Abstract
Background
The prognosis of stage IIIA colorectal cancer (CRC) is much better than that of stage II CRC in Japan. This study aimed to investigate the clinical implications of lymph node metastasis (LNM) in patients with pT1-2 CRC and explore the potential for downstaging pT1-2N+ CRC.
Methods
This retrospective cohort study took place at Saitama Medical University International Medical Center in Japan. We stratified patients with pT1-2 CRC (n=1,288) by presence (LNM+) or absence (LNM−) of LNM, assessing overall survival (OS), cancer-specific survival (CSS), and relapse-free survival (RFS) in both groups before and after propensity score matching (PSM). Cox multivariate analysis served for screening of prognostic risk factors.
Results
LNM+ was ultimately confirmed in 256 study subjects (19.9%). Before matching, tumors of the LNM+ (vs. LNM−) group were more inclined to be large (≥2 cm: 76.6% vs. 61.2%; P<0.001), with greater propensity for infiltrating or ulcerative features (55.1% vs. 36.2%; P<0.001) and histotypes of lesser differentiation (moderately differentiated adenocarcinoma/poorly differentiated adenocarcinoma/signet-ring carcinoma/mucinous carcinoma: 65.6% vs. 45.8%; P<0.001). Likewise, they showed greater tendency for aggressive growth (91.0% vs. 81.1%; P<001), lymphatic (44.5% vs. 19.4%; P<0.001) or vascular (59.0% vs. 35.1%; P<0.001) invasion, and prolific lymph node harvesting (23.6±12.2 vs. 21.7±12.3; P=0.02). Although similar in terms of OS (LNM−, 94.2%; LNM+, 91.8%; P=0.33), the LNM− (vs. LNM+) group displayed significantly better CSS (99.5% vs. 96.9%; P<0.001) and RFS (97.2% vs. 89.5%; P<0.001). After matching, RFS still proved significantly better in the LNM− (vs. LNM+) group (95.9% vs. 89.8%; P=0.01), with multivariate analysis identifying LNM+ as an independent risk factor for RFS before and after PSM. A higher recurrence rate was also evident in the LNM+ (vs. LNM−) group [before matching: 10.5% vs. 2.8% (P<0.001); after matching: 10.2% vs. 4.1% (P=0.008)], involving liver and lymph nodes primarily. Neither OS nor CSS differed significantly by group.
Conclusions
LNM+ pT1-2 CRC patients had a higher risk of hepatic and nodal recurrence, but long-term OS and CSS were unaffected. Perhaps an appropriate downstaging of pT1-2N+ CRC from stage IIIA is a reasonable prospect.
Keywords: pT1-2 colorectal cancer (pT1-2 CRC), lymph node metastasis (LNM), propensity score matching (PSM)
Highlight box.
Key findings
• Lymph node metastasis positive (LNM+) pT1-2 colorectal cancer (CRC) patients had a higher risk of hepatic and nodal recurrence, but long-term overall survival (OS) and cancer-specific survival (CSS) were unaffected.
What is known, and what is new?
• In the Japanese guidelines for the treatment of gastric cancer, patients with stage T1N1 (stage IB) gastric cancer do not require adjuvant chemotherapy. However, the T1N1 CRC is staged IIIA, and adjuvant chemotherapy is routinely required. In Japan, 5-year OS of stage IIIA CRC patients (pT1-2 N+) are 92.5%, 93% for stage I (pT1-2 N0), 88.2% for stage II (pT3-4 N0), the prognosis of stage IIIA CRC is much better than that of stage II CRC.
• The current study shows that LNM+ was not an independent risk factor for CSS and OS after propensity score matching (PSM).
What is the implication, and what should change now?
• Whether the current classification of stage IIIA for T1-2N+ CRC really reflects the true prognosis.
• Perhaps an appropriate downstaging of pT1-2N+ CRC from stage IIIA is a reasonable prospect.
Introduction
In recent years, with the development of surgical techniques and chemotherapy, the prognosis of patients with primary colorectal cancer (CRC) has improved significantly. However, in CRC patients with lymph node metastasis (LNM), 30–40% of them eventually metastasize or relapse, even after curative resection (1). The Japanese General Rules for Clinical and Pathological Studies on Cancer of the Colon, Rectum and Anus, issued by the Japanese Society for Cancer of the Colon and Rectum (JSCCR) once emphasized the importance of the location of LNM, classifying the stage and extent of lymph nodes. N1 was the lymph node that metastasized to supracolonic and paracolonic lymph nodes within 5 cm from the tumor; N2 was the lymph node that metastasized to paracolonic lymph nodes 5–10 cm from the tumor and along the main blood supplying artery; N3 was the apical lymph node that metastasized to the root of the main blood supplying artery (2). Studies have shown that the farther the metastatic lymph nodes are from the tumor, the worse the prognosis (3,4). But other study has shown that the number of LNM is more important to the prognosis of patients (5). Therefore, Japan is now also switching to CRC staging based on the number of LNM (1).
The tumor-nodes-metastasis (TNM) classification system, as defined by the Union for International Cancer Control (UICC), is currently the gold standard for clinical staging of cancer worldwide (6). Both TNM and Japanese systems categorize T1N1 and T2N1 subsets of gastric cancer as stages IB and IIA (2,7), respectively; whereas T1-2N1 esophageal cancer is staged correspondingly as IIB and II (8,9). Thus, inconsistencies are apparent in the pathologic staging of cancer for various organs.
