Abstract
Protein quality control (PQC) plays a vital role in maintaining normal heart function, as cardiomyocytes are relatively sensitive to misfolded or damaged proteins, which tend to accumulate under pathological conditions. Ubiquitin-specific protease (USP) is the largest deubiquitinating enzyme family and a key component of the ubiquitin proteasome system (UPS), which is a non-lysosomal protein degradation machinery to mediate PQC in cells. USPs regulate the stability or activity of the target proteins that involve intracellular signaling, transcriptional control of inflammation, antioxidation, and cell growth. Recent studies demonstrate that the USPs can regulate fibrosis, lipid metabolism, glucose homeostasis, hypertrophic response, post-ischemic recovery and cell death such as apoptosis and ferroptosis in cardiomyocytes. Since myocardial cell loss is an important component of cardiomyopathy, therefore, these findings suggest that the UPSs play emerging roles in cardiomyopathy. This review briefly summarizes recent literature on the regulatory roles of USPs in the occurrence and development of cardiomyopathy, giving us new insights into the molecular mechanisms of USPs in different cardiomyopathy and potential preventive strategies for cardiomyopathy.
Keywords: Cardiomyopathy, USP, Deubiquitination, PQC
Introduction
Cardiomyopathy encompasses a heterogeneous group of heart muscle diseases with poor prognosis and high mortality. According to the American Heart Association (AHA) classification, cardiomyopathies categorized into two major types. Primary cardiomyopathies are characterized by their predominantly limited involvement in heart muscle, which are grouped into types of genetic, mixed (genetic and nongenetic), and acquired; Secondary cardiomyopathies are those that show pathological myocardial involvement based on a variety of generalized systemic (multiorgan) disorders, including ischemic, metabolic, infectious, toxic, auto-immunogenic, and neuromuscular [1]. Although the manifestations and causes of cardiomyopathies are varied, they often lead to myocardial cell death and develop progressive heart failure. Multiple pathophysiological mechanisms contribute to cardiomyopathy, among them, protein quality control (PQC) plays a critical role in heart function because mature cardiomyocytes have limited proliferation and regeneration capacities. Proteasome dysfunction has been viewed as a pathogenic factor in cardiac dysfunction [2]. The ubiquitin-proteasome system (UPS) is an ATP-dependent, non-lysosomal pathway that degrades misfolded or damaged proteins. In normal conditions, misfolded proteins undergo ubiquitylation and timely degradation via UPS. However, UPS may misidentify newly synthesized proteins with aggregation-prone structures before they reach a stable folded state [3]. Over 80% of intracellular proteins are degraded by UPS [4]. Earlier studies revealed the important effect of ubiquitylation in UPS-mediated PQC, and the defect of ubiquitylation contributes to many diseases, including cardiomyopathy [2, 5, 6].
Ubiquitination is one of major post-translational modifications of proteins, and it is essential for protein maturation and function [7]. The ubiquitination process is a cascade of enzymatic reactions catalyzed by three main enzymes: the ubiquitin-activating enzyme (E1), the ubiquitin-conjugating enzyme (E2) and the ubiquitin ligase (E3) [8]. Ubiquitination reactions comprise both mono-ubiquitination and polyubiquitination. The monoubiquitination is the process of a single ubiquitin molecule attaching to a lysine residue of substrate protein, while polyubiquitinnation refers to the binding of multi ubiquitin molecules to lysine residue of substrate proteins. Eight potential linkage sites, including seven lysine sites (K6, K11, K27, K29, K33, K48, and K63) and one N-terminal methionine residue (M1) for the ubiquitin molecules binding [9]. The ubiquitin chains formed by M1 are called linear ubiquitin chains, which form the linear ubiquitin chain assembly complex (LUBAC) with the ubiquitin-conjugating enzyme E2L3 (UBE2L3). LUBAC consists of three subunits, including two RING-between-RING (RBR)-type ubiquitin ligases, heme-oxidized IRP2 ubiquitin ligase 1L (HOIL-1L), HOIL-1-interacting protein (HOIP), and one adaptor protein SHANK-associated RH domain-interacting protein (SHARPIN) [10]. Linear ubiquitin chains on protein substrates can be removed by the DUBs such as OUT deubiquitinase with linear linkage specificity (OTULIN) and cylindromatosis gene (CYLD) [11]. Additional forms of ubiquitin chains and substrates serve important signals for regulation of cellular processes, such as monoubiquitination mediates DNA repair and chromatin structure. K48-linked polyubiquitination facilitates 26S proteasome degradation of targeted proteins. K63-linked polyubiquitination promotes autophagic degradation of protein substrates through the autophagy-lysosome pathway. Linear ubiquitin plays vital roles in regulation of NF-κB, EGFR signaling pathways to regulate innate and adaptive immunity and cell death [10].
However, protein ubiquitylation can be reversibly changed by deubiquitinating enzymes (DUBs). DUBs cleave the covalent bonds, removing ubiquitin molecules from substrates and thereby protecting target proteins from proteasome-dependent degradation [12, 13]. Therefore, the balance between ubiquitination and deubiquitination is crucial for UPS to maintain protein homeostasis and cellular physiological processes. DUBs can be divided into two main classes: cysteine proteases and metalloproteases. Most of DUBs are cysteine proteases, which can be further categorized into ubiquitin-specific proteases (USPs), ovarian tumor proteases (OTUs), ubiquitin C-terminal hydrolases (UCHs), Machado-Joseph domain protease (MJDs), motif interacting with ubiquitin-containing novel DUB family (MINDYs), monocyte chemotactic proteins (MCPIPs), zinc finger with UFM1-specific peptidase domain protein (ZUFSP/ZUP1). Metalloproteases contain DUB named JAB1/MPN/MOV34 (JAMM) [13, 14].
Ubiquitin-specific peptidases (USPs), the largest family of DUBs with approximately 58 members in humans, were first identified and cloned in the yeast Saccharomyces cerevisiae [15]. USPs play a crucial role in maintaining cardiac PQC. A recent study indicates that USP5 is essential for cardiac proteostasis by stabilizing proteasome 26S subunit, non-ATPase 14 (PSMD14). Loss of USP5 enhances UPS activity and autophagic flux in cardiomyocytes, while cardiac-specific overexpression of hUSP5 mitigates pathological remodeling in mice [16]. Studies also have linked many USPs to specific biological pathways functioning in cell cycle progression, DNA damage repair, endocytosis, immune response, and metabolic regulation [17–19]. Increasing evidence suggests that USP dysfunction contributes significantly to the development of cardiac diseases, such as myocardial ischemia-reperfusion injury and cardiomyopathy [20–22]. Several articles reviewed the roles of DUBs and USPs in cancers, inflammatory disorders by regulating the key signaling pathways such as NF-κB, PI3K/AKT/mTOR, MAPK, and discussed their therapeutic potential in diseases [18, 23–26]. However, the association of USPs in cardiomyopathies has not been reviewed.
In this review, we summarize recent literature on USPs and their roles in multiple signaling pathways, including TGF-β, Keap1/Nrf2, p53, NF-κB, and Notch, all of which are critical to the progression of various cardiomyopathies. By elucidating these mechanisms, we aim to highlight the therapeutic potential of targeting USPs in the treatment of cardiomyopathy.
USPs and signaling pathways
Cardiomyopathies are caused by various pathogenesis, but oxidative stress (OS) is considered a key factor in different cardiomyopathies. The production of reactive oxygen species (ROS) in cardiomyocytes has been revealed to affect various signaling pathways, which involved in the mitochondrial damage, lipid peroxidation, inflammation, the post-translational modification of proteins, cardiac hypertrophy and fibrosis, leading to cell death and cardiac dysfunction. ROS also regulates calcium homeostasis and impairs ATP production. For instance, ROS participates in NF-κB, TGF-β, Nrf2, p53 and Notch signaling pathway to mediate inflammation, cardiac remodeling, antioxidant and angiogenesis in the development of heart diseases and cardiomyopathy [27–32]. These pathways also have been shown to be affected by USPs. This review highlights the critical role of USPs in the aforementioned cellular signaling pathways and cardiomyopathies, thereby enhancing our understanding of their biological functions in these diseases.
USPs and NF-κB signaling pathway
Nuclear factor-kB (NF-κB) is a family of transcription factors including p50/p105, p52/p100, p65/ RelA, c-Rel and RelB [33], and mediate responses to a remarkable diversity of external stimuli, thus plays a crucial role in multiple physiological and pathological processes [34], such as cell maturation, cell survival, and inflammation in many cell types, including cardiac myocytes [35]. The roles of NF-κB in cardiovascular diseases, such as atherosclerosis, myocardial ischemia/reperfusion injury, cardiac hypertrophy, cardiac remodeling (fibrosis) and heart failure, have been confirmed in several studies [35–37].
USPs are important for the regulation of NF-κB pathway. Cylindromatosis (CYLD) is a member of the USP family characterized by the specific conjugation of its target proteins with K63-linked and the linear ubiquitin chains [38, 39]. CYLD plays a vital role in the regulation of the NF-κB pathway via targeting its upstream signaling molecules, such as TAK1, TRAF and IKKγ (also known as NEMO) [38, 40]. The activation of NF-κB is modulated by tumor necrotic factor-alpha (TNFα)-induced formation of signaling complex I (RIPK1, cIAP1/2, TRADD and TRAF2/5) at the cell membrane. TRAF2 functions as a K63-linked ubiquitin ligase and acts as a molecular trigger for initiation downstream signaling pathways such as c-Jun N-terminal kinase (JNK) and IKK [41]. Under normal conditions, the ubiquitination of TRAF2 is suppressed by CYLD. However, under external stimuli, TRAF2 undergoes ubiquitination and activates downstream NF-κB or JNK signaling pathways due to its phosphorylation [41]. CYLD suppresses the activation of IKK complex by cleaving K63-linked polyubiquitin chains from TAK1 [42] and linear ubiquitin chains from NEMO [43]. Once the IKK complex is active, it leads to the phosphorylation and K48-linked ubiquitylation for the degradation of IkB, resulting NF-κB translocation to the nucleus and inducing transcription of target genes.
While CYLD is strongly implicated in NF-κB inhibition, other USPs also regulate NF-κB signaling. USP4 directly targets to TRAF2/TRAF6 or TAK1 by deubiquitinating K63-linked ubiquitin chains, thereby negatively regulating TNFα—induced NF-κB activation [44, 45]. USP7 regulates NF-κB transcriptional activity and NF-κB subunit p65 [46]. In response to the receptor activation, USP7 is recruited to NF-κB promoters, where it interacts with NF-κB in a DNA-binding–dependent manner; its deficiency leads to reduced expression of Toll-like receptor (TLR)- and TNFR-induced genes [46]. USP10 inhibits NF-κB activation via disassembling K63-linked NEMO linear ubiquitylation by monocyte chemotactic protein-1-induced protein-1(MCPIP1) [47]. USP11 positively regulates TRAF3 via its deubiquitination activity in ischemia-reperfusion mice model [43]. USP15 enhances NF-κB p65 activation in a TNFα-induced, IκB-α-dependent or independent manner, while NF-κB p65, in turn, upregulates USP15 promoter activity and protein expression [48]. USP18 and USP19 suppress the activation of NF-κB by deubiquitinating TAK1–TAB1 complex via K63- and K27-linked polyubiquitin chains from TAK1 [49, 50]. USP25 acts as a regulator of TLR signaling and regulates TLR4-dependent innate immune responses via targeting and deubiquitinating TRAF3. Upon TLR4 activation, USP25 is recruited to remove K48-linked ubiquitin chains from TRAF3, thereby activating NF-κB and mitogen-activated protein kinase (MAPK) signaling and promoting the production of proinflammatory cytokines [51] (Fig. 1).
Fig. 1.
USPs and NF-κB signaling pathway. NF-κB signaling is activated via canonical or noncanonical pathway. A The canonical pathway is activated by stimulation of TLRs and TNFRs. TLRs recruits MyD88 to form a complex with IRAK1 and IRAK4. Then IRAK1 interacts with E3 ligase TRAF6. TRAF6 functions with E2 enzymes to promote K63-linked polyubiquitination of target proteins, including TRAF6 itself. Ubiquitinated TRAF6 subsequently recruits TAK1 and TABs, which then activates the IKK complex. IKK complex phosphorylates the inhibitory NF-κB subunit IκB, leading to IκB ubiquitination through the SCFβTrCP ligase-dependent proteasome degradation. As a result, NF-κB members NF-κB1 (also named p50), RelA (also named p65), and c-Rel are released and translocated from the cytoplasm to the nucleus for DNA binding and regulation of downstream gene expression. While TNFRs recruit the E3 ligases c-IAP1 and c-IAP2 to promote Lys63 polyubiquitylation of RIP1 and themselves to form a platform with TRAF2/5. This platform interacts with TAK1-TABs complex for further NF-κB activation. Thus, the TAK1-TABs complex is critical for TLRs and TNFRs mediated NF-κB canonical signaling activation. B The noncanonical pathways is mainly dependent on the activation of NF-κB2 (p100)/ RelB complex, which specifically responds to TNFRs receptors such as BAFF and CD40 and recruitment of TRAF2 and TRAF3. The receptor complex interacts with TBK1 and phosphorylates NIK. Ikkα is activated by NIK to phosphorylates p100. Then, p100 is processed to its active form, p52 and translates to the nucleus with RelB for target genes expression. Different USPs mediate deubiquitylations in the process of NF-κB canonical and noncanonical pathways. NF-κB, Nuclear factor-κB; TLRs, Toll-like receptors; TNFRs, tumor Necrosis Factor Receptors; IRAK1/4, Interleukin-1 receptor-associated kinase1/4; TRAF2/3/5/6; TNFR-associated factor 2/3/5/6; TAK1, TGFβ-activated kinase 1; TAB, TAK1-binding protein; TRADD, TNFR1-associated DEATH domain protein; c-IAP1/2, cellular inhibitor of apoptosis 1/2; RIPK1, receptor-interacting protein kinase 1; IKK, NF-κB (IκB) kinase; NIK, NF-κB-inducing kinase; TBK1, TANK-binding kinase 1; BAFF, B-cell activating factor belonging to TNF family; SCFβTRCP, Skp–cullin–F-box–βTRCP; CYLD, cylindromatosis; OTUB1, OTU Deubiquitinase, Ubiquitin Aldehyde Binding 1; USP4/7, Ubiquitin-specific protease 4/7; Ub, ubiquitin; P, phosphorylation
USPs and TGF-β signaling pathway
The transforming growth factor beta (TGF-β) plays important roles in cell differentiation, proliferation, immune regulation, tissue fibrosis, cancer and heart diseases such as cardiomyopathies [52–54]. TGF-β signaling pathway consists of TGF-β isoforms, activins, growth and differentiation factors (GDFs), and bone morphogenetic proteins (BMPs) [55]. This signaling is activated by canonical smad pathway and non-canonical pathway (or p38/JNK pathway) [56–58]. Ubiquitination and deubiquitination regulates TGF-β signaling pathway to affect the occurrence and development of cardiomyopathy. For instance, BAG3 deficiency leads to ubiquitination and proteasomal degradation of transforming growth factor-β receptor 2 (TGFBR2), elevating TGF-β signaling activity and cardiac fibrosis formation, which cause the development of dilated cardiomyopathy (DCM) [59].
Although ubiquitination regulates TGF-β signaling via different E3 ubiquitin ligases [60], the role of USP-mediated deubiquitination in the regulation of TGF-β signaling has been paid more attention in the past decades. USP4 was the first deubiquitination enzyme identified in mammalian cells. USP4 can directly deubiquitylates TβRI, stabilizing it in the plasma membrane [61]. Furthermore, USP4 promotes the phosphorylation of SMAD2, thereby activating the TGF-β signaling pathway by upregulating Relaxin [62]. USP10 has been found to directly interact with SMAD4 to promote SMAD4 deubiquitination in DCM mice [63]. USP11 deubiquitylates activin receptor-like kinase 5 (ALK5), a type I TGF-β receptor that compete with SMAD-specific E3 ubiquitin protein ligase 2 (SMAD-SMURF2) for TβRI and override the negative effects of SMAD7, resulting in the enhancement of TGF-β signaling [64]. USP15 is another positive regulator of TGF-β signaling by binding and deubiquitinating SMURF2 [65, 66]. USP9X, also known as FAM, was reported to deubiquitinate SMURF1, which induces the degradation of SMAD proteins and TβRI. Intriguingly, the USP9X-SMURF1 interaction cannot lead to the USP9X degradation [67]. It has been reported that USP9X also acts as a positive modulator of TGF-β signaling by USP9X-SMAD4 interaction. USP9X selective binds to SMAD4 in competing with the E3 ubiquitin-protein ligase TIF1γ to stabilize nuclear SMAD4, resulting in TGF-β activation, which is facilitated by plasma free fatty acids (FFA) [68]. CYLD and USP26 are found to interact with SMAD7 for its K63-linked polyubiquitination to control TGF-β [69, 70], USP26 deficiency enhances the activation of TGF-β [70].
