Abstract
Background and objectives
Systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) are complex autoimmune diseases that present with a range of systemic and oral manifestations including dental decay and alterations in the oral microbiome profile. The purpose of this study was to compare the fungal and bacterial profile of supragingival dental plaque and dental caries in patients with SLE and RA.
Methods
The present descriptive-cross-sectional-analytical study was conducted on 91 participants (31 RA, 30 lupus, and 30 control groups). Decayed, Missing, and Filled teeth (DMFT) and The International Caries Detection Assessment System (ICDAS) indices were used to investigate tooth decay. The DAS-28 index was used to assess the severity of RA, and the SLEDAI-2 K index was used to evaluate the severity of lupus. The number of supragingival dental plaque Streptococcus mutans, Lactobacillus spp. and Candida albicans colonies were evaluated using Mitis Salivarius Agar (MSA), deMan-Rogosa-Sharpe agar (MRS) and Sabouraud Dextrose Agar with Chloramphenicol (SC) culture medium, respectively. Data were analyzed using one-way ANOVA, Kruskal -Wallis, k2, Fisher’s tests, and Spearman’s correlation coefficient.
Results
A statistically significant relationship was observed between the education level (P = 0.030), mean of DMFT, ICDAS, MSA and SC indices (P < 0.001) with the type of disease. The control and RA group showed significantly higher MRS index than the lupus group (P < 0.001). There was significant and positive correlation between the severity of the disease in lupus patients and SC (Candida albicans) (P < 0.001, R = 0.698) and MRS (P = 0/020, R = 0.408) indices.
Conclusion
Dental decay and the fungal and bacterial flora of supragingival dental plaque patients are higher than in the healthy group. It is recommended that these patients pay more attention to their oral hygiene and undergo periodic oral examinations.
Keywords: Lupus erythematosus, Rheumatoid arthritis, Tooth decay, Candida albicans, Streptococcus mutans, Lactobacillus spp.
Introduction
Systemic lupus erythematosus (SLE) is a complex, multifactorial autoimmune disorder influenced by both genetic and environmental factors [1]. Gender significantly affects susceptibility to this condition, with a prevalence ratio of 8:1 favoring females over males [2]. The precise etiology of lupus remains inadequately understood. Additionally, the role of bacteria has been implicated in the development of SLE, as their products, such as lipopolysaccharides and nucleic acids, can activate the immune system response. This activation occurs through interactions with Toll-like receptors, which subsequently stimulate B cells, T cells, and antigen-presenting cells, leading to the secretion of pro-inflammatory cytokines and the production of antibodies [3]. The mortality rate associated with SLE is approximately three to five times higher than that of the general population, primarily due to chronic inflammatory processes [4]. SLE is characterized by various systemic manifestations, as well as distinct oral lesions including reduced salivary flow rate, salivary glands dysfunction, halitosis and increasing in the prevalence of dental caries which has been corroborated by multiple independent clinical studies [5–7].
Rheumatoid arthritis (RA) represents another systemic autoimmune disorder characterized by chronic systemic inflammation of unknown etiology and is marked by synovial hyperplasia, persistent inflammation, and joint swelling, which can lead to alterations in organ morphology and function. RA is also multifactorial, with genetic and environmental factors contributing to its pathogenesis. Recognized environmental risk factors include obesity, dietary habits, smoking, infections, and the microbiota [8]. Compelling evidence suggests a potential association between the immune response to oral infections and the onset of RA, mediated by enzymes produced by oral pathogens [9].
This disease is frequently linked to oral manifestations, including temporomandibular joint disorders and xerostomia. Xerostomia is a significant contributor to the development of dental caries, as saliva plays a crucial role in caries prevention. Saliva possesses substantial buffering capacity and antimicrobial properties, and maintains the balance between enamel demineralization and remineralization [10]. Oral lesions, such as angular cheilitis, oral candidiasis, lichen planus-like lesions and leukoplakia are also more prevalent among patients with RA, adversely impacting both their systemic health and quality of life. Patients with RA may struggle to maintain proper oral hygiene due to physical limitations, particularly in wrist and finger joint mobility, which increases their susceptibility to periodontal disease and dental caries [11–13]. Dental caries is a multifactorial condition influenced by various factors, including microbial activity [14]. The World Health Organization’s Global Oral Health Status Report highlights the urgent need for action regarding the alarming state of global oral health, with approximately 2 billion individuals affected by caries in permanent teeth and 514 million children suffering from caries in primary teeth [15]. Streptococcus mutans and Lactobacillus spp. species are known to form biofilms that contribute to caries development [16, 17]. Although the prevalence of caries is primarily associated with elevated levels of Streptococcus mutans and Lactobacillus in dental plaque and saliva, Candida species are also implicated in caries development [18]. Candida albicans (C. Albicans) can act as an opportunistic pathogen, potentially causing caries in immunocompromised individuals and in healthy individuals through the use of medical devices and dental implants [19]. The colonization rate of Candida species in healthy individuals ranges from 20 to 40%, while it becomes the predominant flora in over 60% of immunocompromised individuals [20].
The existing literature regarding the oral health conditions and the composition of supragingival dental plaque microbiome in patients diagnosed with SLE and RA is very limited. Some studies have indicated a correlation between the oral microbiota, specifically salivary levels of Streptococcus mutans and Lactobacillus, and the presence of RA and lupus [5, 7, 21–24]. Additionally, a small number of investigations have explored the relationship between salivary and subgingival plaque levels of Candida albicans and these autoimmune diseases [25, 26]. Due to the lack of comprehensive and consistent data regarding the association between the supragingival dental plaque microbiome and certain autoimmune diseases, the aim of the current study was to analyze and compare the fungal and bacterial profiles present in supragingival dental plaque and dental caries between SLE, RA patients and healthy individuals. The underlying hypothesis of this investigation is that there may be positive correlation between the levels of cariogenic microbiome in supragingival dental plaque, the incidence of dental caries and these autoimmune diseases.
