Abstract
Introduction
While tuberosity reinsertion significantly improves the short-term functional outcomes of reverse shoulder arthroplasty done for proximal humerus fracture, we do not know how well these results hold over the long term. The objective of this study was to analyze the effect of tuberosity reinsertion on the quality of life of patients and the functional outcomes of the operated limb after a minimum follow-up of 5 years.
Methods
Sixty-two patients were included. Their mean age at the final review was 79 ± 10 years. The Katz and Lawton scales, Constant score, DASH and SSV were collected. Radiographs were made at the final assessment to analyze the position of the tuberosities and to look for radiological signs of implant loosening.
Results
The mean follow-up was 6.7 ± 1.5 years. The tuberosities had been reinserted in 35 patients (56%). There were no statistically significant differences between groups in the Katz (p = 0.60) and Lawton (p = 0.49) scales, nor the DASH (p = 0.45) or SSV (p = 0.49) at the final review. The Constant score was significantly better in the patients who had their tuberosities reinserted (p = 0.01), also the active forward flexion (p = 0.02), the internal rotation (p = 0.01), and the external rotation arm abduction (p = 0.02), but there was no significant difference for external rotation elbow at side (p = 0.14). None of the patients underwent revision surgery for implant loosening.
Conclusion
Tuberosity reinsertion has a functional benefit beyond 5 years postoperative, although it does not appear to have a significant effect on the geriatric outcomes or the subjective clinical scores. The patients regained satisfactory independence for an orthogeriatric population.
Level of evidence
Level IV—Retrospective study.
Keywords: Reverse shoulder arthroplasty, Tuberosities, Constant score, Geriatric evaluation
Introduction
Proximal humerus fractures (PHF) make up 6% of all fractures in adults and 10% of fractures after 65 years of age [1]. The humerus is the third most common fracture location in older adults, after the proximal femur and distal radius [2]. The incidence of PHF in France is 30,000 cases per year [1]. Reverse shoulder arthroplasty (RSA) has better clinical outcomes [3] and a lower revision rate than hemiarthroplasty for the treatment of displaced PHF [4]. Grammont and Baulot both suggested that the action of the deltoid muscle on the RSA was sufficient to compensate for the lack of rotator cuff muscles, especially on the recovery of forward flexion and abduction [5]. In the early studies by Cazeneuve [6] and Gallinet [7], reinserting the tuberosities was not recommended. However, more recent studies have found a significant improvement in the Constant score when the tuberosities are reinserted (60–79%) versus not reinserted (50–56%) [8–13]. But these studies did not report the effect of tuberosity reinsertion on the patient’s quality of life or independence [10, 12]. Also, very few studies have investigated the functional benefits of tuberosity reinsertion in the long term [11].
The primary objective of our study was to determine if tuberosity reinsertion improves shoulder function in the long term. The secondary objectives were to determine the consequences on the patients’ quality of life, determine the condition of the tuberosities on radiographs and implant survival. The null hypothesis was that there is no significant difference in the Constant score more than 5 years after RSA whether or not the tuberosities are reinserted.
Methods
Patients
This was a retrospective, single-center, comparative, multisurgeon cohort study. Five surgeons were involved (one level 4, threeseven level 3 and five level 2 according Tang and Giddins [14]).
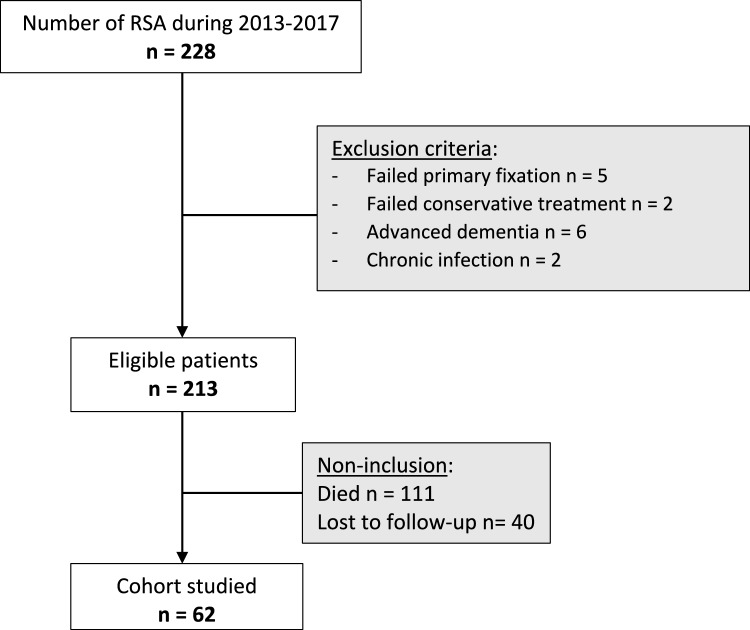
Eligible were patients with a Neer 3 or 4 PHF [15] treated by RSA between January 2013 and December 2017 and who had a minimum follow-up of 5 years (n = 228). After applying the exclusion criteria (Fig. 1), 213 patient records were reviewed. Of these, 111 patients had died, and 40 patients were lost to follow-up.
