Abstract
Introduction
Anemia is a common global health problem, particularly in impoverished regions, with a high incidence rate. The condition is multifactorial, with iron deficiency being one of the most prevalent causes. Current treatment for anemia often relies on iron supplements or erythropoiesis-stimulating agents, although these therapies may show limited efficacy for some patients. Recent evidence suggests that probiotics, prebiotics, and synbiotics, as microbiome modulators, hold significant potential in the treatment of anemia. These interventions may enhance iron absorption and improve overall blood health through their impact on gut microbiota, thus providing an alternative or complementary approach to conventional treatments.
Methods
Six databases, including the Cochrane Central Register of Controlled Trials, Embase, PubMed, Web of Science (WOS), China National Knowledge Infrastructure (CNKI), and WangFang data library, were searched up to November 20, 2024. Studies published in English and Chinese were included. We included randomized controlled trials (RCTs) evaluating the effects of probiotics, prebiotics, or synbiotics in treating anemia. The experimental groups received probiotics, prebiotics, or synbiotics, while the control groups received placebo, alternative treatments, or no treatment. The primary outcome was hemoglobin (Hb) levels. Secondary outcomes included serum iron (SI) and serum ferritin (SF). A descriptive analysis was conducted for studies where meta-analysis was not feasible. The GRADE tool was used to assess the quality of evidence, and the Cochrane guidelines were employed to evaluate the risk of bias in each study.
Results
Seven studies were included comprising a total of eight RCTs, with the main types of anemia being iron deficiency anemia (IDA) and anemia of chronic kidney disease (CKD), involving 632 patients. The analysis revealed that probiotics, prebiotics, or synbiotics significantly improved Hb levels in patients with anemia (WMD = 10.760, 95% CI: 4.593 to 16.747, p = 0.001), though heterogeneity was high (I² = 96.5%). Two RCTs (n = 120 participants) reported significant increases in serum iron levels in the probiotic group (WMD = 3.835, 95% CI: 3.271 to 4.400), with moderate heterogeneity (I² = 38.7%). Two RCTs (n = 192 participants) reported no significant differences were observed between the groups in serum ferritin levels (WMD = 8.048, p = 0.115), and heterogeneity remained high (I² = 62.6%). Subgroup analyses revealed that probiotics improved Hb levels in renal and iron-deficiency anemia, as well as across different doses. The synbiotic group showed consistent efficacy (I² = 0%), while the prebiotic group did not exhibit significant effects, with extremely high heterogeneity (I² = 99.3%). This indicates that heterogeneity may stem from variations in intervention types, and the results should be interpreted with caution.
Conclusion
There is moderate-quality evidence suggesting that probiotics, prebiotics, and synbiotics may improve anemia management, particularly by enhancing Hb levels. Further high-quality RCTs are required to explore the specific role of synbiotics in anemia management, including their comparative efficacy against probiotics and prebiotics alone, and their impact on gastrointestinal factors such as gut microbiota modulation and inflammation reduction.
Systematic review registration
PROSPERO CRD42024590073.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12876-024-03562-8.
Keywords: Anemia1, Probiotic2, Prebiotic3, Synbiotic4, Meta-analysis5, Randomized controlled trial6
Introduction
Anemia is a globally prevalent health issue, affecting approximately one-third of the population [1–3], with IDA being the most common type [4]. IDA affects approximately 136 million children, 16 million pregnant women, and 248 million non-pregnant women [5]. Nearly half of patients with end-stage renal disease (ESRD) also experience anemia [6, 7], which increases the risk of cardiovascular complications, mortality, decreased quality of life, and impaired cognitive and physical functions [8]. Iron plays a crucial role in energy metabolism, oxygen transport, and Adenosine Triphosphate synthesis [9, 10]and iron deficiency can lead to both short-term and long-term health problems, including impaired cognitive and physical development, severe fatigue, and reduced work capacity. Anemia particularly affects children, women, and the elderly in both developing and developed countries [11–13]. Anemia is highly prevalent among men, particularly in the elderly population [14]. Studies have shown that the incidence of anemia significantly increases with age, especially among men aged 80 years and older. In individuals aged 65 years and above, the prevalence of anemia ranges from 11–65% [15, 16], depending on the population studied and the diagnostic criteria applied. study indicate that anemia is more common in older men than in women [17], a study conducted in Poland reported anemia prevalence rates of 20.8% in men compared to 13.6% in women [16].
Probiotics are non-pathogenic microorganisms that, when administered in sufficient amounts in a live form, can confer health benefits to the host by restoring gut microbial balance. Probiotics modulate inflammatory responses by balancing pro-inflammatory and anti-inflammatory cytokines in the body as gut flora changes [18, 19] The anti-inflammatory effects of probiotics have been shown to be beneficial in treating various inflammatory diseases, including diabetes, non-alcoholic fatty liver disease, chronic intestinal inflammation, rheumatoid arthritis, and obesity [20–23]. Probiotics may even alleviate systemic inflammation in critically ill patients [24]. Vonderheid et al. analyzed how probiotics significantly enhance iron absorption. This may occur through immune modulation, anti-inflammatory responses, reduction of ferric iron into bioavailable ferrous iron, and improved iron absorption by intestinal cells [13]. Prebiotics are functional food ingredients that stimulate the growth and colonization of beneficial bacteria in the gut. These microorganisms scavenge iron from the environment by producing high-affinity iron-chelating compounds (siderophores) [25]. Additionally, prebiotics promote iron absorption in the proximal colon and duodenum by producing short-chain fatty acids in the colon [26]. Studies have shown that prebiotics can improve anemia at both clinical and animal model levels, potentially by enhancing iron metabolism [27, 28]. Furthermore, recent studies indicate that prebiotic supplementation improves iron absorption and alleviates anemia in humans [29, 30]. Synbiotics, a combination of probiotics and prebiotics may provide synergistic benefits by simultaneously promoting the growth of beneficial gut bacteria and enhancing their functional effects, including improved iron absorption and metabolism [31].
However, the evidence for the positive effects of probiotics, prebiotics, and synbiotics on anemia patients remains inconclusive. While probiotics and prebiotics have shown potential in improving iron absorption and metabolism, the combined use of these agents, known as synbiotics, has been less extensively studied. A recent review [31] lacked efficacy analyses of RCTs, leaving a gap in understanding the clinical applicability of these interventions for anemia treatment. Therefore, this systematic review and meta-analysis aims to address this gap by incorporating data from multiple RCTs to assess the efficacy and safety of probiotics and prebiotics in treating anemia. Our goal is to provide a comprehensive synthesis of current clinical data, thereby contributing evidence-based insights for developing effective treatment guidelines.
