Abstract
Some bacteria emit dimethyl disulphide (DMDS), one of the bioactive volatile sulphurous compounds (VSCs) of environmental and ecological significance, yet unexplored in combating drug-resistant yeasts. Here, we show the anti-budding and fungicidal activity of volatile DMDS emitted from Burkholderia cepacia LS-044 on the caspofungin-resistant yeast Nakaseomyces glabratus NT2. We identified a gene encoding L-methionine-γ-lyase (mdeA) catalysing DMDS formation in LS-044 and detected volatile DMDS as one of the VSCs (12% peak area) emitted by LS-044 through solid-phase microextraction followed by gas chromatographic-mass spectrometry. Exposure to volatiles of LS-044 resulted in a significant decline in metabolism (~ 98%), media alkalinity (~ 26%), and viable cell count (52‒95%) of NT2. Confocal microscopy of carboxyfluorescein succinimidyl ester- and calcofluor white stained cells revealed significantly high mean fluorescence in NT2 exposed to the volatiles of LS-044 (~ fivefold) and standard DMDS vapour (1.7-fold), suggesting a significant thickening of the cell wall. The surface area-to-volume ratio decreased significantly in NT2 cells exposed to volatiles of LS-044 and DMDS versus unexposed NT cells (6.5‒10.2 vs. 8.3‒13.2). DMDS exhibited a minimum inhibitory concentration of 0.5% (v/v) on NT2 cells in liquid broth dilution assay, and displayed fractional inhibitory concentration index of 0.95 with commercial antifungal clotrimazole reflecting lack of synergy or antagonism during the present combination therapy. The 26S rRNA gene sequence-based phylogeny revealed a tight phylogenetic association between NT2 and N. glabratus of environmental and clinical origins. Our study provided novel mechanistic insights into DMDS-driven bacterial antagonism on drug-resistant budding yeast N. glabratus NT2 that could be exploited in the ecological engineering and therapeutics of drug-resistant fungal infection.
Keywords: Candida glabrata, Volatilome, Volatile sulphurous compounds (VSCs), Fungicide, Antagonism, Volatile organic compounds (VOCs)
Subject terms: Microbial ecology, Infection, Ecology
Introduction
Dimethyl disulphide (DMDS) is a volatile sulphurous compound (VSCs) being widely used in agriculture as a nematicide, fungicide and/or herbicide under field and/or greenhouse conditions1–6. DMDS can be released into the environment through industrial discharge, microbial processes or photochemical means7–9. This volatile compound is known for its pungent odour, refractive nature and typical environmental pollutant attributes10. Continuous efforts have been made to strengthen the regulatory framework regarding the field application of DMDS1. Alternatively, technologies have been developed to remove DMDS from the environment through biological and physicochemical means9,11,12.
Actinobacterial representatives such as Pseudonocardia asaccharolytica and P. sulfidoxydans are known to metabolize DMDS in vitro13. Microbacterium, Gordonia, Dietzia, Rhodococcus, Propionibacterium, and Janibacter colonizing the biofilter can degrade DMDS from the air14. Removal of VSCs from air can also be achieved through Thiobacillus thioparus15, a bacterium capable of metabolizing organic compounds in biotrickling filters16,17 or through microbial fuel cells10. Bacillus cereus GIGAN2 can biodegrade DMDS from aqueous medium17,18.
In contrast, the microbial emission of volatiles could influence co-existing microbes and eukaryotes. For example, the emission of VOCs by Burkholderia pyrrocinia CNUC9 induces systemic salt tolerance in Arabidopsis thaliana19. DMDS discharged by Bacillus sp. B55 enhances the availability of reduced sulphur for Nicotiana attenuata plants growing in sulphur-deficient soils and for sulphur-etr1 plants due to their impaired sulphur uptake/assimilation/metabolism. DMDS is found to be a bioactive molecule produced by B. ambifaria H8 against maize stalk rot20 and an inducer of systemic resistance in plants against necrotrophic pathogens21.
Plants and animals under normal and/or pathophysiological conditions may discharge VSCs. DMDS is emitted through pulmonary excretion in mice22 or from melanoma cells in humans23,24. Emission of DMDS from guava (Psidium guajava L.) can activate the defense responses in eavesdropping orange (Citrus sinensis L. Osbeck) plants and boost their herbivore resistance to Asian citrus psyllid (Diaphorina citri Kuwayama)25. DMDS exhibits vasodilation in mice26 and exerts a neuropathological effect on the desert locust Schistocerca gregaria27. This volatile compound is an insecticidal neurotoxin28 and an attractant pheromone in hamsters29,30. DMDS exhibits fumigant activity against plant parasitic root-knot nematode Meloidogyne incognita31 and could be useful in countering plant foliar disease21. A recent study showed DMDS-driven membrane disruption in phytopathogenic fungi Sclerotinia minor and induction of systemic resistance in tomato32. However, the direct impacts of DMDS produced by a bacterium on the drug-resistant yeasts remains unexplored.
Nakaseomyces glabratus (formerly Candida glabrata), a budding yeast affiliated with the phylum Ascomycota and family Saccharomycetaceae, colonizes different niches within the human host. It is an opportunistic pathogen and an emerging cause of concern during human infections33,34. This species can exhibit resistance to echinocandins34, a class of antifungal drugs that inhibit beta (1, 3)-D-glucan synthase involved in the maintenance of the integrity of the fungal cell wall35. The emergence of echinocandin-resistant strains is alarming, as this class of antibiotics generally has mild adverse effects in humans36. Therefore, there is quest for naturally occurring fungicides to combat N. glabratus without the aid of antibiotics that could contribute to the attainment of Sustainable Development Goal (SDG No. 3; Good Health and Well Being) of the United Nations.
We hypothesized that the VSCs emitted by a biocontrol bacterium may elicit antagonistic effects on the drug-resistant budding yeast N. glabratus. We tested our hypothesis using a caspofungin-resistant N. glabratus NT2 and a biocontrol bacterium B. cepacia LS-044, a member of Burkholderia cepacia complex (Bcc), harbouring a methionine catabolic pathway, using bipartition plate assays followed by detailed molecular level investigations.
Materials and methodology
Chemicals and reagents
DMDS (Cas No. 624–92-0; France) was procured from Sigma-Aldrich. Alamar blue (AB, Cell-Quant™ AlamarBlue Cell Viability Reagent; ABP Biosciences) and phenol red (PR; Loba Chemie) were obtained from respective suppliers and used without further purification. Carboxyfluorescein succinimidyl ester (CFSE) and calcofluor white (CW) were purchased from Sigma Aldrich. High-Performance Liquid Chromatography/Gas Chromatography (HPLC/GC) grade solvents were used for chromatographic analysis.
Microorganisms and culture conditions
Burkholderia cepacia LS-044 was originated from the endosphere of Oryza sativa ssp. japonica cv. TN71 in Taiwan37. The strain was revived from -80 °C on potato dextrose agar (PDA) and incubated at 37 °C under darkness. Yeast strains NT2 and NT5 were isolated, respectively, from sewage samples originating from a hospital in Mangalore using the standard dilution extinction plating technique on PDA. Since LS-044 and NT2/NT5 grew well on PDA, it was used as a common medium for culturing both microbes. Plates were incubated at 37 °C under darkness. Purified colonies were cultured in potato dextrose broth (PDB) and preserved at -80 °C as glycerol (30%, v/v) stocks.
Molecular identification of yeasts
Genomic DNA (gDNA) from yeast strains was isolated through UltraCleanTM Microbial gDNA Isolation Kit (MO BIO, USA) by following the manufacturer’s instructions. A fragment of the gene encoding a large subunit (LSU) of RNA (26S rRNA) was amplified by using a gDNA template with primer pair NL-1 (5′-GCA TAT CAA TAA GCG GAG GAA AAG-3′) and NL-4 (5′-GGT CCG TGT TTC AAG ACG G-3′)38. The gene encoding LSU was amplified using the following polymerase chain reaction (PCR) conditions: 95 °C for 4 min followed by 35 cycles at 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 1.5 min and finally, 1 cycle at 72 °C for 5 min. NL-1 primer was used for sequencing as per earlier descriptions39. Sequences were trimmed using CAP340. Closely related LSU sequences were retrieved from the National Centre for Biotechnology Information (NCBI) for phylogenetic analysis. The nucleotide sequences encoding LSU were analysed by MEGA 5 (Molecular Evolutionary Genetics Analysis, version 5.0;41), after multiple alignment by Clustal_X42. Distance matrix method (distance options according to the Kimura two-parameter model43 and maximum-likelihood44 methods were used for molecular analysis and phylogeny. Tree topology was evaluated by using bootstrap resampling based on 1,000 replications45.
