ABSTRACT
The generation of nitrite by the oral microbiota is believed to contribute to healthy cardiovascular function, with oral nitrate reduction to nitrite associated with systemic blood pressure regulation. There is the potential to manipulate the composition or activities of the oral microbiota to a higher nitrate-reducing state through nitrate supplementation. The current study examined microbial community composition and enzymatic responses to nitrate supplementation in sessile oral microbiota grown in continuous culture. Nitrate reductase (NaR) activity and nitrite concentrations were not significantly different to tongue-derived inocula in model biofilms. These were generally dominated by Streptococcus spp., initially, and a single nitrate supplementation resulted in the increased relative abundance of the nitrate-reducing genera Veillonella, Neisseria, and Proteus spp. Nitrite concentrations increased concomitantly and continued to increase throughout oral microbiota development. Continuous nitrate supplementation, over a 7-day period, was similarly associated with an elevated abundance of nitrate-reducing taxa and increased nitrite concentration in the perfusate. In experiments in which the models were established in continuous low or high nitrate environments, there was an initial elevation in nitrate reductase, and nitrite concentrations reached a relatively constant concentration over time similar to the acute nitrate challenge with a similar expansion of Veillonella and Neisseria. In summary, we have investigated nitrate metabolism in continuous culture oral biofilms, showing that nitrate addition increases nitrate reductase activity and nitrite concentrations in oral microbiota with the expansion of putatively NaR-producing taxa.
IMPORTANCE
Clinical evidence suggests that blood pressure regulation can be promoted by nitrite generated through the reduction of supplemental dietary nitrate by the oral microbiota. We have utilized oral microbiota models to investigate the mechanisms responsible, demonstrating that nitrate addition increases nitrate reductase activity and nitrite concentrations in oral microbiota with the expansion of nitrate-reducing taxa.
KEYWORDS: oral microbiota, multiple Sorbarod device, dietary nitrate, nitrite, nitric oxide, blood pressure, hypertension
INTRODUCTION
Hypertension, defined as blood pressure (BP) equal to or greater than 140/90 mmHg, significantly increases the risk of hemorrhagic stroke, myocardial infarction, heart failure, and chronic renal disease (1, 2). Between 1995 and 2015, there was a global increase in systolic BP (SBP), loss of disability-adjusted life-years and BP-related deaths (3). As one of the most preventable causes of premature morbidity and mortality globally, improved treatment and prevention of hypertension is a high priority. Modern genomic-driven diagnostic and therapeutic technologies may offer new biomarkers and novel pathways in the treatment of hypertension (4). The metabolism of dietary nitrate to nitrite, which may regulate systemic cardiovascular function and BP (5–7), offers a novel route for intervention. There is currently substantial ongoing research into using dietary nitrate [as reviewed by Alzahrani et al. (8)] to target nitrate reduction to improve systemic cardiovascular health, all reporting wide variability in efficacy between agents and target populations (9, 10).
Inorganic nitrate is introduced into the human body via food and drinking water. Dietary nitrates originate from the fixation of environmental nitrogen by bacteria and are accumulated in high abundance in green, leafy vegetables, such as spinach and lettuce (11). Additional sources include nitrate/nitrite ions added to cured meats as a preservative (12). Once ingested, nitrate is rapidly absorbed across the gastrointestinal (GI) tract and into the plasma. Nitrate is actively transported from the plasma into the salivary glands by sialic acid transporters (13, 14), resulting in high nitrate concentrations in saliva, where oral bacteria can reduce the nitrate to nitrite by the actions of nitrate reductase (NaR) enzymes (14). The surface of the tongue is reported to be the location where most of the nitrate reduction within the mouth occurs (15, 16). The nitrite then enters the GI tract and leads to the generation of nitric oxide (NO) in blood and tissues, which is dubbed the alternative enterosalivary pathway. NO is a pleiotropic signaling molecule, with wide-ranging effects in the body, and is critical in cardiovascular homeostasis. Reduced bioavailability of NO has been implicated in the pathology of cardiovascular and renal diseases (17–22). NO regulates vascular tone by relaxing smooth muscle cells, leading to vasodilation of blood vessels. NO therefore has the potential to lower high BP via smooth muscle vasodilation (20).
Disruption of microbial homeostasis in the mouth has been associated with hypertension (23). It has been reported that disturbances of the oral microbial communities as a result of mouthwash use may decrease oral NaR activity and increase BP in both hypertensive (24) and normotensive adults (25–29). The oral microbiota, therefore, may play a critical role in our systemic health and, consequently, manipulation of these communities may offer a novel tool of intervention in cardiovascular disorders (30). There is now an emerging field of microbiome-driven research aimed at increasing NO production, via nitrate supplementation, for the treatment of hypertension (30).
There is currently uncertainty about the clinical benefits of dietary supplementation with nitrate. Studies have generally utilized nitrate-rich beetroot juice as the dietary intervention. Some studies reported a significant lowering of BP and an improvement in vascular function in adults (31–35), while others reported no effects on cardiovascular function from acute or chronic dietary nitrate dosing (24, 36). Higher oral NaR is significantly associated with greater BP decrease following acute nitrate supplementation (37–40). Understanding this variability in the efficacy of nitrate supplementation in the treatment of hypertension may enable the optimization of interventions. Currently, there is little information about the effects of nitrate supplementation on the taxonomic composition of the oral microbiota. In the present study, therefore, we have used the multiple Sorbarod device (MSD) to establish oral microcosms in an in vitro continuous culture system and used this to evaluate the effects of nitrate dosing on bacterial community structure and associated NaR activity. MSDs are based on the establishment of biofilms (BF) on filters through which growth medium is perfused, which enables both biofilm and perfusate (PA) to be monitored. Models were inoculated with samples from the tongue of several healthy volunteers to replicate communities most relevant to the oral microbiome. Bacterial populations were monitored using 16S rRNA gene sequencing. NaR activity and perfusate nitrite concentration were determined longitudinally, following nitrate dosing.
MATERIALS AND METHODS
Volunteers
All reagents were obtained from Sigma-Aldrich (Gillingham, Dorset, UK) unless otherwise stated. Healthy participants (n = 4; subjects 1–4) were recruited via study advertisements. Inclusion criteria included being aged between 18 and 45, no antibiotic use within 6 months, not currently experiencing any self-assessed oral disease and not a current tobacco smoker.
Oral sample collection
Tongue surface samples were collected using a sterile Tongue Cleaner (Superdrug, Surrey, UK) in three motions from the rear to the front of the tongue. The end of the Tongue Cleaner was placed in a sterile vessel containing 2 mL phosphate-buffered saline (PBS, pH = 7.4, M/L = 1.37 M sodium chloride, 0.27 M potassium chloride, 0.1 M phosphate buffer; VWR, Lutterworth, UK) and 1 g of 3.5 mm–5.5 mm sterile glass beads (VWR, Lutterworth, UK). The solution was mixed via vortex (30 seconds), transferred to a microcentrifuge tube (Thermo Scientific, MA, USA), and frozen at −80°C for subsequent
Continuous culture oral microcosms
MSDs are in vitro models which support the growth of biofilms on five cellulose filter cylinders in parallel (10 mm diameter; 20 mm length) within a stainless steel housing (as depicted in Fig. S1). The use of the device to grow oral microcosms has been previously reported (41–45). MSDs can be sampled destructively by removal and processing of individual colonized filters or by sampling the perfusate which is generated continuously. MSDs were contained within a standard incubator maintained at 36°C. In this study, three models were exploited to (i) validate the use of MSDs for assessment of time-dependent changes in nitrate metabolism (subject 1), (ii) explore how nitrate intervention might impact both metabolism and microbial community composition (subject 2), and (iii) compare the effects of nitrate intervention relative to a non-treated control over time (subject 3/4). The models utilized are shown in Fig. 1.
Fig 1.
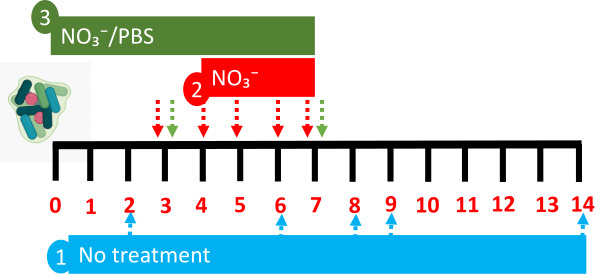
Models utilized to explore nitrate metabolism in vitro. Three models were utilized in this study to (i) validate the use of an oral microcosm model to examine time-dependent changes in microbial community composition and nitrate metabolism, (ii) explore how nitrate (15 mM) intervention manipulates biofilm composition, and (iii) compare the effects of nitrate (15 mM) on biofilm composition and nitrate metabolism relative to a PBS control in oral microcosm communities derived from two individuals. Arrows depict time points for sample collection for 16S rRNA sequencing and nitrate metabolism assays.
