Abstract
Background
Congestive heart failure (CHF) is a critical condition resulting from impaired blood pumping, leading to fluid accumulation. While calcium plays a vital role in skeletal health and muscle contraction, its potential in preventing CHF remains unclear.
Objectives
This study aimed to investigate the relationship between calcium intake and the risk of CHF using data from the National Health and Nutrition Examination Survey (NHANES) collected between 2003 and 2018.
Methods
After completing the questionnaire, participants were classified into CHF and control groups, with calcium intake considered as the primary exposure variable, alongside categorical factors such as age and race. Statistical analyses included t-tests and chi-square tests. A subsequent risk stratification analysis assessed the impact of various covariates on CHF. Additionally, Least Absolute Shrinkage and Selection Operator (LASSO) regression was applied to identify optimal predictor variables, and model performance was evaluated using the receiver operating characteristic curve.
Results
A total of 3,083 participants were included in the study. Baseline characteristics revealed significant differences in race (p = 0.004), education (p = 0.019), fasting glucose (p = 0.047), and calcium intake (p = 0.020) between the CHF and control groups. In model 3, calcium intake was significantly associated with a reduced risk of CHF (p < 0.001, OR = 6.37 × 10–4, 95% CI = 1.47 × 10–4 − 1.13 × 10–3), acting as a protective factor (OR < 1, 95% CI = 1 × 10–6 − 1 × 10–5). LASSO regression further identified calcium intake as a predictor variable.
Conclusion
Calcium intake serves as a protective factor against CHF, potentially lowering its risk. These findings provide theoretical support for the prevention and diagnosis of CHF.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12872-025-05008-9.
Keywords: Congestive heart failure, Calcium intake, Weighted multivariate logistic regression model, NHANES
Introduction
Congestive heart failure (CHF) is a critical pathological condition characterized by the heart’s inability to pump sufficient blood to meet the body’s demands, resulting in fluid accumulation in the lungs or peripheral tissues [1, 2]. Clinically, CHF manifests through symptoms such as dyspnea, fatigue, and peripheral edema, and epidemiologically, it affects millions worldwide, posing a substantial burden on healthcare systems [3]. The European Society of Cardiology (ESC) and the American Heart Association (AHA) guidelines provide clear definitions of congestion, incorporating clinical symptoms (dyspnea, orthopnea, paroxysmal nocturnal dyspnea, and edema), physical signs (jugular venous distension, hepatomegaly, and wet rales), and imaging findings (e.g., chest X-rays revealing pulmonary congestion and echocardiograms showing a dilated inferior vena cava) [1] The etiology of CHF is multifactorial, encompassing genetic predispositions, acquired cardiac abnormalities, and lifestyle factors. Although current therapies, such as pharmacological interventions with diuretics, focus on symptom management and improving quality of life, they do not reverse the underlying condition [4]. Scicchitano P et al. demonstrated that parathyroid hormone (PTH) levels were elevated in acute heart failure compared to chronic heart failure and in patients exhibiting clinical signs of congestion, such as peripheral edema and orthopnea. Additionally, PTH levels were significantly correlated with NYHA class and the HYDRA score [5]. These findings suggest that PTH may be closely linked to the clinical manifestations of CHF and may play a significant role in the pathophysiological mechanisms of both acute and chronic heart failure. Therefore, it is crucial to identify new risk factors for CHF to gain deeper insights into its development and progression. These findings not only offer new perspectives on early disease prevention but also provide valuable clinical insights for the development of innovative therapeutic drugs. Calcium, an essential mineral, plays a critical role in maintaining skeletal integrity, blood coagulation, nerve transmission, and muscle contraction [6]. Adequate calcium intake is essential for preventing diseases such as osteoporosis [7]. Recent studies have highlighted the complex relationship between calcium intake and cardiovascular health, particularly in the context of heart failure [8, 9]. Despite calcium’s importance in cellular functions and cardiac operations, the precise impact of calcium levels on heart failure progression remains unclear, necessitating further exploration. Both calcium deficiency and excess intake have been shown to influence heart failure, emphasizing the importance of balanced calcium consumption for maintaining cardiovascular health [10–12].