Fig. 1.
Flow chart
The remaining 62 patients were invited for a final in-person review more than 5 years after RSA to carry out a full clinical examination of the shoulder and to make follow-up radiographs. All patients provided their written consent after having received clear, accurate information about the study. This study was approved by our facility’s data protection officer (Ref number DEC22-302).
Surgical technique
A cemented UNIC® RSA implant (EVOLUTIS™) was used in all patients. It consists of a 30-mm diameter titanium glenoid base secured with two to four standard cancellous bone screws (5-mm diameter), a 38-mm diameter stainless steel glenosphere, a cemented highly polished titanium alloy humeral stem with 20° retroversion and a polyethylene insert. The RSA was implanted using a superolateral surgical approach [16]. Given the lack of consensus about the benefits of tuberosity reinsertion when these surgeries were done in 2013–2017, the decision to perform reinsertion was left up to the surgeon, based on intraoperative findings and preferences.
After exposing the fracture site and the rotator cuff, any bone fragments without periosteum were excised. The greater and lesser tuberosities were separated using bone scissors in Neer 3 fractures. The tuberosities were captured with two to four Mersuture® #1, PDS II® loop, or Vicryl Plus® #0 sutures. The surgeon was free to do tenodesis or tenotomy with the tendon of the long head of biceps. After glenoid exposure and labrum excision, a threaded guide pin was inserted into the middle of the glenoid, which was then prepared with a motorized burr.
Before cementing the chosen humeral stem, two Mersuture® #1 sutures were positioned in the lateral cortex of the humeral shaft to set the stage for reinserting the tuberosities. Once this was completed, the tuberosities were reinserted using the tension-band wiring technique described by Boileau et al. [17].
The operated limb was immobilized for 3 weeks in a sling. Passive and active-assisted mobilization of the shoulder was initiated in the immediate post-operative period. Active mobilization was initiated after weaning of the sling.
Clinical and radiological assessment
A full clinical examination of the shoulder was done by a surgeon (NA) who was not involved in the RSA implantation. The Constant [18, 19], the Disabilities of the Arm, Shoulder and Hand (DASH) and the Subjective Shoulder Value (SSV) were collected. We documented active range of motion, measured with a goniometer for the forward flexion, abduction and external rotation. The internal rotation (IR) was measured as the highest vertebral level the patient could reach in the back, then conversed into numerical values as in the Constant score.
The Activities of Daily Living (ADL) and instrumental Activities of Daily Living (iADL) scales were completed to determine the patient’s independence and their overall functional prognosis after the surgery.
Conventional radiographs of the operated shoulder were made at the final assessment (AP views in three different rotations and Lamy lateral view) to determine fracture union and the position of the tuberosities if they had been reinserted (Fig. 2). The tuberosities were considered healed if the greater tuberosity was visibly continuous with the humeral shaft on the AP view in neutral rotation. The tuberosities’ position and potential secondary osteolysis were determined on the AP views (all three rotations) and the lateral view [20]. We looked for the presence of periprosthetic radiolucent lines (≥ 2 mm) [21] and notching (Sirvaux classification) [22] suggestive of implant loosening.
Fig. 2.
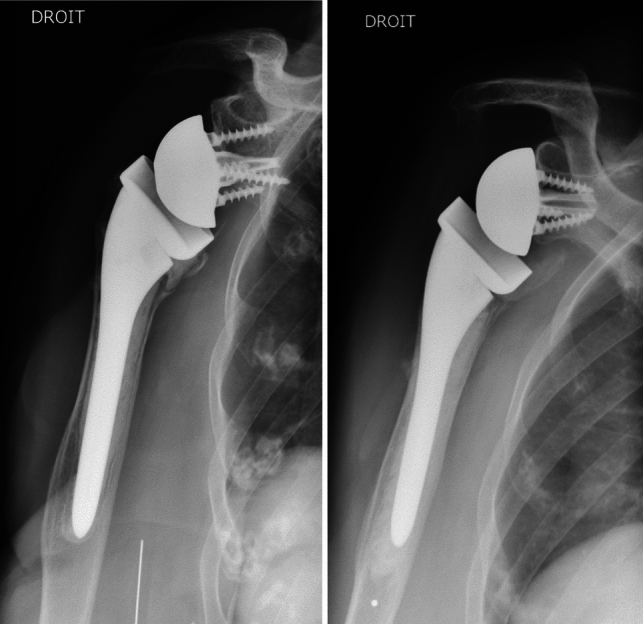
Postoperative radiographs: tuberosities reinserted (left image) or not reinserted (right image)
Statistical analysis
Data were analyzed statistically using SPSS® software (Version 20.0, SPSS IBM, New York, U.S.A). The Shapiro–Wilk test was used to determine if the data was normally distributed. Student’s t test was used to compare the means of normally distributed continuous variables. A non-parametric Mann–Whitney was used to compare continuous variables that were not normally distributed. Due to the small sample size, Fisher’s exact test was used to test hypotheses involving nominative or ordinal parameters. The null hypothesis was rejected when p < 0.05.
