Abstract
Marfan syndrome (MFS) is an inherited connective tissue disorder, with aortic root aneurysm and/or dissection being the most severe and life-threatening complication. These conditions have been linked to pathogenic variants in the FBN1 gene and dysregulated TGFβ signaling. Our objective was to evaluate the mRNA expression of FBN1, TGFBR1, TGFBR2, and TGFB2 in aortic tissue from MFS patients undergoing surgery for aortic dilation. This prospective study (2014–2023) included 20 MFS patients diagnosed according to the 2010 Ghent criteria, who underwent surgery for aneurysm or dissection based on Heart Team recommendations, along with 20 non-MFS controls. RNA was extracted, and mRNA levels were quantified using RT-qPCR. Patients with dissection showed significantly higher FBN1 mRNA levels [79 (48.1–110.1)] compared to controls [37.2 (25.1–79)] (p = 0.03). Conversely, TGFB2 expression was significantly lower in MFS patients [12.17 (6.54–24.70)] than in controls [44.29 (25.85–85.36)] (p = 0.029). A positive correlation was observed between higher FBN1 expression and a larger sinotubular junction diameter (r = 0.42, p = 0.07), while increased FBN1 expression was particularly evident in MFS patients with dissection. Additionally, TGFB2 expression showed an inverse correlation with ascending aortic diameter (r = 0.53, p = 0.01). In aortic tissue, we found decreased TGFB2 and receptor levels alongside increased FBN1 mRNA levels. These molecular alterations may reflect compensatory mechanisms in response to tissue damage caused by mechanical stress, leading to dysregulation of physiological signaling pathways and ultimately contributing to aortic dilation in MFS.
Keywords: Marfan syndrome, aortic dissection, FBN1, TGFβR1, TGFβR2, TGFβ2
1. Introduction
Marfan syndrome (MFS) is a hereditary connective tissue disorder [1]. Its incidence varies, but the estimated prevalence is approximately 1 in 10,000. The condition has high penetrance with variable clinical expression.
The clinical criteria for diagnosing MFS are based on its effects on the most commonly affected tissues, including the musculoskeletal system, eyes, cardiovascular and pulmonary tissues, and skin [2]. One of the most serious and potentially life-threatening complications is aortic root aneurysm, which can lead to dissection and rupture. Other key clinical features include mitral valve prolapse, pneumothorax, dural ectasia, and myopia. A defining characteristic of the affected tissues is their high fibrillin content, particularly in the aorta, musculoskeletal structures, lungs, and cornea.
Fibrillin-1 (FBN1) plays a critical role in TGFβ2 signaling, and genetic variants in FBN1 lead to increased bioavailability and activity of TGFβ2. Pathogenic variants in the FBN1 gene, which encodes the FBN1 protein, have been identified as the primary cause of MFS. FBN1 is the main component of 10–12 nm microfibrils in the extracellular matrix of connective tissue. Most pathogenic variants in FBN1 are missense mutations or nonsense mutations that introduce premature termination codons [3]. These variants can lead to the production of truncated FBN1 proteins that may still be incorporated into extracellular microfibrils. However, truncated transcripts are typically degraded by nonsense-mediated mRNA decay. The resulting loss of elastic fiber integrity weakens the vessel walls, reducing their ability to withstand mechanical stress [4].
Additionally, a significant reduction in aortic contraction has been observed, suggesting potential mechanisms involved in the development and progression of aortic aneurysms in Marfan syndrome (MFS). This may contribute to the deterioration of the wall’s mechanical properties and structural integrity. While aneurysm formation appears to have a genetic basis as a triggering factor, other elements, such as mechanical stress and inflammatory processes, may also play a role [5]. Moreover, MFS patients exhibit markedly lower TGFβ2 mRNA levels, potentially indicating an inflammatory response that further weakens the aortic wall and accelerates its degeneration [6].
Different pathogenic variants in TGFβR1 and TGFβR2, receptors for transforming growth factor beta (TGFβ), have been identified [7]. Pathogenic variants in these genes were later recognized as the cause of a distinct condition, now classified as Loeys–Dietz syndrome [8]. While the idea that abnormally activated TGFβ signaling is responsible for Marfan syndrome and related disorders remains uncertain, it is evident that FBN1 and components of the TGFβ signaling pathway, including TGFβR1 and TGFβR2, share overlapping genetic mechanisms [4,7].
TGFβ2 first binds to its type 2 receptor (TGFβR2) on the cell surface, which subsequently recruits and phosphorylates the type 1 receptor (TGFβR1). Upon activation, TGFβR1 phosphorylates the SMAD proteins (SMAD2 and SMAD3).
The phosphorylated SMAD2 and SMAD3 then bind to SMAD4 and are transported into the nucleus with the help of importins 7 and 8 [9]. Inside the nucleus, they regulate the expression of TGFβ target genes by interacting with transcription factors and cofactors, including coactivators and corepressors [10,11,12]. TGFβ2 can also signal through non-SMAD (non-canonical) pathways, such as those involving mitogen-activated protein kinases (MAPK) [13,14]. Recent studies support the hypothesis that TGFβ signaling promotes the development of thoracic aortic aneurysms (TAAs). This has been demonstrated in animal models and is further supported by findings in human aortas with advanced TAA [15]. While the increase in circulating TGFβ within the extracellular matrix has been widely studied, little is known about whether its expression is also elevated intracellularly in response to genetic or epigenetic modulation in both canonical and non-canonical signaling pathways. Therefore, our objective was to assess the mRNA expression of FBN1, TGFBR1, TGFBR2, and TGFB2 in the aortic tissue of Marfan syndrome patients with aortic dilation.
2. Results
2.1. Characteristics of the Study Population
Table 1 compares demographic characteristics and laboratory findings between patients with Marfan syndrome (MFS) and controls. The study included 40 participants, evenly divided between the MFS group and controls. The MFS group had a significantly younger average age (32 ± 8 years) compared to controls (64 ± 13 years) (p = 0.0001). Regarding medical conditions, systemic arterial hypertension (SAH) was less prevalent in the MFS group (25%) compared to controls (70%) (p = 0.0001).
Table 1.
Demographic characteristics of patients with Marfan syndrome and controls.
| Total n = 40 |
MFS n = 20 |
Controls n = 20 |
p | |
|---|---|---|---|---|
| Gender | ||||
| Women (n) | 14 | 10 | 4 | NS |
| Men (n) | 26 | 10 | 16 | NS |
| Age (years) | 48 ± 19 | 32 ± 8 | 64 ± 13 | 0.0001 |
| BMI (kg/m2) | 25 ± 4 | 24 ± 4 | 26 ± 5 | NS |
| Laboratories | Median (min–max) | |||
| Hemoglobin (g/dL) | 14 (9–28) | 13 (9–18) | 14 (9–28) | NS |
| Total Cholesterol (mg/dL) | 258 (56–279) | 150 (75–222) | 179 (56–279) | 0.03 |
| HDL-C (mg/dL) | 35 (8–68) | 35 (19–54) | 35 (8–68) | NS |
| LDL-C (mg/dL) | 104 (40–202) | 93 (40–132) | 127 (40–202) | 0.02 |
| Triglycerides (mg/dL) | 101 (41–371) | 92 (41–371) | 111 (80–396) | 0.008 |
| Glucose (mg/dL) | 96 (54–209) | 89 (54–209) | 104 (81–271) | 0.001 |
| Serum Creatinine (mg/dL) | 0.05 (0.6–5.0) | 0.80 (0.6–1.7) | 1.04 (0.7–5.0) | 0.03 |
| LVEF (%) | 55 (15–71) | 54 (18–66) | 58 (15–71) | NS |
| Comorbidities | ||||
| Obesity n (%) | 9 (23) | 5 (25) | 4 (20) | NS |
| SAH n (%) | 19 (48) | 5 (25) | 14 (70) | 0.0001 |
| DMTII n (%) | 10 (25) | 4 (20) | 6 (30) | NS |
| Smoking n (%) | 2 (5) | 0 (0) | 2 (10) | NS |
| Hypothyroidism n (%) | 2 (5) | 0 (0) | 2 (10) | NS |
| Aortic dilation n (%) | 33 (83) | 20 (100) | 13 (65) | 0.001 |
| Atrial fibrillation n (%) | 4 (10) | 1 (5) | 3 (15) | NS |
BMI = body mass index, HDL-C = high-density lipoprotein cholesterol, LDL-C = low-density lipoprotein cholesterol, SAH = systemic arterial hypertension, DMTII = Diabetes Mellitus type II, LVEF = left ventricle ejection fraction. Non-normal variables are expressed as median (min–max) and analyzed with the Mann–Whitney U test, while normal variables are mean ± standard deviation and analyzed with the t-test. NS = no significance. p < 0.05.