The clinical import of nodal metastasis in patients with CRC is integral to the historic system devised by Dukes [1932], in which B and C tumor designations reflect either absence (LNM−) or presence (LNM+) of LNM (10,11). Currently, node-positive CRC signals TNM-stipulated stage III disease that is further compartmentalized as IIIA (T1-2N1, ≤3 positive nodes), IIIB (T3-4aN+), or IIIC (T4bN+), based on extent of lymph node involvement (2,6). Hence, pT1-2N+ CRC is seemingly more advanced than pT1-2N+ gastric or esophageal cancer under the present staging hierarchy, which is rarely called into question (10,12).
In a previous study, we found that LNM did not affect cancer-specific survival (CSS) in patients with pT2 CRC (12). This study aims to explore the clinical implications of LNM in pT1-2 CRC and assess the potential for downstaging. We present this article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-2024-982/rc).
Methods
This retrospective cohort study took place at Saitama Medical University International Medical Center in Japan. Between April 2007 and December 2020, a total of 1,570 patients with pT1-2 CRC underwent radical resections. According to the D3 lymph node dissection concept, mobilize the colon along the embryological and anatomical planes to obtain a negative circumferential margin and ligation the root of the primary tumor-supplying artery for resection of an adequate mesentery. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Ethics Committee of Saitama Medical University International Medical Center (No. 19-006) and individual consent for this retrospective analysis was waived. Some candidates (n=282) failed to qualify based on hereditary familial cancer, tumor recurrence, or transanal surgery (i.e., without lymph node dissection). Radical excision was also performed in patients with positive vertical margins or infiltration depth greater than 1,000 microns after ESD/EMR. The remainder (n=1,288) were grouped according to LNM status, either present (LNM+) or absent (LNM−) (Figure 1).
Figure 1.

Schematic of patient allocation/study design. CRCs, colorectal cancers; LNM, lymph node metastasis; LNM−, lymph node metastasis negative; LNM+, lymph node metastasis positive.
All research data were collected as specified in General Rules for Clinical and Pathological Studies on Cancer of the Colon, Rectum and Anus, issued by the Japanese Society for Cancer of the Colon and Rectum (JSCCR). Tumor stage determinations relied on clinical parameters, using UICC TNM classification standards cited in the American Joint Committee on Cancer (AJCC) Cancer Staging Manual (7th edition). Two Japanese pathologists reviewed all CRC specimens.
We first analyzed clinicopathologic patient data in advance of propensity score matching (PSM) to assess prognostic differences by group (LNM+ vs. LNM−), including overall survival (OS), CSS, and relapse-free survival (RFS) rates.
PSM served to balance differences in baseline variables, and multivariate regression models were used to identify potential independent risk factors. The following parameters were incorporated into a multivariate model for PSM: sex, age, preoperative carcinoembryonic antigen (CEA) level, body mass index (BMI), tumor location, family tumor history, neoadjuvant therapy, preoperative endoscopic submucosal dissection/endoscopic mucosal resection (ESD/EMR), surgical approach, lymph node dissection level, operative time, postoperative complications, tumor size, gross features, histologic pattern, infiltrative nature, lymphatic or venous invasion, excised lymph nodes, and pT1:pT2 ratio. After matching, Logistic regression analysis was performed to determine the differences in OS, CSS and RFS.
Statistical analysis
All statistical analytics were driven by standard software (SPSS v22; IBM Corp., Armonk, NY, USA) operated on a Mac computer (Apple Inc., Cupertino, CA, USA). To test for differences in categorical variables, Chi-square and Fisher’s exact tests were applied. We used Kaplan-Meier method to estimate OS, CSS, and RFS, setting significance at P<0.05.
Results
Qualifying patients (n=1,288) were grouped as LNM+ (256/1,288, 19.9%) or LNM− (1,032/1,288, 80.1%) (Table 1). In univariate analyses, there were no significant group-wise differences with respect to sex, age, BMI, preoperative CEA level, tumor location, history of neoadjuvant therapy, surgical approach, operative time, postoperative complications, or operative blood loss.
Table 1. Clinical characteristics and surgical results before and after PSM.
