Abstract
Background
Total Mixed Ration (TMR) is recognized for its balanced nutritional composition, improved feed efficiency, enhanced animal production, and stabilization of the gastrointestinal microbiome. It has been extensively implemented in intensive ruminant farming, particularly for cattle and sheep, with demonstrated positive outcomes. However, its effects on the nutritional health of non-ruminant herbivores, such as horses, remain insufficiently investigated. This study aims to evaluate the comparative effects of TMR feeding versus conventional feeding practices in Akhal-Teke horses while maintaining identical dietary compositions and nutritional levels. By analyzing body weight, growth in body measurements, nutrient metabolism, and faecal microbiome diversity, the study aims to determine the potential advantages of TMR feeding for monogastric herbivores.
Results
Compared The TMR group (S) demonstrated a significant increase in total weight gain and average daily weight gain, surpassing the control group (C) by 47.53% (P < 0.05) and 48.28% (P < 0.05), respectively. Moreover, the S group showed substantial improvements in DM (Dry Matter ), DE (Digestible Energy), CP (Crude Protein), ADF (Acid Detergent Fiber), and P (Phosphorus), with increases of 8.27% (P < 0.01), 11.97% (P < 0.01), 14.30% (P < 0.01), 39.52% (P < 0.01), and 38.35% (P < 0.01), respectively, compared to the C group. No significant differences were observed in serum parameters, including TP (Total Protein), ALB (Albumin), Cre (Creatinine), UA (Uric Acid), UREA (Urea), Glu (Glucose), T-Bil (Total Bilirubin), D-Bil (Direct Bilirubin), TC (Total Cholesterol), ALT (Alanine Aminotransferase), AST (Aspartate Aminotransferase), AST/ALT, ALP (Alkaline Phosphatase), CK (Creatine Kinase), Ca2+ (Calcium), Mg2+ (Magnesium), and Inorganic Phosphorus (P > 0.05). However, UREA was significantly reduced by 14.90% (P < 0.01). While faecal pH and VFA were unaffected (P > 0.05), the abundance of Spirochaetota, Treponema, Christensenellaceae_R-7_group, and Lactobacillus_hayakitensis was significantly elevated (P < 0.05). However, Prevotellaceae abundance was significantly reduced (P < 0.05).
Conclusions
In conclusion, under the conditions of this study, TMR feeding notably improved body weight, nutrient digestibility, gut microbiota composition, and fiber degradation in Akhal-Teke horses when compared to traditional feeding methods.
Keywords: TMR, Apparent digestibility, Blood biochemical parameters, Faecal microbiota, Akhal-Teke horse
Introduction
The Akhal-Teke horse, indigenous to Turkmenistan, is famously known as the “Ferghana horse” in China [1]. Its distinctive body and skeletal structure provide notable advantages in speed, strength, and endurance [2, 3]. Due to its rarity and high value, the purebred Akhal-Teke is especially scarce, with an estimated global population of approximately 5,000 horses as of 2010 [4]. As China’s equine industry continues to expand, there is a growing demand for high-quality foreign breeds and improvements in local Chinese breeds, spurred by sectors such as competition, spectator sports, and equestrian activities [5]. Consequently, the scientific management of Akhal-Teke breeding practices is essential for optimizing growth performance, reproductive success, and the overall development of China’s equine industry [6]. However, research on the feeding and management practices for Akhal-Teke horses remains insufficient. Traditional breeding methods, which predominantly rely on long hay as the main feed supplemented with complementary feeds, dominate under confined housing conditions. Enhancing the composition and nutritional balance of equine diets is vital for the healthy breeding of these horses.
TMR provides a well-balanced nutritional profile, improved feed utilization, and substantial gains in animal performance. It stabilizes the idigestive microbial environment, effectively preventing digestive disorders, and significantly boosts feed conversion efficiency. These benefits make TMR a critical method for large-scale, intensive cattle and sheep production, as well as for scientific feeding management. Developed and promoted in the 1960s by countries such as the United Kingdom, the United States, and Israel, TMR technology saw the introduction of group feeding systems in the 1970s in the U.S., based on mechanized production [7]. The associated processes and equipment have advanced significantly, and TMR adoption has spread to countries like Canada, the Netherlands, and Italy. In housed feeding conditions, TMR’s exceptional palatability, enhanced nutrient digestion and absorption, and resistance to spoilage contribute to its profound impact on herbivorous livestock [8]. While widely implemented in cattle and sheep farming, TMR technology has yet to reach technological maturity in equine farming. Lan et al. [9] compared different feeding sequences-concentrates and roughage-along with TMR feeding in lactating donkeys, finding that donkeys fed the mixed ration exhibited superior growth performance, with markedly higher crude protein (CP) and fat digestibility, compared to those fed concentrate followed by roughage. Furthermore, Firmicutes abundance was significantly greater in the TMR group. Riond et al. [10] demonstrated that horses fed TMR exhibited significantly higher feed efficiency (kg gain/Mcal DE consumption) compared to those fed traditional hay and concentrate, with growth rates exceeding those predicted by the NRC (1989). Despite these studies on TMR in equids, no comprehensive research has yet assessed the effects of TMR on equine growth performance, energy production, nutrient digestion, health, and gut microbial diversity.
Materials and methods
Animals and experimental design
Fourteen Akhal-Teke broodmares, averaging 16.5 ± 2.1 months in age and 315.8 ± 21.5 kg in body weight, were selected for the experiment and randomly assigned to two groups: the Control Group (C group) and the TMR Group (S group). Both groups were fed diets with identical compositions and nutritional levels. The C group received a traditional feeding regimen, consisting of natural, uncut alfalfa hay supplemented with concentrate. In contrast, the S group was provided with a TMR, which included alfalfa hay chopped to an average length of 3 cm. The feeding trial lasted for 45 days, with a 15-day adaptation period followed by a 30-day main feeding phase. Digestibility and metabolism trials were conducted from days 41 to 45. Body weight and body measurements were recorded before the main feeding phase and on day 45. Blood samples were collected from the jugular vein, and faecal samples were collected on day 45 for analysis of blood biochemical parameters, faecal microbiota, pH, and volatile fatty acids (VFA).
Feeding management
Horses in both the C group and S group were provided equivalent daily feed quantities per animal, with diet compositions outlined in Table 1.
Table 1.
Composition and nutritional levels of the diet
| Diet composition | Content | Nutrient level1 | Content |
|---|---|---|---|
| Alfalfa hay | 68.97% | DM, % | 93.75 |
| Corn | 6.21% | OM, % | 84.07 |
| Oats | 6.21% | CP, % | 18.36 |
| Barley | 0.94% | EE, % | 1.89 |
| Bran | 7.76% | Gross energy, MJ/kg | 14.92 |
| Soybean meal | 6.21% | NDF, % | 32.34 |
| Salt | 0.61% | ADF, % | 23.94 |
| Calcium hydrogen phosphate | 0.61% | Ca, % | 1.61 |
| Electrolyte vitamins | 0.31% | P, % | 0.33 |
| Vegetable oil | 1.56% | ||
| Garlic powder | 0.61% | ||
| Total | 100% |
1The nutrient levels are all measured values
The C group received a sequential feeding regimen: non-mixed alfalfa hay (maintained at natural harvest length of 30–50 cm) was administered in four equal portions (2 kg each) at 11:30, 17:30, 23:30, and 05:30, followed by uniformly blended concentrate supplement delivered in two portions (1.6 kg each) at 14:00 and 20:00 daily.
The S group was fed TMR, prepared by precise weighing of all components and homogenization using a TMR mixer (Model 9JGW-5; Shandong Xinxingtai Machinery Manufacturing Co., Ltd., China). Feeding protocols were phased as:
Days 1–5: Alfalfa hay (2 kg) at 11:30, 23:30, 05:30; TMR (1.8 kg) at 14:00, 17:30; concentrate supplement (1.6 kg) at 20:00.
Days 6–10: TMR (1.8 kg) at 11:30, 14:00, 17:30, 20:00; Alfalfa hay (2 kg) at 23:30, 05:30.
Days 16–45: TMR (1.93 kg) at 11:30, 14:00, 17:30, 20:00, 23:30, 05:30 (detailed in Table 2).
Table 2.
