Abstract
Mannheimia Haemolytica (M. haemolytica) is an organism causing pneumonia in ruminants. M. haemolytica causes severe economic losses to the global livestock industry. Several diagnostic methods have been developed, including bacterial culture, bacterial DNA detection and serological assays. Diagnosis of M. haemolytica is based on the bacteriological methods such as isolation of the microorganisms from clinical samples. Available methods are time consuming and not easy to perform. Serological tests based on recombinant proteins may provide higher sensitivity and specificity than culture tests. There is a need for new diagnostic methods to detect M. haemolytica -specific antibodies. In this study, a latex agglutination test (LAT) was developed based on recombinant outer membrane pasteurella lipoprotein E (rPlpE) for detecting specific antibodies against M. haemolytica. Expressed recombinant PlpE was coated with latex particles for a latex agglutination test. The recombinant PlpE was able to detect anti-M. haemolytica IgG in positive sera, but showed no immunoreaction with Pasteurella multocida and negative samples. These results suggest that the rPlpE can be used to detect the specific anti Mannheimia Haemolytica - IgG Antibodies. Because the recombinant proteins can be produced efficiently and are inexpensively, their use in diagnostic kits such as LATs as reagents can reduce the cost of them. This rapid and specific anti M. haemolytica antibody detection method using recombinant proteins can save cost and be widely applied for efficient and practical detection of. M. haemolytica.
Keywords: Antibody, Latex agglutination test, Mannheimia Haemolytica, PlpE, Recombinant protein
1. Introduction
M. haemolytica is a gram-negative bacterium that causes pneumonia, mastitis and septicemia in cattle and small ruminants ( 1 ). Therefore, fast detection of M. haemolytica is critical. e Several diagnostic methods have been developed, including bacterial culture, bacterial DNA detection and serological assays. Conventional M. haemolytica identification methods are based on evaluation of bacterial growth evaluation under optimal conditions. The culture-based method takes days to detect M. haemolytica. The aim is to achieve fast and accurate diagnosis in clinical practice. Since PCR requires less time, it is considered as a valuable technique to identify M. haemolytica in clinical specimens. Several serological tests have been evaluated and used worldwide such, as the tube agglutination test, indirect hemagglutination, dot immunoblotting and ELISA. The latex agglutination test (LAT), as a simpleand fast method, has been widely used for testing of various clinical samples from medicine and veterinary medicine ( 2 - 7 ). M. haemolytica outer membrane proteins (OMPs) have the ability to induce high antibody responses ( 8 ). There are several candidate M. haemolytica proteins as immunogens, such as outer membrane pasteurella lipoprotein E (PlpE), which is found in all serotypes of M. haemolytica ( 9 ). Recombinant antigens have been developed to perform serological studies in using different outer membrane proteins. There is a need for new diagnostic methods for specific and rapid detection of M. haemolytica specific antibodies. In this study, we aimed to generate recombinant PlpE as an antigen to describe its application as an Indigenous latex agglutination assay reagent for the detection of anti- M. haemolytica antibodies.
2. Materials and Methods
2.1. Bacterial isolation
Pneumonic lungs of sheep were collected from slaughterhouse. Lung tissues were examined and sampled.Colonies on blood agar was examined for the presence of hemolysis. Identification of the M. haemolytica was based on biochemical tests ( 1 ).
2.2. Gene Construction of Recombinant Protein
Bacterial DNA was extracted from colonies by the boiling method. The PCR amplification of the Lkt gene with primers (F) GCAGGAGGTGATTATTAAAGTGG and (R) CAGCAGTTATTGTCATACCTGAAC and HP gene with primers (F) CGAGCAAGCACAATTACATTATGC and (R) CACCGTCAAATTCCTGTGGATAAC were performed with PCR master mix (Takara, Japan) and bacterial DNA per reaction. The reaction mixture solution for PCR was prepared using 12.5 μl Mastermix, 0.5 µl each of M. haemolytica primers, 0.2 µl of Taq DNA polymerase, 6.3 μl H2O, and 4 μl of DNA template to give a final volume of 25 μl. PCR thermal cycling conditions consisted of an initial denaturation at 95°C for 5 min for one cycle followed by 30 cycles of denaturation at 94°C for 60s, annealing at 58°C for 45 s, and extension at 72°C for 60 s. A final extension step was performed at 72°C for 10 min (one cycle). The PlpE gene was amplified from the genomic DNA of M. haemolytica using the following pair of oligonucleotide primers: CTCTAATTAGAATTCCGGAGGAAGCGGTAGCGG and GCCGGCCCTCGAGTTTTTTCTCGCTAACCATTAT. The amplified DNA fragment was cloned into the pET26b (+) vector to obtain His-tagged protein (pET26b-plpE) (Novagen, USA) ( 10 ).
