Abstract
Purpose
Breast cancer follow-up (surveillance and aftercare) varies from one-size-fits-all to more personalised approaches. A systematic review was performed to get insight in existing evidence on (cost-)effectiveness of personalised follow-up.
Methods
PubMed, Scopus and Cochrane were searched between 01–01-2010 and 10–10-2022 (review registered in PROSPERO:CRD42022375770). The inclusion population comprised nonmetastatic breast cancer patients ≥ 18 years, after completing curative treatment. All intervention-control studies studying personalised surveillance and/or aftercare designed for use during the entire follow-up period were included. All review processes including risk of bias assessment were performed by two reviewers. Characteristics of included studies were described.
Results
Overall, 3708 publications were identified, 64 full-text publications were read and 16 were included for data extraction. One study evaluated personalised surveillance. Various personalised aftercare interventions and outcomes were studied. Most common elements included in personalised aftercare plans were treatment summaries (75%), follow-up guidelines (56%), lists of available supportive care resources (38%) and PROs (25%). Control conditions mostly comprised usual care. Four out of seven (57%) studies reported improvements in quality of life following personalisation. Six studies (38%) found no personalisation effect, for multiple outcomes assessed (e.g. distress, satisfaction). One (6.3%) study was judged as low, four (25%) as high risk of bias and 11 (68.8%) as with concerns.
Conclusion
The included studies varied in interventions, measurement instruments and outcomes, making it impossible to draw conclusions on the effectiveness of personalised follow-up. There is a need for a definition of both personalised surveillance and aftercare, whereafter outcomes can be measured according to uniform standards.
Keywords: Personalised follow-up, Breast cancer, Surveillance, Aftercare
Introduction
While breast cancer incidence has grown over time—up to 2.3 million diagnoses in 2020 worldwide [1]—mortality rates have declined [2, 3]. Consequently, this results in a large number of breast cancer survivors in follow-up care, consisting of two parts: surveillance and aftercare. Surveillance aims to detect asymptomatic locoregional recurrences (LRR) or second primary breast cancers (SPBC) using mammograms and physical examination. The ultimate aim is to curatively treat patients. The ultimate aim is to curatively treat patients. As distant recurrences are in most cases not curable, and the early detection of distant recurrences does not improve prognosis, surveillance does not actively aim to detect these. Aftercare aims to detect diagnosis- or treatment-related side effects and subsequently use interventions to reduce these and improve quality of life (QoL).
Although in some countries guidelines advise to personalise follow-up [4, 5], current guidelines in, for example, the Netherlands and Belgium still advise similar surveillance schedules for all patients [6, 7]. This is probably due to a lack of clinical evidence that adapting surveillance schedules according to risk profiles is effective. However, it is widely known that differences in individual characteristics largely influence LRR and SPBC risks [8]. Furthermore, about 50% of LRRs and 25% of SPBCs are detected by patients themselves due to symptoms, outside of scheduled surveillance visits [9]. Moreover, patients’ beliefs and expectations of surveillance are often not realistic, including the incorrect assumption that surveillance also aims to detect distant metastases and that breast cancer cannot recur in between scheduled visits [10]. Importantly, overall LRR and SPBC risks are low and largely differ among individual patients [11, 12]. This is expected to lead to unnecessary surveillance visits for many patients and perhaps too little visits for specific high-risk patients.
While surveillance often consists of a one-size-fits-all approach, a large variation in aftercare is present, as this is often arranged according to both clinicians’ and patients’ preferences. It depends on the hospital which health care provider is involved (e.g. surgeon, specialised nurse [13]) and whether they make use of prescheduled consultations. Health care providers are also looking for appropriate tools they can use to personalise aftercare [13]. In addition, there are no guidelines on the specific contents of aftercare plans, resulting in unmet supportive care needs regarding fear of cancer recurrence, daily activity and sexual and psychological well-being [14].
Both these arguments and the fact that an increasing number of breast cancer survivors will receive follow-up care, lead to an increasing belief that surveillance and aftercare should be personalised [15–17]. However, clear evidence is needed about the effectiveness of both personalised surveillance and aftercare. The aim of this review was to identify all studies published from 2010 that investigated the effectiveness of personalised surveillance and/or aftercare in curatively treated nonmetastatic breast cancer patients.
Methods
This review’s protocol has been registered and made available in PROSPERO [18] (CRD42022375770). The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 checklist [19] was used for transparent reporting (Online Resource 1–2).
Search strategy
Databases of PubMed, Scopus (including Medline and keywords of Embase) and Cochrane were searched for relevant publications between January 1st, 2010, and November 10th, 2022. Reference lists of relevant reviews were consulted. Studies published before 2010 were excluded, as we expected these to be less relevant for current clinical practice. The full search strategy is shown in Online Resource 3.
Eligibility criteria
The study population concerned nonmetastatic breast cancer patients ≥ 18 years, starting follow-up after completion of curative treatment (surgery and, if applicable, radiotherapy and/or chemotherapy). Patients could still be treated with endocrine therapy or targeted therapy. We included all intervention-control studies studying personalised surveillance and/or aftercare, including any intervention tailored to a patient’s individual characteristics and designed to be used for the entire follow-up period. Studies that investigated the effectiveness of short-term dietary, physical interventions, cognitive behavioural therapy or psychoeducational interventions, which were not part of a larger intervention designed for use during the entire follow-up period, were therefore excluded. We included studies on all outcomes, except for diagnostic accuracy, feasibility or patient experiences of the intervention only, without evaluating effectiveness.
Inclusion and data extraction
Two reviewers (JvH, MvM) independently screened and judged all identified studies on title and abstract. In case of doubt or disagreement, the study was included for full-text analysis. Both reviewers independently read the full text and decided on definite inclusion. Discrepancies were extensively discussed and resolved. Co-authors were consulted if necessary. Final data extraction was performed by one reviewer (MvM), and the second reviewer (JvH) was consulted in case of doubts. In case of missing or unclear information on interventions, the study’s first author was consulted. Data on population, intervention, control and outcomes were extracted. As multiple types of interventions and outcomes were studied, the data is presented descriptively.
Risk of bias
To assess risk of bias (ROB) of included studies, the Cochrane risk of bias tool for randomised (RoB2) [20] or nonrandomised studies (ROBINS-I) [21] were used, whenever applicable. ROB assessment was independently performed by two reviewers, and discrepancies were discussed.
Results
The search strategy yielded 3708 publications. Sixty-four publications were deemed eligible for full-text analysis. After full-text reading, 16 were included for data extraction. Reasons for the exclusion of the other 48 studies were (1) no intervention designed for the entire follow-up, (2) no control group, (3) no effectiveness measured (feasibility studies) and (4) intervention not personalised. Examples of excluded studies are Wallner et al. [22], Haq et al. [23] and Admiraal et al. [24], because they studied feasibility only, did not use a control group, and studied a short-term psychoeducational intervention (so did not personalise the entire follow-up period), respectively. The entire selection is visualised in Fig. 1. The 16 studies finally included are summarised in Table 1. Fifteen studies presented results of randomised controlled trials (RCTs) and one study of a pretest–posttest design. Three studies concerned the same RCT [25–27] but with different outcome assessments (longer follow-up or cost-effectiveness analyses), so were considered different studies in further analysis.
Fig. 1.
Flow chart of inclusion of publications. *Including Medline and keywords of Embase. **Including studies focusing on short-term dietary, cognitive behavioural, physical or psychoeducational interventions and interventions applied during active treatment
Table 1.
