Abstract
Cutaneous squamous cell carcinoma (cSCC) is the second most common type of skin cancer arising from squamous cells of the epidermis. Most cases of cSCC have a good prognosis if detected and treated early; however, certain cases can be aggressive. The primary risk factor for cSCC is prolonged ultraviolet radiation from sun exposure, leading to DNA mutations. Other risk factors have also been observed, including adverse reactions to medications, particularly immunosuppressants. A query of the Food and Drug Administration Adverse Events Reporting System (FAERS) was done, and all reported events of cSCC as adverse events to medication were recorded along with demographic data of patients affected. A total of 4,792 cases of cSCC as an adverse event to medication were reported between 1997 and 2023. Lenalidomide, a chemotherapeutic drug, had the most cases of cSCC as an adverse event. Nine of the top 10 drugs associated with cSCC had immunosuppressive characteristics. While males had higher odds of cSCC associated with corticosteroids and calcineurin inhibitors, females had higher odds of cSCC related to monoclonal antibodies. Geriatric patients accounted for the majority of cSCC cases at 59.7%. Drawing on data from the FAERS database, there’s been a consistent increase in cSCC cases as a side-effect to certain medications, with most having immunosuppressive characteristics. Since there is a lack of up-to-date literature overviewing the most implicated medications for cSCC, we aimed to illustrate this better, as well as patient demographics, to better guide clinicians when prescribing these medications.
Keywords: General dermatology, Skin cancer, Squamous cell carcinoma, Adverse events, Side-effects, Medications, Drugs, Clinical research
Introduction
Cutaneous squamous cell carcinoma (cSCC) is a type of skin cancer that arises from epidermal squamous cells, typically due to DNA damage of cells leading to a malignant transformation [1]. cSCC is the second most common skin cancer, representing about 20 to 50% of all skin cancers in the United States [2]. While most cSCC are not life-threatening and can be treated with Mohs surgery or surgical excision if detected early, specific subsets of cSCC have more aggressive features associated with increased chances of metastasis and death [3].
Like other cutaneous malignancies, cumulative ultraviolet (UV) radiation from sun exposure is the most common risk factor for developing cSCC, hence why rates are highest in the elderly and fair-skinned individuals with minimal sun-protective melanin [4]. While cSCC can occur on any part of the skin, it most commonly presents on sun-exposed areas such as the head, neck, and arms. Although the most common cause of cSCC is cumulative UV exposure, other risk factors include exposure to arsenic and alkylating agents, oncogenic human papillomavirus, and immunosuppression [5–8]. Immunosuppressive agents increasing the risk of cSCC have been well established in the literature. Solid organ transplant recipients who require chronic immunosuppression have their risk of cSCC increased up to 65 to 250 times that of the general healthy population [9].
There needs to be more comprehensive literature assessing all the specific immunosuppressive agents that increase the risk of developing cSCC. Starting in 1993, the Food and Drug Administration (FDA) created a publicly accessible database, FAERS (Food and Drug Administration Adverse Event Reporting System that contains reports on adverse events suspected to be caused by medications. This database has improved the FDA’s ability to monitor approved drugs’ safety over extended periods. It aids clinicians in evaluating the association between medications and specific adverse reactions. In this report, we aim to explore medications and demographic information associated with the development of cSCC, as reported in the FAERS.
Materials and methods
All adverse events labeled “Squamous Cell Carcinoma of Skin” were searched on the FAERS database. Reported adverse events linked to cSCC were collected, and a statistical analysis was performed. We tallied the aggregate number of adverse events associated with drugs most typically linked with cSCC to gauge its prevalence against other side effects. Cases reported that did not include the age and sex of patients were not included in our demographic analyses. Using patient sex as the primary variable, we used logistic regression to determine odds ratios (ORs). We generated ORs for corticosteroids, including prednisone, dexamethasone, and prednisolone, in the first analysis. We evaluated two monoclonal antibodies, adalimumab and infliximab, for the second analysis. Our third analysis evaluated two calcineurin inhibitors, tacrolimus and cyclosporine.