Carbon source utilization and antifungal susceptibility assay
Yeast isolates were subjected to biochemical and anti-fungal susceptibility testing using fungal identification (VITEK 2 ID, bioMérieux) and AST (VITEK 2 AST-YS08, bioMérieux) cards according to the manufacturer’s protocol. Suspension of freshly grown colonies were prepared in saline (aqueous 0.45% to 0.50% NaCl, pH 4.5 to 7.0) and adjusted OD to a McFarland No. 0.50 to 0.63 using a calibrated VITEK® 2 DensiCHEK™ Plus. The suspension tube and VITEK 2 fungal ID and AST cards were placed in the cassette and incubated at 37 °C for 24 h. Loading the cassette into the instrument and retrieval of raw data were done according to the VITEK instrument user manual. Test results were recorded within 10 h of incubation.
Identification of methionine degradation pathway
The detailed methodology employed in the genome sequencing of LS-044 is given earlier 46. The genome of LS-044 (GCA_004803625.1; BioSample: SAMN11382798) was uploaded to Rapid Annotation using Subsystem Technology (RAST) database47 and screened for genes encoding proteins involved in methionine catabolic pathways through the Basic Local Alignment Search Tool (BLAST). The circular genome map was developed using Proksee48. Chemical structures and the biosynthetic pathway were reconstructed using Marvin (https://chemaxon.com/products/marvin).
Scanning electron microscopy
Scanning electron microscopy for LS-044 was carried out according to the earlier description46. Briefly, the cells grown in PDB was filtered through a 0.2-µm pore size GTBP filter (Millipore; GTBP01300) and fixed with 2.5% glutaraldehyde solution prepared in 0.2 M cacodylate buffer (sodium cacodylate trihydrate, final pH 7.4 adjusted with HCl). Cells were incubated at –20 °C overnight. The pre-fixed cells were treated with gradients of ethanol (50, 70, 80, 90 and 100% ethanol, respectively; 10 min each) for dehydration and finally stored at 4 °C in 100% ethanol. Dehydrated cells were subjected to critical point drying, coated with gold–palladium and observed under a field emission scanning electron microscope (JSM-7401F; JEOL).
SPME GC–MS identification of DMDS emission
The emission of DMDS by LS-044 was validated by solid-phase micro extraction followed by gas chromatography-mass spectrometry (SPME GC–MS) as described earlier31,49. Briefly, cells were cultivated in full-strength nutrient broth (NB, HiMedia) without the exogenous addition of sulfurous compounds in Erlenmeyer flasks, sealed tightly using parafilm and incubated at 30 °C for five days. SPME needle carrying a preconditioned (helium at 280 °C for 30 min before use) CAR/PDMS SPME fiber (75 μm, Supelco, Bellefonte, PA, USA) was pierced through the parafilm and kept for 24 h to extract the volatiles accumulated at the headspace. SPME fibers were subsequently mounted onto the injection port of the GC–MS instrument (QP2010 SE, Shimadzu Corp., Kyoto, Japan) equipped with an RTx-5MS column (30 m length × 0.25 mm diameter × 0.50 μm thickness). The components of volatiles were separated using helium (flow rate: 1 ml/min). GC conditions were maintained as follows: 40 °C for 5 min, 40‒120 °C at a rising rate of 3 °C/min, 120‒180 °C at 4 °C/min, 180‒280 °C at 20 °C/min, and held at 280 °C for 5 min. MS data were acquired under the following settings: start time, 0.10 min; end time, 35.0 min; Acquisition mode, Scan; event time, 0.3 s; scan speed, 2500; start m/s, 35.0; end m/z, 650. The DMDS peak was identified using retention time (RT) and mass-to-charge ratio (m/z) while refering to the National Institute of Standards and Technology (NIST20) database.
Bipartition plate assay
Bipartition plate assays were carried out to understand the impacts of DMDS emitting from the LS-044 on the cell morphology, metabolic activity, cell viability and budding process of N. glabratus NT2. The schematic representation of treatments used in bipartition plate experiments is shown in Fig. S1. The partition plates were labeled as NaCl, NaCl + X, NT2 and NT2 + X where X refers to either LS-044 or DMDS standard. Yeast cell suspension (OD600, 0.1) was prepared in 0.85% NaCl to be placed in the case compartment. Experiments were initiated by growing LS-044 on precast agar at the case1 compartment of the bipartition plate overnight (~ 16 h). Ten μl of DMDS taken in a 200 μl microfuge tube was placed in a compartment of case 2. The next day, 100 μl yeast suspension taken in 200 μl microfuge tube was placed in the opposite compartment of the case 1 and case 2 plates. Yeast cells (100 μl in 200 μl microfuge tube; OD600, 0.1) without LS-044/DMDS exposure were maintained as controls. Alternatively, yeast-free NaCl (100 μl in 200 μl microfuge tube) with and without exposure to LS-044/DMDS was used as respective blanks. The plates were sealed using cellotape and incubated at 37 °C under darkness for 72 h. Experiments were done in triplicate.
Alamar blue assay
After 72 h of incubation at the bipartition plate, 10% (v/v) AB dye was added to the culture suspension and incubated at 37 °C for three hours. The absorbance was measured at 600 and 570 nm. AB dye reduction (%) was quantified according to the manufacturer’s protocol (Cell-Quan AB Cell Viability Reagent; ABP Biosciences).
Phenol red assay
After 72 h of incubation at the bipartition plate, cultures were subjected to centrifugation and 100 μl of cell-free media were treated with 5 μl PR (0.5% w/v) indicator. Changes in acidity and alkalinity were read at 415 and 560 nm, respectively.
Viable cell count
After 72 h of incubation at the bipartition plate, viable yeast cells were quantified either by the conventional spread plate method or by spot assay on PDB. Colonies were manually counted after 24 h of incubation at 37 °C and the viable cell count was expressed as colony-forming unit (CFU) per ml.
Determination of minimum inhibitory concentration (MIC), fractional inhibitory concentration (FIC) and impact of DMDS on biofilm formation
Minimum inhibitory concentration (MIC) of DMDS on NT2 was determined by standard broth dilution antifungal susceptibility testing (M27-A2) as per Clinical and Laboratory Standard Institute (CLSI) guidelines50. Fractional inhibitory concentration (FIC) was determined as per earlier description51 using clotrimazole as an antifungal agent. NT2 cells were grown in a MicroPlate containing PDB for 24 h at 37 °C with an initial OD of 0.1 at 600 nm in the presence and absence of DMDS (0.5%) and planktonic growth was estimated. The impact of DMDS on the biofilm formation was assessed in the presence and absence of DMDS using microplate crystal violet staining52.
Epifluorescence cell counting and confocal microscopy
After 72 h of incubation at the bipartition plate, cells were stained with CFSE and CW while following the methods published elsewhere53 with minor modifications. One-hundred μl culture suspension was centrifuged, supernatants were removed and the pellets were mixed with 100 μl of 70% ethanol and incubated at room temperature for 15 min. Ethanol was removed by centrifugation (10,000 RPM, 10 min, 25 °C) and cell pellets were washed with 100 μl of phosphate buffered saline (PBS, pH 7.4), resuspended in 10 μl of PBS through the brief vortex, Ten μl CFSE stain (working stock: 4 ml PBS and 25 μg/ml CFSE) was added to the cell pellets and incubated for 10 min at room temperature. The suspension was centrifuged (10,000 RPM, 10 min, 25 °C) to harvest the cells. Next, 10 μl BSA (working stock: 10 ml PBS and 2% BSA) was added. In the same tube, add 10 μl CW stain (working stock: 10% calc. white sol.) and incubated for 5 min under RT. Excessive stains were removed by centrifugation (10,000 RPM, 10 min, 25 °C). Pellets were resuspended in 100 μl of PBS, and a 5 μl suspension was mounted on the slide for microscope observation under blue and green light excitations using fluorescence microscopy (Zoe) and confocal microscopy (Zeiss). Fluorescence measurements were done using the ImageJ analysis plugin of Fiji54,55 while randomly selecting minimal fifteen cells for the mean fluorescence.
Statistical analysis
Statistical significance was determined by one-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test or t-test using GraphPad Prism (version 8).
Sequence deposition
The GenBank/EMBL/DDBJ accession number for the 26S rRNA gene D1/D2 domain sequences of strain NT2 and NT5 are PQ846718 and PQ846721, respectively.