USPs are also involved in the regulation of TGF-β non-canonical signaling activation. It has been reported that USP4 acts as a negative regulator to inhibit IL-1β-, LPS- and TGF-β-induced NF-κB activation by deubiquitinating TAK1 [45]. USP10 induces activation of TAK1-JNK/p38 signaling pathways to promote hepatocyte inflammation and apoptosis in hepatic I/R injury mouse model [71]. USP10 is also recruited by HSP47 for the deubiquitinating of SMAD4 and activating of TGF-β signaling in myocardial ischemia/reperfusion injury (MIRI) model [72]. While USP18 and USP19 exert antihypertrophic effects by suppressing TAK1-(JNK1/2)/p38 signaling axis based on their deubiquitinating activity of TAK1 [73, 74]. USP22 promotes the pathological process of myocardial hypertrophy by deubiquitinating HIF-1a which further activate the TAK1-(JNK1/2)/p38 signaling pathway [75]. CYLD enhanced ERK- and p38/c-jun pathways in cardiomyocytes [76] (Fig. 2).
Fig. 2.
USPs and TGF-β signaling pathway. The TGF-β signaling pathway is activated via the binding of TGF-β ligands to TGF-β type II receptors (TβRII). After the binding of TβRII, TβRI is recruited to form a functional heterologous complex and is activated as TβRI kinase by TβRII, which transphosphorylases the glutamine synthetase domain of TβRI. Activated TβRI phosphorylates and activates Smad2 and Smad3 (Smad2/3). Activated Smad2/3 interacts with Smad4 to produce an activation complex, which then translocate into nucleus to control the transcription of target genes. Smad7, an I-Smad, blocks the phosphorylation and activation of Smad2/3 by competing with the binding to TβRI. Smad7 also binds to Smurf2 to form an E3 ubiquitin ligase, causing the degradation of receptors. Smurf1 and ALK5 function as competitive with Smurf2 and enhance the turnover of receptors. In addition, TRAF6 and TAK1 facilitate the activation of p38 /MAPK and JNK, resulting in the control of cell growth and proliferation. Different USPs mediate deubiquitinations in the process of TGF-β canonical and noncanonical pathways. TGF-β, transforming growth factor beta; TβRI/II, TGF-β type I/II receptors; SMAD2/3/4, small mothers against decapentaplegic 2/3/4; TRAF6, tumor necrosis factor receptor-associated factor 6; TAK1, TGF-β-activated kinase 1; JNK, c-Jun N-terminal kinase; Smurf1/2, Smad ubiquitin regulatory factor 1/2; ALK5, activin receptor-like kinase 5; I-Smad, inhibitory Smad; CYLD, cylindromatosis; USP4/9X, Ubiquitin-specific protease 4/9X; Ub, ubiquitin; P, phosphorylation
USPs and Nrf2 signaling pathway
Nuclear factor erythroid 2-related factor 2 (NFE2L2/Nrf2) is a transcription factor that plays a central role in cellular antioxidant responses by regulating downstream genes containing antioxidant response elements (AREs) in their promoters [77]. Beyond its antioxidative functions, Nrf2 has emerged as a key regulator of intracellular protein quality control (PQC) and cardiac pathophysiology [78], with accumulating evidence supporting its protective effects in cardiovascular disease progression [79]. Lack of Nrf2 leads to increase oxidative stress in cardiomyocytes and causes cardiac hypertrophy and diabetic cardiomyopathy [22, 80]. Nrf2 activity is highly regulated by Kelch-like ECH-associating protein 1 (Keap1), which act as an adaptor in an E3 ubiquitin ligase complex with the scaffold protein Cullin 3 (Cul3) and Ring box protein 1 (Rbx1) [81]. Under basal conditions, Keap1 and NRF2 form Keap1/Nrf2 complex for proteasome degradation via ubiquitination to ensure Nrf2 maintain at a low level. However, under oxidative stress, Keap1 occurs structural change upon modification by electrophilic molecules so that Nrf2 separates from the Keap1/Nrf2 complex, leading the accumulation of Nrf2 in nucleus [82]. Additionally, two alternative E3 ubiquitin ligase complexes—β-TrCP-SKP1-CUL1-RBX1 and HRD1—have been identified as regulators of Nrf2 stability through distinct ubiquitination mechanisms [83].
Emerging research highlights the critical regulatory roles of deubiquitinating enzymes (USPs) in modulating Nrf2 signaling. Several USP family members target Keap1 to inhibit its K48-linked ubiquitination, including USP7, USP15, USP16, and USP25, a mechanism that accelerates Nrf2 degradation and exacerbates myocardial/hepatic ischemia-reperfusion injury [77, 84–86]. Intriguingly, fibroblast growth factor 18 (FGF18) counteracts this process by suppressing USP16 activity, thereby enhancing Nrf2 levels and protecting against hepatic ischemia-reperfusion injury [85]. In addition to Keap1, USPs can directly target Nrf2 for its deubiquitination. USP8, USP11, and USP29 deubiquitinate and stabilize Nrf2 by removing K48-linked polyubiquitin chains from Nrf2, inhibiting ferroptosis and reducing spinal cord injury [87–89]. Beyond direct interactions, USP28 exerts indirect control by stabilizing TRIM21 (a negative regulator of Nrf2) through deubiquitination, ultimately increasing oxidative stress and driving cardiac hypertrophy [22]. Non-canonical Nrf2 activation pathways involve competitive disruption of the Keap1-Nrf2 complex. The SQSTM1/p62 protein (hereafter p62) binds Keap1 via its Keap1-interacting region (KIR), sequestering Keap1 and preventing Nrf2 ubiquitination [90]. Recent studies reveal USP13 enhances p62 oligomerization by deubiquitinating its PB1 domain at Lys7, thereby strengthening Keap1 binding and promoting Nrf2 nuclear translocation [91]. Similarly, USP8 facilitates p62 activity by removing K11-linked ubiquitination at Lys420 in its ubiquitin-associated (UBA) domain [92] (Fig. 3).
Fig. 3.
USPs and Nrf2 signaling pathway. The key components of the Nrf2 pathway are Nrf2, Keap1 and CUL3 scaffold protein (with Rbx1). CUL3 and Keap1 form dimers for the E3 ubiquitin ligase complex, which binds to one Nrf2 with two binding motifs (DLG and ETGE) and degrades Nrf2 protein. Phosphorylation of p62 remarkedly increases the binding affinity of P62 with Keap1, leading the separation of Nrf2 from Keap1 and accumulation of cytoplasmic Nrf2. Nrf2 is then translocated into nucleus and targets ARE genes. Nrf2, Nuclear factor erythro2-related factor 2 (NFE2L2); Keap1, Kelch-like ECH-associating protein 1; CUL3, Cullin 3; Rbx1, Ring box protein 1; ARE, antioxidant response element; USP, Ubiquitin-specific protease; Ub, ubiquitin; P, phosphorylation
USPs and p53 signaling pathway
P53 is a sequence-specific DNA binding protein which binds to more than 500 target genes to regulate a variety of cellular functions, including DNA repair, cell differentiation, cell-cycle progression, cellular senescence, energy metabolism, and apoptosis [93]. Renowned as the "guardian of the genome," p53 is a pivotal tumor suppressor, with its dysfunction implicated in nearly all cancers. Notably, over 50% of human cancers harbor p53 mutations [94, 95]. Beyond oncology, p53 also exerts critical roles in cardiovascular pathologies [96, 97].
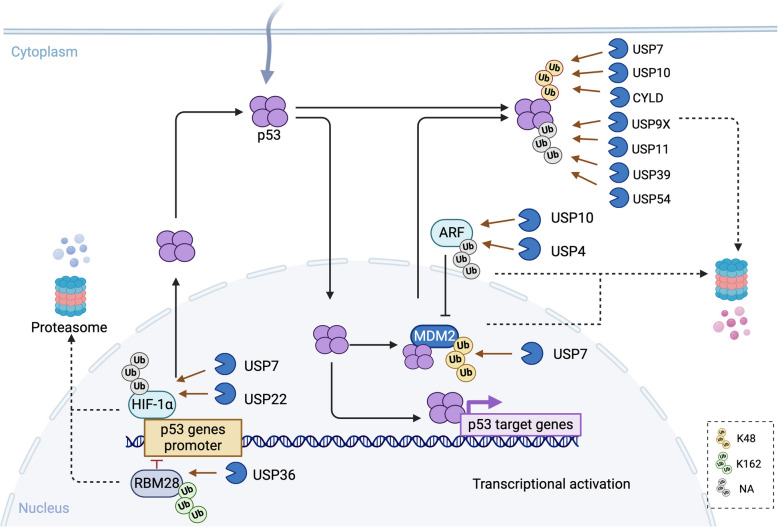
Under basal conditions, p53 is maintained at low levels due to proteasomal degradation mediated by MDM2, an E3 ubiquitin ligase. MDM2 binds p53’s transcriptional activation domain, promoting its ubiquitination and degradation. Intriguingly, MDM2 itself is transcriptionally regulated by p53, forming a tightly controlled autoregulatory feedback loop [98]. In response to stress, p53 increasing is mainly dependent on its stabilization through the posttranslational modification such as deubiquitination, or its transcriptional gene activation. HAUSP (also known as USP7) is the first, but the only identified USP to deubiquitinate both p53 and MDM2. USP7 strongly stabilizes p53 by deubiquitinating p53 both in vitro and in vivo [99], it also binds and deubiquitinates MDM2 in a p53-independent manner and decreasing p53 levels in normal cells [20, 99, 100]. USP10, another key regulator, stabilizes cytoplasmic p53 by reversing MDM2-driven nuclear export [101]. Additionally, USP10 and USP4 deubiquitinate p14ARF—a product of the INK4/ARF locus—which antagonizes MDM2 to enhance p53 stability [102, 103]. USP13 further modulates this network by regulating USP10 degradation, as demonstrated in the heart, lung and liver of newborn mice [104]. Other deubiquitinases, including CYLD, USP9X, USP11, USP39, and USP54, stabilize p53 by cleaving K48- or K63-linked ubiquitin chains, promoting p53 nuclear translocation and activation [105–109]. Conversely, USP36 indirectly suppresses p53 by stabilizing RBM28, a transcriptional repressor of the p53 promoter [110]. Hypoxia inducible factor-1α (HIF-1α) regulates p53 expression. Both USP7 and USP22 stabilize HIF-1α via deubiquitination, amplifying p53 levels in fibroblasts and hepatocellular carcinoma models [111, 112] (Fig. 4).
Fig. 4.
USPs and p53 signaling pathway. p53 forms tetramers and translocate into nucleus, where p53 binds and activates target genes transcription to regulate many different biological processes in response to cellular stress. p53 protein keeps at a very level under normal condition, it is tightly regulated by MDM2, which promotes ubiquitination and proteasome-dependent degradation of p53. p53 itself binds to MDM2 to form a feedback loop. ARF binds to MDM2, leading to the rapid degradation of MDM2 and accumulation of p53. p53 proteins and p53 transcriptional activators HIF-1α can be ubiquitinated and proteasomal degraded in normal condition, whereas it is oppositely regulated by DUBs in stress condition. ARF, alternate open reading frame; MDM2, Mouse double minute 2; HIF-1α, hypoxia inducible factor-1α; RBM28, RNA-binding protein; CYLD, cylindromatosis; USP, Ubiquitin-specific protease; Ub, ubiquitin; P, phosphorylation
USPs and other signaling pathways
The Notch signaling pathway plays a crucial role in regulating cell proliferation, differentiation, development, and homeostasis. Specifically, it regulates cardiac development and is involved in modulating myocardial injury processes, including neovascularization, myocardial fibrosis, cardiomyocyte apoptosis, oxidative stress, and stem cell differentiation [113]. The Notch pathway functions via canonical or non-canonical signaling pathway. In the canonical pathway, activation occurs via Notch ligands and receptors, which ultimately form the DNA-binding transcription factor CBF1/suppressor of hairless/Lag1 (CSL, also called RBPJ) to initiate downstream target gene expression [114]. Subsequently, the disintegrin and metalloproteinase (ADAM) proteins and the γ-secretase complex sequentially cleave the Notch receptor, releasing the Notch intracellular domain (NICD). The NICD then translocates into the nucleus to interact with CSL, forming a transcriptional activation complex through recruitment of mammalian mastermind-like 1–3 (MAML1–3) proteins [115]. In contrast, the non-canonical pathway mediates crosstalk between Notch and other signaling pathways, such as the PI3K/AKT and NF-κB pathways, thereby activating their respective target genes [116].
Emerging evidence indicates that ubiquitin-specific proteases (USPs) critically regulate Notch signaling. For instance, USP10 deubiquitinates NICD1 to promote Notch signaling and regulate myocardial fibrosis in diabetic mice [117]. In endothelial cells (ECs), USP10 directly interacts with Notch1-NICD1, enhancing receptor activation; conversely, endothelial-specific Usp10 knockout mice exhibit excessive vessel sprouting [118]. Similarly, Zhai et al. demonstrated that USP10 stabilizes NICD by removing K11- and K48-linked polyubiquitination chains [119]. USP8 is found to deubiquitinate and stabilize NICD for Notch activation in sepsis mouse model. Upregulation of USP8 expression reduces lipopolysaccharide (LPS) -induced ECs injury. Downregulation of USP8 retardates cellular growth, wound closure, and colony forming [120, 121]. Additionally, USP5 enhances Notch/receptor tyrosine kinase (RTK) signaling via NICD stabilization [122], while USP7 and USP12 directly stabilize Notch proteins through deubiquitination [123, 124]. Notably, USP9X regulates Notch signaling indirectly by stabilizing mindbomb homolog 1 (MIB1), an E3 ligase required for NOTCH ligand endocytosis, thereby enhancing Notch1 activity in porcine aortic valve interstitial cells (pAVICs) [125].
Roles of USPs in cardiomyopathies
USPs in Hypertrophic Cardiomyopathy
Hypertrophic cardiomyopathy (HCM), the most common primary cardiomyopathy, affects individuals across all sexes and ethnic groups. With an estimated global prevalence of 1:200–1:500, this condition affects approximately 750,000 individuals in the United States and 15–20 million worldwide [126]. Pathologically, HCM is characterized by compensatory cardiomyocyte hypertrophy, which initially preserves ventricular wall stress and maintains ejection fraction. However, sustained pathological cardiac hypertrophy eventually leads to an increase of heart failure and mortality [127]. The pathogenesis of cardiac hypertrophy is not fully understood but a complex process of myocyte molecular modification has been involved in HCM [128]. Multiple signaling pathways, such as MAPK, NF-𝜅B, TGF-β, TNF-α, the insulin-like growth factor-I (IGF-I) activated PI3K/AKT/mTOR, and Ca2 + -dependent signaling pathways, have been implicated in HCM pathogenesis [129–131]. Considered that cardiomyocytes are terminally differentiated, the PQC is critical in HCM, giving the importance of DUBs to precisely control protein function, localization, and degradation [132]. Accumulating evidence demonstrates that USPs, the largest member of DUBs with high substrate specificity, are crucial to cardiac pathophysiology in HCM.
TAK1, a key member of the mitogen-activated protein kinase kinase kinase (MAPKKK) family, mediates TGF-β-induced activation of NF-κB, JNK, and p38 signaling [133–135] and has been identified as a central driver of cardiac hypertrophy [136, 137]. Stress-activated MAPKs p38 and JNK1/2 are critically involved in cardiac remodeling progression [138–140], whereas their pharmacological inhibition attenuates hypertrophic development and heart failure [141]. TAK1 activation is regulated by USP18 and USP19, which exert anti-hypertrophic effects by suppressing TAK1-(JNK1/2)/P38 signaling axis based on their deubiquitinating activity of TAK1 [73, 74]. USP18 also suppresses the activation of NF-κB by deubiquitinating TAK1–TAB1 complex [49] via Lys63-linked polyubiquitination of TAK1, triggers the activation of IKK/NF-κB [142]. USP4, a prominently expressed USP in normal heart but significantly reduced in human failing heart and murine HCM model, targets TAK1 to regulate its downstream pathways. Yang et al. reveals that USP4 can deubiquitinate TAK1 timely to downregulate TNFα-mediated activation of NF-κB when TNFα rapidly induced Lys63-linked TAK1 polyubiquitnation [45]. Pu et al. also proves that USP4 can suppress autophosphorylation of TAK1 for deubiquitination and then phosphorylation of JNK and p38-mitogen-activated kinase [143, 144]. CYLD enhances MAPK/ERK mediated p38/c-jun activation, suppressing Nrf2 expression and antioxidative capacity, therefor promoting cardiac remodeling in TCA mouse model [76]. USP22 is expressed in the heart and associated with HCM. A new study reveals that USP22 promotes the pathological process of myocardial hypertrophy by deubiquitinating HIF-1a and further activate the TAK1-(JNK1/2)/P38 signaling pathway [75].
