Abstract
Osteosarcoma (OS) is the most common primary bone malignancy, with lung metastasis being the leading cause of mortality. The metastatic process is driven by complex biological mechanisms, including tumor cell-specific adaptations of growth pathways, immune modulation within the tumor microenvironment, and reactivation of metastatic cells from dormancy. This scoping review captures overlooked and under researched pathways, supporting mainstream therapeutic targets while shedding light on novel ones, reinforcing and revising conclusions drawn in previous literature, and guiding future research. MEDLINE, Embase, and Cochrane CENTRAL were searched with a publication date limit from 2019 onwards using relevant MeSH terms combined with Boolean operators, truncations, and keyword searches. The search culminated in 43 reports, including 30 in vivo, 8 in vitro, and 5 observational studies. This study conforms to the PRISMA-ScR guidelines. Tumor cell adaptations, including epithelial-mesenchymal transition (EMT) and enhanced migratory and proliferative signaling via JAK/STAT and TGF-β pathways, are critical drivers of OS lung metastasis. Manipulated upstream ligand-driven signaling promotes transcriptional changes that increase cell cycle proteins and mesenchymal markers, conferring chemoresistance and advancing OS cells toward a metastatic state. The tumor microenvironment also plays a key role; interactions between OS cell-derived cytokines and tumor-infiltrating immune cells lead to tumor associated macrophages and neutrophils (TAMs/TANs), which help establish a pre-metastatic niche and provoke immune remodeling. However, the impact of TAMs on OS survival remains ambiguous due to their dual pro- and anti-tumor roles. Lung-induced dormancy links tumor intrinsic and immune-driven mechanisms, allowing tumor cells to evade immunity or pause progression. Inflammatory pathways and immune activation can reverse dormancy, promoting further OS dissemination. The reviewed evidence supports targeting intracellular signaling and immune pathways to mitigate OS metastasis. The paucity of longitudinal data on lung dormancy warrants caution, emphasizing integrated approaches and better controlled studies with focus on combinatorial therapies for more conclusive outcomes.
Keywords: osteosarcoma, sarcoma, metastasis, translational biology, lung colonization
Introduction
Osteosarcoma (OS) is the most common primary bone tumor, accounting for almost 50% of malignant bone tumors. 1 It is a condition characterized by the abnormal formation of osteoid and bone tissue from tumor cells, with epidemiological observations indicating that younger age is a significant risk factor associated to the onset of OS.1,2 The survival rate of OS is approximately 60–70% and if diagnosed early, localized OS has a 5-year overall survival of >70%. 3 Up to 20% of individuals with osteosarcoma present with metastasis, most commonly to the lungs, and this cohort of patients face a 5-year overall survival rate of less than 30%, with a large incidence of relapse in initially successfully treated patients. 3
Currently, the cornerstone of treatment for OS lung metastasis is surgical resection and a chemotherapy regimen, however, the gold standard management of both synchronous OS lung metastasis and relapse is unclear. 4 Study of the biological mechanisms that underscore the metastasis of OS to the lungs, and determinants of the survival of OS within the lung parenchyma have been postulated, in pursuit of synthesising biomarkers to help flag early micro metastasis, while also streamlining the introduction of less invasive forms of therapy, particularly immunotherapies. Commonly discussed biological pathways in the literature include: the adaptations of intracellular mechanisms and their interplay with metastatic lung progression; immune modulation in OS lung metastasis within the tumor microenvironment; and lung-induced metastatic dormancy. These all present targets for novel therapies to combat OS lung metastasis, however, conflicting evidence appears throughout the literature when studying the efficacy of immunotherapies specific to elements of these pathways.
In 2019, the Children's Oncology Group articulated a variety of inquiries regarding OS lung metastasis. 5 They sought to understand the intrinsic adaptations or signals that permit OS cells to survive and thrive within pulmonary tissues, and the mechanism by which lung-derived signals activate survival pathways in these tumor cells. These pivotal questions have helped guide the themes of our review, honing in on key intracellular signaling pathways and the interaction between tumor and microenvironment within the lungs to help address these gaps in the current literature available. By focusing on extracting relevant data from contemporary studies surrounding the topic, we provide an up-to-date evaluation of the biological drivers of lung colonization in OS and factors actively inhibiting the encroachment of metastatic cells upon the lung tissue while touching upon newly researched therapeutic interventions throughout where suitable.
Methods
PubMed, Embase, and Cochrane CENTRAL were searched for full text articles in the English language from the years 2019–2025 that focused on biological mechanisms underscoring lung metastasis in OS, with the most recent search conducted on 23/02/2025. The Children's Oncology Group article in 2019 guides the objectives of this article as alluded to earlier, justifying the publication date of our search strategy. Non-English articles were excluded due to the core literature base being in English and the lack of resources to adequately translate literature while ensuring minimal misinterpretation of key findings.
Studies focusing on non-lung metastases, unless findings also address lung involvement, were excluded. Purely clinical studies without biological insights or mechanistic focus were also excluded. As there is sufficient volume and diversity in published research to identify key mechanisms, map research trends and highlight knowledge gaps, only published articles have been included. Using only credible, rigorously reviewed studies may help improve the reliability of thematic synthesis.
Our MeSH terms with appropriate truncations and Boolean operators ensured a high sensitivity of detection of relevant articles, with the following terms used for search purposes: “Osteosarcoma”, “Bone Neoplasms”, “Lung Neoplasms”, “Neoplasm Metastasis”, “Pulmonary Neoplasms”, “Biological Factors”, “Signal Transduction”, “Cell Movement”, “Angiogenesis Inducing Agents”, “Epithelial-Mesenchymal Transition”. These terms were paired with key words, “osteosarcoma”, “lung” and “metastasis”, to cast a wide net and account for any human error in indexing medical terms. 6 These terms and limits are conducive to capturing the most relevant studies on the subject at hand while filtering out irrelevant clinical or therapeutic-only studies. The described approach ensured a balance between a manageable dataset for review, with concurrent maximal coverage of high-quality, recently conducted research.
The title-abstract screening was undertaken blindly by three reviewers, A.B, A.I and A.M, to reduce the chance of error and to add more scrutiny during the screening process. 1908 articles were eligible for initial screening process after duplicates were removed. Once articles satisfying our eligibility assessment were determined, a total of 43 studies were included for data retrieval, comprising 30 in vivo studies, 8 in vitro studies, and 5 observational studies.
Among the studies retrieved and included, the reference lists of pertinent papers were also scrutinized to identify additional articles of interest for our scoping review.
These articles were subsequently selected for data extraction, using a data collection spreadsheet finalised after being piloted by two reviewers, A.B and A.I, on five of the articles (randomly selected by random number generator) and thereby refined for clarity before full use. The data extraction process was completed independently by A.B, A.I and A.M, with reviewers retrieving data separately and then comparing to catch errors. The main biological mechanisms outlined in each article reviewed were highlighted in the data extraction process and used to thematically group, aswell as summarise data in a manner, streamlining integration of the condensed information into the finalised review under an appropriate set of headings.
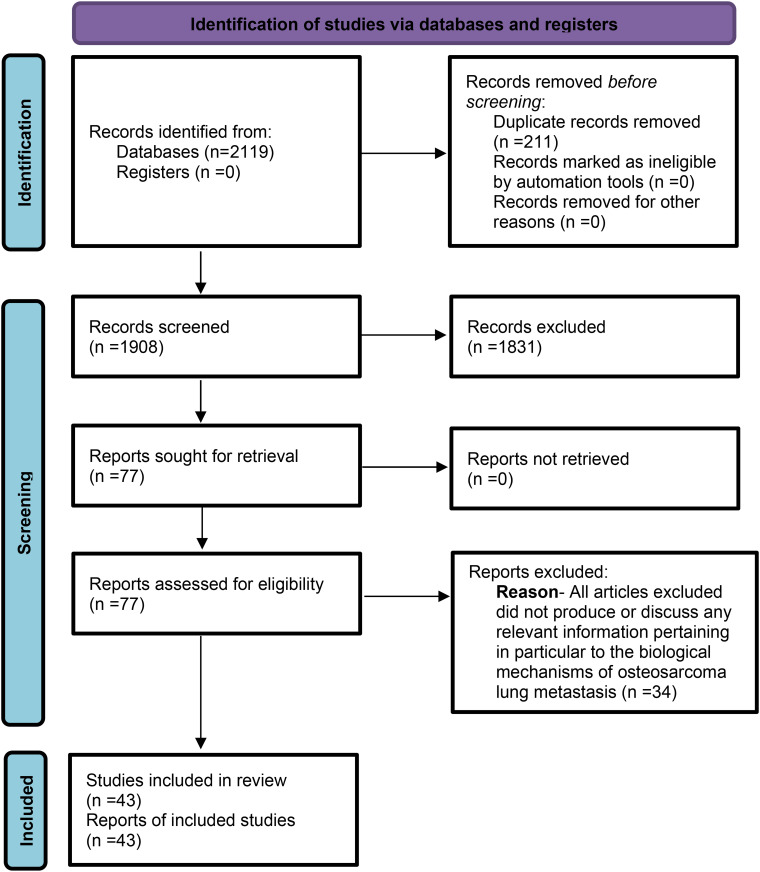
To ensure an objectively thorough reporting of our findings and methodological transparency, the structure of the scoping review was modeled in compliance with the PRISMA-ScR guidelines, which helped also form our review protocol that was followed throughout, however this protocol has not been registered or published (Figure 1). 7 As touched upon, OS lung metastasis is an evolving field of study, underpinned by a multitude of intersecting biological processes that each require careful examination. A scoping review affords the opportunity to map this widely heterogenous topic by allowing a collation of research produced from varying research study designs. 8 In the midst of complex, poorly defined concepts, this framework enables the consolidation of key research findings to formally clarify them and identify gaps in current understanding, serving as a stepping stone to guide future research.
Figure 1.
PRISMA-SCR Flowchart Illustrating the Process of Article Identification, Screening, Eligibility Assessment, and Inclusion for Data Extraction. This Diagram Outlines the Number of Records Retrieved, Screened, Excluded, and Ultimately Included in the Final Review.
