Abstract
The topological attributes of structural covariance networks (SCNs) based on fractal dimension (FD) and changes in brain network connectivity were investigated using graph theory and network-based statistics (NBS) in patients with noise-induced hearing loss (NIHL). High-resolution 3D T1 images of 40 patients with NIHL and 38 healthy controls (HCs) were analyzed. FD-based Pearson correlation coefficients were calculated and converted to Fisher’s Z to construct the SCNs. Topological attributes and network hubs were calculated using the graph theory. Topological measures between groups were compared using nonparametric permutation tests. Abnormal connection networks were identified using NBS analysis. The NIHL group showed a significantly increased normalized clustering coefficient, normalized characteristic path length, and decreased nodal efficiency of the right medial orbitofrontal gyrus. Additionally, the network hubs based on betweenness centrality and degree centrality were both the right transverse temporal gyrus and left parahippocampal gyrus in the NIHL group. The NBS analysis revealed two subnetworks with abnormal connections. The subnetwork with enhanced connections was mainly distributed in the default mode, frontoparietal, dorsal attention, and somatomotor networks, whereas the subnetwork with reduced connections was mainly distributed in the limbic, visual, default mode, and auditory networks. These findings demonstrate the abnormal topological structure of FD-based SCNs in patients with NIHL, which may contribute to understand the complex mechanisms of brain damage at the network level, providing a new theoretical basis for neuropathological mechanisms.
Keywords: Noise-induced hearing loss, Structural covariance network, Fractal dimension, Graph theory analysis, Network-based statistical analysis
Subject terms: Biomedical engineering, Magnetic resonance imaging
Introduction
Noise-induced hearing loss (NIHL) is sensorineural hearing loss caused by long-term exposure to occupational, environmental and other loud sound sources1. The World Health Organization has estimated that one-third of hearing loss could be attributed to noise exposure, and hearing loss has become a global public health problem2,3. NIHL is permanent and irreversible in most cases, which causes many damages to the auditory structure and contributes to a great negative impact on the quality of life of individuals, such as depression, anxiety, and cognitive disorders1,4. Given its widespread impact, it is crucial to understand the neuropathological mechanisms underlying NIHL. Owing to its non-invasiveness and high resolution, magnetic resonance technology has been widely used in clinics and has become a powerful tool for probing the human brain neuroscience. The researchers found that hearing loss was associated not only with auditory brain regions, but also with structural and functional changes in other regions. The structural changes were found in children with congenital bilateral deep SNHL after hearing deprivation5. Functional networks altered in infants with severe bilateral congenital sensorineural hearing loss6. Zou et al. found that the topological structure of the white matter network was disarranged in patients with unilateral sudden deafness, and the changes of brain node centers in SSNHL patients involved auditory network, visual network, attention network, default mode network, sensorimotor network and subcortical network7. These findings emphasize the necessity of comprehensively exploring the changes in brain structure and function in patients with NIHL. To explore the impact of NIHL on the brain more deeply and comprehensively, this study comprehensively uses multiple methods, such as fractal dimension (FD), graph theory, and network-based statistics (NBS) to study the changes in structural covariant networks (SCNs) in patients with NIHL and further explore the neuropathological mechanism of NIHL.
As a nonlinear measure of complexity, FD has unique advantages in evaluating the complexity of brain structure and can provide an important perspective for our research. FD is a nonlinear metric based on fractal geometry and has been defined as the complexity index for a long time. It summarizes the morphological characteristics of objects with providing the value of self-similarity, and then provides the value of its overall complexity8. FD has been used to evaluate the complexity of multiple scales on the brain9,10. Studies have shown that in various psychiatric and nervous system diseases, FD is more sensitive to detect morphological changes related to pathological and developmental changes, and also can more sensitively characterize structural differences of brain11–13. In the study of NIHL, FD also shows unique value. Compared with other methods that simply measure the size or morphology of the brain structure, FD focuses more on the complexity and self-similarity of the structure. It can quantify the subtle and complex changes that occur in the microstructure of brain, which are difficult to detect by traditional methods.
In addition, SCNs also have an important role in studying the impact of NIHL on the brain. SCNs have been applied to the study of various central nervous system diseases, such as schizophrenia, anxiety, and depression14–16. Neuroimaging studies have shown that the theoretical basis of SCNs model is the common morphological changes of interconnected brain regions, which is a valuable tool for the study of brain topology17. SCNs can explore how different brain regions throughout the brain interact and affect their structural morphology, rather than just focusing on individual regions or structures18,19. Compared with functional networks, SCNs have lower computational intensity and noise sensitivity, therefor, they have stronger persuasiveness and wider application in constructing group-level networks20. Since NIHL may trigger synergistic changes among multiple brain regions, these changes may not be limited to auditory-related regions, but may also involve other regions related to functions such as cognition and emotion. Through SCNs analysis, it is possible to better understand how NIHL affects the structural covariant relationships among brain regions and then reveal the influence mechanism on overall brain function.
In order to better understand structural covariant relationship and its effect on brain function, researchers introduced graph theory to analyze brain networks14,21,22. A complex network is formed in structure and function on the human brain, which can promote the effective processing and integration of information. Graph theory provides a powerful tool to model brain tissue, building a large-scale network of nodes and edges. Graph theory-based complex network analysis provides a powerful and general framework, which can directly measure the global and local organizational properties of networks, while also provides a range of metrics23. Graph theory can help quantitatively analyze changes in the topological properties of the brain network in patients with NIHL. By comparing the topological differences of the brain network between NIHL patients and the normal population, we can deeply understand the impact of NIHL on the integration and separation of overall brain function. NBS analysis is used to solve multiple comparison problems when analyzing connectivity graphs and allowed to identify subnetworks and connections with abnormal connections. It can also reveal changes in the strength of connections in brain networks and provides a complementary approach for assessing interbrain connectivity differences24. Since the brain network connections are complex and there may be multiple potentially abnormal connections in patients with NIHL. When processing such complex network data, traditional statistical methods may lead to a high false positive rate due to multiple comparison problems, whereas NBS can more accurately locate abnormal connections in brain networks. Graph theory and the NBS method have been widely used to treat hearing loss5,6,12.
This study conducted a complex network analysis by comprehensively employing FD, graph theory, and NBS methods. It not only focused on regional changes, but also considered the overall interaction of the brain network, revealing the specific impacts of NIHL on the brain network structure. This study solved the deficiencies of existing studies on the changes in brain network structure and function in patients with NIHL, but also deeply explored the neural mechanism of NIHL from micro to macro and from local to global dimensions. This provides a new theoretical basis for revealing neuropathological mechanisms in patients with NIHL.
Materials and methods
Subjects
According to the national occupational health standard GBZ 49-2014 “Diagnosis of Occupational Noise Deafness” of the People’s Republic of China, 40 patients with NIHL diagnosed in the occupational department of Yantaishan Hospital from 2014 to 2020 were selected. In the same period, 38 healthy controls (HCs) with matching age and education level were selected. All subjects were tested using the Mini-Mental State Examination (MMSE) and Hamilton Anxiety Scale (HAMA). The inclusion criteria for the NIHL group were as follows: adult male; han nationality; education level from primary school to university level; normal mental state (MMSE ≥ 27), no other neurological, psychiatric history and family history; no history of psychotropic drugs. Exclusion criteria: illiterate or non-cooperative, or MRI scan contraindications.
The study protocol was approved by the Hospital Ethics Committee (Yanshanlun 2023014). Written informed consent was obtained from each subject. All methods were performed in accordance with the relevant guidelines and regulations.
MRI data acquisition and data preprocessing
MRI data acquisition
The subjects were scanned using GE Discovery MR 750 3.0T (GE Company, USA). MRI parameters were as follows: repetition time (TR) = 6.9 ms, echo time (TE) = 3.4 ms, flip angle = 12°, matrix size = 256 × 256, field of view (FOV) = 25.6 cm × 25.6 cm, scanning time = 258 s, 172 sagittal slices, a thickness of 1 mm without a gap. During the scanning process, the subjects maintained a natural supine position, closed their eyes, relaxed and reduced mental activity and head movement.
Data preprocessing
Data preprocessing was done in the software package SPM12 (https://www.fil.ion.ucl.ac.uk/spm/) and its CAT12 toolkit (https://neuro-jena.github.io/cat/). First, the original images in DICOM format were converted to NIFTI format, and all T1 images were manually checked for quality by the researchers. Secondly, the 3D-MRI images were segmented by the segment function of CAT12 software to obtain gray matter, white matter and cerebrospinal fluid images. Next, the Dartel algorithm was used to standardize the segmented images into the Montreal Neurological Institute (MNI) standard space to eliminate individual differences in the brain. Subsequently, segmented images were smoothed using a 20 mm full-width-at-half-maximum (FWHM) Gaussian kernel13,25 to reduce the image registration error and improve the signal to noise ratio and image quality. Finally, the FD values of 68 brain regions were extracted using the CAT12 toolkit based on the Desikan-Killiany map26, and were found to be normally distributed according to the Shapiro-Wilk test.
