Abstract
Aim:
The present study examined the protective potential of human adipose tissue-derived mesenchymal stem cells (hASCs) modified to overexpress alpha-1 antitrypsin (AAT), in a mouse model of the liver fibrosis.
Background:
For the treatment of end-stage liver diseases, cell therapy has emerged as a promising noninvasive alternative to liver transplantation. Mesenchymal stem cells (MSCs) are being evaluated due to their dual capabilities of promoting liver regeneration and modulating the pathogenic inflammation of the immune system.
Methods:
Liver fibrosis was induced in mice via the intraperitoneal injection of carbon tetrachloride (CCl4). MSCs were extracted from the human adipose tissue. After stemness confirmation, the cells were transduced with the lentiviruses containing the AAT gene, and then injected into the mice’s tail vein. Fourteen days’ post-transplantation, mice were sacrificed, and blood and tissue samples were collected for analysis. Important liver enzymes, including alanine transaminase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), albumin, and total bilirubin (TB), were measured. Histological studies were carried out using the hematoxylin and eosin (H&E), as well as Masson’s trichrome (MT) staining.
Results:
Compared to hASCs, treatment with AAT-hASCs resulted in greater reductions in ALT, AST, ALP, and TB, as well as normalized albumin levels. AAT-hASCs promoted enhanced liver regeneration histologically, likely attributable to anti-inflammatory and anti-proteolytic properties of AAT.
Conclusion:
These findings indicate AAT-engineered hASCs as a promising cell-gene therapy candidate for further study in liver cirrhosis models.
Key Words: Liver fibrosis, Adipose tissue-derived mesenchymal stem cells, Alpha-1 antitrypsin, Lentiviral vectors, Carbon tetrachloride
Introduction
Chronic tissue damage elicits an aberrant wound-healing program that drives progressive organ dysfunction via the cumulative disruption of tissue homeostasis and normal cell physiology (1, 2). Liver fibrosis is a widespread problem worldwide, with a high occurrence and a tendency to develop into cirrhosis. This makes it a significant public health concern that is linked to a significant number of deaths. Epidemiological data indicate approximately 1.03 million deaths worldwide per annum stemming from the severe sequelae of cirrhosis. These sobering statistics underscore urgent need to develop novel efficacious anti-fibrotic therapies to halt or reverse fibrosis for improved clinical outcomes (3).
Extracellular matrix (ECM) aggregation, especially collagen released by hepatic stellate cells, is a cardinal feature of liver fibrosis (1). Hepatocyte cell death, inflammatory cell infiltration, inflammatory cytokine production, the proliferation of non-parenchymal cells, and collagen deposition produce an ECM that seems to be involved in the complex process underlying liver fibrosis (4). At present, liver transplantation continues to be the sole conclusive therapeutic approach for advanced cirrhosis; however, its efficacy is hindered by donor scarcity, surgical complications, immune rejection, and exorbitant expenses. (5).
Stem cell-based therapies were introduced as promising strategies to treat end-stage liver diseases. The potential of stem cells in reducing the pathological complications, and clinical symptoms of cirrhosis was previously reported (6-11). In this regard, mesenchymal stem cells (MSCs) were extensively studied (12, 13). Beyond their ability to self-renew, they also have the power to differentiate into distinct cells such as chondrocytes, adipocytes, and osteoblasts. This ability is linked to their capacity to repair tissue. (12, 14).
Several cell-based clinical trials have applied MSCs to treat multiple disorders, like liver disease (12, 15). MSCs are achievable from different sources, including adipose tissue, bone marrow, and umbilical cord (16-18). Paracrine factors’ secretion is one of the main mechanisms through which these cells induce tissue repair (19-21). It is well-known that MSCs can migrate to injury sites, and provide protection and tissue repair (19, 20, 22). Due to these advantageous characteristics, mesenchymal stem cells (MSCs) are presently recognized as a viable vehicle for delivering therapeutic genes into impaired tissues in vivo and as a therapeutic strategy for tissue repair. (22, 23).
Alpha-1 antitrypsin (AAT), mainly synthesized in the liver, is a multifunctional acute-phase protein characterized by the anti-inflammatory and anti-protease activities (24). AAT increases the production of interleukin-10 (IL-10), and suppresses tumor necrosis factor-alpha (TNF-α) and IL-1β release (25, 26). Moreover, it was shown that AAT is effectively associated with regulating inflammation, proteolysis, and cell cycle arrest (27, 28). Additional research has shown that AAT contributes to intracellular anti-proteolytic effects by binding and inactivating caspase 3, therefore shielding lung microvascular endothelial cells and pancreatic β-cells from death. (29, 30).
This study evaluated the effects of human adipose-derived MSCs (hASCs), and hASCs engineered to express AAT (AAT-hASCs) in a mouse model of liver fibrosis induced by carbon tetrachloride. The results will provide insight into the therapeutic potential of naïve, and AAT-expressing MSCs for treating cirrhosis and end-stage liver disease.