Calcium ion (Ca2 +) plays a vital role in the contraction and relaxation of cardiomyocytes. the sarcoplasmic reticulum (SR) coordinates Ca2⁺ cycling through L-type Ca2⁺ channels (LTCCs) and ryanodine receptor 2 (RyR2)-mediated Ca2⁺ release, with SERCA2a actively replenishing SR Ca2⁺ stores [145, 146]. Many studies have reported that the changes in SERCA2a activity contribute to the pathogenesis of overload-induced cardiac hypertrophy [147, 148], and SERCA2a is regulated by several posttranslational modifications (PTMs) such as deubiquitination [149]. Recently, the role of USP25 in myocardial hypertrophy via deubiquitinating SERCA2a is reported by Ye et al. [21]. They find that USP25 is markedly increased in angiotensin II (Ang II) and transverse aortic constriction (TAC) induced HCM mouse models, while USP25 deficiency can aggravate the cardiac injury in those models. USP25 targets the SERCA2a protein at cysteine 178 to keep it from degradation via K48 ubiquitin chain deconjugation [21]. USP2 overexpression decreases the concentration of Ca2+ and increases SERCA2a by deubiquitinating mitochondrial protein Mitofusin-2 (Mfn2), alleviating mitochondrial dysfunction, and apoptosis in the Ang II-induced cardiac hypertrophy mouse model [150]. USP2 also is reported to stabilize junction plakoglobin (JUP) protein, which block the AKT/β-catenin signaling cascade and alleviate cardiac hypertrophy in ISO-induced mouse model [151].
Highlight the emerging role of protein modification such as Sirtuins protein acetylation and N6-methyladenosine (m6A) modification in cardiomyopathy [152–154]. Notably, the deubiquitination regulation related to these processes holds great importance. For instance, USP10 deubiquitinates Sirt6, which blocks AKT signaling and subsequent Ang II-induced cardiac hypertrophy. Cardiac-specific overexpression of USP10 protects against cardiac hypertrophy [130]. The natural compound Limonin protect USP10 antihypertrophic effect in the same model [155]. Conversely, USP12 is regarded as a positive regulator of Ang II-induced cardiac hypertrophy by deubiquitinating and stabilizing p300 to activate methyltransferase-like 3 (METTL3), which catalyzes m6A modification on messenger RNA and promotes cardiac hypertrophy. Overexpression USP12 aggravates cardiac hypertrophy, whereas inhibition of USP12 ameliorates Ang II-induced myocardial hypertrophy [156]. USP18 is associated with myocardial hypertrophy in a TAC mouse model. In AAV9-cTNT-Usp18 myocardial-specific knockout mice, USP18 downregulation aggravates myocardial remodeling [157]. Similarly, recent studies by Han et al. revealed that USP28 deletion protects against Ang II- and TAC-induced hypertrophy by stabilizing TRIM21 via K48-linked deubiquitination, which suppresses Nrf2-mediated antioxidant responses and reduces oxidative stress [22]. Elevations of cardiomyocyte length and maladaptive eccentric hypertrophy are found by Jean-Charles et al. in USP20 konckout -TAC mice. In this mouse model, they find that USP20 deubiquitinates and prevents the protein degradation of Myosin Heavy Chain 7 (MYH7) through lysine-48 polyubiquitination [158]. Strikingly, USP7 has been found to associate with myocardial hypertrophy. Homozygous of cardiomyocyte-specific USP7 conditional knockout mice died approximately three weeks after birth, and heterozygous knockout mice develops severe hypertrophy, fibrosis and cardiac dysfunction due to disrupted mitochondrial dynamics [159]. Recently, USP38 has been reported to be a harmful factor in the progression of hypertrophic heart failure by removing K48-linked polyubiquitination of TBK1, resulting the stabilization of p-TBK1 and activation of AKT signaling pathway in both Ang II-induced myocardial hypertrophy and pressure overload-induced left ventricular electrical remodeling mouse models [160, 161]. Despite these advances, the precise molecular mechanisms linking USPs to HCM remain incompletely understood, necessitating further investigation (shown in Table 1).
Table 1.
USPs in HCM
| USPs | Molecule targets | Cleaved ubiquitin chain(s) | Mechanisms | Model | Ref |
|---|---|---|---|---|---|
| USP2 | Mfn2 | NA | interacts with Mfn2 for deubiquitination in the Ang II-induced cardiac hypertrophy | Ang II mouse model | [150] |
| JUP | NA | stabilizes JUP protein to block the Akt/β-catenin pathway in the ISO-induced hypertrophic cardiomyopathy | ISO mouse model | [151] | |
| USP4 | TAK1 | K63 | suppresses autophosphorylation of TAK1 for deubiquitination | Human failing heart and murine HCM model | [143, 144] |
| USP10 | Sirt6 | NA | promotes Sirt6 levels by inhibiting its ubiquitination degradation | Ang II mouse model | [130, 155] |
| USP12 | p300 | NA | deubiquitinates p300 to activate METTL3 | Ang II mouse model | [156] |
| USP14 | GSK-3β | NA | increases the phosphorylation of GSK-3β | Ang II or TAC rat model | [162] |
| USP18 | TAK1 | NA | suppresses TAK1-(JNK1/2)/P38 signaling axis based on its deubiquitinating activity to exert antihypertrophic effects | TCA mouse model | [73] |
| USP20 | MYH7 | K48 | deubiquitinates and prevents the protein degradation of MYH7 through lysine-48 polyubiquitination | TCA mouse model | [158] |
| USP22 | HIF-1a | NA | deubiquitinates HIF-1a and further activated the TAK1-(JNK1/2)/P38 signaling pathway | Ang II mouse model | [75] |
| USP25 | SERCA2a | K48 | targets to the cysteine at position 178 of SERCA2a protein and deconjugates the K48 ubiquitin chain | Ang II and TAC mouse model | [21] |
| USP28 | TRIM21 | K48 | deubiquitinating and stabilizing TRIM21 to negatively regulate Nrf2 antioxidant response, leading to increasing oxidative stress in cardiomyocytes and promoting cardiac hypertrophy | Ang II or TAC mouse model | [22] |
| USP38 | TBK1 | K48 | removes K48-linked polyubiquitination of TBK1 to stabilize p-TBK1 | Ang II and TAC mouse model | [160, 161] |
| CYLD | ERK | NA | enhances MAPK/ERK mediated p38/c-jun activation to suppress Nrf2 expression and antioxidative capacity | TCA mouse model | [76] |
USPs in Dilated Cardiomyopathy
Dilated cardiomyopathy (DCM), characterized by left ventricular (LV) or biventricular enlargement as well as systolic dysfunction (ejection fraction < 45%), represents the the structural and compositional alterations of cardiomyocytes and and serves as the final phenotype of multiple underlying disease entities [163]. The estimated prevalence of DCM can be as high as 1:250—1:400 [163, 164]. In 2019, there were 388,388 reported cases in the United States, though the global incidence remains unclear [165]. The symptoms of DCM are severe, often leading to heart failure and sudden death. Approximately 20–30% DCM caused by genetic mutation of about hundred genes such as TTN, LMNA, FLNC, SCN5A, BAG3, MYH7, PLN, RBM20 [166–169], and the acquired causes can include infectious, toxins, metabolic, immunologic, neuromuscular, and radiation [170]. The molecular basis of DCM pathology primarily involves impairment of proteins, especially those produced from gene mutations. These changes affect such as the energy generation (TTN, MYH7 mutation), nuclear envelope (LMNA mutation), spliceosome defect (RBM20 mutation), Ion abnormalities (SCN5A mutation) and force transmission (FLNC mutation) in cardiomyocytes. The alteration of proteins involves abnormal signaling pathways, cytoskeletal structure modifications, and cardiomyocyte dysfunction, where the UPS holds an essential role in cardiomyocyte growth, cell death, fibrosis and inflammation [170].
p53 is a transcription factor that induces pro-apoptotic molecules (e.g., Bax) and activates caspases in cardiomyocytes. It has been reported that p53 highly express in DCM [96, 171, 172]. MDM2, an E3 ubiquitin ligase, mediates p53 ubiquitination and subsequent proteasomal degradation. USP7 cleaves MDM2-mediated p53 ubiquitin forms for p53 stabilization [99, 172]. Birks et al. observed increased levels of both USP7 and p53 in left ventricular myocardial tissues of DCM patients, suggesting that USP7 may contribute to DCM progression [172]. Additionally, DCM heart tissues exhibit high expression of polyubiquitinated proteins and 20S-proteasome chymotrypsin-like proteins, while caspase substrate Poly-ADP-ribose polymerase 1 (PARP-1) is downregulated [172]. Sin et al. showed that USP7 was suppressed by resveratrol via activation of SIRT1 (Sirtuin 1) to reduce p53 and protect doxorubicin (DOX)-induced DCM [173]. Moreover, USP36 has been reported to be a new target molecule to alleviate DOX-induced cardiomyopathy. Wang et al. finds that USP36 expression is increased in the DOX-induced cardiomyopathy, and silencing USP36 can alleviate oxidative stress injury and apoptosis induced by DOX. Mechanistically, USP36 targets PARP1 for its deubiquitination and this function can be abolished by the C131A mutant in USP36 [174].
As is well-known, TGF-β signaling plays a pivotal role in promoting endothelial to mesenchymal transition (EndMT) [175], which has been reported to involve in the cardiac remodeling during DCM development [176]. SMAD proteins, act as the main mediators in the primary signaling of TGF-β family, are phosphorylated in response to activated TGF-β receptors to form homomeric complexes and heteromeric complexes with SMAD4 so that these complexes can accumulate in the nucleus and target downstream genes to regulate transcription [56, 57]. Zhao et al. demonstrated that EndMT and epidermal growth factor‐like repeats and discoidin I‐like domains 3 (EDIL3) are activated in patients and mice with DCM. EDIL3 depletion attenuates EndMT by reducing TGF-β1 and SMAD4, which are the targets of the ubiquitination and degradation by USP10 [63]. USP dysfunction also directly develop DCM [16]. USP5 interacts with the components of the cellular PQC machinery PSMD14 to regulate PQC in adult cardiomyocytes. Deletion of USP5 increases both K48 and K63-linked ubiquitin chains protein accumulation and aggregation, resulting the DCM mice with enlarged ventricles, dilated atria, and substantial reduction of ventricular wall thickness as well as increased left ventricular end-systolic (LVESV) and end-diastolic volumes (LVEDV), highlighting its critical role in maintaining cardiac structure and function [16] (shown in Table 2).
Table 2.
USPs in DCM
| USPs | Molecule targets | Cleaved ubiquitin chain(s) | Mechanisms | Model | Ref |
|---|---|---|---|---|---|
| USP5 | PSMD14 | K48 and K63 | interacts with the components of the cellular PQC machinery PSMD14 to regulate PQC in adult cardiomyocytes | USP5KO and hUSP5 overexpression mouse model | [16] |
| USP7 | p53 | NA | cleaves ubiquitin from modified forms of p53 for stabilization | DCM patients | [99, 172] |
| NA | be suppressed via activation of SIRT1 to reduce p53 and protect DOX-induced DCM by resveratrol | DOX-induced mouse model | [173] | ||
| USP10 | SMAD4 | NA | directly interacts with SMAD4 to maintain its activity in HUVEC, promoting EndMT and exacerbating DCM-induced cardiac dysfunction and remodeling | DOX-induced mouse model | [63] |
| USP36 | PARP1 | NA | targetes PARP1 for deubiquitination and this function could be abolished by the C131A mutant in USP36 | DOX-induced mouse model | [174] |
USPs in Ischemic Cardiomyopathy
Ischemic cardiomyopathy (ICM) is a term that refers to damaged heart muscle that cannot pump blood properly due to myocardial damage caused by ischemia. The term has represented a broader meaning which refers to the cardiomyopathies secondary to coronary artery diseases (CHD) [177]. CHD is characterized by formation of plaques in the coronary arteries, decreasing their capacity to supply nutrients and oxygen, causing ischemia/hypoxia and even myocardial cell death called myocardial infarction (MI). MI is attributed to the thrombotic occlusion of the coronary artery, which remains the leading cause of morbidity and mortality worldwide. Currently, the most effective treatment of MI is percutaneous coronary intervention (PCI), which rapidly restores blood flow to the heart. However, this also leads to the damage of myocardium which is termed as MIRI [178]. Upon ischemic or post-I/R injury, the cardiac cell death initiates the inflammatory phase by promoting the migration of neutrophils and monocytes and release of inflammatory factors, subsequently activating cardiac fibroblasts and leading to cardiac fibrosis and cardiac remodeling [179, 180]. Cardiac fibrosis is characterized by collagen fiber deposition and cell proliferation. After infarction or other cardiomyocyte injury, cardiac fibroblasts are activated and subsequently differentiate into myofibroblasts to repair the damage site [181, 182]. Although the repair process can maintain the integrity of the ventricular structure, it results in cardiac remodeling and further deterioration of cardiac function. TGF-β signaling is well-known in cardiac fibrosis by its stimulating collagen production through promoting the transition of cardiac fibroblast into myofibroblast [183–185]. Recently, Tang et al. finds that cardiac fibroblast USP10 target SMAD4 for its deubiquitination to facilitate the activation of TGF-β/SMAD4 signaling pathway in post-I/R injury, which ultimately leading to cardiac fibrosis [72].
Recently, there is growing evidence that USP plays an important role in the pathogenesis of MIRI by regulating apoptosis, ferroptosis and calcium overload. Ferroptosis, which is characterized by iron accumulation and lipid peroxidation, has been shown to play a vital role in MIRI [166, 186]. Keap1/Nrf2 pathway is one of the most important defense mechanisms against oxidative stress [187] and ferroptosis [166]. There are several studies that have shown the relationship between USP and ferroptosis after MIRI. USP7 and Keap1 are elevated in the MIRI mouse model. Inhibiting USP7 can restore the expression of Keap1 to lower level, which increase Nrf2 accumulation as well as protect myocardium ferroptotic damage induced by I/R injury [84]. Another study by Peng et al. demonstrated that USP7 promotes ferroptosis in MIRI by deubiquitinating and stabilizing p53, which in turn regulates transferrin receptor 1 (TfR1) levels in I/R-treated rat hearts [20]. TfR1 is responsible for iron uptake in cells as well as supposes to be a specific ferroptosis marker [188]. According to several reports, p53 has an effect on iron uptake while played a dual role in regulating ferroptosis as a transcription factor by targeting SLC7A11 to promote ferroptosis [189] or inhibiting DPP4 activity to suppress ferroptosis [190]. Liu et al. demonstrates USP11 can relieve myocardial injury in ischemia-reperfusion rat model. Its mechanism is realized by positively regulating TRAF3 via its deubiquitination activity [43]. CYLD deubiquitinates and stabilizes p53, which serves as a transcriptional factor to positively regulate m7G Methyltransferase1 (Mettl1) in MIRI mouse model. Mettl1 promotes cardiac remodeling during I/R injury. Inhibition of CYLD alleviates cardiomyocytes apoptosis induced by Mettl1 overexpression or oxidative stress [191].
c-Jun N-terminal kinase (JNK)1/2 pathway, as one of the MAPK signaling pathways, takes part in cell cycle, cell growth, and differentiation, as well as mediating cell apoptosis. JNK1/2 can be phosphorylated by dual-specificity protein phosphatases (DUSP), which is known as MAPK phosphatases (MKPs). USP49 positively regulates DUSP1 expression through deubiquitinating DUSP1 in AC16 cardiomyocytes and I/R-induced mouse model. USP49 knockdown Inhibits JNK1/2 activation and cell apoptosis [192]. USP47 has been implicated in the progression of MIRI through NF-κB pathway activation [193]. However, this USP47-mediated NF-κB activation is due to UP47 promoting NF-κB promoter activity [193]. Song et al. finds that USP22 deubiquitinates and stabilizes Lysine Demethylase 3A (KDM3A), and KDM3A inhibits histone 3 lysine 9 di-methylation (H3K9me2) levels in the Yes1 Associated Transcriptional Regulator ( YAP1) promoter to elevate YAP1 transcription, ultimately mitigating MIRI in rat models [194] (shown in Table 3).
Table 3.
USPs in ICM
| USPs | Molecule targets | Cleaved ubiquitin chain(s) | Mechanisms | Model | Ref |
|---|---|---|---|---|---|
| USP7 | Keap1 | NA | binds Keap1 for deubiquitination to reduce Nrf2 accumulation in the nucleus and increase ROS production | MIRI mouse model | [84] |
| p53 | NA | targets p53 which regulated TfR1 protein level | MIRI rat model | [20] | |
| USP10 | SMAD4 | NA | targetes SMAD4 for deubiquitination to facilitate the activation of TGF-β/SMAD4 signaling pathway | MIRI mouse model | [72] |
| USP11 | TRAF3 | NA | positively regulates TRAF3 via its deubiquitination activity | MIRI rat model | [43] |
| USP22 | KDM3A | NA | deubiquitinates and stabilizes KDM3A protein levels to elevate YAP1 transcription | MIRI rat model | [194] |
| USP47 | NF-kB | NA | promotes apoptosis in rat myocardial cells after ischemia/reperfusion injury via NF-kB activation | MIRI mouse model | [193] |
| USP49 | DUSP1 | NA | stabilize DUSP1 expression to activate JNK1/2 signaling | MIRI mouse model | [192] |
| CYLD | p53 | NA | deubiquitinates and stabilizes p53 to positively regulate m7G Methyltransferase1 (Mettl1) in MIRI mouse model | MIRI mouse model | [191] |
USPs in Diabetic Cardiomyopathy
Diabetes mellitus (DM) is a group of metabolic disorders with high morbidity worldwide. In 2017, there are 425 million people suffering from this disease and the number is projected to rise to 693 million by 2045 [195]. defined by leading cardiology societies as ventricular dysfunction in diabetic patients without coronary atherosclerosis or hypertension, is driven by multifactorial mechanisms including mitochondrial dysfunction, metabolic dysregulation, oxidative stress, endothelial dysfunction, and autonomic dysfunction [196–198]. In the diabetic heart, fatty acid (FA) oxidation becomes almost the only source of ATP production [199], which produces high level of oxidative stress, thereby exacerbate the progression of DiaCM.
