Abstract
Background
Trigeminal neuralgia (TN) is a severely painful condition that is challenging to treat effectively. For patients who do not respond well to oral medications, percutaneous radiofrequency thermocoagulation (PRT) has emerged as a safe and minimally invasive procedure for treating TN. This study aimed to assess the safety and effectiveness of PRT in treating elderly patients with ITN.
Methods
A prospective cohort study was conducted among 134 elderly patients with ITN subjected to PRT at the Pioneer Medical Center for Neurosurgery and Pain Management in Sana’a city, Yemen, from September 2022 to October 2024. PRT was performed by foramen ovale puncture, and repeat PRT was conducted for all patients with recurrent pain. The Barrow Neurological Institute (BNI) pain intensity scale was used to assess the degree of pain after PRT. The outcome was considered successful if the patient’s BNI score decreased to I-IIIb or failed if a patient’s BNI pain score was IV or V. Recurrence of pain and complications were also recorded. Data about the safety and effectiveness of PRT were then summarized using descriptive statistics.
Results
PRT was successful in 125 of 134 patients (93.3%), with 95 patients (71.4%) achieving complete pain relief (BNI score I) and 30 patients (22.4%) achieving adequate pain control (BNI scores II–IIIb). Pain recurrence was observed in 27 patients (20.1%) within two years, and repeat PRT was successful in all recurrent cases, with 19 patients (70.4%) achieving complete pain relief. Complications were minimal, with intrabuccal hematoma being the most common (4.5%) and dysesthesia the least common (1.5%).
Conclusion
PRT is a highly effective and safe treatment for elderly patients with ITN in Yemen, achieving an immediate success rate of over 90% in pain relief. While pain recurrence is a notable concern, repeat PRT proves to be a reliable solution for managing recurrent pain. PRT is associated with a low rate of mild and transient complications, but long-term monitoring and follow-up for recurrence and complications are essential to optimize outcomes.
Keywords: Percutaneous radiofrequency thermocoagulation, Idiopathic trigeminal neuralgia, Effectiveness, Safety, Yemen
Introduction
Trigeminal neuralgia (TN) is the most common cranial neuralgia, which is characterized by repeated episodes of unilateral electric shock-like pain that occurs abruptly and lasts briefly [1, 2]. It is triggered by harmless stimuli and can develop without apparent cause or as a consequence of another underlying condition [1, 2]. TN is a manifestation of orofacial neuropathic pain often confined to one or more branches of the fifth cranial nerve [3]. Since TN can refer to several related but distinct conditions, a number of classification systems have been used, including classical vs. secondary, idiopathic vs. iatrogenic, typical vs. atypical, type I vs. II, related to multiple sclerosis (MS) or herpes zoster, etc [4]. It is estimated that the annual incidence of TN ranges from 4.3 to 28.9 cases per 100,000 people, increasing with age [5]. The female-to-male incidence ratio of TN is about 3:2 [4].
The pathophysiology of TN remains unclear, but according to the “ignition theory,” TN is thought to result from abnormalities in the afferent neurons of the trigeminal root or ganglion [6]. Currently, the etiology of TN is explained by three main theories. The first theory attributes TN to underlying disease (MS, diabetes mellitus, and hypertension), while the second attributes it to direct nerve trauma (neurovascular compression), and the third suggests a polyetiological origin [7]. However, the cause of TN remains unknown for the majority of patients [8, 9].
TN must be diagnosed based on the clinical history and physical examination, but sub-categorization and differential diagnosis often require imaging assessment such as magnetic resonance imaging or computed tomography (CT) scans [10]. There are three considerations when diagnosing TN. First, the location and distribution of the trigeminal nerve. Most patients suffer pain in the areas where maxillary and mandibular branches innervate, while a few cases may involve the ophthalmic branch [11]. When pain occurs at the base of the tongue, it is essential to differentiate TN from glossopharyngeal neuralgia (GPN), as the trigeminal and glossopharyngeal nerves can overlap [12]. Second, the characteristics of TN, which is severe in intensity, electric shock-like, sharp in quality, and paroxysmal in occurrence, with each episode lasting less than 2 min with definite refractory periods [13]. Third, TN can be triggered by harmless stimuli such as chewing, brushing teeth, or cold wind. However, these triggers can also be similar to those seen in GPN, making it essential to rely on additional diagnostic criteria to differentiate between the two conditions [12]. The diagnosis of TN can be made when the above three conditions are met, and no other conditions better explain the symptoms according to the criteria of the International Classification of Headache Disorders, third edition (ICHD-3) [14].