Methods
Isolating and cell culture of human adipose tissue-derived stem cells (hASCs)
Adipose tissue was obtained with informed consent from three patients undergoing cosmetic surgery procedures. The study protocol was approved by Institutional Medical Ethics Committee of Tarbiat Modares University (IR.TMU.REC.1396.672). The hASCs were isolated using the methodology that had been previously documented (31). In brief, the adipose tissue was subjected to digestion at 37 ºC for 30 minutes using collagenase I (0.075%; Sigma-Aldrich). The resulting cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Gibco), containing 10% fetal bovine serum (FBS, Gibco), and 1% penicillin/streptomycin (Sigma-Aldrich), followed by incubation in a humidified atmosphere containing 5% CO2 at 37 ºC (32, 33).
Immunophenotyping
The isolated hASCs were detached at the second passage and washed in a PBS buffer two times. Fluorochrome-conjugated antibodies were used for flow cytometric immunophenotyping study of the isolated hASCs, based on previously described study (32). Fluorescein isothiocyanate (FITC) mouse anti-human CD44, CD45, and HLA-DR, and FITC mouse anti-human CD105 (conjugated to phycoerythrin), mouse anti-human CD90, FITC mouse anti-human CD34 (conjugated to phycoerythrin), and mouse anti-human CD73, were mixed with the cells, and then incubated (20 min /4°C). After two PBS washes, the cells were reconstituted in the identical buffer. The mean fluorescent intensities were detected using a BD FACS Calibur (BD biosciences, USA)
Osteogenic and adipogenic lineage differentiation potential
In vitro lineage potential of isolated hASCs was conducted based on previous study (33). The hASCs, detached from the second passage, were seeded (1×103 cells/cm2) in 4-well plates, and treated using an osteogenic and adipogenic differentiation induction media. The media were replaced with the fresh one every three days. At day 14, the presence of calcium deposition and liquid vacuoles was detected using Alizarin Red and Oil Red O staining, respectively.
Lentivirus production and cell transduction
The synthetic AAT gene sequence (1.4 kb) was cloned in PlexJred transfer vector (Openbiosystems, USA) using XhoI and MluI restriction enzymes. The transfer vector expresses Jred fluorescent protein and puromycin-resistance gene under the control of CMV promoter. Addgenes Co. (USA) supplied the envelope plasmid pMD2G, which encodes the vesicular stomatitis virus G (VSV-G) envelope, and the packaging plasmid psPAX2. The viral particles were generated by calcium phosphate-mediated transfection of HEK-293T cells as described previously (34). 6 ×106 293T cells were cultured in a 10 cm2 plate containing 10 ml medium (DMEM, 10% FBS, and 1% penicillin/ streptomycin). On the following day, the cells were co-transfected with PlexJred lentiviral vector (21 μg), pMD2G plasmid (10.5 μg), and psPAX2 plasmid (21 μg). The culture medium containing lentiviral particles was collected 24 h post-transfection, concentrated using Amicon® Ultra 15 ml Centrifugal filters – 100000 MWCO (Millipore, USA), and kept at -80 °C. The hASCs (from passage three) were transferred into the cell culture plat of 24 wells (1×104 cells/cm2). After reaching 70% confluency, the cells were transduced with lentiviral particles (multiplicity of infection (MOI) = 80) using the polybrene (8 ug/ml). The medium was renewed after 24 h, and the number of transduced cells was counted using an inverted fluorescent microscope (Nikon Eclipse TE2000-S, Japan).
RNA extraction and quantitative real-time PCR (qRT-PCR) assay
EZ-10 Animal Total RNA Miniprep Kit (BioBasic, Canada) was used for total RNA extraction on day seven, based on the guidelines. The cDNA synthesis kit (PrimeScriptTM RT Reagent Kit, TaKaRa, Japan), and SYBR Premix Ex Taq II (TaKaRa, Japan) were applied for reverse transcription of RNAs and cDNA amplification, respectively. A qRT-PCR assay with three steps was carried out as follows: denaturation (95°C / 30 s), annealing (56°C / 30 s), and extension (72°C / 60 s) for 40 cycles. The expression of β-actin (reference gene) was used for normalizing the target gene (AAT) expression. The used primers in the experiments were as follows: AAT (forward: 5-ACCTGGAAAATGAACTCACCCACG-3, reverse: 5-CTTGGAGAGCTTCAGGGGTGCCTC-3), and β-actin (forward: 5-CTGGAACGGTGAAGGTGACA-3, reverse: 5-AAGGGACTTCCTGTAACAATGCA-3).
Animals
Male C57BL/6 mice (Six weeks) were prepared by Histogenotech Co. (Iran), and kept in cages at 24 °C in a 12:12 h light-dark cycle and available food and water. The research was authorized by Tarbiat Modares University's Institutional Medical Ethics Committee (IR.TMU.REC.1396.672), which also approved all animal studies. Liver fibrosis induction
After an acclimation period of 1 week, the mice were assigned to four groups (each group contained five mice) at random; (I) negative control group, without any treatment; (II) positive control group received CCl4, 12 times over eight weeks (1 ml/kg of CCl4 two times weekly for four weeks, IP); and (III and IV) treatment group received CCl4 (with the same dose used for positive control) which were treated with 1×106 hASCs or AAT-hASCs (infused slowly through the tail vein) after four weeks. Animals were administered intraperitoneal injections of cyclosporine solution at a rate of 20 mg/kg/day for a duration of five days following cell transplantation and five days prior to it.
