Abstract
Heterogeneous extracellular vesicles (EVs) from various types of tumours are acknowledged for inducing the formation of pre‐metastatic “niches” in draining lymph nodes (LNs) to promote lymphatic metastasis. In order to identify the specific subpopulations of EVs involved, we performed high‐resolution proteomic analysis combined with nanoflow cytometry of bladder cancer (BCa) tissue‐derived EVs to identify a novel subset of tumour‐derived EVs that contain integrin α6 (ITGA6+EVs) and revealed the positive correlation of ITGA6+EVs with the formation of pre‐metastatic niche in draining LNs and lymphatic metastasis in multicentre clinical analysis of 820‐case BCa patients. BCa‐derived ITGA6+EVs induced E‐selectin (SELE)‐marked lymphatic remodelling pre‐metastatic niche and promoted metastasis in draining LNs through delivering cargo circRNA‐LIPAR to lymphatic endothelial cells in vivo and in vitro. Mechanistically, LIPAR linked ITGA6 to the switch II domain of RAB5A and sustained RAB5A GTP‐bound activated state, thus maintaining the production of ITGA6+EVs loaded with LIPAR through endosomal trafficking. ITGA6+EVs targeted lymphatic vessels through ITGA6‐CD151 interplay and released LIPAR to induce SELE overexpression‐marked lymphatic remodelling pre‐metastatic niche. Importantly, we constructed engineered‐ITGA6 EVs to inhibit lymphatic pre‐metastatic niche, which suppressed lymphatic metastasis and prolonged survival in preclinical models. Collectively, our study uncovers the mechanism of BCa‐derived ITGA6+EVs mediating pre‐metastatic niche and provides an engineered‐EV‐based strategy against BCa lymphatic metastasis.
Keywords: bladder cancer, extracellular vesicles, ITGA6, lymphatic metastasis, pre‐metastatic niche
In BCa, LIPAR linked ITGA6 to RAB5A to form a ternary complex, which blocked GDI‐mediated RAB5A inactivation to trigger the sustained production of ITGA6+EVs loaded with LIPAR. Subsequently, BCa‐derived ITGA6+EVs targeted lymphatic vessels via ITGA6‐CD151 interplay between BCa cells and lymphatic endothelial cells, and delivered LIPAR to activate SELE transcription, thus mediating pre‐metastatic niche formation in draining LNs and promoting lymphatic metastasis.

1. INTRODUCTION
Bladder cancer (BCa) preferentially spreads from its primary site into draining lymph nodes (LNs) through lymphatic systems (Shariat et al., 2012; Wu et al., 2023). Accumulating evidence indicates that draining LNs are not passive receivers of metastatic cells, but are instead selectively and actively modified by the primary tumour before metastatic spread has even occurred (García‐Silva et al., 2021). Previous studies hold that these pre‐metastatic “niches” created by malignant tumours preceded metastasis in draining LNs feature active lymphangiogenesis and immunosuppression, which provide more approaches for the metastasis of tumour cells into lymphatic systems and protect them from immune response (Li et al., 2020; Liu & Cao, 2016; Morrissey et al., 2021). Yet, recent studies reveal that blocking immunosuppression or lymphangiogenesis is unable to completely prevent disseminated BCa cells from metastasizing into BCa draining LNs (Gómez de Liaño Lista et al., 2020; He et al., 2018; Zheng et al., 2024a). Lymphatic remodelling triggered by overexpressing adhesion molecules on the surface of lymphatic endothelial cells (LECs) has been considered as another major characteristic in lymphatic pre‐metastatic niche, to which metastatic cells can adhere strongly and then extravasate into the lymphatic system, leading to the metastatic outgrowth in draining LNs (Bieniasz‐Krzywiec et al., 2019; Shen et al., 2019). However, the determined existence and molecular regulatory mechanisms triggering lymphatic remodelling pre‐metastatic niche formation in BCa draining LNs remain incompletely unclear.
Extracellular vesicles (EVs) are nanovesicles with lipid bilayer secreted by most cells (Teng & Fussenegger, 2020). Tumour‐derived EVs that contain pro‐metastatic factors travel far from their original site through the circulation to facilitate pre‐metastatic niche formation and increase metastasis burden of tumour (Chen et al., 2020; Costa‐Silva et al., 2015; Liu et al., 2016). These tumour‐derived EVs “dock” onto other tissues through molecules on their surface, after which they fuse with the tissues and release their pro‐metastatic cargoes (Leary et al., 2022; Xie et al., 2022). Subsequently, pro‐metastatic cargoes released by EVs reshape gene expression pattern and metabolism pattern of recipient cells to trigger function reprogramming and morphology remodelling, reinforcing pre‐metastatic niche formation (Liu et al., 2016; Wang et al., 2021). However, the surface molecules that confer selectivity of the tumour‐derived EVs for different target recipient cells as well as on the protein and nucleic acid cargoes within the EVs that influence processes of pre‐metastatic niche formation in target draining LNs to promote lymphatic metastasis warrant further exploration.
In the present study, we analysed the membrane‐protein expression signature of BCa‐derived EVs and found a distinct EV subset containing integrin α6 (ITGA6). We demonstrated that BCa‐derived ITGA6‐containing EVs (ITGA6+EVs) targeted anchored to lymphatic vessels through the interplay of ITGA6‐CD151 and released cargo circRNA‐LIPAR to induce E‐selectin (SELE) overexpression, thus creating lymphatic remodelling associated pre‐metastatic niche to recruit BCa cells metastasis into draining LNs. Importantly, we constructed engineered‐ITGA6 EVs for LN targeting delivery to inhibit lymphatic pre‐metastatic niche and effectively prevent lymphatic metastasis of BCa to extend survival in mice. These findings elucidate the precise mechanism of BCa‐derived ITGA6+EVs promoting lymphatic metastasis via inducing lymphatic remodelling pre‐metastatic niche in targeting draining LNs and represent a potential strategy of inhibiting pre‐metastatic niche formation to intervene BCa lymphatic metastasis.
2. MATERIALS AND METHODS
2.1. Cell lines and cell culture
Human BCa cell lines UM‐UC‐3, 5637, T24 and human embryonic kidney cell line HEK‐293T were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). Human lymphatic endothelial cells (HLECs) were obtained from ScienCell Research Laboratories (Carlsbad, CA, USA, Cat#2500). The UM‐UC‐3 and HEK‐293T cells were cultured in Dulbecco's modified Eagle's medium (Invitrogen, Waltham, MA, USA). The 5637 and T24 cells were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium (Invitrogen, Waltham, MA, USA, Cat#1640TS). HLECs were cultured in an endothelial cell medium (ECM; ScienCell Research Laboratories, Cat#1001). The ECM was added with 5% FBS, and the other culture medium was added with 10% FBS (Gibco, Indianapolis, IN, USA, Cat#10093). All cells were cultured in a humidified incubator with 5% CO2 at 37°C and the authentication as well as mycoplasma testing were qualified by STR DNA profiling.
2.2. Patients and clinical samples
All pairs of BCa tissues and normal adjacent tissues (NATs) were obtained from patients who had undergone surgery at the five independent hospitals in Guangzhou, Guangdong province, among which 348 cases from Sun Yat‐sen Memorial Hospital of Sun Yat‐sen University, 189 cases from Zhujiang Hospital of Southern Medical University, 114 cases from the Third Affiliated Hospital of Sun Yat‐sen University, 57 cases from the Third Affiliated Hospital of Southern Medical University and 112 cases from Nanfang Hospital of Southern Medical University. The normal LNs from non‐tumour patients were obtained from patients with tuberculous contracture of the bladder who underwent pelvic lymphadenectomy or patients with enlarged inguinal or retroperitoneal LNs predicted by imaging analysis and diagnosed as non‐malignant LNs by B‐ultrasound‐guided LN puncture biopsy (Table S1). These specimens were acquired and gained approval by the Ethics Committees of each participating hospital and conducted in accordance with recognized ethical guidelines (SYSKY‐2023‐366‐01). The pathologic state of each tissue was diagnosed as BCa by three pathologists independently.
2.3. Isolation, purification and characterization of EVs
The tissue‐derived EVs were isolated according to a centrifugation‐based protocol. Tumour tissues and NATs were cut into small pieces lightly and incubated with RPMI‐1640 media supplemented with DNase I (0.1 mg/mL, Roche) and collagenase (1 mg/mL, Sigma Aldrich) in shaking incubators at 37°C for 30 min. After filtration through a 70 µm pore PES filter (Millipore), the dissociated tissues and single cell were removed through centrifugation at 300 × g for 10 min and 2000 × g for 20 min. Next, the supernatant was centrifuged at 10,000 × g for 45 min and then centrifuged at 120,000 × g for 70 min to isolate EVs that resuspended in PBS for further analysis. All centrifugations were performed at 4°C.
The cell‐derived EVs were isolated through the centrifugation‐based protocol. Briefly, we collected the culture medium with EV‐depleted FBS and administrated them into centrifugation sequentially at 2000 × g for 10 min, 10,000 × g for 30 min and 120,000 × g for 70 min. Subsequently, the pellets were rinsed in PBS and then centrifuged at 120,000 × g for 70 min to obtain EVs that resuspended in PBS again. The isolated EVs were then labelled with 3 µL infrared fluorescent dye DiD (Molecular Probes), after which labelled EVs were washed twice with 20 mL PBS followed by resuspending in PBS for further use. We further identified the average diameter and concentration of isolated EVs through nanoparticle tracking analysis (NTA), in which EVs were diluted in 1 mL PBS and then measured by NanoSight NS300 (Malvern Panalytical).
2.4. 4D label‐free quantitative proteomics
To detect different expression proteins in isolated EVs, the 4D label‐free quantitative proteomics were conducted by Shanghai Bioprofile Technology Company (China). In brief, the total proteins from EVs were extracted and filtered into sample preparation to prepare the peptide fragments for LC‐MS/MS analysis. Subsequently, the appropriate amounts of peptides taken from each sample were used for chromatographic separation by the Easy nLC 1200 chromatography system (Thermo Scientific, USA), after which the peptides were analysed by the data‐dependent acquisition of parallel accumulation serial fragmentation mass spectrometry (MS) using timsTOF mass spectrometer (Bruker, Germany). The MSFragger version 2.4 software was used to search the proteins following the Uniprot protein database.
2.5. Electron microscopy analysis and immunogold staining
The transmission electron microscopy (TEM) analysis was performed to measure the isolated particle characteristics following the manufacturer's protocol. Briefly, we deposited the isolated particles on the grid for 60 min drying and then fixed them with 2.5% glutaraldehyde for 10 min. After washing with PBS for five times, the systems were stained with uranyl acetate for 5 min. Next, the grids were washed with PBS again and dried in the air, after which JEOL transmission electron microscope 1400EX (JEOL, Tokyo, Japan) was used to image the particles.
For immunogold staining, the grids were blocked with 10% FBS for 20 min and then were incubated with anti‐ITGA6 antibody diluted in 1:10 ratio in blocking solution at 4°C overnight. Subsequently, the grids with anti‐rat IgG were conjugated with 10 nm gold particles for 60 min and then were rinsed with PBS. Then the grids were fixed in 1% glutaraldehyde for 5 min and washed with distilled water. After contrasting and embedding in a mixture of 4% uranyl acetate and 2% methylcellulose in 1:9 ratio, the grids were measured using the EOL transmission electron microscope 1400EX (JEOL, Tokyo, Japan) at 80 kV.
2.6. Nanoflow cytometry of EVs
Nanoflow cytometry of EVs was conducted by CytoFLEXS instrument (Beckman Coulter) that utilized the violet laser to detect EVs by side scatter according to the manufacturer's instructions (SpittlerA., Set‐up of the CytoFLEX for EVs measurement, Application Information Bulletin 2015). Briefly, EVs were diluted in PBS and incubated with directly conjugated antibodies (anti‐CD49f‐FITC). Gating EVs with a mixture of 100 nm, 200 nm (both iZON), and 500 nm yellow‐green beads (Polysciences). Unstained EVs, isotype‐stained EVs and antibodies alone were used as negative controls. Subsequently, we performed a dilution analysis to identify potential particle swarming. The FlowJo v10 (BD) was used for FACS data analysis.
2.7. Education and LN colonization studies
We performed experimental procedures with the approval of the Sun Yat‐sen Memorial Hospital Institutional Animal Care and Use Committee (AP20230211). To analyse the role of ITGA6+EVs in mediating pre‐metastatic niche formation in draining LNs, 10 µg EVs that resuspended in 20 µL PBS were injected into the footpad of 4−6 week old nude mice purchased from the Sun Yat‐Sen University Experimental Animal Center (Guangzhou, China) every 2 days for 10 days. Then we euthanized the mice and dissected popliteal LNs which were fixed in 4% paraformaldehyde and embedded in paraffin for immunostaining. To verify the effect of different treatment with indicated EVs in BCa lymphatic metastasis, mCherry‐labelled UM‐UC‐3 cells were implanted into the footpads of the mice after pretreating with indicated EVs for 10 days, and the status of the popliteal LNs were monitored and imaged weekly through In Vivo Imaging System (IVIS) (Xenogen Corporation) until the day 21. The mice were fasted for 8 hours before the scanning and anesthetized with pentobarbital. Next, we enucleated primary footpad tumours and popliteal LNs and embedded isolated tissues with paraffin for further analysis.
As for the orthotopic xenograft model, the mice were randomly divided into three groups following treatment with the indicated EVs from day 1 to day 10. The mice were anesthetization with pentobarbital. Then we gently inserted an angiocatheter (24‐gauge; Terumo Medical Products) lubricated with paraffin oil into the urethra of mice to evacuate the urine and then inoculated 5×105 UM‐UC‐3 cells suspended in 50 µL of PBS into the bladder of mice. The catheter was left in place for 60 min. After tumorigenesis in the bladder of mice, the pelvic LNs were enucleated for further detection on day 14.
