Abstract
Background/Objective
The neutralizing monoclonal antibody against SARS-CoV-2 is regarded as one of the most effective therapies for COVID-19.: This study was a randomized, double-blinded, placebo-controlled Phase II trial conducted to evaluate the efficacy of neutralizing monoclonal antibody (SCTA01) in high-risk outpatients diagnosed with COVID-19.
Methods
The primary endpoint was the proportion of patients who experienced COVID-19-related hospitalization (defined as at least 24 h of acute care) or death (all causes) by Day 29.
Results
109 patients were randomly assigned to and received SCTA01 750 mg (n = 25), 1500 mg (n = 29), 3000 mg (n = 30), or placebo (n = 25). Only two experienced COVID-19-related hospitalization by Day 29, one from the 750 mg group and the other from the 3000 mg group. Statistical analysis revealed no significant differences in viral load reduction (p = 0.20) or symptom score reduction (p = 0.37) between the SCTA01 total and placebo groups. Additionally, the incidence of adverse events was comparable between the SCTA01 group (23.8 %) and the placebo group (24.0 %). Notably, no treatment-related serious adverse events (SAEs) were reported.
Conclusions
There was no significant difference in clinical outcome between SCTA01 and placebo in the treatment of high-risk outpatients diagnosed with COVID-19, and it was well tolerated.
CLINICAL TRIAL
The trial was registered at ClinicalTrial.gov (NCT04709328).
Keywords: COVID-19, SARS-CoV-2, Neutralizing antibody
1. Introduction
Coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), poses an ongoing threat to public health [1]. While most individuals infected with SARS-CoV-2 remain asymptomatic or exhibit mild to moderate symptoms, specific populations are at a heightened risk of developing severe illness or experiencing fatal outcomes. This is particularly true for older adults and individuals with specific medical conditions, including cancer, cerebrovascular disease, chronic kidney disease, chronic liver disease, chronic lung disease, diabetes, heart disease, or those who are immunocompromised [[2], [3], [4]].
Several antiviral medications, including oral drugs and monoclonal antibodies (mAbs), have been employed in treating patients with mild to moderate COVID-19 [[5], [6], [7], [8], [9]]. SARS-CoV-2 is known for its high mutation rate, particularly in the spike protein, which is a primary target for many monoclonal antibodies and vaccines against SARS-CoV-2. These mutations can lead to structural changes in viral proteins, potentially reducing the binding efficiency of therapeutic agents or neutralizing antibodies. Therefore, it is important to track emerging variants and evaluate their resistance to current therapeutic strategies to adjust therapy strategies [10].
SCTA01 is a human IgG1 monoclonal antibody targeting the receptor binding domain (RBD) of SARS-CoV-2 and featuring a LALA-modified Fc region. This modification aimed to eliminate the function of antibody-dependent cell cytotoxicity (ADCC) and to mitigate antibody-dependent enhancement (ADE) [11]. In non-clinical studies, SCTA01 inhibited viral replication in both the trachea and lungs, thus preventing pulmonary damage with no significant adverse events (AEs), even at a dosage ten times greater than the effective dose of SCTA01 [12]. In the Phase I study of SCTA01, healthy adults received SCTA01 at doses of 5 mg/kg, 15 mg/kg, 30 mg/kg, 50 mg/kg, or placebo. The frequency of AEs in the SCTA01 group was comparable to that in the placebo groups [11].
The trial was designed to evaluate the safety and efficacy of monoclonal antibody SCTA01 in high-risk outpatients diagnosed with COVID-19 who were at higher risk of hospitalization or death.
2. Materials and methods
2.1. Trial design, treatment, and oversight
A randomized, double-blinded, placebo-controlled Phase II trial was conducted to evaluate SCTA01 in high-risk outpatients diagnosed with COVID-19. The final protocol is provided in Supplement 2. The study was conducted in accordance with the Declaration of Helsinki. The institutional review board approved the protocol at all participating centers, and written informed consent was obtained from all participants. The study was terminated early following the completion of the Phase II portion due to the emergence of Omicron variants resistant to SCTA01.
