Abstract
Background
Respiratory muscle training (RMT) aims to improve inspiratory and/or expiratory muscle function in neuromuscular disorders (NMDs). A comprehensive overview of the available literature is lacking. This scoping review explores methodological characteristics, (adverse) effects, and adherence of RMT studies in NMDs. Moreover, it identifies limitations and research gaps in the literature and provides future research directions.
Summary
Eligible studies were identified using MEDLINE, Embase, Cochrane Database of Systematic Reviews, and Cochrane Central Register of Controlled Trials databases. Three reviewers independently selected articles. Inclusion criteria were English language, original research articles on RMT using a device, patients with an NMD, and pulmonary function tests or respiratory muscle strength as outcome measures. We included NMDs with slow, intermediate and fast progression. Exclusion criteria were critically ill patients, weaning from mechanical ventilation, other neurological disorders, and RMT combined with non-respiratory interventions. One reviewer extracted the data on patients’ characteristics, methodological characteristics, results of outcome measures, adverse events, and patient adherence. Forty-five studies were identified. We found a large diversity in study designs and training protocols. The effects of RMT on respiratory muscle strength and/or endurance are variable. Patient adherence was high and no serious adverse events were reported.
Key Messages
The diversity in studies across the available literature precludes definitive conclusions regarding the effects of RMT on respiratory muscle function and clinically relevant outcomes in NMDs. Therefore, well-powered and -designed studies that focus on clinically relevant outcomes and assess whether RMT can improve or offset deterioration of respiratory muscle weakness in NMDs are needed.
Keywords: Respiratory muscle training, Neuromuscular disorders, Scoping review
Introduction
Respiratory muscle weakness is a common feature in many patients with a neuromuscular disorder (NMD) [1]. Both inspiratory and expiratory muscles can be affected. Respiratory muscle weakness leads to (exertional) dyspnoea, orthopnoea, fatigue, morning headache, excessive daytime sleepiness, impaired ventilation and ineffective cough [1]. Moreover, respiratory failure is a major cause of morbidity and mortality in patients with an NMD [1, 2]. Application of (nocturnal) non-invasive mechanical ventilation (NIV) and invasive mechanical ventilation is the cornerstone of therapy for patients with an NMD and respiratory failure, and has substantially improved survival and quality of life [3, 4]. Adjunctive lung volume recruiting therapies, like air-stacking and mechanical insufflation-exsufflation, could improve lung- and chest wall mechanics and aid in clearing secretions [5, 6]. This is important as patients with an NMD are prone to develop respiratory complications such as lower respiratory tract infections and atelectasis [7]. However, the effect on clinical outcomes of these interventions is subject to debate [5, 8]. The common factor among the aforementioned therapies is that they aim to unload or assist the weakened respiratory muscle pump. In contrast, there are limited interventions aimed at improving the capacity of the respiratory muscles in NMDs.
Respiratory muscle training (RMT) is an intervention that aims to improve inspiratory and/or expiratory muscle function by increasing the load on the respiratory muscles during training sessions. The physiological rationale for the application of RMT is that skeletal muscles have a high degree of plasticity to facilitate adaptation to the loads imposed on them [9, 10]. This includes recruitment of additional motor units, increase in the frequency of muscle fibre contraction and hypertrophy of the muscle fibres. High-load, low-flow training yields strength gains, while low-load, high-flow training improves endurance [11]. Intermediate load and flow improve respiratory muscle strength as well as endurance [11, 12]. Moreover, RMT is an effective method for improving cough function, leading to avoiding mucus congestions and improved oxygenation [13, 14]. Expiratory muscle training (EMT) is able to increase expiratory strength and thus cough strength. Inspiratory muscle training (IMT) focusses on improving inspiratory strength. Both types of training are able to improve respiratory muscle endurance. The structure of respiratory muscles is comparable to other skeletal muscles [15, 16]. However, adaptations to their specific function of breathing make them different from other skeletal muscles in several aspects, including higher fatigue resistance, higher oxidative capacity, greater capillary density, and greater maximal blood flow [17]. The effects of RMT on respiratory muscle fibres in NMDs have not been studied.
In the past, health-care providers were reluctant to recommend any form of physical exercise to patients with an NMD due to the concerns of inducing muscle injury [18]. However, multiple studies in the last decade demonstrated that whole-body aerobic exercise training is generally safe and well tolerated, and improves the oxidative capacity of locomotor muscles and functional outcomes in patients with an NMD [18]. This has led to the incorporation of aerobic exercise training in standard clinical care, although high-intensity training is usually discouraged because of the risk of muscle damage. Moreover, targeted resistance training addressing specific muscles with weakness in various NMDs is more effective than overall aerobic training [19]. This suggests that the respiratory muscles in patients with an NMD and respiratory muscle weakness may also benefit from targeted exercise to prevent respiratory complications.
Despite a strong physiological rationale, recent systematic reviews on RMT in NMDs did not support the routine use of RMT in clinical practice due to data heterogeneity, small sample sizes, and a large risk of bias in a limited number of randomised controlled trials [20–23]. The strict inclusion criteria inherent to a systematic review limit the comprehensive exploration of the available literature. In contrast, a scoping review can be used to summarise findings from a body of knowledge that is heterogeneous in methods or discipline [24]. This specifically enables us to comprehensively map the study designs, training protocols (including frequency, intensity, duration, and type of training), outcome measures, adherence, and adverse events observed in RMT studies involving patients with NMD. Thus, the objectives of this scoping review were to explore: (1) methodological characteristics of RMT studies in patients with an NMD, (2) (adverse) effects of RMT in patients with an NMD, including the main study results, and (3) patient adherence to RMT. This approach facilitates the identification of research gaps and potential limitations in the available literature, aiding in the planning and commissioning of future research to advance RMT in patients with an NMD.
Methods
We used the 5-stage scoping review method published by Arksey and O’Malley [25] and further recommendations made by Levac et al. [26]. In addition, we followed the PRISMA-ScR guidelines (online suppl. material B; for all online suppl. material, see https://doi.org/10.1159/000539726). Our review protocol was not published in advance.
Search Strategy
We performed a systematic search in the MEDLINE, Embase, CDSR (Cochrane Database of Systematic Reviews), and CENTRAL (Cochrane Central Register of Controlled Trials) databases on June 23, 2023. The search strategy was developed with the guidance of an experienced librarian from the Radboud University Library, Nijmegen, the Netherlands. We used two main categories: respiratory muscle training and neuromuscular diseases. There were no limitations, including on the date of publication. The search strategy in the different databases is provided in online supplementary material A. Grey literature was not searched, and authors of the studies were contacted if the full text was not accessible via the Radboud University Library.
Study Selection
We applied the following eligibility criteria. (1) The studies had to be original research articles on RMT using a device focused on improving respiratory muscle strength and/or endurance. The device used either normocapnic hyperpnoea, flow resistive loading, or pressure threshold loading [27]. Normocapnic hyperpnoea is used for improving endurance (low load and high flow stimulus), while other devices can be used to improve both strength and endurance [11]. (2) The studies included one type of NMD or multiple NMDs. (3) Pulmonary function tests or respiratory muscle strength had to be one of the outcome measures. (4) A full-text English article had to be available.
We had no restrictions on the type of study, hence including randomised controlled trials (RCTs), quasi-randomised controlled trials (including cross-over trials and non-RCTs), pretest-posttest trials and case reports. Articles were excluded if they investigated: (1) critically ill patients, (2) weaning from mechanical ventilation, (3) other neurological disorders next to NMDs, or (4) RMT combined with non-respiratory interventions. Three reviewers (M.C.P., D.P., E.S.B.K.) independently removed the duplicates and screened the studies on title and abstract for eligibility in the Covidence online software (Veritas Health Innovation Ltd, Melbourne, VIC, Australia). Subsequently, the eligibility of the remaining studies was assessed by reading full texts. Disagreements were resolved by discussion or by consulting a fourth and fifth senior reviewer (D.L., J.D.).
Charting the Data
A data-charting form was built by one reviewer (E.S.B.K.) and evaluated by two other reviewers (D.P., M.C.P.). One reviewer (E.S.B.K.) extracted the data on demographics, inclusion and exclusion criteria, study protocol, inclusion of control groups, results of all assessed outcome measures, adverse events, adherence, and raw data on the respiratory muscle function tests. A second reviewer (M.L.S.) checked the extracted data from all tables in this review. A senior reviewer (J.D.) was contacted in case the data interpretation was challenging. Quality of the included studies was not systematically assessed as the objective was to conduct a scoping review and not a systematic review.
Synthesis of Results
For the result synthesis we divided the included studies into subgroups based on the type of NMD and explored the following data.
Patient characteristics.
Methodological characteristics, including study design, and frequency, intensity, time, and type of RMT (FITT principle).
Effects of RMT on respiratory muscle strength assessed by maximal inspiratory pressure (MIP), maximal expiratory pressure (MEP) and sniff nasal pressure (SNIP); pulmonary function consisting of forced vital capacity (FVC) or vital capacity (VC); cough strength, including peak cough flow (PCF); respiratory muscle endurance consisting of time trials (including maximal voluntary ventilation [MVV]), constant load testing or incremental load testing; functional outcome measures; and patient-reported outcomes.
Adverse events.
Patient adherence reported as percentages.
Characteristics and results of the studies are displayed in Tables 1–5. The mean (SD) or median (interquartile range) difference is shown for respiratory function outcome measures. The tables are ordered based on the type of NMD and on level of evidence. Only statistically significant differences (p < 0.05) are shown. We calculated the SD in case only the standard error of the mean was stated in the study, and we calculated the mean and SD in case this was not provided and we disclosed whether we had access to the raw data.
Table 1.