The characteristics of the total Ghent criteria are shown in Table 2. Ten patients (50%) had no known family history of Marfan syndrome (MFS) or aortic dissection, while the other ten (50%) had a family history of both. All patients met at least two criteria; 7 (35%) met two criteria, 7 (35%) met three, 5 (25%) met four, and 1 (5%) met five.
Table 2.
Characteristics of the total Ghent criteria with which the patients were classified and frequency of variables of the systemic score.
| Patient | Age | Sex | Ghent Criteria | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Total Ghent Criteria | ||||
| FH | EL | AD | Points SS | SS 7/20 | FBN1 | ||||
| 1 | 32 | F | + | − | + | 15 (SWM, FF, PC; PN, DE, ER. HF, MS, SM, M, MVP) | + | TNP | 3 (FH + AD + SS) |
| 2 | 25 | F | + | − | + | 14 (SWM, FF, PC, HF, DE, MS, SM, AS/H) | + | + | 4 (FH + AD + SS + FBN1) |
| 3 | 36 | F | + | − | + | 13 (SWM, FF, PC, MS, ER, MVP) | + | TNP | 3 (FH + AD + SS) |
| 4 | 23 | M | − | − | + | 15 (SWM, PC, HF, RUS/LS, MS, M; MVP) | + | + | 3 (AD + SS + FBN1) |
| 5 | 35 | F | − | + | + | 7 (SWM, RUS/LS, MS, M, MVP) | + | TNP | 3 (EL + AD + SS) |
| 6 | 35 | F | − | − | + | 7 (FF, HF, RUS/LS, S M, MS, MVP) | + | TNP | 2 (AD + SS) |
| 7 | 52 | F | − | − | + | 7 (SWM, FF, HF, MS, SM, M, MVP) | + | TNP | 2 (AD + SS) |
| 8 | 51 | M | − | − | + | 8 (FF, MS, HF, ER, M, MS, DE, MVP) | + | TNP | 2 (AD + SS) |
| 9 | 35 | F | + | + | + | 10 (SWM, FF, PE, HF, DE, MS, MS) | + | TNP | 4 (FH + EL + AD + SS) |
| 10 | 33 | M | − | − | + | 4 (DE, MS, PC) | + | TNP | 2 (AD + SS) |
| 11 | 33 | F | − | − | + | 5 (PE, FF, HF, DE) | − | + | 2 (AD + FBN1) |
| 12 | 21 | F | + | − | + | 5 (PE, FF, DE; ER, MS) | − | + | 3 (FH + AD + FBN1) |
| 13 | 28 | M | − | + | + | 12 (SWM, FF, PE, HF, DE, MS, RUS/LS, ER, MS) | + | TNP | 3 (EL + AD + SS) |
| 14 | 37 | M | + | + | + | 9 (SWM, PE, HF, DEMS, SM) | + | + | 5 (FH + EL + AD + SS + FBN1) |
| 15 | 32 | M | + | + | + | 10 (SWM, FF, HF, DE, RUS/LS, MS, SM) | + | TNP | 4 (FH + EL + AD + SS) |
| 16 | 32 | M | + | − | + | 14 (SWM, FF, PE, HF, DE, RUS/LS, MS, ER, MS, M) | + | TNP | 3 (FH + AD + SS) |
| 17 | 30 | M | − | − | + | 11 (SWM, FF, PE, DE, ER; MS, M, MVP) | + | TNP | 2 (AD + SS) |
| 18 | 33 | M | + | + | + | 17 (SWM, FF, PE, HF, PN, DE, MD, AS/H, ER, MS, M, MVP) | + | TNP | 4 (FH + EL + AD + SS) |
| 19 | 22 | M | + | + | + | 9 (SWM, FF, PC, HF, MS, M, MVP) | + | TNP | 4 (FH + EL + AD + SS) |
| 20 | 51 | F | − | − | + | 7 (SWM, FF, MS, SM, MVP) | + | TNP | 2 (AD + SS) |
| Frequency of systemic score items in patients with Marfan syndrome. | |||||||||
| Total 20 | |||||||||
| Stretch marks n (%) | 19 (95) | ||||||||
| Facial features n (%) | 17 (85) | ||||||||
| Cavus foot n (%) | 14 (70) | ||||||||
| Scoliosis n (%) | 13 (65) | ||||||||
| Reduction in the upper/lower segment n (%) | 11 (55) | ||||||||
| Valve prolapse n (%) | 11 (55) | ||||||||
| Dural ectasia n (%) | 10 (50) | ||||||||
| Myopia n (%) | 10 (50) | ||||||||
| Walker Murdock sign n (%) | 9 (45) | ||||||||
| Pectum excavatum n (%) | 7 (35) | ||||||||
| Pectum carinatum n (%) | 6 (30) | ||||||||
| Steinberg sign n (%) | 5 (25) | ||||||||
| Reduced elbow extension n (%) | 3 (15) | ||||||||
| Pneumothorax n (%) | 2 (10) | ||||||||
| Acetabular protrusion n (%) | 0 (0) | ||||||||
F = female, M = male, FH = family history, EL = ectopia lentis, AD = aortic dilatation, SS = systemic score, TNP = test not performed, SWM = Steinberg–Walker–Murdock sign, FF = facial feature, PC = pectum carinatum, PN = pneumothorax, DE = dural ecstasy, SM = stretch marks, M = myopia, MVP = mitral valve prolapse, HF = hollow foot, MS = mild scoliosis, ER = elbow reduction, R US/LS = reduction in the upper segment over the lower one, PE = pectum excavatum, AS > H = measurement of the arm span greater than the height.
The genetic variants identified in FBN1 in five patients follow the Human Genome Variation Society (HGVS) nomenclature. Patient 2 harbors NM_000138.5:c.3697C>T (p.Gln1233Ter), a stop-gain mutation. Patient 4 has NM_000138.5:c.2258G>T (p.Gly753Val), a non-synonymous SNV. Patient 11 has NM_000138.5:c.1539T>G (p.Cys513Trp), a non-synonymous SNV. Patient 12 carries NM_000138.5:c.4621C>T (p.Arg1541Ter), a stop-gain variant. Patient 14 presents NM_000138.5:c.7133G>A (p.Cys2378Tyr), a non-synonymous SNV.
A comparison of surgical conditions and procedures between MFS patients and controls is presented in Table 3. The study included 40 patients, 20 with MFS and 20 controls. Aortic dilation was significantly more frequent in MFS patients (85%) compared to controls (45%), with p = 0.01. Aortic dissection occurred in 17.5% of the total population, affecting 10% of MFS patients and 25% of controls. Clamping time was notably longer in MFS patients (median 152 min, range 110–224) compared to controls (median 131 min, range 66–254), with p = 0.03. Lastly, mortality was significantly higher in the control group (20%) compared to the MFS group (0%), with p = 0.05.
Table 3.
Type of surgery and type of surgical condition between cases and controls.
| Total | MFS | Controls | p | |
|---|---|---|---|---|
| Type of surgery | n = 40 | n = 20 | n = 20 | |
| Elective n (%) | 31 (77.5) | 16 (80) | 15 (75) | NS |
| Urgent n (%) | 9 (22.5) | 4 (20) | 5 (25) | NS |
| CSEC (minutes) | 184 (122–440) | 182 (122–352) | 160 (94–440) | NS |
| Clamping time (minutes) | 136 (66–254) | 152 (110–224) | 131 (66–254) | 0.03 |
| Postoperative Survival Duration (days) | 19 (0–116) | − | 16 (0–100) | NS |
| Without aortic dilation or dissection n (%) | 7 (17.5) | 1 (5) | 6 (30) | NS |
| Aortic dilation n (%) | 26 (65) | 17 (85) | 9 (45) | 0.01 |
| Aortic dissection n (%) | 7 (17.5) | 2 (10) | 5 (25) | NS |
| Survivors n (%) | 36 (90) | 20 (100) | 16 (80) | NS |
| They stopped coming to the institution | 9 (22.5) | 7 (35) | 2 (10) | NS |
| Transfer to another institution n (%) | 1 (2.5) | 0 (0) | 1 (5) | NS |
| Death n (%) | 4 (10) | 0 (0) | 4 (20) | 0.05 |
MFS = Marfan Syndrome, CSEC = cardiac surgery with extracorporeal circulation. Non-normal variables are expressed as median (min–max) and analyzed with the Mann–Whitney U test. NS = no significance. p < 0.05.
Seven patients with MFS stopped attending the institution. We contacted them by phone, confirmed they were alive, and invited them to return for follow-up. In the control group, two patients stopped attending. One was successfully located and confirmed to be alive. The other was also alive, but we could not contact him directly. Many patients were from other countries and did not provide contact information, making follow-up difficult.