2.8. Adhesion assays
The cell adhesion assays were performed to detect the attachment capacity between BCa cells and HLECs. Briefly, 2 × 105 HLECs pretreated by indicated EVs were seeded into 24‐well plates to culture about 100% of confluency. Next, 5 × 104 UM‐UC‐3 cells stained by PKH‐67 green fluorescent dye (Sigma Aldrich, St Louis, USA, Cat#MINI67) were resuspended in 500 µL conditioned ECM and were seeded onto the precoated HLECs cells for 2 h incubation. Subsequently, the non‐attached cells were washed and removed with PBS. The attached tumour cells were then scanned by an inverted fluorescence microscope (Olympus IX73, Japan) and counted by ImageJ (NIH, Bethesda, MD, USA, RRID:SCR_003070).
2.9. Immunofluorescence
We fixed the tissues from BCa patients or mice experiments with formalin and then embedded them in paraffin for further analysis. After dewaxing and hydration, the tissue sections were mixed with indicated primary antibodies for 1 h incubation. The sections were incubated with indicated fluorescence conjugated secondary antibodies at room temperature for 20 min and then DAPI was used to stain the nuclei in the sections. The laser scanning confocal microscopy (Zeiss, Pleasanton, CA, USA) was used to capture the images of sections. As for the definition with high or low expression level of ITGA6, the staining index (SI) was used and the median value, SI = 8, was defined as the cut‐off value. And samples with a SI ≥ 8 were defined as high expression and samples with a SI < 8 were low expression. The SI was calculated by multiplying the positive percentage with staining intensity with possible scores of 0, 1, 2, 3, 4, 6, 8, 9, 12 and 16, among which the percentage of positive staining tumour cells was designated as follows: 0 (no positive), 1 (0%–10% positive), 2 (10%–30% positive), 3 (30%−70% positive) and 4 (over 70% positive) and the staining intensity was graded as follows: 1 (no staining), 2 (weak staining), 3 (moderate staining) and 4 (strong staining).
For cell immunofluorescence analysis, the pretreated cells were seeded in the confocal dish followed by fixing with 4% paraformaldehyde and were blocked with 5% BSA, respectively. Next, treating the cells with primary antibodies for overnight at 4°C, followed by the incubation with indicated fluorescence‐conjugated secondary antibodies, and then staining the nuclei with DAPI. The indicated cells were captured through a laser scanning confocal microscopy (Zeiss, Pleasanton, CA, USA).
2.10. His‐pull down assay
The ITGA6‐interacted proteins were detected by His‐pulldown. Briefly, 1 × 107 amount of HLECs were harvested and lysed in 300 µL lysis buffer for 30 min and centrifuged at 12,000 × g for another 30 min to obtain the total protein samples. Then, the lysates were incubated with purified His‐labelled recombinant ITGA6 at 4°C overnight. Subsequently, the target proteins were pulldown through magnetic beads conjugated by anti‐His antibodies. The binding proteins were eluted from the beads and subjected to further analysis.
2.11. RNA pull‐down assay
We performed RNA pull‐down assays to examine the proteins interacting with LIPAR following the manufacturer's protocol of Magnetic RNA‐Protein Pull‐Down Kit (Thermo Scientific). Biotinylated LIPAR probes were synthesized by GenePharma (Suzhou, China). Briefly, a total of 2 × 107 5637 cells or UM‐UC‐3 cells were combined with 200 µL lysis buffer and then were frozen in liquid nitrogen immediately, which were then refrigerated at −80°C for complete lysing overnight. Subsequently, we incubated 50 pmol biotinylated LIPAR probe with 50 µL washed streptavidin labelled magnetic beads (Invitrogen) for 30 min. Next, we obtained the supernatant of lysate by centrifugation for 10 min at 12,000 × g and then mixed lysate with preprocessed magnetic beads for incubation overnight at 4°C. After treating with wash buffer for five times, the binding proteins from beads were eluted for further detection.
2.12. Endosome isolation
We isolated the endosomes from BCa cells using a Minute Endosome Isolation and Cell Fractionation kit (Invent Biotechnologies, ED‐028) following the manufacturer's protocol. Briefly, 2 × 107 UM‐UC‐3 cells or 5637 cells were harvested and suspended in lysis buffer A supplemented with protease inhibitor cocktail. After removing intact cells, larger organelles and plasma membranes from cell extracts by filtration, the cell lysates were incubated with supplied precipitation buffer B. Next, the endosomes were enriched in pellets through centrifugation to remove the supernatants, which were resuspended in TRIZOL Regent or RIPA lysis buffer for further analysis. The purified endosomes were validated by western blotting analysis with acknowledged markers including EEA1 (an early endosome marker), GM130 (a Golgi marker), RAB7A (a late endosome marker), Na/K ATPase (a plasma marker) and CALNEXIN (an endoplasmic reticulum marker) to confirm that they were early endosomes.
2.13. Statistics and reproducibility
In experiments where mice or cultures received treatment, these were randomized before dosing. The SPSS 26.0 (IBM, Chicago, IL, USA) and GraphPad Prism 9 (GraphPad Software, La Jolla, CA, USA) were used for statistical analysis. All quantitative data were calculated as mean ± standard deviation of at least three independent experiments. Cumulative survival time was evaluated with Kaplan‐Meier analysis and tested by log‐rank method. Multivariate Cox proportional hazards models were used to determine independent prognostic factors. The relationship between unpaired variables was evaluated by the chi‐square test, and relationship between paired variables was evaluated by the two‐tailed Student's t‐test and one‐way ANOVA test. p < 0.05 was considered as statistically significant.
3. RESULTS
3.1. BCa‐derived EVs induce formation of pre‐metastatic niche in draining LNs
BCa‐derived EVs potentially travel far from primary tumours to act as potential mediators for inducing pre‐metastatic niches (Urabe et al., 2021). To determine the role of tumour‐derived EVs in mediating the pre‐metastatic niche for LN metastasis of BCa, an EV‐education experiment model that mimicked the process of primary tumour‐derived EVs mediating the pre‐metastatic niche in draining LNs before metastasis was conducted. We first isolated BCa cell‐derived EVs (UM‐UC‐3‐EVs) and normal bladder epithelial cell‐derived EVs (SV‐HUC‐1‐EVs), which showed the typical EV structure and a diameter of 30–150 nm (Figure 1a,b). Mice were repeatedly given UM‐UC‐3‐EVs or SV‐HUC‐1‐EVs as a negative control into the footpad for 10 days, then injected with mCherry‐labelled UM‐UC‐3 cells into the footpad. Notably, mice that received UM‐UC‐3‐EV‐education showed significantly higher fluorescence in popliteal LNs than those educated with SV‐HUC‐1‐EVs (Figure S1A,B). Moreover, the metastatic rate of popliteal LNs was higher in UM‐UC‐3‐EV‐education group than in the control group (Figure S1C,D), confirming that BCa‐derived EVs induce pre‐metastatic niche formation in draining LNs to promote lymphatic metastasis.
FIGURE 1.
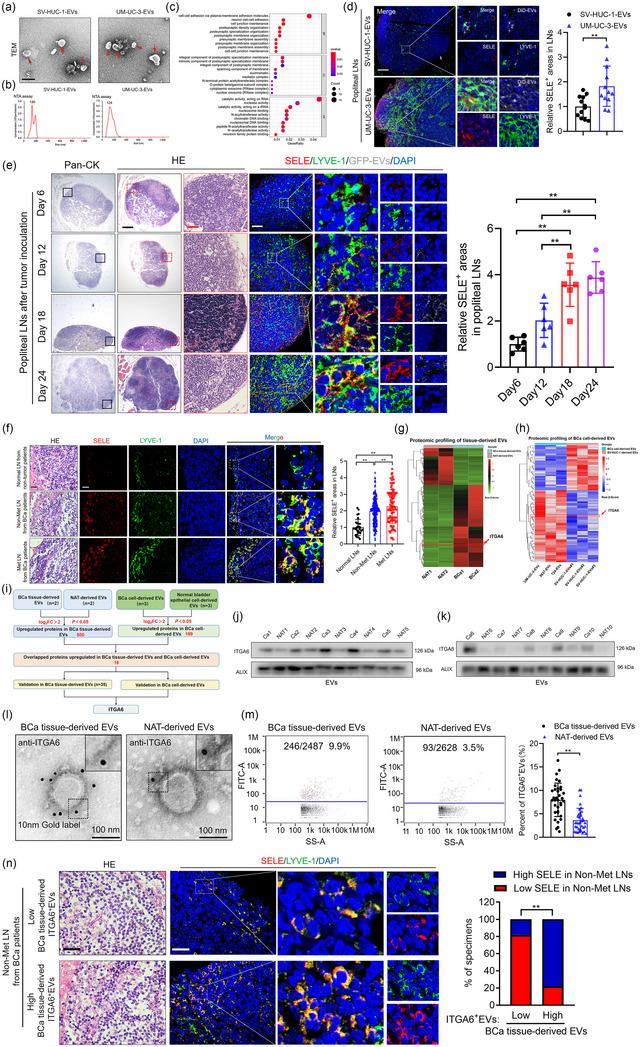
BCa‐derived ITGA6+EVs correlate with pre‐metastatic niche in draining LNs. (a)–(b) Representative TEM images and NTA analysis of EVs isolated from UM‐UC‐3 cells and SV‐HUC‐1 cells. Scale bars: 100 nm. (c) GO analysis of the enriched pathways in UM‐UC‐3‐EV‐educated popliteal LNs. (d) Representative images and quantifications of SELE and LYVE‐1‐indicated lymphatic vessel in popliteal LNs from the mice (n = 12 per group). Scale bar: 50 µm. (e) Representative images and quantifications of SELE and LYVE‐1‐indicated lymphatic vessel in popliteal LNs from the mice after footpad tumour inoculation (n = 6 per group). H&E, haematoxylin and eosin. Scale bar: 500 µm (black), 50 µm (red), 50 µm (white). (f) Representative images and quantifications of SELE and LYVE‐1‐indicated lymphatic vessel in LNs from the BCa patients (n = 348). Scale bar: 50 µm (black), 50 µm (white). (g)–(h) Heatmap of the differentially expressed proteins in BCa tissue‐derived EVs compared with NAT‐derived EVs (g) or in BCa cell‐derived EVs compared with SV‐HUC‐1 derived EVs (h). (i) Schematic illustration for screening the co‐upregulated proteins in BCa tissue‐derived EVs and BCa cell‐derived EVs. (j)–(k) Western blotting analysis of ITGA6 expression in BCa tissue‐derived EVs and NAT‐derived EVs. (l)–(m) Representative immunogold‐labelling electron microscopy images (l) and flow cytometry plots (m) of BCa tissue‐derived EVs and NAT‐derived EVs (n = 35). Scale bar: 100 nm. (n) Representative images and percentages of SELE positive areas in non‐metastatic LNs from BCa patients with different expression level of ITGA6 in BCa tissue‐derived EVs (n = 35). Scale bar: 50 µm (black), 50 µm (white). The statistical difference was assessed by two‐tailed Student's t‐test in (d), (m) one‐way ANOVA followed by Dunnett's tests in (e), (f) the χ2 test in (n). Error bars represent the SD of three independent experiments. ANOVA, analysis of variance, BCa, bladder cancer; EVs, extracellular vesicles; LNs, lymph nodes; NAT, normal adjacent tissue; NTA, nanoparticle tracking; SD, standard deviation; SELE, E‐selectin; TEM; transmission electron microscopy. * p < 0.05, ** p < 0.01.
To explore the phenotype of pre‐metastatic niche in draining LNs, we performed next‐generation sequencing (NGS) of LNs exposed to BCa‐derived EVs and identified 242 genes that were upregulated and 215 that were downregulated relative to their expression in LNs exposed to control EVs, and Gene Ontology analysis linked the upregulated genes to cell adhesion (GSE246070) (Figure 1c). Further validation of these adhesion‐associated genes in mice draining LNs revealed that SELE, an endothelial cell‐specific adhesion molecule, was remarkedly overexpressed in lymphatic vessel endothelial hyaluronan receptor 1 (LYVE‐1) positive areas in LNs educated with UM‐UC‐3‐EVs compared with those educated with control EVs (Figure 1d and Figure S1E), indicating that BCa‐derived EV‐educated pre‐metastatic niche is characterized by the overexpression of SELE in lymphatic vessels.
To achieve real‐time monitoring of BCa‐induced pre‐metastatic niche in draining LNs through EVs, we constructed a popliteal lymphatic metastasis model by implanting a GFP‐CD63 UM‐UC‐3 cell line into footpad (Figure S1F). SELE‐overexpression‐induced massive lymphatic remodelling started around day 6 after tumour inoculation and progressed until day 18, which is consistent with the accumulation of tumour‐derived EV signal in the popliteal LNs (Figure 1e), indicating that BCa‐derived EV‐induced lymphatic remodelling pre‐metastatic niche is formed within 18 days after tumour cells injection. Moreover, SELE was more abundant in LYVE‐1 positive areas in metastatic LNs and non‐metastatic LNs from BCa patients than in normal LNs from non‐tumour patients (Figure 1f).
We further detected whether BCa‐derived EVs induced the upregulation of SELE in LECs helping to recruit metastatic tumour cells to the LNs. The results showed that pretreating monolayers of HLECs with BCa‐derived EVs promoted subsequent adhesion of BCa cells on the monolayer, which was not observed when SELE was knocked down in the HLECs (Figure S1G–J). Collectively, our findings reveal that BCa‐derived EVs induce lymphatic remodelling associated pre‐metastatic niche to promote lymphatic metastasis via promoting the adhesion and colonization of BCa cells in draining LNs in a SELE dependent manner.