Liver function tests
Blood sampling was done from all experimental groups two weeks following cell transplantation. The serum was removed, and stored for further assessment. Serum levels of albumin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TB), and alkaline phosphatase (ALP) were quantified using commercially available colorimetric assay kits (Pars Azmon, Iran).
Liver pathology
For histological analysis, liver tissues were fixed in 10% neutral buffered formalin within 24 hours of collection. The tissues were then processed and embedded in paraffin, and 4 μm thick sections were cut. The sections were stained using hematoxylin and eosin (H&E) to observe the general structure, and Masson's trichrome (MT) to examine collagen deposition and evaluate fibrosis. The stained sections were evaluated by light microscopy for histopathological changes. Fibrosis was quantified on MT-stained sections using ImageJ software to calculate the percent area of collagen staining.
Statistical analysis
GraphPad Prism 5 was used for data analysis. Three sections for each mouse and three animals for each experimental group were used for the pathological analysis. The statistical evaluation of qPCR and functional assays was conducted using a one-way ANOVA followed by Tukey's test. Unpaired two-sample student’s t-test was used to assess differences among the groups. Values are provided as mean± SEM, and p<0.05 was considered significant.
Results
Characterization of hASCs
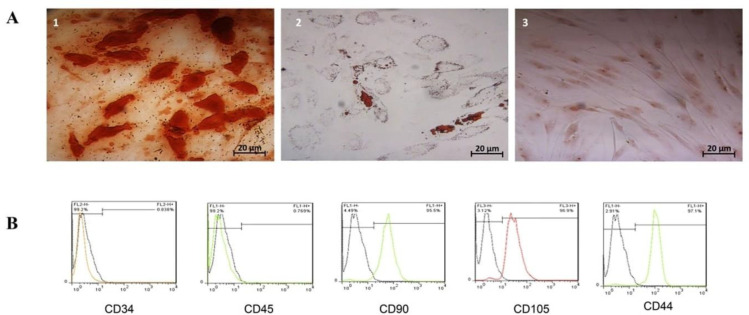
The isolated hASCs showed a fibroblast-like morphology and readily adhered to the tissue flask’s plastic surface. The osteogenic and adipogenic differentiation was approved through morphological alterations, as well as staining results (Figure 1A). Immunophenotypic analysis by flow cytometry demonstrated that the isolated hASCs were positive for mesenchymal markers CD105 (96.9%), CD90 (95.5%), and CD44 (97.1%) and negative for hematopoietic markers CD45 (0.76%) and CD34 (0.838%) (Figure 1B).
Figure 1.
A) Adipocyte and osteocyte differentiation of isolated hASCs. (1) The intracellular accumulated lipid droplets show adipogenic differentiation, (2) the intracellular calcium deposits show osteogenic differentiation, and (3) the undifferentiated hASCs serve as a negative control. B) Flow cytometry of hASCs surface marker was positive for CD90 (95.5%), CD105 (96.9%), and CD44 (97.1%) but negative for CD45 (0.769%) and CD34 (0.838%).
In vitro transduction of hASCs with the lentiviral-AAT vector
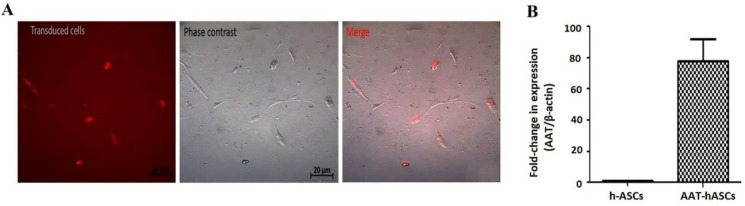
Lentiviral transduction was used to generate hASCs stably expressing AAT. Using a fluorescent microscope and Jred expression as a guide, the cell transduction efficiency was calculated to be 80% (Figure 2A). Quantification of AAT gene expression on day 7, using RT-PCR, showed an 80-fold increase in the AAT expression in transduced hASCs cells (Figure 2B).
Figure 2.
Transduced hASCs stably expressing AAT and Jred. (A) The transduction efficacy of transduced hASCs was assessed using a fluorescent microscope and based on the expression of Jred. (B) qPCR expression analysis of AAT expression levels indicated 80-fold increase in the AAT-hASCs. The expression levels were normalized to β-actin, and the values are expressed as mean±SEM (n=3).