Method and materials
Study design and participants
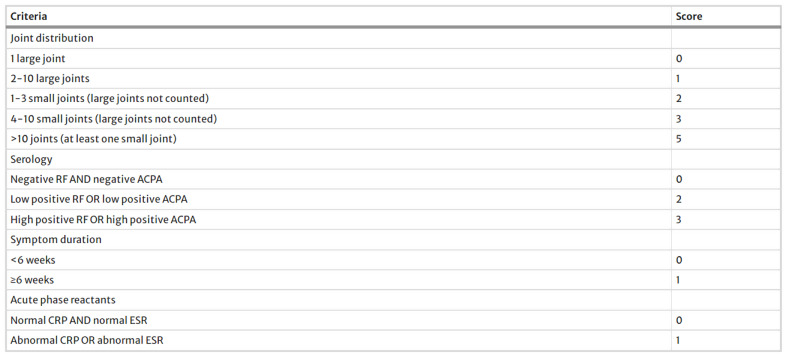
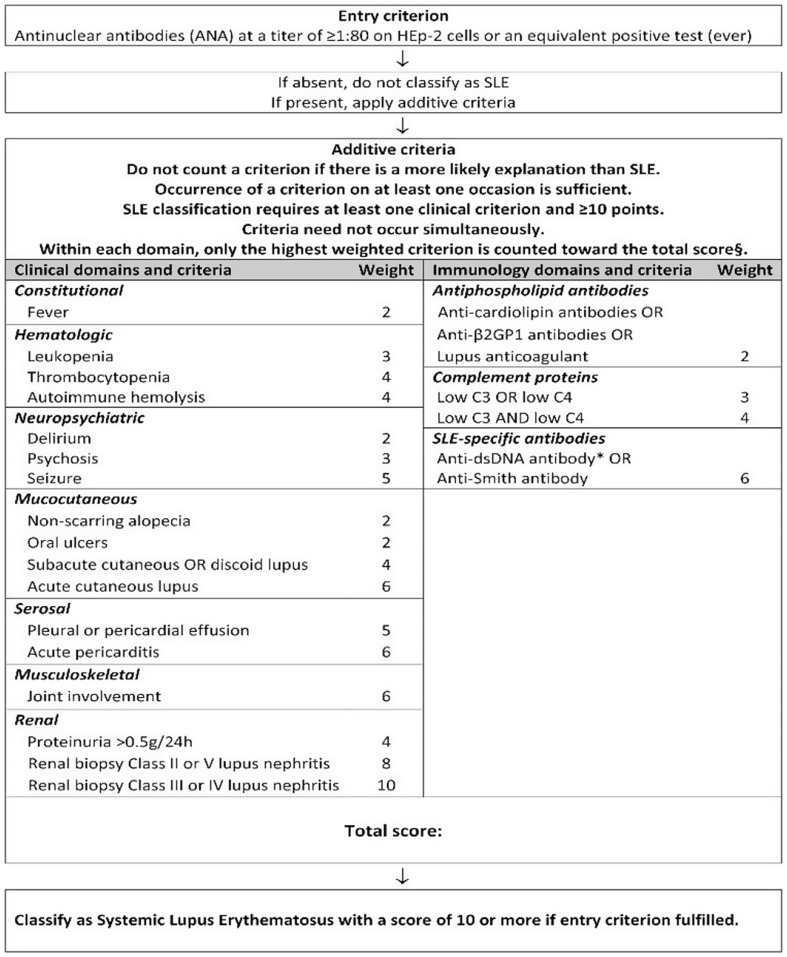
The present cross-sectional-descriptive study was conducted in 2024 with the ethics committee code IR.MUQ.REC.1402.077 at Shahid Beheshti Hospital, Qom, Iran. The diagnosis of rheumatoid arthritis was carried out by a rheumatologist, people who fulfilled the American and European Society of Rheumatologists (EULAR/ACR) 2010 criteria were enrolled (Fig. 1). 28 Disease Activity Score (DAS-28) index was used to determine the disease severity and activity of RA in recovering patients [27]. In this way, it was divided into four categories: The illness was considered in the remission mode if DAS28 < 3.2, and moderate if 3.2 ≤ DAS28 ≤ 5.1. In addition, the disease activity was considered high if DAS28 > 5.1 [28]. DAS-28 was calculated using an online DAS-Score calculator based on four criteria, including the number of tender joints, the number of swollen joints, the ESR blood level, and the visual analog scale (Available from: www.das-score.nl). The improved version of SLEDAI (SLE Disease Activity Index), called SLEDAI-2 K, was used to evaluate the activity and severity of lupus disease (Fig. 2) [29]. The sample size was calculated based on the 80% power and 0.05 alpha and participants were selected from three groups. A total of 91 volunteers were included in the study. They were selected by stratified convenience sampling [30]. Inclusion criteria were people over the age of 18 who, based on clinical evidence and diagnostic criteria, have been definitively diagnosed with RA and lupus erythematosus for at least 12 months by the rheumatologist. The control group comprised of healthy volunteers over the age of 18 who visited the vaccination clinic to have their children vaccinated. Their overall health status in periodic checkup was validated by qualified internal medicine specialists at the hospital. People who brush their teeth at least twice a day use dental floss at least once daily and participants with at least one first permanent molar in the oral cavity.
Fig. 1.
2010 ACR/EULAR classification criteria for rheumatoid arthritis
Fig. 2.
2019 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Systemic Lupus Erythematosus
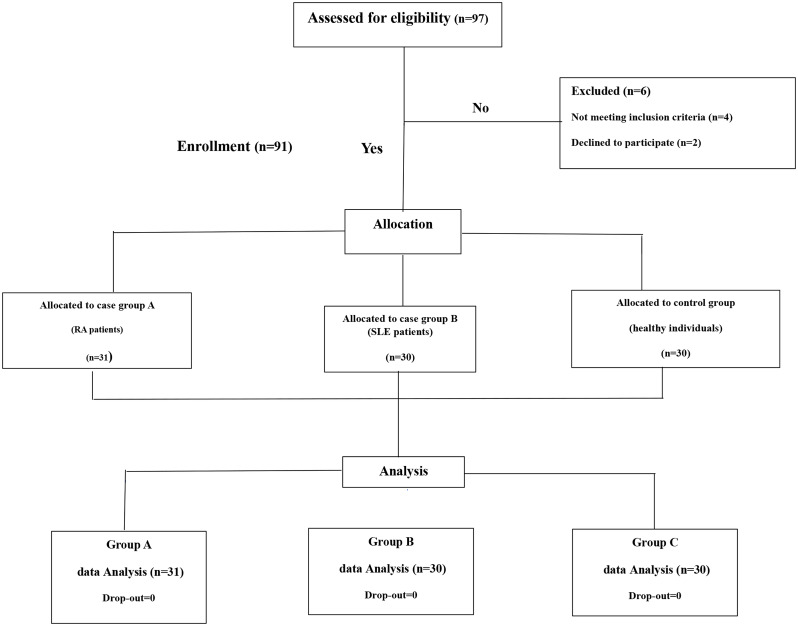
Exclusion criteria were the presence of any other systemic or autoimmune diseases except RA and systemic lupus erythematosus, inflammatory conditions, active infection, participants presenting < 8 teeth in their mouth, endocrine disorders or malignancies, pregnancy, the use of any systemic and topical antibiotics and antifungals during the past month, individuals underwent periodontal therapy or tooth cleansing within the last 6 months before sample collection and smoking. Study groups were matched based on age, gender. Participants were selected based on their medical history and then examined and interviewed by a dentist and specialist rheumatologist regarding demographics (age, gender, education, marital status), medical history, and clinical information. One experienced dentist was calibrated for DMFT and ICDAS index assessment and caries diagnosis. All of the eligible participants completed the “Informed Consent Form” (Fig. 3).
