Abstract
AIM
To compare the effects of manipulating light levels versus manipulating the spectral content of short wavelengths (blue light) of ambient lighting on refractive development in young rabbits.
METHODS
A total of 32 healthy 3-week-old rabbits were randomly assigned to one of the four groups with 8 in each group for 12wk: Control group (NC) under low blue light (output ratio of blue light 1.8%) at low illuminance (341 lx), HI group under low blue light (output ratio of blue light 1.6%) at high illuminance (5057 lx), simulating natural light (S-NL) group under high blue light (output ratio of blue light 4.9%) at high illuminance (5052 lx), and MB group under high blue light (output ratio of blue light 5.2%) at low illuminance (342 lx). The lighting in each group were provided by light emitting diode (LED) lamps emitting visible light (range 380-780 nm) in addition to (or not) LED lamps only emitting short wavelength (range 380-500 nm). Refraction, axial length, and corneal curvature radius were assessed by retinoscopy, ultrasonography and keratometry, respectively. Average data of both eyes for each animal were used as single values and compared among groups.
RESULTS
During the 12-week intervention, all animals had an emmetropization period. The decrease of refraction in rabbits in HI group was similar to S-NL group, both slower than that of NC group (P<0.001). At the 12th week, the refraction (3.000±0.267 D) and vitreous cavity depth (7.421±0.168 mm) of S-NL was similar to HI group (3.250±0.267 D, 7.264±0.256 mm), significantly different from NC group (1.937±0.291 D, 7.825±0.313 mm; P<0.001 for both). High blue light at low illuminance had little effect on refraction change. At the end of intervention, the difference of refraction (2.219±0.281 D) and vitreous cavity depth (7.785±0.229 mm) in MB group were not statistically significant (P=0.311, P=0.749) compared with NC group. The other components were less affected by lighting conditions (P>0.05).
CONCLUSION
The light levels per se but not the rich in spectral content of short wavelengths determine the inhibitory effect of ambient lighting on myopia development in rabbits.
Keywords: emmetropization, myopia, refractive error, illuminance, short wavelengths
INTRODUCTION
Myopia, also known as nearsightedness, is a common eye condition with an increasing prevalence in recent years[1]. In 2020, Wang et al[2] reported that the prevalence of myopia in primary school students in China had reached 63.1% and increased with grade in a non-linear manner to 90% by grade 10 or above. Furthermore, it is estimated that the global prevalence of myopia will account for 52% by 2050[3]. Previous studies believed that only high myopia will add to the burden of sight-threatening ocular complications such as cataracts, retinal detachment, macular degeneration and glaucoma, but a current systematic analysis showed that even moderate or low myopia also had considerable risks[4]. Because of the higher incidence and serious complications, myopia has been recognized as an important public health concern[5]–[6].
It is well established that time spent indoors increases the risk of myopia onset in children, whereas time spent outdoors reduces the risk of myopia. This notion is supported by results from human[7]–[8] and various laboratory animal studies, including those involving chickens[9]–[10], guinea pigs[11], primates[12], and tree shrews[13]. These studies indicated that the significantly higher illuminance encountered outdoors contributed to a reduced risk of myopia. Furthermore, the differences in the spectral composition of lighting between typical indoor and outdoor environments are still responsible. Typical outdoor sunlight contains a preponderance of short wavelengths (blue light)[14], while tungsten, fluorescent lights and light emitting diode (LED) lamps were weaker in blue light. In order to maximize luminance contrast, the eye would become relatively myopic in typical indoor scenes that are dominated by relatively long-wavelength lighting[15]. Then, animals kept in blue light, such as fish[16], chicks[17], and guinea pigs[18] had a lower refraction change or remained more hyperopic compared to those kept in long wavelengths (red light).
However, the difference between light levels and spectral content of short wavelengths on refractive development has not been reported. A study performed by Smith et al[19] assumed that increasing light intensity did not alter the final amount of myopia change in monkeys wearing monocular -3.0 D lenses. Furthermore, in recent years, studies also indicated that red light can effectively alter the process of emmetropization[20]–[24] and blue light appeared to act as a cue that should increase the eye's elongation rate[20], which strongly impact the view that more spectral content of blue light was beneficial to reduce the myopia change. These contradictory findings of the effect of light on refractive development, uncertain risk factors outdoors and potentially detrimental effects caused by long-term exposure to short wavelengths, such as sunburn, macular degeneration and increased risk of skin cancers[25], all urge us to further explore the effects of lighting levels and the spectral content of short wavelengths on refractive development and axial growth and guide us to optimize potential treatment strategies for myopia.
