Abstract
AKI is common in patients with liver cirrhosis (LC) affecting (30 to 50%). Our study aimed to evaluate the role of urinary biomarkers as predictors of AKI, its etiology and mortality. We performed a prospective cohort study of patients with LC during 1 year. Urine samples for biomarkers dosage were collected within 48 h of hospital admission. Diagnosis of AKI was performed according to KDIGO 2012 criteria. The results were presented using Chi-square, T test, AUC-ROC and logistic regression (p < 0.05). We included 100 patients, 58.5 ± 16.1 years, main etiologies of LC were alcohol and and metabolic disfunction associated with steatohepatitis. Infection was the main cause of LC decompensation. AKI occurred in 53% of patients and mortality was 20%. CHILD C, infectious as cause of decompensation, baseline creatinine, need for mechanical ventilation and noradrenaline use and urinary were associated with AKI. We found no association between AKI and KIM-1 and IL-18. The main etiologies of AKI were transient ischeamia (49%), renal (43.4%), and hepatorenal syndrome (HRS) (7.5%). There was difference between the groups in hematuria, proteinuria, FENa, FEUr and uNGAL which were higher in renal AKI when compared to transient ischaemia and HSR. FENa and FEUr were excellent predictors of AKI etiology (AUC-ROC > 0.80, sensitivity and specificity > 0.80), while only uNGAL was good predictor of AKI etiology (AUC-ROC, sensitivity and specificity > 0.70). Regarding death, CHILD C, baseline creatinine, KDIGO 3, septic AKI, need for mechanical ventilation and IL-18 were identified as associated variables. Only NGAL was predictor of AKI and its etiology, anticipating AKI diagnosis in 2.5 ± 1.1 days, while IL-18 was predictor of death. We highlight the importance of lower-cost biochemical tests as FENa and FEUr and clinical information as CHILD and cause of LC descompensation which were relevant in predicting AKI, its etiology and death.
Keywords: Acute kidney injury, Liver cirrhosis, Biomarkers
Subject terms: Hepatology, Liver cirrhosis, Nephrology
Introduction
Acute kidney injury (AKI) is very common in cirrhotic patients affecting from 30 to 50% of patients with liver cirrhosis1. While multiple etiologies can lead to AKI, pre-renal azotemia also named as transient ischemia seems to be the most frequent cause of AKI. Regardless of the cause, AKI is associated with worse survival with the poorest outcomes observed in those with hepatorenal syndrome (HRS) and acute tubular necrosis (ATN)1,2.
In recent years, new definitions and classifications of AKI in cirrhosis have emerged based on increase in serum creatinine (Scr) and reduction in urine output3,4. It is important to mention that all definitions of AKI in cirrhotic patients still use creatinine despite its multiple limitations in liver cirrhosis4–6. One of the major drawbacks of SCr is the time lag between the onset of kidney injury and the rise in SCr levels. Another important limitation is that SCr lacks discriminatory ability with regards to the etiology of kidney injury. Moreover, patients with liver disease have sarcopenia, decreased hepatic synthesis of creatinine, and increased tubular secretion of creatinine which translates into overestimation of the actual GFR6–8. These factors justify the need for finding novel biomarkers of early injury, such as neutrophil gelatinase-associated lipocalin (NGAL), Interleukin-18 (IL-18) and kidney injury molecule-1 (KIM-1)9–13.
Furthermore, diagnostic tools such as urinary biomarkers (fractional excretion of sodium (FENa) and urea (FEUr) and point-of-care ultrasound (POCUS) can be used to differ between different causes of AKI and direct management of AKI in these patients.
Measurement of the FENa < 1% to differentiate ATN from HRS-AKI or pre renal has been thought to be unhelpful since FENa < 1% is common in all kind of patients with cirrhosis (non-AKI and any AKI etiology). However, if using a lower threshold of FENa < 0.5%, for example, in combination with clinical judgment and other urinary biomarkers such as proteinuria, hematuria or novel biomarkers, the test may have improved specificity in identifying AKI etiology, differentiating ATN from HRS-AKI and pre renal10–15.
Given the higher mortality rate of patients with liver cirrhosis and AKI and lack of studies, this research aimed to investigate the role of urinary biomarkers as predictor of AKI diagnostic and etiology and patients mortality.
Patients and methods
This was an observational study. All patients with liver cirrhosis admitted to the Clinical Hospital at Botucatu School of Medicine, Sao Paulo, Brazil (University of Sao Paulo State–UNESP) were followed prospectively from the time of admission to discharge. Nephrology fellows visited emergency rooms and wards daily from January 2023 to January 2024 and collected data on all patients with liver cirrhosis admitted in the hospital. We have excluded chronic kidney disease (CKD) patients stages 4 and 5 (clearance de creatinine < 30 ml/min), kidney transplantation, hospital stay < 24 h, in palliative care, patients already admitted in hospital with AKI and no urinary exams within the first 48 h after admission. Complete data on inclusions and exclusions are shown in (Fig. 1).
Fig. 1.
Patients included in the study (flowchart).
AKI in patients with cirrhosis was defined and classified according to Kidney Disease Improving Global Outcomes (KDIGO) 2012 and ADQI and ICA consensus 2024 criteria based on increase of serum creatinine > 0.3 mg/dl within 48 h or > 50% from baseline value known or presumed to have occurred within the prior 7 days5,10,16. Baseline creatinine was defined as the lowest serum creatinine value in the last 6 months before AKI or, for those without this measurement, the lowest value achieved during hospitalization in the absence of dialysis17.