Methods
This study is registered in PROSPERO under the registration number: CRD42024590073. We adhered to and reported according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines [32] (the PRISMA 2020 Checklist is available in the supplementary materials).
Eligibility criteria
Studies that met the following inclusion criteria were selected for analysis: (1) Participants: Adults diagnosed with anemia, irrespective of the cause, meeting local diagnostic standards for anemia; (2) Intervention: Subjects received probiotics, prebiotics, or synbiotics; (5) Comparison: Placebo without probiotics, prebiotics and synbiotics; (6) Outcomes: Primary outcome was Hb levels, with secondary outcomes including indicators of iron metabolism; (7) Study Design: RCTs with either a crossover or parallel design.
Exclusion criteria
Reviews, conference papers, animal studies, non-peer-reviewed articles, retrospective studies, case-control studies, and self-controlled studies were excluded. RCTs that did not report outcomes related to the efficacy of probiotics, prebiotics and synbiotics in treating anemia were also excluded.
A search was conducted on PubMed, Embase, Cochrane Library, WOS, CNKI, and WanFang for studies published in English or Chinese from January 1, 2000, to November 20, 2024. We also searched for gray literature, including clinical trial registries (ClinicalTrials.gov and Google scholar), conference proceedings, dissertations (ProQuest and CNKI), and reference lists of relevant articles. The detailed search strategy and exclusion list are provided in the supplementary materials.
Study selection
Search records were imported into Endnote 21 to remove duplicates. Titles and abstracts were screened for initial study selection, followed by a full-text review to confirm the final inclusion. Two reviewers (QH and YL) independently conducted the literature search and screening process. The inter-rater agreement for study selection was substantial, with a Cohen’s kappa of 0.65. Disagreements were resolved through discussion, and if no consensus was reached, a third reviewer (FYZ) made the final decision.
Data extraction
Two reviewers independently extracted data using a predefined standardized form. Extracted data included author and publication year, cause of anemia, duration of disease, sample size, participants’ age and sex, type and dosage of supplements, and outcome measures. If original study data were unavailable in the articles, the corresponding authors were contacted for additional information. Data extraction was cross-checked, and any discrepancies were resolved by a third reviewer (FYZ).
Assessment of risk of bias
The risk of bias was evaluated using the Cochrane RoB 2 tool, assessing five domains: randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. For each RCT, two reviewers (QH and YL) independently evaluated each domain, assigning ratings of “high risk,” “some concerns,” or “low risk.” Any discrepancies were resolved through discussion or consultation with a third reviewer (SY).
Data synthesis and statistical analysis
Statistical analyses were conducted using Stata 17 software. Continuous variables were evaluated through weighted mean differences (WMD) and 95% confidence intervals (CI). A random-effects model was employed when substantial heterogeneity was detected (I² ≥ 50% or P < 0.05), while a fixed-effects model was chosen in cases of low heterogeneity. Sensitivity analyses were performed to assess the stability of the findings, and publication bias was evaluated using Egger’s and Begg’s tests. A threshold of P < 0.05 was deemed statistically significant.
Results
Results of literature search and selection
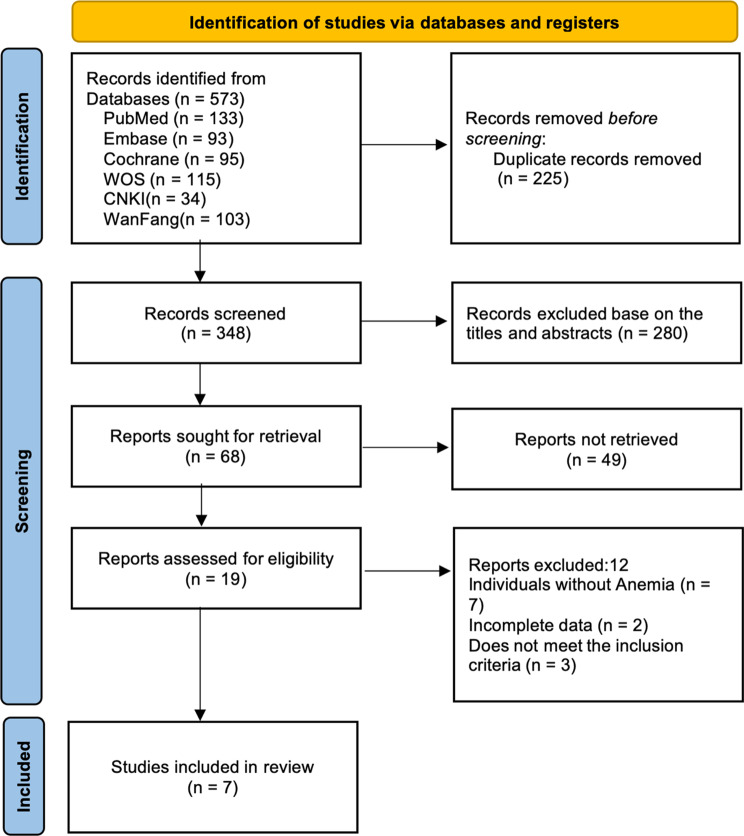
A total of 573 relevant articles were identified from six databases. After removing 225 duplicates, titles, abstracts, and full texts were reviewed, ultimately including 7 eligible studies. The detailed flowchart is shown in Fig. 1.
Fig. 1.
The screening flowchart
Characteristics of included studies
This review included 7 studies [25, 33–38], comprising 8 RCTs with 632 participants, Four of the studies were conducted in China [35–38], two in Iran [33, 34], and one in Pakistan [25]. The publication years ranged from 2018 to 2023. A total of 105 patients received probiotics, 96 received prebiotics, and 150 received synbiotics. Three studies examined IDA [25, 34, 37], while four focused on Anemia of CKD [33, 35, 36, 38]; four of the renal studies included patients undergoing hemodialysis [33, 35, 39, 40]. three studies [33, 39, 41] reported adverse effects, primarily nausea, diarrhea, and altered taste. All studies reported Hb levels. Detailed characteristics of the included studies are shown in Table 1.
Table 1.