Feeding regimen of the diet
| Group | Time | 11:30 | 14: 00 | 17: 30 | 20: 00 | 23: 30 | 05: 30 |
|---|---|---|---|---|---|---|---|
| Control group | 0–45 d | Alfalfa 2 kg | Concentrate Supplement 1.6 kg | Alfalfa 2 kg |
Concentrate Supplement 1.6 kg |
Alfalfa 2 kg | Alfalfa 2 kg |
| TMR group | 1–5 d | Alfalfa 2 kg | TMR1.8 kg | TMR1.8 kg | Concentrate Supplement 1.6 kg | Alfalfa 2 kg | Alfalfa 2 kg |
| 6–10 d | TMR1.8 kg | TMR1.8 kg | TMR1.8 kg | TMR1.8 kg | Alfalfa 2 kg | Alfalfa 2 kg | |
| 11–15 d | TMR1.84 kg | TMR1.84 kg | TMR1.84 kg | TMR1.84 kg | TMR1.84 kg | Alfalfa 2 kg | |
| 16–45 d | TMR1.93 kg | TMR1.93 kg | TMR1.93 kg | TMR1.93 kg | TMR1.93 kg | TMR1.93 kg |
All horses had ad libitum access to water and feed. Daily management included stall sanitation, bedding replacement, and two-hour rotational paddock exercise sessions alternating between C group and S group.
Sample collection and analysis
Blood sampling and analysis
Every 15 days during the trial, body weight was recorded using a weighbridge, while body height, body length, chest circumference, and cannon circumference were measured with a tape measure. On the morning of the final day, fasting blood samples were collected from the jugular vein following restraint of the horses and site disinfection with an alcohol swab. A 5 mL blood sample was drawn using a sodium heparin vacuum blood collection tube, centrifuged at 3,500 rpm for 15 min, and the supernatant was transferred to centrifuge tubes for storage at -20 °C, pending analysis of blood biochemical parameters. From days 41 to 45, a 5-day nutrient digestibility trial was conducted. During the trial, 500 g of TMR feed was sampled daily before the morning feeding (11:30) using a five-point sampling method. The feed samples were air-dried, weighed, crushed, and sealed in airtight bags for later analysis. Faecal samples were collected daily for nutrient digestibility analysis through total faecal collection. Faecal material was gathered from each horse using collection buckets containing 10% sulfuric acid to prevent nitrogen loss. The daily faecal output per horse was recorded, and the faeces were thoroughly mixed in the bucket using sterile rubber gloves. A 10% sample of the total faecal output was collected daily using the secondary methyl method, ensuring samples were taken from the upper, middle, and lower layers of the faeces in the bucket. On the final day of the experiment, fresh faecal samples were collected using disposable sterile gloves. The samples were mixed, and 20 g from each sample were placed into two separate 10 mL sterile RNA preservation tubes. The samples were immediately frozen in liquid nitrogen and stored at − 80 °C for subsequent analysis of faecal pH, VFA, and faecal microbiota diversity.
Determination of nutrient digestibility
All chemical analyses in this study were conducted at the Laboratory of Herbivore Nutrition for Meat & Milk Production (Autonomous Region Key Laboratory, licensed facility, Urumqi, Xinjiang, China). The faecal subsamples, previously stored at -20 °C, were thawed and transferred to aluminum boxes before being placed in an electric thermostatic blast drying oven at 65 °C for 48 h. The collected samples, including concentrate supplements, forage, forage leftovers, and dried faeces, were homogenized using a multifunctional high-speed grinder (400 g upright type; Tohe Electromechanical Technology Co., Ltd., Shanghai, China) and subsequently sieved through a 1 mm mesh. Following the international AOAC method [11] for sample drying and in accordance with Chinese national standards for routine nutrient analysis, we determined the contents of Dry Matter (DM), Crude Protein (CP), Calcium (Ca), and Phosphorus (P). Digestible Energy (DE) was measured using a high-precision OR2014 calorimeter (Shanghai Ou Rui Instrument Equipment Co., Ltd., Shanghai, China). Neutral Detergent Fiber (NDF) and Acid Detergent Fiber (ADF) concentrations were analyzed according to the method described by Van Soest et al. [12] using an automated fiber analyzer (ANKOM-2000; Shanghai Longjie Instrument Equipment Co., Ltd., Shanghai, China).
Determination of faecal pH and volatile fatty acids
A 10 g sample of freshly collected faecal was mixed with 10 mL of ultrapure water and vortexed for 3 min using a vortex mixer. The mixture was then filtered through four layers of gauze, and the pH of each sample was measured using a calibrated pH meter (FiveEasy22-Meter, Mettler-Toledo International Trade (Shanghai) Co., Ltd.). The concentrations of VFA, including acetate, propionate, butyrate, isobutyrate, valerate, and isovalerate, were determined by gas chromatography. Faecal subsamples were stored at -80 °C, then thawed and homogenized prior to analysis. For the VFA determination, 10 g of the subsample was mixed with 10 mL of ultrapure water, vortexed at 4 °C for 30 min, and filtered through four layers of gauze. The filtrate was centrifuged at 5,000×g for 10 min, and the supernatant was incubated at 4 °C for 12 h. A 0.6 mL aliquot of the supernatant was mixed with 0.6 mL of 19% trichloroacetic acid (Sigma Aldrich, Shanghai, China) and 0.1 mL of a 60 mmol internal standard solution (crotonic acid, Sigma Aldrich, Shanghai, China). The mixture was vortexed and allowed to stand at 4 °C for 20 min, followed by centrifugation at 20,000 × g for 15 min. A 1 µL sample of the supernatant was injected for analysis. The gas chromatograph (Agilent 7890 A, Agilent Technologies (China) Ltd., Beijing, China) was equipped with an HP-FFAP capillary column (50 m × 0.20 mm internal diameter × 0.33 μm film thickness, Agilent Technologies, Beijing, China). The chromatographic conditions were as follows: the oven temperature was increased at 10 °C/min from 60 °C to 220 °C, where it was held for 12 min. The injector and detector were set at 240 °C and 280 °C, respectively. Nitrogen was used as the carrier gas at a flow rate of 5.0 mL/min.
DNA extraction, PCR amplification, and 16 S RNA sequencing
(1) Total genomic DNA was extracted from all samples using an optimized CTAB/SDS protocol. DNA concentration and purity were verified through 1% agarose gel electrophoresis, followed by dilution to a standardized concentration of 1 ng/µL using sterile water. The V3-V4 hypervariable regions of bacterial 16S rRNA genes were amplified via PCR using barcoded universal primers: 341F (5’-CCTAYGGGRBGCASCAG-3’) and 806R (5’-GGACTACNNGGGTATCTAAT-3’). All PCR reactions (30 µL total volume) contained 15 µL Phusion® High-Fidelity PCR Master Mix (New England Biolabs), 0.2 µM of each primer, and approximately 10 ng template DNA. The thermal cycling protocol consisted of: initial denaturation at 98℃ for 1 min; 30 cycles of denaturation (98℃, 10 s), annealing (50℃, 30 s), and extension (72℃, 30 s); with final extension at 72℃ for 5 min [13]. Sequencing libraries were prepared using the Illumina TruSeq DNA PCR-Free Library Preparation Kit (Illumina, USA) following manufacturer’s instructions, with unique index codes incorporated. Library quality was assessed using both Qubit 2.0 Fluorometer (Thermo Scientific) and Agilent Bioanalyzer 2100 system. Final sequencing was performed on Illumina NovaSeq platform generating 250 bp paired-end reads, which were subsequently merged using FLASH.
(1) Paired-end reads were demultiplexed according to their unique barcodes. Subsequent sequence analysis was performed using QIIME pipeline [14, 15].
(2) Microbial diversity analysis was performed using the QIIME software package (Quantitative Insights into Microbial Ecology) complemented by custom Perl scripts for assessing both alpha- (intra-sample) and beta- (inter-sample) diversity. Initial quality control involved filtering raw reads through QIIME’s built-in quality filters. Operational Taxonomic Units (OTUs) were delineated at a 97% sequence similarity threshold. For subsequent taxonomic classification, a representative sequence from each OTU cluster was selected and analyzed using the RDP classifier [16, 17].
