Highlights
-
•
Multiple IPNs in pediatric osteosarcoma indicate poor prognosis with 5-year OS of 30%.
-
•
Solitary IPNs show survival outcomes comparable to patients without pulmonary nodules.
-
•
Extensive bone involvement (>1/3) is a significant risk factor for IPN development (p = 0.03).
-
•
Multidisciplinary team (MDT) approach ensures personalized treatment for IPN-positive cases.
Keywords: Osteosarcoma, Adolescents, Indeterminate Pulmonary Nodules (IPNs), Prognosis
Abstract
Background
Osteosarcoma is the most common primary malignant bone tumor in pediatric and adolescent patients. Although pulmonary metastasis is a key driver of prognosis, the role of IPNs in risk stratification remains inadequately defined.
Objective
This study aims to assess the incidence, progression, and prognostic significance of IPNs in pediatric and adolescent osteosarcoma patients, providing insights for clinical staging and treatment strategy development.
Methods
We retrospectively analyzed clinical data from 126 osteosarcoma patients aged 20 years or younger who were treated at Henan Cancer Hospital between January 2012 and January 2022. Pre-treatment thin-slice computed tomography (CT) scans of lung were used to categorize patients into three groups: no IPN (n = 100), solitary IPN (n = 16), and multiple IPNs (n = 10). Baseline characteristics, primary tumor parameters, treatment modalities, and follow-up data were collected. Univariate and multivariate analyses were conducted to assess risk factors and survival outcomes.
Results
The overall incidence of IPNs was 20.6 %, with multiple IPNs accounting for 38.5 % of the IPN-positive cases. A significantly higher proportion of patients in the IPN-positive group had bone involvement exceeding one-third of the total affected bone compared to the no-IPN group (57.7 % vs. 34.0 %, p = 0.016). While univariate analysis suggested a potential association between tumor diameter > 8 cm and IPN occurrence (odds ratio [OR] = 2.08, 95 % confidence interval [CI]: 0.83–5.21, p = 0.120), this was not statistically significant in multivariate analysis (OR = 3.61, p = 0.283). Kaplan–Meier survival analysis revealed that the 3-year metastasis-free survival (MFS) and overall survival (OS) rates in the IPN-positive group were significantly lower than those in the no-IPN group (MFS: 57.7 % vs. 64.0 %, p = 0.03; OS: 65.4 % vs. 76.0 %, p = 0.04). Further subgroup analysis indicated that while solitary IPN cases had survival outcomes comparable to those without IPNs, multiple IPN cases exhibited a markedly reduced 5-year OS (30.0 % vs. 69.0 %, p = 0.045). Cox regression analysis demonstrated that multiple IPNs increased the risk of death by 2.87-fold (hazard ratio [HR] = 2.87, p = 0.020).
Conclusion
Indeterminate Pulmonary Nodules are relatively common in pediatric osteosarcoma patients. In particular, multiple IPNs are strongly associated with a higher tumor burden and increased metastatic potential, serving as an independent indicator of poor prognosis. These findings emphasize the importance of preoperative IPN assessment and risk stratification in guiding individualized treatment strategies.
1. Introduction
Osteosarcoma is the most common primary malignant bone tumor in children and adolescents, with approximately 60 % of cases occurring between the ages of 10 and 20 [1], [2]. Despite advances in multimodal treatments—such as chemotherapy and surgical resection—the overall prognosis remains highly dependent on early diagnosis and the effective management of metastatic lesions [3]. Patients with localized osteosarcoma have a 5-year survival rate exceeding 70 %, whereas those with metastatic disease experience a significant decline in survival, with rates below 30 % [4].
The lungs are the most common site of osteosarcoma metastasis, with approximately 85 % of metastatic cases initially presenting with pulmonary lesions. Furthermore, 15–20 % of patients exhibit detectable pulmonary metastases at the time of diagnosis [5], [6]. Therefore, the early identification and precise assessment of lung involvement play a critical role in staging, treatment planning, and prognostic evaluation. With advances in imaging technology, high-resolution thin-slice computed tomography (CT) has become a standard tool for pulmonary evaluation. This modality has enabled the frequent detection of small pulmonary lesions, known as Indeterminate Pulmonary Nodules (IPNs), during pre-treated screening. However, the clinical significance of these nodules remains uncertain, as they may represent either benign findings or early micrometastatic disease.
Current literature lacks consensus on the interpretation of IPNs, and the proportion of nodules that eventually develop into overt metastatic lesions is not well established [7], [8]. Recent guidelines, including those from the NCCN and the Chinese Society of Clinical Oncology (CSCO), have not addressed the potential prognostic significance of pulmonary IPNs in osteosarcoma patients, highlighting the need for further investigation into their role in risk stratification and treatment planning. The limited research on the natural history, risk factors, and prognostic implications of IPNs underscores this gap [8]. Determining whether IPNs represent early signs of metastasis or incidental findings is crucial for refining treatment strategies and enhancing patient outcomes.
Against this background, the present study retrospectively examines the incidence, progression, and prognostic significance of pulmonary IPNs in pediatric osteosarcoma patients. By integrating clinical and imaging data, we aim to provide valuable insights into the role of IPNs in risk stratification and personalized treatment decision-making, ultimately enhancing survival outcomes in this vulnerable population.
1.1. Clinical data
This single-center retrospective cohort study aimed to analyze the incidence, evolution, and prognostic impact of IPNs in pediatric and adolescent osteosarcoma patients.
