Abstract
Purpose
Cardiovascular-kidney-metabolic (CKM) syndrome is a newly recognized multisystem disorder, with over 90% of cases occurring in the early stages (0–3). However, there is a lack of evidence for dietary interventions during this phase. This study is the first to examine the connection between the composite dietary antioxidant index (CDAI) and mortality risk in early-stage CKM patients, aiming to support early intervention strategies based on nutrition.
Methods
We analyzed data from 7,642 CKM 0–3 stage patients from the NHANES 2007–2018 cohort. Multivariable weighted Cox regression was used to assess the association between CDAI and all-cause/cardiovascular disease (CVD) mortality. A restricted cubic spline was applied to explore dose-response relationships, with subgroup analyses to explore population heterogeneity.
Results
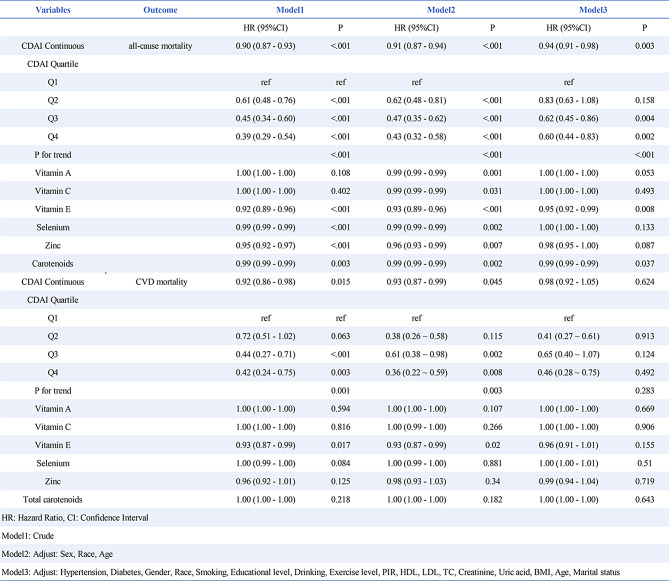
During a median follow-up of 94 months, 594 all-cause deaths and 151 CVD deaths were recorded. Each unit increase in CDAI was substantially linked to a 6% decrease in all-cause mortality after controlling for covariates (HR = 0.94, 95% CI 0.91–0.98). Mortality risk was 40% lower in the highest CDAI quartile than in the lowest quartile (HR = 0.60, 95% CI 0.44–0.83). Dose-response analysis revealed a linear decreasing trend (p < 0.001). Subgroup analyses further supported these findings. Carotenoids and vitamin E were also substantially linked to a decreased all-cause mortality rate in CKM patients. For CVD mortality, a significant association was observed in unadjusted models (HR = 0.92, 95% CI 0.86–0.98, P < 0.05), but no significant association was found between CDAI and CVD mortality in the fully adjusted model (HR = 0.98, 95% CI 0.92–1.05, P = 0.62), suggesting potential mediation by covariates such as comorbidities and lifestyle factors.
Conclusion
Enhanced dietary antioxidant capacity significantly reduces all-cause mortality risk in early-stage CKM patients (stages 0–3), with vitamin E and carotenoids potentially playing key protective roles. These findings provide dose-response evidence for early nutritional interventions in CKM syndrome, suggesting that multi-nutrient strategies targeting oxidative stress pathways may offer new directions for prevention and management.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12986-025-00974-5.
Keywords: CDAI, CKM, Diet, NHANES
Introduction
In 2023, the American Heart Association (AHA) presented Cardiovascular-Kidney-Metabolic (CKM) Syndrome as a newly recognized clinical syndrome [1]. By combining the pathophysiological relationships among metabolic dysfunction, CKD, and CVD, it offers a new approach for the holistic prevention and treatment of related conditions [1]. The incidence of CKM is increasing in tandem with the growing frequency of CKD and metabolic syndrome. Epidemiological data indicate that approximately 44% of U.S. adolescents are in CKM stages 1–2, which is associated with food insecurity and nutrient deficiencies [2]. Crucially, the risk across CKM stages is strongly linked to cardiovascular mortality [3], underscoring the need for early intervention. Key drivers include glucose abnormalities, dyslipidemia, CKD, and abdominal obesity [4–6]. The interplay of these factors drives metabolic disturbances, chronic inflammation, oxidative stress, and neuroendocrine dysregulation, central to CKM pathophysiology and its progression to target organ damage (heart, kidneys) [7, 8]. For effective management, CKM is staged (0–4), emphasizing early identification and intervention to halt progression [9]. During 2011–2020, approximately 90.8% of U.S. adults met criteria for stages 0–3 [10]. The AHA has emphasized the importance of risk prediction and management during this early stage, recommending preventive strategies to avert cardiovascular events [1].
One of the main mechanisms in the pathophysiology of CKM, oxidative stress, aggravates heart, renal, and metabolic dysfunction by causing cellular damage and inflammation [8, 11–13]. Modifiable nutritional factors, particularly dietary antioxidants, play a crucial role in mitigating oxidative stress [14]. There is substantial epidemiological data linking dietary antioxidant intake to a lower chance of dying from chronic diseases [15, 16]. Vitamins C and E, β-carotene, and various trace elements, which are dietary antioxidants, effectively counteract free radicals, reduce oxidative damage, and maintain immune function, offering potential protective effects against cardiovascular diseases, metabolic syndrome, CKD, and other chronic conditions [17–22]. Research involving prospective cohorts has revealed a strong inverse link between the consumption of flavonoids and mortality due to cardiovascular issues [23], whereas vitamin C supplements have been related to increased cognitive function and better prognoses in individuals with cardiovascular issues [24, 25].
The Composite Dietary Antioxidant Index (CDAI) serves as a comprehensive measure of overall antioxidant intake, reflecting both the antioxidant status and dietary quality. It offers a distinct advantage over indices focused on single nutrients or other dietary patterns by specifically quantifying the cumulative antioxidant potential from multiple dietary sources, which is particularly relevant given the synergistic actions of antioxidants. Previous studies have associated CDAI with numerous health outcomes, highlighting its importance in preventing chronic diseases [26–28]. However, its association with mortality risk in early-stage CKM patients remains unexplored. While some research examines antioxidant-rich diets in high-risk groups [29, 30], there is still a lack of research examining CKM patients at different stages.
