Abstract
Clinical trials frequently include multiple end points that mature at different times. The initial report, typically based on the primary end point, may be published when key planned co-primary or secondary analyses are not yet available. Clinical Trial Updates provide an opportunity to disseminate additional results from studies, published in JCO or elsewhere, for which the primary end point has already been reported.
Mesenchymal-epithelial transition (MET) signaling pathway plays a role in the pathogenesis of selected patients with papillary renal cell carcinoma (PRCC). In the phase II PAPMET trial (ClinicalTrials.gov identifier: NCT02761057), cabozantinib significantly prolonged progression-free survival and improved objective response rate compared with sunitinib in patients with advanced PRCC. Here, we present the final overall survival (OS) analysis. In this multicenter, randomized phase II, open-label trial, 147 patients with advanced PRCC who have received up to one previous therapy (excluding vascular endothelial growth factor–directed agents) were assigned to sunitinib, cabozantinib, crizotinib, or savolitinib. Ultimately, savolitinib and crizotinib arms were closed because of futility. With a median follow-up of 17.5 months, the median OS was 21.5 months (95% CI, 12.0 to 28.1) with cabozantinib and 17.3 months (95% CI, 12.8 to 21.8) with sunitinib (hazard ratio, 0.83; 95% CI, 0.51 to 1.36; P = .46). The OS landmark estimates for cabozantinib and sunitinib were 50% versus 39% at 24 months and 32% versus 28% at 36 months. In conclusion, we observed no significant difference in OS across treatment arms. Although cabozantinib represents a well-supported option for advanced PRCC, the lack of survival benefit underscores the need to develop novel therapies for this disease.
In S1500, no OS advantage was seen with cabozantinib versus sunitinib in patients with PRCC, underscoring the need for new therapies.
INTRODUCTION
Approximately 15%-20% of patients with renal cell carcinoma (RCC) are diagnosed with papillary RCC.1 In clear cell RCC, the most common subtype of the disease, survival has significantly improved with the emergence of checkpoint inhibition–based regimens. By contrast, there has yet to be a randomized study demonstrating a significant survival advantage in the setting of advanced papillary RCC (PRCC).2,3
It has been increasingly recognized that PRCC is a heterogeneous disease with multiple potential drivers. However, it is well accepted that a subset of PRCC is driven by alterations in the mesenchymal-epithelial transition (MET) proto-oncogene.4,5 We designed SWOG 1500 to determine if MET-directed therapies might supersede the activity of vascular endothelial growth factor (VEGF)–directed therapies in advanced PRCC.6 Patients in this study were randomly assigned to receive either sunitinib or the experimental arms of cabozantinib, crizotinib, or savolitinib. Arms containing savolitinib and crizotinib were closed after meeting the prespecified futility boundary. We have previously reported the primary end point of progression-free survival (PFS), which was 9.0 months versus 5.6 months (hazard ratio [HR], 0.60 [95% CI, 0.37 to 0.97]; P = .019, one-sided) favoring therapy with cabozantinib, a dual VEGF/MET inhibitor, compared with sunitinib.6 Overall response rate was also higher for cabozantinib versus sunitinib (23% v 4%; two-sided P = .010). However, overall survival (OS) was immature at the time of our original report. With extended follow-up, we report herein updated OS analyses from SWOG 1500.
METHODS
Study Design and Participants
Study design was detailed in the original report (full protocol: Protocol, online only).6 Patients with metastatic PRCC who had received ≤1 previous therapy (excluding VEGF- and MET-directed agents) were randomly assigned 1:1:1:1 to receive sunitinib, cabozantinib, crizotinib, or savolitinib. Sunitinib was dosed at 50 mg oral once daily 4 weeks on, 2 weeks off, cabozantinib was dosed at 60 mg oral once daily, crizotinib was dosed at 250 mg oral twice daily, and savolitinib was dosed at 600 mg oral once daily.
End Points and Assessments
The primary objective of the study was to compare PFS with sunitinib against each of the arms (cabozantinib, crizotinib, and savolitinib) separately, representing the time from random assignment to the time of radiographic or clinical progression, symptomatic deterioration, or death from any cause. Assessments occurred at baseline and every 12 weeks after random assignment until radiographic progression or discontinuation of study treatment, with further limited follow-up requested up to 3 years after random assignment. Objective response rate (RECIST v.1.1), OS, and safety (NCICTCAE v.4.0) were secondary end points.
Statistical Analyses
With 164 enrolled patients, we had 85% power to detect a 75% improvement in median PFS in any one of the three experimental arms versus sunitinib with a one-sided α of 0.10. Kaplan-Meier curves were used to present time-to-event data, and the two groups were compared using log-rank tests (Fig 1A). The HRs and 95% CIs were calculated using a stratified Cox proportional hazards model. A type I error rate of 0.05 was set; all tests were two-sided. SAS, version 9.4, was used for statistical analyses.