Specific Adaptations and Intracellular Signals of Tumor Cells
WNT/ β - Catenin Signaling
The G-protein coupled Frizzled (FZD) receptors have an established role in β-catenin signaling. Activated by wingless integrated (WNT) ligands, there are ten FZD receptors (FZD1-FZD10) which facilitate the subsequent induction of canonical and non-canonical pathways. 9 These signaling pathways contain many biological targets relevant to the tumorigenesis of OS such as MYC, cyclin D1 and axin, however upstream biological targets, in particular the initial signaling steps are also a point of interest. 10 Therapeutic targeting of the canonical WNT/β-catenin signaling pathway is particularly important due to its role in stem cell renewal and bone regulation during embryogenesis, along with its activation in OS, which is associated with invasion and metastasis.11,12
In OS, FZD7 expression is increased, with its upregulation associated with epithelial-to-mesenchymal transition (EMT). 13 EMT is characterized by a loss of epithelial cell markers, eg E-cadherin, with subsequent upregulated expression of mesenchymal cell markers, eg Fibronectin and Vimentin.14–16 This leads to a loss of cell polarity and intercellular adhesion, alongside concurrent increased cell motility and invasiveness. This is significant, as increasing EMT expression is associated with a higher capacity for primary tumor cell dissemination, migration, cluster survival in circulation, extravasation, metastatic colonization and chemoresistance. 16
One of the other FZD receptors, FZD8, is reported to be activated by WNT2 ligand and downregulated by miR-100 or miR-520b, acting to promote cell proliferation, migration, and invasion via activation of WNT/β-catenin pathway. 17 FZD9, also implicated in OS progression, has an essential role in osteoblast differentiation for bone formation and pre-B cell development. Depending on the tumor, FZD9 interacts with several WNT ligands including WNT2, WNT5a, and WNT3a, to promote EMT and invasiveness. 18 It has been discovered that proto-oncogene c-Fos determines FZD9 and WNT2 expression which as alluded to, are associated with an aggressive OS phenotype, with FZD9 in particular frequenting later stages of OS. 19 Other molecular targets of the WNT/β-catenin pathway include chemokine receptor-9, COL5A2, also associated with EMT and OS metastasis when overly expressed. 20 Discovery of these molecular targets has prompted further research into therapeutic agents to combat them, and promising synthetic and naturally derived agents include: metillin, tegavivint and echinatin.21–23
JAK/STAT Signaling
The JAK/STAT signaling pathway strongly influences immune regulatory processes in tumor cell recognition and its role can be described as a double-edged sword, either directing towards a tumor progressive-state or a tumor inhibitory state. 24 STAT1 and 2 are recognised as maintaining anti-tumor defence, while STAT3 and 5 are associated with tumor initiation and progression. 24
Interleukin 6 (IL-6) as a prominent activator of the JAK/STAT signaling pathway is well recognised, and it is highly expressed in OS. 25 IL6 interacts with STAT3 in a positive feedback loop-like pattern, conducive to excessive activation of chronic inflammatory pathways, provoking tumor progression. 25 Additionally, anti-apoptotic proteins are also influenced by STAT, in particular STAT5, which works to inhibit Bcl-2, reducing tumor cell sensitivity to apoptosis. 26 STAT3 alternatively, when activated, upregulates PD-L1 expression, leading to immune evasion, and its phosphorylation enhances OS cell survival through resistance to chemotherapy.25,27 The relationship between STAT3 and PD-L1 is further highlighted by the effects of sunitinib, a STAT3 inhibitor, that resulted in significant reductions in PD-L1 expression and OS progression, effectively remodeling the immune system by restoring immune surveillance. 27
The overarching relationship of STAT with OS progression is delineated by novel STAT3-specific inhibitory immunotherapies such as TPCA-1. This therapy in particular was shown to halt OS progression by reducing MMP-2 and cyclin-D1 levels, which under normal circumstances both promote tumor progression through cell cycle maintenance and EMT.28,29 Other interesting immunotherapies include curcumin, reflected by the effects of curcumin analog L48H37 on suppressing OS cell migration and invasion by manipulating & inhibiting the JAK/STAT signaling pathway. 30 Additional immunotherapies targeting this pathway include the JAK2 inhibitors AG490 and telocinobufagin.31,32 These therapies result in a reduction of OS cell proliferation, migration, and invasion by inhibiting the JAK2/STAT3 pathway in particular. Combination therapies inhibiting both JAK/STAT and PD-L1 may enhance therapeutic efficacy when compared to monotherapies, by stifling the downstream effects of JAK/STAT activation too. Future directions of therapy may include modulation of the delivery system of these drugs to enhance bioavailability, such as nanoparticle drug delivery systems or alternatively using gene editing techniques such as CRISPR-Cas9 to silence STAT3. 33
In OS, several long non-coding RNAs are altered, mediating tumor progression and metastasis through modulating the JAK/STAT pathway. 34 An example of this is microRNA-101, which inactivates the PI3K/AKT and JAK/STAT3 signaling pathways, through the downregulation of ROCK-1 (Rho-associated kinase 1), subsequently inhibiting OS tumor growth and metastasis. In the absence of inhibition, ROCK-1 and STAT3 engage in a positive feedback loop, with ROCK-1 promoting STAT3 phosphorylation and STAT3 promoting ROCK-1 transcription, in turn resulting in EMT. 35 Similarly, microRNA-126 inhibits proliferation, migration, invasion in OS by targeting and silencing the ZEB1 (Zinc Finger E-box Binding Homeobox 1) gene consquently inactivating the JNK and JAK1/STAT3 pathways, which under normal circumstances suppress epithelial markers (eg, E-cadherin/CDH1) to promote mesenchymal traits (eg, N-cadherin/CDH2, vimentin). 36 Notably, unlike carcinomas, which undergo EMT as a key step in metastasis, OS is a mesenchymal-origin tumor, however, suppressing epithelial markers and promoting mesenchymal traits in OS can still influence tumor progression through mechanisms analogous to EMT.
NOTCH Signaling
The NOTCH signaling pathway is found to promote OS tumor growth and metastasis through promoting cell cycle expression and EMT, with NOTCH-1 displaying the strongest association with poorer outcomes in OS cases.37,38
The NOTCH target genes, HES1 and HEY2, both belonging to the “Hairy and Enhancer of Split” family of transcription factors, were significantly upregulated in OS patient samples and associated with a poor prognosis. 39 HES1 specifically correlates with a worse response to chemotherapy and thereby can be used as a potential marker to stratify patients with regards to their prognosis post-chemotherapy, guiding individualised therapy. 40 A ligand of NOTCH and activator of this pathway, JAG1, was found to be positively associated with the metastasis and recurrence of OS. 41 CEMIP, a protein found to regulate OS progression by manipulating the NOTCH pathway, like JAG1, promotes OS growth and metastases when overexpressed. 42
The expressions of NOTCH1, NOTCH2, HES1, and Delta-like 1 gene (DLL1) (a ligand in NOTCH signaling pathway) were significantly upregulated in a highly invasive and metastatic LM7 OS cell line, compared to normal human osteoblasts and the SaOS-2 cell line, which has a lower metastatic potential. 43 This highlights the contribution of NOTCH pathway to initiating, maintaining and enhancing OS progression, and conferring a higher metastatic potential to OS cells. Additionally, JAG1 expression was significantly higher in the highly metastatic F5M2 cell lines compared to the less metastatic F4 OS cells, exhibiting its function as a potent activator of the NOTCH pathway and the deleterious effects this poses on OS progression. 41
Therapeutic interventions focusing on this pathway for the purposes of halting OS progression centre upon combatting NOTCH-1 signaling. Naturally derived compounds such as curcumin and oleanolic acid both proved to halt OS progression by varying mechanisms including modulating microRNA expression to help suppress biological mechanisms promoting OS. 38 MiRNAs were shown to have variable effects on NOTCH signaling, with some such as miR-34a downregulating DLL1, NOTCH 1 and NOTCH 2 thereby regulating OS invasiveness, while others such as miR-199b-5p and miR-135b advance OS progression.44–46
Receptor Tyrosine Kinase (RTK) Signaling
Multiple receptor tyrosine kinases (RTK) are implicated in aggressive forms of OS. 47 Through fibrogenic programming, FGFR (fibroblast growth factor receptor), an RTK, was found to aid the growth of metastatic OS in the lung without impacting the growth of the primary tumor. 48 Fibronectin from resulting fibrosis initiated by FGFR promoted fibrogenic programming and OS growth within lung microenvironment; notably fibroblastic programming was found to confer a huge advantage in the synthesis of macrometastes. 48 Through the use of nintendinab, a known antifibrotic drug used in idiopathic pulmonary fibrosis, the myofibroblastic programming process was limited by targeting the FGFR-fibronectin axis in its entirety, thereby presenting itself as a druggable target to halt progression of OS metastasis within the lungs.48,49
IGF1R, a marker used to indicate OS invasiveness and metastasis is commonly out of balance with IGF binding proteins (IGFBPs) in aggressive OS cell lines. 50 Despite IGF1R's prominent role in advancing the spread of OS to distant sites, therapeutic agents targeting this receptor signaling pathway have shown little success, mainly secondary to resistance. 51 Research suggests that the use of dual- therapies, with the inhibition of multiple targets, is more effective, illustrated by experiments conducted on a orthotopic murine xenograft model, wherein both receptors, IGF1R and EGFR were targeted concurrently in a 143B human OS cell line, resulting in reduction in both tumor growth and the number of metastases. 51
AXL is part of a subgroup family of Receptor tyrosine kinases, ‘TAM’, which is made up of Tyro3, AXL, and MerTK. 52 AXL has been associated with recurrence and metastasis of OS and is an independent predictor of worse outcomes and poorer prognoses in OS patients. 53 Overexpression in OS is conducive overactivation of PI3K/AKT and MAPK/ERK pathways, which promote Hippo signaling, allowing YAP1/TAZ activation in a positive feedback loop with AXL expression, aiding OS migration and contributing to chemoresistance.54–58 An experiment using a model replicating the lung microenvironment studied circulating tumor cells (CTC's) in pulmonary vasculature and other areas of lung, away from the primary loci (PT cells), where human derived OS cells were first injected into the trachea (2D cells) and travel to in the lung. 59 This report found significant upregulation of EPHB2, FGFR2, and RET mRNA in CTC's compared to PT cells, but also in PT cells compared to 2D cells (except here, FGFR2 was downregulated in comparison), showing the effect of tumor microenvironment in lungs on altering RTK profiles that influence the OS metastatic cascade. 59 The same experiment also showed upregulation of YAP1 and TAZ in CTC's, which are commonly overexpressed in OS due to Hippo pathway dysregulation, which as alluded to, results in AXL overexpression. 59 It was found here that metastasis-prone OS as compared to cells with lower metastatic ability expressed higher levels of AXL, and that SGI-7079, a Gas-6 (ligand of AXL) inhibitor, slowed migration of metastasis prone OS cells. 59 Additionally, CTC's were found to express EMT-related transcription factors such as ZEB-1, suggesting these cells had undergone EMT, and thereby there is potential interaction between YAP/TAZ/AXL and EMT. 59 Associations between the AXL pathway itself and EMT has also been highlighted, wherein one study showed strong positive correlation between a form of AXL and MMP-9 expression in those OS patients, where MMP-9 through extracellular matrix degradation causes loss of E-cadherin-based adhesion allows epithelial cells to transition into mesenchymal-like invasive cells. 60
Overexpression of MET, another RTK, and its ligand HGF (hepatocyte growth factor), was found to be involved in the proliferation, invasiveness and metastasis of OS cells. 61 Immunohistochemical analysis revealed MET overexpression in 82% of OS metastases. 62 A multitude of various MET inhibitors have been tested with success, one being K252a, that was found to reverse migratory capability induced by HGF activation of MET and consequently PKB/AKT growth pathway in two OS cell lines. 63 Other inhibitors include anlotinib, PHA-665752 and Cabozantinib, that all work through various mechanisms to inhibit MET and successfully demonstrate efficacy in hindering OS progression.64–66 Cabozantinib, an inhibitor of RET posited to contribute to OS migration, was shown to promote OS migration as opposed to cell growth in aggressive cell lines, which celebrated a 71.4% progression-free survival rate in OS patients at 4 months. 67
RANK/RANKL/OPG Signaling
OS has been associated with a deregulated RANKL/OPG balance resulting in pathological bone features, but also, tumor invasiveness and dissemination. 68 Under normal conditions, RANKL, a protein produced by osteoblasts stimulates osteoclastogenesis via binding to RANK on osteoclastic surface, whereas OPG antagonises this effect, competing with RANK as a receptor binding RANKL, thereby inhibiting RANK activation and associated osteoclastogenesis. 69
