Abstract
Ventricular arrhythmias (VA) can be life‐threatening arrhythmias that result in significant morbidity and mortality. Catheter ablation (CA) is an invasive treatment modality that can be effective in the treatment of VA where medications fail. Recurrence occurs commonly following CA due to an inability to deliver lesions of adequate depth to cauterise the electrical circuits that drive VA or reach areas of scar responsible for VA. Stereotactic body radiotherapy is a non‐invasive treatment modality that allows volumetric delivery of energy to treat circuits that cannot be reached by CA. It overcomes the weaknesses of CA and has been successfully utilised in small clinical trials to treat refractory VA. This article summarises the current evidence for this novel treatment modality and the steps that will be required to bring it to the forefront of VA treatment.
Keywords: ablation, non‐invasive, radiosurgery, stereotactic body radiation therapy, ventricular arrhythmias
Introduction
Ventricular arrhythmia (VA) is a life‐threatening condition whereby frequent or continuous beats from the bottom chamber of the heart can cause syncope, heart failure or death. Implantable cardioverter defibrillators (ICD) save lives by terminating VA but do not modify the underlying mechanisms that drive this arrhythmia. Recurrent ICD shocks are associated with decreased quality of life and increased mortality. 1 , 2 , 3 VA results from two different mechanisms: abnormal focal activity leading to automaticity or triggered activity, and re‐entry circuits. 4 Abnormal focal activity results from membrane instability in poorly coupled myocytes and Purkinje cells. 5 Re‐entry results from small bridges of viable myocytes within scar that are capable of sustaining continuous electrical circuits. 5 Re‐entry is the predominant mechanism that causes VA in structural heart disease. 6
Catheter ablation (CA) with radiofrequency energy (RF) is the subsequent line of treatment when medications fail in VA. CA aims to find and cauterise viable myocytes within scar that form re‐entry circuits. Although CA has been demonstrated to be more effective than medication to control VA, the current success rate remains modest ranging from 50% to 70% in contemporary studies, with an overall major complication rate including death of 8.8%. 7 , 8 , 9 , 10 Treatment failure is due to several different factors (Fig. 1). Fibrosis and fat that develops within scar tissue shields deep underlying circuits that drive VA from effective eradication with RF energy. 11 The area of scar may be inaccessible to catheters due to anatomical constraints such as mechanical valves. 12 Alternative methods have been developed to improve lesion depth with RF such as combined epicardial ablation, use of bipolar ablation and novel ablation catheters such as the retractable needle tip electrode catheter. 13 , 14 , 15 Neuromodulation of the sympathetic system through stellate ganglion block and bilateral cardiac sympathetic denervation has been utilised to successfully reduce VT burden. 16 , 17 There has been significant interest in different energy sources to treat refractory VT such as ethanol ablation, cryoablation and more recently electroporation or pulsed field ablation which has shown promise in achieving deeper lesions compared to RF. 18 , 19
Fig. 1.
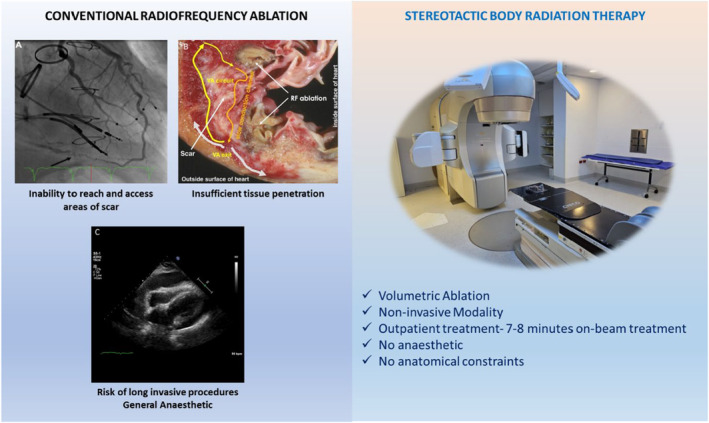
Weaknesses of conventional radiofrequency ablation compared to strengths of stereotactic radiotherapy. (a) Double mechanical valve limiting catheter access into left ventricle. (b) Inability of RF ablation to achieve ablation through scar. (c) Cardiac tamponade as a rare life‐threatening complication from CA. Reprinted [adapted] from Divakara et al. 128
Another alternative energy source is stereotactic body radiation therapy (SBRT), a mainstay treatment in oncology has now been adopted for use in the myocardium whereby clinicians can deliver radiation deep within myocardial tissue. Notably, it is a non‐invasive treatment modality, and there are promising early data from non‐randomised retrospective and prospective trials that it can treat VA in patients with advanced structural heart disease who are not amenable to further CA attempts. However, there are major gaps in knowledge regarding radiation dosing and delivery, long‐term adverse effects of SBRT and the underlying mechanisms that lead to VA suppression that need to be answered.
Antiarrhythmic effect of cardiac SBRT
Preclinical studies
Stereotactic body radiotherapy (SBRT) utilises the convergence of multiple intersecting beams of radiation to deliver high doses of ablative radiotherapy with sub‐millimetre precision whilst minimising damage to surrounding tissue through the sharp dose gradient it achieves. 20 In 2010, Sharma et al. published a seminal preclinical study investigating the use of SBRT in a porcine model. 21 They utilised the CyberKnife platform to deliver SBRT to the atrioventricular node, cavotricuspid isthmus and pulmonary veins. 21 Doses of 25 Gy or higher were able to produce lesions similar to ablation in each of these areas. Histological analysis at 90 days following the treatment revealed well‐circumscribed, transmural lesions with loss of myocyte architecture and increased fibrin with minimal collateral damage. 21 Subsequent studies have confirmed dose dependency with increasing fibrosis and earlier conduction block with increasing dosage. 22 , 23 These studies found that 25 Gy was the minimum dosage required to achieve myocardial fibrosis and conduction block in normal atrial tissue. 21 , 22 , 23 Hence, 25 Gy has since been employed in subsequent clinical trials; however, the optimal dosage required to achieve VA suppression in myocardial scar remains unknown.
Clinical outcomes
Clinical studies
Building on the initial case report data, 24 Cuculich et al. published a case series of five patients treated with SBRT. 25 Median treatment time was 12 min. A marked 99.9% reduction in VA burden was observed after a 6‐week blanking period at a median of follow‐up of 12 months. 2 patients had recurrence of VA during follow‐up at 4 weeks and 9 months, respectively. One patient passed away 3 weeks post‐treatment from a fatal stroke; however, this patient had a history of atrial fibrillation and was not anticoagulated due to bleeding risk. Therefore, it is unclear whether SBRT contributed to this patient's death. Post‐mortem 3 weeks after treatment showed no acute myocardial inflammation, acute cellular necrosis or fibrosis.
A prospective single‐centre trial with 19 patients was published by Robinson et al., following this case series. 4 17 patients had refractory VA, and 2 patients had pre‐ventricular ectopic (PVC)‐induced cardiomyopathy. Median treatment time was 15.3 min. At 24 months, this cohort demonstrated a reduction to a median of 2 VA episodes and a persistent reduction of VA episodes in 16 out of 18 patients. 26 An early reduction in VA episodes was noted in the 6‐week blanking period. At 3 months, there was 1 case of pericarditis which was conservatively managed and 1 exacerbation of heart failure that resulted in hospitalisation. Late side effects (>2 years) included a pericardial effusion which was medically managed and gastropericardial fistula requiring surgical repair. At 5 years, 15 of the 19 (79%) patients had passed away and freedom from any VT was 13%. 27 Most deaths were due to cardiac failure (38%). Whilst this may represent the natural history of cardiomyopathy patients, whether SBRT accelerated heart failure progression in these patients is unknown.
To date, clinical outcome data for SBRT of cardiac arrhythmias continue to be limited to small prospective and retrospective clinical studies and case reports. There are currently 41 trials published enrolling a total of 162 patients (Table 3). Most patients enrolled have refractory ventricular tachycardia (VT) resulting from non‐ischaemic cardiomyopathy (NICM) or ischaemic cardiomyopathy (ICM). Rarer aetiologies of VT such as cardiac sarcoid (n = 4), arrhythmogenic right ventricular cardiomyopathy (n = 1), hypertrophic cardiomyopathy (n = 2) and cardiac tumours (n = 2) have been treated with SBRT. 28 , 29 , 30 , 31 , 32 A small number of patients with PVC‐induced cardiomyopathy (n = 3) and ventricular fibrillation (n = 1) have also been treated with SBRT. 4 , 33 , 34 Of note, SBRT has been used in patients with contraindications to CA, that is mechanical valves (n = 9) and LV thrombus (n = 5), as well as critically ill patients presenting with electrical storm. 12 , 35 Atrial fibrillation has been treated by SBRT in small case series to varying success; however, the main focus of research has been on VA due to the clinical need for alternate more efficacious therapies in this cohort of patients. 36 , 37
Table 3.
