ABSTRACT
Plastids are crucial for fuelling and regulating plant growth and development. Photosynthesising chloroplasts provide energy for growth, while other plastids play additional key roles in various aspects of plant physiology. For function and development, plastids greatly depend on nucleus‐encoded proteins, and they can modulate the synthesis of these proteins by sending retrograde signals to the nucleus. These signals communicate the developmental and operational status of the plastid, both of which are sensitive to the environment. Abiotic stressors such as drought, salinity, and suboptimal light and temperature conditions can induce changes in chloroplast metabolism, ultrastructure and cellular positioning. In response to specific environmental triggers, retrograde signals reprogramme nuclear gene expression to fine‐tune plastid form and function, but also influence whole‐plant morphology. Over the past years, the chloroplast responses to stress have become clearer. Various sources of retrograde signals, derived from plastid metabolism, plastid gene expression and altered photosynthetic redox balance, are now known to directly interfere with canonical signalling pathways. However, most of what is known about retrograde signalling originates from studies using artificial stressors, such as chemical treatments or genetic mutations, and its importance in natural environments is still poorly understood. This review highlights the understanding of plastid responses to the environment, as well as the impact generated downstream of retrograde signals, to better understand the role of plastids in abiotic stress resilience of flowering plants.
Keywords: abiotic stress, chloroplasts, environmental adaptation, gene expression regulation, genomes uncoupled, photosynthesis, plant development, retrograde signalling
Summary statement
Plastids can sense environmental stress and improve whole plant adaptation through retrograde communication to the nucleus. Recent studies have advanced our understanding of integrated canonical and retrograde signalling during abiotic stress, which fine‐tune gene expression and development.
1. Introduction
Plant life as we know it depends on plastids, whether it be for photosynthesis, producing essential metabolites or even steering plant development. Plastids, such as chloroplasts, are generally believed to have evolved during a single endosymbiotic event, where a free‐living photosynthetic cyanobacterium was engulfed by an ancestral eukaryotic host. The transition from free‐living microbes to organelles involved a drastic reduction of genome size, which nowadays amounts to only 5%–10% of that in free‐living cyanobacteria (Martin et al. 2002). The remaining plastid genome, the plastome, encodes anywhere from 120 genes in plants, up to approximately 200 genes in certain red algae (Pfannschmidt et al. 2015). The dependency of plastids on nuclear transcription allows the host cell to steer plastid development, resulting in a range of different plastid types.
Both the development and functionality of plastids are sensitive to environmental signals. For example, in newly germinated seedlings, etioplast‐to‐chloroplast biogenesis depends on the presence of light (Pipitone et al. 2021). For mature chloroplasts, any environmental signal that impacts photosynthesis has concomitant effects on its functionality, including not only light but also salinity, temperature and drought.
For plants to succeed in a dynamic natural environment, chloroplast development and operation must be plastic. Having said that, chloroplasts cannot operate alone, but rather in conjunction with the nucleus. Additionally, alterations in plastid metabolism can have significant effects on the host cell. To coordinate the coupling of chloroplast well‐being and nuclear gene expression, signalling systems communicate from the plastids to the nucleus (via retrograde signals) and vice versa (via anterograde signals) (Hernández‐Verdeja et al. 2020). Significant progress has been made in deducing the nature of chloroplast‐derived retrograde signals (reviewed in [Chan et al. 2016b]). Notwithstanding these findings, the majority of these studies relied on the use of chemical stressors or genetic mutations that induce chloroplast malfunction. The contribution of chloroplast signals to more natural stress responses and plant development remains to be comprehensively reviewed. Here, we summarise the latest developments in the understanding of how chloroplasts are affected by abiotic stress, how this steers chloroplast‐derived retrograde signals and how these contribute to whole‐plant acclimation to the environment. Despite our efforts to include all recent developments, we apologise to those whose work could not be added due to space restrictions.
2. The Impact of Abiotic Stress on Chloroplast Form and Functionality
Plants are continuously exposed to fluctuating and possibly detrimental environmental conditions that may affect cellular homoeostasis by inducing reactive oxygen species (ROS), limiting water availability or altering metabolic processes. Such stress‐induced cellular changes can affect various aspects of chloroplast form and functionality.
2.1. Chloroplast Ultrastructure and Positioning
Abiotic stresses can induce changes in chloroplast ultrastructure such as reduced grana stacking, swelling of the thylakoid lumen and a loss of thylakoid organisation (Wang et al. 2014; Wang et al. 2019). Thylakoid disassembly leads to the mobilisation of lipophilic compounds that accumulate in lipid reservoirs, the plastoglubules. These plastoglobules increase in number and size in response to stress conditions (Wang et al. 2019; Zhang et al. 2010). Additionally, abiotic stressors can compromise chloroplast envelope membrane integrity, which is associated with increased stromule formation (Figure 1A) (Gray et al. 2012). While their function hasn't been fully resolved, stromules have been suggested to be involved in inter‐organellar communication.
Figure 1.
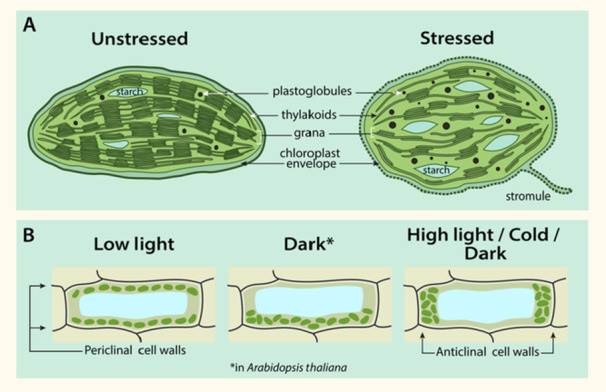
Abiotic stressors affect chloroplast ultrastructure (A) and chloroplast positioning within the cell (B). Chloroplast ultrastructure changes under stress include rounding of the chloroplast, increased plastoglobuli size and number, stromule formation, dismantlement of the thylakoid network, grana unstacking, altered numbers of starch granules and chloroplast envelope damage (as indicated by dashed line). (B) Chloroplast positioning is altered under different light and temperature conditions. Under low light, chloroplasts orient along periclinal cell walls, but under high light, they orient along anticlinal cell walls. Positioning in the dark conditions differs between species, with the chloroplast moving to the bottom of the cell in Arabidopsis, but aligning along the anticlinal cell walls in other species. In response to cold under low‐light conditions, chloroplasts also move to the anticlinal cell walls. Representative cells show a side view.
While the aforementioned responses to stress are relatively consistent, studies have yielded inconsistent findings related to the presence of starch granules following stress treatment. Chilling stress causes both a decrease and an accumulation of starch granules. The decrease has been attributed to either a reduction in photosynthesis rate or the breakdown of starch granules to facilitate the biosynthesis of osmoprotectants (Ribeiro et al. 2022). Other studies have attributed an accumulation of starch granules in response to decreased respiration and starch hydrolysis rates following cold exposure (Zhuang et al. 2019). This disparity in findings likely reflects a dynamic response to stress conditions in terms of starch metabolism (Ristic and Ashworth 1993).
The shape and positioning of chloroplasts within the cell vary substantially between stress and non‐stress conditions. Chloroplasts often become more spherical or thicker in stress (Meng et al. 2016; Zhang et al. 2010) (Figure 1A). Additionally, chloroplasts alter their positioning under different environmental conditions so as to optimise light absorption for photosynthesis and avoid damage to the photosynthetic machinery (Figure 1B). These movements have been best studied in the context of light, where chloroplasts position themselves along the anticlinal (side) cell walls under potentially harmful high light intensities, but accumulate in layers along the periclinal (top and bottom) cell walls under dim light (Schramma et al. 2023; Davis and Hangarter 2012). Interestingly, under dark conditions, chloroplasts accumulate along the bottom of cells in Arabidopsis (Suetsugu et al. 2005), but along the anticlinal walls in non‐angiosperms such as the liverwort Apopellia endiviifolia (Yong et al. 2021) (Figure 1B).
Temperature stress also alters chloroplast positioning. For example, in dim light at low temperatures (approximately 5°C), chloroplasts orient along the anticlinal cell walls (Ogasawara et al. 2013), likely as a means to reduce cold‐induced photosystem II (PSII) photoinhibition. The movement of chloroplasts in response to light and temperature depends on the blue light photoreceptor phototropin (Fujii et al. 2017). In addition to light and temperature, abiotic stressors, including drought and salinity, can alter chloroplast positioning (Yamada et al. 2009). Although chloroplast movements under these stresses likely play a role in photoprotection or the maintenance of photosynthesis as well, their function is yet to be fully elucidated.
2.2. Chloroplast Functionality: Photosynthesis
The photosynthetic system is incredibly sensitive to fluctuating environmental conditions. Many of the earlier‐mentioned changes in chloroplast ultrastructure reflect this. Additionally, abiotic stresses can impose rapid disruptions to photosynthesis by affecting enzyme activity, redox homoeostasis and pigment biosynthesis and directly damaging the photosystems. The impact on photosynthesis, however, can vary considerably depending on the nature and intensity of the stress. Here we summarise the effects of drought, salinity, heat stress and high/low light conditions.
Drought markedly reduces photosynthetic efficiency by a number of mechanisms. In response to drought, plants typically close their stomata and reduce transpiration to conserve water reserves (Sperry et al. 2017). This entails a trade‐off whereby CO2 intake is reduced, which slows the Calvin cycle and limits NADP+ availability to the electron transport chain (Noctor et al. 2014). Additionally, excess O2, resulting from continued photosynthesis and closed stomata, can induce the wasteful process of photorespiration, which concurrently acts as a source of oxidative stress (Noctor 2002). At a biochemical level, drought leads to over‐reduction of the electron transport chain (Noctor et al. 2014), which can cause over‐excitation of the PSII reaction centre. Excess energy may be passed on to O2 (which becomes enriched when stomata are closed) and yield singlet oxygen (Dmitrieva et al. 2020). Additionally, O2 may act as an alternative electron acceptor, being reduced to superoxide (O2 −), and subsequently hydrogen peroxide (H2O2) and the hydroxyl radical (•OH), which induces oxidative stress (Gururani et al. 2015). These ROS may further decrease photosynthetic efficiently by directly damaging the D1 and D2 proteins of PSII (Krieger‐Liszkay 2005), disrupting photosynthetic pigments (Shin et al. 2021), as well as inducing lipid peroxidation of thylakoid membranes (Killi et al. 2020). The damage to PSII further reduces its quantum yield efficiency through photoinhibition (Moustakas et al. 2022). To counteract these deleterious effects, plants have evolved photoprotective mechanisms such as antioxidant enzymes (Logan et al. 2006), PSII damage repair (Theis and Schroda 2016), non‐photochemical quenching (Lu et al. 2022) and PSI cyclic electron flow (Huang et al. 2018). The balance between damage imposed by abiotic stressors and the photoprotective potential within the chloroplast dictates the harmfulness to plant productivity.
Photosynthetic damage imposed by drought also occurs under salt stress. Even so, the toxic effects of sodium (Na+) and chloride (Cl−) ions add to the osmotic effects. For example, high ion concentrations affect plastid membrane stability (Wang et al. 2014). The uptake of Na+ into the cell via non‐selective cation channels induces cellular electrolyte leakage, which results in a dramatic loss in cellular K+ levels, which inhibits photosynthesis at multiple levels (Van Zelm et al. 2020).
The impact of ionic stress induced by salinity is difficult to study independently of the accompanying osmotic stress without using proper controls. The detrimental effects of Na+ on photosynthesis, however, have been exhibited through the use of Na+ channel blockers, which can partially rescue the photosynthetic perturbations inflicted by salinity (Zhang and Xing 2008). On a similar note, overexpression of the Arabidopsis vacuolar Na+/H+ antiporter NHX1 (Sodium/Hydrogen Exchanger 1) improved photosynthesis via Na+ sequestration (He et al. 2005). Intriguingly, while ion toxicity is typically attributed to Na+ ions, Chen and Yu (2007) found inhibition of photosynthesis in soybean to be impacted by Cl− rather than Na+ ions. Interestingly, some halophytic species require high Cl− concentrations to maintain optimal photosynthesis (Preston and Pace 1985), which suggests that many of these responses differ between species.