Results
Chromatographic evidence and genetic basis for DMDS emission in Burkholderia cepacia LS-044
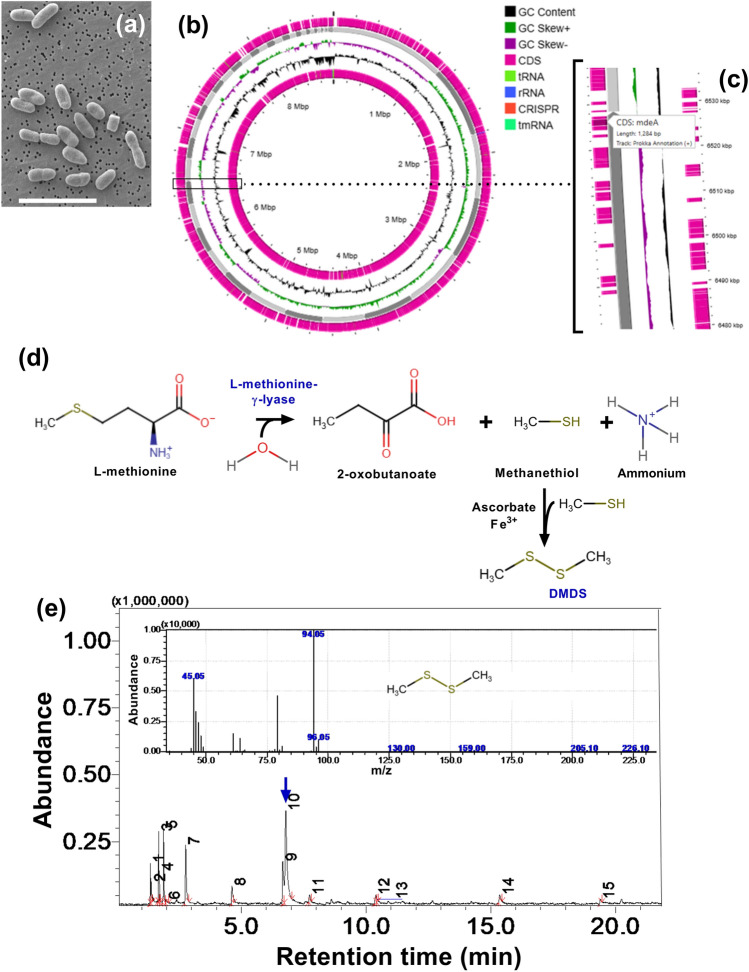
The morphological features and circular genome plot of LS-044 are shown in Fig. 1a and b, respectively. Genome mining of LS-044 at RAST revealed the presence of a gene (mdeA) encoding L-methionine-γ-lyase (Fig. 1c), catalyzing the first step of methionine degradation leading to methanethiol. As formed methanethiol can be transformed into DMDS in the presence of Fe3+ and/or the availability of ascorbate (Fig. 1d)8. The emission of volatile DMDS by LS-044 was substantiated by SPME GC–MS, where we found the elution of DMDS (~ 12% peak area) at 6.8 min as verified through the NIST10 database (Fig. 1e). The identification of mdeA explains the DMDS production in LS-044 as validated through SPME GC–MS.
Fig. 1.
Identification of dimethyl disulphide (DMDS) biosynthetic pathway and gas chromatographic evidence for the emission of DMDS in Burkholderia cepacia LS-044. Scanning electron microscopic view of LS-044 (a), circular plot of its genome (b), enlarged view of the circular genome showing localization of mdeA (c), which encodes L-methionine-γ-lyase involved in the methionine degradation (d); subsequent non-enzymatic reactions results in the formation of DMDS that emits from bacterial biomass as evidenced through SPME GC–MS (blue arrow) (e). The MS fingerprint of DMDS is shown in the inset.
Biochemical characterization and antifungal susceptibility testing of NT2
A study conducted on the Irish tertiary hospital using the VITEK 2 system revealed that the isolates of N. glabratus exhibit Inter-laboratory variability in terms of resistance to caspofungin MICs33. We investigated nutritional requirements and antibiotic resistance patterns in NT2 and NT5 using VITEK 2 fungal ID and VITEK 2 AST-YS08 kits due to the strains proximity to the clinical setting. In VITEK 2 fungal ID, NT2 and NT5 were positive for leucine arylamidase activity and for the assimilation of L-malate. D-glucose, d-mannose, D-trehalose, L-glutamate, acetate, L-proline and D-gluconate. While NT2 was positive for gamma-glutamyl transferase and assimilation of L-arabinose and DL-lactate, NT2 was positive for arginine gp. Both strains were negative for other reactions at VITEK 2 fungal ID. In VITEK 2 AST-YS08, NT2 and NT5 were resistant to caspofungin. The resistance level was graded by fluorescence-based automated AST system of VITEK under default mode. We found variability in terms of strains’ nutritional requirements within the species glabratus. Subsequent experiments were exclusively done on NT2 due to its high resistance to caspofungin as compared to NT5.
DMDS-producing LS-044 hampered metabolism and viable cell count in Nakaseomyces glabratus NT2
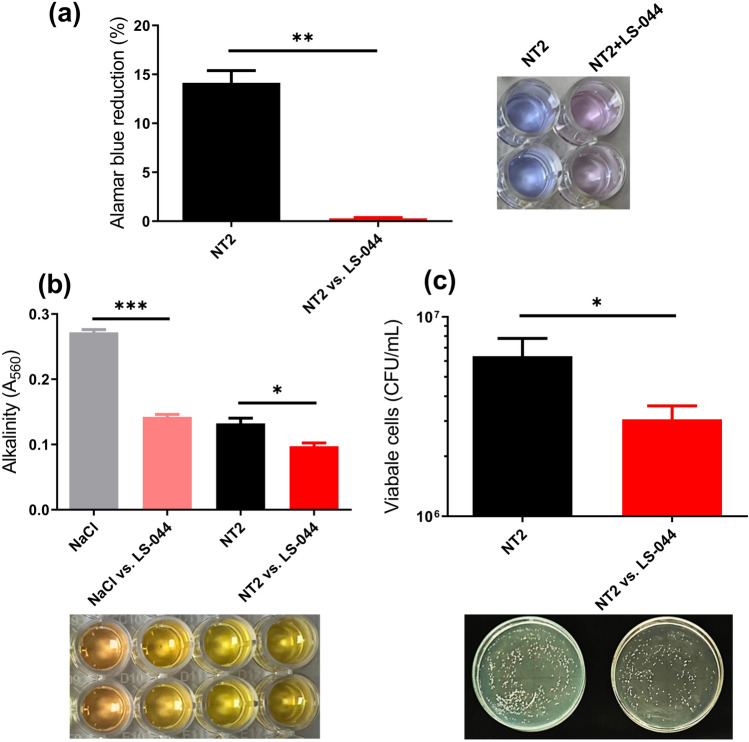
The influence of volatiles emitted from LS-044 was tested on the metabolism of N. glabratus NT2, change of media pH and cell viability using AB dye reduction, PR and CFU assays, respectively. We found ~ 98% decline (p = 0.0040) in the dye reduction in NT2 exposed to LS-044 in the bipartition plate (Fig. 2a). Interestingly, we observed a significant decline in media alkalinity (~ 48.0%, p < 0.0009) in blank NaCl exposed to LS-044 volatiles as compared to blank unexposed NaCl (Fig. 2b). Similarly, a significant decline in media alkalinity (~ 52.0%, p < 0.0009) was found in unexposed NT2 as compared to blank unexposed NaCl. Media alkalization further decreased significantly in the NT2 exposed to volatiles of LS-044 as compared to unexposed NT2 (~ 26.0%, p < 0.0330). We also found a significant decline in the viability of NT2 as a function of exposure to volatiles of LS-044 (~ 52%, p = 0.0209) (Fig. 2c). Thus, these data indicated direct anti-fungal impacts of volatiles emitted from LS-044 on N. glabratus NT2.
Fig. 2.
Bipartition plate assay results revealed the lethal impact of the volatiles of Burkholderia cepacia LS-044 on the budding yeast Nakaseomyces glabratus NT2. The impact of volatiles of LS-044 on the metabolic activity (a), alkalinity (b) and viable cell count of N. glabratus NT2 (c) are shown. Statistical significance (*p < 0.1, **p < 0.05, ***p < 0.01) was determined by t-test using GraphPad Prism, ns, non-significant.