The National Health and Nutrition Examination Survey (NHANES), conducted by the CDC, provides comprehensive cross-sectional data on the health and nutritional status of the U.S. population. This dataset includes demographic, dietary, health, and laboratory data, which are essential for shaping public health policies and health service planning. By leveraging the NHANES database, this study aims to explore the relationship between dietary calcium intake and the incidence and severity of CHF. The findings derived from this dataset will contribute to a better understanding of how calcium intake influences CHF, potentially informing future therapeutic strategies and dietary recommendations for individuals at risk of or living with CHF.
Materials and methods
Study design and participants
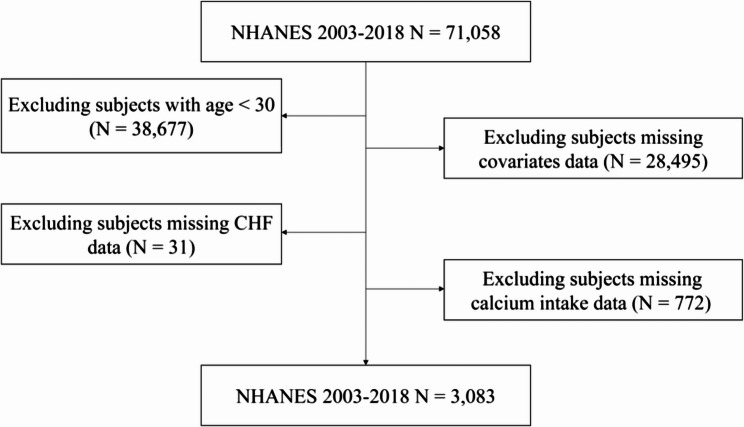
This study utilized data from the NHANES (https://www.cdc.gov/nches/nhanes), with participants selected from the 2003 to 2018 survey period. The exclusion criteria for participants were as follows: (1) As CHF is primarily observed in individuals over 60 years old and is relatively rare in those under 30 [13], subjects aged under 30 were excluded (n = 38,677); (2) missing covariate data (n = 28,495); (3) missing CHF (MCQ160B) data (n = 31); and (4) missing calcium intake data (n = 772). After applying these exclusions, 3,083 subjects were included in the final analysis (Fig. 1). All participants provided written informed consent, underwent comprehensive measurements, and participated in standardized interviews. The study protocol received approval from the Ethics Review Board of the National Center for Health Statistics (NCHS).
Fig. 1.
A flowchart the inclusion and exclusion criteria
Assessment of outcome and exposure
CHF was the primary outcome of the study. Based on the response to question MCQ160B from the questionnaire: “Has your doctor or other health professional ever told you that you have CHF?” individuals who answered “yes” were classified as having a diagnosis of CHF and were included in the disease group [14]. Those who answered “no” were categorized as controls. Calcium intake, considered the exposure variable, was assessed through a 24-hour recall questionnaire (DR1ICALC).
Assessment of covariates
Additional covariates examined in the study included age (RIDAGEYR), gender (RIAGENDR), race (RIDRETH1), education (DMDEDUC2), smoking status (SMQ020), body mass index (BMI) (BMXBMI), alcohol consumption (ALQ110), high blood pressure (BPQ020), and fasting glucose levels (LBDGLUSI). Detailed information on these covariates is presented in Table 1.
Table 1.