Results
Clinical outcomes
The tuberosities had been reinserted in 35 patients (56%). The mean follow-up was 7.2 ± 1.4 years in patients whose tuberosities had not been reinserted (group 1) and 6.5 ± 1.3 years in patients whose tuberosities had been reinserted (group 2) (p = 0.07). The general characteristics of the patients were comparable between the two groups. (Table 1) The Constant (absolute and adjusted) was significantly better in the group with tuberosity reinsertion (absolute Constant of 69.8 ± 17.1) than in the group without reinsertion (56.7 ± 19.7) (p = 0.01). There was a statistically significant difference in favor of reinsertion for external rotation arm abduction (ER2) (p = 0.02), internal rotation (p = 0.01), active forward flexion (p = 0.02) and active abduction (p = 0.04). There was no significant difference for external rotation elbow at side (ER1) (p = 0.14), DASH (p = 0.45) or SSV (p = 0.49) (Table 2).
Table 1.
Patient demographics
| Total (n = 62) | Group 1 Tuberosities not reinserted (n = 27) | Group 2 Tuberosities reinserted (n = 35) | p | ||
|---|---|---|---|---|---|
| Follow-up (years) mean ± SD | 6.7 ± 1.5 | 7.2 ± 1.4 | 6.5 ± 1.3 | 0.07 | |
| Age at surgery (years) mean ± SD | 73 ± 10 | 74 ± 10 | 71 ± 9 | 0.17 | |
| Age at last follow-up (years) mean ± SD | 79 ± 10 | 82 ± 10 | 78 ± 9 | 0.08 | |
| Females n (%) | 50 (80.6%) | 93% | 71% | ||
| BMI (mean ± SD) | 28.1 ± 5.4 | 28.3 ± 5.8 | 27.8 ± 5.2 | 0.72 | |
| Smoker, n (%) | 5 (8.1%) | 1 (3.7%) | 4 (11.4%) | 0.38 | |
| Alcohol, n (%) | 10 (16.1%) | 5 (19%) | 5 (14%) | 0.74 | |
| Dominant arm operated on n (%) | 35 (56.5%) | 17 (63%) | 18 (51.4%) | 0.36 | |
| Fracture context n (%) | Low energy | 54 (87.1%) | 25 (92.6%) | 29 (83%) | 0.45 |
| High energy | 8 (12.9%) | 2 (7.4%) | 6 (17%) | ||
| Living situation n (%) | Home | 41 (66.1%) | 18 (66.7%) | 23 (65.7%) | 0.94 |
| At home with assistance | 8 (12.9%) | 3 (11.1%) | 5 (14.3%) | ||
| Nursing home | 13 (21%) | 6 (22.2%) | 7 (20%) | ||
| Neer classification n (%) | Neer 3 | 25 (40%) | 11 (40.7%) | 14 (40%) | 0.95 |
| Neer 4 | 37 (60%) | 16 (59.3%) | 21 (60%) | ||
| Time to surgery (days) mean ± SD | 2.1 ± 3 | 2 ± 3 | 2 ± 3 | 0.95 | |
| Length of hospital stay (days) mean ± SD | 6.4 ± 4.5 | 6.9 ± 4.4 | 6 ± 4.6 | 0.48 | |
| ASA score 3 or 4 n (%) | 1 | 4 (6.5%) | 1 (3.7%) | 3 (8.6%) | 0.9 |
| 2 | 23 (37.1%) | 10 (37%) | 13 (37.1%) | ||
| 3 | 29 (46.8%) | 13 (48.1%) | 16 (45.7%) | ||
| 4 | 6 (9.6%) | 3 (11.1%) | 3 (8.6%) | ||
| Complications, n (%) | Glenoid loosening | 1 (1.6%) | 0 (0%) | 1 (2.9%) | 0.4 |
| Periprosthetic fracture | 2 (3.2%) | 1 (3.7%) | 1 (2.9%) | ||
| Dislocation | 2 (3.2%) | 2 (7.4%) | 0 (0%) | ||
Table 2.