2.2. Patients
All patients were 18 years of age or older, had laboratory-confirmed evidence of SARS-CoV-2 infection, with at least one of the high-risk factors associated with COVID-19, and presented with at least two COVID-19-related symptoms that started less than ten days prior to randomization. Patients were not hospitalized. High-risk factors for COVID-19 included a) age ≥60 years, b) BMI ≥30 kg/m2, c) presence of an immunosuppressive condition, d) current receipt of immunosuppressant or systemic corticosteroid treatments, and e) being ≥45 years old with underlying conditions. The investigator reviewed the eligibility criteria (Supplement 2).
2.3. Randomization and intervention
Eligible patients were randomized in a 1:1:1:1 ratio using stratified block randomization approach to receive either 750 mg group, 1500 mg group, 3000 mg group of SCTA01, or placebo during Phase II of the trial. Randomization was stratified by country, age (<45 vs. 45 to 59 vs. ≥ 60 years old), presence of shortness of breath (Yes vs. No), and vaccination or reinfection status (Yes vs. No). All patients received a single intravenous dose of SCTA01 or placebo. The standard of care for COVID-19 treatment served as the baseline therapy, and patients were managed according to the local clinical routine for treating high-risk outpatients diagnosed with COVID-19. Randomization and treatment took placed on Day 1. After randomization, COVID-19 symptoms were recorded before treatment on Day 1.
2.4. Endpoints
The primary endpoint was the proportion of patients who experienced COVID-19-related hospitalization (defined as at least 24 h of acute care) or death (all-cause) by Day 29. Secondary endpoints included: (1) changes in symptom score (total of ratings) from baseline to Day 3, 5, 7, 11, 15, 22, and 29; (2) time to symptom improvement by Day 29; (3) time to symptom resolution by Day 29; (4) change in SARS-CoV-2 viral shedding from baseline to Day 4, 8, 11, 15 and 29 measured by quantitative reverse transcription polymerase chain reaction (RT-qPCR) in nasopharyngeal (NP) or oropharyngeal (OP) samples. AEs and serious adverse events (SAEs) were also analyzed in this trial. All patients randomly assigned and having received at least one dose of SCTA01 or placebo were included in the intention-to-treat analysis.
2.5. Statistical analysis
2.5.1. Analysis of primary endpoints
The primary efficacy analysis utilized the Cochran-Mantel-Haenszel (CMH) test, stratified by country, age (<45 vs. 45 to 59 vs. ≥ 60 years old), presence of shortness of breath (Yes vs. No), and vaccination or reinfection status (Yes vs. No), at a significance level of 0.025 (one-sided). Combining country strata was the preferred approach, guided by medical opinion on the standard of care (SOC) in each country. If certain strata were too small, they were combined to ensure the statistical method functioned appropriately under anonymous review. Fisher's exact test method was used when less than 80 % of the expected cell counts were ≥5.
2.5.2. Analysis of secondary endpoints
The symptom scores on the day of infusion were used as the baseline for calculating the change in symptom scores. The change in ordinal scores was summarized by frequency and proportions by day and treatment group. Linear models were employed to analyze the change in symptom scores. Similarly, the change in viral load from baseline was analyzed using the same methods.
Time to symptom improvement and resolution were summarized using Kaplan-Meier curves and 95 % confidence bounds. A stratified Cox model was used to estimate the hazard ratio and two-sided 95 % confidence intervals. P-values were calculated using a non-parametric stratified log-rank test.
3. Results
3.1. Patients
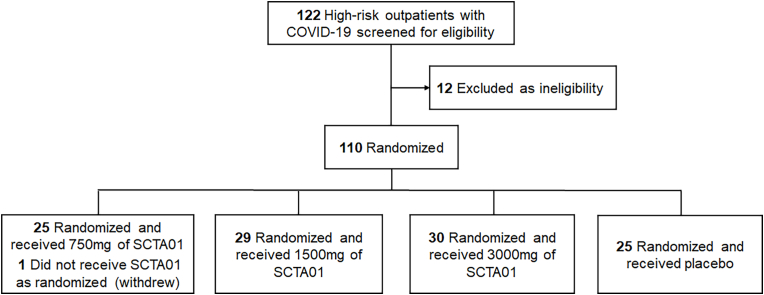
A total of 110 patients were randomized into two groups: SCTA01 total (85 patients) and the placebo (25 patients) from April 9, 2021 to April 25, 2022 (Fig. 1). Within the SCTA01 group, 25 received 750 mg, 29 received 1500 mg, and 30 received 3000 mg. One patient assigned to the 750 mg group did not receive the drug due to early withdrawal. All treated patients completed the Day 29 visit, except for one in the 1500 mg group, who withdrew from the study.