Results
The FAERS database was queried on January 7th, 2024, and there were a total of 4,792 reported cases of cSCC as adverse events from medications. The distribution of adverse events of cSCC is displayed by year in Fig. 1, tracing the first report of cSCC back to 1997. Since then, there has been an apparent increase in reports, peaking at 470 cases in 2019. In Fig. 2, we highlight the twenty drugs most frequently implicated in reports of cSCC as an adverse event. Within the top ten drugs, lenalidomide is the most common at 712 (14.9%), followed by tacrolimus (8.5%), adalimumab (8.0%), mycophenolate mofetil (6.5%), voriconazole (6.2%), prednisone (5.6%), dexamethasone (5.5%), cyclosporine (4.5%), prednisolone (4.4%), and infliximab (4.1%). Nine of these ten medications had immunosuppressive components, and only voriconazole, an antifungal, did not.
Fig. 1.
Reported cases of cutaneous squamous cell carcinoma as an adverse event
Fig. 2.
The top 20 medications associated with cutaneous squamous cell carcinoma as an adverse event
Three of the drugs were categorized as corticosteroids, two were monoclonal antibodies, and two others were calcineurin inhibitors. Squamous cell carcinoma constituted 0.18% of all adverse events associated with prednisone, 0.27% for dexamethasone, and 0.27% for prednisolone. Our analysis found that males had higher odds of developing cSCC as a side effect from corticosteroids: prednisone (OR: 1.19, P < 0.05), prednisolone (OR: 1.54, P < 0.05), and dexamethasone (OR: 2.30, P < 0.05).
Among the top two monoclonal antibodies, cSCC represented 0.06% of all adverse events from adalimumab use and 0.10% for infliximab. Contrasting with corticosteroids, we found that cSCC as an adverse event from monoclonal antibodies were more likely to present in females: adalimumab (OR: 2.17, P < 0.05) and infliximab (OR: 1.55, P < 0.05). The top two calcineurin inhibitors, tacrolimus, and cyclosporine, had 0.39% and 0.26% cases of cSCC as a proportion of all adverse events, respectively. Like corticosteroids, males were more likely to experience cSCC as an adverse event: tacrolimus (OR: 1.85, P < 0.05) and cyclosporine (OR: 2.03, P < 0.05).
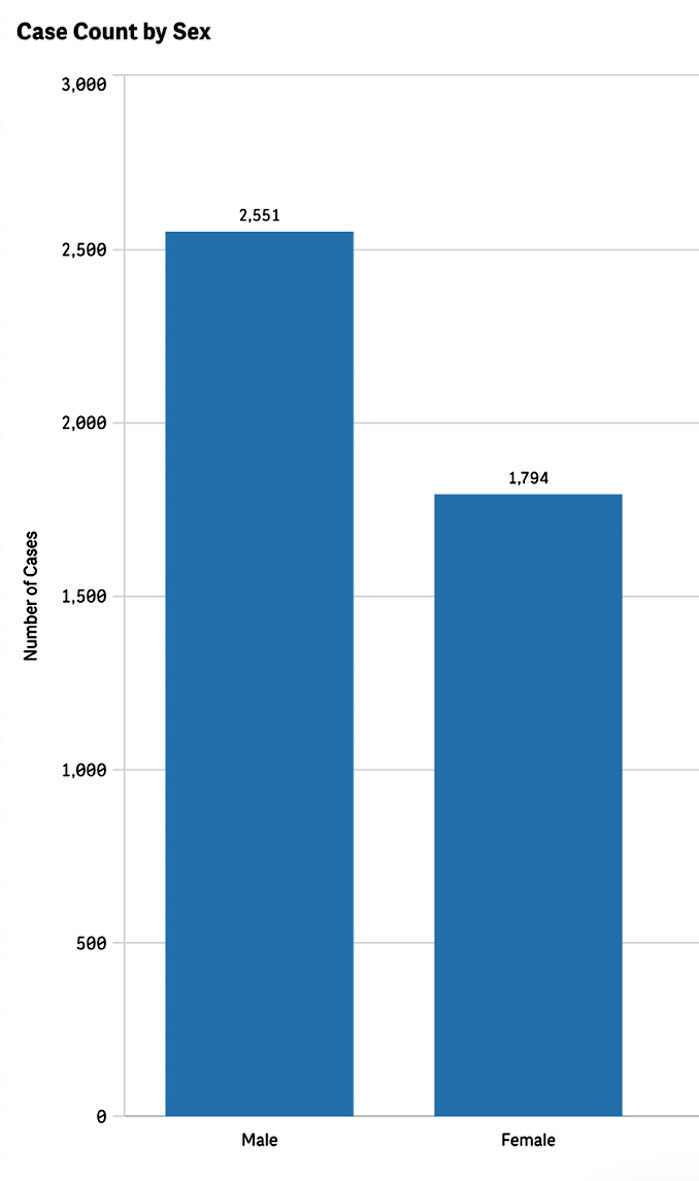
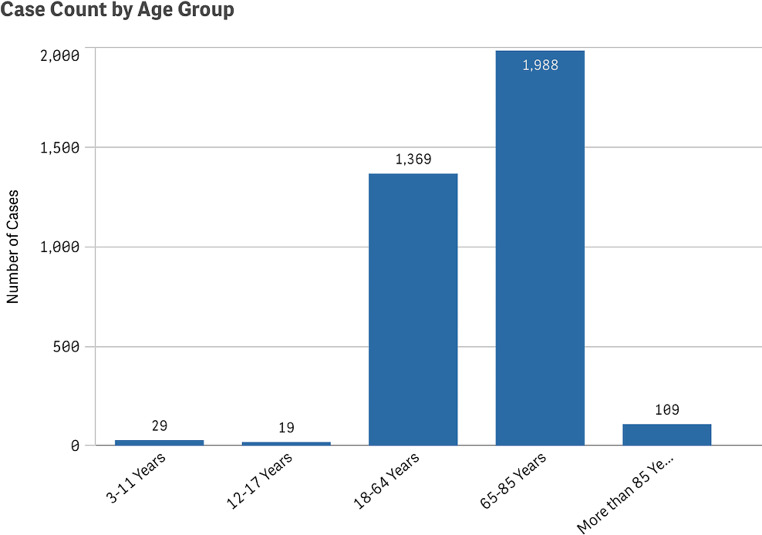
Additionally, we analyzed patient demographics and found that most reported cSCC cases occurred in males at 58.7%, compared to 41.3% in females (Fig. 3). There were cases of cSCC reported as an adverse event in all age groups. However, the proportion of cases reported among pediatric patients was low at 1.37%, whereas elderly patients over the age of 65 represented 59.7% of all cases (Fig. 4).