Detailed overview of included publications with the most important study characteristics
| Authors and reference | Type of personalised intervention and design | N | Patients | Intervention | Control | Outcome(s) | Conclusion(s) | Risk of bias |
|---|---|---|---|---|---|---|---|---|
| Grunfeld et al. [26] | Aftercare, randomised controlled trial | 408 | Women with early-stage breast cancer who completed primary treatment at least 3 months previously, except for endocrine therapy | An SCP including a personalised treatment summary, a patient version of the Canadian national follow-up guideline, a summary table of the guideline that served as a reminder system and a personalised health care resource list. The documents were reviewed during a 30-min educational session with a nurse who made explicitly clear that surveillance and aftercare were now the responsibility of the primary care physician | All routine surveillance and aftercare were transferred to the patient’s own primary care physician |
Primary: change in cancer-specific distress total score at 12 months Secondary: change in total score and subscales of general psychological distress, health-related QoL, patient satisfaction, continuity/coordination of care, the frequency with which patients declined transfer to the primary care physician, the frequency and types of post-discharge visits to the oncologist, awareness of which physician was primarily responsible for follow-up care |
No benefit of the SCP on any of the outcomes was found More patients correctly identified the primary care physician as primarily responsible for follow-up care (both surveillance and aftercare) |
With concerns |
| Hershman et al. [28] | Aftercare, randomised controlled trial | 141 | Women who had a history of stage 0-III breast cancer and were within 6 weeks of completion of initial adjuvant treatment (radiation or chemotherapy) | A National Cancer Institute publication, Facing Forward: life after cancer treatment (a guide for people treated for cancer) plus a personal one-hour meeting with a nurse practitioner and nutritionist to receive a personalised treatment summary, general surveillance recommendations, discussion of risks of side effects and screening and lifestyle recommendations | Only the National Cancer Institute publication, Facing Forward: life after cancer treatment (a guide for people treated for cancer) | Treatment satisfaction, unique and multidimensional aspects of long-term cancer survivorship (impact of cancer), survivor concerns (cancer worry and health worry) after 3 and 6 months | No difference in cancer worry, treatment satisfaction, survivor concerns, depression or impact of cancer. A significant improvement was found in health worry scores at 3 months, but not at 6 months | Low risk |
| Coyle et al. [25] | Aftercare, a follow-up on Grunfeld et al. [26] | 408 | See 1 | See 1 | See 1 | Costs and utilities (a standard measure of QoL on a 0 to 1 scale) after 24 months | The SCPs were not cost-effective | With concerns |
| Rocque et al. [29] | Aftercare, pilot randomised controlled trial | 38 | Patients diagnosed with stage 0-III breast cancer who completed active treatment and had an email account | Patients first filled in an existing survey on satisfaction with knowledge. Then patients received immediate access of an SCP including all of the recommended Institute of Medicine (IOM) elements (IOM recommends a record of all care received and important disease characteristics, information about the likely course of recovery from acute treatment toxicities as well as the need for ongoing health maintenance or any recommended chemopreventive therapies, including available resources on psychosocial and other practical issues. IOM states that survivorship care needs to be patient-centred and tailored to the patient’s clinical situation and preferences) | Usual care (not further specified). Patients first filled in an existing survey on satisfaction with knowledge. Patients received delayed access to the SCP, after having filled in the survivor knowledge survey (WISDOM-B, study outcome, so after final data collection) | Change in survivor knowledge after 4 weeks | A small difference in survivor knowledge was found, in favour of the intervention group, but it was not significant (the study was not powered to detect a difference of less than 10%) | With concerns |
| Wheelock et al. [30] | Aftercare, randomised controlled trial | 102 | Non-metastatic breast cancer who completed active treatment and had access to a computer | SIS-NET: Patients received three breast cancer-related clinic visits with care providers of their choice with additional appointments scheduled later (after the study period) as needed. The intervention included the integration of online health questionnaires at 3-month intervals evaluating symptoms monitored and followed by telephone as necessary by a designated nurse practitioner | Usual care (not further specified). Patients were scheduled for clinic visits based on patient and clinician preference and were invited to complete a routine online health questionnaire before each appointment |
Primary: number of days between symptom reporting and remote evaluation and potential management of symptoms after 18 months Secondary: use of health care resources, including the number of breast cancer-related clinic visits, number of total medical appointments and number of laboratory tests and imaging studies |
Integration of online health questionnaires with remote review by a nurse practitioner facilitated symptom reporting and may provide a means of convenient symptom assessment, but did not reduce health care resource use | High risk |
| Boekhout et al. [27] | Aftercare, randomised controlled trial (follow-up on Grunfeld et al. [26]) | 408 | See 1 | See 1 | See 1 |
See 1, but with two extensions: - follow-up to 24 months instead of 12 months - additional outcome: adherence to guidelines on follow-up care |
No benefit on any of the outcomes was found, including adherence to follow-up guidelines | With concerns |
| Ruddy et al. [31] | Surveillance and aftercare, randomised phase II trial | 100 | Non-metastatic female breast cancer patients ≥ 18 years, after treatment | SCP including details on tumour, therapies, providers, screening recommendations and recommended visit frequencies. Patient navigators called/met with patients every 3 months and tried to dissuade patients from scheduling routine surveillance visits within 3 months of each other and made suggestions for visits that could be postponed based on their SCP | Usual care (no SCP or contact with a patient navigator) which was at the discretion of each care provider and not dictated by the study |
Primary: the proportion of patients who had at least two breast or chest wall examinations within 30 days of each other without a new related complaint (reflecting potentially unnecessary care) after 12 months Secondary: frequency of visits in general, adherence to standard screening recommendations, QoL, satisfaction with care |
SCPs did not lead to statistically significant improvements in patient care. This shows that it is difficult to implement SCPs and patient navigation in a way that meaningfully improves care | With concerns |
| Kvale et al. [32] | Aftercare, randomised controlled trial | 79 | Female breast cancer patients diagnosed with stage 0-IIIb breast cancer who were within 1 year of completing active treatment | A POSTCARE intervention: A single coaching encounter utilising motivational interviewing techniques to engage patients in the development of a patient-owned SCP that incorporates a treatment summary, health goals and strategies related to cancer follow-up, surveillance, symptom management and health behaviour | Usual care (not further specified) | QoL, depressive symptoms, self-management, self-efficacy, care coordination at 3 months | The intervention group reported better scores for self-reported health, physical and emotional function roles and demonstrated trends towards improvement in other domains. The intervention group also demonstrated clinically significant improvement in depressive symptoms | With concerns |
| Maly et al. [33] | Aftercare, randomised controlled trial | 219 | Female breast cancer patients ≥ 21 years, diagnosed with stage 0-III breast cancer 10 to 24 months earlier, had their last definitive treatment (surgery, chemotherapy, radiation) at least 1 month earlier and were English or Spanish speaking | Receipt of an individually tailored treatment summary and SCP (including specific side-effects of these treatments) and one in-person counselling session with a trained, bilingual, bicultural nurse to review the contents | Usual care (not further specified) |
Primary: physician implementation of treatment summaries and SCP Secondary: patient adherence to recommended survivorship up to 12 months and health-related QoL (mental and physical health) at 6 and 12 months |
Treatment summaries and SCPs have a significantly positive impact on physician implementation score, but no difference was found for patient adherence and health-related QoL after 12 months | With concerns |
| Tevaarwerk et al. [34]* | Aftercare, randomised controlled trial | 127 | Non-metastatic breast cancer patients within 2 years of completing primary treatment | Immediate receipt of an individualised SCP, including relevant breast screening based on patient-specific risk (actual follow-up visits were not tailored), information on bone health and cardiovascular health tailored to patients’ risk, information about treatment in case a patient received it, and specific symptoms and concerns, reported on questionnaires, were covered at regular visits by nurse practitioners or physician assistants | Usual care (not further specified) and delayed receipt of a SCP, after final data collection |
Primary: change in survivor knowledge after 12 weeks Secondary: satisfaction with communication and knowledge |
A modest improvement in survivor knowledge after 12 weeks was seen in both groups. In the intervention group, this seemed related to repeated administration of the survey rather than receipt of the SCP. No effect on satisfaction | High risk |
| Ramirez et al. [35] | Aftercare, randomised controlled trial | 120 | Non-metastatic female Latina primary breast cancer patients ≥ 18 years, deficit in either cancer screening (pap smear/colonoscopy) or a positive comorbidity screening (BMI ≥ 25, diabetes or high glucose level, high blood pressure or current smoker), after treatment | Active support by patient navigators trained in motivational interviewing, providing patients with regular personalised assistance (including phone calls, home visits, transportation assistance, coordination of care), cancer screenings appointments, educational classes, referral to community resources, educational opportunities and help with insurance applications | Patients only received a fact sheet of study services with contact information of patient navigators | QoL after 6 months | Patient navigation led to improved QoL as compared to usual care | With concerns |