Functional and clinical outcomes at last follow-up
| Mean ± SD n = 62 | Tuberosities not reinserted n = 27 | Tuberosities reinserted n = 35 | p | |
|---|---|---|---|---|
| Constant-Murley score (/100) | 64.1 ± 19.2 | 56.7 ± 19.7 | 69.8 ± 17.1 | 0.01 |
| Adjusted Constant score (/100) | 64.2 ± 19 | 64.1 ± 19.4 | 77.1 ± 16.5 | < 0.01 |
| AFF (°) | 116 ± 32 | 105 ± 32 | 125 ± 31 | 0.02 |
| Abduction (°) | 99 ± 26 | 92 ± 25 | 105 ± 25 | 0.04 |
| ER1 (°) | 47 ± 24 | 42 ± 22 | 51 ± 25 | 0.14 |
| ER2 (°) | 38 ± 23 | 30 ± 20 | 44 ± 24 | 0.02 |
| IR (°) | 2 ± 1 | 2 ± 1 | 3 ± 1 | 0.01 |
| DASH (%) | 41.7 ± 22.0 | 44.6 ± 22.0 | 34.1 ± 15.5 | 0.45 |
| SSV (%) | 69.0 ± 25.4 | 66.1 ± 27.6 | 70.9 ± 24.0 | 0.49 |
| ADL (/6 points) | 4.8 ± 1.6 | 4.7 ± 1.7 | 4.9 ± 1.6 | 0.60 |
| iADL (/8 points) | 5 ± 2.9 | 4.7 ± 2.9 | 5.2 ± 2.9 | 0.49 |
AFF, active forward flexion; ER1, external rotation arm at side; ER2, external rotation in 90° abduction; IR, internal rotation; DASH, Disabilities arm shoulder and hand; SSV, subjective shoulder value; ADL, activities of daily living; iADL, instrumental activities of daily living
There was no significant difference between groups in the ADL (p = 0.60) and iADL (p = 0.49).
Radiological outcomes
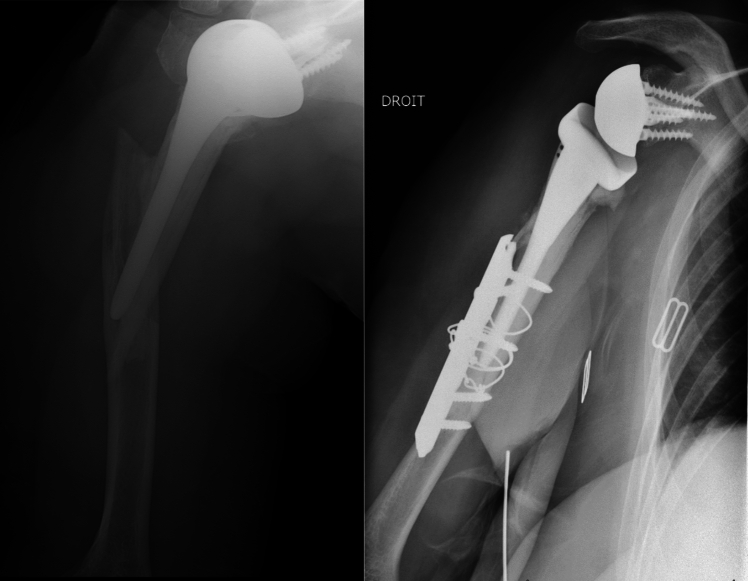
Among the 35 patients in group 2, 13 had secondary lysis of the tuberosities visible on the final radiographs (Fig. 3). One patient in group 2 had glenoid implant loosening associated with fracture of the metaglenoid screw 5 years after RSA implantation (Fig. 4), with a periprosthetic radiolucent line visible. We found the glenosphere to be too high, leading to notching (grade 1), which may have been the cause of this loosening [22]. This patient chose not to undergo revision surgery.
Fig. 3.
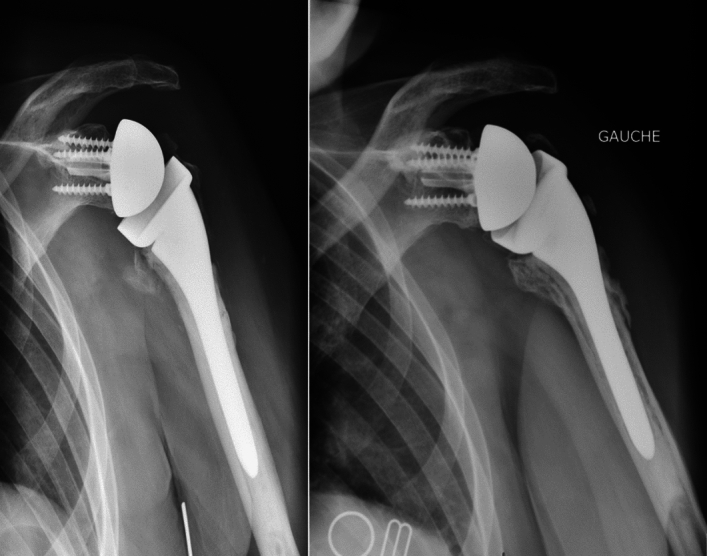
AP radiographs of a left shoulder showing osteolysis of the tuberosities: 1-month radiograph (left image) and 5-year radiograph (right image) showing greater tuberosity osteolysis
Fig. 4.