Heat stress affects photosynthesis at different levels. First of all, in most plant species, warm temperatures cause a reduction in photosynthetic pigments (chlorophylls and carotenoids), which results in reduced light harvesting and should prevent ROS accumulation (Jespersen et al. 2016). Evergreen grasses that lack a Chla catabolic protein accumulate more ROS and experience severe photodamage when exposed to heat stress (Zhang et al. 2022). Heat causes dismantling of PSII–LHCII complexes and the degradation of many PSII‐ and PSI‐associated proteins, leading to reduced photosynthetic activity (Zhang et al. 2022). In addition, carbon fixation is strongly impacted by heat stress, due to slower reactivation of Rubisco by activase enzymes (Salvucci and Crafts‐Brandner 2004).
One other abiotic factor that affects chloroplast primary metabolism is light. Different aspects of light can impact photosynthesis, including fluctuations in light intensity (Morales and Kaiser 2020) and spectral composition (Liu and Van Iersel 2021). Akin to drought stress, a sudden increase in light intensity can induce over‐reduction of the photosynthetic electron transport chain (Yamori et al. 2016), which inevitably results in ROS formation and photoinhibition (Shi et al. 2022). The aforementioned photoprotective mechanisms come into play to maintain redox homoeostasis under these over‐reducing conditions. In addition, maintaining photosynthesis under high light stress requires repair of PSII (Shi et al. 2022).
3. Plastid‐Derived Retrograde Signals
Given the limited capacity for self‐regulation via the plastid genome, the ability of chloroplasts to adapt to local conditions requires tight communication with the nucleus. Chloroplast‐to‐nucleus retrograde signals can be categorised at either biogenic or operational control levels. ‘Biogenic control’ refers to signalling that occurs during plastid development, whereas ‘operational control’ refers to signals released by mature chloroplasts in response to environmental fluctuations or stress (Chan et al. 2016b). The retrograde signalling pathways annotated to date are complex, and many signals remain to be fully described. Nevertheless, four major categories of signals exist: (1) intermediates of the tetrapyrrole biosynthesis pathway; (2) signals from altered plastid gene expression (PGE); (3) photosynthesis‐derived signals arising from altered thylakoid redox homoeostasis and (4) signals derived from other metabolic pathways (Figure 3). In the following sections, we will review the recent understanding of how these different classes of plastid‐derived signals act as retrograde signals.
Figure 3.
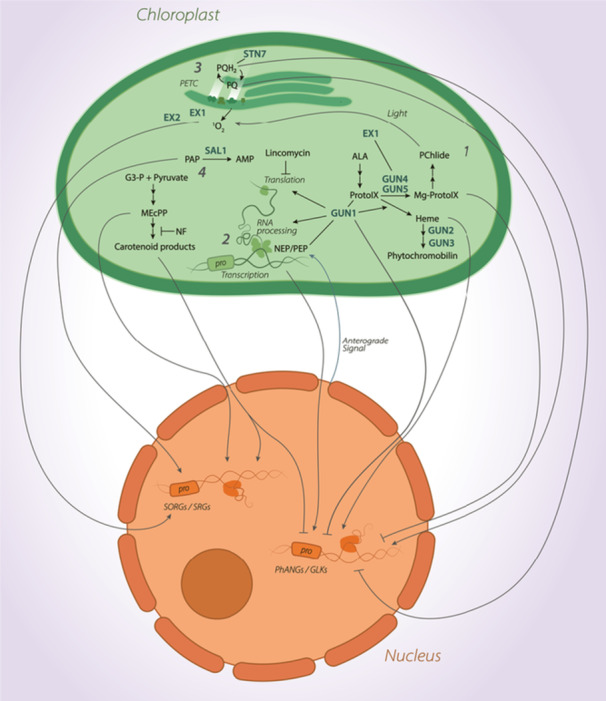
Simplified schematic representing four major retrograde signalling sources: (1) intermediates of the tetrapyrrole biosynthesis pathway, (2) signals from altered plastid gene expression, (3) photosynthesis‐derived signals and (4) signals arising from other metabolic pathways. Grey arrows indicate retrograde signals with either promotion (arrowhead) or repression (block arrow) of Singlet Oxygen Responsive Genes/Stress Responsive Genes or Photosynthesis Associated Nuclear Genes/Golden Like transcription factors. The chloroplast is shown in green, and the nucleus is shown in orange. [Color figure can be viewed at wileyonlinelibrary.com]
3.1. Coupling of Genomes and the Tetrapyrrole Biosynthesis Pathway
The discovery that Photosynthesis Associated Nuclear Gene (PhANG) expression relies on functional chloroplasts provided insights into the biogenic control of retrograde signals. Forward genetic screens in Arabidopsis using the carotenoid biosynthesis inhibiting herbicide norflurazon led to the identification of the gun (genomes uncoupled) mutants. Exogenous treatment of light‐exposed plants with norflurazon typically triggers a strong repression of PhANGs. The gun mutants fail to exhibit this response, suggesting an impairment in chloroplast‐to‐nucleus communication (Susek et al. 1993; Mochizuki et al. 2001; Adhikari et al. 2011). Notably, five of the six GUN genes/alleles (GUN2–GUN6) directly regulate the tetrapyrrole synthesis pathway, thereby presenting this pathway as a source of biogenic retrograde signals.
GUN2 and GUN3 encode the enzymes haem oxygenase and protochromobilin synthase, respectively, and mutations in these genes lead to haem accumulation (Mochizuki et al. 2001). gun6 also alters haem, but by encoding a gain‐of‐function allele for FERROCHELATASE1, which directly synthesises haem from protoporphyrin IX (Woodson et al. 2011) (Figure 2). Contrastingly, GUN4 and GUN5 are involved in synthesising the first committed chlorophyll precursor, Mg‐protoporphyrin IX (Mg‐ProtoIX) (Adhikari et al. 2011). GUN5 encodes the H‐subunit of Mg‐chelatase, while GUN4 encodes a Mg‐chelatase activator that binds its H‐subunit, substrate and product (Mochizuki et al. 2001; Larkin et al. 2003) (Figure 2).
Figure 2.
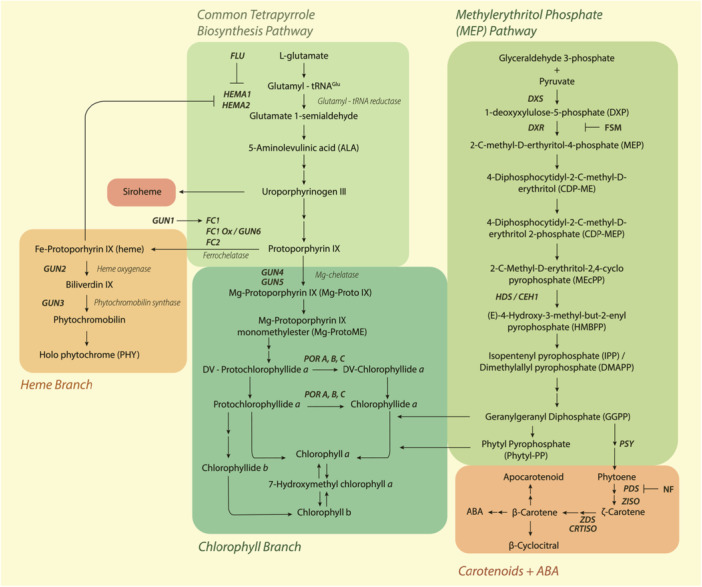
Simplified model for tetrapyrrole biosynthesis, the methylerythritol phosphate pathway and carotenoid biosynthesis pathways in plants. Single arrows indicate direct steps, double arrows indicate indirect steps, enzymes are in italics, and gene names are in bold italics. Boxes correspond to labels directly above/below. Abbreviations: ABA abscisic acid, CEH1 Constitutively Expressing HYDROPEROXIDE LYASE 1, CRTISO CAROTENOID ISOMERASE, DXR 1‐DeoxyXylulose‐5‐phosphate Reductoisomerase, DXS 1‐DeoxyXylulose‐5‐phosphate Synthase, FLU FLUORESCENT IN BLUE LIGHT, FSM fosmidomycin, GUN GENOMES UNCOUPLED, HEMA HEME A, NF norflurazon, PDS phytoene desaturase, POR protochlorophyllide oxidoreductase, PSY phytoene synthase, ZDS ζ ‐carotene desaturase. [Color figure can be viewed at wileyonlinelibrary.com]
Following the identification of the gun mutants, researchers set out to determine whether the tetrapyrrole intermediates encoded by these genes act as mobile retrograde signals. Of these, the porphyrin intermediates Mg‐ProtoIX and Mg‐ProtoIX monomethylester (Mg‐ProtoIXme) have been proposed as putative retrograde signals (Figure 3), although this proposition has been subject to scrutiny (Larkin 2016).
In Arabidopsis, Strand et al. (2003) initially suggested that Mg‐ProtoIX acts as a repressor of PhANG expression by recording increased levels of Mg‐ProtoIX in seedlings where chloroplast biogenesis was blocked by norflurazon. The increase was less pronounced in treated gun2 and gun5 mutants, which also kept high PhANG expression. In subsequent years, a number of different publications provided evidence to support a model whereby Mg‐ProtoIX or Mg‐ProtoIXme downregulates PhANG expression (Alawady and Grimm 2005; Ankele et al. 2007). This model was challenged by later studies, which did not record a similar correlation between Mg‐ProtoIX levels and PhANG repression (Mochizuki et al. 2008; Moulin et al. 2008).
Some of the mentioned contradictories can be explained by the fact that the accumulation of porphyrin intermediates following stress is short‐lived and transient (Zhang et al. 2011). As persistent accumulation of chlorophyll intermediates results in photodynamic as well as physiological damage (Meskauskiene et al. 2001), the high turnover of Mg‐ProtoIX and Mg‐ProtoIXme could circumvent toxicity. Additionally, it is known that both Mg‐ProtoIX and Mg‐ProtoIXme levels oscillate in a photoperiodic light regime, peaking during the light period and dropping during the dark (Norén et al. 2016). It is therefore hard to rule out the impact of the experimental set‐up on the regulation of PhANG expression.
Another proposition about the effect of the gun5 mutation is that it diverts protoporphyrin IX from Mg‐ProtoIX, towards Fe‐protoporphyrin, thereby increasing haem levels (Woodson et al. 2011) (Figure 2). Indeed, haem can act as a positive biogenic retrograde signal that controls nuclear gene expression. Evidence supporting this arose from the discovery of the gun6‐1D mutant that overexpresses FC1 (FERROCHELATASE 1). Akin to the other GUN alleles, gun6‐1D plants exhibit a derepression of PhANG expression under NF (Woodson et al. 2011). This study failed to observe a gun phenotype in plants overexpressing a second ferrochelatase isoform (FC2), thus indicating that only FC1‐derived haem acts as a retrograde signal. Moreover, in the dark‐grown Chlamydomonas reinhardtii cultures, haem feeding induced transcription of the nuclear gene HEAT SHOCK PROTEIN 70 A (HSP70A), further suggesting haem as a positive regulator of nuclear gene expression (Von Gromoff et al. 2008). Despite GUN2 encoding haem oxygenase (Figure 2), conflicting data exist relating to whether gun2 accumulates more or less haem relative to wild‐type plants after NF treatment (Woodson et al. 2011).
One of the primary issues with correlating haem accumulation to PhANG expression relates to quantification methods. Haem is associated with many different proteins known as hemoproteins, which carry out vital biological functions. It is generally believed that a non‐specifically bound pool of ‘free’ haem acts as a retrograde signal. Due to poor solubility, it is unlikely that free haem accumulates in the cytosol, but it is more likely present in membranes or bound non‐specifically to proteins (Thomas and Weinstein 1990). Efforts have been made to establish a methodology for distinguishing this pool of free haem from haem associated with hemoproteins (Espinas et al. 2012). Nonetheless, inconsistencies in protocols used in different studies may account for contradictory findings.
Perhaps the strongest evidence favouring haem as a retrograde signal is the fact that it is exported from plastids (Thomas and Weinstein 1990). Moreover, haem has been recognised as a signalling molecule in different types of organisms, among which are yeast (Zhang and Hach 1999) and animals (Bottino‐Rojas et al. 2015). In that regard, many researchers consider haem as the primary tetrapyrrole‐derived mobile retrograde signal arising during chloroplast biogenesis (Larkin 2016).
