FRET-based macromolecular crowding sensor.17,66 Cell growth already resumes before the crowding stabilizes at a new level. Hyperosmotic stress is one of the most frequently used perturbants in the laboratory to generate phase separation in mammalian cells. Phase separation may be induced by increased concentration of phase-separating proteins, macromolecular crowding, a change in ionic strength, or an active response of the cell. For example, the eukaryotic protein WNK1 kinase phase separates due to the macromolecular crowding in cells with hypertonicity, which activates a signaling pathway for cell volume recovery.290 As protein condensation has been less investigated in bacteria, osmotic stress-induced phase separation has not been described yet to the best of our knowledge. It is, however, known that hypertonic stress leads to nucleoid condensation.291
5.5. Stickiness
Weak and native associative interactions can alter protein stability or trigger formation of (transient) protein assemblies, as has been shown for purinosomes or G bodies in eukaryotic cells. Stickiness can also arise from nonspecific (hydrophobic, electrostatic) interactions between macromolecules,292 where, for example, chaperones bind to exposed hydrophobic surfaces or unfolded proteins expose their hydrophobic regions to stick to the cell’s biomacromolecules. Human cell lines possess a more extensive and complex chaperone and proteostasis system compared to prokaryotes and may have a different stickiness profile than bacteria, which are, on the other hand, more crowded.
When biomacromolecular surface chemistries are incompatible, it can cause misfolding, aggregation, and phase separation. Generic nonspecific interactions, or stickiness, lead to lowered diffusion, which can be tuned by the charge of biomacromolecules, as shown for the set of charged GFPs.88 The internal ionic strength depends on the bacterial species (section 2.1.3) and further tunes these interactions. The regulation of protein surface properties through mutation, i.e., the tuning of protein stickiness, is required in the presence of macromolecular crowding. Cellular macromolecules have coevolved over many generations, which may have led to “optimal stickiness”, but this is not the case when new proteins are introduced (e.g., by heterologous expression). In-cell NMR measurements have shown that amino acid substitutions in a Cu/Zn superoxide dismutase (SOD1) did not significantly impact its stability in eukaryotes but did in bacteria.293 Differences in stability of macromolecules in mammalian and bacterial cell lines can be related to the lower macromolecular crowding in eukaryotes.
Translational diffusion modulates diffusion-limited reactions and depends strongly on the physicochemical characteristics of the macromolecules such as stickiness and crowding, and these differ for numerous bacterial and mammalian cell types. Indeed, the less crowded mammalian cells allow faster translational motion of fluorescent proteins than bacteria do: fluorescent protein diffusion in various bacteria is in the range of 3–12 μm2/s, whereas it is 27 μm2/s in fibroblast cells and 24 μm2/s in Dictyostelium discoideum,68 compared with 87 μm2/s in aqueous media. The diffusion in bacteria is more in the range of that in the ER lumen, which is 5–10 μm2/s.294 In both eukaryotes and E. coli, diffusion depends on stickiness, where supercharged cationic GFPs have been shown to stick to ribosomes. In human cells, a positively charged peptide fused to GFP has a lower diffusion coefficient in the vicinity of F-actin.88,142 The diffusivity in E. coli shows stronger dependence on the charge of an introduced protein than in mammalian cells,295 probably due to higher crowding providing shorter distances for sticky, electrostatic interactions. Also, rotational diffusion (i.e., the rotation of a molecule along its own axes) of human SOD1 barrel is lower in E. coli than mammalian cells,296 but the same rotational diffusion coefficient is found for GFP.294 Homologously expressed bacterial TTHA rotates freely in E. coli, whereas heterologously expressed HAH1 does not. Here, the most important factor is the intracellular context, which has coevolved with the native protein, whereas a protein that is not in its native environment may experience enhanced stickiness and thus a slowed rotation.295 Amino acid substitutions have been shown to increase the rotational diffusion coefficients of HAH1 and SOD1 in bacteria, apparently by reducing their stickiness in the cell.296
Aside from stickiness and additional weak and transient molecular interactions, there are significant differences in other structural levels of cellular protein organization. Whereas prokaryotes have smaller proteins on average than mammalian cells,63 recent predictions based on Alphafold2 indicate a higher degree of protein homo-oligomerization in bacteria than in mammalian cells. According to Schweke et al.,297 45% of the E. coli proteome forms homo-oligomers, compared to 20% in human cells. We suggest that bacteria use more noncovalent homo-oligomerization, such as ATP-binding cassette (ABC) transporters consisting of self-assembled dimers that originate from a single short gene, whereas equivalent genes in eukaryotes have been duplicated and fused. Indeed, most ABC transporters in E. coli are homodimers, whereas they are fused in mammalian cells.298,299
5.6. Ribosomes
Cells contain a high concentration of ribosomes, and it has been proposed that they reduce diffusion of particles in the range of 20–40 nm. Cryo-TEM measurements suggest that the cytosolic concentration of ribosomes in yeast cells is exceptionally high, at 23 μM or 20% of the cytosolic volume.162 The concentration drops to 13 μM when cells are treated with rapamycin. Rapamycin targets mTORC1, which prevents mTORC1 from sensing amino acids and controlling ribosome concentration. The ribosome concentration estimated for E. coli is 10 μM, which is close to that of yeast.8 The ribosome concentration for mammalian cells has been estimated at 1 μM,300 but this concentration may be less accurate, as the cell volume was not measured precisely. Rapamycin reduces both diffusion and protein phase separation in yeast and mammalian cells; in yeast, the diffusion coefficient increases 1.8-fold, and in human HEK293 cells, 1.25-fold. In addition, there is an 80% and 50% decrease in SUMO10-SIM6 droplet area in yeast and HEK293 cells, respectively. As the cytoplasmic ribosome concentration may be very low in mammalian cells, this would suggest an additional mechanism, such as the presence of mRNA, that determines the viscosity. Indeed, a recent study by Xie et al. shows that mRNA condensation upon stress, such as carbon depletion, increases the diffusivity of proteins in the cytosol.301 Barriers presented by mRNA organization may in fact dominate over the ribosome crowding effects proposed earlier.
By analogy with rapamycin treatment in eukaryotic cells, ATP depletion in E. coli cells reduces the diffusion of particles larger than 30 nm,155 although the mobility of GFP is not. The size dependence is similar to that of a colloidal glass transition. The same reduction in diffusion can be seen in yeast, where energy depletion reduces the diffusion of the same viral matrix particles as in E. coli.192 Munder et. al suggested this is caused by an acidification of the cytoplasm, and Joyner et al. suggested an increase in macromolecular crowding.158,160 Later TEM images showed major changes in the yeast cytoplasm upon energy depletion, including more lipid droplets, membrane invaginations, membranous structures, and fibrillar aggregates,302 each of which could present roadblocks for larger diffusing particles. This is likely similar to an aging yeast cell, where similar large ultrastructural changes were seen.303 Of note, the direction of the diffusion change of these particles is strongly dependent on the particle identity,301 which may be due to a change in proteomic stickiness upon ATP depletion.304 This suggests that other phenomena may play a role.
5.7. Ionic Strength
In the cell, ionic strength plays a crucial role in organizing biomacromolecules. As biomacromolecules are charged, ion pairing between their residues increases affinity and specificity in protein–protein and protein–polynucleotide interactions. However, counterions screen the charge of these residues and need to be replaced during binding. Hence, counterions play a role in protein–protein and protein–polynucleotide interactions, as well as complex coacervate formation. E. coli has a cytoplasmic ionic strength of ∼300 mM, which compares to ∼140 mM for the cytosol of mammalian cells.18,63 Counterions need to be removed for charged proteins to interact, which costs energy and is less favorable at higher counterion concentration. Furthermore, a higher ionic strength leads to increased Debye screening of the protein charge, resulting in shorter-range attraction between opposite charges. This assumes the ions to be inert point charges, but the identity of small molecule anions also matters, as it can determine preferential interactions as given by the Hofmeister series. Glutamate is the predominant anion in E. coli, whereas chloride is the most abundant anion in mammalian cells. Glutamate is more kosmotropic (Terminology) than chloride and should interact less with proteins.305 Indeed, preferential exclusion of glutamate from a single-stranded DNA binding protein enhances condensation of this protein, whereas chloride interacts with the protein and therefore reduces condensation.306 Moreover, kosmotropic salts such as sodium fluoride can enhance the phase separation of the RNA-binding protein FUS, whereas chaotropic salts such as sodium bromide and sodium iodide inhibit it.307 Therefore, the specific ion interactions and ionic strength together may alter the biochemical organization of the cytoplasm in eukaryotes in a different manner than in prokaryotes.
Next to ionic effects, interactions with species-specific and electrostatically neutral osmolytes also have the potential to affect solvent quality and potentially trigger phase separation. Here, the effect of the solutes is highly solute specific, which is determined by how well they are hydrated and mostly how much they directly interact with a protein and with which moieties (amide or side chain).308 Common kosmotropes such as glycine betaine and trehalose are thus excluded from the protein surface, stabilizing the proteins. These are thus called compatible solutes and used in the different domains of life.309 They are vital when the intracellular solute concentration needs to be increased upon hypertonic stress. Chaotropic solutes such as urea are less common in cells. Urea is a waste-product in mammals but can be a nitrogen source for bacteria.310 Indeed, buffer experiments show that TMAO, which is a common cosolvent in deep sea fish and highly kosmotropic, enhances phase separation of γ-d-crystallin, whereas urea inhibits it.311 Because the cell’s small molecule composition is highly species- and (stress) condition-dependent, the interactions of small molecules with the biomacromolecules that drive biochemical organization will vary in different species and conditions.
6. The Bacterial Cell Cycle Machinery
6.1. Effects of Crowding and LLPS on Chromosome Replication
Replication of the bacterial chromosome is an essential cell cycle process that ensures faithful duplication of the genetic material to pass onto daughter cells, which is followed by segregation and completion of cell division. Chromosome replication is driven by a protein machine, the replisome, that acts bidirectionally to duplicate the DNA, from the chromosomal origin of replication (oriC) to the terminus of replication (ter), in three stages: initiation, elongation, and termination.312 The replication process in bacteria is exquisitely coordinated by crosstalk mechanisms with chromosome segregation and cell division, partially overlapping with them, as a means to rapidly proliferate and survive.313 The impact of macromolecular crowding on some of the multiple systems involved directly or indirectly in replication has been described. So far, a protein involved in the process, (ss)DNA-binding protein (SSB), has been shown to form biomolecular condensates. It would not be surprising if other proteins participating in chromosome replication will also be found to undergo phase separation, given their multiple domains of homo- and heteroassociation and their ability to form complexes with long DNA chains (single or double stranded) or to bind membranes, features commonly observed in proteins prone to phase separation.
6.1.1. Crowding and Chromosome Replication
Various studies in bacteria and other microorganisms have indicated that DNA replication has a strong dependence on macromolecular crowding.314−316 One of these studies demonstrated that crowding increases the activity of E. coli DNA polymerase I, a processive enzyme that participates in the joining of Okazaki fragments during lagging-strand replication and in repair of damaged DNA.317 Addition of PEG 8000, dextran T-70, Ficoll 70, or bovine plasma albumin as crowding agents enhances the reaction rates of nick-translation and gap-filing by the enzyme, counteracting the ionic strength-dependent reduction of activity observed at KCl > 0.1 M in dilute solution, or with other salts. Smaller molecules such as glucose, sucrose, or low molecular weight PEG have lower or no effect on DNA polymerase I activity. Crowding remarkably decreases the apparent KM values of DNA polymerase I for DNA, counterbalancing the increase in KM observed at high ionic strength in dilute solution and presumably enhancing the binding of the polymerase to DNA. These early results suggest that crowding could act as a metabolic buffer on macromolecular interactions, extending the range of intracellular conditions to which bacteria can adapt.
Crowding also seems to play a crucial role in regulating the precise timing of chromosomal replication initiation by the protein DnaA.318 This protein is an ATPase that binds to specific DNA sequences within oriC, leading to the assembly of the replication complex in all known eubacterial species. DnaA is active when bound to ATP and inactive in its ADP-bound form. The exchange of ADP for ATP is stimulated upon interaction of the protein with the lipid membrane or with specific sequences on the chromosome.319−321 By using fluorescent analogs of ATP, it was found that high concentrations of Ficoll 70 accelerate the exchange of ATP on the membrane-bound DnaA.318 Thus, a crowding effect at the interface between the membrane and the aqueous phase, where the protein is located, accounts for the highly cooperative shift from a relatively slow to a rapid nucleotide exchange. Crowding would probably enhance DnaA oligomerization, consistent with the known tendency of this protein to self-associate,322 although stabilization of a compact conformation cannot be ruled out. In addition, it is possible that interactions with other proteins are facilitated by crowding in vivo, but this remains to be confirmed.318
6.1.2. Phase Separation and Chromosome Replication
Bacterial SSB, essential for chromosomal DNA replication and repair, has been shown to form biomolecular condensates.253 The intrinsically disordered linker of SSB is required for condensation, and the interactions of its conserved ssDNA binding domain and C-terminal peptide upon self-association of the protein enhance the process, as do glutamate ions.306 SSB condensation occurs in the absence of DNA (Figure 13). In contrast to many other examples of phase separation either aided or disfavored by nucleic acid binding, in this case the role of DNA depends on the ssDNA:SSB stoichiometry. In vitro, SSB phase separates at low ssDNA:SSB ratios and, under these conditions, DNA partitions into the condensates. Increasing the ssDNA:SSB ratio inhibits phase separation, caused by competition between ssDNA and the SSB C-terminal domain for binding to the ssDNA binding domain. SSB partner proteins such as the DNA repair protein RecQ strongly partition into the SSB condensates, including those with low binding affinity (Kd in the tenths of micromolar range), although specific interaction is required. Small molecules such as nucleotides also accumulate to a slight degree inside the condensates, and the diffusion of clients within them scales with their size. Besides its canonical interaction with ssDNA, SSB enrichment inside the condensates also enables binding to RNA despite its lower affinity for SSB compared to ssDNA. This suggests that SSB may have a role in RNA metabolism.
Figure 13.

Formation of biomolecular condensates by SSB and regulation by ssDNA. (A) Multifaceted interactions of SSB structural regions are required for efficient LLPS. Schematic domain structures of SSB constructs are shown at the top, with numbers indicating amino acid positions at boundaries of structural regions. The SSBdC construct lacks the C-terminal peptide region. Below, turbidity is shown as a function of protein concentration, in the absence and presence of BSA (150 g/L), along with a model of LLPS-driving interactions. (B) SsDNA regulates SSB phase separation, as shown at the left by fluorescence microscopy of samples containing SSB, fluorescein-labeled SSB, and increasing concentrations of unlabeled dT79, and, on the right, a schematic model for the LLPS-inhibiting effect of ssDNA (black line). (C) Proposed model for the in vivo role of SSB LLPS, based on data from refs (253) and (250). Figure adapted from ref (253). Copyright 2020 the Authors. Published by PNAS under Creative Commons Attribution-NonCommercial-NoDerivatives License 4.0 (CC BY-NC-ND) [CC BY-NC-ND 4.0 Deed | Attribution-NonCommercial-NoDerivs 4.0 International | Creative Commons].
Formation of SSB condensates has been analyzed in vitro, in dilute solutions containing glutamate and with BSA or PEG as crowding agents, and in cell extracts.253 As is typical for condensates assembled in bacteria, in vivo confirmation of these condensates remains challenging due to their small size. Theoretical estimations by the authors253 show that the maximum diameter of an intracellular SSB condensate would be ∼120 nm, if the entire pool of cellular SSB molecules (∼2,000 SSB tetramers) formed a single droplet. Although super-resolution microscopy approaches allow visualization of particles of this size, assessing their dynamic properties for compelling demonstration of LLPS behavior is not straightforward. Nonetheless, in vivo reports show the formation of SSB foci at replication forks and also near the cytoplasmic membrane,250 presumably reflecting the known interaction of SSB with membrane lipids. This suggests that condensation may provide a means to regulate SSB function, favoring its storage near the membrane at low local ssDNA concentration when DNA repair needs are minimal (Figure 13). When the free SSB pool exceeds the DNA-bound fraction, condensation would be expected to occur even at genomic DNA sites. An increase in cytoplasmic ssDNA, reflecting a demand for SSB in DNA repair, would dissolve the condensates and “release the guards” of the genome, enabling SSB to repair damaged DNA.
6.2. Effects of Crowding and LLPS on Bacterial Plasmid and Chromosome Segregation
Prior to division, each future daughter bacterial cell inherits a fully replicated chromosome as a consequence of chromosome segregation. In many bacteria, this crucial process begins during replication with migration of the duplicated replication origins to opposite cell poles, followed by bulk segregation of the chromosome toward each cell pole and the resolution and transport of the replication termini at the division septum.214 Segregation is promoted by chromosomal macrodomains that further organize the DNA.323 Segregation mechanisms vary within bacterial species.324C. crescentus, B. subtilis, and Vibrio cholerae use an active pulling mechanism (Figure 14) that directs the chromosomes toward the bacterial poles through forces and directionality, using the dynamic parABS system. During growth of C. crescentus and V. cholerae, the ParB CTPase binds the oriC-proximal parS sequences in the chromosome, and the nucleoprotein complex thus formed moves poleward through interaction with the ParA ATPase, which seems to form a concentration gradient within the cell. In bacteria devoid of these active segregation systems, such as E. coli, spontaneous demixing of chromosomes occurs by entropic forces exerted on the replicating DNA.325
Figure 14.

DNA segregation and effects of phase separation. (A) (top) Plasmid segregation by parABS following a pulling mechanism (Type I). ParB binds parS sequences on the plasmids, and the ParB-parS nucleoprotein complex moves poleward, with its attached plasmid, through interactions with ParA that is localized between ParB-parS and the poles. Dashed arrows depict the path of the plasmids. (bottom) Plasmid segregation by ParMRC through a pushing mechanism (Type II). ParR binds parC sequences on the plasmids. A ParM filament polymerizes from soluble monomers between the ParR-parC nucleoprotein complexes on a pair of plasmids and pushes them apart toward the poles. Reprinted in part and adapted from ref (324). Copyright 2021 Gogou, Japaridze, and Dekker under the Creative Commons Attribution License (CC BY) [CC BY 4.0 Deed | Attribution 4.0 International | Creative Commons]. (B) Dynamic properties of ParB condensates. (left) Representative image of a live cell (cell contour represented by a white line) with low-mobility (blue) and high-mobility (red) trajectories of single ParB molecules. Magnified views of each ParB condensate with different low-mobility trajectories are shown with different colors. (right) Histogram of apparent diffusion coefficients for low-mobility (blue) and high-mobility (red) trajectories. Reprinted in part with permission from ref (148). Copyright 2020 Elsevier Inc.
Segregation of plasmids, which are much smaller than bacterial chromosomes (1–1,000 kbp vs 1–10 Mbp326) is simpler and more widely studied. Segregation of most high-copy-number plasmids occurs through random Brownian motion.327 Low-copy-number plasmids, on the other hand, often encode dedicated segregation systems to maintain inheritance.328,329 One such system, plasmid P1 of E. coli, uses parABS(324) (vide infra). The other, plasmid R1 of E. coli, uses a mitotic-like mechanism powered by two proteins encoded by the plasmid, ParR and ParM; the latter is a homologue of actin. In this system, parC DNA sequences near the replication origin of the plasmids bind to the ParR protein, which in turn interacts with the ParM actin. As a result, R1 plasmid segregation is driven by ParM polymers, which connect a pair of plasmids through the ParR-parS interaction, pushing them toward opposite cell poles.328
6.2.1. Crowding Promotes DNA Segregation
Entropy-driven segregation of the two replicated daughter chromosomes in rod-shaped bacteria has been modeled using two flexible ring polymers in the presence of cylindrical confinement and crowding agents.330 Crowders were simulated as spherical particles of MW 67 kDa, and the volume fraction of crowders (Φ) ranged between 0 and 0.3 to mimic the cell’s response to external osmolarity changes that cause dehydration of the cytoplasm, resulting in increased macromolecular crowding.67 In unsegregated polymers, contacts between them increase with higher Φ due to slower polymer dynamics. However, stronger crowding induces crowding particles to localize between polymer rings, enhancing ring–ring separation and increasing the mean residence time of separated rings at the cylinder ends. This is in agreement with theoretical predictions of entropic repulsion between overlapping segments of long polymer chains.331 According to Langevin dynamics simulations using a similar model, the segregation time was determined to increase with increasing Φ due to slower chain diffusion, whereas, for a fixed volume fraction, the segregation time decreases with increasing size of the crowders.332 Experiments with E. coli showed that protein oscillations exerted by the Min system can guide demixing of the chromosomes through interactions between MinD and DNA. Such interactions can enhance the entropic effects that, according to simulations, do not seem to be sufficient to drive full segregation on their own.333
Although the influence of crowding for each specific segregation step remains largely unknown, there are several reports that characterize the effects of crowding on overall segregation. For example, bacterial actin-like proteins, known as Alps, form polymers to promote segregation of various plasmids, and molecular crowding enhances their organization into complex structures.334 Supramolecular structures are also formed by AlfA protein from B. subtilis to segregate pBET131 plasmids during bacterial growth and sporulation,335 ParM polymers to segregate plasmid pSK41 in Staphylococcus aureus, as well as ParM polymers to segregate plasmid R1 in E. coli. All of these structures exhibit a multiplicity of states depending on nucleotide association, ionic strength, and pH. Besides electrostatic interactions through counterions with like-charged filaments,336 excluded volume effects from macromolecular crowding shift the equilibrium between single filaments and bundles.337,338In vitro studies have determined that R1-ParM bundling results mainly from molecular crowding, with a random distribution of filament polarity within the bundles stabilized by long-range electrostatic attractive forces between patches of residues.339 These properties presumably result in equally efficient DNA capture at both ends of the bundle. The increased stiffness of the filaments, their ability to handle large DNA cargos, and their structural plasticity340 are all factors that allow segregation to occur. Interestingly, ATP-triggered filament bundles formed by AlfA over a wide range of ionic strengths and pH values in dilute buffers were similar to supramolecular structures formed in the presence of crowding agents.341 As with pSK41-ParM, which also spontaneously forms bundles in the absence of crowders,342 the formation of the different kinds of polymorphic structures is thought to be mostly mediated by counterions.343,344 It is notable that bacterial actins work with a small number of associated regulatory proteins compared with the multiplicity of eukaryotic actin- or microtubule-associated protein modulators. Thus, it is attractive to postulate that in bacterial polymerizing systems, the greater functional degrees of freedom conferred by molecular crowding and counterions result in a greater diversity of filament–filament interactions, which obviates the need for numerous accessory proteins.334
6.2.2. Direct and Condensate-Driven Effects of Phase Separation on Segregation
Phase separation-related demixing of the multiple DNA molecules found in a typical prokaryotic cell345 affects its internal organization and function. An artificial nanofluidic model has allowed quantification of the interactions of two dsDNA molecules in cavities with controlled anisotropy. The conclusion was that the two molecules spontaneously demix in elliptical cavities and orient along the poles with increasing cavity anisotropy.346 Mixing a large dsDNA molecule with a plasmid results in the exclusion of the plasmid toward the poles. Such an uneven distribution is enhanced by molecular crowding and is reminiscent of similar nonuniformity observed for high-copy-number plasmids in bacterial cells.347 Interestingly, a variety of large structures in bacterial cells, described as biomolecular condensates, foci, aggregates, etc., seem to often localize in zones excluded by the nucleoid. These structures appear at the cell poles, form in response to internal and environmental stresses348,349 (see section 7), and freely diffuse in the regions of the cytoplasm devoid of nucleoid,139,350 suggesting their localization might be influenced by segregation-induced entropic forces. One example of a protein involved in chromosome segregation351 that forms such structures under starvation conditions is the E. coli GTPase ObgE,348 which localizes in the cytoplasm and partly associates with the membrane.351
Phase separation has been described in vivo for the aforementioned E. coli parABS system that segregates plasmid P1.148 Similar to the chromosome segregation systems in other species, it consists of the DNA site parS, the DNA binding protein ParB, and the ATPase ParA. In E. coli cells plasmid parS-associated ParB forms nanometer-sized condensates whose fusion is prevented by the ATPase activity of the ParA motor.148 Two different dynamic behaviors have been found by using single-molecule tracking photoactivated localization microscopy (sptPALM) within these condensates: a low-mobility fraction of immobile ParB dimers bound to parS, and a high-mobility fraction of ParB dimers nonspecifically interacting with the DNA (Figure 14). Distribution of the replicated DNA along the cell length occurs upon ParA binding to the condensates that, accordingly, appear segregated. In a separate study, the effect of high pressure on the ParB condensates has been addressed in live E. coli cells by fluorescence intensity fluctuation-based methods, namely two-photon scanning number and brightness (sN&B) and raster scanning imaging correlation spectroscopy (RICS).352 Application of 100 MPa of pressure disrupts ParB condensates, some of which reassemble upon pressure release, indicating that they are reversible. Brightness analysis shows that the protein forms dimers in the condensates, disrupted by the application of pressure.
The ParB-parS partition complex of the parABS system was demonstrated to undergo LLPS in vitro.(353) In the presence of crowders such as PEG, dynamic round condensates of ParB from C. glutamicum are stabilized by the interaction with parS. Electrostatic interactions regulating ParB self-association seem to be involved in the formation of these condensates, because, as for many others, an increase in the ionic strength of the solution increases the saturation concentration needed for LLPS. As mentioned above, ParB binds and hydrolyzes the nucleotide CTP, and this CTPase activity is enhanced by interaction with parS. Interestingly, CTP stabilizes ParB condensates, significantly decreasing the saturation concentration for phase separation. This effect is specific for CTP, since nucleotides such as ATP or GTP, not recognized by ParB, disfavor condensation. This constitutes another example of phase separation promoted by the nucleotide CTP, as is the case for the nucleoid occlusion factor Noc of B. subtilis (see section 6.3). ParB homologues from other bacteria also form biomolecular condensates with analogous CTP regulation, suggesting an evolutionarily conserved mechanism for this protein in segregation. In C. crescentus, specific association of ParB to parS sites is controlled by the ATPase ParA, with the latter pulling the duplicated origin region toward the opposite cell pole. ParA concentrations at the new pole become thus slightly higher, triggering polymerization into a liquid phase-condensate of PopZ, the polar organizing protein that anchors ParBS to the pole.354−356
Chromosome segregation may be also affected by crowding effects and phase separation in other organizational systems that contribute to this essential process,357 its regulation, or its coordination with other cell cycle steps. For example, SMC (Structural Maintenance of the Chromosome) proteins, which are present in all bacteria as well as eukaryotes, organize and compact the DNA and probably mediate segregation by organizing replicated DNA into individual chromosomes prior to segregation. In B. subtilis and C. crescentus, SMC condensins interact with ParB358,359 bound to parS sequences. Distribution of SMC proteins in B. subtilis is modulated by XerC and XerD recombinases, which bind to the dif site at the chromosome replication terminus (ter) and catalyze the resolution of chromosome dimers that arise from replication.360 In E. coli, the SMC homologue MukB along with its partner proteins MukE and MukF organize in axial cores, including in cells with lower molecular crowding.361 The MukBEF complex binds to chromosomal sites everywhere except in the ter macrodomain, as a result of the antagonistic action of MatP protein,362 a key organizer of the ter macrodomain. In B. subtilis and E. coli, SMC proteins also interact with bacterial topoisomerases that contribute to chromosomal organization and segregation.363,364
Other factors driving segregation include systems that coordinate chromosome segregation with cell division. Among them are the divisome spatial positioning systems in E. coli such as the Ter-linkage mediated in part by MatP;365 a similar system has been characterized in C. crescentus.(366) The nucleoid occlusion effector in E. coli, SlmA protein, also coordinates cell division and chromosome segregation when bound to its specific sequences in MatP-free DNA regions outside of the Ter macrodomain.367 SlmA has been shown to form heterotypic condensates with the central division protein FtsZ in vitro (see section 6.3) that might ultimately affect its role in the coordination of segregation with division.