Microcosm growth conditions
The microcosms were formed in modified culture medium comprising (g/L in distilled water: 2.5 g mucin (type II; porcine; gastric), 2.0 g bacteriological peptone, 2.0 g tryptone, 1.0 g yeast extract, 0.35 g sodium chloride, 0.2 g potassium chloride, 0.2 g calcium chloride, 0.1 g cysteine hydrochloride, 0.001 g hemin, 0.002 g vitamin K1) under gentle agitation using a magnetic stirrer (42). Models were preconditioned with growth medium for 2 h via a peristaltic pump (Minipulse 3; Gilson, Villiers-Le-Bel, France) and throughout model runs (flow rate = 4 mL/min.). The pumps were switched off during primary inoculation. For each experiment, fermenters were inoculated on two occasions, 4 h apart, by removing the upper section of the MSD and depositing 200 µL of freshly collected saliva from the same donor directly into the upper equilibration chamber as described by McBain et al. (42, 46). Sodium nitrate [15 mM; selected based upon the maximum human salivary concentration following dietary sodium nitrate dosing and use in clinical studies investigating the effects of nitrate intervention on hypertension in vivo (38, 47, 48)] or PBS (control) were applied to each model system as depicted in Fig. 1 (49). The inoculum was frozen at −80°C for subsequent 16S rRNA gene sequencing and nitrate metabolism studies as described below.
Analysis of biofilms and perfusate
After 3 days of microcosm equilibration, 5 mL perfusate (PA) was collected from the perfusate equilibrium chamber (Fig. S1) by placing a sterile Universal bottle (Medline Scientific, Oxfordshire, UK) underneath the MSD waste tube. After sample collection, the area was manually sterilized using ethanol (70% vol/vol) and the waste collection vessel was reattached. To collect the biofilms (BF), the peristaltic pump was halted, and the model was opened aseptically. A single Sorbarod filter was removed and replaced with a sterile filter. Sorbarod filter samples were macerated via placement in half-strength thioglycolate medium (14.5 g/L; Oxoid, Basingstoke, UK) and 1 g of 3.5 mm–5.5 mm sterile glass beads (VWR, Lutterworth, UK). Samples were vortexed (30 seconds), transferred to a sterile tube (Thermo Scientific, MA, USA), and frozen at −80°C for subsequent molecular analysis. BF samples were then serially diluted 1:10 in PBS (101 – 106), and 200 µL was plated in duplicate onto selective and nonselective media: Wilkins-Chalgren agar, incubated both aerobically and anaerobically, and Mitis-Salivarius Agar with Tellurite (Oxoid, Basingstoke, UK). Aerobic samples were incubated at 37°C in a standard incubator for 7 days. Anaerobic plates were placed in an anaerobic chamber (atmosphere H2, 10%; CO2, 10%; N2, 80%) (Don Whitely Scientific, Shipley, UK) held at 37°C for 7 days. Colonies were counted and expressed as log10 colony-forming units (CFU) per milliliter of filter homogenate.
NaR and nitrite assays
The NaR assay was adapted from Lowe and Evans (50) and Allison and Macfarlane (51). Test inocula (0.1 mL) were equilibrated to 36°C in a buffer comprising 25 mM potassium phosphate buffer, 10 mM potassium nitrate, 0.05 mM ethylenediaminetetraacetic acid (1.8 mL), and 2 mM β-nicotinamide adenine dinucleotide (0.1 mL; MP Biomedicals, CA, USA). In parallel, baseline levels were determined via resuspension of test inocula (0.1 mL) without nitrate supplementation in 58 mM (1 mL) sulfanilamide stop solution. The baseline and test inocula were then incubated at 36°C under gentle agitation (100 RPM) for 1 h; stop solution was added to the test inocula (2 minutes). Colorimetric assessment was undertaken by the addition of 0.77 mM N-(1-Naphthyl)ethylenediamine dihydrochloride solution to test and baseline samples (10 minutes). Solutions were dispensed into a sterile 96-well plate (Corning, NY, USA), alongside a nitrite standard curve (0–36 mM), and absorbance was acquired at 540 nm (BioTek, VT, USA). To quantify nitrate reductase activity, relative difference between baseline and sample nitrite concentration was recorded and values were normalized against sample volume (100 µL).
16S rRNA gene sequencing and bioinformatics
DNA was extracted from test inocula, Sorbarod filter, and MSD perfusate samples using a DNeasy PowerSoil Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Extracted DNA samples were adjusted to a concentration of 1 ng/µL and subsequent amplification was achieved through 16S rRNA gene polymerase chain reaction (PCR) with Illumina (San Diego, USA) adapted 515F and 806R primers targeting the V4 region of the 16S (52) and NEBNext High-Fidelity 2X PCR Master Mix (New England Biolabs, Ipswich, USA) at 98°C (2 minutes), followed by 25 cycles of 95°C (20 seconds), 62°C (15 seconds), 70°C, (30 seconds), and a final elongation step of 72°C (5 minutes), which have been previously validated in oral microbiome studies (53–56). The amplified 16S rRNA gene was purified using a QIAquick purification kit (Qiagen, Hilden, Germany) and amplicons confirmed via gel electrophoresis.
Sequencing of the 16S rRNA gene V4 amplicons was performed on the Illumina MiSeq platform (Illumina Inc, Cambridge, UK). Raw sequence data were imported into the quantitative insights into microbial ecology (QIIME) version 2 (2020.2) (57, 58). The sequences were de-replicated, similarity clustered, analyses for chimeras, demultiplexed, and quality filtered using the d2-demux plug-in followed by denoising with DADA2 (q2-dada2) (59). Amplicon sequence variants (ASVs) were aligned via the q2-alignment plug-in and taxonomy assigned to the ASVs using the q2-feature-classifier (60) against the Greengenes (v13.8) 97% ASVs reference sequences (61) for the generation of biological observation matrix (BIOM) tables comprising the sample metadata. ASVs present in the negative controls were subtracted from the remaining samples. Following the importation of data using the qiime2R package (62), analysis was performed using the Phyloseq package (63) converting data into relative abundance, and ggPlot2 (64) was applied to generate figures in R version 3.6.2 (65). Statistical investigation of differential analysis of count data was accomplished with DESeq2 (66).
RESULTS
Validation of a model for reliable assessment of temporal changes in nitrate metabolism of oral microcosms in vitro
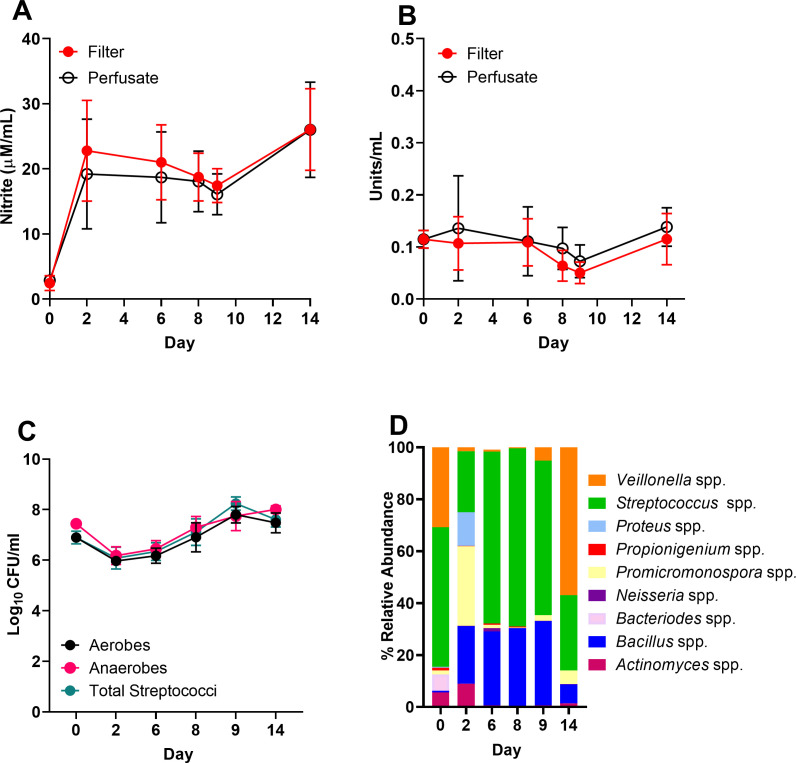
Oral biofilm formation is a dynamic process, in which pioneer colonizers including Streptococcus spp. initiate adhesion to the salivary pellicle, facilitating the recruitment of Veillonella spp. and subsequent biofilm maturation (67). Members of this dynamic community, including Veillonella spp., reduce nitrate to nitrite, the elevated circulation of which is implicated in reduced blood pressure (68). However, methods to reliably assess temporal changes in basal nitrate metabolism remain lacking. Initial validation of nitrate metabolism assays (experimental setup 1, Fig. 1 and 2A and B) revealed both nitrite secretion and nitrate reductase activity were detectable in microcosms not supplemented with sodium nitrate. However, no significant changes in the relative levels of nitrate metabolism were observed throughout this period, with nitrite quantification proving least variable 6–8 days post-inoculation (Fig. 2B). These nitrate metabolism assays were accompanied by differential CFU counts, in which the relative proportion of aerobes, anaerobes, and total streptococci remained stable throughout the 14-day incubation period (Fig. 2C). Therefore, a 7-day incubation period was selected for assessing the effects of nitrate intervention on nitrate metabolism. To complement nitrate metabolism assays, the use of 16S rRNA sequencing was exploited to ascertain time-dependent changes in oral microcosm community composition (Fig. 2D; Table S1). Monitoring temporal changes in oral biofilm composition indicated diminished diversity relative to the initial inocula (Fig. 2D; Table S1), although this was not significant for either alpha or beta diversity (P > 0.05, data not shown). Microbial community composition fluctuated across the 14-day culture period, but Streptococcus spp. proved most abundant across the study until day 14, when the relative abundance of Veillonella spp. accounted for 57.1% of the population (Fig. 2A through C; Table S1). Over the 14-day period, detectable levels of Bacillus spp. were observed and comprised 0.7% of the initial inoculum, the presence of which is associated with yellow tongue coating [Fig. 2A (69)].