| Riis et al. [36] | Surveillance and aftercare, pilot randomised controlled trial | 134 | Female postmenopausal nonmetastatic hormonal receptor-positive breast cancer patients ≥ 50 years, after surgery and scheduled for at least 5 years of endocrine therapy | No mandatory consultations. PROs were collected 3 months and used actively as (1) screening tool to assess problems and requirements and (2) as dialogue tool to map symptoms and concerns in order to focus the discussion on what mattered most to the individual patient | Standard follow-up care, including pre-scheduled consultations at 6 monthly intervals for 5 years |
Primary: Satisfaction with care, unmet needs after 24 months Secondary: use of consultations, adherence to treatment, self-reported symptoms, functioning, QoL |
Individualised follow-up led to equal satisfaction, unmet needs, adherence to treatment and QoL as compared to usual care. However, the number of consultations in the intervention group was lower | With concerns |
| van der Hout et al. [37] | Aftercare, randomised controlled trial | 625 | Multiple cancer types (including female breast cancer), both metastatic and non-metastatic patients > 18 years and 3–5 months after curative treatment | Direct access to the web-based eHealth application Oncokompas that supports cancer survivors in self-management by monitoring cancer-generic and tumour-specific symptoms and QoL, providing feedback and information on the scores and a personalised overview of supportive care options | Waitlist – access to Oncokompas after 6 months (after final data collection) |
Primary: patient activation after intervention, 3 and 6 months Secondary: QoL, mental adjustment to cancer, supportive care needs, self-efficacy, personal control, perceived efficacy in patient-physician interaction |
No significant effect on all outcomes except for a positive effect on QoL and tumour-specific symptom burden | With concerns |
| Fang et al. [6] | Aftercare, randomised controlled trial | 202 | Female breast cancer patients ≥ 20 years, diagnosed in the last 5 years. Primary treatment had to be completed with no sign of recurrence, and patients had to speak Mandarin or Taiwanese | Access to a web-based personalised SCP computerised application: Healthy living with breast cancer. Tailored information (e.g. treatments and specific side effects) was presented in seven modules using texts or videos based on literature conducted in Taiwan identifying the needs of women with breast cancer. In addition, a push notification reminded women to take their prescribed medicine, participate in a module, seek remedies for side effects, and the date of next outpatient follow-up | Standard follow-up care. Patients were provided with an educational leaflet with information about timing of follow-up visits, recommended examinations, and special considerations related to years since diagnosis and cancer pathology. They attended routine clinic follow-ups |
Primary: unmet needs after 12 months Secondary: fear of recurrence, symptoms, depression, anxiety, QoL |
The intervention was effective in decreasing women’s unmet needs after 6 months and decreasing their fear of recurrence after 12 months. QoL was improved. However there was no evidence of an intervention effect on symptom distress, anxiety and depression. NB: women in the intervention group more often received chemotherapy, which can have contributed to the differences | High risk |
| Rutkowski et al. [38]** | Aftercare, pretest–posttest design with sequential inclusion of intervention and control group | 87 | Breast cancer survivors discharged from the Wellness Beyond Cancer Programme who received either a standardised or personalised SCP | The personalised aftercare care plan included a treatment summary (i.e. diagnosis, medications received, surgeries, etc.) and follow-up surveillance guidelines specific to breast cancer survivors (e.g. mammogram, breast self-check) with the next follow-up test due date indicated | Similar follow-up surveillance guidelines. The difference with the intervention was the absence of treatment summaries and recommended next follow-up test due dates | Perceived knowledge, perceived efficacy in patient-physician interactions, patient activation pre- and post-reception of the personalised plan | Standardised SCPs offer comparable outcomes on self-efficacy in patient-physician interactions and patient activation to those of personalised plans. Survivors with a personalised care plan reported greater perceived knowledge, however the standardised plans resulted in a significant increase in perceived knowledge from pre to post | High risk |
| O’Hea et al. [39] | Aftercare, randomised controlled trial | 200 | Female non-metastatic breast cancer patients ≥ 18 years with an active treatment plan who were alert and oriented and could read and comprehend English at a sixth grade level, and were available for follow-up assessments | Polaris Oncology Survivorship Transition (POST) SCP consisting of 10 components: (1) demographic information, (2) diagnosis and status, (3) medical care team, (4) surgery and therapy details, (5) side effects, (6) dates of recent tests, exams and scans, (7) current medications, (8) barriers, (9) upcoming appointments and/or appointments that needed to be scheduled and (10) libraries of information and references on relevant resource | Standard follow-up care | Physical and emotional symptoms, perceptions of quality of oncology care related to SCPning after 1, 3 and 6 months | In terms of QoL, the present study does not support our theory that the POST would significantly impact QoL in women ending treatment for breast cancer | With concerns |
N, number of patients included; SCP, survivorship care plan; QoL, quality of life
*In the paper, it was unclear to what degree the intervention was personalised, and the information was obtained after having contact with the first author
**The first author of the paper was consulted to get more insight in the degree of personalisation of the intervention, which was not entirely clear from the paper. Apart from the personalised treatment summaries, the surveillance was similar for all patients, but personal needs (physical, social, emotional, spiritual and practical) were addressed. Therefore, for this review, we considered only aftercare to be personalised in this study
Populations
All studies included nonmetastatic breast cancer patients who completed primary treatment, except for adjuvant targeted or endocrine therapy. Two studies focused on very specific populations. The first included Latina breast cancer patients with a deficit in either cancer screening (PAP smear or colonoscopy) or a positive comorbidity screening [35]. The second only included hormonal receptor-positive breast cancer patients ≥ 50 years old undergoing endocrine therapy [36]. One study included multiple cancer types [37].
Interventions
Interventions could consist of the use of personalised aftercare plans or a combined surveillance and aftercare plans, either or not supplemented with (1) educational or counselling sessions, (2) active support by patient navigators and/or (3) monitoring patient-reported outcomes (PROs).
Personalised surveillance
Although nine out of 16 studies included general recommended surveillance guidelines in aftercare plans [25–29, 31, 32, 34, 39], only one study evaluated a form of personalised surveillance [31]: all patients were dissuaded from scheduling routine follow-up visits and received suggestions for visits that could be postponed based on their aftercare plan (including personal tumour and treatment history).
Personalised aftercare
Aftercare often concerned a plan containing general information supplemented with personalised information. Of all 16 studies, 12 incorporated personalised treatment summaries [6, 25–29, 31–34, 38, 39], six contained lists of available supportive care resources [25–27, 29, 31, 39], four incorporated PRO measures (PROMS) [30, 34, 36, 37], two contained reminders on next follow-up dates [6, 38], one contained a general guide for people treated for cancer [28] and one contained, additional to a complete overview of individual patient-, tumour- and treatment-related details, information on the medical care team, potential side effects, dates of recent visits, current medications, barriers, upcoming appointments and libraries with further information [39].
Five studies additionally included a 30-min to 1-h educational session with a nurse and/or nutritionist [25–28, 33]. This sometimes consisted of an explanation that follow-up care was now the responsibility of the primary care physician [25–27], and mostly included additional information on the contents of a general aftercare plan. One study included motivational interviewing to engage patients in the development of a patient-owned aftercare plan [32].
One study included active support by patient navigators providing patients personalised assistance including phone calls, home visits, transportation assistance and care coordination, and help with practical things [35]. In three studies, patients regularly met/called with a patient navigator, nurse practitioner or a physician assistant at predefined intervals [31, 34, 35].
Two studies integrated PROMs evaluating symptoms, followed by telephone consults [30] or visits with a nurse or physician assistant [34]. One study had no mandatory consultations, but regularly collected PROs that were used as screening and dialogue tool [36]. Two studies included web-based applications, one supporting cancer survivors in self-management by monitoring symptoms and QoL, providing feedback and personalised supportive care options [37], and one included tailored information on treatment and side effects and included push notifications to remind women to take medicine (e.g. endocrine therapy), participate in a module or seek remedies for side effects [6].
Controls
Most studies included routine follow-up care as control [6, 25–33, 35, 36, 39], which could differ per hospital and between studies. In one study, this routine follow-up care consisted of outpatient clinic visits which were based on patient and clinician experiences, which could be seen as personalised surveillance. However, this study specifically focused on the use of online health questionnaires that were monitored by a nurse practitioner (intervention) vs. no monitoring (control) and did not describe the potential effectiveness of personalised surveillance [30].