1.2. Study Design and patient selection
A total of 126 pathologically confirmed osteosarcoma patients aged ≤ 20 years, admitted to Henan Cancer Hospital from January 2012 to January 2022, were included in this study. All patients met the following criteria:
1.3. Inclusion criteria
The inclusion criteria were as follows: patients presenting for their first visit and diagnosed with osteosarcoma by pathological biopsy and surgical resection, aged ≤ 20 years, who had completed a thin-slice lung CT scan with complete imaging data before treatment, who were classified at diagnosis as Enneking Stage IIa–IIb with no definitive evidence of pulmonary or other distant metastases, and who underwent a standardized comprehensive treatment protocol—including neoadjuvant chemotherapy, surgical resection, and adjuvant chemotherapy—with a follow-up period exceeding half one year.
1.4. Exclusion criteria
The exclusion criteria were as follows: patients with a prior history of other malignant tumors or those who had received treatment (including chemotherapy or surgery) at other institutions; patients suspected of having pulmonary or other organ metastases at the first visit who were subsequently confirmed to have metastases within one months of initiating treatment (i.e., Enneking Stage III patients); and patients with poor treatment compliance, chemotherapy intolerance, or those who discontinued treatment or had incomplete follow-up data.
1.5. Imaging evaluation
All patients included in this study had CT reports documenting pulmonary nodules that met the definition of IPNs as outlined in international radiological guidelines [9]. These nodules were characterized as small, circumscribed lesions lacking definitive imaging features to classify them as either malignant or benign. A solitary IPN was defined as a single pulmonary nodule identified on imaging, with no evidence of additional nodules in either the ipsilateral or contralateral lung. To ensure consistency and reproducibility, all cases were independently re-evaluated by experienced radiologists at the time of study inclusion. This re-evaluation confirmed that the pulmonary nodules met the established criteria for IPNs, including solitary IPNs. All patients underwent routine thin-slice lung CT examinations during treatment and follow-up (see Fig. 1, Fig. 2, Fig. 3). Based on the imaging reports, these pulmonary nodules were evaluated as follows:
Fig. 1.
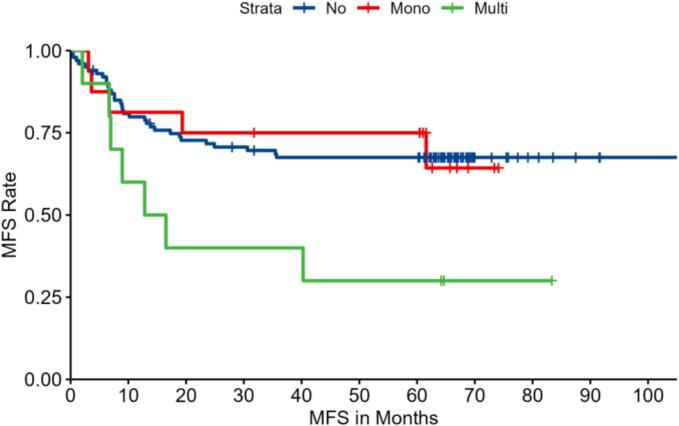
Kaplan–Meier Curve for Metastasis-Free Survival (MFS) of pediatric osteosarcoma patients in three groups: no IPNs (no), with a solitary IPN (Mono), and with multiple IPNs (Muti). The log-rank test indicates a significant difference among the groups (p = 0.04).
Fig. 2.
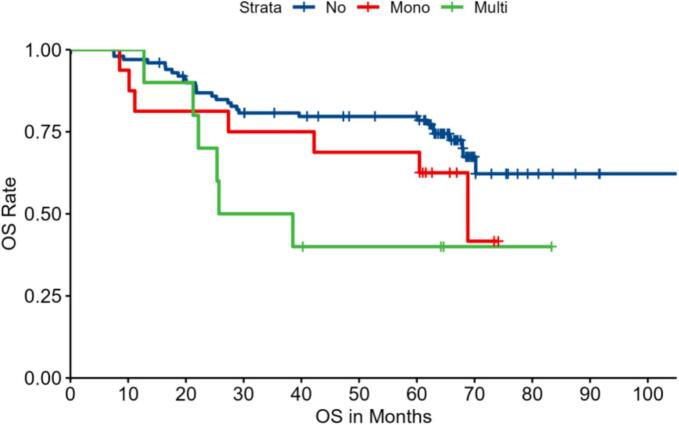
Kaplan–Meier Curve for Overall Survival (OS) of pediatric osteosarcoma patients in three groups: no IPNs (no), with a solitary IPN (Mono), and with multiple IPNs (Muti). The log-rank test indicates a significant difference among the groups (p = 0.045).
Fig. 3.
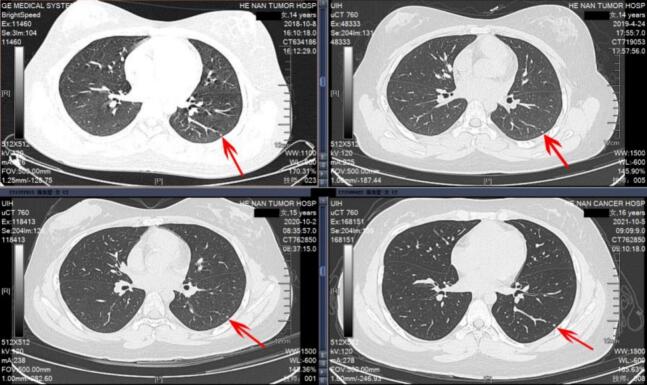
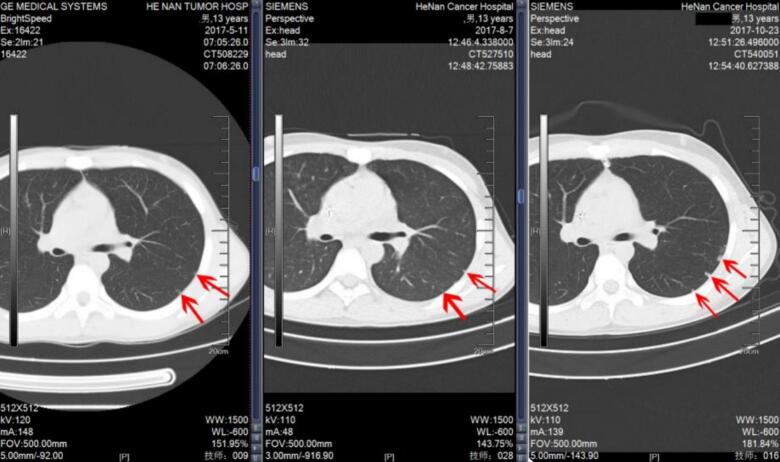
Case 1. A 14-year-old female patient with distal femoral osteosarcoma. At initial presentation, an Indeterminate Pulmonary Nodule (IPN) was detected in the left lower lung, which remained unchanged over a 3-year follow-up period.