Information on confounding factors and their numbers
| Variable | Data set and definition number | Year |
|---|---|---|
| Age | DEMO_C-DEMO_J(RIDAGEYR) | 2003–2018 |
| Gender | DEMO_C-DEMO_J(RIAGENDR) | 2003–2018 |
| Race | DEMO_C-DEMO_J(RIDRETH1) | 2003–2018 |
| Education | DEMO_C-DEMO_J(DMDEDUC2) | 2003–2018 |
| Alcohol Consumption | ALQ_C-ALQ_J(ALQ110) | 2003–2018 |
| Smoke | SMQ_C-SMQ_J(SMQ020) | 2003–2018 |
| High blood pressure | BPQ_C-BPQ_J(BPQ020) | 2003–2018 |
| Fasting glucose | L10AM_C-OGTT_J(LBDGLUSI) | 2003–2018 |
| BMI | BMX_C-BMX_J(BMXBMI) | 2003–2018 |
Statistical analysis
Differences in participant characteristics between CHF and control groups were assessed using the Student’s t-test for continuous variables and the chi-square test for categorical variables. Three models were developed: Model I (unadjusted), which examined the relationship between calcium intake and CHF; Model II (minimally adjusted), which adjusted for age, race, and gender; and Model III (fully adjusted), which included additional adjustments for education, smoking status, BMI, alcohol consumption, high blood pressure, and fasting glucose, building upon Model II. Weighted multivariate logistic regression was employed to investigate the potential association between calcium intake and CHF in all three models. Furthermore, the effect of covariates on CHF was examined in Model III through risk stratification analysis. An odds ratio (OR) greater than 1 indicated a risk factor, while an OR less than 1 suggested a protective factor. The accuracy and reliability of Model III were evaluated using the receiver operating characteristic (ROC) curve, employing the pROC (v 1.18.0) package [15], with an area under the curve (AUC) greater than 0.7 indicating good predictive performance. Additionally, Least Absolute Shrinkage and Selection Operator (LASSO) analysis was conducted on the exposure and covariates using the glmnet (v 4.1-8) package [16] to identify the optimal predictor variables based on the selected lambda value. The reliability of the LASSO results was further assessed using the LASSO-ROC curve (AUC > 0.7). These identified predictor variables were then used to construct a nomogram model for CHF diagnosis. The results were presented as OR with 95% confidence intervals (CI). P < 0.05 was indicated to be significant.
Results
Differences in subjects’ baseline characteristics
Based on the pre-established inclusion and exclusion criteria, a total of 3,083 participants were included in the final analysis, comprising 136 subjects in the CHF group and 2,947 subjects in the control group. Among all participants, Non-Hispanic Whites represented the largest demographic, accounting for 40.54% of the total cohort. In terms of gender, females constituted the majority, making up approximately 70.72% of the participants. Most subjects were between 30 and 80 years old and were non-smokers. Baseline characteristic analysis revealed significant differences between the CHF and control groups in terms of race, age, education level, BMI, hypertension, fasting blood glucose, and calcium intake (p < 0.05) (Table 2).
Table 2.
Baseline characteristics of study participants
| Characteristics | Level | Congestive heart failure patterns | p.value | |
|---|---|---|---|---|
| Yes | No | |||
| n | 136 | 2947 | ||
| Race (%) | MexicanAmerican | 12 (8.8) | 515 (17.5) | 0.004 |
| OtherHispanic | 11 (8.1) | 269 (9.1) | ||
| Non-HispanicWhite | 74 (54.4) | 1176 (39.9) | ||
| Non-HispanicBlack | 31 (22.8) | 671 (22.8) | ||
| OtherRace | 8 (5.9) | 316 (10.7) | ||
| Age (%) | 30–80 | 90 (66.2) | 2647 (89.8) | < 0.001 |
| > 80 | 46 (33.8) | 300 (10.2) | ||
| Gender (%) | Male | 38 (27.9) | 855 (29.0) | 0.863 |
| Female | 98 (72.1) | 2092 (71.0) | ||