2.2. Surgical Characteristics
Table 4 summarizes the surgical characteristics and procedures performed. Measurements of the aortic ring diameter (ARD) and sinus of Valsalva diameter (SVD) demonstrated considerable variability, with ARD ranging from 22 mm to 62 mm and SVD from 26 mm to 94 mm. Similarly, the diameter of the sinotubular junction (STJ) ranged from 26 mm to 100 mm.
Table 4.
Average aortic diameters, type of surgery and procedures performed in patients with aortic dilatation and MFS.
| No | Sex | Age | CEC | ACT | ARD | SVD | STJ | TAscA | TS | D | Surgery |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 32 | No | No | 22 | 44 | 26 | 29 | E | No | Aortic Replacement + Revascularization of Visceral Trunks |
| 2 | F | 25 | 184 | 160 | 23 | 51 | 23 | 28 | E | No | Post-Asc Ao Tube Abdominal Aorta Replacement + Mesenteric and Renal Trunks |
| 3 | F | 36 | 272 | 197 | 22 | 40 | 34 | 28 | E | No | Bentall + Replacement of Arch with 3 Trunks + Packing |
| 4 | M | 23 | 149 | 113 | 43 | 91 | 95 | 30 | E | No | Bentall + CVM INC 30 |
| 5 | F | 35 | 209 | 154 | 41 | 80 | 82 | 93 | E | No | Bentall + REVAS HVSI-CD |
| 6 | F | 35 | 187 | 149 | 44 | 50 | 45 | 53 | E | No | Bentall |
| 7 | F | 42 | 142 | 110 | 32 | 46 | 34 | 32 | E | No | Bentall + Packing |
| 8 | M | 51 | 192 | 145 | 30 | 75 | 60 | 68 | E | No | Bentall + Tricuspid Valve Repair INC 28 + Packing |
| 9 | F | 35 | 22 | 200 | 62 | 60 | 70 | 40 | E | No | Bentall + CVM INC 34 + Tricuspid Valve Repair INC 28 + Left Atrial Redx + Arch Wrapping with Dacron Tube |
| 10 | M | 33 | 151 | 122 | 38 | 44 | 36 | 36 | E | No | Bentall |
| 11 | F | 33 | 162 | 118 | 27 | 48 | 58 | 67 | E | No | Bentall |
| 12 | F | 21 | 210 | 115 | 38 | 57 | 72 | 54 | U | yes | Bentall |
| 13 | M | 28 | 184 | 159 | 36 | 94 | 100 | 107 | E | No | Bentall + CVM INC 30 + REVAS HVSI-CD |
| 14 | M | 37 | 179 | 150 | 37 | 44 | 45 | 38 | E | No | Aortic Valve Resuspension (David Type) |
| 15 | M | 32 | 186 | 136 | 46 | 77 | 77 | 76 | E | No | Bentall |
| 16 | M | 32 | 352 | 205 | 34 | 56 | 50 | 56 | U | yes | Aortic Valve Replacement Bentall and Bono |
| 17 | M | 30 | 290 | 224 | 35 | 82 | 37 | 37 | U | yes | Aortic Valve Replacement Bentall and Bono |
| 18 | M | 33 | 243 | 194 | 43 | 84 | 32 | 29 | U | No | Aortic Valve Replacement Bentall and Bono |
| 19 | M | 22 | 230 | 117 | 39 | 50 | 34 | 30 | E | No | David |
| 20 | F | 53 | 440 | 164 | 26 | 38 | 43 | 20 | E | No | Bentall + Revascularization of Supra-Aortic Trunks + Elephant Trunk + Packing |
CEC = extracorporeal circulation, ACT = aortic clamping time, ARD = aortic ring diameter, SVD = sinus of valsalva diameter, STJ = sinotubular junction, TAscA = tubular ascending aorta, TS = type of surgery, E = elective, U = urgent, D = dissection, F = female, M = male.
The most frequently performed surgical procedures included the Bentall technique, undertaken in 12 patients, and aortic replacement, conducted in 4 patients. Less common interventions included supra-aortic trunk revascularization and aortic valve resuspension (David technique), each performed in a single patient. Additionally, combined procedures were performed in 3 patients, such as the Bentall technique combined with tricuspid valve repair and surgical packing.
A comparative analysis of aortic diameters between patients with Marfan syndrome (MFS) and controls revealed significant differences in key measurements. The aortic root diameter was significantly larger in the MFS group (37.55 ± 7.5 mm) compared to controls (30.8 ± 6.1 mm; p = 0.004). Similarly, the sinus of Valsalva and sinotubular junction diameters were markedly increased in MFS patients (61.3 ± 20.9 mm and 57.1 ± 21.4 mm, respectively) compared to controls (38.4 ± 5.2 mm and 38.2 ± 9.9 mm, respectively; p = 0.0001 for both). While the ascending aorta diameter was larger in MFS patients (58.8 ± 24.3 mm) than in controls (47.4 ± 15.5 mm), this difference did not reach statistical significance (p = 0.08). Detailed comparisons are presented in Table 5.
Table 5.
Comparative analysis of aortic diameters in patients with Marfan syndrome and control group.
| Diameters | Marfan | Control | p |
|---|---|---|---|
| Aortic root | 37.55 ± 7.5 | 30.8 ± 6.1 | 0.004 |
| Sinus of Valsalva | 61.3 ± 20.9 | 38.4 ± 5.2 | 0.0001 |
| Sinotubular junction | 57.1 ± 21.4 | 38.2 ± 9.9 | 0.0001 |
| Ascending aorta | 58.8 ± 24.3 | 47.4 ± 15.5 | 0.08 |
Data are expressed as mean ± standard deviation.
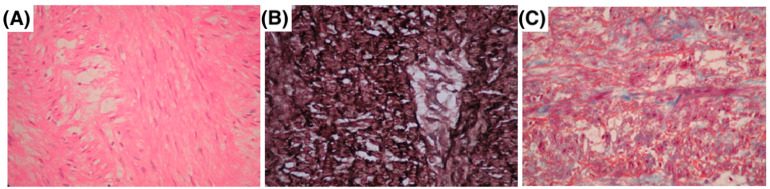
Figure 1 illustrates the histological findings in MFS patients with aortic pathology. Panel A shows a representative photomicrograph of a 28-year-old male with a giant ascending aortic aneurysm, revealing ruptured elastic fibers in the adventitia and minimal inflammatory infiltrate, stained with hematoxylin and eosin (H&E). Panel B depicts a 36-year-old female with Stanford type A aortic dissection extending into the supra-aortic trunks, highlighting the loss of elastic fiber continuity, cystic medial necrosis, vascular medial scarring, wall dissection, and focal thrombosis, stained with Weigert’s method for elastic fibers. Panel C presents findings from a 33-year-old male with DeBakey type II aortic dissection, showing extensive destruction of elastic fibers and cystic medial necrosis, stained with Masson’s trichrome.
Figure 1.
Histopathology of aortic aneurysms and dissections in three MFS patients. (A) A 28-year-old male with a giant ascending aorta aneurysm, showing rupture of elastic fibers in the adventitia with minimal inflammatory infiltrate hematoxylin–eosin. (B) A 36-year-old female with Stanford type A aortic dissection extending to the supra-aortic trunks, Weigert’s method. (C) A 33-year-old male with DeBakey type II aortic dissection, showing destruction of elastic fibers with cystic medial necrosis Masson’s trichrome staining.
2.3. FBN1, TGFβR1, TGβR2 and TGFβ2 mRNA Level Expression
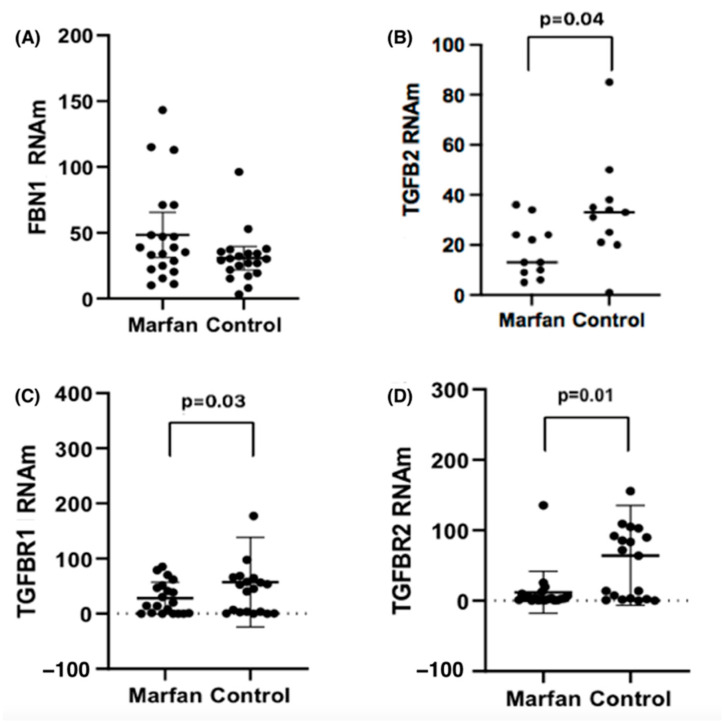
With the purpose of knowing the expression levels of the genes studied and knowing if there are differences between the values with respect to the patients considered as controls, the measurement was carried out in aortic tissues of our study population. Figure 2 shows the expression levels of mRNA for FBN1, TGFβR1, TGFβR2, and TGFβ2, evaluated in both groups, comparing individuals with Marfan syndrome to the control group. The mRNA levels were compared between controls and Marfan patients, both with and without aortic dilation, aneurysm, or dissection; see Table 6.