Clinical studies of SBRT for VA to date
| Reference | Patient characteristics (sample size, gender, age) | Dose, procedure length | Delivery system | PTV size (median, range) | ITV to PTV expansion | Follow‐up | Recurrence | SBRT complications | Mechanistic findings |
|---|---|---|---|---|---|---|---|---|---|
| Cvek et al. (2014) 112 | N = 1 female; Age 72 | 25 Gy in 1 fraction; 114 min | CyberKnife | N.R. | N.R. | 4 months | No recurrence at 4 months | None | Minimal elevation of troponin T serum levels detected 10 days after treatment |
| Loo et al. (2015) 24 | N = 1 male; Age 71 | 25 Gy in 1 fraction; 90 min | CyberKnife | N.R. | N.R. | 9 months, 2 month blanking period | Reduction in VT burden from 562 to 52 episodes per month at 3 months |
None Patient died 9 months later due to exacerbation of COPD and recurrent VT |
Cycle length of VT decreased from 380–411 ms to 470 ms post‐SBRT |
| Cuculich et al. (2017) 25 | N = 5 (4 male, 1 female); Age 66 (60–83) | 25 Gy in 1 fraction; 15 min | Varian TrueBeam | 51.3 mL, 17.3–81 mL | 5 mm expansion | 12 months, 6‐week blanking period |
99% reduction in total VT episodes seen in ¾ evaluable patients Recurrence in ¼ evaluable patients |
1 patient died of a stroke 3‐weeks after SBRT. Remains unclear if this was related to SBRT |
No CT evidence of changes to myocardium or coronary arteries 12 months post‐treatment in patients Post‐mortem pathological assessment of the deceased patient revealed viable myocardium with ectatic blood vessels on the interface of dense scar – often explained by the acute vascular injury. However, no signs of acute vasculitis, tissue oedema, acute myocyte necrosis, haemorrhage or acute inflammation |
| Jumeau et al. (2018) 113 | N = 1 male; Age 75 | 25 Gy in 1 fraction; 45 min | CyberKnife | 21 mL | N.R. | 4 months | No recurrence at 4 months | None | N/A |
| Haskova et al. (2019) 31 | N = 1 male; Age 34 | 25 Gy in 1 fraction; N.R. | CyberKnife | N.R. | N.R. | 8 months |
Gradual reduction in VT episodes. No recurrence at 8 months |
None | N/A |
| Zeng et al. (2019) 32 | N = 1 male; Age 29 | 24 Gy in 3 fractions; N.R. | CyberKnife | 71.2 mL | N.R. | 4 months |
Complete reduction from 189 to 0 VT episodes/day after 2 months No recurrence at 4 months |
None | N/A |
| Scholz et al. (2019) 34 | N = 1 male; Age 53 | 24 Gy in 1 fraction; 5 min | Elekta Versa | 55.8 mL | 2 mm expansion | 2 months |
No VT episodes at 2 weeks post‐SBRT No recurrence at 2 months |
None | N/A |
| Neuwirth et al. (2019) 38 | N = 10 (9 male, 1 female); Mean age 66 (61–78) | 25 Gy in 1 fraction; 68 min (45–80 min range) | CyberKnife | 22.1 mL, 14.2–29.6 mL | 0 mm expansion | 28 months, 90‐day blanking period |
An average of 87.9% reduction in VT episodes from 70.7 to 8.7 episodes/month. 2 patients with no response; Recurrence in 8/10 patients |
4 patients with nausea associated with acute toxicity 1 patient with possible grade 3 late toxicity (mitral regurgitation at 17 months) 3 unrelated deaths |
No signs of significant elevation of troponin during follow‐up |
| Krug et al. (2019) 114 | N = 1 male; Age 78 | 25 Gy in 1 fraction; 15 min | Varian TrueBeam | 42.2 mL | 5 mm expansion | 2 months | Reduction from 20 to 6.4 VT episodes/month; Recurrence at 2 months |
Periprocedural nausea with a single episode of vomiting; Patient died 57 days post‐treatment due to sepsis associated cardiac circulatory failure. |
Clinical autopsy revealed diffuse fibrosis throughout heart (no evident differences between target tissue and the control: posterior cardiac wall tissue). Specific to the target area, there was a 2 mm region of fresh necrosis and no evidence of a demarcating inflammatory reaction |
| Robinson et al. (2019) 4 | N = 17 (15 males, 2 females); Median age 66 (49–81) | 25 Gy in 1 fraction; 15.3 min (5.4–32.3 min range) | Varian TrueBeam (n = 3) and Varian Edge (n = 16) | 98.99 mL, 60–298.8 mL | 5 mm expansion | 24 months, 6‐week blanking period |
97% reduction from median of 119 to 3 VT episodes/month Recurrence in 11/16 evaluable patients at 6 months |
1 patient with pneumonitis 2 patients with delayed pericarditis/ Effusion 1 patient died from unrelated accident 17 days post‐SBRT |
N/A |
| Lloyd et al. (2019) 48 |
N = 10 (7 males, 3 females), Median age 61 (51–78) |
25 Gy in 1 fraction; 30 min appointment | Varian Edge | 81.4 mL, 45–238 mL | 1–5 mm expansion | 6 months |
A mean reduction of 94% in total seconds of detected VT in 9/9 evaluable patients. One patient with no response to SBRT. Most of patients responding in first 2 weeks No recurrence at 6 months |
2 patients with mild pneumonitis. 1 patient requiring resuscitation with slow VT (below treatment zone of her device) during SBRT treatment; 1 patient required heart transplant after no response to SBRT |
Observed disruption of gap junctions on electron microscopy specimens (possibly explaining the relatively acute treatment response) |
| Qian et al. (2019) 36 | N = 2 (1 male and 1 female), Age 59 and 55 | 25 Gy in 1 fraction; 90 min for each treatment | CyberKnife | 48.87 mL and 54.5 mL | N.R. | 6 months | Recurrence of atrial fibrillation at 6 months in 1st patient. No recurrence in 2nd patient at 6 months | Nil complications | |
| Park et al (2020) 29 | N = 1; Male; Age 76 | 24 Gy three fractions; N.R. | Varian Clinac iX | N.R. | 10 mm expansion | 6 months | 2 recurrences of VT resolving with ATP. No further ICD shocks | Mild pulmonary fibrosis on CXR | N/A |
| Qian et al. (2020) 50 | N = 1 male; Age 77 | 25 Gy in 1 fraction; N.R. | N.R. | N.R. | N/A | 4 months | Reduction from 12 to 4 cardioverter‐defibrillator shocks in 3 months. No recurrence at 4 months | Tapering of antiarrhythmic medications after 4 months revealed new recurrent VT at treatment border zone | N/A |
| Shoji et al. (2020) 37 | N = 3 (2 male, 1 female), Age 76, 67 and 81 | 25–35 Gy in 1 fraction, N.R. | CyberKnife | 78.5 mL, 51.5–111 mL | 3 mm expansion | 24 months | AF in patients with metastatic cancer. Recurrence of atrial fibrillation occurred in remaining 2 patients who survived until 24 months | 1 unrelated patient death 4 days after procedure from cancer progression | |
| Gianni et al. (2020), 44 | N = 5 males, Mean Age 63 (45–76) | 25 Gy in 1 fraction; 82 min (45–80 min range) | CyberKnife | 173 mL, 96–184 mL | 3 mm expansion | 12 months |
Reduction in 4/5 patients from 6.4 to 4.9 VT episodes /month; Recurrence in all patients 6 months later |
None; 2 patients died of advanced heart failure |
N/A |
| Mayinger et al. (2020) 115 | N = 1 male; Age 71 | 25 Gy in 1 fraction; 24 min | MR Linac | 73.6 mL | 2 mm | 3 months |
100% reduction in episodes at 1 week; No recurrence at 3 months |
Aggravation of VT and prolonged ES 24 h post‐SBRT, ceasing after 48 h following high dose of dexamethasone | N/A |
| Yugo et al. (2020) 40 | N = 3 (2 males, 1 female), Age 72 (68–83) | 25 Gy in 1 fraction; N.R. | Varian TrueBeam | 92.95 mL. 63.9–106.6 mL | 2 mm | 13 months, 6 week blanking period |
Mean reduction from 140 to 54 VT episodes in 2/3 patients Recurrence in 3/3 patients at 6 weeks |
None; 3/3 unrelated patient deaths due to comorbidities |
N/A |
| Peichl et al. (2020) 81 | N = 1; Male; Age 66 | 2 doses of 25 Gy; N.R. | CyberKnife. | 21 and 18 mL respectively for each case | 3 mm | 12 months | 1st SBRT guided by electroanatomical mapping and CT failed to suppress ventricular tachycardia. Subsequent electroanatomical mapping showed evidence of scar adjacent to area of earliest activation of VT. 2nd SBRT delivered with new integration software. Smaller target volume allowed addition of 3 mm margin to PTV increasing the probability of achieving transmural lesion. No further VT at follow‐up at 12 months following 2nd SBRT | None | Nil |
| Chin et al. (2021) 116 | N = 8 males; Mean age 70 (65–86) | 25 Gy in 1 fraction; 18.2 ± 6 min | Brainlab Novalis Tx | 84.9 mL, 21.1–190.7 mL | 6–8 mm margin | 8 months |
Mean reduction from 17 to 4.1 episodes/month. Resolution in 3/6 evaluable patients by 3 months; Recurrence in 4/6 evaluable patients at 8 months |
No acute complications 3 unrelated patient deaths |
N/A |
| Dusi et al. (2021) 117 | N = 1 male; Age 73 | 25 Gy in 1 fraction; N.R. | N.R. | 27.7 mL | N.R. | 2 months | Reduction from 121 episodes/month to 5.5 episodes/month | No acute complications. Patient passed away from septic shock 2 months post‐SBRT | N/A |
| Carbucicchio et al. (2021) 33 | N = 7 males; Mean Age 70 (63–77) | 25 Gy in 1 Fraction; 31 ± 6 min | Varian trilogy | 198.3 mL, 138–225 mL | N.R. | 6 months | 4/4 evaluable patients showed significant decrease from 29 to 2 VT episodes/month at 6 months |
1 episode of pulmonary fibrosis. 1 episode of nausea and vomiting 3 unrelated patient deaths |
N/A |
| Lee et al. (2021) 118 | N = 1; Male; Age 11 | 25 Gy single fraction; N.R. | Elekta Versa HD | N.R | 5 mm | 3 months | No VT at 3 months after SBRT and bilateral stellate ganglion blockade | None | N/A |
| Donnelly et al. (2021) 119 | N = 1; Male; 51 M | 25 Gy single fraction; N.R. | N.R. | N.R. | 5 mm | Not documented | Decrease in PVC burden from 21% to 5% on follow‐up with improvement of LV function to 45%–50% (decreased due to PVC induced cardiomyopathy) | None | |
| Ho et al. (2021) 30 | N = 7 (6 males, 1 female); Age 55 (23–80) | 25 Gy in 1 Fraction; N.R. | Varian TrueBeam | 52.1 mL, 14.4–92.6 mL | 5 mm | 14.5 months |
Significant reduction in VT episodes Recurrence in 4/6 evaluable patients |
No acute complications; Left ventricular apical thrombus prevented one patient from receiving SBRT. One patient with grade 1 pericardial effusion 6 months post‐treatment One unrelated death due to hepatic failure |
N/A |
| Gerard et al. (2021) 120 | N = 2; Male; Age Both 65 | 25 Gy single fraction; 20 and 24 min respectively | Varian TrueBeam | 103 and 66.4 mL respectively for each case | N.R. | 17 and 12 months |
Case 1 had ES in context of pneumonia 10 months after treatment. At 17 months one recorded VT event and no ICD shocks. Case 2 had no further VT at 12 month follow‐up |
Case 1 had mild oesophagitis treated with antacids | N/A |
| Aras et al. (2021) 12 | N = 1; Male; Age 58 | 25 Gy single fraction; 7.5 min | Varian TrueBeam. | N.R. | 5 mm | 10 months | Significant reduction in VT episodes following SBRT | No complications. | N/A |
| Lee et al. (2021) 45 | N = 7 (4 males, 3 females); N.R. (60–70s) | 25 Gy in 1 Fraction; 33 min (30–60 min) | Varian medical systems and Elekta | 89.5 mL, 57.5–139 mL | 3–5 mm margin | 6 months |
85% reduction in VT episodes at 6 months post‐SBRT in 5/5 observable patients Short term recurrence observed |
None; Three patient deaths from progressive heart failure, two dying within 4 weeks post‐treatment |
Autopsy obtained from patient that died within 4 weeks of treatment demonstrating increased vascularity in viable myocardium and previous fibrosis due to myocardial ischaemia No other histologic features of acute necrosis, thrombosis, changes to blood vessel wall, fibroblast proliferation or nuclear atypia to suggest radiation exposure were evident |