| Education (%) | LessThan9thGrade | 24 (17.6) | 454 (15.4) | 0.019 |
| 9-11thGrade | 27 (19.9) | 437 (14.8) | ||
| High School Grad | 35 (25.7) | 699 (23.7) | ||
| Some College | 37 (27.2) | 729 (24.7) | ||
| College Graduate or above | 13 (9.6) | 628 (21.3) | ||
| BMI (%) | <=25 | 20 (14.7) | 736 (25.0) | < 0.001 |
| 26–29 | 35 (25.7) | 949 (32.2) | ||
| >=30 | 81 (59.6) | 1262 (42.8) | ||
| Alcohol consumption (%) | Morethan12Cups/Day | 75 (55.1) | 1561 (53.0) | 0.682 |
| Lessthan12Cups/Day | 61 (44.9) | 1386 (47.0) | ||
| Smoke (%) | Yes | 47 (34.6) | 787 (26.7) | 0.055 |
| No | 89 (65.4) | 2160 (73.3) | ||
| High_blood_pressure (%) | Yes | 108 (79.4) | 1342 (45.5) | < 0.001 |
| No | 28 (20.6) | 1605 (54.5) | ||
| Fasting_Glucose (mean (SD)) | 6.62 (1.84) | 6.23 (2.24) | 0.047 | |
| Calcium_intake (mean (SD)) | 724.22 (380.63) | 836.91 (558.80) | 0.02 | |
Correlation between CHF and calcium intake
Three continuous weighted multivariable generalized linear models (GLM) were subsequently constructed to explore the association between calcium intake and CHF. In Model 1, calcium intake was significantly associated with an increased risk of CHF (OR = 7.85 × 10–4, 95% CI = 3.4 × 10–4 − 1.23 × 10–3, p = 6.8800e-04). Model 2, which adjusted for gender, age, and race, showed that these factors did not significantly alter the association between calcium intake and CHF (OR = 7.40 × 10–4, 95% CI = 2.74 × 10–4 − 1.21 × 10–3, p = 2.1729e-03). In Model 3, further adjustments for education, BMI, alcohol consumption, hypertension, and fasting glucose revealed no significant interference with the effect of calcium intake on CHF (OR = 6.37 × 10–4, 95% CI = 1.47 × 10–4 − 1.13 × 10–3, p = 1.1345e-02) (Table 3). The consistency of the association across all models suggests a robust and reliable relationship between calcium intake and CHF, largely unaffected by the confounding variables included in these models.
Table 3.
Association analysis between exposure factors and outcomes
| SII | OR | 95% CI | p.value |
|---|---|---|---|
| Model 1 | 7.8500e-04 | 3.4000e-04–1.2300e-03 | 6.8800e-04 |
| Model 2 | 7.4038e-04 | 2.7357e-04–1.2072e-03 | 2.1729e-03 |
| Model 3 | 6.3660e-04 | 1.4731e-04–1.1259e-03 | 1.1345e-02 |
Risk stratification analysis and LASSO analysis
Model 3 was used to assess the stability of the association between calcium intake and CHF risk across different populations. The findings revealed a strong association between calcium intake and CHF, with calcium intake serving as a protective factor (OR = 3 × 10–6). Additionally, individuals over the age of 80 (OR = 4.623) and those with a BMI greater than 30 (OR = 2.571) were identified as risk factors for CHF (Fig. 2A). ROC analysis, with an AUC of 0.802, demonstrated that calcium intake is highly accurate in predicting CHF risk (Fig. 2B).
Fig. 2.
Associations between dietary calcium intake and the risk of CHF. A Weighted multivariate logistic regression analysis of dietary calcium and CHF risk. B Exposure factors ROC curve C LASSO model residual sum of squares plot and LASSO regression coefficient plot. D Constructing a column chart based on the seven best predictor variables E LASSO-ROC curve. AUC: Area under curve; BMI: Body mass index; LASSO: Least absolute shrinkage and selection operator; OR: Odds ratio
At an optimal lambda value of 0.0003309, age, BMI, alcohol consumption, smoking status, high blood pressure, fasting glucose, and calcium intake were identified as the most significant predictive variables for CHF (Fig. 2C). A risk prediction nomogram was then constructed based on these seven key variables, each contributing significantly to the model’s predictive accuracy (Fig. 2D). The LASSO results were further evaluated through ROC analysis, yielding an AUC of 0.76, indicating good predictive performance (Fig. 2E).