Reduction of liver fibrosis by AAT-hASCs
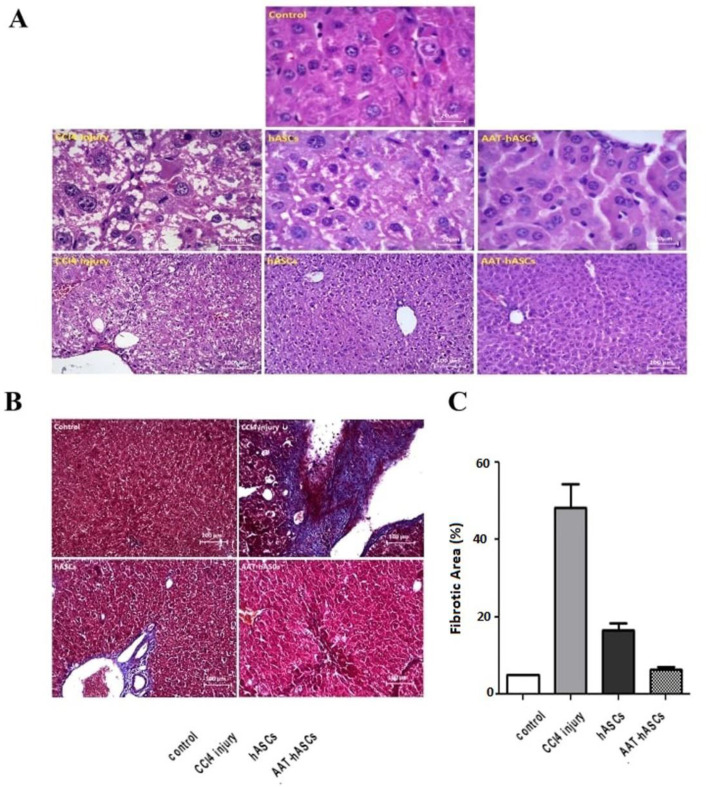
Histopathological analysis using H&E staining indicated that collagen was accumulated in ECM after eight CCl4 injections in four weeks; thus, fibrotic livers were whiter than normal livers. The control slides’ evaluation indicated that healthy hepatocytes have a parallel arrangement, radiate from the central vein, which are characterized by huge and bright vesicular nuclei. In the absence of inflammatory cells, discernible hepatic lobules and well-defined hepatic cords are noted, alongside vesicular and healthy nuclei that contain acidophilic granules within their cytoplasm. On the other hand, the liver slides of CCl4-treated mice show necrosis and inflammation. Lobular inflammation is detected along with swollen, and enlarged hepatocytes with highly diluted cytoplasm (balloon cells), which destroyed hepatocyte cords (lobular disarray). Moreover, fat droplets appear in balloon cells (steatosis). Acidophil bodies are observable, and the nuclei are highly polymorphic, with karyorrhexis (nucleus fragmentation) and karyolysis (nucleus obliteration). The healing benefits are clearly visible in the liver sections of mice treated with hASCs and AAT-hASCs, and all indications of cell inflammation and necrosis are diminished (Figure 3A). The quantitative assessment of liver fibrosis in MT-stained sections using ImageJ software indicated a significant reduction (near the control group) in the AAT-hASCs group’s fibrosis percentage after two weeks (Figure 3B). A significant difference was found between the hASCs and AAT-hASCs groups within the same interval (p<0.05).
Figure 3.
Histopathological analysis of livfer fibrosis. (A) H&E staining, and (B) MT staining of the liver tissue slides. (C) Using ImageJ software, a quantitative analysis of liver fibrosis in MT-stained sections was performed. Treatment of liver fibrosis with hASCs and AAT-hASCs had significant positive effects (p<0.05) on the reduction of fibrosis. The values are presented as mean±SEM (n=3).
Quantification of serological liver fibrosis markers
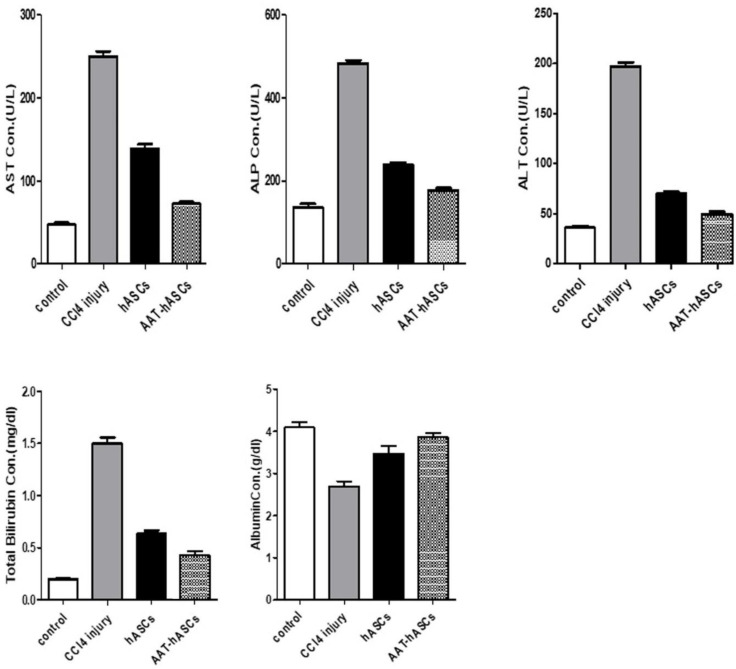
To further evaluate the effect of hASCs and AAT-hASCs on liver function, the serum concentrations of ALT, AST, ALP, TB, and albumin were assessed in different groups. The serum ALT, AST, ALP, and TB levels were markedly elevated in animals treated with CCl4 than in the control group, while albumin level was reduced (p<0.05) (Figure 4). The measurement of liver function-related serological markers is dramatically impacted by the administration of hASCs and AAT-hASCs to mice with liver damage produced by CCl4. In particular, using AAT-hASCs improved serological marker values close to the control group’s level.
Figure 4.