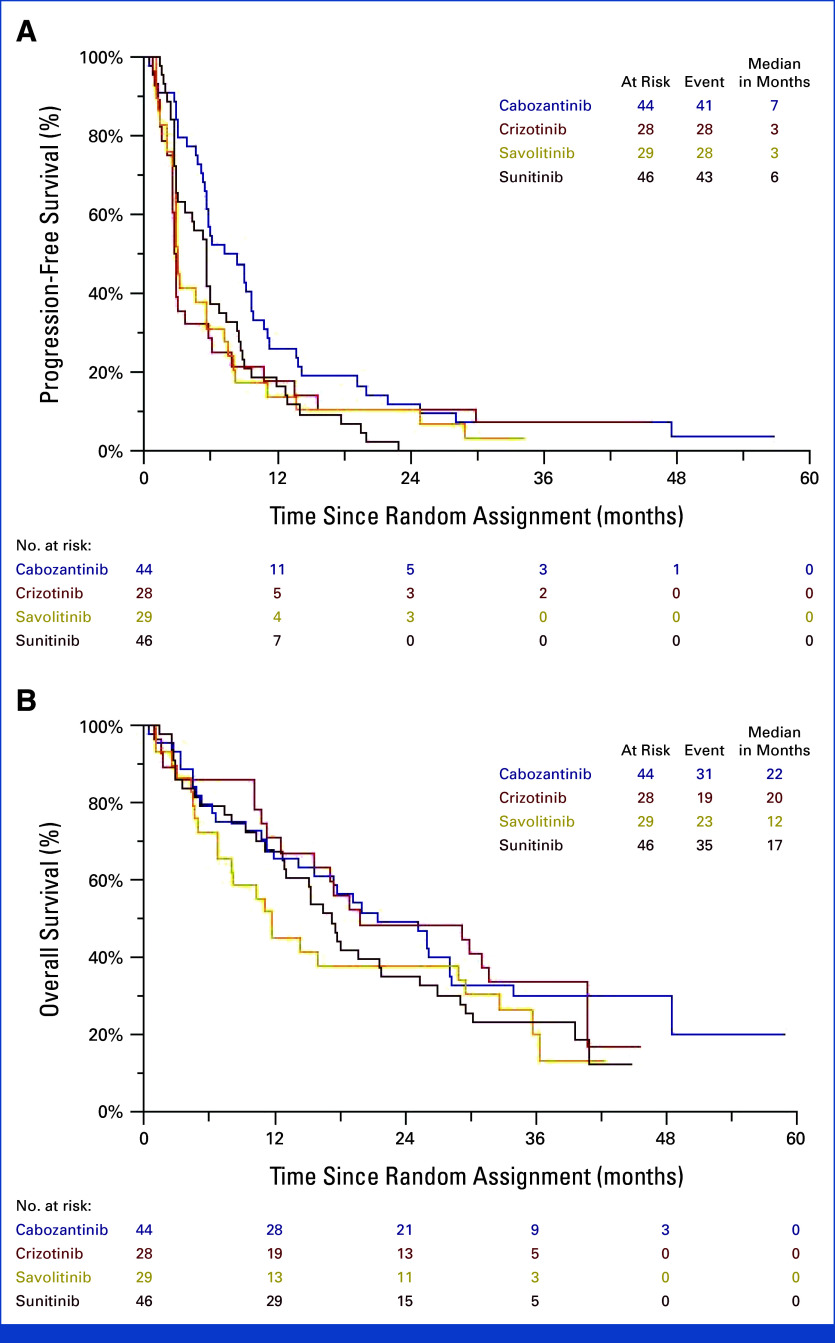
FIG 1.
Kaplan-Meier analysis of (A) progression free-survival and (B) overall survival.
RESULTS
Survival Outcomes
A total of 152 patients were randomly assigned 1:1:1:1 to the four arms. After five patients were excluded because of no evidence of metastatic disease, 147 patients remained evaluable, with 46, 44, 28, and 29 patients assigned to receive sunitinib, cabozantinib, crizotinib, and savolitinib, respectively. The median follow-up was 17.5 months in this report; further patient characteristics are presented in Table 1.
TABLE 1.
Patient Characteristics
| Characteristic | All Patients | Sunitinib (n = 46) | Cabozantinib (n = 44) | Crizotinib (n = 28) | Savolitinib (n = 29) |
|---|---|---|---|---|---|
| Median age, years (range) | 68 (58-75) | 65 (58-73) | 65 (58-75) | 68 (61-75) | 67 (58-72) |
| Males, No. (%) | 112 (76) | 35 (76) | 36 (82) | 22 (79) | 19 (86) |
| Race, No. (%) | |||||
| White | 114 (78) | 39 (85) | 32 (73) | 22 (79) | 21 (72) |
| Black | 21 (14) | 5 (11) | 9 (20) | 4 (14) | 3 (10) |
| Other | 12 (4) | 2 (4) | 3 (7) | 2 (8) | 5 (16) |
| Previous systemic therapy, No. (%) | 10 (7) | 3 (7) | 2 (5) | 2 (7) | 3 (10) |
| Histologic subtype (central assessment), No. (%) | |||||
| Type I | 41 (28) | 12 (26) | 14 (32) | 9 (32) | 6 (21) |
| Type II | 63 (43) | 21 (46) | 16 (36) | 13 (46) | 13 (45) |
| IMDC risk group, No. (%) | |||||
| Favorable | 38 (26) | 14 (30) | 10 (23) | 8 (29) | 6 (30) |
| Intermediate | 89 (61) | 26 (57) | 28 (64) | 16 (57) | 19 (66) |
| Poor | 20 (14) | 6 (13) | 6 (14) | 4 (14) | 4 (14) |
| Zubrod PS | |||||
| 0 | 91 (62) | 29 (63) | 29 (66) | 18 (64) | 15 (52) |
| 1 | 56 (38) | 17 (37) | 15 (34) | 10 (36) | 14 (48) |
| Metastatic sites of interest, No. (%) | |||||
| Bones | 26 (18) | 7 (15) | 6 (14) | 5 (18) | 8 (28) |
| CNS | 1 (<1) | 0 (0) | 0 (0) | 1 (<1) | 0 (0) |
| Liver | 38 (26) | 13 (9) | 13 (9) | 4 (3) | 8 (5) |
| Lung | 25 (17) | 7 (5) | 11 (7) | 3 (2) | 4 (3) |
Abbreviations: IMDC, International mRCC Database Consortium; PS, performance status.
Since the original report and as of the September 30, 2023 data cutoff for OS, there were an additional 25 deaths. With 108 deaths, median OS was 21.5 months (95% CI, 12.0 to 28.1) with cabozantinib and 17.3 months (95% CI, 12.8 to 21.8) with sunitinib (covariate-adjusted HR for OS: 0.83 [95% CI, 0.51 to 1.36]; P = .46; Fig 1B). The median OS was 19.9 months (95% CI, 11.2 to 40.8) with crizotinib and 11.7 months (95% CI, 6.7 to 29.5) with savolitinib. OS landmark estimates for cabozantinib and sunitinib were 50% versus 39% at 24 months and were 32% versus 28% at 36 months. Seventeen of 44 patients (39%) on cabozantinib and 20 of 46 (43%) on sunitinib received subsequent anticancer therapy, including 18 of 48 regimens that were anti-VEGF inhibitors (38%), 18 of 48 (38%) PD(L)-1 checkpoint inhibitors, and seven of 48 (15%) mammalian target of rapamycin inhibitors (Appendix Table A1, online only).