Fig 2.
Detectable levels of nitrite and nitrate reductase in oral biofilm consortia. An MSD model was established and inoculated with tongue material collected from subject 1 inoculum (n = 2) . Nitrate metabolism of biofilm (filter) and perfusate samples was assessed via nitrite secretion (A) and nitrate reductase assays (B). Differential counts of aerobes, anaerobes (Wilkins-Chalgren agar (WCA)), or total streptococci (Mitis Salivarius agar (MSA)) in biofilm samples were surveyed via plate counting at each time point (C). 16S rRNA sequencing was then exploited to evaluate biofilm community composition (D) over 14 days. CFU, colony-forming unit.
Exogenous nitrate application elevates nitrite production in filtered but not perfused biofilms
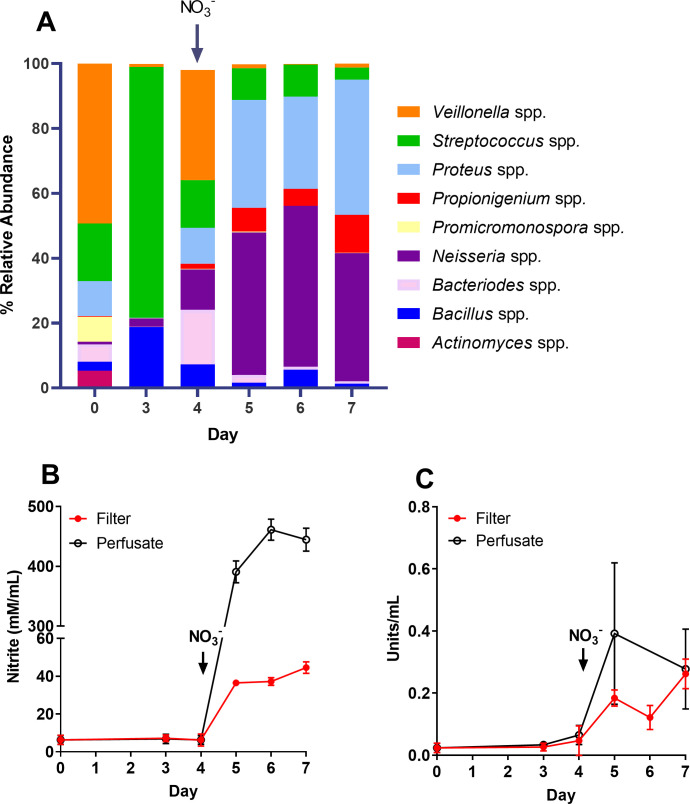
As observed when validating this system, nitrite and nitrate reductase are present at detectable levels in oral microcosm consortia. However, the acute exogenous application of nitrate has proven to impact not only biofilm nitrate metabolism but also composition. Nitrate-reducing genera including Neisseria spp. are enriched, while periopathogens including Porphyromonas spp. are diminished 9 h post nitrate application (70, 71). However, oral biofilm maturation can occur over a period of days (42, 72, 73). Therefore, as described in Fig. 1 (experimental setup 2), nitrate was applied to pre-formed biofilms (4 days) and, subsequently, time-dependent changes in nitrate metabolism and biofilm composition were assessed. Intriguingly, assessment of nitrate metabolism revealed nitrate intervention resulted in an increase in nitrite production in collected perfusate (Fig. 3B). Nitrate reductase activity also appeared to spike 24 h post intervention but stabilized on days 6–7 (Fig. 3C). Nitrate application was also accompanied by a shift in community composition (Fig. 3A). Prior to nitrate application (day 3), biofilm was dominated by Streptococcus spp. but, following nitrate intervention on day 4, shifted toward an increasing abundance of Neisseria and Proteus spp., a finding previously observed by Rosier et al. (70). To verify that this shift was due to nitrate intervention, changes in composition and nitrate metabolism were assessed in biofilms treated with nitrate or PBS (control) throughout culture.
Fig 3.
Elevated abundances of Neisseria and Proteus spp. following exposure of pre-formed biofilms to nitrate. Oral biofilm microcosms derived from subject 2 were established for 4 days on cellulose filters in a continuous culture flow-through system, and subsequently exposed to nitrate. Changes in biofilm composition (A), nitrite secretion (B), and nitrate reductase activity (C) were assessed over 7 days from both biofilm (filter) and perfusate samples pre- and post-exposure to sodium nitrate (15 mM, day 4).
Nitrate intervention may result in the elevated abundance of Neisseria spp. in some individuals
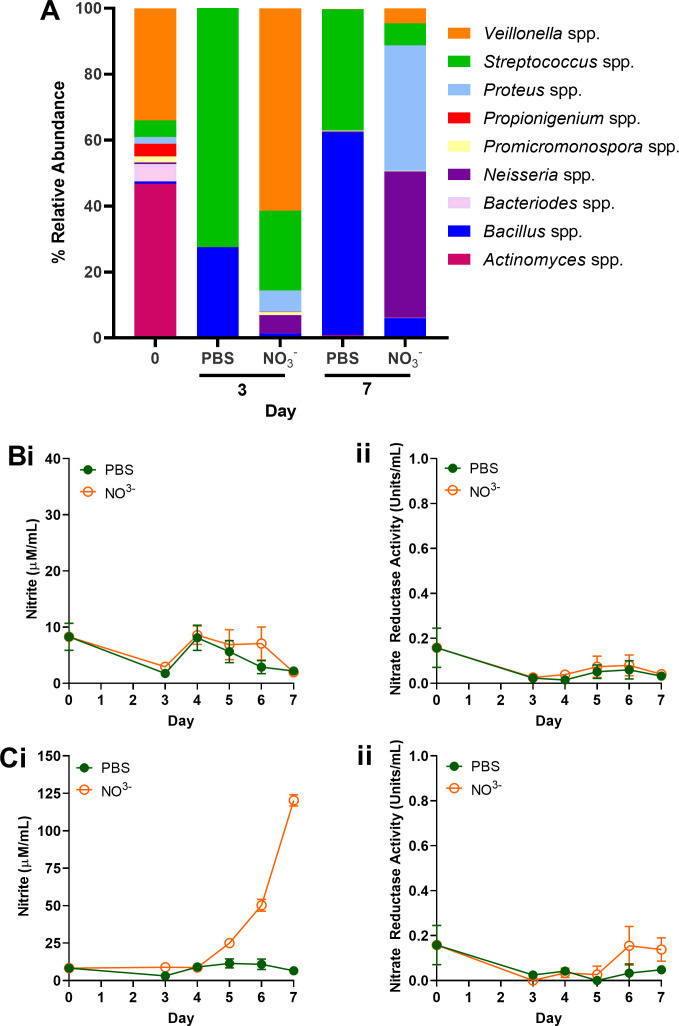
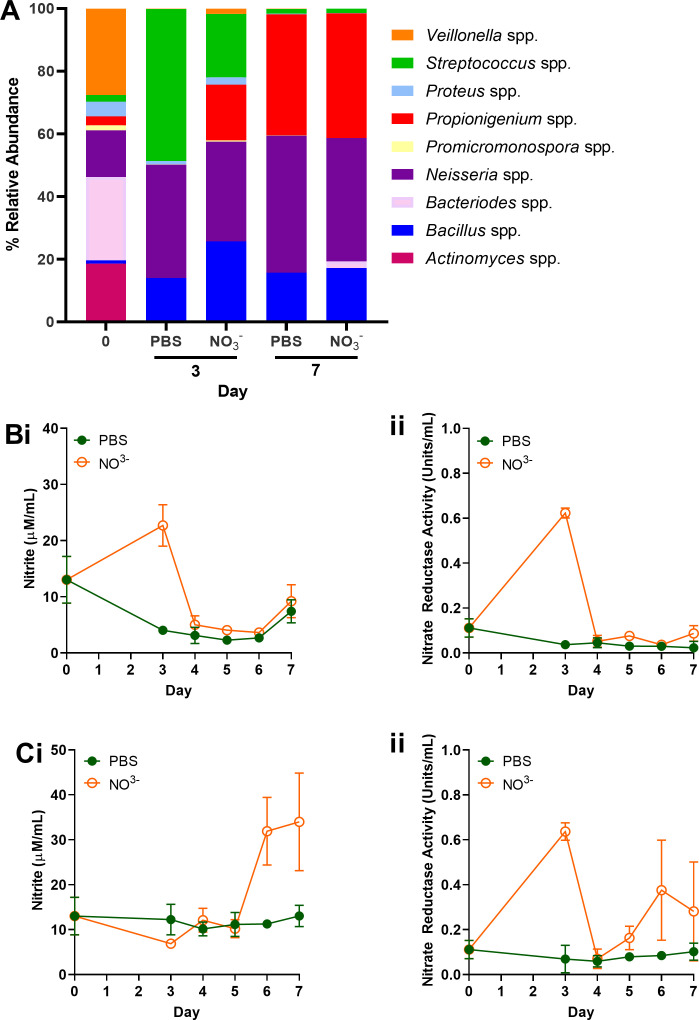
There are significant inter-individual differences in oral microbiota composition, including the abundance of nitrate-reducing species such Neisseria, Rothia, and Veillonella (74). Therefore, a third experimental setup (Fig. 1) was exploited to evaluate inter-individual differences in responses to continuous sodium nitrate supplementation relative to a PBS-treated control. Initial oral microcosm communities in two individuals used in this study proved divergent (Fig. 4 and 5), in which subject 3 (Fig. 4A; Table S3A) exhibited an initial community dominated by Veillonella and Actinomyces spp. Comparatively, Actinomyces, Bacteroides, Veillonella, and Neisseria spp. proved the most abundant genera of inocula derived from subject 4 (day 0, Fig. 5A; Table S3B). Culture with continuous nitrate appeared to shift the community composition of subject 3 (Fig. 4A), and an increased abundance of nitrate-reducing Proteus and Neisseria spp. was observed 7 days post-inoculation relative to the untreated control. This increased abundance was also accompanied by an 18.4-fold increase in nitrite secretion in nitrate-treated perfusate samples relative to the PBS-treated (Fig. 4Ci). No significant changes in nitrite metabolism following nitrate intervention were observed in biofilm samples relative to the PBS control (Fig. 4Bi). Comparatively, while elevated levels of nitrite secretion could be observed following 6 days of nitrate application in perfusate samples (2.83-fold relative to PBS control, Fig. 5Ci), intervention exerted no significant effect on microcosm composition of subject 4. The abundance of Neisseria spp. remained stable (37.7% ± 5.1) across treatment groups on both days 3 and 7. Initial inoculum comprised higher levels of Neisseria spp. in subject 4 (Fig. 5A, 14.9%) when compared to subject 3 (Fig. 4A, 0.5%). Assessment of pooled ASVs from days 3 and 7 revealed significant increases in Veillonella spp. and Neisseria spp. following nitrate intervention in subject 3 (Fig. 4A) relative to the PBS control (Fig. 6A, P < 0.05), but inconsistent effects in subject 4 (Fig. 6B).
Fig 4.
Nitrate intervention elevates the abundance of Neisseria and Proteus spp. ASVs. Oral microcosm (subject 3) was inoculated into the continuous flow-through system and exposed to either 15 mM nitrate (NO₃⁻) or PBS (control) continuously for 7 days. Changes in control (PBS) or NO₃⁻treated biofilm composition (A) were assessed via 16S rRNA sequencing. Nitrate metabolism from collected biofilms (filter; B) or perfusate (C) was examined via nitrite secretion (i) and nitrate reductase activity (ii) assays.