Fig. 3.
Study design flowchart. RA: Rheumatoid arthritis, SLE: Systemic Lupus Erythematosus
Oral examination
Oral examinations were performed to assess the DMFT (decayed, missing, filled teeth) and the ICDAS index (The International Caries Detection Assessment System). After drying the teeth, the DMFT value was obtained based on WHO (World Health Organization) criteria from the total number of decayed, missing, and filled teeth due to decay [31]. It should be noted that teeth that have been extracted due to orthodontics or trauma were not calculated in DMFT [31]. Also, the ICDAS system (ICDAS II), which was developed to evaluate dental caries (visually) on clean and dry teeth, was used. This system was used to record the status of dental caries. In this method, a code was considered for each tooth, and this number was related to the severity of caries (code 0 = Sound tooth surface: No evidence of caries after 5-sec air drying; code 1 = First visual change in enamel: Opacity or discoloration (white or brown) is visible at the entrance to the pit or fissure seen after prolonged air drying; code 2 = Distinct visual change in enamel visible when wet, lesion must be visible when dry; code 3 = Localized enamel breakdown (without clinical visual signs of dentinal involvement) seen when damp and after prolonged drying; code 4 = underlying Dark shadow from dentin with or without enamel breakdown; code 5 = Obvious cavity with visible dentin; code 6 = Extensive (more than half the surface) distinct cavity with visible dentine) [32, 33].
Fungal and bacterial profile evaluation
In order to evaluate the fungal and bacterial profile of the mouth, supra-gingival dental plaque samples were collected from the lower or upper permanent molars in all groups with the manual scaling instruments (Hu-Friedy, Chicago, IL, USA). Plaque samples were immediately transferred into a microtube containing 1 ml of sterile distilled water and sent to the microbiology laboratory under sterile conditions until used for assessing. To evaluate the bacterial profile, Mitis Salivarius Agar culture medium with Bacitracin and 10% Sucrose (MSA) was used to isolate Streptococcus mutans in the laboratory. The plates were incubated in an environment containing 5% CO2 at 37ºC for 48 h. Streptococcus mutans colonies were differentiated and confirmed using morphological and biochemical tests. To isolate Lactobacillus spp. deMan-Rogosa-Sharpe agar (MRS) Broth culture medium was used and kept for 48 h at 37ºC and under anaerobic conditions (using a palladium catalyst-based anaerobic chamber (COY)). Lactobacillus spp. colonies were differentiated and confirmed using morphological tests and biochemical characteristics [31]. In order to evaluate the fungal profile of supragingival dental plaque and to isolate Candida, the samples were cultured in Sabouraud Dextrose Agar with Chloramphenicol (SC) culture medium. Then, it was incubated at 37ºC for 48 h. C. Albicans colonies were examined microscopically [34].
Variables
The main outcomes of the study were oral parameters including supra gingival dental plaque Streptococcus mutans, Lactobacillus spp. and Candida albicans levels. The secondary outcomes were DMFT, ICDAS indices values.
Statistical analysis
In the present study, statistical analysis was performed using SPSS version 17 software. Descriptive statistical analysis (mean, standard deviation, frequency, and percentage) was used. Data distribution was checked using the KS test and P-P diagram. The one-way ANOVA test and the Kruska-Wallis test were used to compare the oral variables between the three study groups. The k2 test and, if necessary, the Fisher’s test were used to compare the qualitative variables between the groups. P < 0.05 was considered as a significant level.
Results
Participants characteristics
The current study included 91 eligible participants (46 female and 45 male), including 31 RA, 30 lupus, and 30 healthy people (Table 1). The demographic information of these people was such that the mean and standard deviation of the age of all participants was 44 ± 13.27. In terms of education, most participants had High school (29.7%) and bachelor’s degrees (23.1%) (Table 2). Demographic variables of participants in gender are shown in Table 3; also, 70.3% of participants were married (Table 4).
Table 1.
Demographic variables of participants in the type of disease
| Variable | Participant features | Frequency | Percentage% |
|---|---|---|---|
| Type of Diseases | Lupus erythematosus | 30 | 33 |
| Rheumatoid Arthritis | 31 | 34 | |
| Control | 30 | 33 | |
| Total | 91 | 100.0 |
Table 2.
Demographic variables of participants in terms of education
| Variable | Participant features | Frequency | Percentage% |
|---|---|---|---|
| Education | Elementary | 12 | 13.2 |
| High school | 27 | 29.7 | |
| Diploma | 18 | 19.8 | |
| Associate’s | 10 | 11 | |
| Bachelor’s | 21 | 23.1 | |
| Master’s and PhD | 3 | 3.3 | |
| Total | 91 | 100.0 |
Table 3.
Demographic variables of participants in terms of gender
| Variable | Participant features | Frequency | Percentage% |
|---|---|---|---|
| Gender | Male | 45 | 49.5 |
| Female | 46 | 50.5 | |
| Total | 91 | 100.0 |
Table 4.
Demographic variables of participants in terms of marriage
| Variable | Participant features | Frequency | Percentage% |
|---|---|---|---|
| Marital Status | Single | 27 | 29.7 |
| Married | 64 | 70.3 | |
| total | 91 | 100.0 |
The most used Medications in Lupus erythematosus patients were prednisolone (73.33%), hydroxychloroquine (20%), cyclophosphamide (3.33%), azathioprine (3.33%), and in RA patients were Methotrexate (63.33%), Hydroxychloroquine (16.66%) and prednisolone (13.33%). Disease duration in Lupus erythematosus and RA patients was 5/4 ± 6.2 and 5/5 ± 6.5 years, respectively.
The Chi-square test was used to investigate the relationship between the type of disease and gender, education level and marital status. The results showed no statistically significant relationship between these variables and the type of disease (P = 0.860, P = 0.030, P = 0.830).