As a kind of mammal, young rabbits have been used in ophthalmic research for a long time[26]–[27]. The transmittance of electromagnetic radiation through the ocular media of the rabbit eyes[28] was similar to the transmittance spectra of the human crystalline lens[29]. The evidence that rabbits use visual cues to emmetropize also has been provided[30]. Therefore, in this study, we analyzed and clarified the changes in refractive development and axial components in young, pigmented rabbits after rearing them in different ambient lighting conditions to provide reference for clarifying the effects of light levels and spectral content on refractive development.
MATERIALS AND METHODS
Ethical Approval
This study was approved and supervised by the Experimental Animal Ethics Committee of North Sichuan Medical College (NSMC Appl. No. 2021 [24]).
Animals
In this study, 32 healthy pigmented young male/female rabbits, aged 3-week with weight of 250-450 g provided by the Experimental Animal Centre of North Sichuan Medical College were used. Animals with refractive medium opacity or fundus abnormalities were excluded. The animals were then raised in rabbit cages, which were surrounded by black shading cloth to simulate an independent rearing space, in the North Sichuan Medical College Experimental Animal Centre for 12wk. Food was regularly supplied in the morning, at noon and in the evening every day with unrestricted access to water. The room temperature was kept at 24°C±2°C with air circulation. All these conditions remained unchanged during treatment.
Grouping and Lighting
All rabbits were randomly divided into 4 groups (randomly designated by non-breeders), 8 for each group, namely less blue light at low illuminance (control, NC) group, less blue light at high illuminance (HI) group, more blue light at high illuminance (simulating natural light, S-NL) group and more blue light at low illuminance light (MB) group. At the top of each rearing space, lighting equipment was installed on the simulated ceiling with a 12h light/12h dark cycle (light from 07:00 to 19:00). NC group was fed in space which was composed by 3 LED lamps emitting visible light range 380-780 nm (white LED; OPPLE12-LE-47026, 5 W) to simulate the lighting with less short wavelengths at low illuminance (300-350 lx). HI group was put into space with 20 white LED lamps to simulate the lighting with less short wavelengths at high illuminance (5000-5100 lx). MB group was reared in space with 2 white LED lamps and 1 LED lamp which emit only short wavelengths 380-500 nm (blue LED) to simulate the lighting with more short wavelengths at low illuminance (300-350 lx), and the S-NL group was raised in space with 17 white LED lamps and 9 blue LED lamps to simulate the lighting with more short wavelengths at high illuminance (5000-5100 lx). Before the start of the experiment, the lighting parameters and the relative spectral distribution of each group were measured at the horizontal position of the eyes of rabbits in the feeding cage with the spectral illuminance analyzer (OHSP-350, HOPOOCOLOR). The lighting parameters and relative spectral distribution were shown in Table 1 and Figure 1.
Table 1. Lighting parameters of light conditions.
| Group | Illuminance (lx) | Irradiance (mW/cm2) | Output ratio of blue light (%) |
| NC | 341 | 118.09 | 1.8 |
| MB | 342 | 179.48 | 5.2 |
| HI | 5057 | 1553.94 | 1.6 |
| S-NL | 5052 | 1705.49 | 4.9 |
Illuminance, irradiance, output ratio (%) all measured by spectral illuminance analyzer (OHSP-350, HOPOOCOLOR). Blue light: Wavelength between 380 and 500 nm; NC: Less blue light at low illuminance group; MB: More blue light at low illuminance group; HI: Less blue light at high illuminance group; S-NL: More blue light at high illuminance group.
Figure 1. Relative spectral distribution of light conditions.
A, C: Spectral distribution of less blue light at low illuminance and less blue light at high illuminance were the same. The proportion of short-wavelength light with wavelength below 500 nm was small. B: The spectral distribution of more blue light at high illuminance. The relative power ratio of short wavelengths with wavelength below 500 nm were higher than that of the control group. D: The spectral distribution of more blue light at low illuminance. The relative power ratio of short wavelengths with wavelength below 500 nm were similar to more blue light at high illuminance, significantly higher than control group and less blue light at high illuminance group.