Figure 2.
Expression levels of (A) FBN1, (B) TGFβ2, (C) TGFβR1 and (D) TGβR2 mRNA, normalized with 18 s mRNA, in the study groups.
Table 6.
FBN1, TGFβR1, TGFβR2 and TGFβ2 levels between patients with Marfan syndrome and controls.
| With Dilatation Aneurysm | With Dissection | |||||
|---|---|---|---|---|---|---|
| mRNA | Controls n = 8 |
MFS n = 16 |
p1 | Controls n = 3 |
MFS n = 4 |
p2 |
| FBN1 | 33.04 (18.5–98.2) | 34.5 (11–143.2) | NS | 37.2 (25.1–79) | 79 (48.1–110.1) | 0.03 |
| TGFβR1 | 60.6 (0.57–177.2) | 35.3 (0.17–47.5) | NS | 52.5 (6.8–65.8) | 70.6 (30.1–111.1) | NS |
| TGFβR2 | 96.3 (0.05–282.02) | 4 (0.40–41.3) | 0.05 | 83.5 (71.8–135.9) | 5.4 (3.6–7.2) | NS |
| TGFβ2 | 31.20 (1.12–35) | 22.30 (5.60–36.86) | NS | 44.29 (25.85–85.36) | 12.17 (6.54–24.70) | 0.02 |
MFS = Marfan syndrome; p1, statistical significance of comparative group that had dilatation, MFS vs. controls; p2, statistical significance of group with dissection, MFS vs. controls. Data are expressed as median (min–max) and analyzed with the Mann–Whitney U test. NS: no significance. p < 0.05.
For FBN1, MFS patients with dissection (n = 4) had levels of 79 (48.1–110.1), compared to controls with dissection (n = 3), who had levels of 37.2 (25.1–79), with (p = 0.03). For TGFβR1 mRNA levels, we found no differences between MFS and controls who had dilation or dissection.
For TGFβR2 mRNA levels in patients with dilatation, we observed [Control 96.3 (0.05–282.02) vs. MFS [4 (0.40–41.3) p = 0.05]. In the case of the TGFβ2 mRNA in dissection, MFS patients had markedly lower levels of TGFβ2, with a median of 12.17 (6.54–24.70) vs. controls 44.28 (25.85–85.36), with (p = 0.02).
The results presented in Table 7 show the correlation between mRNA expression levels of FBN1, TGFβR1, TGFβR2, and TGFβ2 and different aortic segments for both Marfan patients and controls. Pearson correlation coefficients (r) and p-values are provided for each aortic segment, including the aortic root, sinus of Valsalva, sinotubular junction, and ascending aorta. In patients with Marfan syndrome, a positive correlation was observed between mRNA FBN1 expression and the diameter of the sinotubular junction (r = 0.42, p = 0.07), as well as between mRNA TGFβ2 expression and the diameter of the ascending aorta (r = 0.53, p = 0.01), suggesting a possible relationship between these molecular markers and aortic enlargement in these patients. In the control group, a positive and significant correlation was detected between mRNA TGFβ2 expression and the diameter of the aortic root (r = 0.62, p = 0.004), as well as between mRNA TGFβR1 expression and the diameter of the ascending aorta (r = 0.81, p = 0.001). Additionally, mRNA TGFβ2 expression showed a significant negative correlation with the diameter of the sinus of Valsalva (r = −0.46, p = 0.039).
Table 7.
Correlation between mRNA expression levels and different aortic segments in Marfan patients and controls.
| Aortic Segment Site | FBN1 | TGFβR1 | TGFβR2 | TGFβ2 | ||||
|---|---|---|---|---|---|---|---|---|
| Marfan | r | p | r | p | r | p | r | p |
| Aortic root | 0.02 | 0.92 | 0.02 | 0.92 | 0.11 | 0.64 | −0.03 | 0.87 |
| Sinus of Valsalva | 0.39 | 0.09 | 0.34 | 0.14 | 0.18 | 0.44 | 0.02 | 0.95 |
| Sinotubular junction | 0.42 | 0.07 | 0.22 | 0.35 | 0.02 | 0.90 | 0.44 | 0.05 |
| Ascending aorta | 0.07 | 0.74 | 0.42 | 0.07 | 0.14 | 0.55 | 0.53 | 0.01 |
| Control | r | p | r | p | r | p | r | p |
| Aortic root | −0.20 | 0.52 | 0.21 | 0.91 | 0.33 | 0.28 | 0.62 | 0.004 |
| Sinus of Valsalva | 0.36 | 1.0 | 0.21 | 0.50 | 0.38 | 0.22 | −0.46 | 0.039 |
| Sinotubular junction | 0.22 | 0.48 | 0.64 | 0.02 | 0.01 | 0.96 | 0.03 | 0.85 |
| Ascending aorta | 0.01 | 0.95 | 0.81 | 0.001 | 0.16 | 1.0 | 0.008 | 0.92 |
r: Pearson correlation coefficient; p: p-value.
To evaluate whether the presence of FBN1 variants influences the expression of genes related to the TGFβ2 pathway, patients with Marfan syndrome were stratified into two groups: those with identified FBN1 mutations (MFS-Variants) and those without genetic testing (MFS-TNP). Both groups were compared to healthy controls.
Table 8 show a significant reduction in TGFβR2 expression in MFS-Variants compared to controls [Control 67.86 (0.05–282.02) vs. MFS-Variants 2.03 (0.03–3.61), p = 0.02]. Similarly, MFS-TNP also exhibited lower TGFβR2 expression than controls [4.03 (0.34–25.62), p = 0.01]; however, no significant difference was observed between MFS-Variants and MFS-TNP (p = 0.16).
Table 8.
Differential expression of mRNA genes in Marfan syndrome patients with and without identified FBN1 variants.
| mRNA | Controls n = 20 |
MFS-Variants n = 5 |
MFS-TNP n = 15 |
p1 | p2 | p3 |
|---|---|---|---|---|---|---|
| FBN1 | 29.53 (3.20–96.20) | 33.86 (19–112.8) | 29.08 (1.43–143.2) | 0.27 | 0.75 | 0.45 |
| TGFβR1 | 48.82 (0.10–347.5) | 1.12 (0.25–61.79) | 17.30 (0.7–78.79) | 0.22 | 0.27 | 0.71 |
| TGFβR2 | 67.86 (0.05–282.02) | 2.03 (0.03–3.61) | 4.03 (0.34–25.62) | 0.02 | 0.01 | 0.16 |
| TGFβ2 | 33.68 (1.12–85.36) | 9.63 (5.60–13.67) | 22.30 (6.54–36.86) | 0.07 | 0.08 | 0.23 |
TNP = test not performed, p1 = Control vs. MFS-Variants, p2 = Control vs. MFS-TNP, p3 = MFS-Variants vs. MFS-TNP. Data are expressed as median (min–max) and analyzed with the Mann–Whitney U test. p < 0.05.
3. Discussion
For over two decades, research has shown that some patients who meet the Ghent criteria for Marfan syndrome but lack FBN1 pathogenic variants instead carry genetic variants in TGFβR1 or TGFβR2. This discovery revealed significant clinical overlap between type 1 and type 2 Marfan syndrome (MFS1 and MFS2) and Loeys–Dietz syndrome (LDS), emphasizing the need for precise genetic and clinical differentiation [16].
The pleiotropic nature of TGFβ signaling has led to divergent findings regarding its function. As part of a larger signaling superfamily, it includes 30 different ligands, 7 distinct type I receptors, 5 type II receptors, and 8 Smad proteins [17,18]. Consequently, TGFβ signaling is tightly regulated at multiple levels, including extracellular modulation [19,20], transcriptional initiation by coactivators, corepressors, and transcriptional terminators [21], as well as various feedback mechanisms and cross-talk pathways that either terminate or redirect the intracellular signal [22,23].