Fig. 1.
Patient enrollment and treatment allocation.
a: included in the efficacy analysis and safety analysis.
Among 109 patients, 39.5 % were aged 60 or older, and 55.0 % were female. The racial distribution included 19.3 % Asian and 61.5 % White patients. 66.1 % of the patients had obesity (BMI ≥30 kg/m2), and 47.7 % had at least one high-risk medical condition. Additionally, 57.8 % of the patients had a high viral load (≥105 copies/mL), and the median duration of symptoms before randomization was four days. Patients’ demographics and baseline characteristics were balanced across all groups (Table 1).
Table 1.
Patient demographics and baseline clinical characteristics.
| SCTA01 |
||||
|---|---|---|---|---|
| 750 mg (N = 25) n (%) | 1500 mg (N = 29) n (%) | 3000 mg (N = 30) n (%) | Placebo (N = 25) n (%) | |
| Age (years) | ||||
| Median (min, max) | 54.0 (23, 83) | 55.0 (21, 98) | 58.0 (29, 77) | 54.0 (25, 85) |
| >=60 years | 11 (42.3) | 9 (31.0) | 14 (46.7) | 9 (36.0) |
| Sex | ||||
| Male | 13 (52.0) | 8 (27.6) | 14 (46.7) | 14 (56.0) |
| Female | 12 (48.0) | 21 (72.4) | 16 (53.3) | 11 (44.0) |
| Race | ||||
| Asian | 5 (20.0) | 5 (17.2) | 7 (23.3) | 4 (16.0) |
| Black or African American | 0 | 0 | 1 (3.3) | 2 (8.0) |
| White | 17 (68.0) | 17 (58.6) | 18 (60.0) | 15 (60.0) |
| NA | 3 (12.0) | 6 (10.3) | 3 (10.0) | 4 (16.0) |
| BMI | ||||
| <30 kg/m^2 | 8 (32.0) | 7 (24.1) | 9 (30.0) | 13 (52.0) |
| >=30 kg/m^2 | 17 (68.0) | 22 (75.9) | 21 (70.0) | 12 (48.0) |
| Viral load | ||||
| >=10^5 copies/mL | 10 (40.0) | 15 (51.7) | 12 (40.0) | 11 (44.0) |
| <10^5 copies/mL | 12 (48.0) | 10 (34.5) | 14 (46.7) | 10 (40.0) |
| NA | 3 (12.0) | 4 (13.8) | 4 (13.3) | 4 (16.0) |
| Duration of Symptoms before randomization | ||||
| Median (min, mix) | 4 (1,9) | 4 (1,10) | 4 (1,9) | 3 (1,8) |
| High-risk medical history# | ||||
| Yes | 15 (60.0) | 12 (41.4) | 18 (60.0) | 12 (48.0) |
| No | 10 (40.0) | 17 (58.6) | 12 (40.0) | 13 (52.0) |
| Immunosuppressant or corticosteroid treatments | ||||
| Yes | 2 (8.0) | 2 (6.9) | 3 (10.0) | 3 (12.0) |
| No | 23 (92.0) | 27 (93.1) | 27 (90.0) | 22 (88.0) |
# High-risk medical history included immunosuppressive diseases, hypertension, cardiovascular disease, history of stroke and other cerebral disease (neurological disease), diabetes, chronic obstructive pulmonary disease, or other chronic respiratory disease or chronic kidney disease. NA, not applicable.
3.2. Efficacy
3.2.1. Primary endpoint
By Day 29, COVID-19-related hospitalization occurred in 1 out of 25 patients in the 750 mg group and 1 out of 30 patients in the 3000 mg group. There were no such events in the 1500 mg or placebo groups. No deaths reported by Day 29. Additionally, one patient in the placebo group died on Day 52 due to COVID-19 after visiting the emergency room on Day 35. Consequently, the small sample size of patients enrolled was insufficient to determine the efficacy of SCTA01 in high-risk outpatients diagnosed with COVID-19.