Metabolic myopathies
| Study design | First author, year of publication | NMD | N | Intervention according to FITT principle of training: Frequency, Intensity, Time, and Type | Results | ||
|---|---|---|---|---|---|---|---|
| RCT | Jones et al. [28] (2020) | LOPD
|
22 | F | 75 IMT and EMT repetitions, 5 days a week | MIP, MEP, PCF | No change |
| I | 70% of MIP and MEP | ||||||
| T | 12 weeks | Diaphragm thickness at rest | |||||
| T | Threshold IMT and EMT (threshold PEP, threshold IMT-Respironics; EMST 150-Aspire Products) | Diaphragm thickening ratio | |||||
| Control group (n = 10) performed sham-RMT (15% of MIP and MEP) | |||||||
| Pretest-posttest trial | Aslan et al. [29] (2016) | LOPD
|
8 | F | 15 min, twice a day, at least 5 days a week | MIP | Increase from 30 (22; 48) to 39 (31; 57) cmH2O |
| I | 30% of MIP | ||||||
| T | 8 weeks | MEP, FVC, PCF | No change | ||||
| T | Threshold IMT (Respironics) | ||||||
| Jevnikar et al. [30] (2015) | LOPD
|
8 | F | 15 training cycles a day for 45 min (15 min IMT and 30 min deep slow breathing) | MIP | Increase from 32 (18) to 37 (19) cmH2O | |
| I | 30% of MIP | ||||||
| T | 24 months | MEP, FVC | No change | ||||
| T | Threshold IMT (Respironics) | ||||||
| Jones et al. [31] (2016) | LOPD
|
8 | F | 3 sets of 25 IMT and EMT repetitions, 5 days a week | MIP | Increase from 48 (21) to 57 (23) cmH2O1 | |
| I | 60–70% of MIP and MEP | MEP | Increase from 78 (27) to 90 (33) cmH2O1 | ||||
| T | 12 weeks | PCF | Increase from 8 (1) to 9 (3) L/s1 | ||||
| T | Threshold IMT and EMT (threshold PEP, threshold IMT – Respironics; EMST 150-Aspire Products) | ||||||
| Smith et al. [32] (2017) | IOPD
|
9 | F | 3-4 sets of 6–10 inspiratory efforts, 3 days a week | Rest peak inspiratory flow (partial MV) | Increase (raw data N/A) | |
| I | Highest tolerated load, at least 50% of unassisted tidal volume | ||||||
| T | 90 days | Rest peak inspiratory flow (full-time MV), tidal volume | No change | ||||
| T | Threshold IMT (Threshold PEP or AccuPEEP) | ||||||
| Wenninger et al. [33] (2019) | LOPD
|
11 | F | 7 intervals with 15 inhalations each, 5 days a week | MIP | Increase from 49 (18) to 61 (29) cmH2O | |
| I | 30% of MIP, increase dependent of the Borg score | ||||||
| T | Period A: 6 weeks | ||||||
| Period C: 40 weeks | MEP, FVC | No change | |||||
| T | Threshold IMT (digital Respifit S) | ||||||
| Period B was a 6-week non-training period | |||||||
| Case report | Crisp et al. [34] (2020) | IOPD
|
1 | F | 3 sets of 25 IMT and EMT repetitions, 5 days a week | MIP, MEP, PCF | Increase |
| I | 60–70% of MIP and MEP | ||||||
| T | Two times 12 weeks (7 months inbetween) | ||||||
| T | Threshold IMT and EMT (Threshold PEP, Threshold IMT – Respironics; EMST 150-Aspire Products) | ||||||
| Jones et al. [35] (2011) | LOPD
|
2 | F | 25 IMT and EMT repetitions, 6 days a week | MIP, MEP | Increase | |
| I | Approximately 60% of MIP and MEP | ||||||
| T | 16 and 32 weeks | FVC | No change | ||||
| T | Threshold IMT and EMT (pressure threshold respiratory trainers) | ||||||
| Jones et al. [36] (2014) | IOPD
|
2 | F | 3 sets of 25 IMT and EMT repetitions, 5 days a week | MIP, MEP, PCF (tested in subject 2) | Increase | |
| I | 60–70% of MIP and MEP | ||||||
| T | 12 weeks | FVC | No change | ||||
| T | Threshold IMT and EMT (Threshold PEP, Threshold IMT – Respironics; EMST 150-Aspire Products) | ||||||
| Martin et al. [37] (1983) | LOPD
|
1 | F | 15 min, twice a day | MIP, MEP, FVC | Increase | |
| I | Resistance dependent of endurance tests | ||||||
| T | 7 months | Constant load endurance testing | |||||
| T | Resistive IMT (breathing against resistors) |
Overview of the studies in metabolic myopathies. Available baseline patient characteristics are stated under the type of NMD. In case children are included, their age at inclusion is also mentioned. The number of patients included in the analysis is shown. The training device is stated between parentheses after the type of training. In case of an improvement in outcome measures compared to either baseline or to a control group, values are provided either by mean (SD) or median (Interquartile range). Values of improvements in case reports are not provided.
EMT, expiratory muscle training; ERT, enzyme replacement therapy; FVC, forced vital capacity; IMT, inspiratory muscle training; IOPD, infantile-onset Pompe disease; LOPD, late-onset Pompe disease; MEP, maximal expiratory pressure; MIP, maximal inspiratory pressure; MV, mechanical ventilation; N/A, not available; NIV, non-invasive ventilation; NMD, neuromuscular disorder; PCF, peak cough flow; RMT, respiratory muscle training. 1Calculated from raw data.
Table 5.
Multiple neuromuscular disorders
| Study design | First author, year of publication | NMD | N | Intervention according to FITT principle of training: Frequency, Intensity, Time, and Type | Results | ||
|---|---|---|---|---|---|---|---|
| RCT | Aslan et al. [65] (2014) | DM OPDM Desminopathy Multiminicore disease FSHD Congenital myopathy LGMD SMA Type 3
|
24 | F | 15 min IMT and 15 min EMT, twice a day, at least 5 days a week | MIP | Increase from 50 (10) to 75 (17) cmH2O |
| I | 30% of MIP and MEP | MEP | Increase from 46 (19) to 60 (25) cmH2O | ||||
| T | 8 weeks | SNIP | Increase from 45 (12) to 61 (17) cmH2O | ||||
| T | Threshold IMT and EMT (threshold PEP, Threshold IMT – Respironics) | FVC, PCF | No change | ||||
| Control group (n = 10) performed sham-RMT (minimal intensity) | |||||||
| Gozal and Thiriet [67] (1999) | DMD SMA type 3
|
21 | F | Twice a day | MIP | Increase of 20 (4) cmH2O (baseline 63 (11) cmH2O) | |
| I | 30% of MIP or MEP | MEP | Increase of 27 (5) cmH2O (baseline 57 (8) cmH2O) | ||||
| T | 6 months | ||||||
| T | Threshold IMT and EMT (Threshold IMT and Threshold PEP, Healthdyne) | FVC | No change | ||||
| Control group (n = 10) performed similar exercises with no load, not further specified. Healthy subjects (n = 20) also performed the training with or without respiratory loads | |||||||
| Cross-over trial | Estrup et al. [66] 1986 | Progressive muscular dystrophy SMA type 2 and 3
|
9 | F | 4 times a day | MIP, MEP | No change |
| I | 70% of MIP (for endurance training) | FVC | Increase (raw data N/A) | ||||
| T | 4 weeks | ||||||
| T | Endurance training by resistive RMT and respiratory muscle strength training by maximal inspiration and expiration (in-house developed) | MVV in 15 s | Increase to median 141 (range 70;295) % | ||||
| Six healthy subjects were included too | |||||||
| Pretest-posttest trial | Human and Morrow [68] (2021) | DMD SMA Myopathy Degenerative neuropathy
|
3 | F | 3 sets of 30 breaths, twice a day, 5 days a week | MIP | Increase from 38 (15) to 48 (16) cmH2O |
| 3 | I | 30% of MIP | |||||
| 1 | T | 6 weeks | SNIP, PCF, FVC, VC | No change | |||
| 1 | T | Tapered flow resistive loading IMT (POWERbreathe K3) | |||||
| Klefbeck et al. [69] (2000) | Post-polio syndrome
|
7 | F | 20 min, once a day | MIP, MEP, VC, MVV in 15 s | No change | |
| I | Approximately 30% of MIP, adjusted to perceived exertion | ||||||
| T | 10 weeks | Incremental load endurance testing | Increase from mean 11 (range 5–17) to 17 (range 14–21) cmH2O and from mean 12 (range 6–18) to 18 (range 15–22) cmH2O | ||||
| T | Threshold IMT (Healthscan) | ||||||
| Koessler et al. [70] (2001) | DMD SMA
|
18 | F | 10 loaded breathing cycles of resistive training and 10 maximal static inspiratory efforts, twice a day | MIP (Group A) | Increase from 51 (21) to 87 (13) cmH2O | |
| 9 | I | 70–80% of MIP for resistive training, 90% of MIP for static efforts | MIP (Group B) | Increase from 59 (19) to 94 (30) cmH2O | |||
| T | 24 months | MIP (Group C) | Increase from 71 (23) to 99 (27) cmH2O | ||||
| T | Resistive IMT (in-house developed) | VC | No change | ||||
| Group A: VC range of 27–50% of predicted | MVV in 12 s (Group A) | Increase from 52.7 (30.5) to 69.5 (21.8) L/min | |||||
| Group B: VC range of 51–70% of predicted | MVV in 12 s (Group B) | Increase from 53.2 (22.9) to 62.4 (28.4) L/min | |||||
| Group C: VC range of 71–96% of predicted | MVV in 12 s (Group C) | Increase from 59.5 (16.1) to 70.5 (10.6) L/min | |||||
| Winkler et al. [71] (2000) | DMD SMA
|
13 | F | Twice a day | MIP | Increase (raw data N/A) | |
| I | A variable inspiratory resistance for endurance training and maximal static inspiratory efforts against an almost occluded resistance for strength training | VC | No change | ||||
| 3 | T | 9 months | MVV in 12 s | Increase (raw data N/A) | |||
| T | Resistive IMT (in-house developed) |
Overview of the studies in multiple neuromuscular disorders. Available baseline patient characteristics are stated under the type of NMD. The number of patients included in the analysis is shown. The training device is stated between parentheses after the type of training. In case of an improvement in outcome measures compared to either baseline or to a control group, values are provided by mean (SD) unless stated otherwise.