Two studies included elements of the intervention in the control group, like a guide for cancer survivorship [28] or a fact sheet with contact information of patient navigators [35]. One study used similar intervention and control conditions except for personalised treatment summaries and recommended the next surveillance due dates, which were only provided in the intervention group [38].
Outcomes
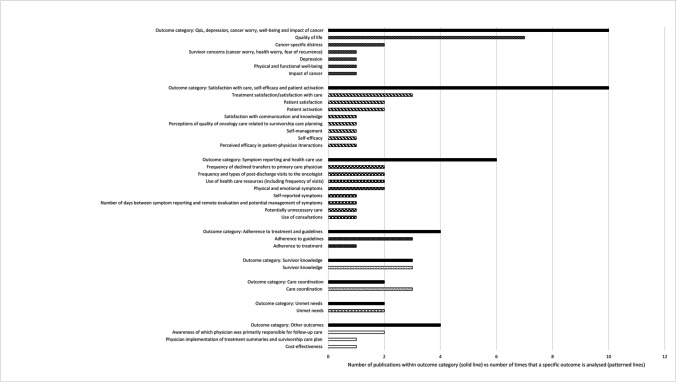
A detailed overview of all studied outcomes can be found in Table 1. Below, a summary of the most studied outcome categories is given, while Fig. 2 shows all of the specific evaluated outcomes.
Fig. 2.
Overview of all outcome(s) (categories) in the 16 included studies. The solid lines indicate the number of studies that analysed a specific outcome category, and the patterned lines indicate the number of times the specific outcome is analysed. The latter numbers do not add up to the numbers in the solid lines, because in one study, multiple outcomes could have been analysed
QoL/depression/cancer worry/well-being/impact of cancer
Ten studies evaluated one of these outcomes. Seven studies evaluated QoL [26, 27, 31–33, 35, 39], and four found a significant positive effect of personalised aftercare on QoL [6, 32, 35, 37]. One of these evaluated active support by patient navigators (differences on subscales ranging from 1.6 to 8.1, all significant except for well-being after 6 months, based on FACT-B/FACT-G questionnaires) [35], one evaluated a web-based eHealth application supporting self-management (summary score difference of 2.3 after 6 months using EORTC QLQ-C30) [37], one evaluated provision of tailored information using a web-based application (summary score difference 6.9 after 12 months, based using WHOQOL-BREF) [6] and one evaluated a coaching encounter to engage patients in the development of a patient-owned aftercare plan (proportion of clinically meaningful improvement in physical role 55 vs.18%, bodily pain 47 vs. 24% and emotional role 42 vs.21% for intervention and control group, respectively, based on SF-36). The latter also found a small significant improvement in depressive symptoms (mean difference of − 1.6 in the intervention group between baseline and 3 months, based on PHQ-9) [32]. Three studies evaluated distress/worries [6, 28, 36], and one found a significant decrease in fear of recurrence (mean difference of − 1.6 after 12 months, based on cancer worry scale) after access to a web-based aftercare plan (high ROB, see the ‘Risk of bias’ section) [6], and one found a significant improvement in health worry after three (mean scores of 2.7 vs. 2.3, respectively, based on ASC), but not after 6 months, for patients who received a personal educational meeting, compared to the control group. They did not find any difference between intervention and control on physical and functional well-being and impact of cancer [28].
Satisfaction with care/self-efficacy/patient activation
Ten studies evaluated forms of satisfaction, self-efficacy/self-management or patient activation [26–28, 31, 32, 34, 36–39]. One found a nonsignificant trend towards improvement of self-efficacy and self-management after a coaching encounter to engage patients in a patient-owned aftercare plan [32].
Symptom reporting/health care use
Six studies evaluated symptom(s) (reporting) or outcomes related to health care use [26, 27, 30, 31, 36, 39]. One reported a significant positive effect of symptom monitoring using online questionnaires in between standard surveillance visits on symptom reporting (mean of 7.4 vs 3.2 new or changed symptoms within 18 months, respectively), but not on health care resource use [30]. This study had a high ROB (see the ‘Risk of bias’ section). Another study found a significantly lower number of consultations in the intervention group—where PROs were collected and used as screening and dialogue tools—compared to the control group (2.1 vs 4.3 within 2 years, respectively) [36]. All other studies focusing on symptoms, type and/or frequency of care use did not find any significant or clinically relevant differences between intervention and control groups.
Adherence to treatment/guidelines
Four studies evaluated treatment/guideline adherence [27, 31, 33, 36], focusing on adherence to recommended visits or adherence to use of endocrine therapy. None of the studies found significant or clinically relevant differences between intervention and control groups.
Survivor knowledge
Three studies evaluated survivor knowledge [29, 34, 38], of which two found a significant positive effect of the intervention [34, 38]. One of these evaluated the effect of an individualised aftercare plan but hypothesised that the effect was more related to repeated administration of the survey than receipt of the aftercare plan [34]. The other—which was the only pretest–posttest study included in this review—showed that patients who received a personalised survivorship care plan reported greater perceived knowledge, but that the standardised plans resulted in a significant increase in perceived knowledge from pre to post [38]. Importantly, both of these studies were considered high ROB.
Care coordination
Three studies evaluated care coordination [26, 27, 32], but none found significant differences between intervention and control groups. Two of these reported on the same RCT, but with different follow-up times [26, 27]. One study reported a trend towards a positive effect with a mean score of 47.4 vs 35.1 on ‘discussion of survivorship care with primary care physician’ for intervention and control group, respectively [32].
Unmet needs
Two studies evaluated unmet needs [6, 36], which were measured by either the Patient Experiences Questionnaire [36] (including open questions on certain procedures that were not offered or concerns that were not discussed with care providers) or the Cancer Survivor Unmet Needs-Chinese Scale [6] (including questions on communication, information, physical/psychological, medical care and communication needs). The latter found a significant decrease in unmet needs after access to a web-based personalised aftercare plan, compared to the control group (mean difference of − 3.6 after 6 months using CaSUN-C, high ROB) [6].
Other
Two studies (based on the same RCT) evaluated patients’ awareness of which physician was primarily responsible for follow-up care, as follow-up care was transferred to primary care [26, 27]. Both did not find any significant or relevant effect of a personalised aftercare plan plus an educational session. One study evaluated physician implementation of treatment summaries and a personalised aftercare plan (score based on the number of needs addressed by physicians), and found a significant positive effect (mean difference of 16 (scale of 1–100) after 12 months) [33]. Finally, one study evaluated the cost-effectiveness (based on the same RCT as two other studies that did not report any intervention effects [26, 27]) of a personalised aftercare plan plus an educational session and concluded it was not cost-effective [25].
Risk of bias
Of all 16 studies, one study (6.3%) was classified as low [28], four (25%) as high ROB [6, 30, 34, 38] and 11 (68.8%) with concerns [25–27, 29, 31–33, 35–37, 39]. The three studies based on one RCT were all rated as with concerns [25–27]. There were some discrepancies between reviewers which could primarily be explained by different interpretations of signalling questions of domains two and four of RoB2 [20]. This regarded mostly discrepancies between low ROB or having concerns. After careful discussion, the most stringent outcomes were used for the final assessment (Fig. 3, Table 1).
Fig. 3.
Risk of bias assessment. Upper panel: RoB-2, risk of bias tool for randomised controlled trials; D1, randomisation process; D2, deviations from the intended interventions; D3, missing outcome data; D4, measurement of the outcome; D5, selection of the reported results. Lower panel: ROBINS-E, risk of bias tool for nonrandomised studies; D1, confounding; D2, measurement of the exposure; D3, selection of participants; D4, post-exposure interventions; D5, missing data; D6, measurement of the outcome; D7, selection of the reported results
Discussion
In this review, 16 studies were identified that evaluated the effectiveness of a personalised surveillance and/or aftercare plan in non-metastatic breast cancer patients after curative treatment. A wide range of personalised interventions and different outcomes were studied. Only one study examined a form of personalised surveillance, which did not find any significant or relevant effect on the frequency of visits, adherence to guidelines, QoL and satisfaction with care [31]. Most studies evaluating aftercare plans included individual treatment summaries, overviews of standard follow-up guidelines and/or overviews of available supportive care resources. QoL was most frequently studied, and four out of seven studies found a significantly positive effect of a personalised aftercare intervention. However, most of these studies found small absolute effects. Importantly, only one study was considered to have low ROB, and this study did not find any effect of personalised aftercare. A wide range of other outcomes was studied, with conflicting results. Surprisingly, only one study found a significant effect of personalised aftercare on the outcome category of satisfaction with care/self-efficacy/patient activation, which seems counterintuitive. However, as all studies used different personalised interventions and studied different outcomes using different measurement instruments, it is impossible to compare all studies and to draw conclusions on the effectiveness of personalised follow-up. The fact that three studies were based on one RCT did not affect the conclusions of this review.