3.2.2. Key secondary endpoints
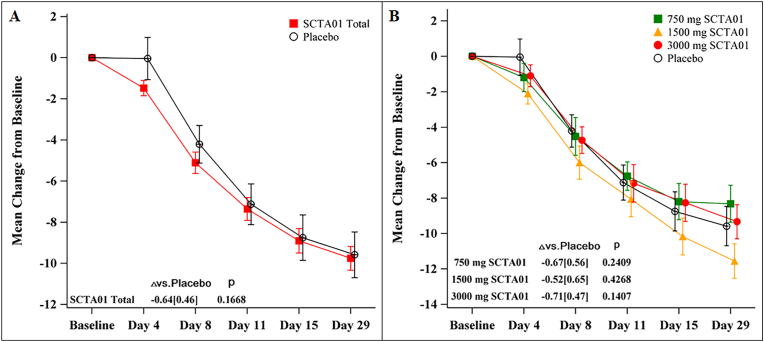
There were no statistically significant differences between the SCTA01 groups and the placebo group in the mean change of total symptom scores from baseline (SCTA01 total vs. placebo: mean difference = −0.64, p = 0.17) (Fig. 2A). Similar improvement trends across different SCTA01 dose groups are shown in Fig. 2B.
Fig. 2.
Symptom scores from baseline to Day 29.
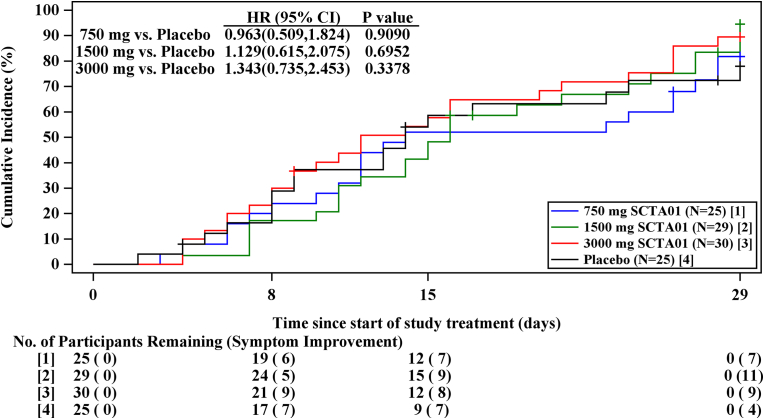
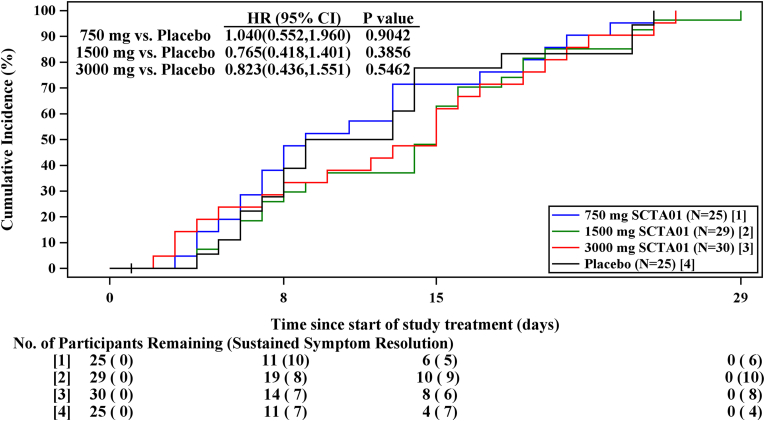
Although, there were no significant differences between individual dose of SCTA01 and the placebo group regarding the time to symptom improvement (Fig. 3) or time to sustained symptom resolution (Fig. 4), with all p-values exceeding 0.05. The proportion of patients experiencing symptom improvement by Day 29 was as follows: 84.5 % in the SCTA01 total group, with specific improvements of 80.0 % in the 750 mg dose, 86.2 % in the 1500 mg dose, and 86.7 % in the 3000 mg dose. In comparison, only 72.0 % of patients in the placebo group reported symptom improvement. Similarly, the proportion of patients achieving sustained symptom resolution by Day 29 was 89.3 % in the SCTA01 total group, 88.0 %, 93.1 %, and 86.7 % in the 750 mg, 1500 mg, and 3000 mg SCTA01, respectively, compared to 76.0 % in the placebo group. These results showed a numerical advantage in the SCTA01 groups relative to the placebo group (Supplement 1, Table S1).
Fig. 3.
Time for symptom improvement.
Fig. 4.
Time to sustained symptom resolution.