(3) Alpha diversity metrics were computed following rarefaction normalization of the OTU table, incorporating three key indices: Chao1 (estimating species richness), Observed OTUs (quantifying unique taxonomic units per sample), and Shannon index (evaluating community diversity). Corresponding rarefaction curves were generated to validate sampling adequacy. Beta diversity was assessed through both weighted and unweighted UniFrac distance metrics, which incorporate phylogenetic relationships. For comparative analysis of inter-sample microbial composition differences, multiple statistical approaches were employed: Student’s t-test, MetaStat analysis, Linear Discriminant Analysis Effect Size (LEfSe), Analysis of Similarities (ANOSIM), and Multi-Response Permutation Procedures (MRPP) [18, 19].
Measurement of plasma biochemical indicators
Blood biochemical parameters were measured using an automated biochemical analyzer (Mindray Bio-Medical Electronics Co., Ltd., Shenzhen, China; Model: BS-240VET) with reagent kits from the same company, following the provided protocols.The measured parameters included Total Protein (TP), Albumin (ALB), Uric Acid (UA), Urea (UREA), Creatinine (Cre), Glucose (Glu), Total Cholesterol (TC), Total Bilirubin (T-Bil), Direct Bilirubin (D-Bil), Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST), Alkaline Phosphatase (ALP), Creatine Kinase (CK), Calcium (Ca2+), Inorganic Phosphorus (P), Magnesium (Mg2+), and the AST/ALT ratio.
Data analysis
Data analysis was performed using independent sample t-tests in SPSS version 18.0. Results are presented as mean ± standard deviation (Mean ± SD). A P-value < 0.05 was considered statistically significant, and a P-value < 0.01 was considered highly significant. Body weight, measurements, and other parameters, were graphically presented using GraphPad Prism version 8.0.2. Correlation analysis was performed and visualized using Spearman’s rank correlation method (implemented in R version 4.2.0).
Results
Growth performance
Figure 1 illustrates the effects of TMR and traditional feeding on the body weight and body measurements of Akhal-Teke horses. As shown in Fig. 1C, D, E, F and G, there were no significant differences (P > 0.05) in body weight, body height, body oblique length, tube circumference, or chest Circumference between horses fed the two different diets. However, the S group exhibited a significant increase in both total weight gain and average daily weight gain compared to the C group on traditional diets, with increases of 4.1 kg (P < 0.05) and 0.1 kg (P < 0.05), respectively.
Fig. 1.
Horse Weight and Body Size A: Average daily gain; B: Total weight gain; C: Weight; D: Body height; E: Body oblique length; F: Tube circumference; G: Chest Circumference (C: Control group; S: TMR group)
Nutrient digestibility
Table 3 summarizes the effects of TMR and traditional feeding on apparent nutrient digestibility. The S group demonstrated highly significant improvements in the apparent digestibility of dry matter (DM), digestible energy, CP, acid detergent fiber (ADF), and phosphorus (P < 0.01). Specifically, the apparent digestibility of DM in the S group increased by 8.28% (P < 0.01) compared to the C group. Digestible energy increased by 12.01% (P < 0.01), CP by 14.30% (P < 0.01), ADF by 39.52% (P < 0.01), and phosphorus by 38.35% (P < 0.01).
Table 3.
Effects of TMR Diet on Apparent Nutrient Digestibility in Akhal-Teke Horses
| Item | C | S | P-value |
|---|---|---|---|
| DM (%) | 70.94 ± 1.31 | 76.81 ± 1.07 | 0.005 |
| Digestible Energy (MJ/kg) | 9.52 ± 0.31 | 10.66 ± 0.19 | 0.008 |
| CP (%) | 56.91 ± 1.99 | 65.05 ± 1.76 | 0.010 |
| NDF (%) | 75.86 ± 1.94 | 80.55 ± 1.94 | 0.112 |
| ADF (%) | 36.46 ± 3.88 | 50.86 ± 2.27 | 0.008 |
| Ca (%) | 30.86 ± 3.92 | 38.80 ± 4.38 | 0.202 |
| P (%) | 18.48 ± 1.79 | 25.57 ± 3.18 | 0.010 |
Blood biochemical indicators
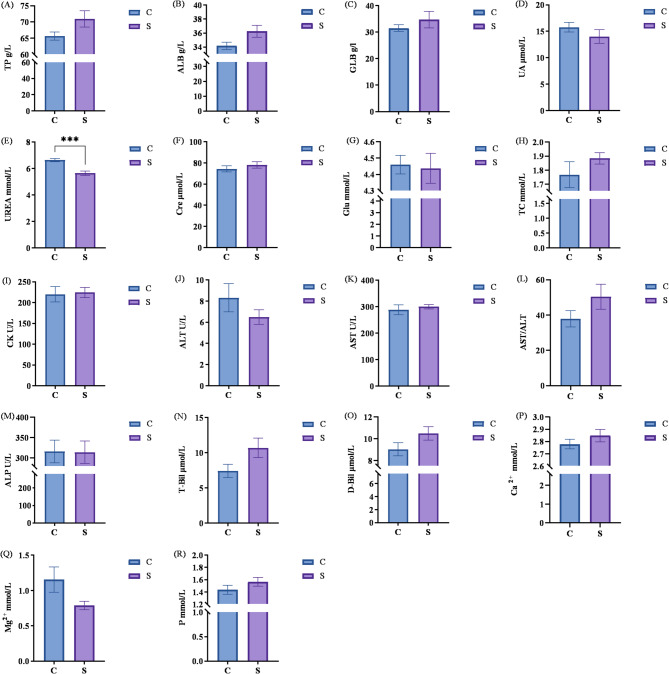
Figure 2 presents the effect of TMR and conventional feeding on serum biochemical indices. No significant differences were observed between the two groups for serum TP, ALB, UA, Cre, Glu, T-Bil, D-Bil, TC, ALT, AST/ALT, ALP, CK, Ca2+, Mg2+, and inorganic phosphorus (P > 0.05). However, serum levels of T-Bil, D-Bil, AST/ALT, and inorganic P in the S group increased by 44.32%, 16.19%, 33.23%, and 9.03% respectively compared with the C group, though no significant differences were observed (P > 0.05). Additionally, the TMR diet led to a significant reduction in serum urea concentration, with a 14.90% decrease in urea nitrogen (P < 0.01).
Fig. 2.
Effects of TMR Diet and Traditional Feeding on Serum Biochemical Parameters in Akhal-Teke Horses A: TP (Total Protein); B: ALB (Albumin); C: GLB (Globulin); D: UA (Uric Acid); E: UREA (Urea); F: Cre (Creatinine); G: Glu (Glucose); H: TC (Total Cholesterol); I: CK (Creatine Kinase); J: ALT (Alanine Aminotransferase); K: AST (Aspartate Aminotransferase); LK: AST/ALT; M: ALP (Alkaline Phosphatase); N: T-Bil (Total Bilirubin); O: D-Bil (Direct Bilirubin); P: Ca2+ (Calcium); Q: Mg2+ (Magnesium); R: P (Inorganic Phosphorus);
Faecal pH and volatile fatty acids
Figure 3 demonstrates the effects of TMR and traditional feeding on VFA and pH. Feeding TMR to Akhal-Teke horses improved the carbohydrate degradation rate, resulting in higher levels of acetic acid, propionic acid, isobutyric acid, butyric acid, isovaleric acid, valeric acid, and total VFA in the faecal (P > 0.05). Furthermore, there was no significant difference in faecal pH between the S group and the C group (P > 0.05).
Fig. 3.
Faecal pH and Volatile Fatty Acids A: Acetic acid; B: Propionic acid; C: Butyric acid; D: Isobutyric acid; E: Valeric acid; F: Isovaleric acid; G: Total VFA; H: pH
Alpha diversity of faecal Bacteria
Table 4 presents the effects of TMR and traditional feeding on the alpha diversity of faecal microbiota in Akhal-Teke horses. Good’s coverage index indicated that sequencing coverage exceeded 99% for both the C and S groups, suggesting high accuracy in the faecal microbiota composition data. Based on the observed operational taxonomic units (OTUs), the S group exhibited higher species richness compared to the C group, though this difference was not statistically significant (P > 0.05). Additionally, there were no significant differences in the Chao1, Pielou_e, and Shannon indices between the S group and the C group (P > 0.05).
Table 4.
