Abstract
Lumbar spinal stenosis (LSS) occurs when bony, ligamentous, and synovial elements of the lower axial spine degenerate and overgrow, compressing neural and vascular elements in the spinal canal. Compression can cause static back pain, radicular lower extremity pain, or neurogenic claudication. Radiological and clinical findings are needed to diagnose lumbar stenosis. In this framework, caudal epidural steroid injection (ESI) is a standard treatment. The volume injected and needle positioning are the main issues that could compromise the effectiveness of the epidural injection. However, ultrasound-guided caudal epidural injections have become more common in recent years. Since Klocke and colleagues described the ultra-sound-guided caudal block in 2003, it has grown in popularity. Multiple ethnic studies have reported ultrasound-guided caudal injection success rates of 96.9–100%. Color Doppler ultrasonography can also determine if a drug solution reaches the lumbosacral region. We enrolled 42 patients with lumbar spinal stenosis, persistent lumbosciatalgia, and neurogenic claudicatio unresponsive to painkillers who were not surgical candidates. Each patient receives four weekly injections for four weeks. If the patient responds to treatment but still has pain, monthly injections are needed to reach and maintain the benefit. Treatment will be changed if the patient does not respond after 4 caudal injections. Sterile caudal epidural injections are performed with ultrasound guidance and a spinal needle 21G. Triamcinolone 40 mg, levobupivacaine 10 mg, and physiological solution 10 ml are delivered. Each patient received an average of 4 injective treatments (4±2, Means 4, SD 2). Data analysis shows that the treatment reduced pain significantly before and after therapy, which persisted after 3 months. Caudal epidural injection is one of the most common conservative treatments for chronic low back pain with radiculopathy, and lidocaine alone or with steroids is effective. In this framework, the two main literature issues about caudal epidural injection effectiveness on lumbar pain are correct. Therefore, 10 ml is suitable and effective for treating symptoms without side effects. Pain reduction of over 50% from start to finish and three-month follow-up have shown significant results in pain control and disability improvement. Finally, caudal epidural injection for lumbar spinal stenosis symptoms is effective, safe, and provides long-term pain relief.
Key Words: caudal epidural injection, lumbar spinal stenosis, ultrasound guidance
Lumbar Spinal Stenosis (LSS) is a pathological process where bony, ligamentous, and synovial elements of the lower axial spine degenerate and overgrow, progressively compressing the neural and vascular elements in the spinal canal.1 This degenerative process consists of one of the most diagnosed spinal disorders in older adults and may result in the impingement of the nerve roots of the cauda equina. Although the pathophysiology of the clinical syndrome is not well understood, a narrow central canal or intervertebral foramen is an essential defining feature. In the case of degenerative lumbar spinal stenosis, the most common form of lumbar stenosis, disc degeneration, thickening and buckling of the ligamentum flavum and facet hypertrophy contribute to canal narrowing.
Congenital factors may predispose some individuals to this condition: disc degeneration has a substantial genetic component, but less is known about the degree to which genes influence other contributors to degenerative stenosis or associated central spinal canal dimensions.
Findings on imaging are scarcely correlated with symptoms and disability. There may be neurovascular or inflammatory factors or other mediators that cause symptoms to manifest in association with a narrow canal, which may have different genetic and environmental influences.2
The compression can be either asymptomatic if mild or it can result in a variable combination of static back pain, radicular lower extremity pain, or neurogenic claudication. The diagnosis of lumbar stenosis can be difficult and involves a combination of radiological and clinical findings.
Treatment ranges from conservative measures with physical therapy and core strengthening, to steroid injections in the facet joints or in the epidural space, to a more radical solution with surgical decompression.1
In this framework, caudal epidural steroid injection (ESI) is an established treatment for spine-related problems.3,4 Caudal access to epidural space is considered a rapid and advantageous technique:3 i) caudal entry into the epidural space is relatively easy and there is very little risk of dural puncture; ii) it is not expensive; iii) it can be used in patients suffering from coagulopathies.