Fig. 3.
Reported cases of cutaneous squamous cell carcinoma by patient sex
Fig. 4.
Reported cases of cutaneous squamous cell carcinoma by patient age group
Discussion
This investigation provides an in-depth analysis of medications and patient demographics linked to the development of cSCC as an adverse reaction using the FAERS database. The FDA’s database has cataloged over 28 million adverse events over the years, with cases of cSCC only making up 0.017% of these, underscoring that it is relatively infrequent. Nonetheless, beginning in 2011, there has been a sharp increase in the number of cSCC reports to the FAERS database, which coincides with the rates of cSCC increasing in the general population. (10–11) As managing many debilitating diseases relies on immunosuppressive medications, clinicians and researchers must understand epidemiological trends and rates of adverse events associated with these drugs to assess their safety and better guide decisions when prescribing regimens.
The association of immunosuppression and increased risk of cSCC is well established in the literature. However, the precise mechanism by which this occurs is incompletely understood. Immunocompetence is vital in preventing cancer as the immune system can identify and eliminate cells that have undergone malignant transformation due to DNA damage [12, 13]. Thus, taking immunosuppressive drugs blunts this protection and permits cancer cells to proliferate more easily. This is consistent with our findings in the FAERS database analysis, as 17 of the top 20 medications associated with cSCC have immunosuppressive characteristics. Although these drugs have a relatively low rate of cSCC as an associated adverse event compared to other side effects, it is important to highlight these as patients who develop cSCC secondary to immunosuppressive regimens have been found to have more aggressive and rapidly progressing variants of cSCC. (14–15)
Two of the top 20 medications implicated in the development of cSCC did not have immunosuppressive properties: voriconazole and vemurafenib. The pathophysiology of voriconazole-associated cSCC is not well understood. Some suggested mechanisms include direct phototoxicity from voriconazole metabolites and the upregulation of COX-2, an essential enzyme in developing cSCC [16–18]. Vemurafenib is a BRAF inhibitor that is typically used to treat metastatic melanoma; however, this molecular mechanism can trigger RAS mutations due to a paradoxical activation of mitogen-activated protein kinase (MAPK), which can lead to the development of cSCC [19–21].