Characteristics of the randomized controlled studies
| study | country | sample size(T, C) | age [mean(SD)] (T, C) |
gender (male/female) (T, C) | intervention | Basic treatment | Type of anemia | Duration | Adverse events(T/C) | outcome indicator | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| T | C | ||||||||||
| Shang BJ 2018 | China | 32, 32 | 61.5(8.7), 58.9(12.4) | 18/14, 17,15 | Prebiotic capsules(420 mg) Including the following strains: Bifidobacterium longiformis,Lactobacillus acidophilus,Enterococcus faecalis/Tid | Placebo, same size starch capsules | Weekly injections of 12,000 units of erythropoietin α, along with daily supplementation of folic acid and vitamin B12. | Renal Anemia | 12 W | None | Hb |
| YangY 2018 | China | 50, 40 |
31.2(10.5), 31.8(9.6) |
27/23, 23/17 | Prebiotic capsules(4 g) Including the following strains: Bifidobacterium longiformis,Lactobacillus bulgaricus and Streptococcus thermophilus/Bid | None | Ferrous fumarate 30.0 mg/ time, once/week | iron deficiency anemia | 8 W |
8(4 cases of nausea, 2 cases of diarrhea and 2 cases of oral malodor )/ 17(7 cases of nausea, 4 cases of diarrhea, 4 cases of constipation and 2 cases of oral malodor) |
Hb, SI |
| Haghighat N 2021 | Iran | 23,19 |
40.08(10.11), 45.47(10.76) |
12/11, 10/9 | Synbiotic(5 g) Including the following strains: Each 5 g sachet contains four probiotic strains Lactobacillus acidophilus strain T16, Bifidobacterium bifidum strain BIA-6, Bifidobacterium lactis strain BIA-7, Bifidobacterium longum strain BIA-8, each at 2.7 × 10^7 CFU/g) and 15 g of prebiotics (including 5 g fructooligosaccharides, 5 g galactooligosaccharides, and 5 g inulin)/Qid |
Placebo(5 g) Contains only excipients primarily maltodextrin, matched to the intervention supplements in appearance and taste/qid |
Thrice-weekly Hemodiafiltration | Renal Anemia | 12 W | None | Hb |
| Haghighat N 2021 | Iran | 23,19 |
46.21(11.49) 45.47(10.76) |
12/11, 10/9 | Probiotic(5 g): Each 5 g sachet contains the same four probiotic strains as the synbiotic, with an additional 15 g of placebo ingredients per three 5 g sachets/Qid |
Placebo(5 g) Contains only excipients primarily maltodextrin, matched to the intervention supplements in appearance and taste/qid |
Thrice-weekly Hemodiafiltration | Renal Anemia | 12 W | None | Hb |
| Iqbal S 2022 | Pakistan | 15, 15 | 18–25 | All female | 963 mg/kg GOS + 30 ppm FeSO4 | iron fortified flour without any prebiotics | NR | iron deficiency anemia | 12 W | NR | Hb, SI, SF |
| Li Y 2022 | China | 81, 81 |
51.15 ± 8.13 49.62 ± 8.47 |
45/36, 42/39 | DF(10 g) primarily composed of galactomannan,resistant dextrin,fructooligosaccharide, and starch/Qd | Potato starch(10 g) derived from potatoes and prepacked identically to the DF mixture, differing only in the color of the packaging/qd | NR | Renal Anemia | 8 W | NR | Hb, SF |
| Wang FZ 2022 | China | 104, 52 |
52.6(10.4), 52.2(7.7) |
40/64, 21/31 | Compound prebiotic (3 g) Including the following strains: Lactobacillus paracentriformis,Lactobacillus plantarum,Bifidobacterium youth,Bifidobacterium animalis,Fructooligosaccharides,maltodextrin,lactose,α-amylase/Tid | Control agent without added strains(3 g) Including the following: fructooligosaccharides,maltodextrin,lactose,and α-amylase/tid | Hemodiafiltration once a week and hemoperfusion once a month | Renal Anemia | 8 W | NR | Hb |
| Kooshki A 2023 | Iran | 23, 23 | 62.83(16.62),62.92(16.80) | 11/12, 10/13 | Synbiotic(400 mg) Including the following strains:Lactobacillus coagulans and fructooligosaccharides | Placebo, same size farina capsules | Thrice-weekly Hemodiafiltration | iron deficiency anemia | 8 W | NR | Hb |
Abbreviations RCT,randomized controlled trial; T,treatment group; C,control group; NR,Not reported; W,week; D,day. Hb,hemoglobin; SI,Serum iron; SF,Serum ferritin; GOS: Galacto oligosaccharides; FOS: fructo-oligosaccharides; DF: Dietary fiber; NR,not reports; Qd,Once a day; Bid,Twice a day; Tid,Three times a day; Qid,quater in die; Synbiotics,combination of probiotics and prebiotics
Risk of bias
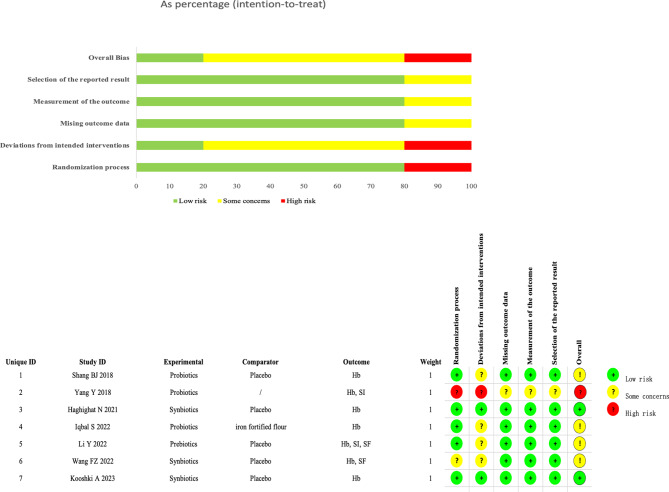
We assessed the risk of bias for the 7 articles using the Cochrane RoB 2.0. Four studies [25, 35, 36, 38] were rated as having “some concerns” due to unclear or incomplete reporting of allocation concealment and blinding. One study [37] was rated as “high risk” due to unclear blinding and allocation, while two studies [33, 34] were rated as “low risk.” Detailed risk of bias assessments is presented in Fig. 2.
Fig. 2.