Serological Liver function markers. ALT, ALP, AST, albumin, and TB concentrations were measured in experimental groups. One-way ANOVA was applied to determine the significance of values in the groups (n=3 per group).
In general, the results indicated the superior potential of hASCs, and more notably AAT-hASCs, to improve liver function (Figure 4).
Discussion
The present study showed that the administration of genetically modified hASCs with a lentiviral vector encoding the human AAT gene could protect against CCl4-related liver fibrosis in a mouse model. This method may provide a unique treatment for human fibrosis, since it capitalizes on the protective properties of hAAT and hASCs. Several previous in vivo studies evaluated the therapeutic potential of MSCs for liver fibrosis. Jang et al. showed attenuation of hepatic fibrosis and improved liver function in rats treated with thioacetamide after treatment by bone marrow-derived MSCs (35). Among different types of MSCs, hASCs are easily harvested, besides possessing the ability of differentiation into the hepatic lineage and self-renewing capacity. Furthermore, induced pluripotent stem cells and embryonic stem cells are a trustworthy alternative cell source for clinical research and applications since they don't raise any ethical concerns (36). The hASCs secrete multiple cytokines and chemokines, including IL-6, IL-8, granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor, monocyte chemotactic protein-1 (MCP1), nerve growth factor, and hepatocyte growth factor (HGF) (37). Evidence shows that hASCs secretions play roles in enhancing endogenous cell stimulation, and inhibiting apoptosis, angiogenesis, and anti-inflammatory responses (38).
MSCs are not sufficient to treat liver fibrosis given the complex pathological changes involved. As a result, the therapeutic potential of MSCs could be increased through genetic manipulation to express therapeutic transgenes (39). Several studies have used various genes and molecules to enhance the therapeutic efficiency of MSCs. In 2014, a study evaluated the therapeutic effects of embryonic cord-derived MSCs expressing HGF on CCl4-related liver cirrhosis. The concentrations of ALT, AST, and ALP were recovered in animals after treatment using HGF-expressing cells. While treatment with native MSCs made slight differences (40). Jin et al. (2016) conducted a research to investigate the effects of Bcl2-expressing MSCs on the regeneration of the liver in rats with cirrhosis. The finding indicated significant improvement in histological and biochemical markers at weeks two, three, and four post-transplantation (41). Hence, we evaluated the antifibrotic potential of AAT- hASCs genetically modified to deliver AAT in a mouse model.
Human AAT is a multifunctional acute-phase protein with known anti-proteinase, anti-inflammatory, and cytoprotective properties (42). The anti-inflammatory effects of AAT may be particularly useful for treating liver fibrosis. AAT reduces the levels of leukotriene B4, nitric oxide, and pro-inflammatory cytokines, like TNF-α, IL-1β, IL-6, IL-8, IL-32, and MCP1 (43). Jedicke et al. showed the effectiveness of AAT in preventing the acute liver injury. In vitro, they detected direct suppression of active caspase-3 in liver homogenates and a cell-free system. Moreover, treatment of mice with AAT resulted in a significant decrease in the serum concentrations of TNF-α, besides the reduced activity of ADAM metallopeptidase domain 17 (ADAM17 or TACE). Moreover, elevated survival and reduced apoptotic hepatocytes in mouse models of α-amanitin and acetaminophen-related liver injury were reported (44).
Thus, we found that treatment with hASCs and AAT- hASCs promoted liver function recovery in CCL4-induced liver fibrosis mice. Our results further confirmed the efficacy of AAT and demonstrated that AAT-hASCs had a stronger therapeutic potential than MSCs in treating fibrosis and restoring liver function.
Functional improvement, as well as reduced fibrosis, are key factors in determining liver tissue reconstruction. Sections obtained from CCl4-treated mice showed progressive inflammatory cell infiltration, and congested blood vessels. Treating with hASCs decreased the amount of cellular infiltration and congestion. Meanwhile, AAT-hASCs showed a more notable reduction in fibrosis, and the physical tissue appearance returned to normal.
MT staining results showed that AAT-hASCs significantly reduced the fibrotic area percentage compared to hASCs group. Our findings also showed substantial decreases in ALT, AST, TB, and ALP levels, as well as the restoration of albumin levels in the AAT-hASCs group, indicating that damaged livers may be functionally improved. These parameters were applied in different investigations as indices of improved liver function (45-47).
Conclusion
In conclusion, the present study showed the effective reconstruction of damaged liver tissue, and indicated the reduced level of fibrosis in the CCl4 liver fibrosis-induced mice treated with AAT-hASCs. The effects of AAT-hASCs were superior to hASCs alone. As a result, combining cell and gene therapy with AAT-hASCs is proposed as a viable therapeutic treatment for fibrotic liver disease. Additional studies are warranted to further optimize this therapeutic strategy, and facilitate translation toward clinical use for treating fibrotic liver diseases in patients.
Ethical approval
This study protocol was reviewed and approved by the Institutional Medical Ethics Committee of Tarbiat Modares University, approval number IR.TMU.REC.1396.672.
Conflict of interests
The authors declare that they have no competing interests.