Concerning phenotypes of AKI, they were classified in pre renal, renal (ATN) or HRS according to clinical manifestations as insult identification, laboratory exams and prompt recovery of kidney function right after 24 h following adequate volume resuscitation (when clinically indicated)9–11. In pre renal, ischemic insult was identified, there was no hematuria and proteinuria in urine exams and the kidney function improved after 24 h following volume resuscitation. Criteria for HRS diagnosis were cirrhosis with ascites, absence of improvement in serum creatinine and/or urine output within 24 h following adequate volume resuscitation (when clinically indicated), absence of strong clinical or lab evidences for an alternative explanation as the primary cause of AKI (sepsis, nephrotoxins, hemodynamic instability, proteinuria, hematuria or changes in ultrasound)18.
Variables previously reported to be associated with AKI, its etiology and death in other populations were included in the risk factor analysis. Baseline data, including demographics, medical history, and severity, were collected prospectively on each patient by review of the medical record. Key risk factors analyzed are displayed in (Table 1). Laboratory characteristics were recorded for the first hospitalized day and laboratory data were recorded each day during hospitalization. Kidney function was assessed daily based on creatinine levels and urine output (when available). Urine was analyzed for hematuria, proteinuria, FENa, FEUr, NGAL, KIM-1, IL-18 within the first 48 h after admission. The samples were centrifuged and stored at minus 80-degree Celsius and were analyzed subsequently. NGAL, IL-18 and KIM-1 were measured by the enzyme linked immunosorbent assay (ELISA). We performed level of NGAL, IL-18 and KIM1-1 in 10 healthy subjects between 30 and 45 years old and the mean was 52.2 ± 12.29 pg/ml, 28.2 ± 2.25 pg/ml and 56.2 ± 15.19 pg/ml respectively.
Table 1.
Clinical and laboratory characteristics of patients based on the presence of acute kideny injury and patients outcome.
| Variables | General (n = 100) | No AKI | AKI (n = 53) | P value |
|---|---|---|---|---|
| Male gender (%) | 72 (72) | 34 (72.3) | 38 (71.6) | 0.28 |
| Age | 58.5 ± 16.1 | 57.5 ± 16.6 | 59.4 ± 14.6 | 0.08 |
| Cause of cirrhosis (%) | ||||
| Alcoholic | 41 (41) | 21 (44.6) | 21(39,6) | 0.23 |
| MASH | 33 (33) | 16 (34) | 17 (32) | 0.52 |
| MELD | 18.96 ± 3.1 | 14.29 ± 4.27 | 20.29 ± 5.9 | < 0.01 |
| CHILD A (%) | 26 (26) | 19 (40.4) | 7 (13.2) | 0.006 |
| CHILD B (%) | 39 (39) | 18 (38.3) | 21 (51.6) | 0.008 |
| CHILD C | 35 (35) | 11 (23.4) | 24 (45.3) | 0.007 |
| Infection as cause of decompensation | 39 (39) | 14 (29.7) | 25 (47.2) | 0.004 |
| Baseline creatinine (mg/dl) | 0.91 ± 036 | 0.79 ± 0.21 | 1.09 ± 0.42 | < 0.01 |
| Diabetes (%) | 56 (56) | 25 (53.2) | 31 (58.5) | 0.22 |
| Hypertension (%) | 52 (52) | 20 (42.5) | 32 (60.4) | 0.43 |
| CKD (%) | 21 (21) | 6 (12.8) | 15 (28.3) | 0.04 |
| Baseline e GFR (ml/min) | 91.6 ± 23.3 | 108.3 ± 14.69 | 92.8 ± 26.64 | 0.013 |
| Hematuria (%) | 36 (36) | 12 (25.6) | 24 (45.3) | 0.003 |
| Proteinuria (%) | 26 (16) | 6 (12.8) | 16 (30.2) | 0.02 |
| Albumin (g/dl) | 3.39 ± 0.5 | 3.19 ± 0.4 | 3.42 ± 0.6 | 0.6 |
| C – reactive protein | 3.31 ± 2.5 | 2.5 ± 3.2 | 3.9 ± 1.35 | 0.47 |
| Leukocytes | 5.31 ± 2.8 | 4.8 ± 3.2 | 6.2 ± 1.33 | 0.04 |
| Platelets | 133.31 ± 22.5 | 139.9 ± 33.2 | 122.5 ± 21.35 | 0.07 |
| Thrombin time | 3.61 ± 1.5 | 3.12 ± 1.2 | 3.85 ± 1.35 | 0.08 |
| Partial thromboplastin time | 19.31 ± 12.5 | 18.9 ± 13.2 | 20.3 ± 12.35 | 0.47 |
| Hemoglobin (mg/dl) | 8.407 ± 5.27 | 8.65 ± 4.5 | 8.2 ± 5.8 | 0.78 |
| Neef for mechanical ventilation (%) | 19 (19) | 4 (8.5) | 15 (28.3) | 0.04 |
| Need for noradrenalin (%) | 22 (22) | 5 (10.6) | 17 (32) | 0.037 |
| Variables | General (n = 100) | Survival (n = 80) | No survival (n = 20) | P value |
|---|---|---|---|---|
| Male gender (%) | 72 (72) | 57 (71,2) | 15 (75) | 0.91 |
| Age | 58.5 ± 16.1 | 56 ± 16.6 | 62.4 ± 14.6 | 0.03 |
| Cirrhosis cause (%) | ||||
| Alcoholic | 41 (41) | 31 (38.7) | 10 (50) | 0.04 |
| MASH | 33 (33) | 28 (35) | 5 (25) | 0.09 |
| MELD | 18.96 ± 3.1 | 17.51 ± 6.27 | 22.29 ± 8.9 | 0.04 |
| CHILD A (%) | 26 (26) | 23 (28.7) | 3 (15) | 0.04 |
| CHILD B (%) | 39 (39) | 30 (37.5) | 9 (45) | 0.09 |