Analysis of variance was used to investigate the relationship between the type of disease and age. No statistically significant relationship was observed between these two variables (P = 0.070).
Dental decay and oral microbiota status assessment
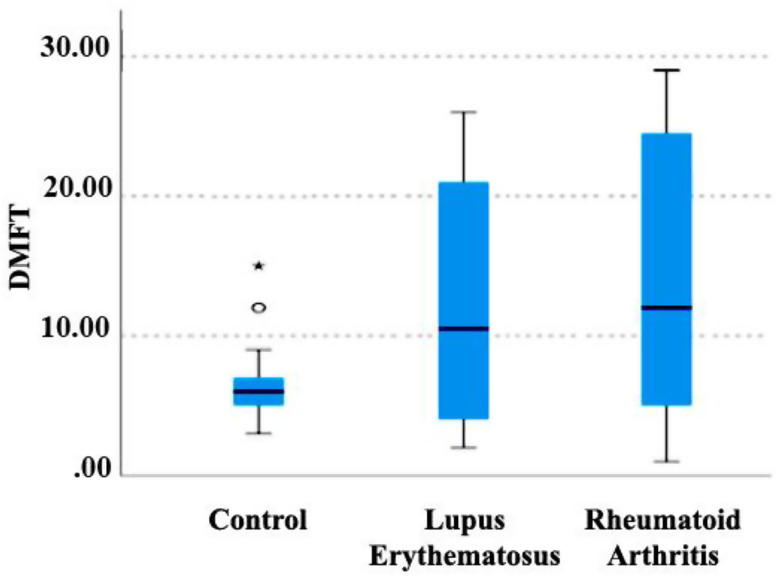
Kruskal -Wallis non-parametric test was used to investigate the relationship between the mean DMFT index and the type of disease (Fig. 4). A significant relationship was observed between the type of disease and this index. DMFT index in lupus and RA groups was higher than in the control group, and this difference was statistically significant (P < 0.001). Although the DMFT index in the RA group was higher than the lupus group, no significant difference was observed between the two groups (P = 0.480).
Fig. 4.
Relationship between disease type and DMFT index. DMFT; (Decayed, Missing, and Filled teeth)
The one-way ANOVA test was used to investigate the relationship between the type of disease and the ICDAS index. There was a statistically significant relationship between the type of disease and the mentioned index. The ICDAS index for lupus and RA patients was significantly higher than the control group (P < 0.001). In the comparison between lupus and RA groups, although the ICDAS index was higher in the RA group, there was no statistically significant difference between these two groups (P = 0.460).
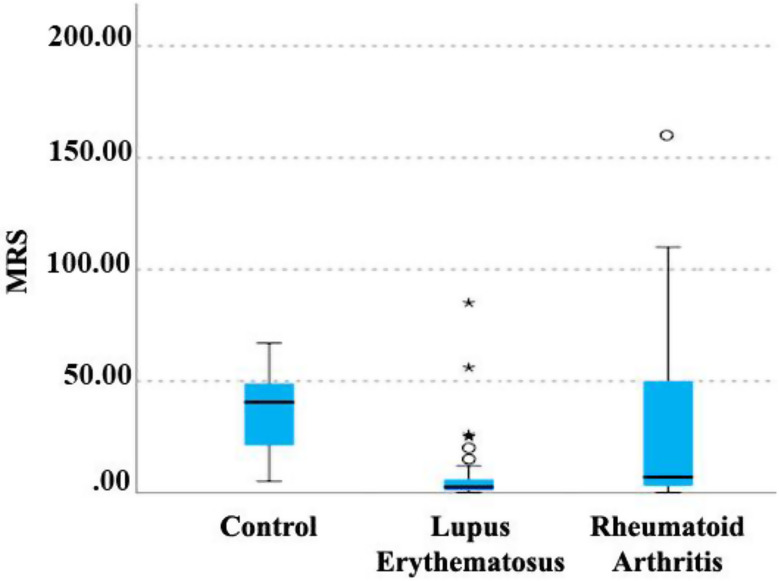
Kruskal-Wallis non-parametric test was used to investigate the relationship between the MRS index (culture medium for the isolation of Lactobacillus) and the type of disease (Fig. 5). A significant relationship was observed between the kind of disease and this index. The control and RA group had a higher MRS index than the lupus group, and this difference was statistically significant (P < 0.001). Also, the control group showed a higher average MRS index than the RA group, which was statistically significant (P < 0.001).
Fig. 5.
Relationship between disease type and MRS index. MRS; (deMan, Rogosa, and Sharpe)
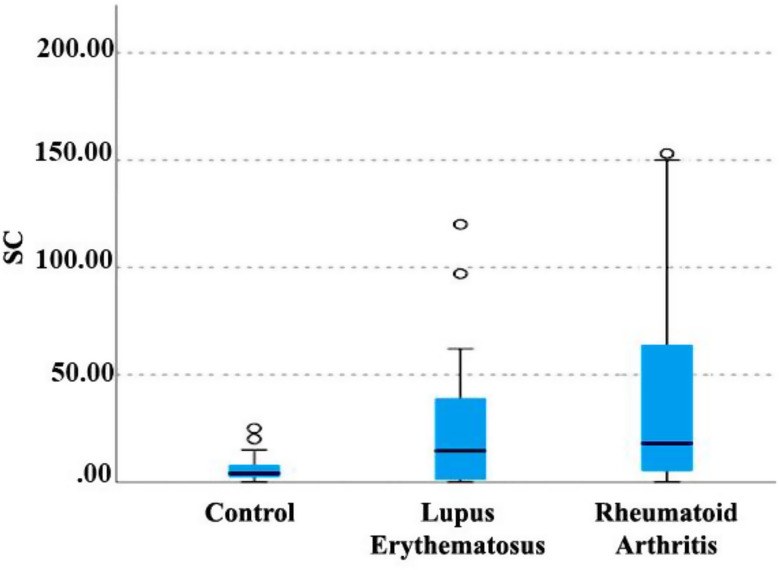
Kruskal-Wallis non-parametric test was used to investigate the relationship between the SC index (culture medium for the isolation of Candida) and the type of disease (Fig. 6). A significant relationship was observed between the type of disease and this index. SC index for lupus and RA patients was significantly higher than the control group, and this difference was statistically significant (P < 0.001). Although the SC index in the lupus and RA groups was higher, no significant difference was observed between the two groups (P = 0.240).
Fig. 6.