Safety of Cabozantinib and Sunitinib Arms
The rates of all-grade adverse events remained comparable between cabozantinib (42 patients, 98%) and sunitinib (43 patients, 96%; Table 2). Grade ≥3 adverse events occurred in 69% and 67% of patients receiving sunitinib and cabozantinib, respectively. The most common grade 3 or 4 adverse events with sunitinib were anemia (13%), hypertension (20%), and decreased white blood cell count (11%). The most common grade 3 or 4 adverse events with cabozantinib were hypertension (33%), hand-foot syndrome (21%), hypophosphatemia (14%), and fatigue (14%).
TABLE 2.
Adverse Events Reported With an Attribution of Being Possibly, Probably, or Definitely Related to Protocol Treatment and Occurring in 10% or More of Patients in the Sunitinib or Cabozantinib Arms
| Adverse Event | Cabozantinib (n = 43), No. (%) | Sunitinib (n = 45), No. (%) | ||
|---|---|---|---|---|
| Grade 3-4 | Any grade | Grade 3-4 | Any grade | |
| Maximum grade any adverse event | 29 (67) | 42 (98) | 31 (69) | 43 (96) |
| Nonhematologic adverse events | ||||
| Abdominal pain | 3 (7) | 6 (14) | 1 (2) | 3 (7) |
| Constipation | 0 (0) | 8 (19) | 0 (0) | 5 (11) |
| Diarrhea | 3 (7) | 24 (56) | 3 (7) | 22 (49) |
| Dry mouth | 0 (0) | 6 (14) | 0 (0) | 5 (11) |
| Dysgeusia | 0 (0) | 18 (42) | 0 (0) | 14 (31) |
| Dyspepsia | 0 (0) | 3 (7) | 0 (0) | 5 (11) |
| Mucositis oral | 1 (2) | 16 (37) | 0 (0) | 13 (29) |
| Nausea | 0 (0) | 16 (37) | 4 (9) | 20 (44) |
| Vomiting | 0 (0) | 6 (14) | 1 (2) | 11 (24) |
| GI disorders—others | 1 (2) | 6 (14) | 0 (0) | 3 (7) |
| Hand-foot syndrome | 9 (21) | 21 (49) | 0 (0) | 11 (24) |
| Headache | 0 (0) | 5 (12) | 0 (0) | 4 (9) |
| Fatigue | 6 (14) | 30 (70) | 4 (9) | 28 (62) |
| Dyspnea | 0 (0) | 6 (14) | 0 (0) | 3 (7) |
| Dizziness | 0 (0) | 1 (2) | 0 (0) | 5 (11) |
| Thromboembolic event | 5 (12) | 8 (19) | 0 (0) | 1 (2) |
| Pain in extremity | 1 (2) | 7 (16) | 0 (0) | 3 (7) |
| Peripheral sensory neuropathy | 0 (0) | 6 (14) | 0 (0) | 0 (0) |
| Dehydration | 0 (0) | 1 (2) | 1 (2) | 5 (11) |
| Hoarseness | 0 (0) | 7 (16) | 0 (0) | 2 (4) |
| Hyperthyroidism | 0 (0) | 7 (16) | 0 (0) | 2 (4) |
| Hypothyroidism | 0 (0) | 17 (40) | 0 (0) | 9 (20) |
| Skin/subcutaneous tissue | 0 (0) | 5 (12) | 0 (0) | 8 (18) |
| Rash acneiform | 0 (0) | 5 (12) | 0 (0) | 0 (0) |
| Rash maculopapular | 0 (0) | 9 (21) | 0 (0) | 3 (7) |
| Hematologic adverse events | ||||
| WBC decreased | 0 (0) | 9 (21) | 5 (11) | 13 (29) |
| Neutrophil count decreased | 0 (0) | 7 (16) | 4 (9) | 11 (24) |
| Lymphocyte count decreased | 0 (0) | 6 (14) | 2 (4) | 10 (22) |
| Anemia | 0 (0) | 11 (26) | 6 (13) | 17 (38) |
| Platelet count decreased | 0 (0) | 9 (21) | 2 (4) | 19 (42) |
| Laboratory adverse events | ||||
| ALT increased | 1 (2) | 13 (30) | 1 (2) | 6 (13) |
| AST increased | 0 (0) | 15 (35) | 1 (2) | 8 (18) |
| Creatinine increased | 0 (0) | 7 (16) | 0 (0) | 14 (31) |
| Hypoalbuminemia | 0 (0) | 7 (16) | 1 (2) | 6 (13) |
| Hypocalcemia | 1 (2) | 11 (26) | 0 (0) | 1 (2) |
| Hypokalemia | 0 (0) | 6 (14) | 0 (0) | 0 (0) |
| Hypomagnesemia | 2 (5) | 10 (23) | 0 (0) | 0 (0) |
| Hyponatremia | 3 (7) | 3 (7) | 2 (4) | 5 (11) |
| Hypophosphatemia | 6 (14) | 13 (30) | 0 (0) | 3 (7) |
| Proteinuria | 2 (5) | 9 (21) | 1 (2) | 8 (18) |
At the time of this analysis, no patients remain on protocol treatment. The median time on sunitinib and cabozantinib was 2.9 months and 5.7 months, and dose reductions and discontinuation rates were observed in 38% and 24% with sunitinib compared with 34% and 23% with cabozantinib, respectively.
DISCUSSION
As with our original report, we observed no difference in OS across treatment arms in SWOG 1500. Although we feel that cabozantinib represents a well-supported option for advanced PRCC on the basis of our findings and irrespective of PRCC subtype, the lack of survival benefit underscores the need for novel therapies for this disease.7 Our updated analyses did not yield any new findings with respect to PFS and response rate (both still favoring cabozantinib), and no new safety signals compared with our previous report.