Construction of structural covariance networks
The FD values with 68 cortical regions were used to construct the SCNs. The 68 brain regions (ROIs) were defined as nodes of SCNs, and the Pearson correlation coefficients were defined as edges of SCNs. With age and education level as covariates, 68 × 68 correlation matrixes were established by calculating the Pearson correlation coefficients between FD values of each brain regions for each group22,27.
In order to enhance the normality of the correlation coefficients, the values of 68 × 68 correlation matrix were transformed with Fisher’s Z. Subsequently, the correlation matrixes after Fisher’s Z were transformed to binary adjacency matrixes using various sparsity thresholds. Considering the absolute threshold at a single sparsity resulting in different numbers of edges, so the sparsity thresholds were defined as a range of network sparsity, ranging from 0.06 to 0.3 with an interval of 0.01. In this study, the minimum sparsity should meet the requirement that both SCNs were fully connected without any isolated nodes, so the minimum sparsity is 0.0619,28. Simultaneously, 100 random networks were generated and used to calculate network properties to determine whether the SCNs of the two groups had significant non-random topology in the above process.
Graph-based network analysis
Network topology attributes
Network topology attributes of the SCNs were calculated using Brain Connectivity Toolbox (BCT, https://www.nitrc.org/projects/bct). Global network topology attributes include clustering coefficient (Cp), characteristic path length (Lp), normalized clustering coefficient (γ), normalized characteristic path length (λ), small-worldness (σ), local efficiency (Elocal) and global efficiency (Eglobal). Nodal topology attributes include betweenness centrality (BC), degree centrality (DC) and nodal efficiency (NE). The definitions of these network topology attributes were the same as in previous studies21,29. The γ (γ = Cp-real / Cp-rand) and λ (λ = Lp-real / Lp-rand) were obtained by the ratio of Cp and Lp of 100 random networks to the real network respectively. The σ (σ = γ / λ) was obtained by the ratio of γ and λ.
Network hubs
The network hubs are the most interconnected region in the whole brain, which play a key role in the transmission and exchange of network information. The network hubs were defined as regions where the values of BC or DC were at least two standard deviations greater than the mean values22,29.
Statistical analysis
Baseline characteristics
Demographic and clinical data were analyzed with SPSS 26 software. An independent two-sample t-test was performed to analyze differences in age, education level, HAMA score and MMSE score between NIHL and HCs groups (p < 0.05 was statistically significant).
Network topology attributes statistic
The nonparametric permutation test is a statistical method that does not depend on the assumption of the data distribution. Given the typically unknown distribution of network topology attribute values, we used the non-parametric permutation test to analyze between-group differences in all network topology attributes between NIHL and HCs groups19,29,30. Initially, the real network topology attributes were calculated separately for NIHL and HCs groups within network sparsity range (0.06–0.3, with an interval of 0.01). Next, an independent permutation was performed for each sparsity level. The FD values of all subjects were randomly reallocated to NIHL and HCs groups with the same number of samples as the original groups, and the network topology attributes were recalculated within the same network sparsity range. This process was repeated 1000 times to obtain the permutation distribution. In each permutation test, the differences in network topology properties between NIHL and HCs groups were calculated and a null distribution of these differences was generated, which reflected the range of differences that could occur under the null hypothesis. Subsequently, the actual between-group differences were placed into the null distribution generated above, and p-values were calculated based on the position of the actual differences in the percentile of the null distribution, thereby assessing the statistical significance of the differences. Within the sparsity range, significant differences were observed in a consistent direction. Significant differences were confirmed when between-group differences were less than 2.5% or greater than 97.5%. Furthermore, because the area under the curve (AUC) is an integrated scalar of indicators under a group of thresholds, it is not affected by the randomness and bias of a single threshold selection, and is more sensitive to subtle and complex changes in brain function and structure caused by diseases31. Therefore, this study used AUC analysis to quantify the topological attributes of each network. Considering that evaluating network topological attributes at different network sparsity will produce multiple comparisons, we used the false discovery rate (FDR) method to correct the AUC differences to improve the reliability of statistical inference. Finally, the AUC differences of network topology attributes in real SCNs were compared with those in random SCNs (1000 repetitions), and the FDR method was applied for multiple comparisons with a threshold of 0.05.
Network-based statistic
The NBS is a nonparametric statistics approach to identify abnormal subnetworks of altered structural connectivity in the brain24. First, setting the threshold to 3.1 (t-test statistic threshold, correspond to p < 0.001), all edges were globally searched to identify any connected subnetworks of between-group differences with exceeding threshold value. Secondly, corrected p values for each subnetwork previously identified were calculated using permutation tests. P < 0.01 was set as the threshold of significance analysis. The null distribution for the largest subnetwork was generated under the null hypothesis of 1000 permutations, and then the statistical significances were evaluated. The subnetworks with increased and decreased connections were obtained by respective calculating NIHL-HCs and HCs-NIHL through the above process. Finally, the significant subnetworks were reported after corrected at p < 0.001, and were visualized using BrainNet Viewer32 and circos (https://circos.ca/).
In addition, based on previous research33, the brain regions according to the Desikan–Killiany atlas were divided into eight networks, which were showed in Table 1.
Table 1.
Abbreviations of networks.
| Network | Abbreviation | Network | Abbreviation |
|---|---|---|---|
| Visual network | VSN | Dorsal attention network | DAN |
| Somatomotor network | SMN | Frontoparietal network | FPN |
| Auditory network | AUN | Default mode network | DMN |
| Ventral attention network | VAN | Limbic network | LBN |
Results
Demographic and clinical characteristics and FD values
The demographic and clinical characteristics of NIHL and HCs groups are shown in Table 2. There were no significantly differences in age (p = 0.930), education level (p = 0.281), MMSE (p = 0.253) between NIHL and HCs groups. The HAMA (p < 0.001) in NIHL group was significantly greater than HCs group. FD values in 68 brain regions of NIHL and HCs groups are shown in Table 3.
Table 2.
Demographic and clinical characteristics of all participants (mean ± SD).
| NIHL(n = 40) | HCs(n = 38) | t | p | |
|---|---|---|---|---|
| Age(years) | 45.50 ± 8.17 | 45.66 ± 7.74 | − 0.088 | 0.930 |
| Education level(years) | 11.20 ± 1.86 | 11.74 ± 2.48 | − 1.086 | 0.281 |
| HAMA score | 12.79 ± 12.32 | 3.76 ± 1.05 | 4.53 | < 0.001* |
| MMSE score | 29.15 ± 1.37 | 28.82 ± 1.18 | 1.15 | 0.253 |
NIHL noise-induced hearing loss, HCs healthy controls, HAMA Hamilton Anxiety Scale, MMSE mini-mental state examination.
*Represented significant differences (p < 0.05).
Table 3.
FD values of all participants (mean ± SD).