3.2. Plastid Signal Integration via GUN1
The original gun mutant screen of Susek et al. (1993) yielded five genes encoding enzymes involved in the tetrapyrrole synthesis pathway, as described above. The sixth candidate from this study is GUN1, which encodes a chloroplast‐localised pentatricopeptide repeat (PPR) protein and received particular interest, as it seems to integrate many different signals during plastid stress. The GUN1 protein is specifically active during the early phases of chloroplast biogenesis and destabilises when chloroplasts mature (Hernández‐Verdeja et al. 2022).
Similar to GUN2–6, GUN1 coordinates the flux of the tetrapyrrole synthesis pathway at two different levels: First, it inhibits the pathway via to‐date unknown binding to enzymes or porphyrins, resulting in reduced haem and Pchlide levels after ALA (aminolevulinic acid) feeding in the presence of GUN1 (Shimizu et al. 2019). Second, it promotes FC1 affinity with its substrate, likely by directly interacting with Proto‐IX (Figures 2 and 3). This results in increased haem synthesis in darkness, when GUN1 is active. Due to GUN1's low abundance, these mechanisms will likely not significantly reduce tetrapyrrole levels in darkness, but rapid GUN1 degradation in the light could give the pathway a boost (Shimizu et al. 2019). Gun1 mutants of Arabidopsis maintained PhANG gene expression not only after NF but also after lincomycin treatment, which suggests a role in retrograde signalling beyond the tetrapyrrole synthesis pathway.
The PPR domains of the GUN1 protein could interact with nucleic acids. In vitro studies confirmed GUN1 binding to DNA a long time ago, but later work additionally identified a function in RNA editing and stability via interaction with MULTIPLE ORGANELLAR RNA EDITING FACTOR 2 (MORF2) (Zhao et al. 2019). Initially, it was thought that only MORF2 bound to RNA molecules, but recent work revealed that GUN1 can directly interact with and stabilise target transcripts, rRNA and tRNA, as well (Tang et al. 2024).
Besides its role in post‐translational RNA editing, GUN1 steers the plastid‐encoded protein pool at other levels as well. GUN1 regulates mRNA levels, the accumulation of the PLASTID RIBOSOMAL PROTEIN S1 (PRPS1), and interacts with proteins involved in plastid protein homoeostasis (Tadini et al. 2016; Marino et al. 2019). Lastly, by binding to chloroplast HEAT SHOCK PROTEIN70‐1, GUN1 regulates the import of cytosolic (pre‐)proteins into the chloroplast. As a consequence, accumulation of these proteins in the cytosol causes gun‐like phenotypes (Wu et al. 2019).
The molecular function of GUN1 seems diverse and interferes with many different plastid pathways that release retrograde signals. This might make GUN1 the most pronounced integrator of retrograde signals upon stress. Indeed, GUN1 seems to be involved in the responses to high light, leading to altered development and increased photoprotection (Martín et al. 2016; Richter et al. 2020).
3.3. Signals From the Plastid Transcription and Translational Machinery
Plastid transcription is carried out by two primary RNA polymerases: nuclear‐encoded RNA polymerase (NEP) and plastid‐encoded RNA polymerase (PEP). During chloroplast biogenesis, NEP is the main RNA polymerase in etioplasts, while PEP is the primary regulator of transcription in mature chloroplasts (Hernández‐Verdeja et al. 2020). It is worth noting that, though less active, a fully assembled PEP complex has recently been shown in both proplastids and etioplasts of dark‐grown Arabidopsis cell cultures and seedlings, respectively (Ji et al. 2021). It is the general shift in polymerase activity rather than existence that characterises chloroplast biogenesis. This shift is initiated when light is perceived by nuclear photoreceptors (Hernández‐Verdeja et al. 2020). Thus, the initiation of PGE requires anterograde signalling for the full activation of PEP. The use of chemical inhibitors that directly block plastid transcription or translation elegantly showed the role of retrograde signals arising from the plastid protein synthesis machinery in regulating chloroplast development. Upon treatment with inhibitors such as lincomycin, rifampicin or tagetoxin, the expression of various PhANGs is downregulated (Rapp and Mullet 1991).
Repression of PhANG expression is also present in mutants for genes encoding components of the transcriptional machinery of chloroplasts. One example is the sigma factors, which are required for the correct promotor binding of PEP (Lerbs‐Mache 2011). Especially, mutations in SIG2 and SIG6 lead to a pale‐green leaf phenotype, accompanied by a reduction in PhANG and plastid transcription, further suggesting a relationship between plastid and nuclear gene expression (Woodson et al. 2013).
During chloroplast biogenesis, the PEP core forms a complex with 12 nuclear‐encoded PEP‐ASSOCIATED PROTEINs (PAPs), which are required for PEP functionality. Inactivation of the PAP genes results in low PEP activity, as well as an albino or pale leaf phenotype, thus confirming the PAPs' involvement in chloroplast development (Pfalz and Pfannschmidt 2013). Interestingly, expression profiling of a pap7 mutant revealed disturbances in both plastid and nuclear gene expression (Grübler et al. 2017). Pap7 and pap8 mutants showed defects in the accumulation of the GOLDEN‐LIKE transcription factors (GLK1 and GLK2), responsible for the expression of nuclear photosynthetic genes, further linking PEP function to nuclear expression (Grübler et al. 2017; Liebers et al. 2020).
3.4. Photosynthesis‐Derived Signals
Photosynthesis is a highly sensitive process that requires coordinated PhANG and PhAPG expression to respond to fluctuations in the environment. Alterations in photosynthesis can induce a variety of retrograde signals (Figure 3). These include those generated directly from the PETC, redox‐active enzymes, ROS and sugars. In general, these signals are induced by disturbances to the photosynthetic redox balance.
Of the different photosynthesis‐derived signalling components, ROS are perhaps the best known. The most common include 1O2, O2 ●−, H2O2 and •OH. They all possess unique properties, for example, •OH is the most reactive and short‐lived, whereas H2O2 is the most stable and long‐lived of these four (Mittler 2017). Though long regarded as toxic by‐products of aerobic metabolism, research has revealed that ROS should be maintained at a basal level for optimal plant health (Schieber and Chandel 2014; Mittler 2017). One of the best annotated retrograde signals inducing ROS is 1O2, on which we will elaborate further in this review.
The first evidence supporting the role of 1O2 as a retrograde signalling component arose from the identification of the protochlorophyllide (Pchlide) accumulating fluorescent in blue light (flu) mutant (Meskauskiene et al. 2001). The flu mutant offered an ideal system for exploring ROS‐derived stress responses, as Pchlide, accumulating in etioplasts, can transfer light energy to ground‐state oxygen, yielding a 1O2 burst upon seedling illumination (Op Den Camp et al. 2003). Shortly after a dark‐to‐light shift, flu plants exhibit necrotic lesions, as well as increases in 1O2‐responsive gene (SORG) expression (Op Den Camp et al. 2003). These responses may appear to be induced by 1O2 toxicity, but subsequent studies suggested an alternative hypothesis. Inactivation of EXECUTER1 (EX1) and its homologue EX2 abolished the necrotic phenotype of flu, despite maintaining elevated 1O2 (Lee et al. 2007). Moreover, the upregulation of SORG expression in flu is abolished in an ex1 ex2 flu triple mutant, providing evidence for EX1 and EX2 as active 1O2 signalling components (Lee et al. 2007). These findings provided evidence for a retrograde signalling system that controls necrosis in response to 1O2 elevation in plastids.
EX1 is localised to the chloroplast grana margins, in the same region where chlorophyll synthesis and PSII repair take place (Wang et al. 2016). PSII repair is carried out by an ATP‐dependent zinc metalloprotease, FtsH (Kato and Sakamoto 2018). Moreover, degradation of EX1 shortly following light exposure appears to be dependent on FtsH, suggesting EX1 may be a target of proteolysis (Dogra et al. 2017; Wang et al. 2016). FtsH inactivation in flu background led to a strong downregulation of 1O2‐responsive genes, similar to that of the ex1 flu mutant. A later study showed that EX1 is a target of oxidation by 1O2, which primes EX1 for FtsH degradation (Dogra et al. 2019). A recent paper revealed that EX2 also undergoes 1O2‐dependent oxidation and FtsH proteolysis, which in turn further stimulates EX1 oxidation, offering the first evidence for the nature of the EX2–EX1 relationship (Dogra et al. 2022).
Multiple lines of evidence have revealed EX1 and EX2 as regulators of biotic and abiotic stress responses following 1O2 accumulation. For example, treatment of Arabidopsis seedlings with the toxin tenuazonic acid (TeA), isolated from the fungal pathogen Alternaria alternata, resulted in an increase in 1O2, EX1/EX2‐mediated gene expression. TeA blocks the electron transport chain, which leads to an over‐accumulation of reduced plastoquinone (PQ) and the excitation of chlorophyll to a triplet state (3Chl) (Chen et al. 2015). In a similar manner, high light stress generates 3Chl (Krieger‐Liszkay 2005). With that in mind, it is unsurprising that light stress‐induced photoinhibition was diminished in ex1 ex2 double mutants (Kim et al. 2012). Finally, a recent study found that mutating ex1 ex2 completely rescues the high‐light‐induced photobleaching phenotype of de‐etiolating pif1 pif3 double mutants (Li et al. 2023). EX1 interacts with GUN4 and GUN5 in etioplasts, upon light exposure and the release of 1O2. Initially, Li et al. (2023) proposed migration of EX1 to the nucleus following 1O2 exposure; however, this theory has since been rebutted by Liu et al. (2025). Instead, it was suggested that EX1 adheres to the outside of the nucleus during cell fractionation due to methodology‐induced chloroplast rupture (Liu et al. 2025). Nonetheless, these findings further demonstrate the light‐responsive signalling potential of 1O2.
Whilst ROS are one source of signals arising from altered redox balance during photosynthesis, numerous other components of the photosynthetic redox regulatory system can be impacted by environmental changes. As light drives electron transport during photosynthesis, altered redox homoeostasis can act as an important signal conferring information about light quality to the nucleus. One of the best‐known redox‐active compounds that has been linked to retrograde signalling is PQ (Pfannschmidt et al. 2003).
PQ carries out a range of functions within the chloroplast, including electron transfer from PSII to cytochrome b 6 f (Havaux 2020), cyclic electron flow around PSI (Johnson 2011), and facilitating the light‐driven reduction of oxygen at PSII to H2O2 via O2 ●− (Vetoshkina et al. 2017). PQ localises to a range of chloroplast lipid structures (Ksas et al. 2018), but is largely found in thylakoid membranes where it associates with PSII (Havaux 2020). Here, each PSII reaction centre is associated with approximately 5–15 PQ molecules, coining the term ‘plastoquinone pool’ to describe the collective dynamics of these molecules. During electron transport, PQ reduces to PQH2 (plastoquinol) molecules. PQH2 later releases protons and electrons to return to its oxidised state, allowing fluid electron transport (Havaux 2020). In theory, the ratio of PQH2 to PQ represents a putative redox sensor that can transmit information relating to photosynthetic electron transport to downstream (retrograde) signalling pathways (Figure 3).
This proposition was tested for some time, and indeed, the redox state of the PQ pool has been associated with changes in PhANG and PhAPG expression. A study in Dunaliella tertiolecta found that increased LHCB1 expression, when shifting from high to low light conditions, depends on PQ redox state (Escoubas et al. 1995). Blocking electron transport from PSII to PQ by DCMU (3‐[3,4‐dichlorophenyl]‐1,1‐dimethylurea) under high light led to a partial increase in LHCB expression. Similarly, the inhibition of PQH2 oxidation by DBMIB (2,5‐dibromo‐3‐methyl‐6‐isopropyl‐p‐benzoquinone) in low light induced a higher‐light‐adjusted transcriptional response for LHCB1 (Escoubas et al. 1995). The redox state of PQ is not only impacted by shifts in light intensity; however, unbalanced excitation of PSII and PSI (e.g., by shifts in the light spectrum) can likewise have an effect (Tullberg et al. 2000). A recent study found the redox state of PQ to regulate stomatal development by altering the expression of SPEECHLESS and MUTE transcription factors, which directly links photosynthesis‐derived retrograde signals to physiological adaptation (Zoulias et al. 2021).