The peroxisome proliferator-activated receptors (PPARs), a family of transcription factors composed of three tissue-specific distribution isoforms including PPARα, PPARβ/δ, and PPARγ, modulate various biological processes such as lipid metabolism, glucose homeostasis, Ca2 + handling, inflammation and oxidative stress [200, 201]. A network of PPARs plays an essential role in the regulation of myocardial energy and fatty acid homeostasis [202]. In T2DM, activation of PPARα promotes mitochondrial FA oxidation [203]. FA in the cytoplasm can activate PPARγ, which in turn increases expression of the fatty acid translocase CD36 to transport free fatty acid (FFA) to adipose tissue, thereby reducing the lip toxicity of the myocardium [204]. CD36 also uptakes long-chain fatty acids (LCFAs) and involves in the regulation of metabolism during DiaCM [204, 205]. Emerging evidence highlights the role of deubiquitination in PPAR regulation. For instance, USP7 has been reported to be upregulated in DiaCM and deubiquitinate PGC-1β to increase PPARα. USP7 knockout resists mitochondria dysfunction and attenuates diabetic cardiomyopathy [206]. Mfn2 has been shown to ameliorate mitochondrial dysfunction by promoting mitochondrial fusion and PPARα promotes Mfn2 transcription by binding to Mfn2 promoter. USP28 deubiquinates and stabilizes PPARα (Lys152) and therefore promote Mfn2 activation to maintain mitochondria homeostasis [203]. USP28 is decreased significantly in the db/db mouse hearts and diabetic patients’ hearts, USP28 overexpression had a cardioprotective effect on the high-fat diet/streptozotocin–induced type 2 diabetes mice [203]. PPARγ is deubiquitinated by USP22 via K48-linked deubiquitination chains to regulate lipid accumulation [207]. CD36 is the target of the E3 ligase [205, 208] and can be regulated by USP. USP14 can stabilize CD36 protein via cleaving ubiquitin chains from ubiquitinated CD36 proteins in macrophages [209], and USP11 mediates the deubiquitination of CD36 in heart to protect it from degradation [210].
Advanced glycation end products (AGEs) are long-lasting molecules from advanced, nonenzymatic glycosylation by excess sugars during DM. The accumulation of AGEs induces the development and progression of DiaCM via receptor dependent pathways [197, 211]. On the one hand, AGEs interact with AGE receptor (RAGE) to activate NF-κB signaling to induce oxidative stress and enhance extracellular matrix accumulation [197]. AGEs can promote collagen degradation by causing crosslinks of collagen molecules. The crosslinked AGEs are found in SERCA2a, which may contribute to the impairment of sarcoplasmic reticulum Ca2 + re-uptake in cardiac myocytes [212, 213]. SERCA2a is regulated by ubiquitination and deubiquitination [21]. USP25 is involved in the maintenance of intracellular calcium handling in cardiomyocytes by deubiquitinating and stabilizing SERCA2a [21].
It has been shown that USP24 promotes ferroptosis by activating NF-κB in DiaCM model [214]. In a high-fat diet and streptozotocin-induced mouse DCM model, increased expression of USP24, NF-κB, and ferroptosis marker are detected. However, it is not clear whether this effect is mediated through direct or indirect USP24- NF-κB regulation [214]. USP10 has been shown that it can activate.
Notch1 to exert protective effect on MI in T2DM mice [117], and mediates the deubiquitination and activation of AMPKα to alleviate the progression of diabetic vascular calcification (VC) [215]. Although the exact mechanisms need to be further explored (shown in Table 4).
Table 4.
USPs in DiaCM
| USPs | Molecule targets | Cleaved ubiquitin chain(s) | Mechanisms | Model | Ref |
|---|---|---|---|---|---|
| USP7 | PGC-1β | NA | deubiquitinates PGC-1β to increase PPARα levels | high-fat diet /STZ-induced mouse model | [206] |
| USP10 | Notch1 | NA | activates Notch1 to exert protective effect on MI in T2DM mice | high-fat diet /STZ-induced mouse model | [117] |
| USP24 | NF-κB | NA | activates NF-κB to promote ferroptosis in DiaCM models | high-fat diet /STZ-induced mouse model | [214] |
| USP28 | PPARα | NA | interacts with PPARα for deubiquination to promote Mfn2 transcription and maintain mitochondria homeostasis | high-fat diet /STZ-induced mouse model | [203] |
Therapeutic potential of USP in cardiomyopathy
USPs play critical roles in deubiquitinating and stabilizing proteins, changing protein level, protein localization, protein activity, and protein–protein interactions. These regulatory effects are essential for signal transduction pathways that govern cellular proliferation, growth, and apoptosis in both physiological and pathological conditions. Individual USP selectively recognize and stabilize the target proteins, exerting either positive or negative regulatory effects on various signaling pathways. Given their involvement in numerous diseases, USPs have emerged as promising therapeutic targets in recent years. Notably, USP inhibitors have demonstrated significant anti-cancer potential [18, 26, 216]. However, the application of USP inhibitors, agonists or other modulatory compounds used in cardiomyopathy treatment remain limited and have not been summarized.
Therapeutic potential in HCM
In a transverse aortic constriction (TAC) mouse model, USP18 is found to be associated with myocardial hypertrophy through TAK1/p38/JNK1/2 pathway, USP18 deficiency enhances TAK1 activity in response to pressure overload [73], whereas USP18 overexpression attenuates pathological cardiac remodeling [157]. The natural flavonoid cardamonin (CAR), a specific USP18 agonist, significantly improves cardiac function in TAC mice by enhancing USP18-mediated anti-hypertrophic effects, as evidenced by increased ejection fraction (EF%), reduced B-type natriuretic peptide (BNP) levels, and attenuated myocardial inflammation/fibrosis [157]. 5Z-7-Oxozeaenol, a specific TAK1 inhibitor, reverses the prohypertrophic effect of USP18 deficiency in TAC mouse model [73]. In angiotensin II (Ang II)-induced hypertrophy models, the citrus-derived compound limonin promotes nuclear translocation of USP10, thereby activating SIRT6-dependent signaling to suppress hypertrophy [130]. Mechanistically, limonin administration elevates nuclear/cytoplasmic USP10 levels, enhances PPARα expression, and increases mice EF (%) and fractional shortening(%). Amlexanox, an inhibitor of TBK1, has been found to be associated with USP38 mediated cardiac remodeling. USP38 overexpression promotes polyubiquitination of TBK1, resulting in the stabilization of p-TBK1 and activation of AKT signaling pathway in Ang II-induced myocardial hypertrophy mouse model. Amlexanox protects USP38 mediated cardiac remodeling, decreasing the protein expression of USP38-mediated TBK1/AKT-GSK3β/mTOR signaling pathway [158]. Otilonium Bromide (OB), a USP28 inhibitor, protects mouse heart against Ang II- or TAC-induced cardiac dysfunction and hypertrophy by inhibiting USP28 whose activity related K48-linked deubiquitinating and stabilizing TRIM21. TRIM21 negatively regulates Nrf2 antioxidant response, leading to increasing oxidative stress in cardiomyocytes and promoting cardiac hypertrophy [22].
Therapeutic potential in DCM
Resveratrol, a natural compound found in red grape peanuts and berries, has been reported to decrease USP7 and p53 levels via SIRT1 activation in DOX-induced cardiotoxicity mouse model [173].
Therapeutic potential in ICM
Col003, a heat shock protein 47 (HSP47) inhibitor, inhibits hypoxia-induced fibrogenesis via impeding the HSP47-mediated USP10/TGFβ1/Smad4 pathway in adult mouse cardiac fibroblasts [72]. In this study, USP10 is recruited by HSP47 to deubiquitinate SMAD4, thereby activating TGF-β signaling in a left anterior descending coronary artery (LAD) ligation MIRI model. HSP47 is highly expressed in both patients and mice cardiac fibroblast cells from ischemic cardiac pathogenesis. In this context, HSP47 facilitates USP10 recruitment to deubiquitinate SMAD4, which leads to increased activation of TGFβ1. This process promotes fibrosis and fibroblast proliferation in ischemic hearts. Targeting HSP47 with Col003 disrupts fibrogenesis in cardiac fibroblasts, suggesting its potential as a therapeutic strategy to combat fibrosis in ischemic heart conditions [72]. However, this drug has only been tested in vitro, and its anti-fibrosis effects in vivo still need to be further validated.
Therapeutic potential in DiaCM
Adeno-associated virus (AAV) therapy might be a potential approach in diabetic hearts. Follistatin-like protein 1 (FSTL1) has been shown to activate the USP10/Notch1 signaling pathway, which is associated with myocardial apoptosis in T2DM mice. AAV9-FSTL1 is established to improve cardiac function following MI by reducing serum lactate dehydrogenase (LDH), apoptosis and fibrosis in T2DM mice [117]. Furthermore, USP28 plays a role in attenuating DiaCM progression through the PPARα-Mfn2 axis in diabetic hearts [203]. AAV9-USP28 administration attenuates cardiac remodeling and dysfunction, lipid accumulation, and mitochondrial impairment in high-fat diet/streptozotocin-induced type 2 diabetes mice [203]. These findings suggest the clinical relevance and therapeutic potential of AAV in T2DM-associated MI and cardiovascular diseases.
Conclusion and perspectives
USPs are a major class of DUBs with the ability to deubiquitinate substrate proteins. However, the balance between ubiquitination and deubiquitination becomes such important because the properly coordinated proteostasis is critical in response to cellular stimuli and stressors. PQC, now known to be regulated by USPs, is even more important in heart. USPs regulate many biological processes, including cell differentiation, DNA damage repair, receptor internalization, and immune response. These are the basis for cell physiology and homeostasis. In the past decades, abundant evidence has been gathered to show the link between USPs and diseases such as cancer. More recently, an increasing number of studies showed an emerging role of USPs in the occurrence and development of heart diseases, especially cardiomyopathies. The regulatory mechanisms of USPs are crucial for us to understand their function in cardiomyopathy development. The relationships between USPs and cardiomyopathies are often complex and multifaceted. Abnormal expression of USPs leads to imbalances in ubiquitin modification, which can have significant implications in disease progression. Several USPs are involved in regulating multiple substrate proteins, or an individual USP may influence multiple disease-associated pathways, sometimes exhibiting contrasting effects—disease-promoting or disease-protecting—depending on the specific context and type of cardiomyopathy.
A prime example is USP10, which plays a critical role in various cellular processes and many human diseases [217]. In HCM, USP10 deubiquitinates Sirt6, which blocks AKT signaling and protects against cardiac hypertrophy [130]. Conversely, in dilated cardiomyopathy (DCM), USP10 activates SMAD4/TGF-β1 signaling to promote endothelial-mesenchymal transition, exacerbating disease progression [63]. In ICM, cardiac fibroblast USP10 also targets SMAD4 for its deubiquitination to facilitate the activation of TGF-β/SMAD4 signaling pathway, which ultimately leading to cardiac fibrosis [72]. In DiaCM, USP10 has been shown that it can activate Notch1 to exert protective effect on MI in T2DM mice [117]. These findings are not limited to cardiomyopathy, as USP10 has been shown to regulate cellular metabolism, DNA damage, and tumorigenesis. USP10 mediates AMPKα deubiquitination, facilitating its phosphorylation at Thr172, thus, regulates metabolic homeostasis [218]. USP10 regulates Notch signaling by stabilizing the Notch1 intracellular domain (NICD1) in endothelial cells of mouse retina, influencing retinal angiogenesis and vascular homeostasis [118]. In cancer, USP10 primarily acts as a tumor suppressor, though it can also function as an oncogene. The most important targets of USP10 in cancer is p53 [101]. Additionally, USP10 can suppress SIRT6 ubiquitination, thus protecting it from proteasomal degradation, which in turn inhibits cell-cycle progression and tumor formation [219]. USP10 has been shown to correlate with cancer progression and poor overall patient survival, particularly in prostate cancer, where it stabilizes G3BP2 through polyubiquitination. Increased G3BP2 activity inhibits p53 functionality [220]. Another USP, called USP7 (also known as HAUSP), plays a critical role in the development of different cardiomyopathy. Its associated targets include p53 and SIRT1/p53 axis in DCM [99, 172, 173], Keap1/Nrf2 and p53/TfR1 in ICM [20, 84], PGC-1β and PPARα signaling in DiaCM [206]. Despite significant advances in characterizing USP functions in cardiomyopathy, the molecular mechanisms underlying their context-dependent regulatory duality—wherein individual USPs exert opposing inhibitory or activating effects across distinct cardiomyopathy subtypes or disease stages—remain incompletely elucidated.
Detailly, USPs regulate several signaling pathways associated with cardiomyopathy pathogenesis such as ROS, fibrosis, chronic inflammation and abnormal metabolism, leading to progression of cardiomyopathy. We have summarized the regulatory roles of USPs in several signaling pathways regulated by USPs, including NF-κB pathway (mainly in HCM, ICM and DiaCM), TGF-β pathway (mainly in HCM, DCM and ICM), Nrf2 signaling pathway (in ICM). Various other relevant pathways involved in the development of cardiomyopathies also have been reported recently, such as PPAR signaling pathways regulated by USP7 and USP28 in DiaCM, PARP1 related pathway regulated by USP36 in DCM, Notch1 pathway regulated by USP10 (in DiaCM). The interaction between certain USPs and target proteins that participate in signaling pathways involved in different cardiomyopathies was summarized in Tables 1, 2, 3 and 4 and Fig. 5. However, our summarization is limited from adequate, and thus, more regulatory roles of USPs in cardiomyopathy need to be elucidated. Targeting USPs may be a therapeutic potential to treat cardiomyopathy in the future.
Fig. 5.