Risk of Bias of RCTs
Results of the meta-analysis
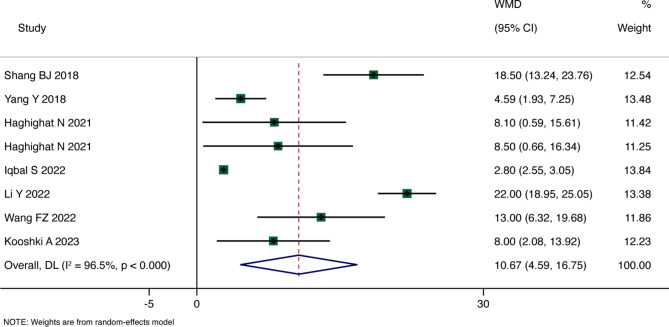
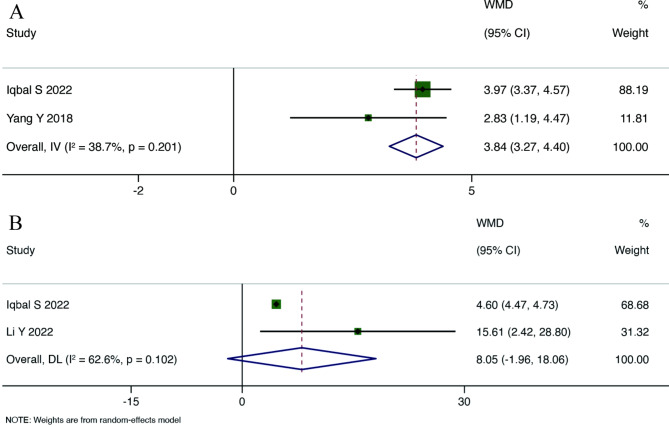
Main Results: Eight RCTs reported Hb levels. The overall WMD analyzed through a random-effects model indicated a statistically significant improvement in Hb levels among anemia patients receiving probiotics, prebiotics, or synbiotics compared to the control group (WMD = 10.760, 95% CI: 4.593 to 16.747, p = 0.001), with high heterogeneity (I²=96.5%, p < 0.001). Detailed results are shown in Fig. 3. Two RCTs reported SI levels, and the overall summary WMD analyzed through a fixed-effects model demonstrated that SI levels in the probiotic group were higher than those in the control group (WMD = 3.835; 95% CI: 3.271 to 4.400), with moderate heterogeneity (I²=38.7%, p = 0.201). Two RCTs reported SF levels, and the overall summary WMD from a random-effects model indicated no statistically significant difference between the probiotic group and the control group (WMD = 8.048, 95% CI: -1.960 to 18.056, p = 0.115), with high heterogeneity (I²=62.6%, p = 0.102), as depicted in Figs. 3 and 4.
Fig. 3.
Forest plot for Hb
Fig. 4.
Forest plot for SI (A), SF (B)
Subgroup Analysis: Due to the high heterogeneity of the primary outcome Hb (I²=96.5%), we conducted subgroup analyses based on causes of disease, duration of treatment, dosage, and probiotic type to further explore the sources of heterogeneity.
The results of the subgroup analysis by different etiologies indicated that Hb levels were higher in Anemia of CKD compared to the control group (WMD = 14.611, 95% CI: 8.847 to 20.474, p < 0.001), with high heterogeneity (I²=81.1%, p < 0.001). In IDA, Hb levels were also higher than those in the control group (WMD = 3.917, 95% CI: 1.761 to 6.073, p < 0.001), with moderate heterogeneity (I²=57.1%, p = 0.097).
Further analysis based on treatment duration revealed that at 8 weeks, Hb levels were higher in the intervention group (WMD = 11.927, 95% CI: 2.040 to 21.813, p = 0.018), with high heterogeneity (I²=95.9%, p < 0.001). Similarly, at 12 weeks, Hb levels exceeded those in the control group (WMD = 9.346, 95% CI: 0.913 to 17.780, p = 0.030), with high heterogeneity (I²=92.1%, p < 0.001).
Regarding dosage, subgroup analysis showed that Hb levels were significantly higher in the group receiving more than 5 g of probiotics (WMD = 9.800, 95% CI: 2.800 to 16.799, p = 0.006), with high heterogeneity (I²=97.0%, p < 0.001). Similarly, in the group receiving less than 5 g of probiotics, Hb levels were also higher than those in the control group (WMD = 13.341, 95% CI: 3.053 to 23.629, p = 0.011), but with moderate heterogeneity (I²=85.2%, p = 0.009). Both groups exhibited high heterogeneity, suggesting that dosage may not be the only influencing factor influencing therapeutic efficacy.
In the subgroup analysis by probiotic type, both the probiotic and synbiotic groups showed significant improvements in Hb levels (WMD = 7.917 and WMD = 9.787, respectively). Notably, the synbiotic group exhibited low heterogeneity (I²=0%), indicating more consistent efficacy. In contrast, the prebiotic group did not show significant efficacy (WMD = 12.338, 95% CI: -6.478 to 31.153, p < 0.001) and exhibited extremely high heterogeneity (I²=99.3%). This variability suggests that the effects of prebiotics may vary considerably across studies, or that their role in anemia treatment may be limited.
Based on the results of the subgroup analyses, we can conclude that the source of overall heterogeneity may stem from the different types of microbiota modulators used. More detailed results are shown in Table 2 and Supplementary Materials Fig. 4.
Table 2.
Subgroup analyses of Outcome indicators
Adverse reactions
Three studies [33, 39, 41] reported adverse reactions; four studies [25, 34, 35, 40] were not mentioned, while one study [41]reported eight cases in the probiotic group (nausea in 4 cases, diarrhea in 2 cases, oral malodor in 2 cases). No serious adverse reactions related to probiotic, prebiotic and synbiotics treatment were observed.
Sensitivity analysis and publication bias
We performed sensitivity analysis and publication bias assessment on the meta-analysis results, which indicated stability. The results of the Begg’s and Egger’s tests showed no significant publication bias Supplementary Materials Figs. 2 and 3.
Grade
In this study, the quality of evidence for Hb levels was rated as moderate, while SI and SF were rated as low quality. Detailed reasons are shown in Table 3.
Table 3.
Summary of methodological quality of evidence according to GRADE
| Certainty assessment | No of patients | Effect | Certainty | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Experimental | Placebo | Relative (95% CI) |
Absolute (95% CI) |
|
| Hemoglobin | |||||||||||
| 8 | randomised trials | not serious | seriousa | not seriousb | not serious | none | 351 | 281 | - |
WMD 10.76 0 higher (4.593 higher to 16.747 higher) |
⨁⨁⨁◯ Moderatea, b |
| Serum iron | |||||||||||
| 2 | randomised trials | very seriousc, d | not seriouse | not seriousb | not serious | none | 65 | 55 | - |
WMD 3.835 higher (3.271 higher to 4.4 higher) |
⨁⨁◯◯ Lowb, c,d, e |
| Serum ferritin | |||||||||||
| 2 | randomised trials | seriousc | seriousa | not seriousb | not serious | none | 96 | 96 | - |
WMD 8.048 higher (1.96 lower to 18.056 higher) |
⨁⨁◯◯ Lowa, b,c |
CI: confidence interval; WMD: weighted mean difference
GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate
Very low quality: We are very uncertain about the estimate
Explanations
a. The overall sample is small and the I2 is large (> 50%)
b. No indirectness of evidence was found in any study
c. level for small sample size and wide confdence interval
d. There were serious problems with the randomization process and blinding
e. I2 is small (< 50%)
Discussion
This meta-analysis summarized the results of eight RCTs, showing that probiotics, prebiotics, and synbiotics significantly improved Hb levels in patients with anemia. While sensitivity analysis indicated stable results, subgroup analysis revealed that the high heterogeneity might be attributed to different microbiota modulators. Additionally, the variation in diagnostic criteria, dosage, and the limited number of included studies may also contribute to the observed heterogeneity. The high heterogeneity and the small sample size, along with varying treatment modalities, resulted in moderate-quality evidence for Hb improvement and low-quality evidence for iron metabolism markers.