This study provides important insights into the dysregulation of the TGFβ signaling pathway in Marfan syndrome (MFS). We observed overexpression of FBN1 and underexpression of TGFβ2, TGFβR1, and TGFβR2 in MFS patients, aligning with previous findings suggesting TGFβ signaling dysfunction in the context of MFS [24]. This altered gene expression may contribute to progressive aortic dilation and the high risk of aneurysms and aortic dissection observed in these patients [25].
Our findings regarding FBN1 overexpression are consistent with the structural role of fibrillin in maintaining the integrity of the extracellular matrix (ECM) [26]. In MFS, pathogenic variants in FBN1 impair fibrillin function, leading to the structural abnormalities observed in the aorta [27,28]. The increased FBN1 expression we observed likely represents a compensatory response aimed at stabilizing the ECM to counteract these defects. However, despite this upregulation at the transcript level, the presence of pathogenic FBN1 variants suggests that this compensation is functionally insufficient. In our cohort, we identified different types of FBN1 variants, including stop-gain mutations (p.Gln1233Ter, p.Arg1541Ter) and non-synonymous (p.Gly753Val, p.Cys513Trp, p.Cys2378Tyr) SNVs which likely impact fibrillin-1 function and contribute to ECM instability [29]. Previous studies have proposed that defective fibrillin incorporation into microfibrils may lead to increased gene expression as an attempt to restore ECM integrity, yet this overproduction does not necessarily result in functional protein assembly [15]. An apparent contradiction in our findings is the expected upregulation of TGFβ2, TGFβR1, and TGFβR2 signaling in MFS, while FBN1 is downregulated at the mRNA level. This paradox may stem from a failed feedback mechanism, where cells attempt to compensate for ECM dysfunction and increased mechanical stress by modulating gene expression [30]. This interplay between FBN1 deficiency and dysregulated TGFβ2 signaling may be a crucial factor in the vascular complications observed in MFS. While tissue inflammation and mechanical stress are known to activate TGFβ2 release from the ECM, the transcriptional suppression of key pathway components in our study suggests a disruption in intracellular regulatory mechanisms. This impairment may exacerbate the progression of aortic dilation, as TGFβ2 signaling plays a crucial role in ECM remodeling and vascular homeostasis [31].
Histopathological studies of aortas in MFS patients show significant inflammatory cellular infiltration, which can promote the release of pro-inflammatory cytokines that interfere with TGFβ signaling. The downregulation of TGFβ2 observed in our study suggests that chronic inflammation in MFS may lead to a paradoxical suppression of key TGFβ signaling components, disrupting normal feedback loops [32]. The inflammatory infiltrate, including macrophages, T cells, and neutrophils, contributes to matrix degradation through the secretion of matrix metalloproteinases (MMPs), leading to further disruption of the ECM and abnormal TGFβ activation [33,34]. In particular, macrophages can release TGFβ2 trapped in the ECM, exacerbating aortic damage and initiating a cycle of inflammation and tissue degradation [35,36].
Previous studies have shown that mechanical stress from aortic dilation in MFS [37] can activate TGFβ signaling by releasing latent TGFβ2 bound to the extracellular matrix (ECM), which is then activated through mechanoreceptors [38]. However, in MFS, where the aortic wall is already compromised, this activation may be insufficient or dysregulated, failing to maintain structural integrity and instead contributing to aneurysm and dissection development. Consistent with these findings, research in murine models deficient in TGFβ2 receptors has demonstrated increased susceptibility to aortic complications, reinforcing the essential role of TGFβ in preserving aortic stability [39] and structural integrity [40,41,42]. These models also exhibit dysregulated ECM-related gene expression following TGFβ signaling disruption [43,44], further promoting aneurysm formation [45].
Our findings align with this evidence but reveal a key distinction; while TGFβ2 signaling is often reported as upregulated at the extracellular level in various pathological conditions, we observed a significant downregulation of TGFβ2 expression in the aortic tissue of MFS patients [46,47,48]. This suggests that mechanical stress, rather than consistently amplifying TGFβ signaling, may instead impair its intracellular regulation, disrupting normal mechanobiological responses and contributing to disease progression [49].
An important aspect of this study is the relationship between the expression levels of the evaluated mRNAs and the dilation of different aortic regions. In patients with Marfan syndrome (MFS), a positive correlation was observed between FBN1 expression and the diameter of the sinotubular junction [50]. Similarly, a positive correlation was found between TGFβ2 levels and the diameter of the ascending aorta, suggesting that TGFβ2-mediated signaling may play a role in the mechanisms leading to aortic dilation. It is possible that TGFβ2 expression is altered as part of the cellular response to biomechanical stress on the aortic wall caused by dilation [51].
These findings align with studies showing the involvement of TGFβ in extracellular matrix remodeling and the fibrotic response in affected vascular tissues [52,53]. The increased FBN1 levels observed in MFS may represent a compensatory response to ECM dysfunction, yet this compensation appears to be ineffective due to the presence of pathogenic variants [54].
Clinical manifestations of MFS vary depending on disease severity, with molecular alterations in key pathways influencing these manifestations. Elevated levels of active TGFβ are a prominent feature in this dysfunction, leading to reduced aortic wall distensibility and contributing to aneurysmal dilation [55]. This increases the risk of severe vascular complications.
The classic aortic aneurysm associated with Marfan syndrome, known as “annuloaortic ectasia”, involves dilation of the aortic ring and the sinuses of Valsalva. If not treated promptly, this condition can lead to aortic insufficiency, dissection, and congestive heart failure. As aortic dilation progresses, the risk of rupture and dissection increases. In MFS, the probability of dying from aortic disease is 200 times higher than in the general population [56].
In addition to aortic damage, patients may also have valve pathology, including a bicuspid aortic valve, which increases the risk of both aortic and cardiac valve dysfunction. Biomechanical studies have shown greater stiffness in the aortic tissue, which can contribute to early dilation and dissection. In adolescents and young adults, aortic valve regurgitation and aortic root disease are the most relevant cardiac pathologies, though mitral valve prolapse and regurgitation may also occur during aortic damage [57].
Given the complex pathophysiology at the cardiovascular level, it is crucial to analyze these intertwined damage mechanisms in detail to identify potential sites for therapeutic intervention. Our findings suggest that Marfan syndrome involves a maladaptive feedback loop, where compensatory mechanisms at the transcriptional level fail to counteract ECM dysfunction. This highlights the need for targeted therapies that can restore proper TGFβ signaling regulation and prevent progressive vascular damage.
4. Materials and Methods
4.1. Patient Population
A prospective comparative study was conducted between cases with MFS with aortic or aortic dissection and control subjects without MFS at the Ignacio Chavez National Institute of Cardiology between March 2014 and March 2023, including adults over 18 years of age of either sex who agreed to participate in the study. Twenty patients with MFS were included. In addition to the assessment consultation, conducted in the cardiology outpatient clinic, they were evaluated by a geneticist and were also re-evaluated by a rheumatology specialist expert in the subject, and the MFS was classified based on the 2010 Ghent criteria [17], which require more than two criteria for classification. These criteria are (1) a positive family history of Marfan syndrome (FH), (2) aortic dilatation and/or dissection (AoD), (3) ectopia lentis (EL), (4) a systemic score (SS) greater than 7/20, and/or (5) a mutation in the FBN1 gene demonstrated by genetic testing. Fulfillment of any two criteria strongly suggests the syndrome. Echocardiogram, computed tomography or magnetic resonance imaging was performed to evaluate aortic enlargement or dissection, mitral and aortic valve prolapse, and tricuspid valve prolapse. Control subjects included (C) had aortic stenosis and Tri leaflet valve damage and did not have any syndromic or autoimmune pathology. The aortic dilatation that the controls presented is secondary to valvular dysfunction. The controls, like the patients with Marfan syndrome, required valve replacement surgery and aortic reconstruction. All patients and controls who presented aortic dilatation went to a medical–surgical session (Heart team) to standardize the requirements of the type of aortic surgery to be considered, according to the pathology of each patient. They were treated at the National Institute of Cardiology “Ignacio Chávez”. The criteria for exclusion were doubtful diagnosis and/or lack of agreement to sign the informed consent for the research study. The elimination criteria were considered as insufficient tissue sample taken at the time of surgery even when the patients met the inclusion criteria or inadequate conditions for obtaining the sample considering the requirements for the research process.
Mexican patients were defined as individuals with at least three generations born in Mexico.