The initial approach to treating trigeminal neuralgia (TN) involves conservative medical management [15]. In the twentieth century, TN has witnessed significant advancements in treatment since the introduction of antiepileptic drugs, such as diphenylhydantoin and carbamazepine, and the introduction of microvascular decompression (MVD) [15, 16]. When medical treatment proves ineffective or leads to significant side effects, surgical interventions may be considered. Different types of surgery are used to treat TN, such as neurectomy, percutaneous radiofrequency thermocoagulation (PRT), radiosurgery, and MVD [17]. In certain cases, surgical decompression of the blood vessel compressing the trigeminal nerve has been shown to alleviate pain effectively [18–20]. In elderly patients over 60 years with ITN unresponsive to medical therapy, current recommendations advise the use of PRT over other modalities like MVD due to its non-destructive and neuromodulatory nature [21]. There is a lack of studies on PRT in the treatment of idiopathic TN among Yemeni patients. Therefore, this study aimed to assess the safety and effectiveness of PRT in treating elderly patients with ITN.
Methods
Study design, setting and patients
A prospective, descriptive cohort study was carried out at the Pioneer Medical Center for Neurosurgery and Pain Management in Sana’a city, the sole facility in Yemen offering PRT for TN. From September 2022 to October 2024, 187 patients were assessed at the center. Among them, 158 patients met the criteria and participated in the study, while 29 had non-ITN. Of the 158 patients who initially met the inclusion criteria and received PRT, 15 were lost to follow-up by the end of the first year, and an additional 9 were lost by the end of the second year. Thus, 134 patients completed the full follow-up and were included in final analysis (Fig. 1). These patients had ITN involving the maxillary division (V2), mandibular division (V3), or a combination of V2 and V3, as well as cases involving V1 + V2 or V1 + V2 + V3.
Fig. 1.
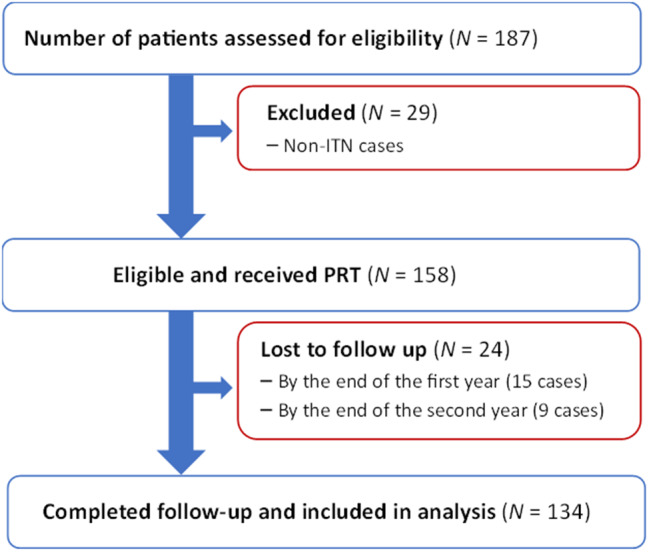
Flowchart of participants in the prospective cohort study
The study included patients aged 58 years or older with ITN who did not experience facial numbness, had previously undergone drug treatment for TN with inadequate pain control despite maximal tolerated carbamazepine (1200 mg/day) for more than three months or experienced side effects, and were unwilling to undergo microvascular decompression. Additionally, patients were included if they had ischemic cardiac disease, were severely affected by pain, and did not achieve pain relief even with high doses of carbamazepine. Patients with vascular contact without clear nerve root compression who were ineligible for general anesthesia due to comorbid conditions were also included in the study.
Patients with isolated ophthalmic nerve (V1) involvement were excluded from the study because the treatment for V1-related TN primarily involves RFT at low temperatures or pulsed radiofrequency. However, those with combined involvement of V1 and V2 and/or V3 branches were included if the primary pain focus was in the V2/V3 branches. Additionally, the study excluded patients with TN due to herpes zoster, patients who had undergone minimally invasive procedures such as balloon compression, and those who refused to participate voluntarily or did not adhere to medical advice. Patients who had skin or deep tissue infections at the site of puncture, bleeding disorders, anticoagulant use, or bilateral TN or secondary TN (STN) were also excluded.
Neuroimaging and diagnosis of ITN
All patients were diagnosed following the diagnostic criteria for ITN or STN as outlined in the ICHD-3 [14] and the third version of the International Headache Society in 2013 [22]. A 1.5 Tesla MRI scan was performed according to a TN protocol pre-defined for the trigeminal nerve to rule out STN and evaluate the neurovascular relations, degree, localization, and type of any neurovascular contact [23]. For patients with claustrophobia, severe obesity or a pacemaker, an open 1.5 Tesla MRI scanner was used without the predefined TN protocol. Head CT scans were performed preoperatively to rule out alternative causes of facial pain such as chronic maxillary sinusitis, dental cysts, or periodontitis. Additionally, brain CT was used in cases of pain recurrence to exclude rare complications related to previous procedures, such as intracranial hemorrhage. An experienced neuroradiologist unaware of the patients’ condition evaluated all MRI scans. ITN was diagnosed in patients whose MRI scans showed no evidence of neurovascular compression affecting the trigeminal nerve and ruled out other secondary causes of TN.