DM, myotonic dystrophy; DMD, Duchenne muscular dystrophy; EMT expiratory muscle training; FSHD, facioscapulohumeral muscular dystrophy; FVC, forced vital capacity; IMT, inspiratory muscle training; LGMD, limb-girdle muscular dystrophy; MEP, maximal expiratory pressure; MIP, maximal inspiratory pressure; MVV, maximal voluntary ventilation; N/A, not available; NIV, non-invasive ventilation; NMD, neuromuscular disorder; OPDM, oculopharyngodistal myopathy; PCF, peak cough flow; RMT, respiratory muscle training; SMA, spinal muscular atrophy; SNIP, sniff nasal pressure; VC, vital capacity. 1Calculated from raw data.
Results
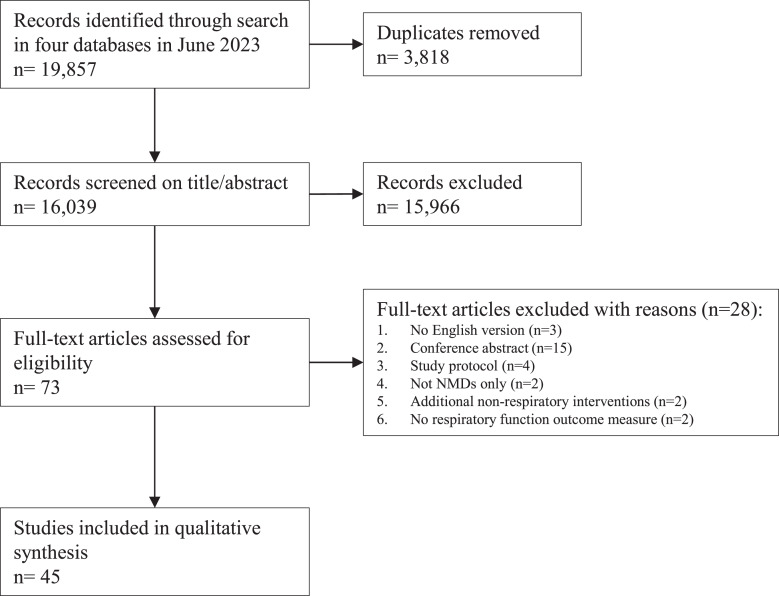
Figure 1 shows the flowchart for the selection of studies. 19,857 records were identified using the search strategy on June 23, 2023. Based on the abstract, 73 articles were selected for full-text screening. Forty-five articles met the inclusion and exclusion criteria for this review. The studies were divided based on the type of NMD: metabolic myopathies (n = 10; Table 1), disorders of the neuromuscular junction (n = 6; Table 2), motor neuron disease (n = 8; Table 3), muscular dystrophies (n = 14; Table 4), and multiple NMDs/other types of NMDs (n = 7; Table 5). Study designs were RCTs (n = 8), non-randomised controlled trials (n = 3), cross-over trials (n = 4), pretest-posttest trials (n = 21), and case reports (n = 9). The frequency, intensity, time, and type, referred to as the FITT principle [72], are used to specify the RMT. IMT (n = 24) was most frequently performed, followed by studies combining IMT and EMT(n = 12), endurance training (n = 5), and EMT only (n = 4). The duration of the training was in most studies 16 weeks or less (n = 31) and the other studies continued training for 6 months or more (n = 14) (range: 18 days–24 months). A global summary of the most important results per type of NMD is given in the next paragraphs.
Fig. 1.
Flowchart for the study selection process. NMD, neuromuscular disorder.
Table 2.
Disorders of the neuromuscular junction
| Study design | First author, year of publication | NMD | N | Intervention according to FITT principle of training: Frequency, Intensity, Time, and Type | Results | ||
|---|---|---|---|---|---|---|---|
| RCT | Fregonezi et al. [38] (2005) | Mild to moderate generalised MG
|
27 | F | 45 min, 3 times a week | MIP | Increase from 56 (22) to 71 (27) cmH2O |
| I | Increased until 60% of MIP | MEP | Increase from 103 (41) to 115 (40) cmH2O | ||||
| T | 8 weeks | FVC, MVV | No change | ||||
| T | Threshold IMT (Threshold IMT – Respironics) combined with diaphragmatic breathing and pursed lip breathing | ||||||
| Control group (n = 13) was encouraged to use breathing retraining techniques | |||||||
| Non-RCT | Freitag et al. [39] (2018) | Mild to moderate generalised MG
|
17 | F | Phase 1: 30 min, 5 days a week | MIP, VC | No change |
| Phase 2: 30 min, 5 times per 2 weeks | |||||||
| I | 50–60% of individual MVV15 | ||||||
| T | Phase 1: 4 weeks | MVV in 15 s | Increase to 109% (18.0)1 after phase 2 | ||||
| Phase 2: 12 months | Constant load endurance testing | Increase from 10.5 (4.5) to 29.5 (10.7)1 min after phase 2 | |||||
| T | Normocapnic hyperpnoea training (the device consisted of a rebreathing bag and tubing with a hole allowing to breathe in and out to fresh air) | ||||||
| Control group (n = 5) selected based on personal requirements and received no training | |||||||
| Pretest-posttest trial | Hsu et al. [40] (2020) | Mild to moderate generalised MG
|
34 | F | 30 min, twice a day, 5 days a week | MIP, MEP | No change |
| I | IMT: 30–60% of MIP | ||||||
| EMT: 15–75% of MEP | |||||||
| T | 12 weeks | FVC | Increase from 78 (12.6) to 84 (17.7) % of predicted | ||||
| Threshold IMT and EMT (Dofin Breathing Trainer) | |||||||
| T | Control group (n = 16) received no training | ||||||
| Rassler et al. [41] (2007) | Mild to moderate generalised MG
|
10 | F | 30 min, 5 days a week | MIP, VC, MVV | No change | |
| I | 50–60% of MVV | ||||||
| T | 4–6 weeks | Constant load endurance testing | Increase from 8.4 (2.8) to 17.1 (4.1) min1 | ||||
| T | Normocapnic hyperpnoea training (the device consisted of a rebreathing bag and tubing with a hole allowing to breathe in and out to fresh air) | ||||||
| Rassler et al. [42] (2011) | Mild to moderate generalised MG
|
10 | F | Phase 1: 30 min, 5 days a week | MIP, VC, MVV | No change | |
| Phase 2: 30 min, 5 times per 2 weeks | |||||||
| I | 50–60% of MVV | ||||||
| T | Phase 1: 4–6 weeks | Constant load endurance testing | Increase from 6.1 (2.5) to 20.3 (9.5) min1 | ||||
| Phase 2: 3 months | |||||||
| T | Normocapnic hyperpnoea training (the device consisted of a rebreathing bag and tubing with a hole allowing to breathe in and out to fresh air) | ||||||
| Weiner et al. [43] (1998) | Mild to severe Generalised MG
|
18 | F | 30 min, 6 times a week | MIP-mild to moderate | Increase from 57 (12) to 87 (17) cmH2O1 | |
| I | Increased until 60% of MIP or MEP | MIP-severe | Increase from 29 (17) to 46 (19) cmH2O1 | ||||
| T | 3 months | MEP-mild to moderate | Increase from 72 (11–87 (15) cmH2O1 | ||||
| T | Threshold IMT for all patients and threshold EMT only for mild to moderately affected group (Threshold Inspiratory Muscle Trainer- Healthscan; EMT with the same trainer held on the reversed side) | MEP-severe | No change | ||||
| FVC-mild to moderate | Increase from 76 (5.7) to 88 (6.3) % of predicted1 | ||||||
| FVC-severe | Increase from 35 (15.8) to 54 (14.1) % of predicted1 | ||||||
| Incremental load endurance testing-mild to moderate | Increase from 47.9 (12.6) to 72.0 (13.3) PmPeak/MIP % for 60 s1 | ||||||
| Incremental load endurance testing-severe | Increase from 26.0 (8.2) to 43.4 (10.7) %1 |
Overview of the studies in disorders of the neuromuscular junction. Available baseline patient characteristics are stated under the type of NMD. The number of patients included in the analysis is shown. The training device is stated between parentheses after the type of training. In case of an improvement in outcome measures compared to either baseline or to a control group, values are provided by mean (SD).
AZA, Azathioprine; ChEI, cholinesterase inhibitors; EMT, expiratory muscle training; FVC, forced vital capacity; IMT, inspiratory muscle training; MEP, maximal expiratory pressure; MG, myasthenia gravis; MIP, maximal inspiratory pressure; MVV, maximal voluntary ventilation; NMD, neuromuscular disorder; PmPeak, peak pressure (pressure achieved with the heaviest load, tolerated for at least 60 s); VC, vital capacity. 1SD calculated from standard error of the mean. 2Calculated from raw data.
Table 3.