2.3. Production of the Recombinant Protein
The recombinant plasmid encoding the PlpE protein was expressed in E. coli BL21 (DE3). Protein expression was induced with a final concentration of 1 mM isopropyl-D-1- thiogalactopyranoside (IPTG). His6-tagged PlpE was purified using a nickel affinity column (Qiagen, Germany). The purified protein was evaluated by SDS-PAGE and Western blotting. Recombinant protein was electrophoresed by SDS-PAGE using 4% stacking and 12% separating acrylamide gels, and then transferred to nitrocellulose membrane using a semi-dry system. The membrane was blocked with 3% skim milk in PBS and then incubated overnight. After three rounds of washing with PBS containing Tween 20 (PBST buffer), the membrane was incubated with a 1:10000 dilution of mouse antihistidine monoclonal antibody (Sigma Aldrich, USA) for 1 hour at room temperature. After washing with PBST, the membrane was developed with substrate (0.5 mg/ml Diaminobenzidine, DAB, 0.005% H2O2) (Sigma Aldrich, USA) ( 10 ).
2.4. Rabbit Polyclonal Antibody Production
Four adult male New Zealand White rabbits (2 ± 0.2 kg) were acclimated in the animal house for at least two weeks in the animal house. On day zero,1 ml of the inactivated M. haemolytica (4 × 109 CFU/mL) emulsified with 1 ml of Freund’s complete adjuvant (Sigma-Aldrich, St. Louis, MO, USA) was injected subcutaneously. From the second to the fourth immunization Freund’s complete adjuvant was replaced by Freund’s incomplete adjuvant. The control group (four rabbits) received 1 mL of sterile PBS plus 1 mL adjuvant. Blood samples were collected from the rabbits before each immunization and one week after the last booster ( 11 ).
2.5. ELISA
ELISA plates (Greiner Bio-One, Austria) were coated with 100 μl/well of protein fractions of whole M. haemolytica at 0.1 μg/ml in 50mM carbonate / bicarbonate buffer (PH 9.6) at 4°C overnight. After blocking with 3 % skim milk and washing three times with PBS containing 0.05% (v/v) Tween 20, 100 μl of rabbit sera diluted 1:50 in 1% BSA were incubated for one hour at 37°C. Horseradish peroxidase anti-rabbit IgG conjugate (Sigma-Aldrich, St. Louis, MO, USA) diluted at 1:6000 was added and incubated for 1hour at 37 °C. 100 μl of the substrate buffer containing O-phenylenediamine dihydrochloride (OPD) (Sigma Aldrich, USA) and 30% H2O2 were added to the plate wells. Finally, the optical density (OD) was read on an ELISA reader (Immunoskan BDSL, Thermo Lab. Systems, Finland) at 450 nm ( 11 ).
2.6. Western Blot Analysis
Crude cell lysate of M. haemolytica and purified rPlpE were resolved by 12% SDS-PAGE and transferred to a nitrocellulose membrane. After blocking with 5% skim milk in PBS overnight at room temperature (RT), themembrane was washed with PBST and incubated with rabbit polyclonal antibody at 1:50 dilution for 1 hour at RT. After washing, horseradish peroxidase anti-rabbit IgG conjugate (Sigma-Aldrich, St. Louis, MO, USA) diluted at 1:2000 was added and incubated with shaking for 1 hour at RT. Finally, the membrane was washed and placed into a substrate solution (H2O2/ DAB) (Sigma-Aldrich, St. Louis, MO, USA) ( 11 ).