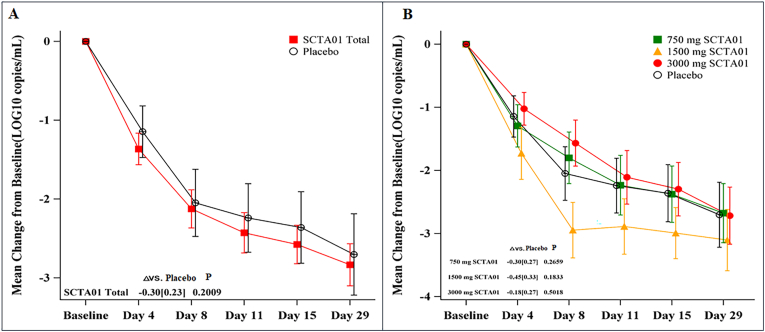
There were no significant differences between the SCTA01 groups and the placebo group in the mean change of viral load from baseline (SCTA01 total: mean difference = −0.30, p = 0.20) (Fig. 5A). Similar results for individual dose groups are displayed in Fig. 5B.
Fig. 5.
Viral load from baseline to Day 29.
Fig. 2A shows the mean change of total symptom scores from baseline. Each of the 14 symptoms was graded on a scale of 0 (no symptom) to 3 (severe symptom) or 0 (no symptom) to 2 (severe symptom). Detailed score information is provided in the Supplement 2. Fig. 2B shows the change of symptoms in each group. The I bar represents the standard error. P represents the probability value.
Fig. 3 shows the proportion of patients who achieve symptom improvement over time. Symptom improvement was defined as (1) symptoms scored as severe or moderate on the COVID-19-related symptom questionnaire at baseline are scored as mild or absent, AND (2) symptoms scored as mild or absent at baseline are scored as absent. HR, Hazard ratio, CI, confidence interval.
Fig. 4 shows the proportion of patients who achieve sustained symptom resolution over time. Sustained symptom resolution was defined as all COVID-19-related symptoms remaining absent or no worse than mild for select symptoms that may take longer to resolve (e.g., cough, fatigue, loss of smell or taste) for a sustained period of 48 h by Day 29: HR, Hazard ratio, CI, confidence interval.
Fig. 5A shows the mean viral load change between the SCTA01 total group and the placebo group from baseline to Day 29. The limit of detection for the SARS-CoV-2 Viral Load Quantitation assay is 500 copies/mL in Viral Transport Media. Fig. 5B shows the change of symptoms in each group. The I bar represents the standard error. P represents the probability value.
3.3. Safety
The incidence of AEs was comparable across the SCTA01 and placebo groups, with 32.0 % in the 750 mg group, 20.7 % in the 1500 mg group, 20.0 % in the 3000 mg group, and 24.0 % in the placebo group) (Table 2). The most commonly reported AE was headache, occurring in 8.0 % of patients in the 750 mg group, 3.3 % in the 3000 mg group, and 8.0 % in the placebo group. Notably, no infusion-related AEs were reported.
Table 2.
Adverse events.
| SCTA01 |
||||
|---|---|---|---|---|
| 750 mg (N = 25) n (%) | 1500 mg (N = 29) n (%) | 3000 mg (N = 30) n (%) | Placebo (N = 25) n (%) | |
| TEAE | 8 (32.0) | 6 (20.7) | 6 (20.0) | 6 (24.0) |
| Grade 1 | 3 (12.0) | 5 (17.2) | 2 (6.7) | 1 (4.0) |
| Grade 2 | 3 (12.0) | 1 (3.4) | 2 (6.7) | 1 (4.0) |
| ≥ Grade 3 | 2 (8.0) | 0 | 2 (6.7) | 4 (16.0) |
| TEAE, according to PT | ||||
| Headache | 2 (8.0) | 0 | 1 (3.3) | 2 (8.0) |
| Urinary tract infection | 1 (4.0) | 1 (3.4) | 0 | 0 |
| Fibrin D dimer increased | 1 (4.0) | 0 | 1 (3.3) | 0 |
| Pneumonia | 0 | 0 | 1 (3.3) | 0 |
| Pyrexia | 1 (4.0) | 0 | 0 | 1 (4.0) |
| Chills | 0 | 1 (3.4) | 0 | 0 |
| Injection site swelling | 0 | 1 (3.4) | 0 | 0 |
| Diabetes mellitus | 0 | 0 | 1 (3.3) | 0 |
| Hyperglycaemia | 1 (4.0) | 0 | 0 | 0 |
| Hypokalaemia | 1 (4.0) | 0 | 0 | 0 |
| Oropharyngeal pain | 0 | 0 | 1 (3.3) | 0 |
| Acute abdomen | 0 | 0 | 1 (3.3) | 0 |
| Diarrhoea | 1 (4.0) | 0 | 0 | 0 |
| Epigastric discomfort | 1 (4.0) | 0 | 0 | 0 |
| Haematochezia | 1 (4.0) | 0 | 0 | 0 |
| Hypertension | 0 | 0 | 1 (3.3) | 1 (4.0) |
| Hypertensive crisis | 0 | 1 (3.4) | 0 | 0 |
| Sinus tachycardia | 1 (4.0) | 0 | 0 | 0 |
| Back pain | 1 (4.0) | 0 | 0 | 1 (4.0) |
| Anaemia | 0 | 0 | 1 (3.3) | 0 |
| Multiple fractures | 1 (4.0) | 0 | 0 | 0 |