Fig 5.
Effects of nitrate intervention may depend upon initial microcosm composition. An oral microcosm (subject 4) was inoculated into the continuous flow-through system and exposed to either 15 mM nitrate (NO₃⁻) or PBS (untreated) continuously for 7 days. Changes in biofilm composition (A) were assessed via 16S rRNA sequencing. Nitrate metabolism from collected biofilms (filter; B) or perfusate (C) was evaluated via nitrite secretion (i) and nitrate reductase activity (ii) assays.
Fig 6.
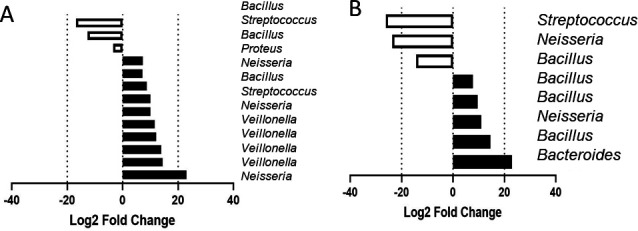
Shift toward high nitrate-reducing organisms following nitrate dosing. Oral microcosms derived from two individuals (subject 3, A and subject 4, B) were either dosed with 15 mM nitrate or PBS and data pooled following the acquisition of 16S rRNA data on collection days 3 and 7. Figures depict combined changes in ASVs on days 3 and 7 in nitrate-treated samples, relative to PBS control.
DISCUSSION
The enterosalivary nitrogen oxide cycle involves the conversion of diet-derived nitrate into nitrite by oral bacteria and the reduction of nitrite to NO in host circulation and tissues (6, 75). Recent research has identified this metabolic pathway as an important link between oral and systemic health (22, 76). The composition of the oral microbiota and the associated nitrate-reducing metabolic potential therefore could provide an opportunity to intervene. We hypothesized that dosing with inorganic nitrate would provide a competitive advantage for certain taxa and may result in their clonal expansion but that responses to nitrate exposure would vary between individual microbiota. Nitrate dosing resulted in a shift in bacterial profiles toward a higher relative abundance of putative nitrate-reducing genera such as Neisseria, coupled with an increase in the rate of nitrite formation, that was affected by inter-individual variation in the initial inoculum.
The efficacy of inorganic nitrate supplementation and the downstream effects of the enterosalivary pathway have been reported to vary considerably between individuals (76, 77) although the reasons for this variability are not fully understood. In a systematic review investigating the use of inorganic nitrate for treatment of adults with high BP, Remington and Winters (78) reported that of the 12 studies identified, five reported a significant reduction (P < 0.05) in both SBP and diastolic blood pressure. One study reported a significant reduction in SBP only, and six reported no reduction in BP. Another review of the role of oral microbiota in plasma nitrite concentrations reported similar variability between study outcomes when assessing the disruption of the oral microbiota using antiseptic mouthwashes, with four of the six studies reporting a reduction of plasma nitrite concentrations, while for two studies, there was no difference (39). Furthermore, three studies out of five studies reported higher SBP in mouthwash groups compared to controls (39). Following dietary nitrate supplementation, BP reductions have been correlated with increased plasma nitrite concentrations, implicating nitrate reduction as important in the efficacy of the intervention (38, 79). Although there is a role for oral bacteria in in vivo nitrate cycling, the impact on plasma nitrite and NO, as well as subsequent cardiovascular responses, remains poorly understood.
A similar degree of unpredictability in responses to acute nitrate challenges was observed within the current study. Some oral microcosms displayed a clear increase in NaR activity and thus higher nitrite within the perfusate, alongside a shift in bacterial profiles toward high relative abundances of known nitrate reducers, while others did not respond. These increases in nitrate metabolism were accompanied by the significant expansion of Neisseria spp. and Proteus spp. relative to initial abundance within inocula, while no significant effect on the abundance of these species across the time course was observed in communities exhibiting no response to nitrate intervention. We thus hypothesize that initial community composition may dictate sensitivity to nitrate intervention. The results of this study reflect previous in vivo results regarding the unpredictability surrounding the efficacy of nitrate as a prebiotic in the treatment of cardiovascular disorders (39, 76). In a previous in vitro investigation, which aimed to identify nitrate-reducing candidate probiotics, oral microcosms that did not initially respond to nitrate did respond once a high nitrate-reducing probiotic bacterium was introduced in tandem with the nitrate in short period (4–7 h) experiments (71). Preliminary identification of high NaR-producing bacterium with a strong safety profile, a minimal effective dose, and the potential for large-scale industrial production with the possibility of incorporation in consumer dietary products should be studied in vitro and in vivo (80). These studies should examine populations with both high and low baseline NaR activity to further investigate the preliminary evidence that those with lowest salivary NaR respond most to prebiotic nitrate that is presented in this current study. Furthermore, high nitrate-reducing oral bacterium has been suggested to have an anti-cariogenic effect, mediated by alkali production and lactate consumption limiting a drop in pH as carbohydrates are fermented (70, 81, 82). Monitoring of medium pH in vitro would allow this hypothesis to be investigated further.