Ocular Biometry
All measures were taken while the animals were awake. Refractive state was measured in darkness every two weeks at the same time of day (around 10:00 a.m.) during the intervention by retinoscopy and the data were recorded as the mean of three measurements. No cycloplegic agent was used during all the examinations because the data tested before found no difference between using cycloplegic agents and not using cycloplegic agents and that McBrien et al[31] reported cycloplegic agents may interfere with refractive development. The anterior radius of curvature of the cornea was measured at the beginning (3-week-old) and end of intervention at the same time of day (around 15:00 p.m.) by keratometry (OM-4; Topcon Co., Japan) and the data also calculated from the average of three readings. Axial dimensions were measured in all animals at the beginning (3-week-old) and end of intervention at the same time of day (around 19:00 p.m.) with A-scan ultrasonography (11 MHz, Cinescan A/B, Quantel Co., France) after topical anesthesia with one drop of 0.4% oxybuprocaine hydrochloride (Santen Co., Osaka, Japan) and the data were recorded as the mean of ten measurements. The velocities of sound were assumed as 1532 m/s in the aqueous and vitreous humour and 1641 m/s in the lens. The A-mode ultrasound provides and stores waveforms with peaks that correspond to the front and back of the cornea, front and back of the lens and the internal limiting membrane of the retina. Off-line, the analysis cursors were moved to each pair of peaks to provide measures of anterior chamber depth, lens thickness and vitreous cavity depth. Among all the animals participating in the experiment, the refractive state and other ocular component dimensions were not significantly different in both eyes. Therefore, average data of both eyes for each animal were used as single values in the statistical analysis[32].
Statistical Analysis
The Statistical Package for the Social Sciences V.22 (SPSS 22.0) was used to describe statistics and analysis data. Data were expressed as mean±standard deviation (SD). Data on refractions were plotted as a function of time. One-way ANOVA, followed by LSD-test for post hoc analysis was used to examine between-group differences in refraction and ocular parameters at the start and end of intervention, between-group differences in changes of refraction and axial components. The same groups differences in refraction at the start and end of intervention were analyzed by paired t-test. Differences were defined as being significant at values of P less than 0.05.
RESULTS
At the beginning of intervention, there was no significant difference in refraction and other ocular components among the four groups (P>0.05; Table 2). During the different lighting exposure regimens, all animals emmetropized towards less hyperopic refraction (P<0.001, paired t-test), as showed in Table 2.
Table 2. Refraction and ocular parameters at the beginning and end of the intervention.
| Parameters | NC | MB | HI | S-NL | F (31,3) | P |
| Refraction (D) | ||||||
| Begin | 4.688±0.417 | 4.750±0.423 | 4.688±0.417 | 4.656±0.421 | 0.070 | 0.975 |
| 12wk | 1.937±0.291 | 2.219±0.281 | 3.250±0.267a,b | 3.000±0.267a,b | 40.549 | 0.001 |
| t/P | 29.103/0.000 | 15.842/0.000 | 10.286/0.000 | 14.387/0.000 | ||
| R (mm) | ||||||
| Begin | 5.476±0.234 | 5.500±0.179 | 5.405±0.207 | 5.450±0.232 | 0.298 | 0.827 |
| 12wk | 6.730±0.158 | 6.721±0.178 | 6.745±0.151 | 6.728±0.192 | 0.027 | 0.994 |
| ACD (mm) | ||||||
| Begin | 2.120±0.060 | 2.144±0.043 | 2.160±0.048 | 2.090±0.050 | 2.875 | 0.054 |
| 12wk | 2.346±0.029 | 2.296±0.059 | 2.270±0.058 | 2.300±0.069 | 2.547 | 0.076 |
| LT (mm) | ||||||
| Begin | 5.011±0.078 | 4.996±0.079 | 5.005±0.082 | 5.021±0.072 | 0.147 | 0.931 |
| 12wk | 6.041±0.078 | 5.994±0.094 | 6.040±0.104 | 5.970±0.041 | 1.417 | 0.259 |
| VCD (mm) | ||||||
| Begin | 6.109±0.283 | 6.195±0.252 | 6.017±0.305 | 6.278±0.265 | 1.305 | 0.292 |
| 12wk | 7.825±0.313 | 7.785±0.229 | 7.264±0.256a,b | 7.421±0.168a,b | 9.901 | 0.001 |
D: Dioptor; R: Radius of curvature of cornea; ACD: Anterior chamber depth; LT: Lens thickness; VCD: Vitreous cavity depth; NC: Less blue light at low illuminance group; MB: More blue light at low illuminance group; HI: Less blue light at high illuminance group; S-NL: More blue light at high illuminance group. aP<0.001 compared to NC group, bP<0.001 compared to MB group by LSD-test.
mean±SD
Figure 2A showed the refractive development of young rabbits in response to less blue light at low illuminance (NC group), the eyes (principally the vitreous chamber) grew rapidly so that at the end of intervention they were less than two diopters hyperopic (1.937±0.291 D). As shown in Table 2, the depth of vitreous chamber was the largest of the four groups at the end of intervention (7.825±0.313 mm).
Figure 2. Refractive development in young rabbit over 12wk.

During the intervention, all rabbits had an emmetropization period with the decreasing of hyperopia. A: Refractive response of animals in NC group; B: Refraction of 8 young rabbits raised in HI group was higher hyperopia than that of animals raised in NC group; C: Refraction in MB group was closer to NC group; D: Refraction of animals in S-NL group was higher than NC group. NC: Less blue light at low illuminance group; MB: More blue light at low illuminance group; HI: Less blue light at high illuminance group; S-NL: More blue light at high illuminance group.