| Insomnia | 1 (4.0) | 0 | 0 | 0 |
| Proteinuria | 0 | 1 (3.4) | 0 | 0 |
| Brain stem infarction | 0 | 0 | 0 | 1 (4.0) |
| Metabolic encephalopathy | 0 | 0 | 0 | 1 (4.0) |
| Decreased appetite | 0 | 0 | 0 | 1 (4.0) |
| Hypernatraemia | 0 | 0 | 0 | 1 (4.0) |
| Cough | 0 | 0 | 0 | 3 (12.0) |
| Catarrh | 0 | 0 | 0 | 1 (4.0) |
| Acute myocardial infarction | 0 | 0 | 0 | 1 (4.0) |
| SAE | 1 (4.0) | 0 | 1 (3.3) | 1 (4.0) |
Three patients experienced treatment-unrelated SAEs. One patient in the 750 mg group experienced worsening of acute abdomen and anemia. Another patient in the 3000 mg group sustained multiple physical injuries, including a fracture of the transverse distal radius with dorsal displacement of the distal fragments secondary to a fall. One patient in the placebo group died due to complications of COVID-19, suffering from metabolic encephalopathy, cerebrovascular disease infarct pontine area, cardiopulmonary arrest secondary to acute myocardial infarction, and hypernatremia.
4. Discussion
The primary endpoint of this trial was to evaluate whether SCTA01 could reduce the risk of COVID-19-related hospitalization or all-cause death by Day 29. As there were only 2 primary endpoint events, statistical analysis could not be performed. These two patients were older (≥55 years old) and had pre-existing cardiac disease, hypertension, or diabetes at baseline. They also received medication and oxygen treatment. Additionally, baseline viral loads were also high in these two patients, consistent with the findings from the REGEN-COV antibody study, which reported that higher viral loads were associated with an increased risk of hospitalization or death [7].
Viral clearance plays a crucial role in treating COVID-19 diseases, as it is linked with better clinical outcomes [7]. Among the secondary endpoints in this study, there were no statistical differences between the SCTA01 groups and the placebo group regarding clinical outcomes or viral load. However, the proportion of patients with clinical improvement and resolution was numerically higher in the SCTA01 group than in the placebo group.
The lack of significant therapeutic outcomes observed in this trial may be attributed to mutations in the SARS-CoV-2 virus, as the trial was conducted during the Delta and Omicron waves of the pandemic [13,14]. The neutralizing capacity of SCTA01 against the Delta decreased, with a 3.8-fold reduction in the half-maximal inhibitory concentration compared to the SARS-CoV-2 variant with the D614G mutation (Supplement 1, Fig. S1). SCTA01 was unable to neutralize the Omicron variant (Supplement 1, Fig. S2). The US-FDA revoked the emergency use authorization for mAbs once the emerging variants became resistant to already approved antibody therapies [[15], [16], [17]].
SCTA01 was well tolerated, and no safety signals were observed in this study. A low incidence of Grade 3 or higher AEs occurred during post-treatment. No treatment-related SAEs were observed. There was also no evidence of ADE with SCTA01. ADE is a process where antibodies can inadvertently worsen a viral infection; and it is known to be mediated by virus-antibody immune complexes via interactions with Fc and Fc receptors or the complement system [18,19]. Given the potential for ADE, some antibodies with LALA-modified Fc regions have been developed and approved for the treatment of certain diseases, including Spesolimab, Teplizunb, LYCo555 and LYCoV016 [6,[20], [21], [22], [23]]. However, there is no evidence to suggest that ADE has been a significant factor in causing severe COVID-19 or COVID-19-related deaths.