Akin to many sources of retrograde signals, the nature of signal transmission from PQ remains elusive. One putative signalling component of the PQ redox state is the serine/threonine protein kinase STATE TRANSITION 7 (STN7), a known regulator of LHCII phosphorylation within PSII (Bellafiore et al. 2005). Over‐excitation of PSII relative to PSI leads to PQ reduction, activating STN7, in a manner that is dependent on the docking of PQH2 within the Qo site of the Cyt b 6 f complex (Zito 1999). Active STN7 phosphorylates LHCB1 and LHCB2, leading to their dissociation from PSII (Lemeille et al. 2009), as well as the relocation of primarily LHCB2 to PSI, thus altering photosystem antenna sizes (Longoni et al. 2015). Mutants lacking functional STN7 fail to perform this state transition (Bellafiore et al. 2005), whilst also exhibiting larger grana diameter and reduced stacking dynamics under different light intensities (Hepworth et al. 2021; Flannery et al. 2023). While STN7's role in regulating phosphorylation is local, changes in thylakoid dynamics suggest a more distant impact on nuclear transcription.
3.5. Non‐Tetrapyrrole‐Derived Metabolic Signals
Plastids are metabolic factories that drive a myriad of biochemical processes, including the synthesis of fatty acids, plant hormones, vitamins and secondary metabolites. As with photosynthesis, plastid primary and secondary metabolism is governed by nuclear gene expression, but is impacted by stressors, thus requiring retrograde signalling to remain in balance. The retrograde signals arising from altered non‐photosynthetic tetrapyrrole‐based metabolism have been studied primarily in the context of operational control, with 2‐C‐Methyl‐d‐erythritol‐2,4‐cyclophosphate (MEcPP), various carotenoid derivatives and 3′‐phosphoadenosine 5′‐phosphate (PAP) being the best known signalling metabolites (Jiang and Dehesh 2021).
Many of the known plastid metabolites implicated in retrograde signalling arise from the methylerythritol phosphate (MEP) pathway—an evolutionarily conserved, indispensable pathway present in all plastid‐bearing organisms (Zeng and Dehesh 2021). This pathway drives the production of the functionally and structurally diverse class of metabolites known as the isoprenoids (Allamand et al. 2023), which include plant hormones, pigments and various secondary metabolites (Rodríguez‐Concepción 2014) (Figure 2). One intermediate of the MEP pathway, MEcPP (Figures 2 and 3), was revealed as a retrograde signalling molecule resulting from a genetic screen for regulators of the stress‐inducible, nuclear‐encoded gene HYDROPEROXIDE LYASE (HPL) (Xiao et al. 2012). An amino acid substitution in the gene encoding HYDROXY‐2‐METHYL‐2‐(E)‐BUTENYL 4‐DIPHOSPHATE SYNTHASE (HDS) generated an MEcPP accumulating mutant (ceh1), which was characterised by a strong transcriptional upregulation of not only HPL, but also ARABIDOPSIS ISOCHORISMATE SYNTHASE 1 (ICS1). ceh1 accumulates higher levels of salicylic acid (SA), which in turn confers enhanced resistance to infection by Pseudomonas syringae (Xiao et al. 2012). In wild‐type plants, MEcPP levels increase in response to stressors such as wounding, pathogen attack and high light (Xiao et al. 2012; Wang et al. 2017; Onkokesung et al. 2019), suggesting its role in stress signalling.
Following accumulation, MEcPP can elicit pleiotropic responses by regulating the transcription of a variety of nuclear‐encoded genes. In spite of this, the exact mechanism by which MEcPP activates stress‐responsive gene expression remains elusive. One study found that MEcPP activated the transcription factor CALMODULIN‐BINDING TRANSCRIPTION ACTIVATOR 3 (CAMTA3) in a Ca2+ manner, although the exact mechanism of CAMTA3 induction is unclear (Benn et al. 2016). Interestingly, MEcPP also accumulates in bacteria in response to oxidative stress (Ostrovsky et al. 1998). In Chlamydia trachomatis, MEcPP has been implicated in chromatin remodelling by disrupting the activity of Histone H1‐like protein (Hc1) required for maintaining genome compaction (Grieshaber et al. 2004). Such studies in bacteria may provide insight into the mechanism of regulation in plants, but should be investigated cautiously due to the complexity of signalling between organelles.
Another set of MEP pathway‐derived metabolites involved in retrograde signalling are the carotenoid derivatives (Figures 2 and 3). Carotenoids are a diverse group of isoprenoid metabolites that play vital roles in photosynthesis and photoprotection (Sun et al. 2022). They are considered to be the main quenchers of 1O2 in the plastids. The role of carotenoids in retrograde signalling is also revealed through the extensive use of NF as a means to alter PhANG expression (Figure 3). NF inhibits PHYTOENE DESATURASE (PDS), which converts phytoene to ζ‐carotene, leading to extensive photooxidation and bleaching of seedlings in light (Foudree et al. 2010) (Figure 2). The role of carotenoids in retrograde signalling spans beyond their antioxidative properties; however, a large body of research has suggested that the carotenoids themselves regulate nuclear gene expression.
β‐Cyclocitral (β‐CC), a volatile apocarotenoid generated through the 1O2‐mediated oxidation of β‐carotene (Ramel et al. 2012), is one well‐studied proposed retrograde signalling molecule (Figure 2). Under high‐light conditions, β‐CC acts by promoting SA synthesis, resulting in the nuclear localisation of NONEXPRESSOR OF PATHOGENESIS‐RELATED GENES 1 (NPR1) and subsequent transcriptional reprogramming of ROS detoxification genes, thereby enhancing plant tolerance to oxidative stress (Lv et al. 2015). Another study found protective effects of β‐CC treatment under cold conditions, which was likewise attributed to changes in stress‐related gene expression (Ramel et al. 2012). Later, it was shown that the resistance of young leaves to high light depended on β‐CC‐mediated SCARECROW LIKE14 (SCL14) upregulation and the induction of associated reactive carbonyl species (RCS) detoxification (D'Alessandro et al. 2018). Furthermore, β‐CC improves drought and salt tolerance, possibly by changing root development (D'Alessandro et al. 2019; Dickinson et al. 2019; Braat et al. 2023). However, the exact nature of β‐CC signalling warrants further investigation.
In addition to β‐CC, other cis‐carotene‐derived apocarotenoids also constitute retrograde signalling responses at both biogenic and operational levels. However, in many cases, the exact apocarotenoid responsible for the retrograde signal remains unknown. For example, mutating the ZDS/CHLOROPLAST BIOGENESIS5 (CLB5) gene induces alterations in PhAPG and PhANG expression, accompanied by albinism and abnormal leaf morphology, through the action of an unknown apocarotenoid (Avendaño‐Vázquez et al. 2014). Similarly, the post‐transcriptional regulation of PSY, the enzyme catalysing the first committed step of cis‐carotene biosynthesis (Figure 2), was proposed to be regulated by plastid‐derived apocarotenoid levels (Alvarez et al. 2016). Another well‐characterised carotenoid biosynthesis mutant is the Arabidopsis carotenoid and chloroplast regulation 2 (ccr2) mutant, affected in the gene encoding the enzyme CAROTENOID ISOMERASE (CRTISO; Figure 2). Etioplasts of dark‐grown ccr2 seedlings lack prolamellar bodies, resulting in perturbed photosynthesis establishment after transitioning to light (Park et al. 2002). This phenotype was attributed to another, undefined, cis‐carotene‐derived apocarotenoid that accumulates under dark or short‐day conditions (Cazzonelli et al. 2020). Such an apocarotenoid must be synthesised from enzymatic steps upstream of CRTISO. Indeed, second mutations in either ζ‐CAROTENE ISOMERASE (Cazzonelli et al. 2020) or PSY rescue plastid abnormalities in ccr2, possibly by altering nuclear transcription of core photomorphogenesis components such as the PIFs and ELONGATED HYPOCOTYL 5 (Cazzonelli et al. 2020; Hou et al. 2024).
Whilst it is clear that altering enzymatic steps of the apocarotenoid biosynthesis pathway has implications for plastid development and nuclear gene expression, further research is needed to better describe the nature of these signals. Whether these apocarotenoids are indeed retrograde signals that are exported from the plastids to regulate nuclear gene expression remains unclear. One may consider the possibility that overaccumulation of specific cis‐apocarotenoids has direct impacts on plastid ultrastructure, which has concomitant effects on nuclear gene expression through unknown signalling pathways. Taken together, this field offers an exciting opportunity for future research in operational retrograde signalling.
One other metabolism‐derived retrograde signal source is that of PAP and the nucleotide phosphatase SAL1 (Figure 3). PAP is generated as a by‐product of sulphur metabolism, but is degraded by SAL1 under normal growth conditions (Chen et al. 2011; Estavillo et al. 2011). Interestingly, early research on SAL1 identified its role in regulating stress responses using a genetic screen for mutants with upregulated expression of the antioxidant ASCORBATE PEROXIDASE 2 (Rossel et al. 2006). This altered expression of APX2 (alx8) mutant holds a null mutation in SAL1, which provides resistance to high light and drought stress (Rossel et al. 2006; Wilson et al. 2009). Such tolerance was later attributed to an accumulation of plastid‐localised PAP (Estavillo et al. 2011). The SAL1‐PAP module, therefore, presented itself as a putative stress‐responsive retrograde signalling module. High light and drought stress induce oxidative stress within the plastids, offering a clue to the regulation of the SAL1‐PAP pathway. Increasing plastid oxidative stress reduces SAL1 activity through dimerisation, disulphide bond formation and glutathionylation (Chan et al. 2016a). This presents SAL1's role outside of sulphur metabolism, as an oxidative stress sensor that regulates the expression of plastid redox‐associated nuclear genes (such as APX2) via PAP.
Under heat stress, PAP accumulates as a result of increased tocopherol levels. In this case, the PAP induction is independent of oxidative stress and SAL1 and induces nuclear miRNAs that promote heat tolerance (Fang et al. 2019). The fact that PAP translocates from the cytosol to plastids (Bohrer et al. 2014) makes it a good candidate for intracellular signalling activity.
4. Stressed Chloroplasts Steer Plant Development Through Retrograde Signalling
So far, we have described how various abiotic conditions cause chloroplast stress and the retrograde signals emitted from stressed chloroplasts that affect nuclear gene expression and stress tolerance. The effects of retrograde signals on stress resilience are often studied using chloroplast stress‐inducing antibiotics or mutants that display constitutive retrograde signalling. Nevertheless, a number of studies have shown that abiotic stress induces retrograde signalling through GUN1, MEcPP, carotenoid derivatives and SAL1‐PAP. In addition to the regulation of PhANG or stress‐responsive gene expression, these chloroplast‐derived signals can strongly affect whole plant physiology, growth and development by interfering with canonical signalling pathways.
4.1. Retrograde Signals Interact With Photoreceptor Signalling
Plants constantly monitor their light environment and adapt their growth to best suit the local conditions. To this end, they possess dedicated wavelength‐specific photoreceptors that together sense most of the light spectrum. Depletion of photosynthetically important blue and red light in darkness or shaded environments leads to inactivation of cryptochrome (cry) and phytochrome (phy) photoreceptors, respectively. This photoreceptor inactivation releases the repression of PIFs, resulting in auxin synthesis and shoot elongation as well as an inhibition of photomorphogenesis. In light, on the other hand, activated cry and phy repress PIFs and inhibit the CONSTITUTIVELY PHOTOMORPHOGENIC 1 (COP1)/SUPPRESSOR OF PHYTOCHROME A‐105 (SPA) E3 ubiquitin ligase complex, which results in stabilisation of the photomorphogenesis‐promoting transcription factor HY5 (Gommers and Monte 2017). Among the HY5‐induced genes are the GOLDEN 2‐LIKE (GLK) transcription factors GLK1 and GLK2, which are oppositely regulated in darkness by repressive PIF binding (Zhang et al. 2024; Martín et al. 2016). These GLKs promote photomorphogenesis by direct stimulation of PhANG expression as well as through enhanced expression of the transcription factor B‐BOX16 (Veciana et al. 2022).
Among the first identified common targets of light and retrograde signalling are cry1 and HY5. A screen for gun mutants revealed that, like gun1, cry1 mutants maintain partial light‐induced LHCB1.1 expression in the presence of chloroplast‐inhibiting antibiotics (Ruckle et al. 2007). Mutation of cry1 or hy5 in a gun1 background further increased LHCB1.1 expression in light with lincomycin, suggesting additive effects for GUN1, cry1 and HY5 in retrograde signalling. Moreover, these mutations severely increased photobleaching in high light, especially in higher‐order mutants, suggesting that retrograde or photoreceptor signalling through these proteins limits photooxidative damage (Ruckle et al. 2007). Later studies showed that GUN1, cry1, HY5 and GLK2 are required for anthocyanin synthesis, to reduce photooxidative damage in high light (Richter et al. 2023).