Discussion
CHF is a major cardiovascular condition characterized by high morbidity and mortality. The progression and patterns of CHF remain challenging to track and predict over time, making early detection crucial for effective management and treatment. Ca2+ plays a vital role in regulating muscle contraction, synaptic transmission, hormone secretion, and cellular processes such as proliferation and apoptosis [17]. Alterations in intracellular Ca2+ signaling in cardiomyocytes have been implicated in the pathogenesis of heart failure, with defects in Ca2+ signaling serving as a central pathogenic mechanism in heart disease [18]. However, the relationship between calcium intake and CHF remains unclear. Baseline characteristics revealed significant differences between the CHF and control groups in terms of race, education, fasting glucose, and calcium intake (p < 0.05). Calcium intake was found to be significantly associated with CHF across all three models (p < 0.001), acting as a protective factor. Therefore, this study explored the association between dietary calcium intake and CHF risk, utilizing robust statistical methods, including risk stratification and LASSO regression analysis. LASSO regression analysis identified calcium intake as a significant predictor variable. The AUC values for both Model III and the LASSO model exceeded 0.7, indicating strong predictive power. The study concluded that calcium intake serves as a protective factor against CHF, with its effects largely unaffected by other covariates such as age or hypertension. The predictive value of calcium intake for CHF risk was further validated using ROC curves, highlighting its potential as a modifiable factor in CHF management and prevention. Optimizing calcium intake may be crucial in reducing the risk of CHF, thus providing theoretical support for its role in the prevention and diagnosis of the disease.
The baseline characteristics also indicated that age and BMI are key factors influencing CHF progression. CHF is a condition resulting from the heart’s inability to meet the body’s metabolic demands. It is primarily a disease of older adults, with a prevalence of 1–2% of the population, and is rare in infancy and childhood [19]. Although the pathophysiology of CHF is similar across age groups, older adults are more likely to develop CHF, even with preserved left ventricular systolic function. This condition, known as diastolic heart failure, accounts for 50% of all CHF cases in individuals over the age of 65 [20]. The present study identified age > 80 years as a risk factor for CHF (OR = 4.62). Similarly, BMI emerged as a critical factor in CHF risk. Notably, 53.48% of patients with CHF had a BMI ≥ 30 kg/m2. In comparison to non-CHF individuals, those with CHF had higher BMI, reduced energy and macronutrient intake, and lower hematocrit and hemoglobin levels [20]. Numerous studies have established a strong link between lower hematocrit and hemoglobin levels and poor clinical outcomes in CHF, further supporting the role of a BMI ≥ 30 kg/m2 as a significant risk factor for CHF. These findings align with the results of the present study.
The relationship between dietary calcium and cardiovascular health remains a subject of ongoing debate. Previous studies have associated higher intakes of calcium, magnesium, and potassium with improved cardiovascular outcomes, although the precise mechanisms through which dietary calcium influences CHF risk remain unclear [21]. Potential mechanisms may involve the prevention of vascular calcification or improved metabolic control in cardiac muscle. This is consistent with findings suggesting that moderate dietary calcium intake protects against cardiovascular mortality, yet its specific role in conditions like CHF requires further investigation [22, 23]. This study revealed significant differences in clinical characteristics between CHF and non-CHF groups, particularly in calcium intake (p < 0.05). Across multiple models, calcium intake consistently demonstrated a protective effect against CHF, independent of confounding factors such as age or hypertension (Model III OR = 6.37 × 10–4, p < 0.001). These findings suggest that adequate calcium intake may reduce the risk of developing CHF.
The robustness of the model, demonstrated through LASSO regression, identified several key predictors of CHF, including age, BMI, and metabolic factors such as fasting glucose and hypertension—well-established CHF risk factors. The high predictive performance of the model, evidenced by AUC values exceeding 0.76, indicates that it effectively captures the complex interactions between these variables in predicting CHF risk. This predictive validity highlights the potential for incorporating dietary and metabolic data into comprehensive risk assessment tools for CHF.
In summary, this study, employing a range of statistical methods to examine the interactions between various covariates and calcium intake, substantiates calcium intake as a protective factor against CHF. These findings suggest promising avenues for developing strategies to mitigate and prevent CHF, potentially influencing future nutritional and therapeutic guidelines. However, the limitations inherent in the cross-sectional design and potential measurement inaccuracies should caution against broad extrapolation of these results. Future longitudinal studies are necessary to deepen our understanding and further confirm the protective role of calcium in CHF pathogenesis, ultimately guiding more effective public health interventions and clinical strategies to address this widespread cardiovascular condition.