Identification of DMDS as a fungicidal agent against Nakaseomyces glabratus NT2
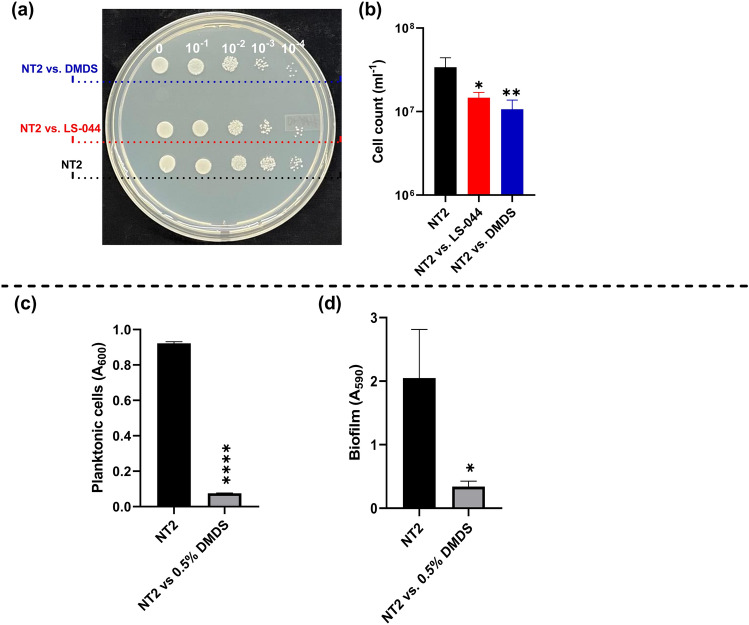
We speculated anti-budding impacts of LS-044 on NT2, possibly achieved through DMDS emission by the former. CFUs were estimated in parallel to see the changes in viable cell count while using unexposed NT2 as control. We observed a significant decline in CFUs of NT2 when exposed to LS-044 volatilome (56.9%, p < 0.031) or DMDS vapour (68.6%, p < 0.0181) as compared to unexposed NT2 as control (Fig. 3a‒b). In liquid broth dilution assay, DMDS exhibited a MIC of 0.5% (v/v) on NT2 cells, and displayed fractional inhibitory concentration index (FICI) of 0.95 with commercial antifungal clotrimazole (MIC: 0.01%, w/v) reflecting the lack of synergism or antagonism during the present combination therapy. The impacts of 0.5% DMDS on planktonic cells and biofilm formation are shown in Fig. 3c‒d, respectively.
Fig. 3.
Identification of DMDS as one of the bioactive volatiles of Burkholderia cepacia LS-044, suppressing growth and biofilm formation in Nakaseomyces glabratus NT2. A PDA plate showing the colonies of control NT2 without volatile exposure and NT2 pre-exposed to volatiles of LS-044 and standard DMDS vapour (a). The corresponding viable cells are shown in the bar chart (b). The optical cell density (c) and biofilm formation (d) when exposed to 0.5% DMDS in liquid cultures. Statistical significance (*p < 0.1 and **p < 0.05) in (b) was determined using One-way ANOVA. Statistical significance (*p < 0.05 and ****p < 0.0001) in (c) and (d) was determined using the t-test.
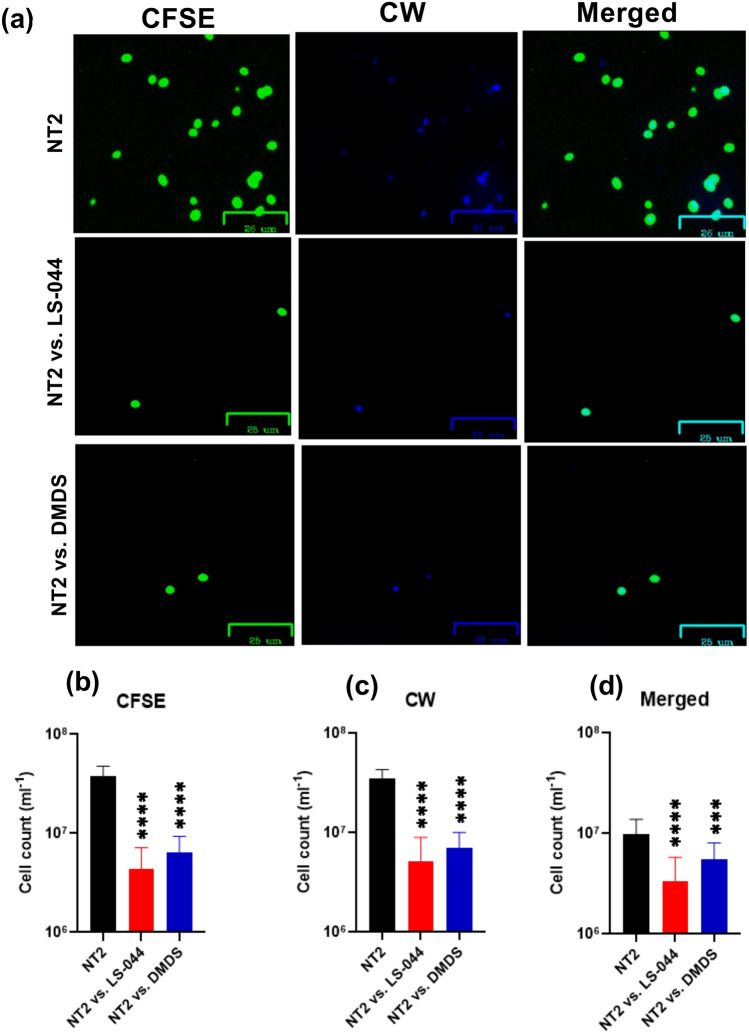
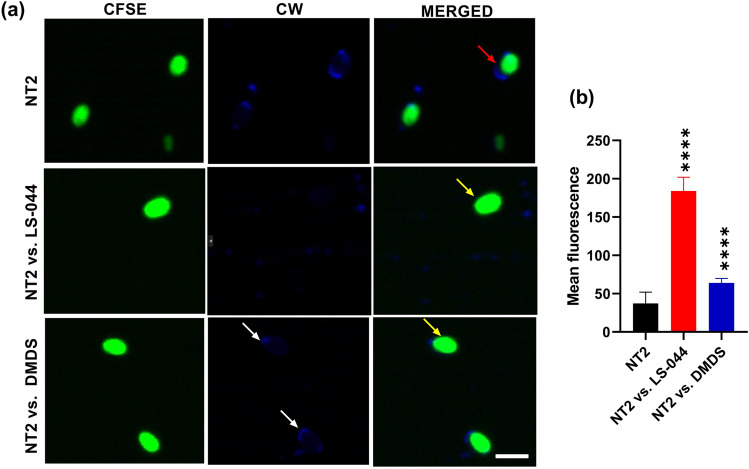
Epifluorescence microscopy was carried out to investigate the influence of DMDS on cell wall architecture using fluorescence dyes CFSE and CW. We found variations in terms of cellular affinity to take up CFSE/CW and identified a significant decline in NT2 cell number when exposed to the volatilome of LS-044 and DMDS vapour (Fig. 4a‒d). A significant decline in NT2 cell count that emits blue was recorded as a consequence of exposure to LS-044 volatiles (88.6%, p < 0.0001) and standard DMDS vapour (83.2%, p < 0.0001), as compared to unexposed NT2. Similarly, a significant decline in NT2 cell count that emits green was detected when exposed to LS-044 volatiles (85.3%, p < 0.0001) and standard DMDS vapour (79.6%, p < 0.0001), as compared to unexposed NT2. Based on the merged image analysis, we detected significantly high fluorescence in NT2 exposed to LS-044 volatiles (66.0%, p < 0.0001) and standard DMDS vapour (43.9%, p < 0.0002), as compared to unexposed NT2. These data indicated the potential ability of volatile DMDS to constrain the budding process of NT2, besides triggering the excessive deposition of cell wall peptidoglycans.
Fig. 4.
Epifluorescence microscopy revealing the susceptibility of Nakaseomyces glabratus NT2 to the volatilome of Burkholderia cepacia LS-044 and standard dimethyl disulphide (DMDS) vapour. Differential cell wall staining of control NT2 cells without exposure to bacterial volatile (uppermost panel) and NT2 exposed to the volatiles of LS-044 (middle panel) and the standard DMDS (lowermost panel) (a). Cells were labeled with carboxyfluorescein succinimidyl ester (CFSE) and calcofluor white (CW) and subjected to excitation at green (491 nm) and blue (405 nm) lasers, respectively. The images were captured with Zoe. Scale bar, 25 μm. The relative abundance of cells strained with CFSE (b), CW (c) and undergone dual straining (d) when exposed to the volatiles of LS-044 and DMDS standard as compared to control NT2 (without exposure to volatiles) are shown. Statistical significance was determined using One-way ANOVA; ***p < 0.01, ****p < 0.0001; ns, non-significant.
DMDS emission from LS-044 inhibits the budding process and decreases the surface area-to-volume ratio
We further carried out confocal microscopy to get deeper insights into structural changes that occurred at NT2 due to exposure to the volatiles of LS-044/DMDS. NT2 readily exhibited the signs of budding (red arrow) and absorbed less amounts of florescent dyes when grown without any volatile exposure (Fig. 5a). In contrast, we found intense fluorescence in NT2 pre-exposed to the volatiles of LS-044 and DMDS (yellow arrows) accounting for significant (p < 0.0001) ~ fivefold and 1.7-fold increase in the fluorescence of cells, respectively (Fig. 5b). The cell size was significantly larger in NT2 pre-exposed to volatiles of LS-044 or DMDS (~ 22%, p < 0.0024) when compared to unexposed NT2. Furthermore, fluorescence microscopy showed constrained budding in NT2 as a function of exposure to volatiles of LS-044 and DMDS (white arrow, scar). The surface area-to-volume ratio decreased significantly in NT2 cells exposed to volatiles of LS-044 and DMDS as compared to unexposed NT cells (6.5‒10.2 vs. 8.3‒13.2, p < 0.05). Taken together, data revealed the potential ability of DMDS to modulate cell wall architecture, decreases the surface area-to-volume ratio and cease budding in NT2.