| CHILD C (%) | 35 (35) | 27 (30.6) | 8 (40) | 0.04 |
| Infection as causeof descompensation | 39 (39) | 28 (33.7) | 11 (55) | 0.03 |
| Baseline creatinine (mg/dl) | 0.91 ± 036 | 0.86 ± 0.21 | 1.10.42 | 0.04 |
| Diabetes (%) | 56 (56) | 49 (61.3) | 7 (70) | 0.22 |
| Hypertension (%) | 52 (52) | 42 (52.6) | 10 (50) | 0.93 |
| CKD (%) | 21 (21) | 18 (22.5) | 3 (15) | 0.13 |
| Baseline GRF (ml/min) | 91.6 ± 23.3 | 108.3 ± 14.69 | 92.8 ± 26.64 | < 0.001 |
| AKI | 53 (53) | 38 (47.5) | 15 (75) | < 0.001 |
| KDIGO 3 | 19 (19) | 9 (11.2) | 10 (50) | 0.04 |
| AKRT | 15 (15) | 3 (3.7) | 12 (60) | < 0.001 |
| AKI etiology | ||||
| Ischaemic transiente | 26 (26) | 22 (27.5) | 4 (20) | 0.25 |
| Non-septic AKI | 11 (11) | 8 (10) | 3 (15) | 0.37 |
| Septic-AKI | 12 (12) | 4 (5) | 8 (40) | 0.04 |
| HRS | 4 (4) | 1 (1.25) | 3 (15) | 0.04 |
| Hematuria (%) | 36 (36) | 28 (35) | 8 (40) | 0.31 |
| Proteinuria (%) | 26 (26) | 19 (23.8) | 7 (35) | 0.06 |
| Albumin (g/dl) | 3.39 ± 0.5 | 3.19 ± 0.4 | 3.42 ± 0.6 | 0.6 |
| C reactive protein (CPR)- mg/dl | 3.31 ± 2.5 | 3.1 ± 2.2 | 4.8 ± 1.35 | 0.04 |
| Leukocytes (103) | 5.31 ± 2.8 | 6.2 ± 3.2 | 9.1 ± 3.33 | 0.04 |
| Platelets | 133.31 ± 22.5 | 141.9 ± 33.2 | 132.5 ± 21.35 | 0.04 |
| Thrombin time | 3.61 ± 1.5 | 3.7 ± 1.2 | 4.15 ± 1.35 | 0.23 |
| Partial thromboplastin time | 19.31 ± 12.5 | 18.9 ± 13.2 | 20.3 ± 12.35 | 0.47 |
| Hemoglobin (g/dl) | 8.407 ± 5.27 | 8.65 ± 4.5 | 8.1 ± 4.8 | 0.18 |
| Neef for mechanical ventilation (%) | 19 (19) | 6 (7.5) | 13 (65) | 0.02 |
| Need for noradrenalin (%) | 22 (22) | 7 (8.75) | 15 (75) | 0.01 |
AKI acute kidney inuury, CKD chronic kidney disease, eGRF estimated glomerular rate filtartion, MASH metabolic disfunction associated with steatohepatitis, HSR hepatorenal syndrome.
The Ethics Committee of the Botucatu School of Medicine, UNESP, analysed and approved the study (CAAE 74344823.4.0000.5411).
Statistical analysis
The sample size was calculated, considering the difference in the urinary value of urinary NGAL between the population with and without AKI would be 50 pg/ml, standard deviation of 80 pg/ml, alpha error of 0.05, study power of 80%, requiring the inclusion of at least 80 patients.
Data analysis was performed using SAS for Windows (version 9.2, SAS Institute, Cary, NC, USA, 2012). Continuous variables with normal distribution were described using means ± standard deviation categorical variables were presented as n (%). For the analysis of continuous variables, Student’s t-test was used for data with a parametric distribution. For the analysis of categorical variables, a chi-square test was used. We also used logistic regression for identifying variables associated with AKI, its etiology and death, Diagnostic characteristics of biomarkers in predicting AKI, its etiology and death were assessed by calculation of the area under the receiver operating characteristic curve (AUC-ROC). The optimal cutoff points were determined by the highest values of sensitivity and specificity shown in AU-ROC analysis. In all tests, differences were considered significant at 5%.
Results
One hundred patients were included in the final analysis (Fig. 1). Mean age was 58.5 ± 16.1 years, 72% were male, most of them had comorbidities (65.4%), and hypertension, diabetes mellitus and chronic kidney disease (CKD) were the most frequent (52, 56 and 21% of patients, respectively). MELD was 18.96 ± 3.1 and CHILD B was the most frequent (39%). The main etiologies of liver cirrhosis were alcohol (41%) and metabolic disfunction associated with steatohepatitis (MASH-33%). The main cause of liver decompensation was infectious (39%).
Fifty three patients (53%) developed AKI and the mean time was 2.5 ± 1.1 days after hospitalization. Most of the patients were classified as KDIGO 1 (43.4%), while KDIGO 3 occurred in 35.8% and KDIGO 2 in 22.6%. ATN-ISS was 0.32 ± 0.11, acute renal replacement therapy was indicated in 15%. Mortality rate was 20%.
The non-AKI (n = 47) and AKI group (n = 53) were similar in gender, age, hypertension, diabetes and serum lab exams at admission. AKI group had higher MELD (), higher baseline creatinine, higher CHILD B and C, higher CKD, higher need for mechanical ventilation and noradrenaline use, infectious was higher as cause of liver cirrhosis decompensation and had higher proteinuria and hematuria at admission (Table 1).