Relationship between disease type and SC index. SC; (Sabouraud Dextrose Agar with Chloramphenicol)
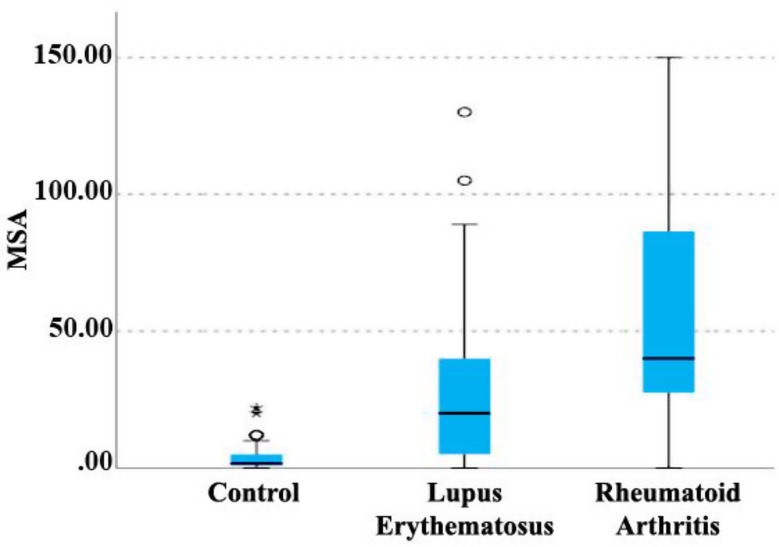
Kruskal-Wallis non-parametric test was used to investigate the relationship between the MSA index (culture medium for isolation of Streptococcus mutans) and the type of disease (Fig. 7). According to the results of this test, a significant relationship was observed between the type of disease and this index. The MSA index in lupus and RA groups was higher than in the control group, and this difference was statistically significant (P < 0.001). Also, in the patient groups, a statistically significant difference was observed between lupus and RA groups. The mean MSA index in the RA group was significantly higher than lupus group (P = 0.020).
Fig. 7.
Relationship between disease type and MSA index. MSA; (Mitis Salivarius Agar)
Association of dental decay and oral microbiota status with disease activity (DAS28) in RA group
The one-way ANOVA test showed that in the RA group, there was no significant association between dental decay, salivary microbiota status, and disease activity (Table 5).
Table 5.
Association of oral parameters with disease activity (DAS28) in RA group
| Variables RA activity | N | Mean | Std. Deviation | Std. Error | 95% Confidence Interval for Mean | Minimum | Maximum | Sum of Squares | df | Mean Square | F | Sig. | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||||||||||
| MRS | remission | 7 | 11.4286 | 17.21295 | 6.50588 | -4.4908 | 27.3479 | 3.00 | 50.00 | 4275.530 | 2 | 2137.765 | 1.174 | 0.324 |
| low activity | 7 | 30.0000 | 40.31129 | 15.23624 | -7.2817 | 67.2817 | 0.00 | 105.00 | ||||||
| moderate activity | 17 | 40.7059 | 49.65854 | 12.04397 | 15.1738 | 66.2379 | 0.00 | 160.00 | ||||||
| Total | 31 | 31.6774 | 42.91805 | 7.70831 | 15.9350 | 47.4199 | 0.00 | 160.00 | 55258.774 | 30 | ||||
| SC | remission | 7 | 14.5714 | 21.15701 | 7.99660 | -4.9955 | 34.1384 | 0.00 | 60.00 | 7045.792 | 2 | 3522.896 | 1.427 | 0.257 |
| low activity | 7 | 51.0000 | 39.55587 | 14.95071 | 14.4169 | 87.5831 | 0.00 | 96.00 | ||||||
| moderate activity | 17 | 50.4706 | 59.70146 | 14.47973 | 19.7749 | 81.1662 | 0.00 | 153.00 | ||||||
| Total | 31 | 42.4839 | 50.38113 | 9.04872 | 24.0039 | 60.9638 | 0.00 | 153.00 | 76147.742 | 30 | ||||
| MSA | remission | 7 | 34.8571 | 30.44355 | 11.50658 | 6.7016 | 63.0127 | 0.00 | 90.00 | 5742.730 | 2 | 2871.365 | 1.796 | 0.185 |
| low activity | 7 | 48.4286 | 29.07380 | 10.98886 | 21.5398 | 75.3173 | 10.00 | 99.00 | ||||||
| moderate activity | 17 | 67.4118 | 46.18720 | 11.20204 | 43.6645 | 91.1590 | 10.00 | 150.00 | ||||||
| Total | 31 | 55.7742 | 41.03146 | 7.36947 | 40.7237 | 70.8247 | 0.00 | 150.00 | 50507.419 | 30 | ||||
| DMFT | remission | 7 | 10.4286 | 6.45128 | 2.43836 | 4.4621 | 16.3950 | 3.00 | 22.00 | 245.162 | 2 | 122.581 | 1.264 | 0.298 |
| low activity | 7 | 12.0000 | 12.83225 | 4.85014 | 0.1321 | 23.8679 | 1.00 | 27.00 | ||||||
| moderate activity | 17 | 16.7647 | 9.60813 | 2.33031 | 11.8247 | 21.7048 | 5.00 | 29.00 | ||||||
| Total | 31 | 14.2581 | 9.93300 | 1.78402 | 10.6146 | 17.9015 | 1.00 | 29.00 | 2959.935 | 30 | ||||
| Total ICDAS | remission | 7 | 27.2857 | 11.65782 | 4.40624 | 16.5040 | 38.0674 | 10.00 | 41.00 | 523.263 | 2 | 261.632 | 0.686 | 0.512 |
| low activity | 7 | 23.5714 | 27.62159 | 10.43998 | -1.9743 | 49.1171 | 4.00 | 78.00 | ||||||
| moderate activity | 17 | 33.2941 | 18.16509 | 4.40568 | 23.9545 | 42.6337 | 1.00 | 64.00 | ||||||
| Total | 31 | 29.7419 | 19.31833 | 3.46967 | 22.6559 | 36.8280 | 1.00 | 78.00 | 11195.935 | 30 | ||||
MRS; (deMan, Rogosa, and Sharpe)
SC; (Sabouraud Dextrose Agar with Chloramphenicol)
MSA; (Mitis Salivarius Agar)
DMFT; (Decayed, Missing, and Filled teeth)
Std; (Standard Deviation)
df; (Degrees of freedom)
Association of dental decay and oral microbiota status with disease activity (SLEDAI-2 K) in the lupus group
Spearman’s non-parametric test was used to investigate the relationship between the activity of lupus disease and the DMFT index (Table 6). There was no statistically significant relationship between the two variables (P = 0.610 and R = 0.220).