Fig. 5.
Confocal microscopy revealing the impacts of the volatilome of Burkholderia cepacia LS-044 and standard dimethyl disulphide (DMDS) vapour on budding and cell wall peptidoglycans of Nakaseomyces glabratus NT2. Differential cellular morphology of control NT2 cells without exposure to bacterial volatiles (uppermost panel) and when exposed to the volatiles of LS-044 (middle panel) and the vapour of standard DMDS (lowermost panel) are shown (a). Cells were labeled with carboxyfluorescein succinimidyl ester (CFSE) and calcofluor white (CW) and subjected to excitation at green (491 nm) and blue (405 nm) lasers, respectively, and observed under a Zeiss confocal microscope. Budding cells, thickened luminous cell wall and scars are highlighted in red, yellow and white arrows, respectively. Scale bar, 5 μm. Differential fluorescence of yeast cells strained with CFSE when exposed to the volatiles of LS-044 and DMDS standard, as compared to control NT2 (without exposure to volatiles), is shown (b). Error bar, mean ± SD (n = 15). Statistical significance was determined using One-way ANOVA. ****p < 0.0001.
Nakaseomyces glabratus NT2 shares phylogenetic neighbourhood with both clinical and environmental isolates
We assessed the taxonomic position of NT2 and its phylogenetic neighbours using phylogenetic analysis based on the maximum likelihood algorithm. NT2 occupied a position in the clade that accommodated the type strain N. glabratus ATCC 2001 T, and various strains of Candida glabrata (misclassification) and N. glabratus isolated from various parts of the world (Fig. 6). The Nakaseomyces clade can be distinguished from the Candida clade in the maximum likelihood tree. We found strains originating from both environmental and clinical samples occupying the clade that accommodated NT2. Furthermore, we found that most of the strains share 100% sequence similarity with NT2. The phylogenetic analysis based on LSU of the 26S rRNA gene D1/D2 domain confirmed the NT2 as an authentic strain of N. glabratus and provided evidence for its widespread occurrence in environmental and clinical settings.
Fig. 6.
Unrooted maximum likelihood tree depicting the phylogenetic position of the strains of Nakaseomyces glabratus (NT2 and NT5, red triangles; accession No. PQ846718 and PQ846721, respectively) used in this study and related strains based on the Internal Transcribed Spacer sequencing targeted to the gene encoding larger subunit of ribosomal RNA (26S rRNA gene D1/D2 domain). Isolation source and/or origin of the isolates are indicated after the accession number. Bootstrap values of > 70% after 1,000 bootstrap replicates are shown at the branch points. Reference sequences were retrieved from GenBank under the accession numbers given in parentheses. Type strains are marked with strain number followed by ‘T’. Bar, 0.05 substitutions per site.
Discussion
In the present study, we explored the possible exploitation of bacterial VSCs as a fungicide to counter the representatives of N. glabratus, a recently established fungal taxon that accommodates misclassified Candida species, including C. glabrata56–58. The infection caused by N. glabratus is of global health concern since the incidence is increasing, both at the population level and as a proportion of all invasive yeast infections, and the increases appear related to the usage of fungicides59. Long-term persistence of acquired drug resistance and resistance-associated mutations were found in N. glabratus60. Strains of N. glabratus were found associated with candidiasis61,62, endocarditis63 and blood infection in humans34,64,65. The epidemiology, susceptibility and intra-species structural plasticity in N. glabratus have been well documented62,64,66,67. A study based on population genomics reveals high diversity, recombination and nosocomial transmission among N. glabratus causing invasive infections65. Distinguishing the representatives of Candida from Nakaseomyces is essential as the latter displays high prevalence and exhibits strong virulence features59. Internal Transcribed Spacer (ITS) sequencing and ascoaited phylogeny employed in this study authenticated the affiliation of NT2 and NT5 as the representatives of N. glabratus, prompting further detailed investigations against VSCs.
The biogenesis of VSCs such as hydrogen sulphide (H2S), methanethiol, dimethyl sulphide (DMS), DMDS and dimethyl trisulphide (DMTS) is reported in some bacteria8. DMDS emission has been found in Proteus68, Serratia marcescens, S. rubidea, Pseudomonas aeruginosa (strains #3 and #6), P. oryzihabitans, Pantoea eucrina, Bacillus oshimensis, Burkholderia gladioli, Burkholderia cepacia31, Burkholderia ambifaria H820, Burkholderia pyrrocinia CNUC919 and Massilia putida69. A study reported DMDS formation in Achromobacter, Pseudomonas acidovorans, P. alcaligenes, P. pseudoalcaligenes, P. testosteroni, Alcaligenes denitrificans subsp. xylosoxydans, A. denitrificans subsp. denitrificans, A. faecalis, and A. odorans70. In bacteria, the first pathway to generate VSCs involves demethiolation of L-methionine to yield methanethiol, ammonia, and 2-oxobutyrate mediated by L-methionine-γ-lyase71. The presence of the gene encoding L-methionine-γ-lyase corroborates the DMDS emission detected in LS-044. The emission of DMDS is most likely contributed to the broad-spectrum antifungal features and plant probiotic characteristics reported earlier in this strain37,46,49,72.
The bacterial DMDS production varies with species/strain and culture conditions31,49. The SPME-based analysis identified DMDS as a major VOC component (65%) produced by B. cepacia when cultivated on LB agar earlier31. We found 12% peak area assigned to DMDS in the volatilome of LS-044 when grown on Cruze’s basal agar, while exogenous amendments altered the amount of DMDS49. Earlier, studies demonstrated the efficacy of pure forms of volatile fungicides on Candida albicans73 and multicellular phytopathogenic fungus Sclerotinia minor32. This study is unique as it demonstrated the detrimental impacts of volatile DMDS emitted from bacteria on the metabolism and cell replication of N. glabratus.
Various mechanisms have been proposed to elucidate the antifungal attributes of VOCs. The membrane damage leading to the leakage of intracellular material and/or cell death was proposed to explain the fungicidal mechanism of VOCs32,74–76. VOCs such as eugenol, 2-methyl butanol and 3-methyl butanol alter the membrane permeability and disrupt the cell wall, leading to enhanced leakage of potassium ions and cellular materials76,77. DMDS-exposed Sclerotinia minor showed altered integrity of cell wall and membrane, leading to ~ 49.2% electrolyte leakage32. Based on our confocal microscopy data, we propose that the DMDS-driven decrease in cell surface area-to-volume ratio and excessive deposition of cell wall peptidoglycan could constrain the nutrient uptake and budding. A recent study showed binding attributes of surface-bound glyceraldehyde 3-phosphate dehydrogenase of N. glabratus to human vitronectin and plasminogen78. Further studies are needed to map the implications of DMDS-driven yeast cell surface modification on host–pathogen interactions.
Volatile compounds can also interfere with fungal metabolism: for example, guaiazulene exhibited a high binding affinity for malate synthase (−8.5 kcal/mol) and isocitrate lyase (−9.3 kcal/mol) and was found to interfere with the glyoxylate shunt in Candida albicans73. While some antibiotics are known to react with thiol groups and cause direct damage to proteins79, thiol- and disulphide-containing derivatives of vancomycin itself are found to be effective in countering microbial resistance and biofilm formation80. We found suppressed metabolic activity and variation in media pH in addition to constraining the budding, reflecting multiple biochemical and metabolic changes in NT2 as a consequence of DMDS exposure. The significance of DMDS-driven excessive deposition of cell wall peptidoglycan and the possible thiol group interference on the metabolic pathways merit further investigation.
The presence of DMDS has been reported in fruits and vegetables2,18,81,82. Emission of VSCs, including DMDS has been considered as a biomarker to trace the consumption of Allium vegetables in humans83,84. The antifungal attributes of DMDS discovered in this study suggest that consuming fruits and vegetables rich in DMDS could be a natural remedy to counter the drug-resistant N. glabratus involved in systemic and urogenital tract infections. On the other hand, the combination of antifungal therapy is predicted to be a general feature of echinocandins, a class of drugs found to be relatively mild in terms of their adverse effects as compared to other antibiotics on humans36. The FICI obtained for DMDS versus NT2 in the presence of clotrimazole, an azole class drug, reflects the indifference due to the lack of synergism or antagonism during the present combination therapy. A recent study showed the requirement of a sulphur compound (S-methyl thioacetate) to fully achieve antifungal effects of a live biotherapeutic microorganism Lactobacillus rhamnosus Lcr35 targeted to vaginal candidiasis85. Antifungal attributes listed in this study makes DMDS as an ideal candidate in the development of novel sulphur-based vaginal suppository to combat N. glabratus. Nonetheless, further studies are warranted to determine the efficacy of utilizing DMDS either singly or in association with echinocandins to develop effective therapeutic formulations.