Investigating the impact of dietary antioxidants (via CDAI) on prognosis in early-stage CKM, characterized by systemic dysregulation involving oxidative stress and chronic inflammation, can enhance pathophysiological understanding and inform prevention strategies [9]. Given the established link between CKM stage and cardiovascular mortality [3], and the critical public health importance of fatal outcomes, we focused on mortality as the primary endpoint. Therefore, this study systematically assesses the association between CDAI and both all-cause and CVD mortality in CKM stages 0–3 patients using the NHANES 2007–2018 dataset. It further explores heterogeneity across different genders, races, and socioeconomic backgrounds, aiming to provide scientific evidence and practical guidance for dietary interventions in the early management of CKM syndrome.
Methods
Study design and population
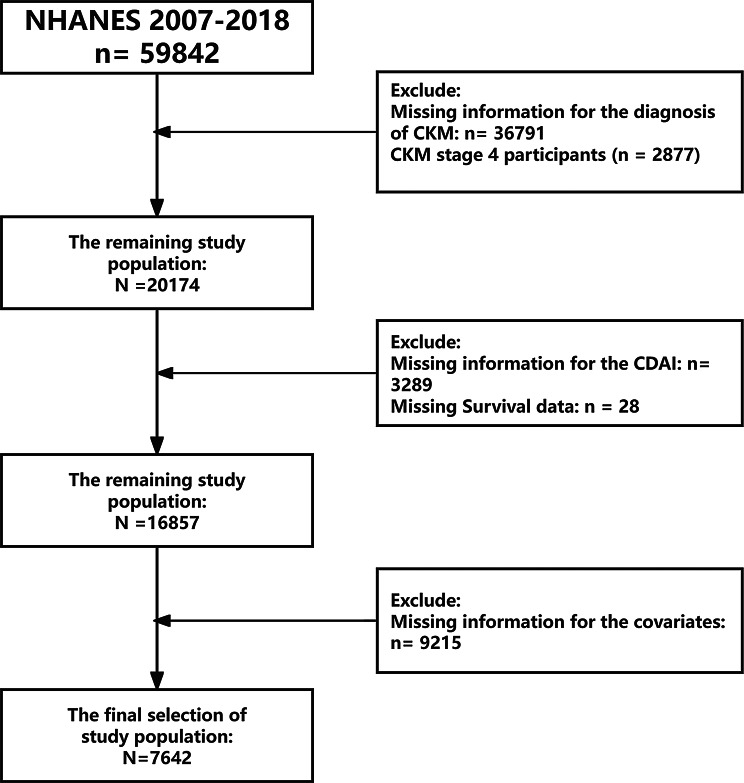
For the purpose of this investigation, data from the NHANES 2007–2018 cohort that is readily available to the public were utilized. Participants who were at least 20 years old and had completed the dietary survey and gave information regarding their CKM diagnosis were included. After excluding individuals with missing key covariates, the final sample size was 7,642. Figure 1 provides a detailed overview of the participant selection process.
Fig. 1.
Research flowchart
Definition of CKM and CDAI
CKM syndrome was categorized into four stages according to the AHA guidelines (Supplementary Tables S1 and S2) [10]. The CDAI combines six nutrients with antioxidant properties: vitamins A, C, E, zinc, β-carotene, and selenium [22]. Nutrient intake was assessed based on two 24-hour dietary recalls (including both food and supplements), as detailed in Supplementary Table S3. The calculation method involves subtracting the mean intake of each nutrient from the individual intake, then dividing by the standard deviation to obtain a Z-score. These standardized Z-scores are then summed with equal weighting to yield the total CDAI score.
Outcomes
The main outcome was all-cause mortality, while CVD mortality served as the secondary outcome. Death causes were categorized using the 10th edition of the ICD-10. All-cause mortality included deaths from any cause, whereas CVD mortality specifically referred to deaths from heart disease-related diagnoses (I00-I09, I11, I13, I20-I51).
Covariates selection and measurement
This analysis considered multiple potential confounders based on NHANES 2007–2018 data, including demographic factors (race, gender, marital status, age, education level, PIR), lifestyle factors (smoking, drinking, exercise level), biochemical parameters (total cholesterol, HDL, LDL, creatinine, uric acid), and major comorbidities (hypertension, diabetes). Data for each variable were collected via direct measurements, physical examinations, and questionnaires. To ensure data integrity, we removed all missing values for covariates. Detailed definitions of each predictor variable are provided in Supplementary Table S4.
Statistical analysis
Given the multistage probability sampling design of NHANES, primary sampling units, sample weights, and stratification were incorporated into the analysis. We performed weighted analyses using the “survey” package in R to generate national estimates, ensuring the results were generalizable to the non-institutionalized U.S. population and preventing overestimation of statistical significance. According to NHANES guidelines, weights prioritize the representativeness of small population subgroups, which are then adjusted accordingly.
To assess the relationship between CDAI and all-cause mortality in CKM stages 0–3, we performed weighted multivariable Cox regression. Three models were constructed: Model 1 (unadjusted), Model 2 (adjusted for gender, age, and race), and Model 3 (further adjusted for poverty income ratio, education level, hypertension, diabetes, marital status, smoking, drinking, exercise level, BMI, creatinine, TC, LDL, uric acid, and HDL). To explore potential non-linear relationships, a Restricted Cubic Spline model was used.
Kaplan-Meier survival curves and log-rank tests were applied to examine differences across CDAI quartiles. A Cox regression-based prediction model was also developed, and a nomogram was generated. To reduce the impact of high-dimensional data on model performance, we first employed Lasso regression for variable selection to identify key predictors (R glmnet package). These selected features were further validated using the Boruta algorithm (R Boruta package), and only those identified by both methods were retained for modeling.
Results
Baseline population characteristics
A total of 7,642 participants were included in this study, weighted to represent 66,663,969 U.S. adults. The average age of the participants was 46.97 (± 0.32) years, with males comprising 47.19% of the cohort. The average CDAI score was 14.08 ± 2.80. Compared to the lowest quartile, participants in the highest CDAI quartile had significantly longer survival times and a markedly reduced all-cause mortality. Differences in CKM stages were statistically significant (P < 0.05), while LDL, age, diabetes, and hypertension did not show significant differences (P > 0.05). Notably, 496 participants were classified as stage 3 CKM (4.01% of the sample), while the majority (59.75%) were in stage 2 (Supplementary Table S5).