4.1. Limitation
The trial had few limitations. First, the sample size was too small thus influencing the generalizability of our results. Second, mutations in the spike protein of SARS-CoV-2 reduced the neutralizing capacity of SCTA01. Third, the ADCC function was eliminated due to the LALA modification of SCTA01, which may have reduced its effectiveness.
5. Conclusion
Neutralizing monoclonal antibodies against SARS-CoV-2 remains a key treatment option for COVID-19 outpatients. Future development of monoclonal antibodies should focus on enhancing binding site specificity, targeting conserved regions. Additionally, combination therapies may offer a more robust approach to combat viral mutations and ensure long-term effectiveness.
CRediT authorship contribution statement
Jorge Diaz: Writing – review & editing, Writing – original draft, Investigation. Allex Fonseca: Writing – review & editing, Writing – original draft, Investigation. Lixin Yan: Writing – review & editing, Supervision, Formal analysis, Data curation. Dongfang Liu: Writing – review & editing, Software, Data curation. Liangzhi Xie: Writing – review & editing, Project administration, Funding acquisition, Data curation.
Funding
The National Key Research and Development Program of China [2021YFE0201700], and the Beijing Science and Technology Planning Project, China [Z201100005420017] funded this research.
Declaration of competing interest
Sinocelltech Ltd. sponsored this work. Lixin Yan, Dongfang Liu, and Liangzhi Xie are employees of Sinocelltech Co., Ltd. and have stock ownership and/or potential stock option interests in the company. All authors declare no other conflicts of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.conctc.2025.101496.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
Data availability
Data will be made available on request.
References
- 1.WHO COVID-19 dashboard. https://data.who.int/dashboards/covid19/deaths?n=c.
- 2.People with Certain Medical Conditions and COVID-19 Risk Factors. https://www.cdc.gov/covid/risk-factors/index.html.
- 3.Petrilli C.M., Jones S.A., Yang J., Rajagopalan H., O'Donnell L., Chernyak Y., Tobin K.A., Cerfolio R.J., Francois F., Horwitz L.I. Br. Med. J. 2020;369 doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Lancet. Lancet (London, England)) 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hammond J., Leister-Tebbe H., Gardner A., Abreu P., Bao W., Wisemandle W., Baniecki M., Hendrick V.M., Damle B., Simon-Campos A., Pypstra R., Rusnak J.M. N. Engl. J. Med. 2022;386(15):1397–1408. doi: 10.1056/NEJMoa2118542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen P., Nirula A., Heller B., Gottlieb R.L., Boscia J., Morris J., Huhn G., Cardona J., Mocherla B., Stosor V., Shawa I., Adams A.C., Van Naarden J., Custer K.L., Shen L., Durante M., Oakley G., Schade A.E., Sabo J., Patel D.R., Klekotka P., Skovronsky D.M. N. Engl. J. Med. 2021;384(3):229–237. doi: 10.1056/NEJMoa2029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinreich D.M., Sivapalasingam S., Norton T., Ali S., Gao H., Bhore R., Xiao J., Hooper A.T., Hamilton J.D., Musser B.J., Rofail D., Hussein M., Im J., Atmodjo D.Y., Perry C., Pan C., Mahmood A., Hosain R., Davis J.D., Turner K.C., Baum A., Kyratsous C.A., Kim Y., Cook A., Kampman W., Roque-Guerrero L., Acloque G., Aazami H., Cannon K., Simon-Campos J.A., Bocchini J.A., Kowal B., Dicioccio A.T., Soo Y., Geba G.P., Stahl N., Lipsich L., Braunstein N., Herman G., Yancopoulos G.D. N. Engl. J. Med. 2021;385(23) doi: 10.1056/NEJMoa2108163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta A., Gonzalez-Rojas Y., Juarez E., Crespo C.M., Moya J., Falci D.R., Sarkis E., Solis J., Zheng H., Scott N., Cathcart A.L., Hebner C.M., Sager J., Mogalian E., Tipple C., Peppercorn A., Alexander E., Pang P.S., Free A., Brinson C., Aldinger M., Shapiro A.E. N. Engl. J. Med. 2021;385(21):1941–1950. doi: 10.1056/NEJMoa2107934. [DOI] [PubMed] [Google Scholar]