PRT procedure
Patients were asked to fast for at least six hours and were administered a prophylactic antibiotic one hour before the procedure. After taking a detailed medical history, conducting routine preoperative blood investigations, and examining MRI and CT scans of the head, PRT was performed via foramen ovale puncture as described by Härtel [24]. All procedures were performed in a fluoroscopy-equipped operating room at the Pioneer Medical Center for Neurosurgery and Pain Management. Initial and repeated PRT procedures for all patients were performed by the same surgeon to avoid operating bias. Briefly, patients were positioned supine with the head and neck in neutral alignment. Using sterile markers, Hartel’s anatomical reference points [25] were outlined on the face, with the primary insertion site established 3 cm from the corner of the mouth. Two directional guides—the inner margin of the pupil and a point 2.5 cm anterior to the ear’s tragus along the zygomatic arch—ensured proper alignment toward the foramen ovale. The procedure was conducted exclusively under local anesthesia, with no sedation administered in order to preserve patient responsiveness. All patients remained fully conscious throughout the procedure, enabling real-time dermatomal sensory mapping prior to lesioning to ensure accurate electrode placement during sensory stimulation and minimize the risk of inadvertent nerve injury. A 0.5% lidocaine solution (10 ml) was injected along the subcutaneous pathway to anesthetize the cannula track. Continuous cardiac monitoring was performed throughout the procedure to detect bradycardia or other autonomic disturbances related to trigemino-vagal reflex. To ensure patient safety, defibrillator pads were placed on the chest before the procedure, atropine ampules were prepared and readily available, and a fully equipped crash cart remained on standby throughout the procedure.
A 22-gauge, 10-cm radiofrequency cannula with a 5-mm active tip was advanced under imaging guidance, aligning with the pupil in frontal views and positioned 3 cm anterior to the ear canal in lateral projections. Fluoroscopy dynamically adjusted the trajectory as the needle approached the skull base, with final placement within the foramen ovale confirmed via real-time imaging, including both anteroposterior and lateral fluoroscopic views to verify proper trajectory and depth. Final cannula tip position and depth within the foramen ovale were verified using C-arm fluoroscopy. To ensure accurate targeting of specific trigeminal divisions, the trajectory and depth of the cannula were adjusted according to the intended branch. For V2 lesions, the cannula was advanced through the foramen ovale with a slightly anterior and superior orientation. For V3 lesions, the cannula was angled more posteriorly and inferiorly, with patient-reported sensations in the lower lip, chin, or mandibular dentition guiding placement. Sensory mapping was employed during stimulation at 50 Hz (0.1–0.2 V) to confirm the correct branch by eliciting paresthesia in the upper lip, cheek, or maxillary teeth for V2 and lower lip, chin, or in mandibular teeth for V3. Fluoroscopic guidance was continuously used to correct cannula positioning, and adjustments in trajectory were made as needed based on patient-reported sensory feedback. Moreover, corneal reflex was monitored throughout the procedure to avoid unintended involvement of the V1 division.
The trigeminal nerve rootlets afferent into the trigeminal ganglion received sensory input from various facial regions. To ensure the correct positioning of the electrode tip, stimulation at 50 Hz and 0.1–0.2 V was applied while patients subjectively reported sensation in response to the stimulation. In cases where only one trigeminal division was involved, three radiofrequency lesions were performed at 70 °C for 90 s (3–5 mm). In cases involving two divisions, two lesions were applied per division to balance efficacy with safety in the elderly population, totaling four lesions. This same protocol was used during repeat procedures in cases of pain recurrence. The 70 °C threshold was used to prioritize the safety and reduce the risk of potential complications in the elderly study population. To effectively relieve pain, the cannula tip was rotated 180° during each temperature adjustment before any additional thermal lesioning. To ensure effective pain management during the lesioning phase, 0.2 mL of 2% lidocaine was delivered directly into the trigeminal ganglion immediately before initiating radiofrequency ablation. To confirm that the observed pain relief was due to the PRT rather than the short-lived effect of local anesthesia, BNI scores were evaluated between 6 and 12 h after the procedure, once the anesthetic effect had subsided. If sufficient pain control was not achieved within 48 h, the procedure was repeated. After each electrode repositioning, the accuracy of the position was verified by sensory stimulation. In addition, corneal reflex was monitored at all stages of the procedure. At the end of the procedure, 1 ml of methylprednisolone (40 mg) was injected around the Gasserian ganglion to reduce post-procedural neuritis and pain flare, in accordance with our institutional protocol. Although it is a particulate steroid, the risk of embolic events was minimized by adhering to meticulous injection technique, including careful aspiration before injection and continuous fluoroscopic guidance to ensure extravascular placement of the medication. After the surgical procedure, the cannula was removed, and the patients were transferred to the ward to take strict bed rest for 24 h, including 6 h of bed rest without a pillow. During this period, the vital signs were monitored, and patients with pain relief and no serious adverse reactions were then discharged. For patients who experienced pain recurrence, repeat PRT procedures were carried out by the same surgeon using the same operative protocol and thermocoagulation parameters as the initial intervention to ensure consistency and minimize operator-related variability.