Relationship between CDAI and mortality outcomes
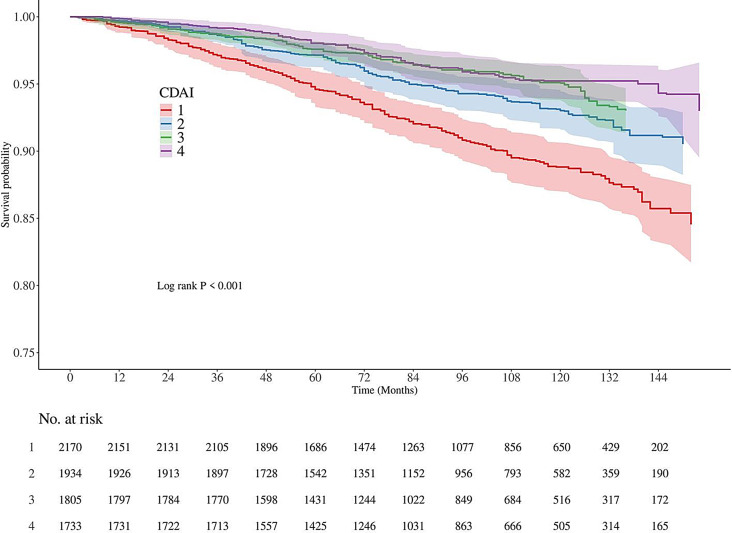
When analyzed as a continuous variable, CDAI was significantly inversely associated with all-cause mortality in unadjusted, partially adjusted, and fully adjusted models. The hazard ratios (HRs) (95% CI) were 0.90 (0.87–0.93), 0.91 (0.87–0.94), and 0.94 (0.91–0.98), respectively (Table 1). A similar trend was observed when participants were grouped by CDAI quartiles. In the fully adjusted model, individuals in the highest quartile exhibited a 60% reduction in all-cause mortality risk compared to those in the lowest quartile, with a significant trend (P < 0.001). Subsequent analysis indicated that both vitamin E and carotenoids were linked to reduced all-cause mortality. Kaplan-Meier survival curves (Fig. 2) indicated that individuals with low CDAI had the highest risk of mortality compared to those with high CDAI (P < 0.001). Finally, CDAI was associated with cardiovascular mortality in Model 2 (P < 0.05), but this association disappeared after further adjustment in Model 3 (Table 1).
Table 1.
Correlation between CDAI, all-cause and CVD mortality in CKM patients based on ICD-10
Fig. 2.
K-M analyses for all-cause mortality
RCS and threshold effects analysis
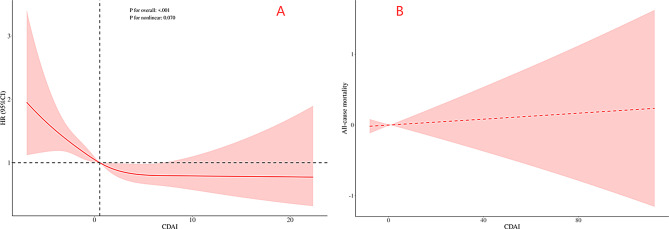
Restricted Cubic Splines (RCS) were employed to evaluate the association between CDAI levels and all-cause mortality in CKM (Fig. 3A). The RCS analysis demonstrated a significant linear association between CDAI, as a continuous variable, and reduced all-cause mortality risk in adjusted CKM (P < 0.001), with no evidence of non-linearity (P > 0.05). The analysis revealed no threshold effect in the relationship between CDAI and all-cause mortality in CKM (refer to Fig. 3B and Supplementary Table S6).
Fig. 3.
The RCS and threshold effect curve of the association between CDAI and all-cause mortality in model 3
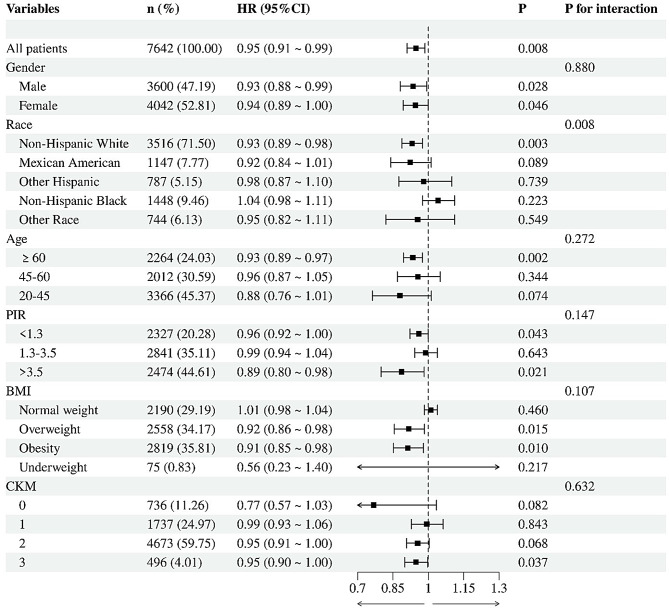
Subgroup analyses
In fully adjusted models, higher CDAI was significantly associated with reduced all-cause mortality overall (HR = 0.95, 95% CI: 0.91–0.99; P = 0.008), with consistent protective effects across most subgroups (Fig. 4). Notable heterogeneity emerged by race (P-interaction = 0.008), where significant risk reduction was observed in Non-Hispanic Whites (HR = 0.93, P = 0.003) but not in Non-Hispanic Blacks (HR = 1.04, P = 0.223). Protective associations were strongest in older adults (≥ 60y: HR = 0.93, P = 0.002), high-income individuals (PIR > 3.5: HR = 0.89, P = 0.021), and overweight/obese subgroups (P < 0.05), while no benefit was seen in normal-weight participants (HR = 1.01, P = 0.46). Effects remained robust across CKM stages (P-interaction = 0.632), supporting CDAI’s role in early intervention.
Fig. 4.