| No. | Regions | Hemisphere | NIHL(n = 40) | HCs(n = 38) | t | p |
|---|---|---|---|---|---|---|
| 1 | Bankssts | L | 2.90 ± 0.23 | 2.87 ± 0.20 | 0.713 | 0.478 |
| R | 2.84 ± 0.15 | 2.87 ± 0.14 | − 0.928 | 0.356 | ||
| 2 | Caudal anterior cingulate gyrus | L | 2.54 ± 0.14 | 2.54 ± 0.17 | 0.059 | 0.953 |
| R | 2.43 ± 0.15 | 2.46 ± 0.14 | − 0.761 | 0.449 | ||
| 3 | Caudal middle frontal gyrus | L | 2.81 ± 0.12 | 2.85 ± 0.12 | − 1.188 | 0.239 |
| R | 2.92 ± 0.14 | 2.91 ± 0.14 | 0.382 | 0.703 | ||
| 4 | Cuneus | L | 2.67 ± 0.16 | 2.69 ± 0.17 | − 0.401 | 0.690 |
| R | 2.71 ± 0.21 | 2.70 ± 0.16 | 0.172 | 0.864 | ||
| 5 | Entorhinal cortex | L | 2.61 ± 0.17 | 2.65 ± 0.19 | − 0.966 | 0.337 |
| R | 2.62 ± 0.17 | 2.61 ± 0.18 | 0.305 | 0.761 | ||
| 6 | Fusiform gyrus | L | 2.45 ± 0.09 | 2.45 ± 0.10 | − 0.126 | 0.900 |
| R | 2.43 ± 0.07 | 2.43 ± 0.08 | − 0.281 | 0.779 | ||
| 7 | Inferior parietal gyrus | L | 2.79 ± 0.09 | 2.79 ± 0.10 | 0.080 | 0.937 |
| R | 2.87 ± 0.10 | 2.89 ± 0.09 | − 1.026 | 0.308 | ||
| 8 | Inferior temporal gyrus | L | 2.53 ± 0.14 | 2.52 ± 0.14 | 0.187 | 0.853 |
| R | 2.50 ± 0.11 | 2.51 ± 0.09 | − 0.467 | 0.642 | ||
| 9 | Isthmus cingulate cortex | L | 2.06 ± 0.11 | 2.08 ± 0.14 | − 0.847 | 0.399 |
| R | 2.04 ± 0.11 | 2.05 ± 0.11 | − 0.497 | 0.621 | ||
| 10 | Lateral occipital gyrus | L | 2.50 ± 0.07 | 2.50 ± 0.07 | 2.610 | 0.011* |
| R | 2.42 ± 0.10 | 2.42 ± 0.08 | − 0.395 | 0.694 | ||
| 11 | Lateral orbitofrontal gyrus | L | 2.28 ± 0.05 | 2.27 ± 0.06 | 0.918 | 0.361 |
| R | 2.27 ± 0.07 | 2.27 ± 0.06 | 0.005 | 0.996 | ||
| 12 | Lingual gyrus | L | 2.64 ± 0.11 | 2.63 ± 0.11 | 0.236 | 0.814 |
| R | 2.69 ± 0.09 | 2.67 ± 0.09 | 0.895 | 0.374 | ||
| 13 | Medial orbitofrontal gyrus | L | 2.60 ± 0.13 | 2.62 ± 0.10 | − 1.085 | 0.281 |
| R | 2.52 ± 0.12 | 2.54 ± 0.10 | − 0.947 | 0.346 | ||
| 14 | Middle temporal gyrus | L | 2.62 ± 0.13 | 2.60 ± 0.12 | 0.619 | 0.538 |
| R | 2.56 ± 0.11 | 2.59 ± 0.10 | − 1.057 | 0.294 | ||
| 15 | Parahippocampal gyrus | L | 2.58 ± 0.16 | 2.60 ± 0.19 | − 0.676 | 0.501 |
| R | 2.46 ± 0.14 | 2.47 ± 0.13 | − 0.449 | 0.654 | ||
| 16 | Paracentral lobule | L | 2.63 ± 0.12 | 2.63 ± 0.12 | − 0.124 | 0.902 |
| R | 2.60 ± 0.10 | 2.59 ± 0.10 | 0.505 | 0.615 | ||
| 17 | Pars opercularis | L | 2.83 ± 0.18 | 2.80 ± 0.19 | 0.733 | 0.466 |
| R | 2.92 ± 0.18 | 2.87 ± 0.16 | 1.139 | 0.258 | ||
| 18 | Pars orbitalis | L | 2.78 ± 0.18 | 2.73 ± 0.15 | 1.242 | 0.218 |
| R | 2.79 ± 0.15 | 2.85 ± 0.20 | − 1.508 | 0.136 | ||
| 19 | Pars triangularis | L | 2.71 ± 0.13 | 2.69 ± 0.17 | 0.566 | 0.573 |
| R | 2.69 ± 0.19 | 2.68 ± 0.18 | 0.366 | 0.716 | ||
| 20 | Pericalcarine cortex | L | 2.66 ± 0.22 | 2.71 ± 0.18 | − 0.972 | 0.334 |
| R | 2.40 ± 0.25 | 2.34 ± 0.22 | 1.148 | 0.254 | ||
| 21 | Postcentral gyrus | L | 2.80 ± 0.11 | 2.80 ± 0.09 | 0.135 | 0.893 |
| R | 2.82 ± 0.10 | 2.79 ± 0.10 | 1.161 | 0.249 | ||
| 22 | Posterior cingulate cortex | L | 2.65 ± 0.16 | 2.65 ± 0.18 | − 0.159 | 0.874 |
| R | 2.52 ± 0.13 | 2.56 ± 0.15 | − 1.034 | 0.304 | ||
| 23 | Precentral gyrus | L | 2.80 ± 0.07 | 2.79 ± 0.06 | 0.854 | 0.396 |
| R | 2.77 ± 0.05 | 2.76 ± 0.07 | 0.685 | 0.496 | ||
| 24 | Precuneus | L | 2.74 ± 0.11 | 2.75 ± 0.10 | − 0.652 | 0.517 |
| R | 2.70 ± 0.09 | 2.67 ± 0.07 | 1.781 | 0.079 | ||
| 25 | Rostral anterior cingulate gyrus | L | 2.07 ± 0.12 | 2.09 ± 0.14 | − 0.593 | 0.555 |
| R | 2.07 ± 0.14 | 2.13 ± 0.14 | − 1.637 | 0.106 | ||
| 26 | Rostral middle frontal gyrus | L | 2.53 ± 0.08 | 2.50 ± 0.08 | 2.029 | 0.046* |
| R | 2.51 ± 0.09 | 2.52 ± 0.09 | − 0.653 | 0.516 | ||
| 27 | Superior frontal gyrus | L | 2.41 ± 0.05 | 2.42 ± 0.06 | − 0.724 | 0.471 |
| R | 2.38 ± 0.06 | 2.37 ± 0.04 | 0.227 | 0.821 | ||
| 28 | Superior parietal gyrus | L | 2.68 ± 0.07 | 2.66 ± 0.08 | 0.757 | 0.451 |
| R | 2.73 ± 0.07 | 2.69 ± 0.08 | 1.786 | 0.078 | ||
| 29 | Superior temporal gyrus | L | 2.76 ± 0.12 | 2.77 ± 0.12 | − 0.107 | 0.915 |
| R | 2.74 ± 0.13 | 2.75 ± 0.10 | − 0.326 | 0.745 | ||
| 30 | Supramarginal gyrus | L | 2.64 ± 0.09 | 2.68 ± 0.09 | − 1.626 | 0.108 |
| R | 2.62 ± 0.09 | 2.65 ± 0.08 | − 1.449 | 0.151 | ||
| 31 | Frontal pole | L | 2.56 ± 0.18 | 2.60 ± 0.24 | − 0.782 | 0.436 |
| R | 2.57 ± 0.19 | 2.57 ± 0.19 | − 0.024 | 0.981 | ||
| 32 | Temporal pole | L | 2.40 ± 0.13 | 2.45 ± 0.12 | − 1.746 | 0.085 |
| R | 2.35 ± 0.13 | 2.39 ± 0.12 | − 1.399 | 0.166 | ||
| 33 | Transverse temporal gyrus | L | 2.48 ± 0.24 | 2.47 ± 0.23 | 0.231 | 0.818 |
| R | 2.36 ± 0.18 | 2.41 ± 0.21 | − 1.189 | 0.238 | ||
| 34 | Insula | L | 2.35 ± 0.07 | 2.39 ± 0.09 | − 2.178 | 0.033* |
| R | 2.35 ± 0.09 | 2.36 ± 0.08 | − 0.780 | 0.438 |
NIHL noise-induced hearing loss, HCs healthy controls, L left, R right.
*Represented significant differences (p < 0.05).
Global network topology attributes
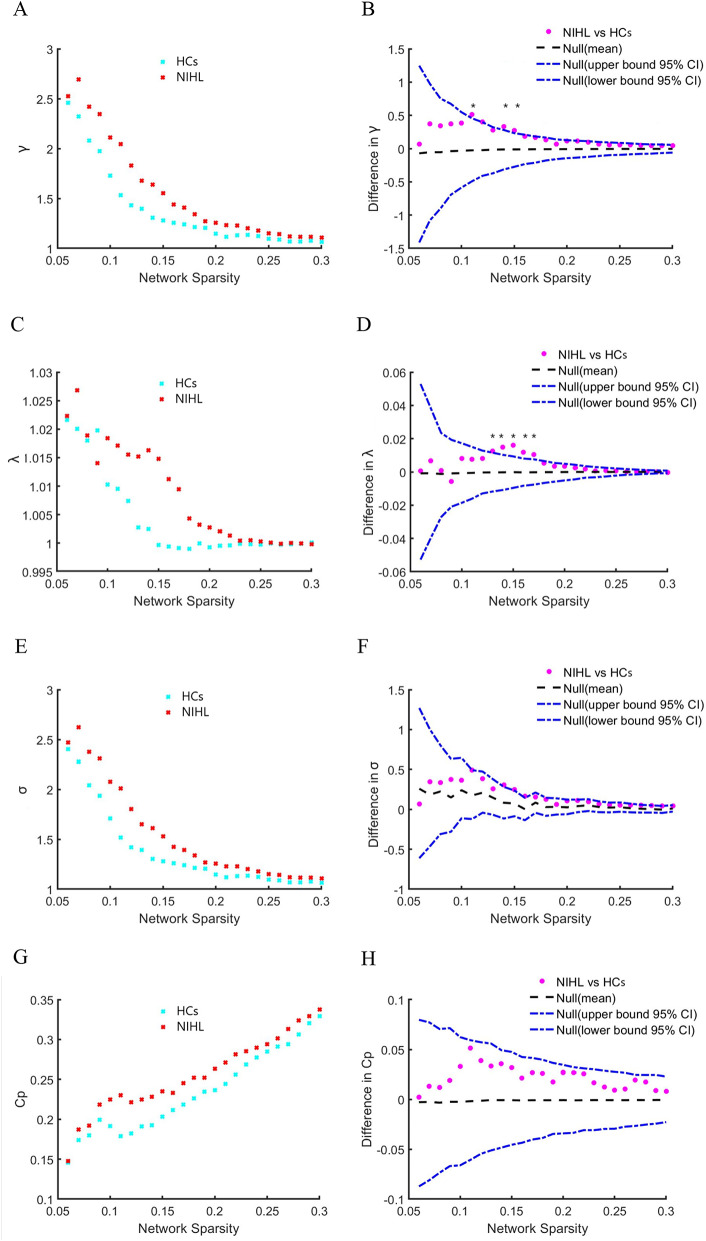
The changes and between-group differences between NIHL and HCs groups in global network attributes at network sparsity range from 0.06 to 0.3 are shown in Fig. 1. Small-world properties were found in both NIHL and HCs groups, with γ > 1, λ ≈ 1 and σ > 1 in Fig. 1A, C,E. Compared with the HCs group, the NIHL group exhibited significantly increased λ and γ at several network densities in Fig. 1B, D. No significant between-group differences were found in σ, Cp, Elocal, Lp and Eglobal attributes in Fig. 1 (F, H, J, L, N).
