Abstract
Temporal Interference Stimulation (TIS) represents a novel non-invasive brain stimulation technique that deeply targets specific brain regions using the differential beat frequency of two high-frequency stimulation pairs. This study investigated the neuromodulatory effects of TIS at different beat frequencies on cortical excitability in the rat motor cortex. Rats were randomly assigned into four groups, receiving TIS at alpha (10 Hz), beta (20 Hz), gamma (70 Hz), or sham frequencies targeting the motor cortex for 20 min under anesthesia. Cortical excitability and inhibition were evaluated by measuring motor-evoked potentials (MEPs), input-output (I/O) curves, and long-interval intracortical inhibition (LICI) before and after TIS. Additionally, immunohistochemistry was performed for neural biomarkers c-Fos and glutamic acid decarboxylase (GAD-65) to confirm targeted neural activation following TIS. We also examined glial fibrillary acidic protein (GFAP)-positive cells in the stimulated region to assess astrocyte responses associated with TIS. Alpha and gamma TIS significantly increased MEP amplitudes compared to sham stimulation. The analysis of I/O curves revealed a significant enhancement in the area under the curve (AUC) post-stimulation in the alpha and gamma TIS groups. Notably, only gamma TIS significantly reduced intracortical inhibition, indicated by an increased LICI ratio post-stimulation. Immunohistochemical analysis demonstrated a significant 35% increase in c-Fos-positive cells in the stimulated motor cortex regions after TIS compared to sham, whereas no significant changes in GAD-65-positive cells or GFAP expression were observed. These findings indicate that a single session of alpha or gamma TIS effectively modulates cortical excitability, highlighting its potential for targeted neuromodulation applications.
Keywords: Temporal interference stimulation, Neuromodulation, Cortical excitability, Intracortical Inhibition, Motor-evoked potential
Subject terms: Neuroscience, Medical research
Background
Neuromodulation, particularly electromagnetic brain stimulation, holds significant potential in modulating neural activity1. Deep brain stimulation (DBS), in particular, is a powerful and widely used technique for targeting deep brain structures to treat various neurological diseases, such as Alzheimer’s or Parkinson’s disease2–4. Despite its benefits, DBS is an invasive technique that requires surgical procedures to implant the stimulating electrodes and stimulator; consequently, it carries a high risk of infection and other surgical complications5,6. In addition, there are numerous non-invasive brain stimulation (NIBS) techniques, including transcranial direct current stimulation (tDCS), transcranial alternating current stimulation (tACS), and repetitive transcranial magnetic stimulation (rTMS), which have demonstrated their neuromodulatory and therapeutic effects on neuroplasticity, movement-related neuroplasticity, motor control, and learning7–10. Nevertheless, these NIBS techniques with low spatial resolution indirectly influence deep structures by stimulating superficial cortical regions, leveraging the interconnected neural networks within the brain10,11. Given the need to overcome the limitations of existing techniques, researchers are particularly focused on advancing novel approaches to reach the deep brain structures with high precision and non-invasively while minimizing side effects.
As first introduced in 2017 by Grossman et al.12, a novel non-invasive deep brain stimulation technique, a so-called temporal interference stimulation (TIS), demonstrates the ability to reach precisely deep brain regions without stimulating the overlaying cortex. TIS technically involves applying two different high-frequency alternating currents in the kilohertz (kHz) range (frequencies f1 and f2, with f2 < f1, where “f” refers to frequency), which interfere to generate an envelope electric field oscillating at a low frequency13. While the kHz high-frequency alternating currents have been observed not to directly cause neural activation, the envelope low-frequency referred to as a beat frequency or difference frequency (∆f = f1 – f2) - within the physiological range of electrical brain activity (< 100 Hz) - can modulate neuronal activity within the specific brain structures as it targets12–14. TIS enables spatially steerable stimulation and is more focal than conventional NIBS techniques, such as tDCS and tACS.
To date, research on TIS applications has garnered considerable attention in the field of neurostimulation, with a vast number of studies spanning from computational models15–17 to preclinical12,18–20 and clinical trials21–24. The findings of these studies highlighted that TIS could be a safe and promising non-invasive approach, capable of producing physiological effects in target brain structures and enhancing motor performance in healthy and disease models. Particularly, in regard to neuromodulation, TIS could be used for exploring the influence of different beat frequencies target distinct brain oscillations like theta (4–8 Hz), alpha (8–13 Hz), beta (13–30 Hz), and gamma (≥ 30 Hz) which are associated with various cognitive and motor functions25–27. Existing evidence shows that stimulating the primary motor cortex at beta or high gamma beat frequencies of TIS in healthy participants can promote cortical excitability and movements23. In addition, a latest study successfully developed and applied the theta burst protocol of TIS to modulate motor cortex neuroplasticity in rodents28.
Despite promising preliminary results from TIS, the underlying mechanisms remain inadequately explored. Given the increasing evidence supporting its neuromodulatory potential, this study aimed to investigate the effects of TIS on cortical excitability and intracortical inhibition in the rat motor cortex using motor-evoked potentials (MEPs). MEPs elicited by epidural stimulation provided an integrated assessment of cortical excitability, thus enhancing the translational relevance of our findings for future clinical applications.
