Abstract
PURPOSE
The phase III RESILIENT trial compared second-line liposomal irinotecan with topotecan in patients with small cell lung cancer (SCLC).
PATIENTS AND METHODS
Patients with SCLC and progression on or after first-line platinum-based chemotherapy were randomly assigned (1:1) to intravenous (IV) liposomal irinotecan (70 mg/m2 every 2 weeks in a 6-week cycle) or IV topotecan (1.5 mg/m2 daily for 5 consecutive days, every 3 weeks in a 6-week cycle). The primary end point was overall survival (OS). Key secondary end points included progression-free survival (PFS) and objective response rate (ORR).
RESULTS
Among 461 randomly assigned patients, 229 received liposomal irinotecan and 232 received topotecan. The median follow-up was 18.4 months. The median OS was 7.9 months with liposomal irinotecan versus 8.3 months with topotecan (hazard ratio [HR], 1.11 [95% CI, 0.90 to 1.37]; P = .31). The median PFS per blinded independent central review (BICR) was 4.0 months with liposomal irinotecan and 3.3 months with topotecan (HR, 0.96 [95% CI, 0.77 to 1.20]; nominal P = .71); ORR per BICR was 44.1% (95% CI, 37.6 to 50.8) and 21.6% (16.4 to 27.4), respectively. Overall, 42.0% and 83.4% of patients receiving liposomal irinotecan and topotecan, respectively, experienced grade ≥3 related treatment-emergent adverse events (TEAEs). The most common grade ≥3 related TEAEs were diarrhea (13.7%), neutropenia (8.0%), and decreased neutrophil count (4.4%) with liposomal irinotecan and neutropenia (51.6%), anemia (30.9%), and leukopenia (29.1%) with topotecan.
CONCLUSION
Liposomal irinotecan and topotecan demonstrated similar median OS and PFS in patients with relapsed SCLC. Although the primary end point of OS was not met, liposomal irinotecan demonstrated a higher ORR than topotecan. The safety profile of liposomal irinotecan was consistent with its known safety profile; no new safety concerns emerged.
INTRODUCTION
Targeted therapies have redefined oncology management for many tumor types in recent years, but novel treatment options have remained elusive for patients with small cell lung cancer (SCLC).1 SCLC is characterized by a rapid doubling time and early metastases,2 and most patients present with extensive-stage or metastatic disease at diagnosis.3,4 The aggressive nature of SCLC means that affected patients face a poorer prognosis than those with any other type of lung cancer. The five-year survival rate for SCLC is 7.2%, compared with 29.8%, 22.5%, and 18.6% for adenocarcinoma, squamous cell carcinoma, and large cell carcinoma, respectively.5
CONTEXT
Key Objective
Does liposomal irinotecan provide an overall survival (OS) benefit versus topotecan as second-line treatment for patients with small cell lung cancer (SCLC)?
Knowledge Generated
Liposomal irinotecan and topotecan demonstrated similar median OS in patients with SCLC who had progressed on or after first-line platinum-based chemotherapy. Although the primary end point of the study was not met, liposomal irinotecan demonstrated similar progression-free survival, a doubling of objective response rate and a reduced incidence of grade ≥3 related treatment-emergent adverse events (TEAEs) and TEAE-related discontinuations compared with topotecan.
Relevance (T.E. Stinchcombe)
-
This trial demonstrated the single activity and the adverse events associated with liposomal irinotecan, and additional trials of liposomal irinotecan are needed to define its role in SCLC. Antibody drug conjugates and bi-specific T-cell engagers are also being investigated in this disease.*
*Relevance section written by JCO Associate Editor Thomas E. Stinchcombe, MD.
First-line therapy for patients with metastatic SCLC (with etoposide and cisplatin or carboplatin, alone or combined with atezolizumab or durvalumab)6 is associated with high response rates, but most patients relapse within 1-2 years,7,8 and subsequent treatment options are limited. Currently, only two drugs are approved for second-line SCLC treatment: the topoisomerase I inhibitor, topotecan,9,10 and the alkylating agent, lurbinectedin.11-13 Topotecan is an established agent, but its modest antitumor activity is transient, and its use is limited by myelosuppression and hematological toxicities.8,14,15 Lurbinectedin was granted accelerated approval in 2020 for second-line use in adults with metastatic SCLC11 on the basis of a manageable safety profile and an overall response rate of 35.2% in a phase II trial.16 In the subsequent phase III ATLANTIS trial, lurbinectedin in combination with doxorubicin also showed activity in patients with relapsed SCLC, but the primary overall survival (OS) end point was not met versus physician's choice of topotecan or cyclophosphamide/doxorubicin/vincristine (hazard ratio [HR], 0.97; P = .70).17 First-line immunotherapy in combination with chemotherapy modestly improved OS in both the CASPIAN and IMpower133 studies,18,19 becoming a new standard of care. However, immunotherapy alone has shown limited efficacy in a small number of patients with SCLC in second-line treatment.6 Thus, there remains an unmet need for novel efficacious second-line treatment options for patients with SCLC.
Nonliposomal irinotecan is an established component of the SCLC treatment landscape and acts by inhibiting the action of topoisomerase I, mainly via its active metabolite SN-38.20,21 However, efficacy of nonliposomal irinotecan is limited by its short half-life and associated duration of exposure.20,21 Liposomal irinotecan (ONIVYDE, ONIVYDE pegylated liposomal; historical names include nal-IRI, MM-398, or PEP02; Ipsen Biopharmaceuticals, Inc, Cambridge, MA) is a liposomal formulation that encapsulates irinotecan in a lipid bilayer vesicle, keeping it in circulation for longer than nonliposomal irinotecan before conversion to SN-38.22,23 At equivalent doses, liposomal irinotecan demonstrates higher and sustained intratumoral levels of irinotecan and SN-38 relative to nonliposomal irinotecan.20,21 Preclinical data suggest that the longer half-life relative to the nonliposomal formulation, and associated prolonged exposure may be more important than high peak concentrations for cytotoxic activity.21
RESILIENT (ClinicalTrials.gov identifier: NCT03088813) is a two-part phase II/III study to assess the safety, tolerability, and efficacy of liposomal irinotecan monotherapy as second-line treatment for patients with SCLC. In the phase II dose-expansion stage (part 1 of the study), liposomal irinotecan demonstrated promising antitumor activity, with no new safety signals.24 The objective response rate (ORR) according to the Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST v1.1)25 and investigator assessment among 25 patients receiving liposomal irinotecan (70 mg/m2 every 2 weeks in a 6-week cycle) was 44.0% (95% CI, 24.4 to 65.1). The liposomal irinotecan dose selected for part 2 of the study was based on the part 1 findings.24
Here, we report results from RESILIENT part 2, a randomized, open-label, phase III study that compared the efficacy and safety of liposomal irinotecan versus topotecan in patients with relapsed SCLC and progression on or after first-line platinum-based chemotherapy.
PATIENTS AND METHODS
Patients
Eligible patients were age ≥18 years with SCLC, confirmed by histopathology or cytology according to the International Association for the Study of Lung Cancer classification and radiologically confirmed disease progression on or after first-line platinum-based chemotherapy. In addition, patients had an Eastern Cooperative Oncology Group Performance Status (ECOG PS) score of 0 or 1 and a life expectancy of more than 12 weeks. Patients who had received one line of immunotherapy (alone or in combination) as first- or second-line therapy were eligible, as were patients with asymptomatic radiologically stable CNS metastases.
A full list of the eligibility criteria is provided in the Data Supplement (online only).
Study Design and Treatment
Patients were randomly assigned (1:1) to receive intravenous (IV) liposomal irinotecan (70 mg/m2 over 90 minutes, every 2 weeks in a 6-week cycle) or IV topotecan (1.5 mg/m2 over 30 minutes daily for 5 consecutive days, every 3 weeks in a 6-week cycle). To manage myelosuppression, prophylactic granulocyte colony-stimulating factor was recommended for all patients receiving topotecan (in all cycles starting 24 hours after the last dose); use in patients receiving liposomal irinotecan was based on investigator discretion.
Treatments were allocated using a computerized interactive response technology system, with stratification by geographical region (North America v Asia v rest of world); platinum sensitivity status (resistant [progression within 90 days of completing first-line platinum-based therapy] v sensitive [all others]); performance status (ECOG PS score of 0 v 1); and receipt of prior immunotherapy (yes v no).
Trial therapies continued until radiologically determined disease progression per local radiology review and/or investigator assessment, per RECIST v1.1 criteria25 (or Response Assessment in Neuro-Oncology Brain Metastases [RANO-BM]26 for CNS lesions) or unacceptable toxicity. All patients completed a 30-day follow-up assessment after permanent discontinuation of study treatment, after which they entered long-term follow-up and their survival status was monitored until death, loss to follow-up, withdrawal of consent, or study closure, whichever occurred first. A full list of reasons for withdrawal and discontinuation is provided in the Data Supplement.
End Points and Assessments
The primary end point in part 2 of RESILIENT was OS for liposomal irinotecan versus topotecan. OS was defined as the number of months from random assignment to the date of death due to any cause. Key secondary end points included progression-free survival (PFS; time from random assignment to first documented disease progression or death due to any cause, whichever occurred first) as per blinded independent central review (BICR) assessment, ORR (proportion of patients achieving complete or partial response) by BICR assessment, and the safety profile of liposomal irinotecan versus topotecan. A list of the per-protocol study end points is provided in the Data Supplement.