The main problems that could impair the effectiveness of the treatment with epidural injection are the volume injected and the correct positioning of the needle.5
Caudal epidural injection could be performed using anatomical landmarks, but the risk of failure is high.6 Therefore, a guide tool is necessary to achieve the aim.7,8
The percentage of unsuccess in the absence of fluoroscopic guidance was reported in 9% to 38% of cases. This has caused many authors to recommend the use of fluoroscopy to perform epidural injections and it is considered the technique of first choice for accurate needle placement,9 even though this does not assure either targeted delivery or accurate placement of the drug.10
Nevertheless, in the last years is even more frequent the use of ultrasound-guided technique to execute the caudal epidural injection. The ultra-sound-guided block was first described by Klocke and colleagues in 2003, and has, since then, gained increasing popularity. Several studies from various ethnic populations have repeatedly reported very high successful rates (96.9– 100%) of ultrasound-guided caudal injection.2
Yoon and colleagues11 used color Doppler ultrasonography guidance to position the needle during ESI and to visualize any vascular intake of the medication. The needle position was then verified using an injection of a contrast dye and fluoroscopy. Under ultrasound guidance, the correct placement of the needle was confirmed by fluoroscopy in 50 of the 52 successfully injected patients.
The accuracy of steroid injection into the caudal space using ultrasound guidance was comparable with that obtained using fluoroscopic guidance. Moreover, Color Doppler ultrasonography guidance allows to determine whether a drug solution reaches the lumbosacral region.12
Another debated issue is the entity of the volume injected: volumetric caudal injections have been examined by epidurography by Kim et al. who found no advantage in terms of cephalic migration of injectate, despite using incremental volumes up to 50 ml,13 but there is evidence that an injected volume of 20 ml spread to a level varying from L5 and T9, with a median value of L3.14
Aim of the study
The study aims to assess the effectiveness of caudal epidural injection with corticosteroid and local anesthetic in a standard volume using ultrasound guidance for the injection to treat symptoms related to lumbar spinal stenosis, not eligible for surgical treatment.
Materials and Methods
We have enrolled 42 patients with the diagnosis of lumbar spinal stenosis, with persistent lumbosciatalgia and neurogenic claudicatio unresponsive to pharmacological therapy.
Exclusion criteria are the following: i) age <18 years; ii) current coagulopathic diseases; iii) injuries of spinal medulla; iv) current infections; v) uncontrolled diabetes mellitus; vi) uncontrolled hypertension; vii) uncontrolled glaucoma; viii) peripheral myelopathy and peripheral neuropathy; ix) previous spine surgery is not an absolute contraindication.
Statistical analysis
The details of the statistical analysis conducted can be seen in Table 1 and Figure 1.
Assessment of the pain
To quantify the pain, we use the NRS scale, and the subjective improvement is measured using the Subjective Rating Scale of function recovery. The above-mentioned scale consists of 5 levels, which correspond to the following points: 0=no recovery, 1=slight recovery, 2=moderate recovery, 3=good recovery, and 4=excellent recovery. The subjective improvement is measured using the Functional Rating Index (FRI), which contains 10 items that measure both pain and function of the spinal musculoskeletal system. Of these 10 items, 8 refer to activities of daily living that might be adversely affected by a spinal condition, and 2 refer to two different attributes of pain. Because many spinal disabilities are most likely a combination of loss of function and pain and/or the fear of pain, using both pain and function allows for a wider view of a patient’s disability.10
Using a 5-point scale for each item, the patient ranks his or her perceived ability to perform a function and/or the quantity of pain at present ("right now") by selecting one of the five response points that are anchored by bipolar statements (0 =no pain or full ability to function); 4= worst possible pain and/or unable to perform this function at all). When all 10 items are completed, the FRI score is calculated as follows: (total score / 40) x 100% (Table 2).15 Furthermore, all patients undergo the Oswestry Disability Index 2.1°, Versione Italiana (ODI-I) before the treatment, after the treatment, and in the follow-up evaluation at 3 months.
Table 1.