Probiotics have been shown to influence various aspects of human health, including iron metabolism [42]. Iron is a crucial micronutrient involved in numerous physiological processes, and its deficiency can lead to anemia and other health issues, It is well known that iron deficiency alters gut microbiota composition and increases the risk of intestinal infections [43]. Recent studies have demonstrated that certain probiotics, such as Lactobacillus acidophilus and Bifidobacterium longum, can improve iron absorption and influence the course of anemia [44]. Garcés et al. 2018. showed [45] that Lactobacillus fermentum can survive the gastric environment and internalize into intestinal cells, delivering iron oxide nanoparticles and supplying adequate iron levels to these cells. This cooperation offers a novel method to enhance iron absorption. Moreover, prebiotics like galacto-oligosaccharides and fructo-oligosaccharides can enhance iron bioavailability and mitigate the harmful effects of iron on the gut microbiome [31]. However, the current evidence remains insufficient to support clinical applications [46, 47]. Animal experiments have shown that prebiotics can enhance iron absorption in iron-deficient growing rats, possibly through regulating protein expression and the gut microbiome [48]. A study on Kenyan infants supplemented with prebiotics [49] also found that prebiotics improved iron absorption and reduced the adverse effects of iron on the gut microbiome and inflammation. Lactobacillus fermentum produces p-hydroxyphenyllactic acid, which reduces ferric iron (Fe(III)) to ferrous iron (Fe(II)), enhancing iron absorption through Divalent Metal Transporter 1 channels in enterocytes [50], Multispecies probiotics have been shown to improve iron bioavailability and enhance duodenal iron absorption. Notably, they exert a dose-independent effect on the shift of iron from serum, while also promoting a dose-dependent increase in pancreatic and liver iron uptake [51, 52]. When combined with iron supplementation, probiotics may be more effective at increasing duodenal iron absorption and tissue iron stores compared to iron supplementation alone [53]. Probiotics such as Lactobacillus acidophilus and Bifidobacterium longum have also been demonstrated to enhance iron absorption, thus positively influencing anemia treatment [13, 54]. Importantly, probiotic supplementation does not disrupt the biochemical and hepatic regulatory processes of iron balance. Instead, it helps maintain homeostasis of key iron transporters and regulatory proteins, such as hepcidin, ferritin, and transferrin receptors [42, 55].
Probiotics, prebiotics, and synbiotics have potential roles in improving clinical outcomes in patients with anemia, with a particular focus on their regulatory effects on gastrointestinal factors. Synbiotics have demonstrated more pronounced efficacy, potentially influencing the pathophysiology of anemia through various mechanisms. These mechanisms include enhancing gut microbiota balance, reducing levels of pro-inflammatory cytokines (such as IL-6, IL-8, IL-17a, and IFN-γ), and decreasing the accumulation of uremic toxins (such as indoxyl sulfate and blood urea nitrogen) [56, 57]. Current literature also suggests that synbiotics effectively alleviate abdominal discomfort and improve systemic inflammation. These effects may contribute to enhanced intestinal absorption of iron and other nutrients, thereby further ameliorating anemia [58].
In patients with kidney disease, factors like long-term dietary control, weakened immunity, and chronic antibiotic use contribute to dysbiosis of the gut microbiota [59, 60]. Anemia in these patients results from decreased erythropoietin production, chronic inflammation, iron homeostasis disorders, reduced red blood cell lifespan due to uremic toxins, and disruption of the hematopoietic microenvironment [61]. Inflammation is considered one of the major factors affecting Hb fluctuations in patients with CKD [62–64]. Pro-inflammatory cytokine production during inflammation not only inhibits erythropoiesis but also reduces the response of bone marrow erythroid progenitor cells to erythropoietin [65–67]. Anemia of CKD is a common complication in patients with CKD and ESRD [68]. It is frequently associated with chronic inflammation and the accumulation of uremic toxins. Probiotics and prebiotics have been explored as potential therapeutic interventions to modulate gut microbiota, reduce inflammation, and improve clinical outcomes in these patients [58]. Some studies indicate that prebiotics, probiotics, and synbiotics can reduce inflammatory markers such as C-reactive protein, interleukin-6 (IL-6), and uremic toxins like indoxyl sulfate and p-cresyl sulfate in CKD and ESRD patients [69–71]. Prebiotics have also been shown to be particularly effective in reducing IL-6, tumor necrosis factor-alpha (TNF-α), IS, blood urea nitrogen, and malondialdehyde [69]. Furthermore, synbiotic interventions have been found to significantly increase beneficial gut bacteria like Bifidobacterium, which may contribute to improved gut health and reduced uremic toxin levels [72].
Despite these promising results, a meta-analysis reached a contrary conclusion, showing that probiotics and synbiotics did not improve inflammatory markers in patients with renal failure, indicating that current evidence is still insufficient. However, probiotics, prebiotics, and synbiotics have shown potential in improving renal function parameters such as serum creatinine, BUN, and cystatin C in patients with diabetic kidney disease [73]. These supplements also appear to reduce oxidative stress and improve lipid profiles, which are beneficial for overall renal health. However, the overall quality of the studies remains variable, and further large-scale, well-designed randomized controlled trials are needed to confirm their safety and efficacy in managing Anemia of CKD and improving renal function [74].
In addition, certain probiotic strains, such as Lactobacillus acidophilus,Bifidobacterium bifidum,Bifidobacterium lactis, and Bifidobacterium longum, have been shown to effectively reduce inflammation markers and exhibit anti-inflammatory properties [75, 76]. However, the aforementioned meta-analysis indicated that probiotics and synbiotics did not improve inflammatory markers in patients with renal failure, further illustrating the need for additional, robust clinical evidence [77].