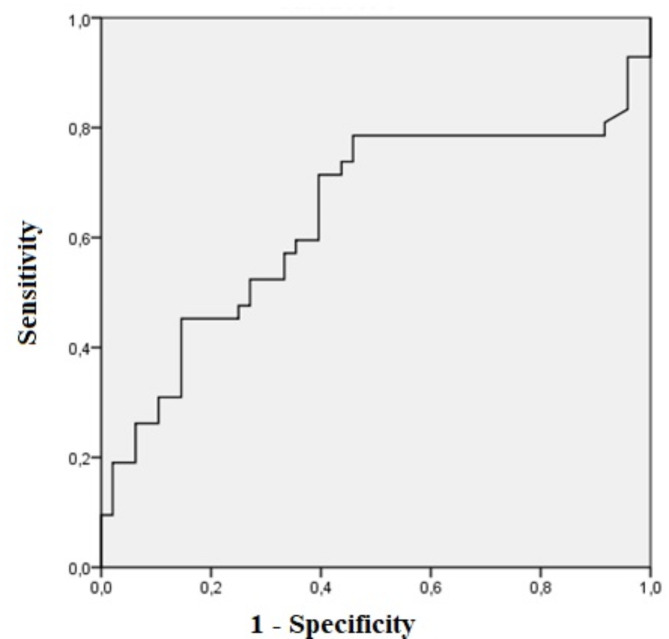
Relating to urinary biomarkers, uNGAL was higher in AKI patients (152.11 ± 67.09 vs. 377.56 ± 57.61, p = 0.01), while there was no difference in KIM-1 and IL-18 between non-AKI and AKI patients (Table 3). The area under the curve for uNGAL was 0.68. The optimal cutoff value was 120 pg/ml and sensitivity and specificity were 72.4 and 70.4%, respectively (p = 0.02, CI 0.51–0.76) as shown in (Fig. 2). uNGAL anticipated AKI diagnosis according to KDIGO criteria in 2.5 ± 1.1 days.
Table 3.
Urinary NGAL, FENa e FEUr sensitivity and specificity in AKI etiology (renal vs non-renal) and outcome (survival vs non survival).
| Urinary biomarkers | AUC-ROC | P value | cutoff | Sensitivity | Specificity | CI (95%) |
|---|---|---|---|---|---|---|
| Renal AKI | ||||||
| FENa (%) | 0.87 | < 0.01 | 0.8% | 93.3 | 76.5 | 0.78–0.95 |
| FEUr(%) | 0.80 | < 0.01 | 32.5% | 82.5 | 73.5 | 0.67–0.93 |
| NGAL (pg/ML) | 0.75 | < 0.01 | 336 | 76.5 | 70.5 | 0.63-0.088 |
| KIM-1 (pg/ML) | 0.54 | 0.09 | 100 | 60.5 | 55.6 | 0.65–1.02 |
| IL-18 (pg/ML) | 0.53 | 0.08 | 85 | 59.5 | 54.6 | 0.64–1.05 |
| Mortality | ||||||
| FENa (%) | 0.57 | 0.08 | 0.9% | 63.3 | 56.5 | 0.78–1.05 |
| FEUr(%) | 0.61 | 0.07 | 31.5% | 62.5 | 63.5 | 0.67–1.03 |
| NGAL (pg/ML) | 0.65 | 0.08 | 240 | 66.5 | 60.5 | 0.63–1.08 |
| KIM-1 (pg/ML) | 0.54 | 0.09 | 105 | 60.5 | 65.6 | 0.65–1.02 |
| IL-18 (pg/ML) | 0.74 | 0.02 | 40.5 | 76.5 | 71.6 | 0.63–0.86 |
Fig. 2.
ROC analysis of uNGAL in pacientes with liver cirrhosis with AKI vs. non-AKI.
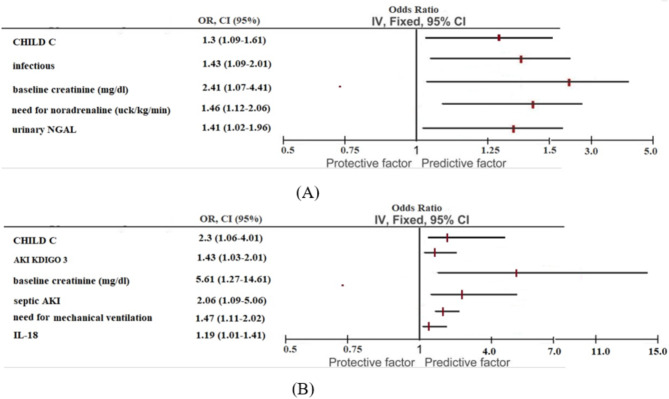
At logistic regression analysis, CHILD C (OR 1.3; 95% CI 1.09–1.61, p = 0.029), infectious as cause of liver cirrhosis decompensation (OR 1.43; 95% CI 1.09–2.01, p = 0.04), baseline creatinine (OR 2.41; 95% CI 1.07–4.41, p = 0.01), need for noradrenaline (OR 1.52; 95% CI 1.13–2.91, p = 0.04), and urinary NGAL (OR 1.41; 95% CI 1.02–1.96, (p = 0.04) were identified as variables associated with AKI (Table 4).
Table 4.
Logistic regression for acute kideny injury and death in liver cirrhosis patients.
| Variables | OR (CI 95%) | p |
|---|---|---|
| AKI | ||
| CHILD C | 1.3 (1.09–1.61) | 0.029 |
| Infectious as cause of decompensation | 1.43 (1.09–2.01) | 0.04 |
| Baseline creatinine (mg/dl) | 2.41 (1.07–4.41) | 0.01 |
| Need for noradrenaline (ucg/kg/min) | 1.41 (1.02–1.96) | 0.04 |
| Urinary NGAL | 1.41 (1.02–1.96) | 0.04 |
| Death | ||
| CHILD C | 2.3 (1.059–4.01) | 0.029 |
| AKI KDIGO 3 | 1.43 (1.03–2.01) | 0.04 |
| Baseline creatinine (mg/dl) | 5.61 (1.27–14.61) | 0.02 |
| Septic AKI | 2.06 (1.09–5.06) | 0.02 |
| Need for mechanical ventilation | 1.47 (1.11–2.02) | 0.03 |
| IL-18 | 1.19 (1.01–1.41) | 0.04 |
The main etiologies of AKI were transient ischaemia (49%), renal (ischaemic tubular necrosis, septic AKI and nephrotoxic tubular necrosis in 43.4%), and HRS (7.5%). The duration of the AKI episode was 8.4 ± 2.5 days, being longer in SHR (13 ± 4 days and shorter in transient AKI (1.8 ± 0.6 days). ATN duration was 9.2 ± 3.2 days. Information was added to result section.