Motor neuron disease
| Study design | First author, year of publication | NMD | N | Intervention according to FITT principle of training: Frequency, Intensity, Time, and Type | Results | ||
|---|---|---|---|---|---|---|---|
| RCT | Cheah et al. [44] (2009) | ALS | 15 | F | 10 min, 3 times a day | MIP, MEP, SNIP, FVC | No change |
| Progressive bulbar palsy | I | Increased until 60% of SNIP | |||||
Primary lateral sclerosis
|
1 | T | 12 weeks | ||||
| T | Threshold IMT (Respironics) | ||||||
| 3 | Control group (n = 10) performed sham-IMT (spring-loaded valve removed in IMT device) | ||||||
| Pinto et al. [45] 2012 | ALS
|
20 | F | 10 min, twice a day | MIP, MEP, SNIP, FVC, MVV in 12 s | No change | |
| I | 30–40% of MIP | ||||||
| T | 8 months | ||||||
| T | Threshold IMT (Respironics) | ||||||
| Control group (n = 9) performed sham-IMT (minimal load value) for 4 months, followed by 4 months of active IMT | |||||||
| Plowman et al. [46] 2019 | ALS
|
46 | F | 20 min, 5 days a week | MEP | Increase from 99 (46) to 126 (61) cmH2O | |
| I | 50% of MEP | ||||||
| T | 8 weeks | FVC, PCF | No change | ||||
| T | Threshold EMT (Threshold PEP – Respironics; EMST 150-Aspire Products) | ||||||
| Control group (n = 23) performed sham-EMT (spring removed) | |||||||
| Non-RCT | Vicente-Campos et al. [47] 2022 | ALS
|
20 | F | 15 repetitions, twice a day, 5 days a week | MIP | Increase of 6 (10) cmH2O (baseline 50.3 (19.9) cmH2O) |
| I | Increased until 60% of MIP | ||||||
| T | 8 weeks | ||||||
| T | Resistive IMT (POWERbreathe) | ||||||
| Control group (n = 10) received usual care | |||||||
| Pretest-posttest trial | Plowman et al. [48] 2016 | ALS
|
15 | F | 20 min, 5 days a week | MEP | Increase of 17 (23) cmH2O1 |
| I | 50% of MEP | ||||||
| T | 5 weeks | PCF | No change | ||||
| T | Threshold EMT (Threshold PEP – Respironics; EMST 150-Aspire Products) | ||||||
| Case report | Human et al. [49] 2019 | SMA type 2
|
1 | F | 15 repetitions, twice a day, 5 days a week | MIP, SNIP, PCF | Decline |
| I | 30% of MIP | ||||||
| T | 6 weeks | FVC | Increase | ||||
| T | Tapered flow resistive loading IMT (POWERbreathe K3) | ||||||
| Robison et al. [50] 2018 | Bulbar-onset ALS
|
1 | F | 25 inspiratory and 25 expiratory repetitions, 5 days a week | MIP, MEP, PCF | Increase | |
| I | 30% of MIP and MEP | ||||||
| T | 24 months | FVC | No change | ||||
| T | Threshold IMT and EMT (threshold IMT – Respironics; EMST 150-Aspire Products) | ||||||
| Tabor et al. [51] 2016 | Spinal-onset ALS
|
1 | F | 25 repetitions, 5 days a week | MEP, cough inspired volume, cough total within cough trial | Increase | |
| I | 50% of MEP | ||||||
| T | Twice 8 weeks (first 8 weeks of sham training, valve removed) | ||||||
| T | Threshold EMT (EMST 150-Aspire Products) |
Overview of the studies in motor neuron diseases. Available baseline patient characteristics are stated under the type of NMD. In case children are included, their age at inclusion is also mentioned. The number of patients included in the analysis is shown. The training device is stated between parentheses after the type of training. In case of an improvement in outcome measures compared to either baseline or to a control group, values are provided by mean (SD). Values of improvements in case reports are not provided.
ALS, amyotrophic lateral sclerosis; ALSFRS-R, Revised Amyotrophic Lateral Sclerosis Functional Rating Scale; EMT expiratory muscle training; FVC, forced vital capacity; IMT, inspiratory muscle training; MEP, maximal expiratory pressure; MIP, maximal inspiratory pressure; MVV, maximal voluntary ventilation; NIV, non-invasive ventilation; NMD, neuromuscular disorder; PCF, peak cough flow; SMA, spinal muscular atrophy; SNIP, sniff nasal pressure.
1SD calculated from standard error of the mean.
Table 4.
Muscular dystrophies
| Study design | First author, year of publication | NMD | N | Intervention according to FITT principle of training: Frequency, Intensity, Time, and Type | Results | ||
|---|---|---|---|---|---|---|---|
| RCT | Wanke et al. [52] (1994) | DMD
|
22 | F | 10 loaded breathing cycles of resistive training and 10 maximal static inspiratory efforts, twice a day | Pesmax | Increase from 3.2 (0.9) to 5.6 (1.0) kPa |
| I | Almost occluded resistance | Pdimax | Increase from 3.9 (1.1) to 6.6 (1.2) kPa | ||||
| T | 6 months | MVV in 12 s | No change | ||||
| T | Resistive IMT (in-house developed) | Endurance time trial | Increase from 14.9 (3.8) to 17.8 (3.4) min | ||||
| Control group (n = 12) received no training | |||||||
| Non-RCT | Yeldan et al. [53] (2008) | LGMD BMD
|
17 | F | 15 min, twice a day, 5 days a week | MIP | Increase of 38 (23) cmH2O (baseline 85 (36) cmH2O) |
| I | 30% of MIP | ||||||
| 6 | T | 12 weeks | MEP, FVC | No change | |||
| T | Threshold IMT (Respironics) | ||||||
| Control group (n = 12) performed breathing exercises | |||||||
| Cross-over trial | Martin et al. [73] (1986) | DMD
|
18 | F | 30 min a day (strength training) and ventilation to exhaustion 3 times (endurance training), 5 days a week | MIP, MEP, VC | No change |
| I | Maximum static inspiratory and expiratory manoeuvres at approximately 20% intervals over the VC range (strength training) and hollow acrylic tube with different central bore diameters (endurance training) | ||||||
| T | Two months | Constant load endurance testing (expiratory and inspiratory, respectively) | Increase of 6.1 (5.1) s (baseline 15.4 (7.4) s) and 8.5 (8.1) s (baseline 11.9 (3.8) s)2 | ||||
| T | Resistive IMT and EMT (in-house developed) | ||||||
| Stern et al. [55] 1991 continued training three boys for more than a year and found an increase in MIP, MEP, and endurance, but a decrease in VC | |||||||
| Rodillo et al. [54] (1989) | DMD
|
20 | F | 20 inspirations a day | MIP, MEP, FVC | No change | |
| I | Forced inspiration against a resistance that increased as inspiratory flow increased | ||||||
| T | 18 days | ||||||
| T | Resistive IMT (Triflow II Inspirometer) | ||||||
| Placebo: 10 expirations a day with a mini peak flow metre | |||||||
| Stern et al. [56] (1989) | DMD
|
12 | F | 20 min, 5 days a week | MIP, MEP, VC, endurance time trial | No change | |
| I | Resistance dependent of increases of heartbeats | ||||||
| T | Six months | ||||||
| T | Resistive IMT (in-house developed) | ||||||
| Pretest-posttest trial | Abe et al. [57] (1998) | DM
|
10 | F | 15 min, 3 times a day | MIP | Increase (raw data N/A) |
| I | 30% of MIP | MEP | Increase (raw data N/A) | ||||
| T | 16 weeks | VC | Increase (raw data N/A) | ||||
| T | Resistive IMT (PFLEX Inspiratory Muscle Trainer, Healthscan) | ||||||
| Araujo et al. [58] (2012) | DM 1 | 6 | F | 4 series of 20 repetitions, 3 days a week | MIP | Increase from 54 (43;65) to 68 (60;77) cmH2O | |
| I | Increased until 70% of MIP and MEP | MEP | Increase from 62 (54;68) to 75 (57;98) cmH2O | ||||
| T | 8 weeks | SNIP | Increase from 67 (56;73) to 69 (58;80) cmH2O | ||||
| T | Threshold IMT and EMT (Healthscan) | FVC | No change | ||||
| DiMarco et al. [59] (1985) | DMD LGMD FSHD
|
5 | F | 15–20 min, twice a day | MIP, MEP, VC, MVV in 15 s | No change | |
| 5 | I | Inspiratory resistance tolerated for at least 3 min, but less than 15 min | |||||
| 1 | T | 6 weeks, 6 patients continued for another 6 weeks | Constant load endurance testing | Increase to 228 (269) %1 | |||
| T | Resistive IMT (in-house developed) | ||||||
| Malarvizhi et al. [60] (2022) | DMD
|
5 | F | 3 repetitions | FVC | No change | |
| I | Minimal resistance | ||||||
| T | 6 weeks | ||||||
| T | Resistive IMT (Ultrabreathe) | ||||||
| Takaso et al. [61] (2010) | DMD
|
17 | F | 3 sets of 15 repetitions a day | FVC | Increase from 22 (3.1) to 26 (2.8)2 % of predicted | |
| I | 30% of MIP | ||||||
| T | 6 weeks | ||||||
| T | Threshold IMT (Respironics) | ||||||
| Topin et al. [62] (2002) | DMD
|
16 | F | 10 min, twice a day | MIP, FVC | No change | |
| I | 30% of MIP | ||||||
| T | 6 weeks | Endurance time trial | Increase from 307.6 (126.6) to 448.4 (176.7) s | ||||
| T | Threshold IMT (Healthscan) | ||||||
| Control group (n = 8) performed sham-IMT (less than 5% of MIP) | |||||||
| Vilozni et al. [63] (1994) | DMD
|
15 | F | 20 min, 6 days a week | FVC | No change | |
| I | A percentage of the individual baseline MVV, increases based on success rate | MVV in 15 s | Increase to 110 (8) % | ||||
| T | 5 weeks | Incremental load endurance testing | Increase to 130 (12) % | ||||
| T | Respiratory muscle endurance training (hyperventilation based, in-house developed) | ||||||
| Case report | Allen et al. [64] (2020) | DM 1
|
1 | F | 5 blocks of 5 repetitions, 5 days a week | MIP, MEP, SNIP, FVC, PCF | Increase |
| I | Increased until 54 cmH2O | ||||||
| T | 32 weeks | ||||||
| T | Threshold EMT (EMST150-Aspire Products) |
Overview of the studies in muscular dystrophies. Available baseline patient characteristics are stated under the type of NMD. In case children are included, their age at inclusion is also mentioned. The number of patients included in the analysis is shown. The training device is stated between parentheses after the type of training. In case of an improvement in outcome measures compared to either baseline or to a control group, values are provided by mean (SD) or median (interquartile range). Values of improvements in case reports are not provided.
BMD, Becker muscular dystrophy; DM, myotonic dystrophy; DMD, Duchenne muscular dystrophy; EMT expiratory muscle training; FSHD, facioscapulohumeral muscular dystrophy; FVC, forced vital capacity; IMT, inspiratory muscle training; LGMD, limb-girdle muscular dystrophy; MEP, maximal expiratory pressure; MIP, maximal inspiratory pressure; MVV, maximal voluntary ventilation; N/A, not available; NMD, neuromuscular disorder; PCF, peak cough flow; Pdimax, transdiaphragmatic pressure; Pesmax, maximal sniff oesophageal pressure; SNIP, sniff nasal pressure.
