Abstract
Aims
Significant advancements have been made in the management of obstructive hypertrophic cardiomyopathy (oHCM), yet the extent of left ventricular outflow tract (LVOT) gradient reduction achieved with commonly used pharmacological therapies [beta-blockers (BBs), calcium channel blockers (CCBs), disopyramide, and cardiac myosin inhibitors (CMIs)] relative to each other is still unclear.
Methods and results
PubMed and Scopus were searched up to September 2024. Clinical trials or observational studies that assessed the changes associated with BBs, CCBs, disopyramide, or CMIs in LVOT gradient at rest or with provocation in patients with oHCM were included. Mean changes in LVOT gradients were pooled as mean differences (MD) with 95% confidence intervals (CIs) in a random-effects model. Thirty-seven studies, with 44 arms and 1898 patients, were included in the analysis. At the therapeutic class level, pooled analysis showed that disopyramide was associated with the highest reduction in LVOT gradient at rest [MD: −43.5 (95% CI, −51.6 to −35.3)], followed by CMIs [MD: −34.8 (95% CI, −40.6 to −29.0)], BBs [MD: −20.7 (95% CI, −29.4 to −12.0)], and then CCBs [MD: −14.7 (95% CI, −23.3 to −6.1)], inter-action P < 0.01. Within CMIs, mavacamten had a higher effect than aficamten on gradient reduction; among the included BBs, metoprolol showed the highest gradient reduction, while among CCBs, verapamil was the most effective (inter-action P < 0.01). Similar results were observed for provocable LVOT gradients.
Conclusion
Pharmacological therapies effectively reduced LVOT gradients in oHCM patients to varying degrees, with disopyramide and CMIs showing the highest effect, followed by BBs and CCBs.
Keywords: Hypertrophic cardiomyopathy, Left ventricular outflow tract obstruction, Medical treatment
Introduction
Hypertrophic cardiomyopathy (HCM) is the most common inherited heart disease and associated left ventricular outflow tract (LVOT) obstruction (LVOTO) can result in symptoms (e.g. dyspnoea, angina, or syncope) and reduced functional capacity.1,2 Approximately two thirds of patients with HCM have LVOTO either at rest or with provocation, termed obstructive hypertrophic cardiomyopathy (oHCM).3 Negative ionotropic therapy with beta-blockers (BBs), calcium channel blockers (CCBs), and disopyramide have traditionally been used as initial medical therapy to treat oHCM. These first line therapies may improve pre-load, have a negative chronotropic and inotropic effects, but may also cause side effects. In cases where maximally titrated doses of these agents fail to alleviate symptomatic LVOTO, septal reduction therapy (SRT) with myectomy or alcohol septal ablation had traditionally been undertaken. In the current era, the novel class of cardiac myosin inhibitors (CMI) have changed the landscape and are now used in HCM patients who have persistent LVOTO despite first-line therapy, offering an alternative to invasive SRT in many patients.
Despite widespread use of negative inotropic therapy and increasing utilization of CMIs, there is, to the best of our knowledge, no study that comprehensively assessed their comparative efficacy in reducing LVOT gradients in oHCM. Each negative inotropic medication has potential side effects, and CMIs also carry additional costs and a greater surveillance burden. In light of this, we conducted a systematic review and meta-analysis to compare current guideline-recommended pharmacological therapies (both by class and as separate agents) to better understand their real-world comparative efficacy and relative reduction in LVOT gradients in patients with oHCM.
Methods
The study was conducted in adherence to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement (see Supplementary material online, Table S1).4 An a priori study protocol was established and applied. No Institutional Review Board approval or patient informed consent was required for the systematic review of the literature and meta-analysis.
Literature search strategy
PubMed/Medline and Scopus were interrogated up to September 2024 for a combination of relevant keywords and Medical Subject Headings (MeSH) terms. The full search strategy is presented in Supplementary material online, Table S2. The search was limited to English and human studies. Multiple publications of retrieved articles (i.e. with the same population) were acquired in order to include the most complete or up-to-date study results. Moreover, a manual search of the reference lists of the included studies was conducted to ensure that no relevant studies were overlooked. The first selection was made by reading titles/abstracts and the final selection by reading the full articles. Articles were screened by two independent reviewers (K.A. and M.P.P.), and any discordance was resolved by the opinion of a third reviewer (C.A. or J.M.F.). Duplicates were identified and removed using Endnote 21 (Clarivate, Philadelphia, PA, USA).
Study selection criteria
Original studies were included if met the following criteria: Clinical trials (including randomized or non-randomized approach), experimental studies (before/after treatment), or observational studies that assessed LVOT maximal instantaneous gradient changes (at rest or with provocation) associated with BBs, CCBs, disopyramide or CMIs (specifically mavacamten or aficamten) in patients with oHCM. Studies that reported a provokable LVOT gradient either post-exercise or with Valsalva maneuver were included, with either value used in the analysis as a provokable LVOT gradient. If a study reported gradients from both provocation methods, the measurement with the higher baseline gradient or assessed in a larger sample was chosen for the analysis. If both methods were assessed in the same number of patients, the one with the higher baseline measurement was included. There is data showing close correlation with invasively measured and echocardiographic peak LVOT gradients in HCM, providing validity for the comparison of both hemodynamic and echocardiographic measures in this meta-analysis.5
Exclusion criteria included one or more of the following: non-English articles, absence of a full peer reviewed published manuscript, reviews, book chapters, case reports, retracted articles, studies conducted in vitro or in animals, studies with insufficient data for analysis of LVOT gradients, studies that included infants or children, and articles that included the same population of a previous study or trial. Studies that included patients that underwent invasive SRT (e.g. alcohol septal ablation or myectomy) were excluded to better assess the actual efficacy of the respective pharmacological treatments. Studies that included practolol or dihydropyridine CCBs (e.g. nifedipine) were also excluded given the former was withdrawn from the market due to reported adverse effects and the latter due to vasodilatory effects is not used clinically for LVOT gradient reduction in oHCM.6,7
Data extraction
Data were extracted independently by 2 reviewers (K.A. and M.P.P.) from publications that met the inclusion criteria from the second read using a standardized data extraction electronic form. Discrepancies were resolved by consensus between the two reviewers and the senior investigator. Data that were abstracted included study and patient-related characteristics as follows: first author’s name, year of publication, study location, study design, sample size, treatment type, dose and duration, age, sex, background/concurrent treatment, baseline and follow-up LVOT gradients, and adverse events (AEs).
Risk of bias assessment
The risk of bias in non-randomized studies of interventions (ROBINS-I) tool was used for the risk of bias assessment of the included non-randomized and observational studies.8 This tool includes seven domains and rates the overall risk of bias as low, moderate, serious, critical, or unclear. Moreover, the Cochrane risk-of-bias tool (RoB2) was used for risk of bias assessment of the included RCTs.9 This tool assesses the following items: randomization process, effect of assignment to intervention, effect of adhering to intervention, missing outcome data, measurement of the outcome, and selection of the reported result. Judgment is either low some concerns or high risk of bias. Two reviewers (M.P.P. and G.P.) independently performed this assessment, and disagreements were resolved by discussion between the two reviewers and the senior investigator.
Quantitative data synthesis
The change in LVOT gradient values (across treatment duration from baseline or across the pharmacological intervention group compared with placebo, whenever reported) were collected in mmHg. If not reported, the mean change in LVOT gradient after treatment were calculated as follows: (LVOT gradient value at end of follow-up)—(measurement at baseline). Their related standard deviations (SD) were also computed as follows: SD = square root [(SDpre-treatment)2 + (SDpost-treatment)2−(2R * SDpre-treatment * SDpost-treatment)], assuming a correlation coefficient (R) of 0.5, as described before.10,11 The method by Wan et al.12 was used to calculate the mean and SD when the data were reported as median and range. Likewise, the method by Altman and Bland was used if values were reported as mean and standard error or confidence interval (CI).11,13
Mean changes in LVOT gradients (either after the treatment compared with the baseline agent or the relative changes in the treatment group compared with placebo, whenever reported) were pooled as mean differences (MD) with 95% CI in a random-effects model, given the assumed heterogeneity among the included studies due to variations in study design and patient characteristics. The pooled analysis was performed based on sub-grouping according to treatments classes (i.e. BBs, CCBs, disopyramide, or CMIs) and the individual drug agents, to address heterogeneity and to quantify the efficacy of each treatment separately. A P value for inter-action below 0.1 implies a statistically significant sub-group effect.14 A sensitivity analysis excluding the acute effects studies (i.e. those that assessed the immediate effects of intravenous treatments) was also conducted to evaluate the long-term, sustained therapeutic effects. However, this sensitivity analysis was applied only to the treatment classes, not to the individual drug agents, due to the low number of studies that assessed each individual drug agent after excluding the acute effects studies.
Inter-study heterogeneity was assessed by visual inspection of the forest plots and quantified by I2 and χ2 tests. Heterogeneity was interpreted according to the Cochrane Handbook recommendations. The I2 test was read as follows: 0% to 40%, low heterogeneity; 30% to 60% may represent moderate heterogeneity; and 50% to 90%, may indicate substantial heterogeneity. A χ2 test P value below 0.1 was considered significant heterogeneity. Potential publication bias was evaluated by visual inspection of Begg’s funnel plot asymmetry and statistically confirmed by Egger’s regression test.15 Analyses were performed using R software (v 4.3.2, R Foundation for Statistical Computing, Vienna, Austria).