There were differences between etiologies of AKI in FENa FEUr, proteinuria, hematuria and uNGAL, wich were higher in ATN when compared to pre renal and HRS, as shown in (Table 2). There was no difference between the groups in IL-18 and KIM-1.
Table 2.
Urinary biomarkers of patients according to the presence of acute kideny injury (AKI), etiology of AKI and outcome.
| AKI | No AKI (n = 47) | AKI (n = 53) | P value | |
|---|---|---|---|---|
| FENa (%) | 0.83 ± 0.11 | 1.03 ± 0,59 | 0.08 | |
| FEUr(%) | 28.31 ± 11.13 | 32.64 ± 9.23* | 0.06 | |
| NGAL (pg/ML) | 152.11 ± 67.09 | 377.56 ± 57.618 | 0.01 | |
| KIM-1 (pg/ML) | 178.9 ± 53.2 | 167.3 ± 22.35 | 0.68 | |
| IL-18 (pg/ML) | 86.9 ± 38.34 | 101.6 ± 43.2 | 0.59 |
| Etiologies of AKI | Transient AKI (n = 26) | Renal AKI (n = 23) | HRS (n = 4) | Value p |
|---|---|---|---|---|
| Hematuria (%) | 2 (7.7) | 19 (82.6) * | 1(25) | < 0.01 |
| Proteinuria > 0.5 g (%) | 2 (7.7) | 17 (73.9) * | 1 (3.8) | < 0.01 |
| FENa (%) | 0.23 ± 0.11 | 1.43 ± 0.59 * | 0.21 ± 0.09 | < 0.01 |
| FEUr(%) | 26.64 ± 7.13 | 40.64 ± 9.23 * | 28.64 ± 11.21 | < 0.01 |
| NGAL (pg/ML) | 389.12 ± 170.61 | 553.471 ± 117.09 | 377.56 ± 168.5 | 0.05 |
| KIM-1 (pg/ML) | 173.3 ± 42.5 | 188.91 ± 153.2 | 177.3 ± 22.35 | 0.68 |
| IL-18 (pg/ML) | 99.9 ± 41.84 | 186.9 ± 88.34 | 101.6 ± 43.2 | 0.59 |
| Patients outcome | Survival (n = 80) | Non-survival (n = 20) | VALOR p | |
|---|---|---|---|---|
| FENa (%) | 0,93 ± 0,21 | 1,02 ± 0,39 | 0.29 | |
| FEUr(%) | 29.31 ± 10.13 | 30.64 ± 12.20 | 0.36 | |
| NGAL (pg/ML) | 252.11 ± 68.09 | 305.56 ± 67.618 | 0.51 | |
| KIM-1 (pg/ML) | 193.9 ± 48.2 | 171.3 ± 32.15 | 0.19 | |
| IL-18 (pg/ML) | 69.9 ± 38.34 | 96.6 ± 43.2 | 0.01 |
FENa fractional excretion of sodium, FEUr fractional excretion of urea, NGAL neutrophil gelatinase-associated lipocalin, KIM-1 kidney injury molecule-1, IL-18 Interleukin-18, AKI acute kidney inury, HRS hepatorenal syndrome.
FENa and FEUr were excellent predictors of AKI etiology (AUC-ROC > 0.80, sensitivity and specificity > 0.80), while uNGAL was a good predictor (AUC-ROC, sensitivity and specificity > 0.70) as shown in (Table 3). The optimal cutoff values were 0.7% for FENa, 32,5% for FeUr and as 336 pg/ml for NGAL.
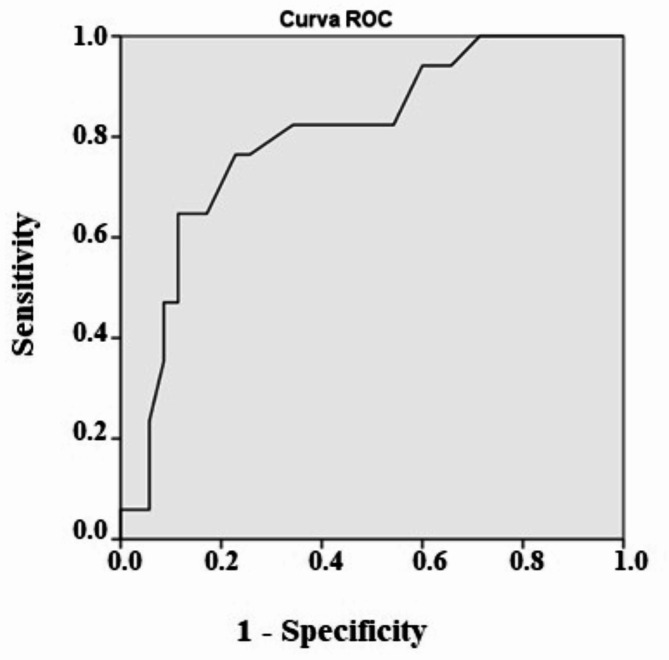
Table 1 shows the clinical and laboratory characteristics of the population according to the hospital outcome. The groups were similar in gender, comorbidities, cirrhosis etiology and lab exams at hospital admission. The two groups were statistically different in age, CHILD C, MELD, alcohol as cirrhosis etiology AKI ), KDIGO 3 acute kidney replacement therapy (3.7 vs. 60%, p < 0.01), baseline creatinine ( leucocytes and reactive protein C at hospital admission, need for mechanical ventilation and noradrenaline use, wich were higher in non-survival patients. HRS and septic AKI were associated with death (Table 1). The uIL-18 was a good predictor of death (AUC-ROC 0.74, cutoff 40.5, sensitivity 0.8 and specificity 0.70), FENa, FEUr, KIM-1 and NGAL were not associated with death (Table 3; Fig. 3).