Conclusion
The present study detected a key gene (mdeA) encoding L-methionine-γ-lyase, involved in the methionine catabolic pathway in LS-044, governing DMDS emission. The bipartition plate assays identified DMDS as an antagonistic molecule of LS-044 that suppressed N. glabratus. DMDS constrained the metabolic activity and budding process in N. glabratus through disruption of cell wall architecture and diminishing the cell surface area-to-volume ratio. Internal Transcribed Spacer (ITS) sequencing and phylogenetic analysis substantiated NT2 and NT5 as authentic representatives of N. glabratus and provided evidence for their widespread occurrence of the present taxon. The susceptibility of N. glabratus to microbial DMDS is significant since the yeast strains co-exist with bacteria and share both environmental and clinical niches. Further studies are needed to understand the contribution of DMDS originating from diet in combating the drug-resistant N. glabratus involved in systemic and urogenital tract infections.
Supplementary Information
Acknowledgements
Authors would like to thank Dr. Rajesh P. Shastry and Dr. Shamprasad Varijaraghu for helping us in ITS sequencing and confocal microscopy, respectively.
Abbreviations
- AB
Alamar blue
- ANOVA
One-way analysis of variance
- B. cepacia
Burkholderia cepacia
- Bcc
Burkholderia cepacia complex
- BLAST
Basic local alignment search tool
- CFSE
Carboxyfluorescein succinimidyl ester
- CFU
Colony-forming unit
- CLSI
Clinical and laboratory standards institute
- CW
Calcofluor white
- DMDS
Dimethyl disulphide
- DMS
Dimethyl sulphide
- DMTS
Dimethyl trisulphide
- FIC
Fractional inhibitory concentration
- FICI
Fractional inhibitory concentration index
- GC
Gas chromatography
- GC–MS
Gas chromatography-mass spectrometry
- gDNA
Genomic DNA
- H2S
Hydrogen sulphide
- HCl
Hydrochloric acid
- HPLC
High-performance liquid chromatography
- ITS
Internal transcribed spacer
- LS-044
B. Cepacia LS-044
- LSU
Large subunit
- MEGA 5
Molecular evolutionary genetics analysis, version 5.0
- MIC
Minimum inhibitory concentration
- N. glabratus
Nakaseomyces glabratus
- NaCl
Sodium chloride
- NCBI
National centre for biotechnology information
- PBS
Phosphate buffered saline
- PCR
Polymerase chain reaction
- PDA
Potato dextrose agar
- PDB
Potato dextrose broth
- PR
Phenol red
- RAST
Rapid annotation using subsystem technology
- RT
Retention time
- SDA
Sabouraud dextrose agar
- SDG
Sustainable development goals
- SPME
Solid-phase micro extraction
- VOCs
Volatile organic compounds
- VSCs
Volatile sulphurous compounds
- 26S rRNA
26S subunit ribosomal RNA
Author contributions
Nishmat Abdul Kadar Rahmath: data curation, formal analysis, investigation, methodology, validation, visualization and writing-original draft, Shih-Yao Lin: data curation, formal analysis, writing-review and editing, Chiu-Chung Young: formal analysis, resources, software and writing-review and editing, Asif Hameed: conceptualization, funding acquisition, project administration, supervision and writing-review and editing. All the authors discussed the results and revised the manuscript.
Funding
AH would like to thank Yenepoya (Deemed to be University) for the Seed Grant (YU/Seed grant/139–2023). NAKR acknowledges Junior Research Fellowship from Yenepoya (Deemed to be University).
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author (Dr. Asif Hameed) on reasonable request.
Declaration
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chiu-Chung Young, Email: ccyoung@mail.nchu.edu.tw.
Asif Hameed, Email: asifhameed@yenepoya.edu.in.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-05383-5.
References
- 1.European Food Safety, A. et al. Peer review of the pesticide risk assessment of the active substance dimethyl disulfide. EFSA J17 e05905 10.2903/j.efsa.2019.5905 (2019). [DOI] [PMC free article] [PubMed]
- 2.Fang, N. et al. Dissipation and residues of dimethyl disulfide in tomatoes and soil under greenhouse and open field conditions. J. Environ. Sci. Health B.55, 566–573. 10.1080/03601234.2020.1740531 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Yan, D. et al. Dimethyl disulfide (DMDS) as an effective soil fumigant against nematodes in China. PLoS ONE14, e0224456. 10.1371/journal.pone.0224456 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu, J., Land, C. J., Vallad, G. E. & Boyd, N. S. Tomato tolerance and pest control following fumigation with different ratios of dimethyl disulfide and chloropicrin. Pest. Manag. Sci.75, 1416–1424. 10.1002/ps.5262 (2019). [DOI] [PubMed] [Google Scholar]
- 5.Yu, J., Sharpe, S. M., Vallad, G. E. & Boyd, N. S. Pest control with drip-applied dimethyl disulfide and chloropicrin in plastic-mulched tomato (Solanum lycopersicum L.). Pest Manag. Sci.76, 1569–1577. 10.1002/ps.5678 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Bhadra, S. et al. Analysis of the soil fumigant, dimethyl disulfide, in swine blood by dynamic headspace gas chromatography-mass spectroscopy. J. Chromatogr. A1638, 461856. 10.1016/j.chroma.2020.461856 (2021). [DOI] [PubMed] [Google Scholar]
- 7.Du, Q. et al. Photochemical production of carbonyl sulfide, carbon disulfide and dimethyl sulfide in a lake water. J. Environ. Sci. (China)51, 146–156. 10.1016/j.jes.2016.08.006 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Schulz, S. & Dickschat, J. S. Bacterial volatiles: The smell of small organisms. Nat. Prod. Rep.24, 814–842. 10.1039/b507392h (2007). [DOI] [PubMed] [Google Scholar]
- 9.Wu, J. et al. Co-regulating the pore structure and surface chemistry of sludge-based biochar for high-performance deodorization of gaseous dimethyl disulfide. Chemosphere364, 142992. 10.1016/j.chemosphere.2024.142992 (2024). [DOI] [PubMed] [Google Scholar]
- 10.Zhao, J. et al. Superior dimethyl disulfide degradation in a microbial fuel cell: Extracellular electron transfer and hybrid metabolism pathways. Environ. Pollut.315, 120469. 10.1016/j.envpol.2022.120469 (2022). [DOI] [PubMed] [Google Scholar]
- 11.Li, W., Han, Z. & Sun, D. Preparation of sludge-based activated carbon for adsorption of dimethyl sulfide and dimethyl disulfide during sludge aerobic composting. Chemosphere279, 130924. 10.1016/j.chemosphere.2021.130924 (2021). [DOI] [PubMed] [Google Scholar]
- 12.Wan, S., Li, G., An, T. & Guo, B. Co-treatment of single, binary and ternary mixture gas of ethanethiol, dimethyl disulfide and thioanisole in a biotrickling filter seeded with Lysinibacillus sphaericus RG-1. J. Hazard Mater.186, 1050–1057. 10.1016/j.jhazmat.2010.11.099 (2011). [DOI] [PubMed] [Google Scholar]
- 13.Reichert, K., Lipski, A., Pradella, S., Stackebrandt, E. & Altendorf, K. Pseudonocardia asaccharolytica sp. nov. and Pseudonocardia sulfidoxydans sp. nov., two new dimethyl disulfide-degrading actinomycetes and emended description of the genus. Int. J. Syst. Bacteriol.48(2), 441–449. 10.1099/00207713-48-2-441 (1998). [DOI] [PubMed] [Google Scholar]
- 14.Kristiansen, A. et al. Butyric acid- and dimethyl disulfide-assimilating microorganisms in a biofilter treating air emissions from a livestock facility. Appl. Environ. Microbiol77, 8595–8604. 10.1128/AEM.06175-11 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanagawa, T. & Mikami, E. Removal of methanethiol, dimethyl sulfide, dimethyl disulfide, and hydrogen sulfide from contaminated air by Thiobacillus thioparus TK-m. Appl. Environ. Microbiol55, 555–558. 10.1128/aem.55.3.555-558.1989 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arellano-Garcia, L., Gonzalez-Sanchez, A., Van Langenhove, H., Kumar, A. & Revah, S. Removal of odorant dimethyl disulfide under alkaline and neutral conditions in biotrickling filters. Water Sci. Technol.66, 1641–1646. 10.2166/wst.2012.365 (2012). [DOI] [PubMed] [Google Scholar]