USPs and cardiomyopathies. The USPs regulate several signaling pathways in cardiomyopathies. Propelled by these signaling pathways, the cardiomyocytes gradually undergo oxidative stress and chronic inflammation and abnormal metabolism, leading to the pathological processes of heart hypertrophy, fibrosis, apoptosis, and cell death, resulting in cardiac dysfunction and advancing the development of cardiomyopathies
Abbreviations
- ADAM10
A disintegrin and metalloprotease 10
- Ang II
Angiotensin II
- ALK5
Activin receptor-like kinase 5
- ARE
Antioxidant response element
- BAFF
B-cell activating factor
- BAG3
BAG cochaperone 3
- CSL
CBF1/suppressor of hairless/Lag1
- Cul3
Cullin 3
- CYLD
Cylindromatosis
- DCM
Dilated cardiomyopathy
- DiaCM
Diabetic cardiomyopathy
- DOX
Doxorubicin
- DUBs
Deubiquitinating enzymes
- EndMT
Endothelial to mesenchymal transition
- ER
Endoplasmic reticulum
- FGF18
Fibroblast Growth Factor 18
- FLNC
Filamin C, Gamma
- FOXO
Forkhead box subfamily O
- GLUT1
Glucose transporter 1
- GSK-3β
Glycogen synthase kinase 3β
- HAUSP
Herpesvirus-associated ubiquitin-specific protease
- H3K9me2
Histone 3 lysine 9 di-methylation
- HCC
Hepatocellular carcinoma
- HCM
Hypertrophic cardiomyopathy
- HIF-1α
Hypoxia inducible factor-1α
- ICM
Ischemic cardiomyopathy
- IGF-I
Insulin-like growth factor-I
- IRAK1/4
Interleukin-1 receptor-associated kinase1/4
- JUP
Junction Plakoglobin
- PFKFB3
6-Phosphofructo-2-Kinase/Fructose-2,6-Biphosphatase 3
- PI3K
Phosphoinositide 3-kinase
- PSMD14
Proteasome 26S Subunit, Non-ATPase 14
- c-JNK
C-Jun N-terminal kinase
- Keap1
Kelch-like ECH-associating protein 1
- KDM3A
Lysine Demethylase 3A
- KLF2
Kruppel-like factor 2
- LPS
Lipopolysaccharide
- LTCC
L-type Ca 2 + channel
- LUBAC
Linear ubiquitin chain assembly complex
- MAPK
Mitogen-activated protein kinase
- MAML1–3
Mammalian mastermind-like 1–3
- MCPIPs
Monocyte chemotactic protein-induced proteins
- MDM2
Mouse double minute 2
- Mfn2
Mitofusin-2
- MYH7
Myosin heavy chain 7
- MI
Myocardial infarction
- MINDYs
Motif interacting with ubiquitin-containing novel DUB family
- MIB1
Mindbomb homolog 1
- MIRI
Myocardial ischemia/reperfusion injury
- MJDs
Machado-Joseph disease protein domain protease
- MYH7
Myosin Heavy Chain 7
- NeXT
Notch extracellular truncation
- NECD
Notch extracellular domain) and
- NICD
Notch intracellular domain
- NF-κB
Nuclear factor-kB
- Nrf2
Nuclear factor erythro2-related factor 2
- eNOS
Endothelial nitric oxide synthase
- OTUs
Ovarian tumor proteases
- OTUB1
Ubiquitin Aldehyde Binding 1
- PARP-1
Poly -ADP-ribose polymerase 1
- PDK1
Phosphoinositide dependent kinase 1
- PIP2
Phosphatidylinositol 4,5-bisphosphate
- PIP3
Phosphatidylinositol 3,4,5-trisphosphate
- PQC
Protein quality control
- PLN
Phospholamban
- PPAR
Peroxisome proliferator-activated receptors
- PTEN
Phosphatase and tensin homolog
- PTM
Posttranslational modification
- RBM20
RNA binding motif protein 20
- Rbx1
Ring box protein 1
- RIPK1
Receptor-interacting protein kinase 1
- ROS
Reactive oxygen species
- SCFβTRCP
Skp–cullin–F-box–βTRCP
- SERCA2a
Sarcoplasmic/endoplasmic reticulum Ca2 + ATPase 2a
- SIRT1
Sirtuin 1
- SMAD
Small mothers against decapentaplegic
- SMURF1/2
SMAD-specific E3 ubiquitin protein ligase 1/2
- mTORC1/2
Mammalian target of rapamycin complex 1/2
- TAC
Transverse aortic constriction
- TAK1
TGF-ꞵ-activated kinase 1
- TBK1
TANK-binding kinase 1
- TGF-ꞵ
Transforming growth factor beta
- TβRI
TGF-β type I serine/threonine kinase receptor
- TMD
Transmembrane domain
- TLR
Toll-like receptor
- TNF-α
Tumor necrotic factor-alpha
- TNFR
Tumor necrosis factor receptor
- TRAF2
TNF receptor-associated factor 2
- UCHs
Ubiquitin C-terminal hydrolases
- UPS
Ubiquitin proteasome system
- USP
Ubiquitin-specific protease
- UBE2L3
Ubiquitin-conjugating enzyme E2L3
- VEGF
Vascular endothelial growth factor
- YAP1
Yes1 Associated Transcriptional Regulator
- ZUFSP/ZUP1
Zinc finger with UFM1-specific peptidase domain protein
Authors’ contributions
Conceptualization and design: D.L. and Q.M. Writing of first draft: D.L. Figures and tables: D.L. Final editing of text: Q.M. All authors reviewed the manuscript.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, Moss AJ, Seidman CE, Young JB. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association scientific statement from the council on clinical cardiology, heart failure and transplantation committee; quality of care and outcomes research and functional genomics and translational biology interdisciplinary working groups; and council on epidemiology and prevention. Circulation. 2006;113:1807–16. [DOI] [PubMed] [Google Scholar]
- 2.Gilda JE, Gomes AV. Proteasome dysfunction in cardiomyopathies. J Physiol. 2017;595:4051–71. 10.1113/JP273607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dantuma NP, Lindsten K. Stressing the ubiquitin-proteasome system. Cardiovasc Res. 2010;85:263–71. 10.1093/cvr/cvp255. [DOI] [PubMed] [Google Scholar]
- 4.Li Y, Reverter D. Molecular mechanisms of DUBs Regulation in signaling and disease. Int J Mol Sci. 2021;22. 10.3390/ijms22030986. [DOI] [PMC free article] [PubMed]
- 5.Han D, Wang L, Jiang S, Yang Q. The ubiquitin-proteasome system in breast cancer. Trends Mol Med. 2023;29:599–621. 10.1016/j.molmed.2023.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Tsukamoto O, Minamino T, Kitakaze M. Functional alterations of cardiac proteasomes under physiological and pathological conditions. Cardiovasc Res. 2010;85:339–46. 10.1093/cvr/cvp282. [DOI] [PubMed] [Google Scholar]
- 7.Popovic D, Vucic D, Dikic I. Ubiquitination in disease pathogenesis and treatment. Nat Med. 2014;20:1242–53. 10.1038/nm.3739. [DOI] [PubMed] [Google Scholar]
- 8.Scheffner M, Nuber U, Huibregtse JM. Protein ubiquitination involving an E1–E2-E3 enzyme ubiquitin thioester cascade. Nature. 1995;373:81–3. 10.1038/373081a0. [DOI] [PubMed] [Google Scholar]
- 9.Yau R, Rape M. The increasing complexity of the ubiquitin code. Nat Cell Biol. 2016;18:579–86. 10.1038/ncb3358. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Liu S, Li S. Mechanisms underlying linear ubiquitination and implications in tumorigenesis and drug discovery. Cell Commun Signal. 2023;21:340. 10.1186/s12964-023-01239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao L, Zhang W, Shi XH, Chang X, Han Y, Liu C, Jiang Z, Yang X. The mechanism of linear ubiquitination in regulating cell death and correlative diseases. Cell Death Dis. 2023;14:659. 10.1038/s41419-023-06183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dewson G, Eichhorn PJA, Komander D. Deubiquitinases in cancer. Nat Rev Cancer. 2023;23:842–62. 10.1038/s41568-023-00633-y. [DOI] [PubMed] [Google Scholar]
- 13.Clague MJ, Urbé S, Komander D. Breaking the chains: deubiquitylating enzyme specificity begets function. Nat Rev Mol Cell Biol. 2019;20:338–52. 10.1038/s41580-019-0099-1. [DOI] [PubMed] [Google Scholar]
- 14.Cruz L, Soares P, Correia M. Ubiquitin-Specific proteases: players in cancer cellular processes. Pharmaceuticals (Basel). 2021;14. 10.3390/ph14090848. [DOI] [PMC free article] [PubMed]
- 15.Tobias JW, Varshavsky A. Cloning and functional analysis of the ubiquitin-specific protease gene UBP1 of Saccharomyces cerevisiae. J Biol Chem. 1991;266:12021–8. [PubMed] [Google Scholar]
- 16.Eibach Y, Kreher S, Poetsch MS, Kho AL, Gaertner U, Clemen CS, Schröder R, Guo K, Milting H, Meder B, et al. The deubiquitinase USP5 prevents accumulation of protein aggregates in cardiomyocytes. Sci Adv. 2025;11:eado3852. 10.1126/sciadv.ado3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lill JR, Wertz IE. Toward understanding ubiquitin-modifying enzymes: from pharmacological targeting to proteomics. Trends Pharmacol Sci. 2014;35:187–207. 10.1016/j.tips.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Gao H, Xi Z, Dai J, Xue J, Guan X, Zhao L, Chen Z, Xing F. Drug resistance mechanisms and treatment strategies mediated by Ubiquitin-Specific Proteases (USPs) in cancers: new directions and therapeutic options. Mol Cancer. 2024;23:88. 10.1186/s12943-024-02005-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitamura H. Ubiquitin-Specific Proteases (USPs) and metabolic disorders. Int J Mol Sci. 2023;24. 10.3390/ijms24043219. [DOI] [PMC free article] [PubMed]
- 20.Tang LJ, Zhou YJ, Xiong XM, Li NS, Zhang JJ, Luo XJ, Peng J. Ubiquitin-specific protease 7 promotes ferroptosis via activation of the p53/TfR1 pathway in the rat hearts after ischemia/reperfusion. Free Radic Biol Med. 2021;162:339–52. 10.1016/j.freeradbiomed.2020.10.307. [DOI] [PubMed] [Google Scholar]
- 21.Ye B, Zhou H, Chen Y, Luo W, Lin W, Zhao Y, Han J, Han X, Huang W, Wu G, et al. USP25 ameliorates pathological cardiac hypertrophy by stabilizing SERCA2a in cardiomyocytes. Circ Res. 2023;132:465–80. 10.1161/CIRCRESAHA.122.321849. [DOI] [PubMed] [Google Scholar]
- 22.Han J, Lin L, Fang Z, Ye B, Han X, Xu J, Han B, Min J, Qian J, Wu G, et al. Cardiomyocyte-derived USP28 negatively regulates antioxidant response and promotes cardiac hypertrophy via deubiquitinating TRIM21. Theranostics. 2024;14:6236–48. 10.7150/thno.99340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xian Y, Ye J, Tang Y, Zhang N, Peng C, Huang W, He G. Deubiquitinases as novel therapeutic targets for diseases. MedComm. 2020;2024(5):e70036. 10.1002/mco2.70036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samuel VP, Moglad E, Afzal M, Kazmi I, Alzarea SI, Ali H, Almujri SS, Abida, Imran M, Gupta G, et al. Exploring Ubiquitin-specific proteases as therapeutic targets in Glioblastoma. Pathol Res Pract. 2024;260:155443. 10.1016/j.prp.2024.155443. [DOI] [PubMed]
- 25.Xia G, Guo Y, Zhang J, Han M, Meng X, Lv J. An overview of the deubiquitinase USP53: a promising diagnostic marker and therapeutic target. Curr Protein Pept Sci. 2024;25:708–18. 10.2174/0113892037292440240518194922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao H, Yin J, Ji C, Yu X, Xue J, Guan X, Zhang S, Liu X, Xing F. Targeting ubiquitin specific proteases (USPs) in cancer immunotherapy: from basic research to preclinical application. J Exp Clin Cancer Res. 2023;42:225. 10.1186/s13046-023-02805-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morgan MJ, Liu Z-g. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011;21:103–15. 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu R-M, Desai LP. Reciprocal regulation of TGF-β and reactive oxygen species: a perverse cycle for fibrosis. Redox Biol. 2015;6:565–77. 10.1016/j.redox.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris IS, DeNicola GM. The complex interplay between antioxidants and ROS in cancer. Trends Cell Biol. 2020;30:440–51. 10.1016/j.tcb.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Liu B, Chen Y, St Clair DK. ROS and p53: a versatile partnership. Free Radic Biol Med. 2008;44:1529–35. 10.1016/j.freeradbiomed.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xuan W, Khan M, Ashraf M. Extracellular vesicles from notch activated cardiac mesenchymal stem cells promote myocyte proliferation and neovasculogenesis. Front Cell Dev Biol. 2020;8:11. 10.3389/fcell.2020.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alzahrani AM, Rajendran P, Veeraraghavan VP, Hanieh H. Cardiac protective effect of kirenol against doxorubicin-induced cardiac hypertrophy in H9c2 cells through Nrf2 signaling via PI3K/AKT pathways. Int J Mol Sci. 2021;22. 10.3390/ijms22063269. [DOI] [PMC free article] [PubMed]
- 33.Ghosh S, Hayden MS. New regulators of NF-kappaB in inflammation. Nat Rev Immunol. 2008;8:837–48. 10.1038/nri2423. [DOI] [PubMed] [Google Scholar]
- 34.Oeckinghaus A, Ghosh S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol. 2009;1:a000034. 10.1101/cshperspect.a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gordon JW, Shaw JA, Kirshenbaum LA. Multiple facets of NF-kappaB in the heart: to be or not to NF-kappaB. Circ Res. 2011;108:1122–32. 10.1161/CIRCRESAHA.110.226928. [DOI] [PubMed] [Google Scholar]
- 36.Van der Heiden K, Cuhlmann S, le Luong A, Zakkar M, Evans PC. Role of nuclear factor kappaB in cardiovascular health and disease. Clin Sci (Lond). 2010;118:593–605. 10.1042/CS20090557. [DOI] [PubMed] [Google Scholar]
- 37.Li Y, Ha T, Gao X, Kelley J, Williams DL, Browder IW, Kao RL, Li C. NF-kappaB activation is required for the development of cardiac hypertrophy in vivo. Am J Physiol Heart Circ Physiol. 2004;287:H1712-1720. 10.1152/ajpheart.00124.2004. [DOI] [PubMed] [Google Scholar]
- 38.Sun S-C. Deubiquitylation and regulation of the immune response. Nat Rev Immunol. 2008;8:501–11. 10.1038/nri2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gerlach B, Cordier SM, Schmukle AC, Emmerich CH, Rieser E, Haas TL, Webb AI, Rickard JA, Anderton H, Wong WWL, et al. Linear ubiquitination prevents inflammation and regulates immune signalling. Nature. 2011;471:591–6. 10.1038/nature09816. [DOI] [PubMed] [Google Scholar]
- 40.Haas TL, Emmerich CH, Gerlach B, Schmukle AC, Cordier SM, Rieser E, Feltham R, Vince J, Warnken U, Wenger T, et al. Recruitment of the linear ubiquitin chain assembly complex stabilizes the TNF-R1 signaling complex and is required for TNF-mediated gene induction. Mol Cell. 2009;36:831–44. 10.1016/j.molcel.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 41.Reiley W, Zhang M, Wu X, Granger E, Sun S-C. Regulation of the deubiquitinating enzyme CYLD by IkappaB kinase gamma-dependent phosphorylation. Mol Cell Biol. 2005;25:3886–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reiley WW, Jin W, Lee AJ, Wright A, Wu X, Tewalt EF, Leonard TO, Norbury CC, Fitzpatrick L, Zhang M, et al. Deubiquitinating enzyme CYLD negatively regulates the ubiquitin-dependent kinase Tak1 and prevents abnormal T cell responses. J Exp Med. 2007;204:1475–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y, Hailati J, Ma X, Midilibieke H, Liu Z. Ubiquitin-specific protease 11 aggravates ischemia-reperfusion-induced cardiomyocyte pyroptosis and injury by promoting TRAF3 deubiquitination. Balkan Med J. 2023;40:205–14. 10.4274/balkanmedj.galenos.2023.2022-12-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiao N, Li H, Luo J, Wang R, Chen H, Chen J, Wang P. Ubiquitin-specific protease 4 (USP4) targets TRAF2 and TRAF6 for deubiquitination and inhibits TNFα-induced cancer cell migration. Biochem J. 2012;441:979–86. 10.1042/BJ20111358. [DOI] [PubMed] [Google Scholar]