Results
Flow and characteristics of included studies
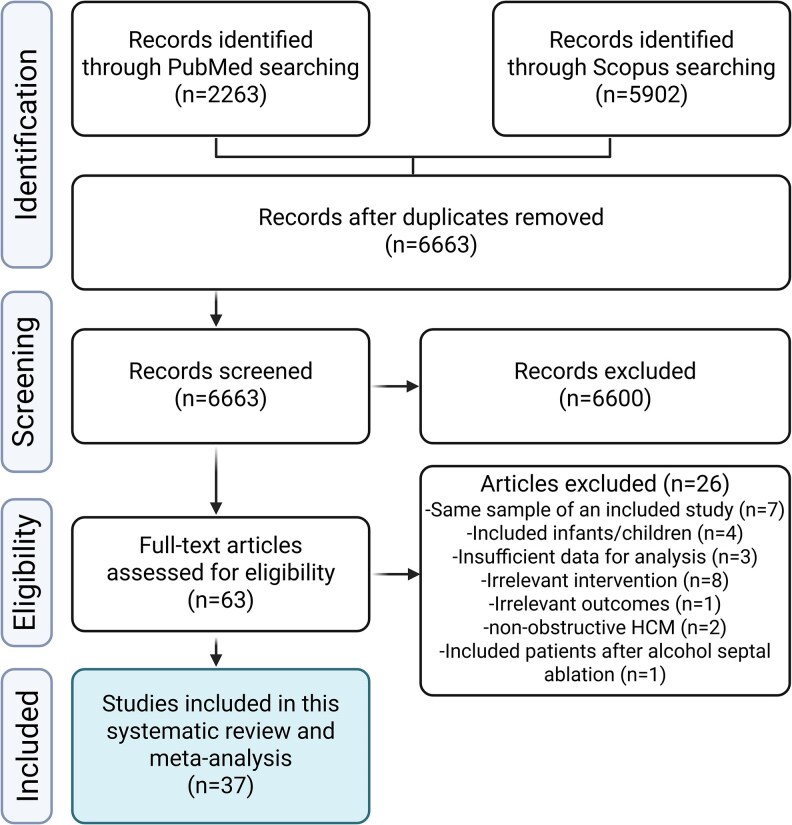
A total of 8165 records were identified through the literature search. After removal of duplicates and the two-step screening approach, 37 articles [with 44 arms, including 11 clinical trials (7 randomized and 4 non-randomized), 13 experimental studies (before/after treatment), and 13 observational studies (7 prospective and 6 retrospective cohorts), Supplementary material online, Table S3] met our inclusion criteria and were included in the analysis. Figure 1 shows the PRISMA flow diagram for the current study along with the reasons for excluded studies. The current analysis included 1898 patients with oHCM. The average age of the included sample was 51 years (9.7) and 57% of subjects were male. The median follow-up was 13.6 months (IQR 7.4). The publication year of the included studies ranged from 1979 up to 2024. Table 1 shows the baseline characteristics of the included studies. In addition, Supplementary material online, Table S4 summarize the reported AEs of the included drugs.
Figure 1.
Study flow diagram showing the selection process of studies included in the systematic review and meta-analysis.
Table 1.
Characteristics and baseline parameters of the studies included in the meta-analysis
| Study | Study design | Sample | Intervention | Dose | Concurrent treatments | Follow-up time | Age** | Males** | Baseline rest LVOT gradient | Baseline provokable LVOT gradient |
|---|---|---|---|---|---|---|---|---|---|---|
| Monda et al.16 | Observational retrospective cohort study | 92 | Bisoprolol | 5.7 (3.2) mg | None | 6 months (3 to 7) | 49.9 (16.5) | 54 (59%) | 69 (15) | NA |
| Dybro et al.17 | RCT | 29 | Metoprolol | 50, 100, 150 mg | None | 6 weeks | 60 (11) | 18 (62%) | 62.3 (46) | 113.66 (90.44) |
| Haruki et al.18 | Observational retrospective cohort study | 33 | Disopyramide | 271 (81) mg/day | 27 BBs and 9 CCBs | 9.58 (7.14) years | 56.8 (16.1) | 10 (30.3%) | 74.5 (26.4) | NA |
| Sherrid et al.19 | Observational prospective cohort study | 141 | Disopyramide | 600 mg/day | BBs or verapamil | 4.5 (3.6) years | 56.7 (15) | 153 (51%) | 59 (46) | NA |
| Nistri et al.20 | Observational prospective cohort study | 27 | Nadolol | 80 mg/day | None | 12 (4) months | 36 (15) | 22 (81%) | NA | 87 (29) |
| Kajimoto et al.21 | Non-randomized trial | 24 | Disopyramide | 1.0 mg/kg/5min | None | 5 min post-administration | 55 (42–58)* | NA | 92.3 (32.9) | NA |
| Kajimoto et al.21 | 12 | Propranolol | 0.20 mg/kg/5min | None | 5 min post-administration | 55 (42–58)* | NA | 93.9 (23.1) | NA | |
| Kajimoto et al.21 | 12 | Verapamil | 0.10 mg/kg/5min | None | 5 min post-administration | 55 (42–58)* | NA | 86.9 (23.3) | NA | |
| Sherrid et al.22 | Observational retrospective cohort study | 78 | Disopyramide | 600 mg/day | 70 BBs, 35 CCBs, and 11 both | 3.1 (2.6) years | 47 (20) | 60 (51%) | 75 (33) | NA |
| Sherrid et al.22 | 40 | Disopyramide | 600 mg/day | 73 (35) | NA | |||||
| Betocchi et al.23 | Experimental study (before/after treatment) | 16 | Diltiazem | 0.25 mg/kg over 2 min, then 0.014/mg/kg/min over 10 min | None (withdrawn before the study) | After 10 min of the infusion | 38 | 11 (68.7%) | 46 (36) | NA |
| Matsubara et al.24 | Experimental study (before/after treatment) | 6 | Disopyramide | 50 mg or 100 mg | None (withdrawn before the study) | After administration of the drug | 55 (8) | 3 (50.0%) | 100 (45) | NA |
| Kimball et al.25 | Experimental study (before/after treatment) | 25 | Disopyramide | 100 mg | None (withdrawn before the study) | After administration of the drug | 40 (18–70)* | 12 (48%) | 86 (34) | 124 (33) |
| Dimitrow and Dubiel26 | Experimental study (before/after treatment) | 20 | Pindolol | 5 mg/twice daily | Verapamil | 6 months | 37 (8) | 15 (75%) | 29 (30) | NA |
| Millaire et al.27 | Experimental study (before/after treatment) | 9 | Disopyramide | 500 (50) mg/day | NA | 1 month | 39 (14) | 6 (66.7%) | 83 (51) | 107 (37) |
| Pollick et al.28 | Experimental study (before/after treatment) | 43 | Disopyramide | 2 mg/kg | NA | After completion of drug infusion | 44 | 25 (58.1%) | 74 (34) | 138 (44) |
| Pollick et al.29 | RCT | 10 | Disopyramide | 150 mg four times a day | NA | 4 days | 48 | 8 (80%) | 61 (20) | NA |
| Pollick et al28 | 10 | Propranolol | 40 mg four times a day | NA | 4 days | 48 | 8 (80%) | 61 (20) | NA | |
| Sherrid et al.30 | Experimental study (before/after treatment) | 7 | Disopyramide | 150 mg every 6 h | None | 23 days | 64 | 3 (42.8%) | 63.57 (14.66) | NA |
| Anderson et al.31 | Experimental study (before/after treatment) | 15 | Verapamil | 690 mg/day | All on propranolol | 6 months | 52 | 11 (73.3%) | 57 (155) | 114 (232.37) |
| Bonow et al.32 | Experimental study (before/after treatment) | 10 | Verapamil | 0.007, 0.014, and 0.021 mg/kg/min for 10 min each | None | At the completion of drug infusion | 47 | 7 (50.0%) | 62 (27) | NA |
| Tendera et al.33 | Experimental study (before/after treatment) | 8 | Verapamil | 0.1 mg/kg over two minutes, followed by infusion of 0.01 mg/kg/min. | None | After 10 min of the infusion | 33 (10) | 13 (81.2%) | 50 (37) | NA |
| Landmark et al.34 | Non-randomized trial | 6 | Propranolol | 5 mg intravenously over 5 min | None | After 10 min of the injection | 45 (26–67) | NA | 81 (22) | NA |
| Pollick et al.35 | Experimental study (before/after treatment) | 4 | Disopyramide | 100 mg (IV), 300 mg (acute/24 h), 200 mg 4 times day (maintenance) | None | After the injection | 23–62 | 3 (60.0%) | 50.25 (24.68) | 120.5 (54.24) |
| Storstein et al.36 | non-randomized trial | 7 | Pindolol | 10 mg | None | After the injection | 39.1 | 4 (57.1%) | 35.4 (35.8) | NA |
| Kaltenbach et al.37 | Experimental study (before/after treatment) | 9 | Verapamil | 480 mg/day | None | 15 months (range 4–24 months) | 19–44 | 18 (81.8%) | 21.55 (27.35) | 51.11 (19.83) |
| Rosing et al.38 | Experimental study (before/after treatment) | 14 | Verapamil | 0.007, 0.014 and 0.021 mg/kg/min. | Propranolol | After 10 min of the infusion | 44 (3) | 16 (59.2%) | 86 (26.2) | 90 (27.71) |