RANKL is found to be produced by OS tumors and cell lines that also express RANK. 68 RANKL-RANK signaling in osteoclast precursors, promoted by the production of RANK-L from OS cells triggers osteoclastogenesis and bone destruction. 70 In turn, this process releases growth factors to attract and stimulate tumor cell growth, propagating a “vicious cycle” between bone destruction and tumor expansion with more OS cells producing RANK-L and thereby promoting further bone destruction, reinforcing RANK-L's role in OS growth and migration. 69 The significance of RANK-L in spurring on OS lung metastasis is illustrated by the reduction in tumor growth displayed when an OS cell line did not express RANK-L, demonstrating the dependence of this axis on RANK-L to promote OS lung metastasis through bone destruction. 68 This axis however does not solely rely on direct osteoclastogenesis, and instead, it's been shown that OS cells are capable of intrinsically produce cytokines, chemokines, growth factors, and hormones to encourage RANKL- RANK interactions through PTHrP-induced OPG suppression, resulting in further bone destruction, thereby indirectly causing OS growth. 69
Autocrine interactions involving the RANK-RANKL axis have also been observed, separating this from the previously discussed OS cell-osteoclast interactions that promote osteoclastogenesis. These autocrine interactions involving RANK expressing OS cells that release RANKL which then in turn directly binds to the RANK receptor on OS cells, is proposed to stimulate EMT through enhanced expression of the NF-kB pathway. This conclusion is supported by the reduced migration and invasion of OS cells following treatment with the NF-κB inhibitor dimethyl fumarate. 71
An autocrine-like activation of OS cells however is disputed by one study suggesting that RANK expression was absent when studying OS cells but present in other cells within the OS samples taken, for instance osteoclast precursors, whereas in contrast, OS cells within the same sample expressed RANKL with 37% (29/79) of samples exhibiting ≥10% RANKL positive tumor cells. 72 Therefore an argument can be made that a RANK- osteoclast mediated interaction is more likely to contribute to OS progression as opposed to an autocrine-like interaction. This hypothesis is further supported by one study assessing the effect of RANK/RANKL inhibition, with results demonstrating that this axis inhibition is only significant when targeted towards osteoclasts resulting in delayed tumor initiation and prolonged lifespan, whereas when focused upon osteoblasts solely, there is negligible effect exerted upon OS. 73
Although RANK-L does not have a direct impact on proliferation of RANK-expressing OS cells, data suggests there is a direct effect on the cell differentiation process through an autocrine process. 68 Interestingly, RANK expression serves as a factor of good prognosis, due to pro-differentiation effect of RANK-L on these cells, notably however, the same study found in immune-compromised mice, RANK expression by tumor cells favours the occurrence of lung metastases. This finding can be extrapolated to patients undergoing chemotherapy, which is a common case in OS patients, thereby inhibition of this signaling may be of major relevance for preventing metastases. 68 The cell differentiation effect of RANK-L may explain the effects of denosumab, a RANK-L inhibitor, when used both alone and with doxorubicin. This experiment showed an insignificant or discouraging response respective to the two treatment groups, and it stresses the necessity for undertaking pre-clinical studies prior to blocking RANK/RANK-L pathways in OS. 74
Hedgehog/Gli Signaling
The Hh-GLI signaling pathway is important in embryonic development and tumorigenesis. The constituents of Hh signaling comprise of: 3 Hh ligands (Sonic hedgehog (Shh), Desert hedgehog (Dhh) and Indian hedgehog (Ihh)); 2 twelve-pass transmembrane receptors (Patched1 (PTCH1) and Patched2 (PTCH2)); 3 transcription factors (GLI1 (activator), GLI2 (repressor/activator) and GLI3 (repressor/activator)). 75 Under normal conditions, in the absence of Hh ligands, PTCH keeps SMO inactivated, and at this point, GLI (GLI 2 or 3 repressor) is actively inhibiting the levels of the Hh target gene exposure. 76 The interaction between Hh ligand and PTCH induces phosphorylation of SMO, resulting in release of SMO inhibition by PTCH and subsequently activating SMO. This causes eventual activation of GLI transcription factors (activators) and expression of target genes of Hh pathway, 77 which include the cell cycle–related proteins cyclin D/E and anti-apoptotic protein Bcl-2, thereby promoting OS proliferation and tumor invasiveness. 78 This pathway has been implicated in pulmonary metastases by multiple studies, with a report showing that a group of mice treated with cyclopamine, an SMO inhibitor, lowering pulmonary metastasis by 20%. 79
GLI-2 in particular is associated with poorer clinical outcomes in OS patients and can be utilised as a prognostic marker and therapeutic target. 80 Studies have shown a higher GLI-2 expression in OS cell lines in comparison to normal osteoblasts and an associated increase in proliferation and metastasis of OS to the lungs with overexpression of RPS3, a target gene of GLI-2 and a potential marker of invasive OS. 81 Moreover, GLI-2 has been implicated in chemoresistance, reinforced by increased sensitivity to doxorubicin and methotrexate with GLI-2 knockdown. 80 Inhibition of GLI-2 on the other hand, induces apoptosis, which results from the G1 arrest due to reduced cyclin D1, SKP2 and phosphorylated Rb expression. 82
Similar to GLI-2, GLI-1 expression was also seen to be upregulated in OS patients. In the same vein as GLI-2, GLI-1 expression confers chemoresistance to OS cells. 83 Silencing GLI1 was shown to restore sensitivity of OS cells to chemotherapeutic agents, reducing proliferation, migration and cloning capacity of OS cells treated with cisplatin. 83 The reason GLI-1 expression is associated with chemoresistance is the subsequent blockade of y-H2AX, altering DNA repair and damage functions and subsequently reducing the efficacy of cisplatin 83 Additionally, Gankyrin, one of the regulatory subunits of the 26-kD human proteasome complex and a known onco-protein, promotes both migration and invasiveness of OS by regulating GLI-1 associated stemness factors, while suppressing GLI-1 degradation. 84 One study found that Ganykrin overexpression and it's interaction with GLI-1 was found to be associated with pulmonary metastasis of OS, displaying an increased number of metastatic lung nodules and higher lung weight in subjects. 84
Transforming Growth Factor-β Receptor Signaling
Transforming growth factor-β (TGF-β) receptors represents an evolutionarily conserved family of secreted polypeptide factors that mediate a diverse range of embryonic and adult signaling functions. 85 Their influence on cancer progression is dependent on cancer type, either halting or promoting tumor progression, with the latter being true for sarcomas. 86 Their presence is a greater predictor of OS when compared to healthy controls and confers a greater risk of lung metastasis, suggestively due to their ability to induce EMT in OS cells, but other factors have also been reported to contribute. 86 This effect on metastatic potential is illustrated by the inhibition of OS cells’ migratory ability and invasiveness when in the presence of TGF-B signaling pathway inhibitors Smad7, SD-208, and halofuginone. 87
Interestingly, one study has suggested a connection between the Hippo and TGF-B signaling pathway, with the interaction between the two purportedly promoting lung metastasis of OS. 88 YAP, one of the main effectors in the Hippo pathway, was found to promote lung metastasis of OS, namely through EMT as demonstrated by significantly increased expression of N-cadherin, Snail, Twist2, vimentin, MMP-2 and MMP-9, which all play a key role in OS cell detachment from primary tumor, migration and invasion. 88 Under normal conditions, after nuclear translocation, Smad3/4, a TGF-B receptor signaling protein heterodimer complex, binds to the CAGA promoter to amplify Lux gene transcription, a reporter gene to measure transcriptional activity of Smad3/4 in response to TGF-β signaling. YAP interacts with Smad proteins (especially Smad3 and Smad4), facilitating their translocation into the nucleus, and thereby enhancing transcriptional ability as seen by an amplified response of the (CAGA)₉-lux reporting system in the presence of YAP activation. 88 This demonstrates a synergistic role of YAP and TGF-β in driving OS progression, with YAP functioning as a co-activator of Smad signaling in OS cells.
TGFR-B3, one of many TGFB receptors, was found to be protected by SCD4, a target gene transcribed by Smad3/4, which inhibited it's degradation by MMP's under normal conditions. 89 This potentiates a vicious cycle resulting in excessive TGFBR-3 expression and downstream Smad3 activation, consequently increasing metastatic potential of OS cells through EMT enhancement. 89 An increase in ZFP36L1 however is shown to break the feedback loop between SDC4 expression and TGF-β signaling, thereby becoming a potential therapeutic agent for OS-driven lung metastasis. 89
For a summary review of the adaptations of intracellular signaling mechanisms that both promote and hinder OS lung metastasis, see Supplementary information A.
Immune Modulation Promoting Pulmonary Metastasis via the Tumor Microenvironment
The immune system plays a dual role in the recognition and progression of malignancy. Immunosuppression in patients is associated with an increased risk of cancer, inferring a defensive role. 90 Immune cells can confer an antitumor effect, as the quality and quantity of immune infiltrates present in the primary tumor is associated with improved survival. 91
Tumor cells release tumor antigens which are presented by antigen-presenting cells (APCs) to the adaptive immune system in response to activation by immunostimulatory signals such as inflammatory cytokines. Induction of T-cells can then lead to cancer cell killing. 92 The adaptive immune system also has a protective function against malignancy through recognizing ‘driver mutations’ within oncogenes or tumor suppressor genes which promote tumorigenesis. 93 However, tumor cells can evade the host's immune system through manipulation of the tumor microenvironment (TME) via complex mechanisms. 94 The TME comprises tumor cells, immune cells, stromal cells, blood vessels and extracellular matrix (ECM), with immune cells being a critical component. 94 Communication between tumor cells, cytokines, chemokines, growth factors and inflammatory mediators are active promoters of multi-drug resistance, tumorigenesis and metastasis.94,95
Tumor Associated Macrophages (TAMs) and Tumor Associated Neutrophils (TANs)
Cancer-cell derived cytokines can differentiate tumor infiltrating immune cells into tumor promoting, such as TAMs and TANs. 95 These myeloid-derived suppressor cells inhibit the activity of lymphocytes through complex mechanisms leading to the downregulation of T cell receptors, and reduced T-cell survival and proliferation. 96 TAMs can be differentiated into two subtypes: M1 and M2. M1 are typically involved in the anti-tumor response via producing reactive oxygen species and proinflammatory cytokines.97,98 However, M2 TAMs support the metastatic and invasive potential of tumors through the upregulation of proteolytic enzymes and various cytokines and chemokines. 99
TAMs have been implicated in the metastasis of OS through various mechanisms. Gene expression analysis showed TAMs upregulates cycloxygenase-2 (COX-2) pathways, leading to phosphorylation of the STAT3 resulting in the migration and invasion of OS cells. These findings were further supported as inhibition of the COX pathways using aspirin in mice, resulted in a significantly reduced level of lung metastasis. 100 Macrophages can stimulate the invasion of tumor cells in breast cancer and gliomas through the secretion of EGFR ligands. In vitro and in vivo studies have shown that EGFR inhibitors such as gefitinib altered the metastatic effect of macrophages in OS. However, these results were specific to the cell lines used in the study which could limit generalizability. 101
In vitro studies demonstrated TAMS infiltration is significantly higher in lung metastasis of OS compared to the primary lesion. Migration of M2 TAMS in OS cells is promoted by the zinc finger protein ZIM3 and the chemokine CCL25. Mouse models with silenced ZIM3 and CCL25 demonstrated significantly improved survival and reduced pulmonary infiltration by M2 TAMs. Genetic analysis through the R2 database showed CCL25 and ZIM3 expression was marginally higher in patients with OS metastasis and therefore associated with a poorer prognosis. 102 However, Buddingh et.al identified an association with TAMs and improved survival in high-grade OS. TAMs were characterized and quantified in pretreatment biopsies, post chemotherapy resections and metastatic lesions using genome-wide expression analysis. A high expression of macrophage associated genes was associated with a better metastasis-free survival. Survival benefit corresponded to response to chemotherapy. OS cells have both M1 and M2 characteristics. It was proposed that cell death through chemotherapy releases endogenous danger signals, causing polarization of M2 to M1 TAMs. 103 The contrasting effects between the different sub-group of TAMs demonstrates the complexity of their effect on OS outcomes. This is further compounded by the ability of TAMs to switch between different subgroups.