3.2. Niche‐forming BCa‐derived EVs are coated with ITGA6
It is well‐established that tumour‐derived EVs possess functional heterogeneity owing to distinct membrane‐protein expression patterns (Hoshino et al., 2015; Kowal et al., 2016). To identify the subpopulations of BCa‐derived EVs responsible for the observed induction of pre‐metastatic niche, we performed quantitative MS comparison of proteomes from BCa tissue‐derived EVs and paired NAT‐derived EVs to identify that 500 proteins were remarkedly upregulated in BCa tissue‐derived EVs compared with NAT‐derived EVs (PXD052771) (Figure 1g). Subsequently, another quantitative MS analysis in BCa cell‐derived EVs and SV‐HUC‐1‐EVs revealed that 189 proteins were upregulated in BCa cell‐derived EVs compared with control (PXD053106) (Figure 1h). We intersected these overexpressed proteins to identify 16 proteins that were consistently upregulated in both EVs derived from BCa tissues and BCa cells (Figure 1i). Further validation of these 16 proteins expression in BCa tissue‐derived EVs and NAT‐derived EVs revealed that ITGA6, HGS, ADAM10, RAP1B and GNB2 were most significantly overexpressed in BCa tissue‐derived EVs compared with control (Figure 1j,k and Figure S1K). Meanwhile, immunogold‐labelling electron microscopy showed that immunogold staining of ITGA6, ADAM10 and GNB2 was largely outside of the membrane bilayer in BCa tissue‐derived EVs and NAT‐derived EVs, among which ITGA6 was dramatically abundant in BCa tissue‐derived EVs than in NAT‐derived EVs observed by nanoflow cytometry (Figure 1l,m and Figure S1L,M). Importantly, the non‐metastatic LNs from patients with higher enrichment of ITGA6+EVs in corresponding BCa tissues possessed higher SELE expression in lymphatic vessels, which was not observed in GNB2 and ADAM10 enrichment group (Figure 1n), indicating the association between ITGA6+EVs with pre‐metastatic niche formation in draining LNs. Moreover, knocking out ITGA6 from UM‐UC‐3 cells prevented their EVs to upregulate SELE in lymphatic vessels in LNs from mice in the EV‐education experimental model (Figure 2a–g), demonstrating that BCa‐derived ITGA6+EVs play a critical role in inducing SELE overexpression in lymphatic vessels to mediate pre‐metastatic niche formation in draining LNs.
FIGURE 2.

BCa‐derived ITGA6+EVs target HLECs in draining LNs. (a) Schematic illustration for ITGA6 deletion. (b)–(c) Western blotting analysis of ITGA6 expression in UM‐UC‐3 cells (b) and corresponding EVs (c). (d) Representative immunogold‐labelling electron microscopy images of UM‐UC‐3‐EVWT and UM‐UC‐3‐EVITGA6‐KO. Scale bar: 100 nm. (e)–(f) The NTA analysis and quantifications of UM‐UC‐3‐EVWT and UM‐UC‐3‐EVITGA6‐KO. (g) Representative images and quantifications of SELE and LYVE‐1‐indicated lymphatic vessels in popliteal LNs from mice (n = 12 per group). Scale bar: 50 µm (black), 50 µm (white). (h) Representative flow cytometry analysis and quantification of DiD‐labelled EVs uptake by the indicated stromal cells in draining LNs (n = 3 LNs analysed). The gating strategy is shown in Figure 2SA. (i)–(j) Representative images and quantification of BECs, HLECs, FRCs, macrophages, DCs, T cells and B cells after incubation with DiD‐labelled EVs. Scale bars: 5 µm. (k)–(m) Representative flow cytometry analysis (k)–(l) and immunofluorescence images (m) of HLECs after incubation with DiD‐labelled EVs in combination with ITGA6 neutralizing antibody. Scale bars: 5 µm. The statistical difference was assessed by two‐tailed Student's t‐test in (f), (g), (h) and (j); the one‐way ANOVA followed by Dunnett's tests in (l), (m). Error bars represent the SD of three independent experiments. ANOVA, analysis of variance, BCa, bladder cancer; FRCs, fibroblastic reticular cells; HLECs, human lymphatic endothelial cells; LNs, lymph nodes; NTA, nanoparticle tracking; SD, standard deviation; SELE, E‐selectin. * p < 0.05, ** p < 0.01.
Given that ITGA6 usually are stable in the dimer form, we further detected whether ITGA6 in BCa cells and BCa‐derived EVs was in dimer or monomer form. The ITGA6 in BCa cells and BCa‐derived EVs were isolated and subjected to the blue native polyacrylamide gel electrophoresis analysis. The results showed an apparent band with molecular weight larger than 300 kDa in the ITGA6 samples isolated from BCa cells and BCa‐derived EVs (Figure S1N), different from the size of the recombinant ITGA6 monomers that was predicted to be located in 126 kDa, indicating that ITGA6 is not in a monomer form in BCa cells and BCa‐derived EVs. Co‐immunoprecipitation (Co‐IP) assays and followed MS analysis were performed to identify the potential subunit that combined with ITGA6, in which β4‐integrin (ITGB4) was recognized as the top enriched protein by ITGA6 (Figure S1O–P). Further validation by western blotting analysis confirmed the interaction between ITGA6 and ITGB4 in BCa cells and BCa‐derived EVs (Figure S1Q–R). Moreover, proximity ligation assays (PLA) assays confirmed the interaction between ITGA6 and ITGB4 in BCa cells (Figure S1S). Meanwhile, multimodality structure illumination microscopy (Multi‐SIM) analysis showed that ITGA6 and ITBG4 co‐localized in the same EV (Figure S1T), indicating that ITGA6 dimerizes with ITGB4 in BCa cells and BCa‐derived EVs. Together, these results demonstrate that BCa‐derived ITGA6+EVs are associated with lymphatic remodelling associated pre‐metastatic niche in draining LNs.
3.3. BCa‐derived ITGA6+EVs target HLECs to upregulate SELE expression in draining LNs
To identify the targeting cell types of BCa‐derived ITGA6+EVs in draining LNs, we examined the uptake of DiD dye‐labelled EVs from UM‐UC‐3 cells into different cell populations within popliteal LNs. The results showed that most cell populations internalized the EVs to some degree, with LECs internalizing them much more (Figure 2h and Figure S2A). In contrast, EVs from UM‐UC‐3 cells lacking the gene encoding ITGA6 (UM‐UC‐3‐EVITGA6‐KO) were internalized to a much smaller extent by LECs, though they were internalized to similar extents by other cell populations including blood endothelial cells (BECs), fibroblastic reticular cells (FRCs), macrophages, dendritic cells (DCs), T cells and B cells, as measured by flow cytometry or immunofluorescence (Figure 2h–j). Moreover, HLECs internalized larger amounts of EVs secreted from ITGA6‐overexpressing UM‐UC‐3 cells (UM‐UC‐3‐EVITGA6), which was blocked by ITGA6 neutralizing antibody (Figure 2k–m), indicating that BCa‐derived ITGA6+EVs specifically target HLECs in an ITGA6 dependent manner.
To confirm the determined role of ITGA6 in the targeting efficiency of ITGA6+EVs to HLECs, engineered‐ITGA6 EVs (Engineered EVs/E‐EVs) mimicked the ITGA6+EV subset was conducted through engineering ITGA6‐Lamp2b plasmid to decorate ITGA6 in the surface of EVs (Figure 3a). The successful construction of Engineered EVs was confirmed through western blotting analysis, flow cytometry and immunogold‐labelling electron microscopy, which revealed an obvious expression of ITGA6 in the membrane of Engineered EVs, while the control EVs (Control EVs/C‐EVs) were rarely detected (Figure 3b–d and Figure S2B). Importantly, Engineered EV signals accumulated in popliteal LNs to a much greater extent than Control EVs (Figure 3e–f and Figure S2C). Moreover, we treated mice with DiD‐labelled Engineered EVs via intravascular injection and found that the signals of Engineered EVs enriched in pelvic LNs were higher than Control EVs (Figure S2D–E), suggesting that Engineered EVs specifically accumulate in LNs. Additionally, we observed more accumulation of Engineered EVs in HLECs compared with Control EVs through flow cytometry and immunofluorescence (Figure 3g–i), confirming that the targeting functionality of ITGA6+EVs to HLECs in draining LNs attributes to the membrane protein ITGA6.
FIGURE 3.

BCa‐derived ITGA6+EVs upregulate SELE expression to promote BCa cells adhesion. (a) Schematic illustration showing the establishment of Engineered EVs. (b) Representative immunogold‐labelling electron microscopy images of Engineered EVs and Control EVs. Scale bar: 100 nm. (c)–(d) Representative flow cytometry plots (c) and quantification (d) of ITGA6 expression on the surface of Engineered EVs and Control EVs. (e) Representative bioluminescence image and quantification of popliteal LNs from the mice received DiD‐labelled EVs injection in footpad (n = 12 per group). (f) Representative image and quantification of popliteal LNs after footpad injection of DiD‐labelled EVs Scale bar: 50 µm. (g)–(i) Representative immunofluorescence images (g) and flow cytometry plots (h)–(i) of HLECs incubated with DiD‐labelled EVs. Scale bars: 5 µm. (j)–(l) qRT‐PCR analysis (j), western blotting analysis (k), and representative confocal images and quantification (l) of SELE expression in HLECs incubated with UM‐UC‐3‐EVITGA6‐KO or UM‐UC‐3‐EVWT. Scale bars: 5 µm. (m)–(n) qRT‐PCR analysis (m) and representative confocal images and quantification (n) of SELE expression in HLECs incubated with UM‐UC‐3‐EVITGA6 or UM‐UC‐3‐EVVector. Scale bars: 5 µm. (o)–(q) Representative images and quantification of PKH‐67‐labelled UM‐UC‐3 cell attachment on monolayer forming by UM‐UC‐3‐EVITGA6‐KO‐incubated HLECs (o)–(p) and UM‐UC‐3‐EVITGA6‐incubated HLECs with or without knocking down SELE (q). Scale bar: 100 µm. The statistical difference was assessed by two‐tailed Student's t‐test in (d)–(g), (i), (j), (l)–(n), (p); the one‐way ANOVA followed by Dunnett's tests in (q). Error bars represent the SD of three independent experiments. * p < 0.05, ** p < 0.01. ANOVA, analysis of variance; BCa, bladder cancer; EVs, extracellular vesicles; HLECs, Human lymphatic endothelial cells; LNs, lymph nodes; qRT‐PCR, real‐time polymerase chain reaction; SD, Standard deviation; SELE, E‐selectin.
We further tested whether specific interaction between BCa‐derived ITGA6+EVs and HLECs could explain the ability of BCa‐derived EVs to upregulate SELE in draining LNs. Incubation with UM‐UC‐3‐EVITGA6‐KO decreased SELE expression in HLECs compared with UM‐UC‐3‐EVWT (Figure 3j–l). Conversely, UM‐UC‐3‐EVITGA6 treatment upregulated SELE expression in HLECs compared with UM‐UC‐3‐EVVector group (Figure 3n,m). Subsequently, adhesion assay revealed that knocking out ITGA6 abolished the effect of UM‐UC‐3‐EVs in inducing adhesion between HLECs and BCa cells compared with wild‐type group (Figure 3o,p). In addition, we incubated HLECs with UM‐UC‐3‐EVITGA6 and found that overexpressing ITGA6 expression in EVs promoted the attachment of UM‐UC‐3 cells to HLECs compared with control EVs, while inhibiting SELE in HLECs abrogated this effect (Figure 3q and Figure S2F), suggesting that BCa‐derived ITGA6+EVs promote HLECs to attract BCa cells via upregulating SELE. Meanwhile, knocking down ITGB4 suppressed the ability of ITGA6 overexpressing UM‐UC‐3‐derived EVs to mediate the adhesion between HLECs and BCa cells (Figure S2G), indicating that ITGA6 dimerizes with ITGB4 in BCa‐derived EVs for function in BCa. Collectively, these results reveal that BCa‐derived ITGA6+EVs induce SELE overexpression to promote tumour cell adhesion by targeting HLECs in draining LNs.
3.4. BCa‐derived ITGA6+EVs target HLECs by interacting with CD151
Considering that highly targeting efficiency of EVs is determined by specific interactions between membrane‐proteins of EVs and receptors at the plasma membrane of the recipient cells (Gobbo et al., 2015; Leary et al., 2022; Zhang et al., 2022), we further detected the specific receptor of ITGA6 on HLECs membrane. Confocal images revealed that His‐labelled recombinant ITGA6 (His‐rITGA6) proteins significantly bound with the plasma membrane of HLECs (Figure 4a,b), suggesting the existence of specific ITGA6‐targeting molecules on the surface of HLECs. Subsequently, in vitro His pull‐down assays with His‐rITGA6 protein were conducted in HLECs and the silver staining of pull‐down components showed an obvious band with a molecular weight between 35 and 25 kDa in His‐rITGA6 group, which was identified as CD151 by quantitative MS analysis (Figure 4c,d). Moreover, the binding between ITGA6 and CD151 was confirmed through western blotting after His pull‐down or co‐IP assays by ITGA6 antibody with membrane proteins isolated from HLECs exposed to UM‐UC‐3‐EVITGA6 (Figure 4e,f). This interaction was also supported by the immunofluorescence assays showing colocalization of His‐rITGA6 and CD151 on the plasma membrane in HLECs (Figure 4g). Importantly, knocking down CD151 suppressed the internalization of UM‐UC‐3‐EVITGA6 in HLECs (Figure 4h,i). Taken together, BCa‐derived ITGA6+EVs target HLECs through interacting with CD151.