| Qian et al. (2022) 41 | N = 6 Males; Median age 72 (70–83) | 25 Gy in 1 Fraction; 13.8 min (11.0–15.0 min) | Varian TrueBeam | 317.1 mL, 262–345.1 mL | 5 mm margin | 18 months |
Insignificant reduction from 42 (IQR 19–269) to 29 (IQR: 0–81) VT episodes in 6 months Significant reduction in device shocks from 12 (IQR: 3–9) to 0 (IQR 0–1) Recurrence in 4/6 patients at 213 (IQR 105–306) days post‐treatment |
3/6 patients with possible adverse effects including pneumonia, heart failure exacerbation, asymptomatic pericardial effusion; 3 patient deaths due to end‐stage heart failure |
Substrate modification approach targeting regions of wall thinning, myocardial fat and calcification in ischaemic cardiomyopathy utilised |
| Haskova et al. (2022) 121 | N = 3; Male; Age 66 (34–77) | Single dose of 25 Gy; N.R. | CyberKnife | Redo PTVs –21.2, 23.4 and 43.4 mL respectively for each case | 3 mm for case 2. N.R. for case 1 and 3 | 22–32 months |
Patient 1 and 2 underwent redo SBRT at 20 and 24 months post‐index SBRT. No further VT at 22 and 32 months following 2nd SBRT Patient 3 had SBRT for recurrent VT 4 months after index SBRT. Required additional CA for slow VT to render non‐inducible. At 2 month mark died of progressive heart failure with no further VT |
None | N/A |
| Wight et al. (2022) 59 | N = 14 (10 Males, 4 Females); Mean Age 61 (50–78) | 25 Gy in 1 fraction; N.R. | Varian TrueBeam | N.R. | 1–5 mm expansion |
7 months |
Patients had a 59% reduction in VT, 39% reduction in ATP and a 60% reduction in shocks |
Pneumonitis in four of the 14 patients Two patients died shortly after SBRT, one died post‐heart transplant and another had ES and haemodynamic instability leading to death |
N/A |
| Miszczyk et al. (2022) 122 | N = 1 Male; 51 year old | 25 Gy in 1 fraction; 33 min | Varian Edge | 88.7 mL | 3 mm expansion | 12 months | No recurrence | Antiarrhythmic response that lasted almost a year, until a heart failure exacerbation necessitated a heart transplant |
No evidence of fibrosis Despite a complete treatment response, there was no homogenous transmural fibrosis in the irradiated region, and the overall presentation of the heart was similar to other transplanted hearts of patients with advanced heart failure |
| Ninni et al. (2022) 35 | N = 17; Males 13; Females 4; Mean age = 67 ± 12.8 | 25 Gy in 1 fraction; 30 min | CyberKnife | 53.3 mL, 20.0 mL –186 mL | 5 mm expansion | Median 12.5 (10.5–17.8) months | High rate of patients presenting late VT recurrence after SBRT. 40% of these patients presented VT recurrences related to single VT episodes, and 30% of patients presented VT recurrences associated with ICD shocks at 18 months | Two patients died during the blanking period (<6 weeks). 1 patient died of cardiogenic shock and the other with refractory ES.2 patients died beyond 6 weeks. 1 patient died from AKI, metabolic acidosis and electromechanical dissociation. The other died from refractory cardiogenic shock | Late VT recurrences were unrelated to ES and were associated with increase in cycle length in VT in two‐thirds of the patients |
| Bernstein et al. (2022) 123 | N = 1; Male; Age 75 | 25 Gy in single fraction; N.R. | N.R. | 87.9 mL | 5 mm | 6 months | No VT recurrence | N/A | N/A |
| Cozzi et al. (2022) 124 | N = 1; Male; Age 81 | 25 Gy in single fraction; 15 min | Varian True Beam | 122.5 mL | 5 mm | 7 months | No VT recurrence | None | N/A |
| Huang et al. (2022) 125 | N = 1; Male; Age 63 | 12 Gy in single fraction; 24 min | Versa HDTM | 65.75 mL | 5 mm | 15 months | At 6 months, recurrence of VT from different anatomically area requiring ATP and ICD shock. VT was mapped and ablated. Following placement of CRT device and ablation marked decrease in VT burden | N/A | N/A |
| Vaskovskii et al. (2022) 126 | N = 1; Male; Age 57 | Single dose of 25 Gy; N.R. | Varian TrueBeam | 46 mL | 5 mm | 6 months | No further VT episodes after 2 month mark post‐procedure | Nil | N/A |
| Aras et al. (2023) 49 |
N = 8, 8 Males; Mean age 58 ± 14 |
25 Gy in 1 Fraction; 5.6 min (3.6–7.45 min) | Varian TrueBeam | 157.44 mL, 70.5–272.7 mL | 5 mm | 8 months | All patients demonstrated VT recurrences; however, ICD and anti‐tachycardia pacing therapies were significantly reduced with SBRT. The 2 weeks to 3 months period outcomes were favourable. After 6 months, one patient was ICD therapy‐free and the remaining patients demonstrated VT episodes |
2 pericardial effusions. 1 asymptomatic and 1 resolved with medical management Four patients died during follow‐up. 1 patient had end stage heart failure and recurrent VT. 3 deaths due to unrelated causes |
N/A |
| Van Der Ree et al. (2023) 39 | N = 6; 6 Males; Age 73 (54–83) | Single dose 25 Gy; 4.6 min (3.6–5.2 min) | Agility linear accelerator (Elekta) | 187 mL, 93–372 mL | 5 mm | 12 months |
Met primary outcome of ≥50% reduction in VT in 4 patients Median number of treated VT episodes reduced from 31 (range 8–138) before treatment to 9 (range 0–109) after treatment Recurrence occurred in 5 (83%) of patients at 12 months |
1 patient had radiation induced electrical reset of ICD 1 patient developed severe thoracic chest wall within the field requiring steroids and cervical nerve block 2 patients developed pericardial effusions responding to conservative management 1 patient had pneumonitis 1 patient developed intracardiac thrombus which was possibly radiation related |
N/A |
| Scanavacca et al. (2023) 127 | N = 1; Male, Age 53 | Single dose of 25 Gy; 15 min | N.R. | 74.5 mL | N.R. | 12 months | No recurrence in 12 months | None | N/A |
| Van Der Ree et al. (2023) 28 | N = 1, Female, Age 47 | Single dose 20 Gy to proarrhythmic substrate; CyberKnife. 2 x 2 Gy low‐dose whole heart irradiation | Tomotherapy HAD treatment system |
PTV 16 mL 1162 mL for whole heart immunomodulatory radiotherapy. |
3 mm | 55 months |
Reduction in sustained VT episodes by 95% and NSVT episodes by 58%. At 7 months presented in ES due to influenza. Following seventh CA only 2 sustained episodes requiring ATP in following 38 months PET CT demonstrated regression of hypermetabolic activity with no active signs of cardiac sarcoidosis at 55 months |
Moderate aortic regurgitation | Reduction in inflammatory disease activity on PET‐CT |
The workup of patients across clinical studies is not standardised and variable. Most groups utilise invasive electroanatomical maps (EAM) obtained during CA combined with anatomical scar imaging obtained from cardiac CT, cardiac magnetic resonance (CMR) and echocardiography to define VT substrate. 35 , 38 , 39 Some groups have substituted invasive EAMs with dedicated non‐invasive maps, allowing a completely non‐invasive approach. 25 , 33 Other groups have employed nuclear medicine scans such as positron emission tomography (PET‐CT) and single photon emission tomography (SPECT) to assist in identifying areas of myocardial viability within scar. 24 , 40
The reported planned target volume (PTV) differs markedly across current studies ranging from a median of 51.3 mL to 317.1 mL (Table 3). 25 , 41 The large variability in PTV may be explained by several different factors. Firstly, there were differences in anatomical size of VT substrate with initial studies having a higher proportion of NICM than ICM with smaller treatment volumes. 25 , 35 , 41 Secondly, different groups have utilised different strategies in targeting scar. Some studies looked to minimise treatment volumes by targeting SBRT to VT exit sites, whilst others utilised SBRT to target the entire substrate. 4 , 41 Thirdly, manual delineation of VT substrate and target volume (TV) contouring likely results in interobserver and intergroup variability. 42 , 43 Finally, a range of different margin expansions have been utilised across studies to attain both internal target volume (ITV) and PTV. 38 , 44 , 45
Margin expansions to account for treatment uncertainties vary depending on whether Linac or CyberKnife platforms are employed to deliver SBRT. The CyberKnife platform utilises ICD leads, left ventricular (LV) leads or temporary pacing wires as fiducials to allow marker‐based tracking of respiratory motion (Synchrony, Accuray). 24 , 38 , 46 Cardiac motion has been accounted for in some CyberKnife treatments by incorporating cardiac motion on fluoroscopy and using ECG‐gated CTs. 24 , 38 CyberKnife utilises a more conservative 3 mm (range, 0–5 mm) ITV to PTV expansion based on the reported accuracy of CyberKnife for lung/abdomen SBRT. 46 , 47 Linac platforms utilise a combined cardiorespiratory ITV obtained from respiratory gated 4D CT. The majority of Linac treatments utilised 5 mm (range, 0–10 mm) expansion of ITV to PTV. 4 , 25 , 39 , 41
Efficacy of cardiac stereotactic body radiotherapy
Mirroring the significant differences in workup and treatment of patients in these studies is the variable efficacy of SBRT observed across studies. In contrast with 99.9% reduction in VT burden described by Cuculich in his seminal paper, some groups have reported a reduction in VT burden as low as 31%. 41 , 44 Similarly, reduction in ICD therapies has been reported as low as 57% reduction in shocks and 50% reduction in anti‐tachycardia pacing (ATP) therapies. 40 , 48
Early suppression of VA within a 6‐week period has been noted across multiple clinical trials. It is apparent that this suppressive effect occurs within a 6‐week period in the absence of fibrosis from post‐mortem results and animal studies. 4 , 35 , 39 , 49 These data challenge the hypothesis that SBRT achieves its antiarrhythmic effect by solely inducing fibrosis.
Most patients had late recurrence of VA occurring post‐SBRT beyond the 9‐month mark. 35 , 39 , 44 , 49 EAMs and 12 lead ECGs obtained from these patients following recurrence have indicated viable myocytes within the PTV and also identified new VT circuits in areas adjacent to the PTV. 39 , 50 Recurrence within the PTV may reflect resistance of VT substrate to SBRT. Larger PTV size has been correlated with ICM and decreasing efficacy of SBRT for VT, perhaps indicating that 25 Gy is not sufficient to achieve arrhythmia suppression in ischaemic scar. 41 , 44 VT circuits adjacent to the PTV may result from scar development from subthreshold dosing of SBRT in viable myocardium. 50 More consistent and precise clinical target definition with better cardiorespiratory motion management during SBRT delivery may reduce the propensity for late recurrence to occur from VT circuits adjacent to the PTV.
Potential mechanisms of SBRT
Preclinical studies aimed to utilise radiotherapy to create ablative lesions comparable to CA by destroying cells, inducing fibrosis and producing electrically inert tissue. Fibrosis was dose‐dependent with higher doses utilised (30–55 Gy) to achieve strongly fibrotic, non‐conducting lesions at 3–6 months. 21 , 22 A much earlier antiarrhythmic effect has been observed in clinical trials (<6 weeks) utilising a lower 25 Gy dose. The therapeutic effect of radiotherapy therefore cannot be explained by fibrosis alone.