Table 6.
Disease activity index (SLEDAI-2 K) status in systemic lupus erythematosus group
| N | Minimum | Maximum | Mean | Std. Deviation | |
|---|---|---|---|---|---|
| Disease activity | 30 | 18.00 | 40.00 | 27.2000 | 7.34096 |
| Valid N (listwise) | 30 |
Spearman’s non-parametric test was used to investigate the relationship between the activity of lupus disease and the TOTAL ICDAS index. There was no statistically significant relationship between the two variables (P = 0.070 and R = 0.323).
Spearman’s non-parametric test was used to investigate the relationship between the activity of lupus disease and MRS (culture medium for the isolation of Lactobacillus). The results showed a statistically significant relationship between the two variables (P = 0.020 and R = 0.408).
Spearman’s non-parametric test was used to investigate the relationship between the activity of lupus disease and SC (culture medium for the isolation of Candida). There was a statistically significant relationship between these two variables (P < 0.001 and R = 0.698). Spearman’s non-parametric test was used to investigate the relationship between the activity of lupus disease and MSA (culture medium for the isolation of Streptococcus mutans. There was no statistically significant relationship between the two variables (P = 0.190 and R = 0.245).
Discussion
The findings of this study showed that the mean DMFT index was significantly higher in people with lupus and RA than in the control group. In the study of Kreher et al. (2023), Medrano et al. (2022), and Mehdipour et al. (2022), it has been reported that RA patients had higher DMFT compared to the index control group [21, 35, 36]. Also, the study by Rodriguez et al. (2016) and Perng et al. (2023) showed that the DMFT index was higher in lupus erythematosus patients than in the control group [7, 37], which is in line with the results of this study. The rationale for the increase in the incidence of dental caries in these patients seems to be the salivary gland dysfunction and decreased salivary flow rate, increased acidity level of saliva, the appearance of some oral microorganisms, and the difficulties associated with maintaining oral hygiene practices as a result of general arthritis, particularly affecting the fingers of the hands and arms [21].
The findings of this study showed that there was a statistically significant relationship between the type of disease and the ICDAS index. Thus, the mean ICDAS index values for lupus and RA patients were significantly higher than those of the control group. This finding was not consistent with the survey conducted by Roccuzzo et al. (2023), in which the patients with RA and the control group had almost the same mean ICDAS index values [38]. One potential explanation for the discrepancies in findings may be attributed to variations in study design. Additionally, the duration of the disease, which may influence the incidence of tooth decay, could represent another contributing factor [39]. In the findings of the present study, it was reported that a statistically significant relationship was observed between the MRS index (culture medium for the isolation of Lactobacillus) and the type of disease. The MRS index was significantly higher in the control group than in the people with RA. The present study was not consistent with the survey conducted by Krutyhołowa et al. (2022) and Zhang et al. (2015), who reported that Lactobacillus salivarius was overexpressed in the saliva and dental plaque of people with rheumatoid arthritis compared to healthy people [22, 23]. Zhang et al., in their comprehensive study of the intestinal and oral microbiomes in individuals diagnosed with rheumatoid arthritis, provided evidence supporting the concept that rheumatoid arthritis is indicative of a chronic inflammatory state. This condition may arise from the proliferation of pathogenic bacteria or a deficiency of modulating bacteria, both of which can stimulate or exacerbate the immune system responses [22]. This study showed that the SC index (culture medium for the isolation of Candida) was significantly higher in people with lupus and RA than in the control group. Stähli et al. (2022), reported that the presence of C. Albicans in subgingival biofilm samples of RA patients is higher than in the control group. The study of Atika et al. (2016) showed that C. albicans colonization is found in the saliva of RA patients. Also, in the study of Sojod et al. (2021), it was reported that the prevalence of C. Albicans is significantly greater in patients with lupus in comparison to the control group, which is consistent with the results of our study [40–42]. This research is not in agreement with the study conducted by Paksoy et al. (2023), who did not observe any relationship between the presence of Candida in the oral cavity of RA patients compared to the control group. Also, it is not consistent with the study by Navas et al. (2012), who reported that although among the various species examined, oral C. Albicans was identified as the most frequently isolated fungus in the group of lupus patients, there was no significant difference compared to the control group [25, 26]. Variations in outcomes may be ascribed to the diverse medications administered to patients, the specific sites of oral sample collection, and the methodologies employed in processing these samples within the studies.
The present research showed that the MSA index (culture medium for the isolation of Streptococcus mutans) showed a substantial increase in oral cavity of people with lupus and RA compared to the control group. This finding is in line with the study of Rodriguez et al. (2016), and Gofur et al. (2020), who reported that a higher prevalence of Streptococcus mutans species were observed in the salivary microbiome of lupus patients compared to healthy individuals, as well as with the study of Yang et al. (2018), who showed that the prevalence of salivary Streptococcus mutans and Streptococcus sobrinus species was significantly higher in patients with lupus when compared to the control group [5, 7, 24]. They stated that the probable rationale for this fact was the diminished immune system response in lupus patients against oral microbiomes, which was associated with elevated susceptibility to dental caries in this population. Also, the present study is in line with the study of Kreher et al. (2023), who reported that RA patients exhibit a higher prevalence of root caries, less salivary flow rate, and a higher concentration of Streptococcus mutans in saliva [21]. In justification of the findings, It is important to highlight that infections in patients with lupus and rheumatoid arthritis may progress rapidly as a result of the underlying disease or the immunosuppressive effects associated with their treatment [43].
In the present investigation, we reported that there was a significant positive relationship between the severity of lupus, MRS (culture medium for the isolation of Lactobacillus), and SC (culture medium for the isolation of Candida) in lupus patients. The present study was consistent with the studies of Calderaro DC et al. (2017) and Gofur et al. (2019) on SLE patients, which observed a strong correlation between the oral health score and the severity of the disease using the SLEDAI index [44, 45]. One potential mechanism underlying this phenomenon may be attributed to the influence of disease severity on the quality of oral health assessments, oral health parameters, and the composition of the oral cavity microbiota.
The present study reported that there was no statistically significant relationship between disease severity and tooth decay in lupus and RA patients. Our study was not in line with the studies conducted by Äyräväinen et al. (2018) and Almasi et al. (2020), who reported that in RA patients, the severity of disease was found to be positively correlated with elevated dental caries indices [46, 47]. Also, our study was not consistent with the studies of Aurlene et al. (2020) and Rodriguez et al. (2016), which reported that the dental caries index showed significant direct relationship with disease severity in lupus patients [6, 7]. Regarding the relation between lupus and RA disease severity and tooth decay, some studies reported that the severity of this disease was related to poor oral hygiene and a high incidence of dental caries [24]. However, certain studies have not indicated a relationship between the two variables [48], These studies are limited in scale, and further research is necessary to determine whether there is any biological plausibility associated with the findings.