Tumor assessments were performed by computed tomography or brain magnetic resonance imaging at screening (baseline), every 6 weeks until progressive disease using RECIST v1.1 guidelines or RANO-BM for CNS lesions. Progressive disease was determined by local radiology review and/or by investigator assessment.
Adverse events (AEs) were recorded and coded using MedDRA (version 25.0), and severity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0.27
Trial Oversight
The study was performed in accordance with the Declaration of Helsinki and the International Conference on Harmonization Consolidated Guideline on Good Clinical Practice and the requirements of the US Food and Drug Administration and/or local regulatory authorities regarding the conduct of human clinical trials. The protocol was approved by the local institutional review board and independent ethics committees of the participating centers (Appendix Table A1, online only). Patients provided written informed consent at screening. Protocol amendments made after the study started are described in the protocol. The sponsor collaborated with senior authors on study design, gathering, analyzing, and interpreting results. The authors had access to all study data, reviewed and edited the manuscript, and had final responsibility for the decision to submit. The sponsor funded medical writing and editorial assistance.
Statistical Analysis
Efficacy was assessed in all randomly assigned patients according to the intention-to-treat (ITT) principle. Safety was assessed in all patients who received at least one dose of the trial regimen. The primary end point of OS was evaluated when at least 350 events were observed in 450 patients across the two treatment arms to provide at least 87% power to detect a HR of ≤0.714 (anticipated median OS 10.5 months for liposomal irinotecan v 7.5 months for topotecan) at an overall one-sided type level of 0.025. At the primary analysis, the one-sided type 1 error was controlled and allocated alpha of 0.024 per the Hwang-Shih-DeCani method.
The family-wise type 1 error rate was strictly controlled for secondary end points in a hierarchical approach. The statistical inference for PFS (by BICR) was only performed if the primary OS end point was statistically significant and the ORR if the PFS secondary end point was statistically significant.
Between-group differences in OS and PFS were assessed using a stratified log-rank test. Kaplan-Meier analysis was used to estimate median (95% CI) survival estimates, and HRs (95% CI) were estimated using stratified Cox proportional hazards models. Prespecified sensitivity analyses and subgroup analyses were conducted for OS and PFS. For the OS analysis, patients without observed death were censored according to the last recorded date alive. For the PFS analysis, patients with documented progressive disease or death after two consecutive missed assessments, new anticancer therapy, treatment discontinuation, or loss to follow-up were censored at the time of the last adequate tumor assessment.
ORR and accompanying 95% CI were calculated and compared for the two treatment groups using the Cochran-Mantel-Haenszel method, incorporating region and platinum sensitivity stratification factors. Analyses were carried out using SAS software, version 9.4 or higher (SAS Institute, Inc, Cary, NC).
RESULTS
Patients
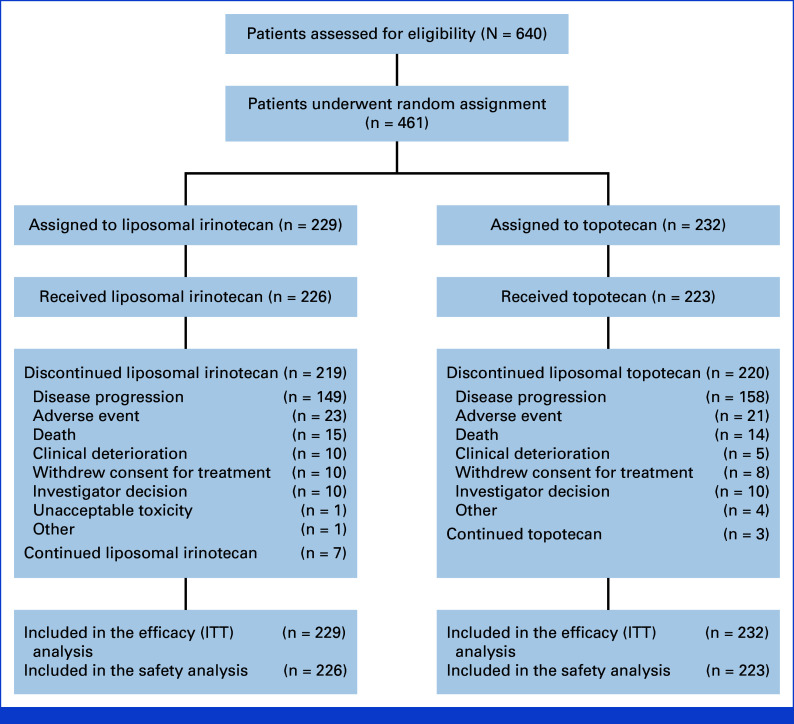
Between August 2019 and February 2021, 461 patients were randomly allocated to receive liposomal irinotecan 70 mg/m2 (every 2 weeks in a 6-week cycle) (n = 229) or topotecan 1.5 mg/m2 (for 5 consecutive days, every 3 weeks in a 6-week cycle) (n = 232); these patients comprised the ITT population (Fig 1). The safety population comprised 449 patients, of whom 226 received liposomal irinotecan and 223 received topotecan. As of data cutoff on February 8, 2022, seven patients (3.1%) in the liposomal irinotecan group and three (1.3%) in the topotecan group were still following the assigned trial regimen. The most common reason for premature discontinuation of the study medication was disease progression (149 patients [65.1%] in the liposomal irinotecan group and 158 [68.1%] in the topotecan group). Baseline demographics and clinical characteristics were generally balanced between groups; however, the proportion of patients with brain and/or CNS lesions was 24.5% in the liposomal irinotecan arm compared with 32.8% in the topotecan arm (Table 1).
FIG 1.
CONSORT diagram: eligibility, random assignment, and follow-up. ITT, intention-to-treat.
TABLE 1.
Demographic and Disease Characteristics at Baseline
| Characteristic | Liposomal Irinotecan (n = 229) | Topotecan (n = 232) | All Patients (N = 461) |
|---|---|---|---|
| Age, years | |||
| Mean (SD) | 62.9 (8.1) | 61.7 (7.5) | 62.3 (7.8) |
| Median (range) | 63.0 (37.0-82.0) | 62.0 (28.0-81.0) | 62.0 (28.0-82.0) |
| Women, No. (%) | 79 (34.5) | 69 (29.7) | 148 (32.1) |
| White, No. (%) | 184 (80.3) | 182 (78.4) | 366 (79.4) |
| ECOG PS score, No. (%) | |||
| 0 | 59 (25.8) | 59 (25.4) | 118 (25.6) |
| 1 | 169 (73.8) | 173 (74.6) | 342 (74.2) |
| Smoking status, No. (%) | |||
| Current | 72 (31.4) | 76 (32.8) | 148 (32.1) |
| Former | 134 (58.5) | 132 (56.9) | 266 (57.7) |
| Never | 23 (10.0) | 24 (10.3) | 47 (10.2) |
| Disease status, No. (%) | |||
| Locally advanced | 25 (10.9) | 27 (11.6) | 52 (11.3) |
| Metastatic | 204 (89.1) | 205 (88.4) | 409 (88.7) |
| Key metastatic site(s), No. (%) | |||
| Brain and/or CNS lesions | 56 (24.5) | 76 (32.8) | 132 (28.6) |
| Hepatic | 17 (7.4) | 15 (6.5) | 32 (6.9) |
| Bone and locomotor | 51 (22.3) | 58 (25.0) | 109 (23.6) |
| Time since initial diagnosis, months | |||
| Mean (SD) | 9.9 (7.9) | 8.7 (4.7) | 9.3 (6.5) |
| Median (range) | 7.9 (0.8-72.3) | 7.7 (2.3-32.4) | 7.8 (0.8-72.3) |
| Time since recent progression, months | |||
| Mean (SD) | 0.8 (1.0) | 0.8 (1.1) | 0.8 (1.2) |
| Median (range) | 0.5 (0.0-12.2) | 0.4 (0.0-12.2) | 0.4 (0.0-12.2) |
| Prior radiotherapy, No. (%) | |||
| Yes | 114 (49.8) | 121 (52.2) | 235 (51.0) |
| Previous therapies, No. (%) | |||
| Chemotherapy | 229 (100.0) | 232 (100.0) | 461 (100.0) |
| Immunotherapy | 42 (18.3) | 43 (18.5) | 85 (18.4) |
| Targeted therapy | 1 (0.4) | 1 (0.4) | 2 (0.4) |
| Other | 0 (0) | 0 (0) | 0 (0) |
| Best response to previous therapies, No. (%) | |||
| Complete response | 9 (3.9) | 3 (1.3) | 12 (2.6) |
| Partial response | 104 (45.4) | 114 (49.1) | 218 (47.3) |
| Stable disease | 48 (21.0) | 43 (18.5) | 91 (19.7) |
| Progressive disease | 47 (20.5) | 49 (21.1) | 96 (20.8) |
| Nonevaluable | 3 (1.3) | 2 (0.9) | 5 (1.1) |
| Unknown | 18 (7.9) | 21 (9.1) | 39 (8.5) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; PS, performance status; SD, standard deviation.
All patients in both treatment arms had received prior chemotherapy, whereas 18.3% and 18.5% of patients receiving liposomal irinotecan and topotecan, respectively, had received prior immunotherapy (Table 1). The median (range) relative total dose intensity was 97.7% (1.1%-103.4%) and 88.4% (0.7%-102.7%). The median (range) number of treatment cycles (6-week cycle for both arms) was 2 (1-16) and 2 (1-14) and the median duration of treatment was 12.9 weeks (range 2.0-102.4) and 12.7 weeks (3.0-93.6) for patients receiving liposomal irinotecan and topotecan, respectively.