6.3. Effects of Crowding and LLPS on Bacterial Cell Division
Most bacteria divide by binary fission, relying on a multiprotein machinery, the divisome, whose assembly is subjected to a precise regulation in time and space through the coordinated action of various protein factors (Figure 15).368 The cytokinetic ring is built by polymers of the protein FtsZ, a GTPase engaged in a complex scheme of reversible self-association reactions controlled by nucleotides, cations, and salt.369 Regulation of assembly of this “Z-ring” takes place through interactions of FtsZ with partners and ligands, some of which bind to a conserved C-terminal domain (CCTD) of FtsZ, which in E. coli interacts with at least 6 different proteins.370
Figure 15.

Schematic representation of a dividing E. coli cell showing the FtsZ ring at midcell. (A) Nucleoid occlusion, mediated by the protein SlmA bound to specific DNA sequences (SBSs) on the chromosome, antagonizes Z-ring formation near the chromosome. (B) Two proteins, ZipA and FtsA, anchor the Z-ring to the membrane. (C) The Ter linkage involving the proteins ZapA, ZapB, and MatP, which binds matS sequences at the Ter macrodomain of the chromosome, promotes Z-ring assembly at midcell. (D) The oscillatory MinCDE system, formed by the proteins MinC, MinD, and MinE, prevents Z-ring assembly at the cell poles. Adapted from ref (371). Copyright 2021 by the Authors. Published by MDPI, Basel, Switzerland under the terms and conditions of the Creative Commons Attribution (CC BY) license [https:// creativecommons.org/licenses/by/4.0/].
6.3.1. Macromolecular Crowding and Cell Division
The vast majority of the studies exploring the impact of macromolecular crowding on bacterial cell division have focused on FtsZ, because of its ability to form polymorphic structures of large size, alone or assisted by the many proteins with which it interacts, whose interconversion equilibria are susceptible to modulation by excluded volume effects.372
6.3.1.1. Crowding and FtsZ Oligomers
The impact of crowding on the oligomerization of the GDP-bound form of E. coli FtsZ has been studied using nonideal tracer sedimentation equilibrium, a method in which the dilute species is labeled to distinguish it from the crowders.373 This oligomerization takes place according to an indefinite linear self-association model in which a Mg2+ ion is bound by each protein monomer added to the oligomer, and the affinity for monomer incorporation gradually decreases with oligomer size.374 This mechanism is radically different from that used for the cooperative formation of FtsZ polymers elicited by GTP.375 By using iodinated FtsZ as tracer, equilibrium gradients have been measured and analyzed to retrieve the apparent weight-average molar mass of this protein as a function of its concentration and of those of the crowders, BSA or cyanmethemoglobin.373 The two crowders tested interact with FtsZ exclusively via steric repulsion, having large effects on its association constants in the presence of Mg2+. The effects are particularly pronounced at high crowder concentration, leading to high oligomer sizes. Consequently, decamers and larger oligomeric species of FtsZ, only minimally represented in dilute solution, become more abundant in crowded conditions.373
Brownian dynamic simulations have been applied to study macromolecular crowding effects on the rates of FtsZ dimerization. In this approach, the rate constants in crowding conditions are obtained from the rate constant in the dilute solution, applying a factor that accounts for the crowding effect.376 Simulations show that crowding reduces the diffusion of FtsZ, due to the concomitant increase in viscosity. At crowder excluded volume fractions below 0.3, this reduction is somehow counteracted by crowding-related enhancing effects, resulting in negligible overall changes in the FtsZ dimerization rate constant. At excluded volume fractions of 0.3, however, the enhancing effects prevail and the dimerization rate constant is ∼4 times higher compared to that in dilute solution.
6.3.1.2. Crowding and FtsZ Polymers
Dramatic effects of crowding on GTP-induced E. coli FtsZ polymers, usually one subunit-thick under dilute solution conditions,370 have been reported by different laboratories. Electron microscopy and AFM images of FtsZ in the presence of high concentrations of model crowding agents like Ficoll 70 or dextran T70 evidence the formation of FtsZ bundles through lateral association of single-stranded protofilaments23 (Figure 16). These larger structures are energetically more favorable under crowding conditions than the protofilaments, as they exclude less volume. The bundles are still dynamic, but their disassembly rate and GTPase activity are lower compared to those of the protofilaments. In addition to linear bundles, rings and toroids of E. coli FtsZ have been described in an electron microscopy study, in which methyl cellulose or poly(vinyl alcohol) is used as a crowder.377
Figure 16.

Effect of crowders on the polymerization of FtsZ. (A) Electron microscopy images of GTP-triggered FtsZ polymers in the presence of the specified crowders. (B) Variation of the critical concentration of polymerization (Cc) of FtsZ with the concentration of Ficoll 70, ovomucoid, and RNase A. Lines correspond to simulations according to a volume exclusion model, showing a pure volume exclusion behavior for Ficoll 70 (υFicoll = 0.96 mL/g) and for RNase A (υRNase = 0.703 mL/g). Experimental data in the presence of ovomucoid cannot be explained in terms of a pure volume exclusion behavior (dashed line, υOvo = 0.69 mL/g) or assuming repulsion with like molecules (dotted line, υOvo = 1.61 mL/g), instead being compatible with a model assuming additional effects (solid line, υOvo = 6.6 mL/g). Arrow in the legend depicts increasing volume exclusion. Adapted or reprinted in part from ref (378), copyright 2016 Monterroso et al. Published by PLOS under the terms of the Creative Commons Attribution License [CC BY 4.0 Deed | Attribution 4.0 International | Creative Commons], and ref (23), copyright 2003 Elsevier Inc. under the terms of the Creative Commons CC-BY license [CC BY 4.0 Deed | Attribution 4.0 International | Creative Commons].
Polymerization of Mycobacterium tuberculosis FtsZ has also been scrutinized using these crowding agents.379 Variable arrangements of the same type as those observed with E. coli FtsZ are observed, including rings and toroids, in the presence of KCl. However, when more closely inspected, some structural features of these M. tuberculosis FtsZ assemblies are different from those of E. coli FtsZ, suggesting distinctive assembly mechanisms.379 Moreover, in the presence of Na+ ions, FtsZ from E. coli forms helical spirals, whereas for M. tuberculosis FtsZ the equilibrium is shifted toward long bundles. By using time-lapse TIRF microscopy, the rate of elongation of the FtsZ bundles from M. tuberculosis in crowding conditions has been determined. After an elongation phase, a steady state is reached after which the lengths of the bundles mostly decrease or remain unaltered.
Taken together, these studies indicate that the polymorphic nature of the GTP-induced FtsZ filaments is also maintained in crowding conditions. Their final arrangement strongly depends on conditions such as type and concentration of salts or pH and on the particular FtsZ protein being studied, similar to that usually observed for this protein in dilute solution.380 Importantly, all these studies conclude that crowding favors lateral interactions of FtsZ polymers, known to be a prerequisite for engaging in a functional Z-ring.381,382 Indeed, bacteria have proteins specifically devoted to the cross-linking of FtsZ protofilaments, acting as positive regulators of Z-ring assembly, the most important ones being the Zap proteins (ZapA, B, C, and D). As Z-ring formation needs to be restricted to the cell center at the time of cell division, mechanisms antagonizing the crowding-induced bundling are likely necessary in vivo.(23) Consistent with this, several negative regulatory proteins and systems in bacteria inhibit Z-ring formation at the wrong places in the cell. In E. coli, the two main spatial regulators are the Min system and nucleoid occlusion, which act on lateral interactions between FtsZ filaments as well as longitudinal interactions between FtsZ subunits within protofilaments.
The Min system of E. coli comprises the proteins MinC, MinD, and MinE that together block FtsZ ring assembly at the cell poles (Figure 15). Powered by ATP-driven bulk migration of MinD and MinE from one cell pole to the opposite cell pole, the concentration of the MinD-binding protein MinC over time ends up being highest at the cell poles and lowest at midcell, where the future Z-ring forms.383,384 The key regulatory mechanism is that direct interaction of MinC with FtsZ selectively inhibits Z-ring formation at the cell poles, thus helping to corral FtsZ polymers to midcell. The C-terminal region of MinC recognizes FtsZ,385 interfering with the lateral association of its filaments.386 The N-terminus of MinC, on the other hand, inhibits protofilament assembly,387 resulting in a two-pronged disruption of FtsZ protofilament bundles.
Co-reconstitutions of FtsZ and the Min system on lipid bilayers, together with the membrane tethering protein ZipA that interacts with FtsZ (Figure 15), have shown strong coupling between both systems, which is reflected in the formation of antiphase waves that are enhanced in crowding conditions.388 This behavior is consistent with the antagonistic regulation of FtsZ polymerization and bundling by the Min system, and its corralling of FtsZ to midcell.
FtsZ bundles formed in noncrowding conditions are also disrupted by SlmA,389 the protein that mediates nucleoid occlusion in E. coli.(390) Nucleoid occlusion, mediated by SlmA binding to several SlmA binding sequences (SBSs) on the bacterial chromosomal DNA, prevents FtsZ rings from forming over unpartitioned chromosomes and causing potentially catastrophic chromosome breakage (Figure 15). Notably, the nucleoprotein complexes of SlmA with its specific binding sequences accelerate FtsZ depolymerization in crowding conditions comparable to those found in the cytoplasm247 analogously to that described in dilute solution.391 This suggests that lateral interactions do not confer particular resistance to the antagonistic action of SlmA. Interestingly, the positive regulator ZapA (Figure 15) partially reverses the acceleration of FtsZ disassembly by SlmA/SBS, and it does so more efficiently in the presence of crowding agents.247 These results suggest that excluded volume effects might contribute to the regulation of Z-ring formation, reinforcing the agonistic action of specific factors, while not interfering with the antagonists, as they are designed to counteract crowding-related effects such as lateral interactions and bundling. We speculate that crowding could be one of the missing factors determining Z-ring localization, since in the absence of both negative (the Min system and nucleoid occlusion) and positive regulators, multiple discrete Z-rings still form and are biased toward the cell center.392
The polymerization of FtsZ by GTP occurs through a cooperative mechanism characterized by a critical concentration (Cc), which represents a threshold above which polymers are formed.380 Light scattering and fluorescence anisotropy based determinations of Cc(393) have shown that high concentrations of unrelated proteins, Ficoll, dextran, or sucrose decrease its value378 (Figure 16), consistent with the notion that crowding generally favors self-association. Evolution of the experimentally determined Cc with the concentration of the different crowders is compared in this study with simulations based on volume exclusion theory, assuming that FtsZ polymerization behaves as a first-order phase transition and with activity coefficients defined in terms of the exclusion volume (Vex), concentration, and masses of all species in the solution. The extent of crowding effects on the Cc agrees with the exclusion volume behavior (i.e., simulations using partial specific volumes of the species as Vex are compatible with the experimental data) in the case of neutral inert polymers such as Ficoll or dextran and for proteins like RNase A when its own oligomerization is considered. In contrast, reductions in the Cc larger than expected for a pure exclusion effect are observed for ovomucoid as crowder and for DNA at relatively low concentration. Effects beyond excluded volume predictions may be partially attributed to additional electrostatic repulsion between negatively charged ovomucoid or DNA and FtsZ, which is also negatively charged at neutral pH.
FtsZ polymers have also been studied inside microdroplets generated by microfluidics, through which hundreds of droplets of controlled size and composition are obtained, containing crowding agents coencapsulated with the protein and stabilized by E. coli lipids.394 The distribution of the fibrous protein networks was found to be dependent on FtsZ and crowder concentration. In both cases, increasing concentrations rendered a spread of the FtsZ polymer network, reducing the so-called depletion zone most probably generated by geometric and entropic restraints. Restrictions imposed by the spatial boundaries are also characterized by modifying the container shape. FtsZ has been later encapsulated inside lipid-stabilized microdroplets containing protein crowding agents or E. coli lysates.395 The appearance of bundles is observed in all cases, either from the beginning or after shrinkage of the microdroplets, leading to concentration of the crowding agents but also of FtsZ and, presumably, of the buffer components. Macromolecular crowding is one of the decisive experimental factors in the bottom up reconstitution of a minimal machinery for autonomous division, as shown in a study in which FtsZ and the Min system were encapsulated in lipid vesicles with crowding agents (Figure 17).396 This study provides a showcase of the emergence of cell division in a minimal system.
Figure 17.

Positioning of Z-ring by the Min system in vesicles. (A) 3D maximum projection of a merged confocal image of vesicles containing the MinCDE proteins (mScarlet-I-MinC, magenta) and FtsZ-Venus-MTS (green) in dextran 70, showing that Z-rings are spatially restricted to the vesicle midpoint by the inhibitory action of the Min-oscillatory wave. The MTS is a heterologous amphipathic helix (membrane targeting sequence) fused to FtsZ-Venus that artificially tethers it to the membrane. (B) 3D projections of a Z-ring positioned by the MinCDE system (top) as in panel A, and a Z-ring that is still positioned at the vesicle midpoint, albeit less efficiently, by the Min system lacking MinC (bottom: Min waves are not visible because of the absence of mScarlet-I-MinC). (C) Time-lapse confocal images of the Z-ring (FtsZ-Venus-MTS, green) stabilized by the oscillatory pole-to-pole Min waves (magenta) as reflected by mScarlet-I-MinC. Adapted in part from ref (396). Copyright 2022 the Authors. Published by Springer Nature under a Creative Commons Attribution 4.0 International License [https://creativecommons.org/licenses/by/4.0/].
6.3.1.3. Mixed Macromolecular Crowding and FtsZ
The assembly of FtsZ has also been probed in single-phase systems containing two crowders,378 as a closer approximation of the bacterial cytoplasm, in which crowding effects arise from various macromolecules with different properties rather than from one type of macromolecule. This is one of the few studies available on mixed macromolecular crowding, aimed at discerning whether these mixtures display additive or nonadditive behavior. The Cc of FtsZ assembly in these mixtures is always lower than that in dilute solution, but the effects generally deviate from the plain sum of those exerted by the individual crowders, and either reinforcement or counteraction of each other’s effects occur, depending on their physicochemical properties (Figure 18). Thus, the dramatic effects of negatively charged ovomucoid are strongly potentiated by Ficoll or dextran but counteracted by positively charged RNase A or by the osmolyte sucrose.
Figure 18.
Effect of mixed crowders, involving inert polymers and proteins, on the polymerization of FtsZ. (A and B) Cc values determined in the presence of the specified crowders. F, O, and R are Ficoll 70, ovomucoid, and RNase A, respectively. The numbers in the x-axis are their concentrations in g/L, alone, or in the mixtures. Total crowder concentration in the mixtures is 150 g/L. Long and short dashed lines depict the Cc values in the presence of 150 g/L Ficoll (A and B) and ovomucoid (A) or RNase A (B), respectively. (C) Cc of FtsZ assembly in the presence of the specified individual crowders and their mixtures (50%). Adapted from ref (378). Copyright 2016 Monterroso et al. Published by PLOS under the terms of the Creative Commons Attribution License [CC BY 4.0 Deed | Attribution 4.0 International | Creative Commons].
6.3.1.4. Crowding and Other Division Proteins from E. coli
In addition to the above-described analysis of crowding effects on the Min system reconstituted on supported lipid bilayers alongside FtsZ and ZipA,388 two other crowding studies have focused on this oscillating protein complex. The first study used it as a model system to evaluate the ability of a new multicompartmental reaction-diffusion modeling method, Spatiocyte, to reproduce the effects of volume exclusion associated with crowding.397 This method is applied for the simulation of MinD translational diffusion in a crowded compartment with a 34% volume occupancy and also on a crowded surface with 23% of the area occupied with inert and immobile crowder molecules. Anomalous diffusion is observed in both cases, more pronounced on the crowded surface despite the lower occupancy, which is attributed to the lower dimensionality of the surface space. The results agree with previous studies suggesting that crowding on the cell membrane reduces diffusion of MinD and MinE.398 The second study investigates the impact of sucrose as a crowding agent on MinE amyloid-like structures involving its N-terminal domain. Lateral bending of the protein fibrils on mica surfaces seems to be modulated by crowding and ionic strength, according to AFM imaging.399
6.3.2. LLPS and Cell Division
As with the crowding reports, most studies of LLPS involving bacterial division proteins have been focused on E. coli FtsZ oligomers, polymers, and multiprotein or nucleoprotein complexes with partners. These works address two LLPS-related phenomena: the behavior of FtsZ in model crowding systems displaying aqueous two-phase behavior, and assembly of FtsZ into homotypic and heterotypic phase-separated biomolecular condensates driven by crowding400 (Figure 19). There is also a large body of research on the phase separation of proteins involved in the regulation of asymmetric division in C. crescentus.
Figure 19.
FtsZ and phase separation. (A) FtsZ distributes differently in encapsulated phase-separated binary mixtures of crowders (PEG/dextran 500 and PEG/Ficoll 70 are shown as examples) depending on its association state. Dissociation of polymers upon GTP depletion produces redistribution within phases of FtsZ species, that are no longer found at the lipid membrane confining the microdroplets.401 (B) Under crowding conditions promoting phase separation, FtsZ, alone or in the presence of binding partners, forms biomolecular condensates that congregate at the lipid boundary depending on their composition.247,248
6.3.2.1. LLPS and E. coli FtsZ
The possible impact of the membraneless microenvironments inherent to all kinds of cells, including bacteria, on the reactivity and distribution of FtsZ has been analyzed in binary mixtures of PEG and a second crowder401 (Figure 19). Determinations by fluorescence of the partition coefficient (K) of FtsZ used the following equation:
| 8 |
where the fraction terms are the protein concentrations in both phases. The results show that FtsZ unevenly distributes in systems with two crowders at concentrations at which they demix, forming two compartments with distinct physicochemical properties. Confocal images of the samples are also in line with this observation (Figure 20). K-values < 1 are always obtained, meaning that FtsZ species are generally excluded from the more hydrophobic PEG phase, in which denatured proteins usually partition because of the exposure of their hydrophobic amino acid residues.402 FtsZ strongly accumulates in Ficoll 70 or DNA phases, reflected by K-values < 0.2, while K-values around 0.5 were obtained in LLPS systems with dextran 500, indicating a lower preference for this phase. Being both inert crowders, differences in FtsZ partition between Ficoll 70 and dextran 500 could be ascribed to their different properties rather than their size, as similar partitions are found in dextrans 500 and T40. The asymmetric distribution of FtsZ suggests that microenvironments could contribute to the spatial regulation of FtsZ assembly, facilitated in areas where the protein accumulates above the Cc and hindered in regions of insufficient protein concentration.
Figure 20.

Dynamic relocation of the bacterial division protein FtsZ as a function of its polymerization state in two-phase systems encapsulated inside lipid-stabilized microdroplets. (A) FtsZ filaments preferentially locate in the dextran phase and at the interface of the dextran/PEG system. Upon GTP depletion the filaments disassemble and the protein partitions principally into the dextran phase with no obvious accumulation at the interface. Numbers in the confocal images correspond to time in minutes. A scheme of the association reactions of FtsZ is shown above. (B) Relative amount of FtsZ in each of the phases and at the interface obtained from fluorescence measurements. Reprinted in part from ref (401). Copyright 2016 the Authors. Published by Springer Nature under a Creative Commons Attribution 4.0 International License [CC BY 4.0 Deed | Attribution 4.0 International | Creative Commons].
A significant fraction of the protein locates at the interface of the dextran/PEG compartments when polymers are triggered by GTP.401 This interfacial localization, often observed for large particles because of the concomitant reduction of interfacial tension,403 might serve to concentrate the FtsZ polymers within a defined region and to organize them in two dimensions, perhaps rendering a relative orientation more suitable for constriction than the arrangements in three dimensions. Moreover, the distribution of FtsZ in these systems seems to respond dynamically to the self-association state of the protein, which shifts from one location to another in response to GTP addition and depletion. This has been verified by encapsulation of the LLPS system within water-in-oil microdroplets stabilized by lipid membranes, which provide a more stable platform than the bulk phases (Figure 20). These cell mimics can be generated by manual emulsion, rendering a multiplicity of containers of different sizes401 which may be advantageous in some instances, as with size-associated phenotypes,404 or in a more controlled manner by microfluidics microdroplets with the exact same size and composition.405
FtsZ was later found to self-assemble into biomolecular condensates arising from phase separation facilitated by crowding248,255 (Figure 19). Indeed, FtsZ is a good candidate for LLPS because it contains an IDR that flexibly links the globular core polymerization domain and the CCTD406,407 and exhibits homo- and heteroassociations that confer multivalency.375,380 Addition of SlmA/SBS to FtsZ in the absence of GTP results in dynamic structures enriched in both proteins and SBS DNA that display characteristics of liquid-like condensates248 (Figure 21). Biomolecular condensation is favored by the additional multivalency conferred by the FtsZ/SlmA/SBS system. Notably, SlmA dimerizes and forms SlmA-SBS complexes with a 4:1 stoichiometry under conditions at which FtsZ oligomerization is insufficient to drive its own phase separation. Homotypic FtsZ condensates can be detected only at lower salt, higher Mg2+ and crowder concentrations.255 The intrinsically disordered linker sequence of FtsZ is not essential for condensation in this case, because its removal still permits condensation with similar csat (protein concentration at which condensates start assembling) to that for the full-length protein, as measured by turbidity. Nonetheless, the linker does play a role in condensate assembly kinetics.255
Figure 21.

Dynamic FtsZ-SlmA-SBS condensates in crowded media. (A) Assembly of GTP-triggered FtsZ polymers after addition of nucleotide to FtsZ-SlmA-SBS condensates. The number of condensates decreases with polymer formation. After disassembly of the polymers due to GTP exhaustion, condensates reassemble. Times are in minutes (time zero, GTP addition). Scale bars: 5 μm. A scheme of the dynamic process is shown below. Reprinted in part with permission from ref (248). Copyright 2018 the Authors. (B) Incorporation of ZapA slightly decreases the csat of condensation of FtsZ-SlmA-SBS, monitored using turbidity. Reprinted in part from ref (247). Copyright 2023 the Authors. Published by the Royal Society under the terms of the Creative Commons Attribution License [http://creativecommons.org/licenses/by/4.0/].
Homotypic FtsZ condensates and heterotypic FtsZ/SlmA/SBS condensates recruit the division ring regulator ZapA, an agonist of Z-ring formation that does not display condensation on its own and, contrary to SlmA/SBS, does not promote condensation of FtsZ under conditions disfavoring its oligomerization.247 This regulator shows a minimal reduction in the apparent csat of formation of the FtsZ/SlmA/SBS condensates (Figure 21). Determination of csat for condensates involving more than one macromolecule is not straightforward408 and, in this case, an apparent value is obtained from turbidity measurements in which the ratio between the three elements is kept constant and the total concentration increased.247
Perhaps the most noteworthy feature of FtsZ condensates in vitro is their ability to interconvert with FtsZ polymers when GTP is added, followed by condensate reassembly after GTP depletion due to FtsZ’s GTPase activity. The prevalence of condensates or polymers depends, therefore, on the nucleotide present and is also subject to regulation, with SlmA/SBS strongly favoring condensates vs polymers, and ZapA favoring polymers.247 This suggests that, in vivo, condensates may prevent FtsZ assembly into the bundles that are normally competent for Z-ring assembly at noncentral areas of the cell or under nongrowing conditions, when GTP levels are low. Similarly, accumulation of positive regulators at the cell center could rescue FtsZ from the condensates, favoring the assembly into polymers and, hence, Z-ring formation at midcell.
Interestingly, heterotypic FtsZ/SlmA/SBS condensates preferentially locate at the membrane when reconstituted inside microdroplets generated by microfluidics that display crowding and compartmentalization as cell mimics248 (see section 4; Figure 22). This behavior is consistent with the tendency of SlmA to bind to membranes252 and is also in line with the known enhancement of condensation by surface effects.51 The influence of lipid membranes on the formation of these biomolecular condensates has been further analyzed using supported lipid bilayers as minimal membrane systems in buffers containing glutamate, the most abundant anion in E. coli, which favors formation of condensates of large size.254
Figure 22.
E. coliFtsZ foci suggestive of condensates. (A) FtsZ-GFP foci and filament formation in Chinese hamster ovary cells, after treatment with vinblastine. FtsZ-GFP localization is shown in the same living cell at various times after addition of the drug, in minutes. Arrows indicate growth of filaments from the foci at random locations in the cytoplasm. With time, filaments grow longer, forming a network of filaments, while foci disappear except in the nucleus. Reprinted with permission from ref (409). Copyright 1999 The Company of Biologists Ltd. (B) Representative confocal images of FtsZ-SlmA-SBS condensates (top) and GTP-triggered polymers (bottom) in lipid-stabilized microfluidics-based microdroplets. Also shown are the intensity profiles of the green and red channels, obtained along the line drawn in the images. Reprinted in part from ref (247). Copyright 2023 the Authors. Published by the Royal Society under the terms of the Creative Commons Attribution License [http://creativecommons.org/licenses/by/4.0/].
In light of the current body of knowledge about LLPS behavior, it is likely that the formation of FtsZ condensates has been previously overlooked in in vitro and in vivo studies.247 Indeed, structures compatible with condensates can be observed in images taken upon disassembly of FtsZ polymers reconstituted alongside SlmA/SBS in GUVs, long before they were described as such.391 Similarly, expression of E. coli FtsZ in mammalian cells resulted in formation of dozens of round foci throughout the cytosol that disassembled upon addition of vinblastine, an antitubulin drug, leading to FtsZ polymer assembly409 (Figure 22). FtsZ condensates in bacterial cells have not yet been confirmed, but E. coli cells under long-term nutritional stress form polar foci containing FtsZ (and other divisome proteins) that convert back to polymers upon nutrient addition.410 These reversible foci, along with reversible foci of FtsZ during the nondividing portion of the C. crescentus cell cycle that convert to polymers prior to cell division,411 require further study but are suggestive of condensates.
6.3.2.2. LLPS and B. subtilis Noc Protein
Like SlmA in E. coli, the Noc protein mediates nucleoid occlusion in the Gram-positive species B. subtilis.(412) Unlike SlmA, Noc does not seem to interact with FtsZ directly and instead inhibits FtsZ migration away from the midcell FtsZ ring.413 Intriguingly, however, Noc shares with SlmA the ability to form biomolecular condensates, which have been characterized through reconstitution in GUVs and supported lipid bilayers.147 Phase separation of Noc scales with its concentration, and it is sensitive to the type of salt present in the solution, being favored by potassium glutamate and inhibited by KCl and NaCl. In addition, as observed for other proteins in the DNA-binding ParB family, Noc condensates are strongly promoted by the nucleotide CTP, also known to regulate its membrane binding activity.414 Indeed, these condensates bind to the lipid membrane of water-in-oil microdroplets and GUVs, where they form either film-like structures or round 3D-condensates depending on the protein concentration. Noc condensates induce membrane deformations and preferentially bind to the liquid-disordered phase domains in GUVs exhibiting different membrane domains. Deformation of lipid membranes has also been found in other phase separated systems (see section 4). One interesting observation is that Noc condensates recruit FtsZ, whether the latter is membrane-bound through a membrane targeting sequence or not, despite the lack of any known direct interaction between these proteins. This is probably because of the enhanced concentration of Noc within these condensates, which might potentiate possible weak interactions with FtsZ. Round structures resembling condensates are observed in images taken in vivo in prior work where the interaction of Noc with the membrane was revealed.251
6.3.2.3. LLPS and C. crescentus Cell Division Proteins
PopZ, an intrinsically disordered, oligomerizing protein involved in the cell division of the model Gram-negative species C. crescentus, forms a large biomolecular condensate in the cytoplasm at one cell pole. In addition to their characterization in C. crescentus cells, PopZ condensates have been analyzed in vitro in the presence of divalent cations and after expression in mammalian cells.146 As in other instances, e.g. the eukaryotic stress sensor Pab1415 and E. coli FtsZ,255 condensation is not driven by IDRs of PopZ but by folded regions within the oligomerization domain. Nevertheless, as with FtsZ, the IDRs of PopZ contribute to the regulation of the process. The csat and cD thresholds for the two-phase and the single-dense-phase regimes, respectively,36 and the dynamics of the condensates depend on the length of the unstructured sequence (Figure 23). These key parameters defining condensation behavior could also be tuned by the degree of multivalency of the C-terminal helical region. Interestingly, intrinsically disordered proteins are less common in bacteria compared with eukaryotes (see section 5.3).416,417
Figure 23.