Diversity of horse faecal microbiota1
| Item | C | S | P-value |
|---|---|---|---|
| chao1 | 1810.9965 ± 80.4939 | 1890.3444 ± 76.6565 | 0.489 |
| dominance | 0.0087 ± 0.0024 | 0.015 ± 0.0049 | 0.281 |
| goods_coverage | 0.9993 ± 0.0001 | 0.999 ± 0.0001 | 0.055 |
| observed_features | 1802.1429 ± 80.6085 | 1878.4286 ± 76.4174 | 0.505 |
| pielou_e | 0.844 ± 0.0133 | 0.8304 ± 0.0186 | 0.565 |
| shannon | 9.124 ± 0.1846 | 9.0279 ± 0.2311 | 0.751 |
| simpson | 0.9913 ± 0.0024 | 0.985 ± 0.0049 | 0.281 |
1 Data represent the mean of 14 horses (n = 14)
2 Observed_features, Chao1 and Dominance indices were selected to identify community richness; Shannon and Simpson indices were used to identify community diversity; Good’s coverage index was used to calculate sequencing depth; Pielo_e index was used to calculate species evenness
Venn diagram analysis based on OTUs and β diversity analysis
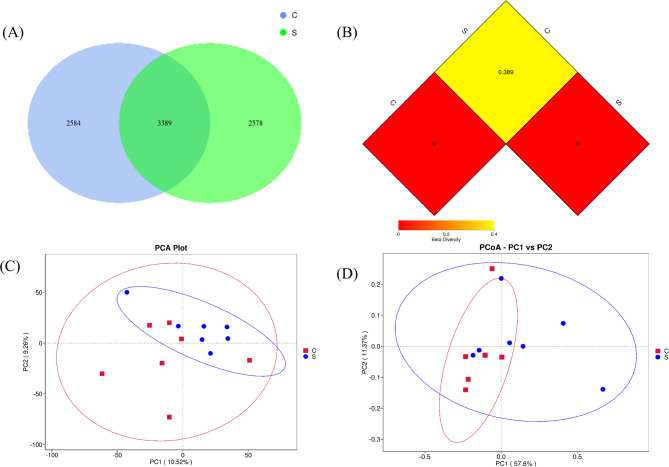
Figure 4 illustrates the effects of TMR and traditional feeding on the faecal microbiota of Akhal-Teke horses, including Venn diagram analysis based on OTUs and β diversity analysis. Subfigure A shows that the two groups shared 3,389 OTUs, with the C group possessing 2,584 OTUs and the S group having 2,578 OTUs.
Fig. 4.
A: Venn diagram analysis based on OTUs. B: Distance matrix heatmap of Beta diversity indices. C: Principal component analysis (PCA). D: Principal coordinate analysis (PCoA). (C: Control group: S: TMR group)
Figure 4B displays the results of distance matrix heatmap analysis, where a smaller dissimilarity coefficient indicates reduced differences in species diversity between the two samples. The dissimilarity coefficient between the C and S groups was 0.389, highlighting a notable disparity in species diversity between the groups.
In Fig. 4C, Principal Component Analysis (PCA)-based weighted analysis reveals that the first and second principal coordinates account for 10.52% and 9.26%, respectively, of the total microbiota variability.
Principal Coordinates Analysis (PCoA)-based weighted ordination analysis (Fig. 4D) further demonstrates that the first and second principal coordinates contribute 57.60% and 11.37%, respectively, to the overall representativeness of the detected microbiota.
Analysis of the relative abundance and variability of faecal Bacteria
Figure 5 displays the effects of TMR and traditional feeding on the abundance of faecal microbiota in Akhal-Teke horses.
Fig. 5.
Relative Abundance of Bacteria in Horse Faecal Relative Abundance Results of Bacteria, A: phylum; B: Outline; C: Eye; D: Science; E: Genus; F: Speciess). (C: Control group; S: TMR group)
As shown in Fig. 5A, the top 10 bacterial phyla include Firmicutes, Bacteroidota, Euryarchaeota, Proteobacteria, Verrucomicrobiota, Spirochaetota, Actinobacteriota, Halobacterota, Fibrobacterota, and Patescibacteria. Notably, the abundance of Spirochaetota in the S group was significantly higher than in the C group (P < 0.05), while Bacteroidota, Verrucomicrobiota and Proteobacteria abundance exhibited a decreasing trend in the S group.
Figure 5B presents the top 10 bacterial classes, which include Clostridia, Bacteroidia, Methanobacteria, Gammaproteobacteria, Bacilli, Verrucomicrobiae, Kiritimatiellae, Spirochaetia, Negativicutes, and Actinobacteriia.
As illustrated in Fig. 5D, the top 10 families include Methanobacteriaceae, Moraxellaceae, Lachnospiraceae, p-251-o5, Streptococcaceae, Akkermansiaceae, F082, Rikenellaceae, Oscillospiraceae, and Prevotellaceae. While most bacterial families showed no significant differences between the two groups, the abundance of the Prevotellaceae family was significantly reduced in the S group compared to the C group (P < 0.05).
At the genus level, the top 10 genera include Methanobrevibacter, Acinetobacter, Streptococcus, Akkermansia, Rikenellaceae_RC9_gut_group, Treponema, Christensenellaceae_R-7_group, Phascolarctobacterium, Lachnospiraceae_UCG-009, and UCG-002. Compared to the C group, the S group showed a significant increase in the abundance of Treponema and Christensenellaceae_R-7_group (P < 0.05), while UCG-002 was significantly reduced in the S group.
At the species level, the top 10 bacterial species include Methanobrevibacter_ruminantium, Lactobacillus_hayakitensis, Fibrobacter_sp_UWH6, Bacterium_enrichment_culture_clone_DPF35, Corynebacterium_stationis, Clostridium_butyricum, Lactobacillus_equi, Bacterium_WCE3006, Ruminococcus_sp_HUN007, and Facklamia_tabacinasalis. While most bacterial species did not show significant differences between the groups, Lactobacillus_hayakitensis was significantly more abundant in the S group compared to the C group (P < 0.05).
LefSe analysis and function prediction of faecal Bacteria
The differential microbial analysis and functional predictions of the gut microbiota in Akhal-Teke horses fed TMR and traditional diets are shown in Fig. 6.
Fig. 6.
Analysis Results of Differential Gut Microbes and Functional Prediction Between TMR Feeding and Traditional Feeding in Akhal-Teke Horses. A: LefSe Analysis; B: KEGG Pathway Annotation; C: Functional Prediction Clustering Analysis D: Correlation Analysis Between Differential Microbes and Predicted Functions
As depicted in Figs. 6A and 17 differentially abundant microbes were shared between the two groups. The differential species in the S group include f_Spirochaetaceae, c_Spirochaetia, o_Spirochaetales, g_Treponema, p_Spirochaetota, f_Christensenellaceae, o_Christensenellales, g_Christensenellaceae_R_7_group, f_Muribaculaceae, f_Lactobacillaceae, g_Ligilactobacillus, and s_Lactobacillus_hayakitensis.
Functional prediction results, shown in Fig. 6B, suggest that the microbial functions are primarily enriched in the following pathways: Cellular Processes, Environmental Information Processing, Genetic Information Processing, Human Diseases, Metabolism, Organismal Systems, and Unclassified pathways.
Figure 6C displays the clustering analysis of functional predictions. In the S group, the microbiota are predominantly concentrated in the following pathways: Metabolism of Cofactors and Vitamins, Membrane Transport, Transcription, Poorly Characterized, Infectious Diseases, Translation, Genetic Information Processing, Metabolism, Xenobiotics Biodegradation and Metabolism, Energy Metabolism, Cell Motility, Nucleotide Metabolism, Cell Growth and Death, Cellular Community, and Prokaryotes.