1SD calculated from standard error of the mean.
2Calculated from raw data.
Metabolic Myopathies
Ten studies on RMT in patients with Pompe disease were identified (Table 1) [28–37]. The participants in these studies were all on enzyme replacement therapy, except for one case report [37]. In total, 71 patients including 11 children were included in one RCT (n = 22), five pretest-posttest trials (n = 44), and four case reports (n = 5). The children were included in one of the pretest-posttest trial and two of the case reports. One case report was a repeat enrolment of a child included in a previous study after 7 months detraining [34, 36]. Nine studies investigated threshold IMT effect of which five studies combined this with threshold EMT. The pretest-posttest threshold IMT trials found an increase in MIP [29–33], and MEP and PCF increased if IMT was combined with EMT [31]. In contrast, there was no increase in MIP and MEP in the RCT on combined threshold IMT and EMT [28]. No change in FVC was found in the studies. One case report investigated resistive IMT [37].
Overall, there were no changes in functional outcome measures and patient-reported outcomes, including the 6-min walk-test (6MWT), dyspnoea symptoms, and quality of life [28–30, 33]. A high adherence to RMT was found in the studies from 80% to 107% [28, 29, 31, 33, 34, 36]. In the RCT [28] and pretest-posttest trials [31, 33], 4 patients with a headache, 3 patients with thoracic pain, 4 patients with muscle myalgia and 1 patient with light-headedness were reported. No serious adverse events directly related to RMT were reported.
Disorders of the Neuromuscular Junction
In patients with generalised myasthenia gravis (MG), we identified six studies investigating the effects of RMT (Table 2) [38–43]. Threshold IMT was performed in one RCT (n = 27) [38] and in two pretest-posttest trials (n = 52) [40, 43]. The latter two studies combined this training with threshold EMT [40, 43]. The RCT and one of the pretest-posttest trials found an increase in MIP and MEP [38, 43] and both pretest-posttest trials found an increase in FVC [40, 43]. MVV did not increase in the RCT, but endurance assessed by incremental load testing increased in one of the pretest-posttest trials [43]. One non-RCT (n = 17) and two pretest-posttest trials (n = 20) investigated normocapnic hyperpnoea training [39, 41, 42]. There was no increase in MIP and VC. Only the non-RCT found an increase in MVV [39]. The three studies did find an increase in constant load respiratory muscle endurance.
In the RCT, the quality of life questionnaire showed an improvement in the physical role domain [38]. The combined threshold IMT and EMT studies showed improvement in functional outcomes (including the 6MWT and MG composite scale), fatigue score, and dyspnoea [40, 43]. In the normocapnic hyperpnoea training studies, the functional outcome (Besinger score) improved after a prolonged maintenance period of three and 12 months [39, 42].
In the studies assessing normocapnic hyperpnoea, several patients with MG did not tolerate the rebreathing technique, the use of the training device, and the training frequency [39, 41, 42]. The training is described as strenuous and time consuming by the patients. No (serious) adverse events directly related to the training were reported.
Motor Neuron Disease
Our search identified eight studies on RMT in patients with motor neuron disease (Table 3) [44–51]. Two RCTs on threshold IMT in patients with ALS (n = 35), progressive bulbar palsy (n = 1), and primary lateral sclerosis (n = 3) found no increase in MIP, MEP, SNIP, FVC, and MVV [44, 45]. One non-RCT on resistive IMT in patients with ALS (n = 20) found an increase in MIP [47]. One RCT (n = 46), one pretest-posttest trial (n = 15), and 2 case reports (n = 2) investigated threshold EMT in patients with ALS [46, 48, 50, 51]. MEP increased in the trials, but PCF did not [46, 48]. The RCT showed no change in FVC [48]. One case report performed tapered flow resistive loading IMT [49].
No effects on quality of life, fatigue, dyspnoea, and functional outcomes (6MWT and ALS Functional Rating Scale-Revised (ALSFRS-R)) were found in the RCTs on threshold IMT [44, 45]. The non-RCT on resistive IMT did find an improvement on the ALSFRS-R, but not on quality of life [47]. The RCT on threshold EMT demonstrated that swallowing function did not deteriorate in comparison to the control group [46]. No improvement in the ALSFRS-R was shown in the threshold EMT studies [46, 48, 50, 51].
The studies showed a high adherence from 82% to 100% [44, 46, 50, 51]. No (serious) adverse events directly related to the training were reported.
Muscular Dystrophies
Fourteen RMT studies in patients with muscular dystrophy were identified (Table 4) [52–64, 73]. One of the studies in patients with Duchenne muscular dystrophy (DMD) was a follow-up [55] of a previous study [73] and thus is not included separately in Table 4. One non-RCT (n = 23) and three pretest-posttest trials (n = 39) performed threshold IMT in patients with DMD (n = 33), limb-girdle muscle dystrophy (LGMD) (n = 17), Becker muscular dystrophy (BMD) (n = 6), and myotonic dystrophy (DM) (n = 6) [53, 58, 61, 62]. The patients with DMD were mostly children. An increase in MIP was found in the non-RCT and one pretest-posttest trial [53, 58]. No change in MEP was found in the non-RCT [53]. One of the pretest-posttest trial combined IMT with EMT in patients with DM and did find an increase in MEP [58]. FVC increased in one pretest-posttest trial [61]. Endurance time increased in one of the pretest-posttest trials [62]. One case report investigated threshold EMT in DM [60].
Seven studies investigated the effect of resistive IMT, including one RCT (n = 22), three cross-over trials (n = 50), and three pretest-posttest trials (n = 26) [52, 54, 56, 57, 59, 60, 73]. Patients with DMD (n = 82), LGMD (n = 5), DM (n = 10) and facioscapulohumeral muscular dystrophy (FSHD) (n = 1) were included. One pretest-posttest trial found an increase in MIP, MEP, and VC [57]. The RCT assessed respiratory muscle strength by maximal oesophageal and transdiaphragmatic sniff pressure and found an increase [52]. The endurance time increased in the RCT, but not in one cross-over trial assessing this outcome [56]. Constant load respiratory muscle endurance increased in a cross-over trial [73] and in a pretest-posttest trial [59].
The effect of a hyperventilation-based training was investigated in 15 boys with DMD in a pretest-posttest trial [63]. FVC did not change, but MVV and incremental load endurance did increase.
One patient complained of light-headedness [61]. No other (serious) adverse events directly related to the training were reported. In the RCT, creatine kinase levels as a sign of muscle injury were not elevated [52].
Studies in Multiple/Other Types of NMDs
Seven studies on multiple types of NMDs were identified (Table 5) [65–68, 70, 71]. An RCT in patients with slowly progressive NMDs (n = 24) [65] and an RCT in children with DMD and spinal muscular atrophy (SMA) type 3 (n = 21) [67] on threshold IMT and EMT found increases in MIP and MEP, but found no change in FVC.
A cross-over trial and two pretest-posttest trials on resistive IMT were performed [66, 70, 71]. The cross-over trial included patients with progressive muscular dystrophy and SMA (n = 9) and found an increase in FVC and MVV but no change in MIP and MEP [66]. The pretest-posttest trials found increases in MIP and MVV in children with DMD (n = 31) and with SMA (n = 12) [70, 71]. No increase in VC was seen in both studies.
Tapered flow resistive loading IMT was performed in a pretest-posttest trial in 8 children with DMD (n = 3), SMA (n = 3), myopathy (n = 1), or degenerative neuropathy (n = 1) [68]. MIP increased, but SNIP, PCF, FVC, and VC did not change. Functional outcomes (Brooke and Vignos scales) and quality of life did not improve, but upper limb function and coordination improved in the motor function measure.
A pretest-posttest trial on threshold IMT in patients with post-polio syndrome (n = 7) found an increase in incremental load endurance and no effect on MIP, MEP, VC, and MVV (Table 5) [69].
Two studies reported an adherence of 85% and 97% [68, 69]. One patient reported fatigue for 3 days after an increased training load [69]. No other (serious) adverse events directly related to the training were reported. No increase in creatine kinase levels was found after the training in one study [71].
Discussion
In this scoping review, we found that: (1) there is a large diversity in study designs and training protocols with varying frequencies of training sessions, intensities, duration and types of training; (2) the effects of RMT on respiratory muscle strength and/or endurance are variable between and within different types of NMDs; and (3) adherence was high and no serious adverse events were reported. Furthermore, we identified the following research gaps: (1) relevant patient characteristics are lacking in most studies. (2) the long-term effects of RMT in NMDs are unknown; (3) clinically relevant outcome measures are underexposed; (4) the association between the degree of respiratory muscle weakness and the effect of RMT is unknown; (5) objective monitoring of training adherence is lacking; and (6) there are no studies that investigated the synergistic effect between RMT and other treatment options.
The variability in the effect of RMT between studies can be explained by several factors. First, there is a large diversity in the design and level of evidence, ranging from RCTs to case reports. Only eight RCTs were performed, whereas most studies (n = 21) used pretest-posttest trial designs. The latter design is sensitive to bias, such as the risk of regression to the mean, sample selection and introducing confounders. Moreover, the absence of a control group lacks the possibility to verify whether the observed effect is caused by the intervention. Second, the sample sizes in most studies are small due to the rare character of NMDs. Third, study populations differ much between studies from rapidly (e.g., ALS) to slowly progressive disorders (e.g., Pompe disease). The effect of RMT on disorders that affect the motor neuron may be different from disorders that affect the muscles. Fourth, the training characteristics vary between studies. Most studies perform IMT, but other studies perform EMT, respiratory muscle endurance training or a combination of these methods. Moreover, the training duration varied much, ranging from 18 days to 2 years. In addition, there was a large variation in the training device used, the training intensity and the number of repetitions per day.