Fig. 1.
Changes and between-group differences in global network attributes at different network sparsity (0.06–0.3) between NIHL and HCs groups. Graphs (A,C,E,G,I,K,M) showed changes, and graphs (B,D,F,H,J,L,N) showed between-group differences under different network sparsity in NIHL and HCs groups. The blue lines (-.) represented a 95% confidence interval, whereas the black lines (-) in the middle denoted the mean differences after 1000 permutations at each network sparsity. The pink lines (·) represented the real between-group differences between NIHL and HCs groups, which fell outside the confidence intervals and indicated significant between-group differences (p < 0.05) under the current threshold. The positive values indicated NIHL > HCs, and the negative values indicated NIHL < HCs. * represented significant differences.
AUC analysis showed that there are no significant differences between NIHL and HCs groups in global network attributes after FDR correction (p < 0.05).
Nodal topology attributes
Significant differences between NIHL and HCs groups were found in regional topological measures after FDR correction (p < 0.05) though AUC analysis. Compared with the HCs group, the NIHL group had decreased NE in the right medial orbitofrontal gyrus (MORG.R). However, no significant differences were found in BC and DC between two groups.
Network hubs analysis
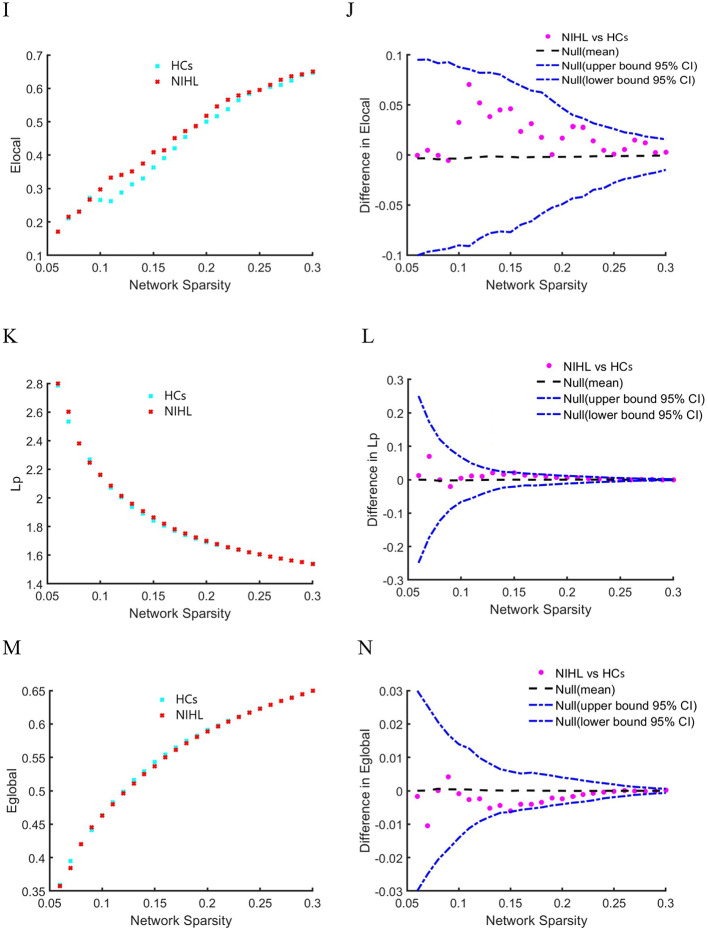
Network hubs based on BC and DC in NIHL and HCs groups are shown in Fig. 2; Table 4. On both BC-based and DC-based network hubs, three hubs were found in the HCs group and two hubs in the NIHL group. The network hubs in the HCs group included left caudal anterior cingulate gyrus (CACG.L), left posterior cingulate cortex (PCC.L), right medial orbitofrontal gyrus (MORG.R) and right transverse temporal gyrus (TTG.R); while in the NIHL group included right transverse temporal gyrus (TTG.R) and left parahippocampal gyrus (PHG.L). In the NIHL group, the network hubs based on DC and BC were both the right transverse temporal gyrus (TTG.R) and the left parahippocampal gyrus (PHG.L), while in the HCs group, the left caudal anterior cingulate gyrus (CACG.L) and the right medial orbitofrontal gyrus (MORG.R) were the consistent network hubs.
Fig. 2.
Network hubs based on BC and DC in NIHL and HCs groups. Hubs based on BC were shown with green in HCs (A) and blue in HINL (B). Hubs based on DC were shown with pink in HCs (C) and yellow in HINL (D).The size of dot represented the values of BC and DC. NIHL noise-induced hearing loss, HCs healthy controls, BC betweenness centrality, DC degree centrality, L left, R right. Abbreviations were listed in Table 5.
Table 4.
Network hubs regions of HINL and HCs groups.
| Hubs regions | BC | DC |
|---|---|---|
| HCs | ||
| CACG.L | 89.77 | 9.18 |
| PCC.L | 85.51 | / |
| MORG.R | 81.20 | 8.86 |
| TTG.R | / | 9.47 |
| NIHL | ||
| PHG.L | 94.40 | 8.85 |
| TTG.R | 101.78 | 9.46 |
\NIHL noise-induced hearing loss, HCs healthy controls, BC betweenness centrality, DC degree centrality, L left, R right. Abbreviations were listed in Table 5.
Table 5.
Abbreviations of brain regions.
| Regions | Abbreviation | Network | Regions | Abbreviation | Network |
|---|---|---|---|---|---|
| Bankssts | BSTS | DMN | Pars orbitalis | PORB | DMN |
| Caudal anterior cingulate gyrus | CACG | DMN | Pars triangularis | PTRI | FPN |
| Caudal middle frontal gyrus | CMFG | FPN | Pericalcarine cortex | PCAL | VSN |
| Cuneus | CUN | VSN | Postcentral gyrus | POSCG | SMN |
| Entorhinal cortex | ENC | LBN | Posterior cingulate cortex | PCC | DMN |
| Fusiform gyrus | FUG | VSN | Precentral gyrus | PRECG | SMN |
| Inferior parietal gyrus | IPG | FPN | Precuneus | PCUN | DMN |
| Inferior temporal gyrus | ITG | DAN | Rostral anterior cingulate gyrus | RACG | DMN |
| Isthmus cingulate cortex | ICC | DMN | Rostral middle frontal gyrus | RMFG | FPN |
| Lateral occipital gyrus | LOG | VSN | Superior frontal gyrus | SFG | DAN |
| Lateral orbitofrontal gyrus | LORG | DMN | Superior parietal gyrus | SPG | DAN |
| Lingual gyrus | LIG | VSN | Superior temporal gyrus | STG | AUN |
| Medial orbitofrontal gyrus | MORG | LBN | Supramarginal gyrus | SMG | VAN |
| Middle temporal gyrus | MTG | DMN | Frontal pole | FPO | DMN |
| Parahippocampal gyrus | PHG | LBN | Temporal pole | TPO | LBN |
| Paracentral lobule | PCL | SMN | Transverse temporal gyrus | TTG | AUN |
| Pars opercularis | POPE | FPN | Insula | INS | VSN |
Abbreviations of network were listed in Table 1.