Methods
Animals
Healthy male Sprague-Dawley (SD) rats (300–500 g) used in the present study were obtained from the Animal Center of Chang Gung University. The animals were housed in a temperature-controlled animal care facility at a temperature of (20 ± 3 °C) with a 12 h light-dark cycle and had ad libitum access to food and water. All experimental procedures were approved by the Institutional Animal Care and Use Committee of Chang Gung University (IACUC No. CGU108-030) and were performed in accordance with the relevant guidelines and regulations. The study design complied with the ethical and methodological standards of the ARRIVE guidelines29. The animals were euthanized by transcardial perfusion fixation under deep anesthesia using 5% isoflurane.
Experimental design
Animals were randomly allocated into the following four groups (n = 8 per group): alpha, beta, gamma, and sham TIS groups. To investigate whether a single session of TIS can induce changes in cortical excitability, electrophysiological recordings were performed pre- and post-intervention up to 30 min. In addition, another set of animals was used to confirm neuronal activation within the targeted stimulated region using immunohistochemical (IHC) staining. The experiment design is shown in Fig. 1A.
Fig. 1.
(A) Schematic representation of the experimental design. Thirty-two rats were divided into four groups of eight animals each. Each group received a respective stimulation protocol for 20 min under anesthesia. Electrophysiological recordings, including motor-evoked potential (MEP), input-output curve (I/O Curve), and long-interval intracortical inhibition (LICI), were measured pre-and post-stimulation. (B) The top view of the rat skull shows the positioning of epidural screw electrodes for stimulation and electrophysiological recordings. (C) A detailed diagram illustrates the electrode placements with specific coordinates on the skull over the targeted motor cortical region.
Experimental setup
Prior to the beginning of each experiment, animals were deeply anesthetized with intraperitoneal injection of Zoletil 50 (50 mg/kg, i.p. Zoletil, Vibac, Carros, France) with xylazine (10 mg/kg, Rompun, Bayer, Barmen, Germany) and were then mounted on a stereotactic apparatus (Model 940, David Kopf Instruments, Tujunga, CA, United States) once the toe-pinch reflex was absent. After hair shaving, a midline incision was made along the scalp, and the implantation area was carefully cleared with H2O2 to expose the bregma.
Two TIS electrode pairs were then implanted on the rat’s skull. Stainless steel epidural screw electrodes (1.6-mm-diameter pole, 0–80 × 1/16, PlasticsOne Inc., Roanoke, VA, United States) were implanted into the four burr holes connecting with the output of the stimulator via wires. The TIS electrode pairs were positioned epidurally surrounding the left motor cortex with the coordinates AP 4.0 mm; ML 1.0 mm and AP -1.0 mm, ML -4.0 mm, on the dura mater (pair 1); AP 4.0 mm, ML -4.0 mm and AP -1.0 mm, ML 1.0 mm, on the dura mater (pair 2), in accordance with functional rat brain mapping30. Electrode placement was confirmed by applying a beat frequency of 10 Hz, which consistently induced contralateral forelimb movement, thus verifying accurate targeting of the motor cortex. Additionally, epidural electrodes positioned on the dura mater over the left motor cortex at coordinates AP 1.5 mm; ML -3.0 mm and AP -1.0 mm; ML -4.0 mm reliably produced MEPs in the contralateral forelimb (Fig. 1B and C).
Temporal interference stimulation (TIS)
TIS was administered while animals were under anesthesia after baseline assessments of electrophysiological recordings. Biphasic TIS was delivered via two electrode pairs using a high definition - interferential stimulation stimulator (Soterix Medical, New Jersey, USA). Each stimulation session was applied for 20 min - a duration comparable to that used in the previous study targeting neuroplasticity effects12, with 30 s ramp-up and 30 s ramp-down periods. We tested three distinct beat frequencies (∆f = 10 Hz, 20 Hz, or 70 Hz) with the frequency of carrier currents fixed at f = 2 kHz (Fig. 2). Accordingly, animals in the alpha group were exposed to ∆f = 10 Hz, those in the beta group to ∆f = 20 Hz, and those in the gamma group to ∆f = 70 Hz. In addition, the sham group underwent a 30 s ramp-up stimulation at randomly either ∆f = 10 Hz, 20 Hz, or 70 Hz, followed by a period of no stimulation; then, at the end of the intervention, a 30 s ramp-down stimulation at the same frequencies was applied. The stimulation intensity of TIS was set at 80% of the resting motor threshold, defined as the minimal electrical stimulation intensity required to induce noticeable contralateral forelimb muscle movements. Preliminary experiments indicated that overt contralateral forelimb movements, such as noticeable twisting, occurred consistently at intensities around 1.8–2 mA. Thus, an intensity of approximately 1.5 mA (80% of the threshold) was selected to ensure effective cortical stimulation without producing overt motor responses.
Fig. 2.
(A) Schematic representation of TIS protocols and (B) TIS stimulator.