Figure 6D presents the correlation analysis between differential microbes and predicted functions.
o_Christensenellales and f_Christensenellaceae are positively correlated with Carbohydrate_metabolism (P = 0.76, r = 0.14).
f_Lactobacillaceae and g_Ligilactobacillus exhibit a positive correlation with Energy _metabolism (P = 0.34, r = 0.43).
f_Muribaculaceae shows a significant positive correlations with Membrane_transport (P < 0.05, r = 0.86), Cell_motility (P < 0.05, r = 0.79), Cell_growth_and_death (P < 0.05, r = 0.86), Environmental_adaptation (P < 0.05, r = 0.71), and Immune_diseases (P < 0.05, r = 0.71).
f_Muribaculaceae demonstrates an extremely significant positive correlation with Signal_transduction (P < 0.01, r = 0.96) and the Endocrine_system (P < 0.01, r = 0.86).
g_Christensenellaceae_R_7_group is significantly positively correlated with Lipid Metabolism (P < 0.05, r = 0.81) and Cellular_processes_and_signaling (P < 0.05, r = 0.79).
f_Bacteroidota and c_Bacteroidia exhibit a negative correlation with Carbohydrate_etabolism (P < 0.05, r = -0.86).
Although most bacterial taxa did not show significant differences between the two groups, the S group demonstrated a significant increase in s_Lactobacillus_hayakitensis compared to the C group (P < 0.05).
Correlation analysis of nutrient digestibility, blood biochemical indicators, and differential Bacteria
Figure 7 presents the correlation analysis between nutrient digestibility, blood biochemical markers, and differential bacteria in Akhal-Teke horses fed TMR. Key indicators that exhibited significant differences compared to the C Group, such as average daily weight gain, total weight gain, DM digestibility, ME, and UREA, alongside distinct microbial variations, were selected for this analysis. The correlation results are as follows:
Fig. 7.
Correlation Analysis Between Nutrients, Blood Biochemical Parameters, and Differential Microbes
o_Christensenellales and f_Christensenellaceae exhibit positive correlations with DM (r = 0.46), CP (r = 0.32), and P (r = 0.46).
f_Muribaculaceae is positively correlated with DM (r = 0.46).
f_Lactobacillaceae and g_Lactobacillaceae both show positive correlations with digestibility (r = 0.57 for each).
f_Lactobacillaceae and g_Lactobacillaceae also demonstrate positive correlations with ADF (r = 0.36 for each).
g_Christensenellaceae_R_7_group is positively correlated with DM (r = 0.43), CP (r = 0.25), total weight gain (r = 0.34), daily weight gain (r = 0.34), and P (r = 0.43).
s_Lactobacillus_hayakitensis is positively correlated with DM (r = 0.36).
Discussion
Effects of total mixed ration on body weight, body measurements, and nutrient digestibility in Akhal-Teke horses
The physical form, processing methods, and feeding strategies of feed are critical in nutrient digestion and absorption, as well as in shaping animals’ feeding behavior, ultimately impacting growth, development, and overall production performance [20]. In horses, the traditional feeding approach-administering roughage before concentrates and separating roughage and concentrate feeds-fails to maintain an optimal rough-to-concentrate ratio. This method not only limits feed intake but also leads to feed wastage [21, 22].
TMR feeding is a key technology for standardized, large-scale feeding management, widely used in ruminant husbandry [23, 24]. Designed to meet the nutritional needs of horses for CP, energy, fiber, minerals, and vitamins, TMR combines finely chopped roughage, concentrates, and various additives to create a nutritionally balanced diet. This approach stabilizes the digestive environment within the horse’s gastrointestinal system, enhances microbial activity, synchronizes the utilization of dietary proteins and carbohydrates, improves feed efficiency, and optimizes roughage use in controlled feeding systems [25]. Therefore, adapting and refining TMR feeding technologies suited to horses in China-drawing from the experiences of cattle and sheep breeding both domestically and internationally-is crucial for promoting the sustainable, healthy, and efficient development of the nation’s horse industry.
TMR feeding enhances the digestibility of DM, CP, NDF, calcium, and phosphorus, resulting in improved growth performance [26]. Research by Mohammad [27] has shown that, compared to separate feeding methods, TMR increases feed intake and milk production in dairy cows. According to JL Riond [10], horses fed TMR demonstrated significantly higher feed efficiency (kg gain/Mcal DE) than those fed conventionally (hay/concentrate), with growth rates exceeding those predicted by the NRC (1989). Furthermore, TMR feeding has been shown to improve daily weight gain in sika deer, enhance rumen fermentation, increase nutrient utilization, and promote protein metabolism in the deer’s body [28].
Feeding Akhal-Teke horses two distinct diets revealed that TMR significantly enhanced both total weight gain and average daily weight gain. Moreover, the apparent digestibility of DM, digestible energy, CP, ADF, and total phosphorus in the diet showed notable improvement. Wang et al. [29] reported an 18% increase in average daily weight gain in beef cattle fed TMR compared to the traditional feeding method (roughage first, then concentrates). By thoroughly mixing various nutritional components, TMR facilitates a more balanced and sustained digestion and absorption process compared to conventional feeding, resulting in higher nutritional absorption and utilization rates of roughage. This mechanism likely explains the observed increases in body weight and nutrient digestibility in horses.
Dong et al. [30] demonstrated that an optimal roughage length (2 cm of bean straw) improved average daily weight gain and final body weight in 12-14-month-old Texas male donkeys, while reducing the feed-to-weight gain ratio. Similarly, Ma et al. [31] examined the effect of roughage length (alfalfa) in TMR on nutrient digestibility in Holstein dairy cows. Their findings indicated that a roughage length of 5 cm resulted in significantly higher digestibility of DM, CP, NDF, and ADF compared to lengths of 1 cm and 9 cm. This suggests that while shorter roughage lengths may reduce feeding and rumination time, excessively short lengths could lower rumen pH, ultimately decreasing digestibility. An appropriate roughage length can therefore optimize nutrient digestibility.
In this study, alfalfa roughage was cut to a 3 cm length. Whether this is the optimal roughage length for TMR feeding in horses remains unclear. Future investigations should explore the effects of different roughage lengths on feeding outcomes under controlled nutritional conditions to identify the ideal length for TMR in horses.
Effects of total mixed ration on blood biochemical indicators in Akhal-Teke horses
Blood biochemical markers are crucial for assessing the physiological state and nutritional metabolism of livestock. These indicators provide valuable insights into animal health while reflecting nutrient digestion, absorption, and metabolism [32]. Critical markers, such as T-Bil, D-Bil, ALT, and AST, are essential for evaluating liver and bile function. As amino acid transferases, ALT and AST play significant roles in amino acid metabolism and the conversion of the body’s three primary nutrients [33, 34]. CK, predominantly found in skeletal muscle, the heart, and brain tissues, serves as a key marker for energy metabolism and muscle contraction [35].
Studies by Hou Fang and Mohri [5, 6] in Akhal-Teke horses revealed blood TP levels ranging from 52.93 to 73.86 g/L, ALB from 27.87 to 44.31 g/L, Cr from 92.2 to 114.04 µmol/L, urea at 4.2 mmol, cholesterol between 1.85 and 2.50 mmol/L, T-Bil from 10.67 to 13.34 µmol/L, D-Bil at 6.2 µmol/L, blood Glu from 4.27 to 5.03 mmol/L, ALP from 133.07 to 459.75 U/L, ALT from 27.93 to 9.22 U/L, CK from 34.0 to 165.6 U/L, Ca2+ between 3.00 and 2.83 mmol/L, and P from 0.92 to 1.26 mmol/L. These values suggest that TMR feeding does not negatively affect the health of Akhal-Teke horses. Our results, which show similar biochemical values in TMR-fed horses, are consistent with the findings of Hou Fang and Mohri [5, 35], further confirming that TMR feeding does not harm the health of these horses.
TP levels reflect dietary protein intake and utilization, while ALB is crucial for metabolite transport and the provision of essential proteins [36]. In this investigation, serum TP, ALB, and GLB levels remained stable in TMR-fed equines (P > 0.05), demonstrating that under isoenergetic and isonitrogenous conditions, neither diet formulation nor feeding management exerted measurable effects on serum protein homeostasis (TP/ALB/GLB) in equids. UREA levels, which indicate nitrogen utilization efficiency, are closely tied to dietary protein intake and the effectiveness of protein breakdown in the digestive system. Elevated serum UREA levels signal reduced nitrogen utilization, which inversely correlates with protein efficiency [37]. In our study under identical dietary composition conditions, while horses exhibited equivalent crude protein intake, the CP digestibility in the S group (TMR diet) was significantly higher than that in the C group (P < 0.05). This indicates that the TMR diet provided abundant nitrogen sources for bodily nitrogen absorption. Furthermore, the significantly reduced serum urea levels (P < 0.01) demonstrated that sufficient nitrogen sources established the material foundation for protein synthesis and metabolism.