FIGURE 4.

ITGA6+EVs target HLECs to transfer circRNA LIPAR by interacting with CD151. (a) Representative image showing the binding of His‐labelled rITGA6 to the plasma membrane of HLECs. Scale bar: 5 µm. (b) Pearson correlation coefficients of His‐labelled rITGA6 and membrane were calculated from multiple individual cells (n = 8 fields analysed). (c)–(d) Sliver staining and MS analysis of the potential ITGA6 interacting proteins. (e) Western blotting analysis for the binding of ITGA6 and CD151 in His pull‐down assay. (f) Western blotting analysis for the binding of ITGA6 and SELE in the co‐IP assays with the membrane proteins from UM‐UC‐3‐EVITGA6‐incubated HLECs. (g) Representative image showing His‐labelled ITGA6 and CD151 colocalized on the membrane of HLECs. Scale bar: 5 µm. (h)–(i) Representative flow cytometry analysis of UM‐UC‐3‐EVITGA6‐incubated HLECs with or without knocking down CD151. (j) Schematic illustration for screening the co‐upregulated circRNAs in BCa‐derived ITGA6+EVs and BCa tissues. (k) Representative image showing ITGA6 and circRNA LIPAR colocalized on the single EV. Scale bar: 100 nm. (l)–(m) Representative images (l) and percentages (m) of SELE positive areas in non‐metastatic LNs from BCa patients with different expression level of LIPAR in BCa tissue‐derived EVs (n = 35). Scale bar: 50 µm (above), 5 µm (down). (n) Schematic illustrating the genetic locus of PLEKHM1P1 gene and circRNA LIPAR derived from exon 4 and exon 5. The statistical difference was assessed by two‐tailed Student's t‐test in (b); the one‐way ANOVA followed by Dunnett's tests in (i); χ 2 test in (m). Error bars represent the SD of three independent experiments. ANOVA, analysis of variance; BCa, bladder cancer; EVs, extracellular vesicles; HLECs, Human lymphatic endothelial cells; LNs, lymph node; SD, standard deviation; SELE, E‐selectin. * p < 0.05, ** p < 0.01.
3.5. circRNA LIPAR is enriched in BCa‐derived ITGA6+EVs
EVs served as crucial mediators preferably achieve their biological effect in recipient cells via delivering distinct biomolecules, among which circRNAs with high stability and abundance in tumour‐derived EVs were under extensive exploration (Almeida et al., 2022; Wang et al., 2022). Moreover, it is well‐established that circRNAs play a significant role in BCa lymphatic metastasis (An et al., 2022; Zhu et al., 2021). To explore the crucial circRNAs in BCa‐derived ITGA6+EVs, the ITGA6+EVs from total UM‐UC‐3‐EVs were isolated by affinity capture using anti‐ITGA6 magnetic beads, and the circRNA expression profile was investigated using NGS (Figure S2H). The results identified 78 circRNAs that were more abundant in ITGA6+EVs than in other EV subpopulations (GSE246659) (Figure S2I). Intersecting these results with our previous NGS of BCa tissues and NATs identified five circRNAs that consistently upregulated in BCa cell‐derived ITGA6+EVs and BCa tissues (GSE191036) (Figure 4j). Further validation of these upregulated circRNAs in BCa tissue‐derived EVs revealed that hsa_circ_0045334 significantly overexpressed in BCa tissue‐derived EVs than in NAT‐derived EVs (Figure S2J–K). Moreover, BCa cell‐derived ITGA6+EVs packaged higher amounts of the hsa_circ_0045334 than BCa‐derived ITGA6 negative EVs (ITGA6−EVs) (Figure S2L). Additionally, we performed Multi‐SIM analysis of UM‐UC‐3‐derived EVs to show that hsa_circ_0045334 was indeed incorporated in the same EVs as ITGA6 positive, while those EVs without ITGA6 possessed an extremely low level of hsa_circ_0045334 (Figure 4k), confirming that ITGA6+EVs possess higher hsa_circ_0045334 expression compared with ITGA6−EVs. Importantly, the non‐metastatic LNs from BCa patients with higher hsa_circ_0045334 expression in parallel BCa tissue‐derived EVs possessed higher SELE expression in LYVE‐1‐indicated lymphatic vessels (Figure 4l,m), indicating that enrichment of hsa_circ_0045334 in BCa‐derived ITGA6+EVs is related to lymphatic remodelling associated pre‐metastatic niche in draining LNs. Therefore, we named hsa_circ_0045334 as the lymphatic metastasis interrelated pre‐metastatic niche associated circRNA (LIPAR).
Sanger sequencing indicated that LIPAR (426 nt) arose through back‐splicing of exons 4 and exons 5 in the PLEKHM1P gene (Figure 4n and Figure S2M). LIPAR was hardly reversed into cDNA using oligo‐dT primers compared with random primers (Figure S2N), indicating that LIPAR lacks a poly‐A tail. Moreover, using complementary DNA (cDNA) and genomic DNA (gDNA) from BCa cells as templates, LIPAR was only amplified in cDNA by divergent primers rather than in the gDNA groups (Figure S2O–P). Treating with RNase R, an exoribonuclease degrading the linear mRNA, decreased the linear PLEKHM1P mRNA level while no variation was observed on LIPAR (Figure S2Q), confirming that LIPAR harbours a loop structure. Consistently, LIPAR showed a significantly longer half‐life than PLEKHM1P mRNA in BCa cells treated with actinomycin D (Figure S2R–S). Taken together, the above results demonstrate that enrichment of LIPAR in BCa‐derived ITGA6+EVs positively correlates with lymphatic remodelling associated pre‐metastatic niche in draining LNs.
3.6. LIPAR forms a ternary complex with RAB5A and ITGA6 in BCa cells
We further explored how LIPAR was packaged into ITGA6+EVs in BCa. Classically, various cargoes directly interacted with specific effector proteins to be selectively sorted into EVs during different steps of the biogenesis process initiated from the endosomal pathway (Lee et al., 2023). Thus, we performed RNA pull‐down assay to explore the interacting proteins of LIPAR in BCa cells. Silver staining revealed two obvious bands with a molecular weight of 100 to 130 kDa and of 15 to 25 kDa in the biotinylated LIPAR group compared with the control group, which were identified as ITGA6 and RAB5A through quantitative MS analysis (Figure 5a,b). The interaction of LIPAR between RAB5A and ITGA6 was confirmed through western blotting after RNA pull‐down (Figure 5c and Figure S3A). Meanwhile, RNA immunoprecipitation (RIP) using anti‐ITGA6 or anti‐RAB5A verified enrichment of LIPAR compared with the IgG group (Figure 5d and Figure S3A). Additionally, immunofluorescence assays showed that LIPAR colocalized with RAB5A and ITGA6 in the cytoplasm of BCa cells (Figure 5e). To analyse the specific interaction sites of RAB5A and ITGA6 on LIPAR, we used catRAPID, a website for indicating the binding sites between non‐coding RNA and targeting proteins, to identify a putative ITGA6‐binding motif located on the 100‐ to 160‐nt region on LIPAR and RAB5A‐binding motif located on the 225‐ to 285‐nt region on LIPAR (Figure 5f). Deleting 100‐ to 160‐nt region on LIPAR significantly decreased LIPAR enrichment by ITGA6, while deleting 225‐ to 285‐nt region remarkedly suppressed LIPAR enrichment by RAB5A (Figure 5g,h and Figure S3B), suggesting that the 100‐ to 160‐nt sequences and 225‐ to 285‐nt sequences are essential for LIPAR‐ITGA6 and LIPAR‐RAB5A interaction, respectively.
FIGURE 5.

LIPAR/ITGA6/RAB5A ternary complex mediates the package of LIPAR into ITGA6+EVs. (a)–(b) Silver staining image and MS analysis of RNA pull‐down assay with LIPAR probes and control probes. (c) Western blotting analysis showing the interaction between LIPAR and RAB5A, and between LIPAR and ITGA6. (d) RIP assays revealing LIPAR enrichment by ITGA6 and RAB5A in UM‐UC‐3 cells. (e) Representative images showing LIPAR colocalized with RAB5A and ITGA6 in UM‐UC‐3 cells. Scale bars: 5 µm. (f) Schematic illustration showing the predicted binding region of LIPAR. (g)–(h) RIP assays after deletion of the 100‐ to 160‐nt and 225‐ to 285‐nt regions of LIPAR in UM‐UC‐3 cells. (i) Schematic illustration showing the structure of LIPAR/ITGA6/RAB5A ternary complex predicted by NPdock. (j) Co‐IP assays showing the interaction of ITGA6 and RAB5A. (k)–(m) Co‐IP (k), PLA (l) and immunofluorescence images (m) showing the interaction of ITGA6 and RAB5A in UM‐UC‐3 cells with or without LIPAR overexpression. Scale bars: 5 µm. (n) Co‐IP showing the interaction between ITGA6 and RAB5A was destroyed in UM‐UC‐3 cells treating with RnaseA. (o) PLA showing the interaction between ITGA6 and RAB5A in UM‐UC‐3 cells with 100‐ to 160‐nt or 225‐ to 285‐nt region of LIPAR mutating. Scale bars: 5 µm. (p) Western blotting analysis of GST‐R5BD pull‐down to detect the RAB5A activity. (q) Co‐IP showing the interaction between GDI and RAB5A was suppressed in UM‐UC‐3 cells with or without LIPAR overexpression. (r)–(s) Western blotting analysis and qRT‐PCR analysis for the expression of ITGA6 and LIPAR in early endosomes isolated from RAB5A‐DN UM‐UC‐3 cells. (t)–(u) Western blotting analysis and immunogold‐labelling electron microscopy detect expression of ITGA6 in UM‐UC‐3‐EVs. Scale bars: 100 nm. (v) qRT‐PCR analysis of LIPAR expression in UM‐UC‐3‐EVs. The statistical difference was assessed by the one‐way ANOVA followed by Dunnett's tests in (d); two‐tailed Student's t‐test in (g), (h), (s), (v). Error bars represent the SD of three independent experiments. ANOVA, analysis of variance; Co‐IP, Co‐immunoprecipitation; EVs, extracellular vesicles; PLA, proximity ligation assays; qRT‐PCR, real‐time polymerase chain reaction; RIP, RNA immunoprecipitation; SD, standard deviation. * p < 0.05, ** p < 0.01.
Interestingly, NPDock, a web server for modelling of RNA‐protein complex structure (Tuszynska et al., 2015), predicted that LIPAR, ITGA6 and RAB5A form a ternary complex (Figure 5i). Given that circRNAs commonly function as a scaffold for protein‐protein interaction to mediate the formation of ternary complex, we explored whether LIPAR facilitated the binding between ITGA6 and RAB5A. Co‐IP assays showed that LIPAR overexpression significantly enhanced the interaction between ITGA6 and RAB5A in BCa cells, while did not change the total expression of ITGA6 and RAB5A (Figure 5j,k and Figure S3C,D). Moreover, PLA and immunofluorescence were performed to confirm that LIPAR overexpression promoted the interaction between ITGA6 and RAB5A (Figure 5l,m ). Conversely, knocking down LIPAR obviously suppressed the interaction between ITGA6 and RAB5A in BCa cells (Figure S3E,F). Treating with an RNA endonuclease, RNase A, severely destroyed the interaction between ITGA6 and RAB5A (Figure 5n and Figure S3G). Importantly, deleting 100‐ to 160‐nt or 225‐ to 285‐nt region of LIPAR obviously suppressed the interaction between ITGA6 and RAB5A (Figure 5o), indicating that LIPAR functions as a scaffold for RAB5A‐ITGA6 connection to form a ternary complex.
3.7. The ternary complex LIPAR/RAB5A/ITGA6 activates RAB5A to package LIPAR into ITGA6+EVs
Strikingly, we analysed the structure of LIPAR/RAB5A/ITGA6 ternary complex and found the predicted binding region of LIPAR on RAB5A was localized in switch II domain, which interacts with the protein “GDP dissociation inhibitor” (GDI) to keep RAB5A in an inactive GDP‐bound state (Cavalli et al., 2001; Cherfils & Zeghouf, 2013). Therefore, we speculated that LIPAR/RAB5A/ITGA6 ternary complex blocked the recognition and interaction of RAB5A by GDI to activate RAB5A via facilitating the domain switch from its GDP‐bound to its active. Consistent with this hypothesis, overexpressing LIPAR in BCa cells increased the formation of ternary complex, suppressed the interaction between RAB5A and GDI, and increased RAB5A activity, while these effects were abolished when the overexpressed LIPAR mutating the 225–285 nt region (Figure 5p,q and Figure S3H–J).
Considering RAB5A activity is crucial for formation of early endosomes and cargo loading (Zeigerer et al., 2012), we explored whether the ternary complex sustained RAB5A activation to package LIPAR and ITGA6 into early endosomes. The early endosomes were separated and validated by western blotting analysis (Figure S3K). Overexpressing dominant‐negative form of RAB5A (RAB5AS34N: RAB5A‐DN) that prevented RAB5A activation decreased the expression of ITGA6 and LIPAR in purified early endosomes compared with RAB5A‐WT group, which reversed the effect of LIPAR overexpression‐induced ternary complex promoting LIPAR and ITGA6 co‐packaging in purified early endosomes (Figure 5r,s and Figure S3L–S), indicating that ternary complex sustains RAB5A activation to package ITGA6 and LIPAR into early endosomes. Immunofluorescence revealed that overexpressing LIPAR significantly enhanced the colocalization of ITGA6 with EEA1 (an early endosome marker), RAB7A (a late endosome marker) and CD63 (a precursor of EV marker), but not with GM130 (Figure S3T–U), suggesting that ternary complex mediates the package of LIPAR and ITGA6 into early endosomes and then were secreted into EVs via endosomal trafficking process. Importantly, we observed that the expression of LIPAR and ITGA6 in BCa‐derived EVs were increased by LIPAR overexpression‐induced ternary complex formation, while this effect was abolished by RAB5A‐DN (Figure 5t–v and Figure S3V–W). These results demonstrate that LIPAR‐mediated formation of the LIPAR/ITGA6/RAB5A ternary complex induces persistent activation of RAB5A to promote the package of LIPAR and ITGA6 into BCa‐derived EVs via endosomal trafficking process.