Among those included in the ITT population, 34.9% of patients receiving liposomal irinotecan and 44.0% of those receiving topotecan received subsequent anticancer therapy (Data Supplement, Table S1).
Efficacy
In the ITT population, the median OS was 7.9 months with liposomal irinotecan versus 8.3 months for topotecan (HR for death, 1.11 [95% CI, 0.90 to 1.37]; P = .3094; Fig 2A). Similar 12- and 18-month OS rates were observed for both arms (31.1% v 32.5% and 16.1% v 20.9% for liposomal irinotecan and topotecan, respectively). In subgroup analyses, OS HRs for liposomal irinotecan versus topotecan were generally consistent with those for the overall population (Data Supplement, Fig S1).
FIG 2.
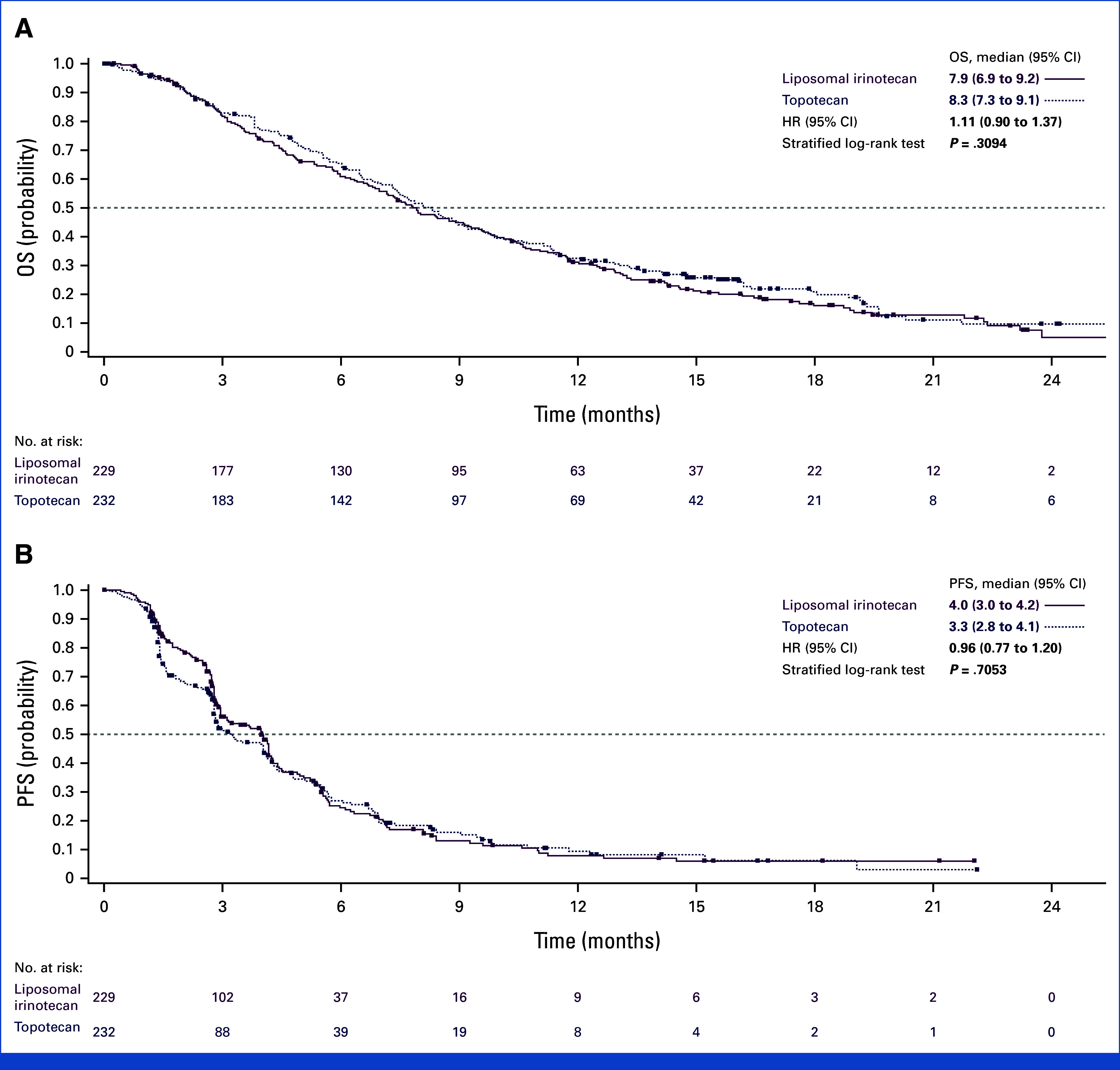
Kaplan-Meier Analysis of (A) OS and (B) PFS. HR, hazard ratio; OS, overall survival; PFS, progression-free survival.
Median PFS per BICR was similar for liposomal irinotecan versus topotecan (4.0 v 3.3 months; HR for disease progression or death, 0.96 [95% CI, 0.77 to 1.20]; nominal P = .7053; Fig 2B).
ORR per BICR was analyzed during the interim analysis (data cutoff August 11, 2021). Liposomal irinotecan was associated with a doubling of ORR compared with topotecan (44.1% v 21.6%; nominal P < .0001). Complete and partial responses were reported in 5.2% and 38.9% of patients receiving liposomal irinotecan, respectively, versus 3.0% and 18.5% of those receiving topotecan (Table 2). Median duration of response was similar for liposomal irinotecan and topotecan (4.1 v 4.2 months; Table 2).
TABLE 2.
Antitumor Activity Outcomes
| Outcome | Liposomal Irinotecan (n = 229) | Topotecan (n = 232) |
|---|---|---|
| Best overall response, No. (%) | ||
| Complete response | 12 (5.2) | 7 (3.0) |
| Partial response | 89 (38.9) | 43 (18.5) |
| Stable disease | 68 (29.7) | 98 (42.2) |
| Progressive disease | 28 (12.2) | 50 (21.6) |
| Not evaluable | 29 (12.7) | 32 (13.8) |
| Undefined | 3 (1.3) | 2 (0.9) |
| ORR, % (95% CI) | ||
| CR + PR | 44.1 (37.6 to 50.8) | 21.6 (16.4 to 27.4) |
| Difference in ORR | 22.3 (14.0 to 30.6); nominal P < .0001 | |
| DoR, months | ||
| Median (95% CI) | 4.1 (3.1 to 4.3) | 4.2 (2.9 to 4.8) |
Abbreviations: CR, complete response; DoR, duration of response; ORR, objective response rate; PR, partial response.
Safety
Any-grade treatment-emergent adverse events (TEAEs) occurred in 96.0% and 99.1% of patients receiving liposomal irinotecan and topotecan, respectively (Table 3). The incidence of grade ≥3 TEAEs was lower in the liposomal irinotecan arm than in the topotecan arm (62.4% v 87.9%; Table 3), and the most common grade ≥3 TEAEs were diarrhea (13.7%), neutropenia (9.3%), and pneumonia (8.0%) for liposomal irinotecan and neutropenia (52.9%), anemia (33.6%), and thrombocytopenia (30.5%) for topotecan. Grade ≥3 related TEAEs occurred in 42.0% of patients receiving liposomal irinotecan and 83.4% receiving topotecan. The most common grade ≥3 related TEAEs were diarrhea (13.7%), neutropenia (8.0%), and decreased neutrophil count (4.4%) in the liposomal irinotecan arm and neutropenia (51.6%), anemia (30.9%), leukopenia, and thrombocytopenia (both 29.1%) in the topotecan arm (Table 3).
TABLE 3.
Duration of Treatment, Cumulative Doses, and Overview of TEAEs
| Outcome | Liposomal Irinotecan (n = 226) | Topotecan (n = 223) | All Patients (N = 449) |
|---|---|---|---|
| Duration of treatment, weeks, median (range) | 12.9 (2.0-102.4) | 12.7 (3.0-93.6) | NR |
| Total dose received, mg, median (range) | 704.5 (1.5-6,195.0) | 50.0 (0.1-359.4) | NR |
| Patients with a TEAE, No. (%) | |||
| Any TEAE | 217 (96.0) | 221 (99.1) | 438 (97.6) |
| Any treatment-related TEAE | 195 (86.3) | 214 (96.0) | 409 (91.1) |
| Grade ≥3 | 141 (62.4) | 196 (87.9) | 337 (75.1) |
| Any TEAE leading to discontinuation | 24 (10.6) | 23 (10.3) | 47 (10.5) |
| Any TEAE leading to dose reduction | 63 (27.9) | 104 (46.6) | 167 (37.2) |
| Any serious TEAE | 105 (46.5) | 88 (39.5) | 193 (43.0) |
| Leading to death | 19 (8.4) | 9 (4.0) | 28 (6.2) |
| Treatment-related TEAE of grade ≥3 occurring in ≥5% of patients in all treatment arms, No. (%) | |||
| Diarrhea | 31 (13.7) | 3 (1.3) | 34 (7.6) |
| Neutropenia | 18 (8.0) | 115 (51.6) | 133 (29.6) |
| Neutrophil count decreased | 10 (4.4) | 39 (17.5) | 49 (10.9) |
| Leukopenia | 9 (4.0) | 65 (29.1) | 74 (16.5) |
| WBC count decreased | 9 (4.0) | 24 (10.8) | 33 (7.3) |
| Anemia | 6 (2.7) | 69 (30.9) | 75 (16.7) |
| Platelet count decreased | 3 (1.3) | 39 (17.5) | 42 (9.4) |
| Febrile neutropenia | 3 (1.3) | 13 (5.8) | 16 (3.6) |
| Lymphopenia | 2 (0.9) | 15 (6.7) | 17 (3.8) |
| Thrombocytopenia | 1 (0.4) | 65 (29.1) | 66 (14.7) |
Abbreviations: NR, not reported; SD, standard deviation; TEAE, treatment-emergent adverse event.