- 17.Chen, X., Liang, Z., An, T. & Li, G. Comparative elimination of dimethyl disulfide by maifanite and ceramic-packed biotrickling filters and their response to microbial community. Bioresour Technol.202, 76–83. 10.1016/j.biortech.2015.11.081 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Liang, Z., An, T., Li, G. & Zhang, Z. Aerobic biodegradation of odorous dimethyl disulfide in aqueous medium by isolated Bacillus cereus GIGAN2 and identification of transformation intermediates. Bioresour Technol.175, 563–568. 10.1016/j.biortech.2014.11.002 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Luo, H., Riu, M., Ryu, C. M. & Yu, J. M. Volatile organic compounds emitted by Burkholderia pyrrocinia CNUC9 trigger induced systemic salt tolerance in Arabidopsis thaliana. Front. Microbiol13, 1050901. 10.3389/fmicb.2022.1050901 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen, X. et al. Burkholderia ambifaria H8 as an effective biocontrol strain against maize stalk rot via producing volatile dimethyl disulfide. Pest Manag. Sci.80, 4125–4136. 10.1002/ps.8119 (2024). [DOI] [PubMed] [Google Scholar]
- 21.Huang, C. J. et al. Dimethyl disulfide is an induced systemic resistance elicitor produced by Bacillus cereus C1L. Pest Manag. Sci.68, 1306–1310. 10.1002/ps.3301 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Susman, J. L., Hornig, J. F., Thomae, S. C. & Smith, R. P. Pulmonary excretion of hydrogen sulfide, methanethiol, dimethyl sulfide and dimethyl disulfide in mice. Drug Chem. Toxicol.1, 327–338. 10.3109/01480547809016045 (1978). [DOI] [PubMed] [Google Scholar]
- 23.Wang, Z., Sun, M. & Wang, C. Detection of melanoma cancer biomarker dimethyl disulfide using cavity ringdown spectroscopy at 266 nm. Appl. Spectrosc70, 1080–1085. 10.1177/0003702816641575 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Kwak, J. et al. Volatile biomarkers from human melanoma cells. J. Chromatogr. B. Analyt. Technol. Biomed Life Sci.931, 90–96. 10.1016/j.jchromb.2013.05.007 (2013). [DOI] [PubMed] [Google Scholar]
- 25.Ling, S. et al. Volatile dimethyl disulfide from guava plants regulate developmental performance of asian citrus psyllid through activation of defense responses in neighboring orange plants. Int. J. Mol. Sci.10.3390/ijms231810271 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han, C. et al. Vasodilation effect of volatile oil from allium macrostemon bunge are mediated by PKA/NO pathway and its constituent dimethyl disulfide in isolated rat pulmonary arterials. Fitoterapia120, 52–57. 10.1016/j.fitote.2017.05.007 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Essawy, A. E., Gaaboub, I. A., Abdel-Moneim, A. M. & El-Sayed, S. A. Neuropathological effect of dimethyl disulfide on neurons of the desert locust Schistocerca gregaria. Toxicol. Ind. Health31, 422–428. 10.1177/0748233713475525 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Dugravot, S. et al. Dimethyl disulfide exerts insecticidal neurotoxicity through mitochondrial dysfunction and activation of insect K(ATP) channels. J. Neurophysiol.90, 259–270. 10.1152/jn.01096.2002 (2003). [DOI] [PubMed] [Google Scholar]
- 29.Macrides, F., Johnson, P. A. & Schneider, S. P. Responses of the male golden hamster to vaginal secretion and dimethyl disulfide: Attraction versus sexual behavior. Behav. Biol.20, 377–386. 10.1016/s0091-6773(77)90931-2 (1977). [DOI] [PubMed] [Google Scholar]
- 30.Singer, A. G. et al. Dimethyl disulfide: An attractant pheromone in hamster vaginal secretion. Science191, 948–950. 10.1126/science.1251205 (1976). [DOI] [PubMed] [Google Scholar]
- 31.Diyapoglu, A. et al. Fumigant activity of bacterial volatile organic compounds against the nematodes Caenorhabditis elegans and Meloidogyne incognita. Molecules10.3390/molecules27154714 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tyagi, S., Lee, K. J., Shukla, P. & Chae, J. C. Dimethyl disulfide exerts antifungal activity against Sclerotinia minor by damaging its membrane and induces systemic resistance in host plants. Sci. Rep.10, 6547. 10.1038/s41598-020-63382-0 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ali, S., Collison, M., McNicholas, S. & McDermott, S. Inter-laboratory variability of caspofungin MICs for Nakaseomyces glabrata isolates - an Irish tertiary hospital experience. Access Microbiol.10.1099/acmi.0.000617.v4 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopez-Marmolejo, A. L. et al. Microevolution of Candida glabrata (Nakaseomyces glabrata) during an infection. Fungal Genet. Biol.172, 103891. 10.1016/j.fgb.2024.103891 (2024). [DOI] [PubMed] [Google Scholar]
- 35.Grover, N. D. Echinocandins: A ray of hope in antifungal drug therapy. Indian J. Pharmacol.42, 9–11. 10.4103/0253-7613.62396 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Denning, D. W. Echinocandin antifungal drugs. Lancet362, 1142–1151. 10.1016/S0140-6736(03)14472-8 (2003). [DOI] [PubMed] [Google Scholar]
- 37.Hameed, A. et al. Diversity and functional characterization of bacterial endophytes dwelling in various rice (Oryza sativa L.) tissues, and their seed-borne dissemination into rhizosphere under gnotobiotic P-stress. Plant Soil394, 177–197. 10.1007/s11104-015-2506-5 (2015). [Google Scholar]
- 38.Kurtzman, C. P. & Robnett, C. J. Identification of clinically important ascomycetous yeasts based on nucleotide divergence in the 5’ end of the large-subunit (26S) ribosomal DNA gene. J. Clin Microbiol.35, 1216–1223. 10.1128/jcm.35.5.1216-1223.1997 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakayan, P. et al. Phosphate-solubilizing soil yeast CC1 improves maize (Zea mays L.) productivity and minimizes requisite chemical fertilization. Plant Soil373, 301–315. 10.1007/s11104-013-1792-z (2013). [Google Scholar]
- 40.Huang, X. & Madan, A. CAP3: A DNA sequence assembly program. Genome Res.9, 868–877. 10.1101/gr.9.9.868 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tamura, K. et al. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol.28, 2731–2739. 10.1093/molbev/msr121 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thompson, J. D., Gibson, T. J., Plewniak, F., Jeanmougin, F. & Higgins, D. G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res.25, 4876–4882. 10.1093/nar/25.24.4876 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol.16, 111–120. 10.1007/BF01731581 (1980). [DOI] [PubMed] [Google Scholar]
- 44.Felsenstein, J. Evolutionary trees from DNA sequences: a maximum likelihood approach. J. Mol. Evol.17, 368–376. 10.1007/BF01734359 (1981). [DOI] [PubMed] [Google Scholar]
- 45.Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution39, 783–791. 10.1111/j.1558-5646.1985.tb00420.x (1985). [DOI] [PubMed] [Google Scholar]
- 46.Hameed, A. et al. Draft genome sequence reveals co-occurrence of multiple antimicrobial resistance and plant probiotic traits in rice root endophytic strain Burkholderia sp. LS-044 affiliated to Burkholderia cepacia complex. J. Glob. Antimicrob Resist20(28), 30. 10.1016/j.jgar.2019.11.017 (2020). [DOI] [PubMed] [Google Scholar]
- 47.Aziz, R. K. et al. The RAST Server: rapid annotations using subsystems technology. BMC Genomics9, 75. 10.1186/1471-2164-9-75 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grant, J. R. et al. Proksee: In-depth characterization and visualization of bacterial genomes. Nucleic Acids Res..51, W484–W492. 10.1093/nar/gkad326 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hameed, A. et al. Hormesis of glyphosate on ferulic acid metabolism and antifungal volatile production in rice root biocontrol endophyte Burkholderia cepacia LS-044. Chemosphere345, 140511. 10.1016/j.chemosphere.2023.140511 (2023). [DOI] [PubMed] [Google Scholar]
- 50.CLSI. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard-third edition; CLSI document M27-A3. Clinical and Laboratory Standards Institute, Wayne. (2008).