Many studies emphasise the need for personalised surveillance [40, 41], but in clinical practice, guidelines still recommend a one-size-fits-all approach [8]. This could be due to many care providers overestimating patients’ recurrence risks [42], or because patients are hesitant about less intensive surveillance [43] due to inadequate risk perceptions, fear of recurrence [44] or unrealistic expectations [10], and could explain that only one of the included studies in this review evaluated a form of personalised surveillance. For both patients and clinicians to get insight in personal risks, a risk prediction tool can be used. INFLUENCE estimates risks of LRR, distant metastases and SPBC [45], and is currently integrated in a decision aid that can be used to personalise surveillance schemes [44]. Recently, this model has been updated to INFLUENCE 3.0 (results not yet published) and is being tested in a large multicentre study on the effectiveness of personalised follow-up, where the model (as part of a decision aid) is used to support the decision regarding the most optimal surveillance scheme [46]. Importantly, the model is based on data from patients that already have been treated for breast cancer, and therefore, the model can explicitly not be used for treatment decision-making. As recurrence rates are generally low [11], it is expected that the frequency of follow-up visits can be reduced for many patients resulting in decreased costs and lower burden on health care [47, 48]. A previous study has already shown that patients are open to the use of risk information in decision-making [43].
The large variety in the type of intervention and outcomes in aftercare suggests that there is a high need for personalisation, but that people are searching for the right way to do so. This is supported by results of several studies, showing large variations in the organisation of aftercare, especially regarding timing, frequency and disciplines of involved care providers [13, 49, 50]. Other studies showed that there are several barriers regarding the integration of PROMs in aftercare [51, 52], which was also evaluated in several of the included studies in this review [30, 34, 36, 37]. It has also been described that promoting engagement and adherence to care plans may lower psychological distress or cancer-related barriers [53]. Studies that evaluated motivational interviewing techniques to increase patient engagement indeed showed significant improvements in QoL [32, 35].
Aftercare is complex and comprises a lot of elements. Ideally, it includes assessment and management of physical and psychosocial effects due to cancer diagnosis and treatment, health promotion and care coordination [54]. In order for patients to get engaged in the management of their own recovery, it is important to empower patients by providing clear information on possible (late) side effects of breast cancer and its treatment—including available self-help and support options—and to give them information on breast awareness (i.e. how to notice potential signs of recurrence in an early state). The relevance of patient empowerment has been acknowledged in literature [55] and has been shown to improve quality of life [56]. In addition, it is crucial for patients to get insight in individual needs. A previous study showed that these individual needs are not always assessed, as only 16.1% asked patients about it [57]. Additionally, many patients have difficulties in expressing their needs [58], and the degree of communication about preferences varies widely between patients with different cultural backgrounds [59]. To support patients to understand their own needs and preferences and to base decisions regarding their health care on it, a patient decision aid or dialogue tool could be used [58, 60], which can form the basis for individual counselling sessions. A prior pilot study showed a newly developed decision aid to have promising effects on shared decision-making, choice evaluation, choice of aftercare and hospital costs, but to substantially increase consultation time [61]. However, one could argue that providing patients with completely individualised aftercare would finally decrease health care use and thus costs. In case a patient timely takes action in case of psychological or physical complaints, or any other concerns, worsening of symptoms and thereby future, more intensive, care use could be prevented. However, this remains to be investigated, as care use might also increase as a result of increased detection of unmet needs. Finally, we can learn from experiences in other cancer types, such as the shared-care survivorship programme for testicular cancer [62] and the Dutch Childhood Oncology Group guideline for follow-up [63].
Strengths and limitations
To our knowledge, this is the first review that included all published intervention-control studies on the effectiveness of personalised follow-up for breast cancer patients. A broad search strategy was used, ensuring a high level of completeness. Title abstract screening and full-text reading were performed independently by two reviewers, which is described to increase the number of relevant studies identified [64]. ROB assessment was also performed by two independent reviewers, which is crucial since ROB judgements can differ substantially between reviewers, especially regarding interpretation on random sequence generation, blinding of participants and personnel and incomplete reporting [65]. The two reviewers extensively discussed discrepancies, and in case a consensus could not be reached, the most stringent judgement was used for final assessment. Data extraction was performed by one reviewer, which could have resulted in higher error rates [66]. However, as the second reviewer had read all publications’ full text, this reviewer could carefully judge the data extraction on completeness. There were two studies [38, 39] included in this review where both reviewers doubted whether only provision of personalised information on treatments, side effects and/or standard surveillance guidelines (without counselling/educational sessions) could really be considered personalised aftercare. To be complete, these papers were included, also to show the inconsistencies in current practice, confirming the belief that one is still searching for the right way to personalise aftercare.
Clinical implications
Fifteen out of 16 studies included in this review solely focus on personalised aftercare, and they all include different types of interventions, studied different outcomes and used different measurement instruments. Besides, in some cases, it could be questioned whether the intervention can be called ‘personalised’. This makes it impossible to draw firm conclusions on the effectiveness of the interventions. First, there is need for a definition of personalised surveillance and aftercare. Ideally, surveillance consists of a decision aid including a prediction tool [45] to jointly discuss personalised surveillance schemes. Besides, personalised aftercare should comprise (1) a patient’s needs assessment (e.g. using PROs), (2) information on potential side effects of cancer (treatment) and available care resources and (3) a personalised aftercare plan, including a diagnosis and treatment summary, decisions on organisation of aftercare (e.g. frequency, involved care providers) and signals to seek care for. A dialogue tool could support the shared decision-making process between care professionals and patients of the development of this personalised aftercare plan. Effectiveness can consequently be measured according to uniform information standards such as the ICHOM initiative [67].
Conclusions and future prospectives
Personalised follow-up varies widely and is not structurally embedded in clinical practice. Therefore, there is still a lack of evidence on its effectiveness. This review shows the current gaps in literature and forms the basis of a large multicentre prospective study on the effectiveness of personalised surveillance and aftercare in breast cancer patients. This prospective study is expected to conquer the problems addressed in this review, and will provide clear evidence on the (cost-)effectiveness of personalised follow-up.
Acknowledgements
The authors thank Julia Poorthuis, MSc, student in health sciences, and Dr. Ir. Hanneke Becht, an information specialist, for their help in the development of the search strategy. Furthermore, the authors thank the members of the NABOR project group who were not involved as a co-author: Retel V, Knottnerus B, van Leeuwen E, Guerrero-Paez C, Burgers J, Vrancken Peeters MJTFD, Honkoop A, Veltman J, Mann R, Wiegersma J, Claassen S, van der Lee ML, van Uden-Kraan CF.