Cases of cSCC in the general population indicate that it is more prevalent in males than females [22]. However, this is likely because men spend more time exposed to the sun without adequate protection [23, 24]. Similarly, our investigation found that reports of cSCC as an adverse event to medications were overall most prevalent amongst males; however, in certain medication classes, such as monoclonal antibodies, cSCC was more prevalent in females. We highlight the need for further investigations into gender-based variations in medication adverse events, as this could help better elucidate which patients may be at higher risk of developing cSCC from certain drug classes.
There are a few limitations present in this study. Although the FDA regulates it, the FAERS database allows patients to self-report adverse events. Thus, we must consider the potential for erroneous reports, as the medical knowledge of the general public may vary significantly. Furthermore, some of the adverse events reported are cases in which the patients are taking multiple medications. Thus, there is the potential for these to serve as confounding factors. Lastly, some of the patients being treated with the reported drugs may have underlying medical conditions that may already predispose them to the development of cSCC.
Acknowledgements
The authors would like to thank the Dr. Phillip Frost Department of Dermatology & Cutaneous Surgery at the University of Miami for continuous support.
Author contributions
PJP and KN wrote the main manuscript text. PJP prepared the figures. All authors reviewed the manuscript.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2018;78(2):237–247. doi: 10.1016/j.jaad.2017.08.059. [DOI] [PubMed] [Google Scholar]
- 2.Rogers HW, Weinstock MA, Feldman SR, Coldiron BM. Incidence Estimate of Nonmelanoma skin Cancer (Keratinocyte Carcinomas) in the US Population, 2012. JAMA Dermatol. 2015;151(10):1081. doi: 10.1001/jamadermatol.2015.1187. [DOI] [PubMed] [Google Scholar]
- 3.Dreyfuss I, Kamath P, Frech F, Hernandez L, Nouri K (2022) Squamous cell carcinoma: 2021 updated review of treatment. Dermatol Ther 35(4). 10.1111/dth.15308 [DOI] [PubMed]
- 4.Wikonkal NM, Brash DE. Ultraviolet Radiation Induced signature mutations in Photocarcinogenesis. J Invest Dermatology Symp Proc. 1999;4(1):6–10. doi: 10.1038/sj.jidsp.5640173. [DOI] [PubMed] [Google Scholar]
- 5.Yuspa SH. Cutaneous chemical carcinogenesis. J Am Acad Dermatol. 1986;15(5):1031–1044. doi: 10.1016/S0190-9622(86)70267-3. [DOI] [PubMed] [Google Scholar]
- 6.Dang C, Koehler A, Forschner T, et al. E6/E7 expression of human papillomavirus types in cutaneous squamous cell dysplasia and carcinoma in immunosuppressed organ transplant recipients: HPV expression in cutaneous dysplasia and SCC. Br J Dermatol. 2006;155(1):129–136. doi: 10.1111/j.1365-2133.2006.07378.x. [DOI] [PubMed] [Google Scholar]
- 7.Torchia D, Massi D, Caproni M, Fabbri P. Multiple cutaneous precanceroses and carcinomas from combined iatrogenic/professional exposure to arsenic. Int J Dermatology. 2008;47(6):592–593. doi: 10.1111/j.1365-4632.2008.03547.x. [DOI] [PubMed] [Google Scholar]
- 8.Jensen P, Hansen S, Møller B, et al. Skin cancer in kidney and heart transplant recipients and different long-term immunosuppressive therapy regimens. J Am Acad Dermatol. 1999;40(2):177–186. doi: 10.1016/S0190-9622(99)70185-4. [DOI] [PubMed] [Google Scholar]
- 9.O’Reilly Zwald F, Brown M. Skin cancer in solid organ transplant recipients: advances in therapy and management. J Am Acad Dermatol. 2011;65(2):253–261. doi: 10.1016/j.jaad.2010.11.062. [DOI] [PubMed] [Google Scholar]