- 51.Fatsis-Kavalopoulos, N., Sanchez-Hevia, D. L. & Andersson, D. I. Beyond the FIC index: the extended information from fractional inhibitory concentrations (FICs). J. Antimicrob Chemother.79, 2394–2396. 10.1093/jac/dkae233 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stepanovic, S., Vukovic, D., Dakic, I., Savic, B. & Svabic-Vlahovic, M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods.40, 175–179. 10.1016/s0167-7012(00)00122-6 (2000). [DOI] [PubMed] [Google Scholar]
- 53.Dagher, Z. et al. Fluorescent tracking of yeast division clarifies the essential role of spleen tyrosine kinase in the intracellular control of Candida glabrata in macrophages. Front .Immunol.9, 1058. 10.3389/fimmu.2018.01058 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods9, 676–682. 10.1038/nmeth.2019 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat Methods9, 671–675. 10.1038/nmeth.2089 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kurtzman, C. P. Phylogenetic circumscription of Saccharomyces, Kluyveromyces and other members of the Saccharomycetaceae, and the proposal of the new genera Lachancea, Nakaseomyces, Naumovia vanderwaltozyma and Zygotorulaspora. FEMS Yeast Res4, 233–245. 10.1016/S1567-1356(03)00175-2 (2003). [DOI] [PubMed] [Google Scholar]
- 57.Borman, A. M. & Johnson, E. M. Name changes for fungi of medical importance, 2018 to 2019. J. Clin. Microbiol.10.1128/JCM.01811-20 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takashima, M. & Sugita, T. Taxonomy of pathogenic yeasts Candida, Cryptococcus, Malassezia, and Trichosporon. Med. Mycol. J.63, 119–132. 10.3314/mmj.22.004 (2022). [DOI] [PubMed] [Google Scholar]
- 59.Beardsley, J. et al. Candida glabrata (Nakaseomyces glabrata): A systematic review of clinical and microbiological data from 2011 to 2021 to inform the World Health organization fungal priority pathogens list. Med. Mycol.10.1093/mmy/myae041 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ksiezopolska, E., Schikora-Tamarit, M. A., Carlos Nunez-Rodriguez, J. & Gabaldon, T. Long-term stability of acquired drug resistance and resistance associated mutations in the fungal pathogen Nakaseomyces glabratus (Candida glabrata). Front. Cell Infect Microbiol.14, 1416509. 10.3389/fcimb.2024.1416509 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Okamoto, M. et al. In Candida glabrata, ERMES component GEM1 controls mitochondrial morphology, mtROS, and drug efflux pump expression resulting in azole susceptibility. J. Fungi. (Basel)10.3390/jof9020240 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Salimi, M. et al. Molecular epidemiology and antifungal susceptibility profile in nakaseomyces glabrata species complex: A 5-Year Countrywide Study. J. Clin. Lab. Anal38, e25042. 10.1002/jcla.25042 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jim, K. K., Daems, J. J. N., Boekholdt, S. M. & van Dijk, K. Nakaseomyces glabrata endocarditis: A therapeutic dilemma. Med. Mycol. Case Rep.40, 54–57. 10.1016/j.mmcr.2023.04.002 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Naicker, S. D. et al. Epidemiology and susceptibility of Nakaseomyces (formerly Candida) glabrata bloodstream isolates from hospitalised adults in South Africa. Med. Mycol.10.1093/mmy/myad057 (2023). [DOI] [PubMed] [Google Scholar]
- 65.Wang, Y. et al. Population genomic analyses reveal high diversity, recombination and nosocomial transmission among Candida glabrata (Nakaseomyces glabrata) isolates causing invasive infections. Microb. Genom.10.1099/mgen.0.001179 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Angoulvant, A., Guitard, J. & Hennequin, C. Old and new pathogenic Nakaseomyces species: Epidemiology, biology, identification, pathogenicity and antifungal resistance. FEMS Yeast Res.10.1093/femsyr/fov114 (2016). [DOI] [PubMed] [Google Scholar]
- 67.Stefanini, I. et al. Genomic assembly of clinical Candida glabrata (Nakaseomyces glabrata) isolates reveals within-species structural plasticity and association with in vitro antifungal susceptibility. Microbiol Spectr10, e0182722. 10.1128/spectrum.01827-22 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hayward, N. J., Jeavons, T. H., Nicholson, A. J. & Thornton, A. G. Methyl mercaptan and dimethyl disulfide production from methionine by Proteus species detected by head-space gas-liquid chromatography. J. Clin. Microbiol6, 187–194. 10.1128/jcm.6.3.187-194.1977 (1977). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Feng, G. D., Yang, S. Z., Li, H. P. & Zhu, H. H. Massilia putida sp. nov., a dimethyl disulfide-producing bacterium isolated from wolfram mine tailing. Int. J. Syst. Evol. Microbiol.66, 50–55. 10.1099/ijsem.0.000670 (2016). [DOI] [PubMed] [Google Scholar]
- 70.Tomita, B. et al. Identification of dimethyl disulfide-forming bacteria isolated from activated sludge. Appl. Environ. Microbiol53, 1541–1547. 10.1128/aem.53.7.1541-1547.1987 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arfi, K., Amarita, F., Spinnler, H. E. & Bonnarme, P. Catabolism of volatile sulfur compounds precursors by Brevibacterium linens and Geotrichum candidum, two microorganisms of the cheese ecosystem. J. Biotechnol.105, 245–253. 10.1016/j.jbiotec.2003.07.003 (2003). [DOI] [PubMed] [Google Scholar]
- 72.Hameed, A. et al. Bacteriostatic stimulus of meropenem on allelochemical-metabolizing Burkholderia sp. LS-044 mitigates ferulic acid autotoxicity in rice (Oryza sativa ssp. japonica cv. Tainung 71). Plant Soil443, 73–86. 10.1007/s11104-019-04195-7 (2019). [Google Scholar]
- 73.Shastry, R. P. et al. Guaiazulene and aureusidin inhibit metabolic adaptability and virulence in by targeting the glyoxylate cycle under alternative carbon conditions. Green Chem. Lett. Rev.10.1080/17518253.2024.2438073 (2024). [Google Scholar]
- 74.Giorgio, A., De Stradis, A., Lo Cantore, P. & Iacobellis, N. S. Biocide effects of volatile organic compounds produced by potential biocontrol rhizobacteria on Sclerotinia sclerotiorum. Front. Microbiol.10.3389/fmicb.2015.01056 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hutchings, M. L., Alpha-Cobb, C. J., Hiller, D. A., Berro, J. & Strobel, S. A. Mycofumigation through production of the volatile DNA-methylating agent -methyl–nitrosoisobutyramide by fungi in the genus. J Biol Chem292, 7358–7371. 10.1074/jbc.M117.779009 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Luo, J. J. et al. Antifungal activity of isoliquiritin and its inhibitory effect against chen through a membrane damage mechanism. Molecules10.3390/molecules21020237 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.OuYang, Q. L., Tao, N. G. & Zhang, M. L. A damaged oxidative phosphorylation mechanism is involved in the antifungal activity of citral against Penicilium digitatum. Front. Microbiol.10.3389/fmicb.2018.00239 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bednarek, A. et al. Glyceraldehyde 3-phosphate dehydrogenase on the surface of Candida albicans and Nakaseomyces glabratus Cells-A moonlighting protein that binds human vitronectin and plasminogen and can adsorb to pathogenic fungal cells via major Adhesins Als3 and Epa6. Int J Mol Sci10.3390/ijms25021013 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Allas, Ü. et al. Antibacterial activity of the nitrovinylfuran G1 (Furvina) and its conversion products. Sci Rep-Uk6, 10.1038/srep36844 (2016). [DOI] [PMC free article] [PubMed]
- 80.Shchelik, I. S. & Gademann, K. Thiol- and disulfide-containing vancomycin derivatives against bacterial resistance and biofilm formation. ACS Med. Chem. Lett.12, 1898–1904. 10.1021/acsmedchemlett.1c00455 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kaium, A. et al. Method Validation and dissipation behaviour of dimethyl disulphide (DMDS) in cucumber and soil by gas chromatography-tandem mass spectrometry. Int. J. Enviro.n Re.s Public Health10.3390/ijerph16224493 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Park, S., Kim, H. W., Lee, C. J., Kim, Y. & Sung, J. Profiles of volatile sulfur compounds in various vegetables consumed in Korea using HS-SPME-GC/MS technique. Front. Nutr.10.3389/fnut.2024.1409008 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pratico, G., Gao, Q., Manach, C. & Dragsted, L. O. Biomarkers of food intake for Allium vegetables. Genes Nutr13, 34. 10.1186/s12263-018-0624-4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sato, S., Sekine, Y., Kakumu, Y. & Hiramoto, T. Measurement of diallyl disulfide and allyl methyl sulfide emanating from human skin surface and influence of ingestion of grilled garlic. Sci .Rep.-Uk10.1038/s41598-019-57258-1 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dausset, C. et al. Identification of sulfur components enhancing the anti-Candida effect of Lactobacillus rhamnosus Lcr35. Sci. Rep.10, 17074. 10.1038/s41598-020-74027-7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author (Dr. Asif Hameed) on reasonable request.