PopZ condensates are regulated by PopZ structural features. Shown at the top are phase diagrams of PopZ expressed in mammalian cells, with PopZ in a dilute phase, two phases, or a dense phase. The nucleoid boundary is represented as a white dotted line. Scale bar, 10 μm. Shown below are phase diagrams of EGFP fused to three PopZ variants with different linker lengths. Each dot represents data from a single cell, and dot color indicates phase. Figure reprinted in part from ref (146). Copyright 2022 the Authors. Published by Springer Nature under a Creative Commons Attribution 4.0 International License [http://creativecommons.org/licenses/by/4.0/].
Alterations in the fluidity of the natural PopZ condensates change their cellular localization and ability to recruit regulatory proteins, implying that modified condensates are often unable to fulfill their role in the orchestration of asymmetric division, which compromises cellular fitness. Notably, not only solid-like but also PopZ condensates that are too liquid are not perfectly suited for their function. From a synthetic biology standpoint, a recent study146 nicely illustrates how synthetic condensates can be rationally designed by dissection of the molecular grammar driving their formation, enabling applications of these structures in biotechnology and biomedicine. It is also proposed that thorough analysis of the material properties of condensates could help us to understand their role in pathologies such as neurodegeneration, mediated by the formation of solid aggregates of proteins like FUS. Another interesting aspect of this study is that the PopZ condensates assembled within mammalian cells are larger than those occurring in their native bacterial cells. These larger condensates retain their intrinsic properties such as the specific partitioning of bacterial proteins and their dynamics, as measured by FRAP. This suggests that mammalian cells, like the microdroplets or GUVs used in other studies, may serve as convenient platforms to reconstitute biomolecular condensates of bacterial origin in order to facilitate their analysis. This also brings up the question of whether components within the crowded cytoplasm of bacteria, such as ribosomes, limit the size of these condensates in the cytoplasm of bacterial cells compared with the eukaryotic cell cytosol.
Other proteins involved in the asymmetric division of C. crescentus have also been described to form biomolecular condensates. For example, SpmX, an integral membrane protein, directly interacts with PopZ at the pole of C. crescentus cells opposite from the FtsZ focus mentioned above. This PopZ-SpmX condensate recruits the cell division protein DivJ to the polar microdomain, stimulating its kinase activity.150 Although SpmX and PopZ form condensates at the same cellular location, they are demixed, forming distinct zones within the condensate (Figure 24). Multivalent interactions between these two proteins are modulated by protein concentrations, temperature, salt, and nutrients. Interestingly, ATP concentrations in the low millimolar range, which occur when nutrients are plentiful, dissolve the condensates of SpmX or PopZ. This behavior is consistent with the previously described role of this nucleotide as a hydrotrope.198 Surprisingly, while SpmX condensation is inhibited by 1,6-hexanediol (1,6-HD), that of PopZ is promoted, despite its demonstrated biomolecular condensation properties.146 This constitutes a good example of the limitations of 1,6-HD to assess biomolecular condensation behavior, as previously discussed.36 In contrast to the dissolution of condensates with ATP, depletion of ATP promotes condensation of the SpmX disordered domain. This leads to compartments with DivJ at higher concentrations, which in turn enhances its activity when its substrate is scarce, for example under low glucose conditions.
Figure 24.

Biomolecular condensates of the proteins SpmX and PopZ from C. crescentus. (A) Localization of PopZ and its associated scaffold proteins PodJ and SpmX, and signaling proteins PleC and DivJ, at specific cell poles of C. crescentus before and after cell division. SpmX recruits DivJ to condensates at the old pole and stimulates the latter’s kinase activity. CtrA-phosphate is a master transcriptional regulator that controls expression of multiple C. crescentus genes and is selectively enriched at the new cell pole. Figure reproduced from ref (249). Copyright 2022 the Authors. Published by Springer Nature under a Creative Commons Attribution 4.0 International License [http://creativecommons.org/licenses/by/4.0/]. (B) Super-resolution images of purified PopZ (labeled with Atto488) and SpmX (ΔTM, labeled with Cy3) with (top) or without its IDR (bottom), showing demixing of the condensates of SpmX within the condensates of PopZ in vitro, driven by the IDR. (C) False-colored images of C. crescentus cells expressing mCherry-PopZ (green) and SpmX-dL5 (magenta) with (top) or without the SpmX IDR (bottom), suggesting that wild-type SpmX forms multiple condensates in the PopZ microdomain in vivo, also promoted by the IDR. The percentage of PopZ condensates enclosing more than one SpmX condensate (B) or cells with more than one SpmX cluster in the PopZ microdomain (C) is indicated on the right. Scale bars, 5 μm. Figure adapted from ref (150). Copyright 2022 the Authors. Published by American Association for the Advancement of Science under a Creative Commons Attribution License 4.0 (CC BY) [https://creativecommons.org/licenses/by/4.0/].
Interestingly, SpmX acts as a negative regulator of phase separation by PodJ, another membrane protein involved in the regulation of cell division,249 and this behavior could have profound implications for the regulation of the cell cycle of C. crescentus. In vitro, biomolecular condensates of PodJ, driven by its disordered domains and coiled-coils, are assembled at relatively low protein concentrations, and they are highly regulated by salt. Below 100 mM NaCl, irreversible structures are observed, whereas above this concentration, liquid droplets form and high salt concentrations dissolve them. Biomolecular condensates of PodJ have also been detected in vivo, and they are less fluid compared with those assembled in vitro. Macromolecular crowding and the cell membrane to which PodJ is tethered are among the factors invoked to explain this difference in fluidity. In fact, as mentioned, macromolecular crowding has been described to play a key role in the assembly of biomolecular condensates,35 including those of the bacterial cell division proteins from E. coli. Hence, it would not be surprising that the condensates from C. crescentus proteins are also affected by crowding.
The inhibition of PodJ phase separation and cell pole targeting by SpmX could have a role in the clearance of PodJ at the old cell pole, since SpmX is expressed after the formation of the PodJ condensates. Moreover, these condensates act as hubs that accumulate client-signaling factors through interaction with different regions of the protein, such as the histidine kinase PleC. Recruitment of PleC by PodJ condensates inhibits PleC activity, suggesting another way that phase separation, in conjunction with allosteric mechanisms, could contribute to the regulation of enzymatic activity in bacteria.418 Some of these studies were conducted by heterologous expression of the proteins of interest in E. coli, exploiting the lack of homologues of C. crescentus polarity proteins in this organism.
7. Connections between Phase Separation and Bacterial Fitness
Since the discovery that bacterial proteins are also able to assemble into biomolecular condensates arising from phase separation, it has become clear that one potential role of these structures is to protect bacterial cells from stressful conditions. For example, the first bacterial condensates identified in vivo, the bacterial ribonucleoprotein bodies (BR-bodies) from C. crescentus assembled by RNase E276 and mRNA-dense bodies at the poles of L. lactis,(185) are thought to be analogous to eukaryotic P-bodies and stress granules. RNase E is crucial for mRNA degradation, and it has been hypothesized that its phase separation, promoted by RNA and reverted by its cleavage, might accelerate mRNA degradation. Typical of many condensate-forming proteins, RNase E harbors an IDR that is necessary and sufficient for its LLPS. In vitro, phase diagrams show that this condensation depends on protein concentration and ionic strength. Cells respond to cellular stress (EDTA or ethanol treatment, or heat shock) by forming BR-bodies, which are subsequently dissolved upon removal of the stress. The BR-bodies increase stress tolerance and overall fitness, as disruption of the RNase E disordered region and inhibition of condensate formation lead to higher susceptibility to stresses.276 Such effects are seemingly not related to the ability of the BR-bodies to recruit degradosome components. The presence of the aberrant polar mRNA foci in L. lactis correlates with cessation of cell division, a heat shock response and loss of nucleoid-occluded ribosomes. The mRNA dense bodies accumulate when transcripts are formed that encode poorly produced membrane proteins, suggesting defects in the coupling of transcription, translation, and membrane insertion.
More recently, it has been proposed that biomolecular condensates called aggresomes increase bacterial fitness, enabling cells to survive stresses such as antibiotic treatment, starvation, oxidative stress, heat shock, or phage infection.149,185 These structures can be found in E. coli but also in other Gram-negatives, and they accumulate proteins such as HslU, a component of the HslVU protease, Kbl, an enzyme that degrades threonine as part of the serine biosynthetic pathway, and AcnB, a cis-aconitase involved in central metabolism. Cells with aggresomes are more resistant to stress because these structures sequester proteins vital for cellular function, thereby shutting down their associated processes and forcing the cell into a dormant state.419,420 This state correlates with a marked change in the physical properties of the cytoplasm, which changes from a fluid state to a more glass-like state.155 Biomolecular condensation is indeed emerging as one of the possible mechanisms behind the intriguing formation of dormant and persister cells in bacterial populations,421 which are able to withstand stresses such as antibiotic treatment, hence representing a threat to human health. Cellular ATP levels decrease markedly upon entry into the stationary phase as a result of the decrease in cellular energy levels. This ATP depletion favors the formation of aggresomes, consistent with ATP acting as a hydrotrope that dissolves aggregates and biomolecular condensates.198 These condensates are heterogeneous in composition and physical properties, and it is hypothesized that these properties might be tuned to respond to stresses of different intensities and durations. Similar to BR-bodies and many other dynamic biomolecular condensates,276 aggresomes form under stress conditions and disassemble when the stress is over. In a recent study of bacterial dormancy in response to antibiotic exposure, faster rates of aggresome disassembly correlated with shorter lag times for cells to exit the dormant state and regrow.422
LLPS also seems to play a role specifically in the protection of bacterial DNA from damage due to stressful conditions such as exposure to UV light. For example, biomolecular condensates enriched in the (ss)DNA-binding protein SSB serve to sequester excess levels of this protein alongside its interacting partners near the membrane.253 Early reports show that SSB levels largely exceed those required to cover the ssDNA sites during replication.423 Storage of this excess in phase-separated compartments would facilitate rapid mobilization of the SSB protein pool when necessary to protect the exposed ssDNA and repair damaged genome loci, as the increase in ssDNA sites dissolves the condensates. In support to this model, cells with mutant SSB unable to efficiently phase separate but still able to bind ssDNA are viable in stress-free conditions but more sensitive to UV light damage than wild-type cells.424
Biomolecular condensation of the DNA protection protein Dps shields DNA under stress conditions by its compaction into a dense complex, also acting as a global regulator of transcription226,425 (Figure 25). Dps condensates do not prevent binding of RNAP, which has access to buried genes, but exclude some other DNA-binding proteins like restriction enzymes, the activity of which decreases with increasing Dps. Upregulation of Dps may also ensure that transcription can continue under conditions of extreme stress. Indeed, Dps deletion reduces survival rates over a diverse range of stress conditions (e.g., heat shock, osmotic shock, starvation, UV exposure, antibiotics, and oxidative stress). Intracellular Dps levels are specifically regulated by the selective ATP-dependent protease ClpXP, which hydrolyzes Dps in the presence of glucose.426 Finally, other NAPs that can form phase separated condensates on DNA are the HU proteins226 (see section 3), implicated in stress response pathways such as the SOS and the osmolarity/supercoiling responses and in the environmental programming of the cellular response during aerobic and acid stress.427 Some NAPs provide an efficient response to various stress conditions, regulating transcription through condensation of the nucleoid.207
Figure 25.
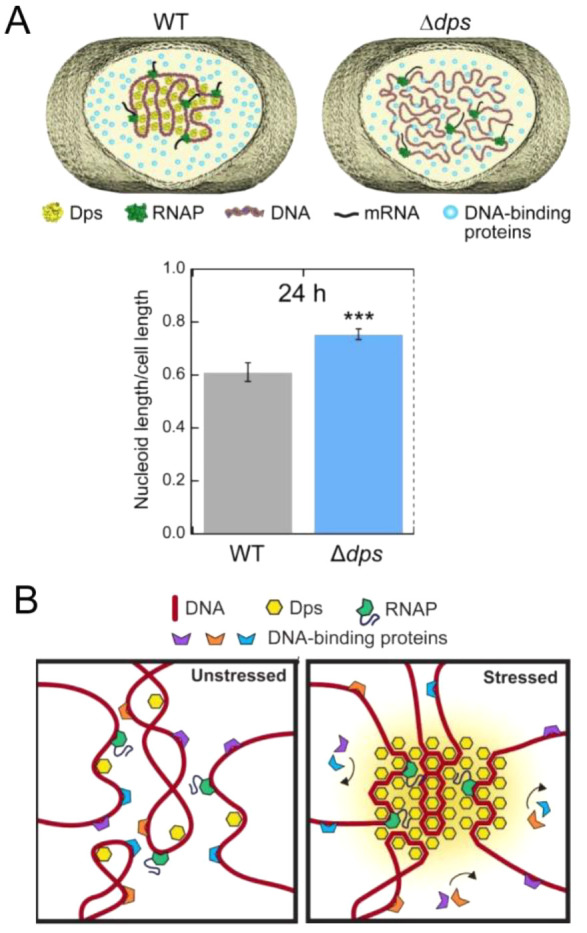
Protection of the DNA by Dps under stress conditions. (A) In wild-type cells, Dps condenses the DNA during the stationary phase (left). This condensation does not take place in the absence of Dps (right). Below, ratios of nucleoid length to cell length in cells with and without Dps. (B) Schematic representation of the model proposed for the protection of DNA by Dps. In the absence of stress conditions, Dps binds to DNA but no major condensation of the nucleoid occurs (left). Under stress conditions, Dps forms biomolecular condensates on a large part of the nucleoid into which RNAP can freely diffuse while other proteins are excluded, which blocks their access to the DNA (right). Figure adapted with permission from ref (425). Copyright 2018 Elsevier Inc.
In contrast to eukaryotic cells, bacteria often have to survive in environments with highly varying nutrient availabilities and types. Biomolecular condensation has been proposed to concentrate enzymes present at low copy numbers, thus enhancing their activity under starving conditions. This is the case of the above-described condensation of C. crescentus SpmX, which at low ATP levels recruits the DivJ kinase as a client to the condensates.150 This recruitment of DivJ concentrates it and results in more efficient kinase activity when ATP levels are low, which is crucial in this aquatic species that often encounters low nutrient densities. Phase separation is also used by the commensal bacterium Bacteroides thetaiotaomicron to maintain fitness in the mammalian gut, a hostile environment with highly variable nutrient levels, multiple competitors, and threats posed by the host immune system.428 Nutrient starvation in this bacterium triggers phase separation of the transcription termination protein Rho, which is driven by its IDR and regulated by protein concentration, salt concentration, and RNA binding. The sequestration of Rho molecules into these membraneless compartments increases Rho transcription termination activity, which in turn modifies the RNA abundance of hundreds of genes, including several required for gut colonization, ultimately promoting bacterial fitness. Finally, photosynthetic cyanobacteria regulate the availability of metabolic enzymes during light–dark cycles by sequestering them in puncta at night and releasing them in a soluble form during the day.429
Other phase-separation related defenses against starvation involve nucleotides and polyphosphate (polyP). PolyP granules are constitutively assembled in some bacteria but also are often formed in response to nutrient limitation.430 In Pseudomonas aeruginosa, in conjunction with the universal starvation alarmone (p)ppGpp, polyP has an additive effect on the nucleoid dynamics and organization, protecting the chromosome during starvation, increasing fitness, and helping cells to survive stresses such as antibiotics. Signaling by (p)ppGpp downregulates enzymes involved in GTP biosynthesis in both B. subtilis and E. coli,431,432 and connections have been established between this signaling and persister cell formation.433 Persister cells display slow or arrested growth,420 and this may be related with their low GTP levels that would shift the equilibrium of FtsZ away from polymers and toward biomolecular condensates.248 Along these lines, foci containing folded FtsZ localize to cell poles in nongrowing late stationary phase E. coli, Salmonella typhimurium, and Shigella flexneri cells and are related to multidrug tolerance.410
8. Conclusions and Future Perspectives
Macromolecular crowding is a key element of the intracellular complexity that potentially modulates the protein–protein, protein–nucleic acid, and protein–lipid interactions in cells. Complexes or assemblies of molecules occurring in bacteria are particularly exposed to crowding effects, because the total concentration of macromolecules in the cytoplasm of these microorganisms is higher than in the cytosol of eukaryotic cells. Crowding in bacteria has been shown to promote the assembly of proteins into larger complexes, to facilitate the binding of proteins to nucleic acids, to stabilize the structure of macromolecules, and to modulate their activity. Macromolecular crowding also elicits the formation of distinct compartments by phase separation, which appears most relevant in the case of bacteria, as they generally lack the membrane-bound organelles that are so crucial for organizing the eukaryotic cytoplasm.
Evaluation of crowding effects on biomolecular interactions and the characterization of biomolecular condensates are technically challenging and further aggravated in vivo by the small size of bacterial cells. Fluorescence methods are among the most useful tools, because of their ability to specifically monitor the molecules of interest in the presence of crowding agents together with the temporal and spatial resolution they provide. The rapid development of fluorescence super-resolution imaging methods and the application of fluorescence microspectroscopy such as fluctuation approaches partially overcome the hurdles resulting from the small size of bacteria, allowing identification of biomolecular condensates and assessment of the dynamics and function of their hallmark components. An alternative to the cellular studies is the reconstitution of the macromolecules in model crowded systems, in bulk solution, or encapsulated within a lipid monolayer or bilayer. Compared with the studies in cells, this strategy allows evaluation of the system in more controlled conditions and a more straightforward interpretation of the results. However, performing quantitative measurements of interactions is still more complex in these reconstituted systems than in the typical dilute solutions.
The rapidly growing number of studies reporting bacterial biomolecular condensates emphasize their importance, but their precise role in bacterial physiology remains elusive. Nevertheless, they seem to potentially participate in the regulation of cell cycle processes, as some factors engaged in cell division, nucleoid replication, and segregation have been shown to undergo phase separation. Biomolecular condensates may therefore be part of a mechanism to provide spatial control of these and other essential processes, a role traditionally attributed principally to the membrane. Moreover, such subtle mechanisms would be particularly appropriate for bacterial cells, which need to rapidly adapt to changes in environmental conditions. There is solid evidence supporting the implications of biomolecular condensates in cellular fitness and protection against adverse environmental conditions.
Despite intensive research during the last years, there are still many unsolved questions concerning the structure, function, and regulation of biomolecular condensates in general and of those assembled by bacterial proteins in particular. Some of the outstanding questions are summarized in Box 1. An interesting aspect is the regulation of biomolecular condensate formation by nucleotides, with CTP often having an enhancing effect and ATP and GTP usually a negative impact, along with other physicochemical factors such as pH, ionic strength, and cosolvents (e.g., compatible solutes). Bacterial condensates are also regulated by supramolecular structures such as DNA, RNA, and the membrane, but the underlying mechanisms are far from well understood. Particularly interesting would be elucidating the role of the nucleoid surface in the modulation of protein phase separation in bacteria. There are studies pointing to post-translational modifications as possible regulators of phase separation, by analogy with eukaryotic condensates, but further studies are needed to evaluate the generality of these observations. The ultrastructure of condensates and the precise arrangement of the components within the condensates, especially in the case of heterotypic ones, is still enigmatic. It also needs to be defined if the stoichiometry of the complexes in dilute solution is maintained when they phase separate to form the condensates. Super-resolution imaging methods together with single-molecule diffusion to probe the dynamics of molecules within the subcompartments of the cell will surely help to answer these questions. In addition, the factors determining the size of these condensates, which in bacterial cells are necessarily smaller than in eukaryotic ones, remain elusive. We hypothesize that the nucleoid-free space of the cell may be a major determinant of condensate size. The cellular amount of the protein forming the condensates and the relative amounts of the different components, in the case of multicomponent condensates, will likely influence their final size.
Box 1. Outstanding Questions.
What are the functions of biomolecular condensates in prokaryotes?
Do ribosomes influence the mobility of native proteins in the cytoplasm of bacteria, archaea, and endosymbionts?
What is the relation between reaction rates, diffusion coefficient, and protein concentrations in the cytoplasm?
What is the mechanistic basis for the fluidization of the cytoplasm under different metabolic conditions?
What is the molecular basis for the differences in protein mobility at the old and new pole of the cell?
Do bacteria (and archaea) age?
What determines the compaction of the nucleoid, given the large differences in the amount of DNA per volume of cytoplasm?
How do the physicochemical characteristics such as crowding and confinement in bacteria affect phase separation differently than in mammalian cells, and does this give unique functional opportunities to bacteria?
What are the factors that determine the size of bacterial biomolecular condensates?
How is condensate assembly regulated by nucleotides in bacteria?
How are the different components arranged within heterotypic bacterial biomolecular condensates?
Is the stoichiometry of heterocomplexes maintained when they phase separate to form condensates in bacteria?
How does crowding affect bacterial biomolecular condensates triggered by other factors?
It is still puzzling why the material properties of condensates such as fluidity need to be maintained within a narrow range to ensure functionality, with deviations toward either lower or higher fluidity seemingly detrimental in the few examples thoroughly analyzed. For natively disordered protein domains, the length of the disordered regions appears to control important material properties of the condensates, but generic physicochemical factors likely also play a role. Although some bacterial biomolecular condensates seem to be driven by macromolecular crowding, the crowding effects in other cases, where condensation is triggered by factors such as ionic strength changes or membrane surfaces, remain to be addressed. It is likely that in many of these cases, crowding will decrease the concentrations of the proteins at which phase separation occurs. Finally, we predict that many structures previously described as foci, bodies, diffusion barriers, or clusters that participate in cell division, SOS response, volume regulation, toxin-antitoxin systems, development of persister cells, and many other cellular processes finally turn out to be biomolecular condensates when viewed in light of the current body of phase separation research.
Acknowledgments
We thank A. Chenal and N. Carvalho (Institut Pasteur) for enlightening and helpful discussion. Research by G.R., S.Z., and B.M was supported by the Spanish Ministerio de Ciencia e Innovación (Grant numbers 2023AEP105 and PID2019-104544GB-I00/AEI/10.13039/501100011033) and by PID2022-136951NB-I00, funded by MCIN/AEI/10.13039/501100011033, and by ERDF “A way of making Europe”. W.M. was supported by the National Institutes of Health, USA (Grant number GM131705). The research of B.P. was funded by the NWO Gravitation program “Building a synthetic cell” (BaSyC) and an ERC Advanced Grant (ABCvolume; #670578). A.J.B. was supported by a ERC Consolidator Grant (PArtCell; no. 864528). The Systems Biochemistry of Bacterial Division group (CIB Margarita Salas) participates in the CSIC Conexiones LifeHUB (Grant number PIE-202120E047). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Glossary
Terminology
- Amphitropic proteins
bind weakly (reversibly) to membrane lipids, and this process regulates their function.
- Biomolecular condensates
are membraneless organelles that form dynamic and compartmentalized structures inside cells and can be described as physical gels. They form through phase separation and can have a wide range of viscoelastic properties. Condensates can bring molecules together that need to interact or segregate molecules from others.
- Chaotropes
are molecules such as urea, guanidinium, and iodide that disrupt the structure of water and biomolecules. They weaken the hydrogen bonding network, leading to increased disorder and reduced structure in the surrounding water molecules.
- Colloids
are mixtures of two or more phases where one substance is dispersed evenly throughout another one. The nucleoid behaves as a colloidal system within the bacterial cytoplasm.
- Cytoplasm
is the intracellular fluid plus membrane-bounded compartments surrounded by the plasma membrane.
- Cytosol
is the aqueous portion of the cytoplasm without organelles. We use the term cytosol in the context of eukaryotic cells and the term cytoplasm to describe the intracellular fluid of bacteria and archaea, which typically do not have organelles.
- Density
is the macromolecule weight per volume, the number density (macromolecule concentration), or volume density (volume fraction). This is not the same as macromolecular crowding, which is the increase in chemical activity due to the colloidal osmotic pressure difference.
- Hydrotropes
increase the solubility of poorly soluble compounds by reducing the surface tension of water.
- Hyperstructures
refer to organized assemblies of cellular macromolecules such as the replication machinery, ribosomes, cytoskeleton, and divisome.
- Inclusion bodies
are intracellular (irreversible) aggregates and typically result from overexpression and misfolding of proteins.
- Intracellular bodies
are specialized structures such as ribosomes or compartments such as the nucleoid or protein-bounded cages in the cytoplasm.
- Kosmotropes
are small molecules such as betaine and K+ and Na+ ions that have a stabilizing effect on the structure of water and biomolecules. They promote the formation of hydrogen bonds and tend to increase the order and structure of the surrounding water molecules.
- Macromolecular crowding
refers to the effects of excluded volume on the energetics and transport properties of macromolecules within a solution containing a high total volume fraction of macromolecules.
- Metabolons
are multienzyme/protein complexes that work in close proximity to enhance the efficiency of metabolic reactions. Examples are the glycolysis and fatty acid synthase metabolons and the pyruvate dehydrogenase complex.
- Micro- and nanocompartments
are protein-bounded structures that serve to sequester specific metabolic processes or enzymatic reactions. An example is the carboxysome that contains enzymes for CO2 fixation.
- Osmoprotectants
(also known as compatible solutes) are small organic molecules that accumulate in cells to high levels (up to molar concentrations) without interfering negatively with metabolic activity. The high and regulated levels of osmoprotectants help maintain osmotic balance by increasing the internal osmotic pressure.
- Plasmolysis
refers to the loss of water from cells in a hypertonic environment (hyperosmotic stress). At high osmotic stress (when the turgor has become zero) the cytoplasmic membrane will shrink away from the cell wall, which is known as plasmolysis.
- Polysomes
are mRNAs loaded with multiple ribosomes, also known as polyribosomes.
- Turgor or turgor pressure
is the hydrostatic pressure difference that balances the difference in internal and external osmolyte concentration (osmolarity).
Biographies
Begoña Monterroso obtained her PhD in Pharmacy (from Universidad Complutense de Madrid) after her studies on the structural and thermodynamic characterization of lytic enzymes degrading and remodeling the pneumococcal cell wall in the group of Profs. Margarita Menéndez and José Laynez, in collaboration with Profs. Juan Hermoso and Armando Albert (Instituto de Química Física, CSIC, Spain). She was later a Fogarty International Postdoctoral Fellow in Prof. Allen Minton’s group at the National Institute of Diabetes and Digestive and Kidney Diseases (NIH, USA), focusing on the quantitative analysis of the effects of macromolecular crowding on protein folding and activity and on the characterization of the association schemes of macromolecular complexes by biophysical techniques such as light scattering. She then joined the group of Prof. Germán Rivas and Dr. Silvia Zorrilla at the Centro de Investigaciones Biológicas Margarita Salas-CSIC as a Senior Research Scientist. Here, she implemented microfluidics technologies, after a stay at the Prof. Wilhelm Huck group at the Institute for Molecules and Materials (Radboud University of Nijmegen, The Netherlands) and initiated the droplets-based reconstitution projects leading to the functional reconstitution of bacterial division elements into cytomimetic crowded systems. She next moved back to Instituto de Química Física Blas Cabrera as a Senior Staff Scientist. Among her main research interests are the characterization of the molecular mechanisms involved in the assembly and functional role of biomolecular condensates, and the biophysical characterization of macromolecular interactions in solution and crowded environments.