| Parameters | Before PSM | After PSM (1:1) | |||||
|---|---|---|---|---|---|---|---|
| LNM− (N=1,032) | LNM+ (N=256) | P value | LNM− (N=244) | LNM+ (N=244) | P value | ||
| Sex | |||||||
| Male | 632 (61.2) | 164 (64.1) | NS | 159 (65.2) | 158 (64.8) | NS | |
| Female | 400 (38.8) | 92 (35.9) | 85 (34.8) | 86 (35.2) | |||
| Age (years) | |||||||
| <70 | 554 (53.7) | 152 (59.4) | NS | 136 (55.7) | 144 (59.0) | NS | |
| ≥70 | 478 (46.3) | 104 (40.6) | 108 (44.3) | 100 (41.0) | |||
| BMI (kg/m2) | 23.1±3.42 | 23.0±3.36 | NS | 23.4±3.44 | 22.9±3.27 | NS | |
| CEA (ng/mL) | |||||||
| <5 | 886 (85.9) | 222 (86.7) | NS | 209 (85.7) | 210 (86.1) | NS | |
| ≥5 | 146 (14.1) | 34 (13.3) | 35 (14.3) | 34 (13.9) | |||
| Tumor location | |||||||
| Left | 729 (70.6) | 197 (77.0) | NS | 177 (72.5) | 187 (76.6) | NS | |
| Right | 303 (29.4) | 59 (23.0) | 67 (27.5) | 57 (23.4) | |||
| Family tumor history | |||||||
| No | 889 (86.1) | 234 (91.4) | 0.03 | 234 (95.9) | 223 (91.4) | NS | |
| Yes | 143 (13.9) | 22 (8.6) | 10 (4.1) | 21 (8.6) | |||
| Abdominal surgery history | |||||||
| No | 638 (61.8) | 165 (64.5) | NS | 150 (61.5) | 156 (63.9) | NS | |
| Yes | 394 (38.2) | 91 (35.5) | 94 (38.5) | 88 (36.1) | |||
| Neoadjuvant chemotherapy | |||||||
| No | 1,010 (97.9) | 253 (98.8) | NS | 240 (98.4) | 241 (98.8) | NS | |
| Yes | 22 (2.1) | 3 (1.2) | 4 (1.6) | 3 (1.2) | |||
| Comorbidity | |||||||
| No | 318 (30.8) | 91 (35.5) | NS | 74 (30.3) | 88 (36.1) | NS | |
| Yes | 714 (69.2) | 165 (64.5) | 170 (69.7) | 156 (63.9) | |||
| EMR/ESD | |||||||
| No | 817 (79.2) | 230 (89.8) | <0.001 | 219 (89.8) | 218 (89.3) | NS | |
| Yes | 215 (20.8) | 26 (10.2) | 25 (10.2) | 26 (10.7) | |||
| cT | |||||||
| T0–T2 | 770 (74.6) | 163 (63.7) | <0.001 | 165 (67.6) | 157 (64.3) | NS | |
| T3–T4 | 262 (25.4) | 93 (36.3) | 79 (32.4) | 87 (35.7) | |||
| Surgical approach | |||||||
| Laparoscopic surgery | 957 (92.7) | 238 (93.0) | NS | 229 (93.9) | 227 (93.0) | NS | |
| Open surgery | 75 (7.3) | 18 (7.0) | 15 (6.1) | 17 (7.0) | |||
| Lymph node dissection | |||||||
| D3 | 678 (65.7) | 197 (77.0) | 0.001 | 186 (76.2) | 185 (75.8) | NS | |
| Non-D3 | 354 (34.3) | 59 (23.0) | 58 (23.8) | 59 (24.2) | |||
| Operative time (min) | 200±77.1 | 208±78.9 | NS | 207±81.8 | 207±78.0 | NS | |
| Post-operational complications | |||||||
| No | 859 (83.2) | 206 (80.5) | NS | 204 (83.6) | 196 (80.3) | NS | |
| Yes | 173 (16.8) | 50 (19.5) | 40 (16.4) | 48 (19.7) | |||
| Operative blood loss (mL) | |||||||
| <100 | 938 (90.9) | 228 (89.1) | NS | 219 (89.8) | 218 (89.3) | NS | |
| ≥100 | 94 (9.1) | 28 (10.9) | 25 (10.2) | 26 (10.7) | |||
| Post-operative hospital stay (days) | 9.23±8.46 | 10.4±13.7 | NS | 9.07±5.82 | 10.5±14.0 | NS | |
| Time to first food intake (days) | 3.46±2.56 | 3.66±2.70 | NS | 3.31±1.30 | 3.67±2.75 | NS | |
Data are presented as n (%) or mean ± standard deviation. BMI, body mass index; CEA, carcinoembryonic antigen; cT, clinical T stage; EMR, endoscopic mucosal resection; ESD, endoscopic submucosal dissection; LNM, lymph node metastasis; LNM−, lymph node metastasis negative; LNM+, lymph node metastasis positive; NS, not significant; PSM, propensity score matching.
Members of the LNM− (vs. LNM+) group more often had family histories of tumor (13.9% vs. 8.6%; P=0.03), undergoing proportionately more ESD/EMR procedures (20.8% vs. 10.2%; P<0.001) and non-D3 radical dissections (34.3% vs. 23.0%; P=0.001). Tumors were otherwise bulkier (≥2 cm: 76.6% vs. 61.2%; P<0.001) and were much more apt to be infiltrating or ulcerative lesions in the LNM+ (vs. LNM−) group, in addition to showing lesser differentiation (moderately differentiated adenocarcinoma/poorly differentiated adenocarcinoma/signet-ring carcinoma/mucinous carcinoma: 65.6% vs. 45.8%; P<0.001); displaying more lymphatic (44.5% vs. 19.4%; P<0.001), vascular (59.0% vs. 35.1%; P<0.001), or perineural (91.0% vs. 81.1%; P<0.001) invasion; and yielding more harvested lymph nodes (23.6±12.2 vs. 21.7±12.3; P=0.02). Lengths of proximal and distal resection margins did not differ significantly by group (Table 2).
Table 2. Pathological characteristics before and after PSM.