NABOR project group
Sabine Siesling1,2, Joke C Korevaar3,4, Constance HC Drossaert10, Marissa C van Maaren1,2, Valesca P Retel11,12, Bart Knottnerus3, Elise van Leeuwen-Stok13, Cristina Guerrero-Paez14, Jako S Burgers,15,16 Anneke M Zeillemaker7, Marie-Jeanne TFD Vrancken Peeters17, Marjan van Hezewijk5, Ester JM Siemerink6, Aafke H Honkoop18, Jeroen Veltman19, Ritse Mann20,21, Jannet Wiegersma22, Saskia Claassen23, Marije L van der Lee24,25, Cornelia F van Uden-Kraan26
1 Department of Health Technology and Services Research, Technical Medical Centre, University of Twente, Enschede, the Netherlands
2 Department of Research and Development, Netherlands Comprehensive Cancer Organisation (IKNL), Utrecht, the Netherlands
3 Netherlands Institute for Health Services Research (NIVEL), Utrecht, the Netherlands.
4 The Hague University of Applied Sciences, The Hague, the Netherlands
5 Radiotherapiegroep, Institution for Radiation Oncology, Arnhem, the Netherlands
6 Department of Internal Medicine, ZGT, Hengelo, the Netherlands
7 Department of Surgery, Alrijne Hospital, Leiden, the Netherlands
8 Department of Surgery, Canisius Wilhelmina Hospital, Nijmegen, the Netherlands
9 Department of Surgery, Diakonessenhuis, Utrecht, the Netherlands
10 Department of Psychology, Health & Technology, University of Twente, Enschede, the Netherlands
11 Division of Psychosocial Research and Epidemiology, Netherlands Cancer Institute-Antoni Van Leeuwenhoek Hospital, Amsterdam, The Netherlands
12 Erasmus School of Health Policy and Management, Erasmus University Rotterdam, Rotterdam, The Netherlands
13 Dutch Breast Cancer Trialists’ Research Group (BOOG), Utrecht, The Netherlands.
14 Dutch Breast Cancer Society (BVN), Utrecht, the Netherlands
15 Department of Family Medicine, School CAPRI, Maastricht University, Maastricht, The Netherlands
16 Dutch College of General Practitioners, Utrecht, The Netherlands
17 Department of Surgical Oncology, Netherlands Cancer Institute, Antoni van Leeuwenhoek & Amsterdam University Medical Center, Netherlands
18 Department of Medical Oncology, Isala Clinics, Zwolle, the Netherlands
19 Department of Radiology, ZGT, Hengelo, the Netherlands
20 Department of Medical Imaging, Radboud University Medical Center, Nijmegen, the Netherlands
21 Department of Radiology, Netherlands Cancer Institute, Amsterdam, the Netherlands
22 Department of Surgical Oncology, Flevoziekenhuis, Almera, the Netherlands
23 Allerzorg, Woerden, the Netherlands
24 Department of Scientific Research, Helen Dowling Institute, Centre for Psycho-Oncology, Bilthoven, the Netherlands
25 Department of Medical and Clinical Psychology, Tilburg University, Tilburg, the Netherlands
26 Santeon, Utrecht, the Netherlands
Abbreviations
- ASC
Assessment of Survivor Concerns questionnaire
- BMI
Body mass index
- CaSUN-C
Cancer Survivor Unmet Needs-Chinese Scale
- EORTC QLQ-C30
European Organisation for the Research and Treatment of Cancer Quality of Life questionnaire-30 questions
- FACT-B
Functional Assessment of Cancer Therapy-Breast
- FACT-G
Functional Assessment of Cancer Therapy-General
- ICHOM
International Consortium for Health Outcomes Measurement
- LRR
Locoregional recurrence
- PAP
Papanicolaou test
- PHQ-9
Patient Health Questionnaire-9 questions
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PROMS
Patient-reported outcome measures
- QoL
Quality of life
- RCT
Randomised controlled trial
- ROB
Risk of bias
- RoB2
Risk of bias tool for randomised studies
- ROBINS-I
Risk of bias tool for nonrandomised studies
- SPBC
Second primary breast cancer
- SF-36
Short Form Health Survey-36 questions
- WHOQOL-BREF
World Health Organisation Quality Of Life-Brief Version
Author contribution
MvM, JvH, JK, CD and SS contributed to the study conception and design. Material preparation, data collection and analysis were performed by MvM and JvH. The first draft of the manuscript was written by MvM and all authors commented on previous versions of the manuscript. All authors have read and approved the final manuscript.
Funding
This review is part of a by ZonMw funded project (number: 10330032010001). The funding body was not involved in the design of the study and collection, analysis, interpretation of data and in writing the manuscript.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
MvM, JK, MvH, ES, AZ, AK, CD and SS are involved in a large multicentre study evaluating (cost-)effectiveness of personalised surveillance and aftercare in the Netherlands (NABOR study, ZonMw project number 10330032010001).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Marissa C. van Maaren, Email: m.c.vanmaaren@utwente.nl
on behalf of the NABOR project group:
Marissa C. van Maaren, Valesca P. Retel, Bart Knottnerus, Elise van Leeuwen-Stok, Cristina Guerrero-Paez, Jako S. Burgers, Anneke M. Zeillemaker, Marie-Jeanne T. F. D. Vrancken Peeters, Marjan van Hezewijk, Ester J. M. Siemerink, Aafke H. Honkoop, Jeroen Veltman, Ritse Mann, Jannet Wiegersma, Saskia Claassen, Marije L. van der Lee, Cornelia F. van Uden-Kraan, J. C. Korevaar, M. van Korevaar, E. Siemerink, A. M. Zeillemaker, A. Klaassen-Dekker, C. H. C. Drossaert, and S. C. Siesling
References
- 1.Arnold M, Morgan E, Rumgay H, Mafra A, Singh D, Laversanne M, et al. Current and future burden of breast cancer: global statistics for 2020 and 2040. Breast. 2022;66:15–23. doi: 10.1016/j.breast.2022.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Meer DJ, Kramer I, van Maaren MC, van Diest PJ, S CL, Maduro JH, , et al. Comprehensive trends in incidence, treatment, survival and mortality of first primary invasive breast cancer stratified by age, stage and receptor subtype in the Netherlands between 1989 and 2017. Int J Cancer. 2021;148(9):2289–303. doi: 10.1002/ijc.33417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giaquinto AN, Sung H, Miller KD, Kramer JL, Newman LA, Minihan A, et al. Breast cancer statistics, 2022. CA Cancer J Clin. 2022;72(6):524–541. doi: 10.3322/caac.21754. [DOI] [PubMed] [Google Scholar]
- 4.Loibl S, Andre F, Bachelot T, Barrios CH, Bergh J, Burstein HJ, et al. Early breast cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2024;35(2):159–182. doi: 10.1016/j.annonc.2023.11.016. [DOI] [PubMed] [Google Scholar]
- 5.West of Scotland Cancer Network. Breast Cancer Regional Follow-up Guideline v3.0. 2024. https://www.woscan.scot.nhs.uk/wp-content/uploads/Final-published_Breast-Follow-up-Regional-Guidance-V3.0_Apr21.pdf. Accessed on 15 Mar 2024
- 6.Fang SY, Wang YL, Lu WH, Lee KT, Kuo YL, Fetzer SJ. Long-term effectiveness of an E-based survivorship care plan for breast cancer survivors: a quasi-experimental study. Patient Educ Couns. 2020;103(3):549–555. doi: 10.1016/j.pec.2019.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Federaal Kenniscentrum voor de Gezondheidszorg. Borstkanker bij vrouwen: diagnose, behandeling en follow-up. 2013. https://kce.fgov.be/sites/default/files/2021-11/KCE_143As_Borstkanker_0.pdf. Accessed on 7 Mar 2024
- 8.De Rose F, Meduri B, De Santis MC, Ferro A, Marino L, Colciago RR, et al. Rethinking breast cancer follow-up based on individual risk and recurrence management. Cancer Treat Rev. 2022;109:102434. doi: 10.1016/j.ctrv.2022.102434. [DOI] [PubMed] [Google Scholar]
- 9.Geurts SM, de Vegt F, Siesling S, Flobbe K, Aben KK, van der Heiden-van der Loo M, , et al. Pattern of follow-up care and early relapse detection in breast cancer patients. Breast Cancer Res Treat. 2012;136(3):859–68. doi: 10.1007/s10549-012-2297-9. [DOI] [PubMed] [Google Scholar]
- 10.Berendsen AJ, Roorda C, Jansen L, de Bock GH. Patients’ beliefs about the aims of breast cancer follow-up: a qualitative study. Maturitas. 2016;91:140–144. doi: 10.1016/j.maturitas.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 11.van Maaren MC, de Munck L, Strobbe LJA, Sonke GS, Westenend PJ, Smidt ML, et al. Ten-year recurrence rates for breast cancer subtypes in the Netherlands: a large population-based study. Int J Cancer. 2019;144(2):263–272. doi: 10.1002/ijc.31914. [DOI] [PubMed] [Google Scholar]