References
- 1.Higashi T, Friedman SL, Hoshida Y. Hepatic stellate cells as key target in liver fibrosis. Adv Drug Deliv Rev. 2017;121:27–42. doi: 10.1016/j.addr.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilkinson HN, Hardman MJ. Wound healing: cellular mechanisms and pathological outcomes. Open Biol. 2020;10:200223. doi: 10.1098/rsob.200223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schuppan D, Kim YO. Evolving therapies for liver fibrosis. J Clin Invest. 2013;123:1887–1901. doi: 10.1172/JCI66028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Odagiri N, Matsubara T, Sato-Matsubara M, Fujii H, Enomoto M, Kawada N. Anti-fibrotic treatments for chronic liver diseases: The present and the future. Clin Mol Hepatol. 2021;27:413. doi: 10.3350/cmh.2020.0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puche JE, Saiman Y, Friedman SL. Hepatic stellate cells and liver fibrosis. Compr Physiol. 2011;3:1473–1492. doi: 10.1002/cphy.c120035. [DOI] [PubMed] [Google Scholar]
- 6.Petrenko O, Königshofer P, Brusilovskaya K, Hofer BS, Bareiner K, Simbrunner B, et al. Transcriptomic signatures of progressive and regressive liver fibrosis and portal hypertension. iScience. 2023 doi: 10.1016/j.isci.2024.109301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singal AK, Duchini A. Liver transplantation in acute alcoholic hepatitis: Current status and future development. World J Hepatol. 2011;3:215. doi: 10.4254/wjh.v3.i8.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terrault NA, Francoz C, Berenguer M, Charlton M, Heimbach J. Liver transplantation 2023: status report, current and future challenges. Clin Gastroenterol Hepatol . 2023 doi: 10.1016/j.cgh.2023.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Ali G, Masoud MS. Bone marrow cells ameliorate liver fibrosis and express albumin after transplantation in CCl4-induced fibrotic liver. Saudi J Gastroenterol. 2012;18:263. doi: 10.4103/1319-3767.98433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agaev B, Agaev R, Popandopoulo A, Jafarli R. Clinical efficacy of autologous mesenchyme multipotential stem cells transplantation in the liver cirrhosis and portal hypertension treatment. Georgian Med News. 2014;39:45. [PubMed] [Google Scholar]
- 11.El-Ansary M, Abdel-Aziz I, Mogawer S, Abdel-Hamid S, Hammam O, Teaema S, et al. Phase II trial: undifferentiated versus differentiated autologous mesenchymal stem cells transplantation in Egyptian patients with HCV induced liver cirrhosis. Stem Cell Rev Rep. 2012;8:972–981. doi: 10.1007/s12015-011-9322-y. [DOI] [PubMed] [Google Scholar]
- 12.Takami T, Terai S, Sakaida I. Stem cell therapy in chronic liver disease. Curr Opin Gastroen. 2012;28:203–208. doi: 10.1097/MOG.0b013e3283521d6a. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Lian F, Li J, Fan W, Xu H, Yang X, et al. Adipose derived mesenchymal stem cells transplantation via portal vein improves microcirculation and ameliorates liver fibrosis induced by CCl4 in rats. J Transl Med. 2012;10:133. doi: 10.1186/1479-5876-10-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang L, Ye JS, Decot V, Stoltz JF, de Isla N. Research on stem cells as candidates to be differentiated into hepatocytes. Biomed Mater Eng. 2012;22:105–111. doi: 10.3233/BME-2012-0695. [DOI] [PubMed] [Google Scholar]
- 15.Squillaro T, Peluso G, Galderisi U. Clinical trials with mesenchymal stem cells: an update. Cell Transplant. 2016;2:829–848. doi: 10.3727/096368915X689622. [DOI] [PubMed] [Google Scholar]
- 16.Tan CY, Lai RC, Wong W, Dan YY, Lim SK, Ho HK. Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models. Stem Cell Res Ther. 2014;5:76. doi: 10.1186/scrt465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samsonraj RM, Raghunath M, Nurcombe V, Hui JH, van Wijnen AJ, Cool SM. Concise review: multifaceted characterization of human mesenchymal stem cells for use in regenerative medicine. Stem Cells Transl Med. 2017;6:2173–2185. doi: 10.1002/sctm.17-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chivukula RR, Mendell JT. Abate and switch: miR-145 in stem cell differentiation. Cell. 2009;137:606–8. doi: 10.1016/j.cell.2009.04.059. [DOI] [PubMed] [Google Scholar]
- 19.Anjos-Afonso F, Bonnet D. Nonhematopoietic/endothelial SSEA-1+ cells define the most primitive progenitors in the adult murine bone marrow mesenchymal compartment. Blood. 2006;109:1298–1306. doi: 10.1182/blood-2006-06-030551. [DOI] [PubMed] [Google Scholar]