Data collection and outcome assessment
Immediate and follow-up evaluations were conducted by a neurologist or an experienced neurosurgery fellow at the Pioneer Medical Center for Neurosurgery and Pain Management. Follow-up assessments were carried out at five intervals: 1 week, 1 month, 6 months, 1 year, and 2 years following the PRT procedure. These evaluations took place either in person at the center’s outpatient clinic or, when necessary, via telephone, and were consistently performed by the same neurologist or neurosurgery fellow. The Barrow Neurological Institute (BNI) pain intensity scale was used to assess the degree of pain after PRT, as scored as in Table 1 [26]. All patients had a preoperative BNI score of ≥ IV. In addition to pain assessment, the incidence and recovery of adverse events, such as facial hematoma, dysesthesia and muscle weakness, were also recorded. Patient-reported improvement, recurrence of pain, and adjustments in oral medication based on pain intensity and characteristics were also recorded.
Table 1.
BNI pain intensity scale
| Pain score | Definition |
|---|---|
| I | No TN, no medication |
| II | Occasional pain, not requiring medication |
| III | Some pain, adequately controlled with medication |
| IIIa | No pain, continued medication |
| IIIb | Persistent pain, controlled with medication |
| IV | Some pain, not adequately controlled with medication |
| V | Severe pain, no pain relief |
BNI Barrow Neurological Institute
Preoperative data, including gender, age, duration and laterality of pain, and affected nerve branches were collected using a data collection sheet. Follow-up evaluations were conducted at the end of the first and second year of PRT in the outpatient department, and the outcome was considered successful if the patient’s BNI score decreased to I-IIIb or failed if a patient’s BNI pain score of IV or V [26, 27]. All adverse events were also recorded, including pain at the site of puncture, facial hematoma or numbness, headache, and bleeding from the external auditory canal. Recurrence was defined as the return of TN with the same characteristics of the preoperative pain and with a frequency of three or more times per day in the absence of medication use [28]. Throughout the follow-up period, patients typically continued their oral medications, with dosages adjusted according to pain intensity and characteristics.
Data analysis
Data were analyzed using IBM SPSS Statistics, version 26 (IBM Corp., Armonk, NY, USA). Continuous data were summarized with the mean and standard deviation (SD), while categorical variables were expressed as frequencies and percentages.
Results
Characteristics of the study cohort
The cohort of patients who completed the study included 55 (41%) males and 79 (59%) females. The mean age of patients was 70.3 ± 9.2 years (range: 58–86). In terms of facial pain laterality, most patients had right-sided TN (61.9%), while 38.1% of patients experienced left-sided pain. On the other hand, most patients had combined V2 and V3 involvement (41.8%), followed by those with isolated V2 involvement (28.4%) and isolated V3 involvement (17.2%). In contrast, combined V1 and V2 involvement was observed in 7 (5.2%) patients, and combined V1, V2, and V3 involvement was observed in 6 (4.5%) patients (Table 2).
Table 2.
Characteristics of patients with ITN and treated with PRT at the pioneer medical center for neurosurgery and pain management in sana’a city, yemen**
| Characteristic | Value |
|---|---|
| Gender (male: female n%) | 55 (41.0): 79 (59.0) |
| Male: female ratio | 1:1.4 |
| Age in years (mean ± SD; range) | 70.3 ± 9.2 (58–86) |
| Facial side affectedn (%) | |
| Right | 83 (61.9) |
| Left | 51 (38.1) |
| Division of TN affectedn (%) | |
| V2 | 38 (28.4) |
| V3 | 27 (20.1) |
| V1 + V2 | 7 (5. 2 ) |
| V2 + V3 | 56 (41.8) |
| V1 + V2 + V3 | 6 (4.5) |
* The total number of patients was 134. ITN idiopathic trigeminal neuralgia; PRT percutaneous radiofrequency thermocoagulation; SD standard deviation; V1 ophthalmic division V2 maxillary division; V3 mandibular division
Immediate pain relief outcomes
Table 3 shows that of the 134 patients, PRT was successful in 125 cases (93.3%), with patients achieving BNI scores from I to III during the early postoperative period. Detailed BNI score distribution is shown in Table 3.
Table 3.
Immediate pain scoring of patients with ITN treated with PRT at the pioneer medical center for neurosurgery and pain management in sana’a city, yemen**
| BNI pain intensity score | n | (%) |
|---|---|---|
| I | 95 | (70.9) |
| II | 11 | (8.2) |
| IIIa | 10 | (7.5) |
| IIIb | 9 | (6.7) |
| IV | 7 | (5.2) |
| V | 2 | (1.5) |
* The total number of patients was 134. ITN idiopathic trigeminal neuralgia; PRT percutaneous radiofrequency thermocoagulation; BNI Barrow Neurological Institute
Recurrence of pain after PRT
Of 134 patients subjected to PRT, 27 (20.1%) experienced pain recurrence within the first two years of PRT. In particular, 2 (1.5%) experienced pain within the first six months of PRT, 14 (10.4%) experienced pain recurrence within the first year of PRT, and 11 (8.2%) experienced pain recurrence within the second year of PRT. All these patients underwent a second PRT.