Figure 2B compared the longitudinal changes in refraction of animals exposed to less blue light at high illuminance (HI group) with the animals exposed to less blue light at low illuminance (NC group). The refractive rate of rabbits in HI group decreased slower than that of animals in NC group over time. At the end of intervention, the group refraction (3.250±0.267 D) was significantly higher than that of NC group at the same age (P=0.001; Table 2). The depth of vitreous chamber was short. It was significantly shorter than vitreous cavity depth in NC group (P<0.001; Table 2). The result indicated that exposure to less blue light at high illuminance significantly reduced the development of myopia in young rabbits by delaying the growth rate of the vitreous cavity depth.
Then, Figure 2C showed that more blue light at low illuminance (MB group) had little effect on refractive development. The refractive response was similar to that with NC group. The refractive state at the end of intervention (2.219±0.281 D) was not significantly different from the refraction in NC group (P=0.311), but significantly different from HI group (P<0.001). Vitreous cavity depth of this group (7.785±0.229 mm) was significantly longer than HI group (P<0.001), but was not significantly longer than that of control group (P=0.749; Table 2).
Figure 2D compared the effects of more blue light at high illuminance (S-NL group) on refractive development: the time-course of the group average refraction were similar to that of the animals in HI group during the intervention period. At the end of intervention, the refractive state (3.000±0.267 D) was significantly hyperopia than NC and MB group (P<0.001 for both), and the vitreous cavity depth (7.421±0.168 mm) was significantly shorter than NC and MB group (P<0.001 for both), both not different from HI group (P=0.491, P=0.971; Table 2).
Then the anterior chamber depth, lens thickness and anterior radius of curvature of the cornea were less affected by light levels and special content. At the end of intervention, there was no significant difference between the four groups (P>0.05; Table 2).
After 12wk, the changes of refraction and axial components showed that myopia changes and vitreous chamber elongation were significantly affected by lighting condition. Myopia changes and vitreous chamber elongation in HI group were significantly lower than that in NC group, so as the S-NL group (Figure 3). The association between the change in refraction and axial components also showed that the correlation between amount of change in vitreous cavity depth and the change in refraction was highly significant (R2=0.271, P=0.002), values of the anterior chamber depth, lens thickness and corneal radius to refraction were small (Figure 4).
Figure 3. Changes in refraction and axial components.

A: Myopia change in HI group was similar to S-NL group, significantly lower than that in NC group; B: Vitreous cavity elongation in HI group was similar to S-NL group, significantly lower than that in NC group. Values are the average of the right and left eyes. aP<0.001 compared with NC group, LSD-t test. NC: Less blue light at low illuminance group; MB: More blue light at low illuminance group; HI: Less blue light at high illuminance group; S-NL: More blue light at high illuminance group.
Figure 4. Association between the change in refraction and ocular components.
Values are the average of the right and left eyes. NC: Less blue light at low illuminance group (black); MB: More blue light at low illuminance group (blue); HI: Less blue light at high illuminance group (red); S-NL: More blue light at high illuminance group (yellow).
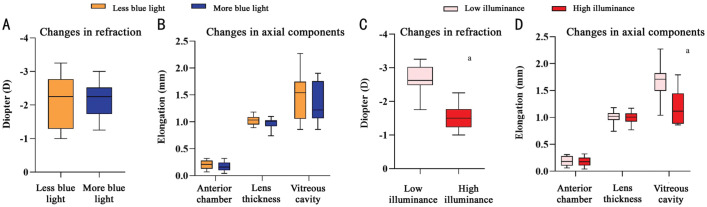
Given the difference of light conditions and eye parameters, we analyzed the effect on the changes in refraction and axial components according to the content of blue light without considering the light levels. The effects of light level on refraction and axial components were also analyzed according to illuminance without considering the content of blue light. Figure 5A, 5B showed that the changes of refraction, vitreous cavity depth, anterior chamber depth and lens thickness in the group of more blue light (S-NL+MB) were relatively smaller than less blue light group (NC+HI), but the difference was not significant. However, the changes in refraction and vitreous cavity depth in high illuminance group (HI+S-NL) were all smaller than that in low illuminance group (NC+MB), and the difference was statistically significant, as showed in Figure 5C, 5D.
Figure 5. Changes of eye parameters grouped by blue light and illuminance.
A, B: There were no significant difference between the changes in refraction and axial components in less or more content of short wavelengths; C, D: High illuminance lighting significantly inhibited the changes in refraction and vitreous cavity depth, and the changes were significantly lower than that in low illuminance. aP<0.05 independent t-test.