The standard of care for advanced PRCC has become a contentious issue. There are now two single-arm phase II studies exploring the combination of cabozantinib with checkpoint inhibitors (one with nivolumab, another with atezolizumab) in non–clear cell RCC. Within the papillary cohort in these studies, response rates were identical at 47%.8,9 Similarly encouraging results were observed with the combination of lenvatinib with pembrolizumab in PRCC in a separate single-arm phase II study, yielding a response rate of 57%.10 These estimates are more than double the 23% response rate observed with cabozantinib in SWOG 1500.6 Similarly, estimated OS landmark with both combination regimens ranged from 70% to 80% at 18 months, which compares favorably with the 57% observed with cabozantinib in PAPMET trial. However, caution must be taken in juxtaposing these single-arm studies against the randomized data from our trial. We strongly support continued enrollment of patients with advanced PRCC in frontline studies, and have recently initiated SWOG 2200, a randomized, phase II study comparing cabozantinib with or without atezolizumab, in this setting.11
More controversial are studies that possess a sunitinib control arm—two such studies are ongoing, SAMETA and STELLAR-304, testing new therapies savolitinib and zanzalintinib, respectively.12,13 Despite the differing designs, had SWOG 1500 shown a survival advantage with cabozantinib in this updated analysis, one could rightly contest the choice of a sunitinib control arm. In fact, estimates of activity of sunitinib vary widely, with response rates ranging from 5% to 13% and PFS ranging from 3 to 7 months.14-16 In the face of our results showing no survival advantage, we support enrollment in these trials as well as SWOG 2200. Firmly establishing superiority of a regimen compared with sunitinib is of particular importance in many parts of the world where health authorities have not yet adopted cabozantinib as a preferred regimen for advanced PRCC.
Limitations of our analysis include closure of multiple study sites at 3 years (the minimum requirement in our protocol), limiting duration of follow-up and censoring of OS. Still, many sites remained open beyond this landmark, and we feel that the median follow-up of 17.5 months for OS is reasonable in this population of patients. Within this span of follow-up, we captured relatively low rates of second-line therapies, amounting to 39% and 43% on the cabozantinib and sunitinib arms, respectively. In clear cell RCC, estimates vary widely regarding the proportion of patients who progress to second-line therapies, but in mature data sets (eg, CheckMate-214 and KEYNOTE-426), the rates of reported second-line therapy use are in excess of 50%.17 The lower rates of second-line therapy in the current study could owe to underreporting, but alternatively could play into the aggressive nature of PRCC, with many patients proceeding to palliative care after frontline treatment. Another limitation specific to the current analysis is that our study was powered to assess PFS; therefore, it is important to acknowledge that our assessment of OS may be underpowered. Our study also does not incorporate biologic criteria (eg, MET status) for enrollment—it is possible that a stronger signal could be seen in such a subpopulation. Genomic characterization of patients in the PAPMET study is ongoing. Of note, we did not observe any difference in outcome on the basis of either locally or centrally designated type 1 or type 2 disease, the former thought to generally be enriched with MET alterations.5,7
In conclusion, our updated analyses continue to support cabozantinib as a reasonable option for patients with advanced PRCC but simultaneously reinforce the need to complete ongoing frontline trials in this disease.
APPENDIX
TABLE A1.
Subsequent Therapies in the Sunitinib and Cabozantinib Arms
| Subsequent Therapy | Sunitinib (n = 20), No. (%) | Cabozantinib (n = 17), No. (%) |
|---|---|---|
| Systemic therapies | ||
| Anti-VEGF alone | 7 (35) | 5 (29) |
| mTOR inhibitor alone | 1 (5) | — |
| PD-1 inhibitor | 9 (45) | 7 (41) |
| CTLA-4 inhibitor | 2 (10) | — |
| Anti-VEGF plus ICI | 3 (15) | 1 (6) |
| Anti-VEGF plus mTOR inhibitor | — | 6 (35) |
| Other | 1 (5) | 3 (18) |
| Local therapies | ||
| Radiation therapy | 1 (5) | 1 (6) |
| Surgery | 1 (5) | — |
NOTE. Six patients on the sunitinib arm received cabozantinib as subsequent therapy, either alone or in combination with PD-(L)1 inhibitors.
Abbreviations: CTLA-4, cytotoxic T-lymphocyte–associated antigen 4; ICI, immune checkpoint inhibitor; mTOR, mammalian target of rapamycin; VEGF, vascular endothelial growth factor.