The variable effects of RMT in NMDs we found are in agreement with the trend found in exercise studies in NMDs. A recently updated Cochrane review assessed the effects of strength and aerobic exercise training in patients with an NMD [74]. The review stated that there is insufficient evidence to conclude that exercise is beneficial, but that there are positive effects in specific NMDs. Despite the absence of clear-cut conclusions, the positive effects of training on locomotor muscles in patients with an NMD that have been found, in combination with the safe and well-tolerated nature of exercise, has led to the recommendation of aerobic exercise training as part of standard care for several muscular dystrophies [75, 76].
Research Gaps
In addition to the evident lack of studies with a control group involving a sufficient number of patients, this scoping review has enabled us to identify further research gaps in the current literature. First, most studies do not report detailed patient characteristics. Characteristics that are often not reported include disability levels, limitations in activities of daily living, and disease duration. It is important to state information on these characteristics as these can affect training results. For example, the effects of RMT may be different in recently diagnosed patients in a relatively good condition compared to patients in a more progressive disease state.
Second, the long-term effects of RMT in NMDs are uncertain. Only a few trials investigated the effect of RMT for a duration of a year or longer [30, 39, 70]. These studies show contradicting results ranging from continued improvement, reaching a plateau phase and a decline after 6 months. It should be noted that disease progression also affects respiratory function and outcome measures.
Third, important clinically relevant outcome measures are underexposed in the current studies, while the ultimate goal of RMT is to improve these outcomes. The lack of effect on pulmonary function questions the clinical relevance of RMT. Functional and patient-reported outcome measures are not assessed in most studies and the studies that investigated these outcomes did not show an improvement [28–30, 33, 44–46, 48, 50, 51, 68]. The effect on the start of NIV or invasive mechanical ventilation and on the frequency of respiratory tract infections or hospitalisation has not been assessed at all.
Fourth, little is known on the association between the degree of respiratory muscle weakness and the effect of RMT. We identified one study that found that the degree of respiratory muscle endurance improvement positively correlated with the baseline respiratory muscle function of the patients [59]. Based on this finding, the authors of this study suggest that the largest improvement in endurance is achieved when the respiratory muscle function is relatively spared. The range of the degree of respiratory muscle weakness to effectively apply RMT is important to know.
Fifth, most studies apply home-based training with a device that does not store the training data or evaluate the quality of the training. Thus, there is no check if the patient applied the technique properly. Moreover, most of the time there is not enough detail on how exact breathing instructions were provided and the adherence is mostly self-reported. Hence, the performance of patients is not assessed objectively in most studies, which limits the possibility of evaluation if this affects the results.
Last, there are no studies that investigated the synergistic effect between RMT and other treatment options. In several studies, patients are using medication at the start of the study, for example, enzyme replacement therapy in Pompe disease, riluzole in ALS, and cholinesterase inhibitors in MG [28–36, 38, 39, 41–45]. This precludes the assessment of a possible synergistic effect between RMT and other treatments, despite its close alignment with clinical practice.
Future Research Directions
The low prevalence of NMDs presents a challenge in recruiting a large number of patients in a single centre. To overcome this limitation (inter)national collaboration to initiate multicentre trials that investigate RMT in specific NMDs should be encouraged. Although challenging, successful attempts have been made to study novel drug therapies in international multicentre connections in relatively large groups of NMDs, such as FSHD and MG [77, 78]. Alternatively, grouping NMDs instead of targeting single disorders can be a viable option to increase recruitment. Careful consideration should be taken when allocating NMDs to a group. It seems rational to select NMDs based on their rate of disease progression, or type of NMD (such as motor neuron disease or muscular dystrophy). Another possible selection could be based on the degree of respiratory muscle weakness, as this could help assess the optimal timing for RMT. Smaller studies focussing on specific NMDs where RMT has not yet been investigated, such as congenital myopathies, may be necessary to provide an initial rationale for RMT in these disorders [79].
Future trials should ideally be designed as a (multicentre) RCT using sham-RMT with no load as a control group since sham-RMT with a low load could cause a positive effect in the control group [28, 65]. However, this effect may also be present in sham-RMT with no load [44]. Additionally, the duration should be extended to at least 1 year, instead of several months, to evaluate the long-term effects and adherence.
The choice of RMT device in future studies should be made carefully. There are many available RMT devices which can be divided in normocapnic hyperpnoea, flow resistive loading, and pressure threshold loading [27]. Patients find normocapnic hyperpnoea training strenuous and difficult to perform [39, 41, 42]. Pressure threshold trainers are most often used [28–32, 34–36, 38, 40, 43–46, 48–51, 53, 58, 61, 62, 64, 65, 67, 69]. One of the advantages of these threshold trainers is that they are not susceptible to variations in the patients’ airflow rate in contrast to flow resistive loading [27]. However, objective monitoring of home-based trainings is not possible with most of these devices. As patient performance is important to evaluate too, we advise the use of electronic RMT devices in future studies. These devices allow performance monitoring by storing the data of the training sessions. Future developments of e-health interventions will be able to keep track of the training even more accurately. Moreover, in case difficulties occur while training, the patients can get into contact with the researchers more easily. In line with this, detailed breathing instructions should be provided and reported [80]. Deep and fast inspirations focussing on volume expansion during inspiration might be useful in this group [80].
The training protocol should be based on the primary aim of the study, i.e., whether to improve respiratory muscle strength, endurance or both. Based on this decision, a high, low or intermediate load should be selected, respectively. To date, no studies investigated the dose-response effects of RMT in NMDs. Therefore, it is challenging to provide a specific training protocol with the appropriate frequency, intensity, and duration of RMT.
To evaluate the effect of RMT on respiratory muscle function in clinical trials several outcome measures can be chosen depending on the type of RMT [81]. IMT focussed on improving strength will most likely improve MIP and SNIP. MEP and cough strength assessed by PCF are most likely to improve in EMT focussed on improving strength. Note that because of a learning effect in the performance of these tests, the patient has to be tested at least two times on two separate occasions before starting RMT to get reliable baseline values [82]. The effect of endurance RMT can be evaluated by (1) time trials of MVV, (2) constant load testing by measuring the maximal time the respiratory muscles can sustain a constant load for each breath [83], or (3) incremental load testing by increasing resistance/threshold load or minute ventilation [81].
Additional outcome measures should be assessed in future studies to evaluate clinically relevant effects. First, functional and patient-reported outcome measures, for example, dyspnoea and quality of life, should be included in future studies to investigate the effect on the daily life of patients. Second, important clinical outcome measures like the occurrence of respiratory tract infections, survival, and hospitalisation should be assessed. Third, future studies should investigate if RMT in NMDs is able to offset respiratory muscle deterioration. In line with this is the question of whether or not the start of NIV or invasive mechanical ventilation could be delayed by RMT and if the hours of ventilation could be reduced.
Study Limitations
Our review has several limitations. First, we restricted our search to English-language full-text studies and did not include grey literature, which may have resulted in the exclusion of relevant publications despite a broad study selection. Second, the data extraction was performed by one reviewer, which may have introduced the risk of misinterpretation of study results or unintentional selection bias. To ensure the accuracy and robustness of our results, the data extraction table was checked by a second reviewer. Third, we did not register the protocol of our scoping review. Protocol registration promotes transparency and helps prevent selective reporting bias. However, it is less important for scoping reviews compared to systematic reviews due to their broader, more exploratory nature, flexible methodology, and focus on mapping literature rather than synthesising specific evidence. Last, we did not perform a formal quality appraisal of the studies included in our review. Quality assessment is a mandatory step in systematic reviews but is optional for scoping reviews. One of the primary objectives of our scoping review was to identify limitations and research gaps in the literature and to provide future research directions. We determined that a quality assessment was not necessary to achieve this objective.
Conclusion
There is a strong physiological rationale for the use of RMT in NMDs. However, the large diversity in study designs, study populations, and training protocols across the available literature precludes the ability to draw definitive and generalisable conclusions on the effects of RMT on respiratory muscle function and clinical outcomes. Nevertheless, health-care providers are faced with the reality of clinical practice and the absence of alternatives to target respiratory muscle function in NMDs. Therefore, it is imperative to conduct future well-powered and -designed sham-controlled studies that focus on the long-term effects of RMT and its impact on clinically relevant outcomes. Electronic RMT devices should be used for objective monitoring. Furthermore, in order to determine the optimal timing and application of RMT, it is crucial to assess its ability to improve or offset deterioration of respiratory muscle weakness in NMDs with varying degrees of respiratory muscle weakness and rates of progression.
Acknowledgements
Several authors are members of the Netherlands Neuromuscular Center (NL-NMD) and the European Reference Network for Rare Neuromuscular diseases (EURO-NMD).
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This work was financially supported by A Foundation Building Strength and the Princess Beatrix Spierfonds (Grant No. W.OR17-08). The funders had no role in the design, data collection, data analysis, and reporting of this study.
Author Contributions
Esmee S.B. van Kleef and Diego Poddighe: collecting and analysing data and writing article. Mayra Caleffi Pereira: collecting and analysing data. Marit L. Schuurbiers: collecting and analysing data and reviewing article. Jan T. Groothuis, Peter J. Wijkstra, Nicol C. Voermans, and. Rik Gosselink: reviewing article. Daniel Langer and Jonne Doorduin: collecting and analysing data and writing article.
Funding Statement
This work was financially supported by A Foundation Building Strength and the Princess Beatrix Spierfonds (Grant No. W.OR17-08). The funders had no role in the design, data collection, data analysis, and reporting of this study.
Supplementary Material.
Supplementary Material.