DISCUSSION
The main finding of this study was that contribution from light levels rather than spectral content of short wavelengths determines the protective effects of lighting on myopia development in young rabbits.
As the studies proved that individuals who spend more time outdoors have more hyperopic refractive errors and a lower prevalence of juvenile-onset myopia[33]–[36], there has been great interest in further exploring this relationship. Lingham et al[37] reviewed the evidence for and against spending time outdoors that may protect against myopia and reported that light levels and spectral composition were relatively significant.
In this study, lighting with high illuminance significantly prevented myopia changes in young rabbits, no matter the spectral content of short wavelengths (Figure 3A). At the low illuminance lighting, even if the blue LED lamps were added to increase the spectral content of short wavelengths, the myopia change and vitreous chamber elongation were similar to that in NC group (Figure 3). The results indicated that light levels determined the protective effects of lighting on refractive development and that intense light was a protective role. Research in chickens provided that high lighting levels, either from sunlight or intense laboratory lights, all reduces the degree of axial myopia produced by form deprivation by 65% over a 4-day treatment period[38]. Smith et al[12] also found that absolute light levels can have a significant impact on vision-dependent ocular growth in primates (rhesus monkeys). Recently, Lanca et al[39] reported that even if certain protective measures were taken to block the short wavelengths, the illuminance of outdoor light was significantly higher than that of indoor light, which played a protective role on the progress of myopia. Results from children also support the positive effects of high light levels for that increasing the light levels in classrooms could reduce the incidence of myopia[40].
However, Smith et al[19] postulated that intense indoor lighting had no significant impact on lens-induced myopia changes in monkeys. Additionally, they observed that exposure to sunlight could markedly decelerate the progression of refractive myopia in both normal eyes and negative lens-induced eyes of young monkeys[41]–[42], thereby establishing a connection between spectral composition and myopia. Over the past decade, numerous studies have further validated that short wavelength light can protect against myopia progression across various species[17],[43]–[45]. Consequently, the hypothesis that short wavelength lights in outdoor scenes are beneficial for myopia mitigating has gained popularity.
In this study, shorter wavelength at low illuminance lighting had little effect on refractive development (Figure 2D), and the refractive state and vitreous cavity depth were all similar to that of NC group at the end of intervention. At high illuminance lighting, the refractive decrease in S-NL group was similar to that seen with HI group, and the refractive state was similar to HI group, significantly hyperopia than that in NC group after 12wk of intervention (P<0.001). The results mean that manipulating the spectral content of short wavelengths had little effect on refractive development in rabbits. In the past, a study performed by Rohrer et al[46] found that refractive development in chickens was not different from controls in white light for either red or near-ultraviolet light. Similarly, animals exposed to ultraviolet light or white light also have no significant difference in compensation for myopia induced by negative lenses[47]. Recently, results from Liu et al[48] also confirmed that no significant difference in mean refraction was observed between the rhesus monkeys raised in blue light and white light. The results of this study were consistent with the research above, supporting no significant difference of light spectral content on refractive development.
As a kind of electromagnetic wave, the propagation of light is also a kind of energy transmission. Outdoor light may inhibit myopia change through light-stimulated dopamine (DA) has been supported by a number of studies[49]–[51]. Experimental studies have confirmed that both intense light and short wavelength light all can promote the secretion of DA, which in turns protects against myopia progression. Combined with the photon energy of different wavelengths and the application of photo biotherapy in recent years[52], we speculate that the irradiance of light and the energy conversion of different wavelengths received by retina might be reasonable for the protective effects of lighting on myopia change. As shown in Table 1, high illuminance always means high irradiance, more short wavelengths also related to more irradiance. The retina of animals exposed to high illuminance lighting or lighting with more short wavelengths received more energy. Experiment conducted by Torii et al[53] confirmed that there was no significant difference in myopia change of chickens reared in lighting with the same irradiance, no matter the spectral content. However, in this study, the output of short wavelengths we controlled in more blue groups were more suitable for outdoor lighting in real life, significantly lower than experimental studies. Therefore, even if the content of short wavelengths was increased, the difference of overall irradiance is still low. This may be reasonable for the lack of statistical difference of refractive development between less blue groups and more blue groups.
In summary, we present evidence demonstrate that contribution from light levels rather than spectral content of short wavelengths determines the protective effect of lighting on myopia development in young rabbits. The result indicates that high light levels rather than the rich in short wavelengths plays the protective role for protecting against myopia change after increasing time outdoors. However, there are limitations in our study (e.g., lacks the data of pupil size during the intervention period, lacks the data of the peak of the rabbit blue cone and ignoring the influence of chromatic aberration), and there is still much to be learned about the mechanism(s) by which intense light prevents the development of myopia.