Network alterations based on NBS analysis
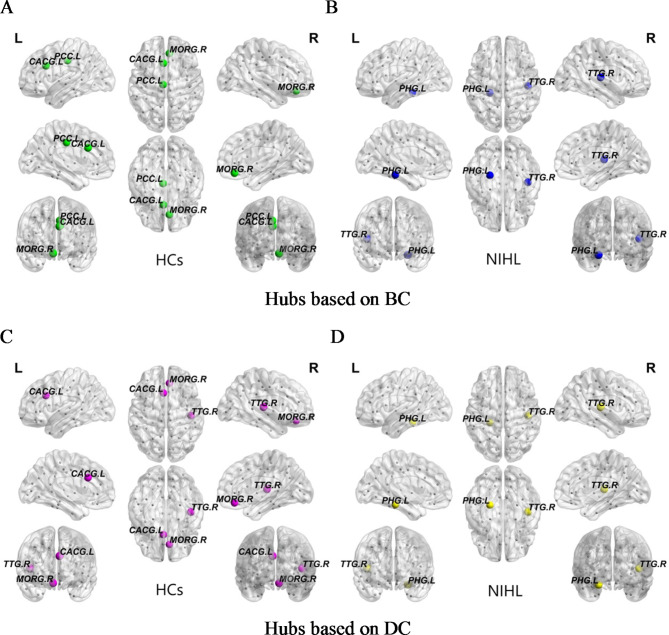
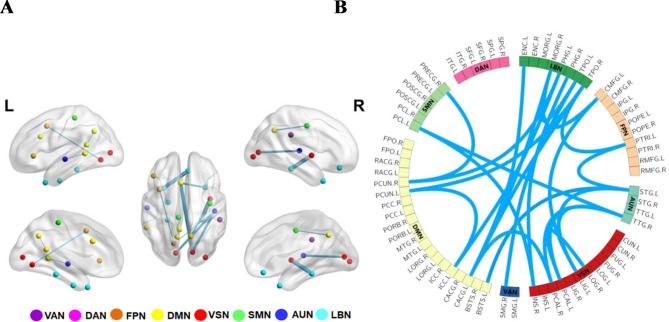
Two abnormal subnetworks were found according to NBS analysis results between NIHL and HCs groups, which were shown in Figs. 3 and 4; Table 6. In the NIHL group, the network of increased connections comprised 26 nodes and 29 connections, which were distributed throughout both hemispheres, in Fig. 3; Table 6. These alterations were primarily distributed in the network connectivity spanning the DMN, FPN, DAN and SMN. Inversely, the NIHL group exhibited network of decreased connections with 23 nodes and 24 connections. These reduced connections mainly involved VSN, LBN, DMN and AUN, in Fig. 4; Table 6.
Fig. 3.
Network of increased connections between NIHL and HCs groups based on NBS (p < 0.001). (A) Nodes and Edges of networks of increased connections. Colored dots represented nodes, and nodes were divided into different colors according to resting-state brain networks which were listed in Table 1. Lines represented edges, and the thickness of lines represented the strength of the connection. (B) The spatial distribution of networks of increased connections. The red lines represented edges with significantly increased connections. L left, R right. Abbreviations were listed in Table 5.
Fig. 4.
Network of decreased connections between NIHL and HCs groups based on NBS (p < 0.001). (A) Nodes and Edges of networks of decreased connections. Colored dots represented nodes, and nodes were divided into different colors according to resting-state brain networks which were listed in Table 1. Lines represented edges, and the thickness of lines represented the strength of the connection. (B) The spatial distribution of networks of decreased connections. The blue lines represented edges with significantly decreased connections. L: left, R: right. Abbreviations were listed in Table 5.
Table 6.
Nodes and edges with abnormal networks in NIHL group.
| Network with increased connections (NIHL > HCs) | Network with decreased connections (NIHL < HCs) | ||||
|---|---|---|---|---|---|
| Network-node | Network-node | Network-node | Network-node | ||
| DMN-BSTS.L | – | DAN-SPG.L | LBN-TPO.L | – | LBN-MORG.R |
| DMN-LORG.L | – | DAN-ITG.R | LBN-ENC.L | – | DMN-CACG.L |
| DMN-LORG.L | – | DMN-BSTS.R | LBN-MORG.R | – | DMN-CACG.L |
| DMN-CACG.L | – | FPN-CMFG.R | LBN-TPO.R | – | DMN-PCUN.L |
| DMN-CACG.L | – | FPN-IPG.R | LBN-PHG.L | – | DMN-ICC.L |
| DMN-MTG.R | – | SMN-PRECG.R | LBN-PHG.R | – | VSN-INS.R |
| DMN-BSTS.R | – | DAN-SPG.R | DMN-BSTS.L | – | AUN-STG.L |
| FPN-IPG.L | – | FPN-POPE.R | DMN-CACG.L | – | LBN-TPO.R |
| FPN-POPE.L | – | FPN-IPG.R | DMN-CACG.L | – | VSN-PCAL.R |
| FPN-PTRI.L | – | DMN-MTG.R | DMN-ICC.L | – | DMN-PCUN.L |
| FPN-RMFG.R | – | SMN-PRECG.R | DMN-PCUN.R | – | LBN-PHG.L |
| FPN-CMFG.R | – | DMN-PCC.L | VSN-INS.R | – | SMN-PCL.L |
| FPN-POPE.R | – | DAN-SPG.L | VSN-PCAL.R | – | AUN-TTG.R |
| FPN-IPG.R | – | AUN-TTG.R | VSN-LIG.L | – | AUN-TTG.R |
| DAN-SPG.L | – | FPN-PTRI.L | VSN-PCAL.L | – | AUN-TTG.R |
| DAN-SFG.L | – | FPN-IPG.R | VSN-LOG.R | – | VSN-PACL.L |
| DAN-SFG.L | – | DAN-SFG.R | AUN-STG.L | – | FPN-PTRI.L |
| DAN-SFG.R | – | FPN-IPG.R | AUN-STG.L | – | VSN-LIG.L |
| DAN-SFG.R | – | SMN-PRECG.R | AUN-TTG.R | – | SMN-PCL.L |
| DAN-ITG.R | – | LBN-PHG.L | FPN-PTRI.L | – | LBN-TPO.R |
| DAN-SPG.R | – | DMN-CACG.L | FPN-CMFG.L | – | VSN-LIG.L |
| SMN-POSCG.L | – | AUN-TTG.R | FPN-CMFG.L | – | LBN-MORG.R |
| SMN-PRECG.L | – | DMN-MTG.R | SMN-PRECG.R | – | DMN-PCUN.R |
| SMN-PRECG.R | – | AUN-TTG.R | VAN-SMG.R | – | LBN-TPO.L |
| SMN-POSCG.R | – | AUN-TTG.R | |||
| LBN-PHG.L | – | FPN-PTRI.L | |||
| LBN-MORG.R | – | FPN-IPG.L | |||
| AUN-TTG.R | – | FPN-POPE.L | |||
| VAN-SMG.R | – | SMN-PRECG.R | |||
Discussion
In this study, we found that the topology characteristics and network connections of FD SCNs were changed in patients with NIHL. The specific performances were as follows: (1) at the global level, the SCNs of both NIHL and HCs groups exhibited small-world properties. Compared with the HCs group, the λ and γ were more increased in the NIHL group. (2) at the nodal level, the NE of some nodes in the NIHL group were lower than those of the HCs group. (3) on BC-based and DC-based network hubs, the centers were located in different regions in NIHL and HCs groups respectively. (4) two abnormally connected networks appeared in the NIHL group.
In general, these studies contribute to our understanding of the neuropathology of NIHL at the network level, and improve our knowledge of the effects of NIHL on brain structure.
Alterations in global attributes of FD SCNs in NIHL
Previous studies had showed that both the structural and functional networks of the human brain followed a small-world topology. Small-world characteristics reflected the best balance between local separation and integration, reflecting a balanced network state34,35. Small-world networks supported local specialization and integrated processing through efficient information transfer while minimizing cabling costs36,37. In this study, the SCNs of NIHL and HCs groups both had small-world characteristics. This suggests that a high level of information interaction in the brain network in patients with NIHL is still present. However, some topological attributes of the NIHL group had altered. Compared with the HCs group, the NIHL group showed significantly increased γ and λ. Both of the unilateral SSNHL groups (lSSNHL and rSSNHL group) showed a significantly increased normalized clustering coefficient (γ) and normalized characteristic path length (λ)38. Wang et al. found that the normalized clustering coefficient and normalized path length increased in patients with mild cognitive impairment, and the abnormality of these structural networks was related to the slow information processing speed39. These are consistent with our findings. The increase in λ usually means that the information transmission path in the brain network becomes more lengthy, resulting in a decrease in information transmission efficiency. In patients with NIHL, this change may be due to the fact that noise exposure leads to a reduction in brain network connections or a weakening of connection strength, so that the information needs to pass through more intermediate nodes during the transmission process, thereby increasing the transmission time and complexity. This indicates that the global information integration ability of the brain network declines in patients with NIHL, which may further affect the speed and accuracy of the information processing in the brain. Meanwhile, the increase in γ may reflect the enhancement of local connections in the brain network. This may be due to noise exposure, which causes the brain to form more tightly connected clusters in local areas in an attempt to maintain or restore local information processing capabilities. This local adaptive response may be a compensation mechanism for noise damage in the brain.