Pain relief outcomes after repeat PRT
Of 27 patients with recurrent pain who underwent a second PRT, treatment was successful in all patients. The distribution of BNI scores following repeated PRT is presented in Table 4.
Table 4.
Pain scoring of patients with recurrent post-PRT ITN treated with a repeat PRT at the pioneer medical center for neurosurgery and pain management in sana’a city, yemen**
| BNI pain intensity score | n | (%) |
|---|---|---|
| I | 19 | (70.4) |
| II | 5 | (18.5) |
| IIIa | 3 | (11.1) |
* The total number of patients was 27. BNI Barrow Neurological Institute; ITN idiopathic trigeminal neuralgia; PRT percutaneous radiofrequency thermocoagulation
Complications of PRT
Non-troublesome facial hypoesthesia was consistently reported by all patients classified within the first BNI categories, and its resolution occurred within two weeks to two months. Table 5 shows that intrabuccal hematoma was the most frequent complication after PRT, observed in six cases (4.5%). Foreign body sensation and masseter muscle weakness were equally reported, each occurring in four cases (3%). In addition, atrophy of temporal muscle and decreased corneal reflex were each observed in three cases (2.2%), while dysesthesia was the least frequent complication, occurring in two cases (1.5%).
Table 5.
Post-PRT complications among patients with ITN at the pioneer medical center for neurosurgery and pain management in sana’a city, yemen**
| Complication | (%) |
|---|---|
| Intrabuccal hematoma | (4.5) |
| Foreign body sensation | (3.0) |
| Masseter muscle weakness | (3.0) |
| Atrophy of temporal muscle | (2.2) |
| Decreased corneal reflex | (2.2) |
| Dysesthesia | (1.5) |
* The total number of patients was 134. PRT percutaneous radiofrequency thermocoagulation; ITN idiopathic trigeminal neuralgia
Discussion
To the best of our knowledge, this is the first study on the effectiveness and safety of PRT in the treatment of ITN in elderly patients in Yemen. The study showed a high success rate of over 93% in the early postoperative period, as measured by BNI pain intensity scores ranging from I to III. Specifically, about two-thirds of patients achieved complete relief of pain without the need for medications, and the remaining third experienced either some occasional pain not requiring medication or varying degrees of pain control with medication. These findings highlight the effectiveness of PRT in substantially reducing pain for most Yemeni patients with ITN. Meanwhile, the high success rate of PRT observed in this study agrees with the findings of studies from other regions, with immediate success rates of 90% or more [7, 29–34]. Therefore, PRT is a reliable and effective modality for treating medically refractory ITN in elderly patients who are unsuitable candidates for invasive surgical interventions. In contrast to the present study, the three-year success rate of PRT among patients with refractory trigeminal neuralgia in the United Kingdom was found to be 70.7% [35]. In South Korea, 86.8% of patients with ITN showed BIN scores of I to III after PRT [36].
The present study also highlighted that a single PRT can be unsuccessful for a small subset of patients with ITN (6.7%), as indicated by BNI scores of IV or V. However, repeating the PRT procedure for these patients succeeded in reducing pain intensity to acceptable scores.
Approximately one-fifth of patients in this study experience a recurrence of pain within the first two years after PRT, which is similar to the recurrence rate observed among 38 patients with ITN in South Korea [36]. In the latter study, the recurrence rate increased to 29.1% within three years. However, patients were followed for two years in the present study. Kanpolat et al. [30] reported a recurrence rate of 25.1% after the first procedure, being early for 7.7% of patients and late for 17.4% of patients. A lower pain recurrence rate of 16% was observed for patients with TN after treatment with PRT followed up from 2 to 10 years in Italy [29]. In a retrospective case series of 21 cases aged over 80 years with severe ITN, pain recurred in one patient 23 months after PRT [37]. The results of the present study show that repeat PRT worked in all cases of recurrence, which supports its use as a treatment option for recurrent ITN. This is particularly important in elderly or high-risk patients and in settings where access to more invasive neurosurgical interventions is limited.
The complications observed following PRT for ITN in this study are consistent with those reported in the literature, highlighting both the effectiveness and potential risks associated with this treatment modality. The finding that intrabuccal hematoma was the most frequent complication could be partially attributed to the proximity of the trigeminal nerve to vascular structures during the procedure. Other post-PRT complications observed among patients in the present study were foreign body sensation, masseter muscle weakness, atrophy of temporal muscle, decreased corneal reflex, and least frequently, dysesthesia. According to Gunduz et al. [7], intrabuccal hematoma was the most common complication observed among 9.1% of patients with TN in Turkey, followed by corneal hypoesthesia and reflex deficit (3.4%) and masseter muscle weakness (2.4%). Kanapolat et al. [30] reported complications among 0.6–5.7% of patients followed for 1 to 25 years, including diminished corneal reflex, masseter weakness/paralysis, dysesthesia, and anesthesia. In South Korea, 15.8% (6/38) of patients with ITN developed pterygoid or masseter muscle weakness after a single PRT, but this weakness resolved completely within six months [36].