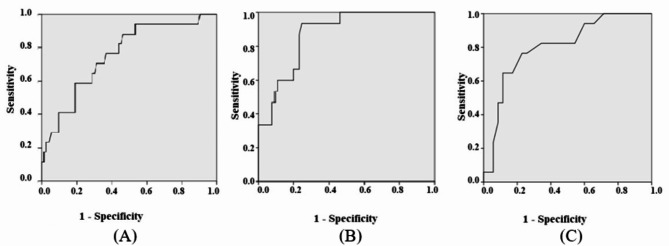
Fig. 3.
(A) ROC analysis of uNGAL in pacientes with liver cirrhosis with AKI renal vs. transient ischaemia or HRS. (B) ROC analysis of FENa in patients with liver cirrhosis with AKI renal vs. transient ischaemia or HRS. (C) ROC analysis of FEUr in patients with liver cirrhosis with AKI renal vs. transient ischemia or HRS.
Logistic regression showed CHILD C, baseline creatinine, KDIGO 3, septic AKI, need for mechanical ventilation and IL-18 were identified as variables associated with death. The uIL-18 was a good predictor of death (AUC-ROC 0,74, sensitivity and specificity > 0.70), KIM-1 and NGAL were not associated with death (Table 4; Figs. 4 and 5).
Fig. 4.
ROC analysis of IL-18 in survival vs. non-survival patients with liver cirrhosis.
Fig. 5.
(A) Forest plot for risk factors for AKI. (B) Forest plot for risk factors for death.
Discussion
It is widely recognized that the incidence of AKI occurs in up to 60% of patients with cirrhosis when hospitalized for acute decompensation and is associated with increased morbidity and mortality1–3,19,20. In 2023, a joint meeting of Acute Disease Quality Initiative (ADQI XXIX) and the International Club Ascites (ICA) refined the diagnostic criteria for AKI and hepatorenal syndrome (HRS), reviewed their epidemiology and pathophysiology and explored the role of biomarkers in the diagnosis and prognostication of AKI. In accordance with other studies, this study showed the incidence of AKI in patients with acute on chronic liver cirrhosis was very high (53%) with most patients having stage 1 disease and we performed it using KDIGO criteria suggested by XXIX ADQI10.
However, it is well established that SCr is not an accurate marker of renal dysfunction in cirrhosis5–9,21. In some patients, the concentrations of SCr remain within normal limits, even in moderate to severe renal dysfunction, resulting in an overestimation of GFR21. Sarcopenia, which is prevalent in patients with advanced cirrhosis, also contributes to low SCr levels. Therefore, AKI diagnosis in liver cirrhotic patients can be difficult or delayed, due to loss of muscle mass, and consequently lower baseline Scr level, masking an increase of its values in kidney injury pathologies, justifying the search for biomarkers of early injury, such as C cystatin, NGAL, KIM-1 and IL-189–13.
The identification of AKI phenotype is also crucial for establishing a treatment strategy and improving clinical outcomes11,22. However, differentiating HRS from ATN is challenging. Reversible AKI in cirrhosis is predominantly a prerenal injury, which resolves with volume administration and discontinuation of diuretics. In contrast, HRS is characterized by its nonresponsiveness to volume expansion. HRS typically represents a continuum of disease, starting with functional changes, followed by structural changes due to prolonged ischemic injury. Intrinsic AKI involves acute tubular necrosis (ATN), acute interstitial nephritis, acute glomerular and vasculitis renal diseases. According to ADQI VIII, HRS-AKI is a phenotype of AKI that is specific to patients with advanced cirrhosis and ascites and it may also occur in the presence of tubular injury, proteinuria, and/or pre-existing CKD.
Our study showed uNGAL was a good predictor of AKI diagnosis, anticipating its diagnoses in 2.5 days, while KIM-1 and IL-18 were not associated with AKI.The transient ischaemia was the main AKI phenotypes, followed by renal AKI (ischaemic tubular necrosis, septic AKI and nephrotoxic tubular necrosis), and HRS.
There were differences between AKI etiologies in FENa, FEUr, proteinuria, hematuria and uNGAL. There was no difference between the groups in IL-18 and KIM-1. FENa and FEUr were excellent predictors of AKI etiology (AUC-ROC > 0.80, sensitivity and specificity > 0.80), while uNGAL was a good predictor (AUC-ROC, sensitivity and specificity > 0.70).
The combined use of functional (e.g., Scr, CysC) and damage (e.g., proteinuria, urinary neutrophil gelatinase-associated lipocalin [uNGAL]) biomarkers enables more accurate differential diagnosis of the etiology and mechanisms of AKI in patients with cirrhosis and potentially enables the identification of AKI sub-phenotypes suitable for specific therapeutic interventions23,24. We believe incorporating them is an important step towards improving our understanding of the mechanisms and pathophysiology of AKI in patients with cirrhosis, selecting time points and targets for interventions and refining the determination of prognosis.