The distribution of participants across groups does not align with epidemiological proportions. The authors have applied statistical methods to address potential biases. The findings may not be broadly generalizable, rather than merely attributing this issue to a relatively low sample size. Ideally, increasing the number of control group participants reflects epidemiological proportions. A notable novelty of this study lies in the utilization of the ICDAS index for caries assessment. This index serves as a qualitative measure for evaluating dental caries and, to our knowledge, has been examined in only a limited number of prior studies involving this patient population. Furthermore, research concerning the structure and profile of the supra gingival plaque microbiota in these patients has been relatively sparse and inconsistent. The current study is subject to several limitations, including a relatively small sample size and constraints in the application of more advanced tools for assessing the dental plaque microbiome. Additionally, the probable influence of some individual habits and life style as a major confounding bias on oral microbial and fungal composition has not been considered within the scope of this research. The cross-sectional design employed in this study represents another additional limitation. Furthermore, the impact of the medications administered during the treatment process on oral parameters represents another potential confounding variable that warrants consideration. Future inquiries into the underlying mechanisms, as well as the development of oral microbiome-assisted diagnostic strategies, prognostic assessments, and therapeutic interventions, may hold significant promise for the effective management of autoimmune disorders such as lupus erythematosus and rheumatoid arthritis.
Conclusion
The findings of the present study indicate that the prevalence of dental caries among patients with systemic rheumatoid arthritis and lupus erythematosus is significantly greater than that observed in the control group. Furthermore, these patients exhibited higher oral microbiome than the control group. It is recommended that enhanced focus be placed on strategies to improve oral health status in this patient population. Additionally, it is advisable for patients to be referred to a dentist for regular oral and dental assessments.
Acknowledgements
The authors would like to thank Clinical Research and development Unit, Shahid Beheshti Hospital, Qom University of Medical Sciences, Qom, Iran.
Abbreviations
- SLE
Systemic lupus erythematosus
- RA
Rheumatoid arthritis
- DMFT
Decayed, Missing Filled teeth
- ICDAS
International Caries Detection Assessment System
- MSA
Mitis Salivarius Agar
- MRS
deMan-Rogosa-Sharpe
- SC
Sabouraud Dextrose Agar with Chloramphenicol
- EULAR/ACR
European league of Rheumatologists/ American college of Rheumatology
- DAS
Disease activity score
- SLEDAI
SLE Disease Activity Index
- WHO
World Health Organization
Author contributions
AM and MM developed and supervised the work. FM, RF and AS performed the data collection. MA performed the data analysis. AR and FK drafted the manuscript. AM, RF, FM and MA contributed to data interpretation. All authors reviewed and edited the manuscript. All authors read and approved the final manuscript.
Funding
This work was funded by a grant from the Vice for Research and Technology of Qom University of Medical Sciences, Qom, Iran.
Data availability
The dataset generated or analyzed during the current study are not publicly available due to the subject matter specialization, but are available from the corresponding author upon reasonable request and with permission of Qom University of Medical Sciences.
Declarations
Ethics approval and consent to participate
The study was performed according to the Helsinki Declaration guidelines after obtaining the approval of the Ethics Committee of Qom University of Medical Sciences (Code No. IR.MUQ.REC.1402.077). All methods were carried out in accordance with the relevant guidelines and regulations. The written informed consent of the participants was also obtained.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Aida Mehdipour, Email: mehdipoor_aida@yahoo.com.
Maryam Masoumi, Email: dr.masoumi2017@gmail.com.
References
- 1.Corrêa JD, et al. Subgingival microbiota dysbiosis in systemic lupus erythematosus: association with periodontal status. Microbiome. 2017;5:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Delis PC. Uncertainty and quality of life in systemic lupus erythematosus: a cross-sectional study. Rehabilitation Nurs J. 2019;44(1):2–10. [DOI] [PubMed] [Google Scholar]
- 3.Rigante D, Mazzoni MB, Esposito S. The cryptic interplay between systemic lupus erythematosus and infections. Autoimmun Rev. 2014;13(2):96–102. [DOI] [PubMed] [Google Scholar]
- 4.Crincoli V, et al. Temporomandibular disorders and oral features in systemic lupus erythematosus patients: an observational study of symptoms and signs. Int J Med Sci. 2020;17(2):153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang L, et al. Saliva dysfunction and oral microbial changes among systemic lupus erythematosus patients with dental caries. Biomed Res Int. 2018;2018(1):8364042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aurlene N, Manipal S, Prabu D. Prevalence of oral mucosal lesions, dental caries, and periodontal disease among patients with systemic lupus erythematosus in a teaching hospital in Chennai, Tamil Nadu. J Family Med Prim Care. 2020;9(7):3374–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loyola Rodriguez J, et al. Frequency of dental caries in active and inactive systemic lupus erythematous patients: salivary and bacterial factors. Lupus. 2016;25(12):1349–56. [DOI] [PubMed] [Google Scholar]
- 8.Croia C, et al. One year in review 2019: pathogenesis of rheumatoid arthritis. Clin Exp Rheumatol. 2019;37(3):347–57. [PubMed] [Google Scholar]
- 9.Esberg A, et al. Oral microbiota identifies patients in early onset rheumatoid arthritis. Microorganisms. 2021;9(8):1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dawes C, Wong D. Role of saliva and salivary diagnostics in the advancement of oral health. J Dent Res. 2019;98(2):133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mandana K, et al. < the > prevalence of oral mucosal lesions and associated factors in 385 patients with rheumatoid arthritis in rheumatology clinics of. Tehran University for a period of one year; 2014.
- 12.Taheri M, et al. Investigation of periodontal conditions in patients with rheumatoid arthritis. J Mash Dent Sch. 2011;35(4):283–8. [Google Scholar]
- 13.de Smit MJ, et al. Commentary: periodontitis and rheumatoid arthritis: what do we know? J Periodontol. 2015;86(9):1013–9. [DOI] [PubMed] [Google Scholar]
- 14.Defta CL, et al. Oral mycobiota: A narrative review. Dentistry J. 2024;12(4):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Organization WH. Global oral health status report: towards universal health coverage for oral health by 2030. World Health Organization; 2022.
