Lipids are essential to the homeostatic structure and function of cells, tissues, and organs. These essential roles of lipids include biosynthesis of biological membranes (permitting organelle compartmentalization), bioenergetics (as an efficient fuel source), and as potent signaling intermediates, both intracellular and extracellular; uniquely, in the lung, lipids are indispensable for surfactant synthesis by type 2 alveolar epithelial cells (AEC2s). AECs serve as facultative stem cells of the alveolus, and their dysfunction is now well recognized as a pivotal event in the pathogenesis of fibrotic lung diseases.
Over the past decade, although there has been growing recognition that lipid metabolism plays an important role in the pathogenesis of lung fibrosis, as reviewed in a recent perspective published online ahead of print in the Journal (1), there remains uncertainty regarding the precise molecular mechanisms (Figure 1). For example, although some studies support augmentation of lipid biosynthetic pathways as serving protective functions against lung fibrosis (2–5), others suggest that particular lipids may be harmful (6, 7). Such differences may be accounted for by differences in the specific lipid species involved, target cells, compartmentalized actions (at both organ-specific and subcellular levels), and experimental protocols.
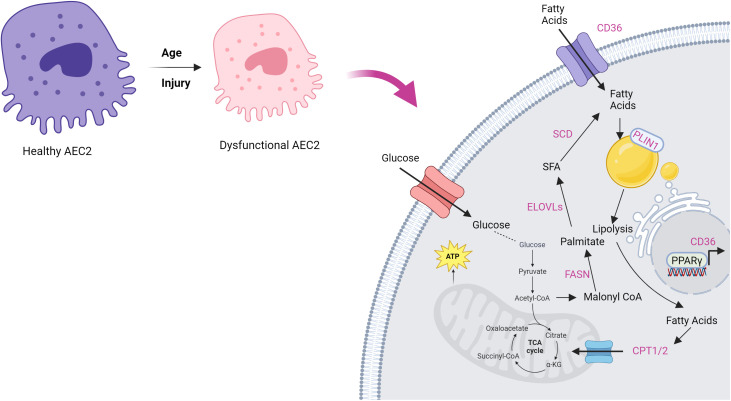
Figure 1.
Lipid metabolic pathways that may be altered in idiopathic pulmonary fibrosis (IPF). Although there are some variations in the role of these pathways in IPF and in experimental models of lung fibrosis, most studies support reductions in both fatty acid biosynthesis and fatty acid oxidation in dysfunctional AEC2s that promote fibrogenesis. Figure adapted by permission from Choi and colleagues in (15) and created with BioRender.com. AEC2 = type 2 alveolar epithelial cell; CPT1/2 = carnitine palmitoyltransferase 1/2; ELOVLs = ELOVL fatty acid elongases; FASN = fatty acid synthase; PLIN1 = perilipin 1; SCD = stearoyl-CoA desaturase; TCA = tricarboxylic acid.
In this issue of the Journal, Liang and colleagues (pp. 242–253) establish a correlation between lipid metabolism and AEC2 dysfunction in the context of experimental injury to a murine aging lung and in idiopathic pulmonary fibrosis (IPF) (8). Using flow cytometry, they isolated AEC2s from both IPF and healthy human donor lungs and subjected them to single-cell RNA sequencing analysis to explore genes associated with lipid metabolism. These studies revealed a downregulation of several genes related to fatty acid biosynthesis (e.g., CHKA, SCD, FASN, CAT, ACOXL, ELOVL6, LPCAT1, LPCAT3) in AEC2s of subjects with IPF compared with healthy human control subjects. Furthermore, genes associated with fatty acid β-oxidation (e.g., ACAT1, ACSL) were also downregulated in IPF AEC2 cells. In addition, genes involved in lipid uptake (e.g., CD36, SLC27A4, SLC27A5) and lipid storage (e.g., PLIN2, MGLL, HILPDA) were found to be downregulated in IPF AEC2s compared with healthy counterparts. Immunofluorescence experiments validated the findings of the single-cell RNA sequencing study, collectively indicating decreased lipid concentrations in the AEC2s of subjects with IPF.
To corroborate their findings in an animal model, the researchers studied an aging mouse model with bleomycin-induced lung injury and three-dimensional (3D) organoids using AEC2s and lung fibroblasts. These in vivo and ex vivo models consistently demonstrated a decrease in AEC2 lipid content in aged, injured mice, as observed in IPF AECs. Interestingly, 3D organoid models of human and mouse AECs supplemented with a lower concentration (2%) of exogenous lipids promoted renewal in young mice but not in older ones. However, a higher lipid concentration (4%) facilitated renewal in both young and aged AEC2 organoids. This enhanced regenerative capacity was evidenced by increased colony size, AEC2 proliferation rate, elevated expression of AEC1 marker genes (AGER, AQP5, T1α), and AEC2-related surfactant proteins in lipid-treated organoids compared with control animals. Furthermore, the researchers used a PPARγ agonist (rosiglitazone) and antagonist (GW9662) in the human organoid model to demonstrate that rosiglitazone augmented colony formation of AEC2s from healthy lungs, whereas GW9662 decreased it. Notably, treatment with 10 μM rosiglitazone significantly increased colony formation of AEC2s from IPF lungs compared with control lungs.