Measurement of FENa to differentiate ATN from HRS-AKI has been thought to be unhelpful since FENa < 1% is common in patients with cirrhosis, even in the absence of AKI [63]. However, if using a lower threshold of FENa of < 0.2% (which may not be possible as many laboratories do not report urine sodium values < 20 mEq/L) in combination with other urinary biomarkers and clinical judgment, the test may have improved specificity in identifying HRS-AKI [54, 63]. In our study, FENa e FEUR were excellent predictors of renal AKI and we identified the cutoff values that differentiated ATN from pre renal or HRS. They were FENa 0.7% and FEUr 32.5%, with AUC-ROC, sensitivity and specificity higher than 80%. uNGAL was also a good predictor of ATN-AKI, cutoff was 230 pg/ml, with AUC-ROC, sensitivity and specificity higher than 70%.
In our study, IL-18 and KIM-1 were not associated with etiology of AKI.
According to literature, although various markers have been shown to improve both diagnosis and prognosis prediction in patients with cirrhosis, a single biomarker is insufficient for having an impact on clinical practice5–9,11,22–26. Whether the target level of uNGAL that would differentiate between the AKI phenotypes, and/or response to terlipressin would be different with the new diagnostic criteria for HRS-AKI set forth by the authors remains to be determined.
It has been suggested that urine NGAL is significantly higher in patients with ATN than those with other causes, including HRS and prerenal AKI27,28. In a study of 112 patients with various phenotypes of AKI, urine NGAL showed the highest diagnostic performance in differentiating ATN from other causes (area under curve [AUC] of 0.79), compared to IL-18, KIM-1, and L-FABP11. A meta-analysis suggested that urine NGAL and IL-18 could discriminate ATN from other AKI phenotypes with AUCs of 0.89 and 0.88, respectively28.
The role of KIM-1 in patients with cirrhosis and AKI has been investigated in few studies, and increased urine KIM-1 levels were observed in patients with ATN compared to those with other causes27. However, in some studies, significant overlaps in urine NGAL, IL-18, and KIM-1 levels were observed between patients with HRS and those with ATN27,29. In addition, various previous studies have defined ATN and other causes of AKI with clinical features, without histological confirmation, which could lead to a misclassification of the AKI phenotype.
Our results agree with previous studies. According to literature, uNGAL is one of the most promising injury biomarkers, with levels significantly increasing in a stepwise manner from HRS-AKI to ATI5–9,25,26,28. A uNGAL value of ∼220–250 pg/ml (Bioporto Diagnostics, Hellerup, Denmark) has been demonstrated to distinguish patients with ATN from other phenotypes13 with response rates to terlipressin seen in 70% of patients with uNGAL < 220 pg/ml compared to only 33% in those with uNGAL > 220 pg/ml25.
The mortality was 20% and logistic regression showed CHILD C, baseline creatinine, KDIGO 3, septic AKI and IL-18 were identified as variables associated with death. The uIL-18 was a good predictor of death (AUC-ROC 0.74, cutoff 40,5 pg/ml, sensitivity 0.76 and specificity 0.71), while KIM-1 and NGAL were not associated with death.
Conflicting results have been reported regarding the association between tubular markers and the risk of mortality11–13,27. In a meta-analysis of five studies, increased urine levels of IL-18 and NGAL identified patients at a higher risk of short-term mortality with an AUC of 0.7628. In two studies investigating the performance of multiple biomarkers, urine NGAL levels showed the best predictive performance in predicting mortality, compared with the urine IL-18, KIM-1, and L-FABP levels and serum cystatin C level11,23. However, two recent prospective studies from South Korea, including patients with decompensated cirrhosis, showed that urine NGAL level was not a significant predictor of mortality; rather, the cystatin C or MELD-cystatin C score were associated with survival outcomes29,24. These discrepancies could be explained by the differences in the clinical characteristics of the patient population. Unlike previous studies that included a wide range of cirrhotic patients, the latter two studies only included patients with decompensated cirrhosis, to investigate the ability of the biomarkers appropriately in patients with a high prevalence of AKI. Another concern is that an increase in these biomarkers is not fully derived from kidneys. As mentioned above, infection or liver disease, the other critical factor of increased mortality, also increase the levels of renal biomarkers, especially NGAL and LFABP. Therefore, it is not clear whether the mortality in the previous studies was driven by AKI itself.
However, there is insufficient data to draw firm conclusions about novel biomarkers role in predicting kidney outcomes in patients with cirrhosis and AKI. Therefore, other novel biomarkers still need to be explored, and the incorporation of various biomarkers should be evaluated in future studies. The availability of these markers in cirrhosis will also help to further develop and improve the performance of certain current treatment strategies, including the early volemic resuscitation or administration of terlipressin.
The present study has some important limitations. It included a small number of patients and was performed in a single center. Due to the small number of patients, no analysis of urinary biomarkers according to the stage of AKI was performed. The role of biomarkers as a predictor of dialysis also was not evaluated. We also included CKD patients stages 1 to 3, so acute on chronic AKI and CKD was defined using only eGFR values. Despite these limitations, its results allow us to conclude that AKI is very frequent in patients with liver cirrhosis and associated with death. uNGAL was a good predictor of AKI, anticipating AKI diagnosis in 2.5 days. FENa, FEUr, proteinuria, hematuria and uNGAL were good urinary biomarkers to differentiate renal AKI from pre renal and HRS. IL-18 was a good predictor of death.
Author contributions
LFS and WSA: Investigation, methodology, formal analysis, writing of the original draft. RSC and DP: methodology, data analysis and data interpretation. WAS, LFS and DP: Conceptualization, review & editing. DP: Funding acquisition, review & editing. All authors reviewed and agreed with the final version of this manuscript.