- 10.Muzic JG, Schmitt AR, Wright AC, et al. Incidence and trends of basal cell carcinoma and cutaneous squamous cell carcinoma. Mayo Clin Proc. 2017;92(6):890–898. doi: 10.1016/j.mayocp.2017.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stang A, Khil L, Kajüter H, et al. Incidence and mortality for cutaneous squamous cell carcinoma: comparison across three continents. Acad Dermatol Venereol. 2019;33(S8):6–10. doi: 10.1111/jdv.15967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vial T. Immunosuppressive drugs and cancer. Toxicology. 2003;185(3):229–240. doi: 10.1016/S0300-483X(02)00612-1. [DOI] [PubMed] [Google Scholar]
- 13.Buchanich JM, Newcomb CW, Washington TL, et al. Use of immunosuppression and subsequent cancer incidence: cohort study. Bmjonc. 2023;2(1):e000037. doi: 10.1136/bmjonc-2023-000037. [DOI] [Google Scholar]
- 14.Elghouche AN, Pflum ZE, Schmalbach CE. Immunosuppression Impact on Head and Neck cutaneous squamous cell carcinoma: a systematic review with Meta-analysis. Otolaryngol–head neck surg. 2019;160(3):439–446. doi: 10.1177/0194599818808511. [DOI] [PubMed] [Google Scholar]
- 15.Willenbrink TJ, Jambusaria-Pahlajani A, Arron S, Seckin D, Harwood CA, Proby CM. Treatment approaches in immunosuppressed patients with advanced cutaneous squamous cell carcinoma. Acad Dermatol Venereol. 2019;33(S8):57–60. doi: 10.1111/jdv.15843. [DOI] [PubMed] [Google Scholar]
- 16.George E, Baranwal N, Kang J, Qureshi A, Drucker A, Cho E. Photosensitizing medications and skin Cancer: a Comprehensive Review. Cancers. 2021;13(10):2344. doi: 10.3390/cancers13102344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikeya S, Sakabe J ichi, Yamada T, Naito T, Tokura Y (2018) Voriconazole-induced photocarcinogenesis is promoted by aryl hydrocarbon receptor-dependent COX-2 upregulation. Sci Rep. ;8(1):5050. 10.1038/s41598-018-23439-7 [DOI] [PMC free article] [PubMed]
- 18.Cowen EW, Nguyen JC, Miller DD, et al. Chronic phototoxicity and aggressive squamous cell carcinoma of the skin in children and adults during treatment with voriconazole. J Am Acad Dermatol. 2010;62(1):31–37. doi: 10.1016/j.jaad.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim A, Cohen MS. The discovery of vemurafenib for the treatment of BRAF-mutated metastatic melanoma. Expert Opin Drug Discov. 2016;11(9):907–916. doi: 10.1080/17460441.2016.1201057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su F, Viros A, Milagre C, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med. 2012;366(3):207–215. doi: 10.1056/NEJMoa1105358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu JH, Cohen DN, Rady PL, Tyring SK. BRAF inhibitor-associated cutaneous squamous cell carcinoma: new mechanistic insight, emerging evidence for viral involvement and perspectives on clinical management. Br J Dermatol. 2017;177(4):914–923. doi: 10.1111/bjd.15348. [DOI] [PubMed] [Google Scholar]
- 22.Tan B, Seth I, Fischer O, et al. Sex disparity for patients with cutaneous squamous cell carcinoma of the Head and Neck: a systematic review. Cancers. 2022;14(23):5830. doi: 10.3390/cancers14235830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adams GJ, Goldstein EK, Goldstein BG, Jarman KL, Goldstein AO. Attitudes and behaviors that impact skin Cancer risk among men. IJERPH. 2021;18(19):9989. doi: 10.3390/ijerph18199989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Green AC, Olsen CM. Cutaneous squamous cell carcinoma: an epidemiological review. Br J Dermatol. 2017;177(2):373–381. doi: 10.1111/bjd.15324. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.