Statistical analysis.
| ODI-I: | ODI-I: | FRI: | FRI | NRS | NRS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| H0 | Score pre= Score post | H0 | Score Follow-up =Score pre | H0 | Score pre= Score post | H0 | Score Follow-up =Score pre | H0 | Score pre= Score post | H0 | Score Follow-up =Score pre |
| H1 | Score pre≠ Score post | H1 | Score Follow-up≠ Score pre | H1 | Score pre≠ Score post | H1 | Score Follow-up≠ Score pre | H1 | Score pre≠ Score post | H1 | Score Follow-up≠ Score pre |
| n | 42 patients | n | 42 patients | n | 42 patients | n | 42 patients | n | 42 patients | n | 42 patients |
| t test | 4.97*10–23 | t test | 2.41*10–20 | t test | 2.75*10–17 | t test | 1.41*10–15 | t test | 1.28*10–21 | t test | 3.55*10–17 |
Table 2.
Functional Rating Index for neck and back problems.
| Pain intensity | 0: no pain | 1: mild pain | 2: severe pain | 3: severe pain | 4: worst possible pain |
| Sleeping | 0: perfect sleep | 1: mildly disturbed sleep | 2: moderated disturbed sleep | 3: greatly disturbed sleep | 4: totally disturbed sleep |
| Personal care | 0: no pain, no restriction | 1: mild pain, no restriction | 2: moderate pain, need to go slowly | 3: moderate pain, need some assistance | 4: severe pain, need 100% assistance |
| Travel | 0: no pain on lingo trips | 1: mild pain on long trips | 2: moderate pain on long trips | 3: moderate pain on short trips | 4: severe pain on short trips |
| Work | 0: can do usual work plus unlimited extra work | 1: can do usual work, no extra work | 2: can do 50% of usual work | 3: can do 25% of usual work | 4: cannot work |
| Recreation | 0: can do all activities | 1: can do most activities | 2: can do some activities | 3: can do a few activities | 4: cannot do any activity |
| Frequency of pain | 0: no pain | 1: occasional pain, 25% of the day | 2: intermittent pain, 50% of the day | 3: frequent pain, 75% of the day | 4: constant pain, 100% of the day |
| Lifting | 0: no pain with any weight | 1: increased pain with heavy weight | 2: increased pain with moderate weight | 3: increased pain with light weight | 4: increased pain with any weight |
| Walking | 0: no pain, any distance | 1: increased pain after 1 mile | 2: increased pain after ½ mile | 3: increased pain after ¼ mile | 4: increased pain with all walking |
| Standing | 0: no pain after several hours | 1: increased pain after several hours | 2: increased pain after 1 hours | 3: increased pain after ½ hour | 4: increased pain with any standing |
The Oswestry Disability Questionnaire score is obtained by summing the scores of each of the 10 sections of the Questionnaire, (from 0 to 5 for each one), then dividing it by the highest score possible, (50 if all the 10 sections have been completed, 45 if one of them has not been completed) then converting this value in percentage (Table 3).16
Figure 1.
Critical values of t.
Patients included and technique employed
The mean age of the above-mentioned 42 patients is 70,67 ± 10,90 years, 12 of whom are female and 30 are male. All patients have already done the electromyography and an MRI of the lumbar vertebral column to confirm the diagnosis. The duration of the pain is 28,7± 2,3 weeks, with an initial high intensity (NRS = 7). All patients included have previously undergone both medical and rehabilitative therapy without any benefit.
The therapeutic scheme consists of 4 injective treatments once a week for 4 weeks on each patient. After these, if the pain relief is not complete but the patient is a responder to the treatment, further injections every month have to be executed: once a month to reach and maintain the benefit.
If after the 4 caudal injections, the patient is a non-responder, he will change therapy.
The caudal epidural injection is a sterile procedure and has been executed using ultrasound guidance, using a spinal needle 21G. The Ultrasound machine employed is Samsung V6. The technique has been performed by the authors, trained in the musculoskeletal US as a guide for interventional treatments.