Immunotherapies that subsequently change tumor associated macrophage polarisation and thereby the tumor microenvironment are being investigated, one of which is a detoxified TLR4 agonist, that appeared to polarise tumor-associated macrophages (TAM) towards the M1 phenotype. 104 However, notably, it was found that the primary mechanism of action of this agonist in reducing lung metastases was dependant on CD8+ T cell recruitment/infiltration, as opposed to the polarisation of macrophages. 104
Chemokines/Cytokines
Chemokines and cytokines have a significant role in activating signaling pathways which mediates angiogenesis and immune cell recruitment and migration. The process is contributed to by the loss of cell-cell adhesion and increased migratory capacity which underpins metastatic potential of malignant cells. Chemokines can also exhibit roles as a growth factor demonstrating an antiapoptotic and proliferative effect. 105 In OS, TAMS have been associated with IL-34 and CCL18 expression which is correlated with increased proliferation and invasion of OS cells. Tissues expressing IL-34 show increased numbers of M2-macrophages. IL-34 induces adhesion of monocytes to endothelial cells and promotes vasculogenic changes which increases the extravasation capability of TAMS.103,106
Chemokines can be classified into four subfamilies: CC, CXC, CX3C and XC according to the variations in the configuration of the two cysteines closest to the N terminus. 106 CXC chemokines are particularly associated with promoting growth and metastasis of tumor cells. CXCL1 and its receptor CXCR2 has been widely implicated in the progression of several cancers, including colorectal, hepatocellular, pancreatic, ovarian and breast cancer.107,108 Finally, CXCL1 has been shown to contribute to lung metastasis in OS through increased expression of vascular cell adhesion molecule 1 (VCAM-1), involved in vascular adhesion and migration of macrophages and T cells. 109 In vivo studies demonstrated reduced pulmonary metastasis in mouse models with suppressed CXCL1 expression. In human OS tissue, higher-stage tumors had significantly raised CXCL1 and VCAM-1 expression compared to lower-stage tumors, suggesting that CXCL1 is a significant regulator in the metastasis of OS. 110 The level of CXCR2 can also have a prognostic value as both VCAM-1 expression and clinical disease progression is positively correlated with higher expression of CXCR2. 111
Figure 2 provides a summary of the mechanisms of immune modulation promoting OS metastasis, highlighting the role of cytokine-driven communication within the tumor microenvironment in facilitating OS cell mobilization and neovascularization.
Figure 2.
Summary of Mechanisms of Immune Modulation Promoting Metastasis of OS. Communication Between the Cytokines and the Tumor Microenvironment Supports Mobilization of OS Cells and Neovascularization, Leading to Worsened Outcomes of OS. This Occurs Alongside Protective Functions of Immune Cells such as M1 TAMs.
Extracellular vesicles (EVs) have been shown to significantly alter the tumor microenvironment, serving as conduits through which primary osteosarcoma (OS) cells communicate with distant organs such as the lungs to promote metastasis. This communication is partly mediated by microRNAs (miRNAs) carried within EVs, which can regulate matrix metalloproteinases (MMPs), leading to extracellular matrix remodeling and the establishment of a pre-metastatic niche. 112 EVs also promote the transformation of normal fibroblasts into cancer-associated fibroblasts (CAFs), further modifying the tumor microenvironment in support of metastasis, phenomena reported in other forms of cancer. 113
In addition, EVs can influence immune cell dynamics by inducing the polarization of M1 macrophages into the pro-tumorigenic M2 phenotype and enhancing neutrophil recruitment. 113 This is achieved through upregulation of toll-like receptor 3 (TLR3) on lung epithelial cells and increased chemokine secretion. EVs also contribute to the infiltration of CD11 + myeloid cells into the tumor microenvironment, a population associated with reduced cytotoxic T cell activity and, consequently, poorer patient survival outcomes.114,115
Moreover, OS-derived exosomes facilitate immune evasion by promoting the differentiation of CD4+ T cells into regulatory T cells (Tregs), which suppress antitumor immune responses. 116 As mentioned earlier, tumor-associated macrophages (TAMs) are the predominant immune cells infiltrating OS tumors; notably alveolar macrophages in particular can suppress dendritic cell maturation within the lung parenchyma by modulating TGF-β expression, thereby further enabling immune evasion and supporting OS metastasis in the lungs. 117
Lung-Induced Dormancy
Cancer cell dormancy, also known as quiescence, is a pivotal aspect of tumor progression, closely associated with the tumor's capacity to withstand cytotoxic treatments, metastasize, and evade immune responses. 118
Clinically, the dormancy phase of a cancer's progression hails significance as a symbol of a critical period of stagnation and beacon of hope before the cataclysmic yet seemingly inevitable deterioration in a patient's condition and a waning of any fruitful expectations with regards to clinical outcomes. This phase, often lasting months to decades, can be viewed as a golden window of opportunity to intervene against future metastases. Despite this theoretical potential, current research suggests the dormancy phase is resistant to current adjuvant chemotherapies due to low mitotic index rate, 119 but the redeeming light at the end of the tunnel remains that this is phenomena necessitates research into two main research avenues, the pathophysiology of organ-induced dormancy, and novel therapeutic strategies to maintain this dormant state. While dormancy mechanisms for OS remain understudied, research into other cancers, such as breast cancer, highlights the lung's ability to modulate metastatic activity.
Currently, there are uncertainties whether pro-colonization characteristics metastatic cells possess are acquired at the primary organ or whether they are developed post-dissemination. Research suggests that pro-colonization traits of metastatic cells may either be pre-acquired at the primary tumor site or developed post-dissemination. 120 However, dormant disseminated tumor cells (DTCs) challenge this view, as they rarely undergo significant genetic alterations due to low proliferative activity. 121 This underscores the critical role of the tumor microenvironment, particularly external, organ-specific factors, in guiding tumor dormancy and potential reactivation.
Growth Factors and Their Inhibitors
The lung microenvironment, rich in stroma-derived bone morphogenetic protein (BMP)-4, a TGFβ superfamily member, induces dormancy in DTCs. 122 BMP antagonism by Coco, a TGF-B receptor ligand inhibitor, suggestively regulated by NDRG1 and KIAA1199 genes, disrupts BMP signaling, reversing dormancy and promoting metastasis. 123 This is illustrated by the slowly imposing nature of the Coco-induced downstream effects taking place, with reduced expression of transcriptional factor GATA3 corrupting the initially quiescent Coco + ve mammary tumor cell line bathing in BMP synthesised by lung epithelial and mesenchymal cells. 124 This process inhibits luminal differentiation, in turn resulting in activation of the following dormant metastatic mammary tumor cell lines, thereby posing as a potential target for therapeutic intervention.
CHRDL2-induced BMP-9 antagonism specifically is a focal point, pivotal to OS tumor formation and metastasis, causing subsequent activation of the PI3K/AKT pathway. 125 This delineates both the importance of BMP and PI3K/AKT pathway in OS pathogenesis, with the latter playing a key role in determining cell cycle progression, evidenced by markedly upregulated AKT expression when whole genome sequencing of OS cells was performed. 126 MicroRNAs like miR-506-3p and miR-126 regulate this pathway, inhibiting OS dissemination and proliferation, marking them as potential therapeutic targets. 126
Insulin growth factor-2, (IGF-2), mirrors the growth factor-led stimulation of lung tumor dormancy in breast cancer metastasis resulting from the actions of BMP, but specifically in OS cells under chemotherapeutic stress. 127 Through cyclin degradation and proteasome-mediated changes, IGF-2 creates the following cell cycle alterations to induce proliferative arrest, such as increased G0–G1 fraction and proportionately increased Ki67-negative (non-proliferating), with contrasting reductions in S and G2–M fractions. 127 The effect of IGF-2 on the induction of dormancy was further reinforced by the exposure of the IGF-2 cultured sample to serum, and the disinhibition of this dormant state.
The maintenance of this dormant state seems to be reliant on autophagy and glutamine levels; seen by a decline in IGF-2 cultured cell survival when Agt7, an E1-like enzyme critical for autophagic flux, is knocked down due to dormancy disruption. 127 Some chemotherapeutic agents were found to enhance the autophagic process, negatively impacting OS dissemination, and promoting dormancy. Targeting this survival mechanism through a combination of chemotherapeutic agents such as methotrexate with an anti-autophagic drug eg chloroquine may work more effectively to limit further OS lung metastasis and progression. These findings reflect the ways in which OS tumor cells can take advantage of cell-autonomous processes to ensure cell survival in adverse conditions by inducing a quiescent phase.
Immune Regulation and Lung Dormancy
The adaptive immune system, particularly CD8+ T cells, plays a critical role in maintaining dormancy of lung metastases originating from fibrosarcoma, melanoma, and breast cancer. 128 Depletion of CD8+ T cells, who's differentiation is dependant on CD4+ T cells, correlates with spontaneous pulmonary metastasis, highlighting immune surveillance's role in preventing metastatic outgrowth. 128 This however conflicts with evidence provided from another study stating that although the induction of dormancy is dependent on CD8+ T cells/IFN-Y, the maintenance of this dormant state does not depend on T cells or immune surveillance. 129 The same study also stated that inflammation, specifically mediated by IL-17A, a cytokine producible by CD4+ T cells, is an important determinant in reversing dormancy in the lungs. 129
The role of the microenvironment in mediating the immune system to trigger or reverse dormancy is a point of interest, and exemplifies it's ever reaching influence over many of the mechanisms involved in creating a pre-metastatic niche. One study showed that the increase of AOC3, an endothelial adhesion molecule, promoting leukocyte extravasation to tissue sites from blood and amplifying the inflammatory response under normal circumstances, can enhance tumor associated neutrophil recruitment in OS lung metastasis. 130 AOC3 was found to be higher in metastatic OS cells compared to cells of the primary tumor, and overall AOC3 was found to promote general inflammatory infiltration in the lung in OS, associated with metastasis. 130 Moreover, where AOC3 was silenced, OS cells showed lower levels of cell proliferation. 130 Together, this implies that AOC3 plays a pro-metastatic role, and its inhibition might be a potential therapeutic strategy for treating osteosarcoma lung metastases.
Inflammation and Dormancy
As mentioned previously, a tumor's microenvironment can influence the progression of metastasis, and inflammation, especially in the chronic inflammatory state, altering the makeup of the extracellular matrix, subsequently resulting in DTC awakening. 131 However, it was shown that short and sustained inflammation, for instance caused by bacterial infections, may also, in a gross manner, disrupt the extracellular matrix architecture, facilitating a transition from a “suppressive” metastatic niche to a ‘growth permissive’ metastatic niche by degrading key proteins in the extracellular matrix to promote disseminated tumor cell (DTC) outgrowth. 132 In a more sophisticated fashion however, lung fibrosis dominated by Collagen I, a condition built up by ongoing long-term inflammatory processes, promotes metastatic growth by activating the PI3K/AKT pathway, resulting in a proliferative state. 131 Also, through COX-2 upregulation, inflammation can activate the PI3K/AKT pathway, promoting the proliferative and migratory ability of OS cells.133,134 Inflammation is also shown to induce EMT transition of dormant DTCs from quiescent, differentiated epithelia to an invasive, dedifferentiated mesenchymal phenotype. This draws parallels with the mechanism of action by which inflammation spurs OS lung metastasis through manipulation of intracellular signaling mechanisms noted previously.
Angiogenesis Suppression
Progression of OS and angiogenesis are tightly linked. 135 In OS, circulating VEGF, a known pro-angiogenic factor released under ischaemic, inflammatory and other adverse conditions, has been associated with the development of lung metastasis, and the advent of this discovery has prompted research into the use of anti-angiogenic agents in advanced OS cases. 136 Inhibiting angiogenesis helps establish a state of balance between tumor cell proliferation and apoptosis, fostering cell cycle arrest within DTCs and inducing dormancy by slowing or preventing the formation of a premetastatic niche. This again highlights the importance of the tumor microenvironment in dictating OS migration, proliferation and invasiveness. 137
AOC3 discussed earlier, a regulator of OS cell dormancy, has been shown to support tumor neovascularisation as a means of reversing OS dormancy within lung parenchyma, thereby modifying the tumor microenvironment, creating a metastatic-progressive niche. 130
Extracellular vesicles (EVs) have been extensively investigated in the context of osteosarcoma (OS) lung metastasis, where they have been shown to play a pivotal role in neovascularisation. 138 Specifically, EVs facilitate vascular permeability and promote OS cell migration, thereby contributing to metastatic progression. 139 These vesicles are enriched with microRNAs (miRNAs) capable of modulating key biological pathways, ultimately reprogramming the tumor microenvironment to resemble a premetastatic niche. Among these, exosomal miR-25–3p has been demonstrated to significantly enhance capillary and venule formation by suppressing the expression of DKK3. 140 Additional miRNAs, including miR-105, miR-210, and miR-3157–3p, have also been implicated in the promotion of neovascularisation through a variety of distinct molecular mechanisms that commonly include MMP's and VEGF. 112
Conclusion
The metastatic progression of OS to the lungs remains a complex and multifaceted process, driven by a myriad of interconnected biological mechanisms. Our review has examined three key dimensions of this process: the adaptation of receptor signaling mechanisms, immune modulation within the tumor microenvironment and lung-induced dormancy. Together, these elements orchestrate the dynamic evolution of OS cells, shaping their ability to colonise and thrive within the pulmonary environment.