| Todde et al.39 | Observational prospective cohort study | 47 | Disopyramide | 220 (29) mg/day | 41 BBs and 6 CCBs | 4.4 (3.6) years | 52 (14) | 36 (57%) | 78 (25) | NA |
| Todde et al.39 | 15 | Disopyramide | 223 (46) mg/day | 11 BBs and 4 CCBs | 50 (13) | 36 (57%) | 79 (38) | NA | ||
| Abood et al.40 | Observational retrospective cohort study | 31 | Mavacamten | 5 mg | 31 BBs, 25 CCBs, and 6 Disopyramide | 12 weeks | 58 (16.5) | 16 (51.6%) | NA | 77.7 |
| Desai et al.41 | Observational prospective cohort study | 150 | Mavacamten | 5 mg | 124 BBs, 36 CCBs, and 7 Disopyramide | 12 weeks | 65 (12) | 71 (47.0%) | 41 (33) | 72 (43) |
| Ramonfaur et al.42 | Observational prospective cohort study | 66 | Mavacamten | 2.5, 5, 10, and 15 mg/day | 59 BBs, 19 CCBs, and 3 Disopyramide | 18 months | 58.8 (15) | 31 (47%) | 56 (43) | 107 (44) |
| Reza et al.43 | Observational retrospective cohort study | 80 | Mavacamten | 5 mg | 31 BBs, 8 CCBs, 3 Disopyramide | 36 weeks | 62.9 (14.3) | 45 (46%) | 56.6 (39.1) | 78.4 (33.0) |
| Roehl et al.44 | Observational retrospective cohort study | 34 | Mavacamten | <2.5 or >2.5 mg | NA | 12 weeks | NA | NA | 72.8(20.2), 0.007 (long-term) | NA |
| Wessly et al.45 | Observational prospective cohort study | 15 | Mavacamten | 5 mg | NA | 30 days | 70 (13) | 6 (40.0%) | 45 (23) | 79 (28) |
| Tian et al.46 | RCT | 81 | Mavacamten | 2.5 mg | BBs (72 in control, 48 in mavacamten), CCBs (6 in control, 4 in mavacamten), others (3 in control, 2 in mavacamten) | 30 weeks | 51.9 (11.9) | 58 (71.6%) | 74.6 (35.1) | 106.8 (43.2) |
| Olivotto et al.47 | RCT | 251 | Mavacamten | 5 mg | BBs (189 in control, 94 in mavacamten), CCBs (42 in control, 25 mavacamten) | 30 weeks | 58.5 (12.2) | 149 (59%) | 52 (59) | 86 (34) |
| Heitner et al.48 | Non-randomized trial | 11 | Mavacamten | 10–20 mg/day | No background therapy | 12 weeks | 56 (22–70)* | 7 (64%) | 60 (28) | 103 (50) |
| Heitner et al.48 | 10 | Mavacamten | 2–5 mg/day | 9 BBs | 12 weeks | 58 (26–67)* | 5 (50%) | 86 (63) | 86 (43) | |
| Desai et al.49 | RCT | 112 | Mavacamten | 2.5, 5, 10, or 15 mm/day | 106 (BBs, CCBs, Disopyramide or combination) | 16 weeks | 59.8 (14.2) | 29 (51.8%) | 51.2 (31.4) | 82.5 (34.7) |
| Maron et al.50 | RCT | 282 | Aficamten | 20 mg | 241 (BBs, CCBs, Disopyramide) | 24 weeks | 59.2 (12.6) | 167 (59%) | 54.8 (27) | 82.9 (32) |
| Maron et al.51 | RCT | 27 | Aficamten | 5–30 mg | All on either BBs or CCBs | 12 weeks | 57 (26–33) | 13 (46.4%) | 53.8 (cohort 1), 58.2 (cohort 2) | 74.4 (cohort 1), 82.3 (cohort 2) |
| Owens et al.52 | Observational prospective cohort study | 13 | Aficamten | 15 mg | 10 BBs, 2 CCBs, and 1 both | 12 weeks | 62 (58–65) | 6 (46.1%) | 45(38–62) | 86 (78–103) |
Continuous variables are reported as mean (standard deviation) or *median (range or inter-quartile range), and categorical variables are reported frequency (percentage). **If the data on the included sub-group for these variables is not reported, we used the data for the whole study sample. RCT, randomized controlled trial; BB, beta-blockers; CCB, calcium channels blockers; LVOT, left ventricular outflow tract; NA, not available.
Risk of bias in the included studies
According to the ROBINS-I tool, most of the included observational/non-randomized studies (22 out of 30) showed a high risk of bias mainly due to the incomplete control for the potential confounders; these studies were predominately older studies from the early oHCM era without control groups. Meanwhile, most of the included clinical trials showed a low risk of bias according to the RoB 2 tool. Details of the risk of bias assessment using both tools are shown in Supplementary material online, Table S5.
Analyses results on the effect of included treatments on LVOT gradients
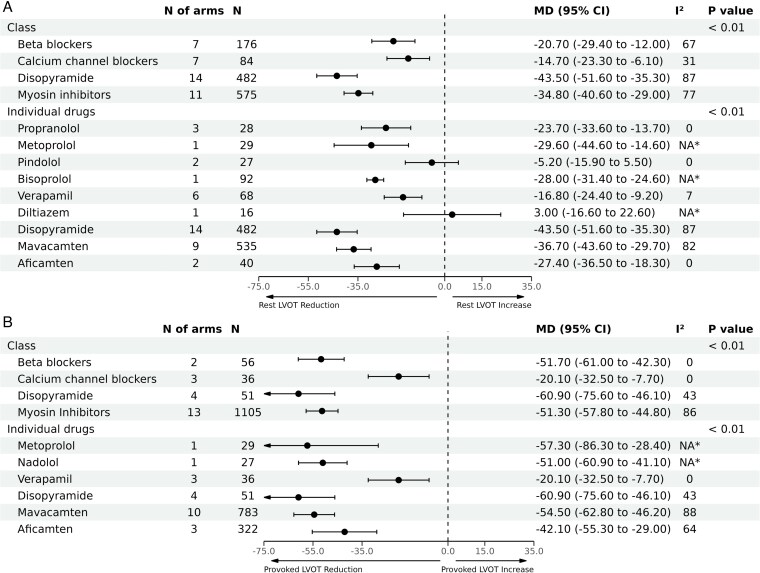
At the therapeutic class level, pooled analyses showed that disopyramide and CMIs were associated with the highest reduction in LVOT gradient at rest [MD: −43.5 mmHg (95% CI, −51.6 to −35.3), n = 482] and [MD: −34.8 mmHg (95% CI, −40.6 to −29.0), n = 575], respectively; followed by BBs [MD: −20.7 mmHg (95% CI, −29.4 to −12.0), n = 176], then CCBs [MD: −14.7 mmHg (95% CI, −23.3 to −6.1), n = 84], inter-action P < 0.01 (Figure 2). A high degree of heterogeneity was observed across the pooled estimates for the included classes (I2 ranged from 67% up to 87%, Figure 2), except for the CCBs (I2 = 31%).
Figure 2.
Pooled analyses by therapeutic class and individual agent for mean difference in left ventricular outflow tract (LVOT) gradient reduction. Panel (A) demonstrates mean difference in reduction of resting LVOT gradients, and Panel (B) demonstrates mean difference in reduction of LVOT gradients with provocation. *Not applicable because only one study was included in this analysis.
On an individual agent level within each class, mavacamten had higher effect on LVOT gradients [MD: −36.7 mmHg (95% CI, −43.6 to −29.7), n = 535] than aficamten, although the latter is still under investigation and has smaller numbers available for pooling at this time. Metoprolol showed the highest reduction in LVOT gradient at rest among BBs [MD: −29.5 mmHg (95% CI, −44.6 to −14.6), n = 29], whilst among CCBs, verapamil was the most effective [MD: −16.8 mmHg (95% CI, −24.4 to −9.2), n = 68], with very small number of patients available for diltiazem. The comparison among the individual agents was significant with inter-action P < 0.01, Figure 2. Significant substantial heterogeneity was observed for disopyramide (87%), and mavacamten (82%), while was insignificant for the other agents. Similar results were observed in terms of LVOT reduction with provocation (Figure 2). However, significant heterogeneity was only observed for CMIs (86%) at the class level and with mavacamten and aficamten at the level of individual agents (88% and 64%, respectively). Summary of the changes in LVOT gradients in each of the included studies is shown in Table 2.
Table 2.