Retrograde and photoreceptor signalling interact downstream of phy signalling as well. In light, phy represses PIFs and thereby inhibits the repressive effect of PIFs on GLK1 expression (Martín et al. 2016). Similarly, GLK1 expression is repressed under stressfully high light conditions or after lincomycin treatment. This suppression is mediated by GUN1 and is independent of PIFs and HY5 (Martín et al. 2016), suggesting that the phy and GUN1 signalling pathways antagonistically regulate GLK1 expression. Interestingly, the repression of GLK1 leads to the inhibition of seedling photomorphogenesis at multiple scales. Besides the suppression of PhANG expression, it prevents the opening and expansion of cotyledons in high light, resulting in a mild skotomorphogenic phenotype (Martín et al. 2016; Veciana et al. 2022). This impact on seedling development is GUN1‐dependent and the result of the inhibition of both GLK1 expression and GLK1‐induced BBX16 expression (Veciana et al. 2022). As a consequence, the overexpression of either GLK1 or BBX16 results in a strongly reduced response to lincomycin or high light, which is largely restored by the bbx16 mutation in GLK1OX.
In contrast to GUN1‐mediated retrograde signalling, the isoprenoid precursor MEcPP affects phy signalling more upstream. As high light causes accumulation of various stress compounds besides MEcPP, its effects are mostly studied by exogenous application or using MEcPP‐accumulating ceh1 mutants (Xiao et al. 2012). These ceh1 mutants have short hypocotyls in light, which could be explained by phyB stabilisation and reduced PIF activity (Jiang et al. 2019). Next to high light, MEcPP accumulates in response to high temperature (Rivasseau et al. 2009). Warm temperatures induce a developmental programme named thermomorphogenesis, which shares many key signalling components with light responses, including phyB and PIFs, so it is likely that MEcPP‐induced retrograde signalling influences this developmental programme as well.
The aforementioned ccr2 mutant, lacking the CRTISO enzyme (Figure 2), accumulates cis‐carotenoids in plants that are shifted from long to short photoperiods (Cazzonelli et al. 2020). These cis‐carotenoids are cleaved to apocarotenoids, introducing a retrograde apocarotenoid signal that inhibits HY5 and PhANG expression, while inducing PIF3 expression (Cazzonelli et al. 2020). The resulting impact on gene expression impairs chloroplast biogenesis and photomorphogenesis, thereby linking another retrograde signal to photoreceptor signalling.
4.2. Retrograde Signals Interact With Hormone Signalling
Phytohormone signalling pathways are sensitive to disruption by stress‐induced retrograde signalling as well. This is perhaps unsurprising, as the previously discussed photoreceptor pathways rely on the synthesis and action of various growth‐related hormones. However, retrograde signalling also affects hormonal pathways that are not directly associated with photoreceptors, indicating a broader impact on growth and stress responses.
As previously mentioned, MEcPP accumulation reduces hypocotyl growth through enhanced phyB abundance and reduced PIF activity. In addition to this, MEcPP also disrupts the abundance and localisation of PIN auxin transporters and reduces the expression of the YUCCA auxin synthesis genes (Jiang et al. 2018; Jiang et al. 2020). As a consequence, MEcPP reduces auxin‐mediated hypocotyl elongation, as visualised using the DR5::GFP auxin signalling marker. Strikingly, DR5::GFP abundance in the hypocotyl is similarly affected in high light, suggesting biological relevance of this chloroplast‐to‐nucleus signalling pathway beyond the MEcPP accumulating ceh1 mutant or exogenous treatment (Jiang et al. 2018).
In addition to auxin, MEcPP accumulation reduces ethylene abundance, probably through reduced expression of various ACC SYNTHASE (ACS) genes that facilitate the production of the ethylene precursor 1‐aminocyclopropane‐1‐carboxylate (ACC) (Jiang et al. 2020). ACC treatment partially rescues hypocotyl length in dwarfed ceh1 plants, but only in seedlings that retain normal auxin signalling, suggesting ethylene is epistatic to auxin in the regulation of hypocotyl elongation (Jiang et al. 2020). Besides hypocotyl elongation, ethylene signalling mediates the lincomycin‐induced inhibition of cotyledon separation in light (Gommers et al. 2020). Although the lincomycin‐induced retrograde signal that targets the ethylene signalling pathway remains elusive, it is GUN1‐independent (Gommers et al. 2020).
The oxidative stress that occurs during high light and drought causes chloroplast accumulation of PAP, through inactivation of the PAP‐degrading enzyme SAL1. Mutation of sal1 in Arabidopsis causes various defects in development and growth, including altered venation, short hypocotyls, compact rosette structure, delayed circadian rhythmicity and disturbed flowering (reviewed in Phua et al. [2018]). These phenotypic defects were identified in a range of forward genetics studies, which gave rise to a plethora of described mutant alleles and at least eight different mutant names, further demonstrating the developmental importance of SAL1 and PAP (Phua et al. 2018). In addition to the onset of specific stress‐responsive genes, PAP accumulation affects the expression levels of ABA, auxin, gibberellin and JA biosynthesis genes, many of which are upregulated (Wilson et al. 2009; Estavillo et al. 2011; Phua et al. 2018). Consequently, changes in hormone levels have been found in 4‐week‐old Arabidopsis sal1 mutants, with reported increases in ABA (Rossel et al. 2006), auxin (Phua et al. 2018) and JA (Rodríguez et al. 2010), but decreased gibberellin levels (Phua et al. 2018).
4.3. Carotenoid Derivatives Modulate Root Development
Besides the previously described effects of β‐CC on SCL14 expression and RCS detoxification, exogenous β‐CC application enhanced root growth in Arabidopsis, rice and tomato (Dickinson et al. 2019). Moreover, β‐CC improves the growth of both the root and shoot of salt‐stressed rice. A study that was published around the same time revealed that β‐CC is converted to water‐soluble β‐cyclocitric acid (β‐CCA) in vivo, which similarly promotes expression of β‐CC‐responsive genes (D'Alessandro et al. 2019). In this study, β‐CCA was shown to accumulate during drought and promote drought tolerance and root growth. In drought‐treated tomato, exogenous β‐CCA application reduced water loss and increased total fruit weight (D'Alessandro et al. 2019).
Although these findings suggest that apocarotenoids increase drought tolerance by enhancing root growth, a later study found a repressive effect of both apocarotenoids on root size, while maintaining the enhanced drought tolerance (Braat et al. 2023). This could be the result of β‐CCA‐induced root suberisation, which forms a water diffusion barrier in the root endodermis and reduces water loss. While these studies appear to be contradictory regarding root growth, the effect on drought tolerance is maintained between them.
5. Perspectives and Conclusions
In this review, we characterised known retrograde signals and their interaction with canonical signalling pathways. While these interactions provide a solution for improving plant stress adaptation, many studies focus on signalling in the context of biogenic control. In terms of high light stress, photomorphogenesis and the initiation of photosynthesis introduce the risk of photo‐oxidative damage. Retrograde signals released in high light mitigate this stress‐induced damage (Jiang and Dehesh 2021). Developmental adaptations such as unexpanded cotyledons are also thought to serve as a protection of the apical meristem of young seedlings (Martín et al. 2016). However, it remains unknown if this really contributes to higher survival.
Another point of interest is that the extent to which metabolic retrograde signalling pathways are simultaneously induced and interact with one another is not really known. Most studies have focussed on individual pathways; however, recent evidence reveals a far more complex and interconnected scenario. This makes it difficult to identify the source of some retrograde signals. For example, recent studies have identified a number of mutations other than flu that can alter 1O2 production in seedlings. To make matters more complex, the MEP pathway can also generate 1O2 following light exposure due to imbalanced isoprenoid and tetrapyrrole flux during chlorophyll synthesis (Kim et al. 2013). Since many abiotic stress conditions alter MEP pathway activity (Rivasseau et al. 2009), the possible involvement of ROS‐derived signalling from this pathway should also be considered.
Another challenge in determining the relevance of described signals under natural conditions lies in the way they have been induced. The use of mutants that over‐accumulate retrograde signals (flu, ceh1, sal1 and ccr2 to name a few), or antibiotics that disrupt plastid functionality at different levels, may trigger signalling cascades that differ significantly from those induced by abiotic stress. Whilst such scenarios have provided necessary insight into the signalling role of plastids, they may not fully reflect the complexity or dynamics of natural environments. Advances in metabolomics may provide a means to find new connections between stress‐induced metabolic changes and PhANG/SRG expression. These tools may enable a more comprehensive understanding of shifts in plastid metabolism in response to abiotic stress and help bridge the gap between experimental models and real‐world conditions.
Finally, this review started by documenting changes in plastid ultrastructure following abiotic stress exposure (Figure 1). At present, it remains unclear to what extent retrograde signals are implicated in these changes. It is well established that stressed plastids generate retrograde signals and that plastid‐induced reprogramming of nuclear transcription promotes the ultrastructural changes that drive chloroplast biogenesis. With this in mind, it seems likely that stress‐responsive ultrastructural changes require coordinated communication between the plastids and the nucleus. This knowledge gap warrants further investigation and offers an insightful new avenue of research into cellular responses to abiotic stress in plants.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgements
This study is supported by the Graduate School for Experimental Plant Sciences (EPS)–Wageningen University (WU) strategic funds for young Tenure Track researchers (granted to C.G.).
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.