ALT, a key aminotransferase present in liver cells, is commonly used to evaluate liver function. Under normal conditions, ALT activity in animal serum remains low. However, when liver cell metabolic balance is disrupted and membrane permeability increases, ALT can leak into the bloodstream [38]. In the present study, no significant difference was observed in ALT levels between TMR-fed and traditionally fed horses (P > 0.05), although all values remained within the normal reference ranges reported in previous studies [5, 6]. This difference may indicate heightened liver metabolism in the traditionally fed horses. In addition, this study observed an increasing trend in blood levels of Cre, T-Bil, D-Bil, TC, AST, AST/ALT, and CK in horses following TMR feeding. Given the liver’s critical role in bilirubin secretion and metabolism, an increase in bilirubin levels typically suggests impairment in liver and bile function [39]. This suggests that TMR feeding enhances liver and bile function, contributing to improved amino acid composition, energy metabolism, and overall physical activity.
Ca²⁺ and P constitute approximately 90% of the mineral content in horses, playing essential roles in skeletal growth, nerve conduction, blood clotting, osmotic pressure maintenance, and enzyme activation. Consequently, horses are particularly vulnerable to deficiencies in calcium and phosphorus [40]. In the current study, there was a trend for TMR-fed horses to have higher levels of Ca2+ and inorganic P compared to the control group. Whether this increase is directly linked to improved mineral digestibility requires further investigation.
Effects of total mixed ration on faecal pH and volatile fatty acids in Akhal-Teke horses
Equines, as single-stomached herbivores, lack a rumen like cattle and sheep. However, their cecum and colon perform fermentation functions similar to the rumen of ruminants. In horses, microbial fermentation primarily occurs in the hindgut, and dietary changes can significantly influence gut microbiota and the fermentation of intestinal polysaccharides [41]. Faecal pH and VFA concentrations are key indicators of hindgut fermentation. Faecal pH reflects the stability of the large intestine (cecum and colon) environment and the normalcy of fermentation. It is influenced by various factors, with diet composition and nutritional intake playing particularly critical roles [42]. A consistent diet offers microbes a uniform fermentation substrate, thus promoting stability in hindgut environment markers [43].
The TMR feeding method can synchronize protein and carbohydrate utilization in the rumens of ruminants [44], stabilize rumen fluid pH, enhance microbial growth, and improve rumen function [45]. Brittany E et al. [46] studied faecal pH in 30 horses (27 Thoroughbreds, 1 Quarter Horse, 1 Standardbred and 1 Paint ×Thoroughbred cross). The results showed that the mean faecal fluid pH was 7.79 (range: 7.28 to 8.07). In our study, faecal pH values in horses were 7.40 (conventional diet) and 7.38 (TMR), which is consistent with the results of Brittany E et al. Moreover, there was no statistically significant difference in faecal pH between the TMR-fed group and the conventionally-fed group. Plant cell wall and fiber degradation result in the production of VFAs [47], with approximately 3 g/L typically produced in the cecum and colon. These fermentation products consist of 70-75% acetate, 18-23% propionate, and 5-7% butyrate, which are absorbed in the cecum and colon, providing 30-70% of the animal’s maintenance energy. The absorption ratio is influenced by diet composition, particularly the concentration of propionate.
Zhang et al. [48] found that when Dezhou donkeys were fed a basal diet with a concentrate-to-forage ratio of 30:70 (using corn stover as forage), the study investigating their hindgut fermentation revealed the following: cecal content pH was 6.85; TVFA concentration was 96.7 mM; acetate concentration was 53.5 mol/100 mol VFA; propionate concentration was 31.8 mmol. Furthermore, the molar proportion of acetate progressively increased in the cecal, dorsal colonic, and ventral colonic contents of the donkeys. Correspondingly, the activities of CMCase (carboxymethyl cellulase), AVI (avicelase), Xlyase (xylanase), and AE (acetylesterase) in the ventral colonic content were higher than those in the cecal and dorsal colonic contents. This indicates that the colon has higher fiber-degrading activity compared to the cecum, enabling it to degrade dietary fiber and produce more acetate. In our study, horses fed TMR exhibited higher levels of acetate, butyrate, and total VFA in their faeces compared to those on traditional diets. This can be attributed to the accelerated carbohydrate degradation in the TMR diet, which increases the surface area available for microbial-substrate contact, enhancing VFA production and lowering faecal pH.
Effects of total mixed ration on faecal microbiota diversity in Akhal-Teke horses
Equine species host a diverse and active microbial community in their large intestine, where undigested food residues from the small intestine are continuously fermented into usable nutrients. The bacterial population in cecal chyme ranges from (5–7) × 109/g, with considerable variation between different sections of the large intestine, such as the cecum and colon. Key bacterial genera include Streptococcus, Bacteroides, and Lactobacillus, while protozoa are present in lower numbers, typically ranging from 102 to 105/g. The concentration of fiber-degrading bacteria in the cecum is six times higher than in the colon, despite the cecum and colon having volumes of approximately 30 L and 180 L, respectively. Protein-degrading bacteria are present at concentrations ranging from (2–8) × 105/g. The physicochemical environment of the large intestine-comprising factors such as pH (6–7), redox potential, anaerobic conditions, temperature, and chyme mixing-significantly influences the composition, structure, and diversity of the intestinal microbiota. These factors are predominantly shaped by dietary structure, with notable differences between traditional forage feeding and TMR feeding, particularly in terms of roughage length and ingredient mixing uniformity. Zhang et al. [49] observed that short roughage (3 ± 0.5 cm) fed to young ruminants helped maintain rumen microbiota homeostasis in pre-weaned buffalo calves. Xu Junzhao [50] demonstrated that TMR diets, compared to fermented TMR diets, significantly increased the relative abundance of Succiniclasticum and Ruminococcaceae_NK4A214_group in the rumen. These findings indicate that roughage length can influence the gastrointestinal microbiota of animals, potentially affecting nutrient digestibility.
Palida Guli·Yushan [51] reported significant seasonal variations in the microbial coverage (Good’s Coverage index) and microbial richness (Chao1 index) of the faecal microbiota in Akhal-Teke horses (P < 0.05), with higher diversity and abundance observed in summer compared to winter, However, the abundance of Verrucomicrobia and Actinobacteria exhibited significant increases during winter. Hou Fang [52] identified 19 phyla in the intestinal microbiota of 21 Akhal-Teke horses, including Fibrobacteres, Spirochaetae, Lentisphaerae, WA-aaa01f12, Cyanobacteria, Synergistetes, Verrucomicrobia, Firmicutes, Tenericutes, Actinobacteria, Armatimonadetes, Bacteria-Unclassified, Saccharibacteria, Chloroflexi, Bacteroidetes, Planctomycetes, Proteobacteria, Elusimicrobia, and Euryarchaeota. Our study confirmed the dominance of these same phyla, with Firmicutes, Bacteroidetes, Euryarchaeota, and Proteobacteria being the most abundant. These findings are consistent with previous research on the intestinal/rumen microbiota of mammals [53–55], where Firmicutes was the predominant phylum, followed by Bacteroidetes, mirroring observations in horse faecal microbiota [56]. An increase in dietary fiber promotes Firmicutes abundance while decreasing Bacteroidetes levels [57, 58]. Given that the roughage-to-concentrate ratio in our diet was approximately 1:9, the high fiber content likely contributed to the elevated abundance of Firmicutes. Additionally, TMR feeding resulted in increased Firmicutes abundance and reduced Bacteroidetes levels. The dietary processing method likely influences fiber digestibility, thereby impacting microbiota composition. Notably, following TMR feeding, a significant increase in the abundance of Spirochaetota, Treponema, Christensenellaceae_R-7_group, and Lactobacillus_hayakitensis was observed, while levels of Prevotellaceae decreased. The sole roughage in our study was alfalfa lucerne, a high-quality forage known for its beneficial effects on animal nutrition and health, especially due to its fiber content, which is essential for gut health, immunity, and microbial diversity. Wang et al. [59] reported that supplementing the diet of weaned piglets with 5% alfalfa powder significantly alleviated diarrhea, reduced serum levels of inflammatory markers (IL-1β and TNF-α), enhanced antioxidant capacity, and promoted the production of short-chain fatty acids (especially butyrate) by modulating the abundance of fiber-degrading bacteria (e.g., Christensenellaceae_R-7_group, Pediococcus, and Weissella), thus improving host health. In our study, a significant increase in fiber-degrading bacteria (such as Treponema, Christensenellaceae_R-7_group, and Lactobacillus_hayakitensis) was observed in the faecal of TMR-fed horses, suggesting that optimizing the length of alfalfa lucerne enhances fiber degradation and promotes the abundance of these bacteria. Prevotellaceae has been associated with the production of VFAs like propionate, which the host utilizes for energy [60]. An increase in Prevotellaceae abundance may also reduce methane production. However, our study found that TMR feeding significantly reduced Prevotellaceae levels in horse faecal without lowering VFA concentrations, a result inconsistent with Strobel et al.‘s [61] findings in ruminants, likely due to the differences in the digestive processes between single-stomached herbivores and ruminants.