2.7. Coating of Polystyrene Particles with Recombinant Protein
Coating of the purified PlpE on the surface of white-colored polystyrene particles of identical size (0.3 μm) was conducted. 100 μL of latex beads were added to 20 μl of purified recombinant PlpE protein. The mixture was then incubated with gentle stirring at 37°C for two hours, after which it was subjected to centrifugation at 5,000 rpm for 10 min. The supernatant was then carefully aspirated out. The pellet was resuspended in blocking buffer (10 mM PBS, pH 7.4, containing 3% BSA) with gentle stirring at 37°C for 45 minutes and then centrifuged at 5,000 rpm for 10 minutes. The blocked beads were washed twice with PBST buffer (pH 7.4) and centrifuged at 5,000 rpm for 10 minutes. Finally, the coated beads were resuspended in a storage buffer (0.1 M glycine saline buffer, pH 8.6, with 0.1% sodium azide,) and stored at 4°C.
2.8. Latex Agglutination Test
A 20 μl volume of sensitized latex suspensions and a 40 μL anti-M. haemolytica IgG-positive serum were mixed on the agglutination card for 2 minutes. The sensitized latex beads were simultaneously mixed with a serum sample that was negative for M. haemolytica. The agglutination results were classified as positive (+) or negative (-). To ascertain the specificity of the LAT, the cross-reactivity was evaluated with an anti- Pasteurella multocida-positive serum. To ascertain the stability of the latex beads, they were stored at 4°C for 1–4 months.
2.9. Statistical Analysis
The statistical analysis was carried out using the IBM SPSS version 16. All data were expressed as the mean ± standard deviation (SD). A p-value of less than <0.05 was considered as statistically significant.
3. Results
3.1. Identification of M. haemolytica
M. haemolytica was distinguished phenotypically by the presence of small gray circle colonies with β-hemolysis and rod-shaped bacilli in the Gram staining. The catalase and oxidase tests yielded positive results, whereas the indole returned a negative outcome. The PCR-amplified products of the M. haemolytica Lkt and HP genes were visualized as 206 and 90 bp bands, respectively (Figure 1). These findings corroborate the hypothesis that the isolated microorganism in the study samples is indeed M. haemolytica.
Figure 1.
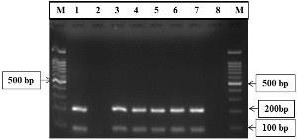
PCR amplification of the Lkt and HP genes. The 100 bp DNA Ladder (Lane M) was used as a reference for the amplicons of Lkt and HP (206 bp and 90bp) (Lanes 1,3,4,5,6,7). Lanes 2 and 8 were used as negative controls.
3.2. Expression and Purification of the Recombinant Protein
The results of the SDS-PAGE analysis showed the rPlpE with a molecular weight of 48 kDa (Figure 2). Western blot analysis revealed that the, anti-His monoclonal antibody was specifically reacted with a protein of approximately 48 kDa, which corresponded to rPlpE (Figure 2).
Figure 2.
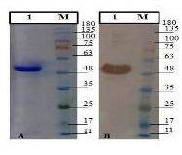
SDS-PAGE of rPlpE (A) and Western blotting with anti-histidine antibody (B). Purified rPlpE (Lane 1). Pre-stained protein ladder (Cinnagen PR911654 [SL7012 ]) (Lane M). The samples derived from the same experiment and gel/blot were processed in parallel
3.3. Indirect ELISA
Specific antibody against M. haemolytica was generated from a heat -inactivated immunogen. The presence of anti-M. haemolytica antibodies was detected in all immunized rabbits, with levels significantly higher than those observed in the control group throughout the experiment. This outcome substantiates the efficacy of the immunization procedure (p<0.05). The presence of anti- M. haemolytica antibodies was seen on days 7, 14, 21 and 28, with a notable increase in antibody levels on day 28 (Figure 3). Non-immunized rabbits showed low antibody titers (p < 0.05).
Figure 3.
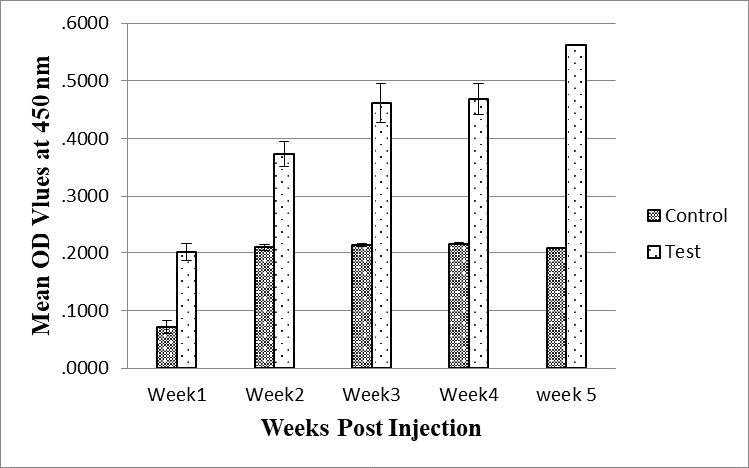
The serum antibody titers in the immunized rabbit with the whole-cell antigen of M. haemolytica were measured by ELISA after five injections (1 mg/animal) with one-week intervals.