Dysesthesia was rare in the cohort of the present study. Similarly, it was observed among 1.4% of patients with TN after PRT in Turkey. In contrast, troublesome dysesthesia occurred in 7.9% (3 of 38) of patients with ITN in South Korea after PRT, but without permanent cranial nerve palsy [36]. The low occurrence of dysesthesia in this study is encouraging and suggests that PRT is generally well-tolerated, but its occurrence underscores the importance of long-term follow-up to address any persistent symptoms. For instance, permanent dysesthesia was reported in 0.6% of 1600 patients with ITN treated with this procedure in Serbia [31]. Loss of facial sensation accounted for over 80% of complications associated with PRT of a cohort of 605 patients with TN in Italy [29].
This study provides important insights into the effectiveness and safety of PRT for treating ITN in Yemeni patients, particularly in a resource-constrained, conflict-affected setting where access to advanced imaging, surgical alternatives, and specialized care is limited. Given Yemen’s lack of comprehensive neurosurgical infrastructure and the fact that our center is the sole facility nationwide offering PRT, the study’s findings provide context-specific evidence that is especially relevant to other low-resource settings facing similar systemic limitations. In a healthcare system strained by economic hardship and conflict, cost-effective outpatient procedures like PRT offer an invaluable alternative. Additionally, by focusing on elderly patients as a group often underrepresented in prior studies, this study addresses a critical gap in the literature and demonstrates the feasibility of PRT in such a high-risk population. Nevertheless, a number of limitations should be acknowledged. First, this study was conducted in a single center, which may limit the generalizability of the findings. However, this is the only center that offers PRT in the country. Second, this study was descriptive in nature, focusing primarily on short-term outcomes such as immediate pain relief and complications, rather than long-term outcomes like survival or recurrence rates beyond two years. The absence of a control group in our study, such as patients receiving saline, local anesthetic or methylprednisolone alone, limits the possibility of a definitive attribution of the PRT therapeutic effect to the intervention itself, as the influence of placebo, regression to mean, or the natural course of ITN cannot be excluded. However, all patients in the study had a refractory ITN and had not achieved adequate relief from high-dose carbamazepine before PRT, making spontaneous remission unlikely. In addition, we did not include a younger comparison group, which limits our ability to determine whether age influences treatment outcomes. Future research should involve age-stratified cohorts to explore whether clinical outcomes, recurrence rates, or complication profiles differ significantly between elderly and younger populations. Prospective cohort studies employing Kaplan-Meier analysis are recommended to analyze longer-term, time-to-event outcomes. In addition, randomized controlled trials with comparator arms are recommended to confirm these findings and evaluate the efficacy of PRT in this patient population more rigorously. Third, motor stimulation (typically 2–5 Hz) was not employed during our procedure to confirm electrode placement within the targeted trigeminal nerve branches. Despite this limitation, our results showed the safety of sensory-guided PRT, as evidenced by the low rate of complications. Fourth, post-procedural facial hypoesthesia was not systematically assessed using a standardized scale such as the BNI Hypoesthesia Score. Instead, sensory disturbances were recorded qualitatively based on patient-reported symptoms and clinical evaluation. Although lidocaine and methylprednisolone were administered intraoperatively to reduce procedural discomfort and inflammation, facial hypoesthesia was assessed after the expected pharmacologic effects had resolved, making it unlikely that these agents masked the sensory effects of thermocoagulation. Future studies should incorporate formal hypoesthesia grading to better quantify sensory outcomes following PRT. Lastly, this study did not include multivariate analysis to identify potential predictors of recurrence or complications due to the sample size and descriptive study design. Future studies with larger cohorts are warranted to perform risk factor analysis using multivariate methods to better understand outcomes and predictors of PRT.
Conclusion
PRT of the Gasserian ganglion is a highly effective and safe treatment modality for treating elderly patients with ITN in Yemen, with an immediate success rate exceeding 90% in pain relief (BNI scores of I to III). While pain recurrence is a notable concern, repeat PRT offers a reliable solution for managing recurrent pain. Therefore, patients should be informed of the possibility of recurrence and the availability of repeat PRT as an effective treatment option. On the other hand, PRT is associated with a low rate of mild and transient complications, but long-term monitoring and follow-up for recurrence and complications are essential.
Acknowledgements
The authors thank the participants who consented to be included in the study, along with the administration and staff of the Pioneer Medical Center for Neurosurgery and Pain Management in Sana’a city for permission and support during the study.