| Parameters | Before PSM | After PSM (1:1) | |||||
|---|---|---|---|---|---|---|---|
| LNM− (N=1,032) | LNM+ (N=256) | P value | LNM− (N=244) | LNM+ (N=244) | P value | ||
| Tumor size (cm) | |||||||
| <2 | 400 (38.8) | 60 (23.4) | <0.001 | 52 (21.3) | 60 (24.6) | NS | |
| ≥2 | 632 (61.2) | 196 (76.6) | 192 (78.7) | 184 (75.4) | |||
| Proximal resection margin (cm) | 12.2±5.97 | 12.4±4.33 | NS | 12.9±8.17 | 12.4±4.37 | NS | |
| Distal resection margin (cm) | 6.53±3.98 | 6.09±4.12 | NS | 6.24±4.04 | 6.17±4.17 | NS | |
| Gross type | |||||||
| Protruding | 658 (63.8) | 115 (44.9) | <0.001 | 118 (48.4) | 113 (46.3) | NS | |
| Infiltrate or ulcerative | 374 (36.2) | 141 (55.1) | 126 (51.6) | 131 (53.7) | |||
| Histology type | |||||||
| Well | 559 (54.2) | 88 (34.4) | <0.001 | 91 (37.3) | 87 (35.7) | NS | |
| Mod, Por, Sig, Muc | 473 (45.8) | 168 (65.6) | 153 (62.7) | 157 (64.3) | |||
| Infiltration form | |||||||
| A | 195 (18.9) | 23 (9.0) | <0.001 | 30 (12.3) | 20 (8.2) | NS | |
| B and C | 837 (81.1) | 233 (91.0) | 214 (87.7) | 224 (91.8) | |||
| Missing | |||||||
| Lymphatic invasion | |||||||
| No | 832 (80.6) | 142 (55.5) | <0.001 | 147 (60.2) | 142 (58.2) | NS | |
| Yes | 200 (19.4) | 114 (44.5) | 97 (39.8) | 102 (41.8) | |||
| Vascular invasion | |||||||
| No | 670 (64.9) | 105 (41.0) | <0.001 | 102 (41.8) | 104 (42.6) | NS | |
| Yes | 362 (35.1) | 151 (59.0) | 142 (58.2) | 140 (57.4) | |||
| Harvested lymph nodes | 21.7±12.3 | 23.6±12.2 | 0.02 | 22.6±15.2 | 23.5±12.2 | NS | |
| pT | |||||||
| 1 | 592 (57.4) | 85 (33.2) | <0.001 | 97 (39.8) | 84 (34.4) | NS | |
| 2 | 440 (42.6) | 171 (66.8) | 147 (60.2) | 160 (65.6) | |||
Data are presented as mean ± standard deviation or n (%). Protruding: type 0, superficial; type 1, protuberant, infiltrative or ulcerative; type 2, expansive ulceration; type 3, infiltrative ulcerating; type 4, diffusely ulcerating. LNM, lymph node metastasis; LNM−, lymph node metastasis negative; LNM+, lymph node metastasis positive; Mod, moderately differentiated adenocarcinoma; Muc, mucinous carcinoma; NS, not significant; Poor, poorly differentiated adenocarcinoma; pT, pathological T stage; PSM, propensity score matching; Sig, signet ring carcinoma; Well, well-differentiated adenocarcinoma.
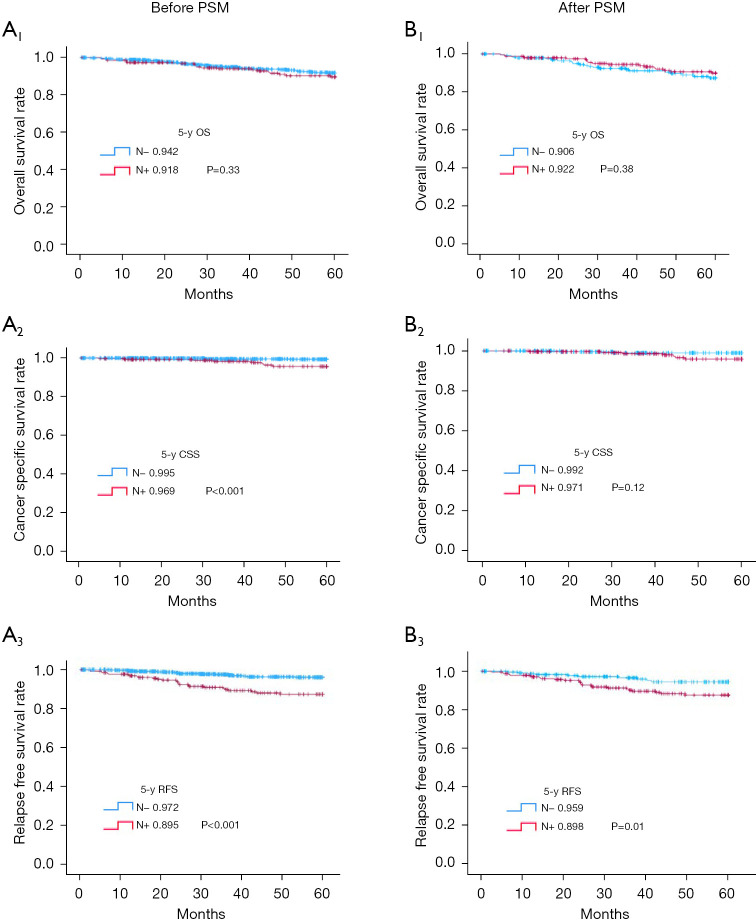
Although 5-year OS of both groups were similar (91.8% vs. 94.2%; P=0.33) (Figure 2, A1), 5-year CSS (Figure 2, A2) and RFS (Figure 2, A3) were significantly worse for the LNM+ (vs. LNM−) group (96.9% vs. 99.5% and 89.5% vs. 97.2%, both P<0.001 respectively).
Figure 2.
Survival outcomes for patients with for pT1–T2 patients with N− and N+ before and after PSM. (A1) 5-y OS before PSM, (A2) 5-y CSS before PSM, (A3) 5-y RFS before PSM, (B1) 5-y OS after PSM, (B2) 5-y CSS after PSM, (B3) 5-y RFS after PSM. CSS, cancer-specific survival; N−, lymph node metastasis negative; N+, lymph node metastasis positive; OS, overall survival; PSM, propensity score matching; pT, pathological T stage; RFS, relapse-free survival; y, year.