Electrophysiological recordings
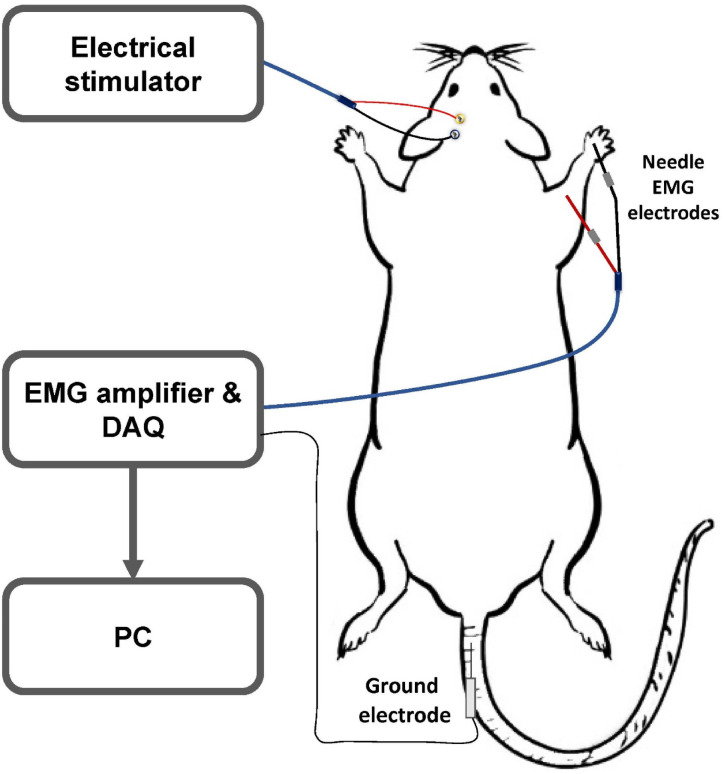
Electromyographic (EMG) was recorded from the biceps brachii muscle contralateral to the stimulated motor cortex via Ag/AgCl needle electrodes (Fig. 3). The raw signals were sampled at 2.5 kHz and band-pass filtered at 50–1000 Hz for offline analysis (MP36, BIOPAC system, California, United States)31,32. Motor-evoked potentials (MEPs), input-output recruitment curve (IOC), and long-interval intracortical inhibition (LICI) were taken before and after the end of the stimulation period. The stimuli were delivered through a two-channel electrical stimulator (STG4002, Multichannel Systems, Reutlingen, Germany) programmed by Multichannel Stimulus II software.
Fig. 3.
Schematic representation of recording electrophysiological responses.
To measure the corticospinal excitability of the left motor cortex, biphasic single pulse stimuli with an inter-stimulus interval (ISI) of 10 s were delivered to the optimal skull position for activation of the right forelimb muscles. We first identified the resting motor threshold (RMT), which was defined as the lowest stimulus intensity eliciting an MEP response of at least 20 uV in 5 out of 10 consecutive trials. Fifteenth single-pulse MEPs (MEPs) at 120% RMT were recorded at different time points: at baseline, immediately after, and up to 30 min after the end of TIS intervention.
The input-output curve (IOC) represents the neural excitability of a stimulation target, which is affected by several factors, including neuromodulators33. It also reflects the recruitment of larger neuronal populations at increased TMS intensities and a change in the balance between GABAergic and glutamatergic activity within M1. In this present study, the IOCs were measured over five intensities of RMT, comprising 90%, 100%, 110%, 120%, and 130% RMT, with each tested once per cycle for three cycles.
Paired-pulse paradigm Long-interval intracortical inhibition (LICI) was investigated using paired-pulse stimuli, including a conditioning stimulus (CS) preceded a test stimulus (TS), to evaluate cortical inhibitory circuitry. Both CS and TS were set at a suprathreshold intensity (120% RMT) with an ISI of 200 ms. Ten conditioning-test stimuli pairs and 10 test stimuli alone were applied before and after TIS (200 ms ISI, 120% RMT, ten pulse pairs, ten single stimuli, 10 s single/paired-pulse interval).
Immunohistochemical staining
We subsequently confirmed whether TIS successfully stimulated the targeted motor cortex by analyzing neuronal activation using the immediate-early expressed gene c-fos as an indicator of activated neurons in rodents34,35. The expression of glutamic acid decarboxylase 65-kilodalton isoform (GAD-65) was also examined for cortical excitability at the cell level36. Additionally, the safety of TIS at the target brain region was assessed by the expression of glial fibrillary acidic protein (GFAP) in astrocytes. In this study, the changes in c-fos, GAD-65, and GFAP expression in the motor cortex following TIS were determined in a different set of animals that received 20 min of alpha TIS.
After 20 min of TIS, the animals were kept resting for an additional 20 min before euthanasia. The animals were anaesthetized under deep sedation with 5% isoflurane and sacrificed by transcardial perfusion fixation with normal saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer solution (PBS). After perfusion was completed, the skull is carefully opened to expose the brain. The brain was then removed and post-fixed in 4% paraformaldehyde at 4 °C overnight. Next, they were immersed in PBS with 30% sucrose for cryoprotection at 4 °C for 5 days, then freezing them at − 81 °C. The brain tissues were sectioned sagittally to a thickness of 30 μm using a cryostat (LEICA CM1950) maintained at − 21 °C. The IHC staining and quantitative analysis were performed similarly to our previous study31. For IHC staining, the primary rabbit antibodies against c-Fos (1:1,000, AB11959, Millipore, United States), GAD-65 (1:100, AB239372, Millipore, United States), and GFAP (1:1,000, AB7260, Millipore, United States) were used.
Data processing and statistical analysis
Data signal processing was performed off-line using Matlab R2021 software (The MathWorks, Inc., Natick, Massachusetts, United States). The peak-to-peak amplitude of all MEP signals was automatically calculated using MATLAB as the absolute difference between the maximum positive peak and the minimum negative peak within a 5–20 ms post-stimulus window and was subsequently verified manually. All data were logarithmically transformed (log10) to achieve normal distribution for the use of parametric tests prior to statistical analysis. However, the figures illustrating the results were based on normalized and raw data. All statistical tests were computed using SPSS Statistics (version 22.0; IBM Corp., Armonk, NY, USA) and Microsoft Excel 2013 (Microsoft, Redmond, WA, USA). The significance level was set at p ≤ 0.05.