Limitations
This study has several limitations. First, the questionnaire relies on data from patients’ self-reports, which may be subjectively biased. The MCQ160B question in the NHANES questionnaire fails to effectively differentiate between different types of heart failure (HF). Additionally, the definition of congestion can be subjective, as it is influenced by individual patient perceptions and the experience of doctors. For example, different doctors may have varying assessments of the degree of congestion, leading to inconsistent diagnoses and potentially affecting the accuracy and comprehensiveness of the research findings. Second, due to the study’s design, it can only establish correlations between variables, not causality. This study, due to its limitations in the variable range of NHANES data, did not include information on NT-proBNP or specific causes and drug use for heart failure. This limitation means that it cannot assess whether low calcium intake precedes CHF, which could introduce bias in the estimation of the association between calcium intake and heart failure. Therefore, future studies should incorporate more detailed clinical features, further analyze baseline drug characteristics, and verify the relationship between calcium intake and different subtypes of heart failure. Additionally, the relatively small sample size (136 patients with CHF) limits the generalizability of the findings, indicating a need for future studies with larger sample sizes to enhance representativeness.
Conclusion
In conclusion, this study identified a significant inverse association between dietary calcium intake and the incidence of CHF, with calcium intake emerging as a protective factor. These findings suggest that optimizing calcium consumption could play a pivotal role in reducing CHF risk. Furthermore, LASSO regression, nomogram modeling, and ROC analyses identified calcium intake as one of the seven most reliable predictive variables for CHF risk assessment. Together, these results offer new insights into CHF prevention and clinical management, highlighting the potential therapeutic benefits of ensuring adequate calcium intake.
Supplementary Information
Acknowledgements
We are grateful to all the people who have given help on this article.
Authors’ contributions
B.Z and L.W: was responsible for designing the study, interpretation of the data, drafting the manuscript, revising the manuscript, and the approval of the final version. X.C: participated in the design of analyses, data analysis, and approval of the final version. Z.W: interpretation of the data and the approval of the final version. H.R: participated in formulating the research question, design of analyses, revising the manuscript, and the approval of the final version. C.H and Q.L: participated in formulating the research question, design of analyses, data analysis, interpretation of the data, and the approval of the final version. All authors read and approved the final manuscript.
Funding
This work was supported by the [grant from the Macao Science and Technology Development Fund] grant number [002/2023/ALC], [The Science and Technology Development Fund, Macau SAR] grant number [File no. 006/2023/SKL] and [General Research Grants of Macau university of Science and Technology] grant number [FRG-21-032-SKL], the [National Natural Science Foundation of China] grant number [82360072] and [Jiangxi Natural Science Foundation] grant number [20232BAB216003].
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This study involves human participants and was approved by NHANES was approved by the NCHS Research Ethics Review Board. Participants gave informed consent to participate in the study before taking part.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Bo Zhu, Li Wan and Xingyu Chen contributed equally to this work and share first authorship.
Contributor Information
Qing Li, Email: liqing199137@126.com.
Chen Huang, Email: chuang@must.edu.mo.