Overall, 10.6% of patients receiving liposomal irinotecan and 10.3% of those receiving topotecan experienced TEAEs leading to treatment discontinuation (Table 3); related TEAEs leading to treatment discontinuation occurred in 4.9% and 4.0% of patients, respectively, and are summarized in the Data Supplement (Table S2). TEAEs leading to dose reduction occurred in 27.9% of patients receiving liposomal irinotecan and 46.6% of those receiving topotecan (Table 3). TEAEs leading to death occurred in 8.4% of patients in the liposomal irinotecan group and 4.0% of patients in the topotecan group; those deemed to be related to treatment occurred in 1.3% and 0.9% of patients, respectively (Data Supplement, Tables S3 and S4).
The details of TEAEs occurring in ≥10% of patients, TEAEs of grade ≥3 occurring in ≥5% of patients, serious TEAEs occurring in ≥2% of patients, and serious related TEAEs occurring in ≥2% of patients are summarized in the Data Supplement (Tables S5-S8, respectively).
DISCUSSION
Part 2 of the RESILIENT study demonstrated similar median OS and PFS for liposomal irinotecan compared with topotecan in patients with SCLC that had progressed on or after first-line platinum-based chemotherapy. Although the primary end point of OS was not met, there was a doubling of ORR (44.1% v 21.6%) with no overlapping confidence intervals in patients receiving liposomal irinotecan compared with those receiving topotecan. These results are consistent with RESILIENT part 1, in which 70 mg/m2 liposomal irinotecan (every 2 weeks in a 6-week cycle) demonstrated a median PFS of 3.98 months (95% CI, 1.45 to 4.24), an ORR of 44.0%, and a median OS of 8.08 months (5.16 to 9.82).24
The safety and tolerability of liposomal irinotecan was consistent with its known safety profile; the most frequent grade ≥3 related TEAE was diarrhea, which is consistent with data reported in RESILIENT part 1.24 In this study, liposomal irinotecan compared with topotecan demonstrated a lower frequency of TEAEs leading to dose reduction, grade ≥3 TEAEs, and grade ≥3 related TEAEs. Overall, the frequency of hematological AEs was lower in patients receiving liposomal irinotecan than in those receiving topotecan. Hematological grade ≥3 related TEAEs such as neutropenia, anemia, thrombocytopenia, and febrile neutropenia occurred at a higher frequency in the topotecan arm; however, the rate of GI grade ≥3 related TEAEs was higher among patients receiving liposomal irinotecan.
The dichotomy between a doubling of response rate with liposomal irinotecan but no significant improvement in survival statistics is striking. Although slight imbalances in demographics were noted between cohorts, these differences are unlikely to contribute substantially to the observed dichotomy. Post-therapy treatment imbalances may also have played a role; however, a more probable explanation relates to the intrinsic biology of SCLC, which is notable for the widespread inactivation of two key cell cycle checkpoint regulators, TP53 and RB1. The concomitant loss of these tumor suppressors promotes chromosomal instability and may contribute to the exceptional intratumoral heterogeneity of SCLC.28 The higher response rate of liposomal irinotecan could reflect a more potent cytotoxic effect in which more sensitive cancer cells are killed but the most resistant survive. The latter of these may give rise to disease recurrence in a similar time course, regardless of the fraction of cancer cells killed. The substantially higher response rate together with less frequent severe treatment-related AEs support liposomal irinotecan as an attractive cytotoxic on which to consider future combination studies—with the goal of combining the high activity of liposomal irinotecan with agents that could extend the durability of the initial response. Further research will be needed to understand how and in which contexts liposomal irinotecan monotherapy, or novel liposomal irinotecan therapy combinations, might benefit patients with SCLC.
RESILIENT part 2 featured a large sample size and randomized study design with outcomes stratified across clinically relevant subgroups to allow the consistency of the results to be evaluated. Importantly, crossover between treatment arms was not permitted during active treatment but was allowed after treatment discontinuation. Furthermore, this trial included a population representative of clinical practice and included patients with platinum-resistant disease, prior immunotherapy, and brain and/or CNS metastases. However, this was an open-label study, which may confer a degree of confounding by indication.
The results of the RESILIENT study underline a persistent need for well-tolerated and efficacious treatment options in the second-line setting. In addition, with increasing uptake of first-line chemoimmunotherapy regimens, there is an emerging requirement to establish the efficacy of second-line therapies in patients who have received these regimens.29 Since key genetic drivers of SCLC have yet to be identified, there is also a need for clinical trials to drive collection of tumor tissue for preclinical and clinical research and facilitate opportunities for targeted therapy. Improved understanding of SCLC biology on the basis of the differential expression of transcription factors may help to optimize treatment strategies and identify patients most likely to benefit from a specific approach.30
In conclusion, the phase III RESILIENT study showed similar median OS for liposomal irinotecan compared with topotecan in patients with SCLC who had progressed on or after first-line platinum-based chemotherapy. Although the primary end point was not met, liposomal irinotecan demonstrated similar PFS, a doubling of ORR, and reduced incidence of grade ≥3 related TEAEs and TEAE-related discontinuations compared with topotecan. This level of activity together with improved tolerability will support future combinatorial therapy research with liposomal irinotecan.
ACKNOWLEDGMENT
The authors thank all patients involved in the study, as well as their caregivers, care team, investigators, and research staff in participating institutions. The authors thank Amber Tear and Emma Bolton, DPhil, of Oxford PharmaGenesis, Oxford, the United Kingdom for providing medical writing support, which was sponsored by Ipsen in accordance with Good Publication Practice guidelines (GPP 2022).
A list of the RESILIENT trial investigators can be found in Appendix Table A1.
APPENDIX
TABLE A1.
RESILIENT Trial Investigator List
| Country | Site Name | Principal Investigator |
|---|---|---|
| Australia | Southern Medical Day Care Center | Philip Clingan |
| Warrnambool & District Base Hospital | Theresa Hayes | |
| Border Medical Oncology Research Unit | Craig Underhill | |
| Belgium | AZ Klina | Wim Demey |
| Center Hospitalier de l'Ardenne | Frederic Forget | |
| AZ Sint-Maarten | Marc Lambrechts | |
| UZ Leuven | Kristiaan Nackaerts | |
| Brazil | Hospital de Caridade de Ijui | Fabio André Franke |
| HGB—Hospital Giovanni Battista—Mãe de Deus Center | Alan Arrieira Azambuja | |
| Oncobio Servicos de Saude | Rodrigo Guimaraes | |
| Hospital de Câncer de Barretos—Fundação Pio XII | Josiane Mourao Dias | |
| INCA—Instituto Nacional de Câncer | Victor Santos | |
| Fundação Faculdade Regional de Medicina de São José do Rio Preto | Bruno Cezar Uchoa Junior | |
| Hospital Nossa Senhora da Conceicao | Gustavo Vasconcelos Alves | |
| CEPHO—Centro de Estudos e Pesquisas de Hematologia e Oncologia | Claudia Vaz de Melo Sette | |
| China | The First Affiliated Hospital of Bengbu Medical College | Minghong Bi |
| The First Hospital of Jilin University | Jiuwei Cui | |
| Beijing Cancer Hospital | Jian Fang | |
| Linyi Cancer Hospital | Jianhua Shi | |
| West China Hospital, Sichuan University | Ke Wang | |
| Guangdong Provincial People's Hospital | Zhen Wang | |
| Zhejiang Cancer Hospital | Xinmin Yu | |
| France | Center Hospitalier de Saint-Quentin | Charles Dayen |
| Hôpital Nord—CHU Marseille | Laurent Greillier | |
| CHU Brest—Hôpital Morvan | Gilles Quere | |