We have previously reported that the abundance and activity of oral nitrate-reducing bacteria impact blood pressure in hypertensive pregnant women and baseline blood pressure can be reduced by dietary nitrate supplementation (40). While the principle that oral nitrate metabolism can positively affect systemic health has been established, there still exists a deficit in systems capable of elucidating mechanisms of inter-individual variation in responses, thus limiting development of prospective interventions that may improve efficacy. The present study introduces an attractive in vitro oral model for assessing the impact of nitrate intervention on both community composition and nitrate metabolism. While the nitrate concentration used to dose the models (15 mM) was broadly representative of physiological concentrations, it was relatively high (38). The typical nitrate concentration range in the saliva is typically 5 mM–8 mM after a nitrate-rich meal (83). Nonetheless, utilization of this concentration proved effective in demonstrating inter-individual variability in response to nitrate intervention. Secondly, fresh saliva as opposed to frozen saliva should be used as inoculum, although there was no change in alpha or beta diversity between the inoculum and the subsequent microcosms in the present study. While losses in microbial diversity may occur when inoculum has been introduced into an in vitro model (15, 47), this was not observed in the current study.
In vitro modeling has previously facilitated the analysis of oral microcosms and ecological perturbations in numerous clinical disorders of the oral cavity, including caries (83) and periodontal disease (84) and subsequent anti-plaque interventions involving probiotics (85, 86), L-arginine (87, 88), and triclosan (89). Continuous culture in vitro models involve the growth and maintenance of a consortium of bacteria following inoculation with representative poly- or mono-species microbial populations (90). These systems enable continuous nutrient availability and the removal of detrimental metabolic waste products, consequently generating persistent and reproducible oral microcosms (43). In vitro studies have been undertaken for the isolation of high nitrate-reducing bacterial species as possible probiotics (live organisms which, when consumed in sufficient quantities, confer a health benefit to the host) (91), alongside the assessment of nitrate itself as a prebiotic (a non-digestible food that can be used as a substrate by host microorganisms to produce energy, metabolites, or micronutrients) (70, 71, 92).
The reduction of nitrate to nitrite in the oral cavity by host bacterium is associated with systemic health, but our current understanding of the underlying mechanisms is incomplete. Inter-individual variability in the ability to reduce nitrate (76, 77) was reproduced in the microcosms. Such variability in this alternative enterosalivary pathway has implications in disorders where NO deficiency is implicated (93). Greater appreciation of this symbiotic relationship and how pre- and probiotic inventions could shift the ecological balance to improve host health offers an opportunity in the treatment of cardiometabolic disorders. Our data suggest that prebiotic nitrate supplementation may be used to alter oral nitrate metabolism, which has been shown in previous in vitro (70) and in vivo studies (94), with dietary interventions an attractive therapeutic avenue for hypertension (95, 96). However, based upon the inter-individual variability in oral microcosm communities utilized across this study, limited conclusions can be drawn. Nonetheless, we present an attractive model that could be utilized alongside communities of known composition to ascertain which effectively boost nitrate metabolism and how this shifts microbiome composition and to assess strain-specific variability in nitrate metabolism as described by Rosier et al. (67, 68). In some cases resulting in the application of combinational approaches, which involve high nitrate-reducing probiotic bacteria alongside prebiotic nitrate supplementation may be neccessary for a certain proportion of individuals. A more complete understanding of the microbiology behind the metabolism of nitrate and nitrite in the oral cavity is now a priority (97).
ACKNOWLEDGMENTS
The authors would like to thank the staff at the Maternal and Fetal Health Research Centre and the School of Pharmacy, University of Manchester.
Thanks to Tommy’s the Baby Charity for funding this project.
Contributor Information
Thomas Willmott, Email: thomas.willmott@manchester.ac.uk.
Andrew J. McBain, Email: Andrew.McBain@manchester.ac.uk.
Christopher A. Elkins, Centers for Disease Control and Prevention, Atlanta, Georgia, USA
DATA AVAILABILITY
The data that support the findings of this study are available from the corresponding author upon reasonable request. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, PRJNA1078588.
ETHICS APPROVAL
Samples were collected following approval from the University of Manchester Research Ethics Committee (Reference: 2019-5929-9344).
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/aem.02035-23.
Fig. S1 and Tables S1 to S3.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. NICE . 2011. Hypertension: the clinical management of primary hypertension in adults. National Institute of Clinical Excellence, National Clinical Guideline Centre, London (UK). [Google Scholar]
- 2. Saiz LC, Gorricho J, Garjón J, Celaya MC, Erviti J, Leache L. 2018. Blood pressure targets for the treatment of people with hypertension and cardiovascular disease. Cochrane Database Syst Rev 7:CD010315. doi: 10.1002/14651858.CD010315.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Forouzanfar MH, Liu P, Roth GA, Ng M, Biryukov S, Marczak L, Alexander L, Estep K, Hassen Abate K, Akinyemiju TF, et al. 2017. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990-2015. JAMA 317:165. doi: 10.1001/jama.2016.19043 [DOI] [PubMed] [Google Scholar]
- 4. Dominiczak A, Delles C, Padmanabhan S. 2017. Genomics and precision medicine for clinicians and scientists in hypertension. Hypertension 69:e10–e13. doi: 10.1161/HYPERTENSIONAHA.116.08252 [DOI] [PubMed] [Google Scholar]
- 5. Lundberg JO, Weitzberg E, Cole JA, Benjamin N. 2004. Nitrate, bacteria and human health. Nat Rev Microbiol 2:593–602. doi: 10.1038/nrmicro929 [DOI] [PubMed] [Google Scholar]
- 6. Lundberg JO, Weitzberg E, Gladwin MT. 2008. The nitrate–nitrite–nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov 7:156–167. doi: 10.1038/nrd2466 [DOI] [PubMed] [Google Scholar]
- 7. Grant MM, Jönsson D. 2019. Next generation sequencing discoveries of the nitrate-responsive oral microbiome and its effect on vascular responses. J Clin Med 8:1110. doi: 10.3390/jcm8081110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alzahrani HS, Jackson KG, Hobbs DA, Lovegrove JA. 2021. The role of dietary nitrate and the oral microbiome on blood pressure and vascular tone. Nutr Res Rev 34:222–239. doi: 10.1017/S0954422420000281 [DOI] [PubMed] [Google Scholar]
- 9. Remington J, Winters K. 2019. Effectiveness of dietary inorganic nitrate for lowering blood pressure in hypertensive adults: a systematic review. JBI Database System Rev Implement Rep 17:365–389. doi: 10.11124/JBISRIR-2017-003842 [DOI] [PubMed] [Google Scholar]
- 10. He Y, Liu J, Cai H, Zhang J, Yi J, Niu Y, Xi H, Peng X, Guo L. 2021. Effect of inorganic nitrate supplementation on blood pressure in older adults: a systematic review and meta-analysis. Nitric Oxide 113–114:13–22. doi: 10.1016/j.niox.2021.04.006 [DOI] [PubMed] [Google Scholar]
- 11. Hobbs DA, George TW, Lovegrove JA. 2013. The effects of dietary nitrate on blood pressure and endothelial function: a review of human intervention studies. Nutr Res Rev 26:210–222. doi: 10.1017/S0954422413000188 [DOI] [PubMed] [Google Scholar]