References
- 1.Chen J, Aronowitz P. Congestive heart failure. Med Clin North Am. 2022;106(3):447–58. 10.1016/j.mcna.2021.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Borlaug BA, Sharma K, Shah SJ, Ho JE. Heart failure with preserved ejection fraction: JACC scientific statement. J Am Coll Cardiol. 2023;81:1810–34. 10.1016/j.jacc.2023.01.049. [DOI] [PubMed] [Google Scholar]
- 3.Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat Rev Cardiol. 2016;13:368–78. 10.1038/nrcardio.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Felker GM, Ellison DH, Mullens W, Cox ZL, Testani JM. Diuretic therapy for patients with heart failure: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:1178–95. 10.1016/j.jacc.2019.12.059. [DOI] [PubMed] [Google Scholar]
- 5.Scicchitano P, Iacoviello M, Passantino A, et al. Plasma levels of intact parathyroid hormone and congestion burden in heart failure: clinical correlations and prognostic role. J Cardiovasc Dev Dis. 2022;9(10):334. 10.3390/jcdd9100334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terrell K, Choi S, Choi S. Calcium’s role and signaling in aging muscle, cellular senescence, and mineral interactions. Int J Mol Sci. 2023;24:17034. 10.3390/ijms242317034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bronner F. Calcium and osteoporosis. Am J Clin Nutr. 1994;60:831–6. 10.1093/ajcn/60.6.831. [DOI] [PubMed] [Google Scholar]
- 8.Dridi H, Kushnir A, Zalk R, Yuan Q, Melville Z, Marks AR. Intracellular calcium leak in heart failure and atrial fibrillation: a unifying mechanism and therapeutic target. Nat Rev Cardiol. 2020;17:732–47. 10.1038/s41569-020-0394-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dridi H, Liu Y, Reiken S, Liu X, Argyrousi EK, Yuan Q, Miotto MC, Sittenfeld L, Meddar A, Soni RK, et al. Heart failure-induced cognitive dysfunction is mediated by intracellular Ca2 + leak through Ryanodine receptor type 2. Nat Neurosci. 2023;26:1365–78. 10.1038/s41593-023-01377-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bourassa MW, Abrams SA, Belizán JM, Boy E, Cormick G, Quijano CD, Gibson S, Gomes F, Hofmeyr GJ, Humphrey J, et al. Interventions to improve calcium intake through foods in populations with low intake. Ann N Y Acad Sci. 2022;1511:40–58. 10.1111/nyas.14743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hodges JK, Cao, Cladis DP, Weaver CM. Lactose intolerance and bone health: the challenge of ensuring adequate calcium intake. Nutrients. 2019;11:718. 10.3390/nu11040718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Daghri NM, Hussain SD, Alnaami AM, Aljohani N, Sabico S. Dietary calcium intake and osteoporosis risk in Arab adults. Nutrients. 2023;15:2829. 10.3390/nu15132829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2017;14(10):591–602. 10.1038/nrcardio.2017.65. [DOI] [PubMed] [Google Scholar]
- 14.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F,… Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American college of cardiology foundation/american heart association task force on practice guidelines. Circulation. 2013;128(16):1810–52. 10.1161/CIR.0b013e31829e8807. [DOI] [PubMed]
- 15.Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez J-C, Müller M. pROC: an open-source package for R and S + to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaksik R, Szumała K, Dinh KN, Śmieja J. Multiomics-based feature extraction and selection for the prediction of lung cancer survival. Int J Mol Sci. 2024;25:3661. 10.3390/ijms25073661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marks AR. A guide for the perplexed: towards an Understanding of the molecular basis of heart failure. Circulation. 2003;107:1456–9. 10.1161/01.cir.0000059745.95643.83. [DOI] [PubMed] [Google Scholar]
- 18.Marks AR. Calcium cycling proteins and heart failure: mechanisms and therapeutics. J Clin Invest. 2013;123:46–52. 10.1172/JCI62834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmaltz AA. Chronic congestive heart failure in infancy and childhood: new aspects of diagnosis and treatment. Klin Padiatr. 2015;227:3–9. 10.1055/s-0034-1389974. [DOI] [PubMed] [Google Scholar]
- 20.Rich MW. Epidemiology, pathophysiology, and etiology of congestive heart failure in older adults. J Am Geriatr Soc. 1997;45:968–74. 10.1111/j.1532-5415.1997.tb02968.x. [DOI] [PubMed] [Google Scholar]
- 21.Pana TA, Dehghani M, Baradaran HR, Neal SR, Wood AD, Kwok CS, Loke YK, Luben RN, Mamas MA, Khaw K-T, et al. Calcium intake, calcium supplementation and cardiovascular disease and mortality in the British population: EPIC-norfolk prospective cohort study and meta-analysis. Eur J Epidemiol. 2021;36:669–83. 10.1007/s10654-020-00710-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levitan EB, Shikany JM, Ahmed A, Snetselaar LG, Martin LW, Curb JD, Lewis CE. Calcium, magnesium and potassium intake and mortality in women with heart failure: the women’s health initiative. Br J Nutr. 2013;110:179–85. 10.1017/S0007114512004667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel Y, Joseph J. Sodium intake and heart failure. Int J Mol Sci. 2020;21:9474. 10.3390/ijms21249474. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.