William Margolin has been studying bacterial cell biology and division for over 30 years, using a combination of genetics, microscopy, and protein biochemistry to advance our understanding of how the simplest cells organize their cytoplasms and duplicate themselves. He received his SB in Biology from the Massachusetts Institute of Technology and his PhD in Molecular Biology from the University of Wisconsin—Madison, where he studied transcriptional regulation during the bacteriophage Mu lytic cycle. Margolin then received a postdoctoral fellowship from the National Science Foundation to study FtsZ and cell division in Rhizobium bacteria, which form a productive symbiosis with legume plants, with Sharon Long at Stanford University. In 1993, Margolin joined the faculty in the Microbiology and Molecular Genetics department at University of Texas Medical School (now McGovern Medical School) in Houston, where he continued his studies of how the simplest cells self-organize and divide, initially with Rhizobium but ultimately with the classic bacterial model system E. coli. Currently a Professor, McGovern Scholar, and Graduate Program Director at his institution, Margolin is also a Fellow of the American Academy of Microbiology.
Arnold J. Boersma studied chemistry at the University of Groningen (The Netherlands), where he also pursued his master’s and doctorate graduate studies in the group of Prof. Ben L. Feringa and Prof. Gerard Roelfes on DNA-based asymmetric catalysis. He then worked as a postdoctoral fellow at Oxford University in the group of Prof. Hagan Bayley, where he engineered protein nanopores for stochastic sensing of neurotransmitters and amino acid enantiomers. He returned to the University of Groningen and worked with Prof. Bert Poolman. Here, he developed novel probes to detect the physicochemical properties inside various types of cells. He next joined the DWI-Leibniz Institute for Interactive Materials in Aachen (Germany), as an Independent Group Leader and a fellow of the Max Planck School “Matter to Life”. He then moved to Utrecht University as an Associate Professor and associate scientist at the DWI-Leibniz Institute. His research focuses on understanding the consequences of macromolecular crowding in cells. To this end, he measures in-cell crowding and its consequences with new molecular probes that he develops and reconstitutes physiological crowding in artificial systems.
Germán Rivas: Doctoral training in the lab of José Gonzalez-Rodríguez (Physical Chemistry Institute, CSIC, Madrid, Spain). PhD in Chemistry (1989, Autonomous University of Madrid, Spain). Postdoctoral training in the laboratories of Allen Minton (NIH, 1990-1992) and Jurgen Engel (1993, Biozentrum, University of Basel, Switzerland). Since 1994, working at the CIB Margarita Salas, CSIC, Madrid. Group leader (since 1996). CSIC Research Professor (since 2015).
GR has devoted his scientific career to quantitatively studying multiprotein systems whose elements dynamically interact to organize functional cellular machines involved in essential processes. During his postdoctoral time in Minton’s lab, he realized the impact of the local microenvironment (background interactions) on the functional energetics of macromolecular associations in physiological (crowded) environments. For these reasons, he and his co-workers developed unique biophysical methods to study protein associations under crowding conditions similar to the natural cell interior, allowing them to experimentally demonstrate that excluded volume effects due to crowding can significantly affect the mode and extent of protein association.
For the last 25 years, the Rivas laboratory has explored the biochemical mechanisms governing the functional interactions of the bacterial division machinery (the divisome) to reconstruct, from the bottom up, operating simplified versions of the divisome in controlled cell-like environments, in the absence of cells. Their research program, framed on the quest to build synthetic cells from scratch, integrates biochemistry, molecular biophysics, membrane reconstitution, and bottom-up synthetic biology approaches.
Bert Poolman was trained in bioenergetics and microbiology and is now active in biochemistry and biophysics. Poolman has a track record in vectorial biochemistry, including metabolic energy conservation, membrane transport, and cell volume regulation as well as the development of innovative technologies in membrane biology and bottom-up synthetic biology. He has advanced the field of ATP-binding cassette and secondary active transporters by combining functional and structural studies. Central questions in the Poolman group are (i) What tasks should a living cell minimally perform and how this can be accomplished with a minimal set of components? (ii) How do molecules permeate biological membranes? (iii) How can one control the volume and physicochemistry of the cell?
The main current research areas include:
• Building of synthetic cells: construction of functional far-from-equilibrium systems for metabolic energy conservation and membrane expansion.
• Cellular homeostasis: elucidation of the (transport) mechanisms that control the physicochemistry and volume of the cell.
• Structure and dynamics of the cytoplasm: understanding of the heterogeneity and spatiotemporal segregation of (macro)molecules in the bacterial cytoplasm.
Poolman has been elected a member of the Royal Netherlands Academy of Arts and Sciences (2009). He has published over 340 publications in international scientific journals that received >33,000 citations and (co)supervised >70 PhD students and 30 post docs. 15 former members of the Poolman group now hold their own academic position (junior to senior professorship); other former group members have been appointed at senior R&D positions at SMEs or companies.
Silvia Zorrilla obtained her BSc and MSc (in Prof. Javier Sancho’s group) in Chemistry from University of Zaragoza (Spain). She conducted her PhD research in Dr. Pilar Lillo’s and Prof. Ulises Acuña’s laboratory at Instituto de Química Física-CSIC, Madrid, on protein interactions and diffusion in crowding conditions by time-resolved fluorescence, complemented with single-molecule methods through a stay in Prof. Antonie Visser’s group (University of Wageningen, The Netherlands). She was a Neuropharma-CSIC fellow in Drs. María Gasset’s and María de los Ángeles Pajares’ laboratories. She received a Marie Curie fellowship to work with Prof. Catherine Royer and Dr. Nathalie Declerck at the Centre de Biochimie Structurale (CNRS/INSERM/University of Montpellier), on the functional genomics of transcriptional regulation in bacteria by fluorescence fluctuation. She obtained a tenured scientist position at Instituto de Química Física-CSIC, starting her independent research on bacterial division by fluorescence, in collaboration with Prof. Rivas’ group. She is currently co-Leader of this group at the Centro de Investigaciones Biológicas Margarita Salas-CSIC, Madrid. Her research is focused in the reconstitution of bacterial division factors in crowded cell-like systems, to understand the process and its regulation and to contribute to the generation of artificial cells. She is interested in the identification of new targets to fight antimicrobials resistance, including biomolecular condensates.
Author Present Address
# Department of Crystallography and Structural Biology, Instituto de Química Física Blas Cabrera, Consejo Superior de Investigaciones Científicas (CSIC), 28006 Madrid, Spain
Author Contributions
B.M.: Conceptualization, Writing—original draft, Writing—review and editing, Visualization, Project administration; W.M.: Conceptualization, Writing—original draft, Writing—review and editing, Visualization, Funding acquisition; A.B.: Conceptualization, Writing—original draft, Writing—review and editing, Funding acquisition; G.R.: Conceptualization, Writing—review and editing, Visualization, Funding acquisition; B.P.: Conceptualization, Writing—original draft, Writing—review and editing, Visualization, Funding acquisition; S.Z.: Conceptualization, Writing—original draft, Writing—review and editing, Visualization, Project administration, Funding acquisition. All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
The authors declare no competing financial interest.
Special Issue
Published as part of Chemical Reviewsvirtual special issue “Molecular Crowding”.
References
- Dewachter L.; Verstraeten N.; Fauvart M.; Michiels J. An Integrative View of Cell Cycle Control in Escherichia Coli. FEMS Microbiol. Rev. 2018, 42 (2), 116–136. 10.1093/femsre/fuy005. [DOI] [PubMed] [Google Scholar]
- Luby-Phelps K. The Physical Chemistry of Cytoplasm and Its Influence on Cell Function: An Update. Mol. Biol. Cell 2013, 24 (17), 2593–2596. 10.1091/mbc.e12-08-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer J.; Poolman B. The Role of Biomacromolecular Crowding, Ionic Strength, and Physicochemical Gradients in the Complexities of Life’s Emergence. Microbiol. Mol. Biol. Rev. 2009, 73 (2), 371–388. 10.1128/MMBR.00010-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas G.; Minton A. P. Toward an Understanding of Biochemical Equilibria within Living Cells. Biophys. Rev. 2018, 10 (2), 241–253. 10.1007/s12551-017-0347-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas G.; Minton A. P. Influence of Nonspecific Interactions on Protein Associations: Implications for Biochemistry In Vivo. Annu. Rev. Biochem. 2022, 91 (1), 321–351. 10.1146/annurev-biochem-040320-104151. [DOI] [PubMed] [Google Scholar]
- Bonucci M.; Shu T.; Holt L. J. How It Feels in a Cell. Trends Cell Biol. 2023, 33, 924. 10.1016/j.tcb.2023.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman S. B.; Trach S. O. Estimation of Macromolecule Concentrations and Excluded Volume Effects for the Cytoplasm of Escherichia Coli. J. Mol. Biol. 1991, 222 (3), 599–620. 10.1016/0022-2836(91)90499-V. [DOI] [PubMed] [Google Scholar]
- Vendeville A.; Larivière D.; Fourmentin E. An Inventory of the Bacterial Macromolecular Components and Their Spatial Organization. FEMS Microbiol. Rev. 2011, 35 (2), 395–414. 10.1111/j.1574-6976.2010.00254.x. [DOI] [PubMed] [Google Scholar]
- Rivas G.; Minton A. P. Macromolecular Crowding In Vitro, In Vivo, and In Between. Trends Biochem. Sci. 2016, 41 (11), 970–981. 10.1016/j.tibs.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. J. Macromolecular Crowding: Obvious but Underappreciated. Trends Biochem. Sci. 2001, 26 (10), 597–604. 10.1016/S0968-0004(01)01938-7. [DOI] [PubMed] [Google Scholar]
- Rivas G.; Ferrone F.; Herzfeld J. Life in a Crowded World: Workshop on the Biological Implications of Macromolecular Crowding. EMBO Rep. 2004, 5 (1), 23–27. 10.1038/sj.embor.7400056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minton A. P.[7] Molecular Crowding: Analysis of Effects of High Concentrations of Inert Cosolutes on Biochemical Equilibria and Rates in Terms of Vol. Exclusion. In Methods in Enzymology; Elsevier, 1998; Vol. 295, pp 127–149. 10.1016/S0076-6879(98)95038-8. [DOI] [PubMed] [Google Scholar]
- Guin D.; Gruebele M. Weak Chemical Interactions That Drive Protein Evolution: Crowding, Sticking, and Quinary Structure in Folding and Function. Chem. Rev. 2019, 119 (18), 10691–10717. 10.1021/acs.chemrev.8b00753. [DOI] [PubMed] [Google Scholar]
- Van Den Berg J.; Boersma A. J.; Poolman B. Microorganisms Maintain Crowding Homeostasis. Nat. Rev. Microbiol. 2017, 15 (5), 309–318. 10.1038/nrmicro.2017.17. [DOI] [PubMed] [Google Scholar]
- Zhou H. X.; Rivas G.; Minton A. P. Macromolecular Crowding and Confinement: Biochemical, Biophysical, and Potential Physiological Consequences. Annu. Rev. Biophys 2008, 37, 375–397. 10.1146/annurev.biophys.37.032807.125817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. J.; Minton A. P.. Protein Aggregation in Crowded Environments. Biol. Chem. 2006, 387 ( (5), ), 10.1515/BC.2006.064. [DOI] [PubMed] [Google Scholar]
- Boersma A. J.; Zuhorn I. S.; Poolman B. A Sensor for Quantification of Macromolecular Crowding in Living Cells. Nat. Methods 2015, 12 (3), 227–229. 10.1038/nmeth.3257. [DOI] [PubMed] [Google Scholar]
- Liu B.; Poolman B.; Boersma A. J. Ionic Strength Sensing in Living Cells. ACS Chem. Biol. 2017, 12 (10), 2510–2514. 10.1021/acschembio.7b00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnutt D.; Gao M.; Brylski O.; Heyden M.; Ebbinghaus S. Excluded-Volume Effects in Living Cells. Angew. Chem., Int. Ed. 2015, 54 (8), 2548–2551. 10.1002/anie.201409847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minton A. P. The Influence of Macromolecular Crowding and Macromolecular Confinement on Biochemical Reactions in Physiological Media. J. Biol. Chem. 2001, 276 (14), 10577–10580. 10.1074/jbc.R100005200. [DOI] [PubMed] [Google Scholar]
- Drenckhahn D.; Pollard T. D. Elongation of Actin Filaments Is a Diffusion-Limited Reaction at the Barbed End and Is Accelerated by Inert Macromolecules. J. Biol. Chem. 1986, 261 (27), 12754–12758. 10.1016/S0021-9258(18)67157-1. [DOI] [PubMed] [Google Scholar]
- Lindner R. A.; Ralston G. B. Macromolecular Crowding: Effects on Actin Polymerisation. Biophys. Chem. 1997, 66 (1), 57–66. 10.1016/S0301-4622(97)00011-2. [DOI] [PubMed] [Google Scholar]
- González J. M.; Jiménez M.; Vélez M.; Mingorance J.; Andreu J. M.; Vicente M.; Rivas G. Essential Cell Division Protein FtsZ Assembles into One Monomer-Thick Ribbons under Conditions Resembling the Crowded Intracellular Environment. J. Biol. Chem. 2003, 278 (39), 37664–37671. 10.1074/jbc.M305230200. [DOI] [PubMed] [Google Scholar]
- Ferrone F. A. Polymerization and Sickle Cell Disease: A Molecular View. Microcirculation 2004, 11 (2), 115–128. 10.1080/10739680490278312. [DOI] [PubMed] [Google Scholar]
- Herzfeld J. Crowding-Induced Organization in Cells: Spontaneous Alignment and Sorting of Filaments with Physiological Control Points. J. Mol. Recognit. 2004, 17 (5), 376–381. 10.1002/jmr.703. [DOI] [PubMed] [Google Scholar]
- Braun M.; Lansky Z.; Hilitski F.; Dogic Z.; Diez S. Entropic Forces Drive Contraction of Cytoskeletal Networks. BioEssays 2016, 38 (5), 474–481. 10.1002/bies.201500183. [DOI] [PubMed] [Google Scholar]
- Ross P. D.; Briehl R. W.; Minton A. P. Temperature Dependence of Nonideality in Concentrated Solutions of Hemoglobin. Biopolymers 1978, 17 (9), 2285–2288. 10.1002/bip.1978.360170920. [DOI] [PubMed] [Google Scholar]
- Minton A. P. The Effect of Volume Occupancy upon the Thermodynamic Activity of Proteins: Some Biochemical Consequences. Mol. Cell. Biochem. 1983, 55 (2), 119–140. 10.1007/BF00673707. [DOI] [PubMed] [Google Scholar]
- Jiao M.; Li H. T.; Chen J.; Minton A. P.; Liang Y. Attractive Protein-Polymer Interactions Markedly Alter the Effect of Macromolecular Crowding on Protein Association Equilibria. Biophys. J. 2010, 99 (3), 914–923. 10.1016/j.bpj.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodeke A. A.; Minton A. P. Quantitative Characterization of Temperature-Independent and Temperature-Dependent Protein–Protein Interactions in Highly Nonideal Solutions. J. Phys. Chem. B 2011, 115 (38), 11261–11268. 10.1021/jp2049266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe T.; Minton A. P. Non-Specific Interactions Between Macromolecular Solutes in Concentrated Solution: Physico-Chemical Manifestations and Biochemical Consequences. Front. Mol. Biosci. 2019, 6, 10. 10.3389/fmolb.2019.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speer S. L.; Stewart C. J.; Sapir L.; Harries D.; Pielak G. J. Macromolecular Crowding Is More than Hard-Core Repulsions. Annu. Rev. Biophys. 2022, 51 (1), 267–300. 10.1146/annurev-biophys-091321-071829. [DOI] [PubMed] [Google Scholar]
- Zimmerman S. B.; Minton A. P. Macromolecular Crowding: Biochemical, Biophysical, and Physiological Consequences. Annu. Rev. Biophys. Biomol. Struct. 1993, 22 (1), 27–65. 10.1146/annurev.bb.22.060193.000331. [DOI] [PubMed] [Google Scholar]
- Shin Y.; Brangwynne C. P.. Liquid Phase Condensation in Cell Physiology and Disease. Science 2017, 357 ( (6357), ), eaaf4382. 10.1126/science.aaf4382. [DOI] [PubMed] [Google Scholar]
- Banani S. F.; Lee H. O.; Hyman A. A.; Rosen M. K. Biomolecular Condensates: Organizers of Cellular Biochemistry. Nat. Rev. Mol. Cell Biol. 2017, 18 (5), 285–298. 10.1038/nrm.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberti S.; Gladfelter A.; Mittag T. Considerations and Challenges in Studying Liquid-Liquid Phase Separation and Biomolecular Condensates. Cell 2019, 176 (3), 419–434. 10.1016/j.cell.2018.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azaldegui C. A.; Vecchiarelli A. G.; Biteen J. S. The Emergence of Phase Separation as an Organizing Principle in Bacteria. Biophys. J. 2021, 120, 1123. 10.1016/j.bpj.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappu R. V.; Cohen S. R.; Dar F.; Farag M.; Kar M. Phase Transitions of Associative Biomacromolecules. Chem. Rev. 2023, 123 (14), 8945–8987. 10.1021/acs.chemrev.2c00814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditlev J. A.; Case L. B.; Rosen M. K. Who’s In and Who’s Out—Compositional Control of Biomolecular Condensates. J. Mol. Biol. 2018, 430 (23), 4666–4684. 10.1016/j.jmb.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Z.-G.; Huang S.-C.; Xia X.-X. Synthetic Protein Condensates for Cellular and Metabolic Engineering. Nat. Chem. Biol. 2022, 18 (12), 1330–1340. 10.1038/s41589-022-01203-3. [DOI] [PubMed] [Google Scholar]
- Dignon G. L.; Best R. B.; Mittal J. Biomolecular Phase Separation: From Molecular Driving Forces to Macroscopic Properties. Annu. Rev. Phys. Chem. 2020, 71 (1), 53–75. 10.1146/annurev-physchem-071819-113553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberti S. Phase Separation in Biology. Curr. Biol. 2017, 27 (20), R1097–R1102. 10.1016/j.cub.2017.08.069. [DOI] [PubMed] [Google Scholar]
- Minton A. P. Simple Calculation of Phase Diagrams for Liquid–Liquid Phase Separation in Solutions of Two Macromolecular Solute Species. J. Phys. Chem. B 2020, 124 (12), 2363–2370. 10.1021/acs.jpcb.0c00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- André A. A. M.; Spruijt E. Liquid–Liquid Phase Separation in Crowded Environments. Int. J. Mol. Sci. 2020, 21 (16), 5908. 10.3390/ijms21165908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin N. Dynamic Synthetic Cells Based on Liquid–Liquid Phase Separation. ChemBioChem. 2019, 20 (20), 2553–2568. 10.1002/cbic.201900183. [DOI] [PubMed] [Google Scholar]
- Alberti S.; Hyman A. A. Biomolecular Condensates at the Nexus of Cellular Stress, Protein Aggregation Disease and Ageing. Nat. Rev. Mol. Cell Biol. 2021, 22 (3), 196–213. 10.1038/s41580-020-00326-6. [DOI] [PubMed] [Google Scholar]
- Musacchio A. On the Role of Phase Separation in the Biogenesis of Membraneless Compartments. EMBO J. 2022, 41 (5), e109952 10.15252/embj.2021109952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruijt E. Open Questions on Liquid–Liquid Phase Separation. Commun. Chem. 2023, 6 (1), 23. 10.1038/s42004-023-00823-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löwe M.; Kalacheva M.; Boersma A. J.; Kedrov A. The More the Merrier: Effects of Macromolecular Crowding on the Structure and Dynamics of Biological Membranes. FEBS J. 2020, 287 (23), 5039–5067. 10.1111/febs.15429. [DOI] [PubMed] [Google Scholar]
- Leonard T. A.; Loose M.; Martens S. The Membrane Surface as a Platform That Organizes Cellular and Biochemical Processes. Dev. Cell 2023, 58 (15), 1315–1332. 10.1016/j.devcel.2023.06.001. [DOI] [PubMed] [Google Scholar]
- Snead W. T.; Gladfelter A. S. The Control Centers of Biomolecular Phase Separation: How Membrane Surfaces, PTMs, and Active Processes Regulate Condensation. Mol. Cell 2019, 76 (2), 295–305. 10.1016/j.molcel.2019.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison T. J. Beyond Langmuir: Surface-Bound Macromolecule Condensates. Mol. Biol. Cell 2020, 31 (23), 2502–2508. 10.1091/mbc.E20-06-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditlev J. A. Membrane-Associated Phase Separation: Organization and Function Emerge from a Two-Dimensional Milieu. J. Mol. Cell Biol. 2021, 13 (4), 319–324. 10.1093/jmcb/mjab010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivi L.; Berger M.; Creyghton R. N. P.; De Franceschi N.; Dekker C.; Mulder B. M.; Claassens N. J.; Ten Wolde P. R.; Van Der Oost J. Towards a Synthetic Cell Cycle. Nat. Commun. 2021, 12 (1), 4531. 10.1038/s41467-021-24772-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwille P.; Spatz J.; Landfester K.; Bodenschatz E.; Herminghaus S.; Sourjik V.; Erb T. J.; Bastiaens P.; Lipowsky R.; Hyman A.; et al. MaxSynBio: Avenues Towards Creating Cells from the Bottom Up. Angew. Chem., Int. Ed. 2018, 57 (41), 13382–13392. 10.1002/anie.201802288. [DOI] [PubMed] [Google Scholar]
- Poolman B. Physicochemical Homeostasis in Bacteria. FEMS Microbiol. Rev. 2023, 47 (4), fuad033 10.1093/femsre/fuad033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth I. R. Bacterial Mechanosensitive Channels: Progress towards an Understanding of Their Roles in Cell Physiology. Curr. Opin. Microbiol. 2014, 18, 16–22. 10.1016/j.mib.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer E.; Krämer R. Responses of Microorganisms to Osmotic Stress. Annu. Rev. Microbiol. 2019, 73 (1), 313–334. 10.1146/annurev-micro-020518-115504. [DOI] [PubMed] [Google Scholar]
- Cox C. D.; Bavi N.; Martinac B. Bacterial Mechanosensors. Annu. Rev. Physiol. 2018, 80 (1), 71–93. 10.1146/annurev-physiol-021317-121351. [DOI] [PubMed] [Google Scholar]
- Drew D.; Boudker O. Shared Molecular Mechanisms of Membrane Transporters. Annu. Rev. Biochem. 2016, 85 (1), 543–572. 10.1146/annurev-biochem-060815-014520. [DOI] [PubMed] [Google Scholar]
- Poolman B.; Spitzer J. J.; Wood J. M. Bacterial Osmosensing: Roles of Membrane Structure and Electrostatics in Lipid–Protein and Protein–Protein Interactions.. Biochim. Biophys. Acta BBA - Biomembr. 2004, 1666 (1–2), 88–104. 10.1016/j.bbamem.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Wood J. M. Bacterial Osmoregulation: A Paradigm for the Study of Cellular Homeostasis. Annu. Rev. Microbiol. 2011, 65 (1), 215–238. 10.1146/annurev-micro-090110-102815. [DOI] [PubMed] [Google Scholar]
- Milo R.; Phillips R.. Cell Biology by the Numbers, 0 ed.; Garland Science, 2015. 10.1201/9780429258770. [DOI] [Google Scholar]
- Cayley S.; Record M. T. Large Changes in Cytoplasmic Biopolymer Concentration with Osmolality Indicate That Macromolecular Crowding May Regulate Protein–DNA Interactions and Growth Rate in Osmotically stressedEscherichia Coli K-12. J. Mol. Recognit. 2004, 17 (5), 488–496. 10.1002/jmr.695. [DOI] [PubMed] [Google Scholar]
- Konopka M. C.; Sochacki K. A.; Bratton B. P.; Shkel I. A.; Record M. T.; Weisshaar J. C. Cytoplasmic Protein Mobility in Osmotically Stressed Escherichia Coli. J. Bacteriol. 2009, 191 (1), 231–237. 10.1128/JB.00536-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B.; Hasrat Z.; Poolman B.; Boersma A. J.. Decreased Effective Macromolecular Crowding in Escherichia Coli Adapted to Hyperosmotic Stress. J. Bacteriol. 2019, 201 ( (10), ), 10.1128/JB.00708-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mika J. T.; Van Den Bogaart G.; Veenhoff L.; Krasnikov V.; Poolman B. Molecular Sieving Properties of the Cytoplasm of Escherichia Coli and Consequences of Osmotic Stress: Molecule Diffusion and Barriers in the Cytoplasm. Mol. Microbiol. 2010, 77 (1), 200–207. 10.1111/j.1365-2958.2010.07201.x. [DOI] [PubMed] [Google Scholar]
- Schavemaker P. E.; Boersma A. J.; Poolman B.. How Important Is Protein Diffusion in Prokaryotes? Front. Mol. Biosci. 2018, 5, 10.3389/fmolb.2018.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang T. Y.; Lercher M. J. Optimal Density of Bacterial Cells. PLOS Comput. Biol. 2023, 19 (6), e1011177 10.1371/journal.pcbi.1011177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikkema H. R.; Gaastra B. F.; Pols T.; Poolman B. Cell Fuelling and Metabolic Energy Conservation in Synthetic Cells. ChemBioChem. 2019, 20 (20), 2581–2592. 10.1002/cbic.201900398. [DOI] [PubMed] [Google Scholar]
- Krulwich T. A.; Sachs G.; Padan E. Molecular Aspects of Bacterial pH Sensing and Homeostasis. Nat. Rev. Microbiol. 2011, 9 (5), 330–343. 10.1038/nrmicro2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levering J.; Musters M. W. J. M.; Bekker M.; Bellomo D.; Fiedler T.; De Vos W. M.; Hugenholtz J.; Kreikemeyer B.; Kummer U.; Teusink B. Role of Phosphate in the Central Metabolism of Two Lactic Acid Bacteria - a Comparative Systems Biology Approach: Modelling Glycolysis of Lactic Acid Bacteria. FEBS J. 2012, 279 (7), 1274–1290. 10.1111/j.1742-4658.2012.08523.x. [DOI] [PubMed] [Google Scholar]