1.5.1. Definition and Grouping
Based on baseline chest CT findings, IPNs can be classified into three groups: No IPN Group (no visible nodules), Solitary IPN Group (a single nodule with the diameter < 10 mm), and Multiple IPNs Group (two or more nodules that initially did not fulfill definitive metastatic criteria).
1.5.2. Evaluation indicators
For each patient, the number, diameter, morphology, margin characteristics, and distribution of the nodules were recorded. According to our radiological reports, if a nodule increased in size by ≥ 25 % within a short interval (1–2 months) or if new nodules ≥ 10 mm appeared and and were suspected of metastasis, further diagnostic evaluations—such as fine-needle aspiration or surgical biopsy—were performed to confirm malignancy.
1.6. Multidisciplinary evaluation
All patients in this study were managed through a multidisciplinary team (MDT) approach. The MDT included specialists from orthopedic oncology, thoracic surgery, medical oncology, radiology, radiation therapy, and pathology. For patients with pulmonary metastases, the MDT carefully evaluated each case and developed individualized treatment plans based on the patient’s clinical condition, overall health status, and family preferences. Treatment options included observation, surgical intervention, radiotherapy, or systemic therapy.
1.7. Follow-Up and endpoints
The follow-up schedule in this study was based on general principles for the follow-up of malignant tumors. Follow-up was conducted through a combination of outpatient visits, telephone interviews, and imaging examinations, with the schedule set at every 3 months within the first two years post-surgery, every 6 months during the third to the fifth year, and annually thereafter. However, due to individual patient circumstances, there were variations in the actual follow-up intervals. The follow-up times recorded in this study represent approximate intervals rather than precise time points.
The primary endpoints were defined as follows: Metastasis-Free Survival (MFS) was the duration from the date of diagnosis to the first detection of pulmonary or other organ metastasis or the last follow-up date, and Overall Survival (OS) was the duration from the date of diagnosis to death from any cause, with surviving patients censored at their last follow-up.
1.8. Data collection
Data were extracted from the hospital information system (HIS) and the Bone and Soft Tissue Tumor Center database, and the collected information included basic patient demographics (age, gender, body mass index, medical history), primary tumor characteristics (tumor location, diameter, proportion of bone involvement, pathological subtype, serum alkaline phosphatase levels, etc.), treatment details (neoadjuvant chemotherapy regimen, surgical method, reconstruction technique, tumor necrosis, adjuvant chemotherapy regimen), and follow-up outcomes (recurrence, metastasis, MFS, OS).
1.9. Statistical analysis
Data analysis was conducted using SPSS (version 26.0) and the R software. Continuous variables were expressed as mean ± standard deviation and compared using t-tests. Categorical variables were presented as frequencies and percentages, and comparisons were made using the chi-square test or Fisher’s exact test as appropriate. Univariate and multivariate logistic regression analyses were conducted to identify risk factors for IPN formation. Survival curves were generated using the Kaplan–Meier method, and group differences were compared using the log-rank test. Prognostic factors influencing MFS and OS were evaluated using univariate and multivariate Cox proportional hazards models. A p-value < 0.05 was considered statistically significant.
Ethical Approval
This study was approved by the ethics committee of our hospital, and all procedures were conducted in strict accordance with the relevant ethical standards and privacy protection requirements.
2. Results
2.1. Patient demographics and baseline characteristics
A total of 126 pediatric and adolescent osteosarcoma patients were included in this retrospective cohort study. Among these patients, 100 (79.4 %) presented with no IPNs on initial CT screening, while 26 (20.6 %) were identified as IPN-positive. The IPN-positive group was further divided into 16 patients with a solitary nodule and 10 with multiple nodules. There were no statistically significant differences between the groups regarding age, gender, body mass index (BMI), incidence of pathological fractures, or abnormal serum alkaline phosphatase (ALP) levels. However, a significantly greater proportion of patients with IPNs exhibited extensive bone involvement—defined as involvement of more than one-third of the affected bone—compared to patients without IPNs (57.7 % vs. 34.0 %, p = 0.016) (Table 1). Although tumors larger than 8 cm were more common in the multiple IPN subgroup (90.0 %) compared to the solitary IPN (56.3 %) and no-IPN groups (52.0 %), this difference did not reach statistical significance (p = 0.07).
Table 1.