Footnotes
Authors' contributions: All authors participated in the design, interpretation of the studies and analysis of the data and review of the manuscript; Zou YC, Tang XP, and Tang ZJ designed the experiment, analyzed the data, wrote and modified the manuscript. Tang XP and Fan HB conducted the experiments and participated in ocular biometry.
Availability of Data and Materials: The datasets used and analyzed during the current study are available from the corresponding author on reasonable request. Or all relevant datasets related to the study can be found in the specified database (https://figshare.com/s/2e9f6420ce220c84a2d4).
Foundations: Supported by Natural Science Foundation of Sichuan Province (No.2022NSFSC0754); Project of Nanchong Science and Technology Bureau (No.22SXQT0350).
Conflicts of Interest: Tang XP, None; Tang ZJ, None; Fan HB, None; Zou YC, None.
REFERENCES
- 1.Spillmann L. Stopping the rise of myopia in Asia. Graefes Arch Clin Exp Ophthalmol. 2020;258(5):943–959. doi: 10.1007/s00417-019-04555-0. [DOI] [PubMed] [Google Scholar]
- 2.Wang J, Ying GS, Fu X, et al. Prevalence of myopia and vision impairment in school students in Eastern China. BMC Ophthalmol. 2020;20(1):2. doi: 10.1186/s12886-019-1281-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holden BA, Fricke TR, Wilson DA, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123(5):1036–1042. doi: 10.1016/j.ophtha.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Haarman AEG, Enthoven CA, Tideman JWL, et al. The complications of myopia: a review and meta-analysis. Invest Ophthalmol Vis Sci. 2020;61(4):49. doi: 10.1167/iovs.61.4.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sankaridurg P, Tahhan N, Kandel H, et al. IMI impact of myopia. Invest Ophthalmol Vis Sci. 2021;62(5):2. doi: 10.1167/iovs.62.5.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Leeuwen R, Haarman AEG, van de Put MAJ, et al. Association of rhegmatogenous retinal detachment incidence with myopia prevalence in the Netherlands. JAMA Ophthalmol. 2021;139(1):85–92. doi: 10.1001/jamaophthalmol.2020.5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J, Wang J, Qi Z, et al. Smartwatch measures of outdoor exposure and myopia in children. JAMA Netw Open. 2024;7(8):e2424595. doi: 10.1001/jamanetworkopen.2024.24595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhakal R, Lawrenson JG, Huntjens B, et al. Light exposure profiles differ between myopes and non-myopes outside school hours. BMJ Open Ophthalmol. 2024;9(1):e001469. doi: 10.1136/bmjophth-2023-001469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarfare S, Yang J, Nickla DL. The effects of brief high intensity light on ocular growth in chicks developing myopia vary with time of day. Exp Eye Res. 2020;195:108039. doi: 10.1016/j.exer.2020.108039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biswas S, Muralidharan AR, Betzler BK, et al. A duration-dependent interaction between high-intensity light and unrestricted vision in the drive for myopia control. Invest Ophthalmol Vis Sci. 2023;64(3):31. doi: 10.1167/iovs.64.3.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang L, Qu X. The effects of high lighting on the development of form-deprivation myopia in guinea pigs. Invest Ophthalmol Vis Sci. 2019;60(13):4319–4327. doi: 10.1167/iovs.18-25258. [DOI] [PubMed] [Google Scholar]
- 12.Smith EL, 3rd, Hung LF, Huang J. Protective effects of high ambient lighting on the development of form-deprivation myopia in rhesus monkeys. Invest Ophthalmol Vis Sci. 2012;53(1):421–428. doi: 10.1167/iovs.11-8652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang L, Zhang S, Schaeffel F, Xiong S, Zheng Y, Zhou X, Lu F, Qu J. Interactions of chromatic and lens-induced defocus during visual control of eye growth in guinea pigs (Cavia porcellus) Vision Res. 2014;94:24–32. doi: 10.1016/j.visres.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 14.Ngo C, Saw SM, Dharani R, et al. Does sunlight (bright lights) explain the protective effects of outdoor activity against myopia? Ophthalmic Physiol Opt. 2013;33(3):368–372. doi: 10.1111/opo.12051. [DOI] [PubMed] [Google Scholar]
- 15.Yoon HH, Taylor CP, Rucker FJ. Indoor illuminants, S-Cone stimulation, and eye growth in chicks. Invest Ophth Vis Sci. 2018;59(9):683. [Google Scholar]
- 16.Kröger RH, Wagner HJ. The eye of the blue acara (Aequidens pulcher, Cichlidae) grows to compensate for defocus due to chromatic aberration. J Comp Physiol A. 1996;179(6):837–842. doi: 10.1007/BF00207362. [DOI] [PubMed] [Google Scholar]