References
- Adhikari, N. D. , Froehlich J. E., Strand D. D., Buck S. M., Kramer D. M., and Larkin R. M.. 2011. “GUN4‐porphyrin Complexes Bind the ChlH/GUN5 Subunit of Mg‐Chelatase and Promote Chlorophyll Biosynthesis in Arabidopsis.” Plant Cell 23: 1449–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alawady, A. E. , and Grimm B.. 2005. “Tobacco Mg Protoporphyrin IX Methyltransferase Is Involved in Inverse Activation of Mg Porphyrin and Protoheme Synthesis.” Plant Journal 41: 282–290. [DOI] [PubMed] [Google Scholar]
- Allamand, A. , Piechowiak T., Lièvremont D., Rohmer M., and Grosdemange‐Billiard C.. 2023. “The Multifaceted MEP Pathway: Towards New Therapeutic Perspectives.” Molecules 28: 1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez, D. , Voss B., Maass D., et al. 2016. “Carotenogenesis Is Regulated by 5'UTR‐Mediated Translation of Phytoene Synthase Splice Variants.” Plant Physiology 172: 2314–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankele, E. , Kindgren P., Pesquet E., and Strand A.. 2007. “In Vivo Visualization of Mg‐Protoporphyrin IX, a Coordinator of Photosynthetic Gene Expression in the Nucleus and the Chloroplast.” Plant Cell 19: 1964–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avendaño‐Vázquez, A.‐O. , Cordoba E., Llamas E., et al. 2014. “An Uncharacterized Apocarotenoid‐Derived Signal Generated in ζ‐Carotene Desaturase Mutants Regulates Leaf Development and the Expression of Chloroplast and Nuclear Genes in Arabidopsis.” Plant Cell 26: 2524–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellafiore, S. , Barneche F., Peltier G., and Rochaix J. D.. 2005. “State Transitions and Light Adaptation Require Chloroplast Thylakoid Protein Kinase STN7.” Nature 433: 892–895. [DOI] [PubMed] [Google Scholar]
- Benn, G. , Bjornson M., Ke H., et al. 2016. “Plastidial Metabolite Mecpp Induces a Transcriptionally Centered Stress‐Response Hub via the Transcription Factor CAMTA3.” Proceedings of the National Academy of Sciences 113: 8855–8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohrer, A. S. , Kopriva S., and Takahashi H.. 2014. “Plastid‐Cytosol Partitioning and Integration of Metabolic Pathways for APS/PAPS Biosynthesis in Arabidopsis thaliana .” Frontiers in Plant Science 5: 751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottino‐Rojas, V. , Talyuli O. A. C., Jupatanakul N., et al. 2015. “Heme Signaling Impacts Global Gene Expression, Immunity and Dengue Virus Infectivity in Aedes aegypti .” PLoS One 10: e0135985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braat, J. , Jaonina M., David P., et al. 2023. “The Response of Arabidopsis to the Apocarotenoid β‐Cyclocitric Acid Reveals a Role for SIAMESE‐RELATED 5 in Root Development and Drought Tolerance.” PNAS Nexus 2: pgad353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazzonelli, C. I. , Hou X., Alagoz Y., et al. 2020. “A Cis‐Carotene Derived Apocarotenoid Regulates Etioplast and Chloroplast Development.” eLife 9: e45310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, K. X. , Mabbitt P. D., Phua S. Y., et al. 2016a. “Sensing and Signaling of Oxidative Stress in Chloroplasts by Inactivation of the SAL1 Phosphoadenosine Phosphatase.” Proceedings of the National Academy of Sciences of the United States of America 113: E4567–E4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, K. X. , Phua S. Y., Crisp P., Mcquinn R., and Pogson B. J.. 2016b. “Learning the Languages of the Chloroplast: Retrograde Signaling and Beyond.” Annual Review of Plant Biology 67: 25–53. [DOI] [PubMed] [Google Scholar]
- Chen, H. , Zhang B., Hicks L. M., and Xiong L.. 2011. “A Nucleotide Metabolite Controls Stress‐Responsive Gene Expression and Plant Development.” PLoS One 6: e26661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, S. , Kim C., Lee J. M., et al. 2015. “Blocking the QB‐Binding Site of Photosystem II by Tenuazonic Acid, a Non‐Host‐Specific Toxin of Alternaria alternata, Activates Singlet Oxygen‐Mediated and EXECUTER‐Dependent Signalling in Arabidopsis.” Plant, Cell & Environment 38: 1069–1080. [DOI] [PubMed] [Google Scholar]
- Chen, X. Q. , and Yu B. J.. 2007. “Ionic Effects of Na+ and Cl‐ on Photosynthesis in Glycine max Seedlings Under Isoosmotic Salt Stress.” Zhi Wu Sheng Li Yu Fen Zi Sheng Wu Xue Xue Bao = Journal of Plant Physiology and Molecular Biology 33: 294–300. [PubMed] [Google Scholar]
- D'Alessandro, S. , Mizokami Y., Légeret B., and Havaux M.. 2019. “The Apocarotenoid β‐Cyclocitric Acid Elicits Drought Tolerance in Plants.” Iscience 19: 461–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Alessandro, S. , Ksas B., and Havaux M.. 2018. “Decoding β‐Cyclocitral‐Mediated Retrograde Signaling Reveals the Role of a Detoxification Response in Plant Tolerance to Photooxidative Stress.” Plant Cell 30: 2495–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, P. A. , and Hangarter R. P.. 2012. “Chloroplast Movement Provides Photoprotection to Plants by Redistributing PSII Damage Within Leaves.” Photosynthesis Research 112: 153–161. [DOI] [PubMed] [Google Scholar]
- Dickinson, A. J. , Lehner K., Mi J., et al. 2019. “β‐Cyclocitral Is a Conserved Root Growth Regulator.” Proceedings of the National Academy of Sciences 116: 10563–10567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitrieva, V. A. , Tyutereva E. V., and Voitsekhovskaja O. V.. 2020. “Singlet Oxygen in Plants: Generation, Detection, and Signaling Roles.” International Journal of Molecular Sciences 21: 3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogra, V. , Duan J., Lee K. P., Lv S., Liu R., and Kim C.. 2017. “FtsH2‐Dependent Proteolysis of EXECUTER1 Is Essential in Mediating Singlet Oxygen‐Triggered Retrograde Signaling in Arabidopsis thaliana .” Frontiers in Plant Science 8: 1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogra, V. , Li M., Singh S., Li M., and Kim C.. 2019. “Oxidative Post‐Translational Modification of EXECUTER1 Is Required for Singlet Oxygen Sensing in Plastids.” Nature Communications 10: 2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogra, V. , Singh R. M., Li M., Li M., Singh S., and Kim C.. 2022. “EXECUTER2 Modulates the EXECUTER1 Signalosome Through Its Singlet Oxygen‐Dependent Oxidation.” Molecular Plant 15: 438–453. [DOI] [PubMed] [Google Scholar]
- Escoubas, J. M. , Lomas M., Laroche J., and Falkowski P. G.. 1995. “Light Intensity Regulation of Cab Gene Transcription Is Signaled by the Redox State of the Plastoquinone Pool.” Proceedings of the National Academy of Sciences 92: 10237–10241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinas, N. A. , Kobayashi K., Takahashi S., Mochizuki N., and Masuda T.. 2012. “Evaluation of Unbound Free Heme in Plant Cells by Differential Acetone Extraction.” Plant and Cell Physiology 53: 1344–1354. [DOI] [PubMed] [Google Scholar]
- Estavillo, G. M. , Crisp P. A., Pornsiriwong W., et al. 2011. “Evidence for a SAL1‐PAP Chloroplast Retrograde Pathway That Functions in Drought and High Light Signaling in Arabidopsis.” Plant Cell 23: 3992–4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang, X. , Zhao G., Zhang S., et al. 2019. “Chloroplast‐to‐Nucleus Signaling Regulates microRNA Biogenesis in Arabidopsis.” Developmental Cell 48: 371–382.e4. [DOI] [PubMed] [Google Scholar]
- Flannery, S. E. , Pastorelli F., Emrich‐Mills T. Z., et al. 2023. “STN7 Is Not Essential for Developmental Acclimation of Arabidopsis to Light Intensity.” Plant Journal 114: 1458–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foudree, A. , Aluru M., and Rodermel S.. 2010. “PDS Activity Acts as a Rheostat of Retrograde Signaling During Early Chloroplast Biogenesis.” Plant Signaling & Behavior 5: 1629–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii, Y. , Tanaka H., Konno N., et al. 2017. “Phototropin Perceives Temperature Based on the Lifetime of Its Photoactivated State.” Proceedings of the National Academy of Sciences 114: 9206–9211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gommers, C. M. M. , and Monte E.. 2017. “Seedling Establishment: A Dimmer Switch‐Regulated Process Between Dark and Light Signaling.” Plant Physiology 176: 1061–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gommers, C. M. M. , Ruiz‐Sola M. Á., Ayats A., Pereira L., Pujol M., and Monte E.. 2020. “GENOMES UNCOUPLED1‐Independent Retrograde Signaling Targets the Ethylene Pathway to Repress Photomorphogenesis.” Plant Physiology 185: 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray, J. C. , Hansen M. R., Shaw D. J., et al. 2012. “Plastid Stromules Are Induced by Stress Treatments Acting Through Abscisic Acid.” Plant Journal 69: 387–398. [DOI] [PubMed] [Google Scholar]
- Grieshaber, N. A. , Fischer E. R., Mead D. J., Dooley C. A., and Hackstadt T.. 2004. “Chlamydial Histone‐DNA Interactions Are Disrupted by a Metabolite in the Methylerythritol Phosphate Pathway of Isoprenoid Biosynthesis.” Proceedings of the National Academy of Sciences 101: 7451–7456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grübler, B. , Merendino L., Twardziok S. O., et al. 2017. “Light and Plastid Signals Regulate Different Sets of Genes in the Albino Mutant Pap7‐1.” Plant Physiology 175: 1203–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gururani, M. A. , Venkatesh J., and Tran L. S. P.. 2015. “Regulation of Photosynthesis During Abiotic Stress‐Induced Photoinhibition.” Molecular Plant 8: 1304–1320. [DOI] [PubMed] [Google Scholar]
- Havaux, M. 2020. “Plastoquinone in and Beyond Photosynthesis.” Trends in Plant Science 25: 1252–1265. [DOI] [PubMed] [Google Scholar]
- He, C. , Yan J., Shen G., et al. 2005. “Expression of an Arabidopsis Vacuolar Sodium/Proton Antiporter Gene in Cotton Improves Photosynthetic Performance Under Salt Conditions and Increases Fiber Yield in the Field.” Plant and Cell Physiology 46: 1848–1854. [DOI] [PubMed] [Google Scholar]
- Hepworth, C. , Wood W. H. J., Emrich‐Mills T. Z., Proctor M. S., Casson S., and Johnson M. P.. 2021. “Dynamic Thylakoid Stacking and State Transitions Work Synergistically to Avoid Acceptor‐Side Limitation of Photosystem I.” Nature Plants 7: 87–98. [DOI] [PubMed] [Google Scholar]
- Hernández‐Verdeja, T. , Vuorijoki L., Jin X., Vergara A., Dubreuil C., and Strand Å.. 2022. “GENOMES UNCOUPLED1 Plays a Key Role During the De‐Etiolation Process in Arabidopsis.” New Phytologist 235: 188–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández‐Verdeja, T. , Vuorijoki L., and Strand Å.. 2020. “Emerging From the Darkness: Interplay Between Light and Plastid Signaling During Chloroplast Biogenesis.” Physiologia Plantarum 169: 397–406. [DOI] [PubMed] [Google Scholar]
- Hou, X. , Alagoz Y., Welsch R., Mortimer M. D., Pogson B. J., and Cazzonelli C. I.. 2024. “Reducing PHYTOENE SYNTHASE Activity Fine‐Tunes the Abundance of a Cis‐Carotene‐Derived Signal That Regulates the PIF3/HY5 Module and Plastid Biogenesis.” Journal of Experimental Botany 75: 1187–1204. [DOI] [PubMed] [Google Scholar]
- Huang, W. , Yang Y. J., Zhang S. B., and Liu T.. 2018. “Cyclic Electron Flow Around Photosystem I Promotes ATP Synthesis Possibly Helping the Rapid Repair of Photodamaged Photosystem II at Low Light.” Frontiers in Plant Science 9: 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jespersen, D. , Zhang J., and Huang B.. 2016. “Chlorophyll Loss Associated With Heat‐Induced Senescence in Bentgrass.” Plant Science 249: 1–12. [DOI] [PubMed] [Google Scholar]
- Ji, Y. , Lehotai N., Zan Y., Dubreuil C., Díaz M. G., and Strand Å.. 2021. “A Fully Assembled Plastid‐Encoded RNA Polymerase Complex Detected in Etioplasts and Proplastids in Arabidopsis.” Physiologia Plantarum 171: 435–446. [DOI] [PubMed] [Google Scholar]
- Jiang, J. , and Dehesh K.. 2021. “Plastidial Retrograde Modulation of Light and Hormonal Signaling: An Odyssey.” New Phytologist 230: 931–937. [DOI] [PubMed] [Google Scholar]