Immediate blockade of conduction in the AV node has been achieved in large animal study with very large doses of SBRT (160 Gy). 51 This functional blockade of conduction may be achieved through disruption of cardiomyocyte electrical conduction in the absence of cell death. Given functional conduction blockade can occur acutely and in the absence of fibrosis, it is plausible that this may contribute to the early antiarrhythmic effect noted in clinical trials. 51 Further investigation will need to be done to determine the minimum dose required to achieve this effect in ventricular tissue and the underlying mechanisms.
Lower doses of radiation (10–25 Gy) in animal infarct models have been associated with improved conductivity within scar tissue reducing vulnerability for re‐entrant arrhythmias such as VT. Animal studies have shown that improved conduction may be mediated by increased connexin 43, a major ventricular gap junction subunit, and voltage‐gated sodium channels (NaV1.5). 52 , 53 , 54 , 55 Connexin 43 upregulation in these studies was notably associated with a decrease in VT inducibility. 53 , 54 The changes above have been observed as earlier as 2 weeks post‐treatment following radiotherapy accounting for the early antiarrhythmic effect observed in current clinical trials. 52 , 55
Reversal of cardiac remodelling and increasing ejection fraction is associated with reduced risk of VA and mortality. 56 CMR data in a subset of patients 3 days (n = 9) pre (EF 18.6% ± 3.9%)‐ and post‐treatment (EF 32.3% ± 4.8%) demonstrated an early but persisting improvement in ejection fraction at 3 months (32.2% ± 7.3%). 26 Retrospective analysis of the ENCORE‐cohort (n = 42) revealed that ≥40% of the heart outside of the PTV received ≥5 Gy. The mean heart dose extrinsic to the non‐targeted areas of myocardium was 5 Gy. 57 The effect of 5 Gy cardiac irradiation was investigated in 3 murine heart failure models by Pedersen et al., who found an improvement in ejection fraction and end diastolic volume in the radiotherapy group compared to sham. 57 Although VT suppression may account for the improvement in ejection fraction observed in ENCORE VT, these data raise the question whether low‐dose radiotherapy also plays a role in reversing cardiac remodelling thereby reducing the risk of VT.
Known early and late toxicities from cardiac stereotactic body radiotherapy
Historically, our knowledge of cardiac toxicity from radiotherapy stems from clinical experience with fractionated treatment in lymphoma and breast cancer. 58 Acute cardiac inflammation from radiotherapy manifests in myocarditis or pericarditis. Most radiation‐induced cardiotoxicity appears to be delayed, usually developing 10–40 years after treatment. 58 The dose of radiation, volume of heart and extent of involvement of coronary arteries are risk factors associated with development of cardiac toxicity. Common toxicities include early onset coronary artery disease, valvular heart disease, complete heart block, heart failure from diastolic dysfunction and pericardial fibrosis. 58
Adverse effects due to cardiac SBRT are summarised in Table 1. Transient early adverse effects such as fatigue, dizziness, nausea and hypotension are common, occurring in up to 23% of patients, and usually resolve spontaneously (grade 1). There has been 1 case of ICD reset to factory settings post‐SBRT which has been reported 39 (grade 2). Rare early delayed adverse effects which have been reported post‐SBRT are fatal heart failure exacerbations and refractory electrical storm (grade 4 and 5). 35 , 45 , 59 These adverse effects may reflect the natural history of disease in this patient cohort, but a SBRT‐related adverse effect is unable to be excluded.
Table 1.
Adverse effects from SBRT
| Adverse events | Manifestation | Clinical grade (CTCAE) | Management |
|---|---|---|---|
| Transient early adverse events | Fatigue, dizziness, nausea and hypotension | 1 | Spontaneous resolution |
| Late delayed toxicities | Pneumonitis | 2 | Steroid therapy |
|
Pericarditis Pericardial effusions |
3–4 | Medical therapy and rarely require pericardiocentesis | |
| Mitral valve regurgitation | 4 | Valve surgery | |
| Aortic regurgitation | 4 | Valve surgery | |
| Left thoracic chest discomfort | 3 | Steroids and cervical nerve root block | |
| Gastroesophageal fistula | 4 | Surgical correction | |
| Oesophageal‐pericardial fistula | 5 | Gastrostomy/mortality from fatal bleeding |
Common late delayed toxicities include radiation‐induced pneumonitis and pericarditis (grade 2 and 3). 33 , 35 , 39 , 44 , 48 , 59 Pneumonitis occurs in 11–29% of patients and generally resolves with steroid therapy. Pericarditis and associated pericardial effusions occur in 17–29% of patients and usually respond to medical therapy and rarely require pericardiocentesis. There have been two cases of severe mitral valve regurgitation which required surgery (grade 4) and one case of moderate aortic regurgitation (grade 3) which were likely radiation‐mediated. 28 , 60 Progress CT scans and echocardiograms post‐SBRT are important to ensure that these complications are detected, monitored and treated. There has been 1 case of left thoracic chest discomfort reported which improved with steroids and cervical nerve root block (grade 3). 39
Rare late delayed toxicities from SBRT are gastroesophageal fistula (grade 4) and oesophagopericardial fistula (grade 5). 26 , 61 The oesophagopericardial fistula patient presented with oesophagitis that responded to antacids 18 days post‐SBRT. At 6 months, the patient presented with dysphagia and a large ulcer was diagnosed in the terminal part of the oesophagus on endoscopy. Endoscopic gastrotomy was performed as surgery was deemed to high risk. The patient had a fatal bleeding event 3 months post‐gastrostomy. The oesophageal dose limits were not exceeded in this case. The gastropericardial fistula occurred 2.4 years post‐SBRT. It was corrected with surgical treatment. Patients receiving radiation therapy to inferior or inferolateral wall, which lies near the gastric tract, must therefore be monitored carefully for this complication.
Current workflow
Patient selection
Currently, SBRT remains an experimental therapy that is offered as part of clinical trials or compassionate access to patients that have VA that is refractory to antiarrhythmic drug escalation and who have failed or are not candidates for catheter ablation. As shown in Table 3, which summarises the data to date supporting use of SBRT, it can be an effective treatment modality in such cases, and its non‐invasive nature makes it particularly suited to treat highly comorbid patients who cannot tolerate general anaesthesia or hospitalisation or have inaccessible scar. The outcome data as demonstrated above remain limited to prospective case studies with no randomised control data to support its use.
Determining clinical target
A combination of anatomical and electrophysiological investigations allows delineation of arrhythmic substrate driving VA. In patients who have failed catheter ablation, EAM information can be integrated with cardiac imaging to provide understanding of arrhythmogenic substrate 62 , 63 (Fig. 2). Electrocardiographic imaging (ECGi) or CardioInsight (Medtronic, Minnesota, United States) utilises an electrode vest, with 60–252 electrodes, in combination with CT scan, can non‐invasively map the VA exit in centres where this is available. 64
Fig. 2.
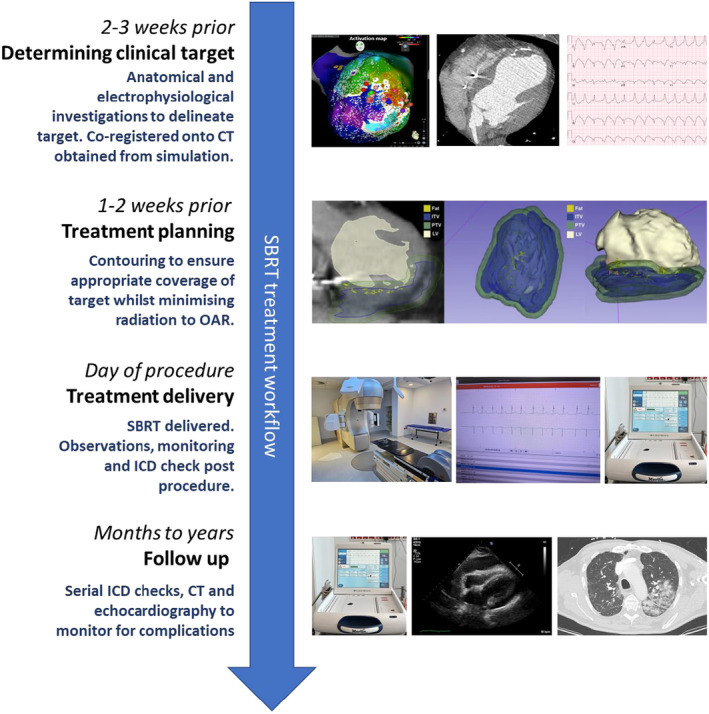
SBRT treatment workflow. Reprinted [adapted] from Qian et al. 129
Imaging techniques available for localisation of scar in structural heart disease include CMR, cardiac CT and radionuclide imaging. CMR provides excellent soft tissue characterisation, visualising myocardial scar with great precision. 65 The utility of CMR can be limited due to artefacts created by ICDs leads; however, wide‐band filters now mitigate this to some extent. 66 Regions of myocardial thinning and putative corridors for VA circuits can be extracted using image processing software (ADAS 3D, Galgo Medical, Barcelona, Spain or In heart, Bordeaux, France) and can be imported into radiotherapy planning software. 67 , 68 Cardiac CT provides higher spatial resolution allowing excellent characterisation of cardiac anatomy. 69 , 70 It can identify areas of significant wall thinning, fibrosis, fat and calcium which are associated with VA substrate. 71 , 72 The anatomical information obtained from cardiac CT can be combined with delayed contrast administration. 70 Radionuclide imaging, such as SPECT and fluorodeoxyglucose‐positron emission tomography (FDG‐PET), can detect areas of scar based on metabolic activity. Areas of rapid transition from low to normal metabolism on FDG‐PET, indicate border zones between normal and scar tissue, have been correlated to VT exit sites on EAMs. 73
Radiotherapy simulation, target volume creation and treatment planning
Patients undergo a treatment simulation session in the radiation oncology CT suite where they are immobilised in the position in which they will receive radiation. Vac‐bags, abdominal compressors and overhead arm extensions are used to assist in immobilisation in a reproducible position. A 4D CT is performed and the average intensity projection typically serves as the anatomic reference for treatment planning to account for respiratory cycle motion in free breathing treatments. 74
Electrophysiological and anatomical information is co‐registered with the simulation CT to define a TV (Fig. 2). This is contoured by a collaborative effort between the electrophysiologist and radiation oncologist utilising the anatomic (MRI, CT, radionuclide) and electrophysiological information (ECG, EAM and ECGi). 42 , 75 An ITV is generated from the TV which accounts for maximum range of cardiorespiratory motion demonstrated from the 4D‐CT. PTV is generated as a small volumetric expansion (≤5 mm) from the ITV which accounts for geometric uncertainties. 42 The contouring process is time‐consuming as it often requires manual comparison between imaging studies and mapping data as well as consideration of adjacent radiosensitive organs. 42 There is continued development in tools to automate integration of electrophysiological maps with radiation planning software; however, these tools require further clinical validation (Table 2). 76
Table 2.