Baseline Characteristics of 126 Pediatric Osteosarcoma Patients.
| Variable | No IPN group (n = 100) | Solitary IPN group (n = 16) | Multiple IPNs group (n = 10) | p-value |
|---|---|---|---|---|
| Age (years, mean ± SD) | 13.7 ± 3.2 | 15.0 ± 2.2 | 13.4 ± 2.1 | 0.220 |
| Male (%) | 61 (61.0 %) | 9 (56.3 %) | 5 (50.0 %) | 0.764 |
| Tumor Diameter > 8 cm (%) | 52 (52.0 %) | 9 (56.3 %) | 9 (90.0 %) | 0.070 |
| Bone Involvement > 1/3 (%) | 34 (34.0 %) | 7 (43.8 %) | 8 (80.0 %) | 0.016* |
| ALP Abnormal (%) | 80 (80.0 %) | 12 (75.0 %) | 8 (80.0 %) | 0.899 |
| Pathological Fracture (%) | 16 (16.0 %) | 1 (6.3 %) | 1 (10.0 %) | 0.540 |
Note: ① All data were derived from a retrospective cohort analysis (N = 126) and analyzed using SPSS version 26.0. ② A two-tailed p-value < 0.05 was considered statistically significant. ALP: Alkaline Phosphatase; SD: Standard Deviation. *P < 0.05 indicates significance.
2.2. Risk factors for IPN development
Univariate logistic regression analysis revealed that extensive bone involvement was a significant risk factor for the development of IPNs, with an odds ratio (OR) of 2.65 (95 % CI: 1.10–6.39, p = 0.030). Although tumors with a diameter greater than 8 cm appeared more frequently in the multiple IPN subgroup (90.0 % in multiple IPNs vs. 56.3 % in solitary IPNs and 52.0 % in the no-IPN group), this trend did not reach statistical significance in either univariate (OR = 2.08, 95 % CI: 0.83–5.21, p = 0.120) or multivariate analyses (OR = 3.61, p = 0.283) (Table 2). Other variables—including age, gender, pathological fractures, and ALP abnormalities—were not significantly associated with the presence of IPNs. These findings suggest that while tumor size may have a contributory role, the extent of bone involvement is a more critical determinant for IPN formation.
Table 2.
Logistic Regression Analysis for the Formation of IPNs.
| Variable | OR (95 % CI) | p-value |
|---|---|---|
| Bone Involvement > 1/3 | 2.65 (1.10–6.39) | 0.030* |
| Tumor Diameter > 8 cm | 2.08 (0.83–5.21) | 0.120 |
| Male (vs. Female) | 0.75 (0.31–1.78) | 0.509 |
| Age 12–17 years (vs. ≤ 12 years) | 1.64 (0.55–4.90) | 0.374 |
| Age 18–20 years (vs. ≤ 12 years) | 0.92 (0.19–4.35) | 0.914 |
Note: ① All data were derived from a retrospective cohort analysis (N = 126) and analyzed using SPSS version 26.0. ② Risk factor analysis was performed using logistic regression. ③ A two-tailed p-value < 0.05 was considered statistically significant. OR: odds ratio; *P < 0.05 indicates statistical significance.
Among the 26 patients who developed pulmonary metastases, tumor necrosis rate data were available for 12 cases. Of these, only 4 patients had a tumor necrosis rate greater than 90 %. Statistical analysis revealed no significant significant differences in MFS or OS between patients with tumor necrosis rates greater than 90 % and those with rates less than 90 % (P > 0.05).
2.3. IPN related recurrence and metastasis
Among the 26 patients with baseline IPNs, 12 developed lung metastases, with a median time of 7.9 months. Among these, 5 out of 16 patients (31.3 %) with solitary nodules experienced progression, including 3 cases where the nodules increased in size and 2 cases where new lesions appeared (median time: 6.7 months). Of the 3 cases with nodule enlargement, only one patient underwent pulmonary tumor resection and remains alive to date. In contrast, 7 out of 10 patients (70.0 %) with multiple nodules showed progression, with a median time of 8.9 months. Additionally, 32 out of 100 patients (32.0 %) without baseline nodules developed metastases, with a median time of 9.0 months. Overall, the mean time from the detection of IPNs to confirmed lung metastasis was 8.55 ± 0.73 months.
Analysis of postoperative outcomes revealed that patients with multiple IPNs developed pulmonary metastases, which was considerably higher than the observed in the IPN-negative group (p = 0.053). Similarly, the combined endpoint of recurrence and metastasis was noted in 70.0 % of the multiple IPN group versus 36.0 % in the no-IPN group (p = 0.053). In contrast, the metastasis rate in the solitary IPN group (31.3 %) was comparable to that of the IPN-negative group (32.0 %, p > 0.99). Moreover, during follow-up, 80 % of patients with multiple IPNs exhibited either a > 25 % increase in nodule size or the emergence of new nodules (≥10 mm), with subsequent pathological confirmation of metastasis (see Table 4).
Table 4.
Postoperative Recurrence and Metastasis Rates of 126 Osteosarcoma Patients.
| Group | Local Recurrence (%) | Postoperative Metastasis (%) | Recurrence + Metastasis (%) |
|---|---|---|---|
| No IPN group | 12.0 % | 32.0 % | 36.0 % |
| Solitary IPN group | 12.5 % | 31.3 % | 31.3 % |
| Multiple IPNs group | 0.0 % | 70.0 % | 70.0 % |
| p-value | 0.506 | 0.053 | 0.053 |
Note: ① All data were derived from a retrospective cohort analysis (N = 126) and analyzed using SPSS version 26.0. ② A two-tailed p-value < 0.05 was considered statistically significant.
2.4. Survival analysis
Kaplan–Meier survival curves were generated to assess both metastasis-free survival (MFS) and overall survival (OS) among the three patient groups. The analysis demonstrated that patients with multiple IPNs had significantly worse outcomes compared to those with no IPNs or a solitary IPN. Specifically, the 3-year MFS was 64.0 % in the no-IPN group, 65.6 % in the solitary IPN group, and only 45.0 % in the multiple IPNs group (log-rank test, p = 0.04) (Fig. 1). Similarly, the 3-year OS was 76.0 % for patients without IPNs versus 65.4 % for the combined IPN-positive group, with a marked decrease to 30.0 % in the multiple IPNs subgroup at 5 years (log-rank test, p = 0.045) (Fig. 2; Table 3). Notably, patients with solitary IPNs exhibited survival outcomes comparable to those without IPNs.