To accurately gauge the influence of each parameter on patient prognosis, Cox regression analysis was done before matching. Age ≥70 years [hazard ratio (HR) =2.1, 95% confidence interval (CI): 1.3–3.5; P=0.002], no comorbidity (HR =1.9, 95% CI: 1–3.5; P=0.04), postoperative complications (HR =2.2, 95% CI: 1.3–3.6; P=0.001), operative blood loss (HR =2.2, 95% CI: 1.4–3.6; P=0.001), and tumor size (HR =1.2, 95% CI: 1.1–1.3; P=0.003) emerged as independent risk factors for OS (Table 3). Independent risk factors for CSS were LNM+ (HR =5.4; 95% CI: 1.7–17; P=0.004) and operative blood loss (HR =4.3, 95% CI: 0.97–19; P=0.05) (Table 4). LNM+ was also an independent risk factor for RFS (HR =2.7; 95% CI: 1.5–4.6; P<0.001), as was age ≥70 years (HR =1.2, 95% CI: 0.7–2.1; P=0.05) and vascular invasion (HR =2.3, 95% CI: 1.3–4.2; P=0.004) (Table 5).
Table 3. Multivariate logistic regression analysis for OS before and after PSM.
| Variables | OS before PSM | OS after PSM | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| LNM+ vs. LNM− | 1.6 | 0.92–2.7 | 0.09 | 1.2 | 0.62–2.3 | 0.60 | |
| Sex (female vs. male) | 0.7 | 0.43–1.2 | 0.17 | 0.44 | 0.19–1 | 0.054 | |
| Age (≥70 vs. <70 years) | 2.1 | 1.3–3.5 | 0.002 | 3 | 1.4–6.3 | 0.004 | |
| Neoadjuvant chemotherapy (no vs. yes) | 2.9 | 0.89–9.2 | 0.07 | 3.9 | 0.86–17 | 0.07 | |
| Comorbidity (no vs. yes) | 1.9 | 1–3.5 | 0.04 | 4 | 1.2–14 | 0.02 | |
| Post-operational complications (no vs. yes) | 2.2 | 1.3–3.6 | 0.001 | 2.4 | 1.2–5 | 0.01 | |
| Operation method (laparoscopic vs. open) | 1.8 | 0.8–4 | 0.16 | 1.1 | 0.39–3.1 | 0.86 | |
| Operative blood loss (≥100 vs. <100 mL) | 2.2 | 1.4–3.6 | 0.001 | 2.8 | 1.4–5.6 | 0.003 | |
| Lymph node dissection (non-D3 vs. D3) | 0.88 | 0.39–2 | 0.76 | 1.6 | 0.61–4.2 | 0.34 | |
| Tumor size (cm) | 1.2 | 1.1–1.3 | 0.003 | 1.3 | 1–1.5 | 0.03 | |
| Lymphatic invasion (no vs. yes) | 1 | 0.57–1.8 | 0.99 | 0.78 | 0.38–1.6 | 0.51 | |
CI, confidence interval; HR, hazard ratio; LNM−, lymph node metastasis negative; LNM+, lymph node metastasis positive; OS, overall survival; PSM, propensity score matching.
Table 4. Multivariate logistic regression analysis for CSS and after PSM.
| Variables | CSS before PSM | CSS after PSM | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| LNM+ vs. LNM− | 5.4 | 1.7–17 | 0.004 | 4.7 | 0.84–27 | 0.07 | |
| Sex (female vs. male) | 0.32 | 0.07–1.5 | 0.15 | 0.21 | 0.023–1.8 | 0.15 | |
| Age (≥70 vs. <70 years) | 2.3 | 0.7–7.9 | 0.17 | 5.2 | 0.88–30 | 0.06 | |
| Neoadjuvant chemotherapy (no vs. yes) | 3.8 | 0.45–33 | 0.22 | 4.2 | 0.43–41 | 0.22 | |
| Comorbidity (no vs. yes) | 1.5 | 0.37–6.4 | 0.55 | 3.3 | 0.3–38 | 0.33 | |
| Operation method (laparoscopic vs. open) | 0.56 | 0.085–3.7 | 0.55 | 0.43 | 0.05–3.6 | 0.43 | |
| Operative blood loss (≥100 vs. <100 mL) | 4.3 | 0.97–19 | 0.05 | 10 | 1.8–61 | 0.009 | |
| Tumor size (cm) | 1.1 | 0.77–1.6 | 0.58 | 1.3 | 0.82–2.1 | 0.25 | |
| pT2 vs. pT1 | 1.9 | 0.44–8.1 | 0.39 | 2.1 | 0.23–19 | 0.51 | |
CI, confidence interval; CSS, cancer-specific survival; HR, hazard ratio; LNM−, lymph node metastasis negative; LNM+, lymph node metastasis positive; PSM, propensity score matching; pT, pathological T stage.
Table 5. Multivariate logistic regression analysis for RFS before and after PSM.
| Variables | RFS before PSM | RFS after PSM | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| LNM+ vs. LNM− | 2.7 | 1.5–4.6 | <0.001 | 2.4 | 1.2–5 | 0.01 | |
| Age (≥70 vs. <70 years) | 1.2 | 0.7–2.1 | 0.05 | 2.1 | 1–4.1 | 0.04 | |
| Tumor location (colon vs. rectum) | 1.4 | 0.78–2.4 | 0.28 | 1.2 | 0.6–2.5 | 0.59 | |
| CEA (≥2 vs. <2 ng/mL) | 1 | 0.48–2.1 | >0.99 | 0.87 | 0.33–2.3 | 0.77 | |
| Operation method (laparoscopic vs. open) | 1.8 | 0.7–4.8 | 0.21 | 1.2 | 0.37–4.1 | 0.73 | |
| Operative blood loss (≥100 vs. <100 mL) | 1.3 | 0.54–3.2 | 0.54 | 2.1 | 0.76–5.8 | 0.15 | |
| Tumor size (cm) | 1.1 | 0.93–1.3 | 0.27 | 1.2 | 0.93–1.4 | 0.19 | |
| pT2 vs. pT1 | 1.3 | 0.7–2.5 | 0.39 | 1.1 | 0.5–2.6 | 0.75 | |
| Vascular invasion (no vs. yes) | 2.3 | 1.3–4.2 | 0.004 | 1.80 | 0.84–3.7 | 0.13 | |
CEA, carcinoembryonic antigen; CI, confidence interval; HR, hazard ratio; LNM−, lymph node metastasis negative; LNM+, lymph node metastasis positive; PSM, propensity score matching; pT, pathological T stage; RFS, relapse-free survival.