Control subjects were 20 randomly selected subjects who underwent thoracic surgery for aortic stenosis and aortic dilatation.
4.2. Surgical Procedure
Patients were considered for surgery if they exhibited an aortic dissection or an aortic dilation of 4.5 cm or larger, confirmed by MRI or CT scans. An interdisciplinary team of cardiac specialists assessed the aortic and valvular conditions to choose the most appropriate surgical method. The procedures used included the Bentall and de Bono techniques, as well as the David-5 procedure, selected according to the specific needs and complexities of each case. The Bentall procedure involves replacing the aortic root and ascending aorta with a Dacron graft, to which both coronary arteries are attached, and one end is fitted with a valve prosthesis. On the other hand, the David Type 5 technique retains the patient’s own aortic valve and commissures, which are then reimplanted within the Dacron graft.
4.3. Blood Samples
Five milliliters of venous blood were collected in tubes with EDTA and without anticoagulant after 12 h of fasting. Plasma was separated by centrifugation for determination of the lipid profile.
4.4. Laboratory Analysis
Total cholesterol, triglycerides, and glucose were determined on a Hitachi 902 autoanalyzer (Böehringer Mannheim, Baden-Württemberg, Germany) using commercial enzymatic kits (Roche Diagnostics, Mannheim, Germany and WakoChemicals, Richmond, VA, USA). HDL-C was determined by a homogeneous enzymatic method (Roche Diagnostics, Mannheim, Germany).
4.5. Aortic Samples
Aortic tissues (2 mm) were collected from MFS patients and placed in sterile containers with RNA later (Quiagen, Hilden, Germany), which stabilizes and protects the tissues during processing and storage (−80 °C) prior to its processing.
4.6. Histology
A segment of 2 mm from the aortic tissues was processed according to conventional histological techniques and stained with hematoxylin and eosin (HE) staining, Weigert’s staining for elastic fibers, and Masson’s trichrome staining. Histological sections were acquired according to the method and equipment described by Zúñiga-Muñoz [58] and examined at 40× magnification.
4.7. Sample Pulverization
Frozen samples were pulverized using liquid nitrogen to maintain RNA integrity and prevent degradation. This process was carried out in a precooled mortar, pulverizing until a fine powder was obtained. The tissue was resuspended in a Tripure solution (a phenol and guanidine thiocyanate-based reagent) for total RNA extraction. The resuspension was performed following the manufacturer’s instructions for Tripure™ (Roche Molecular Biochemicals Welwyn Garden City, Hertfordshire, UK), ensuring proper tissue homogenization. The samples resuspended in Tripure were stored at −80 degrees Celsius until further processing. This storage ensures RNA stability and prevents its degradation.
4.8. mRNA Extraction and Quantification by RT-qPCR
mRNA was extracted using the TriPure Isolation Reagent technique (Roche Molecular Biochemicals, Welwyn Garden City, Hertfordshire, UK). RT-qPCR was then performed, which started with 1 μg of total RNA for cDNA synthesis according to the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster, CA, USA). mRNA was quantified using a Bio-Rad CFX96 Real-Time System (Bio-Rad, Hercules, CA, USA). Expression levels of human FBN1 (Hs00171191_m1), TGFβR1 (Hs00610320_m1), TGFβR2 (Hs00234253_m1), TGFβ2 (Hs00234244_m1) and 18s rRNA (eukaryotic endogenous control 4319413E) (reference gene) were measured using a commercially available kit (TaqMan Gene Expression Assay, Applied Biosystems). Amplifications were performed by starting with a 10 min template denaturation step at 95 °C, followed by 40 cycles at 95 °C for 15 s and 60 °C for 1 min. All analyses were performed in duplicate. Data were expressed relative to each control value, and relative quantification was carried out using the formula 2−ΔΔC [59].
4.9. Statistical Analysis
Data were analyzed using SPSS version 25 (SPSS Inc., Chicago, IL, USA). The risk factors were dichotomized in the form of presence or absence of clinical criteria. A descriptive analysis was conducted for all variables, with results expressed as mean ± standard deviation. The comparison between groups was made using the Student’s t-test for continuous variables. For variables that did not have a normal distribution, the Mann–Whitney U test or the Kruskal–Wallis test was used when we evaluated more groups.
4.10. Ethical Statement
Signed informed consent forms were obtained from each participant after fully explaining the purpose and nature of all procedures used in the study under the Declaration of Helsinki (10). The research was approved by the Ethical, Biosecurity, and Investigation Committees of the National Institute of Cardiology (registration number INCICh 23-1366).
4.11. Data Availability
Due to confidentiality agreements, the data underlying this study are not publicly available. Access to the data can be requested through c_huesca@yahoo.com following their confidentiality protocols.
5. Conclusions
Aortic dilatation in patients with Marfan syndrome is associated with key molecular changes, including decreased levels of TGFβ2 and its receptors, TGFβR1 and TGFβR2. On the other hand, aortic tissue damage is characterized by cystic necrosis and loss of smooth muscle cells, which may be the result of mechanical stress, proteolysis, inflammation and deregulation of oxidative stress, affecting various signaling pathways. Elevated levels of FBN1 mRNA may reflect a compensatory response to this set of mechanisms involved in vascular damage. These findings regarding TGFβ and FBN1 mRNA in tissue support the continuation of molecular analysis in the serum of these patients, to better understand their clinical implications and potential therapeutic approaches.
6. Limitations
One limitation of this study is the small sample size, a common challenge in rare diseases. In MFS, cardiovascular complications are the primary life-threatening risk. As a national reference center, our institute treats most patients with connective tissue disorders. However, some cases are initially identified in ophthalmology clinics due to lens dislocation. These clinics often refer patients to our institute for a comprehensive evaluation. Similarly, pediatric centers transfer patients as they transition into adulthood, particularly when aortic complications arise. Given the incidence of MFS, the number of samples analyzed in this study is substantial. A more comprehensive understanding of the disease would require detailed genetic characterization beyond FBN1, including TGFB2, its receptors (TGFBR1, TGFBR2), and other genes involved in Marfan syndrome and related disorders. Expanding the cohort would necessitate multicenter studies with institutions in other countries that serve similar patient populations. This collaboration would allow for a deeper exploration of how specific genetic variants influence gene expression, signaling pathways, and disease progression. A key strength of this study is that no other national center processes as many samples, and all procedures are performed by expert surgeons.
Acknowledgments
The authors extend their deepest gratitude to all the patients who participated in this research protocol. Your collaboration and trust have been essential in advancing medical knowledge.