Summary of changes in left ventricular outflow tract gradient at rest and with provocation induced by the included interventions
| Study | Intervention | Sample | LVOT gradient at rest | LVOT gradient with provocation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Change [MD (SE)] | ≥50 mmHg pre-treatment | <50 mmHg post-treatment | ≥30 mmHg pre-treatment | <30 mmHg post-treatment | Change [MD (SE)] | ≥50 mmHg pre-treatment | <50 mmHg post-treatment | ≥30 mmHg pre-treatment | <30 mmHg post-treatment | |||
| Monda et al.16 | Bisoprolol | 92 | −28 (1.7) | NA | 57 (62) | NA | 33 (35.9) | NA | 92 (100) | NA | 92 (100) | NA |
| Dybro et al.19 | Metoprolol | 29 | −29.6 (7.7) | NA | NA | NA | NA | −57.3 (14.8) | NA | NA | NA | NA |
| Haruki et al.18 | Disopyramide | 33 | −43.1 (4.5) | NA | NA | 33 (100) | NA | NA | NA | NA | NA | NA |
| Sherrid et al.19 | Disopyramide | 141 | −41 (3.4) | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Nistri et al.20 | Nadolol | 27 | NA | 0 (0) | NA | 0 (0) | NA | −51 (5) | 25 (92.6) | 19 (70.4) | 27 (100) | 14 (51.9) |
| Kajimoto et al.21 | Disopyramide | 24 | −51.7 (6.2) | 21 (87.5) | 18 (75) | 24 (100) | 10 (41.7) | NA | NA | NA | NA | NA |
| Kajimoto et al.21 | Propranolol | 12 | −17.1 (7.8) | 12 (100) | 3 (25) | 12 (100) | 0 (0) | NA | NA | NA | NA | NA |
| Kajimoto et al.21 | Verapamil | 12 | −6.5 (6.7) | 11 (91.7) | 1 (8.3) | 12 (100) | 0 (0) | NA | NA | NA | NA | NA |
| Sherrid et al.22 | Disopyramide | 78 | −35 (3.7) | 78 (100) | NA | 78 (100) | NA | NA | NA | NA | NA | NA |
| Sherrid et al.22 | Disopyramide | 40 | −10 (5.2) | 40 (100) | 11 (27.5) | 40 (100) | NA | NA | NA | NA | NA | NA |
| Betocchi et al.23 | Diltiazem | 16 | 3 (10) | NA | NA | 11 (68.8) | NA | NA | NA | NA | NA | NA |
| Matsubara et al.24 | Disopyramide | 6 | −74 (16.5) | 5 (83.3) | 5 (83.3) | 6 (100) | 5 (83.3) | NA | NA | NA | NA | NA |
| Kimball et al.25 | Disopyramide | 25 | −59 (5.9) | NA | NA | NA | NA | −60 (6.6) | NA | NA | NA | NA |
| Dimitrow et al.26 | Pindolol | 20 | −7 (6) | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Millaire et al.27 | Disopyramide | 9 | −45 (14.9) | 6 (66.7) | 6 (66.7) | 7 (77.8) | 5 (55.6) | −38 (13.6) | 7 (77.8) | 4 (44.4) | 7 (77.8) | 2 (22.2) |
| Pollick et al.28 | Disopyramide | 43 | −62 (4.5) | NA | 41 (95.3) | 40 (93) | 37 (86) | −76 (9.9) | NA | NA | NA | NA |
| Pollick et al.28 | Disopyramide | 10 | −56 (5.7) | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Pollick et al.28 | Propranolol | 10 | −31 (8.4) | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Sherrid et al.30 | Disopyramide | 7 | −50 (5.7) | 6 (85.7) | 7 (100) | 7 (100) | 6 (85.7) | NA | NA | NA | NA | NA |
| Anderson et al.31 | Verapamil | 15 | −23 (43.8) | NA | NA | NA | NA | −29 (68.1) | NA | NA | NA | NA |
| Bonow et al.32 | Verapamil | 10 | −30 (10) | 7 (70) | 7 (70) | 9 (90) | 5 (50) | NA | NA | NA | NA | NA |
| Tendera et al.33 | Verapamil | 8 | −25 (11.8) | 2 (25) | 7 (87.5) | 6 (75) | 5 (62.5) | NA | NA | NA | NA | NA |
| Landmark et al.34 | Propranolol | 6 | −24 (11) | 6 (100) | NA | 6 (100) | NA | NA | NA | NA | NA | NA |
| Pollick et al.35 | Disopyramide | 4 | −48.75 (12) | 2 (50) | 4 (100) | 3 (75) | 4 (100) | −66.75 (23.4) | 4 (100) | 2 (50) | 4 (100) | 1 (25) |
| Storstein et al.36 | Pindolol | 7 | 4.6 (13.9) | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Kaltenbach et al.37 | Verapamil | 9 | −12. 6 (7.9) | 2 (22.2) | 9 (100) | 3 (33.3) | 8 (88.9) | −25 (9.5) | 5 (55.6) | 7 (77.8) | 8 (89) | 6 (66.7) |
| Rosing et al.38 | Verapamil | 14 | −22 (7.5) | NA | NA | 14 (100) | 3 (21.4) | −16 (8.5) | NA | NA | 12 (85.7) | NA |
| Todde et al.39 | Disopyramide | 47 | −35 (4.3) | NA | 35 (74.5) | NA | NA | NA | NA | NA | NA | NA |
| Todde et al.39 | Disopyramide | 15 | −13 (8.8) | NA | 4 (26.7) | NA | NA | NA | NA | NA | NA | NA |
| Abood et al.40 | Mavacamten | 31 | NA | NA | NA | NA | NA | −60.9 (4.5) | 31 (100) | 26 (83.9) | 31 (100) | 26 (83.9) |
| Desai et al.41 | Mavacamten | 150 | −26 (0.2) | NA | NA | 150 (100) | NA | −43 (0.3) | 142 (94.7) | 114 (76) | 150 (100) | 106 (70.7) |
| Ramonfaur et al.42 | Mavacamten | 66 | −37 (4.9) | 61 (92.4) | NA | 61 (92.4) | NA | −80 (7.1) | 61 (92.4) | NA | 61 (92.4) | NA |
| Reza et al.43 | Mavacamten | 56 | −38.6 (4.5) | NA | NA | 56 (100) | 48 (85.7) | −52.8 (3.8) | NA | NA | 56 (100) | 48 (85.7) |
| Roehl et al.44 | Mavacamten | 34 | −26.2 (5.2) | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Wessly et al.45 | Mavacamten | 15 | −40 (5.2) | NA | NA | NA | NA | −65 (6.4) | NA | NA | NA | NA |
| Tian et al.46 | Mavacamten | 81 | −55 (7.2) | NA | NA | NA | NA | −70.29 (9.9) | 81 (100) | 32 (59) mavacamten vs. 3 (11) placebo | 81 (100) | 26 (48) mavacamten vs. 1 (3.7) placebo |
| Olivotto et al.47 | Mavacamten | 251 | NA | NA | NA | NA | NA | −35.6 (3.9) | 251 (100) | 97 (79) mavacamten vs. 44 (34) placebo | 251 (100) | 74 (60) mavacamten vs. 22 (17.2) placebo |
| Heitner et al.48 | Mavacamten | 11 | −47.8 (12.4) | NA | 10 (90.9) | 11 (100) | 10 (90.9) | −89.5 (24.9) | 11 (100) | 10 (91) | 11 (100) | 8 (72.7) |
| Heitner et al.48 | Mavacamten | 10 | −48.5 (17.5) | NA | NA | 10 (100) | NA | −47.1 (17.9) | 10 (100) | 4 (40) | 10 (100) | 0 (0) |
| Desai et al.49 | Mavacamten | 112 | −33.4 (4.5) | NA | NA | NA | NA | −37.2 (5.6) | NA | NA | NA | NA |
| Maron et al.50 | Aficamten | 282 | NA | NA | NA | NA | NA | −50 (3.3 | 282 (100) | NA | 282 (100) | 70 (49) aficamten vs. 5 (3.6) placebo |
| Maron et al.51 | Aficamten | 27 | −28 (7.2) | NA | NA | NA | NA | −43 (9.1) | NA | NA | NA | NA |
| Owens et al.52 | Aficamten | 13 | −27 (6.1) | NA | NA | 13 (100) | 7 (53.8) | −28 (8.9) | NA | NA | NA | NA |
Categorical variables are reported frequency (percentage).
MD, mean difference; SE, standard error; LVOT, left ventricular outflow tract; NA, not available.
Results of the sensitivity analysis excluding the acute effects studies
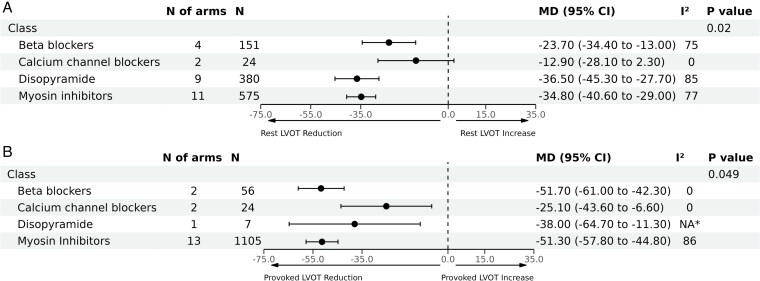
As a sensitivity analysis, we excluded studies with measurement of immediate effects of the relevant treatments, which resulted in 26 articles (with 31 arms) remaining for analysis. Consistent with the previous results, disopyramide and CMIs were associated with the highest reduction in LVOT gradient at rest [MD: −36.5 mmHg (95% CI, −45.3 to −27.7), n = 380] and [MD: −34.8 mmHg (95% CI, −40.6 to −29.0), n = 575], respectively; followed by BBs [MD: −23.7 mmHg (95% CI, −34.4 to −13.0), n = 151]. However, the effect of CCBs on LVOT gradient at rest became insignificant [MD: −12.9 mmHg (95% CI, −28.1 to 2.3), n = 24], inter-action P < 0.01 (Figure 3). Significant heterogeneity was observed across the pooled estimates for the included classes (I2 ranged from 75% up to 85%, Figure 3), except for the CCBs (I2 = 0).