- 20.In 't Anker PS, Scherjon SA, Kleijburg-van der Keur C, de Groot-Swings GM, Claas FH, Fibbe WE, et al. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22:1338–45. doi: 10.1634/stemcells.2004-0058. [DOI] [PubMed] [Google Scholar]
- 21.Davoodian N, Lotfi AS, Soleimani M, Mowla SJ. MicroRNA‐122 overexpression promotes hepatic differentiation of human adipose tissue‐derived stem cells. J Cell Biochem. 2014;115:1582–1593. doi: 10.1002/jcb.24822. [DOI] [PubMed] [Google Scholar]
- 22.Fiore EJ, Mazzolini G, Aquino J. Mesenchymal stem/stromal cells in liver fibrosis: recent findings, old/new caveats and future perspectives. Stem Cell Rev Rep. 2015;11:586–597. doi: 10.1007/s12015-015-9585-9. [DOI] [PubMed] [Google Scholar]
- 23.Garcia MG, Bayo J, Bolontrade MF, Sganga L, Malvicini M, Alaniz L, et al. Hepatocellular carcinoma cells and their fibrotic microenvironment modulate bone marrow-derived mesenchymal stromal cell migration in vitro and in vivo. Mol Pharm. 2011;8:1538–1548. doi: 10.1021/mp200137c. [DOI] [PubMed] [Google Scholar]
- 24.Volarevic V, Nurkovic J, Arsenijevic N, Stojkovic M. Concise review: therapeutic potential of mesenchymal stem cells for the treatment of acute liver failure and cirrhosis. Stem Cells. 2014;32:2818–2823. doi: 10.1002/stem.1818. [DOI] [PubMed] [Google Scholar]
- 25.Aquino JB, Bolontrade MF, García MG, Podhajcer OL, Mazzolini G. Mesenchymal stem cells as therapeutic tools and gene carriers in liver fibrosis and hepatocellular carcinoma. Gene Ther. 2010;17:692–708. doi: 10.1038/gt.2010.10. [DOI] [PubMed] [Google Scholar]
- 26.Jung SC, Park S. New sources, differentiation, and therapeutic uses of mesenchymal stem cells 20. Int J Mol Sci. 2023;24:3938. doi: 10.3390/ijms24043938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stoller JK, Aboussouan LS. α1-antitrypsin deficiency. Lancet. 2005;365:2225–2236. doi: 10.1016/S0140-6736(05)66781-5. [DOI] [PubMed] [Google Scholar]
- 28.Tavasoli T, Arjmand S, Siadat SOR, Shojaosadati SA, Lotfi AS. A robust feeding control strategy adjusted and optimized by a neural network for enhancing of alpha 1-antitrypsin production in Pichia pastoris. Biochem Eng J. 2019;144:18–27. [Google Scholar]
- 29.Janciauskiene SM, Nita IM, Stevens T. α1-Antitrypsin, Old Dog, New Tricks α1-antitrypsin exerts in vitro anti-inflammatory activity in human monocytes by elevating cAMP. J Biol Chem. 2007;282:8573–8582. doi: 10.1074/jbc.M607976200. [DOI] [PubMed] [Google Scholar]
- 30.Zhu H, He J, Liu J, Zhang X, Yang F, Liu P, Wang S. Alpha 1-antitrypsin ameliorates ventilator-induced lung injury in rats by inhibiting inflammatory responses and apoptosis. Exp Biol Med. 2018;243:87–95. doi: 10.1177/1535370217740852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.M Hunt J, Tuder R. Alpha 1 anti-trypsin: one protein, many functions. Current molecular medicine, 2012;12:827–835. doi: 10.2174/156652412801318755. [DOI] [PubMed] [Google Scholar]
- 32.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petrache I, Fijalkowska I, Medler TR, Skirball J, Cruz P, Zhen L, et al. α-1 Antitrypsin inhibits caspase-3 activity, preventing lung endothelial cell apoptosis. Am J Pathol. 2006;169:1155–1166. doi: 10.2353/ajpath.2006.060058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Atkinson M, Song S. Alpha1-antitrypsin protects beta-cells from apoptosis. Diabetes. 2007;56:13161323. doi: 10.2337/db06-1273. [DOI] [PubMed] [Google Scholar]
- 35.Davoodian N, Lotfi AS, Soleimani M, Mola SJ, Arjmand S. Let-7f microRNA negatively regulates hepatic differentiation of human adipose tissue-derived stem cells. J Physiol Biochem. 2014;70:781–789. doi: 10.1007/s13105-014-0346-z. [DOI] [PubMed] [Google Scholar]
- 36.Mohammadpour A, Arjmand S, Lotfi AS, Tavana H, Kabir-Salmani M. Promoting hepatogenic differentiation of human mesenchymal stem cells using a novel laminin-containing gelatin cryogel scaffold. Biochem Biophys Res Commun. 2018;507:15–21. doi: 10.1016/j.bbrc.2018.10.121. [DOI] [PubMed] [Google Scholar]
- 37.Gandomani MG, Lotfi AS, Tamandani DK, Arjmand S, Alizadeh S. The enhancement of differentiating adipose derived mesenchymal stem cells toward hepatocyte like cells using gelatin cryogel scaffold. Biochem Biophys Res Commun. 2017;491:1000–1006. doi: 10.1016/j.bbrc.2017.07.167. [DOI] [PubMed] [Google Scholar]