In normal human brain networks, higher levels of two parameters (Cp and Elocal), which measure functional separation of the network, indicate increased local information processing capacity in the brain. Cp refers to the degree of interconnection between network nodes and neighboring nodes, reflecting the tightness of the connection of the local brain network, and is used to evaluate the efficiency of local information processing in the brain network40. Higher Cp reflects network isolation in brain networks, which is the ability to perform specialized neuronal processing between tightly connected regions41. Elocal is the average of the reverse shortest path lengths between adjacent nodes. It quantifies the efficiency of information transmission between nodes and neighboring nodes and is used to measure the degree of isolation of the network42. Compared with the HCs group, the NIHL group had a higher Cp and Elocal, although there was no significant difference. These also indicated that the information processing ability of local brain areas was enhanced and the local information transmission efficiency didn’t decrease significantly in patients with NIHL. This was consistent with previous studies of age-related hearing loss and unilateral sundden sensorineural hearing loss5,38. The brains of patients with NIHL showed an increase in the clustering coefficient and stability in local efficiency, suggesting that the brain may optimize the efficiency and accuracy of information processing by enhancing the connections between specific brain regions. Simultaneously, the brain may aim to maintain or even improve the efficiency of information transmission by strengthening internal connections. Although the differences in Cp and Elocal between NIHL and HCs groups did not reach a statistically significant level, this trend still provides us with important clues: the brains of patients with NIHL may undergo an adaptive or compensatory adjustment process. This process enables the brain to maintain or enhance the information processing capabilities of local brain regions as well as the efficiency of information transfer, thus compensating for the functional loss caused by impaired auditory input to a certain extent.
This suggests that the increased separation of SCNs in patients with NIHL may be a compensatory mechanism for brain structural damage. In a word, the topological properties of FD SCNs have changed in patients with NIHL. This may provide further evidence that NIHL is a disease associated with brain network alterations.
Alterations in nodal attributes of FD SCNs in NIHL
The abnormal topological properties of nodes indicate that the connectivity and communication efficiency of nodes with other regions are changed, resulting in abnormal regional neural circuits of brain networks, which can obtain additional information that can’t be obtained from global topological networks43. BC refers to the number of shortest paths between all pairs of nodes in the network through a node, which measures the influence of nodes on the overall information flow in the brain network44. DC indicates the number of edges directly connected to the node. The DC is greater, the nodes are connected more. DC evaluates the importance of nodes in the network42. NE indicates the information communication capability of a node in a network35.
At the level of nodes, compared with the HCs group, the node with significant difference in NE was found in the NIHL group, which was MORG.R. This abnormal node was also found in BC and DC, but it was not corrected by FDR (p < 0.05). MORG is involved in higher cognitive processes within the reward circuit that is necessary for reward processing, suppression of emotional responses, and habit formation, and it promotes behavioral outcomes45. Previously, abnormalities in MORG nodes were also found in some anxiety disorders, mental disorders, depression and other diseases. For example: abnormalities of the medial orbitofrontal cortex had been observed in various anxiety disorders, and it was believed that the medial orbitofrontal cortex was involved in the processing of trait anxiety46, functional connections between the right medial orbital gyrus and the dopamine region of the brain are reduced in patients with first-episode schizophrenia47, medial orbitofrontal cortex voxels had lower functional connectivity with temporal cortex areas, the parahippocampal gyrus, and fusiform gyrus in depression48.
In patients with NIHL, changes in the efficiency of MOFG nodes may be due to the remodeling of brain structure and function triggered by long-term noise exposure. The abnormal nodes may mean that some advanced functional interactions of SCNs in patients with NIHL are interrupted, resulting in reduced connectivity and communication efficiency between nodes and other brain areas.
Alterations in nodal hubs
In brain networks, central nodes are at the intersection of many short paths that control the flow of information through the brain42. Central nodes represent areas of the brain that are highly connected and highly centered, which are very important in brain networks but are susceptible to disease-related processes49. The changes of the center are closely related to neurological and psychological diseases50,51.
In this study, the distributions and numbers of BC-based and DC-based hubs were found to be different in the NIHL group than the HCs group. The patients with NIHL had the same nodal hubs based on BC and DC, including the PHG.L and the TTG.R. The PHG belongs to limbic network which is mainly involved in situational cognition, emotion, memory and attention. The limbic network is considered to be able to directly or indirectly accept the input of the auditory signal and produce the corresponding effect52. Hua et al.53 found that the centrality of the parahippocampal gyrus increased in sudden sensory-hearing loss. Moreover, the TTG belongs to the auditory network and is closely related to auditory information processing54. Wen’s study found that the apparent diffusion coefficient value of the temporal transverse gyrus increased55. Elevated ALFF in the temporal transverse gyrus was found in patients with low frequency amplitude senile deafness56. Zhang found that the core nodes of the right deafness group included the parahippocampal gyrus and the superior temporal gyrus related to hearing57. Feng et al. found that ALFF in the transverse temporal gyrus and parahippocampal gyrus in patients with unilateral sensorineural hearing loss were significantly higher than the control group58. These are similar to our findings. In the NIHL group, the PHG.L and TTG.R served as nodal centers, while in the HCs group, the nodal centers were CACG.L and MORG.R. The changes in nodal hubs in the brains of patients with NIHL may be due to brain plasticity and adaptive adjustments caused by long-term noise exposure. The PHG.L and TTG.R, serving as nodal hubs, may be to adapt to the challenges brought by hearing loss and redistribute brain resources to process auditory and other related information more effectively. These obvious changes in nodal hubs suggest that the brain network has been reorganized laterally after NIHL, and the functions of other sensory channels have been compensated and enhanced.
Abnormal network based on NBS
In the NIHL group, the subnetworks with enhanced connections were mainly distributed in DMN, FPN, DAN and SMN. The increased connections between nodes mainly occurred between DMN-DAN, DMN-FPN and DAN-FPN. The DMN is a network that is closely related to the integration of cognitive and emotional processing and is responsible for directing thinking and mental activity59. Li et al. found that patients with SSNHL had increased network connectivity between the DAN, FPN and DMN33. In elderly patients with moderate hearing loss, the auditory cortex showed activation in response to somatosensory stimulus60. Reduced auditory activity led to increased somatosensory activity61. A study of unilateral hearing loss in children found co-activation between multiple FPN and DMN regions, and increased connectivity between DMN and FPN62. The study suggested that the response to sensory deficits was achieved through dynamic reorganization of internetwork connections, possibly reflecting compensatory adaptations to maintain cognitive and sensory functions63,64. Therefore, after the auditory channel is damaged in patients with HINL, the function of other sensory channels is also affected. The compensation of auditory perception may be realized through cross-channel remodeling.
Conversely, the subnetworks with reduced connectivity in the NIHL group were mainly distributed in LBN, VSN, DMN and AUN. The reduced connections between nodes mainly existed between AUN-VSN and LBN-DMN. Ranran et al. found that patients with severe NIHL had reduced functional network connectivity within the AUN, VSN and DAN in the independent component analysis study65. A study using visuospatial working memory tasks found that patients with single-sided deafness had reduced activation in both auditory and visual cortices66. The node connectivity of the default network, visual network, and subcortical network was significantly reduced in patients with long-term unilateral hearing loss67. In patients with SSNHL, connections between VSN, AUN and DAN showed a significant decrease in Internet connection strength33. These results show that NIHL causes significant damage to auditory and sensory cognitive memory related networks. These deficiencies suggest that the effects of NIHL on brain tissue function are complex, and its potential ripple effects may extend to various cognitive and sensory functions.
It is worth noting that we found 41 node connections changed (excluding duplicate nodes), and these nodes were distributed across eight networks. Li et al. found significant between-group differences in the overall functional connectivity of 55 nodes within nine networks in patients with SSNHL, and these changes highlighted the widespread effects of SSNHL on functional connectivity on the brain33. The changes of brain nodes in patients with SSNHL involved auditory network, visual network, attention network, default mode network, sensorimotor network and subcortical network, and the WM network topology changed to randomization7. These changes indicate that there are extensive changes in the internal connectivity of nodes after NIHL, with a larger range of effects than traditional auditory networks.
The abnormal subnetworks suggest that the loss of function coexists with compensatory network recombination in the SCNs in patients with NIHL, suggesting that NIHL is not only a hearing disorder, but also a central nervous system disease.
There are some limitations in this study. First, the SCNs are built on the basis of group level, and each subject doesn’t have a separate network, so the correlation between network parameters and clinical indicators can’t be discussed. Second, the sample size is small, and the results may have statistical bias. Third, this study is a cross-sectional study and cannot reflect the dynamic changes of SCNs in patients with NIHL. In the future, it is necessary to continue to expand the sample size and conduct longitudinal studies to further clarify the relationships between SCNs and functional activity and cognition in patients with HINL.