- 45.Fan YH, Yu Y, Mao RF, Tan XJ, Xu GF, Zhang H, Lu XB, Fu SB, Yang J. USP4 targets TAK1 to downregulate TNFalpha-induced NF-kappaB activation. Cell Death Differ. 2011;18:1547–60. 10.1038/cdd.2011.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Colleran A, Collins PE, O’Carroll C, Ahmed A, Mao X, McManus B, Kiely PA, Burstein E, Carmody RJ. Deubiquitination of NF-κB by Ubiquitin-specific protease-7 promotes transcription. Proc Natl Acad Sci USA. 2013;110:618–23. 10.1073/pnas.1208446110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Niu J, Shi Y, Xue J, Miao R, Huang S, Wang T, Wu J, Fu M, Wu Z-H. USP10 inhibits genotoxic NF-κB activation by MCPIP1-facilitated deubiquitination of NEMO. EMBO J. 2013;32:3206–19. 10.1038/emboj.2013.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou L, Jiang H, Du J, Li L, Li R, Lu J, Fu W, Hou J. USP15 inhibits multiple myeloma cell apoptosis through activating a feedback loop with the transcription factor NF-κBp65. Exp Mol Med. 2018;50. 10.1038/s12276-018-0180-4. [DOI] [PMC free article] [PubMed]
- 49.Liu X, Li H, Zhong B, Blonska M, Gorjestani S, Yan M, Tian Q, Zhang DE, Lin X, Dong C. USP18 inhibits NF-kappaB and NFAT activation during Th17 differentiation by deubiquitinating the TAK1-TAB1 complex. J Exp Med. 2013;210:1575–90. 10.1084/jem.20122327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lei C-Q, Wu X, Zhong X, Jiang L, Zhong B, Shu H-B. USP19 Inhibits TNF-α- and IL-1β-Triggered NF-κB Activation by Deubiquitinating TAK1. J Immunol. 2019;203:259–68. 10.4049/jimmunol.1900083. [DOI] [PubMed] [Google Scholar]
- 51.Zhong B, Liu X, Wang X, Liu X, Li H, Darnay BG, Lin X, Sun S-C, Dong C. Ubiquitin-specific protease 25 regulates TLR4-dependent innate immune responses through deubiquitination of the adaptor protein TRAF3. Sci Signal. 2013;6:ra35. 10.1126/scisignal.2003708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Massagué J, Sheppard D. TGF-β signaling in health and disease. Cell. 2023;186:4007–37. 10.1016/j.cell.2023.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kel A, Thum T, Kunduzova O. Targeting fibroblast phenotype switching in cardiac remodelling as a promising antifibrotic strategy. Eur Heart J. 2025;46:354–8. 10.1093/eurheartj/ehae722. [DOI] [PubMed] [Google Scholar]
- 54.Tuleta I, Hanna A, Humeres C, Aguilan JT, Sidoli S, Zhu F, Frangogiannis NG. Fibroblast-specific TGF-β signaling mediates cardiac dysfunction, fibrosis, and hypertrophy in obese diabetic mice. Cardiovasc Res. 2024;120:2047–63. 10.1093/cvr/cvae210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goumans M-J, Liu Z, ten Dijke P. TGF-beta signaling in vascular biology and dysfunction. Cell Res. 2009;19:116–27. 10.1038/cr.2008.326. [DOI] [PubMed] [Google Scholar]
- 56.Ross S, Hill CS. How the Smads regulate transcription. Int J Biochem Cell Biol. 2008;40:383–408. [DOI] [PubMed] [Google Scholar]
- 57.Umbarkar P, Singh AP, Gupte M, Verma VK, Galindo CL, Guo Y, Zhang Q, McNamara JW, Force T, Lal H. Cardiomyocyte SMAD4-dependent TGF-β signaling is essential to maintain adult heart homeostasis. JACC Basic To Translational Science. 2019;4:41–53. 10.1016/j.jacbts.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sorrentino A, Thakur N, Grimsby S, Marcusson A, von Bulow V, Schuster N, Zhang S, Heldin C-H, Landström M. The type I TGF-beta receptor engages TRAF6 to activate TAK1 in a receptor kinase-independent manner. Nat Cell Biol. 2008;10:1199–207. 10.1038/ncb1780. [DOI] [PubMed] [Google Scholar]
- 59.Wang BZ, Morsink MA, Kim SW, Luo LJ, Zhang X, Soni RK, Lock RI, Rao J, Kim Y, Zhang A, et al. Cardiac fibroblast BAG3 regulates TGFBR2 signaling and fibrosis in dilated cardiomyopathy. J Clin Invest. 2025;135. 10.1172/JCI181630. [DOI] [PMC free article] [PubMed]
- 60.Imamura T, Oshima Y, Hikita A. Regulation of TGF-β family signalling by ubiquitination and deubiquitination. J Biochem. 2013;154:481–9. 10.1093/jb/mvt097. [DOI] [PubMed] [Google Scholar]
- 61.Zhang L, Zhou F, Drabsch Y, Gao R, Snaar-Jagalska BE, Mickanin C, Huang H, Sheppard K-A, Porter JA, Lu CX, et al. USP4 is regulated by AKT phosphorylation and directly deubiquitylates TGF-β type I receptor. Nat Cell Biol. 2012;14:717–26. 10.1038/ncb2522. [DOI] [PubMed] [Google Scholar]
- 62.Cao WH, Liu XP, Meng SL, Gao YW, Wang Y, Ma ZL, Wang XG, Wang HB. USP4 promotes invasion of breast cancer cells via Relaxin/TGF-β1/Smad2/MMP-9 signal. Eur Rev Med Pharmacol Sci. 2016;20:1115–22. [PubMed] [Google Scholar]
- 63.Zhao M, Zheng Z, Peng S, Xu Y, Zhang J, Liu J, Pan W, Yin Z, Xu S, Wei C, et al. Epidermal growth factor-like repeats and discoidin I-like domains 3 deficiency attenuates dilated cardiomyopathy by inhibiting ubiquitin specific peptidase 10 dependent Smad4 deubiquitination. J Am Heart Assoc. 2024;13:e031283. 10.1161/JAHA.123.031283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Al-Salihi MA, Herhaus L, Macartney T, Sapkota GP. USP11 augments TGFβ signalling by deubiquitylating ALK5. Open Biol. 2012;2:120063. 10.1098/rsob.120063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eichhorn PJA, Rodón L, Gonzàlez-Juncà A, Dirac A, Gili M, Martínez-Sáez E, Aura C, Barba I, Peg V, Prat A, et al. USP15 stabilizes TGF-β receptor I and promotes oncogenesis through the activation of TGF-β signaling in glioblastoma. Nat Med. 2012;18:429–35. 10.1038/nm.2619. [DOI] [PubMed] [Google Scholar]
- 66.Iyengar PV, Jaynes P, Rodon L, Lama D, Law KP, Lim YP, Verma C, Seoane J, Eichhorn PJA. USP15 regulates SMURF2 kinetics through C-lobe mediated deubiquitination. Sci Rep. 2015;5:14733. 10.1038/srep14733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xie Y, Avello M, Schirle M, McWhinnie E, Feng Y, Bric-Furlong E, Wilson C, Nathans R, Zhang J, Kirschner MW, et al. Deubiquitinase FAM/USP9X interacts with the E3 ubiquitin ligase SMURF1 protein and protects it from ligase activity-dependent self-degradation. J Biol Chem. 2013;288:2976–85. 10.1074/jbc.M112.430066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu Y, Yu X, Yi X, Wu K, Dwabe S, Atefi M, Elshimali Y, Kemp KT, Bhat K, Haro J, et al. Aberrant phosphorylation of SMAD4 Thr277-mediated USP9x-SMAD4 interaction by free fatty acids promotes breast cancer metastasis. Can Res. 2017;77:1383–94. 10.1158/0008-5472.CAN-16-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao Y, Thornton AM, Kinney MC, Ma CA, Spinner JJ, Fuss IJ, Shevach EM, Jain A. The deubiquitinase CYLD targets Smad7 protein to regulate transforming growth factor β (TGF-β) signaling and the development of regulatory T cells. J Biol Chem. 2011;286:40520–30. 10.1074/jbc.M111.292961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kit Leng Lui S, Iyengar PV, Jaynes P, Isa ZFBA, Pang B, Tan TZ, Eichhorn PJA. USP26 regulates TGF-β signaling by deubiquitinating and stabilizing SMAD7. EMBO Rep. 2017;18:797–808. 10.15252/embr.201643270. [DOI] [PMC free article] [PubMed] [Retracted]
- 71.Jiangqiao Z, Tianyu W, Zhongbao C, Long Z, Jilin Z, Xiaoxiong M, Tao Q. Ubiquitin-specific peptidase 10 protects against hepatic ischaemic/reperfusion injury via TAK1 signalling. Front Immunol. 2020;11. 10.3389/fimmu.2020.506275. [DOI] [PMC free article] [PubMed]
- 72.Xie S, Xing Y, Shi W, Zhang M, Chen M, Fang W, Liu S, Zhang T, Zeng X, Chen S, et al. Cardiac fibroblast heat shock protein 47 aggravates cardiac fibrosis post myocardial ischemia-reperfusion injury by encouraging ubiquitin specific peptidase 10 dependent Smad4 deubiquitination. Acta Pharm Sin B. 2022;12:4138–53. 10.1016/j.apsb.2022.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ying X, Zhao Y, Yao T, Yuan A, Xu L, Gao L, Ding S, Ding H, Pu J, He B. Novel protective role for ubiquitin-specific protease 18 in pathological cardiac remodeling. Hypertension. 2016;68:1160–70. 10.1161/HYPERTENSIONAHA.116.07562. [DOI] [PubMed] [Google Scholar]
- 74.Miao R, Lu Y, He X, Liu X, Chen Z, Wang J. Ubiquitin-specific protease 19 blunts pathological cardiac hypertrophy via inhibition of the TAK1-dependent pathway. J Cell Mol Med. 2020;24:10946–57. 10.1111/jcmm.15724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jiang Y-N, Yang S-X, Guan X, Chen Q, Zhao L, Yu X-Y, Ren F-F, Wu S-J, Wu L-P, Lai T-F, et al. Loss of USP22 alleviates cardiac hypertrophy induced by pressure overload through HiF1-α-TAK1 signaling pathway. Biochim Biophys Acta. 2023;1869:166813. 10.1016/j.bbadis.2023.166813. [DOI] [PubMed] [Google Scholar]
- 76.Wang H, Lai Y, Mathis BJ, Wang W, Li S, Qu C, Li B, Shao L, Song H, Janicki JS, et al. Deubiquitinating enzyme CYLD mediates pressure overload-induced cardiac maladaptive remodeling and dysfunction via downregulating Nrf2. J Mol Cell Cardiol. 2015;84:143–53. 10.1016/j.yjmcc.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 77.Villeneuve NF, Tian W, Wu T, Sun Z, Lau A, Chapman E, Fang D, Zhang DD. USP15 negatively regulates Nrf2 through deubiquitination of Keap1. Mol Cell. 2013;51:68–79. 10.1016/j.molcel.2013.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cui T, Lai Y, Janicki JS, Wang X. Nuclear factor erythroid-2 related factor 2 (Nrf2)-mediated protein quality control in cardiomyocytes. Front Biosci (Landmark Ed). 2016;21:192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reuland DJ, McCord JM, Hamilton KL. The role of Nrf2 in the attenuation of cardiovascular disease. Exerc Sport Sci Rev. 2013;41:162–8. 10.1097/JES.0b013e3182948a1e. [DOI] [PubMed] [Google Scholar]
- 80.Thakur MR, Nachane SS, Tupe RS. Alleviation of albumin glycation-induced diabetic cardiomyopathy by L-Arginine: insights into Nrf-2 signaling. Int J Biol Macromol. 2024;264:130478. 10.1016/j.ijbiomac.2024.130478. [DOI] [PubMed] [Google Scholar]
- 81.Chen QM, Maltagliati AJ. Nrf2 at the heart of oxidative stress and cardiac protection. Physiol Genomics. 2018;50:77–97. 10.1152/physiolgenomics.00041.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cuadrado A, Rojo AI, Wells G, Hayes JD, Cousin SP, Rumsey WL, Attucks OC, Franklin S, Levonen A-L, Kensler TW, et al. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat Rev Drug Discovery. 2019;18:295–317. 10.1038/s41573-018-0008-x. [DOI] [PubMed] [Google Scholar]
- 83.Rojo de la Vega M, Chapman E, Zhang DD. NRF2 and the hallmarks of cancer. Cancer Cell. 2018;34:21–43. 10.1016/j.ccell.2018.03.022. [DOI] [PMC free article] [PubMed]
- 84.Xu Q, Liu M, Gu J, Ling S, Liu X, Luo Z, Jin Y, Chai R, Ou W, Liu S, et al. Ubiquitin-specific protease 7 regulates myocardial ischemia/reperfusion injury by stabilizing Keap1. Cell Death Discov. 2022;8:291. 10.1038/s41420-022-01086-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tong G, Chen Y, Chen X, Fan J, Zhu K, Hu Z, Li S, Zhu J, Feng J, Wu Z, et al. FGF18 alleviates hepatic ischemia-reperfusion injury via the USP16-mediated KEAP1/Nrf2 signaling pathway in male mice. Nat Commun. 2023;14:6107. 10.1038/s41467-023-41800-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cai C, Ma H, Peng J, Shen X, Zhen X, Yu C, Zhang P, Ji F, Wang J. USP25 regulates KEAP1-NRF2 anti-oxidation axis and its inactivation protects acetaminophen-induced liver injury in male mice. Nat Commun. 2023;14:3648. 10.1038/s41467-023-39412-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cui J, Guo Y, Yin T, Gou S, Xiong J, Liang X, Lu C, Peng T. USP8 promotes gemcitabine resistance of pancreatic cancer via deubiquitinating and stabilizing Nrf2. Biomed Pharmacother. 2023;166:115359. 10.1016/j.biopha.2023.115359. [DOI] [PubMed] [Google Scholar]
- 88.Liu W, Tang P, Wang J, Ye W, Ge X, Rong Y, Ji C, Wang Z, Bai J, Fan J, et al. Extracellular vesicles derived from melatonin-preconditioned mesenchymal stem cells containing USP29 repair traumatic spinal cord injury by stabilizing NRF2. J Pineal Res. 2021;71:e12769. 10.1111/jpi.12769. [DOI] [PubMed] [Google Scholar]
- 89.Sowa ME, Bennett EJ, Gygi SP, Harper JW. Defining the human deubiquitinating enzyme interaction landscape. Cell. 2009;138:389–403. 10.1016/j.cell.2009.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Silva-Islas CA, Maldonado PD. Canonical and non-canonical mechanisms of Nrf2 activation. Pharmacol Res. 2018;134:92–9. 10.1016/j.phrs.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 91.Lee B, Kim YH, Lee W, Choi HY, Lee J, Kim J, Mai DN, Jung SF, Kwak MS, Shin J-S. USP13 deubiquitinates p62/SQSTM1 to induce autophagy and Nrf2 release for activating antioxidant response genes. Free Radic Biol Med. 2023;208:820–32. 10.1016/j.freeradbiomed.2023.09.024. [DOI] [PubMed] [Google Scholar]
- 92.Peng H, Yang F, Hu Q, Sun J, Peng C, Zhao Y, Huang C. The ubiquitin-specific protease USP8 directly deubiquitinates SQSTM1/p62 to suppress its autophagic activity. Autophagy. 2020;16:698–708. 10.1080/15548627.2019.1635381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hafner A, Bulyk ML, Jambhekar A, Lahav G. The multiple mechanisms that regulate p53 activity and cell fate. Nat Rev Mol Cell Biol. 2019;20:199–210. 10.1038/s41580-019-0110-x. [DOI] [PubMed] [Google Scholar]
- 94.Liu Y, Su Z, Tavana O, Gu W. Understanding the complexity of p53 in a new era of tumor suppression. Cancer Cell. 2024;42:946–67. 10.1016/j.ccell.2024.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Whibley C, Pharoah PDP, Hollstein M. p53 polymorphisms: cancer implications. Nat Rev Cancer. 2009;9. 10.1038/nrc2584. [DOI] [PubMed]
- 96.Toko H, Takahashi H, Kayama Y, Oka T, Minamino T, Okada S, Morimoto S, Zhan D-Y, Terasaki F, Anderson ME, et al. Ca2+/calmodulin-dependent kinase IIdelta causes heart failure by accumulation of p53 in dilated cardiomyopathy. Circulation. 2010;122:891–9. 10.1161/CIRCULATIONAHA.109.935296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu X, Burke RM, Lighthouse JK, Baker CD, Dirkx RA, Kang B, Chakraborty Y, Mickelsen DM, Twardowski JJ, Mello SS, et al. p53 regulates the extent of fibroblast proliferation and fibrosis in left ventricle pressure overload. Circ Res. 2023;133:271–87. 10.1161/CIRCRESAHA.121.320324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–9. [DOI] [PubMed] [Google Scholar]
- 99.Li M, Chen D, Shiloh A, Luo J, Nikolaev AY, Qin J, Gu W. Deubiquitination of p53 by HAUSP is an important pathway for p53 stabilization. Nature. 2002;416:648–53. [DOI] [PubMed] [Google Scholar]
- 100.Cummins JM, Rago C, Kohli M, Kinzler KW, Lengauer C, Vogelstein B. Tumour suppression: disruption of HAUSP gene stabilizes p53. Nature. 2004;428:1 p following 486. [DOI] [PubMed]
- 101.Yuan J, Luo K, Zhang L, Cheville JC, Lou Z. USP10 regulates p53 localization and stability by deubiquitinating p53. Cell. 2010;140:384–96. 10.1016/j.cell.2009.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ko A, Han SY, Choi CH, Cho H, Lee M-S, Kim S-Y, Song JS, Hong K-M, Lee H-W, Hewitt SM, et al. Oncogene-induced senescence mediated by c-Myc requires USP10 dependent deubiquitination and stabilization of p14ARF. Cell Death Differ. 2018;25:1050–62. 10.1038/s41418-018-0072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang X, Berger FG, Yang J, Lu X. USP4 inhibits p53 through deubiquitinating and stabilizing ARF-BP1. EMBO J. 2011;30:2177–89. 10.1038/emboj.2011.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu J, Xia H, Kim M, Xu L, Li Y, Zhang L, Cai Y, Norberg HV, Zhang T, Furuya T, et al. Beclin1 controls the levels of p53 by regulating the deubiquitination activity of USP10 and USP13. Cell. 2011;147:223–34. 10.1016/j.cell.2011.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang L, Lin Y, Zhou X, Chen Y, Li X, Luo W, Zhou Y, Cai L. CYLD deficiency enhances metabolic reprogramming and tumor progression in nasopharyngeal carcinoma via PFKFB3. Cancer Lett. 2022;532:215586. 10.1016/j.canlet.2022.215586. [DOI] [PubMed] [Google Scholar]