Pedro Barata
Honoraria: UroToday, MJH Life Sciences
Consulting or Advisory Role: Bayer, BMS, Pfizer, EMD Serono, Eisai, Caris Life Sciences, Dendreon (Inst), AstraZeneca, Exelixis, AVEO, Merck, Ipsen, Telix, Seagen Genetics, Guardant Health
Speakers' Bureau: Caris Life Sciences, Bayer, Pfizer/Astellas, AstraZeneca, Merck
Research Funding: Blue Earth Diagnostics (Inst), AVEO (Inst), Merck (Inst), Exelixis, JNJ
Ian M. Thompson
Employment: CHRISTUS Health
Leadership: H-E-B (grocery store chain)
Consulting or Advisory Role: MagForce
Research Funding: MagForce
Patents, Royalties, Other Intellectual Property: I have several patents with colleagues involving novel biomarkers for cancer and two devices for sexual dysfunction and urinary incontinence. No revenues at this time and our University IP office is working with industry to determine if these can be commercialized
Vivek Narayan
Honoraria: Pfizer
Consulting or Advisory Role: Merck, AstraZeneca, Janssen Oncology, Myovant Sciences, Regeneron, Exelixis, Xencor, Pfizer/Astellas, Eisai, Sanofi, Terns Pharmaceuticals
Research Funding: Pfizer (Inst), Bristol Myers Squibb (Inst), Merck (Inst), Janssen (Inst), Regeneron (Inst), Xencor (Inst)
Patents, Royalties, Other Intellectual Property: US Patent No. 18/708,529
Travel, Accommodations, Expenses: Pfizer, Eisai
Daniel J. George
Leadership: Capio BioSciences
Honoraria: Sanofi, Bayer, Exelixis, EMD Serono, OncLive, Pfizer, UroToday, American Association for Cancer Research, Axess Oncology, Janssen Oncology, Millennium Medical Publishing, Novartis, AstraZeneca, Aveo, Eisai, IDEOlogy Health, Myovant Sciences, Medscape, Merck, Nektar, Propella Therapeutics, Seagen
Consulting or Advisory Role: Bayer, Exelixis, Pfizer, Sanofi, Astellas Pharma, Bristol Myers Squibb, Janssen, Merck Sharp & Dohme, Myovant Sciences, AstraZeneca, Michael J. Hennessy Associates, Constellation Pharmaceuticals, Physicans' Education Resource, Propella Therapeutics, RevHealth, Xcures, Novartis
Speakers' Bureau: Sanofi, Bayer, Exelixis
Research Funding: Exelixis (Inst), Janssen Oncology (Inst), Novartis (Inst), Pfizer (Inst), Astellas Pharma (Inst), Bristol Myers Squibb (Inst), Bayer (Inst), Dendreon (Inst), Calithera Biosciences (Inst), Sanofi/Aventis (Inst), Merck (Inst), Corvus Pharmaceuticals (Inst)
Expert Testimony: Exelixis
Travel, Accommodations, Expenses: Exelixis, Pfizer, Novartis, Bayer
Daniel Y.C. Heng
Consulting or Advisory Role: Pfizer, Novartis, Bristol Myers Squibb, Janssen, Astellas Pharma, Ipsen, Eisai, Merck
Research Funding: Pfizer (Inst), Novartis (Inst), Exelixis (Inst), Bristol Myers Squibb (Inst), Ipsen (Inst)
Brian Shuch
Consulting or Advisory Role: Bristol Myers Squibb, Merck, Johnson & Johnson/Janssen, Genentech/Roche, HistoSonics, Veracyte, Telix Pharmaceuticals
Speakers' Bureau: Merck
Patents, Royalties, Other Intellectual Property: UpToDate royalty
Mark Stein
Consulting or Advisory Role: Merck Sharp & Dohme, Exelixis, Xencor, Janssen Oncology, Vaccitech, Bristol Myers Squibb/Medarex
Research Funding: Oncoceutics (Inst), Merck Sharp & Dohme (Inst), Janssen Oncology (Inst), Medivation/Astellas (Inst), Advaxis (Inst), Suzhou Kintor Pharmaceuticals (Inst), Harpoon (Inst), Bristol Myers Squibb (Inst), Genocea Biosciences (Inst), Lilly (Inst), Nektar (Inst), Seagen (Inst), Xencor (Inst), Tmunity Therapeutics, Inc (Inst), Exelixis (Inst), Bellicum Pharmaceuticals, Regeneron (Inst), Bicycle Therapeutics (Inst), AstraZeneca (Inst)
Shuchi Gulati
Honoraria: ASCO, Horizon CME, MashupMedia, Meetings, Events and Conference Coordinators Inc
Consulting or Advisory Role: Puma Biotechnology, EMD Serono, AVEO
Research Funding: AstraZeneca
Patents, Royalties, Other Intellectual Property: Patent pending for: “Diagnostic tools for prediction of survival and responsiveness to treatments.” US17327100
Travel, Accommodations, Expenses: Conquer Cancer Foundation, ASCO, Horizon CME, Meetings, Events and Conference Coordinators Inc
Abhishek Tripathi
Consulting or Advisory Role: AADi, Seattle Genetics/Astellas, Exelixis, Bayer, Gilead Sciences, Pfizer, Deka Biosciences
Speakers' Bureau: Sanofi
Georg A. Bjarnason
Honoraria: Pfizer, Bristol Myers Squibb, Eisai, Ipsen, Merck
Consulting or Advisory Role: Pfizer, Bristol Myers Squibb, Eisai, Ipsen
Research Funding: Pfizer (Inst), Merck (Inst)
Peter Humphrey
Consulting or Advisory Role: Johnson & Johnson/Janssen
Ulka Vaishampayan
Consulting or Advisory Role: Pfizer, Exelixis, Bayer, Bristol Myers Squibb/Medarex, Merck Serono, Gilead Sciences, Sanofi/Aventis, Pfizer/Astellas, Aveo
Speakers' Bureau: Bayer, Exelixis, Astellas Pharma
Research Funding: Bristol Myers Squibb, Merck KGaA
Ajjai Alva
Research Funding: Bristol Myers Squibb (Inst), Merck Sharp & Dohme (Inst), Prometheus (Inst), Mirati Therapeutics (Inst), AstraZeneca (Inst), Roche (Inst), Bayer (Inst), Astellas Pharma (Inst), Arcus Biosciences (Inst), Progenics (Inst), Celgene (Inst), Janssen (Inst)
Tian Zhang
Leadership: Capio BioSciences, Archimmune Therapeutics
Stock and Other Ownership Interests: Capio Biosciences, Archimmune Therapeutics, Nanorobotics
Honoraria: MJH Life Sciences, Aptitude Health, Curio Science, PeerView, Clinical Care Options, Caris Life Sciences
Consulting or Advisory Role: Janssen, Exelixis, Pfizer, Bristol Myers Squibb, Seagen, Eisai, Aravive, Bayer, Lilly, AVEO, Merck, Sanofi/Aventis, Novartis, Gilead Sciences, EMD Serono
Research Funding: Loxo/Lilly (Inst), Tempus (Inst), Pfizer (Inst), ALX Oncology (Inst), Exelixis (Inst), OncoC4
Patents, Royalties, Other Intellectual Property: Circulating tumor cell novel capture by c-MET technology (Inst), Prochelators as Targeted Prodrugs for Prostate Cancer (Inst)
Travel, Accommodations, Expenses: Janssen, Bayer
Other Relationship: ASCO, Kidney Cancer Association, KidneyCan, Myrovlytis Trust
Scott Cole
Consulting or Advisory Role: HERON, Bayer, Seattle Genetics/Astellas
Travel, Accommodations, Expenses: Bayer, HERON
Primo N. Lara Jr
Research Funding: Taiho Pharmaceutical (Inst), AstraZeneca (Inst)
Seth P. Lerner
Stock and Other Ownership Interests: C2i genomics, Aura Biosciences
Honoraria: UroToday, Grand Rounds in Urology
Consulting or Advisory Role: Vaxiion, Verity Pharmaceuticals, Ferring, Pfizer/EMD Serono, C2i Genomics, Aura Biosciences, AstraZeneca, Stimit, Protara Therapeutics, Bristol Myers Squibb Foundation/Janssen, UroGen Pharma, Gilead Sciences, Incyte, ImmunityBio
Research Funding: Endo Pharmaceuticals, FKD Therapies, Viventia Biotech, Roche/Genentech, Japan BCG Laboratory, Vaxiion, QED Therapeutics, Aura Biosciences, SURGE Therapeutics
Patents, Royalties, Other Intellectual Property: TCGA expression subtype single patient classifier
Other Relationship: UpToDate, Bladder Cancer Journal
Naomi Balzer-Haas
Consulting or Advisory Role: Merck Sharp & Dohme, Eisai, Exelixis, AVEO, Roche/Genentech
Sumanta K. Pal
Speakers' Bureau: MJH Life Sciences, IntrinsiQ, PeerView
Travel, Accommodations, Expenses: crispr therapeutics, Ipsen, Exelixis
Open Payments Link: https://openpaymentsdata.cms.gov/physician/259259
No other potential conflicts of interest were reported.