- Booth I. R. Regulation of Cytoplasmic pH in Bacteria. Microbiol. Rev. 1985, 49 (4), 359–378. 10.1128/mr.49.4.359-378.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krulwich T. A.; Agus R.; Schneier M.; Guffanti A. A. Buffering Capacity of Bacilli That Grow at Different pH Ranges. J. Bacteriol. 1985, 162 (2), 768–772. 10.1128/jb.162.2.768-772.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexeeva S.; Hellingwerf K. J.; Teixeira De Mattos M. J. Quantitative Assessment of Oxygen Availability: Perceived Aerobiosis and Its Effect on Flux Distribution in the Respiratory Chain of Escherichia Coli. J. Bacteriol. 2002, 184 (5), 1402–1406. 10.1128/JB.184.5.1402-1406.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy J.; Ekkerman S.; Pliotas C.; Richard M.; Bartlett W.; Grayer S. C.; Morris G. M.; Miller S.; Booth I. R.; Conway S. J.; Rasmussen T. Understanding the Structural Requirements for Activators of the Kef Bacterial Potassium Efflux System. Biochemistry 2014, 53 (12), 1982–1992. 10.1021/bi5001118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliotas C.; Grayer S. C.; Ekkerman S.; Chan A. K. N.; Healy J.; Marius P.; Bartlett W.; Khan A.; Cortopassi W. A.; Chandler S. A.; et al. Adenosine Monophosphate Binding Stabilizes the KTN Domain of the Shewanella Denitrificans Kef Potassium Efflux System. Biochemistry 2017, 56 (32), 4219–4234. 10.1021/acs.biochem.7b00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taglicht D.; Padan E.; Schuldiner S. Overproduction and Purification of a Functional Na+/H+ Antiporter Coded by nhaA (Ant) from Escherichia Coli. J. Biol. Chem. 1991, 266 (17), 11289–11294. 10.1016/S0021-9258(18)99161-1. [DOI] [PubMed] [Google Scholar]
- Winkelmann I.; Uzdavinys P.; Kenney I. M.; Brock J.; Meier P. F.; Wagner L.-M.; Gabriel F.; Jung S.; Matsuoka R.; Von Ballmoos C.; et al. Crystal Structure of the Na+/H+ Antiporter NhaA at Active pH Reveals the Mechanistic Basis for pH Sensing. Nat. Commun. 2022, 13 (1), 6383. 10.1038/s41467-022-34120-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavan T. S.; Cheng R. C.; Jiang T.; Mathews I. I.; Stein R. A.; Koehl A.; Mchaourab H. S.; Tajkhorshid E.; Maduke M.. A CLC-Ec1Mutant Reveals Global Conformational Change and Suggests a Unifying Mechanism for the CLC Cl–/H+ Transport Cycle. eLife 2020, 9, e53479 10.7554/eLife.53479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anantharam V.; Allison M. J.; Maloney P. C. Oxalate:Formate Exchange. J. Biol. Chem. 1989, 264 (13), 7244–7250. 10.1016/S0021-9258(18)83227-6. [DOI] [PubMed] [Google Scholar]
- Poolman B.; Molenaar D.; Smid E. J.; Ubbink T.; Abee T.; Renault P. P.; Konings W. N. Malolactic Fermentation: Electrogenic Malate Uptake and Malate/Lactate Antiport Generate Metabolic Energy. J. Bacteriol. 1991, 173 (19), 6030–6037. 10.1128/jb.173.19.6030-6037.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano A.; Trip H.; Lolkema J. S.; Lucas P. M. Three-Component Lysine/Ornithine Decarboxylation System in Lactobacillus Saerimneri 30a. J. Bacteriol. 2013, 195 (6), 1249–1254. 10.1128/JB.02070-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salema M.; Poolman B.; Lolkema J. S.; Dias M. C. L.; Konings W. N. Uniport of Monoanionic L-Malate in Membrane Vesicles from Leuconostoc Oenos. Eur. J. Biochem. 1994, 225 (1), 289–295. 10.1111/j.1432-1033.1994.00289.x. [DOI] [PubMed] [Google Scholar]
- Sa H. D.; Park J. Y.; Jeong S.-J.; Lee K. W.; Kim J. H. Characterization of Glutamate Decarboxylase (GAD) from Lactobacillus Sakei A156 Isolated from Jeot-Gal. J. Microbiol. Biotechnol. 2015, 25 (5), 696–703. 10.4014/jmb.1412.12075. [DOI] [PubMed] [Google Scholar]
- Gale E. F. Estimation of l(+)-Arginine in Protein Hydrolysates by the Use of l(+)-Arginine Decarboxylase. Nature 1946, 157 (3983), 265–265. 10.1038/157265a0. [DOI] [PubMed] [Google Scholar]
- Suzuki T.; Kobayashi H. Regulation of the Cytoplasmic pH by a Proton-Translocating ATPase in Streptococcus Faecalis (Faecium). A Computer Simulation. Eur. J. Biochem. 1989, 180 (2), 467–471. 10.1111/j.1432-1033.1989.tb14669.x. [DOI] [PubMed] [Google Scholar]
- Schavemaker P. E.; Śmigiel W. M.; Poolman B. Ribosome Surface Properties May Impose Limits on the Nature of the Cytoplasmic Proteome. eLife 2017, 6, e30084 10.7554/eLife.30084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König I.; Zarrine-Afsar A.; Aznauryan M.; Soranno A.; Wunderlich B.; Dingfelder F.; Stüber J. C.; Plückthun A.; Nettels D.; Schuler B. Single-Molecule Spectroscopy of Protein Conformational Dynamics in Live Eukaryotic Cells. Nat. Methods 2015, 12 (8), 773–779. 10.1038/nmeth.3475. [DOI] [PubMed] [Google Scholar]
- Nørby J. G.; Esmann M. The Effect of Ionic Strength and Specific Anions on Substrate Binding and Hydrolytic Activities of Na,K-ATPase. J. Gen. Physiol. 1997, 109 (5), 555–570. 10.1085/jgp.109.5.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syeda R.; Qiu Z.; Dubin A. E.; Murthy S. E.; Florendo M. N.; Mason D. E.; Mathur J.; Cahalan S. M.; Peters E. C.; Montal M.; Patapoutian A. LRRC8 Proteins Form Volume-Regulated Anion Channels That Sense Ionic Strength. Cell 2016, 164 (3), 499–511. 10.1016/j.cell.2015.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biemans-Oldehinkel E.; Mahmood N. A. B. N.; Poolman B. A Sensor for Intracellular Ionic Strength. Proc. Natl. Acad. Sci. U. S. A. 2006, 103 (28), 10624–10629. 10.1073/pnas.0603871103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek P. J.; Patsalo V.; Green D. F.; Raleigh D. P. Ionic Strength Effects on Amyloid Formation by Amylin Are a Complicated Interplay among Debye Screening, Ion Selectivity, and Hofmeister Effects. Biochemistry 2012, 51 (43), 8478–8490. 10.1021/bi300574r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbaum-Garfinkle S.; Kim Y.; Szczepaniak K.; Chen C. C.-H.; Eckmann C. R.; Myong S.; Brangwynne C. P. The Disordered P Granule Protein LAF-1 Drives Phase Separation into Droplets with Tunable Viscosity and Dynamics. Proc. Natl. Acad. Sci. U. S. A. 2015, 112 (23), 7189–7194. 10.1073/pnas.1504822112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Record M. T.; Anderson C. F.; Lohman T. M. Thermodynamic Analysis of Ion Effects on the Binding and Conformational Equilibria of Proteins and Nucleic Acids: The Roles of Ion Association or Release, Screening, and Ion Effects on Water Activity. Q. Rev. Biophys. 1978, 11 (2), 103–178. 10.1017/S003358350000202X. [DOI] [PubMed] [Google Scholar]
- Booth I. R.; Higgins C. F. Enteric Bacteria and Osmotic Stress: Intracellular Potassium Glutamate as a Secondary Signal of Osmotic Stress?. FEMS Microbiol. Lett. 1990, 75 (2–3), 239–246. 10.1111/j.1574-6968.1990.tb04097.x. [DOI] [PubMed] [Google Scholar]
- Dinnbier U.; Limpinsel E.; Schmid R.; Bakker E. P. Transient Accumulation of Potassium Glutamate and Its Replacement by Trehalose during Adaptation of Growing Cells of Escherichia Coli K-12 to Elevated Sodium Chloride Concentrations. Arch. Microbiol. 1988, 150 (4), 348–357. 10.1007/BF00408306. [DOI] [PubMed] [Google Scholar]
- Buda R.; Liu Y.; Yang J.; Hegde S.; Stevenson K.; Bai F.; Pilizota T.. Dynamics of Escherichia Coli ’s Passive Response to a Sudden Decrease in External Osmolarity. Proc. Natl. Acad. Sci. U. S. A. 2016, 113 ( (40), ), E5838–E5846 10.1073/pnas.1522185113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culham D. E.; Shkel I. A.; Record M. T.; Wood J. M. Contributions of Coulombic and Hofmeister Effects to the Osmotic Activation of Escherichia Coli Transporter ProP. Biochemistry 2016, 55 (9), 1301–1313. 10.1021/acs.biochem.5b01169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler C.; Bremer E.; Krämer R. The BCCT Family of Carriers: From Physiology to Crystal Structure: BCCT Carriers. Mol. Microbiol. 2010, 78 (1), 13–34. 10.1111/j.1365-2958.2010.07332.x. [DOI] [PubMed] [Google Scholar]
- Spitzer J. J.; Poolman B. Electrochemical Structure of the Crowded Cytoplasm. Trends Biochem. Sci. 2005, 30 (10), 536–541. 10.1016/j.tibs.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Sundararaman R.; Vigil-Fowler D.; Schwarz K. Improving the Accuracy of Atomistic Simulations of the Electrochemical Interface. Chem. Rev. 2022, 122 (12), 10651–10674. 10.1021/acs.chemrev.1c00800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikkema H. R.; Van Den Noort M.; Rheinberger J.; De Boer M.; Krepel S. T.; Schuurman-Wolters G. K.; Paulino C.; Poolman B. Gating by Ionic Strength and Safety Check by Cyclic-Di-AMP in the ABC Transporter OpuA. Sci. Adv. 2020, 6 (47), eabd7697 10.1126/sciadv.abd7697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Heredia A. Plasma Membrane-Cell Wall Feedback in Bacteria. J. Bacteriol. 2023, 205 (3), e00433–22. 10.1128/jb.00433-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran B. M.; Prabha H.; Iyer A.; O’Byrne C.; Abee T.; Poolman B. Measurement of Protein Mobility in Listeria Monocytogenes Reveals a Unique Tolerance to Osmotic Stress and Temperature Dependence of Diffusion. Front. Microbiol. 2021, 12, 640149 10.3389/fmicb.2021.640149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whatmore A. M.; Reed R. H. Determination of Turgor Pressure in Bacillus Subtilis: A Possible Role for K+ in Turgor Regulation. J. Gen. Microbiol. 1990, 136 (12), 2521–2526. 10.1099/00221287-136-12-2521. [DOI] [PubMed] [Google Scholar]
- Oldewurtel E. R.; Kitahara Y.; van Teeffelen S. Robust Surface-to-Mass Coupling and Turgor-Dependent Cell Width Determine Bacterial Dry-Mass Density. Proc. Natl. Acad. Sci. U. S. A. 2021, 118 (32), e2021416118. 10.1073/pnas.2021416118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott Cayley D.; Guttman H. J.; Thomas Record M. Biophysical Characterization of Changes in Amounts and Activity of Escherichia Coli Cell and Compartment Water and Turgor Pressure in Response to Osmotic Stress. Biophys. J. 2000, 78 (4), 1748–1764. 10.1016/S0006-3495(00)76726-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A. L. Shrinkage of Growing Escherichia Coli Cells by Osmotic Challenge. J. Bacteriol. 1984, 159 (3), 919–924. 10.1128/jb.159.3.919-924.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquina-Lemonche L.; Burns J.; Turner R. D.; Kumar S.; Tank R.; Mullin N.; Wilson J. S.; Chakrabarti B.; Bullough P. A.; Foster S. J.; Hobbs J. K. The Architecture of the Gram-Positive Bacterial Cell Wall. Nature 2020, 582 (7811), 294–297. 10.1038/s41586-020-2236-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J. M.; Bremer E.; Csonka L. N.; Kraemer R.; Poolman B.; Van Der Heide T.; Smith L. T. Osmosensing and Osmoregulatory Compatible Solute Accumulation by Bacteria. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2001, 130 (3), 437–460. 10.1016/S1095-6433(01)00442-1. [DOI] [PubMed] [Google Scholar]
- Yancey P. H.; Clark M. E.; Hand S. C.; Bowlus R. D.; Somero G. N. Living with Water Stress: Evolution of Osmolyte Systems. Science 1982, 217 (4566), 1214–1222. 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]
- Wargo M. J.; Meadows J. A. Carnitine in Bacterial Physiology and Metabolism. Microbiology 2015, 161 (6), 1161–1174. 10.1099/mic.0.000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodsell D. S.Escherichia Coli Bacterium. RCSB Protein Data Bank 2021. 10.2210/rcsb_pdb/goodsell-gallery-028. [DOI] [Google Scholar]
- Van Der Heide T. On the Osmotic Signal and Osmosensing Mechanism of an ABC Transport System for Glycine Betaine. EMBO J. 2001, 20 (24), 7022–7032. 10.1093/emboj/20.24.7022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasawa A.; Swier L. J. Y. M.; Stuart M. C. A.; Brouwers J.; Helms B.; Poolman B. Physicochemical Factors Controlling the Activity and Energy Coupling of an Ionic Strength-Gated ATP-Binding Cassette (ABC) Transporter. J. Biol. Chem. 2013, 288 (41), 29862–29871. 10.1074/jbc.M113.499327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuss M. F.; Wieferig J.-P.; Corey R. A.; Hellmich Y.; Tascón I.; Sousa J. S.; Stansfeld P. J.; Vonck J.; Hänelt I. Cyclic Di-AMP Traps Proton-Coupled K+ Transporters of the KUP Family in an Inward-Occluded Conformation. Nat. Commun. 2023, 14 (1), 3683. 10.1038/s41467-023-38944-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibhardt J.; Hoffmann G.; Turdiev A.; Wang M.; Lee V. T.; Commichau F. M. C-Di-AMP Assists Osmoadaptation by Regulating the Listeria Monocytogenes Potassium Transporters KimA and KtrCD. J. Biol. Chem. 2019, 294 (44), 16020–16033. 10.1074/jbc.RA119.010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundlach J.; Krüger L.; Herzberg C.; Turdiev A.; Poehlein A.; Tascón I.; Weiss M.; Hertel D.; Daniel R.; Hänelt I.; Lee V. T.; Stülke J. Sustained Sensing in Potassium Homeostasis: Cyclic Di-AMP Controls Potassium Uptake by KimA at the Levels of Expression and Activity. J. Biol. Chem. 2019, 294 (24), 9605–9614. 10.1074/jbc.RA119.008774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stülke J.; Krüger L. Cyclic Di-AMP Signaling in Bacteria. Annu. Rev. Microbiol. 2020, 74 (1), 159–179. 10.1146/annurev-micro-020518-115943. [DOI] [PubMed] [Google Scholar]
- Handbook of Electroporation; Miklavčič D., Ed.; Springer International Publishing: Cham, 2017. 10.1007/978-3-319-32886-7. [DOI] [Google Scholar]
- Edwards M. D.; Black S.; Rasmussen T.; Rasmussen A.; Stokes N. R.; Stephen T.-L.; Miller S.; Booth I. R. Characterization of Three Novel Mechanosensitive Channel Activities in Escherichia Coli. Channels 2012, 6 (4), 272–281. 10.4161/chan.20998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukharev S. I.; Sigurdson W. J.; Kung C.; Sachs F. Energetic and Spatial Parameters for Gating of the Bacterial Large Conductance Mechanosensitive Channel. MscL. J. Gen. Physiol. 1999, 113 (4), 525–540. 10.1085/jgp.113.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M. D.; Li Y.; Kim S.; Miller S.; Bartlett W.; Black S.; Dennison S.; Iscla I.; Blount P.; Bowie J. U.; Booth I. R. Pivotal Role of the Glycine-Rich TM3 Helix in Gating the MscS Mechanosensitive Channel. Nat. Struct. Mol. Biol. 2005, 12 (2), 113–119. 10.1038/nsmb895. [DOI] [PubMed] [Google Scholar]
- Rasmussen T.; Rasmussen A.; Singh S.; Galbiati H.; Edwards M. D.; Miller S.; Booth I. R. Properties of the Mechanosensitive Channel MscS Pore Revealed by Tryptophan Scanning Mutagenesis. Biochemistry 2015, 54 (29), 4519–4530. 10.1021/acs.biochem.5b00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boer M.; Anishkin A.; Sukharev S. Adaptive MscS Gating in the Osmotic Permeability Response in E. Coli: The Question of Time. Biochemistry 2011, 50 (19), 4087–4096. 10.1021/bi1019435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moe P.; Blount P. Assessment of Potential Stimuli for Mechano-Dependent Gating of MscL: Effects of Pressure, Tension, and Lipid Headgroups. Biochemistry 2005, 44 (36), 12239–12244. 10.1021/bi0509649. [DOI] [PubMed] [Google Scholar]
- O’Connell J. D.; Zhao A.; Ellington A. D.; Marcotte E. M. Dynamic Reorganization of Metabolic Enzymes into Intracellular Bodies. Annu. Rev. Cell Dev. Biol. 2012, 28 (1), 89–111. 10.1146/annurev-cellbio-101011-155841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovska I.; Nüske E.; Munder M. C.; Kulasegaran G.; Malinovska L.; Kroschwald S.; Richter D.; Fahmy K.; Gibson K.; Verbavatz J.-M.; Alberti S. Filament Formation by Metabolic Enzymes Is a Specific Adaptation to an Advanced State of Cellular Starvation. eLife 2014, 3, e02409 10.7554/eLife.02409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant C. R.; Wan J.; Komeili A. Organelle Formation in Bacteria and Archaea. Annu. Rev. Cell Dev. Biol. 2018, 34 (1), 217–238. 10.1146/annurev-cellbio-100616-060908. [DOI] [PubMed] [Google Scholar]
- Giessen T. W. Encapsulins. Annu. Rev. Biochem. 2022, 91 (1), 353–380. 10.1146/annurev-biochem-040320-102858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greening C.; Lithgow T. Formation and Function of Bacterial Organelles. Nat. Rev. Microbiol. 2020, 18 (12), 677–689. 10.1038/s41579-020-0413-0. [DOI] [PubMed] [Google Scholar]
- Chai Q.; Singh B.; Peisker K.; Metzendorf N.; Ge X.; Dasgupta S.; Sanyal S. Organization of Ribosomes and Nucleoids in Escherichia Coli Cells during Growth and in Quiescence. J. Biol. Chem. 2014, 289 (16), 11342–11352. 10.1074/jbc.M114.557348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F.; Swain P.; Kuijpers L.; Zheng X.; Felter K.; Guurink M.; Solari J.; Jun S.; Shimizu T. S.; Chaudhuri D.; Mulder B.; Dekker C. Cell Boundary Confinement Sets the Size and Position of the E. Coli Chromosome. Curr. Biol. 2019, 29 (13), 2131–2144.e4. 10.1016/j.cub.2019.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman S. B.; Murphy L. D. Macromolecular Crowding and the Mandatory Condensation of DNA in Bacteria. FEBS Lett. 1996, 390 (3), 245–248. 10.1016/0014-5793(96)00725-9. [DOI] [PubMed] [Google Scholar]
- Bakshi S.; Choi H.; Weisshaar J. C.. The Spatial Biology of Transcription and Translation in Rapidly Growing Escherichia Coli. Front. Microbiol. 2015, 6, 10.3389/fmicb.2015.00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakshi S.; Siryaporn A.; Goulian M.; Weisshaar J. C. Superresolution Imaging of Ribosomes and RNA Polymerase in Live Escherichia Coli Cells. Mol. Microbiol. 2012, 85 (1), 21–38. 10.1111/j.1365-2958.2012.08081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler J.; Seybert A.; König L.; Pruggnaller S.; Haselmann U.; Sourjik V.; Weiss M.; Frangakis A. S.; Mogk A.; Bukau B. Quantitative and Spatio-Temporal Features of Protein Aggregation in Escherichia Coli and Consequences on Protein Quality Control and Cellular Ageing. EMBO J. 2010, 29 (5), 910–923. 10.1038/emboj.2009.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coquel A.-S.; Jacob J.-P.; Primet M.; Demarez A.; Dimiccoli M.; Julou T.; Moisan L.; Lindner A. B.; Berry H. Localization of Protein Aggregation in Escherichia Coli Is Governed by Diffusion and Nucleoid Macromolecular Crowding Effect. PLOS Comput. Biol. 2013, 9 (4), e1003038 10.1371/journal.pcbi.1003038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm F. D.; Schroeder K.; Jonas K. Protein Aggregation in Bacteria. FEMS Microbiol. Rev. 2020, 44 (1), 54–72. 10.1093/femsre/fuz026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Śmigiel W. M.; Mantovanelli L.; Linnik D. S.; Punter M.; Silberberg J.; Xiang L.; Xu K.; Poolman B. Protein Diffusion in Escherichia Coli Cytoplasm Scales with the Mass of the Complexes and Is Location Dependent. Sci. Adv. 2022, 8 (32), eabo5387 10.1126/sciadv.abo5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang L.; Chen K.; Yan R.; Li W.; Xu K. Single-Molecule Displacement Mapping Unveils Nanoscale Heterogeneities in Intracellular Diffusivity. Nat. Methods 2020, 17 (5), 524–530. 10.1038/s41592-020-0793-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovanelli L.; Linnik D. S.; Punter M.; Kojakhmetov H. J.; Śmigiel W. M.; Poolman B. Simulation-Based Reconstructed Diffusion Unveils the Effect of Aging on Protein Diffusion in Escherichia Coli. PLOS Comput. Biol. 2023, 19 (9), e1011093 10.1371/journal.pcbi.1011093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dendooven T.; Paris G.; Shkumatov A. V.; Islam Md. S.; Burt A.; Kubańska M. A.; Yang T. Y.; Hardwick S. W.; Luisi B. F. Multi-scale Ensemble Properties of the Escherichia Coli RNA Degradosome. Mol. Microbiol. 2022, 117 (1), 102–120. 10.1111/mmi.14800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladouceur A. M.; Parmar B. S.; Biedzinski S.; Wall J.; Tope S. G.; Cohn D.; Kim A.; Soubry N.; Reyes-Lamothe R.; Weber S. C. Clusters of Bacterial RNA Polymerase Are Biomolecular Condensates That Assemble through Liquid-Liquid Phase Separation. Proc. Natl. Acad. Sci. U A 2020, 117 (31), 18540–18549. 10.1073/pnas.2005019117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasker K.; Boeynaems S.; Lam V.; Scholl D.; Stainton E.; Briner A.; Jacquemyn M.; Daelemans D.; Deniz A.; Villa E.; Holehouse A. S.; Gitler A. D.; Shapiro L. The Material Properties of a Bacterial-Derived Biomolecular Condensate Tune Biological Function in Natural and Synthetic Systems. Nat. Commun. 2022, 13 (1), 5643. 10.1038/s41467-022-33221-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babl L.; Merino-Salomón A.; Kanwa N.; Schwille P. Membrane Mediated Phase Separation of the Bacterial Nucleoid Occlusion Protein Noc. Sci. Rep. 2022, 12 (1), 17949. 10.1038/s41598-022-22680-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilhas B.; Walter J.-C.; Rech J.; David G.; Walliser N. O.; Palmeri J.; Mathieu-Demaziere C.; Parmeggiani A.; Bouet J.-Y.; Le Gall A.; Nollmann M. ATP-Driven Separation of Liquid Phase Condensates in Bacteria. Mol. Cell 2020, 79 (2), 293–303.e4. 10.1016/j.molcel.2020.06.034. [DOI] [PubMed] [Google Scholar]
- Jin X.; Lee J.-E.; Schaefer C.; Luo X.; Wollman A. J. M.; Payne-Dwyer A. L.; Tian T.; Zhang X.; Chen X.; Li Y.; McLeish T. C. B.; Leake M. C.; Bai F. Membraneless Organelles Formed by Liquid-Liquid Phase Separation Increase Bacterial Fitness. Sci. Adv. 2021, 7 (43), eabh2929 10.1126/sciadv.abh2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saurabh S.; Chong T. N.; Bayas C.; Dahlberg P. D.; Cartwright H. N.; Moerner W. E.; Shapiro L. ATP-Responsive Biomolecular Condensates Tune Bacterial Kinase Signaling. Sci. Adv. 2022, 8 (7), eabm6570 10.1126/sciadv.abm6570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins M. J.; Tomares D. T.; Nandana V.; Schrader J. M.; Childers W. S. RNase E Biomolecular Condensates Stimulate PNPase Activity. Sci. Rep. 2023, 13 (1), 12937. 10.1038/s41598-023-39565-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris V.; Blaauwen T. D.; Doi R. H.; Harshey R. M.; Janniere L.; Jiménez-Sánchez A.; Jin D. J.; Levin P. A.; Mileykovskaya E.; Minsky A.; et al. Toward a Hyperstructure Taxonomy. Annu. Rev. Microbiol. 2007, 61 (1), 309–329. 10.1146/annurev.micro.61.081606.103348. [DOI] [PubMed] [Google Scholar]
- Srere P. A. COMPLEXES OF SEQUENTIAL METABOLIC ENZYMES. Annu. Rev. Biochem. 1987, 56 (1), 89–124. 10.1146/annurev.bi.56.070187.000513. [DOI] [PubMed] [Google Scholar]
- Hoang Y.; Azaldegui C. A.; Ghalmi M.; Biteen J. S.; Vecchiarelli A. G.. An Experimental Framework to Assess Biomolecular Condensates in Bacteria. bioRxiv 2023, 10.1101/2023.03.22.533878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry B. R.; Surovtsev I. V.; Cabeen M. T.; O’Hern C. S.; Dufresne E. R.; Jacobs-Wagner C. The Bacterial Cytoplasm Has Glass-like Properties and Is Fluidized by Metabolic Activity. Cell 2014, 156 (1–2), 183–194. 10.1016/j.cell.2013.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka M. C.; Shkel I. A.; Cayley S.; Record M. T.; Weisshaar J. C. Crowding and Confinement Effects on Protein Diffusion In Vivo. J. Bacteriol. 2006, 188 (17), 6115–6123. 10.1128/JB.01982-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Bogaart G.; Hermans N.; Krasnikov V.; Poolman B. Protein Mobility and Diffusive Barriers in Escherichia Coli: Consequences of Osmotic Stress: Protein Diffusion in E. Coli. Mol. Microbiol. 2007, 64 (3), 858–871. 10.1111/j.1365-2958.2007.05705.x. [DOI] [PubMed] [Google Scholar]
- Joyner R. P.; Tang J. H.; Helenius J.; Dultz E.; Brune C.; Holt L. J.; Huet S.; Müller D. J.; Weis K. A Glucose-Starvation Response Regulates the Diffusion of Macromolecules. eLife 2016, 5, e09376 10.7554/eLife.09376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y.; Gresham D.; Holt L. J.. Increased Mesoscale Diffusivity in Response to Acute Glucose Starvation. MicroPublication Biol. 2023, 2023, 10.17912/micropub.biology.000729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munder M. C.; Midtvedt D.; Franzmann T.; Nuske E.; Otto O.; Herbig M.; Ulbricht E.; Muller P.; Taubenberger A.; Maharana S.; Malinovska L.; Richter D.; Guck J.; Zaburdaev V.; Alberti S.. A pH-Driven Transition of the Cytoplasm from a Fluid- to a Solid-like State Promotes Entry into Dormancy. Elife 2016, 5, 10.7554/eLife.09347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Åberg C.; Poolman B. Glass-like Characteristics of Intracellular Motion in Human Cells. Biophys. J. 2021, 120 (11), 2355–2366. 10.1016/j.bpj.2021.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delarue M.; Brittingham G. P.; Pfeffer S.; Surovtsev I. V.; Pinglay S.; Kennedy K. J.; Schaffer M.; Gutierrez J. I.; Sang D.; Poterewicz G.; et al. J. mTORC1 Controls Phase Separation and the Biophysical Properties of the Cytoplasm by Tuning Crowding. Cell 2018, 174 (2), 338–349.e20. 10.1016/j.cell.2018.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losa J.; Leupold S.; Alonso-Martinez D.; Vainikka P.; Thallmair S.; Tych K. M.; Marrink S. J.; Heinemann M. Perspective: A Stirring Role for Metabolism in Cells. Mol. Syst. Biol. 2022, 18 (4), e10822 10.15252/msb.202110822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson L. B.; Ambati V. S.; Brandman O. Cellular Control of Viscosity Counters Changes in Temperature and Energy Availability. Cell 2020, 183 (6), 1572–1585.e16. 10.1016/j.cell.2020.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuenberger P.; Ganscha S.; Kahraman A.; Cappelletti V.; Boersema P. J.; Von Mering C.; Claassen M.; Picotti P. Cell-Wide Analysis of Protein Thermal Unfolding Reveals Determinants of Thermostability. Science 2017, 355 (6327), eaai7825 10.1126/science.aai7825. [DOI] [PubMed] [Google Scholar]
- Di Bari D.; Timr S.; Guiral M.; Giudici-Orticoni M.-T.; Seydel T.; Beck C.; Petrillo C.; Derreumaux P.; Melchionna S.; Sterpone F.; Peters J.; Paciaroni A. Diffusive Dynamics of Bacterial Proteome as a Proxy of Cell Death. ACS Cent. Sci. 2023, 9 (1), 93–102. 10.1021/acscentsci.2c01078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einstein A.Uber Die von Der, 1905.