The patient is positioned in a prone decubitus position with a cushion under the pelvis to better recognize the anatomical landmarks. We use the linear ultrasound transducer (7-13 MHz) firstly with a transversal scan to locate the sacral hiatus to obtain its transverse view where the two sacral cornua appear as two hyperechoic structures, and the sacral hiatus is the hypoechoic region between the 2 band-like hyperechoic structures. At this level, the ultrasound transducer is rotated 90 degrees to obtain a longitudinal scan, to direct the needle using the "in-plane" technique through the sacral hiatus in the epidural space, visualizing the needle in realtime. The confirmation of the correct positioning is verified by visualizing the unidirectional flow of the fluid injected in the epidural space with a color-doppler image (Figure 2,3,4). Moreover, ultrasonography can also provide information regarding the cephalad spreading of injectate during caudal epidural injection. It is suggested that the advancement of the needle tip beyond the apex of the sacral hiatus should be limited to 5 mm to avoid dural puncture because the distance between the apex of the sacral hiatus and dural sac termination can be as short as less than 6 mm.17
Table 3.
Oswestry score related to the assessment of the level of disability.
| Disability assessment | Oswestry score (%) |
|---|---|
| Minimal disability | 0-20 |
| Moderate disability | 21-40 |
| Severe disability | 41-60 |
| Crippled patients | 61-80 |
| Bed-bound patients | 81-100 |
Figure 3.
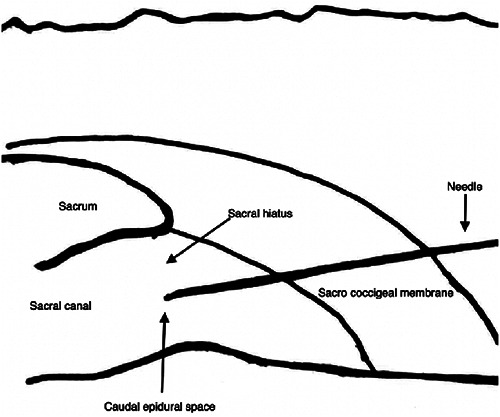
Position of the patient and of the US probe.
Figure 4.
Ultrasonographic imaging of the sacral hiatus and of the needle position (indicated by the white arrow).
The drugs delivered are triamcinolone 40 mg; and levobupivacaine 10 mg; adding physiological solution to obtain a total volume of 10 mL, regardless of the level of stenosis. A solution including Levobupivacaine with a concentration of 0,1% allows the sensory block to avoid the motor block. Neurotoxicity of steroidal drugs is not due to the steroidal molecule but to the Polyethylene glycol present in the formulation. Nevertheless, long-acting delivery formulations contain a percentage of Polyethylene glycol about 3%, inferior to the level of neurotoxicity.
Epidural administration of the steroid appropriately diluted with the local anesthetic and physiological solution guarantees a significant reduction of the neurotoxic effects of the steroid.18
Data analysis
We analyze our results using the T-test for paired data. With a level of significance of 0.001, the scores of all our scales of assessment are different before and after the treatment and also in the follow-up.
Results
We have performed on average 4 injective treatments (4±2, Means 4, SD 2) on each patient. Data analysis shows that the treatment has been effective in pain relief, detecting a significant reduction in pain comparing the conditions before and after the therapy, which also persisted in the follow-up after 3 months (Table 4). In the same way, the results observed in the FRI show a significant improvement comparing the beginning and the end of the treatment, maintained in the 3-month follow-up (Table 5).
Eventually, the results of the ODI-I Questionnaire illustrate a significant improvement in the grade of disability: from a score indicating a grade of severe disability to a level of moderate disability (Table 6).
We do not detect any complication consequent to the treatment performed neither immediately after nor in the follow-up.
Discussion
Caudal epidural injection is one of the most common conservative treatments for chronic low back pain with radiculopathy, and it has been demonstrated that the administration of either Lidocaine alone or lidocaine in conjunction with steroids is significantly effective.19
In this framework, the two main issues discussed in literature concerning the effectiveness of the caudal epidural injection on lumbar pain are the correct positioning of the needle and the volume injected.
Our work confirmed that the ultrasound-guided injection is a safe, precise, and effective technique, representing a valid alternative to fluoroscopy: as Yoon describes, ultrasound is as reliable as fluoroscopy. The choice of the injected volume is very important as the effectiveness of the block depends on this parameter: if too small the block could be ineffective, if too high complications such as increased intracranial pressure may occur.20
The choice to administer 10 ml has been made following data reported in the literature. About this, Kim13 has examined the extension of the distribution of volumes from 10 ml to 50 ml, detecting that the level reached with 10 ml was L3, and it did not vary significantly with higher volumes. Therefore, the volume of 10 ml has been demonstrated to be suitable and effective to treat symptoms without causing side effects.