Li, Yang et al. [35] found that dietary fiber improves Anemia of CKD by regulating the gut microbiota and their metabolites, short-chain fatty acids, suggesting a potential reduction in EPO consumption. This trend could also result from improved iron metabolism or the effects of prebiotics and their metabolites on erythropoietin receptors [78]. A recent study [79] suggested no significant association between dietary fiber intake and biomarkers related to iron absorption/status, but the evidence supports a positive role for dietary fiber in enhancing the bioavailability of dietary iron and drug absorption. Given the complexity of iron metabolism in vivo and the variability in study designs, we cannot overlook the potential role of prebiotics and probiotics in improving iron metabolism, particularly in regulating gut bacterial biomarkers. In conclusion, due to the limited clinical studies and the small overall sample size, in line with the findings of the RCTs included in this review, further research is needed to clarify whether different strains of probiotics or different prebiotic components, and their combinations in synbiotics, have varying impacts on anemia improvement. The studies included in this review highlight the need for large sample sizes and high-quality clinical evidence to more clearly elucidate the efficacy and safety of probiotics, prebiotics and synbiotics in treating anemia.
Limitations and strengths
There are several limitations to this systematic review. First, the number of clinical studies is limited. We included only eight RCTs, involving 632 participants, and conducted meta-analyses on one primary outcome (Hb levels) and two iron metabolism markers (SI and SF). Both Hb and SF outcomes exhibited high heterogeneity. Although subgroup analyses indicated that the types of probiotics and prebiotics might be sources of heterogeneity, the sensitivity analysis of Hb levels was stable. Nevertheless, the high heterogeneity suggests that results should be interpreted with caution. Furthermore, the combined effect of synbiotics in clinical applications did not show greater efficacy compared to probiotics or prebiotics alone.
Our study also has strengths. It is the first meta-analysis and systematic review to clarify the efficacy and safety of probiotics and prebiotics in patients with anemia, providing valuable evidence for evidence-based medicine. We also addressed the limitations of the included studies. Although prebiotics alone did not show significant effects, their combination with probiotics demonstrated notable benefits for patients with anemia. Future research should include high-quality randomized controlled trials to explore the specific role of synbiotics in anemia, particularly their impact on gastrointestinal factors, such as modulating gut microbiota balance and reducing inflammation, and compare their efficacy with probiotics or prebiotics alone. This will help optimize clinical intervention strategies and determine the appropriate dosage and combination of these treatments. Moreover, investigating the interaction between these microbiota modulators and traditional anemia treatments is another important direction for future research.
Conclusion
This systematic review suggests that probiotics, prebiotics, and synbiotics may improve Hb levels and iron metabolism markers in individuals with anemia, based on findings from clinical studies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
QH and YL were responsible for the literature screening and data extraction. SY and FYZ were responsible for risk of bias assessment. YMF, JPZ, and HZ were responsible for statistical analysis and writing up the article. FYZ were responsible for planning and guidance on this paper. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The study was funded by the Acupuncture Neuroscience and Artificial Intelligence Innovation Research Team (ZG-KY-2023-026) and the Traditional Chinese Medicine and Artificial Intelligence Core Faculty Teaching Team (ZG-JX-2023-014).
Data availability
The data are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dunaif GE, Campbell TC. Relative contribution of dietary protein level and aflatoxin B1 dose in generation of presumptive preneoplastic foci in rat liver. J Natl Cancer Inst. 1987;78(2):365–9. [PubMed] [Google Scholar]
- 2.Chaparro CM, Suchdev PS. Anemia epidemiology, pathophysiology, and etiology in low- and middle-income countries. Ann N Y Acad Sci. 2019;1450(1):15–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Milovanovic T, et al. Anemia as a Problem: GP Approach. Dig Dis. 2022;40(3):370–5. [DOI] [PubMed] [Google Scholar]
- 4.Balarajan Y, et al. Anaemia in low-income and middle-income countries. Lancet. 2011;378(9809):2123–35. [DOI] [PubMed] [Google Scholar]
- 5.Stevens GA, et al. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: a systematic analysis of population-representative data. Lancet Glob Health. 2013;1(1):e16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inker LA, et al. Relationship of estimated GFR and Albuminuria to Concurrent Laboratory abnormalities: an Individual Participant Data Meta-Analysis in a Global Consortium. Am J Kidney Dis. 2019;73(2):206–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ku E, et al. Novel anemia therapies in chronic kidney disease: conclusions from a kidney disease: improving global outcomes (KDIGO) Controversies Conference. Kidney Int. 2023;104(4):655–80. [DOI] [PubMed] [Google Scholar]
- 8.Tonelli M, Thadhani R. Anaemia in chronic kidney disease: what do new generation agents offer? Lancet. 2022;399(10326):702–3. [DOI] [PubMed] [Google Scholar]
- 9.Camaschella C. Iron-deficiency anemia. N Engl J Med. 2015;372(19):1832–43. [DOI] [PubMed] [Google Scholar]
- 10.Camaschella C. New insights into iron deficiency and iron deficiency anemia. Blood Rev. 2017;31(4):225–33. [DOI] [PubMed] [Google Scholar]
- 11.Sunuwar DR, et al. Prevalence and factors associated with anemia among women of reproductive age in seven South and Southeast Asian countries: evidence from nationally representative surveys. PLoS ONE. 2020;15(8):e0236449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sunuwar DR, et al. Factors associated with anemia among children in South and Southeast Asia: a multilevel analysis. BMC Public Health. 2023;23(1):343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vonderheid SC et al. A systematic review and Meta-analysis on the effects of Probiotic species on Iron absorption and Iron status. Nutrients, 2019. 11(12). [DOI] [PMC free article] [PubMed]
- 14.Bach V, et al. Prevalence and possible causes of anemia in the elderly: a cross-sectional analysis of a large European university hospital cohort. Clin Interv Aging. 2014;9:1187–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beghé C, Wilson A, Ershler WB. Prevalence and outcomes of anemia in geriatrics: a systematic review of the literature. Am J Med. 2004;116(Suppl 7A):ps3–s10. [DOI] [PubMed] [Google Scholar]
- 16.Styszynski A, et al. Prevalence of anemia in relation to socio-economic factors in elderly Polish population: the results of PolSenior study. J Physiol Pharmacol. 2018;69(1):75–81. [DOI] [PubMed] [Google Scholar]
- 17.Khovasova NO, et al. [The prevalence of anemia and its associations with other geriatric syndromes in subjects over 65 years old: data of Russian epidemiological study EVKALIPT]. Ter Arkh. 2022;94(1):24–31. [DOI] [PubMed] [Google Scholar]
- 18.Cristofori F, et al. Anti-inflammatory and Immunomodulatory effects of Probiotics in Gut inflammation: a door to the body. Front Immunol. 2021;12:578386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isolauri E, et al. Probiotics: effects on immunity. Am J Clin Nutr. 2001;73(2 Suppl):s444–50. [DOI] [PubMed] [Google Scholar]
- 20.Bungau SG et al. Targeting Probiotics in Rheumatoid Arthritis. Nutrients, 2021. 13(10). [DOI] [PMC free article] [PubMed]
- 21.Ferolla SM, et al. Probiotics as a complementary therapeutic approach in nonalcoholic fatty liver disease. World J Hepatol. 2015;7(3):559–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamed Riveros NF, et al. Effect of Bifidobacterium Intake on Body Weight and Body Fat in overweight and obese adult subjects: a systematic review and Meta-analysis. J Am Nutr Assoc. 2024;43(6):519–31. [DOI] [PubMed] [Google Scholar]
- 23.Shadnoush M, et al. Probiotic yogurt affects Pro- and anti-inflammatory factors in patients with inflammatory bowel disease. Iran J Pharm Res. 2013;12(4):929–36. [PMC free article] [PubMed] [Google Scholar]
- 24.Sanaie S, et al. Effect of a multispecies probiotic on inflammatory markers in critically ill patients: a randomized, double-blind, placebo-controlled trial. J Res Med Sci. 2014;19(9):827–33. [PMC free article] [PubMed] [Google Scholar]
- 25.Iqbal S, et al. Effect of inulin, galacto oligosaccharides and iron fortification on iron deficiency anemia among women of reproductive age; a randomized controlled trial. Front Nutr. 2022;9:1028956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ashaolu TJ, Ashaolu JO, Adeyeye SAO. Fermentation of prebiotics by human colonic microbiota in vitro and short-chain fatty acids production: a critical review. J Appl Microbiol. 2021;130(3):677–87. [DOI] [PubMed] [Google Scholar]
- 27.Carvalho L et al. Partially hydrolyzed guar gum increases Ferroportin expression in the Colon of anemic growing rats. Nutrients, 2017. 9(3).