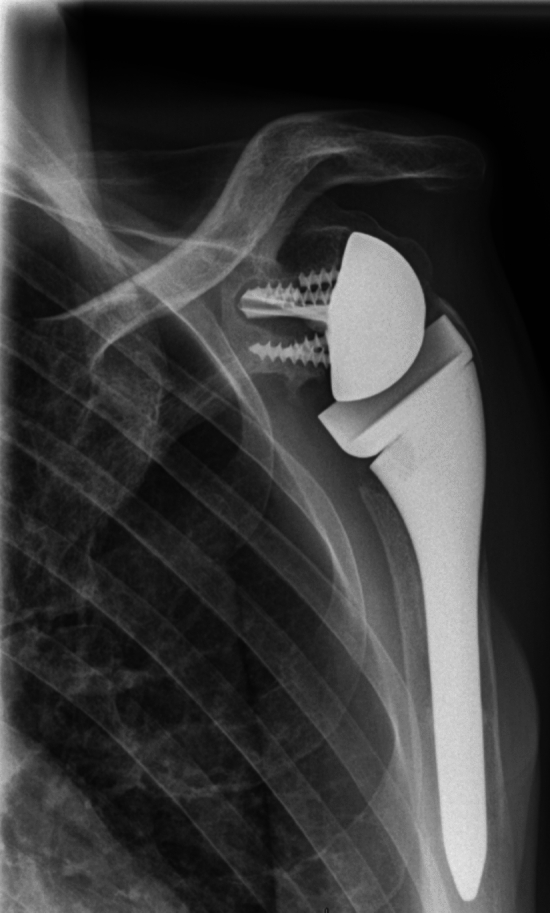
Loosening of glenoid implant
Complications
Two patients had a type A periprosthetic fracture in the Wright & Cofield classification [23]: 1 patient in group 1 at 2 months postoperative and 1 patient in group 2 at 4 years postoperative. In both cases, a new surgical intervention was done for plate fixation, without changing the RSA implants. (Fig. 5).
Fig. 5.
Periprosthetic fracture treated by plate fixation with cerclage wire
Two patients in group 1 had a dislocation episode within the first 3 months after the RSA was implanted. Revision surgery was done to change the polyethylene insert.
Discussion
Reinserting the tuberosities during RSA significantly improves the Constant score (absolute and adjusted) after more than 5 years’ follow-up (p = 0.01). It also improves active forward flexion (p = 0.02), ER2 (p = 0.02), IR (p = 0.01) and active abduction (p = 0.04). These findings are similar to shorter term studies, which found a statistically significant improvement in the Constant score and recovery of joint range of motion when the tuberosities are reinserted [8–10, 12, 13]. However, we found no significant effect on ER1 (42° ± 22° without reinsertion versus 51° ± 25° with reinsertion; p = 0.14), contrary to the studies by Gallinet et al. (p = 0.0015) [10], Ohl et al. (p < 0.001) [8] and Derksen et al. (p = 0.33) [13]. (Table 3) Nevertheless, the improved functional scores and mobility were long-lasting in the patients with PHF who underwent tuberosity reinsertion during RSA treatment.
Table 3.