3.4. Western Blot Detection of Rabbit pAbs Specificity
The specificity of produced the anti-M. haemolytica antibody in the immunized groups was also confirmed using Western blot analysis. The antibody generated against the whole cells of M. haemolytica exhibited reactivity with the protein fractions of M. haemolytica and purified rPlpE protein but not with the control sera. The results showed that the produced anti-M. haemolytica antibodies were able to specifically detect the rPlpE protein.
3.5. Latex Agglutination Test Using Recombinant Protein
The PlpE–LAT demonstrated apositive agglutination pattern with an anti-M. haemolytica IgG-positive serum. The test was confirmed to be highly specific for M. haemolytica, as there was no false-positive reaction with positive serum for Pasteurella multocida. No agglutination was observed for negative samples and PBS after 2 minutes (Figure 4). The stability of the sensitized latex beads was found to be at least 4 months after storage at 4°C.
Figure 4.

Slide latex agglutination test with latex beads coated with rPlpE. (A)The results demonstrated a positive agglutination reaction with anti- M. haemolytica IgG-positive serum. (B) and (C) exhibited negative agglutination with negative samples (serum control) and PBS (D) demonstrated the absence of false-positive reactions with anti-Pasteurella multocida -positive serum.
4. Discussion
Diagnosis of M. haemolytica is based on bacteriological methods such as the isolation of the microorganisms from clinical specimens. The currently available methods are time-consuming and not easy to perform. It is imperative to develop optimized tests for the rapid and simple detection of antibodies against M. haemolytica with high sensitivity and specificity. In a study conducted by Haji Hajikolaei et al. (2010), bacteriological investigations and indirect hemagglutination test (IHA) were compared on the nasopharyngeal and nasal samples from slaughtered cattle.
The results showed the isolation of M. haemolytica from 4 (1.6%) of these animals. The IHA test yielded a positive result for antibody against M. haemolytica in 71.6% of the sera. In comparison with IHA test, the results obtained by culture of M. haemolytica (1.6%) were relatively low ( 12 ). Tabatabaei et al. (2018) reported that culture and PCR methods are practical to identify the bacteria but culture is more time-consuming ( 13 ). In a recent study,Ashrafi et al. (2022) employed the bacteriological examination of the samples obtained from the lungs of slaughtered cattle, in Golestan province, northeastern Iran. Of the isolates, 14 (11.6%) and 22 (18.3%) were positive for P. multocida and M. hemolytica, respectively. The isolates were identified through a combination bacteriological culture, biochemical tests, and polymerase chain reaction analysis ( 14 ). Soliman et al. (2023) identified the presence ofbacteria producing decarboxylase enzymes in minced meat by cultural and biochemical tests. The most isolated bacteria were identified as belonging to theSalmonella species (specifically,Salmonella Typhimurium, and S. Arizonae), E.coli “serotype O44:K74 and O125:K70”, Klebsiella pneumonia, Enterobacter spp, S. aureus, Aeromonas hydrophila, Proteus mirabilis, Pasteurella multocida and Lactobacillus species. The implementation of various were the most isolated bacteria. Various methods for the specific identification of bacteria necessitates the use of expensive laboratory equipment. For example, ELISA can be sensitive for detection but this test is requires special equipment. The LAT is simple, rapid, and much less expensive than other methods which can be for detection in laboratories. LAT is easy to perform with no requirement for expensive equipment and results can be obtained within several minutes ( 2 ). This test can be used as diagnostic tool for the rapid and specific detection of M. haemolytica. Serological tests based on recombinant protein may provide higher sensitivity and specificity than culture tests. Because the production of recombinant proteins is cost-effective, their utilization in LATs can reduce the cost of the diagnostic kits. Outer membrane proteins (OMPs) of M. haemolytica are the best known stimulators of immunity in pasteurellosis ( 5 ). Recombinant PlpE was evaluated to be potent