| Germany | Evangelisches Krankenhaus Hamm gGmbH | Alexander Baraniskin |
| Thoraxklinik Heidelberg gGmbH | Helge Bischoff | |
| Pius-Hospital Oldenburg | Frank Griesinger | |
| Universitaetsklinikum Freiburg | Cornelius Waller | |
| Hungary | Jasz-Nagykun-Szolnok Megyei Hetenyi Geza Korhaz-Rendelointezet | Tibor Csoszi |
| Tudogyogyintezet Torokbalint | Gabriella Galffy | |
| Bekes Megyei Kozponti Korhaz Pandy Kalman Tagkorhaza | Ibolya Laczo | |
| Semmelweis Egyetem | Gyorgy Losonczy | |
| Zala Varmegyei Szent Rafael Korhaz | Sandor Tehenes | |
| Italy | IRCCS Istituto Scientifico Romagnolo Per Lo Studio e La Cura Dei Tumori “Dino Amadori”—IRST | Angelo Delmonte |
| Azienda Sanitaria Universitaria Friuli Centrale | Alessandro Follador | |
| Poland | Szpital Kliniczny im. Heliodora Swiecickiego Uniwersytetu Medycznego im. Karola Marcinkowskiego | Halina Batura-Gabryel |
| KO-MED Centra Kliniczne Biala Podlaska | Piotr Centkowski | |
| Szpitale Pomorskie spółka z ograniczoną odpowiedzialnością | Iwona Danielewicz | |
| Warminsko-Mazurskie Centrum Chorob Pluc w Olsztynie | Andrzej Kazarnowicz | |
| Med-Polonia Sp. z o.o. | Rodryg Ramlau | |
| Romania | Institutul Oncologic “Prof. Dr. Ion Chiricuta” Cluj-Napoca | Alina Simona Muntean |
| Oncomed S.R.L. | Cristina Marinela Oprean | |
| S.C Centrul de Oncologie Sf. Nectarie S.R.L | Michael Schenker | |
| S.C Gral Medical S.R.L | Cristina Tiut | |
| S.C Medisprof S.R.L | Anghel Adrian Udrea | |
| S.C Radiotherapy Center Cluj S.R.L | Andrei Ungureanu | |
| Russia | SPb SBIH “City Clinical Oncological Dispensary” | Nina Karaseva |
| SBIH of Yaroslavl region “Regional Clinical Oncological Hospital” | Nikolay Kislov | |
| “VitaMed” LLC | Elena Poddubskaya | |
| SBHI of Kaluga Region “Kaluga regional clinical oncology dispensary” | Irina Rozhkova | |
| SBIH of Arkhangelsk region “Arkhangelsk Clinical Oncological Dispensary” | Ekaterina Solovyeva | |
| BHI of Omsk region “Clinical Oncology Dispensary” | Anastasia Zimina | |
| Serbia | Clinical Hospital Center “Bezanijska kosa” | Zoran Andric |
| Oncomed System | Vladimir Kovcin | |
| University Clinical Center Kragujevac | Marina Petrovic | |
| General Hospital Uzice | Zorica Radojevic | |
| Institute for Pulmonary Diseases of Vojvodina | Goran Stojanovic | |
| South Korea | The Catholic University of Korea, Seoul St Mary's Hospital | SookHee Hong |
| Asan Medical Center | Sang-We Kim | |
| Chungbuk National University Hospital | Ki Hyeong Lee | |
| The Catholic University of Korea, St Vincent's Hospital | Byoung Yong Shim | |
| Spain | Hospital Universitario Virgen del Rocio | Miriam Alonso Garcia |
| Hospital General Universitario Gregorio Marañon | Antonio Calles Blanco | |
| Hospital Regional Universitario de Malaga | Vanesa Gutierrez Calderon | |
| Hospital Universitari i Politecnic La Fe | Oscar Jose Juan Vidal | |
| Hospital General Universitario de Alicante | Bartomeu Massuti Sureda | |
| Hospital Universitari Vall d'Hebron | Alejandro Navarro Mendivil | |
| ICO l'Hospitalet—Hospital Duran i Reynals | Ramon Palmero Sanchez | |
| Hospital Universitario 12 de Octubre | Luis Paz-Ares Rodriguez | |
| Taiwan | Tri-Service General Hospital | Ching-Liang Ho |
| Changhua Christian Medical Foundation Changhua Christian Hospital | Sheng-Hao Lin | |
| Chang Gung Memorial Hospital, Linkou | Chien-Ying Liu | |
| National Taiwan University Hospital | Chih-Hsin Yang | |
| Turkey | Trakya University Medical Faculty | Irfan Cicin |
| Goztepe Prof Dr Suleyman Yalcin Sehir Hastanesi | Mahmut Gumus | |
| Inonu Uni. Med. Fac. | Hakan Harputluoglu | |
| Istanbul University Cerrahpasa—Cerrahpasa Medical Faculty | Mustafa Ozguroglu | |
| Namik Kemal University | Erdogan Selcuk Seber | |
| Baskent University Adana Application and Research Center | Ahmet Sezer | |
| Ukraine | CI Kryvyi Rih Oncological Dispensary of DRC | Hryhoriy Adamchuk |
| CNE “City Clin Hosp#4” of Dnipro City Council Dept of Chemotherapy SI Dnipropetrovsk MA of MOHU | Igor Bondarenko | |
| CNE CCCH of Uzh CC Oncological Center, Ther Dept, SHEI UNU | Yevhen Hotko | |
| Communal Nonprofit Enterprise Regional Center of Oncology | Oleh Kobziev | |
| Communal Enterprise Kremenchuk Regional Oncology Dispensary of Poltava Regional Council | Oleksandr Koshelenko | |
| RCI Sumy Regional Clinical Oncological Dispensary | Andriy Kurochkin | |
| CI Chernivtsi RC Oncological Dispensary | Yuriy Semegen | |
| Communal Enterprise Volyn Regional Medical Center of Oncology of Volyn Regional Council | Ivan Sinielnikov | |
| Medical Clinic Innovacia, LLC | Tetiana Tarasenko | |
| Odesa Regional Oncologic Dispensary | Dmytro Trukhin | |
| The United States | Winship Cancer Institute of Emory University | Jennifer Carlisle |
| National Jewish Health | Laurie Carr | |
| Summit Cancer Treatment Center | Arvind Chaudhry | |
| Roswell Park Comprehensive Cancer Center | Hongbin Chen | |
| Cancer & Hematology Centers of Western Michigan | Yuanbin Chen | |
| University Hospitals Cleveland Medical Center | Afshin Dowlati | |
| Cancer Research-Atlanta Leader Breast Cancer Institute-CTCA | Herbert Duvivier | |
| Prisma Health Upstate | William Edenfield | |
| Florida Cancer Specialists North | Maen Hussein | |
| Rocky Mountain Cancer Centers, LLP | Robert Jotte | |
| Illinois CancerCare PC | Srinivas Jujjavarapu | |
| Charleston Hematology Oncology Associates, PA | Brian Lingerfelt | |
| Northwest Georgia Oncology Centers | Steven McCune | |
| Tri County Hematology & Oncology Associates, Inc | Nagaprasad Nagajothi | |
| Southern Maine Health Care | Peter Rubin | |
| University of Maryland Medical Group | Katherine Scilla | |
| Tennessee Oncology—Skyline Satellite | David Spigel | |
| Sparrow Regional Cancer Center | Gordan Srkalovic | |
| Henry Ford Hospital | Amy Weise | |
| North Shore Hematology Oncology Associates, PC | Richard Zuniga |
David R. Spigel
Leadership: ASCO (Inst)
Consulting or Advisory Role: Genentech/Roche (Inst), Novartis (Inst), Bristol Myers Squibb (Inst), AstraZeneca (Inst), GlaxoSmithKline (Inst), Jazz Pharmaceuticals (Inst), Sanofi/Aventis (Inst), Ipsen (Inst), Monte Rosa Therapeutics (Inst), AbbVie (Inst), Lyell Immunopharma (Inst), Novocure (Inst), Amgen (Inst), MedImmune (Inst)
Research Funding: Genentech/Roche (Inst), Novartis (Inst), Celgene (Inst), Bristol Myers Squibb (Inst), Lilly (Inst), AstraZeneca (Inst), University of Texas Southwestern Medical Center—Simmons Cancer Center (Inst), Merck (Inst), G1 Therapeutics (Inst), Neon Therapeutics (Inst), Nektar (Inst), Celldex (Inst), Daiichi Sankyo (Inst), Astellas Pharma (Inst), Grail (Inst), Transgene (Inst), Aeglea Biotherapeutics (Inst), Ipsen (Inst), Eisai (Inst), ImClone Systems (Inst), Janssen Oncology (Inst), MedImmune (Inst), Agios (Inst), GlaxoSmithKline (Inst), Tesaro (Inst), Cyteir (Inst), Novocure (Inst), Elevation Oncology (Inst), Calithera Biosciences (Inst), Arcus Biosciences (Inst), Arrys Therapeutics (Inst), Bayer (Inst), BeiGene (Inst), Blueprint Medicines (Inst), Boehringer Ingelheim (Inst), Hutchison MediPharma (Inst), Incyte (Inst), Kronos Bio (Inst), Loxo (Inst), Macrogenics (Inst), PureTech (Inst), Razor Genomics (Inst), Repare Therapeutics (Inst), Rgenix (Inst), Tizona Therapeutics, Inc (Inst), Verastem (Inst), BioNTech (Inst), AbbVie (Inst), Amgen (Inst), Anheart Therapeutics (Inst), Ascendis Pharma (Inst), Endeavor BioMedicines (Inst), Erasca, Inc (Inst), Faeth Therapeutics (Inst), Fujifilm (Inst), Gilead Sciences (Inst), Jazz Pharmaceuticals (Inst), Lyell Immunopharma (Inst), Millennium (Inst), Moderna Therapeutics (Inst), Monte Rosa Therapeutics (Inst), Peloton Therapeutics (Inst), Shenzhen Chipscreen Biosciences (Inst), Stemline Therapeutics (Inst), Synthekine (Inst), Taiho Oncology (Inst), Tango Therapeutics (Inst), Tarveda Therapeutics (Inst), Zai Lab (Inst), Apollomics (Inst), Strata Oncology (Inst), Asher Biotherapeutics (Inst), Denovo Biopharma (Inst), Ellipses Pharma (Inst), EMD Serono (Inst), Evelo Biosciences (Inst), Foundation Bio (Inst), Immunogen (Inst), Janux Therapeutics (Inst), Oncologie (Inst), Pfizer (Inst), Phanes Therapeutics (Inst), PTC Therapeutics (Inst), Seagen (Inst), Takeda (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Genentech, Novartis