3.8. ITGA6+EVs deliver LIPAR into HLECs to enhance SELE transcription
Next, we explored whether LIPAR in BCa‐derived ITGA6+EVs were delivered into HLECs. Incubation with EVs secreted by LIPAR‐overexpressing BCa cells remarkedly upregulated LIPAR expression in HLECs compared with the control EVs, whereas downregulated LIPAR expression in BCa cells impaired the ability of BCa cell‐derived EVs in upregulating LIPAR expression in HLECs (Figure 6a and Figure S4A,B). The ability of ITGA6+EV‐delivered LIPAR to upregulate LIPAR in HLECs was abrogated by treating the HLECs with neutralizing antibody against ITGA6 or by knocking down CD151 (Figure S4C), indicating that LIPAR is precisely transferred to HLECs by BCa‐derived ITGA6+EVs depending on the interaction of ITGA6 and CD151.
FIGURE 6.

ITGA6+EVs deliver LIPAR into HLECs to enhance SELE transcription. (a) qRT‐PCR analysis for the expression of LIPAR in UM‐UC‐3‐EV LIPAR treating HLECs. (b)–(i) qRT‐PCR analysis (b) and (f), western blotting analysis (c) and (g) and representative images (d) and (h) and quantification (e) and (i) of SELE expression in UM‐UC‐3‐EV LIPAR treated HLECs or in combination with ITGA6 neutralizing antibody. Scale bars: 5 µm. (j)–(k) Representative images and quantification of PKH‐67‐labelled UM‐UC‐3 cells attachment on monolayer forming by HLECs with or without UM‐UC‐3‐EV LIPAR incubation. Scale bar: 100 µm. (l) Subcellular fraction analysis of HLEC LIPAR ‐KO incubating with UM‐UC‐3‐EV LIPAR . (m) Transcriptional activity of SELE in UM‐UC‐3‐EV LIPAR ‐treated HLECs transfecting with truncated SELE promoter luciferase plasmids. (n) Schematic diagram of the predicted LIPAR binding sites in the SELE promoter. (o) ChIRP analysis of the LIPAR‐associated chromatin fragments of the SELE promoter in HLECs. (p) Luciferase activity in HLECs incubating with UM‐UC‐3‐EV LIPAR . (q)–(r) ChIP‐qPCR analysis of H3K9ac (q) and p300 (r) enrichment on SELE promoter in HLECs incubating with UM‐UC‐3‐EV LIPA R. (s) qRT‐PCR analysis of SELE expression in UM‐UC‐3‐EV LIPAR treated HLECs with or without inhibiting p300. The statistical difference was assessed by the one‐way ANOVA followed by Dunnett's tests in (a), (f), (i), (k), (p), (s); two‐tailed Student's t‐test in (b), (e), (j), (m), (o), (q), (r); χ2 test in (l). Error bars represent the SD of three independent experiments. ANOVA, analysis of variance; ChIPR, chromatin isolation by RNA purification; HLECs, Human lymphatic endothelial cells; qRT‐PCR, real‐time polymerase chain reaction; SD, standard deviation; SELE, E‐selectin. * p < 0.05, ** p < 0.01.
Then, the role of LIPAR in BCa‐derived ITGA6+EV‐induced lymphatic remodelling associated pre‐metastatic niche in draining LNs was detected. As shown in Figure 6b, qRT‐PCR analysis revealed that incubation with LIPAR‐overexpressing BCa‐derived ITGA6+EVs significantly enhanced the expression of SELE in HLECs compared with those incubated with control EVs, which was also confirmed by immunofluorescence and western blotting analysis (Figure 6c–e). Moreover, neutralizing antibody against ITGA6 significantly suppressed the promoted effect of LIPAR‐overexpressing BCa‐derived ITGA6+EVs in SELE expression in HLECs (Figure 6f–i). The LIPAR‐overexpressing BCa cell‐derived ITGA6+EVs promoted the ability of HLECs to attract BCa cells (Figure 6j–k). We obtained consistent results in HLECs from which endogenous LIPAR had been knocked out (HLEC LIPAR ‐KO) (Figure S4D–M), confirming that the effects are mediated by BCa‐derived ITGA6+EV‐packaged LIPAR rather than by activating endogenous LIPAR expression.
To explore the mechanism of BCa‐derived ITGA6+EV‐packaged LIPAR in upregulating SELE expression in HLECs, the subcellular location of BCa‐derived ITGA6+EV‐packaged LIPAR after internalization by HLEC LIPAR ‐KO was analysed as the subcellular localization of circRNAs determined their biological function (Chen et al., 2023; Misir et al., 2022; Zhang et al., 2023). Abundant LIPAR was detected in the nucleus of HLECs treated with LIPAR‐overexpressing BCa cell‐derived ITGA6+EVs (Figure 6l and Figure S4N), indicating that BCa‐derived ITGA6+EV‐packaged LIPAR may exert its essential functions in the nucleus of HLECs. Then, a series of truncated SELE promoters ranging from −2000 to +200 bp relative to the transcriptional start site were cloned into the luciferase reporter genes and subjected into the luciferase assays. The results revealed that BCa‐derived ITGA6+EV‐packaged LIPAR enhanced transcriptional activity of the −500 to −300 bp region, referred as P2 on SELE promoter in HLECs (Figure 6m and Figure S4O). Moreover, chromatin isolation by RNA purification (ChIRP) assays revealed that BCa‐derived ITGA6+EV‐packaged LIPAR directly interacted with the P2 region on the SELE promoter in HLECs (Figure 6n,o). Mutation of the predicted binding sequence −349 to −337 bp on the P2 region of SELE attenuated the ability of BCa‐derived ITGA6+EV‐packaged LIPAR to upregulate luciferase activity of SELE (Figure 6p and Figure S4P), suggesting that BCa‐derived ITGA6+EV‐packaged LIPAR directly binds with −349 to −337 bp to form a DNA‐RNA triplex and activate SELE transcription.
It has been reported that the targeting gene transcription was mediated by histone modifications on gene promoters (Xia et al., 2019). Therefore, we detected whether the DNA‐RNA triplex formed by BCa‐derived ITGA6+EV‐packaged LIPAR promoted SELE transcription through inducing histone modifications on SELE promoter. Indeed, BCa‐derived ITGA6+EV‐packaged LIPAR significantly increased acetylation of Lys9 on histone 3 (H3K9ac), but not other histone modifications, within the SELE promoter (Figure 6q and Figure S4R–T). Considering the histone modifications are regulated by specific transcriptional factors, we predicted the binding transcriptional factors of SELE promoter region with GTRD, Contra V3 and found three transcriptional factors (GATA2, TBP, p300), among which p300, a histone acetyltransferase (HAT), at the SELE promoter was most significantly increased after treating with BCa‐derived ITGA6+EV‐packaged LIPAR compared with other transcriptional factors confirmed by Chromatin immunoprecipitation (ChIP) assays (Figure 6r and Figure S4Q,U,V), indicating that BCa‐derived ITGA6+EV‐packaged LIPAR recruits p300 to interact with SELE promoter. Moreover, the p300 inhibitor significantly attenuated BCa‐derived ITGA6+EV‐packaged LIPAR to increase H3K9ac enrichment on SELE promoter and to upregulate SELE transcription (Figure 6s and Figure S4W–X). Collectively, these results reveal that BCa‐derived ITGA6+EV‐packaged LIPAR recruits p300 to increase H3K9ac levels on SELE promoter to activate SELE transcription.
3.9. ITGA6+EV‐packaged LIPAR induces formation of pre‐metastatic niche in LNs to promote metastasis of BCa in mice
To verify whether LIPAR transferred by BCa‐derived ITGA6+EVs was necessary and sufficient to elicit pre‐metastatic niche formation and BCa lymphatic metastasis in vivo, LIPAR mimics were composed in vitro and incorporated into Engineered EVs (Figure 7a and Figure S5A,B). Then, we employed the Engineered EVs loading with LIPAR (Engineered EV LIPAR ) and the control empty Engineered EV (Engineered EVEmpty) to EV‐education experiment model as previously described. Treating with Engineered EV LIPAR significantly upregulated SELE and LIPAR expression in lymphatic vessel in popliteal LNs, which was not observed in control groups (Figure 7b–c and Figure S5C). Importantly, pretreating with Engineered EV LIPAR significantly enhanced the metastasis of mCherry‐labelled UM‐UC‐3 cells to the popliteal LNs of mice compared with Engineered EVEmpty or Control EVs (Figure 7d–f). By elucidating the popliteal LNs for further analysis, we observed an increased lymphatic metastasis rate of mice in Engineered EV LIPAR educated group than the other groups (Figure S5D–E), indicating that ITGA6+EV‐packaged LIPAR induces pre‐metastatic niche in draining LNs to promote BCa lymphatic metastasis.
FIGURE 7.
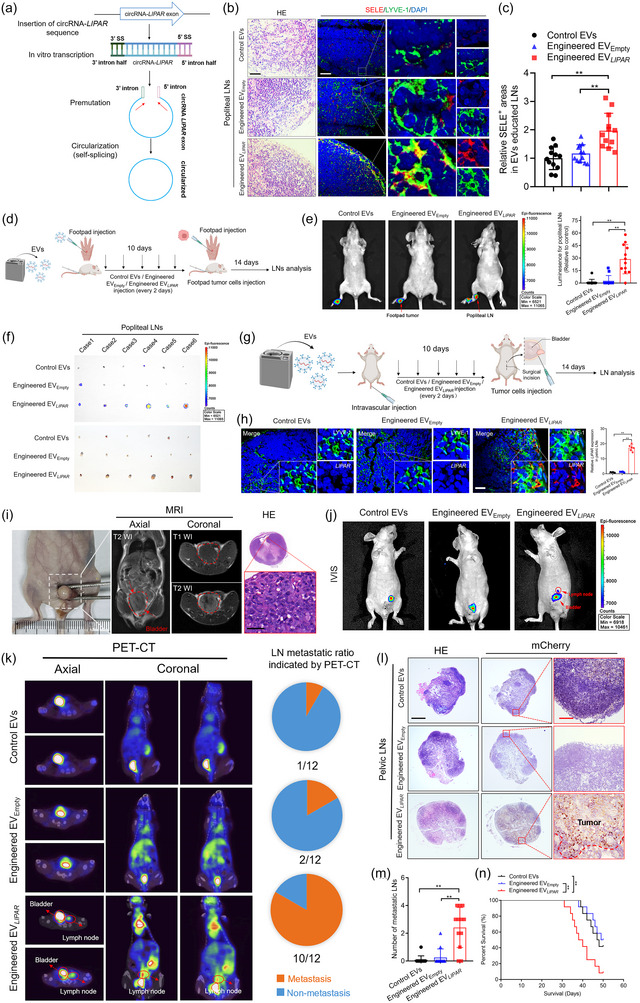
ITGA6+EV‐packaged LIPAR induces pre‐metastatic niche to promote LN metastasis. (a) Schematic illustration showing the in vitro production and circularization of LIPAR. (b)–(c) Representative images (b) and quantifications (c) of SELE positive areas in popliteal LNs from the Engineered EV LIPAR educated mice (n = 12 per group). Scale bar: 50 µm (black), 50 µm (white). (d) Schematic illustration showing the establishment of Engineered EV‐education experiment model via footpad injection. (e) Representative bioluminescence images and quantification of mice educated with Engineered EV LIPAR or Control EVs or Engineered EVEmpty (n = 12 per group). (f) Representative images of the popliteal LNs from mice (n = 12 per group). (g) Schematic illustration showing the establishment of Engineered EV‐education experiment model via intravascular injection. (h) Representative images and quantification of LIPAR in pelvic LNs. (n = 6 per group). Scale bar: 50 µm. (i) Necropsy examination, MRI and HE image of mouse bladders. Scale bar: 5 µm. (j) Representative IVIS showing the metastatic LNs of mice in indicated groups (n = 12 per group). (k) Representative PET‐CT images and pie charts showing the metastatic LNs of mice in indicated groups (n = 12 per group). (l) Representative images of anti‐mCherry analysis in pelvic LNs from the indicated group (n = 12 per group). Scale bars: 500 µm (black), 50 µm (red). (m) Quantification of the metastatic number of pelvic LNs in mice from indicated groups. (n) Kaplan–Meier curves of the OS in mice from indicated groups. The statistical difference was assessed by the one‐way ANOVA followed by Dunnett's tests in (c), (e), (h), (m). Error bars represent the SD of three independent experiments. ANOVA, analysis of variance; EVs, extracellular vesicles; IVIS, in vivo imaging system; LNs, lymph nodes; MRI, magnetic resonance imaging; H&E, hematoxylin and eosin; OS, overall survival; PET‐CT, positron emission tomography‐computed tomography; SD, standard deviation. * p < 0.05, ** p < 0.01.