The association between CDAI and all-cause mortality in different subgroups
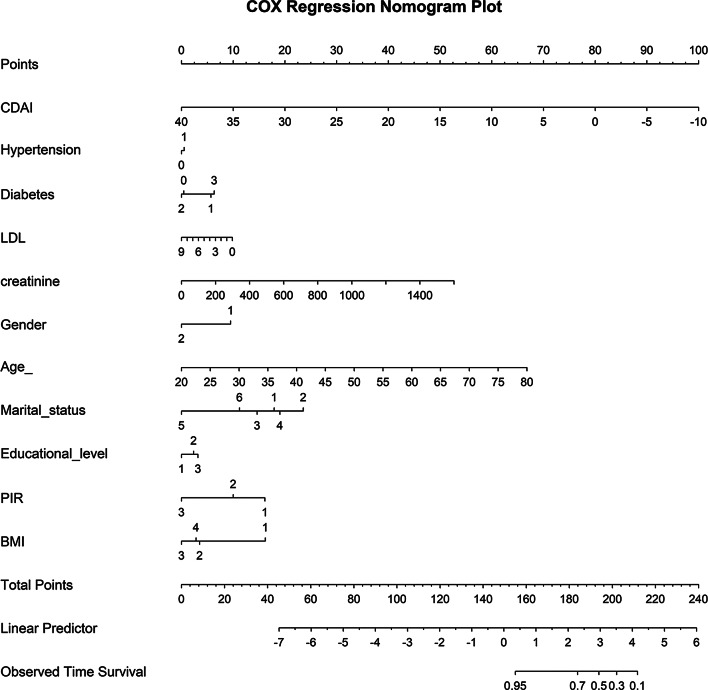
Predictive model for all-cause mortality based on CDAI in CKM
Lasso regression analysis identified 13 significant predictors of all-cause mortality (Supplementary Table S7): CDAI, age, marital status, education level, gender, PIR, exercise level, BMI, diabetes, smoking, hypertension, LDL, and creatinine (Fig. 5A and B). The Boruta algorithm identified 15 key predictors (Supplementary Table S8): CDAI, gender, race, age, marital status, education level, PIR, hypertension, BMI, diabetes, LDL, HDL, TC, uric acid, and creatinine (Fig. 5C and D). By integrating the outcomes of both methods, we identified the 11 key variables for modeling CDAI: age, marital status, gender, education level, PIR, BMI, hypertension, diabetes, LDL, and creatinine. A nomogram was subsequently developed to predict 5-year survival probability based on these variables (Fig. 6). The model’s discriminative ability for all-cause mortality based on CDAI was assessed using the ROC curve, yielding an AUC of 0.85 for the test set and 0.857 for the training set, as shown in Fig. 7. Decision curve analysis (DCA) confirmed the model’s significant net benefit and robust clinical validity (Fig. 7).
Fig. 5.
Feature filtering based on Lasso regression and Boruta algorithm
Fig. 6.
A nomogram model based on CDAI
Fig. 7.
ROC and DCA curves used to assess the nomogram model’s predictive accuracy for all-cause mortality
Discussion
This study systematically analyzed the NHANES 2007–2018 data, including 7,642 participants, to comprehensively evaluate the relationship between CDAI and all-cause mortality associated with CKM syndrome (stages 0–3). According to the findings, there was a considerable decrease in the risk of all-cause mortality as CDAI levels rose. This trend was clearly linear, with the risk of death falling by 6% for every unit increase in CDAI. All-cause mortality was 40% lower for those in the top quartile of CDAI than for those in the bottom quartile. These findings suggest a negative correlation between the overall intake of dietary antioxidant components and the mortality risk in CKM patients, providing a new theoretical basis for dietary interventions in the early prevention and comprehensive management of CKM syndrome.
CKM syndrome is a complex health condition involving the interrelation of obesity, T2D, CVD, and CKD. Oxidative stress plays a critical role in the pathogenesis of these diseases. It results from an overproduction of reactive oxygen species or insufficient antioxidant defenses, causing harm to cells. Oxidative stress is not only a contributing factor to these diseases but may also be a consequence [31, 32]. In CKM syndrome, oxidative stress is particularly linked to cardiovascular disease. Research indicates that oxidative stress exacerbates atherosclerosis by damaging endothelial function, triggering inflammation, and enhancing fibrosis, thus elevating cardiovascular risk [33–35]. Additionally, oxidative stress is associated with insulin resistance, further exacerbating metabolic disorders and cardiovascular risks [7, 36–38]. Beyond the cardiovascular system, oxidative stress adversely affects kidney function. In CKM syndrome, oxidative stress impairs tubular and glomerular function, contributing to the progressive decline in kidney function [39–41]. Increased oxidative stress is strongly linked to CKM severity and mortality risk, as confirmed by machine learning techniques [42]. Antioxidant therapies, such as antioxidant supplementation, have been shown to improve kidney and cardiovascular health to some extent [11, 43, 44]. Oxidative stress is crucial in CKM pathogenesis, and exploring its connection with CKM may lead to novel prevention and treatment strategies. Reducing oxidative stress is not only central to disease mechanisms but also provides potential targets for preventive strategies.
As part of dietary intervention, antioxidants effectively neutralize free radicals and reduce oxidative damage [17, 45, 46]. For example, vitamins E and B have been shown to significantly reduce lipid peroxidation products and mitigate heart and kidney damage [47, 48]. Further studies reveal that vitamin E alleviates inflammation and cellular damage by upregulating GPX4 expression and enhancing Treg cell antioxidant capacity [49]. Furthermore, antioxidants including carotenoids interact with the transcription factors NF-κB and Nrf-2 to modify immune function and reduce oxidative stress, which has anti-inflammatory effects [50, 51]. Some research indicates that high levels of certain antioxidants, like β-carotene, might elevate the risk of particular diseases [52], emphasizing the need to regulate antioxidant consumption and tailor treatments to individuals. On a molecular level, antioxidants modulate various signaling pathways related to cell survival and apoptosis, thereby influencing health outcomes. For example, Chen et al. demonstrated that vitamin D targets KPNA4 to inhibit the NF-κB pathway, reducing vascular inflammation and atherosclerosis, thus slowing coronary artery disease progression [53]. Research indicates that antioxidants can improve endothelial function and enhance vasodilation, thereby helping to reduce cardiovascular events [51, 54]. At the cellular level, antioxidants alleviate oxidative stress-induced cellular damage, maintaining normal cell function. Yadav et al. observed that vitamin B12 supplementation reduced homocysteine levels and oxidative stress in traumatic brain injury mice, enhancing synaptic plasticity and cell survival, ultimately improving neurofunctional outcomes [55]. Similarly, studies show a negative correlation between vitamin D levels and renin levels, suggesting that vitamin D may affect blood pressure via the renin-angiotensin-aldosterone pathway [56]. In addition, through modulation of gut microbiota and enhancement of intestinal barrier function, vitamin C, vitamin E, and β-carotene can provide health benefits and support the immune system’s normal function [57].