SUPPORT
Supported by NIH/National Cancer Institute grants CA180888, CA180819, CA180820, CA180821, CA180863, and CA180868.
CLINICAL TRIAL INFORMATION
NCT02761057 (SWOG 1500).
N.B.-H. and S.K.P. contributed equally to this work.
AUTHOR CONTRIBUTIONS
Conception and design: Pedro Barata, Catherine Tangen, Ian M. Thompson, Daniel Y.C. Heng, Brian Shuch, Mark Stein, Primo N. Lara Jr, Naomi Balzer-Haas, Sumanta K. Pal
Administrative support: Ian M. Thompson, Brian Shuch, Primo N. Lara Jr
Provision of study materials or patients: Vivek Narayan, Daniel J. George, Daniel Y.C. Heng, Mark Stein, Shuchi Gulati, Maria Tretiakova, Abhishek Tripathi, Georg A. Bjarnason, Adebowale Adeniran, Ulka Vaishampayan, Ajjai Alva, Tian Zhang, Scott Cole, Primo N. Lara Jr, Naomi Balzer-Haas, Sumanta K. Pal
Collection and assembly of data: Pedro Barata, Catherine Tangen, Melissa Plets, Vivek Narayan, Daniel J. George, Daniel Y.C. Heng, Brian Shuch, Abhishek Tripathi, Georg A. Bjarnason, Peter Humphrey, Adebowale Adeniran, Ulka Vaishampayan, Ajjai Alva, Tian Zhang, Primo N. Lara Jr, Naomi Balzer-Haas, Sumanta K. Pal
Data analysis and interpretation: Pedro Barata, Catherine Tangen, Melissa Plets, Ian M. Thompson, Vivek Narayan, Daniel J. George, Daniel Y.C. Heng, Brian Shuch, Mark Stein, Shuchi Gulati, Maria Tretiakova, Abhishek Tripathi, Georg A. Bjarnason, Ulka Vaishampayan, Tian Zhang, Scott Cole, Primo N. Lara Jr, Seth P. Lerner, Sumanta K. Pal
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Final Overall Survival Analysis of S1500: A Randomized, Phase II Study Comparing Sunitinib With Cabozantinib, Crizotinib, and Savolitinib in Advanced Papillary Renal Cell Carcinoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Pedro Barata
Honoraria: UroToday, MJH Life Sciences
Consulting or Advisory Role: Bayer, BMS, Pfizer, EMD Serono, Eisai, Caris Life Sciences, Dendreon (Inst), AstraZeneca, Exelixis, AVEO, Merck, Ipsen, Telix, Seagen Genetics, Guardant Health
Speakers' Bureau: Caris Life Sciences, Bayer, Pfizer/Astellas, AstraZeneca, Merck
Research Funding: Blue Earth Diagnostics (Inst), AVEO (Inst), Merck (Inst), Exelixis, JNJ
Ian M. Thompson
Employment: CHRISTUS Health
Leadership: H-E-B (grocery store chain)
Consulting or Advisory Role: MagForce
Research Funding: MagForce
Patents, Royalties, Other Intellectual Property: I have several patents with colleagues involving novel biomarkers for cancer and two devices for sexual dysfunction and urinary incontinence. No revenues at this time and our University IP office is working with industry to determine if these can be commercialized
Vivek Narayan
Honoraria: Pfizer
Consulting or Advisory Role: Merck, AstraZeneca, Janssen Oncology, Myovant Sciences, Regeneron, Exelixis, Xencor, Pfizer/Astellas, Eisai, Sanofi, Terns Pharmaceuticals
Research Funding: Pfizer (Inst), Bristol Myers Squibb (Inst), Merck (Inst), Janssen (Inst), Regeneron (Inst), Xencor (Inst)
Patents, Royalties, Other Intellectual Property: US Patent No. 18/708,529
Travel, Accommodations, Expenses: Pfizer, Eisai
Daniel J. George
Leadership: Capio BioSciences
Honoraria: Sanofi, Bayer, Exelixis, EMD Serono, OncLive, Pfizer, UroToday, American Association for Cancer Research, Axess Oncology, Janssen Oncology, Millennium Medical Publishing, Novartis, AstraZeneca, Aveo, Eisai, IDEOlogy Health, Myovant Sciences, Medscape, Merck, Nektar, Propella Therapeutics, Seagen
Consulting or Advisory Role: Bayer, Exelixis, Pfizer, Sanofi, Astellas Pharma, Bristol Myers Squibb, Janssen, Merck Sharp & Dohme, Myovant Sciences, AstraZeneca, Michael J. Hennessy Associates, Constellation Pharmaceuticals, Physicans' Education Resource, Propella Therapeutics, RevHealth, Xcures, Novartis
Speakers' Bureau: Sanofi, Bayer, Exelixis
Research Funding: Exelixis (Inst), Janssen Oncology (Inst), Novartis (Inst), Pfizer (Inst), Astellas Pharma (Inst), Bristol Myers Squibb (Inst), Bayer (Inst), Dendreon (Inst), Calithera Biosciences (Inst), Sanofi/Aventis (Inst), Merck (Inst), Corvus Pharmaceuticals (Inst)
Expert Testimony: Exelixis
Travel, Accommodations, Expenses: Exelixis, Pfizer, Novartis, Bayer
Daniel Y.C. Heng
Consulting or Advisory Role: Pfizer, Novartis, Bristol Myers Squibb, Janssen, Astellas Pharma, Ipsen, Eisai, Merck
Research Funding: Pfizer (Inst), Novartis (Inst), Exelixis (Inst), Bristol Myers Squibb (Inst), Ipsen (Inst)
Brian Shuch