LefSe analysis of faecal bacteria identified twelve differentially abundant bacteria in TMR-fed horses, including Treponema, Spirochaetota, Christensenellaceae, Christensenellales, Christensenellaceae_R-7_group, and Muribaculaceae, all of which are known for their role in fiber degradation. Christensenellaceae_R-7_group, a key member of the gut microbiota, participates in the butyrate metabolism pathway, essential for gut health, as butyrate is a key metabolic product of gut bacteria. Muribaculaceae, commonly found in healthy individuals, produces acetate and propionate through fermentation [62]. KEGG pathway analysis based on Tax4Fun functional predictions for the TMR-fed group revealed a positive correlation with metabolic and energy pathways. These results suggest that TMR feeding can regulate the composition and abundance of gut microbiota in horses, influencing the metabolism of dietary nutrients.
Conclusions
This study demonstrated that feeding Akhal-Teke horses TMR diets altered gut microbiota diversity, leading to improved apparent digestibility of DM, DE, CP, ADF, thus promoting weight gain in the horses.
Acknowledgements
The authors would like to acknowledge the Zhaosu County Xingzhao Herding Farm racecourse for allowing sample collection from their foal’s, the vet and staff for assistance with for care of theanimals and samples collection.” to: “The authors sincerely thank Xinjiang YEMA Group Co., Ltd. for providing the experimental animals and are grateful to the staff members for their assistance in horse feeding and sample collection.
Author contributions
HJ and PL: Contributed significantly to article conception and design, data acquisition, data analysis, and interpretation.SZ and KC: Performed animal feeding and sample collection. All authors read and approved the final manuscript.XL: Participated in critical revisions of important knowledge and content in the manuscript.
Funding
The study was supported by Major Science and Technology Special Project of Xinjiang Uygur Autonomous Region (2022A02013-2-3); Xinjiang Key Laboratory of Horse Breeding and Exercise Physiology Open Subjects (XJMFY202403).
Data availability
All data generated or analysed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
All protocols were approved by the Animal Care and Use Committee of Xinjiang Agricultural University (permission number 2018012). Informed consent - Owners gave informed consent for their animals’ inclusion in the study. All methods were carried out in accordance with relevant guidelines and regulations for the use of animal subjects. The study was carried out in compliance with the ARRIVE guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hongxin Jing and Pengshun Liu contributed equally to this article.
References
- 1.Hendricks BL. International encyclopedia of horse breeds. Choice Reviews Online. 1996;33(09):33–4847. [Google Scholar]
- 2.Kuznecova Y. K 70 Letiyu Konnogo Perehoda Ashabad Moskva. Ahal Teke IrIfo. 2005;78–83.
- 3.Mackay-Smith A. Speed and the thoroughbred: the complete history. New York, NY, USA: Derrydale; 2000. [Google Scholar]
- 4.Kätlin K, Priit K, Ülle J, Teet S. Myosin heavy chain pattern in the Akhal-Teke horses animal. 2011;5(5):658–62. [DOI] [PubMed]
- 5.Hou F, Xu XL, Hou Y, Li XG, Zhao HQ, Wang JQ, et al. Study on the stress response of Akhaltegin horses to long-distance transport. China Anim Husb Veterinary Med. 2017;44(11):3156–62. [Google Scholar]
- 6.Mohri M, Sardari K, Farzaneh N. Serum biochemistry of Iranian Turkmen (Akhal-Teke) horses. Comp Clin Pathol. 2005;13:128–31. [Google Scholar]
- 7.Xue B, Wang JX, Zhao Y, Liu X, Hao HS. Application of total mixed ration (TMR) in dairy farming.Today’s Animal Husbandry and Veterinary Medicine,2006;(05):12–3.
- 8.Schingoethe DJ. A 100-Year review: total mixed ration feeding of dairy cows. J Dairy Sci. 2017;100(12):10143–50. [DOI] [PubMed] [Google Scholar]
- 9.Xie LH, Xing JY, Qi XZ, Lu T, Jin YL, Muhammad FA, et al. Effects of concentrate feeding sequence on growth performance, nutrient digestibility, VFA production, and fecal microbiota of weaned donkeys. Animals. 2023;13(18):2893–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riond JL, Leoni S, Wanner M. Comparative study of three feeding methods for draught horses of the Swiss army. Schweiz Arch Tierheilkd. 2000;142(10):570–9. [PubMed] [Google Scholar]
- 11.AOAC. Official methods of analysis. 18th ed. Gaithersburg, MD: Association of Official Analytical Chemists; 2007. [Google Scholar]
- 12.Van Soest PJ, Robertson JB, Lewis BA. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. 1991;74(10):3583–97. [DOI] [PubMed] [Google Scholar]
- 13.Magoč T, Salzberg ST. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27(21):2957–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7(5):335–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73(16):5261–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daniel MD, Morgan N, Price JK, Goodrich EP, Nawrocki TZ, DeSantis AJ, et al. An improved greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME Journa. 2012;6(3):610–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jai RR, Yan H, Navas-Molina JA, Walters WA, Ursell LK, Gibbons SM, et al. Subsampled open-reference clustering creates consistent, comprehensive OTU definitions and scales to billions of sequences. PeerJ. 2014;2(0):e545–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cole JR, Wang Q, Cardenas E, Fish JA, Chai B, Farris RJ, et al. The ribosomal database project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009;37(Database):D141–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicola S, Jacques L, Levi W, Dirk G, Larisa M, Wendy S, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12(6):R60–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morgan GIL, Jesse ZJGC, Daniel M, Dan K, Joshua AR, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol. 2013;31(9):814–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yin F, Ma MB, Tian YM, Yang FY. Effects of different feeding methods on growth performance and blood physiological and biochemical indexes of horses. Breed Feeding. 2023;22(06):22–7. [Google Scholar]
- 22.Jia YY. Effect of different ratios of fast/slow fermenting carbohydrates in the ration on Donkey milk yield and milk composition. Tarim University; 2022.
- 23.Li Y, Zhang L, Niu J. Feeding technology of total mixed ration (TMR) for dairy cows. Anim Husb Veterinary Science(Electronic Edition). 2021;(01):46–7.
- 24.Zhang WQ. Effects of fermented total mixed diets on growth performance and rumen fermentation function of lake sheep. Guangxi University; 2024.