Comparison with previous studies
| Study/Year | Follow-up (years, mean, range) | Number of patients | Absolute Constant (mean) | Forward flexion (degrees, mean) | ER1 (degrees, mean) | IR (numerical value, mean) | SSV (mean) | DASH (mean) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GT + | GT– | GT + | GT– | GT + | GT– | GT + | GT– | GT + | GT– | GT + | GT– | |||
| Gallinet et al. [15] 2013 | 2 (1.1–5.1) | 41 | 60.1 | 51.7 | 117.1 | 95.7 | 14.8 | 0 | 3 | 2 | NR | NR | 31.5 | 39.8 |
| Ohl et al. [13] 2018 | 2.3 (1–5) | 420 | 61 | 53.2 | 126.7 | 100.6 | 22 | 6.6 | 4.8 | 4 | 75.5 | 56.5 | NR | NR |
| Gallinet and Cazeneuve [16] 2019 | 7.6 (5–19) | 119 | 60 | 50 | 126 | 100 | 21 | 6 | 4.4 | 3 | 73 | 6 | NR | NR |
| Luciani et al. [17] 2019 | 5 (5–5.6) | 55 | 72.5 | 52.2 | 135 | 108 | 28 | 5.7 | 3 | 1 | NR | NR | 16.8 | 37.4 |
| Barros et al. [14] 2020 | 4.8 (2.7–7.3) | 28 | 79 | 55 | 135 | 90 | 60 | 30 | NR | NR | NR | NR | NR | NR |
| Derksen et al. [15] 2024 | 1 (0.9–1.3) | 50 | 71.3 | 56.3 | NR | NR | 24.9 | 14 | NR | NR | 82.7 | 68 | 18.4 | 31.9 |
| Our study | 6.7 (5–10) | 62 | 69.8 | 56.7 | 124.7 | 105.4 | 50.9 | 41.9 | 3 | 2 | 70.9 | 66.1 | 34.1 | 44.6 |
GT + : tuberosities repaired; GT–: tuberosities not repaired, NR: not reported; ER1, external rotation arm at side; IR, internal rotation; DASH, Disabilities arm shoulder and hand; SSV, subjective shoulder value
There was no significant difference between groups in the DASH (p = 0.45) and SSV (p = 0.49), although both scores were better in the patients in group 2. (Table 2) Ohl et al. found a statistically significant improvement in the SSV at 2 years’ follow-up in patients whose tuberosities were not reinserted (p < 0.001) [8] (Table 3), as did Derksen et al. in a cohort of 50 patients with a mean follow-up of 1 year (p = 0.016) [13]. Luciani et al. found a significantly lower DASH when the tuberosities were reinserted (p = 0.003) [12]. However, Gallinet et al. found no difference in the DASH score in their case series of 41 patients (p = 0.14) [10] (Table 3). The mean age was similar in these studies, ranging from 76.7 to 78.5 years [8–12]. In our study, the mean age was comparable between groups (82 ± 10 years – group 1; 78 ± 9 years – group 2) at the final review. (Table 1) We assume that the functional demands were less given that patients were older when re-evaluated 5 or more years later.
No matter if the tuberosities were reinserted or not, the RSA procedure allowed the patients to maintain satisfactory independence. There were no significant differences in the geriatric evaluation, with a similar ADL score between the two groups (4.7 ± 1.7 group 1 versus 4.9 ± 1.6 group 2, p = 0.60) and a slightly higher iADL score in group 2 (4.7 ± 2.9 group 1 versus 5.2 ± 2.9 group 2, p = 0.49). This can be explained by the somewhat diminished independence in this orthogeriatric population, with 13 patients (21%) residing in a nursing home and 8 patients (13%) receiving home aid, evidence of some loss of independence.
At the final review, secondary osteolysis was found in 13 of the 35 patients whose tuberosities were reinserted. We did not have enough patients in this study for a subgroup analysis. Papadopoulos et al. found a significant improvement in the Constant score when the tuberosities had healed versus when they had not (p < 0.001) [24]. Gunst et al. also found a significant clinical improvement in the Constant score (p = 0.04), forward flexion (p = 0.04) and external rotation (p = 0.01) when the greater tuberosity had healed compared to secondary lysis [25].
No revision surgery was done in our cohort for implant loosening. The single patient who had loosening of the glenoid implant did not want to be re-operated. Two patients had a periprosthetic fracture of the humeral shaft and required another surgery to fix the fracture; their implants did not need to be changed. Maugendre et al. mentioned the low functional demands in these patients who did not survive long enough to wear out the implants [26]. Two patients in group 1 had a dislocation within 3 months of the RSA implantation. Not reinserting the tuberosities may have contributed to this instability. Gallinet and Cazeneuve suggested that the only true factor responsible for instability was excision of the tuberosities (p < 0.0001) [11].
The current study has several strengths. All patients were operated on using the same surgical technique and the same implants. Few studies have reported a long-term follow-up [11, 12], with a mean follow-up of 6.7 ± 1.2 years in our study. Our study is also the only one to have done a geriatric assessment, finding no consequences on patient independence whether or not the tuberosities were reinserted.
The limitations of our study are its retrospective and single-center design, high mortality rate of 52% (Fig. 1), which is attributed to the patients’ advanced age and the follow-up being more than 5 years after the RSA implantation. The multisurgeon nature may also have reduced the comparability of the groups. A comparative analysis of patients who developed secondary osteolysis of the tuberosities would have provided a better comparison with pre-existing studies. Also, a CT scan would have provided a more thorough analysis of tuberosity healing and easier detection of loosening compared to radiographs [27]. However, a CT scan is more complex and less ethical in an orthogeriatric population who may be hesitant to undergo additional diagnostic tests.
Conclusion
Tuberosity reinsertion when implanting RSA for PHF significantly improves the Constant score (absolute and adjusted) and the shoulder’s mobility at more than 5 years’ follow-up. We found no significant difference in the Katz and Lawton geriatric scales, nor in the DASH or SSV scores. The patients regained satisfactory independence given that this was an orthogeriatric population with lower functional demands.