- 12.Kurian AW, McClure LA, John EM, Horn-Ross PL, Ford JM, Clarke CA. Second primary breast cancer occurrence according to hormone receptor status. J Natl Cancer Inst. 2009;101(15):1058–1065. doi: 10.1093/jnci/djp181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ankersmid JW, van Hoeve JC, Strobbe LJA, van Riet YEA, van Uden-Kraan CF, Siesling S, et al. Follow-up after breast cancer: variations, best practices, and opportunities for improvement according to health care professionals. Eur J Cancer Care (Engl) 2021;30(6):e13505. doi: 10.1111/ecc.13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan R, Wang L, Bu X, Wang W, Zhu J. Unmet supportive care needs of breast cancer survivors: a systematic scoping review. BMC Cancer. 2023;23(1):587. doi: 10.1186/s12885-023-11087-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chopra I, Chopra A. Follow-up care for breast cancer survivors: improving patient outcomes. Patient Relat Outcome Meas. 2014;5:71–85. doi: 10.2147/PROM.S49586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Ligt KM, van Egdom LSE, Koppert LB, Siesling S, van Til JA. Opportunities for personalised follow-up care among patients with breast cancer: a scoping review to identify preference-sensitive decisions. Eur J Cancer Care (Engl) 2019;28(3):e13092. doi: 10.1111/ecc.13092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cardoso F, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rubio IT, et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-updagger. Ann Oncol. 2019;30(8):1194–1220. doi: 10.1093/annonc/mdz173. [DOI] [PubMed] [Google Scholar]
- 18.National Institute for Health Research. PROSPERO: International prospective register of systematic reviews. https://www.crd.york.ac.uk/prospero/. Accessed on 03 Oct 2022
- 19.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:14898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 21.Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wallner LP, Abrahamse P, Gargaro JG, Radhakrishnan A, Mullins MA, An LC, et al. Improving the delivery of team-based survivorship care after primary breast cancer treatment through a multi-level intervention: a pilot randomized controlled trial. Breast Cancer Res Treat. 2021;189(1):81–92. doi: 10.1007/s10549-021-06257-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haq R, Kong A, Gulasingam P. A multidisciplinary approach to implement personalized breast cancer treatment and care plans. Curr Oncol. 2021;28(1):767–782. doi: 10.3390/curroncol28010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Admiraal JM, van der Velden AWG, Geerling JI, Burgerhof JGM, Bouma G, Walenkamp AME, et al. Web-based tailored psychoeducation for breast cancer patients at the onset of the survivorship phase: a multicenter randomized controlled trial. J Pain Symptom Manage. 2017;54(4):466–475. doi: 10.1016/j.jpainsymman.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 25.Coyle D, Grunfeld E, Coyle K, Pond G, Julian JA, Levine MN. Cost effectiveness of a survivorship care plan for breast cancer survivors. J Oncol Pract. 2014;10(2):e86–92. doi: 10.1200/JOP.2013.001142. [DOI] [PubMed] [Google Scholar]
- 26.Grunfeld E, Julian JA, Pond G, Maunsell E, Coyle D, Folkes A, et al. Evaluating survivorship care plans: results of a randomized, clinical trial of patients with breast cancer. J Clin Oncol. 2011;29(36):4755–4762. doi: 10.1200/JCO.2011.36.8373. [DOI] [PubMed] [Google Scholar]
- 27.Boekhout AH, Maunsell E, Pond GR, Julian JA, Coyle D, Levine MN, et al. A survivorship care plan for breast cancer survivors: extended results of a randomized clinical trial. J Cancer Surviv. 2015;9(4):683–691. doi: 10.1007/s11764-015-0443-1. [DOI] [PubMed] [Google Scholar]
- 28.Hershman DL, Greenlee H, Awad D, Kalinsky K, Maurer M, Kranwinkel G, et al. Randomized controlled trial of a clinic-based survivorship intervention following adjuvant therapy in breast cancer survivors. Breast Cancer Res Treat. 2013;138(3):795–806. doi: 10.1007/s10549-013-2486-1. [DOI] [PubMed] [Google Scholar]
- 29.Rocque GB, Wisinski KB, Buhr KA, Froeschner JL, Jones N, Donohue S, et al. Development and evaluation of a survey to assess survivor knowledge change after survivorship care plans: WiSDOM-B (Wisconsin Survey of cancer DiagnOsis and Management in Breast cancer) J Cancer Educ. 2014;29(2):270–277. doi: 10.1007/s13187-013-0591-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wheelock AE, Bock MA, Martin EL, Hwang J, Ernest ML, Rugo HS, et al. SIS.NET: a randomized controlled trial evaluating a web-based system for symptom management after treatment of breast cancer. Cancer. 2015;121(6):893–9. doi: 10.1002/cncr.29088. [DOI] [PubMed] [Google Scholar]
- 31.Ruddy KJ, Guo H, Baker EL, Goldstein MJ, Mullaney EE, Shulman LN, et al. Randomized phase 2 trial of a coordinated breast cancer follow-up care program. Cancer. 2016;122(22):3546–3554. doi: 10.1002/cncr.30206. [DOI] [PubMed] [Google Scholar]
- 32.Kvale EA, Huang CS, Meneses KM, Demark-Wahnefried W, Bae S, Azuero CB, et al. Patient-centered support in the survivorship care transition: outcomes from the Patient-Owned Survivorship Care Plan Intervention. Cancer. 2016;122(20):3232–3242. doi: 10.1002/cncr.30136. [DOI] [PubMed] [Google Scholar]
- 33.Maly RC, Liang LJ, Liu Y, Griggs JJ, Ganz PA. Randomized controlled trial of survivorship care plans among low-income, predominantly Latina breast cancer survivors. J Clin Oncol. 2017;35(16):1814–1821. doi: 10.1200/JCO.2016.68.9497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tevaarwerk AJ, Hocking WG, Buhr KA, Gribble M, Seaborne LA, Wisinski KB, et al. A randomized trial of immediate versus delayed survivorship care plan receipt on patient satisfaction and knowledge of diagnosis and treatment. Cancer. 2019;125(6):1000–1007. doi: 10.1002/cncr.31875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramirez AG, Munoz E, Long Parma D, Perez A, Santillan A. Quality of life outcomes from a randomized controlled trial of patient navigation in Latina breast cancer survivors. Cancer Med. 2020;9(21):7837–7848. doi: 10.1002/cam4.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riis CL, Jensen PT, Bechmann T, Moller S, Coulter A, Steffensen KD. Satisfaction with care and adherence to treatment when using patient reported outcomes to individualize follow-up care for women with early breast cancer - a pilot randomized controlled trial. Acta Oncol. 2020;59(4):444–452. doi: 10.1080/0284186X.2020.1717604. [DOI] [PubMed] [Google Scholar]
- 37.van der Hout A, van Uden-Kraan CF, Holtmaat K, Jansen F, Lissenberg-Witte BI, Nieuwenhuijzen GAP, et al. Role of eHealth application Oncokompas in supporting self-management of symptoms and health-related quality of life in cancer survivors: a randomised, controlled trial. Lancet Oncol. 2020;21(1):80–94. doi: 10.1016/S1470-2045(19)30675-8. [DOI] [PubMed] [Google Scholar]
- 38.Rutkowski N, MacDonald-Liska C, Baines KA, Samuel V, Harris C, Lebel S. Standardized versus personalized survivorship care plans for breast cancer survivors: a program evaluation. Can Oncol Nurs J. 2021;31(4):451–456. doi: 10.5737/23688076314451456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Hea EL, Creamer S, Flahive JM, Keating BA, Crocker CR, Williamson SR, et al. Survivorship care planning, quality of life, and confidence to transition to survivorship: a randomized controlled trial with women ending treatment for breast cancer. J Psychosoc Oncol. 2022;40(5):574–594. doi: 10.1080/07347332.2021.1936336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Witteveen A, de Munck L, Groothuis-Oudshoorn CGM, Sonke GS, Poortmans PM, Boersma LJ, et al. Evaluating the age-based recommendations for long-term follow-up in breast cancer. Oncologist. 2020;25(9):e1330–e1338. doi: 10.1634/theoncologist.2019-0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Ligt KM, de Rooij BH, Walraven I, Heins MJ, Verloop J, Siesling S, et al. Varying severities of symptoms underline the relevance of personalized follow-up care in breast cancer survivors: latent class cluster analyses in a cross-sectional cohort. Support Care Cancer. 2022;30(10):7873–7883. doi: 10.1007/s00520-022-07229-6. [DOI] [PubMed] [Google Scholar]