Comparison of cardiac fusion papers
| Cardiac fusion papers | Software translation | EAM software | Plug in | File conversion | Significance |
|---|---|---|---|---|---|
| Brett et al. (2020) 77 | EAM to radiation planning software | CARTO 3 | SlicerRT | Mesh→VTK→DICOM | Robust performance when compared to ADAS VT |
| Hohman et al. (2020) 78 | EAM to radiation planning software | CARTO 3, EnSite, Rhythmia | EAMapReader (3D slicer) | Mesh/XML→DICOM | Compatible with multiple EAM systems |
| Mayinger et al. (2023) 79 | EAM to radiation planning software | CARTO 3, Rhythmia, EnSite | CARDIO‐RT | Mesh→DICOM | Compatible with multiple EAM systems |
| Santos‐Ortega et al. (2022) 80 | EAM to radiation planning software | EnSite | ADAS‐3D | N.R. | MRI fused with EAM and radiotherapy planning CT |
| Qian et al. (2022) 41 | Cardiac CT segmentation to radiation planning software | N/A | MUSIC (3D slicer) | N.R.→DICOM | 4D CT segmented into 3D volumes |
| Peichel et al. (2021) 81 | EAM to radiation planning software | CARTO 3 | In house tool (3D slicer) | Mesh→VAK→DICOM | |
| Lee et al. (2021) 45 | EAM to radiation planning software | Unspecified EAM software | Mimics innovation suite | Mesh→VAK→DICOM | |
| Van der Ree et al. (2022) 82 | Cardiac CT segmentation to radiation planning software | N/A | In house tool | N.R. | |
| Hohman et al. (2023) 83 | EAM to radiation planning software | CARTO 3, EnSite Velocity/Precision, Rhythmia HDx |
EAMapReader (3D slicer) Cardio‐RT |
Mesh/XML→DICOM | Good agreement between Cardio‐RT and ADAS‐3D |
| Wang et al. (2023) 76 | EAM to radiation planning software | CARTO 3, Rhythmia HDx, ECGi | HeaRTmap (3D slicer) | Mesh/XML→DICOM | ECGi compatible |
| Oh et al. (2023) 84 | EAM to radiation planning software | CARTO 3 | In house tool | Mesh→DICOM | |
| Rigal et al. (2023) 85 | EAM/CT‐PET to radiation planning software | CARTO 3 | In house tool | Mesh→VTK→DICOM | Integration of cardiac CT‐PET with EAM to delineate target |
Treatment planning is then performed following standard SBRT principles. Dose is highly conformal with prescribed dose covering an isodose allowing for steep gradients and a high maximum inside ITV. Both intra‐ and extracardiac organs‐at‐risk (OAR) dose tolerances adhere to known constraints. 86 Example beam arrangements and dose‐volume histograms for conventional linacs were reported by Knutson et al. 86 Technical details of reported radiotherapy treatments have been summarised by Lydiard et al. 87
Accounting for cardiorespiratory motion
Accounting for cardiac motion during the delivery of SBRT remains a challenge. 42 , 88 Reducing or tracking cardiac motion during SBRT will allow for decreased PTV and reduced collateral damage to structures at risk. Recent studies looking at cardiac motion during systole and diastole during 4D CT estimate average displacements of 2–3 mm with maximum displacements of 5–6 mm during breath holding conditions. 89 , 90 , 91 During free breathing treatment, the target motion envelope is typically dominated by respiratory rather than cardiac motion. 92 , 93 ITV expansions like those used in thoracic SBRT then encapsulate motion sufficiently. Organs at risk (OAR) such as stomach may require a planning risk volume (PRV) to ensure they are kept within dose tolerances during treatment delivery. If PRV cannot be planned to tolerance, additional motion management strategies may be required (breath hold, beam gating, etc.) or re‐simulation after fasting. 94
Abdominal compression is used commonly in thoracic SBRT to force shallow breathing, thus reducing tumour motion in thoracic and abdominal tumour sites. Mannerberg et al. assessed the effect of abdominal compression on cardiac movement in lung cancer patients undergoing SBRT. 95 Most patients had a large (≥3 mm) motion reduction in LV wall movement in the superior/inferior axis. However, a small number of patients had increased heart motion. Further to this, upward movement of the stomach towards the inferior wall may lead to an increased risk of gastric complications.
Feasibility studies on gel phantoms have demonstrated that it is technically feasible to safely deliver effective radiotherapy gated to cardiac cycle using ECG signals. 96 , 97 Image acquisition in CT, CMR and echocardiography is often gated to the ECG. 98 , 99 , 100 CT and CMR images are often acquired in mid diastole where cardiac motion is minimal to reduce the amount of motion artefact. 101 Integration of ECG‐gated CMR, echocardiography and CT images during the delivery of ECG‐gated SBRT may improve target definition and tracking accuracy, allowing for further reduction in ITV margins. 102 , 103 Validation of these technologies in larger clinical trials will be required.
Treatment delivery
Patients attend radiotherapy treatment as outpatients. They are positioned and immobilised on the linear accelerator (Linac) couch to replicate the simulation session. Alignment of the patient can be verified by fluoroscopic imaging and cone beam CT with fine adjustments made. Treatment is delivered in conscious patients who then undergo post‐therapy observations and monitoring prior to discharge (Fig. 2).
Conventional Linac‐based systems have the advantage of being readily accessible and allowing rapid delivery of therapy in 5–6 min. 104 , 105 For highly comorbid patients with concomitant heart failure who are unable to lie flat, patient tolerability is a crucial factor, especially since movement during SBRT procedures can affect the efficacy and safety of the treatment. In a recent head‐to‐head retrospective comparison, Linac‐based systems showed superiority in sparing distal critical structures compared to CyberKnife due to lower treatment times. 105 CyberKnife systems have limited availability, but have the advantage of tracking patient movement in real time utilising internal fiducials (such as an RV defibrillator lead) to make minute adjustments to the accelerometer that delivers radiotherapy. CyberKnife can access multiple oblique angles allowing greater flexibility in target volume conformations. Hence, CyberKnife has better dose coverage and dose homogeneity with steeper dose gradient which may give it the edge over conventional Linac‐based systems when targeting basal structures in close proximity to critical structures.
ICD considerations
Ionising radiation can oxidise insulators within semiconductors that are housed in contemporary ICDs. Newer devices are therefore more susceptible to the effects of radiation which results in a malfunction rate of 3%–7%. 106 , 107 , 108 Malfunctions mainly consist of resets of the device which can be avoided by minimising neutron producing radiation (≤10 mV). 106 , 107 , 108 High dose rates and cumulative dose can also cause transient or permanent damage and these should be kept within manufacturer and best practice guidelines. 109 High dose rate flattening filter free (FFF) beams are commonly used for cardiac SBRT, and care should be taken to avoid primary beam passing through the ICD and consider using <10MV beam energy as a conservative approach. 110 Patients presenting for SBRT should have device interrogation prior to undergoing radiotherapy to determine baseline settings of the device and lead parameters. They should be monitored on telemetry during the delivery of SBRT to monitor for any signs of device malfunction. They should undergo a repeat device interrogation following SBRT. One important consideration is that VA recurrence post‐SBRT is often at a slower cycle length. 33 , 35 , 39 , 42 , 111 Correspondingly, monitor and therapy zones on ICDs must be adjusted down to allow detection and appropriate treatment.
Follow‐up
Regular surveillance follow‐up with electrophysiologists and radiation oncologists is necessary post‐SBRT treatment (Fig. 2). From an electrophysiology perspective, patients require an ICD check to assess for arrhythmia response with appropriate adjustment of antiarrhythmics and therapy zones. Transthoracic echocardiography will be required to monitor LV function and for pericardial effusions. Up titration of medications and optimisation of heart failure is also paramount to this cohort of patients. From a radiation oncology perspective, monitoring for the development of pneumonitis both clinically and with CT scans where appropriate.
Areas for future research
Originally, SBRT was thought to mediate conduction block through radiation‐induced fibrosis; however, current clinical data question this hypothesis. Mechanistic understanding will be important in determining the optimal dose to achieve VA suppression and exactly what volume of scar needs to be targeted. Standardisation of treatment from patient selection, workup and radiation planning to delivery of radiotherapy will be necessary to further develop this novel treatment modality. Development of automated tools that allow integration of EAM, anatomic and functional imaging during radiation planning will play an important role in ensuring quality assurance through the generation of precise and reproducible TVs. Improved tracking of cardiac motion and ECG‐gated radiotherapy will minimise inaccuracies and ensure critical structures are incorporated into the PTV during radiation delivery. Biomarker verification and development may establish endpoints, other than arrhythmia recurrence, that are predictive of biological and electrophysiological response in scar and a long‐term therapeutic effect. Longer term follow‐up will be important to evaluate for toxicity and impact on cardiac function (Fig. 3).
Fig. 3.

Future directions for the development of SBRT balancing the risks of adverse effects and efficacy.
SBRT must therefore be tested in larger randomised clinical trials with longer follow‐up to better understand the risks and benefits associated with this novel approach. In patients presenting with electrical storm, management and resolution of the cascade of recurrent ICD shocks, sympathetic tone and worsening dyssynchrony that drives further VT may have partially accounted for the reduction in VT observed in these single arm studies, rather than the efficacy of SBRT. Randomisation in comparison with standard of care (CA) will be critical to adjust for this and other important confounders to give a true measure of SBRT efficacy. STAR VT (NCT04612140), Radiate VT (NCT05765175) and Radioablate VT (TBD), led by our centre, are three randomised controlled trials which will look to compare SBRT to CA outcomes in patients who have failed CA. CARA VT (NCT05047198) is another randomised controlled trial which will look to compare SBRT to CA outcomes in patients who have failed CA or who have VT requiring intervention with a PAINESD score ≥ 15. In the CARA VT trial, ECGi will be leveraged in the SBRT arm to allow completely non‐invasive electrical mapping of substrate and treatment of patients.
Conclusions
SBRT is a novel, attractive treatment modality for VA that can be achieved with a completely non‐invasive approach sparing patients from the risks of invasive procedures and general anaesthetic which are often poorly tolerated. The short duration of therapy improves the tolerability of the procedure especially in those with concomitant heart failure and frailty. Future mechanistic and clinical studies will be essential to improve long‐term efficacy and reduce the risk of adverse effects.
Conflict of interest
Verity Ann Ahern is an Editorial Board member of JMIRO and a co‐author of this article. To minimise bias, they were excluded from all editorial decision‐making related to the acceptance of this article for publication.