Author Contributions
Conception and design of the research: M.E.S. and M.R.-B.; acquisition of data: M.E.S., M.R.-B., I.P.-T., V.H.-A. and H.M.-H.; participation in patient enrolment, procedures, and sample collection: M.E.S., M.R.-B., I.H., L.M.-P., R.G., G.F.-A., V.C.-T. and B.A.P.-O.; E.A.-L. and C.H.-G. statistical analysis and interpretation of data: M.E.S., M.R.-B. and G.F.-A.; writing of the manuscript: M.E.S. and G.F.-A.; critical revision of the manuscript for intellectual content: M.E.S., M.R.-B. and G.F.-A. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki; the research was approved by the Ethical, Biosecurity, and Investigation Committees of the National Institute of Cardiology (registration number INCICh 23-1366).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Due to confidentiality agreements, the data underlying this study are not publicly available. Access to the data can be requested through c_huesca@yahoo.com following their confidentiality protocols.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received supported by Instituto Nacional de Cardiologia Ignacio Chavez to pay for the open-access manuscript.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Dietz H.C., Cutting C.R., Pyeritz R.E., Maslen C.L., Sakai L.Y., Corson G.M., Puffenberger E.G., Hamosh A., Nanthakumar E.J., Curristin S.M., et al. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature. 1991;352:337–339. doi: 10.1038/352337a0. [DOI] [PubMed] [Google Scholar]
- 2.Faivre L.C.B.G., Collod-Beroud G., Loeys B.L., Child A., Binquet C., Gautier E., Callewaert B., Arbustini E., Mayer K., Arslan-Kirchner M., et al. Effect of Mutation Type and Location on Clinical Outcome in 1,013 Probands with Marfan Syndrome or Related Phenotypes and FBN1 Mutations: An International Study. Am. J. Hum. Genet. 2007;81:454–466. doi: 10.1086/520125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neptune E.R., Frischmeyer P.A., Arking D.E., Myers L., Bunton T.E., Gayraud B., Ramirez F., Sakai L.Y., Dietz H.C. Dysregulation of TGF-β activation contributes to pathogenesis in Marfan syndrome. Nat. Genet. 2003;33:407–411. doi: 10.1038/ng1116. [DOI] [PubMed] [Google Scholar]
- 4.Loeys B.L., Chen J., Neptune E.R., Judge D.P., Podowski M., Holm T., Meyers J., Leitch C.C., Katsanis N., Sharifi N., et al. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat. Genet. 2005;37:275–281. doi: 10.1038/ng1511. [DOI] [PubMed] [Google Scholar]
- 5.Van Hemelrijk C., Renard M., Loeys B. The Loeys–Dietz syndrome: An update for the clinician. Curr. Opin. Cardiol. 2010;25:546–551. doi: 10.1097/HCO.0b013e32833f0220. [DOI] [PubMed] [Google Scholar]
- 6.Takeda N., Hara H., Fujiwara T., Kanaya T., Maemura S., Komuro I. TGF-β Signaling-Related Genes and Thoracic Aortic Aneurysms and Dissections. Int. J. Mol. Sci. 2018;19:2125. doi: 10.3390/ijms19072125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mizuguchi T., Collod-Beroud G., Akiyama T., Abifadel M., Harada N., Morisaki T., Allard D., Varret M., Claustres M., Morisaki H., et al. Heterozygous TGFBR2 mutations in Marfan syndrome. Nat. Genet. 2004;36:855–860. doi: 10.1038/ng1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuentevilla-Álvarez G., Soto M.E., Torres-Paz Y.E., Meza-Toledo S.E., Vargas-Alarcón G., González-Moyotl N., Pérez-Torres I., Manzano-Pech L., Mejia A.M., Huesca-Gómez C., et al. The usefulness of the genetic panel in the classification and refinement of diagnostic accuracy of Mexican patients with Marfan syndrome and other connective tissue disorders. Biomol. Biomed. 2024;24:302–314. doi: 10.17305/BB.2023.9578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao X., Chen X., Cottonham C., Xu L. Preferential Utilization of Imp7/8 in Nuclear Import of Smads. J. Biol. Chem. 2008;283:22867–22874. doi: 10.1074/jbc.M801320200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinard A., Jones G.T., Milewicz D.M. Genetics of Thoracic and Abdominal Aortic Diseases. Circ. Res. 2019;124:588–606. doi: 10.1161/CIRCRESAHA.118.312436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi Y., Massagué J. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/S0092-8674(03)00432-X. [DOI] [PubMed] [Google Scholar]
- 12.Asano K., Cantalupo A., Sedes L., Ramirez F. Pathophysiology and Therapeutics of Thoracic Aortic Aneurysm in Marfan Syndrome. Biomolecules. 2022;12:128. doi: 10.3390/biom12010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iakoubova O.A., Tong C.H., Rowland C.M., Luke M.M., Garcia V.E., Catanese J.J., Moomiaie R.M., Sotonyi P., Ascady G., Nikas D., et al. Genetic variants in FBN-1 and risk for thoracic aortic aneurysm and dissection. PLoS ONE. 2014;9:e91437. doi: 10.1371/JOURNAL.PONE.0091437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y.E. Non-Smad Signaling Pathways of the TGF-β Family. Cold Spring Harb. Perspect. Biol. 2017;9:a022129. doi: 10.1101/CSHPERSPECT.A022129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones J.A., Spinale F.G., Ikonomidis J.S. Transforming growth factor-beta signaling in thoracic aortic aneurysm development: A paradox in pathogenesis. J. Vasc. Res. 2009;46:119–137. doi: 10.1159/000151766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh K.K., Rommel K., Mishra A., Karck M., Haverich A., Schmidtke J., Arslan-Kirchner M. TGFBR1 and TGFBR2 mutations in patients with features of Marfan syndrome and Loeys-Dietz syndrome. Hum. Mutat. 2006;27:770–777. doi: 10.1002/HUMU.20354. [DOI] [PubMed] [Google Scholar]
- 17.Feng X.H., Derynck R. Specificity and versatility in tgf-beta signaling through Smads. Annu. Rev. Cell Dev. Biol. 2005;21:659–693. doi: 10.1146/ANNUREV.CELLBIO.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- 18.Suszko M.I., Woodruff T.K. Cell-specificity of transforming growth factor-beta response is dictated by receptor bioavailability. J. Mol. Endocrinol. 2006;36:591–600. doi: 10.1677/JME.1.01936. [DOI] [PubMed] [Google Scholar]
- 19.López-Casillas F., Payne H.M., Andres J.L., Massagué J. Betaglycan can act as a dual modulator of TGF-beta access to signaling receptors: Mapping of ligand binding and GAG attachment sites. J. Cell Biol. 1994;124:557–568. doi: 10.1083/JCB.124.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X.F., Lin H.Y., Ng-Eaton E., Downward J., Lodish H.F., Weinberg R.A. Expression cloning and characterization of the TGF-beta type III receptor. Cell. 1991;67:797–805. doi: 10.1016/0092-8674(91)90074-9. [DOI] [PubMed] [Google Scholar]
- 21.Xu L. Regulation of Smad activities. Biochim. Biophys. Acta. 2006;1759:503–513. doi: 10.1016/J.BBAEXP.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Massagué J. How cells read TGF-beta signals. Nat. Rev. Mol. Cell Biol. 2000;1:169–178. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- 23.Moustakas A., Heldin C.H. Non-Smad TGF-beta signals. J. Cell Sci. 2005;118:3573–3584. doi: 10.1242/JCS.02554. [DOI] [PubMed] [Google Scholar]
- 24.Gomez D., Al Haj Zen A., Borges L.F., Philippe M., Gutierrez P.S., Jondeau G., Michel J.B., Vranckx R. Syndromic and non-syndromic aneurysms of the human ascending aorta share activation of the Smad2 pathway. J. Pathol. 2009;218:131–142. doi: 10.1002/PATH.2516. [DOI] [PubMed] [Google Scholar]
- 25.Holm T.M., Habashi J.P., Doyle J.J., Bedja D., Chen Y., Van Erp C., Lindsay M.E., Kim D., Schoenhoff F., Cohn R.D., et al. Noncanonical TGFβ signaling contributes to aortic aneurysm progression in Marfan syndrome mice. Science. 2011;332:358–361. doi: 10.1126/SCIENCE.1192149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coelho S.G., Almeida A.G. Marfan syndrome revisited: From genetics to the clinic. Rev. Port. Cardiol. 2020;39:215–226. doi: 10.1016/J.REPC.2019.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Cohn R.D., Van Erp C., Habashi J.P., Soleimani A.A., Klein E.C., Lisi M.T., Gamradt M., Ap Rhys C.M., Holm T.M., Loeys B.L., et al. Angiotensin II type 1 receptor blockade attenuates TGF-beta-induced failure of muscle regeneration in multiple myopathic states. Nat. Med. 2007;13:204–210. doi: 10.1038/NM1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Honjo Y., Bian Y., Kawakam K., Molinolo A., Longenecker G., Boppana R., Larsson J., Karlsson S., Gutkind J.S., Puri R.K., et al. TGF-beta receptor I conditional knockout mice develop spontaneous squamous cell carcinoma. Cell Cycle. 2007;6:1360–1366. doi: 10.4161/CC.6.11.4268. [DOI] [PubMed] [Google Scholar]
- 29.Li L., Huang J., Liu Y. The extracellular matrix glycoprotein fibrillin-1 in health and disease. Front. Cell Dev. Biol. 2024;11:1302285. doi: 10.3389/FCELL.2023.1302285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Granata A., Serrano F., Bernard W.G., McNamara M., Low L., Sastry P., Sinha S. An iPSC-derived vascular model of Marfan syndrome identifies key mediators of smooth muscle cell death. Nat. Genet. 2017;49:97–109. doi: 10.1038/NG.3723. [DOI] [PubMed] [Google Scholar]