- 28.Marciano R, et al. Effects of prebiotic supplementation on the expression of proteins regulating iron absorption in anaemic growing rats. Br J Nutr. 2015;113(6):901–8. [DOI] [PubMed] [Google Scholar]
- 29.Momo Cabrera P, et al. Comparative prebiotic potential of galacto- and fructo-oligosaccharides, native inulin, and acacia gum in Kenyan infant gut microbiota during iron supplementation. ISME Commun. 2024;4(1):ycae033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paganini D, et al. Prebiotic galacto-oligosaccharides mitigate the adverse effects of iron fortification on the gut microbiome: a randomised controlled study in Kenyan infants. Gut. 2017;66(11):1956–67. [DOI] [PubMed] [Google Scholar]
- 31.Zakrzewska Z et al. Prebiotics, Probiotics, and Postbiotics in the Prevention and Treatment of Anemia. Microorganisms, 2022. 10(7). [DOI] [PMC free article] [PubMed]
- 32.Page MJ, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haghighat N, et al. The Effect of Synbiotic and Probiotic supplementation on Mental Health parameters in patients undergoing hemodialysis: a Double-blind, randomized, placebo-controlled trial. Indian J Nephrol. 2021;31(2):149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kooshki A, et al. Synbiotic supplement for treatment of iron deficiency anaemia in haemodialysis patients: a randomized controlled trial. Nephrol (Carlton). 2023;28(4):234–9. [DOI] [PubMed] [Google Scholar]
- 35.Li Y, et al. The prebiotic effects of soluble dietary fiber mixture on renal anemia and the gut microbiota in end-stage renal disease patients on maintenance hemodialysis: a prospective, randomized, placebo-controlled study. J Transl Med. 2022;20(1):599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shang B, Tang L. Effect of probiotics on hemoglobin in hemodialysis patients with chronic renal failure: a randomized clinical trial. Chin J Integr Traditional Western Nephrol. 2018;19(10):894–6. [Google Scholar]
- 37.Yang Y. Efficacy of probiotics combined with ferrous fumarate in the treatment of iron deficiency anemia. China Practical Med. 2018;13(23):103–5. [Google Scholar]
- 38.Wang F, et al. Application effect of probiotics in hemodialysis patients. China Mod Med. 2022;29(31):112–6. [Google Scholar]
- 39.Shang B, Tang L. A randomized clinical study of the effect of probiotics on hemoglobin in hemodialysis patients with chronic renal failure. Chin J Integr Nephrop. 2018;19(10):894–6. [Google Scholar]
- 40.Wang F, et al. Application effect of probiotics in hemodialysis patients. Chin Contemp Med. 2022;29(31):112–6. [Google Scholar]
- 41.Yang Y. Therapeutic effect of probiotics combined with ferrous fumarate on iron deficiency anemia. Chin Practical Med. 2018;13(23):103–5. [Google Scholar]
- 42.Rusu IG et al. Iron supplementation influence on the Gut Microbiota and Probiotic Intake Effect in Iron Deficiency-A Literature-based review. Nutrients, 2020. 12(7). [DOI] [PMC free article] [PubMed]
- 43.Hadadi N, et al. Intestinal microbiota as a route for micronutrient bioavailability. Curr Opin Endocr Metab Res. 2021;20:100285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Husmann FMD, Zimmermann MB, Herter-Aeberli I. The Effect of Prebiotics on Human Iron absorption: a review. Adv Nutr. 2022;13(6):2296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garcés V, et al. Bacteria-carried Iron oxide nanoparticles for treatment of Anemia. Bioconjug Chem. 2018;29(5):1785–91. [DOI] [PubMed] [Google Scholar]
- 46.Rosen GM, et al. Use of a probiotic to enhance Iron absorption in a Randomized Trial of Pediatric patients presenting with Iron Deficiency. J Pediatr. 2019;207:192–e1971. [DOI] [PubMed] [Google Scholar]
- 47.Meléndez-Illanes L, et al. Does the scientific evidence support the advertising claims made for products containing Lactobacillus casei and Bifidobacterium lactis? A systematic review. J Public Health (Oxf). 2016;38(3):e375–83. [DOI] [PubMed] [Google Scholar]
- 48.Chen JH, et al. Prebiotic oligosaccharides enhance Iron absorption Via Modulation of protein expression and gut microbiota in a dose-response manner in Iron-Deficient growing rats. Mol Nutr Food Res. 2022;66(10):e2101064. [DOI] [PubMed] [Google Scholar]
- 49.Mikulic N, et al. Prebiotics increase iron absorption and reduce the adverse effects of iron on the gut microbiome and inflammation: a randomized controlled trial using iron stable isotopes in Kenyan infants. Am J Clin Nutr. 2024;119(2):456–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.González A, et al. Identification of the key excreted molecule by Lactobacillus fermentum related to host iron absorption. Food Chem. 2017;228:374–80. [DOI] [PubMed] [Google Scholar]
- 51.Skrypnik K, et al. The Effect of multispecies Probiotic supplementation on Iron Status in rats. Biol Trace Elem Res. 2019;192(2):234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Skrypnik K et al. Hepcidin and Erythroferrone correlate with hepatic Iron transporters in rats supplemented with multispecies Probiotics. Molecules, 2020. 25(7). [DOI] [PMC free article] [PubMed]
- 53.Skrypnik K, et al. Influence of multistrain probiotic and iron supplementation on iron status in rats. J Trace Elem Med Biol. 2021;68:126849. [DOI] [PubMed] [Google Scholar]
- 54.Axling U et al. The effect of Lactobacillus plantarum 299v on Iron Status and physical performance in female Iron-deficient athletes: a Randomized Controlled Trial. Nutrients, 2020. 12(5). [DOI] [PMC free article] [PubMed]