We verified these results in an orthotopic xenograft model of BCa metastasis, in which mice were first pretreated with Engineered EV LIPAR or control EVs by intravascular injection for 10 days (Figure 7g). FISH experiments and qRT‐PCR analysis showed that LIPAR was significantly overexpressed in LNs after treatment with Engineered EV LIPAR compared with control groups (Figure 7h, Figure S5F), suggesting that Engineered EVs specifically deliver LIPAR to draining LNs. And then mCherry‐labelled UM‐UC‐3 cells were implanted directly into the bladder, where the tumorigenesis in the bladder was confirmed as shown in Figure 7i. Pretreating with Engineered EV LIPAR remarkedly increased the metastasis of UM‐UC‐3 cells to LNs around the bladder as indicated by PET‐CT scanning and IVIS, but not observed in the Engineered EVEmpty or Control EVs group (Figure 7j–k). Considering that the pelvic LNs around the common iliac, internal iliac, external iliac and obturator represent the typical drainage LNs of BCa in mice (Hoffman, 2002; Van den Broeck et al., 2006), these LNs were enucleated for further analysis and showed that Engineered EV LIPAR education significantly enhanced the pelvic LN metastasis of BCa compared with control groups (Figure 7l–n).
Moreover, pretreating mice with LIPAR‐overexpressing BCa‐derived ITGA6+EVs or Engineered EV LIPAR via footpad injection significantly promoted the popliteal LN metastasis of BCa compared with control groups (Figure 8a–c). Meanwhile, we confirmed these results in the orthotopic xenograft model and found that LIPAR‐overexpressing BCa‐derived ITGA6+EVs or Engineered EV LIPAR pretreatment enhanced pelvic LN metastasis of BCa compared with control groups (Figure 8d–h), indicating that LIPAR triggers BCa‐derived EVs to induce the pre‐metastatic niche for lymphatic metastasis. Taken together, our findings suggest that ITGA6+EV‐packaged LIPAR upregulates SELE expression to create a lymphatic remodelling associated pre‐metastatic niche and promote BCa lymphatic metastasis.
FIGURE 8.

LIPAR triggers BCa‐derived EVs to induce the pre‐metastatic niche for LN metastasis. (a)–(b) Representative bioluminescence images and quantification of LNs from mice (n = 12 per group). (c) The popliteal LN metastatic rates of the mice from the indicated group (n = 12 per group). (d) Representative MRI images and pie charts showing the metastatic LNs of mice in indicated groups (n = 12 per group). (e)–(f) Representative IVIS images and pie charts showing the metastatic LNs of mice in indicated groups (n = 12 per group). (g)–(h) Representative images of anti‐mCherry analysis in pelvic LNs from the indicated group (n = 12 per group). Scale bars: 500 µm (black), 50 µm (red), 50 µm (white). (i) Representative image of LIPAR and ITGA6 expression in BCa tissue and SELE expression in LYVE‐1 indicated lymphatic vessels in paired non‐metastatic LNs (n = 257). Scale bars: 50 µm. (j) Proposed model of BCa‐derived ITGA6+EVs in inducing lymphatic pre‐metastatic niche to promote lymphatic metastasis via delivering LIPAR to HLECs in draining LNs. The statistical difference was by one‐way ANOVA followed by Dunnett's tests in (b), (h); χ2 test in (c). Error bars represent the SD of three independent experiments. ANOVA, analysis of variance; BCa, bladder cancer; EVs, extracellular vesicles; HLECs, Human lymphatic endothelial cells; LNs, lymph nodes; LYVE 1, lymphatic vessel endothelial hyaluronan receptor 1; SD, standard deviation; SELE, E‐selectin. * p < 0.05, ** p < 0.01.
3.10. Targeting blocking SELE via Engineered EVs suppress pre‐metastatic niche and inhibits LN metastasis in preclinical models
Since BCa‐derived ITGA6+EV‐packaged LIPAR prepared a pre‐metastatic niche to promote lymphatic metastasis via sustaining SELE overexpression, we then verified whether blocking SELE could suppress the pre‐metastatic niche formation and inhibit lymphatic metastasis of BCa in vivo. To achieve the targeting blocking of SELE in draining LNs, the intact Engineered EV loading with SELE selective inhibitor (Engineered EVSELE‐inhibitor), was subjected into the footpad injection of mice to decrease the expression of SELE in lymphatic vessel (Figure S5G–K). Engineered EVSELE‐inhibitor treatment significantly suppressed the popliteal LN metastasis of BCa compared with the control group (Figure S5L–O). To further confirm the effect of blocking SELE in preventing pre‐metastasis niche to inhibit BCa lymphatic metastasis, the orthotopic xenograft model was conducted as shown in Figure S5P. Engineered EVSELE‐inhibitor treatment significantly suppressed the pelvic LN metastasis of BCa (Figure S5Q–U). Moreover, treatment with Engineered EVSELE‐inhibitor prolonged the mice's survival than those were treated with control EVs (Figure S5V). Together, these findings suggest that targeting inhibition of SELE by Engineered EVSELE‐inhibitor suppresses the pre‐metastatic niche formation and inhibits lymphatic metastasis of BCa in preclinical models.
3.11. Multicentre analysis of clinical relevance of ITGA6+EV‐packaged LIPAR in BCa
Considering the critical regulatory role of LIPAR in BCa‐derived ITGA6+EVs in upregulating SELE expression to create lymphatic remodelling associated pre‐metastatic niche formation and promote BCa lymphatic metastasis, we detected the clinical relevance of ITGA6+EV‐packaged LIPAR in BCa, which found that LIPAR was overexpressed in BCa tissues compared with NATs through qRT‐PCR analysis (Figure S6A). Moreover, overexpression of LIPAR was confirmed in lymphatic metastatic BCa tissues than in those without lymphatic metastasis in 348 tissue sections from Sun Yat‐sen Memorial Hospital of Sun Yat‐sen University, which was confirmed in four other clinical cohorts of 472 BCa cases (Figure S6B–H). Interestingly, we found that the expression of LIPAR in urinary EVs positively correlated with that in paired tumour tissues, indicating that urinary EV‐packaged LIPAR plays a critical role in LIPAR‐related lymphatic metastasis in BCa. We further explored the clinical relevance of urinary EV‐packaged LIPAR and lymphatic metastasis in BCa. The qRT‐PCR analysis demonstrated a significantly higher expression of LIPAR in urinary EVs from BCa patients than in healthy control urinary EVs (Figure S6I). In addition, the expression of LIPAR in urinary EVs in BCa patients with lymphatic metastasis was significantly higher than in patients without lymphatic metastasis (Figure S6J), suggesting that positive association between urinary EV‐packaged LIPAR and BCa lymphatic metastasis. Moreover, Kaplan–Meier analysis found that higher level of LIPAR in urinary EVs from BCa patients correlated with significantly shorter overall survival (OS) and disease‐free survival (DFS) rates (Figure S6K–N). The urinary EV‐packaged LIPAR was identified as an independent predictor for the poor prognosis of patients with BCa (Table S2–S10). Importantly, higher LIPAR and ITGA6 expression levels in BCa tissues were accompanied by more SELE expression in lymphatic vessels indicated by LYVE‐1 in non‐metastatic LNs (Figure 8i and Figure S6O–P). Collectively, our results demonstrate that ITGA6/LIPAR/SELE axis is widely involved in the pre‐metastatic niche formation and lymphatic metastasis of BCa.
4. DISCUSSION
Cancer cells deliver heterogeneous EVs to particular recipient cells in draining LNs and subsequently alter their gene expression patterns that creating a supportive pre‐metastatic niche to promote lymphatic metastasis (Chen et al., 2018; García‐Silva et al., 2021; Lin et al., 2023). However, the specific EV subsets identified by membrane‐protein signature determining pre‐metastatic niche formation in draining LNs remain largely unknown. Here, we analysed the membrane‐protein expression pattern of BCa cell‐derived EVs as well as BCa tissue‐derived EVs to identify that the lymphatic remodelling pre‐metastatic niche‐forming EV subset was coated with ITGA6. Furthermore, we clarified the clinical correlation of BCa‐derived ITGA6+EVs with pre‐metastatic niche formation in draining LNs and lymphatic metastasis through a large multicentre BCa patient cohort analysis. Previous studies consider that integrin as the most highly expressed receptor on EVs determines tissue‐specific metastasis via mediating EVs in creating specific pre‐metastatic niches (Dong et al., 2014; Hoshino et al., 2015). Here, we comprehensively elucidated the biological function and mechanisms of ITGA6 mediating BCa‐derived EVs in priming lymphatic remodelling pre‐metastatic niche and promoting lymphatic metastasis in draining LNs. Therefore, our findings provide new insight into the EV subpopulation heterogeneity and clarify the role of BCa‐derived ITGA6+EVs in regulating pre‐metastatic niche formation and lymphatic metastasis.
Previous studies consider lymphangiogenesis and immune‐suppressive as the major characteristics of the pre‐metastatic niche in draining LNs, which provide more approaches for metastatic tumour cells to invade draining LNs and promote metastatic tumour cells overcome immunological elimination, while this traditional view hardly explains how the metastatic tumour cells prefer to invade and firm colonize in the draining LNs (Patras et al., 2023). Recently, further researches demonstrate that lymphatic remoulding mediated by overexpression of adhesion molecules in LECs is another key characteristic, which promotes lymphatic vessels to attract disseminated tumour cells to extravasation and firm colonization, thus leading to the metastatic outgrowth in draining LNs (Commerford et al., 2018; García‐Silva et al., 2021). However, the expression pattern of adhesion molecules and specific regulation mechanism in the pre‐metastatic niche of BCa draining LNs remain unclear. In the present study, we analysed the dynamic change of BCa‐derived EV‐induced pre‐metastatic niche and demonstrated that SELE overexpression in lymphatic vessels created lymphatic remodelling pre‐metastatic niche in draining LNs, which might ‘hijack’ the process that inflamed endothelium mediating immune cells adhesion and transmigration for BCa cells metastasizing into draining LNs. Moreover, we clarified the regulatory mechanism of SELE overexpression in draining LNs, where BCa‐derived ITGA6+EVs delivered LIPAR into HLECs to activate SELE transcription. Our findings provide new insight into key characteristics of SELE‐marked lymphatic remodelling in pre‐metastatic niche and explain why BCa cells prefer to metastasize into draining LNs.
Accumulating researches have shown that EVs are promising drug delivery systems owing to their intrinsic biocompatibility, their ability to cross physiological barriers, and low immunogenicity; however, targeting properties deficiency limits the clinical application of natural EVs (Guo et al., 2023; Liu et al., 2020; Zhao et al., 2020). Recent studies reveal that the EV‐membrane‐protein expression pattern determines tissue tropism, thus modifying EV‐membrane‐proteins could endow them with targeting efficiency (Alvarez‐Erviti et al., 2011; Kamerkar et al., 2017). Here, we showed that BCa‐derived EVs possessed the capability of targeting HLECs in draining LNs attributing to the membrane‐protein ITGA6, which enabled EVs to target HLECs via interacting with CD151 on the plasma membrane of HLECs. The increasing success of engineered EV‐packaging small molecule therapeutics offers striking insights into exploring engineered EVs as feasible therapeutic targets for antitumor strategies (Cui et al., 2023; Huang et al., 2023). Consistently, we constructed engineered EVs for LN targeting delivery by decorating with ITGA6 in the EV surface. Loading specific SELE inhibitor into Engineered EVs enabled targeting inhibition of lymphatic pre‐metastatic niche to effectively prevent BCa lymphatic metastasis in preclinical models. Our findings develop a potential approach for intervening BCa lymphatic metastasis via targeting inhibition of pre‐metastatic niche in draining LNs.
Recent studies reveal that the proteins and nucleic acids enriched in tumour‐derived EVs are distinct from their original cells, indicating a specific cargo‐sorting mechanism associated with EV biogenesis and selective content loading, which is still unclear (Dong et al., 2024; Zheng et al., 2024b). Traditional views hold that selective cargo loading correlated with EV biogenesis, which generates within the endosomal system from early endosome to intraluminal vesicles and is released through the fusion of multivesicular bodies with the cell surface (Kalluri & LeBleu, 2020; Teng & Fussenegger, 2020). The RAB protein family recruits the endosomal sorting complex required for transport proteins to trigger membrane inward budding and cargo sorting into the endosomal system, leading to EV biogenesis and selective cargo loading (Lee et al., 2023; Vietri et al., 2020). Here, we demonstrated the critical role of RAB5A in mediating EV biogenesis and specific cargo loading, by which RAB5A initiated the early endosome biogenesis to mediate ITGA6 and LIPAR loading into BCa‐derived EVs. It is well‐established that the biological functions of RAB5A depend on the switch between GTP‐bound and GDP‐bound states, which is under the control of the GDI that can be recognized and bind with RAB5A thus maintaining GDP‐bound inactive states (Cavalli et al., 2001; Zeigerer et al., 2012). However, the mechanism underlying the imbalance of GDI‐mediated RAB5A activation in EV‐content packaging of BCa cells remains unclear. We found that LIPAR linked ITGA6 to the switch II domain of RAB5A and blocked GDI‐mediated RAB5A inactivation to maintain the sustained production of ITGA6+EVs loaded with LIPAR. Our results provide a potential mechanism underlying cargo packaging into tumour‐derived EVs and the biogenesis of ITGA6+EVs in BCa cells.
In conclusion, the present study identifies the biological function and precise mechanism of BCa‐derived ITGA6+EVs in creating the pre‐metastatic niche in draining LNs and develops an engineered EV‐based approach for LN targeting delivery to prevent BCa lymphatic metastasis via inhibiting the lymphatic pre‐metastatic niche.