For MEP (120% RMT), the MEP averages were calculated for each time point and each group before and after stimulation. To reduce variability, the averaged MEPs at each time point (pre, post 0, post 10, post 20, and post 30) were normalized to the averaged baseline (pre-stimulation). A two-way repeated measures ANOVA was then conducted, with Group (alpha TIS, beta TIS, gamma TIS, sham TIS) as the between-subject factor and Time (pre, post-0, post-10, post-20, post-30) as the within-subject factor. If significant main effects or interactions emerged, the t-test with Bonferroni-corrected post-hoc analyses were used to examine specific pairwise differences among groups and time points.
For the I/O curve, the averaged MEP amplitude at each stimulation intensity was computed and compared before and after stimulation using paired t-tests. We also analyzed the area under the I/O curve (AUC) to measure the overall corticospinal output. Two-way ANOVA was employed with factors Time (pre- and post-stimulation) and Group (alpha TIS, beta TIS, gamma TIS, and sham TIS groups). Post-hoc comparisons for significant ANOVA results were performed using the t-test with Bonferroni corrections. To measure intracortical inhibition, LICI was expressed as the ratio of conditioned MEP to unconditioned MEP, where a higher LICI value indicates reduced inhibition and a lower LICI value suggests increased inhibition. To investigate the difference in inhibition among groups over time, two-way repeated measure of ANOVA was performed with Time (pre- and post-stimulation) and Group factors (alpha TIS, beta TIS, gamma TIS, and sham TIS groups). Significant ANOVA results were further explored with Bonferroni-corrected post-hoc pairwise comparisons. Furthermore, differences in histology data between and within groups were investigated using independent and paired t-tests.
Results
Effects of TIS on motor cortical excitability
To investigate the beat frequency-dependent effects of TIS on motor cortical excitability, we conducted a two-way repeated measure of ANOVA on normalized MEP amplitudes across four intervention groups over 30 min. The ANOVA indicated a significant main effect of Time (F(4,112) = 5.036, p = 0.001), but no significant main effect of Group (F(3,28) = 2.327, p > 0.05) or Group × Time interaction (F(12,112) = 1.019, p > 0.05). Figure 4A illustrates the time-course changes in raw MEP amplitudes for alpha TIS, beta TIS, gamma TIS, and sham TIS groups. Subsequent Bonferroni-corrected post-hoc t-tests revealed significant increases in MEP amplitudes compared with the sham TIS group, specifically for the alpha TIS group at post-0 (p = 0.027), post-10 (p = 0.002), and post-20 (p = 0.040), as well as for the overall changes from post-0 to post-30 (p = 0.017). Similarly, significant increases were observed in the gamma TIS group at post-0 (p = 0.027), post-10 (p = 0.025), post-20 (p = 0.012), post-30 (p = 0.034), and for overall changes from post-0 to post-30 (p = 0.008) compared to sham TIS (Fig. 4B and C).
Fig. 4.
(A) Time-course changes in raw MEP signals across four groups. MEP amplitudes increased after alpha TIS, beta TIS, and gamma TIS but did not after sham TIS compared to baseline. (B) The average normalized MEP amplitudes for the contralateral limb across the four intervention protocols (alpha, beta, gamma, and sham TIS). (C) The total responses of cortical excitability were calculated for each intervention group within 30 min following stimulation on the contralateral limb. Asterisks (*) indicate statistically significant differences when comparing alpha TIS, beta TIS, and gamma TIS with the sham group at the same time point. The data are presented as means, with error bars representing the standard error of the mean (SEM); * p ≤ 0.05.
Compared to baseline, paired t-tests with Bonferroni-correction showed statistically significant increases in MEP amplitudes at post 0 (p = 0.026) and post 10 (t = -4.657, p = 0.002) for the alpha TIS group, as well as at post 0 (p = 0.025) and post 10 (p = 0.028), post 20 (p = 0.018), and post 30 (p = 0.010) for the gamma TIS group. Although there were increases in MEP amplitudes following beta TIS, no significant differences were found compared to pre-intervention.
We further analyzed data from IO curves and found that MEP amplitudes significantly increased at higher intensities independently after alpha or gamma TIS but not following beta TIS and sham TIS. Figure 5A represents the amplitude of MEP acquired with five different stimulation intensities for four groups. The alpha TIS group showed significant increases in MEP amplitudes at stimulation intensities of 90% (p = 0.008), 100% (p = 0.041), and 120% (p = 0.036) RMT. In the gamma TIS group, significant increases in MEP amplitudes were observed at 90% (p = 0.007), 100% (p = 0.027), 110% (p = 0.004), 120% (p = 0.037), and 130% (p = 0.048) RMT compared to baseline (Fig. 5B). Additionally, the statistical analysis of AUC data showed a significant main effect in Time (F(1,28) = 11,537, p = 0.002) and no significant effect in Group (F(3,28) = 0.160, p > 0.05) or interaction (F(3,28) = 2.302, p > 0.05). The paired t-tests with Bonferroni-correction showed that there was a significant increase in area after alpha (p = 0.021) and gamma TIS (p = 0.008), while there was no change after beta and sham TIS (p > 0.05) when compared to pre-stimulation (Fig. 5C).
Fig. 5.
(A) Representations of MEP responses at five thresholds (90%, 100%, 110%, 120%, and 130% RMT) pre- and post- stimulation in all groups. (B) Pre- and post-TIS changes in the I/O curve. Average MEP amplitudes across the five thresholds (90%, 100%, 110%, 120%, and 130% RMT) in the four conditions (sham, alpha, beta, and gamma TIS). (C) AUC was calculated for each TIS condition before and after stimulation. The data are presented as means, with error bars representing the standard error of the mean (SEM); *p ≤ 0.05, **p ≤ 0.01.