Conclusion
In this study, we used graph theory, NBS and FD to study changes of SCNs in patients with NIHL. We found that SCNs in patients with NIHL had small-world characteristics, but had abnormal topological properties. There were different network hubs in NIHL and HCs groups. In addition, based on NBS analysis, two abnormal subnetworks were found with extensive changes in patients with NIHL. These findings may contribute to understand the complex underlying mechanisms of brain damage at the network level in patients with NIHL, and will provide new theoretical basis for the neuropathological mechanisms.
Author contributions
Wang Liping: Conceptualization, Investigation, Methodology, Project Management, Writing-original draft, Software. Lv Minghui: Data curation, Methodology, Investigation, Writing-review & editing. Zhang Jiayuan: Investigation, Software, Visualization. Wai Aijie: Data curation, Methodology, Investigation, Visualization. Huang Ranran: Conceptualization, Methodology, Writing-review & editing. Zhang zengcai: Conceptualization, Methodology, Software, Writing-review & editing, Supervision.Zhang guowei: Conceptualization, Funding acquisition, Resources, Writing-review & editing, Supervision.
Data availability
Due to privacy or ethical restrictions, the data of this study are not publicly available. But data are available upon reasonable request from the corresponding author.
Declarations
Competing interests
The authors declare no competing interests.
Ethical statement
The study protocol was approved by the Hospital Ethics Committee (Yanshanlun 2023014). Written informed consent was obtained from each subject. All methods were performed in accordance with the relevant guidelines and regulations.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zhang Zengcai, Email: zhangzengcai@163.com.
Zhang Guowei, Email: zhanggw0535@163.com.
References
- 1.Natarajan, N., Batts, S. & Stankovic, K. M. Correction: Natarajan et al. Noise-Induced Hearing Loss. J. Clin. Med.13(4). 10.3390/jcm13040944 (2024). [DOI] [PMC free article] [PubMed]
- 2.Ding, T., Yan, A. & Liu, K. What is noise-induced hearing loss? Br. J. Hosp. Med.80 (9), 525–529. 10.12968/hmed.2019.80.9.525 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Le, T. N., Straatman, L. V., Lea, J. & Westerberg, B. Current insights in noise-induced hearing loss: a literature review of the underlying mechanism, pathophysiology, asymmetry, and management options. J. Otolaryngol. Head Neck Surg.46 (1), 41. 10.1186/s40463-017-0219-x (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basner, M. Auditory and non-auditory effects of noise on health: an ICBEN perspective. J. Acoust. Soc. Am.137 (4), 2246–2246. 10.1121/1.4920192 (2015). [Google Scholar]
- 5.Yong, W. et al. Disrupted Topological Organization of resting-state functional brain networks in age-related hearing loss. Front. Aging Neurosci. 14907070. 10.3389/fnagi.2022.907070 (2022). [DOI] [PMC free article] [PubMed]
- 6.Cui, W., Wang, S., Chen, B. & Fan, G. Altered functional network in infants with profound bilateral congenital Sensorineural hearing loss: a Graph Theory Analysis. Front. Neurosci.15, 810833. 10.3389/fnins.2021.810833 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zou, Y. et al. Disrupted Topological Organization in White Matter Networks in Unilateral Sudden Sensorineural hearing loss. Front. Neurosci. 15666651. 10.3389/fnins.2021.666651 (2021). [DOI] [PMC free article] [PubMed]
- 8.Meregalli, V. et al. Cortical complexity estimation using fractal dimension: a systematic review of the literature on clinical and nonclinical samples. Eur. J. Neurosci.55 (6), 1547–1583. 10.1111/ejn.15631 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Ieva, A., Esteban, F. J., Grizzi, F., Klonowski, W. & Martín-Landrove, M. Fractals in the neurosciences, part II: clinical applications and future perspectives. Neuroscientist21 (1), 30–43. 10.1177/1073858413513928 (2013). [DOI] [PubMed] [Google Scholar]
- 10.Di Ieva, A., Grizzi, F., Jelinek, H., Pellionisz, A. J. & Losa, G. A. Fractals in the Neurosciences, Part I: General principles and Basic Neurosciences. Neuroscientist20 (4), 403–417. 10.1177/1073858413513927 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Madan, C. R. & Kensinger, E. A. Cortical complexity as a measure of age-related brain atrophy. Neuroimage134, 617–629. 10.1016/j.neuroimage.2016.04.029 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, J. H., Huang, N. X., Zou, T. X. & Chen, H. J. Brain cortical complexity alteration in amyotrophic lateral sclerosis: a preliminary Fractal Dimensionality Study. Biomed. Res. Int.2020 (1521679). 10.1155/2020/1521679 (2020). [DOI] [PMC free article] [PubMed]
- 13.Trevisan, N. et al. The complexity of cortical folding is reduced in chronic cocaine users. Addict. Biol.28 (3), e13268. 10.1111/adb.13268 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim, S., Kim, Y. W., Jeon, H., Im, C. H. & Lee, S. H. Altered cortical thickness-based Individualized Structural Covariance networks in patients with Schizophrenia and Bipolar Disorder. J. Clin. Med.9 (6). 10.3390/jcm9061846 (2020). [DOI] [PMC free article] [PubMed]
- 15.Zhang, X. et al. Disrupted brain gray matter connectome in social anxiety disorder: a novel individualized structural covariance network analysis. Cereb. Cortex. 33 (16), 9627–9638. 10.1093/cercor/bhad231 (2023). [DOI] [PubMed] [Google Scholar]
- 16.Wu, B. et al. Altered single-subject gray matter structural networks in first-episode drug-naïve adolescent major depressive disorder. Psychiat. Res. 329115557. 10.1016/j.psychres.2023.115557 (2023). [DOI] [PubMed]
- 17.Deng, L. et al. Alteration in topological organization characteristics of gray matter covariance networks in patients with prediabetes. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 47 (10), 1375–1384. 10.11817/j.issn.1672-7347.2022.220085 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gong, G., He, Y., Chen, Z. J. & Evans, A. C. Convergence and divergence of thickness correlations with diffusion connections across the human cerebral cortex. Neuroimage59 (2), 1239–1248. 10.1016/j.neuroimage.2011.08.017 (2011). [DOI] [PubMed] [Google Scholar]
- 19.Xu, H., Li, J., Huang, H., Yin, B. & Li, D. D. Abnormal developmental of structural covariance networks in young adults with heavy cannabis use: a 3-year follow-up study. Transl. Psychiatry. 14 (1), 45. 10.1038/s41398-024-02764-8 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bethlehem, R. A. I., Romero-Garcia, R., Mak, E., Bullmore, E. T. & Baron-Cohen, S. Structural Covariance networks in children with autism or ADHD. Cereb. Cortex. 27 (8), 4267–4276. 10.1093/cercor/bhx135 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang, Y. et al. Altered cortical thickness-based structural covariance networks in type 2 diabetes mellitus. Front. Neurosci.18, 1327061. 10.3389/fnins.2024.1327061 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li, S. et al. Abnormal cortical thickness and structural covariance networks in systemic lupus erythematosus patients without major neuropsychiatric manifestations. Arthritis Res. Ther.24 (1), 259. 10.1186/s13075-022-02954-z (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nigro, S. et al. Characterizing structural neural networks in de novo Parkinson disease patients using diffusion tensor imaging. Hum. Brain Mapp.37 (12), 4500–4510. 10.1002/hbm.23324 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zalesky, A., Fornito, A. & Bullmore, E. T. Network-based statistic: identifying differences in brain networks. Neuroimage53 (4), 1197–1207. 10.1016/j.neuroimage.2010.06.041 (2010). [DOI] [PubMed] [Google Scholar]
- 25.Yuan, J. et al. Alterations in cortical volume and complexity in Parkinson’s disease with depression. CNS Neurosci. Ther.30 (2), e14582. 10.1111/cns.14582 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Desikan, R. S. et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage31 (3), 968–980. 10.1016/j.neuroimage.2006.01.021 (2006). [DOI] [PubMed] [Google Scholar]