References
- 1. Boentert M, Wenninger S, Sansone VA. Respiratory involvement in neuromuscular disorders. Curr Opin Neurol. 2017;30(5):529–37. [DOI] [PubMed] [Google Scholar]
- 2. Ambrosino N, Carpenè N, Gherardi M. Chronic respiratory care for neuromuscular diseases in adults. Eur Respir J. 2009;34(2):444–51. [DOI] [PubMed] [Google Scholar]
- 3. Simonds AK, Muntoni F, Heather S, Fielding S. Impact of nasal ventilation on survival in hypercapnic Duchenne muscular dystrophy. Thorax. 1998;53(11):949–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bourke SC, Tomlinson M, Williams TL, Bullock RE, Shaw PJ, Gibson GJ. Effects of non-invasive ventilation on survival and quality of life in patients with amyotrophic lateral sclerosis: a randomised controlled trial. Lancet Neurol. 2006;5(2):140–7. [DOI] [PubMed] [Google Scholar]
- 5. Sheers N, Howard ME, Berlowitz DJ. Respiratory adjuncts to NIV in neuromuscular disease. Respirology. 2019;24(6):512–20. [DOI] [PubMed] [Google Scholar]
- 6. Pfeffer G, Povitz M. Respiratory management of patients with neuromuscular disease: current perspectives. Degener Neurol Neuromuscul Dis. 2016;6:111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Voulgaris A, Antoniadou M, Agrafiotis M, Steiropoulos P. Respiratory involvement in patients with neuromuscular diseases: a narrative review. Pulm Med. 2019;2019:2734054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morrow B, Argent A, Zampoli M, Human A, Corten L, Toussaint M. Cough augmentation techniques for people with chronic neuromuscular disorders. Cochrane Database Syst Rev. 2021;4(4):Cd013170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Qaisar R, Bhaskaran S, Van Remmen H. Muscle fiber type diversification during exercise and regeneration. Free Radic Biol Med. 2016;98:56–67. [DOI] [PubMed] [Google Scholar]
- 10. Welch JF, Kipp S, Sheel AW. Respiratory muscles during exercise: mechanics, energetics, and fatigue. Curr Opin Physiol. 2019;10:102–9. [Google Scholar]
- 11. Tzelepis GE, Vega DL, Cohen ME, Fulambarker AM, Patel KK, McCool FD. Pressure-flow specificity of inspiratory muscle training .J Appl Physiol. 1994;77(2):795–801. [DOI] [PubMed] [Google Scholar]
- 12. Romer LM, McConnell AK. Specificity and reversibility of inspiratory muscle training. Med Sci Sports Exerc. 2003;35(2):237–44. [DOI] [PubMed] [Google Scholar]
- 13. Andrani F, Aiello M, Bertorelli G, Crisafulli E, Chetta A. Cough, a vital reflex. mechanisms, determinants and measurements. Acta Biomed. 2019;89(4):477–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. He Y, Zhao C, Liu Y. Effects of respiratory muscle training on cough function in neurological disorders: a systematic review with meta-analysis. NeuroRehabilitation. 2021;48(4):441–9. [DOI] [PubMed] [Google Scholar]
- 15. Ramirez-Sarmiento A, Orozco-Levi M, Guell R, Barreiro E, Hernandez N, Mota S, et al. Inspiratory muscle training in patients with chronic obstructive pulmonary disease: structural adaptation and physiologic outcomes. Am J Respir Crit Care Med. 2002;166(11):1491–7. [DOI] [PubMed] [Google Scholar]
- 16. Gransee HM, Mantilla CB, Sieck GC. Respiratory muscle plasticity. Compr Physiol. 2012;2(2):1441–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Edwards Rht FJ. Structure and function of the respiratory muscles. In: Roussos C, editor. The thorax, Part B: applied physiology. 2nd ed. New York, Basel, Hong Kong: Marcel Dekker; 1995. p. 185–217. [Google Scholar]
- 18. Preisler N, Orngreen MC. Exercise in muscle disorders: what is our current state? Curr Opin Neurol. 2018;31(5):610–7. [DOI] [PubMed] [Google Scholar]
- 19. Anziska Y, Sternberg A. Exercise in neuromuscular disease. Muscle Nerve. 2013;48(1):3–20. [DOI] [PubMed] [Google Scholar]
- 20. Watson K, Egerton T, Sheers N, Retica S, McGaw R, Clohessy T, et al. Respiratory muscle training in neuromuscular disease: a systematic review and meta-analysis. Eur Respir Rev. 2022;31(166):220065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Human A, Corten L, Jelsma J, Morrow B. Inspiratory muscle training for children and adolescents with neuromuscular diseases: a systematic review. Neuromuscul Disord. 2017;27(6):503–17. [DOI] [PubMed] [Google Scholar]
- 22. Silva IS, Pedrosa R, Azevedo IG, Forbes AM, Fregonezi GA, Dourado Junior ME, et al. Respiratory muscle training in children and adults with neuromuscular disease. Cochrane Database Syst Rev. 2019;9:Cd011711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Williamson E, Pederson N, Rawson H, Daniel T. The effect of inspiratory muscle training on duchenne muscular dystrophy: a meta-analysis. Pediatr Phys Ther. 2019;31(4):323–30. [DOI] [PubMed] [Google Scholar]
- 24. Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for Scoping Reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–73. [DOI] [PubMed] [Google Scholar]
- 25. Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32. [Google Scholar]
- 26. Levac D, Colquhoun H, O'Brien KK. Scoping studies: advancing the methodology. Implement Sci. 2010;5:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sapienza CM. Respiratory muscle strength training applications. Curr Opin Otolaryngol Head Neck Surg. 2008;16(3):216–20. [DOI] [PubMed] [Google Scholar]
- 28. Jones HN, Kuchibhatla M, Crisp KD, Hobson-Webb LD, Case L, Batten MT, et al. Respiratory muscle training in late-onset Pompe disease: results of a sham-controlled clinical trial. Neuromuscul Disord. 2020;30(11):904–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Aslan GK, Huseyinsinoglu BE, Oflazer P, Gurses N, Kiyan E. Inspiratory muscle training in late-onset Pompe disease: the effects on pulmonary function tests, quality of life, and Sleep quality. Lung. 2016;194(4):555–61. [DOI] [PubMed] [Google Scholar]
- 30. Jevnikar M, Kodric M, Cantarutti F, Cifaldi R, Longo C, Della Porta R, et al. Respiratory muscle training with enzyme replacement therapy improves muscle strength in late: onset Pompe disease. Mol Genet Metab Rep. 2015;5:67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jones HN, Crisp KD, Robey RR, Case LE, Kravitz RM, Kishnani PS. Respiratory Muscle Training (RMT) in Late-Onset Pompe Disease (LOPD): effects of training and detraining. Mol Genet Metab. 2016;117(2):120–8. [DOI] [PubMed] [Google Scholar]
- 32. Smith BK, Martin AD, Lawson LA, Vernot V, Marcus J, Islam S, et al. Inspiratory muscle conditioning exercise and diaphragm gene therapy in Pompe disease: clinical evidence of respiratory plasticity. Exp Neurol. 2017;287(Pt 2):216–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wenninger S, Greckl E, Babačić H, Stahl K, Schoser B. Safety and efficacy of short- and long-term inspiratory muscle training in Late-Onset Pompe Disease (LOPD): a pilot study. J Neurol. 2019;266(1):133–47. [DOI] [PubMed] [Google Scholar]
- 34. Crisp KD, Case LE, Kravitz RM, Kishnani PS, Jones HN. Training, detraining, and retraining: two 12-week respiratory muscle training regimens in a child with infantile-onset Pompe disease. J Pediatr Rehabil Med. 2020;13(1):71–80. [DOI] [PubMed] [Google Scholar]
- 35. Jones HN, Moss T, Edwards L, Kishnani PS. Increased inspiratory and expiratory muscle strength following Respiratory Muscle Strength Training (RMST) in two patients with late-onset Pompe disease. Mol Genet Metab. 2011;104(3):417–20. [DOI] [PubMed] [Google Scholar]
- 36. Jones HN, Crisp KD, Moss T, Strollo K, Robey R, Sank J, et al. Effects of Respiratory Muscle Training (RMT) in children with infantile-onset Pompe disease and respiratory muscle weakness. J Pediatr Rehabil Med. 2014;7(3):255–65. [DOI] [PubMed] [Google Scholar]
- 37. Martin RJ, Sufit RL, Ringel SP, Hudgel DW, Hill PL. Respiratory improvement by muscle training in adult-onset acid maltase deficiency. Muscle Nerve. 1983;6(3):201–3. [DOI] [PubMed] [Google Scholar]
- 38. Fregonezi GA, Resqueti VR, Güell R, Pradas J, Casan P. Effects of 8-week, interval-based inspiratory muscle training and breathing retraining in patients with generalized myasthenia gravis. Chest. 2005;128(3):1524–30. [DOI] [PubMed] [Google Scholar]
- 39. Freitag S, Hallebach S, Baumann I, Kalischewski P, Rassler B. Effects of long-term respiratory muscle endurance training on respiratory and functional outcomes in patients with Myasthenia gravis. Respir Med. 2018;144:7–15. [DOI] [PubMed] [Google Scholar]
- 40. Hsu CW, Lin HC, Tsai WC, Lai YR, Huang CC, Su YJ, et al. Respiratory muscle training improves functional outcomes and Reduces fatigue in patients with myasthenia gravis: a single-center hospital-based prospective study. BioMed Res Int. 2020;2020:2923907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rassler B, Hallebach G, Kalischewski P, Baumann I, Schauer J, Spengler CM. The effect of respiratory muscle endurance training in patients with myasthenia gravis. Neuromuscul Disord. 2007;17(5):385–91. [DOI] [PubMed] [Google Scholar]
- 42. Rassler B, Marx G, Hallebach S, Kalischewski P, Baumann I. Long-term respiratory muscle endurance training in patients with myasthenia gravis: first results after four months of training. Autoimmune Dis. 2011;2011:808607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Weiner P, Gross D, Meiner Z, Ganem R, Weiner M, Zamir D, et al. Respiratory muscle training in patients with moderate to severe myasthenia gravis. Can J Neurol Sci. 1998;25(3):236–41. [DOI] [PubMed] [Google Scholar]