The CDAI index, which integrates multiple antioxidant components, including vitamins, minerals, and phytochemicals, provides a more accurate assessment of the body’s antioxidant status. Prior research indicates a notable inverse relationship between CDAI and the risk of chronic diseases. Specifically, CDAI has been negatively correlated with the risks of hypertension [27], heart failure [58], and stroke [59]. Furthermore, CDAI is closely linked to the risk of diabetes and CVD [60, 61], further supporting the potential value of antioxidants in chronic disease prevention. Nevertheless, current research on the link between dietary antioxidants and mortality shows inconsistencies. Consistent with our conclusions, research indicates that while high antioxidant intake is linked to lower CVD and all-cause mortality [62–65], while others suggest that excessive intake of certain antioxidants may increase cardiovascular disease risk [66]. The differences might be due to the complex actions of antioxidants, personal differences, and environmental factors. Therefore, further longitudinal studies and clinical trials are essential to clarify the long-term effects of dietary antioxidants on the health outcomes of CKM patients. However, an interesting finding was the non-significant association between CDAI and CVD mortality in fully adjusted models. There are several potential explanations for this attenuation: (1) Mediation by Other Factors: The attenuation of the association between CDAI and CVD mortality after adjustment may suggest the mediation of this relationship by other covariates such as comorbidities (e.g., diabetes, hypertension), lifestyle factors (e.g., smoking, exercise), and kidney function, which may exert a stronger effect on cardiovascular outcomes. In particular, renal failure, common in CKM, might overshadow the impact of antioxidants on CVD-specific mortality. (2) Reverse Causality: It is possible that reverse causality plays a role. Patients with advanced CKM may have impaired dietary intake due to the progressive nature of kidney and cardiovascular diseases, leading to lower antioxidant consumption, which could confound the observed association. (3) Residual Confounding: Despite controlling for a broad range of confounders, residual confounding from unmeasured factors such as genetic variations in antioxidant pathways or environmental exposures could still influence the relationship between CDAI and CVD mortality. These factors, not captured in NHANES, could impact both antioxidant status and cardiovascular outcomes. (4) Insufficient Follow-up: Another limitation is the duration of follow-up. While the median follow-up period was 94 months, this might not be long enough to capture the full impact of antioxidants on CVD outcomes, especially given the chronic and slowly progressing nature of CKM-related cardiovascular diseases.
This study verified the negative correlation between CDAI and all-cause mortality through multivariable weighted Cox regression and restricted cubic spline models. This finding aligns with recent studies on the link between dietary antioxidants and chronic disease outcomes. A prospective cohort study revealed that increased antioxidant intake correlates with reduced all-cause and CVD mortality among adults with diabetes [67]. Ha and Kyungho’s study, utilizing dietary questionnaires, suggests that diets high in antioxidants could lower the risk of all-cause mortality and cancer mortality [68].
Vitamin E and carotenoids, essential antioxidants, significantly reduce oxidative stress, alleviate inflammation, and maintain metabolic stability. In this study, these antioxidants showed significant associations with CKM-related outcomes, aligning with previous findings. For instance, Chen and colleagues discovered that higher serum carotenoid levels were inversely related to the prevalence of late-stage CKM, depending on the dose [69]. According to Zhu et al., adults with hypertension who had higher serum carotenoid levels experienced reduced all-cause and CVD mortality [70]. Song et al. concluded that a daily dose of 300 mg vitamin E significantly enhanced liver histology in patients with metabolic dysfunction-associated fatty liver disease by reducing fat degeneration, lobular inflammation, and fibrosis [48]. Similarly, a study indicated that increased vitamin E consumption correlates with lower CVD mortality and reduced all-cause mortality [71].
These findings translate into actionable dietary guidance for early-stage CKM management. To achieve the observed 40% reduction in all-cause mortality associated with the highest CDAI quartile, we recommend: (1) Prioritizing vitamin E-rich foods through daily consumption of nuts, seeds, and vegetable oils; (2) Increasing carotenoid intake, orange vegetables (carrots, sweet potatoes), and tomatoes; and (3) Adopting an antioxidant-focused dietary pattern emphasizing Mediterranean-style diets rich in fruits, vegetables, and whole grains while limiting processed foods. As this study suggests a potential protective role of antioxidants against all-cause mortality but not CVD-specific mortality, future studies should focus on exploring how specific antioxidants influence cardiovascular outcomes in CKM patients. This can help determine if antioxidants mediate these effects through indirect pathways (e.g., kidney function, metabolic health) rather than direct cardiovascular protection. The interaction between various dietary antioxidants (e.g., vitamin E, carotenoids, and selenium) may have synergistic effects, which are not captured by examining single-nutrient interventions. Future research should investigate these multi-nutrient effects to refine dietary guidelines for CKM patients.
Nonetheless, this research presents certain limitations. First, NHANES 24-hour dietary recalls are susceptible to recall bias and systematic underreporting in obese populations, potentially attenuating true CDAI-mortality associations. Second, reverse causation remains possible, as residual symptoms in advanced CKM stages may reduce dietary intake. Third, residual confounding from unmeasured factors (e.g., environmental pollutants, genetic polymorphisms in antioxidant pathways) cannot be excluded. While our cohort represents diverse U.S. demographics, generalizability to non-Western populations with distinct dietary patterns (e.g., Asian diets rich in polyphenols or Mediterranean diets) may be limited. Validation in cohorts spanning varied cultural and socioeconomic contexts is warranted. Finally, the cross-sectional design precludes causal inference, warranting validation in longitudinal cohorts.
In conclusion, this study underscores the potential role of dietary antioxidants in the prevention and management of CKM syndrome, providing a valuable research foundation and clinical insights for future multidimensional, personalized disease interventions.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors express gratitude to all participants and investigators of the NHANES.