- 12. Kalaycıoğlu Z, Erim FB. 2019. Nitrate and nitrites in foods: worldwide regional distribution in view of their risks and benefits. J Agric Food Chem 67:7205–7222. doi: 10.1021/acs.jafc.9b01194 [DOI] [PubMed] [Google Scholar]
- 13. Qin L, Liu X, Sun Q, Fan Z, Xia D, Ding G, Ong HL, Adams D, Gahl WA, Zheng C, Qi S, Jin L, Zhang C, Gu L, He J, Deng D, Ambudkar IS, Wang S. 2012. Sialin (SLC17A5) functions as a nitrate transporter in the plasma membrane. Proc Natl Acad Sci U S A 109:13434–13439. doi: 10.1073/pnas.1116633109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Qu XM, Wu ZF, Pang BX, Jin LY, Qin LZ, Wang SL. 2016. From nitrate to nitric oxide: the role of salivary glands and oral bacteria. J Dent Res 95:1452–1456. doi: 10.1177/0022034516673019 [DOI] [PubMed] [Google Scholar]
- 15. Doel JJ, Benjamin N, Hector MP, Rogers M, Allaker RP. 2005. Evaluation of bacterial nitrate reduction in the human oral cavity. Eur J Oral Sci 113:14–19. doi: 10.1111/j.1600-0722.2004.00184.x [DOI] [PubMed] [Google Scholar]
- 16. Ahmed KA, Nichols AL, Honavar J, Dransfield MT, Matalon S, Patel RP. 2017. Measuring nitrate reductase activity from human and rodent tongues. Nitric Oxide 66:62–70. doi: 10.1016/j.niox.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Panza JA, Quyyumi AA, Brush JE, Epstein SE. 1990. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med 323:22–27. doi: 10.1056/NEJM199007053230105 [DOI] [PubMed] [Google Scholar]
- 18. Chowienczyk PJ. 1992. Impaired endothelium-dependent vasodilation of forearm resistance vessels in hypercholesterolaemia. Lancet 340:1430–1432. doi: 10.1016/0140-6736(92)92621-L [DOI] [PubMed] [Google Scholar]
- 19. Bhupathiraju SN, Wedick NM, Pan A, Manson JE, Rexrode KM, Willett WC, Rimm EB, Hu FB. 2013. Quantity and variety in fruit and vegetable intake and risk of coronary heart disease. Am J Clin Nutr 98:1514–1523. doi: 10.3945/ajcn.113.066381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ahmad A, Dempsey SK, Daneva Z, Azam M, Li N, Li P-L, Ritter JK. 2018. Role of nitric oxide in the cardiovascular and renal systems. Int J Mol Sci 19:2605. doi: 10.3390/ijms19092605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hsu CN, Tain YL. 2019. Regulation of nitric oxide production in the developmental programming of hypertension and kidney disease. Int J Mol Sci 20:681. doi: 10.3390/ijms20030681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alzahrani HS, Jackson KG, Hobbs DA, Lovegrove JA. 2021. The role of dietary nitrate and the oral microbiome on blood pressure and vascular tone. Nutr Res Rev 34:222–239. doi: 10.1017/S0954422420000281 [DOI] [PubMed] [Google Scholar]
- 23. Koch CD, Gladwin MT, Freeman BA, Lundberg JO, Weitzberg E, Morris A. 2017. Enterosalivary nitrate metabolism and the microbiome: intersection of microbial metabolism, nitric oxide and diet in cardiac and pulmonary vascular health. Free Radic Biol Med 105:48–67. doi: 10.1016/j.freeradbiomed.2016.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bondonno CP, Liu AH, Croft KD, Ward NC, Shinde S, Moodley Y, Lundberg JO, Puddey IB, Woodman RJ, Hodgson JM. 2015. Absence of an effect of high nitrate intake from beetroot juice on blood pressure in treated hypertensive individuals: a randomized controlled trial. Am J Clin Nutr 102:368–375. doi: 10.3945/ajcn.114.101188 [DOI] [PubMed] [Google Scholar]
- 25. Kapil V, Haydar SMA, Pearl V, Lundberg JO, Weitzberg E, Ahluwalia A. 2013. Physiological role for nitrate-reducing oral bacteria in blood pressure control. Free Radic Biol Med 55:93–100. doi: 10.1016/j.freeradbiomed.2012.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McDonagh STJ, Wylie LJ, Winyard PG, Vanhatalo A, Jones AM. 2015. The effects of chronic nitrate supplementation and the use of strong and weak antibacterial agents on plasma nitrite concentration and exercise blood pressure. Int J Sports Med 36:1177–1185. doi: 10.1055/s-0035-1554700 [DOI] [PubMed] [Google Scholar]
- 27. Sundqvist ML, Lundberg JO, Weitzberg E. 2016. Effects of antiseptic mouthwash on resting metabolic rate: a randomized, double-blind, crossover study. Nitric Oxide 61:38–44. doi: 10.1016/j.niox.2016.10.003 [DOI] [PubMed] [Google Scholar]
- 28. Woessner M, Smoliga JM, Tarzia B, Stabler T, Van Bruggen M, Allen JD. 2016. A stepwise reduction in plasma and salivary nitrite with increasing strengths of mouthwash following a dietary nitrate load. Nitric Oxide 54:1–7. doi: 10.1016/j.niox.2016.01.002 [DOI] [PubMed] [Google Scholar]
- 29. Senkus KE, Crowe-White KM. 2020. Influence of mouth rinse use on the enterosalivary pathway and blood pressure regulation: a systematic review. Crit Rev Food Sci Nutr 60:2874–2886. doi: 10.1080/10408398.2019.1665495 [DOI] [PubMed] [Google Scholar]
- 30. Bryan NS, Tribble G, Angelov N. 2017. Oral microbiome and nitric oxide: the missing link in the management of blood pressure. Curr Hypertens Rep 19:33. doi: 10.1007/s11906-017-0725-2 [DOI] [PubMed] [Google Scholar]
- 31. Webb AJ, Patel N, Loukogeorgakis S, Okorie M, Aboud Z, Misra S, Rashid R, Miall P, Deanfield J, Benjamin N, MacAllister R, Hobbs AJ, Ahluwalia A. 2008. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension 51:784–790. doi: 10.1161/HYPERTENSIONAHA.107.103523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hobbs DA, Kaffa N, George TW, Methven L, Lovegrove JA. 2012. Blood pressure-lowering effects of beetroot juice and novel beetroot-enriched bread products in normotensive male subjects. Br J Nutr 108:2066–2074. doi: 10.1017/S0007114512000190 [DOI] [PubMed] [Google Scholar]
- 33. Kapil V, Khambata RS, Robertson A, Caulfield MJ, Ahluwalia A. 2015. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: a randomized, phase 2, double-blind, placebo-controlled study. Hypertension 65:320–327. doi: 10.1161/HYPERTENSIONAHA.114.04675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Velmurugan S, Gan JM, Rathod KS, Khambata RS, Ghosh SM, Hartley A, Van Eijl S, Sagi-Kiss V, Chowdhury TA, Curtis M, Kuhnle GGC, Wade WG, Ahluwalia A. 2016. Dietary nitrate improves vascular function in patients with hypercholesterolemia: a randomized, double-blind, placebo-controlled study. Am J Clin Nutr 103:25–38. doi: 10.3945/ajcn.115.116244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. van der Avoort CMT, Ten Haaf DSM, Bongers C, van Oorschot F, Verdijk LB, van Loon LJC, Hopman MTE. 2021. Increasing nitrate-rich vegetable intake lowers ambulatory blood pressure in (pre)hypertensive middle-aged and older adults: a 12-wk randomized controlled trial. J Nutr 151:2667–2679. doi: 10.1093/jn/nxab157 [DOI] [PubMed] [Google Scholar]
- 36. Gilchrist M, Winyard PG, Aizawa K, Anning C, Shore A, Benjamin N. 2013. Effect of dietary nitrate on blood pressure, endothelial function, and insulin sensitivity in type 2 diabetes. Free Radic Biol Med 60:89–97. doi: 10.1016/j.freeradbiomed.2013.01.024 [DOI] [PubMed] [Google Scholar]
- 37. Burleigh MC, Liddle L, Monaghan C, Muggeridge DJ, Sculthorpe N, Butcher JP, Henriquez FL, Allen JD, Easton C. 2018. Salivary nitrite production is elevated in individuals with a higher abundance of oral nitrate-reducing bacteria. Free Radic Biol Med 120:80–88. doi: 10.1016/j.freeradbiomed.2018.03.023 [DOI] [PubMed] [Google Scholar]
- 38. Ormesher L, Myers JE, Chmiel C, Wareing M, Greenwood SL, Tropea T, Lundberg JO, Weitzberg E, Nihlen C, Sibley CP, Johnstone ED, Cottrell EC. 2018. Effects of dietary nitrate supplementation, from beetroot juice, on blood pressure in hypertensive pregnant women: a randomised, double-blind, placebo-controlled feasibility trial. Nitric Oxide 80:37–44. doi: 10.1016/j.niox.2018.08.004 [DOI] [PubMed] [Google Scholar]