Receptor signaling pathways play a pivotal role in regulating cellular proliferation, survival, and migration, ultimately influencing the metastatic trajectory of OS cells. By upregulating mesenchymal markers and downregulating epithelial markers, OS cells undergo an EMT-like transition, and this is one of the most common methods of promotion of OS lung metastasis which is cited and pervasive throughout current literature surrounding this area of research. Multiple studies show that in circulating tumor cells which translocated to the lungs from the primary OS expressed high levels of EMT-promoting transcriptional factors eg ZEB-1 and mesenchymal markers, the proven significance of which have been replicated across the board. Fibronectin, heavily upregulated in the EMT process, also plays a significant role in aiding fibrogenic programming, associated with OS lung metastasis progression, thereby illustrating the overlap in pathophysiological mechanisms that promote lung metastasis. Aside from altering the morphology of the OS cells, manipulating the characteristics of the cell cycle and growth pathways remains one of the cornerstone's of promoting OS lung metastasis, with multiple intracellular signaling mechanisms eventually culminating in enhanced transcriptional expression of cell cycle-related proteins. Frequently described throughout the literature studying various intracellular signaling mechanisms and their contribution to OS lung metastasis, is a cross-talk between inflammatory and growth cascades. One instance is IL6, and its pro-tumorigenic actions through the JAK/STAT3 receptor complex, promoting the cell cycle downstream. Other instances include toll-like receptors, and their supposedly protective effect that has been briefly mentioned in literature, by altering the tumor microenvironments susceptibility to T helper cell infiltration. In contrast to the other cell-signaling mechanisms described, the RANK/RANKL/OPG axis acts through a vicious cycle of bone destruction to cause a release of growth factors, and in fact is a cell-to-cell signaling mechanism, instead of a typical intracellular signaling mechanism, relying on the interaction of varying cells and the RANK ligand (RANK-L) to stimulate osteoclast differentiation, bone destruction and subsequent growth factor release.
Cross talk also appears on a macro-scale between different receptor signaling pathways, indicating convergence and overlap in the ways they advance metastasis. The Hippo-Pathway, characterised by YAP-TAZ interactions, is heavily associated with the Hedgehog pathway, contributing to the shared discourse that works to promote metastasis. This is particularly important, as cross talk often contributes to compensatory signaling that allows cancer cells to bypass the effects of targeted therapies. Inhibiting multiple nodes in these networks may prevent or overcome resistance. Aside from focusing solely on receptor inhibition for therapeutic benefit, a mixed approach with emphasis on downstream growth pathway inhibitors is seen to be more efficacious. Gene editing, a more precise approach to halt OS lung metastasis has been explored, but literature is currently limited to assessing the effect of limiting potential therapeutic targets with Crispr-Cas9, including STAT3 and PLK-1.
Immune modulation within the tumor microenvironment further dictates the metastatic potential of OS, evidenced by the role of cytokines and TAMs in progressing OS metastasis. Research has consistently shown that lungs more prominently affected by OS metastasis express an immunosuppressive microenvironment with increased tumor-associated macrophages (TAMs), synthesised by interactions of components of the innate immune system and cytokines, inhibiting effective anti-tumor immune responses. The chemokines/cytokines IL6 and CCL25 in particular are conducive to an immunosuppressive microenvironment characterised by M2 macrophage dominance. Manipulation of aspects of the innate immune system eg TLR's, can mediate the polarisation of M2 macrophages (pro-tumorigenic) towards an M1 phenotype (anti-tumorigenic). However, therapeutic targets catered to immune suppressor cells does not address the T cell exhaustion triggered by checkpoint proteins eg PD-1 and excessive antigen stimulation. Reduced T cell activity in turn drives OS cell immune evasion of defence mechanisms in the lung microenvironment and eventual progression to OS lung metastasis. 141 Immune checkpoint inhibitors eg PD-L1 can help reduce immune-inhibitory signals released downstream by inflammatory and growth cascades, providing some hope in stimulating exhausted T cells. 142 Currently, CAR-T cell therapy is also being explored, with aims of morphing the tumor microenvironment from immunosuppressive to immunostimulatory. 143 The genetic modification of new primary T cells with tumor-specific immunoreceptors eg chimeric antigen receptors (CARs), works to reroute T cells back to targeting tumor cells, thereby combatting T cell exhaustion and ineffective T cell activity. Interestingly, this therapy was found to be successful in haematological malignancies but less so in solid tumors, however, tumor microenvironment surveillance may be useful to determine optimal treatment timing for maximal success with CAR-T cell therapy, as the microenvironment can contribute to CAR-T cell exhaustion. 143 Other therapies such as detoxified TLR4 were shown to enhance CD8+ T helper cell infiltration, transforming the microenvironment, evolving to become more immunostimulatory and contributing to inducing a period of dormancy thereby halting metastatic progression.
Lung-induced dormancy represents a critical phase in metastasis, and this period of quiescence is influenced by inflammatory processes in both the acute and chronic phase, angiogenic balance, and BMPs, which collectively regulate the survival and eventual reactivation of dormant metastatic cells. In a similar vein to other biological mechanisms examined throughout, dormancy is also dependant on the activity of growth pathways, cell cycle protein expression, immune modulation and EMT, which work to transform quiescent epithelial cells into hyperproliferative de-differentiated mesenchymal cells. Angiogenesis however is unique in that it poses an extracellular influence on the tumor microenvironment and subsequently metastatic progression. VEGF expression, influenced by extracellular vesicles and other various factors contained within the tumor microenvironment is associated with a higher risk of lung metastasis due to their ability to facilitate OS cell extravasation, thereby heralding the angiogenic process as a potential therapeutic target in OS lung metastasis. One of multiple clinical studies trialling Pazopanib, a VEGF inhibitor, in three different patients, showed that two of the patients experienced positive responses including initial necrosis of their initial lung and gluteal metastasis, as well as improving from reportedly ‘bed ridden with intractable pains’ to ‘walking into the next clinic appointment’. 144 This evidence can potentially help spur a future randomised control trial with Pazopanib in OS lung metastasis patients.
Current literature also supports interventions targeting extracellular vesicles, which, can promote a pre-metastatic niche in a myriad of different ways, through angiogenesis, immune modulation, and inflammatory pathways, all ways to acclimate the tumor microenvironment. Treatment centres around limiting EV uptake and synthesis, changing the bioactive molecules contained within them eg miRNAs or targeting their fusion with recipient cells. Modulating GTPase pathways, for instance, by accentuating ciliary G-protein RAB28 function, may reduce EV secretion. 145 On the other hand, shifting molecular content of EV's from pro-tumorigenic to anti-tumorigenic may work to reduce metastatic potential, for instance, enriching EVs with miR-101 can limit tumor migration and dissemination by the enhancer of zeste homolog 2 (EZH2) regulation. 146 Alternatively, targeting tetraspanins, which are transmembrane proteins forming complexes with integrins and facilitating adhesion of EV's with target cells, can be useful way to reduce metastatic progression, preventing EVs delivering their cargo into recipient cells, inhibiting the reprogramming process to limit the creation of a pre-metastatic niche (PMN). 147
All in all, the tumor microenvironment plays a crucial role in promoting osteosarcoma (OS) lung metastasis, making it a promising target for therapeutic intervention. In addition to its involvement in immune modulation and maintaining lung dormancy, it can also alter receptor tyrosine kinase phenotypes to further OS lung metastasis. This ability to manipulate and adapt cell signaling pathways highlights its multifaceted nature, serving as a channel through which the primary tumor extends its influence and function to distant sites like the lungs.
Taken together, these insights underscore the need for a more integrated perspective on OS metastasis, incorporating receptor-driven oncogenic signaling, immune interactions, and dormancy regulation. More research with additional preclinical studies is needed to discover the broad biological effects and implications of pathway inhibition with therapeutic agents, to better ascertain their side effect profiles and efficacy. Our review framework has allowed for the rigorous study of the various biological mechanisms described that contribute to and protect against OS lung metastasis, ensuring all the relevant aspects of this phenomena are captured in detail, subsequently revealing areas which the pre-existing literature fails to address. However, it's important to consider that the scoping review process does not include a formal critical appraisal of the included studies. As a result, the quality and risk of bias of the evidence provided are not evaluated, limiting the ability to assess the strength or reliability of the findings. Moreover, casting a wide net to capture many concepts consequentially results in less depth of analysis of each article's findings. We hope off the back of our review, a systematic review and meta-analysis can be completed on this topic to effectively ascertain the efficacy of interventions discussed in articles, helping to guide the level emphasis placed on concepts underlined throughout and conclusions drawn from other experiments, in hopes of producing more robust recommendations with regards to future perspectives.
Supplemental Material
Supplemental material, sj-docx-1-tct-10.1177_15330338251359716 for Pathobiology and Molecular Pathways Implicated in Osteosarcoma Lung Metastasis: A Scoping Review by Ala Bashir, MD, Ayden Ismail, MD, Avenie Mavadia, MD, Aruni Ghose, MD, Saak Victor Ovsepian, PhD, and Stergios Boussios, MD, MSc, PhD, FRCP in Cancer Research & Treatment
Footnotes
ORCID iD: Stergios Boussios https://orcid.org/0000-0002-2512-6131
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Xu H, Zhu X, Bao H, et al. Genetic and clonal dissection of osteosarcoma progression and lung metastasis. Int J Cancer. 2018;143(5):1134-1142. doi: 10.1002/ijc.31389 [DOI] [PubMed] [Google Scholar]
- 2.Zhang B, Zhang Y, Li R, Li J, Lu X, Zhang Y. The efficacy and safety comparison of first-line chemotherapeutic agents (high-dose methotrexate, doxorubicin, cisplatin, and ifosfamide) for osteosarcoma: A network meta-analysis. J Orthop Surg Res. 2020;15(1):51. doi: 10.1186/s13018-020-1576-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang X, Zhao J, Bai Jet al. et al. Risk and clinicopathological features of osteosarcoma metastasis to the lung: A population-based study. J Bone Oncol. 2019;16:100230. doi: 10.1016/j.jbo.2019.100230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Odri GA, Tchicaya-Bouanga J, Yoon DJY, Modrowski D. Metastatic progression of osteosarcomas: A review of current knowledge of environmental versus oncogenic drivers. Cancers, [Online]. 2022;14(2):360. doi: 10.3390/cancers14020360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts RD, Lizardo MM, Reed DR, et al. Provocative questions in osteosarcoma basic and translational biology: A report from the children’s oncology group. Cancer. 2019;125(20):3514-3525. doi: 10.1002/cncr.32351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Columbia.edu. How to use Medical Subject Headings (MeSH) to refine and expand searches | Augustus C. Long Health Sciences Library. [online]. 2022. Available at: https://library.cumc.columbia.edu/kb/how-use-medical-subject-headings-mesh-refine [Accessed 21 May 2025].