Table 3.
Kaplan–Meier Survival Analysis of 126 Osteosarcoma Patients.
| Group | n | 3-Year MFS (%) | 5-Year MFS (%) | p-value (MFS) | 3-Year OS (%) | 5-Year OS (%) | p-value (OS) |
|---|---|---|---|---|---|---|---|
| No IPN group | 100 | 64.0 | 64.0 | − | 76.0 | 69.0 | − |
| Solitary IPN group | 16 | 65.6 | 65.6 | 0.931 | 72.0 | 62.4 | 0.931 |
| Multiple IPNs group | 10 | 45.0 | 35.0 | 0.04* | 65.4 | 30.0 | 0.045* |
| Overall IPN (Total) group | 26 | 57.7 | 53.8 | 0.03* | 65.4 | 53.8 | 0.040* |
Note: ① All data were derived from a retrospective cohort analysis (N = 126) and analyzed using SPSS version 26.0. ② Survival analysis was performed using the Kaplan–Meier method. ③ A two-tailed p-value < 0.05 was considered statistically significant. MFS: metastasis-free survival; OS: overall survival; *P < 0.05 indicates statistical significance.
2.5. Prognostic impact on overall survival
Multivariate Cox proportional hazards analysis confirmed the prognostic significance of multiple IPNs. Patients presenting with multiple IPNs had a 2.87-fold increased risk of death compared to those without IPNs (HR = 2.87, 95 % CI: 1.18–6.97, p = 0.020). In contrast, the presence of a solitary IPN did not significantly impact survival (HR = 1.75, 95 % CI: 0.76–4.01, p = 0.186). Interestingly, the surgical approach had a notable effect on outcomes: patients who underwent prosthetic reconstruction experienced a significant survival advantage, with a 5-year OS of 76.5 % versus 52.9 % in those who underwent amputation (HR = 0.43, 95 % CI: 0.19–0.96, p = 0.040) (Table 5).
Table 5.
Cox Proportional Hazards Analysis for Metastasis-Free Survival (MFS) and Overall Survival (OS).
| Variable | MFS | OS | ||
|---|---|---|---|---|
| HR (95 % CI) | p-value | HR (95 % CI) | p-value | |
| IPN: Solitary vs. None | 0.96 (0.37–2.47) | 0.934 | 1.75 (0.76–4.01) | 0.186 |
| IPN: Multiple vs. None | 2.73 (1.20–6.21) | 0.016* | 2.87 (1.18–6.97) | 0.020* |
| Bone Involvement > 1/3 | 1.27 (0.70–2.30) | 0.436 | 1.48 (0.80–2.74) | 0.212 |
| Tumor Diameter > 8 cm | 0.99 (0.55–1.79) | 0.965 | 1.07 (0.58–1.99) | 0.829 |
| Pathological Fracture | 1.38 (0.64–2.98) | 0.406 | 1.88 (0.90–3.96) | 0.094 |
| Male (vs. Female) | 0.93 (0.51–1.70) | 0.822 | 1.11 (0.59–2.10) | 0.743 |
| Age 12–17 years (vs. ≤ 12 years) | 1.70 (0.78–3.72) | 0.183 | 2.19 (0.96–4.99) | 0.061 |
| Age 18–20 years (vs. ≤ 12 years) | 1.34 (0.49–3.70) | 0.570 | 0.69 (0.18–2.65) | 0.584 |
| ALP Abnormal | 2.26 (0.89–5.73) | 0.087 | 1.74 (0.73–4.15) | 0.208 |
| Prosthetic Reconstruction (vs. Amputation) | 0.36 (0.17–0.78) | 0.010* | 0.43 (0.19–0.96) | 0.040* |
Note: ① All data were derived from a retrospective cohort analysis (N = 126) and analyzed using SPSS version 26.0. ② Survival analysis was conducted using the Kaplan–Meier method and Cox proportional hazards model. ③ A two-tailed p-value < 0.05 was considered statistically significant. HR: hazard ratio; *P < 0.05 indicates statistical significance.
2.6. Case Presentations
Case 1. A 14-year-old female was diagnosed with distal femoral osteosarcoma (Enneking Stage IIb). The primary tumor measured 5 cm in diameter and involved less than one-third of the affected bone. There were no pathological fractures, although her serum alkaline phosphatase (ALP) level was elevated to twice the normal value. At initial presentation, a solitary IPN was detected in the left lower lung on thin-slice CT. The patient underwent six cycles of MAPI neoadjuvant chemotherapy (methotrexate, doxorubicin, cisplatin, and ifosfamide) with prosthetic replacement surgery. During a three-year follow-up period, serial CT examinations demonstrated that the nodule remained stable in size, effectively ruling out pulmonary metastasis (see Fig. 3).