Acknowledgements
This project was fundedby a NHMRC Investigator Emerging Leadership 1 Grant (GNT2018376), NHMRC Synergy Grant (2031477), Heart Foundation Future Leader Fellowship Level 1 with Paul Korner Award (106780) and Westmead Charitable Trust Grant (Australia) to PCQ. SK has received research grants from NHMRC (Synergy Grant 2018592), Heart Foundation (Vanguard Grant 106934), Biosense Webster, Abbott Medical, Medtronic and Sanofi Aventis. EH has previously received honoraria from Novartis as well as honoraria and research funding from AstraZeneca, but these are not relevant to the present work. KD has received a NHMRC postgraduate scholarship. KH and PB have received Unabbreviated Western Sydney Local Health District (WSHLD) research and education grants. The other authors have no disclosures. XL, PB, KD, JB, TC, EH and PCQ were responsible for writing the paper. The paper was critically reviewed by TM, AH, JH, VA, HG and SK. Open access publishing facilitated by The University of Sydney, as part of the Wiley ‐ The University of Sydney agreement via the Council of Australian University Librarians.
X Liulu BSc(Adv), MBBS Hons; P Balaji BSc, MAnimSc, PhD; J Barber MSc, PhD; K De Silva Bmed, MD, BSc MED Hons; T Murray BMRSc; A Hickey BMRSc; T Campbell BSc, PhD; J Harris DCR (RT); H Gee MBBS, DPhil; V Ahern MBBS; S Kumar BSc(Med), MBBS, PhD; E Hau BSc (Med), MBBS, Grad Cert Biostat, PhD; PC Qian BSc (Med) Hons, MBBS, PhD.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- 1. Poole JE, Johnson GW, Hellkamp AS et al. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med 2008; 359: 1009–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Viles‐Gonzalez JF, Arora S, Deshmukh A et al. Outcomes of patients admitted with ventricular arrhythmias and sudden cardiac death in the United States. Heart Rhythm 2019; 16: 358–366. [DOI] [PubMed] [Google Scholar]
- 3. Wei C, Boeck M, Qian PC et al. Cost of cardiac stereotactic body radioablation therapy versus catheter ablation for treatment of ventricular tachycardia. Pacing Clin Electrophysiol 2022; 45: 1124–1131. [DOI] [PubMed] [Google Scholar]
- 4. Robinson CG, Samson PP, Moore KMS et al. Phase I/II trial of electrophysiology‐guided noninvasive cardiac radioablation for ventricular tachycardia. Circulation 2019; 139: 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Antzelevitch C, Burashnikov A. Overview of basic mechanisms of cardiac arrhythmia. Card Electrophysiol Clin 2011; 3: 23–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lopez EM, Malhotra R. Ventricular tachycardia in structural heart disease. J Innov Card Rhythm Manag 2019; 10: 3762–3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sapp JL, Wells GA, Parkash R et al. Ventricular tachycardia ablation versus escalation of antiarrhythmic drugs. N Engl J Med 2016; 375: 111–121. [DOI] [PubMed] [Google Scholar]
- 8. Kuck KH, Schaumann A, Eckardt L et al. Catheter ablation of stable ventricular tachycardia before defibrillator implantation in patients with coronary heart disease (VTACH): a multicentre randomised controlled trial. Lancet 2010; 375: 31–40. [DOI] [PubMed] [Google Scholar]
- 9. Tung R. Evolution of ventricular tachycardia ablation in structural heart disease. J Arrhythmia 2014; 30: 250–261. [Google Scholar]
- 10. Ding WY, Pearman CM, Bonnett L et al. Complication rates following ventricular tachycardia ablation in ischaemic and non‐ischaemic cardiomyopathies: a systematic review. J Interv Card Electrophysiol 2022; 63: 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barkagan M, Leshem E, Shapira‐Daniels A et al. Histopathological characterization of radiofrequency ablation in ventricular scar tissue. JACC Clin Electrophysiol 2019; 5: 920–931. [DOI] [PubMed] [Google Scholar]
- 12. Aras D, Ozturk HF, Ozdemir E et al. Use of stereotactic radioablation therapy as a bailout therapy for refractory ventricular tachycardia in a patient with a no‐entry left ventricle. J Innov Card Rhythm Manag 2021; 12: 4671–4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stevenson WG, Tedrow UB, Reddy V et al. Infusion needle radiofrequency ablation for treatment of refractory ventricular arrhythmias. J Am Coll Cardiol 2019; 73: 1413–1425. [DOI] [PubMed] [Google Scholar]
- 14. Romero J, Cerrud‐Rodriguez Roberto C, Di Biase L et al. Combined endocardial‐epicardial versus endocardial catheter ablation alone for ventricular tachycardia in structural heart disease. JACC Clin Electrophysiol 2019; 5: 13–24. [DOI] [PubMed] [Google Scholar]
- 15. Koruth JS, Dukkipati S, Miller MA, Neuzil P, d'Avila A, Reddy VY. Bipolar irrigated radiofrequency ablation: a therapeutic option for refractory intramural atrial and ventricular tachycardia circuits. Heart Rhythm 2012; 9: 1932–1941. [DOI] [PubMed] [Google Scholar]
- 16. Assis Fabrizio R, Sharma A, Shah R et al. Long‐term outcomes of bilateral cardiac sympathetic denervation for refractory ventricular tachycardia. JACC Clin Electrophysiol 2021; 7: 463–470. [DOI] [PubMed] [Google Scholar]
- 17. Fudim M, Qadri YJ, Waldron NH et al. Stellate ganglion blockade for the treatment of refractory ventricular arrhythmias. JACC Clin Electrophysiol 2020; 6: 562–571. [DOI] [PubMed] [Google Scholar]
- 18. Younis A, Zilberman I, Krywanczyk A et al. Effect of pulsed‐field and radiofrequency ablation on heterogeneous ventricular scar in a swine model of healed myocardial infarction. Circ Arrhythm Electrophysiol 2022; 15: e011209. [DOI] [PubMed] [Google Scholar]
- 19. Martin CA, Zaw MT, Jackson N, Morris D, Costanzo P. First worldwide use of pulsed‐field ablation for ventricular tachycardia ablation via a retrograde approach. J Cardiovasc Electrophysiol 2023; 34: 1772–1775. [DOI] [PubMed] [Google Scholar]
- 20. Suh JH. Stereotactic radiosurgery for the management of brain metastases. N Engl J Med 2010; 362: 1119–1127. [DOI] [PubMed] [Google Scholar]
- 21. Sharma A, Wong D, Weidlich G et al. Noninvasive stereotactic radiosurgery (CyberHeart) for creation of ablation lesions in the atrium. Heart Rhythm 2010; 7: 802–810. [DOI] [PubMed] [Google Scholar]
- 22. Lehmann HI, Graeff C, Simoniello P et al. Feasibility study on cardiac arrhythmia ablation using high‐energy heavy ion beams. Sci Rep 2016; 6: 38895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zei PC, Wong D, Gardner E, Fogarty T, Maguire P. Safety and efficacy of stereotactic radioablation targeting pulmonary vein tissues in an experimental model. Heart Rhythm 2018; 15: 1420–1427. [DOI] [PubMed] [Google Scholar]
- 24. Loo BW Jr, Soltys SG, Wang L et al. Stereotactic ablative radiotherapy for the treatment of refractory cardiac ventricular arrhythmia. Circ Arrhythm Electrophysiol 2015; 8: 748–750. [DOI] [PubMed] [Google Scholar]
- 25. Cuculich PS, Schill MR, Kashani R et al. Noninvasive cardiac radiation for ablation of ventricular tachycardia. N Engl J Med 2017; 377: 2325–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Robinson C, Samson P, Moore K et al. Longer term results from a phase I/II study of EP‐guided noninvasive cardiac radioablation for treatment of ventricular tachycardia (ENCORE‐VT). Int J Radiat Oncol Biol Phys 2019; 105: 682. [Google Scholar]
- 27. Cuculich P, Moore K, Cooper DH et al. LB‐456090‐3 long‐term results from a prospective phase 1/2 trial of electrophysiology‐guided noninvasive cardiac radioablation for ventricular tachycardia. Heart Rhythm 2023; 20: 1083–1084. [Google Scholar]
- 28. Van Der Ree MH, Herrera Siklody C, Le Bloa M et al. First‐in‐human combined low‐dose whole heart irradiation and highdose stereotactic arrhythmia Radioablation for immunosuppressiverefractory cardiac sarcoidosis and ventricular tachycardia. Front Cardiovasc Med 2023;10: 1213165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Park J‐S, Choi Y. Stereotactic cardiac radiation to control ventricular tachycardia and fibrillation storm in a patient with apical hypertrophic cardiomyopathy at burnout stage: case report. J Korean Med Sci 2020; 35: e200–e206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ho L‐T, Chen JL‐Y, Chan H‐M et al. First Asian population study of stereotactic body radiation therapy for ventricular arrhythmias. Sci Rep 2021; 11: 10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Haskova J, Peichl P, Pirk J, Cvek J, Neuwirth R, Kautzner J. Stereotactic radiosurgery as a treatment for recurrent ventricular tachycardia associated with cardiac fibroma. HeartRhythm Case Rep. 2019; 5: 44–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zeng LJ, Huang LH, Tan H et al. Stereotactic body radiation therapy for refractory ventricular tachycardia secondary to cardiac lipoma: a case report. Pacing Clin Electrophysiol 2019; 42: 1276–1279. [DOI] [PubMed] [Google Scholar]
- 33. Carbucicchio C, Andreini D, Piperno G et al. Stereotactic radioablation for the treatment of ventricular tachycardia: preliminary data and insights from the STRA‐MI‐VT phase Ib/II study. J Interv Card Electrophysiol 2021; 62: 427–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Scholz EP, Seidensaal K, Naumann P, André F, Katus HA, Debus J. Risen from the dead: cardiac stereotactic ablative radiotherapy as last rescue in a patient with refractory ventricular fibrillation storm. HeartRhythm Case Rep 2019; 5: 329–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ninni S, Gallot‐Lavallée T, Klein C et al. Stereotactic radioablation for ventricular tachycardia in the setting of electrical storm. Circ Arrhythm Electrophysiol 2022; 15: e010955. [DOI] [PubMed] [Google Scholar]
- 36. Qian PC, Azpiri JR, Assad J et al. Noninvasive stereotactic radioablation for the treatment of atrial fibrillation: first‐in‐man experience. J Arrhythm 2020; 36: 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shoji M, Inaba K, Itami J et al. Advantages and challenges for noninvasive atrial fibrillation ablation. J Interv Card Electrophysiol 2021; 62: 319–327. [DOI] [PubMed] [Google Scholar]
- 38. Neuwirth R, Cvek J, Knybel L et al. Stereotactic radiosurgery for ablation of ventricular tachycardia. Europace 2019; 21: 1088–1095. [DOI] [PubMed] [Google Scholar]
- 39. van der Ree MH, Dieleman EMT, Visser J et al. Non‐invasive stereotactic arrhythmia radiotherapy for ventricular tachycardia: results of the prospective STARNL‐1 trial. Europace 2023; 25: 1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yugo D, Lo LW, Wu YH et al. Case series on stereotactic body radiation therapy in non‐ischemic cardiomyopathy patients with recurrent ventricular tachycardia. Pacing Clin Electrophysiol 2021; 44: 1085–1093. [DOI] [PubMed] [Google Scholar]