PSM was carried out at 1:1 ratio, ensuring 244 patients in each group for univariate analysis. These matched groups did not differ significantly in clinicopathologic characteristics (Tables 1,2), and 5-year OS rates were similar (LNM+, 92.2%; LNM−, 90.6%; P=0.38) (Figure 2, B1). CSS differed significantly before matching but failed to do so after matching (LNM+, 97.1%; LNM−, 99.2%; P=0.12) (Figure 2, B2). However, 5-year RFS in the LNM+ (vs. LNM−) group was still significantly poorer (89.8% vs. 95.9%; P=0.01) (Figure 2, B3).
Multivariate analysis of the matched groups produced the same independent risk factors for OS as before matching (Table 3). Operative blood loss (HR =10, 95% CI: 1.8–61; P=0.009) was an independent prognostic factor for CSS (Table 4), with LNM+ (HR =2.4, 95% CI: 1.2–5; P=0.01) and age ≥70 years (HR =1.2, 95% CI: 1–4.1; P=0.04) identified for RFS (Table 5).
As a final effort, we analyzed recurrence distributions of the two groups. Before matching, overall recurrences in the LNM+ (vs. LNM−) group were significantly more frequent (10.5% vs. 2.8%; P<0.001), with significantly more liver (5.5% vs. 0.6%; P<0.001) and nodal (3.5% vs. 0.5%; P<0.001) involvement. After matching, overall recurrences in the LNM+ (vs. LNM−) group again were significantly greater (10.2% vs. 4.1%; P=0.008), with significant disparities observed for both liver (4.9% vs. 0.8%; P=0.007) and lymph node (3.7% vs. 0%; P=0.002) metastases (Table 6).
Table 6. Tumor recurrence status before and after PSM.
| Variables | Before PSM | After PSM | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total (n=1,288) | LNM− (n=1,032) | LNM+ (n=256) | P value | Total (n=488) | LNM− (n=244) | LNM+ (n=244) | P value | ||
| Total metastasis | 56 (4.3) | 29 (2.8) | 27 (10.5) | <0.001 | 35 (7.2) | 10 (4.1) | 25 (10.2) | 0.008 | |
| Liver | 20 (1.6) | 6 (0.6) | 14 (5.5) | <0.001 | 14 (2.9) | 2 (0.8) | 12 (4.9) | 0.007 | |
| Lymph node | 14 (1.1) | 5 (0.5) | 9 (3.5) | <0.001 | 9 (1.8) | 0 (0.0) | 9 (3.7) | 0.002 | |
| Lung | 21 (1.6) | 14 (1.4) | 7 (2.7) | NS | 14 (2.9) | 8 (3.3) | 6 (2.5) | NS | |
| Peritoneum | 2 (0.2) | 2 (0.2) | 0 (0.0) | NS | 0 (0.0) | 0 (0.0) | 0 (0.0) | NS | |
| Local recurrence | 10 (0.8) | 8 (0.8) | 2 (0.8) | NS | 4 (0.8) | 2 (0.8) | 2 (0.8) | NS | |
| Bone | 5 (0.4) | 3 (0.3) | 2 (0.8) | NS | 4 (0.8) | 2 (0.8) | 2 (0.8) | NS | |
| Brain | 1 (0.1) | 1 (0.1) | 0 (0.0) | NS | 1 (0.2) | 1 (0.4) | 0 (0.0) | NS | |
| Adrenal gland | 1 (0.1) | 0 (0.0) | 1 (0.4) | NS | 1 (0.2) | 0 (0.0) | 1 (0.4) | NS | |
| Mediastinum | 1 (0.1) | 0 (0.0) | 1 (0.4) | NS | 1 (0.2) | 0 (0.0) | 1 (0.4) | NS | |
Data are presented as n (%). LNM−, lymph node metastasis negative; LNM+, lymph node metastasis positive; NS, not significant; PSM, propensity score matching.
It may be suspected that five years of follow-up is not enough to fully capture the difference in long-term survival among pT1-2 CRC patients, therefore, we compared the ten years of follow-up between the two groups again. The 10-year OS of both groups were not significantly difference (89.5% vs. 93.5%; P=0.13) (Figure S1, A1). But the CSS (Figure S1, A2) and 10-year RFS (Figure S1, A3) were significantly worse for the LNM+ (vs. LNM−) group (96.5% vs. 99.2%; P=0.003 and 88.3% vs. 96.9%; P<0.001), but. After matching, the 10-year OS were similar (LNM+, 89.8%; LNM−, 90.6%; P=0.92) (Figure S1, B1). The 10-year CSS differed significantly before matching but failed to do so after matching (LNM+, 96.7%; LNM−, 99.2%; P=0.08) (Figure S1, B2). However, 10-year RFS in the LNM+ (vs. LNM−) group was still significantly worse (88.5% vs. 95.1%; P=0.01) (Figure S1, B3).