Figure 3.
Pooled analyses by therapeutic class for the mean difference in left ventricular outflow tract (LVOT) gradient reduction after a sensitivity analysis excluding studies that measured the immediate effects of the relevant treatments. Panel (A) demonstrates mean difference in reduction of resting LVOT gradients, and Panel (B) demonstrates mean difference in reduction of LVOT gradients with provocation. *Not applicable because only one study was included in this analysis.
In a similar sensitivity analysis for LVOT gradients with provocation, CMIs and BBs were associated with the highest reduction [MD: −51.3 mmHg (95% CI, −57.8 to −44.8), n = 1105] and [MD: −51.7 mmHg (95% CI, −61.0 to −42.3), n = 56], respectively; followed by disopyramide [MD: −38.0 mmHg (95% CI, −64.7 to −11.3), n = 7], then CCBs [MD: −25.1 mmHg (95% CI, −43.6 to −6.6), n = 24], inter-action P = 0.049 (Figure 3). Significant heterogeneity was observed only in terms of CMIs (I2 = 86%).
Publication bias
The funnel plots for LVOT gradient at rest and during provocation appeared symmetrical, suggesting no significant publication bias (see Supplementary material online, Figure S1). This observation was further supported by the Egger’s test, which yielded P-values of 0.74 for the resting gradient and 0.5 for the provoked gradient, indicating no evidence of publication bias.
Discussion
In this systematic review and meta-analysis comparing the effect of currently available pharmacological therapies on LVOT gradient reduction in patients with oHCM, disopyramide and CMIs were associated with the highest mean reduction in LVOT gradient followed by BBs, and CCBs, both on resting and provokable gradients. These results were maintained in a sensitivity analysis assessing only the oral treatment regimens on the resting LVOT gradient, except for CCBs where the effect became insignificant in the setting of the limited number of studies that assessed CCBs in the sensitivity analysis (i.e. only 2 studies). Whereas for LVOT gradients with provocation, CMIs and BBs were associated with the highest reduction, followed by disopyramide then CCBs.
Although prior meta-analyses have assessed the effect of CMIs on LVOT gradients53 and compared the overall effect of structured physical exercise programmes with pharmacologic therapy in general and invasive therapies on LVOT gradient reduction,54 to our knowledge this is the first systematic review and meta-analysis to assess and report the degree of reduction of LVOTO among all available pharmacologic classes and agents with inter-action testing for oHCM management including negative inotropic agents and CMIs.
Guideline directed clinical practice utilizes negative ionotropic therapy for treatment of symptomatic oHCM in the first instance, and if LVOTO persists despite maximal dose up-titration, then either a second negative inotropic agent or a CMI are added. Specifically, American Heart Association/American College of Cardiology (AHA/ACC) and European Society of Cardiology (ESC) guidelines recommend BBs as a first line to relieve symptoms caused by LVOTO.2,55,56 Non-dihydropyridine CCBs (either verapamil or diltiazem) are recommended as alternatives if BBs are ineffective despite using maximum tolerated doses, or if not tolerated because of side effects.57 If symptomatic LVOTO persists despite first line therapy, the AHA/ACC 2024 guideline recommend that treatment with disopyramide, CMI, and SRT (either percutaneous alcohol septal ablation or surgical myectomy) should all be discussed with the patient.2 The advent of CMIs has been suggested to reduce eligibility or need for SRT.58 The ESC guidelines follow a similar approach, but they reserve SRT for patients who continue to experience symptoms despite receiving maximal medical therapy.56 In addition, surgical myectomy may need to be considered in patients who have other indications for surgery, such as primary mitral valve disease.
Choosing between these treatment options should involve shared decision-making with the patient and is often influenced by clinician preference and experience.55 Although these classes and several agents therein are used pharmacologically to treat LVOTO, there has been no study to date comparing their relative efficacy on the degree of LVOT gradient reduction. Such data are of clinical importance, as it may guide clinician decision-making regarding therapy. The findings from this meta-analysis suggest that disopyramide and CMIs are associated with a greater reduction in LVOT gradient compared with BBs and CCBs. BBs appeared to have a greater effect on LVOT gradient reduction than CCBs within the negative inotrope group of medications, and among BBs, metoprolol was associated with the greatest LVOT gradient reduction. Among CCBs, there was data to support the use of verapamil (albeit with lower comparative effect on LVOT gradients compared with other agents); interestingly diltiazem, a commonly used CCB in oHCM, did not show significant reduction in LVOT gradient at rest. However, this finding is restricted by limited data with only one study evaluating the effects of this specific agent and should be considered with caution.
Disopyramide appeared to have a slightly greater effect on LVOT gradient reduction than CMIs, and this data are important as disopyramide represents a cost-effective alternative to CMIs for patients who do not achieve a satisfactory response with BBs and CCBs.59 CMIs in addition to greater cost also have an associated significant burden of echocardiographic surveillance for ejection fraction during treatment. However, precautions are required with the use of disopyramide, as it prolongs the QT interval, thereby increasing the risk of arrhythmias55; in addition, disopyramide may have anti-cholinergic gastrointestinal side effects.60
Among CMIs, this study also found that mavacamten may result in a greater mean reduction in LVOT gradients compared with aficamten. However, this finding is limited by the smaller number of studies currently available for aficamten. Aficamten has been suggested to have a superior pharmacologic profile given its shorter half-life and fewer drug inter-actions compared with mavacamten. This in turn may make aficamten an acceptable alternative option once further data accrues for its efficacy.61 CMIs, particularly mavacamten, in addition to LVOT gradient reduction and symptoms relief, may additionally improve cardiac remodelling, with potential disease modifying effect by specifically targeting the underlying pathophysiologic mechanisms in oHCM62; such a benefit over and above degree of LVOT gradient reduction may not be present in the negative inotropic therapy group, and is beyond the scope of this meta-analysis to assess.
A higher efficacy of metoprolol was noted in our study; however, prior data on direct head-to-head comparisons between this agent and other BBs for LVOT reduction is scarce. The greater effectiveness of metoprolol in reducing LVOT gradients is likely related to both its pharmacokinetic and pharmacodynamic properties, including its cardioselectivity and predominant action as a selective beta-1 receptor blocker. As such, it may exert stronger negative chronotropic and inotropic effects, thereby enabling a longer diastolic filling period and a greater reduction in LVOT gradients.63,64 Similarly, the potential higher efficacy of verapamil in reducing LVOT gradients in oHCM compared with the other non-dihydropyridine CCB can be explained by pre-clinical studies demonstrating that verapamil exerts a stronger negative inotropic and chronotropic effect.65 Verapamil may also increase myocardial oxygen delivery and decrease sympathetic nervous system activity to a greater extent, which could further enhance its ability to reduce the LVOT gradient compared with the other CCB.66
Findings herein have relevant clinical implications. This comprehensive and relative comparison of all available agents for oHCM in this meta-analysis may help guide physician decision-making regarding specific agent and classes of drugs for initial therapy for oHCM. It may also inform planning of future trials for oHCM therapies for comparison arms. Specifically, within BBs, which are first line agents, metoprolol appears to have the greatest effect on LVOTO gradients, and pindolol, which is occasionally used has weaker evidence for its efficacy. CCBs appear to be less efficacious than BBs, and the findings of this study as such support guidelines recommendations for BB as first line agents; within CCBs, verapamil has more data for efficacy than diltiazem. Disopyramide and CMIs appear to be effective add-on agents to BBs, with disopyramide appearing to have greater effect.
This meta-analysis has several limitations, and overall observations need to be considered cautiously due to the observational nature of the studies evaluating negative inotrope therapies (BBs, CCBs, and disopyramide), with these studies being older, with small sample sizes and lacking randomization, blindness, and a control group. In contrast, CMIs which were studied in the contemporary era had randomized controlled trials to support their efficacy. Indirect treatment comparison methods (such as matching-adjusted indirect comparison or network analysis) were not possible because of inability to access individual participate data with the older studies. Additionally, multiple studies evaluating disopyramide and CMIs included background/concurrent treatments with other agents (e.g. BBs or CCBs) that may have contributed to the observed reduction in LVOT gradient. However, for the placebo-controlled trials, we assessed the relative reduction in LVOT gradient induced by the relevant intervention (i.e. compared with placebo) in our analysis to adjust for the background treatments. For the single-arm studies, the baseline LVOT gradient represented a residual gradient after treatment with the first-line medications. Therefore, subsequent changes from these baseline gradients can be mainly attributed to the addition of the new drug that is being evaluated. Of note, this effectively reflects real-world clinical practice, as outlined in the most recent guidelines.
There was significant heterogeneity among the included studies including differing drug doses and duration, follow-up times between baseline and follow-up measurements of LVOT gradient and the methods used to measure the LVOT gradient (invasive vs. Doppler by echocardiogram, with considerable technical differences due to the progress in the last decades). However, published data suggests a close correlation between invasive and echocardiographic measurements of LVOT gradient.5 In addition, because of the small number of studies that investigated each individual drug, further sub-group analyses (e.g. based on drug doses) to assess the mentioned heterogeneity were not feasible. The results for some agents, such as diltiazem and some BBs, were constrained by the limited number of studies available. Finally, the influence of different HCM genotypes was not assessed in most of the included studies, except in three mavacamten trials. However, the response to treatment across these trials was similar between individuals with known genetic variants and those without identified mutations.