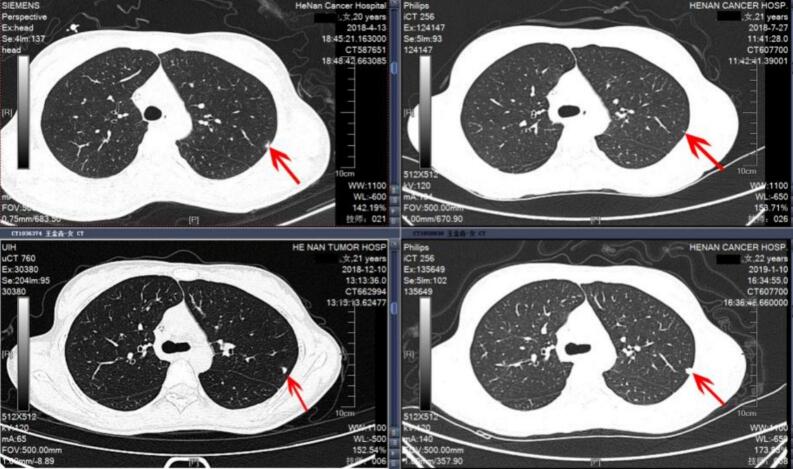
Case 2. A 20-year-old female presented with proximal humeral osteosarcoma (Enneking Stage IIb). The primary tumor was large, measuring 10 cm in diameter, and involved more than one-third of the affected bone. Although there were no pathological fractures, her serum ALP level was significantly elevated—approximately four times the normal value. At diagnosis, a solitary IPN was identified in the left upper lung. After two months of MAP neoadjuvant chemotherapy, the nodule showed a marked reduction in size. However, following prosthetic reconstruction and the completion of six chemotherapy cycles, the nodule began to enlarge one month after treatment cessation. Its progressive growth continued over the subsequent two months, and a biopsy eventually confirmed pulmonary metastasis. Metastatic spread was detected seven months post-treatment, and the patient’s clinical course deteriorated, leading to her death 17 months after the initial diagnosis (see Fig. 4).
Fig. 4.
Case 2. A 20-year-old female patient with proximal humeral osteosarcoma. At initial presentation, an IPN was identified in the left upper lung; following 2 months of chemotherapy, the nodule markedly decreased in size, but after 6 months of treatment and a 1-month treatment pause, the IPN increased in size and continued to enlarge after a subsequent 2-month pause, with biopsy confirming osteosarcoma metastasis.
Case 3. A 13-year-old boy was diagnosed with distal tibia osteosarcoma (Enneking Stage IIb). His primary tumor was extensive, measuring 15 cm in diameter and involving more than one-third of the sacrum. Similar to the other cases, there were no pathological fractures, but his serum ALP level was elevated to twice the normal value. Multiple scattered IPNs were observed in the left lower lung at initial presentation. The patient received three cycles of MAPI neoadjuvant chemotherapy. Five months into treatment, significant enlargement of the pulmonary nodules was noted on follow-up CT imaging. Subsequent studies confirmed the development of pulmonary and rib metastasis. Despite aggressive treatment, the patient’s condition worsened, and he ultimately succumbed to the disease 13 months after diagnosis (see Fig. 5).
Fig. 5.
Case 3. A 13-year-old male patient with distal tibial osteosarcoma. At initial presentation, multiple scattered IPNs were detected in the left lower lung; after treatment and a 5-month follow-up, these nodules significantly increased in size, and imaging studies confirmed pulmonary metastasis.
3. Discussion
This study systematically evaluated the incidence and prognostic implications of IPNs in pediatric and adolescent patients with osteosarcoma, revealing relatively high prevalence (20.6 %) and underscoring the particularly poor outcomes associated with multiple IPNs. Specifically, a 5-year overall survival (OS) rate of only 30.0 % in patients with multiple IPNs—markedly lower than the 69.0 % observed in those without IPNs—highlights the pressing need for vigilant monitoring and targeted management strategies in this high-risk subgroup.
3.1. Prognostic significance of IPNs
A key finding of this analysis is the sharp prognostic dichotomy between solitary and multiple IPNs. While patients presenting with solitary nodules demonstrated outcomes comparable to those without IPNs (e.g., similar 3-year metastasis-free survival rates), the presence of multiple nodules was linked to a notable decrease in both MFS (45.0 % at 3 years) and OS (30.0 % at 5 years). These data indicate that solitary nodules may frequently represent benign or subclinical lesions adequately controlled by standard chemotherapy, whereas multiple nodules likely reflect an advanced degree of hematogenous dissemination, translating into a significantly higher risk of recurrence and systemic spread.
Moreover, the extent of bony involvement—particularly cases exceeding one-third of the affected bone’s length—emerged as a more potent indicator of potential IPN development than tumor diameter alone. This observation suggests that local architectural disruption and elevated tumor burden may intensify vascular invasion and raise the probability of circulating tumor cell dissemination.
In this study, tumor necrosis rate data were incomplete, as only a subset of patients underwent evaluation. Furthermore, the small number of patients with a tumor necrosis rate greater than 90 % (n = 4) limited the statistical power of the analysis. These limitations may have impacted the ability to detect significant correlations between tumor necrosis rate and MFS or OS.
3.2. Molecular mechanisms and Multi-Omics integration
Accumulating evidence, including findings from this study and previous investigations, highlights the complex molecular mechanisms underlying multiple IPNs. These mechanisms involve genomic instability, enhanced angiogenic activity, and immune evasion strategies [10], [11], [12]. Transcriptomic studies analyzing lung metastases in osteosarcoma have identified dysregulated pathways, such as Wnt/β-catenin and PI3K/Akt, which not only drive aggressive tumor behavior but also present potential therapeutic targets [13]. Future research utilizing multi-omics approaches—integrating genomics, transcriptomics, proteomics, and immunomics—is essential to unravel the contributions of these pathways to IPN initiation and progression [14], [15]. Leveraging large-scale datasets through cloud-based platforms and advanced bioinformatics algorithms will enable researchers to more effectively identify actionable biomarkers and enhance risk stratification models.
3.3. Clinical management and advanced techniques
Given the strong association between multiple IPNs and poorer survival outcomes, implementing a tiered treatment strategy is essential. The MDT approach played a pivotal role in the diagnosis and management of patients with pulmonary metastases. By bringing together expertise from various specialties, including oncology, radiology, pathology, and surgery, the MDT ensured that each patient received a personalized treatment plan tailored to their clinical condition and individual needs. For patients with solitary IPNs and stable imaging findings, continuing standard chemotherapy combined with vigilant imaging follow-up may help avoid overtreatment while ensuring timely detection of disease progression. Conversely, patients presenting with multiple IPNs may require more aggressive treatment strategies, such as dose-dense chemotherapy regimens, immunotherapy, or targeted therapies, to address the heightened risk of widespread micrometastatic disease.