The studies by Liang and colleagues (8) advance the concept that abnormal lipid metabolism contributes to AEC2 dysfunction, a hallmark of IPF. An exciting aspect of this work is the demonstration that regenerative capacity of aged AEC2s in the 3D organoid model can be rejuvenated with lipid supplementation or with PPARγ agonists. Although subpopulations of fibroblasts are known to support the AEC2 stem cell niche (9), the role of lipids in their interactions may be complex. For example, a recent study showed that exosomes from IPF lungs carry micro-RNAs that inhibit the de novo fatty acid synthesis pathway in AEC2s (4, 10). In addition, AEC2-supporting fibroblasts have been known to transfer lipids to adjacent AEC2s to support surfactant production (11, 12), and the aging of the mesenchyme itself may contribute to AEC2 dysfunction (10, 13). Thus, defects in cell autonomous lipid metabolism or in niche-supporting cells such as fibroblasts and macrophages (14) may cooperatively account for the AEC2 dysfunction in IPF.
Future investigations must clarify whether (and how) specific variations in lipid species and their compartmentalized actions alter AEC2 function. The metabolic flux of lipids controlled by dynamic changes in lipid biosynthesis, storage, transport, and consumption (via fatty acid oxidation) in AEC2s and their niche-supporting cells remains unclear. Although the “rescue” studies with lipid supplementation promises to open up new therapeutic strategies, it would be important to decipher if the mechanistic basis for more robust regenerative responses is due to lipid support of membrane biosynthesis (critical for proliferating cells), cellular bioenergetics (which wanes with aging), or epigenetic programs (regulated by lipid signaling intermediates). It could not simply be due to more surfactant synthesis, could it?
Footnotes
Supported by National Institute of General Medical Sciences of the National Institutes of Health award R35GM150564 (M.R.G.); National Heart, Lung, and Blood Institute grant P01-HL114470 (V.J.T.); National Institutes of Health grants R01-HL139617 and R01-HL173154 (V.J.T.); and U.S. Department of Veterans Affairs Merit Award I01BX003056 (V.J.T.).
Originally Published in Press as DOI: 10.1165/rcmb.2024-0187ED on May 7, 2024
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. O’Callaghan M, Tarling EJ, Bridges JP, Redente EF, Byrne AJ, Keane MP, et al. Re-examining the role of pulmonary lipids in the pathogenesis of pulmonary fibrosis. Am J Respir Cell Mol Biol . doi: 10.1165/rcmb.2024-0124PS. [DOI] [PubMed] [Google Scholar]
- 2. Romero F, Hong X, Shah D, Kallen CB, Rosas I, Guo Z, et al. Lipid synthesis is required to resolve endoplasmic reticulum stress and limit fibrotic responses in the lung. Am J Respir Cell Mol Biol . 2018;59:225–236. doi: 10.1165/rcmb.2017-0340OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kamp DW. Mitigating lung fibrosis by targeting dysfunctional alveolar epithelial cell lipid metabolism. Am J Respir Cell Mol Biol . 2018;59:139–140. doi: 10.1165/rcmb.2018-0070ED. [DOI] [PubMed] [Google Scholar]
- 4. Hayek H, Rehbini O, Kosmider B, Brandt T, Chatila W, Marchetti N, et al. The regulation of fatty acid synthase by exosomal miR-143-5p and miR-342-5p in idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol . 2024;70:259–282. doi: 10.1165/rcmb.2023-0232OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chung KP, Hsu CL, Fan LC, Huang Z, Bhatia D, Chen YJ, et al. Mitofusins regulate lipid metabolism to mediate the development of lung fibrosis. Nat Commun . 2019;10:3390. doi: 10.1038/s41467-019-11327-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chu SG, Villalba JA, Liang X, Xiong K, Tsoyi K, Ith B, et al. Palmitic acid–rich high-fat diet exacerbates experimental pulmonary fibrosis by modulating endoplasmic reticulum stress. Am J Respir Cell Mol Biol . 2019;61:737–746. doi: 10.1165/rcmb.2018-0324OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Summer R, Mora AL. Lipid metabolism: a new player in the conundrum of lung fibrosis. Am J Respir Cell Mol Biol . 2019;61:669–670. doi: 10.1165/rcmb.2019-0098ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liang J, Huang G, Liu X, Zhang X, Rabata A, Liu N, et al. Lipid deficiency contributes to impaired alveolar progenitor cell function in aging and idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol . 2024;71:242–253. doi: 10.1165/rcmb.2023-0290OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR, et al. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest . 2013;123:3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu X, Zhang X, Yao C, Liang J, Noble PW, Jiang D. Transcriptomics analysis identifies the decline in the alveolar type II stem cell niche in aged human lungs. Am J Respir Cell Mol Biol . 2024;71:229–241. doi: 10.1165/rcmb.2023-0363OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McGowan SE, Torday JS. The pulmonary lipofibroblast (lipid interstitial cell) and its contributions to alveolar development. Annu Rev Physiol . 1997;59:43–62. doi: 10.1146/annurev.physiol.59.1.43. [DOI] [PubMed] [Google Scholar]
- 12. El Agha E, Thannickal VJ. The lung mesenchyme in development, regeneration, and fibrosis. J Clin Invest . 2023;133:e170498. doi: 10.1172/JCI170498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chanda D, Rehan M, Smith SR, Dsouza KG, Wang Y, Bernard K, et al. Mesenchymal stromal cell aging impairs the self-organizing capacity of lung alveolar epithelial stem cells. eLife . 2021;10:e68049. doi: 10.7554/eLife.68049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Romero F, Shah D, Duong M, Penn RB, Fessler MB, Madenspacher J, et al. A pneumocyte-macrophage paracrine lipid axis drives the lung toward fibrosis. Am J Respir Cell Mol Biol . 2015;53:74–86. doi: 10.1165/rcmb.2014-0343OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Choi SYC, Ribeiro CF, Wang Y, Loda M, Plymate SR, Uo T. Druggable metabolic vulnerabilities are exposed and masked during progression to castration resistant prostate cancer. Biomolecules . 2022;12:1590. doi: 10.3390/biom12111590. [DOI] [PMC free article] [PubMed] [Google Scholar]