- 16.Mehdipour A, et al. Comparing the prevalence of Helicobacter pylori and virulence factors CagA, VacA, and dupa in supra-gingival dental plaques of children with and without dental caries: a case–control study. BMC Oral Health. 2022;22(1):170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehdipour A, et al. Prevalence of oral parafunctional habits in children and related factors: an observational cross-sectional study. Int J Clin Pediatr Dentistry. 2023;16(2):308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Srivastava B, et al. Comparative evaluation of oral Candida albicans carriage in children with and without dental caries: a Microbiological in vivo study. Int J Clin Pediatr Dentistry. 2012;5(2):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He Y, et al. An evaluation of Norspermidine on anti-fungal effect on mature Candida albicans biofilms and angiogenesis potential of dental pulp stem cells. Front Bioeng Biotechnol. 2020;8:948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Signoretto C, et al. Support for the role of Candida spp. In extensive caries lesions of children. New Microbiol. 2009;32(1):101. [PubMed] [Google Scholar]
- 21.Kreher D, et al. Dental caries in adult patients with rheumatoid Arthritis—A systematic review. J Clin Med. 2023;12(12):4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X, et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat Med. 2015;21(8):895–905. [DOI] [PubMed] [Google Scholar]
- 23.Krutyhołowa A, et al. Host and bacterial factors linking periodontitis and rheumatoid arthritis. Front Immunol. 2022;13:980805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gofur NRP, et al. Oral hygiene and dental caries status on systemic lupus erythematosus patients: A cross-sectional study. Pesquisa Brasileira Em Odontopediatria E Clínica Integrada. 2020;20:e0013. [Google Scholar]
- 25.Paksoy T et al. Assessment of Epstein–Barr virus, Candida albicans, and some periodontal pathogens in rheumatoid arthritis patients with periodontitis. North Clin Istanbul, 2023. 10(4). [DOI] [PMC free article] [PubMed]
- 26.de Araújo Navas E, et al. Oral microbial colonization in patients with systemic lupus erythematous: correlation with treatment and disease activity. Lupus. 2012;21(9):969–77. [DOI] [PubMed] [Google Scholar]
- 27.Kay J, Upchurch KS. ACR/EULAR 2010 rheumatoid arthritis classification criteria. Rheumatology. 2012;51(suppl6):vi5–9. [DOI] [PubMed] [Google Scholar]
- 28.van der Heijde DM, et al. Judging disease activity in clinical practice in rheumatoid arthritis: first step in the development of a disease activity score. Ann Rheum Dis. 1990;49(11):916–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohmura K. Which is the best SLE activity index for clinical trials? Mod Rheumatol. 2021;31(1):20–8. [DOI] [PubMed] [Google Scholar]
- 30.Brik R, et al. Salivary antioxidants and metalloproteinases in juvenile idiopathic arthritis. Mol Med. 2010;16:122–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chuang C-J, et al. Increased risk of developing dental diseases in patients with primary Sjögren’s syndrome—A secondary cohort analysis of population-based claims data. PLoS ONE. 2020;15(9):e0239442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malek Mohammadi T, Hajizamani A. A review on traditional caries diagnostic systems and introduction of new international caries detection and assessment system (ICDAS). J Dent. 2011;12(1):67–83. [Google Scholar]
- 33.Banava S, et al. Clinical comparison of dental caries by DMFT and ICDAS systems. J Iran Dent Association. 2012;24(3):146–51. [Google Scholar]
- 34.Nadig SD, et al. A relationship between salivary flow rates and Candida counts in patients with Xerostomia. J Oral Maxillofacial Pathol. 2017;21(2):316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sánchez-Medrano AG, et al. Association between subjects with newly diagnosed rheumatoid arthritis and dental caries. Odovtos-International J Dent Sci. 2022;24(2):186–96. [Google Scholar]
- 36.Mehdipour A, et al. Oral health-related quality of life and dental caries in rheumatoid arthritis patients: a cross-sectional observational study. J Med Life. 2022;15(6):854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perng W-T, et al. Dental caries and risk of newly-onset systemic lupus erythematosus: a nationwide population-based cohort study. Curr Med Res Opin. 2023;39(2):307–17. [DOI] [PubMed] [Google Scholar]
- 38.Roccuzzo A, et al. Evaluation of the oral health conditions and oral health-Related quality of life in a Community-Dwellers population aged ≥ 45 years in the Canton of Bern: A preliminary pilot study. Int J Environ Res Public Health. 2023;20(5):4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mühlberg S, et al. Oral health-related quality of life depending on oral health in patients with rheumatoid arthritis. Clin Oral Invest. 2017;21:2661–70. [DOI] [PubMed] [Google Scholar]
- 40.Stähli A, et al. In vitro activity of anti-rheumatic drugs on release of pro-inflammatory cytokines from oral cells in interaction with microorganisms. Front Oral Health. 2022;3:960732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alahmed AM, et al. Correlation between the oral manifestations of rheumatoid arthritis patients on different treatments with the clinical disease activity. J Dent Med Sci. 2016;15(9):132–8. [Google Scholar]
- 42.Sojod B et al. Systemic lupus erythematosus and periodontal disease: a complex clinical and biological interplay. J Clin Med, 2021;10(9):1957. [DOI] [PMC free article] [PubMed]
- 43.Lam DK, Clokie CM, Sándor GK. Systemic lupus erythematosus: a review for dentists. J Can Dent Assoc, 2007. 73(9). [PubMed]
- 44.Calderaro DC, et al. Is chronic periodontitis premature in systemic lupus erythematosus patients? Clin Rheumatol. 2017;36:713–8. [DOI] [PubMed] [Google Scholar]
- 45.Gofur NR, et al. Periodontitis is associated with disease severity and anti-double stranded DNA antibody and interferon-gamma levels in patients with systemic lupus erythematosus. J Taibah Univ Med Sci. 2019;14(6):560–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Äyräväinen L, et al. Activity of rheumatoid arthritis correlates with oral inflammatory burden. Rheumatol Int. 2018;38:1661–9. [DOI] [PubMed] [Google Scholar]
- 47.Almasi S, et al. Relationship between clinical and laboratory findings of rheumatoid arthritis patients with their oral status and disease activity. Caspian J Intern Med. 2021;12(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Joseph R, et al. Association between chronic periodontitis and rheumatoid arthritis: a hospital-based case–control study. Rheumatol Int. 2013;33:103–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset generated or analyzed during the current study are not publicly available due to the subject matter specialization, but are available from the corresponding author upon reasonable request and with permission of Qom University of Medical Sciences.