Afshin Dowlati
Consulting or Advisory Role: AbbVie/Stemcentrx, AstraZeneca, Bristol Myers Squibb, Ipsen, Merck, Tempus
Research Funding: Lilly/ImClone (Inst), Amgen (Inst), Bristol Myers Squibb (Inst), Tesaro (Inst), Takeda (Inst), Mirati Therapeutics (Inst), AbbVie/Stemcentrx (Inst), Bayer (Inst), Seagen (Inst), Ipsen (Inst), Pionyr (Inst), Coordination Pharmaceuticals (Inst), Astellas Pharma (Inst), Bicycle Therapeutics (Inst), Gilead Sciences (Inst)
Yuanbin Chen
Honoraria: AstraZeneca, Amgen, Takeda, Guardant Health, Bristol Myers Squibb/Pfizer, Jazz Pharmaceuticals
Consulting or Advisory Role: Bristol Myers Squibb, AstraZeneca
Speakers' Bureau: AstraZeneca, Bristol Myers Squibb, Takeda, Guardant Health, Amgen, Jazz Pharmaceuticals
Research Funding: AstraZeneca (Inst), Bristol Myers Squibb (Inst), Amgen (Inst), Genentech (Inst), Merck (Inst), Daiichi Sankyo/Astra Zeneca (Inst)
Expert Testimony: AstraZeneca, Takeda
Alejandro Navarro
Consulting or Advisory Role: Boehringer Ingelheim, Bristol Myers Squibb Foundation, Pfizer, Amgen, Takeda, Adium Pharma, Eczacibasi
Speakers' Bureau: Roche, AstraZeneca Spain
Expert Testimony: Oryzon Genomics, Medsir, Hengenix
Travel, Accommodations, Expenses: Boehringer Ingelheim, Pfizer, Roche
James Chih-Hsin Yang
Honoraria: Boehringer Ingelheim, Roche, MSD, AstraZeneca, Novartis, Bristol Myers Squibb, Ono Pharmaceutical, Takeda, Lilly, Pfizer, Amgen (Inst), AstraZeneca/MedImmune (Inst), Boehringer Ingelheim (Inst), Dizal Pharma (Inst), Taiho Pharmaceutical (Inst), Pfizer (Inst), Takeda (Inst), Roche/Genentech (Inst), Daiichi Sankyo/Astra Zeneca (Inst), MSD Oncology (Inst), BeiGene (Inst), Gilead Sciences (Inst), Sanofi/Regeneron (Inst)
Consulting or Advisory Role: Boehringer Ingelheim, Novartis, AstraZeneca, Clovis Oncology, Lilly (Inst), MSD Oncology, Celgene, Bayer, Pfizer, Ono Pharmaceutical, Bristol Myers Squibb, Boehringer Ingelheim (Inst), Yuhan, Hansoh, Blueprint Medicines, Daiichi Sankyo, G1 Therapeutics, AbbVie, Takeda, Amgen, Incyte, GlaxoSmithKline (Inst), Amgen (Inst), Takeda (Inst), AstraZeneca (Inst), Novartis (Inst), MSD Oncology (Inst), Janssen Oncology (Inst), Merck KGaA (Inst), Daiichi Sankyo/Astra Zeneca (Inst), Puma Biotechnology (Inst), Gilead Sciences (Inst), Pfizer (Inst), Taiho Pharmaceutical (Inst), Bayer (Inst), Roche/Genentech (Inst), Sanofi (Inst)
Research Funding: AstraZeneca (Inst)
Travel, Accommodations, Expenses: Pfizer
Maria Jove
Speakers' Bureau: AstraZeneca
Travel, Accommodations, Expenses: Roche
Yi-Long Wu
Honoraria: AstraZeneca, Roche, Pfizer, Boehringer Ingelheim, MSD Oncology, Bristol Myers Squibb/China, Hengrui Pharmaceutical, BeiGene Beijing
Consulting or Advisory Role: AstraZeneca, Roche, Boehringer Ingelheim, Takeda
Research Funding: Boehringer Ingelheim (Inst), Roche (Inst), Pfizer (Inst), BMS (Inst)
Charles M. Rudin
Consulting or Advisory Role: Harpoon Therapeutics, Genentech/Roche, AstraZeneca, Bridge Medicines, Amgen, Jazz Pharmaceuticals, Earli, AbbVie, Daiichi Sankyo/UCB Japan, Kowa, Merck, D2G Oncology, Auron Therapeutics, DISCO
Research Funding: Merck (Inst), Roche/Genentech (Inst), Daiichi Sankyo (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/111056
Huanyu Chen
Employment: Ipsen, Modern Biosciences
Stock and Other Ownership Interests: Ipsen, Moderna Therapeutics
Li Zhang
Employment: Ipsen
Stock and Other Ownership Interests: Ipsen
Stanley Yeung
Employment: Ipsen, GlaxoSmithKline
Stock and Other Ownership Interests: GlaxoSmithKline, Ipsen
Fawzi Benzaghou
Employment: Ipsen
Stock and Other Ownership Interests: Ipsen
Luis Paz-Ares
Leadership: Altum Sequencing, Stab Therapeutics
Stock and Other Ownership Interests: Altum Sequencing, Stab therapeutics
Honoraria: Roche/Genentech, Lilly, Pfizer, Bristol Myers Squibb, MSD, AstraZeneca, Merck Serono, PharmaMar, Novartis, Amgen, Sanofi, Bayer, Takeda, Mirati, Daiichi Sankyo, BeiGene, GSK, Janssen, Medscape, Regeneron, Boehringer Ingelheim
Consulting or Advisory Role: Lilly, MSD, Roche, PharmaMar, Merck, AstraZeneca, Novartis, Amgen, Pfizer, Sanofi, Bayer, BMS, Mirati, GSK, Janssen, Takeda, Regeneron, AbbVie
Speakers' Bureau: MSD Oncology, BMS, Roche/Genentech, Pfizer, Lilly, AstraZeneca, Merck Serono
Research Funding: BMS (Inst), Astra Zeneca (Inst), PharmaMar (Inst), MSD (Inst), Pfizer (Inst)
Other Relationship: Novartis, Ipsen, Pfizer, Servier, Sanofi, Roche, Amgen, Merck, Roche
Paul A. Bunn
Leadership: Verastem
Honoraria: CStone Pharmaceuticals, Ascentage Pharma, VieCure, Genentech/Roche
Consulting or Advisory Role: CStone Pharmaceuticals, Ascentage Pharma, Genentech/Roche, Ipsen
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at the European Lung Cancer Congress 2023, Copenhagen, Denmark, March 29-April 1, 2023.
SUPPORT
Supported by Ipsen. The sponsor was involved in the design of the study, analysis and interpretation of the data, and review of the manuscript.
CLINICAL TRIAL INFORMATION
NCT03088813; EudraCT 2017-004261-26
D.R.S. and A.D. joint first senior author.
Contributor Information
Collaborators: Philip Clingan, Theresa Hayes, Craig Underhill, Wim Demey, Frederic Forget, Marc Lambrechts, Kristiaan Nackaerts, Fabio André Franke, Alan Arrieira Azambuja, Rodrigo Guimaraes, Josiane Mourao Dias, Victor Santos, Bruno Cezar Uchoa Junior, Gustavo Vasconcelos Alves, Claudia Vaz de Melo Sette, Minghong Bi, Jiuwei Cui, Jian Fang, Jianhua Shi, Ke Wang, Zhen Wang, Xinmin Yu, Charles Dayen, Laurent Greillier, Gilles Quere, Alexander Baraniskin, Helge Bischoff, Frank Griesinger, Cornelius Waller, Tibor Csoszi, Gabriella Galffy, Ibolya Laczo, Gyorgy Losonczy, Sandor Tehenes, Angelo Delmonte, Alessandro Follador, Halina Batura-Gabryel, Piotr Centkowski, Iwona Danielewicz, Andrzej Kazarnowicz, Rodryg Ramlau, Alina Simona Muntean, Cristina Marinela Oprean, Michael Schenker, Cristina Tiut, Anghel Adrian Udrea, Andrei Ungureanu, Nina Karaseva, Nikolay Kislov, Elena Poddubskaya, Irina Rozhkova, Ekaterina Solovyeva, Anastasia Zimina, Zoran Andric, Vladimir Kovcin, Marina Petrovic, Zorica Radojevic, Goran Stojanovic, SookHee Hong, Sang-We Kim, Ki Hyeong Lee, Byoung Yong Shim, Miriam Alonso Garcia, Antonio Calles Blanco, Vanesa Gutierrez Calderon, Oscar Jose Juan Vidal, Bartomeu Massuti Sureda, Alejandro Navarro Mendivil, Ramon Palmero Sanchez, Luis Paz-Ares Rodriguez, Ching-Liang Ho, Sheng-Hao Lin, Chien-Ying Liu, Chih-Hsin Yang, Irfan Cicin, Mahmut Gumus, Hakan Harputluoglu, Mustafa Ozguroglu, Erdogan Selcuk Seber, Ahmet Sezer, Hryhoriy Adamchuk, Igor Bondarenko, Yevhen Hotko, Oleh Kobziev, Oleksandr Koshelenko, Andriy Kurochkin, Yuriy Semegen, Ivan Sinielnikov, Tetiana Tarasenko, Dmytro Trukhin, Jennifer Carlisle, Laurie Carr, Arvind Chaudhry, Hongbin Chen, Yuanbin Chen, Afshin Dowlati, Herbert Duvivier, William Edenfield, Maen Hussein, Robert Jotte, Srinivas Jujjavarapu, Brian Lingerfelt, Steven McCune, Nagaprasad Nagajothi, Peter Rubin, Katherine Scilla, David Spigel, Gordan Srkalovic, Amy Weise, and Richard Zuniga
DATA SHARING STATEMENT
Qualified researchers may request access to patient-level study data that underlie the results reported in this publication. Additional relevant study documents, including the clinical study report, study protocol with any amendments, annotated case report form, statistical analysis plan, and data set specifications may also be made available. Patient-level data will be anonymized, and study documents will be redacted to protect the privacy of study participants. Where applicable, data from eligible studies are available 6 months after the studied medicine and indication have been approved in the United States and European Union or after the primary manuscript describing the results has been accepted for publication, whichever is later. Further details on Ipsen's sharing criteria, eligible studies, and process for sharing are available here (https://vivli.org/members/ourmembers/). Any requests should be submitted to www.vivli.org for assessment by an independent scientific review board.