Conclusions
Most currently used pharmacological therapies reduced LVOT gradient in oHCM patients, with disopyramide and CMIs showing the highest effect, followed by BBs and CCBs. Different agents within each class had differing efficacy on LVOT gradient reduction. These findings may be used to guide medical management for patients with oHCM.
Supplementary Material
Acknowledgements
A/Prof Chadi Ayoub is supported by the Mayo Clinic Clinician Engaged in Research award. This publication was supported by Mayo Clinic Arizona Cardiovascular Clinical Research Center (MCA CV CRC). We are thankful for their generous support. Contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the MCA CV CRC.
Contributor Information
Kamal Awad, Department of Cardiovascular Medicine, Mayo Clinic, 5777 E Mayo Blvd, Phoenix, AZ 85054, USA.
Milagros Pereyra Pietri, Department of Cardiovascular Medicine, Mayo Clinic, 5777 E Mayo Blvd, Phoenix, AZ 85054, USA.
Juan M Farina, Department of Cardiovascular Medicine, Mayo Clinic, 5777 E Mayo Blvd, Phoenix, AZ 85054, USA.
Girish Pathangey, Department of Cardiovascular Medicine, Mayo Clinic, 5777 E Mayo Blvd, Phoenix, AZ 85054, USA.
Mohammed Tiseer Abbas, Department of Cardiovascular Medicine, Mayo Clinic, 5777 E Mayo Blvd, Phoenix, AZ 85054, USA.
Isabel G Scalia, Department of Cardiovascular Medicine, Mayo Clinic, 5777 E Mayo Blvd, Phoenix, AZ 85054, USA.
David Le Couteur, ANZAC Research Institute, Concord Hospital, Hospital Road, Concord, NSW 2139, Australia.
Susan Wilanksy, Department of Cardiovascular Medicine, Mayo Clinic, 5777 E Mayo Blvd, Phoenix, AZ 85054, USA.
Steven J Lester, Department of Cardiovascular Medicine, Mayo Clinic, 5777 E Mayo Blvd, Phoenix, AZ 85054, USA.
Steve R Ommen, Department of Cardiovascular Medicine, Mayo Clinic, 200 First St. SW, Rochester, MN 55905, USA.
Jeffrey B Geske, Department of Cardiovascular Medicine, Mayo Clinic, 200 First St. SW, Rochester, MN 55905, USA.
Reza Arsanjani, Department of Cardiovascular Medicine, Mayo Clinic, 5777 E Mayo Blvd, Phoenix, AZ 85054, USA.
Chadi Ayoub, Department of Cardiovascular Medicine, Mayo Clinic, 5777 E Mayo Blvd, Phoenix, AZ 85054, USA.
Supplementary material
Supplementary material is available at European Heart Journal—Cardiovascular Pharmacotherapy online.
Author contributions
Drs K.A. and C.A. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: C.A., J.M.F., K.A., and M.P.P.; Acquisition, analysis, or interpretation of data: K.A., M.P.P., J.M.F., G.P., M.T.A., and I.G.S.; Drafting of the manuscript: K.A., M.P.P., J.M.F., G.P., M.T.A., and I.G.S.; Critical review of the manuscript for important intellectual content: D.L.C., S.W., S.J.L., S.R.O., J.B.G., R.A., and C.A.; Statistical analysis: K.A. and J.M.F.; Administrative, technical, or material support: C.A.; Supervision: R.A. and C.A.
Funding
None.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Ommen SR, Mital S, Burke MA, Day SM, Deswal A, Elliott P, Evanovich LL, Hung J, Joglar JA, Kantor P, Kimmelstiel C, Kittleson M, Link MS, Maron MS, Martinez MW, Miyake CY, Schaff HV, Semsarian C, Sorajja P. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. J Am Coll Cardiol 2020;76:e159–e240. [DOI] [PubMed] [Google Scholar]
- 2. Ommen SR, Ho CY, Asif IM, Balaji S, Burke MA, Day SM, Dearani JA, Epps KC, Evanovich L, Ferrari VA, Joglar JA, Khan SS, Kim JJ, Kittleson MM, Krittanawong C, Martinez MW, Mital S, Naidu SS, Saberi S, Semsarian C, Times S, Waldman CB. 2024 AHA/ACC/AMSSM/HRS/PACES/SCMR guideline for the management of hypertrophic cardiomyopathy: a report of the American Heart Association/American College of Cardiology joint committee on clinical practice guidelines. Circulation 2024;149:e1239–e1311. [DOI] [PubMed] [Google Scholar]
- 3. Ayoub C, Geske JB, Larsen CM, Scott CG, Klarich KW, Pellikka PA. Comparison of valsalva maneuver, amyl nitrite, and exercise echocardiography to demonstrate latent left ventricular outflow obstruction in hypertrophic cardiomyopathy. Am J Cardiol 2017;120:2265–2271. [DOI] [PubMed] [Google Scholar]
- 4. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Open Med 2009;3:e123–e130. [PMC free article] [PubMed] [Google Scholar]
- 5. Geske JB, Cullen MW, Sorajja P, Ommen SR, Nishimura RA. Assessment of left ventricular outflow gradient: hypertrophic cardiomyopathy versus aortic valvular stenosis. JACC Cardiovasc Interv 2012;5:675–681. [DOI] [PubMed] [Google Scholar]
- 6. Fedor JM, Stack RS, Pryor DB, Phillips HR. Adverse effects of nifedipine therapy on hypertrophic obstructive cardiomyopathy. Chest 1983;83:704–706. [DOI] [PubMed] [Google Scholar]
- 7. Pfeifer HJ, Greenblatt DJ, Koch-Weser J. Adverse reactions to practolol in hospitalized patients: a report from the Boston collaborative drug surveillance program. Eur J Clin Pharmacol 1977;12:167–170. [DOI] [PubMed] [Google Scholar]
- 8. Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan A-W, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JPt. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng H-Y, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. Rob 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 10. Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol 1992;45:769–773. [DOI] [PubMed] [Google Scholar]
- 11. Methods Guide for Effectiveness and Comparative Effectiveness Reviews. AHRQ Methods for Effective Health Care. Rockville, MD: Agency for Healthcare Research and Quality (US); 2008. [PubMed]
- 12. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Altman DG, Bland JM. Standard deviations and standard errors. BMJ 2005;331:903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Richardson M, Garner P, Donegan S. Interpretation of subgroup analyses in systematic reviews: a tutorial. Clin Epidemiol Glob 2019;7:192–198. [Google Scholar]
- 15. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Monda E, Lioncino M, Palmiero G, Franco F, Rubino M, Cirillo A, Verrillo F, Fusco A, Caiazza M, Mazzella M, Moscarella E, Dongiglio F, Sepe J, Pacileo G, Calabrò P, Limongelli G. Bisoprolol for treatment of symptomatic patients with obstructive hypertrophic cardiomyopathy. The BASIC (bisoprolol AS therapy in hypertrophic cardiomyopathy) study. Int J Cardiol 2022;354:22–28. [DOI] [PubMed] [Google Scholar]
- 17. Dybro AM, Rasmussen TB, Nielsen RR, Andersen MJ, Jensen MK, Poulsen SH. Randomized trial of metoprolol in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol 2021;78:2505–2517. [DOI] [PubMed] [Google Scholar]
- 18. Haruki S, Minami Y, Suzuki A, Hagiwara N. Effects of flecainide on left ventricular pressure gradient and symptoms in obstructive hypertrophic cardiomyopathy: a comparison of flecainide and disopyramide. Heart Vessels 2015;30:604–610. [DOI] [PubMed] [Google Scholar]
- 19. Sherrid MV, Shetty A, Winson G, Kim B, Musat D, Alviar CL, Homel P, Balaram SK, Swistel DG. Treatment of obstructive hypertrophic cardiomyopathy symptoms and gradient resistant to first-line therapy with beta-blockade or verapamil. Circ Heart Fail 2013;6:694–702. [DOI] [PubMed] [Google Scholar]
- 20. Nistri S, Olivotto I, Maron MS, Ferrantini C, Coppini R, Grifoni C, Baldini K, Sgalambro A, Cecchi F, Maron BJ. Beta blockers for prevention of exercise-induced left ventricular outflow tract obstruction in patients with hypertrophic cardiomyopathy. Am J Cardiol 2012;110:715–719. [DOI] [PubMed] [Google Scholar]
- 21. Kajimoto K, Imai T, Minami Y, Kasanuki H. Comparison of acute reduction in left ventricular outflow tract pressure gradient in obstructive hypertrophic cardiomyopathy by disopyramide versus pilsicainide versus cibenzoline. Am J Cardiol 2010;106:1307–1312. [DOI] [PubMed] [Google Scholar]