- 106.Chen E, Li E, Liu H, Zhou Y, Wen L, Wang J, Wang Y, Ye L, Liang T. miR-26b enhances the sensitivity of hepatocellular carcinoma to Doxorubicin via USP9X-dependent degradation of p53 and regulation of autophagy. Int J Biol Sci. 2021;17:781–95. 10.7150/ijbs.52517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang X, Liu T, Xu S, Gao P, Dong W, Liu W, Gao M, Song L, Cui L, Dong X. A pro-inflammatory mediator USP11 enhances the stability of p53 and inhibits KLF2 in intracerebral hemorrhage. Mol Ther Methods Clin Dev. 2021;21:681–92. 10.1016/j.omtm.2021.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yuan J, Zhang G, Li X, Ma Q, Cheng W, Wang W, Zhang B, Hu T, Song G. Knocking down USP39 inhibits the growth and metastasis of non-small-cell lung cancer cells through activating the p53 pathway. Int J Mol Sci. 2020;21. 10.3390/ijms21238949. [DOI] [PMC free article] [PubMed]
- 109.Chen L, Zhang L, He H, Shao F, Yu Z, Gao Y, He J. Ubiquitin-specific protease 54 regulates GLUT1-mediated aerobic glycolysis to inhibit lung adenocarcinoma progression by modifying p53 degradation. Oncogene. 2024;43:2025–37. 10.1038/s41388-024-03047-8. [DOI] [PubMed] [Google Scholar]
- 110.Xu H, Wang T, Nie H, Sun Q, Jin C, Yang S, Chen Z, Wang X, Tang J, Feng Y, et al. USP36 promotes colorectal cancer progression through inhibition of p53 signaling pathway via stabilizing RBM28. Oncogene. 2024. 10.1038/s41388-024-03178-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jurisic A, Sung P-J, Wappett M, Daubriac J, Lobb IT, Kung W-W, Crawford N, Page N, Cassidy E, Feutren-Burton S, et al. USP7 inhibitors suppress tumour neoangiogenesis and promote synergy with immune checkpoint inhibitors by downregulating fibroblast VEGF. Clin Transl Med. 2024;14:e1648. 10.1002/ctm2.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ling S, Shan Q, Zhan Q, Ye Q, Liu P, Xu S, He X, Ma J, Xiang J, Jiang G, et al. USP22 promotes hypoxia-induced hepatocellular carcinoma stemness by a HIF1α/USP22 positive feedback loop upon TP53 inactivation. Gut. 2020;69:1322–34. 10.1136/gutjnl-2019-319616. [DOI] [PubMed] [Google Scholar]
- 113.Zhang Q, Wang L, Wang S, Cheng H, Xu L, Pei G, Wang Y, Fu C, Jiang Y, He C, et al. Signaling pathways and targeted therapy for myocardial infarction. Signal Transduct Target Ther. 2022;7:78. 10.1038/s41392-022-00925-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schroeter EH, Kisslinger JA, Kopan R. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature. 1998;393:382–6. [DOI] [PubMed] [Google Scholar]
- 115.Shi Q, Xue C, Zeng Y, Yuan X, Chu Q, Jiang S, Wang J, Zhang Y, Zhu D, Li L. Notch signaling pathway in cancer: from mechanistic insights to targeted therapies. Signal Transduct Target Ther. 2024;9:128. 10.1038/s41392-024-01828-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sanalkumar R, Dhanesh SB, James J. Non-canonical activation of Notch signaling/target genes in vertebrates. Cell Mol Life Sci. 2010;67:2957–68. 10.1007/s00018-010-0391-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lu L, Ma J, Liu Y, Shao Y, Xiong X, Duan W, Gao E, Yang Q, Chen S, Yang J, et al. FSTL1-USP10-notch1 signaling axis protects against cardiac dysfunction through inhibition of myocardial fibrosis in diabetic mice. Front Cell Dev Biol. 2021;9:757068. 10.3389/fcell.2021.757068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lim R, Sugino T, Nolte H, Andrade J, Zimmermann B, Shi C, Doddaballapur A, Ong YT, Wilhelm K, Fasse JWD, et al. Deubiquitinase USP10 regulates Notch signaling in the endothelium. Science. 2019;364:188–93. 10.1126/science.aat0778. [DOI] [PubMed] [Google Scholar]
- 119.Zhai S, Lin J, Ji Y, Zhang R, Zhang Z, Cao Y, Liu Y, Tang X, Liu J, Liu P, et al. A microprotein N1DARP encoded by LINC00261 promotes Notch1 intracellular domain (N1ICD) degradation via disrupting USP10-N1ICD interaction to inhibit chemoresistance in Notch1-hyperactivated pancreatic cancer. Cell Discov. 2023;9:95. 10.1038/s41421-023-00592-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu T, Zhang C, Ying J, Wang Y, Yan G, Zhou Y, Lu G. Inhibition of the intracellular domain of Notch1 results in vascular endothelial cell dysfunction in sepsis. Front Immunol. 2023;14:1134556. 10.3389/fimmu.2023.1134556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shin S, Kim K, Kim H-R, Ylaya K, Do S-I, Hewitt SM, Park H-S, Roe J-S, Chung J-Y, Song J. Deubiquitylation and stabilization of Notch1 intracellular domain by ubiquitin-specific protease 8 enhance tumorigenesis in breast cancer. Cell Death Differ. 2020;27:1341–54. 10.1038/s41418-019-0419-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ling X, Huang Q, Xu Y, Jin Y, Feng Y, Shi W, Ye X, Lin Y, Hou L, Lin X. The deubiquitinating enzyme Usp5 regulates Notch and RTK signaling during Drosophila eye development. FEBS Lett. 2017;591:875–88. 10.1002/1873-3468.12580. [DOI] [PubMed] [Google Scholar]
- 123.Jin Q, Martinez CA, Arcipowski KM, Zhu Y, Gutierrez-Diaz BT, Wang KK, Johnson MR, Volk AG, Wang F, Wu J, et al. USP7 cooperates with NOTCH1 to drive the oncogenic transcriptional program in T-cell leukemia. Clin Cancer Res. 2019;25:222–39. 10.1158/1078-0432.CCR-18-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Moretti J, Chastagner P, Liang C-C, Cohn MA, Israël A, Brou C. The ubiquitin-specific protease 12 (USP12) is a negative regulator of notch signaling acting on notch receptor trafficking toward degradation. J Biol Chem. 2012;287:29429–41. 10.1074/jbc.M112.366807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Majumdar U, Manivannan S, Basu M, Ueyama Y, Blaser MC, Cameron E, McDermott MR, Lincoln J, Cole SE, Wood S, et al. Nitric oxide prevents aortic valve calcification by S-nitrosylation of USP9X to activate NOTCH signaling. Sci Adv. 2021;7. 10.1126/sciadv.abe3706. [DOI] [PMC free article] [PubMed]
- 126.Maron BJ, Desai MY, Nishimura RA, Spirito P, Rakowski H, Towbin JA, Rowin EJ, Maron MS, Sherrid MV. Diagnosis and evaluation of hypertrophic cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2022;79:372–89. 10.1016/j.jacc.2021.12.002. [DOI] [PubMed] [Google Scholar]
- 127.Pagan J, Seto T, Pagano M, Cittadini A. Role of the ubiquitin proteasome system in the heart. Circ Res. 2013;112:1046–58. 10.1161/CIRCRESAHA.112.300521. [DOI] [PubMed] [Google Scholar]
- 128.He B, Zhao YC, Gao LC, Ying XY, Xu LW, Su YY, Ji QQ, Lin N, Pu J. Ubiquitin-specific protease 4 is an endogenous negative regulator of pathological cardiac hypertrophy. Hypertension. 2016;67:1237–48. 10.1161/HYPERTENSIONAHA.116.07392. [DOI] [PubMed] [Google Scholar]
- 129.Gupta I, Varshney NK, Khan S. Emergence of members of TRAF and DUB of ubiquitin proteasome system in the regulation of hypertrophic cardiomyopathy. Front Genet. 2018;9:336. 10.3389/fgene.2018.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang DH, Zhang JL, Huang Z, Wu LM, Wang ZM, Li YP, Tian XY, Kong LY, Yao R, Zhang YZ. Deubiquitinase ubiquitin-specific protease 10 deficiency regulates Sirt6 signaling and exacerbates cardiac hypertrophy. J Am Heart Assoc. 2020;9:e017751. 10.1161/JAHA.120.017751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.van Empel VPM, De Windt LJ. Myocyte hypertrophy and apoptosis: a balancing act. Cardiovasc Res. 2004;63:487–99. [DOI] [PubMed] [Google Scholar]
- 132.Lino CA, Demasi M, Barreto-Chaves ML. Ubiquitin proteasome system (UPS) activation in the cardiac hypertrophy of hyperthyroidism. Mol Cell Endocrinol. 2019;493:110451. 10.1016/j.mce.2019.110451. [DOI] [PubMed] [Google Scholar]
- 133.Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412:346–51. 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- 134.Sato S, Sanjo H, Takeda K, Ninomiya-Tsuji J, Yamamoto M, Kawai T, Matsumoto K, Takeuchi O, Akira S. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol. 2005;6:1087–95. 10.1038/ni1255. [DOI] [PubMed] [Google Scholar]
- 135.Shim JH, Xiao C, Paschal AE, Bailey ST, Rao P, Hayden MS, Lee KY, Bussey C, Steckel M, Tanaka N, et al. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 2005;19:2668–81. 10.1101/gad.1360605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang D, Gaussin V, Taffet GE, Belaguli NS, Yamada M, Schwartz RJ, Michael LH, Overbeek PA, Schneider MD. TAK1 is activated in the myocardium after pressure overload and is sufficient to provoke heart failure in transgenic mice. Nat Med. 2000;6:556–63. 10.1038/75037. [DOI] [PubMed] [Google Scholar]
- 137.Watkins SJ, Borthwick GM, Oakenfull R, Robson A, Arthur HM. Angiotensin II-induced cardiomyocyte hypertrophy in vitro is TAK1-dependent and Smad2/3-independent. Hypertens Res. 2012;35:393–8. 10.1038/hr.2011.196. [DOI] [PubMed] [Google Scholar]
- 138.Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–69. 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 139.van Berlo JH, Maillet M, Molkentin JD. Signaling effectors underlying pathologic growth and remodeling of the heart. J Clin Invest. 2013;123:37–45. 10.1172/JCI62839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liao P, Georgakopoulos D, Kovacs A, Zheng M, Lerner D, Pu H, Saffitz J, Chien K, Xiao RP, Kass DA, et al. The in vivo role of p38 MAP kinases in cardiac remodeling and restrictive cardiomyopathy. Proc Natl Acad Sci U S A. 2001;98:12283–8. 10.1073/pnas.211086598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Marber MS, Rose B, Wang Y. The p38 mitogen-activated protein kinase pathway–a potential target for intervention in infarction, hypertrophy, and heart failure. J Mol Cell Cardiol. 2011;51:485–90. 10.1016/j.yjmcc.2010.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fan Y, Yu Y, Shi Y, Sun W, Xie M, Ge N, Mao R, Chang A, Xu G, Schneider MD, et al. Lysine 63-linked polyubiquitination of TAK1 at lysine 158 is required for tumor necrosis factor alpha- and interleukin-1beta-induced IKK/NF-kappaB and JNK/AP-1 activation. J Biol Chem. 2010;285:5347–60. 10.1074/jbc.M109.076976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chen IT, Hsu PH, Hsu WC, Chen NJ, Tseng PH. Polyubiquitination of transforming growth factor beta-activated kinase 1 (TAK1) at lysine 562 residue regulates TLR4-mediated JNK and p38 MAPK activation. Sci Rep. 2015;5:12300. 10.1038/srep12300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hirata Y, Takahashi M, Morishita T, Noguchi T, Matsuzawa A. Post-translational modifications of the TAK1-TAB complex. Int J Mol Sci. 2017;18. 10.3390/ijms18010205. [DOI] [PMC free article] [PubMed]
- 145.Zhihao L, Jingyu N, Lan L, Michael S, Rui G, Xiyun B, Xiaozhi L, Guanwei F. SERCA2a: a key protein in the Ca2+ cycle of the heart failure. Heart Fail Rev. 2020;25:523–35. 10.1007/s10741-019-09873-3. [DOI] [PubMed] [Google Scholar]
- 146.Marks AR. Calcium cycling proteins and heart failure: mechanisms and therapeutics. J Clin Invest. 2013;123:46–52. 10.1172/JCI62834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Korf-Klingebiel M, Reboll MR, Polten F, Weber N, Jackle F, Wu X, Kallikourdis M, Kunderfranco P, Condorelli G, Giannitsis E, et al. Myeloid-derived growth factor protects against pressure overload-induced heart failure by preserving sarco/endoplasmic reticulum Ca(2+)-ATPase expression in cardiomyocytes. Circulation. 2021;144:1227–40. 10.1161/CIRCULATIONAHA.120.053365. [DOI] [PubMed] [Google Scholar]
- 148.Asahi M, Nakayama H, Tada M, Otsu K. Regulation of sarco(endo)plasmic reticulum Ca2+ adenosine triphosphatase by phospholamban and sarcolipin: implication for cardiac hypertrophy and failure. Trends Cardiovasc Med. 2003;13:152–7. 10.1016/s1050-1738(03)00037-9. [DOI] [PubMed] [Google Scholar]
- 149.Yu S, Sun Z, Wang X, Ju T, Wang C, Liu Y, Qu Z, Liu K, Mei Z, Li N, et al. Mettl13 protects against cardiac contractile dysfunction by negatively regulating C-Cbl-mediated ubiquitination of SERCA2a in ischemic heart failure. Sci China Life Sci. 2023. 10.1007/s11427-022-2351-1. [DOI] [PubMed] [Google Scholar]
- 150.Fu D, Luo J, Wu Y, Zhang L, Li L, Chen H, Wen T, Fu Y, Xiong W. Angiotensin II-induced calcium overload affects mitochondrial functions in cardiac hypertrophy by targeting the USP2/MFN2 axis. Mol Cell Endocrinol. 2023;571:111938. 10.1016/j.mce.2023.111938. [DOI] [PubMed] [Google Scholar]
- 151.Gao W, Guo N, Yan H, Zhao S, Sun Y, Chen Z. Mycn ameliorates cardiac hypertrophy-induced heart failure in mice by mediating the USP2/JUP/Akt/β-catenin cascade. BMC Cardiovasc Disord. 2024;24:82. 10.1186/s12872-024-03748-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tu C, Caudal A, Liu Y, Gorgodze N, Zhang H, Lam CK, Dai Y, Zhang A, Wnorowski A, Wu MA, et al. Tachycardia-induced metabolic rewiring as a driver of contractile dysfunction. Nat Biomed Eng. 2024;8:479–94. 10.1038/s41551-023-01134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lin L-C, Liu Z-Y, Yang J-J, Zhao J-Y, Tao H. m6A epitranscriptomic modification in diabetic microvascular complications. Trends Pharmacol Sci. 2023. 10.1016/j.tips.2023.09.013. [DOI] [PubMed]
- 154.Palomer X, Aguilar-Recarte D, García R, Nistal JF, Vázquez-Carrera M. Sirtuins: to be or not to be in diabetic cardiomyopathy. Trends Mol Med. 2021;27:554–71. 10.1016/j.molmed.2021.03.004. [DOI] [PubMed] [Google Scholar]
- 155.Liu LB, Huang SH, Qiu HL, Cen XF, Guo YY, Li D, Ma YL, Xu M, Tang QZ. Limonin stabilises sirtuin 6 (SIRT6) by activating ubiquitin specific peptidase 10 (USP10) in cardiac hypertrophy. Br J Pharmacol. 2022;179:4516–33. 10.1111/bph.15899. [DOI] [PubMed] [Google Scholar]
- 156.Lu P, Xu Y, Sheng Z-Y, Peng X-G, Zhang J-J, Wu Q-H, Wu Y-Q, Cheng X-S, Zhu K. De-ubiquitination of p300 by USP12 critically enhances METTL3 expression and Ang II-induced cardiac hypertrophy. Exp Cell Res. 2021;406:112761. 10.1016/j.yexcr.2021.112761. [DOI] [PubMed] [Google Scholar]
- 157.Feng Z, Pan L, Qiao C, Yang Y, Yang X, Xie Y. Cardamonin intervenes in myocardial hypertrophy progression by regulating Usp18. Phytomedicine. 2024;134:155970. 10.1016/j.phymed.2024.155970. [DOI] [PubMed] [Google Scholar]
- 158.Jean-Charles P-Y, Roy B, Yu SM-W, Pironti G, Nagi K, Mao L, Kaur S, Abraham DM, Maudsley S, Rockman HA, et al. USP20 deletion promotes eccentric cardiac remodeling in response to pressure overload and increases mortality. Am J Physiol Heart Circ Physiol. 2024;327:H1257-H1271. 10.1152/ajpheart.00329.2024. [DOI] [PMC free article] [PubMed]
- 159.Yan M, Mei Y, Zhang T, Liu Z, Su L, Xiao Y, Zhong X, Lu Y. USP7 cardiomyocyte specific knockout causes disordered mitochondrial biogenesis and dynamics and early neonatal lethality in mice. Int J Cardiol. 2024;408:132149. 10.1016/j.ijcard.2024.132149. [DOI] [PubMed] [Google Scholar]