- Bellotto N.; Agudo-Canalejo J.; Colin R.; Golestanian R.; Malengo G.; Sourjik V.. Dependence of Diffusion in Escherichia Coli Cytoplasm on Protein Size, Environmental Conditions, and Cell Growth. eLife 2022, 11, e82654. 10.7554/eLife.82654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elowitz M. B.; Surette M. G.; Wolf P.-E.; Stock J. B.; Leibler S. Protein Mobility in the Cytoplasm of Escherichia Coli. J. Bacteriol. 1999, 181 (1), 197–203. 10.1128/JB.181.1.197-203.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M.; Mommer M. S.; Sourjik V. Mobility of Cytoplasmic, Membrane, and DNA-Binding Proteins in Escherichia Coli. Biophys. J. 2010, 98 (4), 552–559. 10.1016/j.bpj.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullineaux C. W.; Nenninger A.; Ray N.; Robinson C. Diffusion of Green Fluorescent Protein in Three Cell Environments in Escherichia Coli. J. Bacteriol. 2006, 188 (10), 3442–3448. 10.1128/JB.188.10.3442-3448.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mika J. T.; Poolman B. Macromolecule Diffusion and Confinement in Prokaryotic Cells. Curr. Opin. Biotechnol. 2011, 22 (1), 117–126. 10.1016/j.copbio.2010.09.009. [DOI] [PubMed] [Google Scholar]
- Xiang Y.; Surovtsev I. V.; Chang Y.; Govers S. K.; Parry B. R.; Liu J.; Jacobs-Wagner C. Interconnecting Solvent Quality, Transcription, and Chromosome Folding in Escherichia coli. Cell 2021, 184 (14), 3626–3642.e14. 10.1016/j.cell.2021.05.037. [DOI] [PubMed] [Google Scholar]
- Tran B. M.; Linnik D. S.; Punter C. M.; Śmigiel W. M.; Mantovanelli L.; Iyer A.; O’Byrne C.; Abee T.; Johansson J.; Poolman B. Super-Resolving Microscopy Reveals the Localizations and Movement Dynamics of Stressosome Proteins in Listeria Monocytogenes. Commun. Biol. 2023, 6 (1), 51. 10.1038/s42003-023-04423-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florea M. Aging and Immortality in Unicellular Species. Mech. Ageing Dev. 2017, 167, 5–15. 10.1016/j.mad.2017.08.006. [DOI] [PubMed] [Google Scholar]
- Mika J. T.; Schavemaker P. E.; Krasnikov V.; Poolman B. Impact of Osmotic Stress on Protein Diffusion in L Actococcus Lactis: Protein Diffusion in L. Lactis. Mol. Microbiol. 2014, 94 (4), 857–870. 10.1111/mmi.12800. [DOI] [PubMed] [Google Scholar]
- Alsallaq R.; Zhou H.-X. Electrostatic Rate Enhancement and Transient Complex of Protein–Protein Association. Proteins Struct. Funct. Bioinforma. 2008, 71 (1), 320–335. 10.1002/prot.21679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber G.; Fersht A. R. Interaction of Barnase with Its Polypeptide Inhibitor Barstar Studied by Protein Engineering. Biochemistry 1993, 32 (19), 5145–5150. 10.1021/bi00070a025. [DOI] [PubMed] [Google Scholar]
- Wallis R.; Moore G. R.; James R.; Kleanthous C. Protein-Protein Interactions in Colicin E9 DNase-Immunity Protein Complexes. 1. Diffusion-Controlled Association and Femtomolar Binding for the Cognate Complex. Biochemistry 1995, 34 (42), 13743–13750. 10.1021/bi00042a004. [DOI] [PubMed] [Google Scholar]
- Klumpp S.; Scott M.; Pedersen S.; Hwa T. Molecular Crowding Limits Translation and Cell Growth. Proc. Natl. Acad. Sci. U. S. A. 2013, 110 (42), 16754–16759. 10.1073/pnas.1310377110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G.; Fedyunin I.; Miekley O.; Valleriani A.; Moura A.; Ignatova Z. Global and Local Depletion of Ternary Complex Limits Translational Elongation. Nucleic Acids Res. 2010, 38 (14), 4778–4787. 10.1093/nar/gkq196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loose M.; Kruse K.; Schwille P. Protein Self-Organization: Lessons from the Min System. Annu. Rev. Biophys. 2011, 40 (1), 315–336. 10.1146/annurev-biophys-042910-155332. [DOI] [PubMed] [Google Scholar]
- McGuffee S. R.; Elcock A. H. Diffusion, Crowding & Protein Stability in a Dynamic Molecular Model of the Bacterial Cytoplasm. PLoS Comput. Biol. 2010, 6 (3), e1000694 10.1371/journal.pcbi.1000694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis P. J.; Thaker S. D.; Errington J. Compartmentalization of Transcription and Translation in Bacillus Subtilis. EMBO J. 2000, 19 (4), 710–718. 10.1093/emboj/19.4.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gijtenbeek L. A.; Robinson A.; Van Oijen A. M.; Poolman B.; Kok J. On the Spatial Organization of mRNA, Plasmids, and Ribosomes in a Bacterial Host Overexpressing Membrane Proteins. PLOS Genet. 2016, 12 (12), e1006523 10.1371/journal.pgen.1006523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odijk T. Osmotic Compaction of Supercoiled DNA into a Bacterial Nucleoid. Biophys. Chem. 1998, 73 (1–2), 23–29. 10.1016/S0301-4622(98)00115-X. [DOI] [PubMed] [Google Scholar]
- Zimmerman S. B. Shape and Compaction of Escherichia Coli Nucleoids. J. Struct. Biol. 2006, 156 (2), 255–261. 10.1016/j.jsb.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Murphy L. D.; Zimmerman S. B. Condensation and Cohesion of λ DNA in Cell Extracts and Other Media: Implications for the Structure and Function of DNA in Prokaryotes. Biophys. Chem. 1995, 57 (1), 71–92. 10.1016/0301-4622(95)00047-2. [DOI] [PubMed] [Google Scholar]
- Bailoni E.; Partipilo M.; Coenradij J.; Grundel D. A. J.; Slotboom D. J.; Poolman B. Minimal Out-of-Equilibrium Metabolism for Synthetic Cells: A Membrane Perspective. ACS Synth. Biol. 2023, 12 (4), 922–946. 10.1021/acssynbio.3c00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M.; Marinov G. K. The Bioenergetic Costs of a Gene. Proc. Natl. Acad. Sci. U. S. A. 2015, 112 (51), 15690–15695. 10.1073/pnas.1514974112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaljević J.; Saaki T. N. V.; Govers S. K.; Remy O.; Van Raaphorst R.; Lamot T.; Laloux G. Chromosome Choreography during the Non-Binary Cell Cycle of a Predatory Bacterium. Curr. Biol. 2021, 31 (17), 3707–3720.e5. 10.1016/j.cub.2021.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munder M. C.; Midtvedt D.; Franzmann T.; Nüske E.; Otto O.; Herbig M.; Ulbricht E.; Müller P.; Taubenberger A.; Maharana S.; et al. A pH-Driven Transition of the Cytoplasm from a Fluid- to a Solid-like State Promotes Entry into Dormancy. eLife 2016, 5, e09347 10.7554/eLife.09347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakshi S.; Choi H.; Mondal J.; Weisshaar J. C. Time-Dependent Effects of Transcription- and Translation-Halting Drugs on the Spatial Distributions of the E Scherichia Coli Chromosome and Ribosomes: Time-Dependent Drug Effects on E. Coli Chromosome Spatial Distribution. Mol. Microbiol. 2014, 94 (4), 871–887. 10.1111/mmi.12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittas T.; Zuo W.; Boersma A. J. Cell Wall Damage Increases Macromolecular Crowding Effects in the Escherichia Coli Cytoplasm. iScience 2023, 26 (4), 106367 10.1016/j.isci.2023.106367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlodarski M.; Mancini L.; Raciti B.; Sclavi B.; Lagomarsino M. C.; Cicuta P. Cytosolic Crowding Drives the Dynamics of Both Genome and Cytosol in Escherichia Coli Challenged with Sub-Lethal Antibiotic Treatments. iScience 2020, 23 (10), 101560 10.1016/j.isci.2020.101560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y.; Mohapatra S.; Weisshaar J. C. Rigidification of the Escherichia Coli Cytoplasm by the Human Antimicrobial Peptide LL-37 Revealed by Superresolution Fluorescence Microscopy. Proc. Natl. Acad. Sci. U. S. A. 2019, 116 (3), 1017–1026. 10.1073/pnas.1814924116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhailov A. S.; Kapral R.. Hydrodynamic Collective Effects of Active Protein Machines in Solution and Lipid Bilayers. Proc. Natl. Acad. Sci. U. S. A. 2015, 112 ( (28), ), E3639– E3644 10.1073/pnas.1506825112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A.; Malinovska L.; Saha S.; Wang J.; Alberti S.; Krishnan Y.; Hyman A. A. ATP as a Biological Hydrotrope. Science 2017, 356 (6339), 753–756. 10.1126/science.aaf6846. [DOI] [PubMed] [Google Scholar]
- He Y.; Kang J.; Song J. ATP Antagonizes the Crowding-Induced Destabilization of the Human Eye-Lens Protein γS-Crystallin. Biochem. Biophys. Res. Commun. 2020, 526 (4), 1112–1117. 10.1016/j.bbrc.2020.04.014. [DOI] [PubMed] [Google Scholar]
- Pandey M. P.; Sasidharan S.; Raghunathan V. A.; Khandelia H. Molecular Mechanism of Hydrotropic Properties of GTP and ATP. J. Phys. Chem. B 2022, 126 (42), 8486–8494. 10.1021/acs.jpcb.2c06077. [DOI] [PubMed] [Google Scholar]
- Nishizawa M.; Walinda E.; Morimoto D.; Kohn B.; Scheler U.; Shirakawa M.; Sugase K. Effects of Weak Nonspecific Interactions with ATP on Proteins. J. Am. Chem. Soc. 2021, 143 (31), 11982–11993. 10.1021/jacs.0c13118. [DOI] [PubMed] [Google Scholar]
- Golestanian R. Enhanced Diffusion of Enzymes That Catalyze Exothermic Reactions. Phys. Rev. Lett. 2015, 115 (10), 108102 10.1103/PhysRevLett.115.108102. [DOI] [PubMed] [Google Scholar]
- Riedel C.; Gabizon R.; Wilson C. A. M.; Hamadani K.; Tsekouras K.; Marqusee S.; Pressé S.; Bustamante C. The Heat Released during Catalytic Turnover Enhances the Diffusion of an Enzyme. Nature 2015, 517 (7533), 227–230. 10.1038/nature14043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta S.; Dey K. K.; Muddana H. S.; Tabouillot T.; Ibele M. E.; Butler P. J.; Sen A. Enzyme Molecules as Nanomotors. J. Am. Chem. Soc. 2013, 135 (4), 1406–1414. 10.1021/ja3091615. [DOI] [PubMed] [Google Scholar]
- Chen Z.; Shaw A.; Wilson H.; Woringer M.; Darzacq X.; Marqusee S.; Wang Q.; Bustamante C. Single-Molecule Diffusometry Reveals No Catalysis-Induced Diffusion Enhancement of Alkaline Phosphatase as Proposed by FCS Experiments. Proc. Natl. Acad. Sci. U. S. A. 2020, 117 (35), 21328–21335. 10.1073/pnas.2006900117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.; Guo P.; Jin D.; Peng Y.; Sun X.; Chen Y.; Liu X.; Chen W.; Wang W.; Yan X.; Ma X. Enzyme-Powered Tubular Microrobotic Jets as Bioinspired Micropumps for Active Transmembrane Drug Transport. ACS Nano 2023, 17 (5), 5095–5107. 10.1021/acsnano.3c00291. [DOI] [PubMed] [Google Scholar]
- Hołówka J.; Zakrzewska-Czerwińska J. Nucleoid Associated Proteins: The Small Organizers That Help to Cope With Stress. Front. Microbiol. 2020, 11, 590. 10.3389/fmicb.2020.00590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyeux M. Organization of the Bacterial Nucleoid by DNA-Bridging Proteins and Globular Crowders. Front. Microbiol. 2023, 14, 1116776 10.3389/fmicb.2023.1116776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worcel A.; Burgi E. On the Structure of the Folded Chromosome of Escherichia Coli. J. Mol. Biol. 1972, 71 (2), 127–147. 10.1016/0022-2836(72)90342-7. [DOI] [PubMed] [Google Scholar]
- Kavenoff R.; Bowen B. C. Electron Microscopy of Membrane-Free Folded Chromosomes from Escherichia Coli. Chromosoma 1976, 59 (2), 89–101. 10.1007/BF00328479. [DOI] [PubMed] [Google Scholar]
- Cunha S.; Woldringh C. L.; Odijk T. Polymer-Mediated Compaction and Internal Dynamics of Isolated Escherichia Coli Nucleoids. J. Struct. Biol. 2001, 136 (1), 53–66. 10.1006/jsbi.2001.4420. [DOI] [PubMed] [Google Scholar]
- De Vries R. DNA Condensation in Bacteria: Interplay between Macromolecular Crowding and Nucleoid Proteins. Biochimie 2010, 92 (12), 1715–1721. 10.1016/j.biochi.2010.06.024. [DOI] [PubMed] [Google Scholar]
- Pelletier J.; Halvorsen K.; Ha B.-Y.; Paparcone R.; Sandler S. J.; Woldringh C. L.; Wong W. P.; Jun S.. Physical Manipulation of the Escherichia Coli Chromosome Reveals Its Soft Nature. Proc. Natl. Acad. Sci. U. S. A. 2012, 109 ( (40), ), E2649–E2656 10.1073/pnas.1208689109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.; Llopis P. M.; Rudner D. Z. Organization and Segregation of Bacterial Chromosomes. Nat. Rev. Genet. 2013, 14 (3), 191–203. 10.1038/nrg3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray W. T.; Govers S. K.; Xiang Y.; Parry B. R.; Campos M.; Kim S.; Jacobs-Wagner C. Nucleoid Size Scaling and Intracellular Organization of Translation across Bacteria. Cell 2019, 177 (6), 1632–1648.e20. 10.1016/j.cell.2019.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F.; Japaridze A.; Dorier J.; Racko D.; Kwapich R.; Burnier Y.; Dietler G.; Stasiak A. Effects of Physiological Self-Crowding of DNA on Shape and Biological Properties of DNA Molecules with Various Levels of Supercoiling. Nucleic Acids Res. 2015, 43 (4), 2390–2399. 10.1093/nar/gkv055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonejuie P.; Burkart M.; Pogliano K.; Pogliano J. Bacterial Cytological Profiling Rapidly Identifies the Cellular Pathways Targeted by Antibacterial Molecules. Proc. Natl. Acad. Sci. U. S. A. 2013, 110 (40), 16169–16174. 10.1073/pnas.1311066110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojanowski D.; Kołodziej M.; Hołówka J.; Müller R.; Zakrzewska-Czerwińska J. Watching DNA Replication Inhibitors in Action: Exploiting Time-Lapse Microfluidic Microscopy as a Tool for Target-Drug Interaction Studies in Mycobacterium. Antimicrob. Agents Chemother. 2019, 63 (10), e00739–19. 10.1128/AAC.00739-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaval K. G.; Chimalapati S.; Siegel S. D.; Garcia N.; Jaishankar J.; Dalia A. B.; Orth K. Membrane-Localized Expression, Production and Assembly of Vibrio Parahaemolyticus T3SS2 Provides Evidence for Transertion. Nat. Commun. 2023, 14 (1), 1178. 10.1038/s41467-023-36762-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojanowski D.; Hołówka J.; Zakrzewska-Czerwińska J. Where and When Bacterial Chromosome Replication Starts: A Single Cell Perspective. Front. Microbiol. 2018, 9, 2819. 10.3389/fmicb.2018.02819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feric M.; Misteli T. Phase Separation in Genome Organization across Evolution. Trends Cell Biol. 2021, 31 (8), 671–685. 10.1016/j.tcb.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyeux M. A Segregative Phase Separation Scenario of the Formation of the Bacterial Nucleoid. Soft Matter 2018, 14 (36), 7368–7381. 10.1039/C8SM01205A. [DOI] [PubMed] [Google Scholar]
- Finkelstein I. J.; Greene E. C. Molecular Traffic Jams on DNA. Annu. Rev. Biophys. 2013, 42 (1), 241–263. 10.1146/annurev-biophys-083012-130304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabaka M.; Kalwarczyk T.; Holyst R. Quantitative Influence of Macromolecular Crowding on Gene Regulation Kinetics. Nucleic Acids Res. 2014, 42 (2), 727–738. 10.1093/nar/gkt907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojkova P.; Spidlova P.; Stulik J. Nucleoid-Associated Protein HU: A Lilliputian in Gene Regulation of Bacterial Virulence. Front. Cell. Infect. Microbiol. 2019, 9, 159. 10.3389/fcimb.2019.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A.; Joshi A.; Arora K.; Mukhopadhyay S.; Guptasarma P. The Bacterial Nucleoid-Associated Proteins, HU and Dps, Condense DNA into Context-Dependent Biphasic or Multiphasic Complex Coacervates. J. Biol. Chem. 2023, 299 (5), 104637 10.1016/j.jbc.2023.104637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali Azam T.; Iwata A.; Nishimura A.; Ueda S.; Ishihama A. Growth Phase-Dependent Variation in Protein Composition of the Escherichia Coli Nucleoid. J. Bacteriol. 1999, 181 (20), 6361–6370. 10.1128/JB.181.20.6361-6370.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portz B.; Lu F.; Gibbs E. B.; Mayfield J. E.; Rachel Mehaffey M.; Zhang Y. J.; Brodbelt J. S.; Showalter S. A.; Gilmour D. S. Structural Heterogeneity in the Intrinsically Disordered RNA Polymerase II C-Terminal Domain. Nat. Commun. 2017, 8 (1), 15231. 10.1038/ncomms15231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stracy M.; Lesterlin C.; Garza De Leon F.; Uphoff S.; Zawadzki P.; Kapanidis A. N.. Live-Cell Superresolution Microscopy Reveals the Organization of RNA Polymerase in the Bacterial Nucleoid. Proc. Natl. Acad. Sci. U. S. A. 2015, 112 ( (32), ), 10.1073/pnas.1507592112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakshi S.; Dalrymple R. M.; Li W.; Choi H.; Weisshaar J. C. Partitioning of RNA Polymerase Activity in Live Escherichia Coli from Analysis of Single-Molecule Diffusive Trajectories. Biophys. J. 2013, 105 (12), 2676–2686. 10.1016/j.bpj.2013.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacometti S. I.; MacRae M. R.; Dancel-Manning K.; Bhabha G.; Ekiert D. C. Lipid Transport Across Bacterial Membranes. Annu. Rev. Cell Dev. Biol. 2022, 38 (1), 125–153. 10.1146/annurev-cellbio-120420-022914. [DOI] [PubMed] [Google Scholar]
- Rojas E. R.; Billings G.; Odermatt P. D.; Auer G. K.; Zhu L.; Miguel A.; Chang F.; Weibel D. B.; Theriot J. A.; Huang K. C. The Outer Membrane Is an Essential Load-Bearing Element in Gram-Negative Bacteria. Nature 2018, 559 (7715), 617–621. 10.1038/s41586-018-0344-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J.; Rutherford S. T.; Silhavy T. J.; Huang K. C. Physical Properties of the Bacterial Outer Membrane. Nat. Rev. Microbiol. 2022, 20 (4), 236–248. 10.1038/s41579-021-00638-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramkamp M. Fluidity Is the Way to Life: Lipid Phase Separation in Bacterial Membranes. EMBO J. 2022, 41 (5), e110737 10.15252/embj.2022110737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sezgin E.; Levental I.; Mayor S.; Eggeling C. The Mystery of Membrane Organization: Composition, Regulation and Roles of Lipid Rafts. Nat. Rev. Mol. Cell Biol. 2017, 18 (6), 361–374. 10.1038/nrm.2017.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickels J. D.; Chatterjee S.; Stanley C. B.; Qian S.; Cheng X.; Myles D. A. A.; Standaert R. F.; Elkins J. G.; Katsaras J. The in Vivo Structure of Biological Membranes and Evidence for Lipid Domains. PLOS Biol. 2017, 15 (5), e2002214 10.1371/journal.pbio.2002214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajerski W.; Ochonska D.; Brzychczy-Wloch M.; Indyka P.; Jarosz M.; Golda-Cepa M.; Sojka Z.; Kotarba A. Attachment Efficiency of Gold Nanoparticles by Gram-Positive and Gram-Negative Bacterial Strains Governed by Surface Charges. J. Nanoparticle Res. 2019, 21 (8), 186. 10.1007/s11051-019-4617-z. [DOI] [Google Scholar]
- Gohrbandt M.; Lipski A.; Grimshaw J. W.; Buttress J. A.; Baig Z.; Herkenhoff B.; Walter S.; Kurre R.; Deckers-Hebestreit G.; Strahl H. Low Membrane Fluidity Triggers Lipid Phase Separation and Protein Segregation in Living Bacteria. EMBO J. 2022, 41 (5), e109800 10.15252/embj.2021109800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay R.; Huang K. C.; Wingreen N. S. Lipid Localization in Bacterial Cells through Curvature-Mediated Microphase Separation. Biophys. J. 2008, 95 (3), 1034–1049. 10.1529/biophysj.107.126920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai F.; Shoda M.; Harashima R.; Sadaie Y.; Hara H.; Matsumoto K. Cardiolipin Domains in Bacillus Subtilis Marburg Membranes. J. Bacteriol. 2004, 186 (5), 1475–1483. 10.1128/JB.186.5.1475-1483.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romantsov T.; Helbig S.; Culham D. E.; Gill C.; Stalker L.; Wood J. M. Cardiolipin Promotes Polar Localization of Osmosensory Transporter ProP in Escherichia Coli: Cardiolipin and Osmoregulation in Escherichia Coli. Mol. Microbiol. 2007, 64 (6), 1455–1465. 10.1111/j.1365-2958.2007.05727.x. [DOI] [PubMed] [Google Scholar]
- Benn G.; Mikheyeva I. V.; Inns P. G.; Forster J. C.; Ojkic N.; Bortolini C.; Ryadnov M. G.; Kleanthous C.; Silhavy T. J.; Hoogenboom B. W. Phase Separation in the Outer Membrane of Escherichia Coli. Proc. Natl. Acad. Sci. U. S. A. 2021, 118 (44), e2112237118 10.1073/pnas.2112237118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M. Antibiotic-Supersusceptible Mutants of Escherichia Coli and Salmonella Typhimurium. Antimicrob. Agents Chemother. 1993, 37 (11), 2255–2260. 10.1128/AAC.37.11.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L.; Li X.; Zhang S.; Dong Y.; Fang W.; Gao L. Polymyxins Induce Lipid Scrambling and Disrupt the Homeostasis of Gram-Negative Bacteria Membrane. Biophys. J. 2022, 121 (18), 3486–3498. 10.1016/j.bpj.2022.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banjade S.; Rosen M. K. Phase Transitions of Multivalent Proteins Can Promote Clustering of Membrane Receptors. eLife 2014, 3, e04123 10.7554/eLife.04123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinkel F.; Abraham L.; Ko M.; Chao J.; Bach H.; Hui L. T.; Li H.; Zhu M.; Ling Y. M.; Rogalski J. C.; Scurll J.; Bui J. M.; Mayor T.; Gold M. R.; Chou K. C.; Av-Gay Y.; McIntosh L. P.; Gsponer J. Phase Separation and Clustering of an ABC Transporter in Mycobacterium Tuberculosis. Proc. Natl. Acad. Sci. U A 2019, 116 (33), 16326–16331. 10.1073/pnas.1820683116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monterroso B.; Robles-Ramos M. Á.; Sobrinos-Sanguino M.; Luque-Ortega J. R.; Alfonso C.; Margolin W.; Rivas G.; Zorrilla S. Bacterial Division Ring Stabilizing ZapA versus Destabilizing SlmA Modulate FtsZ Switching between Biomolecular Condensates and Polymers. Open Biol. 2023, 13 (3), 220324 10.1098/rsob.220324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monterroso B.; Zorrilla S.; Sobrinos-Sanguino M.; Robles-Ramos M. A.; Lopez-Alvarez M.; Margolin W.; Keating C. D.; Rivas G. Bacterial FtsZ Protein Forms Phase-Separated Condensates with Its Nucleoid-Associated Inhibitor SlmA. EMBO Rep 2019, 20 (1), e45946 10.15252/embr.201845946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W.; Cheng S.; Li Y.; Li X.-Y.; Lu N.; Sun J.; Tang G.; Yang Y.; Cai K.; Li X.; et al. Phase Separation Modulates the Assembly and Dynamics of a Polarity-Related Scaffold-Signaling Hub. Nat. Commun. 2022, 13 (1), 7181. 10.1038/s41467-022-35000-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T.; Liu Y.; Wang Z.; He R.; Xiang Zhang J.; Xu F.; Lei M.; Deci M. B.; Nguyen J.; Bianco P. R. Super-resolution Imaging Reveals Changes in Escherichia Coli SSB Localization in Response to DNA Damage. Genes Cells 2019, 24 (12), 814–826. 10.1111/gtc.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams D. W.; Wu L. J.; Errington J. Nucleoid Occlusion Protein N Oc Recruits DNA to the Bacterial Cell Membrane. EMBO J. 2015, 34 (4), 491–501. 10.15252/embj.201490177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles-Ramos M. A.; Margolin W.; Sobrinos-Sanguino M.; Alfonso C.; Rivas G.; Monterroso B.; Zorrilla S. The Nucleoid Occlusion Protein SlmA Binds to Lipid Membranes. mBio 2020, 11, e02094–20. 10.1128/mBio.02094-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harami G. M.; Kovács Z. J.; Pancsa R.; Pálinkás J.; Baráth V.; Tárnok K.; Málnási-Csizmadia A.; Kovács M. Phase Separation by ssDNA Binding Protein Controlled via Protein–protein and protein–DNA Interactions. Proc. Natl. Acad. Sci. U. S. A. 2020, 117 (42), 26206–26217. 10.1073/pnas.2000761117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paccione G.; Robles-Ramos M. Á.; Alfonso C.; Sobrinos-Sanguino M.; Margolin W.; Zorrilla S.; Monterroso B.; Rivas G. Lipid Surfaces and Glutamate Anions Enhance Formation of Dynamic Biomolecular Condensates Containing Bacterial Cell Division Protein FtsZ and Its DNA-Bound Regulator SlmA. Biochemistry 2022, 61 (22), 2482–2489. 10.1021/acs.biochem.2c00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles-Ramos M. A.; Zorrilla S.; Alfonso C.; Margolin W.; Rivas G.; Monterroso B. Assembly of Bacterial Cell Division Protein FtsZ into Dynamic Biomolecular Condensates. Biochim Biophys Acta Mol. Cell Res. 2021, 1868 (5), 118986 10.1016/j.bbamcr.2021.118986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson J. L.; Prautsch J.; Reynvoet F.; Niemeyer F.; Hause G.; Johnston I. G.; Schattat M. H. Stromule Geometry Allows Optimal Spatial Regulation of Organelle Interactions in the Quasi-2D Cytoplasm. Plant Cell Physiol. 2023, pcad098 10.1093/pcp/pcad098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatova Z.; Gierasch L. M. Inhibition of Protein Aggregation in Vitro and in Vivo by a Natural Osmoprotectant. Proc. Natl. Acad. Sci. U. S. A. 2006, 103 (36), 13357–13361. 10.1073/pnas.0603772103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Q.; Mouton S. N.; Veenhoff L. M.; Boersma A. J. A FRET-Based Method for Monitoring Structural Transitions in Protein Self-Organization. Cell Rep. Methods 2022, 2 (3), 100184 10.1016/j.crmeth.2022.100184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brangwynne C. P. Phase Transitions and Size Scaling of Membrane-Less Organelles. J. Cell Biol. 2013, 203 (6), 875–881. 10.1083/jcb.201308087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snead W. T.; Jalihal A. P.; Gerbich T. M.; Seim I.; Hu Z.; Gladfelter A. S. Membrane Surfaces Regulate Assembly of Ribonucleoprotein Condensates. Nat. Cell Biol. 2022, 24 (4), 461–470. 10.1038/s41556-022-00882-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case L. B.; Zhang X.; Ditlev J. A.; Rosen M. K. Stoichiometry Controls Activity of Phase-Separated Clusters of Actin Signaling Proteins. Science 2019, 363 (6431), 1093–1097. 10.1126/science.aau6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale R. D. The Molecular Motor Toolbox for Intracellular Transport. Cell 2003, 112 (4), 467–480. 10.1016/S0092-8674(03)00111-9. [DOI] [PubMed] [Google Scholar]