Acknowledgements
Joanne Archambault, PhD provided English language assistance. Her fees were paid by the GETRAUM.
Author contribution
NA wrote the main manuscript and prepared tables and figures TA helped to write and reviewed the manuscript MS and CC reviewed the manuscript.
Funding
Open access funding provided by Centre Hospitalier Universitaire de Lille.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Conflict of interest
The authors declare no conflict of interest related directly to this work. Outside this study: MS: Associate Editor for Orthopaedics & Traumatology: Surgery & Research, Consulting for Quanta Medical VCLS™ and Cousin Surgery™.
Generative AI and AI-assisted technologies in the writing process
No artificial intelligence software was used.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Roux A, Decroocq L, El Batti S, Bonnevialle N, Moineau G, Trojani C, Boileau P, De Peretti F (2012) Epidemiology of proximal humerus fractures managed in a trauma center. Orthop Traumatol Surg Res 98:715–719. 10.1016/j.otsr.2012.05.013 [DOI] [PubMed] [Google Scholar]
- 2.Palvanen M, Kannus P, Niemi S, Parkkari J (2006) Update in the epidemiology of proximal humeral fractures. Clin Orthop Relat Res 442:87–92. 10.1097/01.blo.0000194672.79634.78 [DOI] [PubMed] [Google Scholar]
- 3.Bonnevialle N, Tournier C, Clavert P, Ohl X, Sirveaux F, Saragaglia D et al (2016) Hemiarthroplasty versus reverse shoulder arthroplasty in 4-part displaced fractures of the proximal humerus: multicenter retrospective study. Orthop Traumatol Surg Res OTSR 102:569–573. 10.1016/j.otsr.2016.02.014 [DOI] [PubMed] [Google Scholar]
- 4.Gallinet D, Ohl X, Decroocq L, Dib C, Valenti P, Boileau P (2018) Is reverse total shoulder arthroplasty more effective than hemiarthroplasty for treating displaced proximal humerus fractures in older adults? A systematic review and meta-analysis. Orthop Traumatol Surg Res 104:759–766 [DOI] [PubMed] [Google Scholar]
- 5.Grammont PM, Baulot E (2011) The classic: delta shoulder prosthesis for rotator cuff rupture. Clin Orthop 469:2424–2424. 10.1007/s11999-011-1960-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cazeneuve JF, Cristofari DJ (2009) Delta III reverse shoulder arthroplasty: radiological outcome for acute complex fractures of the proximal humerus in elderly patients. Orthop Traumatol Surg Res 95:325–329. 10.1016/j.otsr.2009.03.018 [DOI] [PubMed] [Google Scholar]
- 7.Gallinet D, Clappaz P, Garbuio P, Tropet Y, Obert L (2009) Three or four parts complex proximal humerus fractures: hemiarthroplasty versus reverse prosthesis: a comparative study of 40 cases. Orthop Traumatol Surg Res 95:48–55. 10.1016/j.otsr.2008.09.002 [DOI] [PubMed] [Google Scholar]
- 8.Ohl X, Bonnevialle N, Gallinet D, Ramdane N, Valenti P, Decroocq L, Boileau P (2018) How the greater tuberosity affects clinical outcomes after reverse shoulder arthroplasty for proximal humeral fractures. J Shoulder Elbow Surg 27:2139–2144. 10.1016/j.jse.2018.05.030 [DOI] [PubMed] [Google Scholar]
- 9.Barros LH, Figueiredo S, Marques M, Rodrigues C, Ramos J, Claro R (2020) Consolidação dos tubérculos na artroplastia reversa do ombro após fratura proximal do úmero: Existe melhoria nos resultados funcionais? Rev Bras Ortop 55:748–754. 10.1055/s-0039-3402459 [Google Scholar]
- 10.Gallinet D, Adam A, Gasse N, Rochet S, Obert L (2013) Improvement in shoulder rotation in complex shoulder fractures treated by reverse shoulder arthroplasty. J Shoulder Elbow Surg 22:38–44. 10.1016/j.jse.2012.03.011 [DOI] [PubMed] [Google Scholar]
- 11.Gallinet D, Cazeneuve JF, Boyer E, Menu G, Obert L, Ohl X et al (2019) Reverse shoulder arthroplasty for recent proximal humerus fractures: Outcomes in 422 cases. Orthop Traumatol Surg Res 105:805–811. 10.1016/j.otsr.2019.03.019 [DOI] [PubMed] [Google Scholar]
- 12.Luciani P, Farinelli L, Procaccini R, Verducci C, Gigante A (2019) Primary reverse shoulder arthroplasty for acute proximal humerus fractures: a 5-year long term retrospective study of elderly patients. Injury 50:1974–1977. 10.1016/j.injury.2019.09.019 [DOI] [PubMed] [Google Scholar]