Effects of TIS on intracortical inhibition
Tracings representative of this phenomenon are shown in Fig. 6A. The statistical analysis for LICI revealed a significant main effect in Time (F(1,28) = 6.355, p = 0.018), but no main effect in Group (F(3,28) = 0.411, p = 0.747) as well as interaction (F(3,28) = 1.380, p = 0.269). The paired t-test comparisons on LICI data showed a statistical change in inhibition ratio (p = 0.033) when comparing pre- and post-stimulation in the gamma TIS group. There were no significant differences for other groups (Fig. 6B).
Fig. 6.
(A) Representations in long-interval intracortical inhibition (LICI) showing unconditioned and conditioned MEPs response conditioning stimulus (CS) and testing stimulus (TS) pre- and post-stimulation in the sham, alpha TIS, beta TIS, and gamma TIS groups. (B) Changes of long interval intracortical inhibition (LICI). The ratio of conditioned stimulus to test stimulus alone when the inter-stimulus interval was set to 200 ms. The data are presented as means and the standard error of the mean (SEM); *p ≤ 0.05.
Histological confirmation of stimulated target brain region
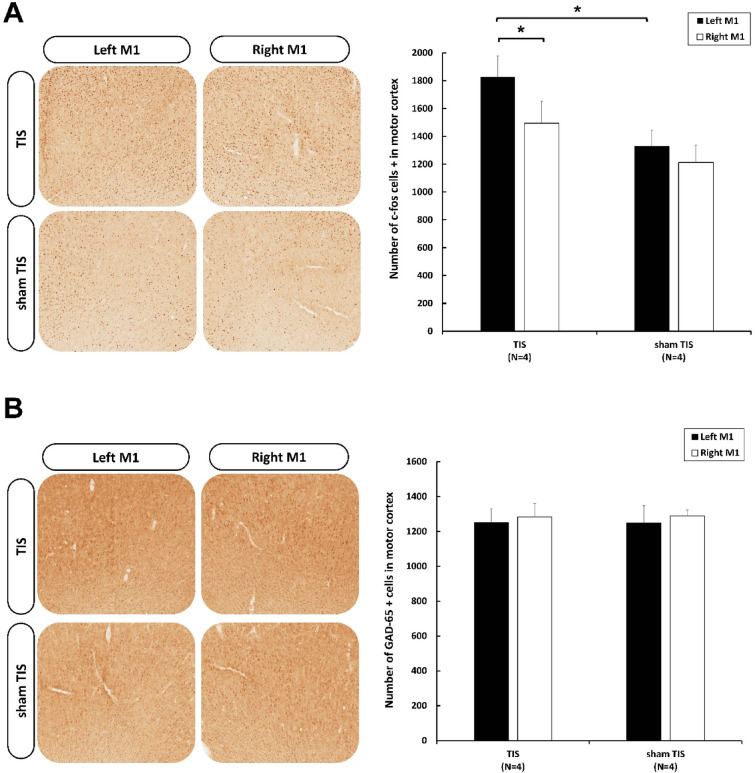
To confirm the positioning of the electrodes targeting the motor cortex (M1), the expression of c-fos and GAD-65 were analyzed after 20 min of alpha TIS.
We observed a significantly higher number of c-fos-positive cells in the stimulated M1 region compared to the sham TIS group (t = -2.582, p = 0.042) and the unstimulated M1 region (t = 10.184, p = 0.002) (Fig. 7A). Statistical analysis also revealed no differences in GAD-65 expression between the two groups (p > 0.05) or between the stimulated and unstimulated brain regions (p > 0.05) (Fig. 7B). Additionally, we found no significant differences in the number of GFAP-positive astrocytes in the stimulated target region compared to the sham group (p > 0.05) and the unstimulated brain region (p > 0.05) (Fig. 8).
Fig. 7.
Changes in cortical expression of (A) c-Fos and (B) GAD-65 following TIS. Asterisks (*) indicate statistically significant differences, either between the stimulated- and non-stimulated regions of the brain within a group, or when compared to the sham group on the same side of the brain. Data are presented as means and the standard error of the mean (SEM); *p ≤ 0.05.
Fig. 8.
Changes in GFAP expression following TIS. Data are presented as means and the standard error of the mean (SEM).
Discussion
The present study aimed to elucidate the neuromodulatory effects of TIS on the motor cortex in rats. To answer this question, we employed different frequencies to determine whether the effects induced by TIS were frequency-dependent. Our results demonstrated that both alpha and gamma TIS significantly increased the excitability of the motor cortex and sustained for 30 min following stimulation, but only gamma TIS could reduce the intracortical inhibition. Furthermore, TIS could regulate neuronal activity at the cellular level within the targeted stimulated cortex region. Taken together, the findings of this study indicated that TIS can effectively modulate motor cortical excitability in rats.