- 42.Ankersmid JW, Spronk PER, Zeillemaker AM, Siesling S. Health care professionals overestimate the risk for locoregional recurrences after breast cancer treatment depending on their specialty. Breast Cancer Res Treat. 2022;193(2):293–303. doi: 10.1007/s10549-022-06549-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ankersmid JW, Drossaert CHC, van Riet YEA, Strobbe LJA, Siesling S, Santeon VBCG (2023) Needs and preferences of breast cancer survivors regarding outcome-based shared decision-making about personalised post-treatment surveillance. J Cancer Surviv 17(5):1471–1479 [DOI] [PMC free article] [PubMed]
- 44.Ankersmid JW, Siesling S, Strobbe LJA, Meulepas JM, van Riet YEA, Engels N, et al. Supporting shared decision-making about surveillance after breast cancer with personalized recurrence risk calculations: development of a patient decision aid using the international patient decision AIDS standards development process in combination with a mixed methods design. JMIR Cancer. 2022;8(4):e38088. doi: 10.2196/38088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Volkel V, Hueting TA, Draeger T, van Maaren MC, de Munck L, Strobbe LJA, et al. Improved risk estimation of locoregional recurrence, secondary contralateral tumors and distant metastases in early breast cancer: the INFLUENCE 2.0 model. Breast Cancer Res Treat. 2021;189(3):817–26. doi: 10.1007/s10549-021-06335-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klaassen-Dekker A, Drossaert CHC, Van Maaren MC, Van Leeuwen-Stok AE, Retel VP, Korevaar JC, et al. Personalized surveillance and aftercare for non-metastasized breast cancer: the NABOR study protocol of a multiple interrupted time series design. BMC Cancer. 2023;23(1):1112. doi: 10.1186/s12885-023-11504-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Draeger T, Voelkel V, Groothuis-Oudshoorn CGM, Lavric M, Veltman J, Dassen A, et al. Applying risk-based follow-up strategies on the dutch breast cancer population: consequences for care and costs. Value Health. 2020;23(9):1149–1156. doi: 10.1016/j.jval.2020.05.012. [DOI] [PubMed] [Google Scholar]
- 48.Bessen T, Keefe DM, Karnon J. Does one size fit all? Cost utility analyses of alternative mammographic follow-up schedules, by risk of recurrence. Int J Technol Assess Health Care. 2015;31(5):281–288. doi: 10.1017/S0266462315000598. [DOI] [PubMed] [Google Scholar]
- 49.Neuman HB, Schumacher JR, Schneider DF, Winslow ER, Busch RA, Tucholka JL, et al. Variation in the types of providers participating in breast cancer follow-up care: a SEER-Medicare analysis. Ann Surg Oncol. 2017;24(3):683–691. doi: 10.1245/s10434-016-5611-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tucholka JL, Jacobson N, Steffens NM, Schumacher JR, Tevaarwerk AJ, Anderson B, et al. Breast cancer survivor’s perspectives on the role different providers play in follow-up care. Support Care Cancer. 2018;26(6):2015–2022. doi: 10.1007/s00520-018-4042-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Riis CL, Bechmann T, Jensen PT, Coulter A, Steffensen KD. Are patient-reported outcomes useful in post-treatment follow-up care for women with early breast cancer? A scoping review. Patient Relat Outcome Meas. 2019;10:117–127. doi: 10.2147/PROM.S195296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Egdom LSE, Oemrawsingh A, Verweij LM, Lingsma HF, Koppert LB, Verhoef C, et al. Implementing patient-reported outcome measures in clinical breast cancer care: a systematic review. Value Health. 2019;22(10):1197–1226. doi: 10.1016/j.jval.2019.04.1927. [DOI] [PubMed] [Google Scholar]
- 53.Szuhany KL, Malgaroli M, Riley G, Miron CD, Suzuki R, Park JH, et al. Barriers and engagement in breast cancer survivorship wellness activities. Breast Cancer Res Treat. 2021;188(1):317–325. doi: 10.1007/s10549-021-06279-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sisler J, Chaput G, Sussman J, Ozokwelu E. Follow-up after treatment for breast cancer: practical guide to survivorship care for family physicians. Can Fam Physician. 2016;62(10):805–811. [PMC free article] [PubMed] [Google Scholar]
- 55.Avery J, Thomas R, Howell D, Dubouloz Wilner CJ. Empowering cancer survivors in managing their own health: a paradoxical dynamic process of taking and letting go of control. Qual Health Res. 2023;33(5):412–425. doi: 10.1177/10497323231158629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shin S, Park H. Effect of empowerment on the quality of life of the survivors of breast cancer: The moderating effect of self-help group participation. Jpn J Nurs Sci. 2017;14(4):311–319. doi: 10.1111/jjns.12161. [DOI] [PubMed] [Google Scholar]
- 57.Rozenblum R, Lisby M, Hockey PM, Levtizion-Korach O, Salzberg CA, Lipsitz S, et al. Uncovering the blind spot of patient satisfaction: an international survey. BMJ Qual Saf. 2011;20(11):959–965. doi: 10.1136/bmjqs-2011-000306. [DOI] [PubMed] [Google Scholar]
- 58.Klaassen L, Dirksen C, Boersma L, Hoving C, of the Bbg Developing an aftercare decision aid; assessing health professionals’ and patients’ preferences. Eur J Cancer Care (Engl) 2018;27(2):e12730. doi: 10.1111/ecc.12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tompkins C, Scanlon K, Scott E, Ream E, Harding S, Armes J. Survivorship care and support following treatment for breast cancer: a multi-ethnic comparative qualitative study of women’s experiences. BMC Health Serv Res. 2016;16(1):401. doi: 10.1186/s12913-016-1625-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fagerlin A, Pignone M, Abhyankar P, Col N, Feldman-Stewart D, Gavaruzzi T, et al. Clarifying values: an updated review. BMC Med Inform Decis Mak. 2013;13(Suppl 2(Suppl 2)):S8. doi: 10.1186/1472-6947-13-S2-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Klaassen LA, Dirksen CD, Boersma LJ, Hoving C, group Bb A novel patient decision aid for aftercare in breast cancer patients: a promising tool to reduce costs by individualizing aftercare. Breast. 2018;41:144–50. doi: 10.1016/j.breast.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 62.Boer H, Lubberts S, Bunskoek S, Nuver J, Lefrandt JD, Steursma G, et al. Shared-care survivorship program for testicular cancer patients: safe and feasible. ESMO Open. 2022;7(3):100488. doi: 10.1016/j.esmoop.2022.100488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sieswerda E, Postma A, van Dalen EC, van der Pal HJH, Tissing WJE, Rammeloo LAJ, et al. The Dutch Childhood Oncology Group guideline for follow-up of asymptomatic cardiac dysfunction in childhood cancer survivors. Ann Oncol. 2012;23(8):2191–2198. doi: 10.1093/annonc/mdr595. [DOI] [PubMed] [Google Scholar]
- 64.Stoll CRT, Izadi S, Fowler S, Green P, Suls J, Colditz GA. The value of a second reviewer for study selection in systematic reviews. Res Synth Methods. 2019;10(4):539–545. doi: 10.1002/jrsm.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bertizzolo L, Bossuyt P, Atal I, Ravaud P, Dechartres A. Disagreements in risk of bias assessment for randomised controlled trials included in more than one Cochrane systematic reviews: a research on research study using cross-sectional design. BMJ Open. 2019;9(4):e028382. doi: 10.1136/bmjopen-2018-028382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Buscemi N, Hartling L, Vandermeer B, Tjosvold L, Klassen TP. Single data extraction generated more errors than double data extraction in systematic reviews. J Clin Epidemiol. 2006;59(7):697–703. doi: 10.1016/j.jclinepi.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 67.Ong WL, Schouwenburg MG, van Bommel ACM, Stowell C, Allison KH, Benn KE, et al. A standard set of value-based patient-centered outcomes for breast cancer: the International Consortium for Health Outcomes Measurement (ICHOM) initiative. JAMA Oncol. 2017;3(5):677–685. doi: 10.1001/jamaoncol.2016.4851. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.