Discussion
Present study findings indicate that despite a significant drop in RFS for the LNM+ (vs. LNM−) group, CSS and OS in patients with pT1-2 CRC remained unaffected by lymph node spread. The sum of nodal metastases is nonetheless an important determinant of patient prognosis and survival imposed by various cancers (13,14). Indeed, the TNM system relies considerably on LNM status in CRC subclassification. A combination of FOLFOX (folinic acid, fluorouracil, and oxaliplatin) is subsequently advised as adjuvant chemotherapy for 6 months after surgery in patients with stage III CRC, using CAPOX (capecitabine and oxaliplatin) instead for just 3 months in those patients at low risk of recurrence; and the benefits achieved are demonstrable (1).
Based on the current classification of colorectal, appendiceal, and anal cancer applied in Japan, 5-year OS rates of patients with CRC are presently at 93% for stage I, 88.2% for stage II, and 92.5% for stage IIIA, dropping sharply to 81.0% for stage IIIB (1). However, these obvious figure fluctuations may be readily explained. Because clinical trials in Japan have shown chemotherapy for stage II CRC to be inconsequential, having no effect on prognosis, adjuvant treatment is generally not recommended. Yet, there is recently published evidence of improved survival in high-risk patients with stage II CRC (defined by clinicopathologic rather than TNM criteria) after adjuvant chemotherapy (15). It is thus incumbent upon guidelines of the EU, United States, and China to better address stage II CRC, acknowledging subsets that warrant postoperative chemotherapy for more favorable outcomes in high-risk patients. As data ostensibly indicate, conventional adjuvant regimens given for stage III (pT1-2N+) CRC do seem beneficial, although confirmation must await relevant prospective clinical studies.
During our assessment, we also found that patient age, preoperative comorbidities or postoperative complications, excessive intraoperative bleeding, and tumor size all impacted OS in patients with CRC. Of particular interest was the finding that operative blood loss remained the sole predictor of CSS after matching, having shared such distinction before matching with LNM+. This underscores the importance of careful hemostasis during surgery, fully respecting embryonic dissection planes (16,17) to bolster patient prognosis.
We similarly determined postoperative CRC recurrence to be an important prognostic factor. According to our data, 10.5% of patients in the LNM+ group experienced recurrences of stage IIIA CRC (primarily hepatic and nodal), even after routine adjuvant therapy. Aggressive secondary surgery to remove resectable metastases of liver or local lymph nodes is often worthwhile (18,19). Such strategies likely account for the lack of impact on OS and CSS documented in recurrence prone LNM+ group members following PSM.
Recent research into RAS and BRAF mutations (20), especially with regard to microsatellite instability, has shown substantial bearing on CRC prognosis (21). Coupled with ongoing advances in circulating tumor DNA (ctDNA) testing, investigators are now equipped with the capacity for genetic analysis of patients, affording classification of CRC without TNM constraints (22). Existing guidelines typically recommend re-examinations every three months in instances of advanced CRC (1), but whether or how to postoperatively monitor stage I CRC is controversial. The findings herein appear to reinforce a need for extended follow-up intervals, reducing visit frequencies in patients with stage I (vs. stage II or III) CRC to mitigate medical costs and radiation exposures.
The biological behavior of pT1-2 CRC is relatively mild, and LNM+ CRC often receive adjuvant chemotherapy (such as FOLFOX or CAPOX regimens), which can effectively reduce the risk of recurrence and extend survival. The application of adjuvant chemotherapy may counteract the negative effects of relapse on OS and CSS. Our study showed that the LNM+ group was most prone to liver metastasis and LNM. These metastases can be effectively controlled by surgical resection or local treatment (such as radiofrequency ablation, radiotherapy, etc.) This aggressive treatment strategy may be one of the reasons why OS and CSS are not significantly affected in LNM+ CRC patients.
A retrospective and non-randomized, single-center study of this sort has inherent limitations, particularly the issue of confounding variables. Although we adjusted for baseline patient differences through PSM, However, since pT1-2N0 CRC patients do not need adjuvant chemotherapy, it is still unknown how much benefit adjuvant chemotherapy can bring to pT1-2N+ CRC patients. For reference, in the guidelines for the treatment of gastric cancer in Japan, adjuvant chemotherapy is not required for patients with gastric cancer in T1N1. Whether patients with T1N1 CRC can also be exempted from adjuvant chemotherapy needs to be further confirmed in the future. Finally, due to the small number of cases, we did not separate rectal cancer from colon cancer, which may lead to some bias. Future prospective multi-center clinical trials are expected to further clarify the significance of LNM in pT1-2 CRC.
Conclusions
LNM+ pT1-2 CRC patients had a higher risk of hepatic and nodal recurrence, but long-term OS and CSS were unaffected. Perhaps an appropriate downstaging of pT1-2N+ CRC from stage IIIA is a reasonable prospect.
Supplementary
The article’s supplementary files as
Acknowledgments
The research results in this paper were presented orally at the SAGES Conference in the United States from March 12–15, 2025.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Ethics Committee of Saitama Medical University International Medical Center (No. 19-006) and individual consent for this retrospective analysis was waived.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-2024-982/rc
Funding: This study was supported by the Shenzhen High-level Hospital Construction Fund.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-2024-982/coif). The authors have no conflicts of interest to declare.