- 41. Qian PC, Quadros K, Aguilar M et al. Substrate modification using stereotactic radioablation to treat refractory ventricular tachycardia in patients with ischemic cardiomyopathy. JACC Clin Electrophysiol 2022; 8: 49–58. [DOI] [PubMed] [Google Scholar]
- 42. Grehn M, Mandija S, Miszczyk M et al. STereotactic Arrhythmia Radioablation (STAR): the Standardized Treatment and Outcome Platform for Stereotactic Therapy Of Re‐entrant tachycardia by a Multidisciplinary consortium (STOPSTORM. eu) and review of current patterns of STAR practice in Europe. Europace 2023; 25: 1284–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. van der Ree MH, Cuculich PS, van Herk M et al. Interobserver variability in target definition for stereotactic arrhythmia radioablation. Front Cardiovasc Med 2023; 10: 1267800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gianni C, Rivera D, Burkhardt JD et al. Stereotactic arrhythmia radioablation for refractory scar‐related ventricular tachycardia. Heart Rhythm 2020; 17: 1241–1248. [DOI] [PubMed] [Google Scholar]
- 45. Lee J, Bates M, Shepherd E et al. Cardiac stereotactic ablative radiotherapy for control of refractory ventricular tachycardia: initial UK multicentre experience. Open Heart 2021; 8: e001770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kilby W, Dooley JR, Kuduvalli G, Sayeh S, Maurer CR. The CyberKnife® robotic radiosurgery system in 2010. Technol Cancer Res Treat 2010; 9: 433–452. [DOI] [PubMed] [Google Scholar]
- 47. Wang Z, Kong QT, Li J et al. Clinical outcomes of cyberknife stereotactic radiosurgery for lung metastases. J Thorac Dis 2015; 7: 407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lloyd MS, Wight J, Schneider F et al. Clinical experience of stereotactic body radiation for refractory ventricular tachycardia in advanced heart failure patients. Heart Rhythm 2020; 17: 415–422. [DOI] [PubMed] [Google Scholar]
- 49. Aras D, Çetin E, Ozturk HF et al. Stereotactic body radioablation therapy as an immediate and early term antiarrhythmic palliative therapeutic choice in patients with refractory ventricular tachycardia. J Interv Card Electrophysiol 2023; 66: 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Qian PC, Quadros K, Aguilar M, Mak R, Zei P, Tedrow UB. Recurrent ventricular tachycardia arising at the treatment borderzone after stereotactic radioablation in a patient with ischemic cardiomyopathy. Europace 2020; 22: 1053. [DOI] [PubMed] [Google Scholar]
- 51. Lehmann HI, Richter D, Prokesch H et al. Atrioventricular node ablation in Langendorff‐perfused porcine hearts using carbon ion particle therapy: methods and an in vivo feasibility investigation for catheter‐free ablation of cardiac arrhythmias. Circ Arrhythm Electrophysiol 2015; 8: 429–438. [DOI] [PubMed] [Google Scholar]
- 52. Amino M, Yoshioka K, Fujibayashi D et al. Year‐long upregulation of connexin43 in rabbit hearts by heavy ion irradiation. Am J Physiol Heart Circ Physiol 2010; 298: H1014–H1021. [DOI] [PubMed] [Google Scholar]
- 53. Amino M, Yoshioka K, Furusawa Y et al. Inducibility of ventricular arrhythmia 1 year following treatment with heavy ion irradiation in dogs with myocardial infarction. Pacing Clin Electrophysiol 2017; 40: 379–390. [DOI] [PubMed] [Google Scholar]
- 54. Amino M, Yoshioka K, Tanabe T et al. Heavy ion radiation up‐regulates Cx43 and ameliorates arrhythmogenic substrates in hearts after myocardial infarction. Cardiovasc Res 2006; 72: 412–421. [DOI] [PubMed] [Google Scholar]
- 55. Zhang DM, Navara R, Yin T et al. Cardiac radiotherapy induces electrical conduction reprogramming in the absence of transmural fibrosis. Nat Commun 2021; 12: 5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Smer A, Saurav A, Azzouz MS et al. Meta‐analysis of risk of ventricular arrhythmias after improvement in left ventricular ejection fraction during follow‐up in patients with primary prevention implantable cardioverter defibrillators. Am J Cardiol 2017; 120: 279–286. [DOI] [PubMed] [Google Scholar]
- 57. Pedersen LN, Valenzuela Ripoll C, Ozcan M et al. Cardiac radiation improves ventricular function in mice and humans with cardiomyopathy. Med 2023; 4: 928–943.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lewis GD, Farach A. Cardiovascular toxicities of radiation therapy. Methodist Debakey Cardiovasc J 2019; 15: 274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wight J, Bigham T, Schwartz A et al. Long term follow‐up of stereotactic body radiation therapy for refractory ventricular tachycardia in advanced heart failure patients. Front Cardiovasc Med 2022; 9: 849113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cvek J, Peichel P, Knybel L et al. Long‐term toxicity of radiosurgery for ablation of ventricular tachycardia. Eur Heart J 2022; 43 (Suppl 2): 43. [Google Scholar]
- 61. Haskova J, Jedlickova K, Cvek J, Knybel L, Neuwirth R, Kautzner J. Oesophagopericardial fistula as a late complication of stereotactic radiotherapy for recurrent ventricular tachycardia. EP Europace. 2022; 24: 969. [DOI] [PubMed] [Google Scholar]
- 62. Rosa G, Quintanilla JG, Salgado R et al. Mapping technologies for catheter ablation of atrial fibrillation beyond pulmonary vein isolation. Eur Cardiol 2021; 16: e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wissner E, Stevenson WG, Kuck KH. Catheter ablation of ventricular tachycardia in ischaemic and non‐ischaemic cardiomyopathy: where are we today? A clinical review. Eur Heart J 2012; 33: 1440–1450. [DOI] [PubMed] [Google Scholar]
- 64. Graham AJ, Schilling RJ. The use of electrocardiographic imaging in localising the origin of arrhythmias during catheter ablation of ventricular tachycardia. Arrhythm Electrophysiol Rev 2021; 10: 211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. von Knobelsdorff‐Brenkenhoff F, Schulz‐Menger J. Cardiovascular magnetic resonance in the guidelines of the European Society of Cardiology: a comprehensive summary and update. J Cardiovasc Magn Reson 2023; 25: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sado DM, Hasleton JM, Herrey AS, Moon JC. CMR in heart failure. Cardiol Res Pract 2011; 2011: 739157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Andreu D, Berruezo A, Ortiz‐Pérez JT et al. Integration of 3D electroanatomic maps and magnetic resonance scar characterization into the navigation system to guide ventricular tachycardia ablation. Circ Arrhythm Electrophysiol 2011; 4: 674–683. [DOI] [PubMed] [Google Scholar]
- 68. Soto‐Iglesias D, Penela D, Jáuregui B et al. Cardiac magnetic resonance‐guided ventricular tachycardia substrate ablation. JACC Clin Electrophysiol 2020; 6: 436–447. [DOI] [PubMed] [Google Scholar]
- 69. Mahida S, Sacher F, Dubois R et al. Cardiac imaging in patients with ventricular tachycardia. Circulation 2017; 136: 2491–2507. [DOI] [PubMed] [Google Scholar]
- 70. Esposito A, Palmisano A, Antunes S et al. Cardiac CT with delayed enhancement in the characterization of ventricular tachycardia structural substrate: relationship between CT‐segmented scar and electro‐anatomic mapping. JACC Cardiovasc Imaging 2016; 9: 822–832. [DOI] [PubMed] [Google Scholar]
- 71. Sasaki T, Calkins H, Miller CF et al. New insight into scar‐related ventricular tachycardia circuits in ischemic cardiomyopathy: fat deposition after myocardial infarction on computed tomography – a pilot study. Heart Rhythm 2015; 12: 1508–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Alyesh DM, Siontis KC, Dabbagh GS et al. Postinfarction myocardial calcifications on cardiac computed tomography. Circ Arrhythm Electrophysiol 2019; 12: e007023. [DOI] [PubMed] [Google Scholar]
- 73. Ghzally Y, Imanli H, Smith M et al. Metabolic Scar Assessment with(18)F‐FDG PET: correlation to ischemic ventricular tachycardia substrate and successful ablation sites. J Nucl Med 2021; 62: 1591–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hugo GD, Rosu M. Advances in 4D radiation therapy for managing respiration: part I – 4D imaging. Z Med Phys 2012; 22: 258–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Krug D, Blanck O, Andratschke N et al. Recommendations regarding cardiac stereotactic body radiotherapy for treatment refractory ventricular tachycardia. Heart Rhythm 2021; 18: 2137–2145. [DOI] [PubMed] [Google Scholar]
- 76. Wang H, Barbhaiya CR, Yuan Y et al. A tool to integrate electrophysiological mapping for cardiac radioablation of ventricular tachycardia. Adv Radiat Oncol 2023; 8: 101272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Brett CL, Cook JA, Aboud AA, Karim R, Shinohara ET, Stevenson WG. Novel workflow for conversion of catheter‐based Electroanatomic mapping to DICOM imaging for noninvasive radioablation of ventricular tachycardia. Pract Radiat Oncol 2021; 11: 84–88. [DOI] [PubMed] [Google Scholar]
- 78. Hohmann S, Henkenberens C, Zormpas C et al. A novel open‐source software‐based high‐precision workflow for target definition in cardiac radioablation. J Cardiovasc Electrophysiol 2020; 31: 2689–2695. [DOI] [PubMed] [Google Scholar]
- 79. Mayinger M, Boda‐Heggemann J, Mehrhof F et al. Quality assurance process within the RAdiosurgery for VENtricular TAchycardia (RAVENTA) trial for the fusion of electroanatomical mapping and radiotherapy planning imaging data in cardiac radioablation. Phys Imaging Radiat Oncol 2023; 25: 100406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Santos‐Ortega A, Rivas‐Gándara N, Pascual‐González G, Seoane A, Granado R, Reyes V. Multimodality imaging fusion to guide stereotactic radioablation for refractory complex ventricular tachycardia. HeartRhythm Case Rep 2022; 8: 836–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Peichl P, Sramko M, Cvek J, Kautzner J. A case report of successful elimination of recurrent ventricular tachycardia by repeated stereotactic radiotherapy: the importance of accurate target volume delineation. Eur Heart J Case Rep 2021; 5: ytaa516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. van der Ree MH, Visser J, Planken RN et al. Standardizing the cardiac radioablation targeting workflow: enabling semi‐automated angulation and segmentation of the heart according to the American Heart Association segmented model. Adv Radiat Oncol 2022; 7: 100928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hohmann S, Xie J, Grehn M et al. Reproducible target transfer from electroanatomic mapping to radiotherapy planning systems for cardiac radioablation – cross‐validation for the RAVENTA trial. Europace 2023; 25 (Supp 1): euad122.303. [Google Scholar]
- 84. Oh S, Liu EH, Trombetta MG et al. A target definition based on electroanatomic maps for stereotactic arrhythmia radioablation. Phys Med 2023; 115: 103160. [DOI] [PubMed] [Google Scholar]