TIS is a promising non-invasive deep brain stimulation as its superior focality, steerability, and tolerability compared to conventional electrical stimulation. While several studies have been conducted to explore the effects of TIS on neurophysiological functions and behaviors in human and animal models, their findings remain inconsistent. In addition, the underlying mechanism of TIS effects has not been fully elucidated, especially with regard to neuromodulation. In regard to the neuromodulatory effects of TIS, it is essential to particularly consider a specific range of stimulation parameters, such as intensity, frequency, and duration, to achieve a comprehensive understanding. For instance, a study in 202320 has employed various beat frequencies (0, 2, 6, 10, 20, 60, 130 Hz) and intensities of TIS (0, 100, 200, 300, 400, 500 µA) to investigate the most influential TIS parameters on modulation of dopamine (DA) releases in the striatum. The authors observed a decline in DA release only after TIS with a beat frequency of 2 Hz and an intensity of 400 µA or greater. Moreover, another study in 2021 has demonstrated that TIS applied on primary motor cortex (M1) at 70 Hz (2 mA, 30 min) significantly promoted reaction time performance in a random reaction time task (SRTT), while TIS at 20 Hz (2 mA, 30 min) enhanced motor learning performance in SRTT and increased motor evoked potential amplitudes compared to sham stimulation23.
To our knowledge, this is the first study to investigate cortical facilitation and inhibition changes following TIS with three different beat frequencies (10, 20, and 70 Hz) targeting the motor cortex in an anesthetized animal model. These frequencies were chosen based on their known physiological relevance in modulating sensorimotor cortical functions37,38. Previous studies have identified alpha and beta frequencies as dominant oscillatory modes in motor cortical areas, closely associated with movement preparation and initiation39–41. Additionally, gamma oscillations have been linked to enhanced cortical activation during significant motor tasks42. Given the mechanistic similarity between TIS and transcranial alternating current stimulation (tACS), it is reasonable to hypothesize that TIS delivered at specific beat frequencies could modulate intrinsic cortical oscillations, thus influencing cortical excitability. Our results indicated that alpha and gamma TIS significantly increased cortical excitability, with lasting effects observed up to 30 min post-stimulation. Although these findings suggest frequency-specific modulation of cortical activity, we acknowledge that the anesthetized state of animals limits definitive conclusions about the direct relationship between induced oscillations and changes in excitability. Interestingly, only gamma TIS induced a significant reduction in intracortical inhibition, consistent with previous findings highlighting the role of gamma oscillations in modulating inhibitory interneuron activity, particularly through the suppression of GABA-A interneurons in the primary motor cortex43. Further investigations, particularly in awake animals, are necessary to confirm and better understand these mechanisms.
Prior research has demonstrated that gamma and beta TIS can facilitate motor learning and enhance motor cortical excitability during various motor tasks, suggesting their potential impact on human motor functions23. Additionally, another study showed that repeated daily sessions of 20 Hz TIS over seven consecutive days could significantly promote motor skills, modulate neurotransmitter metabolism, enhance neuronal calcium influx, neurotransmitter release, and increase synaptic plasticity19. In contrast, our study did not observe significant changes in cortical excitability or inhibition following beta TIS. This discrepancy might reflect fundamental differences in the neural mechanisms activated by beta frequencies compared to alpha and gamma frequencies, or it could stem from variations in experimental parameters such as intensity, duration, electrode configuration, or the anesthetized animal model utilized. Further systematic investigations are necessary to clarify the precise conditions under which beta TIS might be effective and to understand better its distinct neuromodulatory mechanisms.
Neural activation of the stimulated target brain region was validated histologically by examining changes in c-Fos and GAD-65 expression. Numerous studies have demonstrated that c-Fos expression is rapidly induced by various neural stimuli, including sensory input, pharmacological interventions, and neuromodulation techniques such as rTMS, tFUS, tCS, and TIS 12,18,44–48. For instance, Moretti and colleagues revealed that excitatory 10 Hz rTMS caused a significant upregulation of c-fos expression compared to sham stimulation45. In the initial study on TIS, a beat frequency of 10 Hz significantly increased c-fos expression in the hippocampus, indicating strong activation of the targeted brain region12. Likewise, our investigation showed that the increase in the expression of c-fos was significantly observed in the stimulated motor cortex but not in the unstimulated motor cortex or following sham stimulation. In addition, we examined GAD-65, which plays a key role in the synthesis of the inhibitory neurotransmitter GABA49. Our results showed that TIS did not induce changes in the number of GAD-65-positive cells compared to sham stimulation. Further research is needed to elucidate the underlying mechanisms of TIS in modulating inhibitory neuron activity.
Regarding current density, direct comparisons between animal and human TIS studies should consider critical factors such as electrode dimensions, stimulation intensity, and differences in tissue conductivity. Although calculations using standard formulas from conventional tDCS yield high current densities in our animal model (~ 75 mA/cm²), this value does not directly reflect the unique electric field distribution produced by TIS, due to its utilization of interfering high-frequency currents to generate low-frequency modulation at targeted depths50. Recent human TIS studies have safely employed intensities up to 10 mA per channel with minimal perceptible sensation, suggesting different safety thresholds compared to conventional stimulation techniques50,51. Nevertheless, comprehensive research involving advanced computational modeling and human trials is crucial to accurately determine safe operational parameters, ensuring effective translation of preclinical TIS findings to clinical neuromodulation applications. Understanding these nuances is essential for optimizing TIS protocols and bridging animal model insights with human neuromodulatory interventions.
Conclusion
Our findings suggest that TIS can induce neuromodulatory effects with alpha and gamma TIS significantly enhanced cortical excitability in the motor cortex. The TIS technique holds promise as a potential therapy tool for neurological disorders.
Author contributions
TXDN: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing –review & editing. C-WK: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. C-WP: Resources, Writing – original draft, Writing – review & editing. H-LL: Resources, Writing – original draft, Writing – review & editing. M-YC: Resources, Writing – original draft, Writing – review & editing. K-TC: Resources, Writing – review & editing, Funding acquisition, Project administration. T-HH: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
This work was supported by the National Science and Technology Council (NSTC 113-2314-B-182-050-MY3 and NSTC 113-2321-B-002-030) and Chang Gung Medical Foundation, Taiwan (CORPD1P0041, CMRPD1P0141 and CMRPD1N0302).