The advent of radiomics and artificial intelligence (AI) further refines IPN detection and characterization [15], [16], [17]. Through high-dimensional analyses of nodular features such as morphology, texture, and density, these “virtual biopsy” techniques offer enhanced diagnostic consistency and predictive power [18]. Collaborations among MDT are pivotal in translating these tools into individualized treatment plans, thereby balancing therapeutic efficacy with the burden of potential side effects.
3.4. Surgical strategies and prognosis
Our findings also highlight a survival advantage among patients undergoing endoprosthetic reconstruction compared to amputation, with a 5-year OS of 76.5 % versus 52.9 %. Multivariate analysis suggests that prosthetic reconstruction independently provides a protective effect (HR = 0.43). This may be related to selection bias, as patients undergoing amputation often have larger local tumor volumes, poor response to chemotherapy, and involvement of vascular or neural structures, which are typically associated with worse prognoses. In contrast, patients undergoing limb-sparing surgery generally achieve complete local tumor resection, early functional recovery, and a more positive attitude toward treatment. Nevertheless, this result further supports the notion that limb-sparing surgery does not increase the risk of local recurrence or compromise overall survival.
3.5. Limitations and Future directions
This study has several limitations. First, as a retrospective study, it is subject to selection bias and information bias, which may limit the generalizability of the findings. Retrospective data collection relies on the accuracy and completeness of medical records, which may introduce variability and potential inaccuracies. Second, this is a single-center study, and the results may not fully represent broader patient populations or other institutions, where treatment protocols, patient demographics, and follow-up practices may differ. Third, the relatively small sample size, particularly in subgroup analyses (e.g., tumor necrosis rate and outcomes), reduces the statistical power and may have limited the detection of significant differences. Fourth, incomplete data, such as the lack of tumor necrosis rate measurements for all patients, may have introduced bias and restricted the scope of the analysis. Fifth, variability in follow-up intervals due to individual patient circumstances may have influenced the accuracy of survival analyses. Although follow-up was conducted based on general principles for malignant tumors, the actual intervals varied, which may have impacted the consistency of the data. Finally, the study did not include molecular or genetic analyses, which are increasingly recognized as important factors in understanding tumor behavior, metastasis, and prognosis. The lack of such data limits the ability to explore the biological mechanisms underlying pulmonary metastases and their impact on patient outcomes.
In the future, several directions can be pursued to address these limitations and advance the understanding of pulmonary metastases. First, prospective, multi-center studies with larger sample sizes and standardized follow-up protocols are needed to validate the findings and improve the generalizability of the results. Second, incorporating molecular and genetic analyses, such as next-generation sequencing and transcriptomic profiling, could provide deeper insights into the biological mechanisms driving pulmonary metastases and their relationship with chemotherapy response and prognosis. Third, the development of automated, AI-assisted diagnostic protocols may improve the consistency and accuracy of IPN evaluation, reducing variability in clinical decision-making and enhancing early detection of metastases [20], [21]. Fourth, delving deeper into the biology of IPNs through multi-omics datasets—integrating genomics, proteomics, and metabolomics—holds promise for identifying key molecular drivers and potential therapeutic targets [14], [19]. The integration of these datasets with AI-driven analytics could further enhance the identification of predictive biomarkers and therapeutic strategies. Finally, future studies should explore the role of multidisciplinary team (MDT) approaches in optimizing individualized treatment strategies for patients with pulmonary metastases, as well as the impact of different treatment modalities on long-term outcomes.
4. Conclusion
In conclusion, IPNs are not uncommon in pediatric and adolescent osteosarcoma patients, with multiple IPNs constituting a clear independent marker of poor prognosis. These findings emphasize the importance of preoperative IPN assessment and risk stratification in guiding individualized treatment strategies. Future research integrating advanced imaging, multi-omics profiling, and AI technologies is essential to improve prognostication and optimize therapeutic approaches for this vulnerable population.
CRediT authorship contribution statement
Yao Weitao: Writing – original draft. Du Xinhui: Conceptualization. Li Zhehuang: Formal analysis. Hou Jingyu: Formal analysis. Ma Shengbiao: Investigation. Zhang Panhong: Methodology. Niu Xiaohui: Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Yao Weitao, Email: ywtwhm@163.com.
Du Xinhui, Email: drduxinhui@163.com.
Li Zhehuang, Email: zlyylizhehuang4413@zzu.edu.cn.
Hou Jingyu, Email: 15639106961@163.com.
Ma Shengbiao, Email: jrqxnha_m@163.com.
Zhang Panhong, Email: zhangpanhong111@163.com.
Niu Xiaohui, Email: niuxiaohui@263.net.