- 160.Xiao Z, Dai C, Yu T, Zhu J, Pan Y, Shuai W, Kong B, Huang H. Ubiquitin specific protease 38 aggravates pathological cardiac remodeling by stabilizing phospho-TBK1. Int J Biol Sci. 2024;20:1815–32. 10.7150/ijbs.85562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pan Y, Xiao Z, Yang H, Kong B, Meng H, Shuai W, Huang H. USP38 exacerbates pressure overload-induced left ventricular electrical remodeling. Mol Med. 2024;30:97. 10.1186/s10020-024-00846-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Liu N, Chai R, Liu B, Zhang Z, Zhang S, Zhang J, Liao Y, Cai J, Xia X, Li A, et al. Ubiquitin-specific protease 14 regulates cardiac hypertrophy progression by increasing GSK-3beta phosphorylation. Biochem Biophys Res Commun. 2016;478:1236–41. 10.1016/j.bbrc.2016.08.100. [DOI] [PubMed] [Google Scholar]
- 163.Heymans S, Lakdawala NK, Tschöpe C, Klingel K. Dilated cardiomyopathy: causes, mechanisms, and current and future treatment approaches. Lancet (London, England). 2023;402. 10.1016/S0140-6736(23)01241-2. [DOI] [PubMed]
- 164.Reichart D, Magnussen C, Zeller T, Blankenberg S. Dilated cardiomyopathy: from epidemiologic to genetic phenotypes: a translational review of current literature. J Intern Med. 2019;286:362–72. 10.1111/joim.12944. [DOI] [PubMed] [Google Scholar]
- 165.Ababio Y, Kelly SP, Angeli FS, Berghout J, Huang K, Liu K, Burns S, Senerchia C, Moccia R, Brooks GC. Prevalence and clinical burden of idiopathic dilated cardiomyopathy in the United States. Am J Med Open. 2023;10:100038. 10.1016/j.ajmo.2023.100038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Li D, Pi W, Sun Z, Liu X, Jiang J. Ferroptosis and its role in cardiomyopathy. Biomed Pharmacother. 2022;153:113279. 10.1016/j.biopha.2022.113279. [DOI] [PubMed] [Google Scholar]
- 167.Japp AG, Gulati A, Cook SA, Cowie MR, Prasad SK. The diagnosis and evaluation of dilated cardiomyopathy. J Am Coll Cardiol. 2016;67:2996–3010. 10.1016/j.jacc.2016.03.590. [DOI] [PubMed] [Google Scholar]
- 168.Bui QM, Ding J, Hong KN, Adler EA. The genetic evaluation of dilated cardiomyopathy. Struct Heart. 2023;7:100200. 10.1016/j.shj.2023.100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jordan E, Peterson L, Ai T, Asatryan B, Bronicki L, Brown E, Celeghin R, Edwards M, Fan J, Ingles J, et al. Evidence-Based assessment of genes in dilated cardiomyopathy. Circulation. 2021;144. 10.1161/CIRCULATIONAHA.120.053033. [DOI] [PMC free article] [PubMed]
- 170.Spänig S, Kellermann K, Dieterlen M-T, Noack T, Lehmann S, Borger MA, Garbade J, Barac YD, Emrich F. The ubiquitin proteasome system in ischemic and dilated cardiomyopathy. Int J Mol Sci. 2019;20. 10.3390/ijms20246354. [DOI] [PMC free article] [PubMed]
- 171.Xiong S, Van Pelt CS, Elizondo-Fraire AC, Fernandez-Garcia B, Lozano G. Loss of Mdm4 results in p53-dependent dilated cardiomyopathy. Circulation. 2007;115:2925–30. [DOI] [PubMed] [Google Scholar]
- 172.Birks EJ, Latif N, Enesa K, Folkvang T, Luong LA, Sarathchandra P, Khan M, Ovaa H, Terracciano CM, Barton PJR, et al. Elevated p53 expression is associated with dysregulation of the ubiquitin-proteasome system in dilated cardiomyopathy. Cardiovasc Res. 2008;79:472–80. 10.1093/cvr/cvn083. [DOI] [PubMed] [Google Scholar]
- 173.Sin TK, Tam BT, Yung BY, Yip SP, Chan LW, Wong CS, Ying M, Rudd JA, Siu PM. Resveratrol protects against doxorubicin-induced cardiotoxicity in aged hearts through the SIRT1-USP7 axis. J Physiol. 2015;593:1887–99. 10.1113/jphysiol.2014.270101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wang D, Jiang Z, Kan J, Jiang X, Pan C, You S, Chang R, Zhang J, Yang H, Zhu L, et al. USP36-mediated PARP1 deubiquitination in doxorubicin-induced cardiomyopathy. Cell Signal. 2024;117:111070. 10.1016/j.cellsig.2024.111070. [DOI] [PubMed] [Google Scholar]
- 175.Gonzalez DM, Medici D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci Signal. 2014;7:re8. 10.1126/scisignal.2005189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Xie Y, Liao J, Yu Y, Guo Q, Yang Y, Ge J, Chen H, Chen R. Endothelial-to-mesenchymal transition in human idiopathic dilated cardiomyopathy. Mol Med Rep. 2018;17:961–9. 10.3892/mmr.2017.8013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Pantely GA, Bristow JD. Ischemic cardiomyopathy. Prog Cardiovasc Dis. 1984;27:95–114. [DOI] [PubMed] [Google Scholar]
- 178.Fan Z, Cai L, Wang S, Wang J, Chen B. Baicalin prevents myocardial ischemia/reperfusion injury through inhibiting ACSL4 mediated ferroptosis. Front Pharmacol. 2021;12. 10.3389/fphar.2021.628988. [DOI] [PMC free article] [PubMed]
- 179.Dalal S, Shook PL, Singh M, Singh K. Post-ischemic cardioprotective potential of exogenous ubiquitin in myocardial remodeling late after ischemia/reperfusion injury. Life Sci. 2023;312:121216. 10.1016/j.lfs.2022.121216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ong S-B, Hernández-Reséndiz S, Crespo-Avilan GE, Mukhametshina RT, Kwek X-Y, Cabrera-Fuentes HA, Hausenloy DJ. Inflammation following acute myocardial infarction: multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol Ther. 2018;186:73–87. 10.1016/j.pharmthera.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Baehr A, Umansky KB, Bassat E, Jurisch V, Klett K, Bozoglu T, Hornaschewitz N, Solyanik O, Kain D, Ferraro B, et al. Agrin promotes coordinated therapeutic processes leading to improved cardiac repair in pigs. Circulation. 2020;142:868–81. 10.1161/CIRCULATIONAHA.119.045116. [DOI] [PubMed] [Google Scholar]
- 182.Cheng L, Sun X, Zhao X, Wang L, Yu J, Pan G, Li B, Yang H, Zhang Y, Cui W. Surface biofunctional drug-loaded electrospun fibrous scaffolds for comprehensive repairing hypertrophic scars. Biomaterials. 2016;83:169–81. 10.1016/j.biomaterials.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 183.Yousefi F, Shabaninejad Z, Vakili S, Derakhshan M, Movahedpour A, Dabiri H, Ghasemi Y, Mahjoubin-Tehran M, Nikoozadeh A, Savardashtaki A, et al. TGF-β and WNT signaling pathways in cardiac fibrosis: non-coding RNAs come into focus. Cell Commun Signal. 2020;18:87. 10.1186/s12964-020-00555-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Euler-Taimor G, Heger J. The complex pattern of SMAD signaling in the cardiovascular system. Cardiovasc Res. 2006;69:15–25. [DOI] [PubMed] [Google Scholar]
- 185.Hao J, Wang B, Jones SC, Jassal DS, Dixon IM. Interaction between angiotensin II and Smad proteins in fibroblasts in failing heart and in vitro. Am J Physiol Heart Circ Physiol. 2000;279:H3020–30. [DOI] [PubMed] [Google Scholar]
- 186.Fang X, Ardehali H, Min J, Wang F. The molecular and metabolic landscape of iron and ferroptosis in cardiovascular disease. Nat Rev Cardiol. 2022;20:7–23. 10.1038/s41569-022-00735-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sun X, Ou Z, Chen R, Niu X, Chen D, Kang R, Tang D. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology (Baltimore, MD). 2016;63:173–84. 10.1002/hep.28251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Feng H, Schorpp K, Jin J, Yozwiak CE, Hoffstrom BG, Decker AM, Rajbhandari P, Stokes ME, Bender HG, Csuka JM, et al. Transferrin receptor is a specific ferroptosis marker. Cell Rep. 2020;30. 10.1016/j.celrep.2020.02.049. [DOI] [PMC free article] [PubMed]
- 189.Jiang L, Kon N, Li T, Wang S-J, Su T, Hibshoosh H, Baer R, Gu W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520:57–62. 10.1038/nature14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Xie Y, Zhu S, Song X, Sun X, Fan Y, Liu J, Zhong M, Yuan H, Zhang L, Billiar TR, et al. The tumor suppressor p53 limits ferroptosis by blocking DPP4 activity. Cell Rep. 2017;20:1692–704. 10.1016/j.celrep.2017.07.055. [DOI] [PubMed] [Google Scholar]
- 191.Yu S-T, Sun Z-Y, Li N, Qu Z-Z, Wang C-H, Ju T-T, Liu Y-Q, Mei Z-T, Liu K-W, Lu M-X, et al. Mettl1 knockdown alleviates cardiac I/R injury in mice by inactivating the Mettl1-CYLD-P53 positive feedback loop. Acta Pharmacol Sin. 2024. 10.1038/s41401-024-01395-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zhang W, Zhang Y, Zhang H, Zhao Q, Liu Z, Xu Y. USP49 inhibits ischemia-reperfusion-induced cell viability suppression and apoptosis in human AC16 cardiomyocytes through DUSP1-JNK1/2 signaling. J Cell Physiol. 2019;234:6529–38. 10.1002/jcp.27390. [DOI] [PubMed] [Google Scholar]
- 193.Hu Y, Ma Z, Chen Z, Chen B. USP47 promotes apoptosis in rat myocardial cells after ischemia/reperfusion injury via NF-κB activation. Biotechnol Appl Biochem. 2021;68:841–8. 10.1002/bab.2000. [DOI] [PubMed] [Google Scholar]
- 194.Song S, Wang Y, Wang H-Y, Guo L-L. Role of sevoflurane in myocardial ischemia-reperfusion injury via the ubiquitin-specific protease 22/lysine-specific demethylase 3A axis. Bioengineered. 2022;13:13366–83. 10.1080/21655979.2022.2062535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, Malanda B. IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–81. 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 196.Tayanloo-Beik A, Roudsari PP, Rezaei-Tavirani M, Biglar M, Tabatabaei-Malazy O, Arjmand B, Larijani B. Diabetes and heart failure: multi-omics approaches. Front Physiol. 2021;12:705424. 10.3389/fphys.2021.705424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ritchie RH, Abel ED. Basic mechanisms of diabetic heart disease. Circ Res. 2020;126:1501–25. 10.1161/CIRCRESAHA.120.315913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wu S, Lu Q, Ding Y, Wu Y, Qiu Y, Wang P, Mao X, Huang K, Xie Z, Zou M-H. Hyperglycemia-driven inhibition of AMP-activated protein kinase α2 induces diabetic cardiomyopathy by promoting mitochondria-associated endoplasmic reticulum membranes in vivo. Circulation. 2019;139:1913–36. 10.1161/CIRCULATIONAHA.118.033552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Bertero E, Maack C. Metabolic remodelling in heart failure. Nat Rev Cardiol. 2018;15:457–70. 10.1038/s41569-018-0044-6. [DOI] [PubMed] [Google Scholar]
- 200.Wang L, Cai Y, Jian L, Cheung CW, Zhang L, Xia Z. Impact of peroxisome proliferator-activated receptor-α on diabetic cardiomyopathy. Cardiovasc Diabetol. 2021;20:2. 10.1186/s12933-020-01188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lee TI, Kao YH, Chen YC, Huang JH, Hsiao FC, Chen YJ. Peroxisome proliferator-activated receptors modulate cardiac dysfunction in diabetic cardiomyopathy. Diabetes Res Clin Pract. 2013;100:330–9. 10.1016/j.diabres.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 202.Yang Q, Li Y. Roles of PPARs on regulating myocardial energy and lipid homeostasis. J Mol Med (Berl). 2007;85:697–706. [DOI] [PubMed] [Google Scholar]
- 203.Xie S-Y, Liu S-Q, Zhang T, Shi W-K, Xing Y, Fang W-X, Zhang M, Chen M-Y, Xu S-C, Fan M-Q, et al. USP28 serves as a key suppressor of mitochondrial morphofunctional defects and cardiac dysfunction in the diabetic heart. Circulation. 2024;149:684–706. 10.1161/CIRCULATIONAHA.123.065603. [DOI] [PubMed] [Google Scholar]
- 204.Zhang X, Fan J, Li H, Chen C, Wang Y. CD36 signaling in diabetic cardiomyopathy. Aging Dis. 2021;12:826–40. 10.14336/AD.2020.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Shu H, Peng Y, Hang W, Nie J, Zhou N, Wang DW. The role of CD36 in cardiovascular disease. Cardiovasc Res. 2022;118:115–29. 10.1093/cvr/cvaa319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Yan M, Su L, Wu K, Mei Y, Liu Z, Chen Y, Zeng W, Xiao Y, Zhang J, Cai G, et al. USP7 promotes cardiometabolic disorders and mitochondrial homeostasis dysfunction in diabetic mice via stabilizing PGC1β. Pharmacol Res. 2024;205:107235. 10.1016/j.phrs.2024.107235. [DOI] [PubMed] [Google Scholar]
- 207.Ning Z, Guo X, Liu X, Lu C, Wang A, Wang X, Wang W, Chen H, Qin W, Liu X, et al. USP22 regulates lipidome accumulation by stabilizing PPARγ in hepatocellular carcinoma. Nat Commun. 2022;13:2187. 10.1038/s41467-022-29846-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Fang Y, Shen Z-Y, Zhan Y-Z, Feng X-C, Chen K-L, Li Y-S, Deng H-J, Pan S-M, Wu D-H, Ding Y. CD36 inhibits β-catenin/c-myc-mediated glycolysis through ubiquitination of GPC4 to repress colorectal tumorigenesis. Nat Commun. 2019;10:3981. 10.1038/s41467-019-11662-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Zhang F, Xia X, Chai R, Xu R, Xu Q, Liu M, Chen X, Liu B, Liu S, Liu N. Inhibition of USP14 suppresses the formation of foam cell by promoting CD36 degradation. J Cell Mol Med. 2020;24:3292–302. 10.1111/jcmm.15002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zeng M, Wei X, He Y, Yang Y. Ubiquitin-specific protease 11-mediated CD36 deubiquitination acts on C1q/TNF-related protein 9 against atherosclerosis. ESC Heart Failure. 2023;10:2499–509. 10.1002/ehf2.14423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Bugger H, Abel ED. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia. 2014;57:660–71. 10.1007/s00125-014-3171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Bidasee KR, Zhang Y, Shao CH, Wang M, Patel KP, Dincer UD, Besch HR. Diabetes increases formation of advanced glycation end products on Sarco(endo)plasmic reticulum Ca2+-ATPase. Diabetes. 2004;53:463–73. [DOI] [PubMed] [Google Scholar]
- 213.Dillmann WH. Diabetic cardiomyopathy. Circ Res. 2019;124:1160–2. 10.1161/CIRCRESAHA.118.314665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Wu S, Zhou Y, Liang J, Ying P, Situ Q, Tan X, Zhu J. Upregulation of NF-κB by USP24 aggravates ferroptosis in diabetic cardiomyopathy. Free Radical Biol Med. 2024;210:352–66. 10.1016/j.freeradbiomed.2023.11.032. [DOI] [PubMed] [Google Scholar]
- 215.Mushajiang M, Li Y, Sun Z, Liu J, Zhang L, Wang Z. USP10 alleviates Nε-carboxymethyl-lysine-induced vascular calcification and atherogenesis in diabetes mellitus by promoting AMPK activation. Cell Signal. 2024;120:111211. 10.1016/j.cellsig.2024.111211. [DOI] [PubMed] [Google Scholar]
- 216.Chen S, Liu Y, Zhou H. Advances in the development ubiquitin-specific peptidase (USP) inhibitors. Int J Mol Sci. 2021;22. 10.3390/ijms22094546. [DOI] [PMC free article] [PubMed]
- 217.Bhattacharya U, Neizer-Ashun F, Mukherjee P, Bhattacharya R. When the chains do not break: the role of USP10 in physiology and pathology. Cell Death Dis. 2020;11:1033. 10.1038/s41419-020-03246-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Deng M, Yang X, Qin B, Liu T, Zhang H, Guo W, Lee SB, Kim JJ, Yuan J, Pei H, et al. Deubiquitination and activation of AMPK by USP10. Mol Cell. 2016;61:614–24. 10.1016/j.molcel.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Lin Z, Yang H, Tan C, Li J, Liu Z, Quan Q, Kong S, Ye J, Gao B, Fang D. USP10 antagonizes c-Myc transcriptional activation through SIRT6 stabilization to suppress tumor formation. Cell Rep. 2013;5:1639–49. 10.1016/j.celrep.2013.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Takayama K-I, Suzuki T, Fujimura T, Takahashi S, Inoue S. Association of USP10 with G3BP2 inhibits p53 signaling and contributes to poor outcome in prostate cancer. Mol Cancer Res. 2018;16:846–56. 10.1158/1541-7786.MCR-17-0471. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.