Author contributions
Lincheng Duan: Conceptualization, Formal analysis, Methodology, Visualization, Writing – original draft; Hong Yang: Formal analysis, Visualization; ZhuoYang Chen: Formal analysis; Junxin Zhao: Data curation; Jingyi Yang: Validation; Dingjun Cai: Conceptualization, Supervision, Writing – review & editing. All authors reviewed the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (82274653), Sichuan Qihuang Scholars Capacity Enhancement Program (600008241001), and a project grant from Chengdu University of Traditional Chinese Apricot Grove Scholars Program (330024651).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethical approval
The studies involving humans were approved by the NCHS Research Ethics Review Board (ERB). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ndumele CE, Rangaswami J, Chow SL, Neeland IJ, Tuttle KR, Khan SS, et al. Cardiovascular-Kidney-Metabolic health: a presidential advisory from the American heart association. Circulation. 2023;148:1606–35. [DOI] [PubMed] [Google Scholar]
- 2.Baker-Smith CM, Gauen AM, Petito LC, Khan SS, Allen NB. Prevalence of cardiovascular-kidney-metabolic stages in US adolescents and relationship to social determinants of health. J Am Heart Assoc. 2025;14:e040269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao Y, Wang W, Xie S, Xu Y, Lin Z. Joint association of the inflammatory marker and cardiovascular-kidney-metabolic syndrome stages with all-cause and cardiovascular disease mortality: a National prospective study. BMC Public Health. 2025;25:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li W, Shen C, Kong W, Zhou X, Fan H, Zhang Y, et al. Association between the triglyceride glucose-body mass index and future cardiovascular disease risk in a population with cardiovascular-kidney-metabolic syndrome stage 0–3: a nationwide prospective cohort study. Cardiovasc Diabetol. 2024;23:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanbay M, Guldan M, Ozbek L, Copur S, Covic AS, Covic A. Exploring the nexus: the place of kidney diseases within the cardiovascular-kidney-metabolic syndrome spectrum. Eur J Intern Med. 2024;127:1–14. [DOI] [PubMed] [Google Scholar]
- 6.Zhang C, Hao C, Liang W, Hu K, Guo T, Chen Y, et al. Abdominal obesity and frailty progression in population across different cardiovascular-kidney-metabolic syndrome stages: a nationwide longitudinal study. Diabetol Metab Syndr. 2025;17:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masenga SK, Kabwe LS, Chakulya M, Kirabo A. Mechanisms of oxidative stress in metabolic syndrome. Int J Mol Sci. 2023;24:7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pandey S. Metabolomics for the identification of biomarkers in kidney diseases. Nanotheranostics. 2025;9:110–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Claudel SE, Schmidt IM, Waikar SS, Verma A. Cumulative incidence of mortality associated with cardiovascular-kidney-metabolic (CKM) syndrome. J Am Soc Nephrol [Internet]. 2025; Available from: 10.1681/ASN.0000000637 [DOI] [PMC free article] [PubMed]
- 10.Aggarwal R, Ostrominski JW, Vaduganathan M. Prevalence of cardiovascular-kidney-metabolic syndrome stages in US adults, 2011–2020. JAMA. 2024;331:1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sebastian SA, Padda I, Johal G. Cardiovascular-kidney-metabolic (CKM) syndrome: a state-of-the-art review. Curr Probl Cardiol. 2024;49:102344. [DOI] [PubMed] [Google Scholar]
- 12.Sen P, Hamers J, Sittig T, Shashikadze B, d’Ambrosio L, Stöckl JB, et al. Oxidative stress initiates hemodynamic change in CKD-induced heart disease. Basic Res Cardiol. 2024;119:957–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen X, Xie N, Feng L, Huang Y, Wu Y, Zhu H, et al. Oxidative stress in diabetes mellitus and its complications: from pathophysiology to therapeutic strategies. Chin Med J (Engl). 2024;138:15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schulze MB, Martínez-González MA, Fung TT, Lichtenstein AH, Forouhi NG. Food based dietary patterns and chronic disease prevention. Br Med J. 2018;361:k2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karimi E, Gholizadeh M, Abdolahi M, Sedighiyan M, Salehinia F, Siri G, et al. Effect of vitamin B1 supplementation on blood creatinine and lactate levels and clinical outcomes in patients in intensive care units: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev. 2023;82:804–14. [DOI] [PubMed] [Google Scholar]
- 16.Abdollahi H, Salehinia F, Badeli M, Karimi E, Gandomkar H, Asadollahi A, et al. The biochemical parameters and vitamin D levels in ICU patients with covid-19: a cross-sectional study. Endocr Metab Immune Disord Drug Targets. 2021;21:2191–202. [DOI] [PubMed] [Google Scholar]
- 17.Puertollano MA, Puertollano E, de Cienfuegos GÁ, de Pablo MA. Dietary antioxidants: immunity and host defense. Curr Top Med Chem. 2011;11:1752–66. [DOI] [PubMed] [Google Scholar]
- 18.Lee GY, Han SN. The role of vitamin E in immunity. Nutrients. 2018;10:1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin B, Tang S, Sun J, Zhang X, Xu J, Di L, et al. Vitamin C and sodium bicarbonate enhance the antioxidant ability of H9C2 cells and induce HSPs to relieve heat stress. Cell Stress Chaperones. 2018;23:735–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Senousey HK, Chen B, Wang JY, Atta AM, Mohamed FR, Nie QH. Effects of dietary vitamin C, vitamin E, and alpha-lipoic acid supplementation on the antioxidant defense system and immune-related gene expression in broilers exposed to oxidative stress by dexamethasone. Poult Sci. 2018;97:30–8. [DOI] [PubMed] [Google Scholar]
- 21.Apak R, Güçlü K, Ozyürek M, Karademir SE. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J Agric Food Chem. 2004;52:7970–81. [DOI] [PubMed] [Google Scholar]
- 22.Wright ME, Mayne ST, Stolzenberg-Solomon RZ, Li Z, Pietinen P, Taylor PR, et al. Development of a comprehensive dietary antioxidant index and application to lung cancer risk in a cohort of male smokers. Am J Epidemiol. 2004;160:68–76. [DOI] [PubMed] [Google Scholar]