Data Sharing Statement
Available at https://jgo.amegroups.com/article/view/10.21037/jgo-2024-982/dss
References
- 1.Hashiguchi Y, Muro K, Saito Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol 2020;25:1-42. 10.1007/s10147-019-01485-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Japanese Society for Cancer of the Colon and Rectum . Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma: the 3d English Edition [Secondary Publication]. J Anus Rectum Colon 2019;3:175-95. 10.23922/jarc.2019-018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song B, Wang L, Chen Y, et al. The Significance of Skip Lymph Node Metastasis in Colorectal Cancer. Anticancer Res 2023;43:4169-77. 10.21873/anticanres.16608 [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto Y, Takahashi K, Yasuno M, et al. Clinicopathological characteristics of skipping lymph node metastases in patients with colorectal cancer. Jpn J Clin Oncol 1998;28:378-82. 10.1093/jjco/28.6.378 [DOI] [PubMed] [Google Scholar]
- 5.Wang LM, Hirano YM, Ishii TM, et al. The role of apical lymph node metastasis in right colon cancer. Int J Colorectal Dis 2020;35:1887-94. 10.1007/s00384-020-03661-4 [DOI] [PubMed] [Google Scholar]
- 6.O'Sullivan B, Brierley J, Byrd D, et al. The TNM classification of malignant tumours-towards common understanding and reasonable expectations. Lancet Oncol 2017;18:849-51. 10.1016/S1470-2045(17)30438-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011;14:101-12. 10.1007/s10120-011-0041-5 [DOI] [PubMed] [Google Scholar]
- 8.Marom G. Esophageal Cancer Staging. Thorac Surg Clin 2022;32:437-45. 10.1016/j.thorsurg.2022.06.006 [DOI] [PubMed] [Google Scholar]
- 9.Watanabe M, Toh Y, Ishihara R, et al. Comprehensive registry of esophageal cancer in Japan, 2015. Esophagus 2023;20:1-28. 10.1007/s10388-022-00950-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mukai M, Kishima K, Fukumitsu H, et al. Is the T1/2N1 (≤3 nodes) category actually stage IIIA (TNM)/IIIa (Japanese classification) in patients with primary colorectal cancer? Oncol Rep 2011;26:209-14. 10.3892/or.2011.1280 [DOI] [PubMed] [Google Scholar]
- 11.Wang L, Hirano Y, Heng G, et al. Prognostic Utility of Apical Lymph Node Metastasis in Patients With Left-sided Colorectal Cancer. In Vivo 2020;34:2981-9. 10.21873/invivo.12129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L, Cai X, Chen Y, et al. Should pT2N+ Colorectal Cancer Be Downstaged from IIIA to IIA? Anticancer Res 2023;43:5681-8. 10.21873/anticanres.16773 [DOI] [PubMed] [Google Scholar]
- 13.Kotake K, Mizuguchi T, Moritani K, et al. Impact of D3 lymph node dissection on survival for patients with T3 and T4 colon cancer. Int J Colorectal Dis 2014;29:847-52. 10.1007/s00384-014-1885-z [DOI] [PubMed] [Google Scholar]
- 14.Wang L, Hirano Y, Heng G, et al. The Significance of Lateral Lymph Node Metastasis in Low Rectal Cancer: a Propensity Score Matching Study. J Gastrointest Surg 2021;25:1866-74. 10.1007/s11605-020-04825-x [DOI] [PubMed] [Google Scholar]
- 15.Oki E, Ando K, Taniguchi H, et al. Sustainable Clinical Development of Adjuvant Chemotherapy for Colon Cancer. Ann Gastroenterol Surg 2022;6:37-45. 10.1002/ags3.12503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobayashi H, West NP, Takahashi K, et al. Quality of surgery for stage III colon cancer: comparison between England, Germany, and Japan. Ann Surg Oncol 2014;21 Suppl 3:S398-404. 10.1245/s10434-014-3578-9 [DOI] [PubMed] [Google Scholar]
- 17.West NP, Kobayashi H, Takahashi K, et al. Understanding optimal colonic cancer surgery: comparison of Japanese D3 resection and European complete mesocolic excision with central vascular ligation. J Clin Oncol 2012;30:1763-9. 10.1200/JCO.2011.38.3992 [DOI] [PubMed] [Google Scholar]
- 18.Ishihara S, Hayama T, Yamada H, et al. Prognostic impact of primary tumor resection and lymph node dissection in stage IV colorectal cancer with unresectable metastasis: a propensity score analysis in a multicenter retrospective study. Ann Surg Oncol 2014;21:2949-55. 10.1245/s10434-014-3719-1 [DOI] [PubMed] [Google Scholar]
- 19.Katoh H, Yamashita K, Kokuba Y, et al. Surgical resection of stage IV colorectal cancer and prognosis. World J Surg 2008;32:1130-7. 10.1007/s00268-008-9535-7 [DOI] [PubMed] [Google Scholar]
- 20.Díez-Alonso M, Mendoza-Moreno F, Jiménez-Álvarez L, et al. Prognostic factors of survival in stage IV colorectal cancer with synchronous liver metastasis: Negative effect of the KRAS mutation. Mol Clin Oncol 2021;14:93. 10.3892/mco.2021.2255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kubo T, Hirohashi Y, Matsuo K, et al. Mismatch Repair Protein Deficiency Is a Risk Factor for Aberrant Expression of HLA Class I Molecules: A Putative "Adaptive Immune Escape" Phenomenon. Anticancer Res 2017;37:1289-95. 10.21873/anticanres.11446 [DOI] [PubMed] [Google Scholar]
- 22.Merk C, Martling A, Lindberg J, et al. Circulating tumor DNA (ctDNA) in adjuvant therapy of early stage colon cancer: current status and future perspectives. Acta Oncol 2022;61:523-30. 10.1080/0284186X.2022.2033831 [DOI] [PubMed] [Google Scholar]