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ye, H. et al. Neuron matters: neuromodulation with electromagnetic stimulation must consider neurons as dynamic identities. J. Neuroeng. Rehabil. 19(1), 116 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson, M. D. et al. Neuromodulation for brain disorders: challenges and opportunities. IEEE Trans. Biomed. Eng.60(3), 610–624 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fasano, A. & Lozano, A. M. Deep brain stimulation for movement disorders: 2015 and beyond. Curr. Opin. Neurol.28(4), 423–436 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Laxton, A. W. & Lozano, A. M. Deep brain stimulation for the treatment of alzheimer disease and dementias. World Neurosurg.80(3–4), S28e1–S28e8 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Bernstein, J. E. et al. Infections in deep brain stimulator surgery. Cureus11(8), e5440 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fenoy, A. J. & Simpson, R. K. Jr. Risks of common complications in deep brain stimulation surgery: management and avoidance. J. Neurosurg.120(1), 132–139 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Polanía, R., Nitsche, M. A. & Ruff, C. C. Studying and modifying brain function with non-invasive brain stimulation. Nat. Neurosci.21(2), 174–187 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Fregni, F. & Pascual-Leone, A. Technology insight: noninvasive brain stimulation in neurology—perspectives on the therapeutic potential of rTMS and tDCS. Nat. Clin. Pract. Neurol.3(7), 383–393 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Byczynski, G. & Vanneste, S. Modulating motor learning with brain stimulation: Stage-specific perspectives for transcranial and transcutaneous delivery. Prog Neuropsychopharmacol. Biol. Psychiatry. 125, 110766 (2023). [DOI] [PubMed] [Google Scholar]
- 10.Bhattacharya, A. et al. An overview of noninvasive brain stimulation: basic principles and clinical applications. Can. J. Neurol. Sci. / J. Canadien Des. Sci. Neurologiques. 49(4), 479–492 (2022). [DOI] [PubMed] [Google Scholar]
- 11.Davidson, B. et al. Neuromodulation techniques - From non-invasive brain stimulation to deep brain stimulation. Neurotherapeutics21(3), e00330 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grossman, N. et al. Noninvasive deep brain stimulation via temporally interfering electric fields. Cell169(6), 1029–1041e16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mirzakhalili, E. et al. Biophysics of Temporal interference stimulation. Cell. Syst.11(6), 557–572e5 (2020). [DOI] [PubMed] [Google Scholar]
- 14.Hutcheon, B. & Yarom, Y. Resonance, Oscillation and the intrinsic frequency preferences of neurons. Trends Neurosci.23(5), 216–222 (2000). [DOI] [PubMed] [Google Scholar]
- 15.Rampersad, S. et al. Prospects for transcranial Temporal interference stimulation in humans: A computational study. Neuroimage202, 116124 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu, R. et al. Temporal interference stimulation targets deep primate brain. Neuroimage291, 120581 (2024). [DOI] [PubMed] [Google Scholar]
- 17.Lee, S. et al. Feasibility of epidural Temporal interference stimulation for minimally invasive electrical deep brain stimulation: simulation and Phantom experimental studies. J. Neural Eng., 19(5). (2022). [DOI] [PubMed]
- 18.Carmona-Barrón, V. G. et al. Comparing the effects of transcranial alternating current and Temporal interference (tTIS) electric stimulation through whole-brain mapping of c-Fos immunoreactivity. Front Neuroanat., 17. (2023). [DOI] [PMC free article] [PubMed]
- 19.Qi, S. et al. Temporally interfering electric fields brain stimulation in primary motor cortex of mice promotes motor skill through enhancing neuroplasticity. Brain Stimul.17(2), 245–257 (2024). [DOI] [PubMed] [Google Scholar]
- 20.Kwak, Y. et al. Effect of Temporal interference electrical stimulation on phasic dopamine release in the striatum. Brain Stimul. 16(5), 1377–1383 (2023). [DOI] [PubMed] [Google Scholar]
- 21.Piao, Y. et al. Safety evaluation of employing Temporal interference transcranial alternating current stimulation in human studies. Brain Sci., 12(9). (2022). [DOI] [PMC free article] [PubMed]
- 22.Vassiliadis, P. et al. Safety, tolerability and blinding efficiency of non-invasive deep transcranial Temporal interference stimulation: first experience from more than 250 sessions. J. Neural Eng.21(2), 024001 (2024). [DOI] [PubMed] [Google Scholar]
- 23.Ma, R. et al. High Gamma and Beta Temporal Interference Stimulation in the Human Motor Cortex Improves Motor Functions (Cold Spring Harbor Laboratory, 2021). [DOI] [PMC free article] [PubMed]
- 24.Zheng, S. et al. Repetitive Temporal interference stimulation improves jump performance but not the postural stability in young healthy males: a randomized controlled trial. J. Neuroeng. Rehabil.21(1), 38 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karimi, N., Amirfattahi, R., Zeidaabadi, A. & Nezhad Neuromodulation effect of Temporal interference stimulation based on network computational model. Front. Hum. Neurosci., 18. (2024). [DOI] [PMC free article] [PubMed]
- 26.von Conta, J. et al. Characterizing low-frequency Artifacts during Transcranial Temporal Interference Stimulation (tTIS)2p. 100113 (Reports, 2022). 3.