- 7.Tricco AC, Lillie E, Zarin W, et al. PRISMA Extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann Intern Med. 2018;169(7):467-473. doi: 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- 8.Khalil H, Jia R, Moraes EB, et al. Scoping reviews and their role in identifying research priorities. Journal of Clinical Epidemiology, [Online]. 2025; 181: 111712. doi: 10.1016/j.jclinepi.2025.111712 [DOI] [PubMed] [Google Scholar]
- 9.Sompel K, Elango A, Smith AJ, Tennis MA. Cancer chemoprevention through frizzled receptors and EMT. Discov Oncol. 2021;12(1):32. doi: 10.1007/s12672-021-00429-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Q, Deng Z, Yang Y. Metastasis-related signature for clinically predicting prognosis and tumor immune microenvironment of osteosarcoma patients. Mol Biotechnol. 2023;65(11):1836-1845. doi: 10.1007/s12033-023-00681-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bai S, Li Y, Wang Y, et al. Long non-coding RNA MINCR regulates the growth and metastasis of human osteosarcoma cells via Wnt/β-catenin signaling pathway. Acta Biochim Pol. 2022;69(3):551-557. doi: 10.18388/abp.2020_5804 [DOI] [PubMed] [Google Scholar]
- 12.Zhang QH, Hu QX, Xie D, et al. Ganoderma lucidum exerts an anticancer effect on human osteosarcoma cells via suppressing the Wnt/β-catenin signaling pathway. Integr Cancer Ther. 2019;18:1534735419890917. doi: 10.1177/1534735419890917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie C, Chen B, Wu B, Guo J, Shi Y, Cao Y. CircSAMD4A regulates cell progression and epithelial-mesenchymal transition by sponging miR-342-3p via the regulation of FZD7 expression in osteosarcoma. Int J Mol Med. 2020;46(1):107-118. doi: 10.3892/ijmm.2020.4585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng D, Xia K, Yu L, et al. A novel six metastasis-related prognostic gene signature for patients with osteosarcoma. Front Cell Dev Biol. 2021;9:699212. doi: 10.3389/fcell.2021.699212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li S, Peng D, Yin ZQ, Zhu W, Hu XT, Liu CW. Effect of DEC1 on the proliferation, adhesion, invasion and epithelial-mesenchymal transition of osteosarcoma cells. Exp Ther Med. 2020;19(3):2360-2366. doi: 10.3892/etm.2020.8459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mittal V. Epithelial mesenchymal transition in tumor metastasis. Annu Rev Pathol. 2018;13:395-412. doi: 10.1146/annurev-pathol-020117-043854 [DOI] [PubMed] [Google Scholar]
- 17.Zeng CM, Chen Z, Fu L. Frizzled receptors as potential therapeutic targets in human cancers. Int J Mol Sci. 2018;19(5):1543. doi: 10.3390/ijms19051543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li G, Su Q, Liu H, et al. Frizzled7 promotes epithelial-to-mesenchymal transition and stemness via activating canonical wnt/β-catenin pathway in gastric cancer. Int J Biol Sci. 2018;14(3):280-293. doi: 10.7150/ijbs.23756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Q, Liu H, Wang Q, et al. Involvement of c-fos in cell proliferation, migration, and invasion in osteosarcoma cells accompanied by altered expression of Wnt2 and Fzd9. PLoS One. 2017;12(6):e0180558. doi: 10.1371/journal.pone.0180558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han YL, Luo D, Habaxi K, et al. COL5A2 Inhibits the TGF-β and Wnt/β-catenin signaling pathways to inhibit the invasion and metastasis of osteosarcoma. Front Oncol. 2022;12:813809. doi: 10.3389/fonc.2022.813809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu H, Chen D, Xie X, Li Y, Fan T. Melittin inhibits lung metastasis of human osteosarcoma: Evidence of wnt/β-catenin signaling pathway participation. Toxicon. 2021;198:132-142. doi: 10.1016/j.toxicon.2021.04.024 [DOI] [PubMed] [Google Scholar]
- 22.Nomura M, Rainusso N, Lee YC, et al. Tegavivint and the β-catenin/ALDH axis in chemotherapy-resistant and metastatic osteosarcoma. J Natl Cancer Inst. 2019;111(11):1216-1227. doi: 10.1093/jnci/djz026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song P, Gao Z, Bao Y, et al. Wnt/β-catenin signaling pathway in carcinogenesis and cancer therapy. J Hematol Oncol. 2024;17(1):46. doi: 10.1186/s13045-024-01563-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Owen KL, Brockwell NK, Parker BS. JAK-STAT Signaling: A double-edged sword of immune regulation and cancer progression. Cancers (Basel). 2019;11(12):2002. doi: 10.3390/cancers11122002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson DE, O’Keefe RA, Grandis JR. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol. 2018;15(4):234-248. doi: 10.1038/nrclinonc.2018.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Subramaniam D, Angulo P, Ponnurangam S, et al. Suppressing STAT5 signaling affects osteosarcoma growth and stemness. Cell Death Dis. 2020;11(2):149. doi: 10.1038/s41419-020-2335-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duan XL, Guo JP, Li F, Xiu C, Wang H. Sunitinib inhibits PD-L1 expression in osteosarcoma by targeting STAT3 and remodels the immune system in tumor-bearing mice. Future Oncol. 2020;16(24):1815-1824. doi: 10.2217/fon-2019-0725 [DOI] [PubMed] [Google Scholar]
- 28.Reinecke JB, Saraf A, Hinckley J, et al. Metastasis-initiating osteosarcoma subpopulations establish paracrine interactions with both lung and tumor cells to create a metastatic niche. bioRxiv. 2024.06.09.597967. 10.1101/2024.06.09.597967 [DOI]
- 29.Nan J, Du Y, Chen X, et al. TPCA-1 is a direct dual inhibitor of STAT3 and NF-κB and regresses mutant EGFR-associated human non-small cell lung cancers. Mol Cancer Ther. 2014;13(3):617-629. doi: 10.1158/1535-7163.mct-13-0464 [DOI] [PubMed] [Google Scholar]
- 30.Lu KH, Wu HH, Lin RC, et al. Curcumin analogue L48H37 suppresses human osteosarcoma U2OS and MG-63 cells’ migration and invasion in culture by inhibition of uPA via the JAK/STAT signaling pathway. Molecules. 2020;26(1):30. doi: 10.3390/molecules26010030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tu B, Du L, Fan QM, Tang Z, Tang TT. STAT3 Activation by IL-6 from mesenchymal stem cells promotes the proliferation and metastasis of osteosarcoma. Cancer Lett. 2012;325(1):80-88. doi: 10.1016/j.canlet.2012.06.006 [DOI] [PubMed] [Google Scholar]
- 32.Ma X, Xu W, Jin X, et al. Telocinobufagin inhibits osteosarcoma growth and metastasis by inhibiting the JAK2/STAT3 signaling pathway. Eur J Pharmacol. 2023;942:175529. doi: 10.1016/j.ejphar.2023.175529 [DOI] [PubMed] [Google Scholar]
- 33.Gardner HL, Fenger JM, Roberts RD, London CA. Characterizing the metabolic role of STAT3 in canine osteosarcoma. Vet Comp Oncol. 2022;20(4):817-824. doi: 10.1111/vco.12841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Leary VB, Maugg D, Smida J, et al. The long non-coding RNA PARTICLE is associated with WWOX and the absence of FRA16D breakage in osteosarcoma patients. Oncotarget. 2017;8(50):87431-87441. doi: 10.18632/oncotarget.21086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang R, Zhang C, Liu G, Gu R, Wu H. MicroRNA-101 inhibits proliferation, migration and invasion in osteosarcoma cells by targeting ROCK1. Am J Cancer Res. 2017;7(1):88-97. [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang R, Zhang C, Liu G, Gu R, Wu H. MicroRNA-126 inhibits proliferation, migration, invasion, and EMT in osteosarcoma by targeting ZEB1. J Cell Biochem. 2017;118(11):3765-3774. doi: 10.1002/jcb.26024 [DOI] [PubMed] [Google Scholar]
- 37.Yu L, Xia K, Gao T, et al. The notch pathway promotes osteosarcoma progression through activation of ephrin reverse signaling. Mol Cancer Res. 2019;17(12):2383-2394. doi: 10.1158/1541-7786.mcr-19-0493 [DOI] [PubMed] [Google Scholar]
- 38.Zhang Z, Wu W, Shao Z. NOTCH Signaling in osteosarcoma. Curr Issues Mol Biol. 2023;45(3):2266-2283. doi: 10.3390/cimb45030146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanaka M, Setoguchi T, Hirotsu M, et al. Inhibition of notch pathway prevents osteosarcoma growth by cell cycle regulation. Br J Cancer. 2009;100(12):1957-1965. doi: 10.1038/sj.bjc.6605060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dailey DD, Anfinsen KP, Pfaff LE, et al. HES1, A target of notch signaling, is elevated in canine osteosarcoma, but reduced in the most aggressive tumors. BMC Vet Res. 2013;9:130. doi: 10.1186/1746-6148-9-130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang J, Li N, Lu S, et al. The role of notch ligand Jagged1 in osteosarcoma proliferation, metastasis, and recurrence. J Orthop Surg Res. 2021;16(1):226. doi: 10.1186/s13018-021-02372-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng J, Zhang Y, Wan R, et al. CEMIP Promotes osteosarcoma progression and metastasis through activating notch signaling pathway. Front Oncol. 2022;12:919108. doi: 10.3389/fonc.2022.919108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang P, Yang Y, Zweidler-McKay PA, Hughes DP. Critical role of notch signaling in osteosarcoma invasion and metastasis. Clin Cancer Res. 2008;14(10):2962-2969. doi: 10.1158/1078-0432.ccr-07-1992 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Pu Y, Zhao F, Wang H, Cai S. MiR-34a-5p promotes multi-chemoresistance of osteosarcoma through down-regulation of the DLL1 gene. Sci Rep. 2017;7:44218. doi: 10.1038/srep44218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Z, Zhao G, Zhang Y, Ma Y, Ding Y, Xu N. MiR-199b-5p promotes malignant progression of osteosarcoma by regulating HER2. J BUON. 2018;23(6):1816-1824. [PubMed] [Google Scholar]
- 46.Jin H, Luo S, Wang Y, et al. miR-135b stimulates osteosarcoma recurrence and lung metastasis via notch and Wnt/β-catenin signaling. Mol Ther Nucleic Acids. 2017;8:111-122. doi: 10.1016/j.omtn.2017.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zeng M, Liu C, Gong H, et al. Therapeutic potential of tyrosine-protein kinase MET in osteosarcoma. Front Mol Biosci. 2024;11:1367331. doi: 10.3389/fmolb.2024.1367331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang W, Zhao JM, Lin J, et al. Adaptive fibrogenic reprogramming of osteosarcoma stem cells promotes metastatic growth. Cell Rep. 2018;24(5):1266-1277.e5. doi: 10.1016/j.celrep.2018.06.103 [DOI] [PubMed] [Google Scholar]
- 49.Gole S, Bankole A. Nintedanib. 2024. In: StatPearls [Internet]. StatPearls Publishing; 2025. [PubMed] [Google Scholar]
- 50.Rieunier G, Wu X, Macaulay VM, Lee AV, Weyer-Czernilofsky U, Bogenrieder T. Bad to the bone: The role of the insulin-like growth factor axis in osseous metastasis. Clin Cancer Res. 2019;25(12):3479-3485. doi: 10.1158/1078-0432.ccr-18-2697 [DOI] [PubMed] [Google Scholar]
- 51.Gvozdenovic A, Boro A, Born W, Muff R, Fuchs B. A bispecific antibody targeting IGF-IR and EGFR has tumor and metastasis suppressive activity in an orthotopic xenograft osteosarcoma mouse model. Am J Cancer Res. 2017;7(7):1435-1449. [PMC free article] [PubMed] [Google Scholar]
- 52.Post SM, Andreeff M, DiNardo C, Khoury JD, Ruvolo PP. TAM Kinases as regulators of cell death. Biochim Biophys Acta Mol Cell Res. 2021;1868(6):118992. doi: 10.1016/j.bbamcr.2021.118992 [DOI] [PubMed] [Google Scholar]