- 31.Stark V.C., Olfe J., Diaz-Gil D., von Kodolitsch Y., Kozlik-Feldmann R., Reincke J., Stark M., Wiegand P., Zeller T., Mir T.S. TGFβ level in healthy and children with Marfan syndrome—Effective reduction under sartan therapy. Front. Pediatr. 2024;12:1276215. doi: 10.3389/fped.2024.1276215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Türk U.O., Alioğlu E., Nalbantgil S., Nart D. Catastrophic cardiovascular consequences of weight lifting in a family with Marfan syndrome. Turk. Kardiyol. Dern. Ars. 2008;36:32–34. [PubMed] [Google Scholar]
- 33.Seim B.E., Holt M.F., Ratajska A., Michelsen A., Ringseth M.M., Halvorsen B.E., Skjelland M., Kvitting J.P.E., Lundblad R., Krohg-Sørensen K., et al. Markers of extracellular matrix remodeling and systemic inflammation in patients with heritable thoracic aortic diseases. Front. Cardiovasc. Med. 2022;9:1073069. doi: 10.3389/fcvm.2022.1073069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Renard M., Francis C., Ghosh R., Scott A.F., Witmer P.D., Adès L.C., Andelfinger G.U., Arnaud P., Boileau C., Callewaert B.L., et al. Clinical Validity of Genes for Heritable Thoracic Aortic Aneurysm and Dissection. J. Am. Coll. Cardiol. 2018;72:605–615. doi: 10.1016/j.jacc.2018.04.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klopf J., Brostjan C., Neumayer C., Eilenberg W. Neutrophils as regulators and biomarkers of cardiovascular inflammation in the context of abdominal aortic aneurysms. Biomedicines. 2021;9:1236. doi: 10.3390/biomedicines9091236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plana E., Oto J., Medina P., Fernández-Pardo Á., Miralles M. Novel contributions of neutrophils in the pathogenesis of abdominal aortic aneurysm, the role of neutrophil extracellular traps: A systematic review. Thromb. Res. 2020;194:200–208. doi: 10.1016/j.thromres.2020.07.039. [DOI] [PubMed] [Google Scholar]
- 37.Quarto N., Li S., Renda A., Longaker M.T. Exogenous activation of BMP-2 signaling overcomes TGFβ-mediated inhibition of osteogenesis in Marfan embryonic stem cells and Marfan patient-specific induced pluripotent stem cells. Stem Cells. 2012;30:2709–2719. doi: 10.1002/stem.1250. [DOI] [PubMed] [Google Scholar]
- 38.Ikonomidis J.S., Jones J.A., Barbour J.R., Stroud R.E., Clark L.L., Kaplan B.S., Zeeshan A., Bavaria J.E., Gorman J.H., III, Spinale F.G., et al. Expression of matrix metalloproteinases and endogenous inhibitors within ascending aortic aneurysms of patients with Marfan syndrome. Circulation. 2006;114:I-365–I-370. doi: 10.1161/CIRCULATIONAHA.105.000810. [DOI] [PubMed] [Google Scholar]
- 39.Chung A.W.Y., Au Yeung K., Sandor G.G.S., Judge D.P., Dietz H.C., Van Breemen C. Loss of elastic fiber integrity and reduction of vascular smooth muscle contraction resulting from the upregulated activities of matrix metalloproteinase-2 and -9 in the thoracic aortic aneurysm in Marfan syndrome. Circ. Res. 2007;101:512–522. doi: 10.1161/CIRCRESAHA.107.157776. [DOI] [PubMed] [Google Scholar]
- 40.Levéen P., Larsson J., Ehinger M., Cilio C.M., Sundler M., Sjöstrand L.J., Holmdahl R., Karlsson S. Induced disruption of the transforming growth factor beta type II receptor gene in mice causes a lethal inflammatory disorder that is transplantable. Blood. 2002;100:560–568. doi: 10.1182/blood.V100.2.560. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y., Panicker I.S., Anesi J., Sargisson O., Atchison B., Habenicht A.J.R. Animal Models, Pathogenesis, and Potential Treatment of Thoracic Aortic Aneurysm. Int. J. Mol. Sci. 2024;25:901. doi: 10.3390/ijms25020901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei H., Hu J.H., Angelov S.N., Fox K., Yan J., Enstrom R., Smith A., Dichek D.A. Aortopathy in a Mouse Model of Marfan Syndrome Is Not Mediated by Altered Transforming Growth Factor β Signaling. J. Am. Heart Assoc. 2017;6:e004968. doi: 10.1161/JAHA.116.004968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Angelov S.N., Zhu J., D’Ichek D.A. New mouse model of abdominal aortic aneurysm put out to expand. Arterioscler. Thromb. Vasc. Biol. 2017;37:1990–1993. doi: 10.1161/ATVBAHA.117.310177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lareyre F., Clément M., Raffort J., Pohlod S., Patel M., Esposito B., Master L., Finigan A., Vandestienne M., Stergiopulos N., et al. TGFβ (transforming growth factor-β) blockade induces a human-like disease in a nondissecting mouse model of abdominal aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 2017;37:2171–2181. doi: 10.1161/ATVBAHA.117.309999. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y., Ait-Oufella H., Herbin O., Bonnin P., Ramkhelawon B., Taleb S., Huang J., Offenstadt G., Combadière C., Rénia L., et al. TGF-beta activity protects against inflammatory aortic aneurysm progression and complications in angiotensin II-infused mice. J. Clin. Investig. 2010;120:422–432. doi: 10.1172/JCI38136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodríguez J.F., Ruiz C., Doblaré M., Holzapfel G.A. Mechanical stresses in abdominal aortic aneurysms: Influence of diameter, asymmetry, and material anisotropy. J. Biomech. Eng. 2008;130:021023. doi: 10.1115/1.2898830. [DOI] [PubMed] [Google Scholar]
- 47.Vorp D.A., Vande Geest J.P. Biomechanical determinants of abdominal aortic aneurysm rupture. Arterioscler. Thromb. Vasc. Biol. 2005;25:1558–1566. doi: 10.1161/01.ATV.0000174129.77391.55. [DOI] [PubMed] [Google Scholar]
- 48.Haller S.J., Azarbal A.F., Rugonyi S. Predictors of Abdominal Aortic Aneurysm Risks. Bioengineering. 2020;7:79. doi: 10.3390/bioengineering7030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goumans M.J., ten Dijke P. TGF-β Signaling in Control of Cardiovascular Function. Cold Spring Harb. Perspect. Biol. 2018;10:a022210. doi: 10.1101/cshperspect.a022210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sakai L.Y., Keene D.R., Engvall E. Fibrillin, a new 350-kD glycoprotein, is a component of extracellular microfibrils. J. Cell Biol. 1986;103:2499–2509. doi: 10.1083/jcb.103.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Habashi J.P., Judge D.P., Holm T.M., Cohn R.D., Loeys B.L., Cooper T.K., Myers L., Klein E.C., Liu G., Calvi C., et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312:117–121. doi: 10.1126/SCIENCE.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gallo E.M., Loch D.C., Habashi J.P., Calderon J.F., Chen Y., Bedja D., Van Erp C., Gerber E.E., Parker S.J., Sauls K., et al. Angiotensin II-dependent TGF-β signaling contributes to Loeys-Dietz syndrome vascular pathogenesis. J. Clin. Investig. 2014;124:448–460. doi: 10.1172/JCI69666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hasham S.N., Guo D.C., Milewicz D.M. Genetic basis of thoracic aortic aneurysms and dissections. Curr. Opin. Cardiol. 2002;17:677–683. doi: 10.1097/00001573-200211000-00015. [DOI] [PubMed] [Google Scholar]
- 54.Ritelli M., Colombi M. Molecular Genetics and Pathogenesis of Ehlers-Danlos Syndrome and Related Connective Tissue Disorders. Genes. 2020;11:547. doi: 10.3390/genes11050547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Braga M., Bhasin S., Jasuja R., Pervin S., Singh R. Testosterone inhibits transforming growth factor-β signaling during myogenic differentiation and proliferation of mouse satellite cells: Potential role of follistatin in mediating testosterone action. Mol. Cell. Endocrinol. 2012;350:39–52. doi: 10.1016/j.mce.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Growth K.A., Stochholm K., Hove H., Andersen N.H., Gravholt C.H. Causes of Mortality in the Marfan Syndrome (from a Nationwide Register Study) Am. J. Cardiol. 2018;122:1231–1235. doi: 10.1016/j.amjcard.2018.06.034. [DOI] [PubMed] [Google Scholar]
- 57.Yan J., Lehsau A.C., Sauer B., Pieper B., Mohamed S.A., Members M.L.C., Loeys B.L., Dietz H.C., Van Laer L., McCallion A.S., et al. Comparison of biomechanical properties in ascending aortic aneurysms of patients with congenital bicuspid aortic valve and Marfan syndrome. Int. J. Cardiol. 2019;278:65–69. doi: 10.1016/J.IJCARD.2018.11.102. [DOI] [PubMed] [Google Scholar]
- 58.Zúñiga-Muñoz A.M., Pérez-Torres I., Guarner-Lans V., Núñez-Garrido E., Espejel R.V., Huesca-Gómez C., Gamboa-Ávila R., Soto M.E. Glutathione system participation in thoracic aneurysms from patients with Marfan syndrome. Vasa. 2017;46:177–186. doi: 10.1024/0301-1526/a000609. [DOI] [PubMed] [Google Scholar]
- 59.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/METH.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Due to confidentiality agreements, the data underlying this study are not publicly available. Access to the data can be requested through c_huesca@yahoo.com following their confidentiality protocols.