This technique allows to use of a thinner needle than the needle used for the standard lumbar epidural injection, with lower mechanical damage.
The Ultrasound guide indicates the exact positioning of the needle and the exact point of injection. We do not have any short-term and long-term complications. Thus, we can consider this technique safe and effective. The drugs employed with the indicated volume and dosage have shown to be effective and safe.
The control of the pain and the improvement of the entity of the disability have shown significant results, with a reduction of the pain of more than 50% comparing the beginning and the end of the treatment, maintained also at the three-month follow-up.
The judgment of the subjective pain relief and the functional recovery assessed using the Subjective Rating Scale of function recovery has been significant and confirmed by the results observed at the ODI-I (Oswestry Disability Index): the value at the ODI-I indicates the passage from a level of severe disability to a level of moderate disability, with a higher personal autonomy, and a considerable improvement of the quality of life.
This study has the limitations of being a retrospective observational monocentric study, without comparing different groups of patients. Nevertheless, it has been planned to define the adequate volume to inject, to assess the effectiveness and the safety of the technique. The psychological factors that might alter our results, such as the general over-reaction pain, have not been evaluated.
Table 4.
NRS variation before the therapy, after the 1st, 2nd, 3rd treatments and at the 3 months follow-up.
| NRS score | Basal | 1st treatment | 2nd treatment | 3rd treatment | Follow-up 3 months |
|---|---|---|---|---|---|
| Mean | 6.6 | 3.6 | 2.6 | 2.4 | 3.35 |
| SD | 0.84 | 1.26 | 1.17 | 0.96 | 0.94 |
NRS, numerical rating scale; SD, standard deviation.
Table 5.
FRI scale percentage variations before the therapy, at the end of the therapy, and at the 3 months follow-up.
| FRI | % Basal | % End-therapy | Follow-up 3 months |
|---|---|---|---|
| Mean | 31.5 | 19.4 | 21.1 |
| SD | 3.16 | 5.31 | 4.73 |
FRI, functional rating index; SD, standard deviation.
Table 6.
ODI score and percentage before and after the therapy, and at the 3 months follow-up.
| Oswestry score | Score pre | % pre | Score post | % post | Score Follow-up | % Follow-up |
|---|---|---|---|---|---|---|
| Mean | 27.7 | 56.4 | 14 | 28.5 | 14.7 | 29.4 |
| SD | 2.71 | 5.12 | 3.88 | 7.82 | 4.08 | 8.16 |
ODI, oswestry disability index; SD, standard deviation.
Nevertheless, the aim was to assess the functional recovery, as described by the FRI scale.
Conclusions
In conclusion, the results have demonstrated that the treatment of symptoms deriving from lumbar spinal stenosis with caudal epidural injection is effective, free of effects and gives long-lasting pain relief. The ultrasound guidance represents a valid alternative to fluoroscopy, free from ionizing radiation, and could be virtually used in any clinical setting.
This treatment allows patients affected by a pathology without other therapeutic schemes if they are non-responders to the other described treatments, even though it is not effective in the case of myelopathy. It can be performed in an outpatient setting, avoiding the operating room and the radiological equipment.
We can also use the treatment as a maintenance to extend the benefit.
This study defines the ultrasound guide as possible and determines the volume and the dosage of drugs, still controversial in the literature.
List of abbreviations
- LSS
lumbar spinal stenosis
- US
Ultrasound
- ODI-I
Oswestry Disability Index
- MRI
magnetic resonance imaging
- NRS
numeric rating scale
- ESI
epidural steroid injection
- FRI
functional rating index
Footnotes
Conflict of interest
The authors declare no potential conflict of interest, and all authors confirm accuracy.
Contributor Information
Antonello Lovato, Email: lovatoantonello@gmail.com.
Francesco Ceccherelli, Email: fceccherelli@airas.it.