Funding
This research was funded by the São Paulo Research Foundation—FAPESP, grant numbers 2023/13560-3 and CAPES scholarship.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Informed consent
was obtained from study participants or their legal caregiver; and the researchers adhered to the confidentiality of patients’ data and the recommendations in the Declaration of Helsinki throughout the study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Khatua, C. R., Sahu, S. K., Meher, D., Nath, G. & Singh, S. P. Acute kidney injury in hospitalized cirrhotic patients: risk factors, type of kidney injury, and survival. JGH Open5, 199–206 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patidar, K. R. et al. Acute kidney disease is common and associated with poor outcomes in patients with cirrhosis and acute kidney injury. J. Hepatol.77, 108–115 (2022). [DOI] [PubMed] [Google Scholar]
- 3.Rosi, S. et al. New ICA criteria for the diagnosis of acute kidney injury in cirrhotic patients: can we use an imputed value of serum creatinine? Liver Int.35, 2108–2114 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Wong, F. Acute kidney injury in liver cirrhosis: new definition and application. Clin. Mol. Hepatol.22, 415–422 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sherman, D. S., Fish, D. N. & Teitelbaum, I. Assessing renal function in cirrhotic patients: problems and pitfalls. Am. J. Kidney Dis.41, 269–278 (2003). [DOI] [PubMed] [Google Scholar]
- 6.Angeli, P. et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the international club of ascites. J. Hepatol.62, 968–974 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Attieh, R. M. & Wadei, H. M. Acute kidney injury in liver cirrhosis. Diagnostics13, 2361. 10.3390/diagnostics13142361 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caregaro, L. et al. Limitations of serum creatinine level and creatinine clearance as filtration markers in cirrhosis. Arch. Intern. Med.154, 201–205 (1994). [PubMed] [Google Scholar]
- 9.Piano, S., Brocca, A. & Angeli, P. Renal function in cirrhosis: A critical review of available tools. Semin. Liver Dis.38, 230–241 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Nadim, M. K. Acute kidney injury in patients with cirrhosis: acute disease quality initiative (ADQI) and international club of Ascites (ICA) joint multidisciplinary consensus meeting. J. Hepatol.81 (1), 163–183 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belcher, J. M. et al. Kidney biomarkers and differential diagnosis of patients with cirrhosis and acute kidney injury. Hepatology60 (2), 622–632. 10.1002/hep.26980 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Francoz, C., Nadim, M. K. & Durand, F. Kidney biomarkers in cirrhosis. J. Hepatol.65 (4), 809–824. 10.1016/j.jhep.2016.05.025 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Huelin, P. et al. Neutrophil gelatinase-associated lipocalin for assessment of acute kidney injury in cirrhosis: a prospective study. Hepatology70 (1), 319–333. 10.1002/hep.30592 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Asrani, S. K. et al. Role of novel kidney biomarkers in patients with cirrhosis and after liver transplantation. Liver Transpl.28 (3), 466–482. 10.1002/lt.26344 (2022). [DOI] [PubMed] [Google Scholar]
- 15.Juanola, A., Ma, A. T., Pose, E. & Gines, P. Novel biomarkers of AKI in cirrhosis. Semin. Liver Dis.42 (4), 489–500. 10.1055/a-1954-4136 (2022). [DOI] [PubMed] [Google Scholar]
- 16.Kidney Disease. Improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. Suppl., 1–138. (2012).
- 17.Siew, E. D. et al. Commonly used surrogates for baseline renal function affect the classification and prognosis of acute kidney injury. Kidney Int.77, 536–542 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patidar, K. R. et al. Incidence and outcomes of acute kidney injury including hepatorenal syndrome in hospitalized patients with cirrhosis in the US. J. Hepatol. (2023). [DOI] [PMC free article] [PubMed]
- 19.Tariq, R. et al. Incidence, mortality and predictors of acute kidney injury in patients with cirrhosis: a systematic review and metaanalysis. J. Clin. Transl. Hepatol.8 (2), 135–142. 10.14218/JCTH.2019.00060 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Worden, A. et al. The prognostic impact of acute kidney injury recovery patterns in critically ill patients with cirrhosis. Liver Transpl.29 (3), 246–258. 10.1097/LVT.0000000000000008 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maiwall, R. et al. Cystatin C predicts acute kidney injury and mortality in cirrhotics: a prospective cohort study. Liver Int.38 (4), 654–664. 10.1111/liv.13600 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Belcher, J. M. et al. Urinary biomarkers and progression of AKI in patients with cirrhosis. Clin. J. Am. Soc. Nephrol.9 (11), 1857–1867. 10.2215/CJN.09430913 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ariza, X. et al. Analysis of a urinary biomarker panel for clinical outcomes assessment in cirrhosis. PLoS One10, e0128145 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jo, S. K., Yang, J., Hwang, S. M., Lee, M. S. & Park, S. H. Role of biomarkers as predictors of acute kidney injury and mortality in decompensated cirrhosis. Sci. Rep.9, 14508 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gambino, C. et al. Diagnostic and prognostic performance of urinary neutrophil gelatinase-associated lipocalin in patients with cirrhosis and acute kidney injury. Hepatology77 (5), 1630–1638. 10.1002/hep.32799 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lei, L. et al. Value of urinary KIM-1 and NGAL combined with serum Cys C for predicting acute kidney injury secondary to decompensated cirrhosis. Sci. Rep.8 (1), 7962. 10.1038/s41598-018-26226-6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim, T. H. et al. Assessment and prediction of acute kidney injury in patients with decompensated cirrhosis with sérum Cystatin C and urine N-acetyl-beta-D-glucosaminidase. J. Gastroenterol. Hepatol.34, 234–240 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Puthumana, J. et al. Urine Interleukin 18 and Lipocalin 2 are biomarkers of acute tubular necrosis in patients with cirrhosis: a systematic review and metaanalysis. Clin. Gastroenterol. Hepatol.15, 1003–1013e3 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barreto, R. et al. Urinary neutrophil gelatinase-associated Lipocalin predicts kidney outcome and death in patients with cirrhosis and bacterial infections. J. Hepatol.61, 35–42 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.