- Sittewelle M.; Royle S. J. Passive Diffusion Accounts for the Majority of Intracellular Nanovesicle Transport. Life Sci. Alliance 2024, 7 (1), e202302406 10.26508/lsa.202302406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditlev J. A.; Vega A. R.; Köster D. V.; Su X.; Tani T.; Lakoduk A. M.; Vale R. D.; Mayor S.; Jaqaman K.; Rosen M. K. A Composition-Dependent Molecular Clutch between T Cell Signaling Condensates and Actin. eLife 2019, 8, e42695 10.7554/eLife.42695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uversky V. N. Intrinsically Disordered Proteins in Overcrowded Milieu: Membrane-Less Organelles, Phase Separation, and Intrinsic Disorder. Curr. Opin. Struct. Biol. 2017, 44, 18–30. 10.1016/j.sbi.2016.10.015. [DOI] [PubMed] [Google Scholar]
- Sridharan S.; Hernandez-Armendariz A.; Kurzawa N.; Potel C. M.; Memon D.; Beltrao P.; Bantscheff M.; Huber W.; Cuylen-Haering S.; Savitski M. M. Systematic Discovery of Biomolecular Condensate-Specific Protein Phosphorylation. Nat. Chem. Biol. 2022, 18 (10), 1104–1114. 10.1038/s41589-022-01062-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.-W.; Nocka L. M.; Stinger B. L.; DeGrandchamp J. B.; Lew L. J. N.; Alvarez S.; Phan H. T.; Kondo Y.; Kuriyan J.; Groves J. T. A Two-Component Protein Condensate of the EGFR Cytoplasmic Tail and Grb2 Regulates Ras Activation by SOS at the Membrane. Proc. Natl. Acad. Sci. U. S. A. 2022, 119 (19), e2122531119 10.1073/pnas.2122531119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Palacios T. P.; Andersen J. L.. Kinase Regulation by Liquid–Liquid Phase Separation. Trends Cell Biol. 2023, 33, 649. 10.1016/j.tcb.2022.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasker K.; von Diezmann L.; Zhou X.; Ahrens D. G.; Mann T. H.; Moerner W. E.; Shapiro L. Selective Sequestration of Signalling Proteins in a Membraneless Organelle Reinforces the Spatial Regulation of Asymmetry in Caulobacter Crescentus. Nat. Microbiol. 2020, 5 (3), 418–429. 10.1038/s41564-019-0647-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguemaha V.; Zhou H.-X. Liquid-Liquid Phase Separation of Patchy Particles Illuminates Diverse Effects of Regulatory Components on Protein Droplet Formation. Sci. Rep. 2018, 8 (1), 6728. 10.1038/s41598-018-25132-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S.; Chou H.-T.; Matthies D.; Wunder T.; Meyer M. T.; Atkinson N.; Martinez-Sanchez A.; Jeffrey P. D.; Port S. A.; Patena W.; et al. The Structural Basis of Rubisco Phase Separation in the Pyrenoid. Nat. Plants 2020, 6 (12), 1480–1490. 10.1038/s41477-020-00811-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltrogge L. M.; Chaijarasphong T.; Chen A. W.; Bolin E. R.; Marqusee S.; Savage D. F. Multivalent Interactions between CsoS2 and Rubisco Mediate α-Carboxysome Formation. Nat. Struct. Mol. Biol. 2020, 27 (3), 281–287. 10.1038/s41594-020-0387-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.; Yan X.; Aigner H.; Bracher A.; Nguyen N. D.; Hee W. Y.; Long B. M.; Price G. D.; Hartl F. U.; Hayer-Hartl M. Rubisco Condensate Formation by CcmM in β-Carboxysome Biogenesis. Nature 2019, 566 (7742), 131–135. 10.1038/s41586-019-0880-5. [DOI] [PubMed] [Google Scholar]
- Basalla J. L.; Mak C. A.; Byrne J. A.; Ghalmi M.; Hoang Y.; Vecchiarelli A. G. Dissecting the Phase Separation and Oligomerization Activities of the Carboxysome Positioning Protein McdB. eLife 2023, 12, e81362 10.7554/eLife.81362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing W.; Muhlrad D.; Parker R.; Rosen M. K. A Quantitative Inventory of Yeast P Body Proteins Reveals Principles of Composition and Specificity. eLife 2020, 9, e56525 10.7554/eLife.56525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Husini N.; Tomares D. T.; Bitar O.; Childers W. S.; Schrader J. M. Alpha-Proteobacterial RNA Degradosomes Assemble Liquid-Liquid Phase-Separated RNP Bodies. Mol. Cell 2018, 71 (6), 1027–1039 e14. 10.1016/j.molcel.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peskett T. R.; Rau F.; O’Driscoll J.; Patani R.; Lowe A. R.; Saibil H. R. A Liquid to Solid Phase Transition Underlying Pathological Huntingtin Exon1 Aggregation. Mol. Cell 2018, 70 (4), 588–601.e6. 10.1016/j.molcel.2018.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambadipudi S.; Biernat J.; Riedel D.; Mandelkow E.; Zweckstetter M. Liquid–Liquid Phase Separation of the Microtubule-Binding Repeats of the Alzheimer-Related Protein Tau. Nat. Commun. 2017, 8 (1), 275. 10.1038/s41467-017-00480-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A.; Lee H. O.; Jawerth L.; Maharana S.; Jahnel M.; Hein M. Y.; Stoynov S.; Mahamid J.; Saha S.; Franzmann T. M.; et al. Liquid-to-Solid Phase Transition of the ALS Protein FUS Accelerated by Disease Mutation. Cell 2015, 162 (5), 1066–1077. 10.1016/j.cell.2015.07.047. [DOI] [PubMed] [Google Scholar]
- Lipiński W. P.; Visser B. S.; Robu I.; Fakhree M. A. A.; Lindhoud S.; Claessens M. M. A. E.; Spruijt E.. Biomolecular Condensates Can Both Accelerate and Suppress Aggregation of α-Synuclein. Sci. Adv. 2022, 8 ( (48), ), eabq6495. 10.1126/sciadv.abq6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollembeak M. A. M. J. E.; Kurokawa M. Macromolecular Crowding: A Hidden Link between Cell Volume and Everything Else. Cell Physiol Biochem 2021, 55 (S1), 25–40. 10.33594/000000319. [DOI] [PubMed] [Google Scholar]
- Oh S.; Lee C.; Yang W.; Li A.; Mukherjee A.; Basan M.; Ran C.; Yin W.; Tabin C. J.; Fu D.; Xie X. S.; Kirschner M. W. Protein and Lipid Mass Concentration Measurement in Tissues by Stimulated Raman Scattering Microscopy. Proc. Natl. Acad. Sci. U. S. A. 2022, 119 (17), e2117938119 10.1073/pnas.2117938119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayley S.; Lewis B. A.; Guttman H. J.; Record M. T. Characterization of the Cytoplasm of Escherichia Coli K-12 as a Function of External Osmolarity: Implications for Protein-DNA Interactions in Vivo. J. Mol. Biol. 1991, 222 (2), 281–300. 10.1016/0022-2836(91)90212-O. [DOI] [PubMed] [Google Scholar]
- Odermatt P. D.; Miettinen T. P.; Lemière J.; Kang J. H.; Bostan E.; Manalis S. R.; Huang K. C.; Chang F. Variations of Intracellular Density during the Cell Cycle Arise from Tip-Growth Regulation in Fission Yeast. Elife 2021, 10, e64901. 10.7554/eLife.64901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.; Oh S.; Kirschner M. W.. The Uniformity and Stability of Cellular Mass Density in Mammalian Cell Culture. Front. Cell Dev. Biol. 2022, 10, 10.3389/fcell.2022.1017499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.; Chen M.; Hu J.; Sheng R.; Lin Q.; He X.; Guo M. Volumetric Compression Induces Intracellular Crowding to Control Intestinal Organoid Growth via Wnt/β-Catenin Signaling. Cell Stem Cell 2021, 28 (1), 63–78.e7. 10.1016/j.stem.2020.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittas T.; Boersma A. J.. Self-Association of a Nucleoid-Binding Protein Increases with Macromolecular Crowding in Escherichia Coli. bioRxiv 2023, 10.1101/2023.02.23.529735. [DOI] [Google Scholar]
- Burg M. B.; Ferraris J. D.; Dmitrieva N. I. Cellular Response to Hyperosmotic Stresses. Physiol. Rev. 2007, 87 (4), 1441–1474. 10.1152/physrev.00056.2006. [DOI] [PubMed] [Google Scholar]
- Hoffmann E. K.; Lambert I. H.; Pedersen S. F. Physiology of Cell Volume Regulation in Vertebrates. Physiol. Rev. 2009, 89 (1), 193–277. 10.1152/physrev.00037.2007. [DOI] [PubMed] [Google Scholar]
- Boyd-Shiwarski C. R.; Shiwarski D. J.; Griffiths S. E.; Beacham R. T.; Norrell L.; Morrison D. E.; Wang J.; Mann J.; Tennant W.; Anderson E. N.; et al. Kinases Sense Molecular Crowding and Rescue Cell Volume via Phase Separation. Cell 2022, 185 (24), 4488–4506.e20. 10.1016/j.cell.2022.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafiei N.; Cordova M.; Navarre W. W.; Milstein J. N.. Growth Phase-Dependent Chromosome Condensation and Heat-Stable Nucleoid-Structuring Protein Redistribution in Escherichia Coli under Osmotic Stress. J. Bacteriol. 2019, 201 ( (23), ), 10.1128/JB.00469-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth A. J.; Gruebele M. Quinary Protein Structure and the Consequences of Crowding in Living Cells: Leaving the Test-tube Behind. BioEssays 2013, 35 (11), 984–993. 10.1002/bies.201300080. [DOI] [PubMed] [Google Scholar]
- Danielsson J.; Mu X.; Lang L.; Wang H.; Binolfi A.; Theillet F.-X.; Bekei B.; Logan D. T.; Selenko P.; Wennerström H.; Oliveberg M. Thermodynamics of Protein Destabilization in Live Cells. Proc. Natl. Acad. Sci. U. S. A. 2015, 112 (40), 12402–12407. 10.1073/pnas.1511308112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayel M. J.; Hom E. F.; Verkman A. S. Diffusion of Green Fluorescent Protein in the Aqueous-Phase Lumen of Endoplasmic Reticulum. Biophys. J. 1999, 76 (5), 2843–2851. 10.1016/S0006-3495(99)77438-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeb S.; Sörensen T.; Yang F.; Mu X.; Oliveberg M.; Danielsson J. Diffusive Protein Interactions in Human versus Bacterial Cells. Curr. Res. Struct. Biol. 2020, 2, 68–78. 10.1016/j.crstbi.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu X.; Choi S.; Lang L.; Mowray D.; Dokholyan N. V.; Danielsson J.; Oliveberg M. Physicochemical Code for Quinary Protein Interactions in Escherichia Coli. Proc. Natl. Acad. Sci. U. S. A. 2017, 114 (23), E4556–E4563. 10.1073/pnas.1621227114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweke H.; Levin T.; Pacesa M.; Goverde C. A.; Kumar P.; Duhoo Y.; Dornfeld L. J.; Dubreuil B.; Georgeon S.; et al. An Atlas of Protein Homo-Oligomerization across Domains of Life. bioRxiv 2023, 10.1101/2023.06.09.544317. [DOI] [PubMed] [Google Scholar]
- Holland I. B. Rise and Rise of the ABC Transporter Families. Res. Microbiol. 2019, 170 (8), 304–320. 10.1016/j.resmic.2019.08.004. [DOI] [PubMed] [Google Scholar]
- Thomas C.; Aller S. G.; Beis K.; Carpenter E. P.; Chang G.; Chen L.; Dassa E.; Dean M.; Duong Van Hoa F.; Ekiert D.; et al. Structural and Functional Diversity Calls for a New Classification of ABC Transporters. FEBS Lett. 2020, 594 (23), 3767–3775. 10.1002/1873-3468.13935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rospert S.; Dubaquié Y.; Gautschi M. Nascent-Polypeptide-Associated Complex. Cell. Mol. Life Sci. CMLS 2002, 59 (10), 1632–1639. 10.1007/PL00012490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y.; Liu T.; Gresham D.; Holt L. J.. mRNA Condensation Fluidizes the Cytoplasm. bioRxiv 2023, 10.1101/2023.05.30.542963. [DOI] [Google Scholar]
- Marini G.; Nüske E.; Leng W.; Alberti S.; Pigino G. Reorganization of Budding Yeast Cytoplasm upon Energy Depletion. Mol. Biol. Cell 2020, 31 (12), 1232–1245. 10.1091/mbc.E20-02-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton S. N.; Thaller D. J.; Crane M. M.; Rempel I. L.; Terpstra O. T.; Steen A.; Kaeberlein M.; Lusk C. P.; Boersma A. J.; Veenhoff L. M. A Physicochemical Perspective of Aging from Single-Cell Analysis of pH, Macromolecular and Organellar Crowding in Yeast. eLife 2020, 9, e54707 10.7554/eLife.54707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo W.; Huang M.-R.; Schmitz F.; Boersma A. J.. Genetically-Encoded Probes to Determine Nonspecific Hydrophobic and Electrostatic Binding in Cells. bioRxiv 2023, 10.1101/2023.06.27.546658. [DOI] [Google Scholar]
- Sengupta R.; Pantel A.; Cheng X.; Shkel I.; Peran I.; Stenzoski N.; Raleigh D. P.; Record M. T. J. Positioning the Intracellular Salt Potassium Glutamate in the Hofmeister Series by Chemical Unfolding Studies of NTL9. Biochemistry 2016, 55 (15), 2251–2259. 10.1021/acs.biochem.6b00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlov A. G.; Cheng X.; Zhang H.; Shinn M. K.; Weiland E.; Nguyen B.; Shkel I. A.; Zytkiewicz E.; Finkelstein I. J.; Record M. T.; Lohman T. M. How Glutamate Promotes Liquid-Liquid Phase Separation and DNA Binding Cooperativity of E. Coli SSB Protein. J. Mol. Biol. 2022, 434 (9), 167562 10.1016/j.jmb.2022.167562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy A. C.; Dignon G. L.; Kan Y.; Zerze G. H.; Parekh S. H.; Mittal J.; Fawzi N. L. Molecular Interactions Underlying Liquid-Liquid Phase Separation of the FUS Low-Complexity Domain. Nat. Struct. Mol. Biol. 2019, 26 (7), 637–648. 10.1038/s41594-019-0250-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timasheff S. N. Protein-Solvent Preferential Interactions, Protein Hydration, and the Modulation of Biochemical Reactions by Solvent Components. Proc. Natl. Acad. Sci. U. S. A. 2002, 99 (15), 9721–9726. 10.1073/pnas.122225399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey P. H. Organic Osmolytes as Compatible, Metabolic and Counteracting Cytoprotectants in High Osmolarity and Other Stresses. J. Exp. Biol. 2005, 208 (15), 2819–2830. 10.1242/jeb.01730. [DOI] [PubMed] [Google Scholar]
- Sachs G.; Kraut J. A.; Wen Y.; Feng J.; Scott D. R. Urea Transport in Bacteria: Acid Acclimation by Gastric Helicobacter Spp. J. Membr. Biol. 2006, 212 (2), 71–82. 10.1007/s00232-006-0867-7. [DOI] [PubMed] [Google Scholar]
- Cinar H.; Winter R. The Effects of Cosolutes and Crowding on the Kinetics of Protein Condensate Formation Based on Liquid–Liquid Phase Separation: A Pressure-Jump Relaxation Study. Sci. Rep. 2020, 10 (1), 17245. 10.1038/s41598-020-74271-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngren B.; Nielsen H. J.; Jun S.; Austin S. The Multifork Escherichia Coli Chromosome Is a Self-Duplicating and Self-Segregating Thermodynamic Ring Polymer. Genes Dev. 2014, 28 (1), 71–84. 10.1101/gad.231050.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marczynski G. T.; Petit K.; Patel P. Crosstalk Regulation Between Bacterial Chromosome Replication and Chromosome Partitioning. Front. Microbiol. 2019, 10, 279. 10.3389/fmicb.2019.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler-Bistué A.; Aguilar-Pierlé S.; Garcia-Garcerá M.; Val M.-E.; Sismeiro O.; Varet H.; Sieira R.; Krin E.; Skovgaard O.; Comerci D. J.; Rocha E. P. C.; Mazel D. Macromolecular Crowding Links Ribosomal Protein Gene Dosage to Growth Rate in Vibrio Cholerae. BMC Biol. 2020, 18 (1), 43. 10.1186/s12915-020-00777-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akabayov B.; Akabayov S. R.; Lee S.-J.; Wagner G.; Richardson C. C. Impact of Macromolecular Crowding on DNA Replication. Nat. Commun. 2013, 4 (1), 1615. 10.1038/ncomms2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller R. S.; Kaguni J. M.; Kornberg A. Enzymatic Replication of the Origin of the Escherichia Coli Chromosome. Proc. Natl. Acad. Sci. U. S. A. 1981, 78 (12), 7370–7374. 10.1073/pnas.78.12.7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman S. B.; Harrison B. Macromolecular Crowding Increases Binding of DNA Polymerase to DNA: An Adaptive Effect. Proc. Natl. Acad. Sci. U. S. A. 1987, 84 (7), 1871–1875. 10.1073/pnas.84.7.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranovich A.; Gdalevsky G. Y.; Cohen-Luria R.; Fishov I.; Parola A. H. Membrane-Catalyzed Nucleotide Exchange on DnaA. J. Biol. Chem. 2006, 281 (18), 12526–12534. 10.1074/jbc.M510266200. [DOI] [PubMed] [Google Scholar]
- Aranovich A.; Braier-Marcovitz S.; Ansbacher E.; Granek R.; Parola A. H.; Fishov I. N-Terminal-Mediated Oligomerization of DnaA Drives the Occupancy-Dependent Rejuvenation of the Protein on the Membrane. Biosci. Rep. 2015, 35 (5), e00250 10.1042/BSR20150175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner J.; Durrer P.; Kitchen J.; Brunner J.; Crooke E. Membrane-Mediated Release of Nucleotide from an Initiator of Chromosomal Replication, Escherichia Coli DnaA, Occurs with Insertion of a Distinct Region of the Protein into the Lipid Bilayer. J. Biol. Chem. 1998, 273 (9), 5167–5173. 10.1074/jbc.273.9.5167. [DOI] [PubMed] [Google Scholar]
- Hou Y.; Kumar P.; Aggarwal M.; Sarkari F.; Wolcott K. M.; Chattoraj D. K.; Crooke E.; Saxena R. The Linker Domain of the Initiator DnaA Contributes to Its ATP Binding and Membrane Association in E. Coli Chromosomal Replication. Sci. Adv. 2022, 8 (40), eabq6657 10.1126/sciadv.abq6657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felczak M. M.; Simmons L. A.; Kaguni J. M. An Essential Tryptophan of Escherichia Coli DnaA Protein Functions in Oligomerization at the E. Coli Replication Origin. J. Biol. Chem. 2005, 280 (26), 24627–24633. 10.1074/jbc.M503684200. [DOI] [PubMed] [Google Scholar]
- Mercier R.; Petit M.-A.; Schbath S.; Robin S.; El Karoui M.; Boccard F.; Espéli O. The MatP/matS Site-Specific System Organizes the Terminus Region of the E. Coli Chromosome into a Macrodomain. Cell 2008, 135 (3), 475–485. 10.1016/j.cell.2008.08.031. [DOI] [PubMed] [Google Scholar]
- Gogou C.; Japaridze A.; Dekker C. Mechanisms for Chromosome Segregation in Bacteria. Front. Microbiol. 2021, 12, 685687 10.3389/fmicb.2021.685687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Japaridze A.; Gogou C.; Kerssemakers J. W. J.; Nguyen H. M.; Dekker C. Direct Observation of Independently Moving Replisomes in Escherichia Coli. Nat. Commun. 2020, 11 (1), 3109. 10.1038/s41467-020-16946-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani M.; Sanchez Z. K.; Kimbara K.. Genomics of Microbial Plasmids: Classification and Identification Based on Replication and Transfer Systems and Host Taxonomy. Front. Microbiol. 2015, 6, 10.3389/fmicb.2015.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers D. Timing, Self-control and a Sense of Direction Are the Secrets of Multicopy Plasmid Stability. Mol. Microbiol. 1998, 29 (5), 1137–1145. 10.1046/j.1365-2958.1998.01012.x. [DOI] [PubMed] [Google Scholar]
- Garner E. C.; Campbell C. S.; Weibel D. B.; Mullins R. D. Reconstitution of DNA Segregation Driven by Assembly of a Prokaryotic Actin Homolog. Science 2007, 315 (5816), 1270–1274. 10.1126/science.1138527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havey J. C.; Vecchiarelli A. G.; Funnell B. E. ATP-Regulated Interactions between P1 ParA, ParB and Non-Specific DNA That Are Stabilized by the Plasmid Partition Site, parS. Nucleic Acids Res. 2012, 40 (2), 801–812. 10.1093/nar/gkr747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J.; Cherstvy A. G.; Metzler R. Mixing and Segregation of Ring Polymers: Spatial Confinement and Molecular Crowding Effects. New J. Phys. 2014, 16 (5), 053047 10.1088/1367-2630/16/5/053047. [DOI] [Google Scholar]
- Jun S.Polymer Physics for Understanding Bacterial Chromosomes. In Bacterial Chromatin; Dame R. T., Dorman C. J., Eds.; Springer Netherlands: Dordrecht, 2010; pp 97–116. 10.1007/978-90-481-3473-1_6. [DOI] [Google Scholar]
- Chen Y.; Yu W.; Wang J.; Luo K. Polymer Segregation under Confinement: Influences of Macromolecular Crowding and the Interaction between the Polymer and Crowders. J. Chem. Phys. 2015, 143 (13), 134904. 10.1063/1.4932370. [DOI] [PubMed] [Google Scholar]
- Di Ventura B.; Knecht B.; Andreas H.; Godinez W. J.; Fritsche M.; Rohr K.; Nickel W.; Heermann D. W.; Sourjik V. Chromosome Segregation by the Escherichia Coli Min System. Mol. Syst. Biol. 2013, 9 (1), 686. 10.1038/msb.2013.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popp D.; Robinson R. C. Many Ways to Build an Actin Filament: Actin Filament Systems. Mol. Microbiol. 2011, 80 (2), 300–308. 10.1111/j.1365-2958.2011.07599.x. [DOI] [PubMed] [Google Scholar]
- Becker E.; Herrera N. C.; Gunderson F. Q.; Derman A. I.; Dance A. L.; Sims J.; Larsen R. A.; Pogliano J. DNA Segregation by the Bacterial Actin AlfA during Bacillus Subtilis Growth and Development. EMBO J. 2006, 25 (24), 5919–5931. 10.1038/sj.emboj.7601443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong G. C. L.; Pollack L. Electrostatics of Strongly Charged Biological Polymers: Ion-Mediated Interactions and Self-Organization in Nucleic Acids and Proteins. Annu. Rev. Phys. Chem. 2010, 61 (1), 171–189. 10.1146/annurev.physchem.58.032806.104436. [DOI] [PubMed] [Google Scholar]
- Popp D.; Gov N. S.; Iwasa M.; Maéda Y. Effect of Short-Range Forces on the Length Distribution of Fibrous Cytoskeletal Proteins. Biopolymers 2008, 89 (9), 711–721. 10.1002/bip.20999. [DOI] [PubMed] [Google Scholar]
- Salje J.; Zuber B.; Löwe J. Electron Cryomicroscopy of E. Coli Reveals Filament Bundles Involved in Plasmid DNA Segregation. Science 2009, 323 (5913), 509–512. 10.1126/science.1164346. [DOI] [PubMed] [Google Scholar]
- Popp D.; Narita A.; Iwasa M.; Maéda Y.; Robinson R. C. Molecular Mechanism of Bundle Formation by the Bacterial Actin ParM. Biochem. Biophys. Res. Commun. 2010, 391 (4), 1598–1603. 10.1016/j.bbrc.2009.12.078. [DOI] [PubMed] [Google Scholar]
- Kueh H. Y.; Mitchison T. J. Structural Plasticity in Actin and Tubulin Polymer Dynamics. Science 2009, 325 (5943), 960–963. 10.1126/science.1168823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popp D.; Narita A.; Ghoshdastider U.; Maeda K.; Maéda Y.; Oda T.; Fujisawa T.; Onishi H.; Ito K.; Robinson R. C. Polymeric Structures and Dynamic Properties of the Bacterial Actin AlfA. J. Mol. Biol. 2010, 397 (4), 1031–1041. 10.1016/j.jmb.2010.02.010. [DOI] [PubMed] [Google Scholar]
- Popp D.; Xu W.; Narita A.; Brzoska A. J.; Skurray R. A.; Firth N.; Goshdastider U.; Maéda Y.; Robinson R. C.; Schumacher M. A. Structure and Filament Dynamics of the pSK41 Actin-like ParM Protein. J. Biol. Chem. 2010, 285 (13), 10130–10140. 10.1074/jbc.M109.071613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor C. W.; Tabor H. Polyamines in Microorganisms. Microbiol. Rev. 1985, 49 (1), 81–99. 10.1128/mr.49.1.81-99.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor C. W.; Tabor H. 1,4-Diaminobutane (Putrescine), Spermidine, and Spermine. Annu. Rev. Biochem. 1976, 45 (1), 285–306. 10.1146/annurev.bi.45.070176.001441. [DOI] [PubMed] [Google Scholar]
- Egan E. S.; Fogel M. A.; Waldor M. K. MicroReview: Divided Genomes: Negotiating the Cell Cycle in Prokaryotes with Multiple Chromosomes: Multiple Chromosomes in Prokaryotes. Mol. Microbiol. 2005, 56 (5), 1129–1138. 10.1111/j.1365-2958.2005.04622.x. [DOI] [PubMed] [Google Scholar]