References
- 1.Wen M., Ren H., Zhang S., Li T., Zhang J., Ren P. CT45A1 promotes the metastasis of osteosarcoma cells in vitro and in vivo through beta-catenin. Cell Death Dis. 2021;12(7):650. doi: 10.1038/s41419-021-03935-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu S., Yao X. Advances on immunotherapy for osteosarcoma. Mol. Cancer. 2024;23(1):192. doi: 10.1186/s12943-024-02105-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zarghooni K., Bratke G., Landgraf P., Simon T., Maintz D., Eysel P. The Diagnosis and Treatment of Osteosarcoma and Ewing's Sarcoma in Children and Adolescents. Dtsch. Arztebl. Int. 2023;120(24):405–412. doi: 10.3238/arztebl.m2023.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang T.Y., Lan K.C., Wu C.H., Sheu M.L., Yang R.S., Liu S.H. Nepsilon-(1-Carboxymethyl)-L-lysine/RAGE Signaling Drives Metastasis and Cancer Stemness through ERK/NFkappaB axis in Osteosarcoma. Int. J. Biol. Sci. 2024;20(3):880–896. doi: 10.7150/ijbs.90817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu P., Han Y., Meng L., Tian Y., Jin Z., Luo J., et al. Exosomes derived from pulmonary metastatic sites enhance osteosarcoma lung metastasis by transferring the miR-194/215 cluster targeting MARCKS. Acta Pharm Sin B. 2024;14(5):2039–2056. doi: 10.1016/j.apsb.2024.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D., Niu X., Wang Z., Song C.L., Huang Z., Chen K.N., et al. Multiregion Sequencing Reveals the Genetic Heterogeneity and Evolutionary History of Osteosarcoma and Matched Pulmonary Metastases. Cancer Res. 2019;79(1):7–20. doi: 10.1158/0008-5472.CAN-18-1086. [DOI] [PubMed] [Google Scholar]
- 7.Tsoi K.M., Lowe M., Tsuda Y., Lex J.R., Fujiwara T., Almeer G., et al. How Are Indeterminate Pulmonary Nodules at Diagnosis Associated with Survival in Patients with High-Grade Osteosarcoma? Clin Orthop Relat Res. 2021;479(2):298–308. doi: 10.1097/CORR.0000000000001491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hassan M.S., Ariyaratne S., Azzopardi C., Iyengar K.P., Davies A.M., Botchu R. The clinical significance of indeterminate pulmonary nodules in patients with primary bone sarcoma: a systematic review. Br J Radiol. 2024;97(1156):747–756. doi: 10.1093/bjr/tqae040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacMahon H., Naidich D.P., Goo J.M., Lee K.S., Leung A.N.C., Mayo J.R., et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology. 2017;284(1):228–243. doi: 10.1148/radiol.2017161659. [DOI] [PubMed] [Google Scholar]
- 10.Paez R., Kammer M.N., Tanner N.T., Shojaee S., Heideman B.E., Peikert T., et al. Update on Biomarkers for the Stratification of Indeterminate Pulmonary Nodules. Chest. 2023;164(4):1028–1041. doi: 10.1016/j.chest.2023.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szyszko T.A., Yip C., Szlosarek P., Goh V., Cook G.J. The role of new PET tracers for lung cancer. Lung Cancer. 2016;94:7–14. doi: 10.1016/j.lungcan.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 12.He X., Lu M., Hu X., Li L., Zou C., Luo Y., et al. Osteosarcoma immune prognostic index can indicate the nature of indeterminate pulmonary nodules and predict the metachronous metastasis in osteosarcoma patients. Front Oncol. 2022;12 doi: 10.3389/fonc.2022.952228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y., Chen J., Huang Y., Yang S., Tan T., Wang N., et al. Schisandrin B suppresses osteosarcoma lung metastasis in vivo by inhibiting the activation of the Wnt/beta‑catenin and PI3K/Akt signaling pathways. Oncol Rep. 2022;47(3) doi: 10.3892/or.2022.8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao M., Xue G., He B., Deng J., Wang T., Zhong Y., et al. Integrated multiomics signatures to optimize the accurate diagnosis of lung cancer. Nat Commun. 2025;16(1):84. doi: 10.1038/s41467-024-55594-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao Z., Guo S., Han L., Wu L., Zhang Y., Yan B. Altruistic seagull optimization algorithm enables selection of radiomic features for predicting benign and malignant pulmonary nodules. Comput Biol Med. 2024;180 doi: 10.1016/j.compbiomed.2024.108996. [DOI] [PubMed] [Google Scholar]
- 16.Warkentin M.T., Al-Sawaihey H., Lam S., Liu G., Diergaarde B., Yuan J.M., et al. Radiomics analysis to predict pulmonary nodule malignancy using machine learning approaches. Thorax. 2024;79(4):307–315. doi: 10.1136/thorax-2023-220226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J., Sourlos N., Heuvelmans M., Prokop M., Vliegenthart R., van Ooijen P. Explainable machine learning model based on clinical factors for predicting the disappearance of indeterminate pulmonary nodules. Comput Biol Med. 2024;169 doi: 10.1016/j.compbiomed.2023.107871. [DOI] [PubMed] [Google Scholar]
- 18.Marmor H.N., Jackson L., Gawel S., Kammer M., Massion P.P., Grogan E.L., et al. Improving malignancy risk prediction of indeterminate pulmonary nodules with imaging features and biomarkers. Clin. Chim. Acta. 2022;534:106–114. doi: 10.1016/j.cca.2022.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khodayari Moez E., Warkentin M.T., Brhane Y., Lam S., Field J.K., Liu G., et al. Circulating proteome for pulmonary nodule malignancy. J. Natl Cancer Inst. 2023;115(9):1060–1070. doi: 10.1093/jnci/djad122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Headrick J.R., Jr., Parker M.J., Miller A.D. Artificial Intelligence: Can It Save Lives, Hospitals, and Lung Screening? Ann Thorac Surg. 2024;118(3):712–718. doi: 10.1016/j.athoracsur.2024.05.014. [DOI] [PubMed] [Google Scholar]
- 21.Prosper A.E., Kammer M.N., Maldonado F., Aberle D.R., Hsu W. Expanding Role of Advanced Image Analysis in CT-detected Indeterminate Pulmonary Nodules and Early Lung Cancer Characterization. Radiology. 2023;309(1) doi: 10.1148/radiol.222904. [DOI] [PMC free article] [PubMed] [Google Scholar]