immunogen that induced effective immunity against M. haemolytica ( 14 - 19 ). This study aimed to evaluate the efficacy of rPlpE, as one of the outer membrane proteins, is conserved among M. haemolytica strains, in latex agglutination test (LAT) for detection of Mannheimia Haemolytica specific antibodies ( 17 - 19 ). In the present study, PlpE was expressed as recombinant protein and utilized for developing a latex agglutination test. Escherichia coli is one of the best expression systems for the production of recombinant proteins. These proteins can be produced more easily and economically in this expression system. The PlpE gene for high-level expression was sub cloned into pET26b (+) in E. coli. Bacterial expression systems are used frequently due to their rapid growth rate, capacity for continuous fermentation, and relatively low cost ( 19 , 20 ). The pET expression vectors have been widely used for the production of a large number of proteins. ( 20 , 21 ). It was shown that recombinant protein–coated particles were able to give a good agglutination reaction with antibody. Here we describe the development of a simple and and rapid test based on recombinant protein. This test does not require any special equipment. The use of the rPlpE purified recombinant antigen leads to a more standardized diagnostic test with an improved the stability and specificity of the test. The latex agglutination test in association with the bacterial culture and biochemical tests could help the rapid detection of M. haemolytica specific antibodies. In this study, we generated recombinant PlpE and used as antigen for LAT, resulting in high specificity for detecting M. haemolytica antibodies. This rapid and specific anti M. haemolytica antibodies detection method using recombinant proteins can save cost and be widely applied for efficient and practical detection of. M. haemolytica.
Acknowledgment
This research was financially supported by the School of Veterinary Medicine at Shiraz University.
Authors' Contribution
AY and MT contributed to generate the study plan, data analysis and the manuscript preparation. NK and ZH carried out the sample collections and lab work. All authors approved the final draft of the manuscript
Ethics
All experimental protocols were approved by the animal welfare and Ethics Committee in Faculty of Veterinary Medicine, Shiraz University, Iran, (no reference number) and all methods were carried out in accordance with relevant guidelines. All methods are reported in accordance with ARRIVE guidelines
Conflict of Interest
The authors declare that they have no conflict of interest.
Data Availability
The data that support the findings of this study are available on request from the corresponding author.
References
- 1.Quinn PJ, Markey BK, Leonard FC, Hartigan P, Fanning S, Patrick ESF. Veterinary Microbiology and Microbial Disease. 2nd ed. UK: Wiley-Blackwell; 2011. p. 928. [Google Scholar]
- 2.Yap KL. Development of a slide latex agglutination test for rotavirus antigen detection. Malaysian Journal of Pathology. 1994; 16:49–56. [PubMed] [Google Scholar]
- 3.Abdoel TH, Smits HL. Rapid latex agglutination test for the serodiagnosis of human brucellosis. Diagnostic Microbiology and Infectious Disease. 2007;57:123–8. doi: 10.1016/j.diagmicrobio.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 4.Molina-Bolivar JA, Galisteo-Gonzalez F. Latex immunoagglutination assays. Polymer Reviews. 2005; 45:59–98. [Google Scholar]
- 5.Lee JH, Jeong JM, Park YH, Choi SS, Kim YH, Chae JS, et al. Evaluation of the methicillin resistant Staphylococcus aureus (MRSA)-screen latex agglutination test for detection of MRSA of animal origin. Journal of Clinical Microbiology. 2004; 42:2780–2. doi: 10.1128/JCM.42.6.2780-2782.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slotved HC, Kaltoft M, Skovsted IC, Kerrn MB, Espersen F. Simple, rapid latex agglutination test for serotyping of Pneumococci (Pneumotest-Latex) Journal of Clinical Microbiology. 2004; 42:2518–22. doi: 10.1128/JCM.42.6.2518-2522.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.March JB, Kerr K, Lema B. Rapid detection of contagious bovine pleuropneumonia by a Mycoplasma mycoides subsp.mycoides SC capsular polysaccharide-specific antigen detection latex agglutination test. Clinical and Diagnostic Laboratory Immunology. 