- 44. Cheah BC, Boland RA, Brodaty NE, Zoing MC, Jeffery SE, McKenzie DK, et al. INSPIRATIonAL--INSPIRAtory muscle training in amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2009;10(5–6):384–92. [DOI] [PubMed] [Google Scholar]
- 45. Pinto S, Swash M, de Carvalho M. Respiratory exercise in amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2012;13(1):33–43. [DOI] [PubMed] [Google Scholar]
- 46. Plowman EK, Tabor-Gray L, Rosado KM, Vasilopoulos T, Robison R, Chapin JL, et al. Impact of expiratory strength training in amyotrophic lateral sclerosis: results of a randomized, sham-controlled trial. Muscle Nerve. 2019;59(1):40–6. [DOI] [PubMed] [Google Scholar]
- 47. Vicente-Campos D, Sanchez-Jorge S, Chicharro JL, Becerro-de Bengoa-Vallejo R, Rodriguez-Sanz D, García AR, et al. POWERbreathe® inspiratory muscle training in amyotrophic lateral sclerosis. J Clin Med. 2022;11(22):6655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Plowman EK, Watts SA, Tabor L, Robison R, Gaziano J, Domer AS, et al. Impact of expiratory strength training in amyotrophic lateral sclerosis. Muscle Nerve. 2016;54(1):48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Human A, Honey E, Morrow B. Inspiratory muscle training in severe spinal muscular atrophy: a case report. Int J Therapy Rehabilitation. 2019;26(4):1–19. [Google Scholar]
- 50. Robison R, Tabor-Gray LC, Wymer JP, Plowman EK. Combined respiratory training in an individual with C9orf72 amyotrophic lateral sclerosis. Ann Clin Transl Neurol. 2018;5(9):1134–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tabor LC, Rosado KM, Robison R, Hegland K, Humbert IA, Plowman EK. Respiratory training in an individual with amyotrophic lateral sclerosis. Ann Clin Transl Neurol. 2016;3(10):819–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wanke T, Toifl K, Merkle M, Formanek D, Lahrmann H, Zwick H. Inspiratory muscle training in patients with Duchenne muscular dystrophy. Chest. 1994;105(2):475–82. [DOI] [PubMed] [Google Scholar]
- 53. Yeldan I, Gurses HN, Yuksel H. Comparison study of chest physiotherapy home training programmes on respiratory functions in patients with muscular dystrophy. Clin Rehabil. 2008;22(8):741–8. [DOI] [PubMed] [Google Scholar]
- 54. Rodillo E, Noble-Jamieson CM, Aber V, Heckmatt JZ, Muntoni F, Dubowitz V. Respiratory muscle training in Duchenne muscular dystrophy. Arch Dis Child. 1989;64(5):736–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Stern LM, Martin AJ, Jones N, Garrett R, Yeates J. Respiratory training in Duchenne dystrophy. Dev Med Child Neurol. 1991;33(7):649. [DOI] [PubMed] [Google Scholar]
- 56. Stern LM, Martin AJ, Jones N, Garrett R, Yeates J. Training inspiratory resistance in Duchenne dystrophy using adapted computer games. Dev Med Child Neurol. 1989;31(4):494–500. [DOI] [PubMed] [Google Scholar]
- 57. Abe K, Matsuo Y, Kadekawa J, Inoue S, Yanagihara T. Respiratory training for patients with myotonic dystrophy. Neurology. 1998;51(2):641–2. [DOI] [PubMed] [Google Scholar]
- 58. Araujo TL, et al. Effects of respiratory muscle training on respiratory muscle strength and heart rate variability in myotonic dystrophy patients type 1. J Respir CardioVascular Phys Ther. 2012;1(1):3–8. [Google Scholar]
- 59. DiMarco AF, Kelling JS, DiMarco MS, Jacobs I, Shields R, Altose MD. The effects of inspiratory resistive training on respiratory muscle function in patients with muscular dystrophy. Muscle Nerve. 1985;8(4):284–90. [DOI] [PubMed] [Google Scholar]
- 60. Malarvizhi D, Hariharan S. Effectiveness of inspiratory muscle training using Ultrabreathe on pulmonary function in duchenne muscular dystrophy. Res J Pharm Technol. 2022;15(1):193–6. [Google Scholar]
- 61. Takaso M, Nakazawa T, Imura T, Fukushima K, Saito W, Shintani R, et al. Preoperative inspiratory muscle training for patients with severe scoliosis and high-risk pulmonary dysfunction in duchenne muscular dystrophy. Eur J Orthop Surg Traumatol. 2010;20(2):113–21. [Google Scholar]
- 62. Topin N, Matecki S, Le Bris S, Rivier F, Echenne B, Prefaut C, et al. Dose-dependent effect of individualized respiratory muscle training in children with Duchenne muscular dystrophy. Neuromuscul Disord. 2002;12(6):576–83. [DOI] [PubMed] [Google Scholar]
- 63. Vilozni D, Bar-Yishay E, Gur I, Shapira Y, Meyer S, Godfrey S. Computerized respiratory muscle training in children with Duchenne muscular dystrophy. Neuromuscul Disord. 1994;4(3):249–55. [DOI] [PubMed] [Google Scholar]
- 64. Allen J, Astin R, Smith C, Banks D, Turner C. Expiratory muscle strength training improves measures of pressure generation and cough strength in a patient with myotonic dystrophy type 1. Neuromuscul Disord. 2020;30(9):750–5. [DOI] [PubMed] [Google Scholar]
- 65. Aslan GK, Gurses HN, Issever H, Kiyan E. Effects of respiratory muscle training on pulmonary functions in patients with slowly progressive neuromuscular disease: a randomized controlled trial. Clin Rehabil. 2014;28(6):573–81. [DOI] [PubMed] [Google Scholar]
- 66. Estrup C, Lyager S, Noeraa N, Olsen C. Effect of respiratory muscle training in patients with neuromuscular diseases and in normals. Respiration. 1986;50(1):36–43. [DOI] [PubMed] [Google Scholar]
- 67. Gozal D, Thiriet P. Respiratory muscle training in neuromuscular disease: long-term effects on strength and load perception. Med Sci Sports Exerc. 1999;31(11):1522–7. [DOI] [PubMed] [Google Scholar]
- 68. Human A, Morrow BM. Inspiratory muscle training in children and adolescents living with neuromuscular diseases: a pre-experimental study. S Afr J Physiother. 2021;77(1):1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Klefbeck B, Lagerstrand L, Mattsson E. Inspiratory muscle training in patients with prior polio who use part-time assisted ventilation. Arch Phys Med Rehabil. 2000;81(8):1065–71. [DOI] [PubMed] [Google Scholar]
- 70. Koessler W, Wanke T, Winkler G, Nader A, Toifl K, Kurz H, et al. 2 Years' experience with inspiratory muscle training in patients with neuromuscular disorders. Chest. 2001;120(3):765–9. [DOI] [PubMed] [Google Scholar]
- 71. Winkler G, Zifko U, Nader A, Frank W, Zwick H, Toifl K, et al. Dose-dependent effects of inspiratory muscle training in neuromuscular disorders. Muscle Nerve. 2000;23(8):1257–60. [DOI] [PubMed] [Google Scholar]
- 72. ACSM . ACSM’s guidelines for exercise testing and prescription .10th edPhiladelphia: Wolters Kluwer, 2018. [Google Scholar]
- 73. Martin AJ, Stern L, Yeates J, Lepp D, Little J. Respiratory muscle training in Duchenne muscular dystrophy. Dev Med Child Neurol. 1986;28(3):314–8. [DOI] [PubMed] [Google Scholar]
- 74. Voet NB, van der Kooi EL, van Engelen BG, Geurts AC. Strength training and aerobic exercise training for muscle disease. Cochrane Database Syst Rev. 2019;12(12):Cd003907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Narayanaswami P, Weiss M, Selcen D, David W, Raynor E, Carter G, et al. Evidence-based guideline summary: diagnosis and treatment of limb-girdle and distal dystrophies: report of the guideline development subcommittee of the American Academy of Neurology and the practice issues review panel of the American Association of Neuromuscular & Electrodiagnostic Medicine. Neurology. 2014;83(16):1453–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tawil R, Kissel JT, Heatwole C, Pandya S, Gronseth G, Benatar M, et al. Evidence-based guideline summary: evaluation, diagnosis, and management of facioscapulohumeral muscular dystrophy: report of the guideline development, dissemination, and implementation subcommittee of the American academy of neurology and the practice issues review panel of the American association of neuromuscular & electrodiagnostic medicine. Neurology. 2015;85(4):357–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Mellion ML, Ronco L, Berends CL, Pagan L, Brooks S, van Esdonk MJ, et al. Phase 1 clinical trial of losmapimod in facioscapulohumeral dystrophy: Safety, tolerability, pharmacokinetics, and target engagement. Br J Clin Pharmacol. 2021;87(12):4658–69. [DOI] [PubMed] [Google Scholar]
- 78. Howard JF Jr., Bresch S, Genge A, Hewamadduma C, Hinton J, Hussain Y, et al. Safety and efficacy of zilucoplan in patients with generalised myasthenia gravis (RAISE): a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Neurol. 2023;22(5):395–406. [DOI] [PubMed] [Google Scholar]
- 79. van Kleef ESB, Langer D, van Engelen BGM, Ottenheijm CAC, Voermans NC, Doorduin J. Inspiratory muscle training in nemaline myopathy. J Neuromuscul Dis. 2023;10(5):825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Van Hollebeke M, Gosselink R, Langer D. Training specificity of inspiratory muscle training methods: a randomized trial. Front Physiol. 2020;11:576595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Laveneziana P, Albuquerque A, Aliverti A, Babb T, Barreiro E, Dres M, et al. ERS statement on respiratory muscle testing at rest and during exercise. Eur Respir J. 2019;53(6):1801214. [DOI] [PubMed] [Google Scholar]
- 82. Terzi N, Corne F, Mouadil A, Lofaso F, Normand H. Mouth and nasal inspiratory pressure: learning effect and reproducibility in healthy adults. Respiration. 2010;80(5):379–86. [DOI] [PubMed] [Google Scholar]
- 83. Matecki S, Topin N, Hayot M, Rivier F, Echenne B, Prefaut C, et al. A standardized method for the evaluation of respiratory muscle endurance in patients with Duchenne muscular dystrophy. Neuromuscul Disord. 2001;11(2):171–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.