- 39. Zhurakivska K, Troiano G, Caponio VCA, Dioguardi M, Laino L, Maffione AB, Lo Muzio L. 2019. Do changes in oral microbiota correlate with plasma nitrite response? A systematic review. Front Physiol 10:1029. doi: 10.3389/fphys.2019.01029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Willmott T, Ormesher L, McBain AJ, Humphreys GJ, Myers JE, Singh G, Lundberg JO, Weitzberg E, Nihlen C, Cottrell EC. 2023. Altered oral nitrate reduction and bacterial profiles in hypertensive women predict blood pressure lowering following acute dietary nitrate supplementation. Hypertension 80:2397–2406. doi: 10.1161/HYPERTENSIONAHA.123.21263 [DOI] [PubMed] [Google Scholar]
- 41. Greenman J, Spencer P, McKenzie C, Saad S, Duffield J. 2005. In vitro models for oral malodor. Oral Dis 11:14–23. doi: 10.1111/j.1601-0825.2005.01082.x [DOI] [PubMed] [Google Scholar]
- 42. McBain AJ, Sissons C, Ledder RG, Sreenivasan PK, De Vizio W, Gilbert P. 2005. Development and characterization of a simple perfused oral microcosm. J Appl Microbiol 98:624–634. doi: 10.1111/j.1365-2672.2004.02483.x [DOI] [PubMed] [Google Scholar]
- 43. McBain AJ. 2009. Chapter 4: in vitro biofilm models: an overview. Adv Appl Microbiol 69:99–132. doi: 10.1016/S0065-2164(09)69004-3 [DOI] [PubMed] [Google Scholar]
- 44. Ledder RG, Madhwani T, Sreenivasan PK, De Vizio W, McBain AJ. 2009. An in vitro evaluation of hydrolytic enzymes as dental plaque control agents. J Med Microbiol 58:482–491. doi: 10.1099/jmm.0.006601-0 [DOI] [PubMed] [Google Scholar]
- 45. Ledder RG, McBain AJ. 2012. An in vitro comparison of dentifrice formulations in three distinct oral microbiotas. Arch Oral Biol 57:139–147. doi: 10.1016/j.archoralbio.2011.08.004 [DOI] [PubMed] [Google Scholar]
- 46. Ledder RG, Gilbert P, Pluen A, Sreenivasan PK, De Vizio W, McBain AJ. 2006. Individual microflora beget unique oral microcosms. J Appl Microbiol 100:1123–1131. doi: 10.1111/j.1365-2672.2006.02847.x [DOI] [PubMed] [Google Scholar]
- 47. Hughes WE, Treichler DP, Ueda K, Bock JM, Casey DP. 2022. Sodium nitrate supplementation improves blood pressure reactivity in patients with peripheral artery disease. Nutr Metab Cardiovasc Dis 32:710–714. doi: 10.1016/j.numecd.2021.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Morris Jr RC, Pravenec M, Šilhavý J, DiCarlo SE, Kurtz TW. 2019. Small amounts of inorganic nitrate or beetroot provide substantial protection from salt-induced increases in blood pressure. Hypertension 73:1042–1048. doi: 10.1161/HYPERTENSIONAHA.118.12234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Koopman JE, Buijs MJ, Brandt BW, Keijser BJF, Crielaard W, Zaura E. 2016. Nitrate and the origin of saliva influence composition and short chain fatty acid production of oral microcosms. Microb Ecol 72:479–492. doi: 10.1007/s00248-016-0775-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lowe RH, Evans HJ. 1964. Preparation and some properties of a soluble nitrate reductase from Rhizobium japonicum. Biochim Biophys Acta Spec Sect Enzymol Subj 85:377–389. doi: 10.1016/0926-6569(64)90301-3 [DOI] [PubMed] [Google Scholar]
- 51. Allison C, Macfarlane GT. 1989. Dissimilatory nitrate reduction by Propionibacterium acnes. Appl Environ Microbiol 55:2899–2903. doi: 10.1128/aem.55.11.2899-2903.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, Gormley N, Gilbert JA, Smith G, Knight R. 2012. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J 6:1621–1624. doi: 10.1038/ismej.2012.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rullo J, Far PM, Quinn M, Sharma N, Bae S, Irrcher I, Sharma S. 2020. Local oral and nasal microbiome diversity in age-related macular degeneration. Sci Rep 10:3862. doi: 10.1038/s41598-020-60674-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Demmitt BA, Corley RP, Huibregtse BM, Keller MC, Hewitt JK, McQueen MB, Knight R, McDermott I, Krauter KS. 2017. Genetic influences on the human oral microbiome. BMC Genomics 18:659. doi: 10.1186/s12864-017-4008-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Granato DC, Neves LX, Trino LD, Carnielli CM, Lopes AFB, Yokoo S, Pauletti BA, Domingues RR, Sá JO, Persinoti G, Paixão DAA, Rivera C, de Sá Patroni FM, Tommazetto G, Santos-Silva AR, Lopes MA, de Castro G, Brandão TB, Prado-Ribeiro AC, Squina FM, Telles GP, Paes Leme AF. 2021. Meta-omics analysis indicates the saliva microbiome and its proteins associated with the prognosis of oral cancer patients. Biochim Biophys Acta Proteins Proteom 1869:140659. doi: 10.1016/j.bbapap.2021.140659 [DOI] [PubMed] [Google Scholar]
- 56. Su S-C, Chang L-C, Huang H-D, Peng C-Y, Chuang C-Y, Chen Y-T, Lu M-Y, Chiu Y-W, Chen P-Y, Yang S-F. 2021. Oral microbial dysbiosis and its performance in predicting oral cancer. Carcinogenesis 42:127–135. doi: 10.1093/carcin/bgaa062 [DOI] [PubMed] [Google Scholar]
- 57. Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, et al. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336. doi: 10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F, et al. 2019. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 37:852–857. doi: 10.1038/s41587-019-0209-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Callahan BJ, Sankaran K, Fukuyama JA, McMurdie PJ, Holmes SP. 2016. Bioconductor workflow for microbiome data analysis: from raw reads to community analyses. F1000Res 5:1492. doi: 10.12688/f1000research.8986.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bokulich NA, Kaehler BD, Rideout JR, Dillon M, Bolyen E, Knight R, Huttley GA, Gregory Caporaso J. 2018. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 6:90. doi: 10.1186/s40168-018-0470-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. McDonald D, Price MN, Goodrich J, Nawrocki EP, DeSantis TZ, Probst A, Andersen GL, Knight R, Hugenholtz P. 2012. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J 6:610–618. doi: 10.1038/ismej.2011.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bisanz JE. 2018. qiime2R: importing QIIME2 artifacts and associated data into R sessions. 0.99
- 63. McMurdie PJ, Holmes S. 2012. Phyloseq: a bioconductor package for handling and analysis of high-throughput phylogenetic sequence data. Pac Symp Biocomput 17:235–246. doi: 10.1142/9789814366496_0023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wickham H. 2016. ggplot2: elegant graphics for data analysis. Springer-Verlag New York. [Google Scholar]
- 65. RC Team . 2016. R: a language and environment for statistical computing. R Foundation for Statistical Computing. [Google Scholar]
- 66. Love MI, Huber W, Anders S. 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:550. doi: 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kolenbrander PE, Palmer RJ, Periasamy S, Jakubovics NS. 2010. Oral multispecies biofilm development and the key role of cell-cell distance. Nat Rev Microbiol 8:471–480. doi: 10.1038/nrmicro2381 [DOI] [PubMed] [Google Scholar]
- 68. Wicaksono DP, Washio J, Abiko Y, Domon H, Takahashi N. 2020. Nitrite production from nitrate and its link with lactate metabolism in oral Veillonella spp. Appl Environ Microbiol 86:e01255-20. doi: 10.1128/AEM.01255-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chen H, Li Q, Li M, Liu S, Yao C, Wang Z, Zhao Z, Liu P, Yang F, Li X, Wang J, Zeng Y, Tong X. 2021. Microbial characteristics across different tongue coating types in a healthy population. J Oral Microbiol 13:1946316. doi: 10.1080/20002297.2021.1946316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rosier BT, Buetas E, Moya-Gonzalvez EM, Artacho A, Mira A. 2020. Nitrate as a potential prebiotic for the oral microbiome. Sci Rep 10:12895. doi: 10.1038/s41598-020-69931-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rosier BT, Moya-Gonzalvez EM, Corell-Escuin P, Mira A. 2020. Isolation and characterization of nitrate-reducing bacteria as potential probiotics for oral and systemic health. Front Microbiol 11:555465. doi: 10.3389/fmicb.2020.555465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Engel A-S, Kranz HT, Schneider M, Tietze JP, Piwowarcyk A, Kuzius T, Arnold W, Naumova EA. 2020. Biofilm formation on different dental restorative materials in the oral cavity. BMC Oral Health 20:162. doi: 10.1186/s12903-020-01147-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kuboniwa M, Lamont RJ. 2010. Subgingival biofilm formation. Periodontol 2000 52:38–52. doi: 10.1111/j.1600-0757.2009.00311.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Liddle L, Burleigh MC, Monaghan C, Muggeridge DJ, Sculthorpe N, Pedlar CR, Butcher J, Henriquez FL, Easton C. 2019. Variability in nitrate-reducing oral bacteria and nitric oxide metabolites in biological fluids following dietary nitrate administration: an assessment of the critical difference. Nitric Oxide 83:1–10. doi: 10.1016/j.niox.2018.12.003 [DOI] [PubMed] [Google Scholar]