- Liu Z.; Capaldi X.; Zeng L.; Zhang Y.; Reyes-Lamothe R.; Reisner W. Confinement Anisotropy Drives Polar Organization of Two DNA Molecules Interacting in a Nanoscale Cavity. Nat. Commun. 2022, 13 (1), 4358. 10.1038/s41467-022-31398-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Lamothe R.; Tran T.; Meas D.; Lee L.; Li A. M.; Sherratt D. J.; Tolmasky M. E. High-Copy Bacterial Plasmids Diffuse in the Nucleoid-Free Space, Replicate Stochastically and Are Randomly Partitioned at Cell Division. Nucleic Acids Res. 2014, 42 (2), 1042–1051. 10.1093/nar/gkt918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewachter L.; Bollen C.; Wilmaerts D.; Louwagie E.; Herpels P.; Matthay P.; Khodaparast L.; Khodaparast L.; Rousseau F.; Schymkowitz J.; et al. The Dynamic Transition of Persistence toward the Viable but Nonculturable State during Stationary Phase Is Driven by Protein Aggregation. mBio 2021, 12 (4), e00703-21 10.1128/mBio.00703-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govers S. K.; Mortier J.; Adam A.; Aertsen A. Protein Aggregates Encode Epigenetic Memory of Stressful Encounters in Individual Escherichia Coli Cells. PLOS Biol. 2018, 16 (8), e2003853 10.1371/journal.pbio.2003853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A.; Lloyd-Price J.; Neeli-Venkata R.; Oliveira S. M. D.; Ribeiro A. S. In Vivo Kinetics of Segregation and Polar Retention of MS2-GFP-RNA Complexes in Escherichia Coli. Biophys. J. 2014, 106 (9), 1928–1937. 10.1016/j.bpj.2014.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstraeten N.; Fauvart M.; Versées W.; Michiels J. The Universally Conserved Prokaryotic GTPases. Microbiol. Mol. Biol. Rev. 2011, 75 (3), 507–542. 10.1128/MMBR.00009-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourges A. C.; Lazarev A.; Declerck N.; Rogers K. L.; Royer C. A. Quantitative High-Resolution Imaging of Live Microbial Cells at High Hydrostatic Pressure. Biophys. J. 2020, 118 (11), 2670–2679. 10.1016/j.bpj.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babl L.; Giacomelli G.; Ramm B.; Gelmroth A.-K.; Bramkamp M.; Schwille P. CTP-Controlled Liquid–Liquid Phase Separation of ParB. J. Mol. Biol. 2022, 434 (2), 167401 10.1016/j.jmb.2021.167401. [DOI] [PubMed] [Google Scholar]
- Ebersbach G.; Briegel A.; Jensen G. J.; Jacobs-Wagner C. A Self-Associating Protein Critical for Chromosome Attachment, Division, and Polar Organization in Caulobacter. Cell 2008, 134 (6), 956–968. 10.1016/j.cell.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman G. R.; Comolli L. R.; Zhu J.; Eckart M.; Koenig M.; Downing K. H.; Moerner W. E.; Earnest T.; Shapiro L. A Polymeric Protein Anchors the Chromosomal Origin/ParB Complex at a Bacterial Cell Pole. Cell 2008, 134 (6), 945–955. 10.1016/j.cell.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laloux G.; Jacobs-Wagner C. Spatiotemporal Control of PopZ Localization through Cell Cycle–Coupled Multimerization. J. Cell Biol. 2013, 201 (6), 827–841. 10.1083/jcb.201303036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graumann P. L. Chromosome Architecture and Segregation in Prokaryotic Cells. Microb. Physiol. 2015, 24 (5–6), 291–300. 10.1159/000369100. [DOI] [PubMed] [Google Scholar]
- Gruber S.; Errington J. Recruitment of Condensin to Replication Origin Regions by ParB/SpoOJ Promotes Chromosome Segregation in B. Subtilis. Cell 2009, 137 (4), 685–696. 10.1016/j.cell.2009.02.035. [DOI] [PubMed] [Google Scholar]
- Tran N. T.; Laub M. T.; Le T. B. K. SMC Progressively Aligns Chromosomal Arms in Caulobacter Crescentus but Is Antagonized by Convergent Transcription. Cell Rep. 2017, 20 (9), 2057–2071. 10.1016/j.celrep.2017.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaboja X.; Ren Z.; Brandão H. B.; Paul P.; Rudner D. Z.; Wang X. XerD Unloads Bacterial SMC Complexes at the Replication Terminus. Mol. Cell 2021, 81 (4), 756–766.e8. 10.1016/j.molcel.2020.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkelä J.; Sherratt D. J. Organization of the Escherichia Coli Chromosome by a MukBEF Axial Core. Mol. Cell 2020, 78 (2), 250–260.e5. 10.1016/j.molcel.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolivos S.; Upton A. L.; Badrinarayanan A.; Müller J.; Zawadzka K.; Wiktor J.; Gill A.; Arciszewska L.; Nicolas E.; Sherratt D. MatP Regulates the Coordinated Action of Topoisomerase IV and MukBEF in Chromosome Segregation. Nat. Commun. 2016, 7 (1), 10466. 10.1038/ncomms10466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas E.; Upton A. L.; Uphoff S.; Henry O.; Badrinarayanan A.; Sherratt D. The SMC Complex MukBEF Recruits Topoisomerase IV to the Origin of Replication Region in Live Escherichia Coli. mBio 2014, 5 (1), e01001–13. 10.1128/mBio.01001-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadesse S.; Graumann P. L. Differential and Dynamic Localization of Topoisomerases in Bacillus Subtilis. J. Bacteriol. 2006, 188 (8), 3002–3011. 10.1128/JB.188.8.3002-3011.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Männik J.; Castillo D. E.; Yang D.; Siopsis G.; Männik J. The Role of MatP, ZapA and ZapB in Chromosomal Organization and Dynamics in Escherichia Coli. Nucleic Acids Res. 2016, 44 (3), 1216–1226. 10.1093/nar/gkv1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki S.; Jenal U.; Katayama T. Novel Divisome-Associated Protein Spatially Coupling the Z-Ring with the Chromosomal Replication Terminus in Caulobacter Crescentus. mBio 2020, 11 (2), e00487–20. 10.1128/mBio.00487-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H.; McManus H. R.; Dove S. L.; Bernhardt T. G. Nucleoid Occlusion Factor SlmA Is a DNA-Activated FtsZ Polymerization Antagonist. Proc. Natl. Acad. Sci. U. S. A. 2011, 108 (9), 3773–3778. 10.1073/pnas.1018674108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeusser D. P.; Margolin W. Splitsville: Structural and Functional Insights into the Dynamic Bacterial Z Ring. Nat. Rev. Microbiol 2016, 14 (5), 305–319. 10.1038/nrmicro.2016.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du S.; Lutkenhaus J. At the Heart of Bacterial Cytokinesis: The Z Ring. Trends Microbiol 2019, 27 (9), 781–791. 10.1016/j.tim.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz C.; Natale P.; Cueto L.; Vicente M. The Keepers of the Ring: Regulators of FtsZ Assembly. FEMS Microbiol Rev. 2016, 40 (1), 57–67. 10.1093/femsre/fuv040. [DOI] [PubMed] [Google Scholar]
- Zorrilla S.; Monterroso B.; Robles-Ramos M.-Á.; Margolin W.; Rivas G. FtsZ. Interactions and Biomolecular Condensates as Potential Targets for New Antibiotics. Antibiotics 2021, 10 (3), 254. 10.3390/antibiotics10030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas G.; Alfonso C.; Jimenez M.; Monterroso B.; Zorrilla S. Macromolecular Interactions of the Bacterial Division FtsZ Protein: From Quantitative Biochemistry and Crowding to Reconstructing Minimal Divisomes in the Test Tube. Biophys Rev. 2013, 5 (2), 63–77. 10.1007/s12551-013-0115-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas G.; Fernandez J. A.; Minton A. P. Direct Observation of the Enhancement of Noncooperative Protein Self-Assembly by Macromolecular Crowding: Indefinite Linear Self-Association of Bacterial Cell Division Protein FtsZ.. Proc. Natl. Acad. Sci. U A 2001, 98 (6), 3150–3155. 10.1073/pnas.051634398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas G.; López A.; Mingorance J.; Ferrándiz M. J.; Zorrilla S.; Minton A. P.; Vicente M.; Andreu J. M. Magnesium-Induced Linear Self-Association of the FtsZ Bacterial Cell Division Protein Monomer. The Primary Steps for FtsZ Assembly. J. Biol. Chem. 2000, 275 (16), 11740–11749. 10.1074/jbc.275.16.11740. [DOI] [PubMed] [Google Scholar]
- Mingorance J.; Rivas G.; Vélez M.; Gómez-Puertas P.; Vicente M. Strong FtsZ Is with the Force: Mechanisms to Constrict Bacteria. Trends Microbiol 2010, 18 (8), 348–356. 10.1016/j.tim.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Naddaf L.; Sayyed-Ahmad A. Intracellular Crowding Effects on the Self-Association of the Bacterial Cell Division Protein FtsZ. Arch. Biochem. Biophys. 2014, 564, 12–19. 10.1016/j.abb.2014.08.016. [DOI] [PubMed] [Google Scholar]
- Popp D.; Iwasa M.; Narita A.; Erickson H. P.; Maeda Y. FtsZ Condensates: An in Vitro Electron Microscopy Study. Biopolymers 2009, 91 (5), 340–350. 10.1002/bip.21136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monterroso B.; Reija B.; Jimenez M.; Zorrilla S.; Rivas G. Charged Molecules Modulate the Volume Exclusion Effects Exerted by Crowders on FtsZ Polymerization. PLoS One 2016, 11 (2), e0149060 10.1371/journal.pone.0149060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popp D.; Iwasa M.; Erickson H. P.; Narita A.; Maéda Y.; Robinson R. C. Suprastructures and Dynamic Properties of Mycobacterium Tuberculosis FtsZ. J. Biol. Chem. 2010, 285 (15), 11281–11289. 10.1074/jbc.M109.084079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson H. P.; Anderson D. E.; Osawa M. FtsZ in Bacterial Cytokinesis: Cytoskeleton and Force Generator All in One. Microbiol Mol. Biol. Rev. 2010, 74 (4), 504–528. 10.1128/MMBR.00021-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitley K. D.; Jukes C.; Tregidgo N.; Karinou E.; Almada P.; Cesbron Y.; Henriques R.; Dekker C.; Holden S. FtsZ. Treadmilling Is Essential for Z-Ring Condensation and Septal Constriction Initiation in Bacillus Subtilis Cell Division. Nat. Commun. 2021, 12 (1), 2448. 10.1038/s41467-021-22526-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squyres G. R.; Holmes M. J.; Barger S. R.; Pennycook B. R.; Ryan J.; Yan V. T.; Garner E. C. Single-Molecule Imaging Reveals That Z-Ring Condensation Is Essential for Cell Division in Bacillus Subtilis. Nat. Microbiol. 2021, 6 (5), 553–562. 10.1038/s41564-021-00878-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin D. M.; de Boer P. A. MinDE-Dependent Pole-to-Pole Oscillation of Division Inhibitor MinC in Escherichia Coli. J. Bacteriol. 1999, 181 (20), 6419–6424. 10.1128/JB.181.20.6419-6424.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin D. M.; de Boer P. A. Rapid Pole-to-Pole Oscillation of a Protein Required for Directing Division to the Middle of Escherichia Coli. Proc. Natl. Acad. Sci. U. S. A. 1999, 96 (9), 4971–4976. 10.1073/pnas.96.9.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiomi D.; Margolin W. The C-Terminal Domain of MinC Inhibits Assembly of the Z Ring in Escherichia Coli. J. Bacteriol. 2007, 189 (1), 236–243. 10.1128/JB.00666-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dajkovic A.; Lan G.; Sun S. X.; Wirtz D.; Lutkenhaus J. MinC Spatially Controls Bacterial Cytokinesis by Antagonizing the Scaffolding Function of FtsZ. Curr. Biol. 2008, 18 (4), 235–244. 10.1016/j.cub.2008.01.042. [DOI] [PubMed] [Google Scholar]
- Shen B.; Lutkenhaus J. Examination of the Interaction between FtsZ and MinC N in E. Coli Suggests How MinC Disrupts Z Rings. Mol. Microbiol. 2010, 75 (5), 1285–1298. 10.1111/j.1365-2958.2010.07055.x. [DOI] [PubMed] [Google Scholar]
- Martos A.; Raso A.; Jimenez M.; Petrasek Z.; Rivas G.; Schwille P. FtsZ Polymers Tethered to the Membrane by ZipA Are Susceptible to Spatial Regulation by Min Waves. Biophys. J. 2015, 108 (9), 2371–2383. 10.1016/j.bpj.2015.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonthat N. K.; Milam S. L.; Chinnam N.; Whitfill T.; Margolin W.; Schumacher M. A. SlmA Forms a Higher-Order Structure on DNA That Inhibits Cytokinetic Z-Ring Formation over the Nucleoid. Proc. Natl. Acad. Sci. U. S. A. 2013, 110 (26), 10586–10591. 10.1073/pnas.1221036110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt T. G.; de Boer P. A. SlmA, a Nucleoid-Associated, FtsZ Binding Protein Required for Blocking Septal Ring Assembly over Chromosomes in E. Coli. Mol. Cell 2005, 18 (5), 555–564. 10.1016/j.molcel.2005.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabre E. J.; Monterroso B.; Alfonso C.; Sanchez-Gorostiaga A.; Reija B.; Jimenez M.; Vicente M.; Zorrilla S.; Rivas G. The Nucleoid Occlusion SlmA Protein Accelerates the Disassembly of the FtsZ Protein Polymers without Affecting Their GTPase Activity. PLoS One 2015, 10 (5), e0126434 10.1371/journal.pone.0126434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey M. W.; Bisicchia P.; Warren B. T.; Sherratt D. J.; Männik J. Evidence for Divisome Localization Mechanisms Independent of the Min System and SlmA in Escherichia Coli. PLoS Genet. 2014, 10 (8), e1004504 10.1371/journal.pgen.1004504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reija B.; Monterroso B.; Jimenez M.; Vicente M.; Rivas G.; Zorrilla S. Development of a Homogeneous Fluorescence Anisotropy Assay to Monitor and Measure FtsZ Assembly in Solution. Anal. Biochem. 2011, 418 (1), 89–96. 10.1016/j.ab.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Mellouli S.; Monterroso B.; Vutukuri H. R.; te Brinke E.; Chokkalingam V.; Rivas G.; Huck W. T. S. Self-Organization of the Bacterial Cell-Division Protein FtsZ in Confined Environments. Soft Matter 2013, 9, 10493–10500. 10.1039/c3sm51163d. [DOI] [Google Scholar]
- Groen J.; Foschepoth D.; Te Brinke E.; Boersma A. J.; Imamura H.; Rivas G.; Heus H. A.; Huck W. T. S. Associative Interactions in Crowded Solutions of Biopolymers Counteract Depletion Effects. J. Am. Chem. Soc. 2015, 137 (40), 13041–13048. 10.1021/jacs.5b07898. [DOI] [PubMed] [Google Scholar]
- Kohyama S.; Merino-Salomón A.; Schwille P. In Vitro Assembly, Positioning and Contraction of a Division Ring in Minimal Cells. Nat. Commun. 2022, 13 (1), 6098. 10.1038/s41467-022-33679-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arjunan S. N. V.; Tomita M. A New Multicompartmental Reaction-Diffusion Modeling Method Links Transient Membrane Attachment of E. Coli MinE to E-Ring Formation. Syst. Synth. Biol. 2010, 4 (1), 35–53. 10.1007/s11693-009-9047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loose M.; Fischer-Friedrich E.; Ries J.; Kruse K.; Schwille P. Spatial Regulators for Bacterial Cell Division Self-Organize into Surface Waves in Vitro. Science 2008, 320 (5877), 789–792. 10.1126/science.1154413. [DOI] [PubMed] [Google Scholar]
- Chiang Y.-L.; Chang Y.-C.; Chiang I.-C.; Mak H.-M.; Hwang I.-S.; Shih Y.-L. Atomic Force Microscopy Characterization of Protein Fibrils Formed by the Amyloidogenic Region of the Bacterial Protein MinE on Mica and a Supported Lipid Bilayer. PLoS One 2015, 10 (11), e0142506 10.1371/journal.pone.0142506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monterroso B.; Robles-Ramos M. A.; Zorrilla S.; Rivas G. Reconstituting Bacterial Cell Division Assemblies in Crowded, Phase-Separated Media. Methods Enzym. 2021, 646, 19–49. 10.1016/bs.mie.2020.06.012. [DOI] [PubMed] [Google Scholar]
- Monterroso B.; Zorrilla S.; Sobrinos-Sanguino M.; Keating C. D.; Rivas G. Microenvironments Created by Liquid-Liquid Phase Transition Control the Dynamic Distribution of Bacterial Division FtsZ Protein. Sci. Rep 2016, 6, 35140. 10.1038/srep35140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating C. D. Aqueous Phase Separation as a Possible Route to Compartmentalization of Biological Molecules. Acc. Chem. Res. 2012, 45 (12), 2114–2124. 10.1021/ar200294y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich M. R.; El-Kouedi M.; Etherton M. R.; Keating C. D. Partitioning and Assembly of Metal Particles and Their Bioconjugates in Aqueous Two-Phase Systems. Langmuir 2005, 21 (18), 8478–8486. 10.1021/la051220z. [DOI] [PubMed] [Google Scholar]
- Godino E.; Doerr A.; Danelon C. Min Waves without MinC Can Pattern FtsA-Anchored FtsZ Filaments on Model Membranes. Commun. Biol. 2022, 5 (1), 675. 10.1038/s42003-022-03640-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobrinos-Sanguino M.; Zorrilla S.; Keating C. D.; Monterroso B.; Rivas G. Encapsulation of a Compartmentalized Cytoplasm Mimic within a Lipid Membrane by Microfluidics. Chem. Commun. 2017, 53, 4775–4778. 10.1039/C7CC01289F. [DOI] [PubMed] [Google Scholar]
- Gardner K. A. J. A.; Moore D. A.; Erickson H. P. The C-Terminal Linker of Escherichia Coli FtsZ Functions as an Intrinsically Disordered Peptide: FtsZ Linker Is an Intrinsically Disordered Peptide. Mol. Microbiol. 2013, 89 (2), 264–275. 10.1111/mmi.12279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buske P. J.; Levin P. A. A Flexible C-Terminal Linker Is Required for Proper FtsZ Assembly in Vitro and Cytokinetic Ring Formation in Vivo. Mol. Microbiol. 2013, 89 (2), 249–263. 10.1111/mmi.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fare C. M.; Villani A.; Drake L. E.; Shorter J. Higher-Order Organization of Biomolecular Condensates. Open Biol. 2021, 11 (6), 210137 10.1098/rsob.210137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X. C.; Margolin W.; Gonzalez-Garay M. L.; Cabral F. Vinblastine Induces an Interaction between FtsZ and Tubulin in Mammalian Cells. J. Cell Sci. 1999, 112 (14), 2301–2311. 10.1242/jcs.112.14.2301. [DOI] [PubMed] [Google Scholar]
- Yu J.; Liu Y.; Yin H.; Chang Z. Regrowth-Delay Body as a Bacterial Subcellular Structure Marking Multidrug-Tolerant Persisters. Cell Discov 2019, 5, 8. 10.1038/s41421-019-0080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanbichler M.; Shapiro L. MipZ, a Spatial Regulator Coordinating Chromosome Segregation with Cell Division in Caulobacter. Cell 2006, 126 (1), 147–162. 10.1016/j.cell.2006.05.038. [DOI] [PubMed] [Google Scholar]
- Wu L. J.; Errington J. Coordination of Cell Division and Chromosome Segregation by a Nucleoid Occlusion Protein in Bacillus Subtilis. Cell 2004, 117 (7), 915–925. 10.1016/j.cell.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Yu Y.; Zhou J.; Gueiros-Filho F. J.; Kearns D. B.; Jacobson S. C. Noc Corrals Migration of FtsZ Protofilaments during Cytokinesis in Bacillus Subtilis. mBio 2021, 12 (1), e02964-20 10.1128/mBio.02964-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalal A. S. B.; Tran N. T.; Wu L. J.; Ramakrishnan K.; Rejzek M.; Gobbato G.; Stevenson C. E. M.; Lawson D. M.; Errington J.; Le T. B. K. CTP Regulates Membrane-Binding Activity of the Nucleoid Occlusion Protein Noc. Mol. Cell 2021, 81 (17), 3623–3636.e6. 10.1016/j.molcel.2021.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riback J. A.; Katanski C. D.; Kear-Scott J. L.; Pilipenko E. V.; Rojek A. E.; Sosnick T. R.; Drummond D. A. Stress-Triggered Phase Separation Is an Adaptive, Evolutionarily Tuned Response. Cell 2017, 168 (6), 1028–1040 e19. 10.1016/j.cell.2017.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile W.; Salvatore M.; Bassot C.; Elofsson A. Why Do Eukaryotic Proteins Contain More Intrinsically Disordered Regions?. PLOS Comput. Biol. 2019, 15 (7), e1007186 10.1371/journal.pcbi.1007186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Lee R.; Buljan M.; Lang B.; Weatheritt R. J.; Daughdrill G. W.; Dunker A. K.; Fuxreiter M.; Gough J.; Gsponer J.; Jones D. T.; et al. Classification of Intrinsically Disordered Regions and Proteins. Chem. Rev. 2014, 114 (13), 6589–6631. 10.1021/cr400525m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C.; Zhao W.; Duvall S. W.; Kowallis K. A.; Childers W. S. Regulation of the Activity of the Bacterial Histidine Kinase PleC by the Scaffolding Protein PodJ. J. Biol. Chem. 2022, 298 (4), 101683 10.1016/j.jbc.2022.101683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R. A.; Gollan B.; Helaine S. Persistent Bacterial Infections and Persister Cells. Nat. Rev. Microbiol 2017, 15 (8), 453–464. 10.1038/nrmicro.2017.42. [DOI] [PubMed] [Google Scholar]
- Balaban N. Q.; Merrin J.; Chait R.; Kowalik L.; Leibler S. Bacterial Persistence as a Phenotypic Switch. Science 2004, 305 (5690), 1622–1625. 10.1126/science.1099390. [DOI] [PubMed] [Google Scholar]
- Alberti S.; Dormann D. Liquid-Liquid Phase Separation in Disease. Annu. Rev. Genet 2019, 53, 171–194. 10.1146/annurev-genet-112618-043527. [DOI] [PubMed] [Google Scholar]
- Pu Y.; Li Y.; Jin X.; Tian T.; Ma Q.; Zhao Z.; Lin S.-Y.; Chen Z.; Li B.; Yao G.; et al. ATP-Dependent Dynamic Protein Aggregation Regulates Bacterial Dormancy Depth Critical for Antibiotic Tolerance. Mol. Cell 2019, 73 (1), 143–156.e4. 10.1016/j.molcel.2018.10.022. [DOI] [PubMed] [Google Scholar]
- Bobst E. V.; Bobst A. M.; Perrino F. W.; Meyer R. R.; Rein D. C. Variability in the Nucleic Acid Binding Site Size and the Amount of Single-Stranded DNA-Binding Protein in Escherichia Coli. FEBS Lett. 1985, 181 (1), 133–137. 10.1016/0014-5793(85)81128-5. [DOI] [PubMed] [Google Scholar]
- Kozlov A. G.; Weiland E.; Mittal A.; Waldman V.; Antony E.; Fazio N.; Pappu R. V.; Lohman T. M. Intrinsically Disordered C-Terminal Tails of E. Coli Single-Stranded DNA Binding Protein Regulate Cooperative Binding to Single-Stranded DNA. J. Mol. Biol. 2015, 427 (4), 763–774. 10.1016/j.jmb.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janissen R.; Arens M. M. A.; Vtyurina N. N.; Rivai Z.; Sunday N. D.; Eslami-Mossallam B.; Gritsenko A. A.; Laan L.; de Ridder D.; Artsimovitch I.; et al. Global DNA Compaction in Stationary-Phase Bacteria Does Not Affect Transcription. Cell 2018, 174 (5), 1188–1199 e14. 10.1016/j.cell.2018.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephani K.; Weichart D.; Hengge R. Dynamic Control of Dps Protein Levels by ClpXP and ClpAP Proteases in Escherichia Coli: Regulated Proteolysis of Dps Protein. Mol. Microbiol. 2003, 49 (6), 1605–1614. 10.1046/j.1365-2958.2003.03644.x. [DOI] [PubMed] [Google Scholar]
- Oberto J.; Nabti S.; Jooste V.; Mignot H.; Rouviere-Yaniv J. The HU Regulon Is Composed of Genes Responding to Anaerobiosis, Acid Stress, High Osmolarity and SOS Induction. PLoS One 2009, 4 (2), e4367. 10.1371/journal.pone.0004367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krypotou E.; Townsend G. E.; Gao X.; Tachiyama S.; Liu J.; Pokorzynski N. D.; Goodman A. L.; Groisman E. A. Bacteria Require Phase Separation for Fitness in the Mammalian Gut. Science 2023, 379 (6637), 1149–1156. 10.1126/science.abn7229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattanayak G. K.; Liao Y.; Wallace E. W. J.; Budnik B.; Drummond D. A.; Rust M. J. Daily Cycles of Reversible Protein Condensation in Cyanobacteria. Cell Rep. 2020, 32 (7), 108032 10.1016/j.celrep.2020.108032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racki L. R.; Tocheva E. I.; Dieterle M. G.; Sullivan M. C.; Jensen G. J.; Newman D. K. Polyphosphate Granule Biogenesis Is Temporally and Functionally Tied to Cell Cycle Exit during Starvation in Pseudomonas Aeruginosa. Proc. Natl. Acad. Sci. U A 2017, 114 (12), E2440–E2449. 10.1073/pnas.1615575114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriel A.; Bittner A. N.; Kim S. H.; Liu K.; Tehranchi A. K.; Zou W. Y.; Rendon S.; Chen R.; Tu B. P.; Wang J. D. Direct Regulation of GTP Homeostasis by (p)ppGpp: A Critical Component of Viability and Stress Resistance. Mol. Cell 2012, 48 (2), 231–241. 10.1016/j.molcel.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Zbornikova E.; Rejman D.; Gerdes K.. Novel (p)ppGpp Binding and Metabolizing Proteins of Escherichia Coli. mBio 2018, 9 ( (2), ), 10.1128/mBio.02188-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms A.; Maisonneuve E.; Gerdes K.. Mechanisms of Bacterial Persistence during Stress and Antibiotic Exposure. Science 2016, 354 ( (6318), ), 10.1126/science.aaf4268. [DOI] [PubMed] [Google Scholar]