- 17.Chun RK, Choy KY, Li KK, et al. Additive effects of narrowband light and optical defocus on chick eye growth and refraction. Eye Vis (Lond) 2023;10(1):15. doi: 10.1186/s40662-023-00332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Sun Y, Wang K, et al. Effects of blue light exposure on ocular parameters and choroidal blood perfusion in guinea pig. Exp Eye Res. 2023;235:109619. doi: 10.1016/j.exer.2023.109619. [DOI] [PubMed] [Google Scholar]
- 19.Smith EL, 3rd, Hung LF, Arumugam B, et al. Negative lens-induced myopia in infant monkeys: effects of high ambient lighting. Invest Ophthalmol Vis Sci. 2013;54(4):2959–2969. doi: 10.1167/iovs.13-11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gawne TJ, Siegwart JT, Jr, Ward AH, et al. The wavelength composition and temporal modulation of ambient lighting strongly affect refractive development in young tree shrews. Exp Eye Res. 2017;155:75–84. doi: 10.1016/j.exer.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang J, Yang L, Chen R, et al. A role of color vision in emmetropization in C57BL/6J mice. Sci Rep. 2020;10(1):14895. doi: 10.1038/s41598-020-71806-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Zhu M, Yan X, et al. The effect of repeated low-level red-light therapy on myopia control and choroid. Transl Vis Sci Technol. 2024;13(10):29. doi: 10.1167/tvst.13.10.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiang A, He H, Li A, et al. Changes in choroidal thickness and blood flow in response to form deprivation-induced myopia and repeated low-level red-light therapy in guinea pigs. Ophthalmic Physiol Opt. 2025;45(1):111–119. doi: 10.1111/opo.13404. [DOI] [PubMed] [Google Scholar]
- 24.She Z, Ward A, Gawne T. The effects of ambient narrowband long-wavelength light on lens-induced myopia and form-deprivation myopia in tree shrews. Exp Eye Res. 2023;234:109593. doi: 10.1016/j.exer.2023.109593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gieniusz E, Skrzydlewska E, Łuczaj W. Current insights into the role of UV radiation-induced oxidative stress in melanoma pathogenesis. Int J Mol Sci. 2024;25(21):11651. doi: 10.3390/ijms252111651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin Z, Chen X, Ge J, et al. Effects of direct intravitreal dopamine injection on sclera and retina in form-deprived myopic rabbits. J Ocul Pharmacol Ther. 2008;24(6):543–550. doi: 10.1089/jop.2008.0041. [DOI] [PubMed] [Google Scholar]
- 27.Nie HH, Huo LJ, Yang X, et al. Effects of 7-methylxanthine on form-deprivation myopia in pigmented rabbits. Int J Ophthalmol. 2012;5(2):133–137. doi: 10.3980/j.issn.2222-3959.2012.02.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Algvere PV, Torstensson PA, Tengroth BM. Light transmittance of ocular media in living rabbit eyes. Invest Ophthalmol Vis Sci. 1993;34(2):349–354. [PubMed] [Google Scholar]
- 29.Eto T, Teikari P, Najjar RP, et al. A Purkinje image-based system for an assessment of the density and transmittance spectra of the human crystalline lens in vivo. Sci Rep. 2020;10(1):16445. doi: 10.1038/s41598-020-73541-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tong L, Cui D, Zeng J. Topical bendazol inhibits experimental myopia progression and decreases the ocular accumulation of HIF-1α protein in young rabbits. Ophthalmic Physiol Opt. 2020;40(5):567–576. doi: 10.1111/opo.12717. [DOI] [PubMed] [Google Scholar]
- 31.McBrien NA, Stell WK, Carr B. How does atropine exert its anti-myopia effects? Ophthalmic Physiologic Optic. 2013;33(3):373–378. doi: 10.1111/opo.12052. [DOI] [PubMed] [Google Scholar]
- 32.Ederer F. Shall we count numbers of eyes or numbers of subjects? Arch Ophthalmol. 1973;89(1):1–2. doi: 10.1001/archopht.1973.01000040003001. [DOI] [PubMed] [Google Scholar]
- 33.Yang JL, Li DL, Chen J, et al. Effect modification of time spent outdoors on the association between early childhood overweight and myopia: a one-year follow-up study. J Public Health (Oxf) 2024;46(1):107–115. doi: 10.1093/pubmed/fdae006. [DOI] [PubMed] [Google Scholar]
- 34.He X, Lin C, Zhang F, et al. Outdoor time influences VIPR2 polymorphism rs2071623 to regulate axial length in Han Chinese children. Mol Vis. 2023;29:266–273. [PMC free article] [PubMed] [Google Scholar]