- 23.Kim Y, Je Y. Flavonoid intake and mortality from cardiovascular disease and all causes: a meta-analysis of prospective cohort studies. Clin Nutr ESPEN. 2017;20:68–77. [DOI] [PubMed] [Google Scholar]
- 24.Kang JH, Cook NR, Manson JE, Buring JE, Albert CM, Grodstein F. Vitamin E, vitamin C, beta carotene, and cognitive function among women with or at risk of cardiovascular disease: the women’s antioxidant and cardiovascular study. Circulation. 2009;119:2772–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Daghri NM, Khan N, Alkharfy KM, Al-Attas OS, Alokail MS, Alfawaz HA, et al. Selected dietary nutrients and the prevalence of metabolic syndrome in adult males and females in Saudi arabia: a pilot study. Nutrients. 2013;5:4587–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu W, Xu Y, Xiao L, Li K, Liu Q. Composite dietary antioxidant index is associated with the prevalence of metabolic syndrome in females: results from NHANES 2011–2016. Front Nutr. 2025;12:1529332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu M, Si J, Liu Y, Kang L, Xu B. Association between composite dietary antioxidant index and hypertension: insights from NHANES. Clin Exp Hypertens. 2023;45:2233712. [DOI] [PubMed] [Google Scholar]
- 28.Liu C, Lai W, Zhao M, Zhang Y, Hu Y. Association between the composite dietary antioxidant index and atherosclerotic cardiovascular disease in postmenopausal women: a cross-sectional study of NHANES data, 2013–2018. Antioxidants (Basel). 2023;12:1740. [DOI] [PMC free article] [PubMed]
- 29.Tan Z, Meng Y, Li L, Wu Y, Liu C, Dong W, et al. Association of dietary fiber, composite dietary antioxidant index and risk of death in tumor survivors: National health and nutrition examination survey 2001–2018. Nutrients. 2023;15:2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li W, Bai J, Ge Y, Fan Y, Huang Q, Deng Z. Association between compound dietary antioxidant index and all-cause and cancer mortality in patients with chronic obstructive pulmonary disease: results from NHANES 1999–2018. Front Med. 2025;12:1544841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carrier A. Metabolic syndrome and oxidative stress: a complex relationship. Antioxid Redox Signal. 2017;26:429–31. [DOI] [PubMed] [Google Scholar]
- 32.Whaley-Connell A, Sowers JR. Oxidative stress in the cardiorenal metabolic syndrome. Curr Hypertens Rep. 2012;14:360–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Otani H. Oxidative stress as pathogenesis of cardiovascular risk associated with metabolic syndrome. Antioxid Redox Signal. 2011;15:1911–26. [DOI] [PubMed] [Google Scholar]
- 34.Whaley-Connell A, McCullough PA, Sowers JR. The role of oxidative stress in the metabolic syndrome. Rev Cardiovasc Med. 2011;12:21–9. [DOI] [PubMed] [Google Scholar]
- 35.Koumallos N, Sigala E, Milas T, Baikoussis NG, Aragiannis D, Sideris S, et al. Angiotensin regulation of vascular homeostasis: exploring the role of ROS and RAS blockers. Int J Mol Sci. 2023;24:12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen S-J, Yen C-H, Huang Y-C, Lee B-J, Hsia S, Lin P-T. Relationships between inflammation, adiponectin, and oxidative stress in metabolic syndrome. PLoS ONE. 2012;7:e45693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henriksen EJ, Diamond-Stanic MK, Marchionne EM. Oxidative stress and the etiology of insulin resistance and type 2 diabetes. Free Radical Biol Med. 2011;51:993–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Lima EP, Moretti RC, Torres Pomini K, Laurindo LF, Sloan KP, Sloan LA, et al. Glycolipid metabolic disorders, metainflammation, oxidative stress, and cardiovascular diseases: unraveling pathways. Biology (Basel). 2024;13:519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kishi S, Nagasu H, Kidokoro K, Kashihara N. Oxidative stress and the role of redox signalling in chronic kidney disease. Nat Rev Nephrol. 2023;20:101–19. [DOI] [PubMed] [Google Scholar]
- 40.Ravarotto V, Simioni F, Pagnin E, Davis PA, Calò LA. Oxidative stress - chronic kidney disease - cardiovascular disease: a vicious circle. Life Sci. 2018;210:125–31. [DOI] [PubMed] [Google Scholar]
- 41.Verma S, Singh P, Khurana S, Ganguly NK, Kukreti R, Saso L, et al. Implications of oxidative stress in chronic kidney disease: a review on current concepts and therapies. Kidney Res Clin Pract. 2021;40:183–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen Y, Wu S, Liu H, Zhong Z, Bucci T, Wang Y, et al. Role of oxidative balance score in staging and mortality risk of cardiovascular-kidney-metabolic syndrome: insights from traditional and machine learning approaches. Redox Biol. 2025;81:103588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu X, Ma E, Ge Y, Yuan M, Guo X, Peng J, et al. Resveratrol protects against myocardial ischemic injury in obese mice via activating SIRT3/FOXO3a signaling pathway and restoring redox homeostasis. Biomed Pharmacother. 2024;174:116476. [DOI] [PubMed] [Google Scholar]
- 44.Amirkhizi F, Hamedi-Shahraki S, Rahimlou M. Dietary total antioxidant capacity is associated with lower disease severity and inflammatory and oxidative stress biomarkers in patients with knee osteoarthritis. J Health Popul Nutr. 2023;42:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hasanloei MAV, Zeinaly A, Rahimlou M, Houshyar H, Moonesirad S, Hashemi R. Effect of coenzyme Q10 supplementation on oxidative stress and clinical outcomes in patients with low levels of coenzyme Q10 admitted to the intensive care unit. J Nutr Sci. 2021;10:e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hashemi R, Mehdizadeh Khalifani A, Rahimlou M, Manafi M. Comparison of the effect of dietary approaches to stop hypertension diet and American diabetes association nutrition guidelines on lipid profiles in patients with type 2 diabetes: a comparative clinical trial. Nutr Diet. 2019;77:204–11. [DOI] [PubMed] [Google Scholar]