Giuseppe Gagliardi, Email: giuseppe.gagliardi@aulss5.veneto.it.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
References
- 1.Bagley C, MacAllister M, Dosselman L, et al. Current concepts and recent advances in understanding and managing lumbar spine stenosis. F1000Res 20198:F1000 Faculty Rev-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vikram K. Caudal epidural steroid injections-a mini review. Open Access J Neurol Neurosurg 2017;3:555606. [Google Scholar]
- 3.Liu K, Liu P, Liu R, et al. Steroid for epidural injection in spinal stenosis: a systematic review and meta-analysis. Drug Des Devel Ther 2015;9:707-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ibrahim ME, Awadalla MA, Omar AS, Al-Shatouri M. Ultrasound-guided caudal epidural steroid injection in chronic radicular low back pain: short-term electrophysiologic benefits. BJR Open 2020;2:20190006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kao SC, Lin CS. Caudal epidural block: an updated review of anatomy and techniques. Biomed Res Int 2017;2017:9217145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stitz MY, Sommer HM. Accuracy of blind versus fluoroscopically guided caudal epidural injection. Spine (Phila Pa 1976) 1999;24:1371-6. [DOI] [PubMed] [Google Scholar]
- 7.Kao SC, Lin CS. Caudal epidural block: an updated review of anatomy and techniques. Biomed Res Int. 2017;2017:9217145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagpal AS, Vu T-N, Gill B, et al. Systematic review of the effectiveness of caudal epidural steroid injections in the treatment of chronic low back or radicular pain. Intervent Pain Med 2022;4:100149. [Google Scholar]
- 9.Koo BS, Kang WB, Park JW, et al. Analysis of caudal epidurogram in single center: A preliminary study of lumbar radiculopathy management. Medicine (Baltimore) 2018;97:e12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manchikanti L, Cash KA, Pampati V, et al. Evaluation of fluoroscopically guided caudal epidural injections. Pain Physician 2004;7:81-92. [PubMed] [Google Scholar]
- 11.Yoon JS, Sim KH, Kim SJ, et al. The feasibility of color Doppler ultrasonography for caudal epidural steroid injection. Pain 2005;118:210-4. [DOI] [PubMed] [Google Scholar]
- 12.Yoo SW, Ki MJ, Doo AR, et al. Prediction of successful caudal epidural injection using color Doppler ultrasonography in the paramedian sagittal oblique view of the lumbosacral spine. Korean J Pain 2021;34:339-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim KM, Kim HS, Choi KH, Ahn WS. Cephalic spreading levels after volumetric caudal epidural injections in chronic low back pain. J Korean Med Sci 2001; 16:193-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cleary M, Keating C, Poynton AR. The flow patterns of caudal epidural in upper lumbar spinal pathology. Eur Spine J 2011;20:804-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feise RJ, Michael Menke J. Functional rating index: a new valid and reliable instrument to measure the magnitude of clinical change in spinal conditions. Spine (Phila Pa 1976) 2001;26:78-86; discussion 87. [DOI] [PubMed] [Google Scholar]
- 16.Monticone M, Baiardi P, Ferrari S, et al. Development of the Italian version of the Oswestry Disability Index (ODI-I): A cross-cultural adaptation, reliability, and validity study. Spine (Phila Pa 1976) 2009;34:2090-5. [DOI] [PubMed] [Google Scholar]
- 17.Kao SC, Lin CS. Caudal epidural block: an updated review of anatomy and techniques. Biomed Res Int 2017;2017:9217145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marinangeli F, Ciccozzi A, Donatelli F, et al. Uso degli steroidi per via spinale ed epidurale [Clinical use of spinal or epidural steroids]. Minerva Anestesiol 2002;68: 613-20. Italian. [PubMed] [Google Scholar]
- 19.Manchikanti L, Knezevic NN, Boswell MV, et al. Epidural injections for lumbar radiculopathy and spinal stenosis: a comparative systematic review and meta-analysis. Pain Physician 2016;19:E365-410. [PubMed] [Google Scholar]
- 20.Bosscher H. Pressure-volume relationships in the spinal canal and potential neurological complications after epidural fluid injections. Front Pain Res (Lausanne) 2022;3:884277. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