- 85. Rigal L, Simon A, Benali K et al. A novel data integration workflow for target delineation in cardiac radioablation. Arch Cardiovasc Dis Suppl 2023; 15: 106. [Google Scholar]
- 86. Knutson NC, Samson PP, Hugo GD et al. Radiation therapy workflow and dosimetric analysis from a phase 1/2 trial of noninvasive cardiac radioablation for ventricular tachycardia. Int J Radiat Oncol Biol Phys 2019; 104: 1114–1123. [DOI] [PubMed] [Google Scholar]
- 87. Suzanne Lydiard P, Blanck O, Hugo G, O'Brien R, Keall P. A review of cardiac radioablation (CR) for arrhythmias: procedures, technology, and future opportunities. Int J Radiat Oncol Biol Phys 2021; 109: 783–800. [DOI] [PubMed] [Google Scholar]
- 88. Prusator MT, Samson P, Cammin J et al. Evaluation of motion compensation methods for noninvasive cardiac radioablation of ventricular tachycardia. Int J Radiat Oncol Biol Phys 2021; 111: 1023–1032. [DOI] [PubMed] [Google Scholar]
- 89. Wang X, Pan T, Pinnix C et al. Cardiac motion during deep‐inspiration breath‐hold: implications for breast cancer radiotherapy. Int J Radiat Oncol Biol Phys 2012; 82: 708–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ouyang Z, Schoenhagen P, Wazni O et al. Analysis of cardiac motion without respiratory motion for cardiac stereotactic body radiation therapy. J Appl Clin Med Phys 2020; 21: 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Harms J, Schreibmann E, Mccall NS, Lloyd MS, Higgins KA, Castillo R. Cardiac motion and its dosimetric impact during radioablation for refractory ventricular tachycardia. J Appl Clin Med Phys 2023; 24: e13925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bellec J, Rigal L, Hervouin A et al. Cardiac radioablation for ventricular tachycardia: which approach for incorporating cardiorespiratory motions into the planning target volume? Phys Med 2022; 95: 16–24. [DOI] [PubMed] [Google Scholar]
- 93. Prunaretty J, Boisselier P, Aillères N et al. Tracking, gating, free‐breathing, which technique to use for lung stereotactic treatments? A dosimetric comparison. Rep Pract Oncol Radiother 2019; 24: 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Hugo GD, Campbell J, Zhang T, Yan D. Cumulative lung dose for several motion management strategies as a function of pretreatment patient parameters. Int J Radiat Oncol Biol Phys 2009; 74: 593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Mannerberg A, Nilsson MP, Edvardsson A, Karlsson K, Ceberg S. Abdominal compression as motion management for stereotactic radiotherapy of ventricular tachycardia. Phys Imaging Radiat Oncol 2023; 28: 100499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Reis CQ, Robar JL. Evaluation of the feasibility of cardiac gating for SBRT of ventricular tachycardia based on real‐time ECG signal acquisition. J Appl Clin Med Phys 2023; 24: e13814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Poon J, Kohli K, Deyell MW et al. Technical Note: cardiac synchronized volumetric modulated arc therapy for stereotactic arrhythmia radioablation – proof of principle. Med Phys 2020; 47: 3567–3572. [DOI] [PubMed] [Google Scholar]
- 98. Kramer CM, Barkhausen J, Bucciarelli‐Ducci C, Flamm SD, Kim RJ, Nagel E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J Cardiovasc Magn Reson 2020; 22: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Desjardins B, Kazerooni EA. ECG‐gated cardiac CT. Am J Roentgenol 2004; 182: 993–1010. [DOI] [PubMed] [Google Scholar]
- 100. Mitchell C, Rahko PS, Blauwet LA et al. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr 2019; 32: 1–64. [DOI] [PubMed] [Google Scholar]
- 101. Sun Z. Coronary CT angiography with prospective ECG‐triggering: an effective alternative to invasive coronary angiography. Cardiovasc Diagn Ther 2012; 2: 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Perrin R, Maguire P, Garonna A et al. Case report: treatment planning study to demonstrate feasibility of transthoracic ultrasound guidance to facilitate ventricular tachycardia ablation with protons. Front Cardiovasc Med 2022; 9: 849247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kovacs B, Mayinger M, Tanadini‐Lang S et al. First two MRI guided stereotactic body radiation therapy of recurrent sustained ventricular tachycardia. Eur Heart J 2020; 41 (Supplement_2): ehaa946.0758. [Google Scholar]
- 104. Bonaparte I, Gregucci F, Surgo A et al. Linac‐based STereotactic arrhythmia Radioablation (STAR) for ventricular tachycardia: a treatment planning study. Jpn J Radiol 2021; 39: 1223–1228. [DOI] [PubMed] [Google Scholar]
- 105. Weidlich GA, Hacker F, Bellezza D, Maguire P, Gardner EA. Ventricular tachycardia: a treatment comparison study of the CyberKnife with conventional linear accelerators. Cureus 2018; 10: e3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Azraai M, D'Souza D, Lin Y‐H, Nadurata V. Current clinical practice in patients with cardiac implantable electronic devices undergoing radiotherapy: a literature review. Europace 2022; 24: 362–374. [DOI] [PubMed] [Google Scholar]
- 107. Xu B, Wang Y, Tse G et al. Radiotherapy‐induced malfunctions of cardiac implantable electronic devices: a meta‐analysis. Heart Rhythm 2023; 20: 689–698. [DOI] [PubMed] [Google Scholar]
- 108. Yeung C, Chacko S, Glover B et al. Radiotherapy for patients with cardiovascular implantable electronic devices: a review. Can J Cardiol 2018; 34: 244–251. [DOI] [PubMed] [Google Scholar]
- 109. Miften M, Mihailidis D, Kry SF et al. Management of radiotherapy patients with implanted cardiac pacemakers and defibrillators: a report of the AAPM TG‐203. Med Phys 2019; 46: e757–e788. [DOI] [PubMed] [Google Scholar]
- 110. Aslian H, Kron T, Watts T et al. The effect of stereotactic body radiotherapy (SBRT) using flattening filter‐free beams on cardiac implantable electronic devices (CIEDs) in clinical situations. J Appl Clin Med Phys 2020; 21: 121–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Miszczyk M, Jadczyk T, Gołba K et al. Clinical evidence behind stereotactic radiotherapy for the treatment of ventricular tachycardia (STAR)—a comprehensive review. J Clin Med 2021; 10: 1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Cvek J, Neuwirth R, Knybel L et al. Cardiac radiosurgery for malignant ventricular tachycardia. Cureus 2014; 6:e190–e195. [Google Scholar]
- 113. Jumeau R, Ozsahin M, Schwitter J et al. Rescue procedure for an electrical storm using robotic non‐invasive cardiac radio‐ablation. Radiother Oncol 2018; 128: 189–191. [DOI] [PubMed] [Google Scholar]
- 114. Krug D, Blanck O, Demming T et al. Stereotactic body radiotherapy for ventricular tachycardia (cardiac radiosurgery): first‐in‐patient treatment in Germany. Strahlenther Onkol 2020; 196: 23–30. [DOI] [PubMed] [Google Scholar]
- 115. Mayinger M, Kovacs B, Tanadini‐Lang S et al. First magnetic resonance imaging‐guided cardiac radioablation of sustained ventricular tachycardia. Radiother Oncol 2020; 152: 203–207. [DOI] [PubMed] [Google Scholar]
- 116. Chin R, Hayase J, Hu P et al. Non‐invasive stereotactic body radiation therapy for refractory ventricular arrhythmias: an institutional experience. J Interv Card Electrophysiol 2021; 61: 535–543. [DOI] [PubMed] [Google Scholar]
- 117. Dusi V, Vitolo V, Frigerio L et al. First‐in‐man case of non‐invasive proton radiotherapy for the treatment of refractory ventricular tachycardia in advanced heart failure. Eur J Heart Fail 2021; 23: 195–196. [DOI] [PubMed] [Google Scholar]
- 118. Lee Y, Yoon HI, Kim J‐S, Kim A‐Y, Tsevendee S, Uhm J‐S. Incessant ventricular tachycardia treated with cardiac radioablation in an 11‐year‐old boy with dilated cardiomyopathy. HeartRhythm Case Rep 2021; 7: 186–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Donnelly J, Higgins KA, Souza J, Lloyd M. Accessing the inaccessible: stereotactic radioablation of premature ventricular complexes originating in the right ventricle in a patient with a mechanical tricuspid valve. HeartRhythm Case Rep 2021; 7: 229–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Gerard IJ, Bernier M, Hijal T et al. Stereotactic arrhythmia radioablation for ventricular tachycardia: single center first experiences. Adv Radiat Oncol 2021; 6: 100702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Haskova J, Peichl P, Šramko M et al. Case report: repeated stereotactic radiotherapy of recurrent ventricular tachycardia: reasons, feasibility, and safety. Front Cardiovasc Med 2022; 9: 845382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Miszczyk M, Sajdok M, Nozynski J et al. Histopathological examination of an explanted heart in a long‐term responder to cardiac stereotactic body radiotherapy (STereotactic Arrhythmia Radioablation). Front Cardiovasc Med 2022; 9: 919823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Bernstein HM, Leon W, Daly ME et al. Noninvasive stereotactic radiation for refractory ventricular tachycardia after failure of cardiac sympathetic denervation. Case Rep 2022; 4: 1189–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Cozzi S, Bottoni N, Botti A et al. The use of cardiac stereotactic radiation therapy (SBRT) to manage ventricular tachycardia: a case report, review of the literature and technical notes. Pers Med 2022; 12: 1783–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Huang S‐H, Wu Y‐W, Shueng P‐W et al. Case report: stereotactic body radiation therapy with 12 Gy for silencing refractory ventricular tachycardia. Front Cardiovasc Med 2022; 9: 973105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Vaskovskii VA, Taimasova IA, Artyukhina EA et al. Long‐term results of the first clinical application of stereotactic radioablation using a linear electron accelerator for the treatment of ventricular tachycardia. Bull Exp Biol Med 2023; 174: 594–600. [DOI] [PubMed] [Google Scholar]
- 127. Scanavacca MI, Pisani CF, Salvajoli B et al. Stereotactic body radiation therapy for recurrent ventricular tachycardia in Chagas disease: first case in Latin America. Arq Bras Cardiol 2023; 120: e20220614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Menon SD, Whitlock R, Valettas N, Healey JS. Unconventional warfare: successful ablation of ventricular tachycardia by direct ventricular puncture in patients with double mechanical heart valves. HeartRhythm Case Rep 2023; 120: e20220614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Qian PC, Quadros K, Aguilar M et al. Substrate modification using stereotactic radioablation to treat refractory VA in patients with ischaemic cardiomyopathy. Arq Bras Cardiol 2022; 8: 49–58. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