AUTHOR CONTRIBUTIONS
Conception and design: Afshin Dowlati, James Chih-Hsin Yang, Zoran G. Andric, Yi-Long Wu, Huanyu Chen, Fawzi Benzaghou, Luis Paz-Ares, Paul A. Bunn
Administrative support: Afshin Dowlati, Goran Stojanovic, Zoran G. Andric
Provision of study materials or patients: David R. Spigel, Afshin Dowlati, Yuanbin Chen, Alejandro Navarro, James Chih-Hsin Yang, Patricia Rich, Zoran G. Andric, Yi-Long Wu, Huanyu Chen, Li Zhang, Luis Paz-Ares
Collection and assembly of data: Afshin Dowlati, Yuanbin Chen, Alejandro Navarro, James Chih-Hsin Yang, Maria Jove, Patricia Rich, Zoran G. Andric, Yi-Long Wu, Huanyu Chen, Li Zhang, Fawzi Benzaghou, Luis Paz-Ares, Paul A. Bunn
Data analysis and interpretation: David R. Spigel, Yuanbin Chen, Alejandro Navarro, James Chih-Hsin Yang, Goran Stojanovic, Maria Jove, Patricia Rich, Zoran G. Andric, Yi-Long Wu, Charles M. Rudin, Huanyu Chen, Li Zhang, Stanley Yeung, Fawzi Benzaghou, Luis Paz-Ares, Paul A. Bunn
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
RESILIENT Part 2: A Randomized, Open-Label Phase III Study of Liposomal Irinotecan Versus Topotecan in Adults With Relapsed Small Cell Lung Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
David R. Spigel
Leadership: ASCO (Inst)
Consulting or Advisory Role: Genentech/Roche (Inst), Novartis (Inst), Bristol Myers Squibb (Inst), AstraZeneca (Inst), GlaxoSmithKline (Inst), Jazz Pharmaceuticals (Inst), Sanofi/Aventis (Inst), Ipsen (Inst), Monte Rosa Therapeutics (Inst), AbbVie (Inst), Lyell Immunopharma (Inst), Novocure (Inst), Amgen (Inst), MedImmune (Inst)
Research Funding: Genentech/Roche (Inst), Novartis (Inst), Celgene (Inst), Bristol Myers Squibb (Inst), Lilly (Inst), AstraZeneca (Inst), University of Texas Southwestern Medical Center—Simmons Cancer Center (Inst), Merck (Inst), G1 Therapeutics (Inst), Neon Therapeutics (Inst), Nektar (Inst), Celldex (Inst), Daiichi Sankyo (Inst), Astellas Pharma (Inst), Grail (Inst), Transgene (Inst), Aeglea Biotherapeutics (Inst), Ipsen (Inst), Eisai (Inst), ImClone Systems (Inst), Janssen Oncology (Inst), MedImmune (Inst), Agios (Inst), GlaxoSmithKline (Inst), Tesaro (Inst), Cyteir (Inst), Novocure (Inst), Elevation Oncology (Inst), Calithera Biosciences (Inst), Arcus Biosciences (Inst), Arrys Therapeutics (Inst), Bayer (Inst), BeiGene (Inst), Blueprint Medicines (Inst), Boehringer Ingelheim (Inst), Hutchison MediPharma (Inst), Incyte (Inst), Kronos Bio (Inst), Loxo (Inst), Macrogenics (Inst), PureTech (Inst), Razor Genomics (Inst), Repare Therapeutics (Inst), Rgenix (Inst), Tizona Therapeutics, Inc (Inst), Verastem (Inst), BioNTech (Inst), AbbVie (Inst), Amgen (Inst), Anheart Therapeutics (Inst), Ascendis Pharma (Inst), Endeavor BioMedicines (Inst), Erasca, Inc (Inst), Faeth Therapeutics (Inst), Fujifilm (Inst), Gilead Sciences (Inst), Jazz Pharmaceuticals (Inst), Lyell Immunopharma (Inst), Millennium (Inst), Moderna Therapeutics (Inst), Monte Rosa Therapeutics (Inst), Peloton Therapeutics (Inst), Shenzhen Chipscreen Biosciences (Inst), Stemline Therapeutics (Inst), Synthekine (Inst), Taiho Oncology (Inst), Tango Therapeutics (Inst), Tarveda Therapeutics (Inst), Zai Lab (Inst), Apollomics (Inst), Strata Oncology (Inst), Asher Biotherapeutics (Inst), Denovo Biopharma (Inst), Ellipses Pharma (Inst), EMD Serono (Inst), Evelo Biosciences (Inst), Foundation Bio (Inst), Immunogen (Inst), Janux Therapeutics (Inst), Oncologie (Inst), Pfizer (Inst), Phanes Therapeutics (Inst), PTC Therapeutics (Inst), Seagen (Inst), Takeda (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Genentech, Novartis
Afshin Dowlati
Consulting or Advisory Role: AbbVie/Stemcentrx, AstraZeneca, Bristol Myers Squibb, Ipsen, Merck, Tempus
Research Funding: Lilly/ImClone (Inst), Amgen (Inst), Bristol Myers Squibb (Inst), Tesaro (Inst), Takeda (Inst), Mirati Therapeutics (Inst), AbbVie/Stemcentrx (Inst), Bayer (Inst), Seagen (Inst), Ipsen (Inst), Pionyr (Inst), Coordination Pharmaceuticals (Inst), Astellas Pharma (Inst), Bicycle Therapeutics (Inst), Gilead Sciences (Inst)
Yuanbin Chen
Honoraria: AstraZeneca, Amgen, Takeda, Guardant Health, Bristol Myers Squibb/Pfizer, Jazz Pharmaceuticals
Consulting or Advisory Role: Bristol Myers Squibb, AstraZeneca
Speakers' Bureau: AstraZeneca, Bristol Myers Squibb, Takeda, Guardant Health, Amgen, Jazz Pharmaceuticals
Research Funding: AstraZeneca (Inst), Bristol Myers Squibb (Inst), Amgen (Inst), Genentech (Inst), Merck (Inst), Daiichi Sankyo/Astra Zeneca (Inst)
Expert Testimony: AstraZeneca, Takeda
Alejandro Navarro
Consulting or Advisory Role: Boehringer Ingelheim, Bristol Myers Squibb Foundation, Pfizer, Amgen, Takeda, Adium Pharma, Eczacibasi
Speakers' Bureau: Roche, AstraZeneca Spain
Expert Testimony: Oryzon Genomics, Medsir, Hengenix
Travel, Accommodations, Expenses: Boehringer Ingelheim, Pfizer, Roche
James Chih-Hsin Yang
Honoraria: Boehringer Ingelheim, Roche, MSD, AstraZeneca, Novartis, Bristol Myers Squibb, Ono Pharmaceutical, Takeda, Lilly, Pfizer, Amgen (Inst), AstraZeneca/MedImmune (Inst), Boehringer Ingelheim (Inst), Dizal Pharma (Inst), Taiho Pharmaceutical (Inst), Pfizer (Inst), Takeda (Inst), Roche/Genentech (Inst), Daiichi Sankyo/Astra Zeneca (Inst), MSD Oncology (Inst), BeiGene (Inst), Gilead Sciences (Inst), Sanofi/Regeneron (Inst)
Consulting or Advisory Role: Boehringer Ingelheim, Novartis, AstraZeneca, Clovis Oncology, Lilly (Inst), MSD Oncology, Celgene, Bayer, Pfizer, Ono Pharmaceutical, Bristol Myers Squibb, Boehringer Ingelheim (Inst), Yuhan, Hansoh, Blueprint Medicines, Daiichi Sankyo, G1 Therapeutics, AbbVie, Takeda, Amgen, Incyte, GlaxoSmithKline (Inst), Amgen (Inst), Takeda (Inst), AstraZeneca (Inst), Novartis (Inst), MSD Oncology (Inst), Janssen Oncology (Inst), Merck KGaA (Inst), Daiichi Sankyo/Astra Zeneca (Inst), Puma Biotechnology (Inst), Gilead Sciences (Inst), Pfizer (Inst), Taiho Pharmaceutical (Inst), Bayer (Inst), Roche/Genentech (Inst), Sanofi (Inst)
Research Funding: AstraZeneca (Inst)
Travel, Accommodations, Expenses: Pfizer
Maria Jove
Speakers' Bureau: AstraZeneca
Travel, Accommodations, Expenses: Roche
Yi-Long Wu
Honoraria: AstraZeneca, Roche, Pfizer, Boehringer Ingelheim, MSD Oncology, Bristol Myers Squibb/China, Hengrui Pharmaceutical, BeiGene Beijing
Consulting or Advisory Role: AstraZeneca, Roche, Boehringer Ingelheim, Takeda
Research Funding: Boehringer Ingelheim (Inst), Roche (Inst), Pfizer (Inst), BMS (Inst)
Charles M. Rudin
Consulting or Advisory Role: Harpoon Therapeutics, Genentech/Roche, AstraZeneca, Bridge Medicines, Amgen, Jazz Pharmaceuticals, Earli, AbbVie, Daiichi Sankyo/UCB Japan, Kowa, Merck, D2G Oncology, Auron Therapeutics, DISCO
Research Funding: Merck (Inst), Roche/Genentech (Inst), Daiichi Sankyo (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/111056
Huanyu Chen
Employment: Ipsen, Modern Biosciences
Stock and Other Ownership Interests: Ipsen, Moderna Therapeutics
Li Zhang
Employment: Ipsen
Stock and Other Ownership Interests: Ipsen
Stanley Yeung
Employment: Ipsen, GlaxoSmithKline
Stock and Other Ownership Interests: GlaxoSmithKline, Ipsen
Fawzi Benzaghou
Employment: Ipsen
Stock and Other Ownership Interests: Ipsen
Luis Paz-Ares
Leadership: Altum Sequencing, Stab Therapeutics
Stock and Other Ownership Interests: Altum Sequencing, Stab therapeutics
Honoraria: Roche/Genentech, Lilly, Pfizer, Bristol Myers Squibb, MSD, AstraZeneca, Merck Serono, PharmaMar, Novartis, Amgen, Sanofi, Bayer, Takeda, Mirati, Daiichi Sankyo, BeiGene, GSK, Janssen, Medscape, Regeneron, Boehringer Ingelheim
Consulting or Advisory Role: Lilly, MSD, Roche, PharmaMar, Merck, AstraZeneca, Novartis, Amgen, Pfizer, Sanofi, Bayer, BMS, Mirati, GSK, Janssen, Takeda, Regeneron, AbbVie
Speakers' Bureau: MSD Oncology, BMS, Roche/Genentech, Pfizer, Lilly, AstraZeneca, Merck Serono
Research Funding: BMS (Inst), Astra Zeneca (Inst), PharmaMar (Inst), MSD (Inst), Pfizer (Inst)
Other Relationship: Novartis, Ipsen, Pfizer, Servier, Sanofi, Roche, Amgen, Merck, Roche
Paul A. Bunn
Leadership: Verastem
Honoraria: CStone Pharmaceuticals, Ascentage Pharma, VieCure, Genentech/Roche
Consulting or Advisory Role: CStone Pharmaceuticals, Ascentage Pharma, Genentech/Roche, Ipsen
No other potential conflicts of interest were reported.