- 53.Rankin EB, Giaccia AJ. The receptor tyrosine kinase AXL in cancer progression. Cancers (Basel). 2016;8(11):103. doi: 10.3390/cancers8110103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chandhanayingyong C, Kim Y, Staples JR, Hahn C, Lee FY. MAPK/ERK signaling in osteosarcomas, ewing sarcomas and chondrosarcomas: Therapeutic implications and future directions. Sarcoma. 2012;2012:404810. doi: 10.1155/2012/404810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scaltriti M, Elkabets M, Baselga J. Molecular pathways: AXL, a membrane receptor mediator of resistance to therapy. Clin Cancer Res. 2016;22(6):1313-1317. doi: 10.1158/1078-0432.ccr-15-1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang DY, Wu YN, Huang JQ, et al. Hippo/YAP signaling pathway is involved in osteosarcoma chemoresistance. Chin J Cancer. 2016;35:47. doi: 10.1186/s40880-016-0109-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morice S, Danieau G, Rédini F, Brounais-Le-Royer B, Verrecchia F. Targeting the IGF/PI3K/mTOR pathway and AXL/YAP1/TAZ pathways in primary bone cancer. Cancers (Basel). 2020;12(3):645. doi: 10.3390/cancers12030645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Truong DD, Lamhamedi-Cherradi SE, Ludwig JA. Targeting the IGF/PI3K/mTOR pathway and AXL/YAP1/TAZ pathways in primary bone cancer. J Bone Oncol. 2022;33:100419. doi: 10.1016/j.jbo.2022.100419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lamhamedi-Cherradi SE, Mohiuddin S, Mishra DK, et al. Transcriptional activators YAP/TAZ and AXL orchestrate dedifferentiation, cell fate, and metastasis in human osteosarcoma. Cancer Gene Ther. 2021;28(12):1325-1338. doi: 10.1038/s41417-020-00281-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Han J, Tian R, Yong B, et al. Gas6/Axl mediates tumor cell apoptosis, migration and invasion and predicts the clinical outcome of osteosarcoma patients. Biochem Biophys Res Commun. 2013;435(3):493-500. doi: 10.1016/j.bbrc.2013.05.019 [DOI] [PubMed] [Google Scholar]
- 61.Tian Z, Niu X, Yao W. Receptor tyrosine kinases in osteosarcoma treatment: Which is the key target? Front Oncol. 2020;10:1642. doi: 10.3389/fonc.2020.01642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scotlandi K, Baldini N, Oliviero M, et al. Expression of met/hepatocyte growth factor receptor gene and malignant behavior of musculoskeletal tumors. Am J Pathol. 1996;149(4):1209-1219. [PMC free article] [PubMed] [Google Scholar]
- 63.Coltella N, Manara MC, Cerisano V, et al. Role of the MET/HGF receptor in proliferation and invasive behavior of osteosarcoma. FASEB J. 2003;17(9):1162-1164. doi: 10.1096/fj.02-0576fje [DOI] [PubMed] [Google Scholar]
- 64.Wang G, Sun M, Jiang Y, et al. Anlotinib, a novel small molecular tyrosine kinase inhibitor, suppresses growth and metastasis via dual blockade of VEGFR2 and MET in osteosarcoma. Int J Cancer. 2019;145(4):979-993. doi: 10.1002/ijc.32180 [DOI] [PubMed] [Google Scholar]
- 65.Chen W, Wu S, Huang Y, et al. A c-met inhibitor suppresses osteosarcoma progression via the ERK1/2 pathway in human osteosarcoma cells. Onco Targets Ther. 2021;14:4791-4804. doi: 10.2147/ott.s317122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fioramonti M, Fausti V, Pantano F, et al. Cabozantinib affects osteosarcoma growth through A direct effect on tumor cells and modifications in bone microenvironment. Sci Rep. 2018;8(1):4177. doi: 10.1038/s41598-018-22469-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Assi A, Farhat M, Hachem MCR, et al. Tyrosine kinase inhibitors in osteosarcoma: Adapting treatment strategiesa. J Bone Oncol. 2023;43:100511. doi: 10.1016/j.jbo.2023.100511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Navet B, Ando K, Vargas-Franco JW, et al. The intrinsic and extrinsic implications of RANKL/RANK signaling in osteosarcoma: From tumor initiation to lung metastases. Cancers (Basel). 2018;10(11):398. doi: 10.3390/cancers10110398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang Y, Liang J, Liu P, Wang Q, Liu L, Zhao H. The RANK/RANKL/OPG system and tumor bone metastasis: Potential mechanisms and therapeutic strategies. Front Endocrinol (Lausanne). 2022;13:1063815. doi: 10.3389/fendo.2022.1063815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gao YM, Pei Y, Zhao FF, Wang L. Osteoclasts in osteosarcoma: Mechanisms, interactions, and therapeutic prospects. Cancer Manag Res. 2023;15:1323-1337. doi: 10.2147/cmar.s431213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Takeda T, Tsubaki M, Genno S, Tomita K, Nishida S. RANK/RANKL axis promotes migration, invasion, and metastasis of osteosarcoma via activating NF-κB pathway. Exp Cell Res. 2024;436(2):113978. doi: 10.1016/j.yexcr.2024.113978 [DOI] [PubMed] [Google Scholar]
- 72.Branstetter D, Rohrbach K, Huang LY, et al. RANK And RANK ligand expression in primary human osteosarcoma. J Bone Oncol. 2015;4(3):59-68. doi: 10.1016/j.jbo.2015.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen Y, Di Grappa MA, Molyneux SD, et al. RANKL Blockade prevents and treats aggressive osteosarcomas. Sci Transl Med. 2015;7(317):317ra197. doi: 10.1126/scitranslmed.aad0295 [DOI] [PubMed] [Google Scholar]
- 74.Punzo F, Tortora C, Argenziano M, et al. Can denosumab be used in combination with doxorubicin in osteosarcoma? Oncotarget. 2020;11(28):2763-2773. doi: 10.18632/oncotarget.27669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carballo GB, Honorato JR, de Lopes GPF, Spohr TCLSE. A highlight on sonic hedgehog pathway. Cell Commun Signal. 2018;16(1):11. doi: 10.1186/s12964-018-0220-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chaudhry P, Singh M, Triche TJ, Guzman M, Merchant AA. GLI3 Repressor determines hedgehog pathway activation and is required for response to SMO antagonist glasdegib in AML. Blood. 2017;129(26):3465-3475. doi: 10.1182/blood-2016-05-718585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sigafoos AN, Paradise BD, Fernandez-Zapico ME. Hedgehog/GLI signaling pathway: Transduction, regulation, and implications for disease. Cancers (Basel). 2021;13(14):3410. doi: 10.3390/cancers13143410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kumar RM, Fuchs B. Hedgehog signaling inhibitors as anti-cancer agents in osteosarcoma. Cancers (Basel). 2015;7(2):784-794. doi: 10.3390/cancers7020784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Warzecha J, Dinges D, Kaszap B, Henrich D, Marzi I, Seebach C. Effect of the hedgehog-inhibitor cyclopamine on mice with osteosarcoma pulmonary metastases. Int J Mol Med. 2012;29(3):423-427. doi: 10.3892/ijmm.2011.851 [DOI] [PubMed] [Google Scholar]
- 80.Yang W, Liu X, Choy E, Mankin H, Hornicek FJ, Duan Z. Targeting hedgehog-GLI-2 pathway in osteosarcoma. J Orthop Res. 2013;31(3):502-509. doi: 10.1002/jor.22230 [DOI] [PubMed] [Google Scholar]
- 81.Nagao-Kitamoto H, Setoguchi T, Kitamoto S, et al. Ribosomal protein S3 regulates GLI2-mediated osteosarcoma invasion. Cancer Lett. 2015;356(2 Pt B):855-861. doi: 10.1016/j.canlet.2014.10.042 [DOI] [PubMed] [Google Scholar]
- 82.Nagao H, Ijiri K, Hirotsu M, et al. Role of GLI2 in the growth of human osteosarcoma. J Pathol. 2011;224(2):169-179. doi: 10.1002/path.2880 [DOI] [PubMed] [Google Scholar]
- 83.Chen D, Kang X, Li Z, Chen L, Ma Q, Fan P. Hedgehog/GLI1 signaling pathway regulates the resistance to cisplatin in human osteosarcoma. J Cancer. 2021;12(22):6676-6684. doi: 10.7150/jca.61591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang C, Jing J, Hu X, et al. Gankyrin activates the hedgehog signalling to drive metastasis in osteosarcoma. J Cell Mol Med. 2021;25(13):6232-6241. doi: 10.1111/jcmm.16576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tzavlaki K, Moustakas A. TGF-β Signaling. Biomolecules. 2020;10(3):487. doi: 10.3390/biom10030487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ge R, Huang GM. Targeting transforming growth factor beta signaling in metastatic osteosarcoma. J Bone Oncol. 2023;43:100513. doi: 10.1016/j.jbo.2023.100513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lamora A, Talbot J, Mullard M, Brounais-Le Royer B, Redini F, Verrecchia F. TGF-β Signaling in bone remodeling and osteosarcoma progression. J Clin Med. 2016;5(11):96. doi: 10.3390/jcm5110096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Morice S, Danieau G, Tesfaye R, et al. Involvement of the TGF-β signaling pathway in the development of YAP-driven osteosarcoma lung metastasis. Front Oncol. 2021;11:765711. doi: 10.3389/fonc.2021.765711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ma M, Zhuang J, Li H, et al. Low expression of ZFP36L1 in osteosarcoma promotes lung metastasis by inhibiting the SDC4-TGF-β signaling feedback loop. Oncogene. 2024;43(1):47-60. doi: 10.1038/s41388-023-02880-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gonzalez H, Hagerling C, Werb Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes Dev. 2018;32(19-20):1267-1284. doi: 10.1101/gad.314617.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bilotta MT, Antignani A, Fitzgerald DJ. Managing the TME to improve the efficacy of cancer therapy. Front Immunol. 2022;13:954992. doi: 10.3389/fimmu.2022.954992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Blomberg OS, Spagnuolo L, de Visser KE. Immune regulation of metastasis: Mechanistic insights and therapeutic opportunities. Dis Model Mech. 2018;11(10):dmm036236. doi: 10.1242/dmm.036236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Corthay A. Does the immune system naturally protect against cancer? Front Immunol. 2014;5:197. doi: 10.3389/fimmu.2014.00197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Anderson NM, Simon MC. The tumor microenvironment. Curr Biol. 2020;30(16):R921-R925. doi: 10.1016/j.cub.2020.06.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Baghban R, Roshangar L, Jahanban-Esfahlan R, et al. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun Signal. 2020;18(1):59. doi: 10.1186/s12964-020-0530-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fleming V, Hu X, Weber R, et al. Targeting myeloid-derived suppressor cells to bypass tumor-induced immunosuppression. Front Immunol. 2018;9:398. doi: 10.3389/fimmu.2018.00398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen Y, Song Y, Du W, Gong L, Chang H, Zou Z. Tumor-associated macrophages: An accomplice in solid tumor progression. J Biomed Sci. 2019;26(1):78. doi: 10.1186/s12929-019-0568-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lin Y, Xu J, Lan H. Tumor-associated macrophages in tumor metastasis: Biological roles and clinical therapeutic applications. J Hematol Oncol. 2019;12(1):76. doi: 10.1186/s13045-019-0760-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shen Y, Chen JX, Li M, Xiang Z, Wu J, Wang YJ. Role of tumor-associated macrophages in common digestive system malignant tumors. World J Gastrointest Oncol. 2023;15(4):596-616. doi: 10.4251/wjgo.v15.i4.596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Han Y, Guo W, Ren T, et al. Tumor-associated macrophages promote lung metastasis and induce epithelial-mesenchymal transition in osteosarcoma by activating the COX-2/STAT3 axis. Cancer Lett. 2019;440-441:116-125. doi: 10.1016/j.canlet.2018.10.011 [DOI] [PubMed] [Google Scholar]
- 101.Maloney C, Kallis MP, Edelman M, et al. Gefitinib inhibits invasion and metastasis of osteosarcoma via inhibition of macrophage receptor interacting serine-threonine kinase 2. Mol Cancer Ther. 2020;19(6):1340-1350. doi: 10.1158/1535-7163.MCT-19-0903 [DOI] [PubMed] [Google Scholar]
- 102.Li J, Zhao C, Wang D, et al. ZIM3 Activation of CCL25 expression in pulmonary metastatic nodules of osteosarcoma recruits M2 macrophages to promote metastatic growth. Cancer Immunol Immunother. 2023;72(4):903-916. doi: 10.1007/s00262-022-03300-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Buddingh EP, Kuijjer ML, Duim RA, et al. Tumor-infiltrating macrophages are associated with metastasis suppression in high-grade osteosarcoma: A rationale for treatment with macrophage activating agents. Clin Cancer Res. 2011;17(8):2110-2119. doi: 10.1158/1078-0432.CCR-10-2047 [DOI] [PubMed] [Google Scholar]