- Jiang, J. , Rodriguez‐Furlan C., Wang J. Z., et al. 2018. “Interplay of the Two Ancient Metabolites Auxin and MEcPP Regulates Adaptive Growth.” Nature Communications 9: 2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, J. , Xiao Y., Chen H., et al. 2020. “Retrograde Induction of phyB Orchestrates Ethylene‐Auxin Hierarchy to Regulate Growth.” Plant Physiology 183: 1268–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, J. , Zeng L., Ke H., De La Cruz B., and Dehesh K.. 2019. “Orthogonal Regulation of Phytochrome B Abundance by Stress‐Specific Plastidial Retrograde Signaling Metabolite.” Nature Communications 10: 2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, G. N. 2011. “Physiology of PSI Cyclic Electron Transport in Higher Plants.” Biochimica et Biophysica Acta 1807: 384–389. [DOI] [PubMed] [Google Scholar]
- Kato, Y. , and Sakamoto W.. 2018. “FtsH Protease in the Thylakoid Membrane: Physiological Functions and the Regulation of Protease Activity.” Frontiers in Plant Science 9: 855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killi, D. , Raschi A., and Bussotti F.. 2020. “Lipid Peroxidation and Chlorophyll Fluorescence of Photosystem II Performance during Drought and Heat Stress Is Associated With the Antioxidant Capacities of C3 Sunflower and C4 Maize Varieties.” International Journal of Molecular Sciences 21: 4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, C. , Meskauskiene R., Zhang S., et al. 2012. “Chloroplasts of Arabidopsis Are the Source and a Primary Target of a Plant‐Specific Programmed Cell Death Signaling Pathway.” Plant Cell 24: 3026–3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S. , Schlicke H., Van Ree K., et al. 2013. “Arabidopsis Chlorophyll Biosynthesis: An Essential Balance Between the Methylerythritol Phosphate and Tetrapyrrole Pathways.” Plant Cell 25: 4984–4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger‐Liszkay, A. 2004. “Singlet Oxygen Production in Photosynthesis.” Journal of Experimental Botany 56: 337–346. [DOI] [PubMed] [Google Scholar]
- Ksas, B. , Légeret B., Ferretti U., et al. 2018. “The Plastoquinone Pool Outside the Thylakoid Membrane Serves in Plant Photoprotection as a Reservoir of Singlet Oxygen Scavengers.” Plant, Cell & Environment 41: 2277–2287. [DOI] [PubMed] [Google Scholar]
- Larkin, R. M. 2016. “Tetrapyrrole Signaling in Plants.” Frontiers in Plant Science 7: 1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin, R. M. , Alonso J. M., Ecker J. R., and Chory J.. 2003. “GUN4, a Regulator of Chlorophyll Synthesis and Intracellular Signaling.” Science 299: 902–906. [DOI] [PubMed] [Google Scholar]
- Lee, K. P. , Kim C., Landgraf F., and Apel K.. 2007. “EXECUTER1‐ and EXECUTER2‐Dependent Transfer of Stress‐Related Signals From the Plastid to the Nucleus of Arabidopsis thaliana .” Proceedings of the National Academy of Sciences 104: 10270–10275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemeille, S. , Willig A., Depège‐Fargeix N., Delessert C., Bassi R., and Rochaix J. D.. 2009. “Analysis of the Chloroplast Protein Kinase Stt7 During State Transitions.” PLoS Biology 7: e1000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerbs‐Mache, S. 2011. “Function of Plastid Sigma Factors in Higher Plants: Regulation of Gene Expression or Just Preservation of Constitutive Transcription?” Plant Molecular Biology 76: 235–249. [DOI] [PubMed] [Google Scholar]
- Li, Y. , Liu H., Ma T., et al. 2023. “Arabidopsis EXECUTER1 Interacts With Wrky Transcription Factors to Mediate Plastid‐to‐Nucleus Singlet Oxygen Signaling.” Plant Cell 35: 827–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebers, M. , Gillet F.‐X., Israel A., et al. 2020. “Nucleo‐Plastidic PAP8/pTAC6 Couples Chloroplast Formation With Photomorphogenesis.” EMBO Journal 39: e104941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. , and Van Iersel M. W.. 2021. “Photosynthetic Physiology of Blue, Green, and Red Light: Light Intensity Effects and Underlying Mechanisms.” Frontiers in Plant Science 12: 619987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, K. , Zhao H., Lee K. P., et al. 2025. “EXECUTER1 and Singlet Oxygen Signaling: A Reassessment of Nuclear Activity.” Plant Cell 37: koae296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan, B. A. , Kornyeyev D., Hardison J., and Holaday A. S.. 2006. “The Role of Antioxidant Enzymes in Photoprotection.” Photosynthesis Research 88: 119–132. [DOI] [PubMed] [Google Scholar]
- Longoni, P. , Douchi D., Cariti F., Fucile G., and Goldschmidt‐Clermont M.. 2015. “Phosphorylation of the Light‐Harvesting Complex II Isoform Lhcb2 Is Central to State Transitions.” Plant Physiology 169: 2874–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, D. , Zhang Y., Zhang A., and Lu C.. 2022. “Non‐Photochemical Quenching: From Light Perception to Photoprotective Gene Expression.” International Journal of Molecular Sciences 23: 687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv, F. , Zhou J., Zeng L., and Xing D.. 2015. “β‐Cyclocitral Upregulates Salicylic Acid Signalling to Enhance Excess Light Acclimation Inarabidopsis.” Journal of Experimental Botany 66: 4719–4732. [DOI] [PubMed] [Google Scholar]
- Marino, G. , Naranjo B., Wang J., Penzler J.‐F., Kleine T., and Leister D.. 2019. “Relationship of GUN1 to FUG1 in Chloroplast Protein Homeostasis.” Plant Journal 99: 521–535. [DOI] [PubMed] [Google Scholar]
- Martín, G. , Leivar P., Ludevid D., Tepperman J. M., Quail P. H., and Monte E.. 2016. “Phytochrome and Retrograde Signalling Pathways Converge to Antagonistically Regulate a Light‐Induced Transcriptional Network.” Nature Communications 7: 11431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, W. , Rujan T., Richly E., et al. 2002. “Evolutionary Analysis of Arabidopsis, Cyanobacterial, and Chloroplast Genomes Reveals Plastid Phylogeny and Thousands of Cyanobacterial Genes in the Nucleus.” Proceedings of the National Academy of Sciences 99: 12246–12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng, F. , Luo Q., Wang Q., et al. 2016. “Physiological and Proteomic Responses to Salt Stress in Chloroplasts of Diploid and Tetraploid Black Locust (Robinia pseudoacacia L.).” Scientific Reports 6: 23098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meskauskiene, R. , Nater M., Goslings D., Kessler F., Op Den Camp R., and Apel K.. 2001. “FLU: A Negative Regulator of Chlorophyll Biosynthesis in Arabidopsis thaliana .” Proceedings of the National Academy of Sciences 98: 12826–12831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler, R. 2017. “ROS Are Good.” Trends in Plant Science 22: 11–19. [DOI] [PubMed] [Google Scholar]
- Mochizuki, N. , Brusslan J. A., Larkin R., Nagatani A., and Chory J.. 2001. “Arabidopsis Genomes Uncoupled 5 (GUN5) Mutant Reveals the Involvement of Mg‐Chelatase H Subunit in Plastid‐to‐Nucleus Signal Transduction.” Proceedings of the National Academy of Sciences 98: 2053–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki, N. , Tanaka R., Tanaka A., Masuda T., and Nagatani A.. 2008. “The Steady‐State Level of Mg‐Protoporphyrin IX Is Not a Determinant of Plastid‐to‐Nucleus Signaling in Arabidopsis.” Proceedings of the National Academy of Sciences 105: 15184–15189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales, A. , and Kaiser E.. 2020. “Photosynthetic Acclimation to Fluctuating Irradiance in Plants.” Frontiers in Plant Science 11: 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulin, M. , Mccormac A. C., Terry M. J., and Smith A. G.. 2008. “Tetrapyrrole Profiling in Arabidopsis Seedlings Reveals That Retrograde Plastid Nuclear Signaling Is Not Due to Mg‐Protoporphyrin IX Accumulation.” Proceedings of the National Academy of Sciences 105: 15178–15183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustakas, M. , Sperdouli I., and Moustaka J.. 2022. “Early Drought Stress Warning in Plants: Color Pictures of Photosystem II Photochemistry.” Climate 10: 179. [Google Scholar]
- Noctor, G. 2002. “Drought and Oxidative Load in the Leaves of C3 Plants: A Predominant Role for Photorespiration?” Annals of Botany 89, no. Spec No: 841–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor, G. , Mhamdi A., and Foyer C. H.. 2014. “The Roles of Reactive Oxygen Metabolism in Drought: Not So Cut and Dried.” Plant Physiology 164: 1636–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norén, L. , Kindgren P., Stachula P., et al. 2016. “Circadian and Plastid Signaling Pathways Are Integrated to Ensure Correct Expression of the CBF and COR Genes During Photoperiodic Growth.” Plant Physiology 171: 1392–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasawara, Y. , Ishizaki K., Kohchi T., and Kodama Y.. 2013. “Cold‐Induced Organelle Relocation in the Liverwort Marchantia polymorpha L.” Plant, Cell & Environment 36, no. 8: 1520–1528. [DOI] [PubMed] [Google Scholar]
- Onkokesung, N. , Reichelt M., Wright L. P., Phillips M. A., Gershenzon J., and Dicke M.. 2019. “The Plastidial Metabolite 2‐C‐Methyl‐D‐Erythritol‐2,4‐Cyclodiphosphate Modulates Defence Responses Against Aphids.” Plant, Cell & Environment 42: 2309–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Op Den Camp, R. G. L. , Przybyla D., Ochsenbein C., et al. 2003. “Rapid Induction of Distinct Stress Responses After the Release of Singlet Oxygen in Arabidopsis.” Plant Cell 15: 2320–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrovsky, D. , Diomina G., Lysak E., Matveeva E., Ogrel O., and Trutko S.. 1998. “Effect of Oxidative Stress on the Biosynthesis of 2‐C‐Methyl‐D‐Erythritol‐2,4‐Cyclopyrophosphate and Isoprenoids by Several Bacterial Strains.” Archives of Microbiology 171: 69–72. [DOI] [PubMed] [Google Scholar]
- Park, H. , Kreunen S. S., Cuttriss A. J., Dellapenna D., and Pogson B. J.. 2002. “Identification of the Carotenoid Isomerase Provides Insight Into Carotenoid Biosynthesis, Prolamellar Body Formation, and Photomorphogenesis.” Plant Cell 14: 321–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfalz, J. , and Pfannschmidt T.. 2013. “Essential Nucleoid Proteins in Early Chloroplast Development.” Trends in Plant Science 18: 186–194. [DOI] [PubMed] [Google Scholar]
- Pfannschmidt, T. , Blanvillain R., Merendino L., et al. 2015. “Plastid RNA Polymerases: Orchestration of Enzymes With Different Evolutionary Origins Controls Chloroplast Biogenesis During the Plant Life Cycle.” Journal of Experimental Botany 66: 6957–6973. [DOI] [PubMed] [Google Scholar]
- Pfannschmidt, T. , Schütze K., Fey V., Sherameti I., and Oelmüller R.. 2003. “Chloroplast Redox Control of Nuclear Gene Expression—A New Class of Plastid Signals in Interorganellar Communication.” Antioxidants & Redox Signaling 5: 95–101. [DOI] [PubMed] [Google Scholar]
- Phua, S. Y. , Yan D., Chan K. X., Estavillo G. M., Nambara E., and Pogson B. J.. 2018. “The Arabidopsis SAL1‐PAP Pathway: A Case Study for Integrating Chloroplast Retrograde, Light and Hormonal Signaling in Modulating Plant Growth and Development?” Frontiers in Plant Science 9: 1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipitone, R. , Eicke S., Pfister B., et al. 2021. “A Multifaceted Analysis Reveals Two Distinct Phases of Chloroplast Biogenesis During De‐Etiolation in Arabidopsis.” eLife 10: e62709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston, C. , and Pace R. J.. 1985. “The S‐State Dependence of Cl− Binding to Plant Photosystem II.” Biochimica et Biophysica Acta 810: 388–391. [Google Scholar]
- Ramel, F. , Birtic S., Ginies C., Soubigou‐Taconnat L., Triantaphylidès C., and Havaux M.. 2012. “Carotenoid Oxidation Products Are Stress Signals That Mediate Gene Responses to Singlet Oxygen in Plants.” Proceedings of the National Academy of Sciences 109: 5535–5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp, J. C. , and Mullet J. E.. 1991. “Chloroplast Transcription Is Required to Express the Nuclear Genes rbcS and cab. Plastid DNA Copy Number Is Regulated Independently.” Plant Molecular Biology 17: 813–823. [DOI] [PubMed] [Google Scholar]