- 27.Beste, C., Münchau, A. & Frings, C. Towards a systematization of brain oscillatory activity in actions. Commun. Biology, 6(1). (2023). [DOI] [PMC free article] [PubMed]
- 28.Wu, C. W. et al. Pilot study of using transcranial Temporal interfering theta-burst stimulation for modulating motor excitability in rat. J. Neuroeng. Rehabil., 21(1). (2024). [DOI] [PMC free article] [PubMed]
- 29.Kilkenny, C. et al. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. J. Pharmacol. Pharmacotherapeutics. 1(2), 94–99 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fonoff, E. T. et al. Functional mapping of the motor cortex of the rat using transdural electrical stimulation. Behav. Brain. Res.202(1), 138–141 (2009). [DOI] [PubMed] [Google Scholar]
- 31.Nguyen, T. X. D. et al. Transcranial burst electrical stimulation contributes to neuromodulatory effects in the rat motor cortex. Front. Neurosci.17, 1303014 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsieh, T. H. et al. Novel use of Theta burst cortical electrical stimulation for modulating motor plasticity in rats. J. Med. Biol. Eng.35(1), 62–68 (2015). [Google Scholar]
- 33.Mohammad Mahdi Alavi, S., Goetz, S. M. & Saif, M. Input-output slope curve Estimation in neural stimulation based on optimal sampling principles(). J. Neural Eng., 18(4). (2021). [DOI] [PMC free article] [PubMed]
- 34.Lara Aparicio, S. Y. et al. Current opinion on the use of c-Fos in neuroscience. NeuroSci3(4), 687–702 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bullitt, E. Expression of c-fos-like protein as a marker for neuronal activity following noxious stimulation in the rat. J. Comp. Neurol.296(4), 517–530 (1990). [DOI] [PubMed] [Google Scholar]
- 36.Lenz, M. & Vlachos, A. Releasing the cortical brake by Non-Invasive electromagnetic stimulation?? rTMS induces LTD of GABAergic neurotransmission. Front. Neural Circuits. 10, 96 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacKay, W. A. Synchronized neuronal oscillations and their role in motor processes. Trends Cogn. Sci.1(5), 176–183 (1997). [DOI] [PubMed] [Google Scholar]
- 38.Armstrong, S., Sale, M. V. & Cunnington, R. Neural Oscillations and the Initiation of Voluntary Movement9 (Frontiers in Psychology, 2018). [DOI] [PMC free article] [PubMed]
- 39.Alegre, M. et al. Oscillatory changes related to the forced termination of a movement. Clin. Neurophysiol.119(2), 290–300 (2008). [DOI] [PubMed] [Google Scholar]
- 40.Johnstone, D. M. et al. Indirect application of near infrared light induces neuroprotection in a mouse model of parkinsonism - an abscopal neuroprotective effect. Neuroscience274, 93–101 (2014). [DOI] [PubMed] [Google Scholar]
- 41.Tamura, Y. et al. Functional relationship between human Rolandic oscillations and motor cortical excitability: an MEG study. Eur. J. Neurosci.21(9), 2555–2562 (2005). [DOI] [PubMed] [Google Scholar]
- 42.Muthukumaraswamy, S. D. Functional properties of human primary motor cortex gamma oscillations. J. Neurophysiol.104(5), 2873–2885 (2010). [DOI] [PubMed] [Google Scholar]
- 43.Guerra, A. et al. Boosting the LTP-like plasticity effect of intermittent theta-burst stimulation using gamma transcranial alternating current stimulation. Brain Stimul.11(4), 734–742 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aydin-Abidin, S. et al. High- and low-frequency repetitive transcranial magnetic stimulation differentially activates c-Fos and zif268 protein expression in the rat brain. Exp. Brain Res.188(2), 249–261 (2008). [DOI] [PubMed] [Google Scholar]
- 45.Moretti, J. et al. Low intensity repetitive transcranial magnetic stimulation modulates brain-wide functional connectivity to promote anti-correlated c-Fos expression. Sci. Rep.12(1), 20571 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Colmenárez-Raga, A. C. et al. Reversible Functional Changes Evoked by Anodal Epidural Direct Current Electrical Stimulation of the Rat Auditory Cortex13 (Frontiers in Neuroscience, 2019). [DOI] [PMC free article] [PubMed]
- 47.Chu, P. C. et al. Weak ultrasound contributes to neuromodulatory effects in the rat motor cortex. Int. J. Mol. Sci.24(3), 2578 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim, M. S. et al. Repeated anodal transcranial direct current stimulation induces neural plasticity-associated gene expression in the rat cortex and hippocampus. Restor. Neurol. Neurosci.35(2), 137–146 (2017). [DOI] [PubMed] [Google Scholar]
- 49.Lee, S. E., Lee, Y. & Lee, G. H. The regulation of glutamic acid decarboxylases in GABA neurotransmission in the brain. Arch. Pharm. Res.42(12), 1031–1039 (2019). [DOI] [PubMed] [Google Scholar]
- 50.Cassarà, A. M. et al. Recommendations for the safe application of Temporal interference stimulation in the human brain part I: principles of electrical neuromodulation and adverse effects. Bioelectromagnetics, 46(2). (2025). [DOI] [PMC free article] [PubMed]
- 51.Cassarà, A. M. et al. Recommendations for the safe application of Temporal interference stimulation in the human brain part II: biophysics, dosimetry, and safety recommendations. Bioelectromagnetics, 46(1). (2025). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.