- 22. Sherrid MV, Barac I, McKenna WJ, Elliott PM, Dickie S, Chojnowska L, Casey S, Maron BJ. Multicenter study of the efficacy and safety of disopyramide in obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol 2005;45:1251–1258. [DOI] [PubMed] [Google Scholar]
- 23. Betocchi S, Piscione F, Losi MA, Pace L, Boccalatte M, Perrone-Filardi P, Cappelli-Bigazzi M, Briguori C, Manganelli F, Ciampi Q, Salvatore M, Chiariello M. Effects of diltiazem on left ventricular systolic and diastolic function in hypertrophic cardiomyopathy. Am J Cardiol 1996;78:451–457. [DOI] [PubMed] [Google Scholar]
- 24. Matsubara H, Nakatani S, Nagata S, Ishikura F, Katagiri Y, Ohe T, Miyatake K. Salutary effect of disopyramide on left ventricular diastolic function in hypertrophic obstructive cardiomyopathy. J Am Coll Cardiol 1995;26:768–775. [DOI] [PubMed] [Google Scholar]
- 25. Kimball BP, Bui S, Wigle ED. Acute dose-response effects of intravenous disopyramide in hypertrophic obstructive cardiomyopathy. Am Heart J 1993;125:1691–1697. [DOI] [PubMed] [Google Scholar]
- 26. Dimitrow PP, Dubiel JS. Effects on left ventricular function of pindolol added to verapamil in hypertrophic cardiomyopathy. Am J Cardiol 1993;71:313–316. [DOI] [PubMed] [Google Scholar]
- 27. Millaire A, Goullard L, Decoulx E, de Groote P, Houdas Y, Ducloux G. Efficiency of disopyramide in hypertrophic cardiomyopathy during stress states. Am J Cardiol 1992;69:423–424. [DOI] [PubMed] [Google Scholar]
- 28. Pollick C, Kimball B, Henderson M, Wigle ED. Disopyramide in hypertrophic cardiomyopathy. I. Hemodynamic assessment after intravenous administration. Am J Cardiol 1988;62:1248–1251. [DOI] [PubMed] [Google Scholar]
- 29. Pollick C. Disopyramide in hypertrophic cardiomyopathy. II. Noninvasive assessment after oral administration. Am J Cardiol 1988;62:1252–1255. [DOI] [PubMed] [Google Scholar]
- 30. Sherrid M, Delia E, Dwyer E. Oral disopyramide therapy for obstructive hypertrophic cardiomyopathy. Am J Cardiol 1988;62:1085–1088. [DOI] [PubMed] [Google Scholar]
- 31. Anderson DM, Raff GL, Ports TA, Brundage BH, Parmley WW, Chatterjee K. Hypertrophic obstructive cardiomyopathy. Effects of acute and chronic verapamil treatment on left ventricular systolic and diastolic function. Br Heart J 1984;51:523–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bonow RO, Ostrow HG, Rosing DR, Cannon RO 3rd, Lipson LC, Maron BJ, Kent KM, Bacharach SL, Green MV. Effects of verapamil on left ventricular systolic and diastolic function in patients with hypertrophic cardiomyopathy: pressure-volume analysis with a nonimaging scintillation probe. Circulation. 1983;68(5):1062–1073. [DOI] [PubMed] [Google Scholar]
- 33. Tendera M, Polonski L, Kozielska E. Left ventricular end-diastolic pressure-volume relationships in hypertrophic cardiomyopathy. Changes induced by verapamil. Chest 1983;84:54–57. [DOI] [PubMed] [Google Scholar]
- 34. Landmark K, Sire S, Thaulow E, Amlie JP, Nitter-Hauge S. Haemodynamic effects of nifedipine and propranolol in patients with hypertrophic obstructive cardiomyopathy. Br Heart J 1982;48:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pollick C. Muscular subaortic stenosis: hemodynamic and clinical improvement after disopyramide. N Engl J Med 1982;307:997–999. [DOI] [PubMed] [Google Scholar]
- 36. Storstein L, Abrahamsen AM, Storstein O. Hemodynamic effects of strophanthin and pindolol in patients with hypertrophic cardiomyopathy. Eur Heart J 1981;2:297–305. [DOI] [PubMed] [Google Scholar]
- 37. Kaltenbach M, Hopf R, Kober G, Bussmann WD, Keller M, Petersen Y. Treatment of hypertrophic obstructive cardiomyopathy with verapamil. Br Heart J 1979;42:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rosing DR, Kent KM, Borer JS, Seides SF, Maron BJ, Epstein SE. Verapamil therapy: a new approach to the pharmacologic treatment of hypertrophic cardiomyopathy. I. Hemodynamic effects. Circulation 1979;60:1201–1207. [DOI] [PubMed] [Google Scholar]
- 39. Todde G, Dei LL, Polizzi R, Gabrielli D, Canciello G, Romano S, Borrelli F, Halasz G, Ordine L, Di Napoli S, Pacella D, Lombardi R, Esposito G, Re F, Losi M-A. Long-term follow-up study on obstructive hypertrophic cardiomyopathy patients treated with disopyramide: evidences of a notable trend in symptom control within a real-world clinical setting. Front Cardiovasc Med 2024;11:1416600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Abood Z, Jan MF, Ashraf M, Kroboth S, Sanders H, Schweitzer M, Misicka A, Ollerman E, Jahangir A, Galazka P, Tajik AJ. Mavacamten in real-life practice: Initial experience at a hypertrophic cardiomyopathy centre. ESC Heart Fail 2025;12:672–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Desai MY, Hajj-Ali A, Rutkowski K, Ospina S, Gaballa A, Emery M, Asher C, Xu B, Thamilarasan M, Popovic ZB. Real-world experience with mavacamten in obstructive hypertrophic cardiomyopathy: Observations from a tertiary care center. Prog Cardiovasc Dis 2024;86:62–68. [DOI] [PubMed] [Google Scholar]
- 42. Ramonfaur D, Gasperetti A, Blake VE, Rivers B, Kassamali AA, Kasper EK, Barouch LA, Wu KC, Madrazo JA, Carrick RT. Eighteen-Month real-world experience using mavacamten for treatment of obstructive hypertrophic cardiomyopathy in a racially diverse population. J Am Heart Assoc 2024;13:e034069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Reza N, Dubey A, Carattini T, Marzolf A, Hornsby N, de Feria A, Mahmud N, Schuler P, Owens AT. Real-World experience and 36-week outcomes of patients with symptomatic obstructive hypertrophic cardiomyopathy treated with mavacamten. JACC Heart Fail 2024;12:1123–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Roehl KM, Farina JM, Suppah M, Arsanjani R, Lester SJ, Geske JB, Alsidawi S. Predictors of short-term and long-term effects of mavacamten in obstructive hypertrophic cardiomyopathy. Prog Cardiovasc Dis 2024;86:86–88. [DOI] [PubMed] [Google Scholar]
- 45. Wessly P, Lazzara GE, Buergler JM, Nagueh SF. Early observations on effects of mavacamten on left atrial function in obstructive hypertrophic cardiomyopathy patients. JACC Cardiovasc Imaging 2023;16:1633–1634. [DOI] [PubMed] [Google Scholar]
- 46. Tian Z, Li L, Li X, Wang J, Zhang Q, Li Z, Peng D, Yang P, Ma W, Wang F, Jin W, Cheng X, Sun J, Fu Y, Lyu C, Zhang S. Effect of mavacamten on Chinese patients with symptomatic obstructive hypertrophic cardiomyopathy: The EXPLORER-CN randomized clinical trial. JAMA Cardiol 2023;8:957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Olivotto I, Oreziak A, Barriales-Villa R, Abraham TP, Masri A, Garcia-Pavia P, Saberi S, Lakdawala NK, Wheeler MT, Owens A, Kubanek M, Wojakowski W, Jensen MK, Gimeno-Blanes J, Afshar K, Myers J, Hegde SM, Solomon SD, Sehnert AJ, Zhang D, Li W, Bhattacharya M, Edelberg JM, Waldman CB, Lester SJ, Wang A, Ho CY, Jacoby D, Bartunek J, Bondue A, Van Craenenbroeck E, Kubanek M, Zemanek D, Jensen M, Mogensen J, Thune JJ, Charron P, Hagege A, Lairez O, Trochu J-N, Axthelm C, Duengen H-D, Frey N, Mitrovic V, Preusch M, Schulz-Menger J, Seidler T, Arad M, Halabi M, Katz A, Monakier D, Paz O, Viskin S, Zwas D, Olivotto I, Brunner-La Rocca HP, Michels M, Dudek D, Oko-Sarnowska Z, Oreziak A, Wojakowski W, Cardim N, Pereira H, Barriales-Villa R, García Pavia P, Gimeno Blanes J, Hidalgo Urbano R, Rincón Diaz LM, Elliott P, Yousef Z, Abraham T, Afshar K, Alvarez P, Bach R, Becker R, Choudhury L, Fermin D, Jacoby D, Jefferies J, Kramer C, Lakdawala N, Lester S, Marian A, Masri A, Maurer M, Nagueh S, Owens A, Owens D, Rader F, Saberi S, Sherrid M, Shirani J, Symanski J, Turer A, Wang A, Wever-Pinzon O, Wheeler M, Wong T, Yamani M. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2020;396:759–769. [DOI] [PubMed] [Google Scholar]