- Ribeiro, C. , Stitt M., and Hotta C. T.. 2022. “How Stress Affects Your Budget—Stress Impacts on Starch Metabolism.” Frontiers in Plant Science 13: 774060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter, A. S. , Nägele T., Grimm B., et al. 2023. “Retrograde Signaling in Plants: A Critical Review Focusing on the GUN Pathway and Beyond.” Plant Communications 4: 100511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter, A. S. , Tohge T., Fernie A. R., and Grimm B.. 2020. “The Genomes Uncoupled‐Dependent Signalling Pathway Coordinates Plastid Biogenesis With the Synthesis of Anthocyanins.” Philosophical Transactions of the Royal Society, B: Biological Sciences 375: 20190403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristic, Z. , and Ashworth E. N.. 1993. “Changes in Leaf Ultrastructure and Carbohydrates in Arabidopsis thaliana L. (Heyn) cv. Columbia During Rapid Cold Acclimation.” Protoplasma 172: 111–123. [Google Scholar]
- Rivasseau, C. , Seemann M., Boisson A. M., et al. 2009. “Accumulation of 2‐C‐Methyl‐D‐Erythritol 2,4‐Cyclodiphosphate in Illuminated Plant Leaves at Supraoptimal Temperatures Reveals a Bottleneck of the Prokaryotic Methylerythritol 4‐Phosphate Pathway of Isoprenoid Biosynthesis.” Plant, Cell & Environment 32: 82–92. [DOI] [PubMed] [Google Scholar]
- Rodríguez, V. M. , Chételat A., Majcherczyk P., and Farmer E. E.. 2010. “Chloroplastic Phosphoadenosine Phosphosulfate Metabolism Regulates Basal Levels of the Prohormone Jasmonic Acid in Arabidopsis Leaves.” Plant Physiology 152: 1335–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez‐Concepción, M. 2014. “Plant Isoprenoids: A General Overview.” In Plant Isoprenoids: Methods and Protocols. Springer New York. [DOI] [PubMed] [Google Scholar]
- Rossel, J. B. , Walter P. B., Hendrickson L., et al. 2006. “A Mutation Affecting ASCORBATE PEROXIDASE 2 Gene Expression Reveals a Link Between Responses to High Light and Drought Tolerance.” Plant, Cell & Environment 29: 269–281. [DOI] [PubMed] [Google Scholar]
- Ruckle, M. E. , Demarco S. M., and Larkin R. M.. 2007. “Plastid Signals Remodel Light Signaling Networks and Are Essential for Efficient Chloroplast Biogenesis in Arabidopsis.” Plant Cell 19: 3944–3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvucci, M. E. , and Crafts‐Brandner S. J.. 2004. “Inhibition of Photosynthesis by Heat Stress: The Activation State of Rubisco as a Limiting Factor in Photosynthesis.” Physiologia Plantarum 120: 179–186. [DOI] [PubMed] [Google Scholar]
- Schieber, M. , and Chandel N. S.. 2014. “ROS Function in Redox Signaling and Oxidative Stress.” Current Biology 24: R453–R462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramma, N. , Perugachi Israëls C., and Jalaal M.. 2023. “Chloroplasts in Plant Cells Show Active Glassy Behavior Under Low‐Light Conditions.” Proceedings of the National Academy of Sciences 120: e2216497120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, Y. , Ke X., Yang X., Liu Y., and Hou X.. 2022. “Plants Response to Light Stress.” Journal of Genetics and Genomics 49: 735–747. [DOI] [PubMed] [Google Scholar]
- Shimizu, T. , Kacprzak S. M., Mochizuki N., et al. 2019. “The Retrograde Signaling Protein GUN1 Regulates Tetrapyrrole Biosynthesis.” Proceedings of the National Academy of Sciences 116: 24900–24906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, Y. K. , Bhandari S. R., Jo J. S., Song J. W., and Lee J. G.. 2021. “Effect of Drought Stress on Chlorophyll Fluorescence Parameters, Phytochemical Contents, and Antioxidant Activities in Lettuce Seedlings.” Horticulturae 7: 238. [Google Scholar]
- Sperry, J. S. , Venturas M. D., Anderegg W., et al. 2017. “Predicting Stomatal Responses to the Environment From the Optimization of Photosynthetic Gain and Hydraulic Cost.” Plant, Cell & Environment 40: 816–830. [DOI] [PubMed] [Google Scholar]
- Strand, Å. , Asami T., Alonso J., Ecker J. R., and Chory J.. 2003. “Chloroplast to Nucleus Communication Triggered by Accumulation of Mg‐ProtoporphyrinIX.” Nature 421: 79–83. [DOI] [PubMed] [Google Scholar]
- Suetsugu, N. , Kagawa T., and Wada M.. 2005. “An Auxilin‐Like J‐Domain Protein, JAC1, Regulates Phototropin‐Mediated Chloroplast Movement in Arabidopsis.” Plant Physiology 139: 151–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, T. , Rao S., Zhou X., and Li L.. 2022. “Plant Carotenoids: Recent Advances and Future Perspectives.” Molecular Horticulture 2: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susek, R. E. , Ausubel F. M., and Chory J.. 1993. “Signal Transduction Mutants of Arabidopsis Uncouple Nuclear CAB and RBCS Gene Expression From Chloroplast Development.” Cell 74: 787–799. [DOI] [PubMed] [Google Scholar]
- Tadini, L. , Pesaresi P., Kleine T., et al. 2016. “GUN1 Controls Accumulation of the Plastid Ribosomal Protein S1 at the Protein Level and Interacts With Proteins Involved in Plastid Protein Homeostasis.” Plant Physiology 170: 1817–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, Q. , Xu D., Lenzen B., et al. 2024. “Genomes Uncoupled Protein1 Binds to Plastid RNAs and Promotes Their Maturation.” Plant Communications 5, no. 2: 101069. 10.1016/j.xplc.2024.101069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theis, J. , and Schroda M.. 2016. “Revisiting the Photosystem II Repair Cycle.” Plant Signaling & Behavior 11: e1218587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, J. , and Weinstein J. D.. 1990. “Measurement of Heme Efflux and Heme Content in Isolated Developing Chloroplasts.” Plant Physiology 94: 1414–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullberg, A. , Alexciev K., Pfannschmidt T., and Allen J. F.. 2000. “Photosynthetic Electron Flow Regulates Transcription of the psaB Gene in Pea (Pisum sativum L.) Chloroplasts Through the Redox State of the Plastoquinone Pool.” Plant and Cell Physiology 41: 1045–1054. [DOI] [PubMed] [Google Scholar]
- Van Zelm, E. , Zhang Y., and Testerink C.. 2020. “Salt Tolerance Mechanisms of Plants.” Annual Review of Plant Biology 71: 403–433. [DOI] [PubMed] [Google Scholar]
- Veciana, N. , Martín G., Leivar P., and Monte E.. 2022. “BBX16 Mediates the Repression of Seedling Photomorphogenesis Downstream of the GUN1/GLK1 Module During Retrograde Signalling.” New Phytologist 234: 93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetoshkina, D. V. , Ivanov B. N., Khorobrykh S. A., Proskuryakov I. I., and Borisova‐Mubarakshina M. M.. 2017. “Involvement of the Chloroplast Plastoquinone Pool in the Mehler Reaction.” Physiologia Plantarum 161: 45–55. [DOI] [PubMed] [Google Scholar]
- Von Gromoff, E. D. , Alawady A., Meinecke L., Grimm B., and Beck C. F.. 2008. “Heme, a Plastid‐Derived Regulator of Nuclear Gene Expression in Chlamydomonas.” Plant Cell 20: 552–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. Z. , Li B., Xiao Y., et al. 2017. “Initiation of ER Body Formation and Indole Glucosinolate Metabolism by the Plastidial Retrograde Signaling Metabolite.” Molecular Plant 10: 1400–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Kim C., Xu X., et al. 2016. “Singlet Oxygen‐ and EXECUTER1‐mediated Signaling Is Initiated in Grana Margins and Depends on the Protease FtsH2.” Proceedings of the National Academy of Sciences 113: E3792–E3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S. , Uddin M. I., Tanaka K., et al. 2014. “Maintenance of Chloroplast Structure and Function by Overexpression of the Rice MONOGALACTOSYLDIACYLGLYCEROL SYNTHASE Gene Leads to Enhanced Salt Tolerance in Tobacco.” Plant Physiology 165: 1144–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. W. , Jiang D. X., and Chen J. J. H. G. X.. 2019. “Physiological Characterization and Thylakoid Ultrastructure Analysis in Super High‐Yield Hybrid Rice Leaves Under Drought Stress.” Photosynthetica 57: 890–896. [Google Scholar]
- Wilson, P. B. , Estavillo G. M., Field K. J., et al. 2009. “The Nucleotidase/Phosphatase SAL1 Is a Negative Regulator of Drought Tolerance in Arabidopsis.” Plant Journal 58: 299–317. [DOI] [PubMed] [Google Scholar]
- Woodson, J. D. , Perez‐Ruiz J. M., and Chory J.. 2011. “Heme Synthesis by Plastid Ferrochelatase I Regulates Nuclear Gene Expression in Plants.” Current Biology 21: 897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodson, J. D. , Perez‐Ruiz J. M., Schmitz R. J., Ecker J. R., and Chory J.. 2013. “Sigma Factor‐Mediated Plastid Retrograde Signals Control Nuclear Gene Expression.” Plant Journal 73: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, G. Z. , Meyer E. H., Richter A. S., et al. 2019. “Control of Retrograde Signalling by Protein Import and Cytosolic Folding Stress.” Nature Plants 5: 525–538. [DOI] [PubMed] [Google Scholar]
- Xiao, Y. , Savchenko T., Baidoo E. E. K., et al. 2012. “Retrograde Signaling by the Plastidial Metabolite MEcPP Regulates Expression of Nuclear Stress‐Response Genes.” Cell 149: 1525–1535. [DOI] [PubMed] [Google Scholar]
- Yamada, M. , Kawasaki M., Sugiyama T., Miyake H., and Taniguchi M.. 2009. “Differential Positioning of C4 Mesophyll and Bundle Sheath Chloroplasts: Aggregative Movement of C4 Mesophyll Chloroplasts in Response to Environmental Stresses.” Plant and Cell Physiology 50: 1736–1749. [DOI] [PubMed] [Google Scholar]
- Yamori, W. , Makino A., and Shikanai T.. 2016. “A Physiological Role of Cyclic Electron Transport Around Photosystem I in Sustaining Photosynthesis Under Fluctuating Light in Rice.” Scientific Reports 6: 20147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong, L.‐K. , Tsuboyama S., Kitamura R., Kurokura T., Suzuki T., and Kodama Y.. 2021. “Chloroplast Relocation Movement in the Liverwort.” Physiologia Plantarum 173: 775–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, L. , and Dehesh K.. 2021. “The Eukaryotic MEP‐Pathway Genes Are Evolutionarily Conserved and Originated From Chlaymidia and Cyanobacteria.” BMC Genomics 22: 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. , Li H., Huang X., et al. 2022. “STAYGREEN‐Mediated Chlorophyll a Catabolism Is Critical for Photosystem Stability During Heat‐Induced Leaf Senescence in Perennial Ryegrass.” Plant, Cell & Environment 45: 1412–1427. [DOI] [PubMed] [Google Scholar]
- Zhang, L. , and Hach A.. 1999. “Molecular Mechanism of Heme Signaling in Yeast: The Transcriptional Activator Hap1 Serves as the Key Mediator.” Cellular and Molecular Life Sciences: CMLS 56: 415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L. , and Xing D.. 2008. “Rapid Determination of the Damage to Photosynthesis Caused by Salt and Osmotic Stresses Using Delayed Fluorescence of Chloroplasts.” Photochemical & Photobiological Sciences 7: 352–360. [DOI] [PubMed] [Google Scholar]
- Zhang, R. , Wise R. R., Struck K. R., and Sharkey T. D.. 2010. “Moderate Heat Stress of Arabidopsis thaliana Leaves Causes Chloroplast Swelling and Plastoglobule Formation.” Photosynthesis Research 105: 123–134. [DOI] [PubMed] [Google Scholar]
- Zhang, T. , Zhang R., Zeng X.‐Y., et al. 2024. “GLK Transcription Factors Accompany Elongated HYPOCOTYL5 to Orchestrate Light‐Induced Seedling Development in Arabidopsis.” Plant Physiology 194: 2400–2421. [DOI] [PubMed] [Google Scholar]
- Zhang, Z. W. , Yuan S., Feng H., et al. 2011. “Transient Accumulation of Mg‐Protoporphyrin IX Regulates Expression of PhANGs—New Evidence for the Signaling Role of Tetrapyrroles in Mature Arabidopsis Plants.” Journal of Plant Physiology 168: 714–721. [DOI] [PubMed] [Google Scholar]
- Zhao, X. , Huang J., and Chory J.. 2019. “GUN1 Interacts With MORF2 to Regulate Plastid RNA Editing During Retrograde Signaling.” Proceedings of the National Academy of Sciences 116: 10162–10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang, K. , Kong F., Zhang S., et al. 2019. “Whirly1 Enhances Tolerance to Chilling Stress in Tomato via Protection of Photosystem II and Regulation of Starch Degradation.” New Phytologist 221: 1998–2012. [DOI] [PubMed] [Google Scholar]
- Zito, F. 1999. “The Qo Site of Cytochrome b6f Complexes Controls the Activation of the LHCII Kinase.” EMBO Journal 18: 2961–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoulias, N. , Rowe J., Thomson E. E., et al. 2021. “Inhibition of Arabidopsis Stomatal Development by Plastoquinone Oxidation.” Current Biology 31: 5622–5632.e7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.