- 38.Saedi-Marghmaleki M, Moradi M-T, Ghasemi-Dehkordi P, Hashemi L, Karimi A. Evaluation of lentiviral vector-based green fluorescent protein expression in human gastric cancer cell line. J Shahrekord Univ Med Sci. 2019;21:204–209. [Google Scholar]
- 39.Ghaedi M, Sahebghadam LA, Soleymani M, Shamsara M, Arjmand S, Adibi B. Expression of recombinant alpha-1 antitrypsin in CHO and COS-7 cell lines using lentiviral vector. Iran J Biotech. 2009;7:148–156. [Google Scholar]
- 40.Arjmand S, Barzegar A, Mohammadpour A, Rezaei H, Davoodian N, Ghaneialvar H, et al. Raman spectroscopic characterization of hepatic differentiation of mesenchymal stem cells. Int J Med Lab. 2021;8:122–130. [Google Scholar]
- 41.Jang YO, Kim MY, Cho MY, Baik SK, Cho YZ, Kwon SO. Effect of bone marrow-derived mesenchymal stem cells on hepatic fibrosis in a thioacetamide-induced cirrhotic rat model. BMC Gastroenterol. 2014;14:198. doi: 10.1186/s12876-014-0198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang D, Wang ZQ, Deng JQ, Liao JY, Wang X, Xie J, et al. Adipose‐derived stem cells: A candidate for liver regeneration. J Dig Dis. 2015;16:489–498. doi: 10.1111/1751-2980.12268. [DOI] [PubMed] [Google Scholar]
- 43.Cao Y, Yan J, Dong Z, Wang J, Jiang X, Cui T, et al. Adipose-derived mesenchymal stem cells are ideal for the cell-based treatment of refractory wounds: strong potential for angiogenesis. Stem Cell Rev Rep. 2024;20:313–328. doi: 10.1007/s12015-023-10641-y. [DOI] [PubMed] [Google Scholar]
- 44.Banas A, Teratani T, Yamamoto Y, Tokuhara M, Takeshita F, Osaki M, et al. IFATS collection: in vivo therapeutic potential of human adipose tissue mesenchymal stem cells after transplantation into mice with liver injury. Stem Cells. 2008;26:2705–2712. doi: 10.1634/stemcells.2008-0034. [DOI] [PubMed] [Google Scholar]
- 45.D’souza N, Rossignoli F, Golinelli G, Grisendi G, Spano C, Candini O, et al. Mesenchymal stem/stromal cells as a delivery platform in cell and gene therapies. BMC Med. 2015;13:186. doi: 10.1186/s12916-015-0426-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiao W, Shi J. Application of adipose-derived stem cells in ischemic heart disease: theory, potency, and advantage. Front Cardiovasc Med. 2024;11:1324447. doi: 10.3389/fcvm.2024.1324447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lou G, Yang Y, Liu F, Ye B, Chen Z, Zheng M, et al. MiR‐122 modification enhances the therapeutic efficacy of adipose tissue‐derived mesenchymal stem cells against liver fibrosis. J Cell Mol Med. 2017;21:2963–2973. doi: 10.1111/jcmm.13208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seo KW, Sohn SY, Bhang DH, Nam MJ, Lee HW, Youn HY. Therapeutic effects of hepatocyte growth factor‐overexpressing human umbilical cord blood‐derived mesenchymal stem cells on liver fibrosis in rats. Cell Biol Int. 2014;38:106–116. doi: 10.1002/cbin.10186. [DOI] [PubMed] [Google Scholar]
- 49.Seo KW, Sohn SY, Bhang DH, Nam MJ, Lee HW, Youn HY. Mesenchymal stem cells with enhanced Bcl-2 expression promote liver recovery in a rat model of hepatic cirrhosis. Cell Physiol Biochem. 2016;40:1117–1128. doi: 10.1159/000453166. [DOI] [PubMed] [Google Scholar]
- 50.Janciauskiene SM, Bals R, Koczulla R, Vogelmeier C, Köhnlein T, Welte T. The discovery of α1-antitrypsin and its role in health and disease. Resp Med. 2011;105:1129–1139. doi: 10.1016/j.rmed.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 51.De Serres F, Blanco I. Role of alpha‐1 antitrypsin in human health and disease. J Int Med. 2014;276:311–335. doi: 10.1111/joim.12239. [DOI] [PubMed] [Google Scholar]
- 52.Jedicke N, Struever N, Aggrawal N, Welte T, Manns MP, Malek NP, et al. Alpha‐1‐antitrypsin inhibits acute liver failure in mice. Hepatol. 2014;59:2299–2308. doi: 10.1002/hep.27024. [DOI] [PubMed] [Google Scholar]
- 53.Okoro E. Hepatoprotective Effect of ethanolic extract of cnestis ferruginea roots on carbon tetrachloride-induced liver damage in male rats. J Appl Sci Environ Manag. 2024;28:691–698. [Google Scholar]
- 54.Algefare AI, Alfwuaires M, Famurewa AC, Elsawy H, Sedky A. Geraniol prevents CCl4-induced hepatotoxicity via suppression of hepatic oxidative stress, pro-inflammation and apoptosis in rats. Toxicol Rep. 2024;12:128–134. doi: 10.1016/j.toxrep.2024.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hong D, Min JY, Min KB. Association between cadmium exposure and liver function in adults in the United States: a cross-sectional study. J Prev Med Public Health. 2021;54:471. doi: 10.3961/jpmph.21.435. [DOI] [PMC free article] [PubMed] [Google Scholar]