- 47.Ford TC, Downey LA, Simpson T, McPhee G, Oliver C, Stough C. The effect of a high-dose vitamin B multivitamin supplement on the relationship between brain metabolism and blood biomarkers of oxidative stress: a randomized control trial. Nutrients. 2018;10:1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song Y, Ni W, Zheng M, Sheng H, Wang J, Xie S, et al. Vitamin E (300 mg) in the treatment of MASH: a multi-center, randomized, double-blind, placebo-controlled study. Cell Rep Med. 2025;6:101939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luo S, Zeng Y, Chen B, Yan J, Ma F, Zhuang G, et al. Vitamin E and GPX4 cooperatively protect Treg cells from ferroptosis and alleviate intestinal inflammatory damage in necrotizing Enterocolitis. Redox Biol. 2024;75:103303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iddir M, Brito A, Dingeo G, Fernandez Del Campo SS, Samouda H, La Frano MR, et al. Strengthening the immune system and reducing inflammation and oxidative stress through diet and nutrition: considerations during the COVID-19 crisis. Nutrients. 2020;12:1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roşian ŞH, Boarescu I, Boarescu P-M. Antioxidant and anti-inflammatory effects of bioactive compounds in atherosclerosis. Int J Mol Sci. 2025;26:1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qiu Z, Chen X, Geng T, Wan Z, Lu Q, Li L, et al. Associations of serum carotenoids with risk of cardiovascular mortality among individuals with type 2 diabetes: results from NHANES. Diabetes Care. 2022;45:1453–61. [DOI] [PubMed] [Google Scholar]
- 53.Chen S, Swier VJ, Boosani CS, Radwan MM, Agrawal DK. Vitamin D deficiency accelerates coronary artery disease progression in swine. Arterioscler Thromb Vasc Biol. 2016;36:1651–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamagata K, Yamori Y. Inhibition of endothelial dysfunction by dietary flavonoids and preventive effects against cardiovascular disease. J Cardiovasc Pharmacol. 2020;75:1–9. [DOI] [PubMed] [Google Scholar]
- 55.Yadav P, Nasir F, Sivanandam TM. Neuroprotective effect of vitamin B12 supplementation on cognitive functions and neuronal morphology at different time intervals after traumatic brain injury in male Swiss albino mice. Neurochem Int. 2024;180:105869. [DOI] [PubMed] [Google Scholar]
- 56.Han L, Xu X-J, Zhang J-S, Liu H-M. Association between vitamin D deficiency and levels of Renin and angiotensin in essential hypertension. Int J Clin Pract. 2022;2022:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li X-Y, Meng L, Shen L, Ji H-F. Regulation of gut microbiota by vitamin C, vitamin E and β-carotene. Food Res Int. 2023;169:112749. [DOI] [PubMed] [Google Scholar]
- 58.Ma Y, Liu J, Sun J, Cui Y, Wu P, Wei F, et al. Composite dietary antioxidant index and the risk of heart failure: a cross-sectional study from NHANES. Clin Cardiol. 2023;46:1538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Teng T-Q, Liu J, Hu F-F, Li Q-Q, Hu Z-Z, Shi Y. Association of composite dietary antioxidant index with prevalence of stroke: insights from NHANES 1999–2018. Front Immunol. 2024;15:1306059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Muralidharan J, Moreno-Indias I, Bulló M, Lopez JV, Corella D, Castañer O, et al. Effect on gut microbiota of a 1-y lifestyle intervention with mediterranean diet compared with energy-reduced mediterranean diet and physical activity promotion: PREDIMED-plus study. Am J Clin Nutr. 2021;114:1148–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mancini FR, Affret A, Dow C, Balkau B, Bonnet F, Boutron-Ruault M-C, et al. Dietary antioxidant capacity and risk of type 2 diabetes in the large prospective E3N-EPIC cohort. Diabetologia. 2017;61:308–16. [DOI] [PubMed] [Google Scholar]
- 62.Wang X, Hu J, Liu L, Zhang Y, Dang K, Cheng L, et al. Association of dietary inflammatory index and dietary oxidative balance score with all-cause and disease-specific mortality: findings of 2003–2014 National health and nutrition examination survey. Nutrients. 2023;15:3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Y, Meng S, Yu Y, Bi L, Tian J, Zhang L. Associations of dietary selenium intake with the risk of chronic diseases and mortality in US adults. Front Nutr. 2024;11:1363299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim KJ, Choi J, Kim KJ, Kim NH, Kim SG. All-cause and cause-specific mortality risks associated with calcium supplementation with or without vitamin D: a nationwide population-based study. J Intern Med. 2023;294:83–95. [DOI] [PubMed] [Google Scholar]
- 65.Bo Y, Xu H, Zhang H, Zhang J, Wan Z, Zhao X, et al. Intakes of folate, vitamin B6, and vitamin B12 in relation to all-cause and cause-specific mortality: a National population-based cohort. Nutrients. 2022;14:2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang R, Lv M, Yang X, Zhai S. A Mendelian randomized study of Circulating antioxidants in the diet and risk of cardiovascular disease. Sci Rep. 2025;15:10341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang W, Wang X, Cao S, Duan Y, Xu C, Gan D, et al. Dietary antioxidant indices in relation to all-cause and cause-specific mortality among adults with diabetes: a prospective cohort study. Front Nutr. 2022;9:849727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ha K, Liao LM, Sinha R, Chun OK. Dietary total antioxidant capacity, a diet quality index predicting mortality risk in US adults: evidence from the NIH-AARP diet and health study. Antioxid (Basel). 2023;12:1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen M, Cai S, Jia Q, Suo Y, Tang Y, Shi Y, et al. Inverse relationship between serum carotenoid levels and cardiovascular-kidney-metabolic syndrome among the general adult population. J Diabetes. 2025;17:e70046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhu X, Cheang I, Tang Y, Shi M, Zhu Q, Gao R, et al. Associations of serum carotenoids with risk of all-cause and cardiovascular mortality in hypertensive adults. J Am Heart Assoc. 2023;12:e027568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zeng Q, Liao M, Li Y, She F, Zhang P. Association between dietary vitamin E intake and incident cardiovascular disease, cardiovascular, and all-cause mortality: a prospective cohort study using NHANES 2003–2018 data. Int J Cardiol Cardiovasc Risk Prev. 2025;24:200340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.