REFERENCES
- 1.Yuan M, Zhao Y, Arkenau HT, et al. : Signal pathways and precision therapy of small-cell lung cancer. Signal Transduct Target Ther 7:187, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gustafsson BI, Kidd M, Chan A, et al. : Bronchopulmonary neuroendocrine tumors. Cancer 113:5-21, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Wang S, Zimmermann S, Parikh K, et al. : Current diagnosis and management of small-cell lung cancer. Mayo Clin Proc 94:1599-1622, 2019 [DOI] [PubMed] [Google Scholar]
- 4.Xu L, Zhang G, Song S, et al. : Surgery for small cell lung cancer: A Surveillance, Epidemiology, and End Results (SEER) survey from 2010 to 2015. Medicine 98:e17214, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.SEER*Explorer : An interactive website for SEER cancer statistics (2022). Surveillance Research Program, National Cancer Institute. https://seer.cancer.gov/statistics-network/explorer/
- 6.Hiddinga BI, Raskin J, Janssens A, et al. : Recent developments in the treatment of small cell lung cancer. Eur Respir Rev 30:210079, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asai N, Ohkuni Y, Kaneko N, et al. : Relapsed small cell lung cancer: Treatment options and latest developments. Ther Adv Med Oncol 6:69-82, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Brien ME, Ciuleanu TE, Tsekov H, et al. : Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol 24:5441-5447, 2006 [DOI] [PubMed] [Google Scholar]
- 9.HYCAMTIN (topotecan) for injection, for intravenous use [package insert]. U.S. Food and Drug Administration website. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/020671s023lbl.pdf
- 10.HYCAMTIN 1 mg and 4 mg powder for concentrate for solution for infusion [summary of product characteristics]. https://www.ema.europa.eu/en/documents/product-information/hycamtin-epar-product-information_en.pdf
- 11.U.S. Food & Drug Administration : Press release: FDA grants accelerated approval to lurbinectedin for metastatic small cell lung cancer. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-grants-accelerated-approval-lurbinectedin-metastatic-small-cell-lung-cancer
- 12.European Medicines Agency, European Medicines Agency decision, P/0446/2020, 1 December 2020 [product specific waiver for lurbinectedin (EMEA-002846-PIP01-20)]. https://www.ema.europa.eu/en/documents/pip-decision/p/0446/2020-ema-decision-1-december-2020-granting-product-specific-waiver-lurbinectedin-emea-002846-pip01_en.pdf
- 13.Jazz Pharmaceuticals Inc : ZEPZELCA (lurbinectedin) [package insert]. U.S. Food and Drug Administration website. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213702s000lbl.pdf
- 14.Ardizzoni A, Hansen H, Dombernowsky P, et al. : Topotecan, a new active drug in the second-line treatment of small-cell lung cancer: A phase II study in patients with refractory and sensitive disease. The European Organization for Research and Treatment of Cancer Early Clinical Studies Group and New Drug Development Office, and the Lung Cancer Cooperative Group. J Clin Oncol 15:2090-2096, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Horita N, Yamamoto M, Sato T, et al. : Topotecan for relapsed small-cell lung cancer: Systematic review and meta-analysis of 1347 patients. Sci Rep 5:15437, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trigo J, Subbiah V, Besse B, et al. : Lurbinectedin as second-line treatment for patients with small-cell lung cancer: A single-arm, open-label, phase 2 basket trial. Lancet Oncol 21:645-654, 2020 [DOI] [PubMed] [Google Scholar]
- 17.Aix SP, Ciuleanu TE, Navarro A, et al. : Combination lurbinectedin and doxorubicin versus physician's choice of chemotherapy in patients with relapsed small-cell lung cancer (ATLANTIS): A multicentre, randomised, open-label, phase 3 trial. Lancet Respir Med 11:74-86, 2023 [DOI] [PubMed] [Google Scholar]
- 18.Paz-Ares L, Chen Y, Reinmuth N, et al. : Durvalumab, with or without tremelimumab, plus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer: 3-year overall survival update from CASPIAN. ESMO Open 7:100408, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu SV, Reck M, Mansfield AS, et al. : Updated overall survival and PD-L1 subgroup analysis of patients with extensive-stage small-cell lung cancer treated with atezolizumab, carboplatin, and etoposide (IMpower133). J Clin Oncol 39:619-630, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Man FM, Goey AKL, van Schaik RHN, et al. : Individualization of irinotecan treatment: A review of pharmacokinetics, pharmacodynamics, and pharmacogenetics. Clin Pharmacokinet 57:1229-1254, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalra AV, Kim J, Klinz SG, et al. : Preclinical activity of nanoliposomal irinotecan is governed by tumor deposition and intratumor prodrug conversion. Cancer Res 74:7003-7013, 2014 [DOI] [PubMed] [Google Scholar]
- 22.Drummond DC, Noble CO, Guo Z, et al. : Development of a highly active nanoliposomal irinotecan using a novel intraliposomal stabilization strategy. Cancer Res 66:3271-3277, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Ipsen Biopharmaceuticals Inc : Prescribing Information: ONIVYDE™ (irinotecan liposome injection), for intravenous use initial U.S. https://www.ipsen.com/websites/Ipsen_Online/wp-content/uploads/sites/9/2019/01/21083350/ONIVYDE_USPI.pdf
- 24.Paz-Ares L, Spigel DR, Chen Y, et al. : RESILIENT part 1: A phase 2 dose-exploration and dose-expansion study of second-line liposomal irinotecan in adults with small cell lung cancer. Cancer 128:1801-1811, 2022 [DOI] [PubMed] [Google Scholar]
- 25.Eisenhauer EA, Therasse P, Bogaerts J, et al. : New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228-247, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Lin NU, Lee EQ, Aoyama H, et al. : Response assessment criteria for brain metastases: Proposal from the RANO group. Lancet Oncol 16:e270-e278, 2015 [DOI] [PubMed] [Google Scholar]
- 27.National Cancer Institute Division of Cancer Treatment & Diagnosis : Common Terminology Criteria for Adverse Events (CTCAE) v5.0. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_50
- 28.Zhou H, Hu Y, Luo R, et al. : Multi-region exome sequencing reveals the intratumoral heterogeneity of surgically resected small cell lung cancer. Nat Commun 12:5431, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Das M, Padda SK, Weiss J, et al. : Advances in treatment of recurrent small cell lung cancer (SCLC): Insights for optimizing patient outcomes from an expert roundtable discussion. Adv Ther 38:5431-5451, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rudin CM, Brambilla E, Faivre-Finn C, et al. : Small-cell lung cancer. Nat Rev Dis Primers 7:3, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Qualified researchers may request access to patient-level study data that underlie the results reported in this publication. Additional relevant study documents, including the clinical study report, study protocol with any amendments, annotated case report form, statistical analysis plan, and data set specifications may also be made available. Patient-level data will be anonymized, and study documents will be redacted to protect the privacy of study participants. Where applicable, data from eligible studies are available 6 months after the studied medicine and indication have been approved in the United States and European Union or after the primary manuscript describing the results has been accepted for publication, whichever is later. Further details on Ipsen's sharing criteria, eligible studies, and process for sharing are available here (https://vivli.org/members/ourmembers/). Any requests should be submitted to www.vivli.org for assessment by an independent scientific review board.