- 55.Skrypnik K, et al. The effect of multistrain probiotic supplementation in two doses on iron metabolism in obese postmenopausal women: a randomized trial. Food Funct. 2019;10(8):5228–38. [DOI] [PubMed] [Google Scholar]
- 56.Neyrinck AM, et al. Improvement of gastrointestinal discomfort and inflammatory status by a synbiotic in middle-aged adults: a double-blind randomized placebo-controlled trial. Sci Rep. 2021;11(1):2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rauch CE, et al. Effect of prebiotics, probiotics, and synbiotics on gastrointestinal outcomes in healthy adults and active adults at rest and in response to exercise-A systematic literature review. Front Nutr. 2022;9:1003620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu Z, et al. Probiotics, Prebiotics, and Synbiotics improve Uremic, Inflammatory, and gastrointestinal symptoms in end-stage renal Disease with Dialysis: A Network Meta-Analysis of Randomized controlled trials. Front Nutr. 2022;9:850425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koppe L, Mafra D, Fouque D. Probiotics and chronic kidney disease. Kidney Int. 2015;88(5):958–66. [DOI] [PubMed] [Google Scholar]
- 60.Vitetta L, Gobe G. Uremia and chronic kidney disease: the role of the gut microflora and therapies with pro- and prebiotics. Mol Nutr Food Res. 2013;57(5):824–32. [DOI] [PubMed] [Google Scholar]
- 61.Li D, et al. What should be responsible for eryptosis in chronic kidney disease? Kidney Blood Press Res. 2022;47(6):375–90. [DOI] [PubMed] [Google Scholar]
- 62.Begum S, Latunde-Dada GO. Anemia of inflammation with an emphasis on chronic kidney disease. Nutrients, 2019. 11(10). [DOI] [PMC free article] [PubMed]
- 63.Kim YH, et al. The estimated mediating roles of anemia-related variables in the association between kidney function and mortality: a National Health and Nutrition Examination Survey (NHANES) study. Sci Rep. 2024;14(1):6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ueda N, Takasawa K. Impact of inflammation on Ferritin, Hepcidin and the management of Iron Deficiency Anemia in chronic kidney disease. Nutrients, 2018. 10(9). [DOI] [PMC free article] [PubMed]
- 65.Morceau F, Dicato M, Diederich M. Pro-inflammatory cytokine-mediated anemia: regarding molecular mechanisms of erythropoiesis. Mediators Inflamm. 2009;2009:405016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nairz M, et al. The pleiotropic effects of erythropoietin in infection and inflammation. Microbes Infect. 2012;14(3):238–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Paulson RF et al. Stress erythropoiesis is a key inflammatory response. Cells, 2020. 9(3). [DOI] [PMC free article] [PubMed]
- 68.Hanna RM, Streja E, Kalantar-Zadeh K. Burden of Anemia in chronic kidney disease: beyond erythropoietin. Adv Ther. 2021;38(1):52–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nguyen TTU, Kim HW, Kim W. Effects of Probiotics, Prebiotics, and Synbiotics on Uremic Toxins, Inflammation, and Oxidative Stress in Hemodialysis Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J Clin Med, 2021. 10(19). [DOI] [PMC free article] [PubMed]
- 70.Borges NA, et al. Probiotic supplementation in chronic kidney disease: a Double-blind, randomized, placebo-controlled trial. J Ren Nutr. 2018;28(1):28–36. [DOI] [PubMed] [Google Scholar]
- 71.Chen C, et al. Probiotics, Prebiotics, and Synbiotics for patients on Dialysis: a systematic review and Meta-analysis of Randomized controlled trials. J Ren Nutr. 2023;33(1):126–39. [DOI] [PubMed] [Google Scholar]
- 72.Lopes R, et al. Modulation of intestinal microbiota, control of nitrogen products and inflammation by pre/probiotics in chronic kidney disease: a systematic review. Nutr Hosp. 2018;35(3):722–30. [DOI] [PubMed] [Google Scholar]
- 73.Dai Y, et al. Probiotics improve renal function, glucose, lipids, inflammation and oxidative stress in diabetic kidney disease: a systematic review and meta-analysis. Ren Fail. 2022;44(1):862–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McFarlane C, et al. Prebiotic, probiotic, and Synbiotic supplementation in chronic kidney disease: a systematic review and Meta-analysis. J Ren Nutr. 2019;29(3):209–20. [DOI] [PubMed] [Google Scholar]
- 75.Peran L, et al. A comparative study of the preventative effects exerted by three probiotics, Bifidobacterium lactis, Lactobacillus casei and Lactobacillus acidophilus, in the TNBS model of rat colitis. J Appl Microbiol. 2007;103(4):836–44. [DOI] [PubMed] [Google Scholar]
- 76.Saez-Lara MJ et al. The role of probiotic lactic acid bacteria and bifidobacteria in the prevention and treatment of inflammatory bowel disease and other related diseases: a systematic review of randomized human clinical trials. Biomed Res Int, 2015. 2015: p. 505878. [DOI] [PMC free article] [PubMed]
- 77.Kazemi A, et al. Effect of probiotic and synbiotic supplementation on inflammatory markers in health and disease status: a systematic review and meta-analysis of clinical trials. Clin Nutr. 2020;39(3):789–819. [DOI] [PubMed] [Google Scholar]
- 78.Caiado F, Manz MG. A microbiome-macrophage-iron axis guides stressed hematopoietic stem cell fate. Cell Stem Cell. 2022;29(2):177–9. [DOI] [PubMed] [Google Scholar]
- 79.Agrizzi Verediano T, et al. Effects of dietary fiber on intestinal iron absorption, and physiological status: a systematic review of in vivo and clinical studies. Crit Rev Food Sci Nutr. 2023;63(27):9017–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are available from the corresponding author on reasonable request.