Abbreviations
- BNI
Barrow Neurological Institute
- CT
Computed tomography
- GPN
Glossopharyngeal neuralgia
- ICHD
International Classification of Headache Disorders
- ITN
Idiopathic trigeminal neuralgia
- MRI
Magnetic resonance imaging
- MS
Multiple sclerosis
- MVD
Microvascular decompression
- PRT
Percutaneous radiofrequency thermocoagulation
- SD
Standard deviation
- SPSS
Statistical Package for the Social Sciences
- STN
Secondary trigeminal neuralgia
- TN
Trigeminal neuralgia
Author contributions
AAA conceived the study idea and conducted the fieldwork. AAA, KS, and NAA designed the study. SAA analyzed the data and interpreted the results. AAA, KS and NAA drafted the manuscript. All authors reviewed, revised and approved the final manuscript.
Funding
None.
Data availability
Availability of Data and Materials.
Declarations
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki regarding research on human subjects. The study protocol was approved by the Research Ethics Committee of the Faculty of Medicine and Health Sciences at Sana’a University, Yemen. In addition, oral informed consent was obtained from all participants. The privacy and confidentiality of participants were maintained throughout the study. The privacy of participants and the confidentiality of their data were assured.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Maarbjerg S, Di Stefano G, Bendtsen L, Cruccu G. Trigeminal neuralgia - diagnosis and treatment. Cephalalgia. 2017;37(7):648–57. [DOI] [PubMed] [Google Scholar]
- 2.Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38(1):1-211. [DOI] [PubMed]
- 3.Scholz J, Finnerup NB, Attal N, Aziz Q, Baron R, Bennett MI, et al. The IASP classification of chronic pain for ICD-11: chronic neuropathic pain. Pain. 2019;160(1):53–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eller JL, Raslan AM, Burchiel KJ. Trigeminal neuralgia: definition and classification. Neurosurg Focus. 2005;18(5):E3. [DOI] [PubMed] [Google Scholar]
- 5.Mizobuchi Y, Nagahiro S. [Prevalences of trigeminal neuralgia and hemifacial spasm]. No Shinkei Geka. 2024;52(1):22–8. [DOI] [PubMed] [Google Scholar]
- 6.Bharti N, Sujith J, Singla N, Panda NB, Bala I. Radiofrequency thermoablation of the Gasserian ganglion versus the peripheral branches of the trigeminal nerve for treatment of trigeminal neuralgia: a randomized, control trial. Pain Physician. 2019;22(2):147–54. [PubMed] [Google Scholar]
- 7.Gunduz HB, Cevik OM, Asilturk M, Gunes M, Uysal ML, Sofuoglu OE, et al. Percutaneous radiofrequency thermocoagulation in trigeminal neuralgia: analysis of early and late outcomes of 156 cases and 209 interventions. J Korean Neurosurg Soc. 2021;64(5):827–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabalys G, Juodzbalys G, Wang HL. Aetiology and pathogenesis of trigeminal neuralgia: a comprehensive review. J Oral Maxillofac Res. 2013;3(4):e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zakrzewska JM. Diagnosis and differential diagnosis of trigeminal neuralgia. Clin J Pain. 2002;18(1):14–21. [DOI] [PubMed] [Google Scholar]
- 10.Kedarnath NS, Shruthi R. MRI as an essential diagnostic approach for trigeminal neuralgia. J Maxillofac Oral Surg. 2015;14(Suppl 1):462–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rozen TD. Trigeminal neuralgia and glossopharyngeal neuralgia. Neurol Clin. 2004;22(1):185–206. [DOI] [PubMed] [Google Scholar]
- 12.Park JS, Park K. Overview of trigeminal neuralgia. In: Trigeminal neuralgia: a comprehensive guide. Park K, Cho KR, Park K, Cho KR, editors. Singapore: Springer Nature; 2023: 3–8.