- 9.Streinu-Cercel A., Sandulescu O., Preotescu L.L., Kim J.Y., Kim Y.S., Cheon S., Jang Y.R., Lee S.J., Kim S.H., Chang I., Suh J.H., Lee S.G., Kim M.R., Chung D.R., Kim H.N., Streinu-Cercel A., Eom J.S. Open Forum Infect. Dis. 2022;9(4) doi: 10.1093/ofid/ofac053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harvey W.T., Carabelli A.M., Jackson B., Gupta R.K., Thomson E.C., Harrison E.M., Ludden C., Reeve R., Rambaut A., Peacock S.J., Robertson D.L. Nat rev microbiol. Nat. Rev. Microbiol. 2021;19(7):409–424. doi: 10.1038/s41579-021-00573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y., Qi L., Bai H., Sun C., Xu S., Wang Y., Han C., Li Y., Liu L., Cheng X., Liu J., Lei C., Tong Y., Sun M., Yan L., Chen W., Liu X., Liu Q., Xie L., Wang X. Antimicrob. Agents Chemother. 2021;65(11) doi: 10.1128/AAC.01063-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu L., Deng Y.Q., Zhang R.R., Cui Z., Sun C.Y., Fan C.F., Xing X., Huang W., Chen Q., Zhang N.N., Ye Q., Cao T.S., Wang N., Wang L., Cao L., Wang H., Kong D., Ma J., Luo C., Zhang Y., Nie J., Sun Y., Lv Z., Shaw N., Li Q., Li X.F., Hu J., Xie L., Rao Z., Wang Y., Wang X., Qin C.F. Natl. Sci. Rev. 2021;8(3) doi: 10.1093/nsr/nwaa297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World on alert as Omicron spreads. https://multimedia.scmp.com/infographics/news/world/article/3158620/omicron/index.html.
- 14.Map: Tracking the Delta variant. https://www.aljazeera.com/news/2021/7/7/map-tracking-the-covid-19-delta-variant.
- 15.Frequently Asked Questions on the Emergency Use Authorization of REGEN-COV (Casirivimab and Imdevimab), January 24, 2022.
- 16.FDA updates Sotrovimab emergency use authorization. https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-sotrovimab-emergency-use-authorization.
- 17.Coronavirus (COVID-19) Update: FDA Revokes Emergency Use Authorization for Monoclonal Antibody Bamlanivimab. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-monoclonal-antibody-bamlanivimab.
- 18.Lee W.S., Wheatley A.K., Kent S.J., Dekosky B.J. Nat. Microbiol. 2020;5(10):1185–1191. doi: 10.1038/s41564-020-00789-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ajmeriya S., Kumar A., Karmakar S., Rana S., Singh H. J. Indian Inst. Sci. 2022;102(2):671–687. doi: 10.1007/s41745-021-00268-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gottlieb R.L., Nirula A., Chen P., Boscia J., Heller B., Morris J., Huhn G., Cardona J., Mocherla B., Stosor V., Shawa I., Kumar P., Adams A.C., Van Naarden J., Custer K.L., Durante M., Oakley G., Schade A.E., Holzer T.R., Ebert P.J., Higgs R.E., Kallewaard N.L., Sabo J., Patel D.R., Klekotka P., Shen L., Skovronsky D.M. JAMA. 2021;325(7):632–644. doi: 10.1001/jama.2021.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdeldaim D.T., Schindowski K. Pharmaceutics(Pharmaceutics) 2023;15(10) doi: 10.3390/pharmaceutics15102402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.European Medicines Agency. Spevigo EPAR Product Information-Annex. https://www.ema.europa.eu/en/documents/product-information/spevigo-epar-product-information_en.pdf (accessed on 8 August 2023).
- 23.Herold K.C., Bundy B.N., Long S.A., Bluestone J.A., Dimeglio L.A., Dufort M.J., Gitelman S.E., Gottlieb P.A., Krischer J.P., Linsley P.S., Marks J.B., Moore W., Moran A., Rodriguez H., Russell W.E., Schatz D., Skyler J.S., Tsalikian E., Wherrett D.K., Ziegler A.G., Greenbaum C.J. N. Engl. J. Med. 2019;381(7):603–613. doi: 10.1056/NEJMoa1902226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.