- 13.Derksen A, Lill H, Ellwein A, Imrecke J (2024) Tuberosity refixation improves functional outcome following primary reverse shoulder arthroplasty in proximal humeral fracture. Eur J Orthop Surg Traumatol 34:1441–1448. 10.1007/s00590-023-03810-9 [DOI] [PubMed] [Google Scholar]
- 14.Tang JB, Giddins G (2016) Why and how to report surgeons’ levels of expertise. J Hand Surg Eur 41:365–366. 10.1177/1753193416641590 [DOI] [PubMed] [Google Scholar]
- 15.Neer CS (1970) Displaced proximal humeral fractures. I. Classification and evaluation. J Bone Joint Surg Am 52:1077–1089 [PubMed] [Google Scholar]
- 16.Dukan R, Bahman M, Rousseau MA, Boyer P (2020) Outcomes of reverse shoulder arthroplasty using a short stem through a superolateral approach. J Shoulder Elbow Surg 29:1197–1205. 10.1016/j.jse.2019.09.025 [DOI] [PubMed] [Google Scholar]
- 17.Boileau P, Alta TD, Decroocq L, Sirveaux F, Clavert P, Favard L et al (2019) Reverse shoulder arthroplasty for acute fractures in the elderly: is it worth reattaching the tuberosities? J Shoulder Elbow Surg 28:437–444. 10.1016/j.otsr.2016.02.014 [DOI] [PubMed] [Google Scholar]
- 18.Constant CR, Murley AH (1987) A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res 214:160–164 [PubMed] [Google Scholar]
- 19.Tavakkolizadeh A, Ghassemi A, Colegate-Stone T, Latif A, Sinha J (2009) Gender-specific constant score correction for age. Knee Surg Sports Traumatol Arthrosc 17:529–533. 10.1007/s00167-009-0744-x [DOI] [PubMed] [Google Scholar]
- 20.Boileau P, Krishnan SG, Tinsi L, Walch G, Coste JS, Molé D (2002) Tuberosity malposition and migration: reasons for poor outcomes after hemiarthroplasty for displaced fractures of the proximal humerus. J Shoulder Elbow Surg 11:401–412. 10.1067/mse.2002.124527 [DOI] [PubMed] [Google Scholar]
- 21.Deutsch A, Abboud JA, Kelly J, Mody M, Norris T, Ramsey ML et al (2007) Clinical results of revision shoulder arthroplasty for glenoid component loosening. J Shoulder Elbow Surg 16:706–716. 10.1016/j.jse.2007.01.007 [DOI] [PubMed] [Google Scholar]
- 22.Young BL, Cantrell CK, Hamid N (2018) Classifications in brief: the Nerot-Sirveaux classification for scapular notching. Clin Orthop 476:2454–2457. 10.1097/CORR.000000000000044221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wright TW, Cofield RH (1995) Humeral fractures after shoulder arthroplasty. J Bone Jt Surg 77:1340–1346. 10.2106/00004623-199509000-00008 [DOI] [PubMed] [Google Scholar]
- 24.Papadopoulos DV, Kakogeorgou V, Mullen JR, Kontogeorgakos V, Nikolaou VS, Babis G (2024) Non-union of the greater tuberosity in patients undergoing reverse total shoulder arthroplasty for proximal humerus fracture: is it associated with worse outcomes? Eur J Orthop Surg Traumatol. 10.1007/s00590-024-04108-0 [DOI] [PubMed] [Google Scholar]
- 25.Gunst S, Louboutin L, Swan J, Lustig S, Servien E, Nove-Josserand L (2021) Does healing of both greater and lesser tuberosities improve functional outcome after reverse shoulder arthroplasty for fracture? a retrospective study of twenty-eight cases with a computed tomography scan at a minimum of one-year follow-up. Int Orthop 45:681–687. 10.1007/s00264-020-04928-9 [DOI] [PubMed] [Google Scholar]
- 26.Maugendre E, Gadisseux B, Chantelot C, Clavert P, Ramdane N, Werthel JD, Boileau P, (2019) Epidemiology and mortality in older patients treated by reverse shoulder arthroplasty for displaced proximal humerus fractures. Orthop Traumatol Surg Res 105:1509–1513. 10.1016/j.otsr.2019.07.026 [DOI] [PubMed] [Google Scholar]
- 27.Yian EH, Werner CML, Nyffeler RW, Pfirrmann CW, Ramappa A, Sukthankar A, Gerber C (2005) Radiographic and computed tomography analysis of cemented pegged polyethylene glenoid components in total shoulder replacement. J Bone Jt Surg 87:1928–1936. 10.2106/JBJS.D.02675 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.