AUTHOR CONTRIBUTIONS
Yan Lin: Project administration (equal); writing—original draft (equal). Hanhao Zheng: Formal analysis (equal); validation (equal). Linpei Jia: Methodology (equal); validation (equal). Yuming Luo: Methodology (equal); validation (equal). Dingwen Zhang: Investigation (equal); software (equal). Mingjie An: Data curation (equal); resources (equal). Mingrui Pang: Formal analysis (equal); supervision (equal). Xiayao Diao: Project administration (equal); validation (equal). Wenjie Li: Investigation (equal); visualization (equal). Jiancheng Chen: Formal analysis (equal); visualization (equal). Yuanlong Li: Methodology (equal); supervision (equal). Daiyin Liu: Methodology (equal); visualization (equal). Zhicong Liu: Data curation (equal); resources (equal). Jian Huang: Conceptualization (equal); visualization (equal). Tianxin Lin: Project administration (equal); writing – original draft (equal); writing – review and editing (equal). Changhao Chen: Conceptualization (lead); writing—review and editing (lead).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Supporting information
Supporting Information
ACKNOWLEDGEMENTS
The authors thank Prof. J. X. Zhang in the Department of Medical Statistics and Epidemiology of the School of Public Health at Sun Yat‐sen University (Guangzhou, China) for statistical advice and comments on the study. This work was funded by the National Key Research and Development Program of China (2022YFA1305500), the National Natural Science Foundation of China (32322023, 82173272, 81825016, N82173230, 82341018, 82203662, 82173271, 82103416, 82103536, 82173266, 82202276 and 81972385), the Key Areas Research and Development Program of Guangdong (2022B1515120086, 2021B1515020091, 2022A1515140175, 2021A1515010215, 2023A1515011648, 2022A1515012288, 2021A1515010355), the Science and Technology Program of Guangzhou, China (2023A04J2206, 2023QNYXZD003, 2024A04J6560) and Medical Scientific Research Foundation of Guangdong Province, China (A2022117).
Lin, Y. , Zheng, H. , Jia, L. , Luo, Y. , Zhang, D. , An, M. , Pang, M. , Diao, X. , Li, W. , Chen, J. , Li, Y. , Liu, D. , Liu, Z. , Huang, J. , Lin, T. , & Chen, C. (2024). Integrin α6‐containing extracellular vesicles promote lymphatic remodelling for pre‐metastatic niche formation in lymph nodes via interplay with CD151. Journal of Extracellular Vesicles, 13, e12518. 10.1002/jev2.12518
Yan Lin, Hanhao Zheng, Linpei Jia and Yuming Luo contributed equally to this study.
Contributor Information
Tianxin Lin, Email: lintx@mail.sysu.edu.cn.
Changhao Chen, Email: chenchh53@mail.sysu.edu.cn.
DATA AVAILABILITY STATEMENT
All data in the present study are presented in the paper or the Supplementary Materials. The full uncut gels are presented in Figure S7. The primers, probes and antibodies used in the present study are presented in Table S11–S12. The raw data have been deposited in the NCBI GEO, under accession numbers BioProject: GSE246070 and GSE246659. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD052771 and PXD053106.
REFERENCES
- Almeida, A. , Gabriel, M. , Firlej, V. , Martin‐Jaular, L. , Lejars, M. , Cipolla, R. , Petit, F. , Vogt, N. , San‐Roman, M. , Dingli, F. , Loew, D. , Destouches, D. , Vacherot, F. , de la Taille, A. , Théry, C. , & Morillon, A. (2022). Urinary extracellular vesicles contain mature transcriptome enriched in circular and long noncoding RNAs with functional significance in prostate cancer. Journal of Extracellular Vesicles, 11(5), e12210. 10.1002/jev2.12210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez‐Erviti, L. , Seow, Y. , Yin, H. , Betts, C. , Lakhal, S. , & Wood, M. J. (2011). Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nature Biotechnology, 29(4), 341–345. 10.1038/nbt.1807 [DOI] [PubMed] [Google Scholar]
- An, M. , Zheng, H. , Huang, J. , Lin, Y. , Luo, Y. , Kong, Y. , Pang, M. , Zhang, D. , Yang, J. , Chen, J. , Li, Y. , Chen, C. , & Lin, T. (2022). Aberrant nuclear export of circNCOR1 underlies SMAD7‐mediated lymph node metastasis of bladder cancer. Cancer Research, 82(12), 2239–2253. 10.1158/0008-5472.CAN-21-4349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieniasz‐Krzywiec, P. , Martín‐Pérez, R. , Ehling, M. , García‐Caballero, M. , Pinioti, S. , Pretto, S. , Kroes, R. , Aldeni, C. , Di Matteo, M. , Prenen, H. , Tribulatti, M. V. , Campetella, O. , Smeets, A. , Noel, A. , Floris, G. , Van Ginderachter, J. A. , & Mazzone, M. (2019). Podoplanin‐expressing macrophages promote lymphangiogenesis and lymphoinvasion in breast cancer. Cell Metabolism, 30(5), 917–936.e10. 10.1016/j.cmet.2019.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalli, V. , Vilbois, F. , Corti, M. , Marcote, M. J. , Tamura, K. , Karin, M. , Arkinstall, S. , & Gruenberg, J. (2001). The stress‐induced MAP kinase p38 regulates endocytic trafficking via the GDI:Rab5 complex. Molecular Cell, 7(2), 421–432. 10.1016/s1097-2765(01)00189-7 [DOI] [PubMed] [Google Scholar]
- Chen, C. , He, W. , Huang, J. , Wang, B. , Li, H. , Cai, Q. , Su, F. , Bi, J. , Liu, H. , Zhang, B. , Jiang, N. , Zhong, G. , Zhao, Y. , Dong, W. , & Lin, T. (2018). LNMAT1 promotes lymphatic metastasis of bladder cancer via CCL2 dependent macrophage recruitment. Nature Communications, 9(1), 3826. 10.1038/s41467-018-06152-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, C. , Luo, Y. , He, W. , Zhao, Y. , Kong, Y. , Liu, H. , Zhong, G. , Li, Y. , Li, J. , Huang, J. , Chen, R. , & Lin, T. (2020). Exosomal long noncoding RNA LNMAT2 promotes lymphatic metastasis in bladder cancer. The Journal of Clinical Investigation, 130(1), 404–421. 10.1172/JCI130892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Z. , Song, J. , Xie, L. , Xu, G. , Zheng, C. , Xia, X. , Lu, F. , Ma, X. , Zou, F. , Jiang, J. , & Wang, H. (2023). N6‐methyladenosine hypomethylation of circGPATCH2L regulates DNA damage and apoptosis through TRIM28 in intervertebral disc degeneration. Cell Death and Differentiation, 30(8), 1957–1972. 10.1038/s41418-023-01190-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherfils, J. , & Zeghouf, M. (2013). Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiological Reviews, 93(1), 269–309. 10.1152/physrev.00003.2012 [DOI] [PubMed] [Google Scholar]
- Commerford, C. D. , Dieterich, L. C. , He, Y. , Hell, T. , Montoya‐Zegarra, J. A. , Noerrelykke, S. F. , Russo, E. , Röcken, M. , & Detmar, M. (2018). Mechanisms of tumor‐induced lymphovascular niche formation in draining lymph nodes. Cell Reports, 25(13), 3554–3563.e4. 10.1016/j.celrep.2018.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa‐Silva, B. , Aiello, N. M. , Ocean, A. J. , Singh, S. , Zhang, H. , Thakur, B. K. , Becker, A. , Hoshino, A. , Mark, M. T. , Molina, H. , Xiang, J. , Zhang, T. , Theilen, T. M. , García‐Santos, G. , Williams, C. , Ararso, Y. , Huang, Y. , Rodrigues, G. , Shen, T. L. , … Lyden, D. (2015). Pancreatic cancer exosomes initiate pre‐metastatic niche formation in the liver. Nature Cell Biology, 17(6), 816–826. 10.1038/ncb3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, J. , Wang, X. , Li, J. , Zhu, A. , Du, Y. , Zeng, W. , Guo, Y. , Di, L. , & Wang, R. (2023). Immune exosomes loading self‐assembled nanomicelles traverse the blood‐brain barrier for chemo‐immunotherapy against glioblastoma. ACS Nano, Advance online publication. 10.1021/acsnano.2c10219 [DOI] [PubMed] [Google Scholar]
- Dong, L. , Feng, M. , Kuczler, M. D. , Horie, K. , Kim, C. J. , Ma, Z. , Lombardo, K. , Lyons, H. , Amend, S. R. , Kates, M. , Bivalacqua, T. J. , McConkey, D. , Xue, W. , Choi, W. , & Pienta, K. J. (2024). Tumour tissue‐derived small extracellular vesicles reflect molecular subtypes of bladder cancer. Journal of Extracellular Vesicles, 13(2), e12402. 10.1002/jev2.12402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, Y. W. , Wang, R. , Cai, Q. Q. , Qi, B. , Wu, W. , Zhang, Y. H. , & Wu, X. Z. (2014). Sulfatide epigenetically regulates miR‐223 and promotes the migration of human hepatocellular carcinoma cells. Journal of Hepatology, 60(4), 792–801. 10.1016/j.jhep.2013.12.004 [DOI] [PubMed] [Google Scholar]
- García‐Silva, S. , Benito‐Martín, A. , Nogués, L. , Hernández‐Barranco, A. , Mazariegos, M. S. , Santos, V. , Hergueta‐Redondo, M. , Ximénez‐Embún, P. , Kataru, R. P. , Lopez, A. A. , Merino, C. , Sánchez‐Redondo, S. , Graña‐Castro, O. , Matei, I. , Nicolás‐Avila, J. Á. , Torres‐Ruiz, R. , Rodríguez‐Perales, S. , Martínez, L. , Pérez‐Martínez, M. , … Peinado, H. (2021). Melanoma‐derived small extracellular vesicles induce lymphangiogenesis and metastasis through an NGFR‐dependent mechanism. Nature Cancer, 2(12), 1387–1405. 10.1038/s43018-021-00272-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbo, J. , Marcion, G. , Cordonnier, M. , Dias, A. M. M. , Pernet, N. , Hammann, A. , Richaud, S. , Mjahed, H. , Isambert, N. , Clausse, V. , Rébé, C. , Bertaut, A. , Goussot, V. , Lirussi, F. , Ghiringhelli, F. , de Thonel , A. , Fumoleau, P. , Seigneuric, R. , & Garrido, C. (2015). Restoring anticancer immune response by targeting tumor‐derived exosomes With a HSP70 peptide aptamer. Journal of the National Cancer Institute, 108(3). 10.1093/jnci/djv330 [DOI] [PubMed] [Google Scholar]
- Gómez de Liaño Lista, A. , van Dijk, N. , de Velasco Oria de Rueda, G. , Necchi, A. , Lavaud, P. , Morales‐Barrera, R. , Alonso Gordoa, T. , Maroto, P. , Ravaud, A. , Durán, I. , Szabados, B. , Castellano, D. , Giannatempo, P. , Loriot, Y. , Carles, J. , Anguera Palacios, G. , Lefort, F. , Raggi, D. , Gross Goupil, M. , … Van der Heijden, M. S. (2020). Clinical outcome after progressing to frontline and second‐line Anti‐PD‐1/PD‐L1 in advanced urothelial cancer. European Urology, 77(2), 269–276. 10.1016/j.eururo.2019.10.004 [DOI] [PubMed] [Google Scholar]
- Guo, J. , Wang, F. , Hu, Y. , Luo, Y. , Wei, Y. , Xu, K. , Zhang, H. , Liu, H. , Bo, L. , Lv, S. , Sheng, S. , Zhuang, X. , Zhang, T. , Xu, C. , Chen, X. , & Su, J. (2023). Exosome‐based bone‐targeting drug delivery alleviates impaired osteoblastic bone formation and bone loss in inflammatory bowel diseases. Cell Reports. Medicine, 4(1), 100881. 10.1016/j.xcrm.2022.100881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, W. , Zhong, G. , Jiang, N. , Wang, B. , Fan, X. , Chen, C. , Chen, X. , Huang, J. , & Lin, T. (2018). Long noncoding RNA BLACAT2 promotes bladder cancer‐associated lymphangiogenesis and lymphatic metastasis. The Journal of Clinical Investigation, 128(2), 861–875. 10.1172/JCI96218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman, R. (2002). Green fluorescent protein imaging of tumour growth, metastasis, and angiogenesis in mouse models. The Lancet Oncology, 3(9), 546–556. 10.1016/s1470-2045(02)00848-3 [DOI] [PubMed] [Google Scholar]
- Hoshino, A. , Costa‐Silva, B. , Shen, T. L. , Rodrigues, G. , Hashimoto, A. , Tesic Mark, M. , Molina, H. , Kohsaka, S. , Di Giannatale, A. , Ceder, S. , Singh, S. , Williams, C. , Soplop, N. , Uryu, K. , Pharmer, L. , King, T. , Bojmar, L. , Davies, A. E. , Ararso, Y. , … Lyden, D. (2015). Tumour exosome integrins determine organotropic metastasis. Nature, 527(7578), 329–335. 10.1038/nature15756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, C. , Zhou, Y. , Feng, X. , Wang, J. , Li, Y. , & Yao, X. (2023). Delivery of engineered primary tumor‐derived exosomes effectively suppressed the colorectal cancer chemoresistance and liver metastasis. ACS Nano, 17(11), 10313–10326. 10.1021/acsnano.3c00668 [DOI] [PubMed] [Google Scholar]