2003;10:233–40. doi: 10.1128/CDLI.10.2.233-240.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pandher K, Confer AW, Murphy GL. Genetic and immunologic analyses of PlpE, a lipoprotein important in complement-mediated killing of Pasteurella haemolytica serotype 1. Infection and Immunity. 1998; 66:5613–5619. doi: 10.1128/iai.66.12.5613-5619.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pandher K, Murphy GL, Confer AW. Identification of immunogenic, surface-exposed outer membrane proteins of Pasteurella haemolytica serotype 1. Veterinary Microbiology. 1999;65:215–226. doi: 10.1016/s0378-1135(98)00293-4. [DOI] [PubMed] [Google Scholar]
- 10.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 11.Harlow E, Lane D. Antibodies: A Laboratory manual. New York, Cold spring harbor laboratory press. 1988; 726 pages [Google Scholar]
- 12.Haji Hajikolaei MR, Ghorbanpour M, Seyfiabad Shapouri MR, Rasooli A, Ebrahimkhani D, Jabbari AR. Bacteriological and serological studies on Mannheimia haemolytica infection in cattle slaughtered at Ahvaz (southwestern Iran) abattoir. Iranian Journal of Veterinary Research. 2010;11:84–87. [Google Scholar]
- 13.Tabatabaei M, Abdollahi F. Isolation and identification of Mannheimia haemolytica by culture and polymerase chain reaction from sheep’s pulmonary samples in Shiraz, Iran. Veterinary World. 2018;11(5): 636–641. doi: 10.14202/vetworld.2018.636-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ashrafi F, Ahani Azari A, Fozouni L. Prevalence and Antibiotic Resistance Pattern of Mannheima haemolytica and Pasteurella multocida Isolated from Cattle Lung Samples from an Industrial Abattoir: A Study from Northeastern Iran. Iranian Journal of Veterinary Medicine. 2022;16(4):414–422. [Google Scholar]
- 15.Soliman A, Selim A, Abd El-Tawab A. The Effect of Some Nano Plant Extract on Bacteria Producing Biogenic Amines Isolated From Minced Meat. Iran J Vet Med. 2023 [Google Scholar]
- 16.Ayalew S, Confer AW, Blackwood ER. Characterization of immunodominant and potentiallyprotective epitopes of Mannheimia haemolytica serotype 1 outer membrane lipoprotein PlpE. Infection and Immunity. 2004;72:7265–7274. doi: 10.1128/IAI.72.12.7265-7274.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ayalew S, Confer AW, Payton ME, Garrels KD, Shrestha B, Ingram K R, et al. Mannheimia haemolytica chimeric protein vaccine composed of the major surface-exposed epitope of outer membrane lipoprotein PlpE and the neutralizing epitope of leukotoxin. Vaccine. 2008; 26: 4955–4961. doi: 10.1016/j.vaccine.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 18.Batra SA, Shanthalingam S, Donofrio G, Srikumaran S. A chimeric protein comprising the immunogenic domains of Mannheimia haemolytica leukotoxin and outer membrane protein PlpE induces antibodies against leukotoxin and PlpE. Veterinary Immunology and Immunopathology. 2016;175:36–41. doi: 10.1016/j.vetimm.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Confer AW, Ayalew S, Panciera RJ, Montelongo M, Whitworth LC, Hammer JD. Immunogenicity of recombinant Mannheimia haemolytica serotype 1 outer membrane protein PlpE and augmentation of a commercial vaccine. Vaccine. 2003;21:2821–2829. doi: 10.1016/s0264-410x(03)00213-5. [DOI] [PubMed] [Google Scholar]
- 20.Confer AW, Ayalew S, Panciera RJ, Montelongo M, Wray JH. Recombinant Mannheimia haemolytica serotype 1 outer membrane protein PlpE enhances commercial M. haemolytica vaccine-induced resistance against serotype 6 challenge. Vaccine 2006;24 doi: 10.1016/j.vaccine.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 21.Confer AW, Ayalew S, Montelongo M, Step DL, Wray JH, Hansen RD, et al. Immunity of cattle following vaccination with a Mannheimia haemolytica chimeric PlpE-LKT (SAC89) protein. Vaccine. 2009;27:1771–1776. doi: 10.1016/j.vaccine.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 22.Haag AF, Ostermeier C. Positive-selection vector for direct protein expression. Biotechniques. 2009;46:453–457. doi: 10.2144/000113091. [DOI] [PubMed] [Google Scholar]
- 23.Studier FW. Protein production by auto-induction in high density shaking cultures. Protein Expression and Purification. 2005;41:207–234. doi: 10.1016/j.pep.2005.01.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.