- 25.Ma YJ, Wang BY, Li FD, Zhao YZ, Hu X. Effects of different nutrient levels of total mixed diets on production performance, nutrient apparent digestibility and slaughtering performance of housed fattened lambs. J Grass Ind. 2012;21(04):252–8. [Google Scholar]
- 26.Bao K, Wang KY, Wang XX, Yang FH, Li GY. Effects of different feeding methods on growth performance, nutrient digestibility and blood biochemical indexes of Sika deer. Speciality Res. 2015;37(03):1–5. [Google Scholar]
- 27.Mohammad M, Gorgulu M, Goncu S. The effects of total mixed ration and separate feeding on lactational performance of dairy cows. Asian Res J Agric. 2017;5(2):1–7. [Google Scholar]
- 28.Wang KY. Effects of different total mixed ration (TMR) with different concentrate-to-rubber ratio on the digestive metabolism and production performance of Sika deer. Beijing: Chinese Academy of Agricultural Sciences; 2008. [Google Scholar]
- 29.Wang DW, Wu ZF, Liu CB. Comparison of weight gain of beef cattle fed total mixed diets and traditional diets. J Anim Husb Veterinary Med. 2024;43(04):42–4. [Google Scholar]
- 30.Dong BY, Li WQ, Ji CL, Qu HL, Feng YL, Chen YG, et al. Effects of physical properties of roughage on the growth performance of Texas male donkeys and analysis of economic benefits. Feed Ind. 2020;41(17):61–4. [Google Scholar]
- 31.Ma DM, Miao SJ, Liu ZQ, Wan YM. Effects of forage length on feeding behaviour, rumen fermentation and diet digestibility in dairy cows fed a complete mixed diet. China Cattle Sci. 2009;35(04):9–13. [Google Scholar]
- 32.Li JH, Huang XX, He LW, Li C, Jing HJ, Lin J, et al. Effect of ellagic acid on body weight, nutrient digestibility, fecal microbiota, and urolithin A metabolism in thoroughbred horses. J Anim Sci. 2023;101(0):0–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katiuska SA, Juan Carlos GP, Ana MJ. Use of laboratory testing to diagnose liver and biliary dysfunction in the horse. J Gastroenterol Hepatol Res. 2013;2(10):807–13. [Google Scholar]
- 34.Ma P, Wu Y, Zeng Q, Ye G, Chen J, Ye X, et al. Oxidative damage induced by Chlorpyrifos in the hepatic and renal tissue of Kunming mice and the antioxidant role of vitamin E. Food Chem Toxicol. 2013;58(0):177–83. [DOI] [PubMed] [Google Scholar]
- 35.Drent M, Cobben NAM, Henderson RF, Wouters EFM, Dieijen-Visser V. Usefulness of lactate dehydrogenase and its isoenzymes as indicators of lung damage or inflammation. Eur Respir J. 1996;9(8):1736–42. [DOI] [PubMed] [Google Scholar]
- 36.Chen H, Aorigele, Wang C, Simujide, Aricha, Zhang J, et al. Effects of chronic cold stress on the serum enzyme activity, protein metabolism and serum hormone serum hormone secretion of grazing Mongolian cows. J China Agricultural Univ. 2019;24(10):47–54. [Google Scholar]
- 37.Rown MS, Ponce CH, Pulikanti R. Adaptation of beef cattle to high-concentrate diets:performance and ruminal metabolism. J Anim Sci. 2006;84(13):E25–33. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Cao Z, Wang LD, Dong BQ, Qi SZ, Xu XL, et al. Effects of linseed oil supplementation duration on fatty acid profile and fatty acid metabolism-related genes in the muscles of Chinese crested white ducks. Poult Sci. 2023;102(10):102896–102896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benton MC, Lea RA, Macartney-Coxson D, Bellis C, Carless AW, Curran EJ, et al. Serum bilirubin concentration is modified by UGT1A1 haplotypes and influences risk of Type-2 diabetes in the Norfolk Island genetic isolate. BMC Genomic Data. 2015;16(1):0–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caple IW, Doake PA, Ellis PG. Assessment of the calcium and phosphorus nutrition in horses by analysis of urine. Aust Vet. 1982;58(4):125–31. [DOI] [PubMed] [Google Scholar]
- 41.Dicks LT, Botha MH, Dicks E, Botes M. The equine gastro-intestinal tract: an overview of the microbiota, disease and treatment. Livest Sci. 2014;160:69–81. [Google Scholar]
- 42.Wang T. Effects of different dietary concentrate to forage ratios on rumen fluid pH and VFA levels and blood VFA levels in dairy goats. Animal Husbandry & Veterinary Medicine; 2013.
- 43.Mcdonald P, Edwards RA. The influence of conservation methods on digestion and utilization of forages by ruminants. The Proceedings of the Nu-trition Society. 2011;35(2):201–211. [DOI] [PubMed]
- 44.Liu TY. Study on the effect of forage-based total mixed diets on the production performance of meat goats. Doctoral dissertation.Hohhot: Inner Mongolia Agricultural University. 2012.
- 45.Zhu XY, Chen QL, Ni JF. Experiment on the application of TMR technology in the fattening period of lake sheep. China Livest Poult Breed Ind. 2017;13(5):64–5. [Google Scholar]
- 46.Brittany EH, L ML, Andrea SH, Michael DC. DF. Effect of dietary starch source and concentration on equine fecal microbiota. PLoS ONE. 2016;11(4). [DOI] [PMC free article] [PubMed]
- 47.Richardson K, Murray J. Fiber for performance horses: A review. J Equine Vet Erinary Sci. 2016;46:31–9. [Google Scholar]
- 48.Zhang Z, Zhou Q, Zheng R, Zhu M, Wang C. Comparative analysis of cellulolytic enzymes and volatile fatty acids in cecocolonic fluid of Dezhou donkeys. Proceedings of China Animal Agriculture Association. 2021;102–108.
- 49.Zhang J, Wang K, Li H, Chen AQ, Zou CX, Lin B. Effects of roughage source and length on growth performance, nutrient apparent digestibility and rumen flora structure of Buffalo calves. J Anim Nutr. 2021;33(12):6876–88. [Google Scholar]
- 50.Xu JZ. Effects of enzyme-fermented total mixed diets on growth performance, nutrient digestibility, rumen fermentation and bacterial flora of beef cattle. Inner Mongolia University for Nationalities; 2023.
- 51.Palida Guli YS. Study on the intestinal microbial diversity of Ahab-Jiejin and Przewalski’s wild horses in captivity.Xinjiang Agricultural University. 2023.
- 52.Hou F. Detection of five epidemic diseases in imported Akhal-Teke horses and the effects of long-distance transport on their physiology, biochemistry and intestinal microbiology. Xinjiang Agricultural Univ. 2017.
- 53.Pitta D, Pinchak WE, Dowd SE, Osterstock JS, Gontcharova V, Youn E, et al. Rumen bacterial diversity dynamics associated with changing from Bermudagrass hay to grazed winter wheat diets. Microb Ecol. 2009;59(3):511–22. [DOI] [PubMed] [Google Scholar]
- 54.Ochman H, Worobey M, Kuo C, Ndjango JBN, Peeters M, Hahn BH, et al. Evolutionary relationships of wild hominids recapitulated by gut microbial communities. PLoS Biol. 2010;8(11):e1000546–1000546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arumugam M, Raes J, Pelletier E, Le PD, Yamada T, Mende DR, et al. Enterotypes of the human gut Microbiome. Nature. 2011;473(7346):174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brelc JM, Antonopoulos DA, Berg Miller ME, Wilson MK, Yannarell AC, Dinsdale EA, et al. Gene-centric metagenomics of the fiber-adherent bovine rumen Microbiome reveals forage specific glycoside hydrolases. Proc Natl Acad Sci USA. 2009;106(6):1948–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nidhi PJ, Solanki AP, Tejas S, Amrutlal K, Patel S, Parnerkar JK, et al. Metagenome of Mehsani Buffalo rumen microbiota: an assessment of variation in feed-dependent phylogenetic and functional classification. Microb Physiol. 2014;24(4):249–61. [DOI] [PubMed] [Google Scholar]
- 58.Parnell JA, Reimer RA. Prebiotic fiber modulation of the gut microbiota improves risk factors for obesity and the metabolic syndrome. Gut Microbes. 2012;3(1):29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huangfu W, Ma J, Zhang Y, Liu M, Liu B, Zhao J, et al. Dietary fiber-derived butyrate alleviates piglet weaning stress by modulating the TLR4/MyD88/NF-κB pathway. Nutrients. 2024;16:1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu Y. Effects of per-rumen methionine on growth, nutrient digestion and rumen microbial macro-genomics in Yaks. Southwest University for Nationalities; 2022.
- 61.Strobel HJ. Vitamin B12-dependent propionate production by the ruminal bacterium Prevotella ruminicola 23. Appl Environ Microbiol. 1992;58(7):2331–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li LY, Qiu N, Meng YQ, Wang CY, Mine Y, Keast R, et al. Preserved egg white alleviates DSS-induced colitis in mice through the reduction of oxidative stress, modulation of Infl ammatory cytokines, NF-κB, MAPK and gut microbiota composition. Food Sci Hum Wellness. 2023;12(1):312–32. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.