- 13.Maarbjerg S, Gozalov A, Olesen J, Bendtsen L. Trigeminal neuralgia–a prospective systematic study of clinical characteristics in 158 patients. Headache. 2014;54(10):1574–82. [DOI] [PubMed] [Google Scholar]
- 14.Arnold M, Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders. Cephalalgia. 2018;38(1):1–211. [DOI] [PubMed] [Google Scholar]
- 15.Liu JK, Apfelbaum RI. Treatment of trigeminal neuralgia. Neurosurg Clin N Am. 2004;15(3):319–34. [DOI] [PubMed] [Google Scholar]
- 16.Lunsford LD, Apfelbaum RI. Choice of surgical therapeutic modalities for treatment of trigeminal neuralgia: microvascular decompression, percutaneous retrogasserian thermal, or glycerol rhizotomy. Clin Neurosurg. 1985;32:319–33. [PubMed] [Google Scholar]
- 17.Parmar M, Sharma N, Modgill V, Naidu P. Comparative evaluation of surgical procedures for trigeminal neuralgia. J Maxillofac Oral Surg. 2013;12(4):400–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jannetta PJ. Arterial compression of the trigeminal nerve at the Pons in patients with trigeminal neuralgia. J Neurosurg. 1967;26(1):Suppl159–62. [DOI] [PubMed]
- 19.Jannetta PJ. Vascular compression is the cause of trigeminal neuralgia. APS J. 1993;2(4):217–27. [Google Scholar]
- 20.van Loveren H, Tew JM Jr., Keller JT, Nurre MA. A 10-year experience in the treatment of trigeminal neuralgia. Comparison of percutaneous stereotaxic rhizotomy and posterior fossa exploration. J Neurosurg. 1982;57(6):757–64. [DOI] [PubMed] [Google Scholar]
- 21.Latorre G, González-García N, García-Ull J, González-Oria C, Porta-Etessam J, Molina FJ, et al. Diagnosis and treatment of trigeminal neuralgia: consensus statement from the Spanish society of neurology’s headache study group. Neurologia (Engl Ed). 2023;38(Suppl 1):S37–52. [DOI] [PubMed] [Google Scholar]
- 22.Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition (beta version). Cephalalgia. 2013;33(9):629–808. [DOI] [PubMed]
- 23.Maarbjerg S, Wolfram F, Gozalov A, Olesen J, Bendtsen L. Significance of neurovascular contact in classical trigeminal neuralgia. Brain. 2015;138(Pt 2):311–9. [DOI] [PubMed] [Google Scholar]
- 24.Härtel F. Die Leitungsanästhesie und Injections-Behandlung des ganglion gasseri und der trigeminusstämme. 1st ed. Heidelberg: Springer Berlin; 1913. [Google Scholar]
- 25.Saavedra Azcona T, Villaescusa M, Casto F, Paolinelli P, Dover SE, Plou PL et al. Validation of Härtel surface anatomical landmarks for locating the foramen ovale: a computed tomography scan analysis and revised technique description. Oper Neurosurg (Hagerstown). 2025. [DOI] [PubMed]
- 26.Rogers CL, Shetter AG, Fiedler JA, Smith KA, Han PP, Speiser BL. Gamma knife radiosurgery for trigeminal neuralgia: the initial experience of the Barrow neurological Institute. Int J Radiat Oncol Biol Phys. 2000;47(4):1013–9. [DOI] [PubMed] [Google Scholar]
- 27.Chen HI, Lee JY. The measurement of pain in patients with trigeminal neuralgia. Clin Neurosurg. 2010;57:129–33. [PubMed] [Google Scholar]
- 28.Liu G, Du Y, Wang X, Ren Y. Efficacy and safety of repeated percutaneous radiofrequency thermocoagulation for recurrent trigeminal neuralgia. Front Neurol. 2018;9:1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moraci A, Buonaiuto C, Punzo A, Parlato C, Amalfi R. Trigeminal neuralgia treated by percutaneous thermocoagulation. Comparative analysis of percutaneous thermocoagulation and other surgical procedures. Neurochirurgia (Stuttg). 1992;35(2):48–53. [DOI] [PubMed] [Google Scholar]
- 30.Kanpolat Y, Savas A, Bekar A, Berk C. Percutaneous controlled radiofrequency trigeminal rhizotomy for the treatment of idiopathic trigeminal neuralgia: 25-year experience with 1,600 patients. Neurosurgery. 2001;48(3):524–32. [DOI] [PubMed] [Google Scholar]
- 31.Slavik E, Ivanović S, Spaić M, Djurović B. [Idopathic trigeminal neuralgia–radiofrequency rhizotomy of ganglion gasseri]. Acta Chir Iugosl. 2004;51(4):31–8. [DOI] [PubMed] [Google Scholar]
- 32.Tang YZ, Jin D, Bian JJ, Li XY, Lai GH, Ni JX. Long-term outcome of computed tomography-guided percutaneous radiofrequency thermocoagulation for classic trigeminal neuralgia patients older than 70 years. J Craniofac Surg. 2014;25(4):1292–5. [DOI] [PubMed] [Google Scholar]
- 33.Tang YZ, Wu BS, Yang LQ, Yue JN, He LL, Li N, et al. The long-term effective rate of different branches of idiopathic trigeminal neuralgia after single radiofrequency thermocoagulation: a cohort study. Med (Baltim). 2015;94(45):e1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qu S, Zhu X, Wang T, Li D. [The evaluation of curative effect of radiofrequency thermocoagulation on semilunar ganglion of aged patients with recurrent trigeminal neuralgia]. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2016;30(2):135–8. [PubMed] [Google Scholar]
- 35.Haridas A, Mathewson C, Eljamel S. Long-term results of 405 refractory trigeminal neuralgia surgeries in 256 patients. Zentralbl Neurochir. 2008;69(4):170–4. [DOI] [PubMed] [Google Scholar]
- 36.Son BC, Kim HS, Kim IS, Yang SH, Lee SW. Percutaneous radiofrequency thermocoagulation under fluoroscopic image-guidance for idiopathic trigeminal neuralgia. J Korean Neurosurg Soc. 2011;50(5):446–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luo F, Gao S, Zhang L, Dou L. Effect of radiofrequency thermocoagulation guided by spiral CT on severe trigeminal neuralgia in the oldest old patients: 21 cases report. Chin J Rehabil Theory Pract. 2009;15(7):613–4. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Availability of Data and Materials.