Consulting or Advisory Role: Bristol Myers Squibb, Merck, Johnson & Johnson/Janssen, Genentech/Roche, HistoSonics, Veracyte, Telix Pharmaceuticals
Speakers' Bureau: Merck
Patents, Royalties, Other Intellectual Property: UpToDate royalty
Mark Stein
Consulting or Advisory Role: Merck Sharp & Dohme, Exelixis, Xencor, Janssen Oncology, Vaccitech, Bristol Myers Squibb/Medarex
Research Funding: Oncoceutics (Inst), Merck Sharp & Dohme (Inst), Janssen Oncology (Inst), Medivation/Astellas (Inst), Advaxis (Inst), Suzhou Kintor Pharmaceuticals (Inst), Harpoon (Inst), Bristol Myers Squibb (Inst), Genocea Biosciences (Inst), Lilly (Inst), Nektar (Inst), Seagen (Inst), Xencor (Inst), Tmunity Therapeutics, Inc (Inst), Exelixis (Inst), Bellicum Pharmaceuticals, Regeneron (Inst), Bicycle Therapeutics (Inst), AstraZeneca (Inst)
Shuchi Gulati
Honoraria: ASCO, Horizon CME, MashupMedia, Meetings, Events and Conference Coordinators Inc
Consulting or Advisory Role: Puma Biotechnology, EMD Serono, AVEO
Research Funding: AstraZeneca
Patents, Royalties, Other Intellectual Property: Patent pending for: “Diagnostic tools for prediction of survival and responsiveness to treatments.” US17327100
Travel, Accommodations, Expenses: Conquer Cancer Foundation, ASCO, Horizon CME, Meetings, Events and Conference Coordinators Inc
Abhishek Tripathi
Consulting or Advisory Role: AADi, Seattle Genetics/Astellas, Exelixis, Bayer, Gilead Sciences, Pfizer, Deka Biosciences
Speakers' Bureau: Sanofi
Georg A. Bjarnason
Honoraria: Pfizer, Bristol Myers Squibb, Eisai, Ipsen, Merck
Consulting or Advisory Role: Pfizer, Bristol Myers Squibb, Eisai, Ipsen
Research Funding: Pfizer (Inst), Merck (Inst)
Peter Humphrey
Consulting or Advisory Role: Johnson & Johnson/Janssen
Ulka Vaishampayan
Consulting or Advisory Role: Pfizer, Exelixis, Bayer, Bristol Myers Squibb/Medarex, Merck Serono, Gilead Sciences, Sanofi/Aventis, Pfizer/Astellas, Aveo
Speakers' Bureau: Bayer, Exelixis, Astellas Pharma
Research Funding: Bristol Myers Squibb, Merck KGaA
Ajjai Alva
Research Funding: Bristol Myers Squibb (Inst), Merck Sharp & Dohme (Inst), Prometheus (Inst), Mirati Therapeutics (Inst), AstraZeneca (Inst), Roche (Inst), Bayer (Inst), Astellas Pharma (Inst), Arcus Biosciences (Inst), Progenics (Inst), Celgene (Inst), Janssen (Inst)
Tian Zhang
Leadership: Capio BioSciences, Archimmune Therapeutics
Stock and Other Ownership Interests: Capio Biosciences, Archimmune Therapeutics, Nanorobotics
Honoraria: MJH Life Sciences, Aptitude Health, Curio Science, PeerView, Clinical Care Options, Caris Life Sciences
Consulting or Advisory Role: Janssen, Exelixis, Pfizer, Bristol Myers Squibb, Seagen, Eisai, Aravive, Bayer, Lilly, AVEO, Merck, Sanofi/Aventis, Novartis, Gilead Sciences, EMD Serono
Research Funding: Loxo/Lilly (Inst), Tempus (Inst), Pfizer (Inst), ALX Oncology (Inst), Exelixis (Inst), OncoC4
Patents, Royalties, Other Intellectual Property: Circulating tumor cell novel capture by c-MET technology (Inst), Prochelators as Targeted Prodrugs for Prostate Cancer (Inst)
Travel, Accommodations, Expenses: Janssen, Bayer
Other Relationship: ASCO, Kidney Cancer Association, KidneyCan, Myrovlytis Trust
Scott Cole
Consulting or Advisory Role: HERON, Bayer, Seattle Genetics/Astellas
Travel, Accommodations, Expenses: Bayer, HERON
Primo N. Lara Jr
Research Funding: Taiho Pharmaceutical (Inst), AstraZeneca (Inst)
Seth P. Lerner
Stock and Other Ownership Interests: C2i genomics, Aura Biosciences
Honoraria: UroToday, Grand Rounds in Urology
Consulting or Advisory Role: Vaxiion, Verity Pharmaceuticals, Ferring, Pfizer/EMD Serono, C2i Genomics, Aura Biosciences, AstraZeneca, Stimit, Protara Therapeutics, Bristol Myers Squibb Foundation/Janssen, UroGen Pharma, Gilead Sciences, Incyte, ImmunityBio
Research Funding: Endo Pharmaceuticals, FKD Therapies, Viventia Biotech, Roche/Genentech, Japan BCG Laboratory, Vaxiion, QED Therapeutics, Aura Biosciences, SURGE Therapeutics
Patents, Royalties, Other Intellectual Property: TCGA expression subtype single patient classifier
Other Relationship: UpToDate, Bladder Cancer Journal
Naomi Balzer-Haas
Consulting or Advisory Role: Merck Sharp & Dohme, Eisai, Exelixis, AVEO, Roche/Genentech
Sumanta K. Pal
Speakers' Bureau: MJH Life Sciences, IntrinsiQ, PeerView
Travel, Accommodations, Expenses: crispr therapeutics, Ipsen, Exelixis
Open Payments Link: https://openpaymentsdata.cms.gov/physician/259259
No other potential conflicts of interest were reported.