- 27.Yang, Y. et al. Gout is not just arthritis: abnormal cortical thickness and structural Covariance networks in gout. Front. Neurol.12, 662497. 10.3389/fneur.2021.662497 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ottino-González, J. et al. Brain structural covariance network features are robust markers of early heavy alcohol use. Addiction119 (1), 113–124. 10.1111/add.16330 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang, E. et al. Abnormal Topological Organization of Sulcal Depth-Based Structural Covariance Networks in Parkinson’s Disease. Front. Aging Neurosci.12, 575672. 10.3389/fnagi.2020.575672 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayasaka, S. et al. A non-parametric approach for co-analysis of multi-modal brain imaging data: application to Alzheimer’s disease. NEUROIMAGE30 (3), 768–779. 10.1016/j.neuroimage.2005.10.052 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qin, K. et al. Network-level functional topological changes after mindfulness-based cognitive therapy in mood dysregulated adolescents at familial risk for bipolar disorder: a pilot study. BMC Psychiatry. 21 (1), 213. 10.1186/s12888-021-03211-4 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xia, M., Wang, J. & He, Y. BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS One. 8 (7), e68910. 10.1371/journal.pone.0068910 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li, J. et al. Exploring functional connectivity alterations in sudden sensorineural hearing loss: a multilevel analysis. Brain Res. 1824148677. 10.1016/j.brainres.2023.148677 (2023). [DOI] [PubMed]
- 34.Barbey, A. K. Network Neuroscience Theory of Human Intelligence. Trends Cogn. Sci.22 (1), 8–20. 10.1016/j.tics.2017.10.001 (2017). [DOI] [PubMed] [Google Scholar]
- 35.Liao, X., Vasilakos, A. V. & He, Y. Small-world human brain networks: perspectives and challenges. Neurosci. Biobehav. R. 77, 286–300. 10.1016/j.neubiorev.2017.03.018 (2017). [DOI] [PubMed] [Google Scholar]
- 36.Achard, S., Salvador, R., Whitcher, B., Suckling, J. & Bullmore, E. A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs. J. Neurosci.26 (1), 63–72. 10.1523/JNEUROSCI.3874-05.2006 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaiser, M. & Hilgetag, C. C. Nonoptimal component placement, but short processing paths, due to long-distance projections in neural systems. Plos. Comput. Biol.2 (7), e95. 10.1371/journal.pcbi.0020095 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu, H. et al. Disrupted functional brain connectome in unilateral sudden sensorineural hearing loss. Hear. RES.335, 138–148. 10.1016/j.heares.2016.02.016 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Wang, X. B., Zhao, X. H., Jiang, H., Qian, X. I. & Wang, P. J. Small-worldness of brain fMRI network in patients with mild cognitive impairment. Chin. J. Med. Imaging Technol.5, 790–793. 10.19627/j.cnki.cn31-1700/th.2015.01.001 (2014). [Google Scholar]
- 40.Lv, H. et al. Resting-state functional MRI: everything that nonexperts have always wanted to know. Am. J. Neuroradiol.39 (8), 1390–1399. 10.3174/ajnr.A5527 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dai, Z. et al. Disrupted structural and functional brain networks in Alzheimer’s disease. NEUROBIOL. AGING. 75, 71–82. 10.1016/j.neurobiolaging.2018.11.005 (2018). [DOI] [PubMed] [Google Scholar]
- 42.Rubinov, M. & Sporns, O. Complex network measures of brain connectivity: uses and interpretations. NEUROIMAGE52 (3), 1059–1069. 10.1016/j.neuroimage.2009.10.003 (2009). [DOI] [PubMed] [Google Scholar]
- 43.Jin, C. et al. Structural Brain Network Abnormalities in Parkinson’s Disease With Freezing of Gait. Front. Aging Neurosci. 14944925. 10.3389/fnagi.2022.944925 (2022). [DOI] [PMC free article] [PubMed]
- 44.Freeman, L. Centrality in social networks conceptual clarification. Soc. Networks. 1 (3), 215–239. 10.1016/0378-8733(78)90021-7 (1978). [Google Scholar]
- 45.Monteleone, A. M. et al. Neuroendocrinology and brain imaging of reward in eating disorders: a possible key to the treatment of anorexia nervosa and bulimia nervosa. Prog. Neuropsychopharmacol. Biol. Psychiatry. 80 (Pt B), 132–142. 10.1016/j.pnpbp.2017.02.020 (2017). [DOI] [PubMed] [Google Scholar]
- 46.Xue, S. W., Lee, T. W. & Guo, Y. H. Spontaneous activity in medial orbitofrontal cortex correlates with trait anxiety in healthy male adults. J. Zhejiang Univ-SC B. 19 (8), 643–653. 10.1631/jzus.B1700481 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xue, K. K. et al. [Abnormal changes of static and dynamic functional connectivity of dopaminergic midbrain in patients with first-episode schizophrenia and their correlations with clinical symptoms]. Zhonghua Yi Xue Za Zhi. 103 (21), 1623–1630. 10.3760/cma.j.cn112137-20221118-02428 (2023). [DOI] [PubMed] [Google Scholar]
- 48.Rolls, E. T. et al. Functional connectivity of the right inferior frontal gyrus and orbitofrontal cortex in depression. Soc. Cogn. Affect. Neur.. 15 (1), 75–86. 10.1093/scan/nsaa014 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crossley, N. A. et al. The hubs of the human connectome are generally implicated in the anatomy of brain disorders. Brain137 (Pt 8), 2382–2395. 10.1093/brain/awu132 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tian, L. et al. Abnormal functional connectivity of brain network hubs associated with symptom severity in treatment-naive patients with obsessive-compulsive disorder: a resting-state functional MRI study. Prog Neuropsychopharmacol. Biol. Psychiatry. 66, 104–111. 10.1016/j.pnpbp.2015.12.003 (2015). [DOI] [PubMed] [Google Scholar]
- 51.Lin, S. J., Baumeister, T. R., Garg, S. & McKeown, M. J. Cognitive profiles and hub vulnerability in Parkinson’s Disease. Front. Neurol.9, 482. 10.3389/fneur.2018.00482 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bernstein, L. E., Demorest, M. E. & Tucker, P. E. Speech perception without hearing. Percept Psychophys.62 (2), 233–252. 10.3758/bf03205546 (2000). [DOI] [PubMed] [Google Scholar]
- 53.Hua, J. C. et al. Aberrant functional network of small-world in sudden sensorineural hearing loss with tinnitus. Front. Neurosci.16, 898902. 10.3389/fnins.2022.898902 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang Occupational noise-induced Hearing loss Patients: Diffusion Tensor Imaging Study and Resting-state Functional Network Connectivity Study [D] (Shandong University, 2023).
- 55.Zuguang, W. The value of study on the auditory pathway in congenital sensorineural hearing loss children [D]. GuangXi Medical University, (2019).
- 56.Li Investigation of the Neural Mechanisms of Presbycusis with Cognitive Impairment Using Sub-bands Analysis of ALFF and Functional Connectivity [D] (SHANDONG UNIVERSITY, 2021).
- 57.Zhang, L. et al. Aberrant topological properties of whole-brain functional network in chronic right-sided sensorineural hearing loss: a resting-state functional MRI study. Zhonghua Yi Xue Za Zhi. 95 (5), 349–352 (2015). [PubMed] [Google Scholar]
- 58.Yuan, F. E. N. G. et al. Resting-state functional MR Study in Unilateral Sensorineural hearing loss by amplitude of low-frequency Fluctuation[J]. Chin. J. Otol.(2), 224–227. 10.3969/j.issn.1672-2922.2014.02.09 (2014).
- 59.Buckner, R. L., Andrews-Hanna, J. R. & Schacter, D. L. The brain’s default network: anatomy, function, and relevance to disease. ANN. NY ACAD. SCI.1124, 1–38. 10.1196/annals.1440.011 (2008). [DOI] [PubMed] [Google Scholar]
- 60.Cardon, G. & Sharma, A. Somatosensory cross-modal reorganization in adults with Age-Related, early-stage hearing loss. Front. Hum. Neurosci.12, 172. 10.3389/fnhum.2018.00172 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shore, S. E., Koehler, S., Oldakowski, M., Hughes, L. F. & Syed, S. Dorsal cochlear nucleus responses to somatosensory stimulation are enhanced after noise-induced hearing loss. EUR. J. NEUROSCI.27 (1), 155–168. 10.1111/j.1460-9568.2007.05983.x (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jung, M. E., Colletta, M., Coalson, R., Schlaggar, B. L. & Lieu, J. E. C. Differences in interregional brain connectivity in children with unilateral hearing loss. LARYNGOSCOPE127 (11), 2636–2645. 10.1002/lary.26587 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alzaher, M. et al. Brain plasticity and hearing disorders. REV. NEUROL-FRANCE. 177 (9), 1121–1132. 10.1016/j.neurol.2021.09.004 (2021). [DOI] [PubMed] [Google Scholar]
- 64.Auerbach, B. D. & Gritton, H. J. Hearing in Complex environments: auditory Gain Control, attention, and hearing loss. Front. Neurosci.16, 799787. 10.3389/fnins.2022.799787 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ranran, H. et al. Alterations of resting-state functional network connectivity in patients with noise-induced hearing loss: a study based on independent component analysis. EUR. J. NEUROSCI.59 (8), 2029–2045. 10.1111/ejn.16266 (2024). [DOI] [PubMed] [Google Scholar]
- 66.Qiao, Y. et al. Downward cross-modal plasticity in single-sided deafness. NEUROIMAGE197, 608–617. 10.1016/j.neuroimage.2019.05.031 (2019). [DOI] [PubMed] [Google Scholar]
- 67.Zhang, Y. et al. Altered functional networks in long-term unilateral hearing loss: a connectome analysis. Brain Behav.8 (2), e00912. 10.1002/brb3.912 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Due to privacy or ethical restrictions, the data of this study are not publicly available. But data are available upon reasonable request from the corresponding author.