- 104.Oyama R, Nabeshima A, Endo M, et al. A detoxified TLR4 agonist inhibits tumour growth and lung metastasis of osteosarcoma by promoting CD8 + cytotoxic lymphocyte infiltration. BJC Reports, [online]. 2025;3(1):5. doi: 10.1038/s44276-024-00120-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.White GE, Tan TCC, John AE, Whatling C, McPheat WL, Greaves DR. Fractalkine has anti-apoptotic and proliferative effects on human vascular smooth muscle cells via epidermal growth factor receptor signalling. Cardiovascular Research, [Online]. 2010;85(4):825-835. doi: 10.1093/cvr/cvp341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Do HTT, Lee CH, Cho J. Chemokines and their receptors: Multifaceted roles in cancer progression and potential value as cancer prognostic markers. Cancers (Basel). 2020;12(2):287. doi: 10.3390/cancers12020287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hughes CE, Nibbs RJB. A guide to chemokines and their receptors. FEBS J. 2018;285(16):2944-2971. doi: 10.1111/febs.14466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhao H, Wei S, Zhou D, et al. Blocking the CXCL1-CXCR2 axis enhances the effects of doxorubicin in HCC by remodelling the tumour microenvironment via the NF-κB/IL-1β/CXCL1 signalling pathway. Cell Death Discov. 2023;9(1):120. doi: 10.1038/s41420-023-01424-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wu T, Yang W, Sun A, Wei Z, Lin Q. The role of CXC chemokines in cancer progression. Cancers (Basel). 2022;15(1):167. doi: 10.3390/cancers15010167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee CW, Chiang YC, Yu PA, et al. A role of CXCL1 drives osteosarcoma lung metastasis via VCAM-1 production. Front Oncol. 2021;11:735277. doi: 10.3389/fonc.2021.735277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lin SL, Yang SY, Tsai CH, et al. Nerve growth factor promote VCAM-1-dependent monocyte adhesion and M2 polarization in osteosarcoma microenvironment: Implications for larotrectinib therapy. Int J Biol Sci. 2024;20(11):4114-4127. doi: 10.7150/ijbs.95463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhongyu X, Wei X, Hongmei Z, et al. Review of pre-metastatic niches induced by osteosarcoma-derived extracellular vesicles in lung metastasis: A potential opportunity for diagnosis and intervention. Biomed Pharmacother. 2024;178:117203. doi: 10.1016/j.biopha.2024.117203 [DOI] [PubMed] [Google Scholar]
- 113.Wu X, Zhou Z, Xu S, et al. Extracellular vesicle packaged LMP1-activated fibroblasts promote tumor progression via autophagy and stroma-tumor metabolism coupling. Cancer Lett. 2020;478:93-106. doi: 10.1016/j.canlet.2020.03.004 [DOI] [PubMed] [Google Scholar]
- 114.Mazumdar A, Urdinez J, Boro A, et al. Exploring the role of osteosarcoma-derived extracellular vesicles in Pre-metastatic niche formation and metastasis in the 143-B Xenograft mouse osteosarcoma model. Cancers (Basel). 2020;12(11):3457. doi: 10.3390/cancers12113457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jiang K, Li J, Zhang J, et al. SDF-1/CXCR4 axis facilitates myeloid-derived suppressor cells accumulation in osteosarcoma microenvironment and blunts the response to anti-PD-1 therapy. Int Immunopharmacol. 2019;75:105818-105818. doi: 10.1016/j.intimp.2019.105818 [DOI] [PubMed] [Google Scholar]
- 116.Troyer RM, Ruby CE, Goodall CP, et al. Exosomes from osteosarcoma and normal osteoblast differ in proteomic cargo and immunomodulatory effects on T cells. Exp Cell Res. 2017;358(2):369-376. doi: 10.1016/j.yexcr.2017.07.011 [DOI] [PubMed] [Google Scholar]
- 117.Sharma SK, Chintala NK, Vadrevu SK, Patel J, Karbowniczek M, Markiewski MM. Pulmonary alveolar macrophages contribute to the premetastatic niche by suppressing antitumor T cell responses in the lungs. J Immunol. 2015;194(11):5529-5538. doi: 10.4049/jimmunol.1403215 [DOI] [PubMed] [Google Scholar]
- 118.Chao CC, Lee CW, Chang TM, Chen PC, Liu JF. CXCL1/CXCR2 Paracrine axis contributes to lung metastasis in osteosarcoma. Cancers (Basel). 2020;12(2):459. doi: 10.3390/cancers12020459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Francescangeli F, De Angelis ML, Rossi R, et al. Dormancy, stemness, and therapy resistance: Interconnected players in cancer evolution. Cancer Metastasis Rev. 2023;42(1):197-215. doi: 10.1007/s10555-023-10092-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Klauber-DeMore N, Van Zee KJ, Linkov I, Borgen PI, Gerald WL. Biological behavior of human breast cancer micrometastases. Clin Cancer Res. 2001;7(8):2434-2439. [PubMed] [Google Scholar]
- 121.Giancotti FG. Mechanisms governing metastatic dormancy and reactivation. Cell. 2013;155(4):750-764. doi: 10.1016/j.cell.2013.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ghajar CM. Metastasis prevention by targeting the dormant niche. Nat Rev Cancer. 2015;15(4):238-247. doi: 10.1038/nrc3910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Parker AL, Cox TR. The role of the ECM in lung cancer dormancy and outgrowth. Front Oncol. 2020;10:1766. doi: 10.3389/fonc.2020.01766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gao H, Chakraborty G, Lee-Lim AP, et al. The BMP inhibitor Coco reactivates breast cancer cells at lung metastatic sites. Cell. 2012;150(4):764-779. doi: 10.1016/j.cell.2012.06.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen H, Pan R, Li H, et al. CHRDL2 Promotes osteosarcoma cell proliferation and metastasis through the BMP-9/PI3K/AKT pathway. Cell Biol Int. 2021;45(3):623-632. doi: 10.1002/cbin.11507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xiang Y, Yang Y, Liu J, Yang X. Functional role of MicroRNA/PI3K/AKT axis in osteosarcoma. Front Oncol. 2023;13:1219211. doi: 10.3389/fonc.2023.1219211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shimizu T, Sugihara E, Yamaguchi-Iwai S, et al. IGF2 Preserves osteosarcoma cell survival by creating an autophagic state of dormancy that protects cells against chemotherapeutic stress. Cancer Res. 2014;74(22):6531-6541. doi: 10.1158/0008-5472.CAN-14-0914 [DOI] [PubMed] [Google Scholar]
- 128.Wang HF, Wang SS, Huang MC, Liang XH, Tang YJ, Tang YL. Targeting immune-mediated dormancy: A promising treatment of cancer. Front Oncol. 2019;9:498. doi: 10.3389/fonc.2019.00498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pereira P, Panier J, Nater M, et al. Inflammatory cytokines mediate the induction of and awakening from metastatic dormancy. Cell Reports, [Online]. 2025;44(3):115388. doi: 10.1016/j.celrep.2025.115388 [DOI] [PubMed] [Google Scholar]
- 130.Qi L, Gao T, Bai C, et al. AOC3 Accelerates lung metastasis of osteosarcoma by recruiting tumor-associated neutrophils, neutrophil extracellular trap formation and tumor vascularization. Heliyon, [Online]. 2024;10(17):e37070. doi: 10.1016/j.heliyon.2024.e37070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lim AR, Ghajar CM. Thorny ground, rocky soil: Tissue-specific mechanisms of tumor dormancy and relapse. Semin Cancer Biol. 2022;78:104-123. doi: 10.1016/j.semcancer.2021.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Albrengues J, Shields MA, Ng D, et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science. 2018;361(6409):eaao4227. doi: 10.1126/science.aao4227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gómez-Valenzuela F, Escobar E, Pérez-Tomás R, Montecinos VP. The inflammatory profile of the tumor microenvironment, orchestrated by cyclooxygenase-2, promotes epithelial-mesenchymal transition. Front Oncol. 2021;11:686792. doi: 10.3389/fonc.2021.686792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang X, Qu P, Zhao H, Zhao T, Cao N. COX-2 promotes epithelial-mesenchymal transition and migration in osteosarcoma MG-63 cells via PI3K/AKT/NF-κB signaling. Mol Med Rep. 2019;20(4):3811-3819. doi: 10.3892/mmr.2019.10598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Holmgren L, O'Reilly MS, Folkman J. Dormancy of micrometastases: Balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat Med. 1995;1(2):149-153. doi: 10.1038/nm0295-149 [DOI] [PubMed] [Google Scholar]
- 136.Ghajar CM, Peinado H, Mori H, et al. The perivascular niche regulates breast tumour dormancy. Nat Cell Biol. 2013;15(7):807-817. doi: 10.1038/ncb2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xie L, Ji T, Guo W. Anti-angiogenesis target therapy for advanced osteosarcoma (review). Oncol Rep. 2017;38(2):625-636. doi: 10.3892/or.2017.5735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Perut F, Roncuzzi L, Zini N, Massa A, Baldini N. Extracellular nanovesicles secreted by human osteosarcoma cells promote angiogenesis. Cancers (Basel). 2019;11(6):779. doi: 10.3390/cancers11060779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Aslan C, Maralbashi S, Salari F, et al. Tumor-derived exosomes: Implication in angiogenesis and antiangiogenesis cancer therapy. J Cell Physiol. 2019;234(10):16885-16903. doi: 10.1002/jcp.28374 [DOI] [PubMed] [Google Scholar]
- 140.Tao S, Huang J, Wei Z, Li Z, Guo S. EWSAT1 acts in concert with exosomes in osteosarcoma progression and tumor-induced angiogenesis: The ‘double stacking effect’. Advanced Biosystems. 2020;4(9):e2000152. doi: 10.1002/adbi.202000152 [DOI] [PubMed] [Google Scholar]
- 141.McGee LE, Pereira JS, McEachron TA, Mazcko C, LeBlanc AK, Beck JA. The tumor microenvironment of metastatic osteosarcoma in the human and canine lung. Communications Biology, [online]. 2025;8(1):756. doi: 10.1038/s42003-025-07992-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhang Z, Tan X, Jiang Z, Wang H, Yuan H. Immune checkpoint inhibitors in osteosarcoma: A hopeful and challenging future. Front Pharmacol. 2022;13:1031527. doi: 10.3389/fphar.2022.1031527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xia X, Yang Z, Lu Q, et al. Reshaping the tumor immune microenvironment to improve CAR-T cell-based cancer immunotherapy. Mol Cancer. 2024;23(1):175. doi: 10.1186/s12943-024-02079-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Safwat A, Boysen A, Lücke A, Rossen P. Pazopanib in metastatic osteosarcoma: Significant clinical response in three consecutive patients. Acta Oncol (Madr). 2014;53(10):1451-1454. doi: 10.3109/0284186x.2014.948062 [DOI] [PubMed] [Google Scholar]
- 145.Akella JS, Carter SP, Rizvi F, et al. A ciliary BBSome-ARL-6-PDE6D pathway trafficks RAB-28, a negative regulator of extracellular vesicle biogenesis. [online]. 2019. 10.1101/715730 [DOI]
- 146.Zhang K, Dong C, Chen M, et al. Extracellular vesicle-mediated delivery of miR-101 inhibits lung metastasis in osteosarcoma. Theranostics. 2020;10(1):411-425. doi: 10.7150/thno.33482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lu J, Li J, Liu S, et al. Exosomal tetraspanins mediate cancer metastasis by altering host microenvironment. Oncotarget, [Online]. 2017;8(37):62803-62815. doi: 10.18632/oncotarget.19119 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tct-10.1177_15330338251359716 for Pathobiology and Molecular Pathways Implicated in Osteosarcoma Lung Metastasis: A Scoping Review by Ala Bashir, MD, Ayden Ismail, MD, Avenie Mavadia, MD, Aruni Ghose, MD, Saak Victor Ovsepian, PhD, and Stergios Boussios, MD, MSc, PhD, FRCP in Cancer Research & Treatment