REFERENCES
- 1. Sweeney PL, Jang A, Halat SK, et al. Advanced papillary renal cell carcinoma: Epidemiology, genomic drivers, current therapies, and ongoing trials. Cancer Treat Res Commun. 2022;33:100639. doi: 10.1016/j.ctarc.2022.100639. [DOI] [PubMed] [Google Scholar]
- 2. Dizman N, Arslan ZE, Feng M, et al. Sequencing therapies for metastatic renal cell carcinoma. Urol Clin North Am. 2020;47:305–318. doi: 10.1016/j.ucl.2020.04.008. [DOI] [PubMed] [Google Scholar]
- 3. Coppin C, Porzsolt F, Autenrieth M, et al. Immunotherapy for advanced renal cell cancer. Cochrane database Syst Rev. 2015;2015:CD001425. doi: 10.1002/14651858.CD001425.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pal SK, Ali SM, Yakirevich E, et al. Characterization of clinical cases of advanced papillary renal cell carcinoma via comprehensive genomic profiling. Eur Urol. 2018;73:71–78. doi: 10.1016/j.eururo.2017.05.033. [DOI] [PubMed] [Google Scholar]
- 5. Albiges L, Guegan J, Le Formal A, et al. MET is a potential target across all papillary renal cell carcinomas: Result from a large molecular study of pRCC with CGH array and matching gene expression array. Clin Cancer Res. 2014;20:3411–3421. doi: 10.1158/1078-0432.CCR-13-2173. [DOI] [PubMed] [Google Scholar]
- 6. Pal SK, Tangen C, Thompson IM, Jr, et al. A comparison of sunitinib with cabozantinib, crizotinib, and savolitinib for treatment of advanced papillary renal cell carcinoma: A randomised, open-label, phase 2 trial. Lancet. 2021;397:695–703. doi: 10.1016/S0140-6736(21)00152-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tripathi A, Tangen C, Li X, et al. Pathologic concordance rate and outcomes by histologic subtype in advanced papillary renal cell (pRCC) carcinoma: An analysis from the SWOG S1500 (PAPMET) trial. J Clin Oncol. 2023;41 (16_suppl; abstr 4562) [Google Scholar]
- 8. Pal SK, McGregor B, Suárez C, et al. Cabozantinib in combination with atezolizumab for advanced renal cell carcinoma: Results from the COSMIC-021 study. J Clin Oncol. 2021;39:3725–3736. doi: 10.1200/JCO.21.00939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee CH, Voss MH, Carlo MI, et al. Phase II trial of cabozantinib plus nivolumab in patients with non-clear-cell renal cell carcinoma and genomic correlates. J Clin Oncol. 2022;40:2333–2341. doi: 10.1200/JCO.21.01944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Albiges L, Gurney H, Atduev V, et al. 1448O Phase II KEYNOTE-B61 study of pembrolizumab (Pembro)+ lenvatinib (Lenva) as first-line treatment for non-clear cell renal cell carcinoma (nccRCC) Ann Oncol. 2022;33:S1204–S1205. [Google Scholar]
- 11. Maughan BL. Start of a new era: Management of non-clear cell renal cell carcinoma in 2022. Curr Oncol Rep. 2022;24:1201–1208. doi: 10.1007/s11912-022-01269-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chouieri T. Sameta: A phase III study of savolitinib + durvalumab vs sunitinib and durvalumab monotherapy in patients with MET-driven, unresectable, locally advanced/metastatic papillary renal cell carcinoma. Oncologist. 2023;28:S11–S12. [Google Scholar]
- 13.Exelixis A Randomized Open-Label Phase 3 Study of XL092 + Nivolumab vs Sunitinib in Subjects with Advanced or Metastatic Non-Clear Cell Renal Cell Carcinoma. clinicaltrials.gov.
- 14. Tannir NM, Plimack E, Ng C, et al. A phase 2 trial of sunitinib in patients with advanced non–clear cell renal cell carcinoma. Eur Urol. 2012;62:1013–1019. doi: 10.1016/j.eururo.2012.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tannir NM, Jonasch E, Albiges L, et al. Everolimus versus sunitinib prospective evaluation in metastatic non-clear cell renal cell carcinoma (ESPN): A randomized multicenter phase 2 trial. Eur Urol. 2016;69:866–874. doi: 10.1016/j.eururo.2015.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ravaud A, Oudard S, De Fromont M, et al. First-line treatment with sunitinib for type 1 and type 2 locally advanced or metastatic papillary renal cell carcinoma: A phase II study (SUPAP) by the French Genitourinary group (GETUG) Ann Oncol. 2015;26:1123–1128. doi: 10.1093/annonc/mdv149. [DOI] [PubMed] [Google Scholar]
- 17. Iacovelli R, Ciccarese C, Procopio G, et al. Current evidence for second-line treatment in metastatic renal cell carcinoma after progression to immune-based combinations. Cancer Treat Rev. 2022;105:102379. doi: 10.1016/j.ctrv.2022.102379. [DOI] [PubMed] [Google Scholar]