- 75. Sato-Suzuki Y, Washio J, Wicaksono DP, Sato T, Fukumoto S, Takahashi N. 2020. Nitrite-producing oral microbiome in adults and children. Sci Rep 10:16652. doi: 10.1038/s41598-020-73479-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Blekkenhorst LC, Bondonno NP, Liu AH, Ward NC, Prince RL, Lewis JR, Devine A, Croft KD, Hodgson JM, Bondonno CP. 2018. Nitrate, the oral microbiome, and cardiovascular health: a systematic literature review of human and animal studies. Am J Clin Nutr 107:504–522. doi: 10.1093/ajcn/nqx046 [DOI] [PubMed] [Google Scholar]
- 77. Li D, Nishi SK, Jovanovski E, Zurbau A, Komishon A, Mejia SB, Khan TA, Sievenpiper JL, Milicic D, Jenkins A, Vuksan V. 2020. Repeated administration of inorganic nitrate on blood pressure and arterial stiffness: a systematic review and meta-analysis of randomized controlled trials. J Hypertens 38:2122–2140. doi: 10.1097/HJH.0000000000002524 [DOI] [PubMed] [Google Scholar]
- 78. Remington J, Winters K. 2019. Effectiveness of dietary inorganic nitrate for lowering blood pressure in hypertensive adults: a systematic review. JBI Database System Rev Implement Rep 17:365–389. doi: 10.11124/JBISRIR-2017-003842 [DOI] [PubMed] [Google Scholar]
- 79. Kapil V, Milsom AB, Okorie M, Maleki-Toyserkani S, Akram F, Rehman F, Arghandawi S, Pearl V, Benjamin N, Loukogeorgakis S, Macallister R, Hobbs AJ, Webb AJ, Ahluwalia A. 2010. Inorganic nitrate supplementation lowers blood pressure in humans: role for nitrite-derived NO. Hypertension 56:274–281. doi: 10.1161/HYPERTENSIONAHA.110.153536 [DOI] [PubMed] [Google Scholar]
- 80. Fenster K, Freeburg B, Hollard C, Wong C, Rønhave Laursen R, Ouwehand AC. 2019. The production and delivery of probiotics: a review of a practical approach. Microorganisms 7:83. doi: 10.3390/microorganisms7030083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Li H, Thompson I, Carter P, Whiteley A, Bailey M, Leifert C, Killham K. 2007. Salivary nitrate--an ecological factor in reducing oral acidity. Oral Microbiol Immunol 22:67–71. doi: 10.1111/j.1399-302X.2007.00313.x [DOI] [PubMed] [Google Scholar]
- 82. Rosier BT, Palazón C, García-Esteban S, Artacho A, Galiana A, Mira A. 2021. A single dose of nitrate increases resilience against acidification derived from sugar fermentation by the oral microbiome. Front Cell Infect Microbiol 11:692883. doi: 10.3389/fcimb.2021.692883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wu J-S, Zheng M, Zhang M, Pang X, Li L, Wang S-S, Yang X, Wu J-B, Tang Y-J, Tang Y-L, Liang X-H. 2018. Porphyromonas gingivalis promotes 4-nitroquinoline-1-oxide-induced oral carcinogenesis with an alteration of fatty acid metabolism. Front Microbiol 9:2081. doi: 10.3389/fmicb.2018.02081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Velsko IM, Shaddox LM. 2018. Consistent and reproducible long-term in vitro growth of health and disease-associated oral subgingival biofilms. BMC Microbiol 18:70. doi: 10.1186/s12866-018-1212-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Schwendicke F, Korte F, Dörfer CE, Kneist S, Fawzy El-Sayed K, Paris S. 2017. Inhibition of Streptococcus mutans growth and biofilm formation by probiotics in vitro. Caries Res 51:87–95. doi: 10.1159/000452960 [DOI] [PubMed] [Google Scholar]
- 86. Humphreys GJ, McBain AJ. 2019. Antagonistic effects of Streptococcus and Lactobacillus probiotics in pharyngeal biofilms. Lett Appl Microbiol 68:303–312. doi: 10.1111/lam.13133 [DOI] [PubMed] [Google Scholar]
- 87. Kolderman E, Bettampadi D, Samarian D, Dowd SE, Foxman B, Jakubovics NS, Rickard AH. 2015. L-arginine destabilizes oral multi-species biofilm communities developed in human saliva. PLoS One 10:e0121835. doi: 10.1371/journal.pone.0121835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ledder RG, Mistry H, Sreenivasan PK, Humphreys G, McBain AJ. 2017. Arginine exposure decreases acidogenesis in long-term oral biofilm microcosms. mSphere 2:e00295-17. doi: 10.1128/mSphere.00295-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Fernández E, Sánchez MDC, Llama-Palacios A, Sanz M, Herrera D. 2017. Antibacterial effects of toothpastes evaluated in an in vitro biofilm model. Oral Health Prev Dent 15:251–257. doi: 10.3290/j.ohpd.a38526 [DOI] [PubMed] [Google Scholar]
- 90. Brown JL, Johnston W, Delaney C, Short B, Butcher MC, Young T, Butcher J, Riggio M, Culshaw S, Ramage G. 2019. Polymicrobial oral biofilm models: simplifying the complex. J Med Microbiol 68:1573–1584. doi: 10.1099/jmm.0.001063 [DOI] [PubMed] [Google Scholar]
- 91. Rijkers GT, Bengmark S, Enck P, Haller D, Herz U, Kalliomaki M, Kudo S, Lenoir-Wijnkoop I, Mercenier A, Myllyluoma E, Rabot S, Rafter J, Szajewska H, Watzl B, Wells J, Wolvers D, Antoine J-M. 2010. Guidance for substantiating the evidence for beneficial effects of probiotics: current status and recommendations for future research. J Nutr 140:671S–676S. doi: 10.3945/jn.109.113779 [DOI] [PubMed] [Google Scholar]
- 92. Corzo N, Alonso JL, Azpiroz F, Calvo MA, Cirici M, Leis R, Lombó F, Mateos-Aparicio I, Plou FJ, Ruas-Madiedo P, Rúperez P, Redondo-Cuenca A, Sanz ML, Clemente A. 2015. Prebiotics: concept, properties and beneficial effects. Nutr Hosp 31:99–118. doi: 10.3305/nh.2015.31.sup1.8715 [DOI] [PubMed] [Google Scholar]
- 93. Lundberg JO, Carlström M, Weitzberg E. 2018. Metabolic effects of dietary nitrate in health and disease. Cell Metab 28:9–22. doi: 10.1016/j.cmet.2018.06.007 [DOI] [PubMed] [Google Scholar]
- 94. Vanhatalo A, Blackwell JR, L’Heureux JE, Williams DW, Smith A, van der Giezen M, Winyard PG, Kelly J, Jones AM. 2018. Nitrate-responsive oral microbiome modulates nitric oxide homeostasis and blood pressure in humans. Free Radic Biol Med 124:21–30. doi: 10.1016/j.freeradbiomed.2018.05.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ashor AW, Lara J, Siervo M. 2017. Medium-term effects of dietary nitrate supplementation on systolic and diastolic blood pressure in adults: a systematic review and meta-analysis. J Hypertens 35:1353–1359. doi: 10.1097/HJH.0000000000001305 [DOI] [PubMed] [Google Scholar]
- 96. O’Gallagher K, Borg Cardona S, Hill C, Al-Saedi A, Shahed F, Floyd CN, McNeill K, Mills CE, Webb AJ. 2021. Grapefruit juice enhances the systolic blood pressure-lowering effects of dietary nitrate-containing beetroot juice. Br J Clin Pharmacol 87:577–587. doi: 10.1111/bcp.14420 [DOI] [PubMed] [Google Scholar]
- 97. Goh CE, Trinh P, Colombo PC, Genkinger JM, Mathema B, Uhlemann A-C, LeDuc C, Leibel R, Rosenbaum M, Paster BJ, Desvarieux M, Papapanou PN, Jacobs Jr DR, Demmer RT. 2019. Association between nitrate-reducing oral bacteria and cardiometabolic outcomes: results from ORIGINS. J Am Heart Assoc 8:e013324. doi: 10.1161/JAHA.119.013324 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 and Tables S1 to S3.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, PRJNA1078588.