- 48. Heitner SB, Jacoby D, Lester SJ, Owens A, Wang A, Zhang D, Lambing J, Lee J, Semigran M, Sehnert AJ. Mavacamten treatment for obstructive hypertrophic cardiomyopathy: A clinical trial. Ann Intern Med 2019;170:741–748. [DOI] [PubMed] [Google Scholar]
- 49. Desai MY, Owens A, Geske JB, Wolski K, Naidu SS, Smedira NG, Cremer PC, Schaff H, McErlean E, Sewell C, Li W, Sterling L, Lampl K, Edelberg JM, Sehnert AJ, Nissen SE. Myosin inhibition in patients with obstructive hypertrophic cardiomyopathy referred for septal reduction therapy. J Am Coll Cardiol 2022;80:95–108. [DOI] [PubMed] [Google Scholar]
- 50. Maron MS, Masri A, Nassif ME, Barriales-Villa R, Arad M, Cardim N, Choudhury L, Claggett B, Coats CJ, Düngen H-D, Garcia-Pavia P, Hagège AA, Januzzi JL, Lee MMY, Lewis GD, Ma C-S, Michels M, Olivotto I, Oreziak A, Owens AT, Spertus JA, Solomon SD, Tfelt-Hansen J, van Sinttruije M, Veselka J, Watkins H, Jacoby DL, Heitner SB, Kupfer S, Malik FI, Meng L, Wohltman A, Abraham TP. Aficamten for symptomatic obstructive hypertrophic cardiomyopathy. N Engl J Med 2024;390:1849–1861. [DOI] [PubMed] [Google Scholar]
- 51. Maron MS, Masri A, Choudhury L, Olivotto I, Saberi S, Wang A, Garcia-Pavia P, Lakdawala NK, Nagueh SF, Rader F, Tower-Rader A, Turer AT, Coats C, Fifer MA, Owens A, Solomon SD, Watkins H, Barriales-Villa R, Kramer CM, Wong TC, Paige SL, Heitner SB, Kupfer S, Malik FI, Meng L, Wohltman A, Abraham T. Phase 2 study of aficamten in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol 2023;81:34–45. [DOI] [PubMed] [Google Scholar]
- 52. Owens AT, Masri A, Abraham TP, Choudhury L, Rader F, Symanski JD, Turer AT, Wong TC, Tower-Rader A, Coats CJ, Fifer MA, Olivotto I, Solomon SD, Watkins HC, Heitner SB, Jacoby DL, Kupfer S, Malik FI, Meng L, Sohn R, Wohltman A, Maron MS, Aficamten for drug-refractory severe obstructive hypertrophic cardiomyopathy in patients receiving disopyramide: REDWOOD-HCM cohort 3. J Card Fail 2023;29:1576–1582. [DOI] [PubMed] [Google Scholar]
- 53. Yassen M, Changal K, Busken J, Royfman R, Schodowski E, Venkataramany B, Khouri SJ, Moukarbel GV. The efficacy of cardiac myosin inhibitors versus placebo in patients with symptomatic hypertrophic cardiomyopathy: a meta-analysis and systematic review. Am J Cardiol 2024;210:219–224. [DOI] [PubMed] [Google Scholar]
- 54. Bayonas-Ruiz A, Munoz-Franco FM, Sabater-Molina M, Oliva-Sandoval MJ, Gimeno JR, Bonacasa B. Current therapies for hypertrophic cardiomyopathy: a systematic review and meta-analysis of the literature. ESC Heart Fail 2023;10:8–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Writing Committee M, Ommen SR, Ho CY, Asif IM, Balaji S, Burke MA, Day SM, Dearani JA, Epps KC, Evanovich L, Ferrari VA, Joglar JA, Khan SS, Kim JJ, Kittleson MM, Krittanawong C, Martinez MW, Mital S, Naidu SS, Saberi S, Semsarian C, Times S, Waldman CB. 2024 AHA/ACC/AMSSM/HRS/PACES/SCMR guideline for the management of hypertrophic cardiomyopathy: a report of the American Heart Association/American College of Cardiology joint committee on clinical practice Guidelines. J Am Coll Cardiol 2024;83:2324–2405. [DOI] [PubMed] [Google Scholar]
- 56. Arbelo E, Protonotarios A, Gimeno JR, Arbustini E, Barriales-Villa R, Basso C, Bezzina CR, Biagini E, Blom NA, de Boer RA, De Winter T, Elliott PM, Flather M, Garcia-Pavia P, Haugaa KH, Ingles J, Jurcut RO, Klaassen S, Limongelli G, Loeys B, Mogensen J, Olivotto I, Pantazis A, Sharma S, Van Tintelen JP, Ware JS, Kaski JP, Charron P, Imazio M, Abdelhamid M, Aboyans V, Arad M, Asselbergs FW, Asteggiano R, Bilinska Z, Bonnet D, Bundgaard H, Cardim NM, Čelutkienė J, Cikes M, De Ferrari GM, Dusi V, Falk V, Fauchier L, Gandjbakhch E, Heliö T, Koskinas K, Kotecha D, Landmesser U, Lazaros G, Lewis BS, Linhart A, Løchen M-L, Meder B, Mindham R, Moon J, Nielsen JC, Petersen S, Prescott E, Sheppard MN, Sinagra G, Sitges M, Tfelt-Hansen J, Touyz R, Veltrop R, Veselka J, Wahbi K, Wilde A, Zeppenfeld K, Kichou B, Sisakian H, Scherr D, Gerber B, Džubur A, Gospodinova M, Planinc I, Moustra HH, Zemánek D, Jensen MSK, Samir A, Palm K, Heliö T, Wahbi K, Schulze-Bahr E, Haralambos V, Sepp R, Aðalsteinsdóttir B, Ward D, Blich M, Sinagra G, Poniku A, Lunegova O, Rudzitis A, Kassab R, Barysienė J, Huijnen S, Felice T, Vataman E, Pavlovic N, Doghmi N, Asselbergs FW, Kostovska ES, Almaas VM, Biernacka EK, Brito D, Rosca M, Zavatta M, Ristic A, Goncalvesová E, Šinkovec M, Cañadas-Godoy V, Platonov PG, Saguner AM, Saadi ARA, Kammoun I, Celik A, Nesukay E, Abdullaev T, Prescott E, James S, Arbelo E, Baigent C, Borger MA, Buccheri S, Ibanez B, Køber L, Koskinas KC, McEvoy JW, Mihaylova B, Mindham R, Neubeck L, Nielsen JC, Pasquet A, Rakisheva A, Rocca B, Rossello X, Vaartjes I, Vrints C, Witkowski A, Zeppenfeld K. 2023 ESC guidelines for the management of cardiomyopathies: developed by the task force on the management of cardiomyopathies of the European Society of Cardiology (ESC). Eur Heart J 2023;44:3503–3626. [DOI] [PubMed] [Google Scholar]
- 57. Argulian E, Bangalore S, Messerli FH. Misconceptions and facts about Beta-blockers. Am J Med 2019;132:816–819. [DOI] [PubMed] [Google Scholar]
- 58. Desai MY, Owens A, Geske JB, Wolski K, Saberi S, Wang A, Sherrid M, Cremer PC, Naidu SS, Smedira NG, Schaff H, McErlean E, Sewell C, Balasubramanyam A, Lampl K, Sehnert AJ, Nissen SE. Dose-blinded myosin inhibition in patients with obstructive hypertrophic cardiomyopathy referred for septal reduction therapy: outcomes through 32 weeks. Circulation 2023;147:850–863. [DOI] [PubMed] [Google Scholar]
- 59. Beinfeld M, Wasfy JH, Walton S, Sarker J, Nhan E, Rind DM, Pearson SD. Mavacamten for hypertrophic cardiomyopathy: effectiveness and value. J Manag Care Spec Pharm 2022;28:369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Corriveau S, Heydari B, Garceau P. Does disopyramide still have a place in the management of obstructive hypertrophic cardiomyopathy? CJC Open 2024;6:811–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sebastian SA, Padda I, Lehr EJ, Johal G. Aficamten: a breakthrough therapy for symptomatic obstructive hypertrophic cardiomyopathy. Am J Cardiovasc Drugs 2023;23:519–532. [DOI] [PubMed] [Google Scholar]
- 62. Ostrominski JW, Guo R, Elliott PM, Ho CY. Cardiac myosin inhibitors for managing obstructive hypertrophic cardiomyopathy: JACC: heart failure state-of-the-art review. JACC Heart Fail 2023;11:735–748. [DOI] [PubMed] [Google Scholar]
- 63. Taha M, Dahat P, Toriola S, Satnarine T, Zohara Z, Adelekun A, Seffah KD, Salib K, Dardari L, Arcia Franchini AP. Metoprolol or verapamil in the management of patients with hypertrophic cardiomyopathy: a systematic review. Cureus 2023;15:e43197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Seo K, Yamamoto Y, Kirillova A, Kawana M, Yadav S, Huang Y, Wang Q, Lane KV, Pruitt BL, Perez MV, Bernstein D, Wu JC, Wheeler MT, Parikh VN, Ashley EA. Improved cardiac performance and decreased arrhythmia in hypertrophic cardiomyopathy with non-beta-blocking R-enantiomer carvedilol. Circulation 2023;148:1691–1704. [DOI] [PubMed] [Google Scholar]
- 65. Schwinger RH, Bohm M, Erdmann E. Negative inotropic activity of the calcium antagonists isradipine, nifedipine, diltiazem, and verapamil in diseased human myocardium. Am J Hypertens 1991;4:185S–187S. [DOI] [PubMed] [Google Scholar]
- 66. Binggeli C, Corti R, Sudano I, Luscher TF, Noll G. Effects of chronic calcium channel blockade on sympathetic nerve activity in hypertension. Hypertension 2002;39:892–896. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.