- Kalluri, R. , & LeBleu, V. S. (2020). The biology, function, and biomedical applications of exosomes. Science, 367(6478), eaau6977. 10.1126/science.aau6977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamerkar, S. , LeBleu, V. S. , Sugimoto, H. , Yang, S. , Ruivo, C. F. , Melo, S. A. , Lee, J. J. , & Kalluri, R. (2017). Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature, 546(7659), 498–503. 10.1038/nature22341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowal, J. , Arras, G. , Colombo, M. , Jouve, M. , Morath, J. P. , Primdal‐Bengtson, B. , Dingli, F. , Loew, D. , Tkach, M. , & Théry, C. (2016). Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proceedings of the National Academy of Sciences of the United States of America, 113(8), E968–E977. 10.1073/pnas.1521230113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary, N. , Walser, S. , He, Y. , Cousin, N. , Pereira, P. , Gallo, A. , Collado‐Diaz, V. , Halin, C. , Garcia‐Silva, S. , Peinado, H. , & Dieterich, L. C. (2022). Melanoma‐derived extracellular vesicles mediate lymphatic remodelling and impair tumour immunity in draining lymph nodes. Journal of Extracellular Vesicles, 11(2), e12197. 10.1002/jev2.12197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, Y. J. , Shin, K. J. , Jang, H. J. , Ryu, J. S. , Lee, C. Y. , Yoon, J. H. , Seo, J. K. , Park, S. , Lee, S. , Je, A. R. , Huh, Y. H. , Kong, S. Y. , Kwon, T. , Suh, P. G. , & Chae, Y. C. (2023). GPR143 controls ESCRT‐dependent exosome biogenesis and promotes cancer metastasis. Developmental Cell, 58(4), 320–334.e8. 10.1016/j.devcel.2023.01.006 [DOI] [PubMed] [Google Scholar]
- Li, Y. L. , Chen, C. H. , Chen, J. Y. , Lai, Y. S. , Wang, S. C. , Jiang, S. S. , & Hung, W. C. (2020). Single‐cell analysis reveals immune modulation and metabolic switch in tumor‐draining lymph nodes. Oncoimmunology, 9(1), 1830513. 10.1080/2162402X.2020.1830513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, Q. , Zheng, S. , Yu, X. , Chen, M. , Zhou, Y. , Zhou, Q. , Hu, C. , Gu, J. , Xu, Z. , Wang, L. , Liu, Y. , Liu, Q. , Wang, M. , Li, G. , Cheng, H. , Zhou, D. , Liu, G. , Fu, Z. , Long, Y. , … Chen, R. (2023). Standard pancreatoduodenectomy versus extended pancreatoduodenectomy with modified retroperitoneal nerve resection in patients with pancreatic head cancer: A multicenter randomized controlled trial. Cancer Communications, 43(2), 257–275. 10.1002/cac2.12399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, S. , Chen, X. , Bao, L. , Liu, T. , Yuan, P. , Yang, X. , Qiu, X. , Gooding, J. J. , Bai, Y. , Xiao, J. , Pu, F. , & Jin, Y. (2020). Treatment of infarcted heart tissue via the capture and local delivery of circulating exosomes through antibody‐conjugated magnetic nanoparticles. Nature Biomedical Engineering, 4(11), 1063–1075. 10.1038/s41551-020-00637-1 [DOI] [PubMed] [Google Scholar]
- Liu, Y. , & Cao, X. (2016). Characteristics and significance of the pre‐metastatic niche. Cancer Cell, 30(5), 668–681. 10.1016/j.ccell.2016.09.011 [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Gu, Y. , Han, Y. , Zhang, Q. , Jiang, Z. , Zhang, X. , Huang, B. , Xu, X. , Zheng, J. , & Cao, X. (2016). Tumor exosomal RNAs promote lung pre‐metastatic niche formation by activating alveolar epithelial TLR3 to recruit neutrophils. Cancer Cell, 30(2), 243–256. 10.1016/j.ccell.2016.06.021 [DOI] [PubMed] [Google Scholar]
- Misir, S. , Wu, N. , & Yang, B. B. (2022). Specific expression and functions of circular RNAs. Cell Death and Differentiation, 29(3), 481–491. 10.1038/s41418-022-00948-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey, S. M. , Zhang, F. , Ding, C. , Montoya‐Durango, D. E. , Hu, X. , Yang, C. , Wang, Z. , Yuan, F. , Fox, M. , Zhang, H. G. , Guo, H. , Tieri, D. , Kong, M. , Watson, C. T. , Mitchell, R. A. , Zhang, X. , McMasters, K. M. , Huang, J. , & Yan, J. (2021). Tumor‐derived exosomes drive immunosuppressive macrophages in a pre‐metastatic niche through glycolytic dominant metabolic reprogramming. Cell Metabolism, 33(10), 2040–2058.e10. 10.1016/j.cmet.2021.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patras, L. , Shaashua, L. , Matei, I. , & Lyden, D. (2023). Immune determinants of the pre‐metastatic niche. Cancer Cell, 41(3), 546–572. 10.1016/j.ccell.2023.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shariat, S. F. , Ehdaie, B. , Rink, M. , Cha, E. K. , Svatek, R. S. , Chromecki, T. F. , Fajkovic, H. , Novara, G. , David, S. G. , Daneshmand, S. , Fradet, Y. , Lotan, Y. , Sagalowsky, A. I. , Clozel, T. , Bastian, P. J. , Kassouf, W. , Fritsche, H. M. , Burger, M. , Izawa, J. I. , … Gonen, M. (2012). Clinical nodal staging scores for bladder cancer: A proposal for preoperative risk assessment. European Urology, 61(2), 237–242. 10.1016/j.eururo.2011.10.011 [DOI] [PubMed] [Google Scholar]
- Shen, C. N. , Goh, K. S. , Huang, C. R. , Chiang, T. C. , Lee, C. Y. , Jeng, Y. M. , Peng, S. J. , Chien, H. J. , Chung, M. H. , Chou, Y. H. , Hsieh, C. C. , Kulkarni, S. , Pasricha, P. J. , Tien, Y. W. , & Tang, S. C. (2019). Lymphatic vessel remodeling and invasion in pancreatic cancer progression. EBioMedicine, 47, 98–113. 10.1016/j.ebiom.2019.08.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng, F. , & Fussenegger, M. (2020). Shedding light on extracellular vesicle biogenesis and bioengineering. Advanced Science, 8(1), 2003505. 10.1002/advs.202003505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuszynska, I. , Magnus, M. , Jonak, K. , Dawson, W. , & Bujnicki, J. M. (2015). NPDock: A web server for protein‐nucleic acid docking. Nucleic Acids Research, 43(W1), W425–W430. 10.1093/nar/gkv493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urabe, F. , Patil, K. , Ramm, G. A. , Ochiya, T. , & Soekmadji, C. (2021). Extracellular vesicles in the development of organ‐specific metastasis. Journal of Extracellular Vesicles, 10(9), e12125. 10.1002/jev2.12125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Broeck, W. , Derore, A. , & Simoens, P. (2006). Anatomy and nomenclature of murine lymph nodes: Descriptive study and nomenclatory standardization in BALB/cAnNCrl mice. Journal of Immunological Methods, 312(1‐2), 12–19. 10.1016/j.jim.2006.01.022 [DOI] [PubMed] [Google Scholar]
- Vietri, M. , Radulovic, M. , & Stenmark, H. (2020). The many functions of ESCRTs. Nature Reviews. Molecular Cell Biology, 21(1), 25–42. 10.1038/s41580-019-0177-4 [DOI] [PubMed] [Google Scholar]
- Wang, S. , Li, F. , Ye, T. , Wang, J. , Lyu, C. , Qing, S. , Ding, Z. , Gao, X. , Jia, R. , Yu, D. , Ren, J. , Wei, W. , & Ma, G. (2021). Macrophage‐tumor chimeric exosomes accumulate in lymph node and tumor to activate the immune response and the tumor microenvironment. Science Translational Medicine, 13(615), eabb6981. 10.1126/scitranslmed.abb6981 [DOI] [PubMed] [Google Scholar]
- Wang, X. , Chen, T. , Li, C. , Li, W. , Zhou, X. , Li, Y. , Luo, D. , Zhang, N. , Chen, B. , Wang, L. , Zhao, W. , Fu, S. , & Yang, Q. (2022). CircRNA‐CREIT inhibits stress granule assembly and overcomes doxorubicin resistance in TNBC by destabilizing PKR. Journal of Hematology & Oncology, 15(1), 122. 10.1186/s13045-022-01345-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, S. , Hong, G. , Xu, A. , Zeng, H. , Chen, X. , Wang, Y. , Luo, Y. , Wu, P. , Liu, C. , Jiang, N. , Dang, Q. , Yang, C. , Liu, B. , Shen, R. , Chen, Z. , Liao, C. , Lin, Z. , Wang, J. , & Lin, T. (2023). Artificial intelligence‐based model for lymph node metastases detection on whole slide images in bladder cancer: A retrospective, multicentre, diagnostic study. The Lancet Oncology, 24(4), 360–370. 10.1016/S1470-2045(23)00061-X [DOI] [PubMed] [Google Scholar]
- Xia, W. , Xu, J. , Yu, G. , Yao, G. , Xu, K. , Ma, X. , Zhang, N. , Liu, B. , Li, T. , Lin, Z. , Chen, X. , Li, L. , Wang, Q. , Shi, D. , Shi, S. , Zhang, Y. , Song, W. , Jin, H. , Hu, L. , … Sun, Y. P. (2019). Resetting histone modifications during human parental‐to‐zygotic transition. Science, 365(6451), 353–360. 10.1126/science.aaw5118 [DOI] [PubMed] [Google Scholar]
- Xie, Z. , Gao, Y. , Ho, C. , Li, L. , Jin, C. , Wang, X. , Zou, C. , Mao, Y. , Wang, X. , Li, Q. , Fu, D. , & Zhang, Y. F. (2022). Exosome‐delivered CD44v6/C1QBP complex drives pancreatic cancer liver metastasis by promoting fibrotic liver microenvironment. Gut, 71(3), 568–579. 10.1136/gutjnl-2020-323014 [DOI] [PubMed] [Google Scholar]
- Zeigerer, A. , Gilleron, J. , Bogorad, R. L. , Marsico, G. , Nonaka, H. , Seifert, S. , Epstein‐Barash, H. , Kuchimanchi, S. , Peng, C. G. , Ruda, V. M. , Del Conte‐Zerial, P. , Hengstler, J. G. , Kalaidzidis, Y. , Koteliansky, V. , & Zerial, M. (2012). Rab5 is necessary for the biogenesis of the endolysosomal system in vivo. Nature, 485(7399), 465–470. 10.1038/nature11133 [DOI] [PubMed] [Google Scholar]
- Zhang, S. , Wang, X. , Chen, G. , Tong, L. , Dai, T. , Wang, L. , Zhu, L. , Zhang, H. , & Du, D. (2023). CircRNA Galntl6 sponges miR‐335 to ameliorate stress‐induced hypertension through upregulating Lig3 in rostral ventrolateral medulla. Redox Biology, 64, 102782. 10.1016/j.redox.2023.102782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, W. , Zhong, W. , Wang, B. , Yang, J. , Yang, J. , Yu, Z. , Qin, Z. , Shi, A. , Xu, W. , Zheng, C. , Schuchter, L. M. , Karakousis, G. C. , Mitchell, T. C. , Amaravadi, R. , Herlyn, M. , Dong, H. , Gimotty, P. A. , Daaboul, G. , Xu, X. , & Guo, W. (2022). ICAM‐1‐mediated adhesion is a prerequisite for exosome‐induced T cell suppression. Developmental Cell, 57(3), 329–343.e7. 10.1016/j.devcel.2022.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, L. , Gu, C. , Gan, Y. , Shao, L. , Chen, H. , & Zhu, H. (2020). Exosome‐mediated siRNA delivery to suppress postoperative breast cancer metastasis. Journal of Controlled Release : Official Journal of the Controlled Release Society, 318, 1–15. 10.1016/j.jconrel.2019.12.005 [DOI] [PubMed] [Google Scholar]
- Zheng, H. , An, M. , Luo, Y. , Diao, X. , Zhong, W. , Pang, M. , Lin, Y. , Chen, J. , Li, Y. , Kong, Y. , Zhao, Y. , Yin, Y. , Ai, L. , Huang, J. , Chen, C. , & Lin, T. (2024a). PDGFRα+ITGA11+ fibroblasts foster early‐stage cancer lymphovascular invasion and lymphatic metastasis via ITGA11‐SELE interplay. Cancer Cell, 42(4), 682–700.e12. 10.1016/j.ccell.2024.02.002 [DOI] [PubMed] [Google Scholar]
- Zheng, S. , Hu, C. , Lin, Q. , Li, T. , Li, G. , Tian, Q. , Zhang, X. , Huang, T. , Ye, Y. , He, R. , Chen, C. , Zhou, Y. , & Chen, R. (2024b). Extracellular vesicle‐packaged PIAT from cancer‐associated fibroblasts drives neural remodeling by mediating m5C modification in pancreatic cancer mouse models. Science Translational Medicine, 16(756), eadi0178. 10.1126/scitranslmed.adi0178 [DOI] [PubMed] [Google Scholar]
- Zhu, J. , Luo, Y. , Zhao, Y. , Kong, Y. , Zheng, H. , Li, Y. , Gao, B. , Ai, L. , Huang, H. , Huang, J. , Li, Z. , & Chen, C. (2021). circEHBP1 promotes lymphangiogenesis and lymphatic metastasis of bladder cancer via miR‐130a‐3p/TGFβR1/VEGF‐D signaling. Molecular Therapy : The Journal of the American Society of Gene Therapy, 29(5), 1838–1852. 10.1016/j.ymthe.2021.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
All data in the present study are presented in the paper or the Supplementary Materials. The full uncut gels are presented in Figure S7. The primers, probes and antibodies used in the present study are presented in Table S11–S12. The raw data have been deposited in the NCBI GEO, under accession numbers BioProject: GSE246070 and GSE246659. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD052771 and PXD053106.