- 35.Kanclerz P, Lanca C, Radomski SA, et al. The outdoor time in non-myopic children has decreased to that of myopic children during the SARS-CoV-2 pandemic. Rom J Ophthalmol. 2023;67(1):33–40. doi: 10.22336/rjo.2023.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He X, Sankaridurg P, Wang J, et al. Time outdoors in reducing myopia: a school-based cluster randomized trial with objective monitoring of outdoor time and light intensity. Ophthalmology. 2022;129(11):1245–1254. doi: 10.1016/j.ophtha.2022.06.024. [DOI] [PubMed] [Google Scholar]
- 37.Lingham G, MacKey DA, Lucas R, et al. How does spending time outdoors protect against myopia? A review. Brit J Ophthalmol. 2020;104(5):593–599. doi: 10.1136/bjophthalmol-2019-314675. [DOI] [PubMed] [Google Scholar]
- 38.Ashby R, Ohlendorf A, Schaeffel F. The effect of ambient illuminance on the development of deprivation myopia in chicks. Invest Ophthalmol Vis Sci. 2009;50(11):5348–5354. doi: 10.1167/iovs.09-3419. [DOI] [PubMed] [Google Scholar]
- 39.Lanca C, Teo A, Vivagandan A, et al. The effects of different outdoor environments, sunglasses and hats on light levels: implications for myopia prevention. Transl Vis Sci Technol. 2019;8(4):7. doi: 10.1167/tvst.8.4.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suh YW, Ha SG, Kim SH. Effect of classroom illuminance on the development and progression of myopia in school children. Korean J Ophthalmol. 2022;36(3):194–201. doi: 10.3341/kjo.2021.0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y, Ding H, Stell WK, et al. Exposure to sunlight reduces the risk of myopia in rhesus monkeys. PLoS One. 2015;10(6):e0127863. doi: 10.1371/journal.pone.0127863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang X, Yang Y, Wang Y, et al. Protective effects of sunlight exposure against PRK-induced myopia in infant rhesus monkeys. Ophthalmic Physiol Opt. 2021;41(4):911–921. doi: 10.1111/opo.12826. [DOI] [PubMed] [Google Scholar]
- 43.Rucker F, Britton S, Taylor C. Color and temporal frequency sensitive eye growth in chick. Invest Ophthalmol Vis Sci. 2018;59(15):6003–6013. doi: 10.1167/iovs.18-25322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amorim-de-Sousa A, Chakraborty R, Collins MJ, et al. Blue light stimulation of the blind spot in human: from melanopsin to clinically relevant biomarkers of myopia. Bioelectron Med. 2024;10(1):26. doi: 10.1186/s42234-024-00159-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Timucin OB, Arabaci M, Cuce F, et al. The effects of light sources with different spectral structures on ocular axial length in rainbow trout (Oncorhynchus mykiss) Exp Eye Res. 2016;151:212–221. doi: 10.1016/j.exer.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 46.Rohrer B, Schaeffel F, Zrenner E. Longitudinal chromatic aberration and emmetropization: results from the chicken eye. J Physiol. 1992;449:363–376. doi: 10.1113/jphysiol.1992.sp019090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hammond DS, Wildsoet CF. Compensation to positive as well as negative lenses can occur in chicks reared in bright UV lighting. Vision Res. 2012;67:44–50. doi: 10.1016/j.visres.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu R, Hu M, He JC, et al. The effects of monochromatic illumination on early eye development in rhesus monkeys. Invest Ophthalmol Vis Sci. 2014;55(3):1901–1909. doi: 10.1167/iovs.13-12276. [DOI] [PubMed] [Google Scholar]
- 49.Chen S, Zhi ZN, Ruan QQ, et al. Bright light suppresses form-deprivation myopia development with activation of dopamine D1 receptor signaling in the ON pathway in retina. Invest Ophthalmol Vis Sci. 2017;58(4):2306–2316. doi: 10.1167/iovs.16-20402. [DOI] [PubMed] [Google Scholar]
- 50.Cohen Y, Peleg E, Belkin M, et al. Ambient illuminance, retinal dopamine release and refractive development in chicks. Exp Eye Res. 2012;103:33–40. doi: 10.1016/j.exer.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 51.Feldkaemper M, Schaeffel F. An updated view on the role of dopamine in myopia. Exp Eye Res. 2013;114:106–119. doi: 10.1016/j.exer.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 52.Kaynezhad P, Tachtsidis I, Jeffery G. Optical monitoring of retinal respiration in real time: 670 nm light increases the redox state of mitochondria. Exp Eye Res. 2016;152:88–93. doi: 10.1016/j.exer.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Torii H, Kurihara T, Seko Y, et al. Violet light exposure can be a preventive strategy against myopia progression. EBioMedicine. 2017;15:210–219. doi: 10.1016/j.ebiom.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]